Abstract
Background:
Exposure to stressful events related to COVID-19 has been associated with increases in the prevalence of depression and anxiety, raising questions about vulnerabilities that make some individuals most susceptible to internalizing symptoms following stress exposure.
Methods:
The current prospective study examined the effects of neurophysiological reactivity to positive and threatening interpersonal stimuli, indexed by the late positive potential (LPP) event-related potential, in conjunction with exposure to interpersonal pandemic-related stressors in the prediction of internalizing symptom changes from pre- to during the pandemic. Emerging adults (N=75) initially completed measures of internalizing symptoms and an interpersonal emotional images task while electroencephalogram was recorded pre-pandemic and were re-contacted during the COVID-19 pandemic in May 2020 to complete measures of exposure to pandemic-related stressful events and current internalizing symptoms.
Results:
Results indicated that emerging adults experienced numerous stressful events associated with the pandemic, as well as overall increases in symptoms of depression and traumatic intrusions during the pandemic. Furthermore, significant interactions between LPP reactivity to positive and threatening interpersonal stimuli and interpersonal stress exposure emerged in the prediction of internalizing symptoms, controlling for baseline symptoms. Under high exposure to interpersonal stressors, reduced positive LPPs predicted increases in depressive symptoms while enhanced threatening LPPs predicted increases in traumatic intrusions.
Conclusions:
These findings highlight the mental health impacts of the COVID-19 pandemic on emerging adults, and the role of individual differences in neurophysiological reactivity to emotional stimuli in vulnerability for depression and traumatic intrusions following stress exposure.
Keywords: event-related potentials, emotion, depression, internalizing symptoms, stress, pandemic
Introduction
Depressive and anxiety disorders are highly comorbid, prevalent psychopathologies (1,2). Robust evidence demonstrates stressful events, particularly interpersonal events, prospectively predict depression and anxiety (3–7). However, not all individuals who experience stressful events develop depression or anxiety. Understanding factors underlying risk and resilience following exposure to stressful life events is imperative for identifying those at greatest risk and processes to target through intervention.
In addition to constituting an international physical health crisis, the COVID-19 pandemic has had profound mental health impacts, with prevalence estimates of clinical levels of depression and anxiety symptoms far exceeding prior epidemiological research (22.8–45.1% for depression and 20.0–37.1% for anxiety; 8–10). Given the existing stress literature, pandemic-related stressors potentially contributing to the elevation in internalizing symptoms include interpersonal conflicts (11), social isolation and loneliness (12), job loss (13), general life disruption (14), and the uncontrollable nature of these experiences (15).
Emerging adulthood is a time of increasing independence and salience of peer and romantic relationships (16), and a high-risk period for the emergence of psychopathology, particularly mood and anxiety disorders (17,18). Our prior research indicates that emerging adults experienced abrupt disruptions in education, occupations, and relationships due to university closures, economic recession, and social distancing mandates associated with the pandemic, which correlated with depression and anxiety (9). Of these, interpersonal pandemic-related stressors may confer particular risk for internalizing symptoms to this population. Longitudinal data on the pandemic are needed to disentangle pre-existing vulnerability factors making some individuals more susceptible to symptom changes following stress exposure. Considering experiences of stress confound with individuals’ dependent behaviors and cognitions (7,19), the unanticipated, ubiquitous impact of the COVID-19 pandemic provides a unique opportunity to study risk processes that predict individual differences in responses to pandemic-related stressors.
The late positive potential (LPP) is a neurophysiological measure of emotional reactivity that could be applied to understand stress vulnerability. The LPP is an event-related potential (ERP) characterized by a sustained positive deflection beginning approximately 300ms post-stimulus onset (20,21). The LPP reflects the elaborative processing of motivationally salient stimuli and is consistently enhanced in response to emotional compared with neutral stimuli (22). Combined EEG-fMRI studies show the scalp-recorded LPP correlates with activation within a broad network of cortical and subcortical regions, including the amygdala, nucleus accumbens, medial and ventrolateral prefrontal cortices, and visual cortices (23–26). The LPP demonstrates sensitivity to individual differences in emotion processing and is reliably elicited across development (27–29).
