Abstract
Glucocorticoids act through the glucocorticoid receptor (GR), which can function as a transcriptional activator or repressor, to elicit cytostatic and cytotoxic effects in a variety of cells. The molecular mechanisms regulating these events and the target genes affected by the activated receptor remain largely undefined. Using cultured human osteosarcoma cells as a model for the GR antiproliferative effect, we demonstrate that in U20S cells, GR activation leads to irreversible growth inhibition, apoptosis, and repression of Bcl2. This cytotoxic effect is mediated by GR’s transcriptional repression function, since transactivation-deficient mutants and ligands still bring about apoptosis and Bcl2 down-regulation. In contrast, the antiproliferative effect of GR in SAOS2 cells is reversible, does not result in apoptosis or repression of Bcl2, and is a function of the receptor’s ability to stimulate transcription. Thus, the cytotoxic versus cytostatic outcome of glucocorticoid treatment is cell context dependent. Interestingly, the cytostatic effect of glucocorticoids in SAOS2 cells involves multiple GR activation surfaces. GR mutants and ligands that disrupt individual transcriptional activation functions (activation function 1 [AF-1] and AF-2) or receptor dimerization fail to fully inhibit cellular proliferation and, remarkably, discriminate between the targets of GR’s cytostatic action, the cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1. Induction of p21Cip1 is agonist dependent and requires AF-2 but not AF-1 or GR dimerization. In contrast, induction of p27Kip1 is agonist independent, does not require AF-2 or AF-1, but depends on GR dimerization. Our findings indicate that multiple GR transcriptional regulatory mechanisms that employ distinct receptor surfaces are used to evoke either the cytostatic or cytotoxic response to glucocorticoids.
Glucocorticoid hormones regulate a variety of biological processes, including carbohydrate, lipid, and protein metabolism, cell growth and proliferation, development, and reproduction. In most cell contexts, glucocorticoids exert antiproliferative effects, which has prompted their use clinically as a part of anticancer therapy for a diverse range of dysplasias, including lymphoproliferative disorders and several solid tumors (20, 30, 71). The molecular basis of the antiproliferative action of these compounds, however, is not fully understood. Glucocorticoids exert their effects by activating the glucocorticoid receptor (GR), a ligand-dependent transcriptional regulator that transduces the hormonal signal into the nucleus to alter the expression of target genes. Transcriptional responses triggered by the hormone-activated GR include both positive (activation) and negative (repression) regulation, depending on the DNA sequences in the target promoters, termed glucocorticoid response elements (GREs), to which the receptor binds. A canonical or simple GRE is an imperfect palindrome (14, 46) which allows the binding of the GR head-to-head dimer (58), leading to enhanced transcription initiation from the target promoter. Examples of more complex GR-responsive genes include (i) promoters with composite GREs containing an interacting surface both for the receptor and for the nonreceptor enhancer-binding factor(s) and (ii) promoters which lack GR-binding sequences altogether but to which the receptor is tethered through an interacting protein. At composite and tethering elements, GR appears to function as a monomer and can either activate (84, 85) or repress (25, 63, 80) transcription, depending on the promoter and the receptor-interacting protein(s) bound at a site.
Like other steroid receptors, GR is composed of three distinct domains, including an N-terminal transcriptional regulatory region, a central DNA-binding domain (DBD), and a C-terminal signalling domain involved in ligand binding. Two parts of the receptor molecule have been shown to possess an intrinsic ability to activate transcription when tethered to DNA. A hormone-independent activation function 1 (AF-1) is located at the receptor N terminus between amino acids 202 and 264 (using the rat GR numbering scheme); the activity of this domain can be both positively and negatively regulated by phosphorylation (49, 76, 78). Point mutations in this region severely compromise the transcriptional activity of AF-1 (4, 43). The second transcriptional regulatory function, AF-2, maps to the receptor ligand-binding domain (LBD) and is dependent on agonist for activity. The transcriptional activity of AF-2 is mediated by a group of steroid receptor coactivators, including the p160 family (steroid receptor coactivator 1 [SRC1], GR-interacting protein 1 [GRIP1], and ACTR), CREB-binding protein/p300, and PCAF, which associate with the GR LBD in a hormone-dependent manner (7, 17, 41, 68, 82, 91). Thus, ligand induces the LBD to acquire a conformation promoting the interaction with coactivators, which in turn facilitates transcription initiation.
In contrast to transcriptional activation domains, the GR regions responsible for transcriptional repression do not map to a distinct domain. This can be explained in part by the fact that GR-mediated repression involves both protein-DNA and protein-protein interactions between the receptor and a nonreceptor factor, such as AP-1 (25, 101, 103) or NF-κB (11, 23, 53, 99). Therefore, specific promoter contexts dictate which individual GR domains are required for these interactions and thus for transcriptional repression.
In many cell types, GR activation leads to a G1 cell cycle arrest; this cytostatic condition is often reversible, such that upon withdrawal of glucocorticoid the cells reenter the cell cycle (83, 87). In other cell types, glucocorticoid treatment is cytotoxic and irreversible and results in programmed cell death (PCD) or apoptosis (16, 24, 62, 64). The transcriptional regulatory mechanisms underlying the cytostatic versus the cytotoxic effects of GR and the target genes affected by the receptor have not been determined.
Regulation of the G1-to-S phase transition in the mammalian cell cycle can be viewed as a balance between growth-promoting and growth-inhibitory factors. Mitogenic proteins include cyclins, cyclin-dependent kinases (CDKs), CDK regulatory kinase and phosphatase (CAK and CDC25), and transcription factors E2F and cMyc (21, 60, 65, 86). Antimitogenic proteins include Rb (8, 59, 96) and CDK-inhibitory proteins (CDIs), which comprise two distinct subfamilies based on structural and functional homologies: the INK subfamily (p15, p16, p18, and p19) and the Cip/Kip subfamily (p21Cip1, p27Kip1, and p57Kip2) (33). With respect to the antimitogenic effect of GR, it has been demonstrated that receptor activation leads to down-regulation of several growth-promoting factors, including cMyc, cyclin D3, and CDK4 (75). It has recently been shown that in some cell types, the level of p21Cip1 (hereafter referred to as p21) increases in response to GR activation (72, 77). A GR-responsive element in the p21 promoter has been localized to a binding site for CCAAT/enhancer-binding protein α (C/EBPα) (13, 22), whose expression is also GR inducible (73). However, rapid protein synthesis-independent induction of p21 by glucocorticoids suggests a direct transcriptional effect of GR on the p21 promoter, in addition to C/EBPα-dependent stimulation of p21 expression. The tissue and cell specificities of such regulation and the relationships between individual cell cycle regulatory proteins affected by GR are not understood.
Cellular apoptosis appears to be a pathway common to many cell types, including cells of lymphoid and neuronal origin, neutrophils, fibroblasts, and a variety of cultured cell lines (6, 18, 28, 70). GR-induced growth inhibition and apoptosis may result from a failure to differentiate due to a lack of, for example, essential cytokines, growth factors, or extracellular matrix (44). The combined efforts of many laboratories have partially dissected the complex signalling pathways resulting in PCD. Like other signalling cascades, the cell death program involves several protein families, including the interleukin 1β-converting-like enzymes (ICE proteases), cysteine-containing aspartate-specific proteases termed caspases, and the Bcl2 family, which function at distinct steps of the apoptotic pathway (19, 89, 102). Adding to this complexity, the Bcl2 family includes both proapoptotic (Bax, Bad, and BclXS) and antiapoptotic (Bcl2 and BclXL) factors (1). Thus, Bcl2 overexpression can rescue thymocytes from glucocorticoid-induced cell death; however, cell survival depends on the relative abundances of Bcl2 and Bax present in cells (5, 27, 81). With respect to glucocorticoid-induced apoptosis, it has been demonstrated that eliminating caspase 9, but not caspase 3, activity prevents GR-dependent cell death (32, 100). The primary targets responsible for initiating the apoptotic cascade in response to GR activation and transcriptional events that comprise the GR-induced cytotoxic pathway remain elusive.
We have developed a cultured cell system to study GR-mediated cell cycle arrest by using two GR-negative human osteosarcoma cell lines, U2OS and SAOS2. These cell lines provide a powerful tool for dissecting the mechanisms of GR-mediated cell growth inhibition due to the inherent differences in G1 regulatory proteins present in these cells: U2OS cells are p53 and Rb positive and express all three D-type cyclins (D1, D2, and D3), whereas SAOS2 cells lack Rb and p53 and produce only cyclin D3 (26). Ectopic expression and activation of the wild-type (wt) rat GR in both cell lines lead to cell cycle arrest at the G1 stage and morphological alterations, although distinct subsets of genes appear to be affected. GR activation in U2OS cells represses the proliferation-promoting factors CDK4, D-type cyclins, E2F, and cMyc, whereas in SAOS2 cells, it enhances the expression of the CDIs p21 and p27Kip1 (hereafter referred to as p27) (77).
To elucidate the transcriptional mechanisms that determine the choice between the cytostatic and cytotoxic pathways initiated by GR, we generated U2OS and SAOS2 cell lines stably expressing GR mutants that uncouple receptor-dependent transcriptional activation from repression. We examined whether cells were undergoing PCD and assessed the expression of a variety of apoptosis-related gene products in both cell types. Using genetic and pharmacological approaches, we analyzed glucocorticoid induction of p21 and p27 and found that multiple mechanisms of GR transcriptional activation govern cell cycle arrest in SAOS2 cells. We demonstrate that enhanced expression of both p21 and p27, but not either one alone, is necessary to effect GR-mediated cell cycle arrest. Our findings reveal distinct, previously unexpected modes of transcriptional activation involved in CDI induction and the cytostatic effect of glucocorticoids, while the cytotoxic effect of GR is a function of transcriptional repression. Understanding the transcriptional regulatory mechanisms triggering the cytotoxic versus cytostatic effects of GR may ultimately lead to the identification of novel therapies for cancers that exploit the cytotoxic action of glucocorticoids.
MATERIALS AND METHODS
Cell lines and treatments.
Human osteosarcoma cell lines U2OS and SAOS2 were obtained from the American Type Culture Collection (ATCC HTB no. 96 and 85, respectively) and maintained in Dulbecco’s modified Eagle medium (DMEM) (Gibco BRL, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (FBS) (HyClone Laboratories, Inc., Logan, Utah), 50 Units each of penicillin and streptomycin per ml (Gibco BRL), and 2 mM l-glutamine (Gibco BRL). Generation of U2OS and SAOS2 clones expressing the full length wt rat GR [U2OS-GR(+) and SAO2-GR(+) cells] and of control GR-negative clones has been previously described (77). To generate cell lines ectopically expressing GR derivatives, 50% confluent U2OS and SAOS2 cells were transfected with pCMV-30iiB, pCMV-LS7, or pCMV-dim expression vectors (15 μg of DNA per 100-mm-diameter dish) by the calcium phosphate precipitation method. Stable transformants were selected by culturing transfected cells in the presence of 1 mg of Geneticin (G418) (Gibco BRL; 70% active compound) per ml for 2 months and were further maintained in 10% FBS–DMEM supplemented with 500 μg of G418 per ml. The expression of the GR derivatives in selected clones was verified by immunoblotting with GR-specific antibodies (see “Western blotting” below). Based on the results of indirect immunofluorescence with GR-specific antibodies, only clones homogeneously expressing GR were selected for the experiments. To examine the effects of GR activation on cell proliferation, cell death, protein expression, and steady-state mRNA levels, cells were cultured in 10% FBS–DMEM supplemented with 100 nM dexamethasone (Dex) (Sigma, St. Louis, Mo.) (dissolved in 100% ethanol), 100 nM RU 486 (dissolved in 100% ethanol), 100 nM ZK 299 (dissolved in 100% ethanol), or an equal volume of 100% ethanol for the indicated times.
Plasmids and cDNAs.