Previous research shows reduced LPPs to positive stimuli in individuals with and at risk for depression (30–33), but elevated LPPs, generally to negative or threatening stimuli, in individuals with and at risk for anxiety (33–38). The literature on LPPs to negative stimuli in depression is more equivocal, with some evidence of reductions (31) and other studies revealing enhancements (39,40). These discrepancies may be attributable to differences in the orientation of the stimuli (e.g. self-relevant stimuli versus other-oriented stimuli) and/or comorbid anxiety symptoms. However, one study found enhanced LPPs to emotional faces when controlling for comorbid anxiety (41), supporting heterogenous patterns of reactivity within major depressive disorder, potentially due to specific symptom combinations such as the presence or absence of anhedonia. These discrepancies may also relate to developmental differences in reactivity, given evidence for overall reductions in LPPs with age (29). Reduced LPPs in depression fit with emotion context insensitivity theories (42), possibly reflecting the inability to sustain activation in motivational systems (30,43), particularly for positive stimuli (44). Conversely, elevated LPPs associated with anxiety are consistent with the broader literature on heightened attention for threatening stimuli (45,46). This highlights the potential sensitivity of the LPP in distinguishing heterogeneous patterns of emotion processing within depression and between highly comorbid psychopathologies.
Importantly, much of the literature on the LPP and internalizing psychopathologies has relied on cross-sectional designs, but individual differences in emotional reactivity assessed by the LPP may actually reflect an underlying vulnerability for the later emergence of symptoms following stress exposure. Supporting this possibility, we previously showed that an enhanced LPP to unpleasant stimuli and a reduced LPP to pleasant stimuli prospectively predicted increases in psychiatric symptoms in children exposed to greater stress related to a natural disaster (47). This theory is further supported by a larger literature examining ERPs, brain activation, and pupillometry in emotional contexts as moderators of symptom change following stress exposure (14,48–50). Specifically, other ERP research demonstrates an interactive effect between the reward positivity, an ERP marker of positive valence systems function, and exposure to stressful life events in the prediction of depression (48). There is also evidence that amygdala reactivity to threatening stimuli in conjunction with exposure to acute stressful events may be a candidate neural index of stress-vulnerability (14,49). Finally, pupillometry research shows reduced pupil dilation in response to affective stimuli predicted depressive symptoms following exposure to natural-disaster related stress (50).
The majority of the ERP literature on emotion uses broad categories of unpleasant and pleasant stimuli (51) or emotional faces presented out of context. Considering the centrality of social processes to internalizing psychopathologies (52), LPP reactivity to interpersonal stimuli specifically could be highly relevant for understanding vulnerabilities for internalizing symptoms. Research shows the LPP is sensitive to stimulus attributes (53–55) with social aspects of stimuli demonstrating particular salience (55,56). We recently validated a novel set of stimuli to elicit neurophysiological reactivity to interpersonal emotional images (manuscript under review), which may be particularly relevant for understanding symptom changes during the pandemic, given the interpersonal impacts of the pandemic and social distancing. Hyper-reactivity to threat and hypo-reactivity to positive interpersonal stimuli, specifically, may result in tendencies to disengage and withdraw socially (e.g., avoidance of potential threat and low approach motivation towards positive interactions). These tendencies may then be exacerbated by stress exposure, such as stress related to the on-going pandemic where social isolation decreases the availability of positive social interactions, thus contributing to the onset and maintenance of depression.
The current study is among the first to examine prospective predictors of responses to COVID-19 stressful events. Extending findings from Kujawa et al. (2016), we examined LPP reactivity to positive and threatening interpersonal emotional images as predictors of internalizing symptom changes during a global pandemic. In an effort to understand the impact of the COVID-19 pandemic on mental health, we first developed a measure of pandemic-related stressful events in a separate sample of emerging adults (9). In the present study, LPP reactivity to interpersonal images and baseline internalizing symptoms were assessed pre-pandemic. In May 2020, follow-up assessments of exposure to pandemic-related events and internalizing symptoms were completed to examine stress exposure and changes in depressive and anxiety symptoms during the pandemic. Given the potency of interpersonal stressors as predictors of internalizing disorders, the pervasive social disruption associated with the COVID-19 pandemic, and the focus on neurophysiological reactivity to interpersonal emotional images, we examined both total pandemic-related stressors and interpersonal stressors, specifically, as predictors of internalizing symptom change as main effects and interactive effects with the LPP to positive and threatening interpersonal stimuli. We expected significant increases in symptoms of depression and anxiety across time and main effects of both types of stress on symptoms. Furthermore, we predicted that under high exposure to stress, particularly interpersonal stress, reduced LPP reactivity to emotional stimuli would predict changes in depressive symptoms, whereas heightened LPP reactivity to threatening stimuli would predict anxiety symptom changes.
Methods and Materials
Participants
Participants (N=130) were undergraduate students originally recruited as part of a study on emotional and social functioning in emerging adults. At T1, participants completed a series of self-report questionnaires followed by counterbalanced computer-based tasks while EEG was continuously recorded (57). On May 11th-13th, 2020, all participants were contacted with the option to complete additional self-report questionnaires by May 21, 2020. The analyzed sample (N=75) with T1 and T2 data had a mean age of 19.25 years (SD=1.16) at T1, and were 76.0% female, 10.67% Hispanic/Latino, and 54.67% White/Caucasian, 29.33% Asian, 9.33% Black/African American, and 6.67% multiracial. The average time between assessments was 313.14 days (SD=102.26). Participants identifying as female, χ2 (1,113)=6.78, p=.01, Black/African American, χ2 (1,113)=5.47, p=.02, and with higher baseline panic symptoms, t (111)=2.54, p=.02, were more likely to complete the follow-up assessment. The retained sample did not differ from the baseline sample on LPPs or baseline symptoms of depression, social anxiety, or traumatic intrusions (ps>.08). The Institutional Review Board at Vanderbilt University approved this study, and informed consent was obtained from all participants.
Measures
Interpersonal Emotional Interrupt Task
EEG was continuously recorded while participants completed an interpersonal version of an emotional interrupt paradigm, which reliably elicits the LPP (29,58). Stimuli were selected for relevance to the social experiences of emerging adults, including 15 threatening interpersonal images (e.g. bullying by peers, arguing with parents or friends), 15 pleasant interpersonal images (e.g. friends laughing, happy couples), and 15 non-social neutral images (e.g. nature and city scenes). Stimuli were obtained through stock image sites and the Open Affective Standardized Image Set (59). Trials consisted of a fixation cross (+) presented for 800ms, an image presented for 1000ms, a single target arrow (< or >) for 150ms, and the same image presented for an additional 400ms. To ensure attention throughout the task and to measure emotional interference on behavioral performance, participants were instructed to click the right or left mouse button to indicate the target arrow direction on each trial. Only correct trials with responses within 150–2150ms were included in analysis. Inter-trial intervals varied randomly from 1500ms to 2000ms. Participants completed six practice trials followed by two blocks of the task for a total of 90 trials. Behavioral data are presented in Supplemental Information.
Pandemic-Related Stress
Participants completed the college student version of the Pandemic Stress Questionnaire (PSQ; 9), a 24-item measure of perceived exposure and subjective severity of events due to the COVID-19 pandemic (full measure in Supplemental Information). Participants responded “yes/no” to indicate experiencing each event, followed by a perceived severity rating from 1–5 for endorsed events. In addition to PSQ total event scores, we previously tested face valid subscale scores, including an interpersonal subscale which includes 5 items assessing exposure to interpersonal conflicts, unexpected separations, inability to be with close others, loss of a close other due to COVID-19, and experiences of racism and discrimination (9). Only the total and interpersonal PSQ scales were analyzed in the current study.