The 30iiB (E219K/F220L/W234R) (43), LS7 (P493R/A494S) (31), and dimerization-deficient (R479D/D481R) (56) mutants of the full-length rat GR were individually subcloned into the BamHI site of the pCMV-Neor expression vector. For transient transfections, an XG46TL reporter plasmid, containing two consensus GREs upstream of the thymidine kinase promoter (position −109) linked to a luciferase gene was used to assay GR transcriptional activity. An XAP1TL reporter plasmid, containing a single AP-1 binding site upstream of the thymidine kinase promoter fused to a luciferase gene, was used to assay transcriptional repression. The pCMV-LacZ plasmid produced β-galactosidase (β-Gal). For the labeling reaction in Northern blot analysis, full-length human p21Cip1 cDNA was excised from the Bluescript (pBS) XhoI site.
Cell proliferation assays.
U2OS and SAOS2 cell lines ectopically expressing wt GR or receptor substitution derivatives were seeded into six-well plates (15,000 and 20,000 cells/well, respectively) on day 0 and cultured in the presence of ethanol vehicle, 100 nM Dex, or 100 nM RU 486 (see “Cell lines and treatments” above). On the indicated days, cells were trypsinized, resuspended in DMEM, stained by the trypan blue exclusion method, and counted with a hemocytometer.
Assay for PCD.
Coverslips were placed in 24-well plates, precoated with 0.5 ml of 0.1-mg/ml poly-d-lysine (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) in phosphate-buffered saline (PBS) for 5 min, and washed once with PBS. U2OS and SAOS2 GR-expressing cells were seeded onto coverslips; cultured in the absence or presence of 100 nM Dex for 24, 48, or 72 h; washed five times with PBS; and fixed in 4% paraformaldehyde in PBS for 20 min at room temperature. Cells were then permeabilized by incubation with 0.2% Triton X-100 (Bio-Rad Laboratories, Hercules, Calif.) in PBS and subjected to terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay (Promega Corporation, Madison, Wis.) as per the manufacturer’s instructions. Coverslips were mounted onto Citifluor (Ted Pella, Redding, Calif.), and cells were visualized and photographed under a fluorescence microscope with a standard fluorescein filter set at a wavelength of 520 nm to view incorporated fluorescein-12-dUTP and at a wavelength of 620 nm to view red propidium iodide staining.
Transient transfections and reporter activity assays.
U2OS-GR(+) cells were plated on 60-mm-diameter dishes in DMEM–10% FBS. One hour prior to transfection, cells were refed with fresh medium and transfected with the indicated plasmids via the calcium phosphate precipitation method. Five hours later, cells were washed three times with prewarmed PBS to remove calcium phosphate precipitates, allowed to recover for 3 h in DMEM–10% FBS, and incubated with fresh medium containing 100 nM Dex or 100 nM RU 486, where indicated, for an additional 12 h.
Transfected cells were washed twice in PBS and harvested in 1× Reporter Lysis Buffer (Promega). Luciferase activity was quantified in a reaction mixture containing 25 mM glycylglycine (pH 7.8), 15 mM MgSO4, 1 mM ATP, 0.1 mg of bovine serum albumin per ml, and 1 mM dithiothreitol. A Lumat LB 9507 luminometer (EG&G Berthold) was used with 1 mM d-luciferin (Analytical Luminescence Laboratory) as the substrate. Lysates were additionally assayed for β-Gal activity as described elsewhere (2).
Western blotting.
To analyze changes in protein expression, U2OS and SAOS2 cells were treated for 3 days as described above, harvested in PBS, and lysed in 25 to 100 μl of the lysis buffer (150 mM NaCl, 50 mM HEPES [pH 7.5], 1 mM EDTA, 1 mM EGTA, 10% glycerol, 1% Triton X-100, 1 mM NaF, 25 μM ZnCl2) supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride [Sigma] and 1 μg each of aprotinin, pepstatin A, and leupeptin [Boehringer Mannheim Biochemicals, Indianapolis, Ind] per ml) for 15 min on ice. Cell lysates were clarified by centrifugation (10,000 × g for 15 min at 4°C), the total protein concentration was adjusted with the lysis buffer, and samples were boiled in an equal volume of 2× sodium dodecyl sulfate sample buffer. For Western blotting, protein extracts were separated by Tris-glycine–4 to 20% gradient polyacrylamide gel electrophoresis (Novex, San Diego, Calif.), transferred to Immobilon paper (Millipore Corp., Bedford, Mass.), and probed with mouse monoclonal antibodies against GR (BuGR2) (29), Bcl2 (B46620), TIAR (T33520), ICH-1L (I29120), Fas ligand (F37720), p27Kip1 (K25020), p21Cip1 (C24420), and CAS (42920) (Transduction Laboratories, Lexington, Ky.) or rabbit polyclonal antisera against ERK (sc-4024; Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) and CDK4 (06-139; Upstate Biotechnology Inc., Lake Placid, N.Y.). The blots were developed by using either alkaline phosphatase-conjugated goat anti-mouse antibodies (Bio-Rad Laboratories) followed by the addition of the 5-bromo-4-chloro-3-indoyl phosphate–Nitro Blue Tetrazolium phosphatase substrate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) or horseradish peroxidase-coupled goat anti-mouse (Transduction Laboratories) or donkey anti-rabbit antibodies and the enhanced chemiluminescence substrate (Amersham International plc, Amersham, United Kingdom) as per the manufacturer’s instructions.
Northern blotting.
Cells were cultured in 100-mm-diameter dishes for various periods of time with appropriate treatments (see the figure legends), the media were aspirated, and cells were lysed directly on the dishes by adding 3 ml of Ultraspec RNA reagent (BIOTEXC Laboratories, Inc., Houston, Tex.) per dish. Total RNA was isolated from cell homogenates as per the manufacturer’s instructions, denatured at 65°C for 15 min, chilled on ice, and separated on a 1.2% agarose–6% formaldehyde denaturing gel (5 to 8 μg of RNA/lane). Equivalent loading was verified by ethidium bromide staining of rRNA. RNA was transferred to a Duralon membrane (Stratagene, La Jolla, Calif.) as previously described (79), UV-cross-linked to the membrane, and hybridized to a cDNA probe for p21 mRNA by using QuikHyb hybridization mix (Stratagene) as per the manufacturer’s instructions. A cDNA fragment coding for p21 was labeled with [α-32P]dCTP by using a RediPrime random priming labeling kit (Amersham) as per the manufacturer’s instructions. Blots were washed and exposed to Kodak BioMax film for 2 to 24 h at −80°C for autoradiography.
RESULTS
Ectopic expression and activation of wt GR induces PCD in U2OS but not SAOS2 human osteosarcoma cells.
Activation of the ectopically expressed wt rat GR by the glucocorticoid Dex (Fig. 1) in U2OS and SAOS2 human osteosarcoma cells inhibits cell proliferation, inducing G0/G1 cell cycle arrest and morphological alterations in both cell lines (77). To examine whether the effects of Dex were cytostatic or cytotoxic, we first addressed whether cell growth arrest in either cell line was reversible after Dex is withdrawn from the culture medium. Figure 2A (top panel) demonstrates that in U2OS-GR(+) cells, 1 day of Dex treatment is sufficient to delay cell proliferation, whereas longer treatment (2 to 3 days) causes irreversible cell growth arrest. In contrast, SAOS2-GR(+) cells resume cell division as soon as Dex is withdrawn, even after a 3-day course of continuous Dex treatment (Fig. 2B, top panel). These results suggest that a short-term exposure of U2OS-GR(+) cells to the GR agonist Dex commits cells to irreversible cell cycle arrest, whereas inhibition of SAOS2-GR(+) cell proliferation is dependent upon the continuous presence of Dex in the medium.
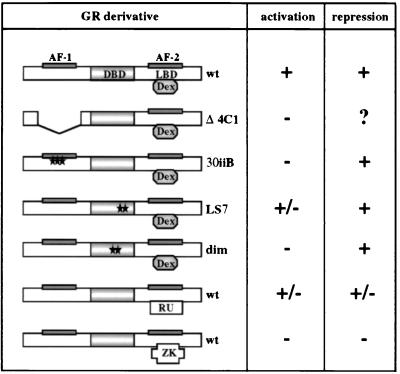
FIG. 1.
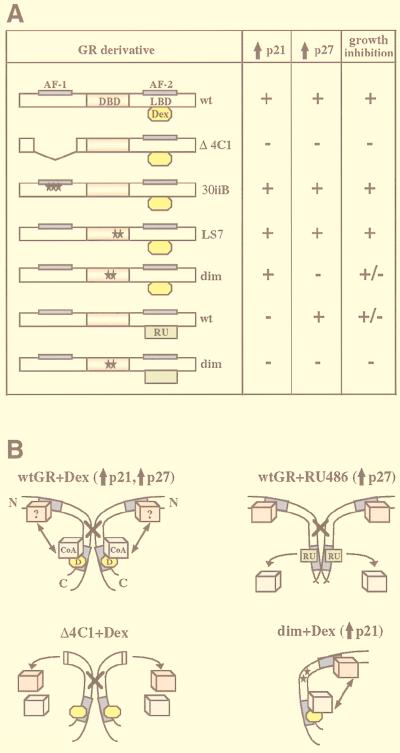
Transcriptional regulatory responses of GR derivatives and ligands. Rat GR derivatives ectopically expressed in U2OS and SAOS2 cells are shown. The GR DBD, LBD, and N-terminal and C-terminal transcriptional activation functions (AF-1 and AF-2, respectively) are indicated. The GR Δ4C1 mutant lacks amino acids 70 to 300. The 30iiB mutant contains three amino acid substitutions in AF-1 (E219K, F220L, and W234R). The LS7 mutant contains two point mutations, P493R and A494S, in the second zinc finger of the GR DBD. The dimer (dim) mutant contains two mutations, R479D and D481R, which disrupt the GR dimerization interface. The locations of the point mutations are shown with stars. The ability of each derivative to activate and repress transcription is summarized based on previously published studies (see text for references); +, −, and +/− represent a transcriptionally competent, a transcriptionally inactive, or a context-dependent phenotype, respectively. A question mark indicates that the phenotype has not been characterized. The transcriptional regulatory responses of the wt GR bound by the full agonist Dex, the partial agonist RU 486 (RU), and the antagonist ZK 299 (ZK) are also shown.
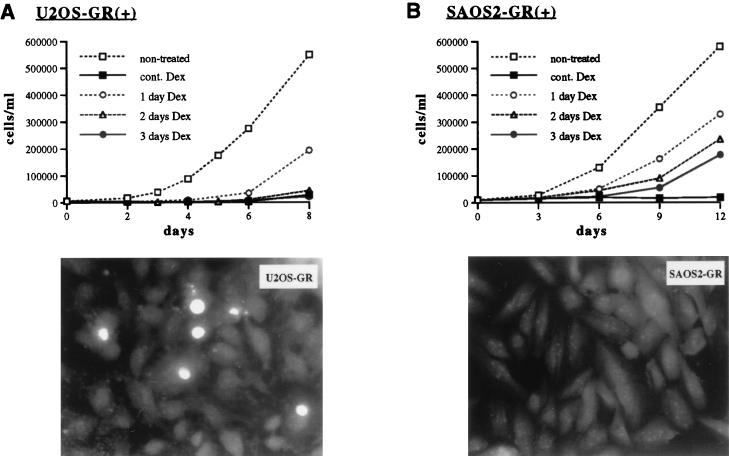
FIG. 2.
GR activation induces apoptosis in U2OS but not SAOS2 cells. (Top panels) U2OS-GR(+) (A) and SAOS2-GR(+) (B) cells were seeded on day 0 into six-well plates (15,000 and 25,000 cells/well, respectively) in the presence of 100 nM Dex where indicated. Cells were refed at 24 (1 day Dex), 48 (2 days Dex), or 72 (3 days Dex) h with hormone-free medium. Nontreated and continuously (cont.) Dex-treated cells were cultured in the absence or presence of Dex, respectively, throughout the experiment. On the indicated days cells were trypsinized, stained with trypan blue, and counted with a hemocytometer. (Bottom panels) GR-expressing U2OS (A) and SAOS2 (B) cells were cultured in the presence of 100 nM Dex for 24, 48, or 72 h and subjected to the TUNEL assay as described in Materials and Methods. Fluorescence microscopy was performed with a standard fluorescein filter set at a wavelength of 520 nm to view fluorescein-12-dUTP incorporated into DNA nicks. Note multiple apoptotic nuclei in U2OS-GR(+) cells treated with Dex for 24 h. No apoptotic nuclear morphology was detected in SAOS2-GR(+) cells treated with Dex for 24, 48 (shown), or 72 h.
Alternative outcomes of hormone-dependent cell growth inhibition suggest that different pathways may underlie the antimitogenic effects of glucocorticoids in U2OS versus SAOS2 cells. To assess whether these cells underwent PCD, U2OS-GR(+) and SAOS2-GR(+) cells were cultured in the presence of 100 nM Dex for 0, 24, 48, and 72 h and subjected to TUNEL assays. Figure 2B (bottom panel) illustrates that U2OS-GR(+) cells undergo apoptosis upon Dex treatment (up to 30% of cells at 48 h). In contrast, no apoptotic nuclear morphology was observed in SAOS2-GR(+) cells (Fig. 2B, bottom panel). Thus, GR activation in U2OS but not SAOS2 cells induces apoptosis.
GR activation in U2OS but not SAOS2 cells results in reduced expression of the antiapoptotic protein Bcl2.
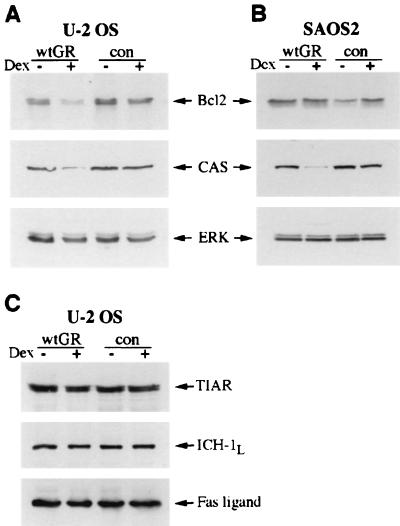
Since U2OS-GR(+) but not SAOS2-GR(+) cells undergo apoptosis in response to GR activation, we compared the expression of proapoptotic and antiapoptotic proteins that may be differentially regulated in the two cell lines. U2OS-GR(+) and GR-negative U2OS cells were cultured in the presence or absence of Dex for 48 h, and the expression of apoptosis-related gene products was examined by immunoblotting. Figure 3A demonstrates that Dex treatment results in reduced expression of the antiapoptotic factor Bcl2 and a cellular apoptosis susceptibility protein (CAS) (9), whereas the levels of several other apoptosis-associated proteins, such as cytotoxic TIA-1-related protein (47), FAS ligand, and caspase 2 (ICH-1L) protease (94) were not altered (Fig. 3C). We have also observed a slight decrease in the concentration of another Bcl2 family member, BclXL, in U2OS-GR(+) cells (data not shown). Interestingly, in SAOS2-GR(+) cells, in which Bcl2 expression was not affected by Dex treatment, the expression of CAS was reduced (Fig. 3B). Since SAOS2 cells do not undergo PCD upon GR activation, decreased CAS expression is apparently not sufficient for apoptosis to occur. Previous studies have indicated that CAS expression closely correlates with cell proliferative potential: CAS protein is highly expressed in actively dividing cells in vivo and in transformed cancer cell lines, whereas in nondividing cells the expression is low (10, 97). Consistent with these observations, GR-induced cell cycle arrest results in a reduction of the CAS level in both cell lines. We have therefore used CAS expression as a marker for cell proliferation in our subsequent experiments. In contrast, reduced Bcl2 expression was specific to U2OS-GR(+) cells, suggesting that repression of antiapoptotic factors may play a role in GR-induced PCD.
FIG. 3.
Expression of apoptosis-related proteins in U2OS-GR(+) and SAOS2-GR(+) cells. U2OS (A and C) and SAOS2 (B) cells expressing wt GR or receptor-deficient control (con) GR-negative cells were cultured in the absence (−) or presence (+) of 100 nM Dex for 2 days, and whole-cell lysates were prepared (see Materials and Methods). Equal amounts of total protein were resolved on a Tris-glycine–4 to 20% gradient polyacrylamide gel, transferred to Immobilon paper, and probed with antibodies against Bcl2, CAS, ERK, TIAR, ICH-1L, and Fas ligand. Equal loading in each lane is demonstrated by probing with an anti-ERK antibody. Each blot is representative of two or more independent experiments.
The GR transcriptional activation-deficient mutants are competent for cell cycle arrest and apoptosis in U2OS cells.
To further elucidate the mechanisms of GR-induced apoptosis in U2OS cells, we sought to uncouple receptor-mediated transcriptional activation from transcriptional repression by utilizing several receptor mutants and ligands. Two GR derivatives that could potentially uncouple activation from repression were individually introduced into U2OS cells (see Materials and Methods). The first mutant, termed LS7, contains two point mutations, P493R and A494S, in the second zinc finger of the GR DBD (Fig. 1) and has previously been used to characterize receptor functions responsible for executing glucocorticoid-mediated apoptosis in human T cells (37). The mutant is competent for transcriptional repression, whereas its ability to activate transcription is impaired under conditions of overexpression (31, 52). The second mutant, termed dim (Fig. 1), contains a different pair of mutated residues in the dimerization loop of the second zinc finger in the GR DBD, R479D and D481R. wt R479 and D481 form arginine-to-aspartate salt bridges between the GR monomers, thereby stabilizing the dimer (56, 58). The R479D/D481R double mutation reduces dimer formation and cooperative DNA binding at a consensus single GRE, leading to a decrease in transcriptional activation relative to that of the wt GR (57). The mutant takes advantage of the fact that GR is recruited to simple GREs as a homodimer, whereas at most GR-repressible elements the receptor functions in its monomeric form. Therefore, partial disruption of a dimerization interface by a point mutation(s) selectively reduces transcriptional activation mediated by classical GREs but preserves transcriptional repression (36, 38). On the basis of the described phenotypes of the LS7 and dim mutants, we speculated that if transcriptional repression by GR is indeed responsible for the cell cycle arrest and apoptosis in U2OS cells, then both mutants should function like the wt GR in this context.
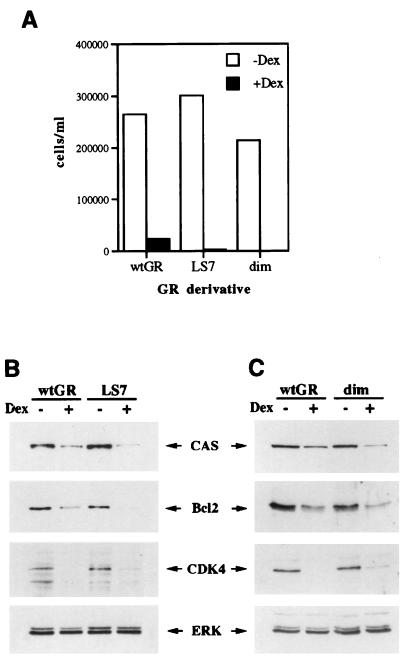
Multiple clones expressing the LS7 and the dim mutants were generated in U2OS cells (where both mutants are competent for GR-mediated transcriptional repression of an AP-1-responsive reporter [data not shown]) and assayed for their ability to proliferate in the presence of Dex along with a U2OS-GR(+) clone. Consistent with our hypothesis, U2OS-LS7 and U2OS-dim clones underwent hormone-dependent inhibition of cell proliferation similar to that in U2OS-GR(+) cells (Fig. 4A). In addition, within 24 h of Dex treatment, we observed morphological alterations in cells expressing the LS7 or the dim mutant identical to those induced by the wt GR in U2OS cells, and TUNEL analysis demonstrated Dex-dependent apoptosis in U2OS-LS7 and U2OS-dim cells (data not shown). The expression of the cell cycle regulatory proteins and cell death mediators was assessed in LS7- and dim-expressing cells and compared to that in U2OS-GR(+) cells. As demonstrated for the wt GR (Fig. 3A) (77), both mutant derivatives reduce the expression of CAS, Bcl2, and CDK4 (Fig. 4B and C), further suggesting that transcriptional repression of survival factors by the LS7 or dimerization-deficient GR mutants is sufficient to execute cell cycle arrest and apoptosis in U2OS cells.
FIG. 4.
The GR LS7 and dimer mutants function like the wt GR in U2OS cells. U2OS-LS7 and U2OS-dim clones were generated as described in Materials and Methods. (A) The GR LS7 and dimerization mutants induce cell cycle arrest in U2OS cells. U2OS-GR(+) (wt GR), U2OS-LS7 (LS7), and U2OS-dim (dim) clones were seeded on day 0 into six-well plates in duplicate and cultured in the absence or presence of 100 nM Dex as indicated. The total numbers of viable cells were determined on days 2, 4, and 6 by the trypan blue exclusion method. The graph represents cell counts on day 6. (B and C) The GR LS7 (B) and dim (C) mutants repress the expression of CAS, Bcl2, and CDK4. U2OS cells expressing the wt GR or the LS7 or dim mutant were cultured in the absence or presence of 100 nM Dex for 40 h and harvested, and the expression of CAS, Bcl2, CDK4, and ERK was assessed by immunoblotting as described in Materials and Methods. Similar results were obtained with at least three independent clones expressing each GR mutant.
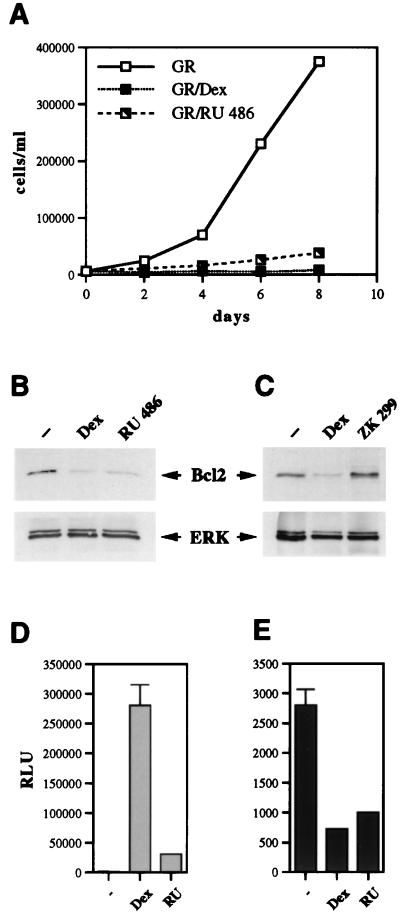
RU 486 functions as a GR agonist in U2OS-GR(+) cells.
We next examined whether GR-mediated cell cycle arrest and apoptosis in U2OS cells were agonist dependent or could also be induced by partial agonists, such as RU 486 (Fig. 1). RU 486-bound GR translocates to the nucleus and binds DNA but does not efficiently activate transcription, whereas its ability to repress transcription is retained in certain cell type and promoter contexts (35, 53, 55). We asked whether RU 486 acts as a GR antagonist or as a partial agonist with respect to the receptor’s cell growth-inhibitory properties in U2OS-GR(+) cells. Interestingly, RU 486 treatment inhibited cell cycle progression in U2OS-GR(+) cells (Fig. 5A) and induced changes in cell morphology similar to those triggered by Dex (data not shown). The cell survival marker Bcl2 was repressed in an RU 486-dependent fashion (Fig. 5B), suggesting that in U2OS-GR(+) cells, this ligand behaves like the GR agonist Dex. In contrast, in the presence of a pure antagonist, ZK 299 (Fig. 1), GR did not inhibit cell proliferation (data not shown) or repress Bcl2 (Fig. 5C). Furthermore, ZK 299 was able to partially counteract the repressive effect of Dex on Bcl2 expression when both ligands were added to U2OS-GR(+) cells simultaneously (data not shown). Thus, GR-mediated growth arrest and apoptosis in U2OS cells occur in the presence of a partial GR agonist, RU 486, but not a pure antagonist, ZK 299. We then compared the effects of Dex and RU 486 on GR-mediated transcriptional activation and repression of transiently introduced GR-inducible and GR-repressible reporter plasmids in the context of U2OS cells. We found that Dex and RU 486 differ markedly in their ability to support GR transcriptional activation, with Dex eliciting a 217-fold induction of the GR-responsive reporter gene, while RU 486 treatment resulted in only a 9-fold induction (Fig. 5D). In contrast, GR-mediated transcriptional repression of the AP-1-responsive reporter gene by RU 486 and that by Dex were virtually identical (Fig. 5E). These results further support our hypothesis that GR-mediated transcriptional repression is the likely mechanism governing receptor-induced apoptosis in U2OS-GR(+) cells.