Internalizing Symptoms
Internalizing symptoms were measured using the 64-item Inventory of Depression and Anxiety Symptoms (IDAS; 60). Participants rated the extent to which each item was experienced in the previous two weeks from 1–5. The IDAS consists of two broad scales, general depression and dysphoria, and ten symptom specific scales. We first examined symptom changes from pre- to during the pandemic in general depression, social anxiety, panic, and traumatic intrusions. To minimize the number of tests conducted, primary analyses of LPPs and interpersonal stress focused on symptom scales that showed an overall increase during the pandemic. Items assessing suicidal ideation (items 7, 9, 14, 15, 41, 43) were not assessed at follow-up and were excluded from the calculation of the general depression scale. Internal consistencies for the analyzed scales at the initial and follow-up assessments were α=.92−.93 for general depression, α=.67−.80 for traumatic intrusions, α=.86−.75 for social anxiety, α=.88−.86 for panic.
EEG Data Collection and Processing
EEG data were continuously recorded using a 64-channel actiCHamp system from BrainProducts (Munich, Germany). Cz served as the online reference and data were collected at a sampling rate of 1000 Hz. Electrooculogram was recorded by facial electrodes placed 1 cm vertically and horizontally around the eyes and referenced to an electrode on the back of the neck, per the BrainProducts bipolar-to-auxiliary adapter design. Offline processing was completed using BrainVision Analyzer software. Data were re-referenced to the linked mastoid recordings (TP9/TP10) and band pass filtered from .01 to 30 Hz. Trials were segmented from −200ms to 1000ms after image onset. Ocular correction and semiautomated procedures identifying voltage steps greater than 50 microvolts per ms between sampling points, voltage differences greater than 175 microvolts within a trial, and lowest allowed activity of .50 microvolts within 100ms intervals were applied. Remaining artifacts were removed through visual inspection. Faulty recordings at single electrodes were resolved through interpolation. Included participants had a minimum of 12 artifact-free trials per condition to obtain a stable LPP (61). Segments were averaged within each condition and baseline corrected to −200ms. LPP was scored from 400–1000ms (47,55,62,63) at a pooling of occipitoparietal sites (POz, PO3, PO4; 34,66,67), consistent with cross-sectional research on this sample (manuscript under review) and the region of overlap in the maximal distributions for both emotional conditions compared to neutral (Figure 1). Exploratory analyses testing a broader occipitoparietal pooling are presented in the Supplemental Information. Split-half reliability for the LPP was acceptable to good (Spearman-Brown coefficients: positive=.83, threatening=.78, neutral=.68). Ten participants were missing EEG data: 1 did not complete the task, 1 had a recording issue, 5 had poor data quality, and 3 had fewer than 12 correct artifact-free trials.
Figure 1.