FIG. 5.
RU 486 functions as a GR agonist in the context of U2OS-GR(+) cells. (A) RU 486 inhibits proliferation of U2OS-GR(+) cells. U2OS-GR(+) cells were seeded on day 0 into six-well plates (15,000 cells/well) in duplicate and cultured in the presence of an ethanol vehicle, 100 nM Dex, or 100 nM RU 486. The total number of viable cells was determined on the indicated days. (B) Expression of Bcl2 is repressed in both Dex-treated and RU 486-treated U2OS-GR(+) cells. U2OS-GR(+) cells were cultured in the presence of an ethanol vehicle, 100 nM Dex, or 100 nM RU 486 for 40 h, and expression of Bcl2 and ERK in whole-cell extracts was examined by immunoblotting as described in Materials and Methods. (C) ZK 299 is a pure GR antagonist in U2OS cells. U2OS-GR(+) cells were cultured in the presence of an ethanol vehicle, 100 nM Dex, or 100 nM ZK 299 for 40 h, and expression of Bcl2 and ERK in whole-cell extracts was examined by immunoblotting. (D) RU 486 is a weak agonist with respect to GR transcriptional activation in U2OS-GR(+) cells. U2OS-GR(+) cells were seeded in 6-cm-diameter dishes (120,000/dish) and transfected the following day via the calcium phosphate precipitation method with an XG46TL reporter plasmid (4 μg/dish) and a pCMV-LacZ plasmid (0.75 μg/dish) as an internal control for transfection efficiency. Transfected cells were treated with an ethanol vehicle (−), 100 nM Dex, or 100 nM RU 486 (RU) for 12 h, and GR transcriptional activation was assessed via luciferase assay, normalized to β-Gal activity, and expressed as relative luminescence units (RLU). (E) RU 486 is a potent agonist with respect to GR-mediated transcriptional repression in U2OS-GR(+) cells. Cells were transfected with an XAP1TL reporter plasmid and a pCMV-LacZ plasmid, and GR transcriptional repression was assessed as described for panel D.
Cell cycle arrest in SAOS2 cells is independent of transcriptional activation by AF-1.
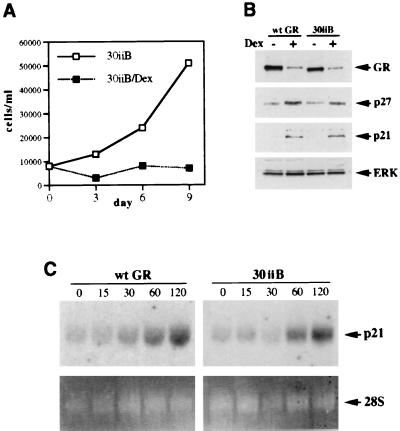
Our previous studies have suggested that the integrity of the GR N terminus was essential for the GR-dependent cell cycle arrest in SAOS2 cells, since deletion of amino acids 70 through 300 (the GR Δ4C1 mutant [Fig. 1]) abolished the receptor’s cytostatic activity and enhancement of p21 and p27 expression (77). However, in addition to eliminating AF-1, this large deletion likely disrupts GR’s ability to interact with putative cofactors necessary for cell cycle arrest in SAOS2 cells. To examine whether transcriptional activation by the N-terminal AF-1 was required for CDI induction and cell cycle arrest in SAOS2 cells, we sought to specifically disrupt the function of AF-1 in the context of the full-length GR. A GR mutant termed 30iiB (Fig. 1) containing three amino acid substitutions (E219K, F220L, and W234R) (43) was previously identified in a genetic screen for GR mutants with reduced ability to activate transcription. While this mutant is competent for transcriptional repression, its ability to activate transcription through AF-1 is severely compromised in both yeast and mammalian cells (43). We have generated multiple stable SAOS2-30iiB clones (see Materials and Methods), and those expressing the 30iiB mutant at levels comparable to the level of the wt GR in SAOS2-GR(+) clones (Fig. 6B, top panel) were analyzed. To evaluate the effects of the 30iiB mutant on cell proliferation, SAOS2-30iiB cells were cultured in the presence or absence of Dex, and the total number of viable cells was determined. Surprisingly, activation of the 30iiB mutant induced complete cell cycle arrest in SAOS2 cells (Fig. 6A). We have also observed the characteristic morphology of Dex-treated SAOS2-30iiB cells, including a significant increase in size, “fried-egg” shape, and vacuolization of the cytoplasm (data not shown), an appearance originally described as “spreading” for Rb-transfected SAOS2 cells (39, 88, 90) as well as hormone-arrested SAOS2-GR(+) cells (77). Activation of both the wt and the mutant receptors results in a decrease of GR protein concentration (Fig. 6B), a process termed homologous down-regulation (12, 66). Importantly, the 30iiB mutant induced both p27 and p21 to levels comparable to those elicited by the wt GR (Fig. 6B); the magnitude and kinetics of the p21 mRNA induction by the wt GR and the 30iiB mutant were indistinguishable (Fig. 6C). Thus, the presence of the GR N terminus, but not transcriptional activation by AF-1 per se, is required for the cell cycle arrest and enhanced expression of CDIs in SAOS2 cells. These findings indicate that additional GR transcriptional regulatory functions are operating to enhance p21 and p27 expression.
FIG. 6.
Transcriptional activation by the GR AF-1 is dispensable for cell cycle arrest in SAOS2 cells. (A) The GR 30iiB mutant induces complete cell cycle arrest in SAOS2 cells. SAOS2-30iiB stable transformants were generated as described in Materials and Methods. SAOS2-30iiB clones were seeded on day 0 into six-well plates (20,000 cells/well) and cultured in the absence or presence of 100 nM Dex. Total numbers of viable cells were determined on the indicated days by using the trypan blue exclusion method. (B) Induction of p21 and p27 by the GR 30iiB mutant. SAOS2-GR(+) (wt GR) and SAOS2-30iiB (30iiB) cells were cultured in the presence of 100 nM Dex or ethanol vehicle for 3 days, and whole-cell extracts were prepared and subjected to immunoblotting for GR, p27, p21, and ERK. Note the induction of p21 and p27 by both the wt GR and the 30iiB mutant. (C) Time course of p21 mRNA induction. SAOS2-GR(+) (wt GR) and SAOS2-30iiB (30iiB) cells were treated with 100 nM Dex for 0, 15, 30, 60, and 120 min, and total RNA was isolated and subjected to Northern blot analysis with a 32P-labeled p21 cDNA probe (top panels). Equal loading in each lane is demonstrated by ethidium bromide staining of the 28S rRNA (bottom panels).
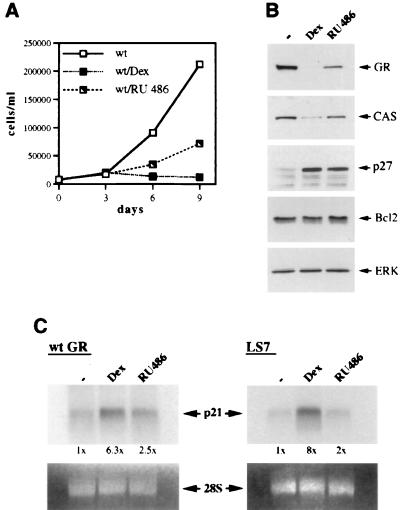
The GR LS7 mutant is competent for cell cycle arrest and induction of CDIs in SAOS2 cells.
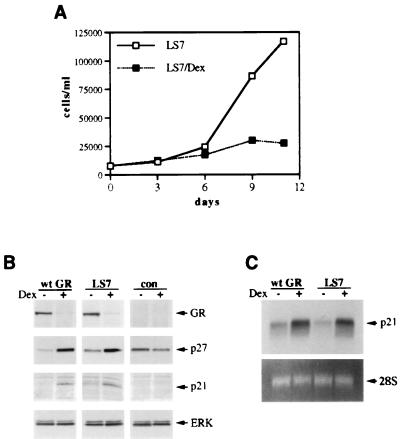
Since functional AF-1 appeared to be dispensable for GR-mediated cell cycle arrest and the induction of CDIs in SAOS2 cells, we examined whether GR transcriptional activation in general is required for these responses. We first employed the GR LS7 mutant (Fig. 1), whose transcriptional activation is reportedly similar to the activity of the wt GR at low levels of receptor expression; however, under conditions of overexpression, LS7 exhibits a transactivation-deficient phenotype due to self-squelching (52). Thus, in the context of stable expression in SAOS2 cells, where the level of ectopically expressed receptor is similar to physiological levels of endogenous GR in mammalian cells, we expected the mutant to induce the expression of CDIs and cell cycle arrest as does the wt GR in SAOS2-GR(+) cells.
The LS7 mutant was stably introduced into SAOS2 cells (see Materials and Methods), and its expression was demonstrated by immunoblotting (Fig. 7B, top panel) and indirect immunofluorescence (data not shown). The LS7 mutant was competent for inhibiting cell proliferation, down-regulating its own expression, or inducing p21 and p27 expression (Fig. 7A and B) and “fried-egg” morphology (data not shown). Figure 7C demonstrates similar levels of p21 mRNA induction by both the wt GR and the activated LS7 mutant within 2.5 h of Dex treatment. Thus, unlike LS7 stably introduced into Jurkat T cells which exhibited reduced transcriptional activation from a transiently transfected reporter construct (37), in the context of SAOS2 cells, the LS7 mutant does not display the self-squelching phenotype and is competent for activating endogenous promoters, thereby acting like the wt GR.
FIG. 7.
The GR LS7 mutant functions like the wt GR when stably expressed in SAOS2 cells. (A) Inhibition of SAOS2 cell proliferation by the GR LS7 mutant. Multiple SAOS2-LS7 clones were generated as described in Materials and Methods. SAOS2-LS7 cells were seeded on day 0 into six-well plates (20,000 cells/well) and cultured in the absence or presence of 100 nM Dex. Total numbers of viable cells were determined on the indicated days by using the trypan blue exclusion method. Similar cell growth kinetics were observed in three independent LS7-expressing clones. (B) The GR LS7 mutant induces the expression of p21 and p27 proteins. SAOS2-GR(+) (wt GR), SAOS2-LS7 (LS7), and GR-negative SAOS2 (con) cells were cultured in the presence of 100 nM Dex or ethanol vehicle for 3 days, and whole-cell extracts were prepared and subjected to immunoblotting for GR, p27, p21, and ERK. Note the increase in p21 and p27 protein in the wt GR- and LS7-expressing cells but not in the GR-deficient control cells. (C) Induction of the steady-state p21 mRNA level by the GR LS7 mutant. SAOS2-GR(+) (wt GR) and SAOS2-LS7 (LS7) cells were treated with 100 nM Dex for 2 h 30 min where indicated, and total RNA was isolated and hybridized to a 32P-labeled p21 cDNA probe (top panel). Equal loading in each lane is demonstrated by ethidium bromide staining of the 28S rRNA (bottom panel).
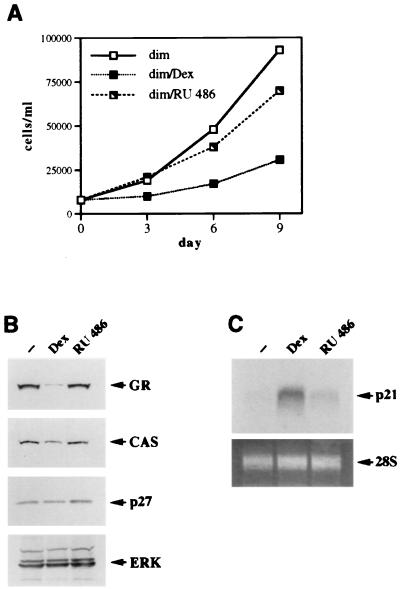
Differential regulation of p21 and p27 in SAOS2 cells as revealed by the GR dimerization-deficient mutant.
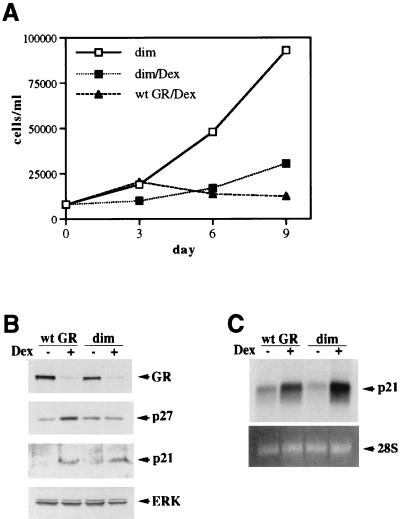
Since the LS7 mutant failed to uncouple transcriptional activation from repression in SAOS2 cells, we utilized the repression-competent dimerization-deficient mutant (dim [Fig. 1]). We reasoned that if the induction of p21 and/or p27 in SAOS2 cells is mediated by GR transcriptional activation through a classical GRE, the dimerization mutant may fail to induce their expression and halt cell cycle progression. The GR dim mutant was therefore stably introduced into SAOS2 cells, and its ability to elicit cell cycle arrest was examined. Interestingly, dim-expressing cells underwent inhibition of cell proliferation but failed to arrest completely [Fig. 8A; compare 96% growth inhibition in SAOS2-GR(+) cells to 76% inhibition in SAOS2-dim cells at day 9], a finding obtained for multiple independent clones. Thus, the phenotypic consequences of the dim activation with respect to the cell growth kinetics were reproducibly milder than those induced by the wt GR or the 30iiB mutant. These results did not reflect a heterogeneity of receptor expression within the population, since based on the results of indirect immunofluorescence with GR-specific monoclonal antibodies, only the clones homogeneously expressing GR (over 95%) were selected for the experiments (data not shown). Furthermore, the expression levels of the dim mutant and the wt GR were similar (Fig. 8B), suggesting that the inability of the dimerization mutant to cause the cell cycle arrest is not a function of reduced expression of the mutant receptor per cell. Since p21 and p27 appeared to be important mediators of GR-dependent cell growth arrest in SAOS2 cells, we analyzed the possible changes in CDI expression triggered by the hormone-activated dim mutant compared to the wt GR. Strikingly, the mutant receptor was fully competent for inducing the expression of p21, whereas p27 induction was abolished (Fig. 8B). The magnitudes of the p21 mRNA increases displayed by the dim mutant and the wt GR were identical (Fig. 8C). To eliminate the possible variability between the individual clones, we have screened 20 independent lines stably expressing the dim mutant and found that in all cases the dimerization-deficient mutant enhanced expression of p21; in contrast, virtually no increase in p27 expression was detected (data not shown). Thus, the R479D/D481R (dim) mutation effectively uncouples induction of p21 from that of p27 in SAOS2 cells, suggesting that (i) enhanced expression of p21 is likely necessary but not sufficient for complete cell cycle arrest, (ii) alterations of cellular morphology correlate with p21 activation, and (iii) induction of p27, but not p21, is dependent on the intact GR dimerization interface.
FIG. 8.
Dissociation of p21 induction from p27 induction in SAOS2 cells through the GR dimerization mutant. (A) Inhibition of cell proliferation by the GR dimerization-deficient mutant. SAOS2 clones stably expressing the GR dim mutant were generated as described in Materials and Methods. SAOS2-GR(+) and SAOS2-dim cells were seeded on day 0 into six-well plates (20,000 cells/well) and cultured in the absence or presence of 100 nM Dex. Total numbers of viable cells were determined on the indicated days. Note incomplete inhibition of cell proliferation by the dim mutant compared to the wt GR-expressing clone. Quantitation of cell counts reveals 76% growth inhibition for the dim mutant and 96% for the wt GR. Identical growth kinetics were demonstrated for three independent clones expressing the GR dim mutant. (B) Induction of p21 but not p27 expression by the dim mutant. SAOS2 cells expressing the wt GR or the dimerization-deficient mutant were cultured in the presence or absence of 100 nM Dex for 3 days, and whole-cell extracts were prepared and subjected to immunoblotting for GR, p27, p21, and ERK. Note the increase in p21 but not p27 protein in the dim-expressing cells. (C) Induction of the steady-state p21 mRNA level by the dim mutant. Hormone treatment, RNA isolation, and Northern hybridization were performed exactly as described for Fig. 7C. Potent Dex-dependent induction of p21 mRNA is observed in both SAOS2-GR(+) (wt GR) and SAOS2-dim (dim) cells.
RU 486 uncouples enhanced expression of p27 from the transcriptional induction of p21 in SAOS2 GR-expressing cells.
Our data on the GR domains responsible for cell cycle arrest in SAOS2 cells suggested that although the presence of the receptor N terminus is essential for cell cycle arrest and CDI induction, transcriptional activation by AF-1 is not required for either. To evaluate the importance of the receptor C-terminal transcriptional regulatory function (AF-2) for the cell cycle arrest and enhanced expression of the CDIs, we used a pharmacological approach and employed RU 486, which prevents the productive interaction of the steroid receptor LBD with the coactivator protein(s) necessary for transcriptional activation via AF-2 (40, 98). SAOS2-GR(+) cells were cultured in the presence of an ethanol vehicle, Dex, or RU 486, and cell growth kinetics in each condition were assessed in a cell proliferation assay. Interestingly, RU 486 inhibited cell proliferation by only 69% at day 9, compared to 98% inhibition observed in the presence of Dex (Fig. 9A). Consistent with the results of the cell proliferation studies, ligand-dependent reduction of GR expression and down-regulation of CAS were still evident in RU 486-treated cells, but to a lesser extent than in Dex-treated SAOS2-GR(+) cells (Fig. 9B). Importantly, RU 486-activated GR enhanced the expression of p27 as did the Dex-activated receptor (Fig. 9B) but failed to efficiently induce p21 at either the protein (data not shown) or mRNA (Fig. 9C, left panel) level. Thus, a rapid increase of steady-state p21 mRNA observed in Dex-treated SAOS2-GR(+) cells was substantially reduced when RU 486 was used as a GR ligand. Furthermore, the receptor LS7 mutant, which mimicked the wt GR with respect to its potency to enhance the expression of CDIs in the presence of Dex, also retained its ability to induce p27 (data not shown), but not p21, when activated with RU 486 (Fig. 9C, right panel). Thus, RU 486 uncoupled p21 induction from p27 induction in the context of both the wt and LS7 GR DBDs, suggesting that the receptor AF-2 and association with coactivator proteins are required for enhanced expression of p21, but not p27. These results further emphasize the differences between the regulation of the two CDK inhibitors: the dimerization mutant was competent for p21 but not p27 induction, whereas wt GR (and LS7) with an antagonist-disabled AF-2 could enhance p27 but not p21 expression.
FIG. 9.
Induction of CDI expression by the wt GR in SAOS2 cells is uncoupled by RU 486. (A) Differential effects of GR ligands on the proliferation of SAOS2-GR(+) cells. SAOS2-GR(+) cells were seeded on day 0 into six-well plates (20,000 cells/well) in duplicate and cultured in the presence of an ethanol vehicle, 100 nM Dex, or 100 nM RU 486. Total numbers of viable cells were determined on the indicated days. Note 69% inhibition of cell proliferation in the presence of RU 486, compared to 98% observed in the presence of Dex. (B) Alterations in protein expression induced by RU 486 in SAOS2-GR(+) cells. SAOS2-GR(+) cells were cultured in the presence of an ethanol vehicle, 100 nM Dex, or 100 nM RU 486 for 3 days, and expression of GR, CAS, p27, Bcl2, and ERK in whole-cell extracts was examined by immunoblotting. Note that comparatively mild repression of GR and CAS is observed in the presence of RU 486, whereas p27 induction is retained whether GR is activated with Dex or RU 486. Similar results were obtained with RU 486 concentrations of 500 nM and 1 μM (data not shown). (C) RU 486-activated wt GR or LS7 mutant fails to efficiently induce p21 mRNA expression in SAOS2 cells. SAOS2-GR(+) (wt GR) and SAOS2-LS7 (LS7) cells were treated with an ethanol vehicle, 100 nM Dex, or 100 nM RU 486 for 2 h 30 min, and total RNA was isolated and subjected to Northern hybridization with a 32P-labeled p21 cDNA probe (top panels). Equal loading in each lane is demonstrated by ethidium bromide staining of 28S rRNA (bottom panels). The results of autoradiography were quantitated by spot densitometry. The densitometric value obtained for mock-treated SAOS2-GR(+) or SAOS2-LS7 cells was arbitrarily set as 1×.
RU 486 loses partial agonist activity in the context of the dimerization-deficient GR mutant.
Since inhibition of cell proliferation by RU 486 in SAOS2 GR-expressing cells occurred primarily through enhanced expression of p27, we speculated that the GR dimerization-deficient mutant, which has lost the ability to induce p27, will not display cytostatic activity in RU 486-treated SAOS2 cells. To test this hypothesis, the SAOS2-dim cells were cultured in the presence of ethanol vehicle, Dex, or RU 486, and the total number of viable cells was determined. Figure 10A demonstrates that Dex treatment of the SAOS2-dim cells inhibited cell proliferation compared to that of their vehicle-treated counterparts (also shown in Fig. 8A), whereas little growth inhibition was observed in the presence of RU 486. These results differed dramatically from the strong antiproliferative effect of this ligand on the SAOS2-GR(+) cells (69% in Fig. 9A). Consistent with the lack of growth inhibition of the dim-expressing cells by RU 486, no morphological alterations were visible in these cells, although spreading was readily detectable in their Dex-treated counterparts (data not shown). To confirm that the dimerization mutant in the presence of RU 486 was unable to induce either p21 or p27 expression, protein and mRNA blots were performed on SAOS2-dim cells treated with an ethanol vehicle, Dex, or RU 486. As shown in Fig. 10B, the protein concentrations of p27, CAS, and GR in RU 486-treated cells are identical to those in vehicle-treated controls. Similarly, the steady-state level of p21 mRNA was strongly induced by the dim mutant in the presence of Dex but not RU 486 (Fig. 10C). Thus, abolishing GR’s ability to induce (i) p27, through specific mutations in the GR dimerization interface, and (ii) p21, through pharmacological manipulation by using a ligand with only partial agonist activity, virtually eliminates GR’s ability to induce cell cycle arrest in SAOS2 cells.
FIG. 10.
RU 486 loses partial agonist activity in the context of the GR dimerization-deficient mutant. (A) The GR dim mutant does not affect cell proliferation in the presence of RU 486. SAOS2-dim cells were seeded on day 0 into six-well plates (20,000 cells/well) in duplicate and cultured in the presence of an ethanol vehicle, 100 nM Dex, or 100 nM RU 486. Total numbers of viable cells were determined on the indicated days. (B) The RU 486-activated GR dimerization-deficient mutant fails to alter the expression of GR, CAS, or p27. SAOS2-dim cells were cultured in the presence of an ethanol vehicle, 100 nM Dex, or 100 nM RU 486 for 3 days, and expression of GR, CAS, p27, and ERK in the whole-cell extracts was examined by immunoblotting. (C) The RU 486-activated dimer mutant does not induce the steady-state level of p21 mRNA. SAOS2-dim cells were treated with an ethanol vehicle, 100 nM Dex, or 100 nM RU 486 for 2 h 30 min, and total RNA was isolated and hybridized to a 32P-labeled p21 cDNA probe (top panel). Equal loading into each lane is demonstrated by ethidium bromide staining of 28S rRNA (bottom panel).