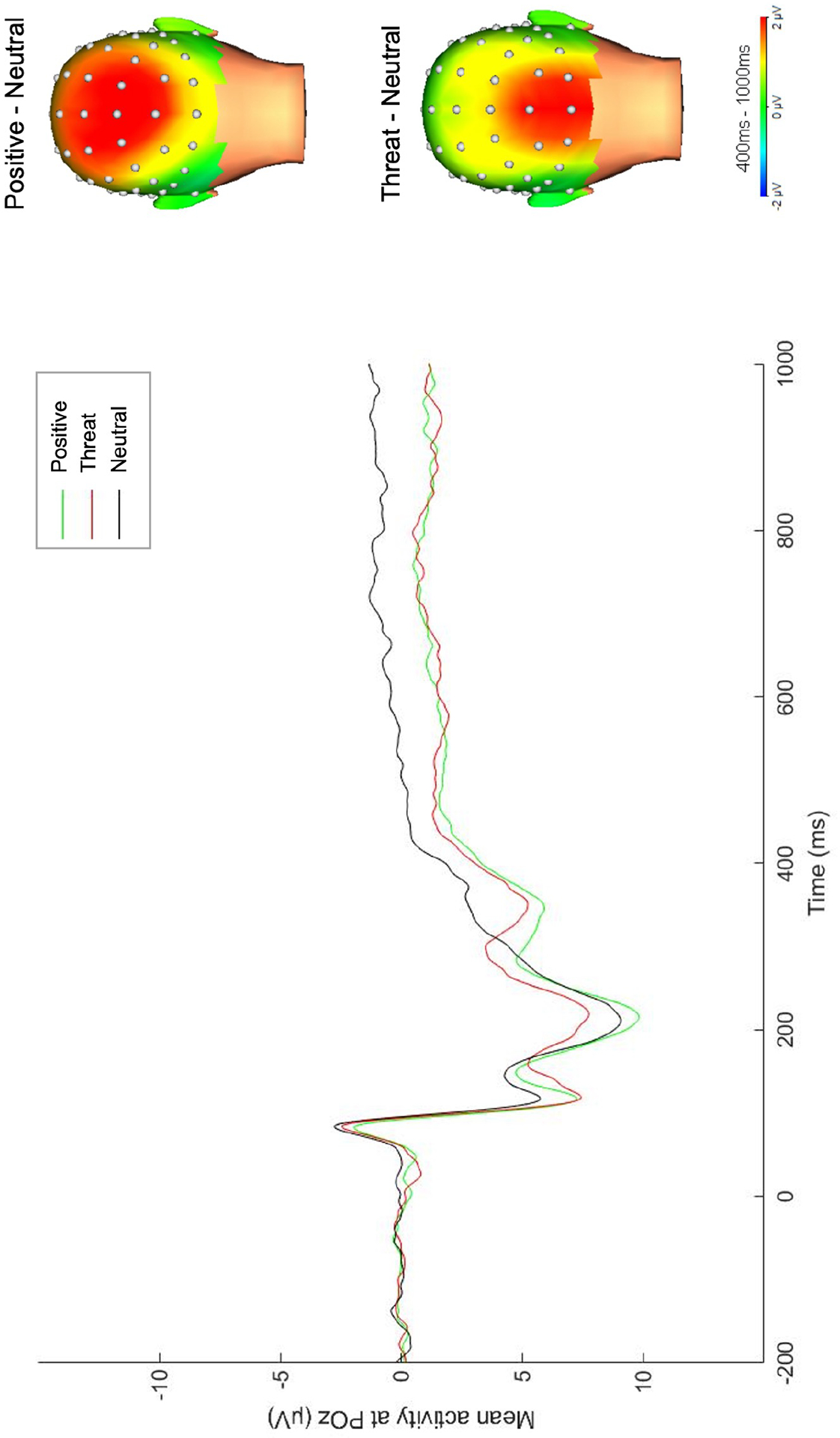
Grand average ERP waveform for the LPP across POz, PO3, and PO4. Scalp distributions reflect the response to the interpersonal emotional condition minus response to the neutral condition.
Data Analysis
Paired-samples t-tests were conducted to analyze internalizing symptom changes from before to during the pandemic. Restricted maximum likelihood was used to estimate missing data using the lme4 package in R (66). Frequencies of endorsed events and event sums were calculated to characterize exposure to pandemic-related stressors.