DISCUSSION
In this report we demonstrate that the cytostatic versus cytotoxic outcome of GR-mediated growth inhibition is cell type dependent. GR activation in U2OS cells is irreversible and results in apoptosis and the down-regulation of antiapoptotic proteins, including Bcl2. This cytotoxic effect in U2OS cells is mediated by GR’s repression function, since transactivation-deficient mutants and ligands also elicit apoptosis. In contrast, GR-mediated cell cycle arrest in SAOS2 cells is reversible and does not result in apoptosis or repression of Bcl2. This cytostatic effect of GR in SAOS2 cells is a function of the receptor’s ability to stimulate transcription of the CDIs p21 and p27, which involves multiple GR transcriptional activation mechanisms.
Why is GR-mediated apoptosis cell type specific?
GR has been previously shown to cause cell death in developing thymocytes, lymphocytes, and a variety of cultured cell lines (18, 54); however, little is known about the molecular mechanisms linking the activated receptor to the cell death program. Several studies have demonstrated that the inhibition of specific caspases, the downstream-most effectors of apoptosis, or ICE-like cysteine proteases, which are activated at earlier stages of the apoptotic pathway, prevents Dex-dependent cell death (15, 34, 42). Overexpression of Bcl2, which rescues thymocytes from apoptosis, prevents activation of certain caspases but not ICE-like proteases (34), suggesting that the Dex-dependent cell death cascade can be interrupted by Bcl2 overexpression only at early stages, before effector caspase activation occurs. It is not clear which proteases are activated in the course of GR-induced apoptosis. Recent studies demonstrate that thymocytes isolated from caspase 9-deficient mice are resistant to glucocorticoid-induced apoptosis but not to apoptosis caused by UV irradiation or Fas and CD3 activation (32, 50). In contrast, T cells depleted of caspase 3 remain Dex sensitive (51) but acquire resistance to the effects of Fas and CD3 activation. These results suggest that in the context of the whole animal, caspase proteases are not redundant and likely perform unique functions which cannot be compensated for by other family members. Perhaps, at a specific step of the apoptotic pathway, activated GR uses caspase 9 as an effector, and this step cannot be bypassed by other proteases. We demonstrate that the expression of ICH-1L (caspase 2) is not altered in U2OS-GR(+) cells undergoing PCD compared to the untreated control cells, consistent with previous findings for glucocorticoid-sensitive lymphocytes. Furthermore, since the levels of Bcl2 were reduced in Dex-treated U2OS cells expressing either the wt GR, LS7, or the dimerization mutant, the same general mechanism appears to operate to induce apoptosis in human glucocorticoid-sensitive T cells and human osteosarcoma cells expressing GR. It would be interesting to examine whether specific inhibition of caspase 9 in U2OS cells prevents GR-dependent cell death. Interestingly, the intact GR transcriptional repression function was found to be sufficient for inducing PCD in human lymphocytes (37, 45). In contrast, thymocytes isolated from transgenic mice homozygous for dimerization-deficient GR are glucocorticoid resistant (74), suggesting that receptor dimerization and transcriptional activation through palindromic GREs are necessary for GR-mediated PCD in these cells. This paradigm emphasizes species-specific and, perhaps, developmental differences responsible for the distinct GR functions required for executing the cell death program in human lymphocytes versus developing murine thymocytes. Alternatively, the LS7 mutant used by Helmberg et al. (37) may not have uncoupled activation from repression.
It is unclear which factors determine the susceptibility of individual cell types to GR-mediated apoptosis. It has been previously reported that forced expression of p21 protects developing myocytes from apoptosis (93), and this protective effect is abrogated in cells lacking functional Rb protein (92). Our data, however, argue that even in the absence of enhanced p21 expression [for example, when the growth of SAOS2-GR(+) cells is inhibited by RU 486], they do not undergo apoptosis but rather retain some ability to proliferate (Fig. 9). This suggests that in the context of SAOS2 cells, p21 functions as a cell cycle inhibitor but not as a survival factor. Clearly, in Rb-negative SAOS2 cells, the cell death program is regulated differently from that in mitogen-deprived myocytes.
Enhanced expression of p21 and p27 in response to GR activation is accomplished through distinct surfaces.
It has previously been demonstrated that the GR-mediated cell cycle arrest in SAOS2 human osteosarcoma cells, rat hepatoma cells, murine fibroblasts, and lymphocytes involves the induction of p21 (13, 72, 77). In some but not all of these cell lines, enhanced expression of p27 has also been observed. In addition, several studies emphasize that increased levels of p27 frequently reflect reduced degradation of the p27 protein via the ubiquitination pathway rather than enhanced expression of the p27 gene (3, 69). In SAOS2-GR(+) cells, we observe a reproducible increase of the level of p27 mRNA in response to Dex treatment, suggesting that enhanced transcription is at least in part responsible for the p27 induction in this cell line. Interestingly, however, this increase is not detectable until after 24 h of continuous Dex treatment (data not shown), whereas induction of the p21 mRNA peaks at 2 h of Dex treatment (77). Such rapid kinetics of p21 induction, combined with the insensitivity of this effect to inhibitors of protein synthesis (e.g., cycloheximide), argue that the p21 promoter is under the direct transcriptional control of GR despite the lack of a consensus GRE. In contrast, the enhanced transcription of p27 likely involves one or more intermediates transducing the glucocorticoid signal from GR to the p27 promoter.
Consistent with the different kinetics of p21 and p27 mRNA induction, different GR transcriptional activation functions appear to be responsible for the enhanced expression of these two CDIs. The GR 30iiB mutant with a transcriptionally inactive AF-1 is competent for increasing the expression of both p21 and p27 (Fig. 11A, 30iiB+Dex) to the level induced by the wt GR. Thus, despite the fact that the presence of the GR N terminus is required for cell cycle arrest and increased expression of CDIs (Fig. 11A, Δ4C1+Dex), AF-1 function is not required for transcriptional induction of either promoter. This unexpected finding suggests that increased CDI expression is accomplished either through transcriptional activation by AF-2 or by an as-yet-unidentified GR transcriptional regulatory domain(s) which, directly in the case of p21 or through an intermediary protein in the case of p27, enhances transcription from the p21 and p27 promoters. Supporting this idea, inactivation of AF-2 by RU 486 largely abolished the induction of p21 (Fig. 11A, wt+RU 486). Interestingly, the GR dimerization-deficient mutant, which fails to activate transcription from single consensus GRE-containing promoters, was competent for inducing the expression of p21 (Fig. 11A, dim+Dex). Thus, transcriptional induction of p21 is likely mediated by AF-2 in a dimerization-independent manner and is unlikely to be driven by a single canonical GRE. Consistent with this idea, the p21 promoter lacks a full consensus GRE but contains several half-GRE-like sequences, which could potentially serve as a platform for a GR-containing protein complex. The mapping of the p21 promoter in hepatoma cells revealed four GR-responsive regions located between bp −1481 and −1184 from the transcription start site (13), one of which corresponds to a C/EBPα binding site whereas the others contain no known regulatory elements. Since the level of C/EBPα is not altered in hormone-treated SAOS2-GR(+) cells (data not shown), it is likely that another element(s) is responsible for transcriptional induction of p21 in this cell context.
FIG. 11.
Regulation of p21 and p27 in SAOS2 cells by different GR derivatives. (A) Effects of GR derivatives on p21 and p27 expression. The GR domains and receptor derivatives stably introduced into SAOS2 cells are described in Fig. 1. The ability of GR derivatives to induce p21 and p27 expression and to effect growth inhibition in SAOS2 cells was assessed in the presence of Dex (yellow ovals) or RU 486 (green rectangles). (B) A model for the differential regulation of CDIs by the wt and mutant GRs in SAOS2 cells. Schematically shown are two wt GR molecules (wtGR+Dex), which in the presence of the full agonist Dex (D, yellow ovals) dimerize through the DBD (indicated by a cross) and recruit a coactivator protein (CoA, blue cubes) to associate with the C-terminal AF-2 (shown in dark blue). An additional putative interacting protein (?, pink cubes) may associate with the GR N terminus and AF-1. Possible communication between the C-terminal coactivator and an unknown N terminus-interacting factor (indicated with an arrow) induces expression of p21 and p27. Deletion of the GR N terminus (Δ4C1+Dex) results in a loss of the unknown interactor, disrupting a putative interaction between the N and the C termini, such that the induction of both p21 and p27 is abolished. wt GR in the presence of the partial agonist RU 486 (wtGR+RU 486) is unable to recruit a C-terminal coactivator due to a conformational change in the LBD. The induction of p21 is abolished, suggesting a requirement for a functional AF-2 for this effect, whereas enhanced expression of p27 occurs in an AF-2-independent manner, possibly through the unknown N-terminal interactor and as-yet-unidentified transcriptional regulatory function at GR N terminus. Disruption of the GR dimerization interface (dim+Dex) eliminates p27 but not p21 induction, indicating that stimulated p27 expression depends on GR dimerization and GRE binding, whereas p21 induction is accomplished by GR in its monomeric form.
In contrast, induction of p27 occurs in the presence of RU 486, suggesting that AF-2 activity is not required for this effect (Fig. 11A, wt+RU 486). It is conceivable that additional transcriptional regulatory domains may be involved in regulating p27 expression. Interestingly, the induction of p27 was sensitive to the mutations R479D and D481R disrupting the GR dimerization interface (Fig. 11A, dim+Dex). Thus, one or more protein intermediates induced by GR to activate p27 transcription may contain a canonical GRE(s) in their promoters, such that the dimerization-deficient mutant no longer activates their expression, thereby eliminating p27 induction. The inability of the dim mutant to efficiently enhance p27 expression also argues that protein intermediates transducing the GR signal to the p27 promoter are GR-induced activators, rather than GR-repressed inhibitors, since the dim mutant is competent for transcriptional repression but fails to stimulate p27 expression. It appears, then, that distinct mechanisms of transcriptional activation are responsible for the increased expression of p21 and p27 in SAOS2 cells in response to GR activation: p21 induction requires transcriptional activation by the GR AF-2 but not an intact dimerization interface, whereas p27 is stimulated when AF-2 is rendered inactive, but GR dimerization is essential.
Requirements for increased expression of p21 and p27 for GR-mediated cell cycle arrest.
We assessed the importance of each CDI for the GR-mediated cell cycle arrest in SAOS2 cells by performing a cell proliferation assay when induction of either p21 (by using RU 486) or p27 (by using the GR dimerization-deficient mutant) was minimized. Our data indicate that although each CDI separately inhibits cell proliferation by 65 to 75%, neither one alone can induce complete cell cycle arrest. Thus, increased expression of both CDIs is necessary for complete growth arrest in SAOS2 cells.
Possible models for differential transcriptional activation of CDIs by GR.
On the basis of our results, we propose a model that accounts for the transcriptional induction of p21 and p27 in SAOS2 cells. Activation of the wt GR by an agonist, such as Dex, can result in dimerization, recruitment of coactivator proteins, and induction of CDIs (Fig. 11B, wt GR+Dex). Previous results also indicate that GR-dependent CDI induction requires the receptor N-terminal residues 70 through 300, since deletion of this region (Fig. 11B, Δ4C1+Dex) eliminates CDI expression and the cytostatic activity of the receptor in SAOS2 cells. However, the AF-1 domain of GR is not required for CDI regulation, since a GR mutant containing a transcriptionally inactive AF-1 (the GR 30iiB mutant) induces CDI expression as efficiently as wt GR. Thus, the GR N terminus, but not AF-1 per se, is necessary for CDI induction. These findings suggest that novel surfaces within the N terminus may interact with another, as-yet-unidentified protein(s) to facilitate transcriptional activation. For some steroid receptors, such as the estrogen receptor, the receptor N- and C-terminal regulatory regions function synergistically to activate transcription, and the coactivator protein SRC1 further promotes a transcriptionally productive interaction between the receptor N terminus and the LBD (48, 61). It has also been shown that in addition to their interaction with the LBDs of most steroid receptors and type II nuclear receptors, p160 family members SRC1 and GRIP1 interact with the estrogen and progesterone receptor N termini (67, 95). Although such interactions between the GR N terminus and GRIP1 have not been detected (82a), this domain may interact with other regulatory proteins. Alternatively, the N-terminal sequences may be critical for preserving the structural integrity of the GR protein.
Our results also indicate that in the presence of RU 486, GR fails to induce p21 expression, whereas p27 induction is preserved (Fig. 11B, wt GR+RU 486). The RU 486-bound GR fails to recruit a coactivator and no longer supports transcriptional activation by AF-2 (40). Thus, additional and as-yet-uncharacterized transcriptional regulatory domains may be involved in enhancing p27 expression. Our findings also suggest that GR transcriptional activation of the p21 promoter is likely dependent on coactivator function mediated by AF-2, while activation of the p27 promoter is not. Finally, mutations that disrupt GR dimerization impair GR’s ability to induce p27 but not p21 (Fig. 11B, dim+Dex), suggesting that monomeric GR is sufficient for inducing p21 expression. Therefore, the requirements for the GR-responsive elements and the promoters responsible for the CDI induction are different for these two regulators. p21 is not induced through a canonical single GRE, and transcriptional activation likely involves protein-protein interactions possibly recruiting GR in its monomeric form to a multiprotein complex. In contrast, p27 induction requires GR dimerization, suggesting that one or more GR-responsive factors that are responsible for enhanced transcription of p27 contain consensus GREs in their promoters.
A fuller understanding of the GR transcriptional regulatory mechanisms and target genes responsible for eliciting the cytotoxic versus cytostatic outcome of GR activation may ultimately lead to the identification of novel compounds that induce the cytotoxic rather than the cytostatic response to glucocorticoids and thus may expand the therapeutic utility of glucocorticoids as chemotherapeutic agents.
ACKNOWLEDGMENTS
We are grateful to Jorge A. Iñiguez-Lluhí, Paul Godowski, and Keith Yamamoto for the GR mutant constructs. We thank Len Freedman, Roland Knoblauch, Janet Trowbridge, and Angus Wilson for critically reading the manuscript.
This work was supported by grants to M.J.G. from the Army Breast Cancer Research Fund (DAMD17-94-J-4454, DAMD17-96-1-6032) and the Irma T. Hirschl Charitable Trust. We also thank the NIH for its support of I.R. (5T32AI07180-17), A.B.H. (2T32GM07308), and D.P. (R29-DK51151-03).
REFERENCES
- 1.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Alam J, Cook J L. Reporter genes: application to the study of mammalian gene transcription. Anal Biochem. 1990;188:245–254. doi: 10.1016/0003-2697(90)90601-5. [DOI] [PubMed] [Google Scholar]
- 3.Alessandrini A, Chiaur D S, Pagano M. Regulation of the cyclin-dependent kinase inhibitor p27 by degradation and phosphorylation. Leukemia. 1997;11:342–345. doi: 10.1038/sj.leu.2400581. [DOI] [PubMed] [Google Scholar]
- 4.Almlof T, Gustafsson J A, Wright A P. Role of hydrophobic amino acid clusters in the transactivation activity of the human glucocorticoid receptor. Mol Cell Biol. 1997;17:934–945. doi: 10.1128/mcb.17.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alnemri E S, Fernandes T F, Haldar S, Croce C M, Litwack G. Involvement of Bcl-2 in glucocorticoid-induced apoptosis of human pre-B-leukemias. Cancer Res. 1992;52:491–495. [PubMed] [Google Scholar]
- 6.Barde Y A. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 7.Blanco J C, Minucci S, Lu J, Yang X J, Walker K K, Chen H, Evans R M, Nakatani Y, Ozato K. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1998;12:1638–1651. doi: 10.1101/gad.12.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brehm A, Miska E A, McCance D J, Reid J L, Bannister A J, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 9.Brinkmann U, Brinkmann E, Gallo M, Pastan I. Cloning and characterization of a cellular apoptosis susceptibility gene, the human homologue to the yeast chromosome segregation gene CSE1. Proc Natl Acad Sci USA. 1995;92:10427–10431. doi: 10.1073/pnas.92.22.10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkmann U, Gallo M, Polymeropoulos M H, Pastan I. The human CAS (cellular apoptosis susceptibility) gene mapping on chromosome 20q13 is amplified in BT474 breast cancer cells and part of aberrant chromosomes in breast and colon cancer cell lines. Genome Res. 1996;6:187–194. doi: 10.1101/gr.6.3.187. [DOI] [PubMed] [Google Scholar]
- 11.Brostjan C, Anrather J, Csizmadia V, Stroka D, Soares M, Bach F H, Winkler H. Glucocorticoid-mediated repression of NFκB activity in endothelial cells does not involve induction of IκBα synthesis. J Biol Chem. 1996;271:19612–19616. doi: 10.1074/jbc.271.32.19612. [DOI] [PubMed] [Google Scholar]
- 12.Burnstein K L, Jewell C M, Sar M, Cidlowski J A. Intragenic sequences of the human glucocorticoid receptor complementary DNA mediate hormone-inducible receptor messenger RNA down-regulation through multiple mechanisms. Mol Endocrinol. 1994;8:1764–1773. doi: 10.1210/mend.8.12.7708063. [DOI] [PubMed] [Google Scholar]
- 13.Cha H H, Cram E J, Wang E C, Huang A J, Kasler H G, Firestone G L. Glucocorticoids stimulate p21 gene expression by targeting multiple transcriptional elements within a steroid responsive region of the p21waf1/cip1 promoter in rat hepatoma cells. J Biol Chem. 1998;273:1998–2007. doi: 10.1074/jbc.273.4.1998. [DOI] [PubMed] [Google Scholar]
- 14.Chandler V L, Maler B A, Yamamoto K R. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983;33:489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- 15.Chandra J, Gilbreath J, Freireich E J, Kliche K O, Andreeff M, Keating M, McConkey D J. Protease activation is required for glucocorticoid-induced apoptosis in chronic lymphocytic leukemic lymphocytes. Blood. 1997;90:3673–3681. [PubMed] [Google Scholar]
- 16.Chapman M S, Askew D J, Kuscuoglu U, Miesfeld R L. Transcriptional control of steroid-regulated apoptosis in murine thymoma cells. Mol Endocrinol. 1996;10:967–978. doi: 10.1210/mend.10.8.8843413. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 18.Cidlowski J A, King K L, Evans-Storms R B, Montague J W, Bortner C D, Hughes F M., Jr The biochemistry and molecular biology of glucocorticoid-induced apoptosis in the immune system. Recent Prog Horm Res. 1996;51:457–490. [PubMed] [Google Scholar]
- 19.Cohen G M. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman R E. Glucocorticoids in cancer therapy. Biotherapy. 1992;4:37–44. doi: 10.1007/BF02171708. [DOI] [PubMed] [Google Scholar]
- 21.Coleman T R, Dunphy W G. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 22.Cram E J, Ramos R A, Wang E C, Cha H H, Nishio Y, Firestone G L. Role of the CCAAT/enhancer binding protein-transcription factor in the glucocorticoid stimulation of p21waf1/cip1 gene promoter activity in growth-arrested rat hepatoma cells. J Biol Chem. 1998;273:2008–2014. doi: 10.1074/jbc.273.4.2008. [DOI] [PubMed] [Google Scholar]
- 23.De Bosscher K, Schmitz M L, Vanden Berghe W, Plaisance S, Fiers W, Haegeman G. Glucocorticoid-mediated repression of nuclear factor-κB-dependent transcription involves direct interference with transactivation. Proc Natl Acad Sci USA. 1997;94:13504–13509. doi: 10.1073/pnas.94.25.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dempster D W, Moonga B S, Stein L S, Horbert W R, Antakly T. Glucocorticoids inhibit bone resorption by isolated rat osteoclasts by enhancing apoptosis. J Endocrinol. 1997;154:397–406. doi: 10.1677/joe.0.1540397. [DOI] [PubMed] [Google Scholar]
- 25.Diamond M I, Miner J N, Yoshinaga S K, Yamamoto K R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990;249:1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- 26.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 27.Farrow S N, Brown R. New members of the Bcl-2 family and their protein partners. Curr Opin Genet Dev. 1996;6:45–49. doi: 10.1016/s0959-437x(96)90009-x. [DOI] [PubMed] [Google Scholar]
- 28.Frasch S C, Nick J A, Fadok V A, Bratton D L, Worthen G S, Henson P M. p38 mitogen-activated protein kinase-dependent and -independent intracellular signal transduction pathways leading to apoptosis in human neutrophils. J Biol Chem. 1998;273:8389–8397. doi: 10.1074/jbc.273.14.8389. [DOI] [PubMed] [Google Scholar]
- 29.Gametchu B, Harrison R W. Characterization of a monoclonal antibody to the rat liver glucocorticoid receptor. Endocrinology. 1984;114:274–279. doi: 10.1210/endo-114-1-274. [DOI] [PubMed] [Google Scholar]
- 30.Gaynon P S, Lustig R H. The use of glucocorticoids in acute lymphoblastic leukemia of childhood. Molecular, cellular, and clinical considerations. J Pediatr Hematol Oncol. 1995;17:1–12. doi: 10.1097/00043426-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Godowski P J, Sakai D D, Yamamoto K R. Signal transduction and transcriptional regulation by the glucocorticoid receptor. UCLA Symp Mol Cell Biol. 1989;95:197–210. [Google Scholar]
- 32.Hakem R, Hakem A, Duncan G S, Henderson J T, Woo M, Soengas M S, Elia A, de la Pompa J L, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman S A, Lowe S W, Penninger J M, Mak T W. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 33.Harper J W, Elledge S J. Cdk inhibitors in development and cancer. Curr Opin Genet Dev. 1996;6:56–64. doi: 10.1016/s0959-437x(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 34.Harvey K J, Blomquist J F, Ucker D S. Commitment and effector phases of the physiological cell death pathway elucidated with respect to Bcl-2 caspase and cyclin-dependent kinase activities. Mol Cell Biol. 1998;18:2912–22. doi: 10.1128/mcb.18.5.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heck S, Bender K, Kullmann M, Gottlicher M, Herrlich P, Cato A C. IκBα-independent downregulation of NF-κB activity by glucocorticoid receptor. EMBO J. 1997;16:4698–4707. doi: 10.1093/emboj/16.15.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heck S, Kullmann M, Gast A, Ponta H, Rahmsdorf H J, Herrlich P, Cato A C. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J. 1994;13:4087–4095. doi: 10.1002/j.1460-2075.1994.tb06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helmberg A, Auphan N, Caelles C, Karin M. Glucocorticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. EMBO J. 1995;14:452–460. doi: 10.1002/j.1460-2075.1995.tb07021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrlich P, Gottlicher M. Transcriptional cross-talk by steroid hormone receptors. In: Freedman L P, editor. The molecular biology of steroid and nuclear hormone receptors. Boston, Mass: Birkhauser; 1998. pp. 191–207. [DOI] [PubMed] [Google Scholar]
- 39.Hinds P W, Mittnacht S, Dulic V, Arnold A, Reed S I, Weinberg R A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 40.Hong H, Kohli K, Garabedian M J, Stallcup M R. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol Cell Biol. 1997;17:2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong H, Kohli K, Trivedi A, Johnson D L, Stallcup M R. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci USA. 1996;93:4948–4952. doi: 10.1073/pnas.93.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes F M, Jr, Cidlowski J A. Glucocorticoid-induced thymocyte apoptosis: protease-dependent activation of cell shrinkage and DNA degradation. J Steroid Biochem Mol Biol. 1998;65:207–217. doi: 10.1016/s0960-0760(97)00188-x. [DOI] [PubMed] [Google Scholar]
- 43.Iniguez-Lluhi J A, Lou D Y, Yamamoto K R. Three amino acid substitutions selectively disrupt the activation but not the repression function of the glucocorticoid receptor N terminus. J Biol Chem. 1997;272:4149–4156. doi: 10.1074/jbc.272.7.4149. [DOI] [PubMed] [Google Scholar]