Consistent with ERP recommendations, unstandardized residual scores were computed to evaluate the LPP in each emotional condition, partialling out variance associated with the neutral condition (67). Multiple linear regressions were conducted to test the effects of LPPs to positive and threatening stimuli and pandemic-related stressful events and their interaction in the prediction of internalizing symptom changes. Interaction terms were calculated by taking the product of LPP residual scores and mean-centered interpersonal stressful events. To isolate change in symptoms from T1 to T2, T1 symptoms were included as covariates. Further, to differentiate effects for depression vs. anxiety, T2 anxiety was included as a covariate when examining predictors of depression, and T2 depression was included as a covariate when examining predictors of anxiety. Full-information maximum likelihood was used to estimate missing data using the lavaan package in R (68). Significant interactions were probed by examining simple slopes at the mean and one standard deviation above and below the mean LPP, region of significance using the Johnson-Neyman technique (69), and confidence bands for the simple slopes through a web-based utility (70). To account for type I errors from multiple comparisons, regression results are also presented with false-discovery rate corrections applied (71).
Results
Frequencies of PSQ Events
Frequencies of endorsed PSQ items are presented in Table 1. Participants endorsed an average of 7.39 total PSQ events (SD=3.38; range 1–17) and 2.24 interpersonal events (SD=.98, range 0–4). Commonly experienced stressors included unexpected separations and moves, inability to be with close others, and cancellation of important events and travel, while the least frequently endorsed stressors included the death of a close other, visa problems, and COVID-19 diagnosis.
Table 1.
Frequency of exposure to events assessed by the Pandemic Stress Questionnaire.
| Subscale/item | % |
|---|---|
| I had difficulty obtaining basic supplies because of the coronavirus pandemic (e.g., food, medicine, toilet paper). | 27.6% |
| I had to move unexpectedly because of the coronavirus pandemic. | 67.1% |
| I had problems with my visa or the Student and Exchange Visitor Information System because of the coronavirus pandemic (e.g., unable to renew). | 2.6% |
| I had to cancel travel or experienced a major disruption in travel plans because of the coronavirus pandemic. | 50.0% |
| I had to cancel or postpone important events because of the coronavirus pandemic (e.g., events for a club, sporting events, major celebrations). | 75.0% |
| I had to take on additional responsibilities caring for others (e.g., siblings, other family members) due to the coronavirus pandemic. | 39.5% |
| I was unexpectedly separated from family, friends, or others close to me because of the coronavirus pandemic. | 89.5% |
| I was unable to be with close family, friends, or partners because of the coronavirus pandemic. | 64.5% |
| I had conflicts or arguments with my partner or family members due to coronavirus (e.g., conflicts about living arrangements, shared work space, schedule expectations). | 59.2% |
| I experienced racism or discrimination due to the coronavirus pandemic. | 5.3% |
| Someone close to me died from COVID-19. | 3.9% |
| I experienced significant financial strain due to the pandemic (e.g., due to travel, purchasing supplies, paying for housing). | 28.9% |
| I temporarily or permanently lost a job or had my work hours greatly reduced due to the coronavirus pandemic. | 35.5% |
| My parent(s) temporarily or permanently lost a job or had their work hours greatly reduced because of the coronavirus pandemic. | 34.2% |
| I was unable to complete important requirements for my education or professional goals due to the coronavirus pandemic (e.g., coursework, taking the SAT or GRE, thesis). | 28.9% |
| I had problems with online courses and/or remote work (e.g., slow connection, no computer or internet access, major differences in time zone). | 48.7% |
| I had symptoms of COVID-19 (e.g., cough, fever, trouble breathing) but was unable to get tested. | 10.5% |
| I was tested for COVID-19. | 10.5% |
| I was diagnosed with COVID-19. | 1.3% |
| I had difficulty accessing or paying for physical or mental health care and/or difficulties with health insurance due to the coronavirus pandemic. | 23.7% |
| I was quarantined for 2 weeks or longer due to possible exposure to COVID-19 or due to international travel. | 31.6% |
| Someone close to me had symptoms of COVID-19 (e.g., cough, fever, trouble breathing) but was unable to get tested. | 14.5% |
| Someone close to me was diagnosed with COVID-19. | 18.4% |
| Someone close to me was quarantined for 2 weeks or longer due to possible exposure to COVID-19 or due to international travel. | 35.5% |
Symptoms of Depression and Anxiety
Paired-samples t-tests revealed significant increases in symptoms of depression, t(74)=4.06, p<.001, and traumatic intrusions, t(74)=4.41, p<.001, a decrease in social anxiety, t(74)=−3.04, p<.01, and no change in panic symptoms, p=.45. At T1, 12.0% of the sample were above the balanced clinical cutoff for major depressive disorder and 6.7% were above the balanced clinical cutoff for post-traumatic stress disorder (PTSD). AT T2, this proportion increased to 32.0% for depression and 26.7% for PTSD (72). Subsequent primary analyses of LPP and stress focused on changes in depression and traumatic intrusions, given that both increased from T1 to T2. Exploratory analyses of social anxiety symptom changes are presented in the Supplemental Information.