- 44.Jacobson M D, Weil M, Raff M C. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 45.Karin M. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell. 1998;93:487–490. doi: 10.1016/s0092-8674(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 46.Karin M, Haslinger A, Holtgreve H, Richards R I, Krauter P, Westphal H M, Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984;308:513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- 47.Kawakami A, Tian Q, Duan X, Streuli M, Schlossman S F, Anderson P. Identification and functional characterization of a TIA-1-related nucleolysin. Proc Natl Acad Sci USA. 1992;89:8681–8685. doi: 10.1073/pnas.89.18.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kraus W L, McInerney E M, Katzenellenbogen B S. Ligand-dependent, transcriptionally productive association of the amino- and carboxyl-terminal regions of a steroid hormone nuclear receptor. Proc Natl Acad Sci USA. 1995;92:12314–12318. doi: 10.1073/pnas.92.26.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krstic M D, Rogatsky I, Yamamoto K R, Garabedian M J. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol. 1997;17:3947–3954. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuida K, Haydar T F, Kuan C Y, Gu Y, Taya C, Karasuyama H, Su M S, Rakic P, Flavell R A. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 51.Kuida K, Zheng T S, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell R A. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 52.Lefstin J A, Thomas J R, Yamamoto K R. Influence of a steroid receptor DNA-binding domain on transcriptional regulatory functions. Genes Dev. 1994;8:2842–2856. doi: 10.1101/gad.8.23.2842. [DOI] [PubMed] [Google Scholar]
- 53.Liden J, Delaunay F, Rafter I, Gustafsson J, Okret S. A new function for the C-terminal zinc finger of the glucocorticoid receptor. Repression of RelA transactivation. J Biol Chem. 1997;272:21467–21472. doi: 10.1074/jbc.272.34.21467. [DOI] [PubMed] [Google Scholar]
- 54.Linette G P, Korsmeyer S J. Differentiation and cell death: lessons from the immune system. Curr Opin Cell Biol. 1994;6:809–815. doi: 10.1016/0955-0674(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 55.Liu W, Hillmann A G, Harmon J M. Hormone-independent repression of AP-1-inducible collagenase promoter activity by glucocorticoid receptors. Mol Cell Biol. 1995;15:1005–1013. doi: 10.1128/mcb.15.2.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu W, Wang J, Sauter N K, Pearce D. Steroid receptor heterodimerization demonstrated in vitro and in vivo. Proc Natl Acad Sci USA. 1995;92:12480–12484. doi: 10.1073/pnas.92.26.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu W, Wang J, Yu G, Pearce D. Steroid receptor transcriptional synergy is potentiated by disruption of the DNA-binding domain dimer interface. Mol Endocrinol. 1996;10:1399–1406. doi: 10.1210/mend.10.11.8923466. [DOI] [PubMed] [Google Scholar]
- 58.Luisi B F, Xu W X, Otwinowski Z, Freedman L P, Yamamoto K R, Sigler P B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991;352:497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- 59.Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain J P, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature. 1998;391:601–605. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- 60.Martin-Castellanos C, Moreno S. Recent advances on cyclins, CDKs and CDK inhibitors. Trends Cell Biol. 1997;7:95–98. doi: 10.1016/S0962-8924(96)10055-6. [DOI] [PubMed] [Google Scholar]
- 61.McInerney E M, Tsai M J, O’Malley B W, Katzenellenbogen B S. Analysis of estrogen receptor transcriptional enhancement by a nuclear hormone receptor coactivator. Proc Natl Acad Sci USA. 1996;93:10069–10073. doi: 10.1073/pnas.93.19.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meagher L C, Cousin J M, Seckl J R, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol. 1996;156:4422–4428. [PubMed] [Google Scholar]
- 63.Miner J N, Diamond M I, Yamamoto K R. Joints in the regulatory lattice: composite regulation by steroid receptor-AP1 complexes. Cell Growth Differ. 1991;2:525–530. [PubMed] [Google Scholar]
- 64.Miyoshi H, Ohki M, Nakagawa T, Honma Y. Glucocorticoids induce apoptosis in acute myeloid leukemia cell lines with A t(8;21) chromosome translocation. Leuk Res. 1997;21:45–50. doi: 10.1016/s0145-2126(96)00089-6. [DOI] [PubMed] [Google Scholar]
- 65.Muller R. Transcriptional regulation during the mammalian cell cycle. Trends Genet. 1995;11:173–178. doi: 10.1016/S0168-9525(00)89039-3. [DOI] [PubMed] [Google Scholar]
- 66.Oakley R H, Cidlowski J A. Homologous down regulation of the glucocorticoid receptor: the molecular machinery. Crit Rev Eukaryot Gene Expr. 1993;3:63–88. [PubMed] [Google Scholar]
- 67.Onate S A, Boonyaratanakornkit V, Spencer T E, Tsai S Y, Tsai M J, Edwards D P, O’Malley B W. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J Biol Chem. 1998;273:12101–12108. doi: 10.1074/jbc.273.20.12101. [DOI] [PubMed] [Google Scholar]
- 68.Onate S A, Tsai S Y, Tsai M J, O’Malley B W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 69.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Del Sal G, Chau V, Yew P R, Draetta G F, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 70.Perlman H, Sata M, Le Roux A, Sedlak T W, Branellec D, Walsh K. Bax-mediated cell death by the Gax homeoprotein requires mitogen activation but is independent of cell cycle activity. EMBO J. 1998;17:3576–3586. doi: 10.1093/emboj/17.13.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pirotte B, Levivier M, Goldman S, Brucher J M, Brotchi J, Hildebrand J. Glucocorticoid-induced long-term remission in primary cerebral lymphoma: case report and review of the literature. J Neurooncol. 1997;32:63–69. doi: 10.1023/a:1005733416571. [DOI] [PubMed] [Google Scholar]
- 72.Ramalingam A, Hirai A, Thompson E A. Glucocorticoid inhibition of fibroblast proliferation and regulation of the cyclin kinase inhibitor p21Cip1. Mol Endocrinol. 1997;11:577–586. doi: 10.1210/mend.11.5.9923. [DOI] [PubMed] [Google Scholar]
- 73.Ramos R A, Nishio Y, Maiyar A C, Simon K E, Ridder C C, Ge Y, Firestone G L. Glucocorticoid-stimulated CCAAT/enhancer-binding protein α expression is required for steroid-induced G1 cell cycle arrest of minimal-deviation rat hepatoma cells. Mol Cell Biol. 1996;16:5288–5301. doi: 10.1128/mcb.16.10.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reichardt H M, Kaestner K H, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 75.Rhee K, Bresnahan W, Hirai A, Hirai M, Thompson E A. C-Myc and cyclin D3 (CcnD3) genes are independent targets for glucocorticoid inhibition of lymphoid cell proliferation. Cancer Res. 1995;55:4188–4195. [PubMed] [Google Scholar]
- 76.Rogatsky I, Logan S K, Garabedian M J. Antagonism of glucocorticoid receptor transcriptional activation by the cJun N-terminal kinase. Proc Natl Acad Sci USA. 1998;95:2050–2055. doi: 10.1073/pnas.95.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rogatsky I, Trowbridge J M, Garabedian M J. Glucocorticoid receptor-mediated cell cycle arrest is achieved through distinct cell-specific transcriptional regulatory mechanisms. Mol Cell Biol. 1997;17:3181–3193. doi: 10.1128/mcb.17.6.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rogatsky I, Waase C M, Garabedian M J. Phosphorylation and inhibition of the rat glucocorticoid receptor transcriptional activity by glycogen synthase kinase-3. J Biol Chem. 1998;273:15315–15321. doi: 10.1074/jbc.273.23.14315. [DOI] [PubMed] [Google Scholar]
- 79.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 80.Schule R, Rangarajan P, Kliewer S, Ransone L J, Bolado J, Yang N, Verma I M, Evans R M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990;62:1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- 81.Sentman C L, Shutter J R, Hockenbery D, Kanagawa O, Korsmeyer S J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 82.Smith C L, Onate S A, Tsai M J, O’Malley B W. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc Natl Acad Sci USA. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82a.Stallcup, M. Personal communication.
- 83.Steffen M, Scherdin U, Duvigneau C, Holzel F. Glucocorticoid-induced alterations of morphology and growth of fibrosarcoma cells derived from 7,12-dimethylbenz(a)anthracene rat mammary tumor. Cancer Res. 1988;48:7212–7218. [PubMed] [Google Scholar]
- 84.Stoecklin E, Wissler M, Gouilleux F, Groner B. Functional interactions between Stat5 and the glucocorticoid receptor. Nature. 1996;383:726–728. doi: 10.1038/383726a0. [DOI] [PubMed] [Google Scholar]
- 85.Stoecklin E, Wissler M, Moriggl R, Groner B. Specific DNA binding of Stat5, but not of glucocorticoid receptor, is required for their functional cooperation in the regulation of gene transcription. Mol Cell Biol. 1997;17:6708–6716. doi: 10.1128/mcb.17.11.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taya Y. RB kinases and RB-binding proteins: new points of view. Trends Biochem Sci. 1997;22:14–17. doi: 10.1016/s0968-0004(96)10070-0. [DOI] [PubMed] [Google Scholar]
- 87.Tchekneva E, Serafin W E. Kirsten sarcoma virus-immortalized mast cell lines. Reversible inhibition of growth by dexamethasone and evidence for the presence of an autocrine growth factor. J Immunol. 1994;152:5912–5921. [PubMed] [Google Scholar]
- 88.Templeton D J, Park S H, Lanier L, Weinberg R A. Nonfunctional mutants of the retinoblastoma protein are characterized by defects in phosphorylation, viral oncoprotein association, and nuclear tethering. Proc Natl Acad Sci USA. 1991;88:3033–3037. doi: 10.1073/pnas.88.8.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 90.Tiemann F, Hinds P W. Induction of DNA synthesis and apoptosis by regulated inactivation of a temperature-sensitive retinoblastoma protein. EMBO J. 1998;17:1040–1052. doi: 10.1093/emboj/17.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Torchia J, Rose D W, Inostroza J, Kamei Y, Westin S, Glass C K, Rosenfeld M G. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 92.Wang J, Guo K, Wills K N, Walsh K. Rb functions to inhibit apoptosis during myocyte differentiation. Cancer Res. 1997;57:351–354. [PubMed] [Google Scholar]
- 93.Wang J, Walsh K. Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science. 1996;273:359–361. doi: 10.1126/science.273.5273.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang L, Miura M, Bergeron L, Zhu H, Yuan J. Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell. 1994;78:739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 95.Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen M P, Chen D, Huang S M, Subramanian S, McKinerney E, Katzenellenbogen B S, Stallcup M R, Kushner P J. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol. 1998;12:1605–1618. doi: 10.1210/mend.12.10.0185. [DOI] [PubMed] [Google Scholar]
- 96.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 97.Wellmann A, Krenacs L, Fest T, Scherf U, Pastan I, Raffeld M, Brinkmann U. Localization of the cell proliferation and apoptosis-associated CAS protein in lymphoid neoplasms. Am J Pathol. 1997;150:25–30. [PMC free article] [PubMed] [Google Scholar]
- 98.Williams S P, Sigler P B. Atomic structure of progesterone complexed with its receptor. Nature. 1998;393:392–396. doi: 10.1038/30775. [DOI] [PubMed] [Google Scholar]
- 99.Wissink S, van Heerde E C, Schmitz M L, Kalkhoven E, van der Burg B, Baeuerle P A, van der Saag P T. Distinct domains of the RelA NF-κB subunit are required for negative cross-talk and direct interaction with the glucocorticoid receptor. J Biol Chem. 1997;272:22278–22284. doi: 10.1074/jbc.272.35.22278. [DOI] [PubMed] [Google Scholar]
- 100.Woo M, Hakem R, Soengas M S, Duncan G S, Shahinian A, Kagi D, Hakem A, McCurrach M, Khoo W, Kaufman S A, Senaldi G, Howard T, Lowe S W, Mak T W. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998;12:806–819. doi: 10.1101/gad.12.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamamoto K R, Pearce D, Thomas J, Miner J N. Combinatorial regulation at mammalian composite response elements. In: McKnight S L, Yamamoto K R, editors. Transcriptional regulation. Vol. 2. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 1169–92. [Google Scholar]
- 102.Yang E, Korsmeyer S J. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 103.Yang-Yen H-F, Chambard J-C, Sun Y-L, Smeal T, Schmidt T J, Drouin J, Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62:1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]