LPP Predicting Internalizing Symptom Changes
Multiple regression results testing the main and interactive effects of LPP to positive and threatening images and interpersonal and total pandemic-related stressors in the prediction of depression and traumatic intrusion symptom changes are presented in Tables 3–6. Interpersonal events predicted change in depressive symptoms (βs=.21−.25, zs=2.21–2.86, ps=.004−.03, FDR-corrected ps=0.01–0.04), but not traumatic intrusions (βs=.09−.16, zs=0.92–1.57, ps=.12−.36). Total events predicted changes in both (βs=.25−.31, zs=2.26–3.38, ps=.001−.02, FDR-corrected ps=0.008–0.04). The main effects of LPPs were not significant (ps=.24−.91). There were no significant interactions between LPPs and total events (ps>.08), however, the interaction between interpersonal stressful events and positive LPPs predicted depressive symptom change (β=−.19, z=−2.05, p=.04, FDR-corrected p=.16) and the interaction between interpersonal events and threatening LPPs predicted change in traumatic intrusion symptoms (β=.25, z=2.39, p=.02, FDR-corrected p=.14). These interactions remained significant at p<.05 when controlling for age, gender, race, and time between assessments.
Table 3.
Multiple regression analyses testing the main and interactive effect of pandemic-related interpersonal stressful events and LPP to emotional interpersonal stimuli in the prediction of depressive symptom changes from pre- to during the pandemic.
| b(SE) | β | b(SE) | β | ||
|---|---|---|---|---|---|
| T1 General Depression | 0.37 (0.10) | 0.32*** | T1 General Depression | 0.36 (0.10) | 0.31*** |
| T2 Traumatic Intrusions | 1.85 (0.37) | 0.45*** | T2 Traumatic Intrusions | 1.85 (0.38) | 0.45*** |
| Positive LPP residuals | −0.04 (0.39) | −0.01 | Threatening LPP residuals | −0.01 (0.41) | −0.00 |
| Interpersonal PSQ Events | 3.65 (1.28) | 0.25** | Interpersonal PSQ Events | 2.92 (1.32) | 0.21* |
| Int. Events X Pos LPP res. | −0.82 (0.40) | −0.19* | Int. Events X Threat LPP res. | −0.66 (0.42) | −0.15 |
| Total model R2=0.49 | Total model R2=0.48 |
p<.001,
p<.01,
p<.05,
p<.10
Note: LPP=late-positive potential; PSQ=Pandemic Stress Questionnaire; Int. Events X Pos LPP res.=The interaction between interpersonal stressful events and LPP residuals to positive images; Int. Events X Threat LPP res.=The interaction between interpersonal stressful events and LPP residuals to threatening images.
Table 6.
Multiple regression analyses testing the main and interactive effect of pandemic-related total stressful events and LPP to emotional interpersonal stimuli in the prediction of traumatic intrusion symptom changes from pre- to during the pandemic.
| b(SE) | β | b(SE) | β | ||
|---|---|---|---|---|---|
| T1 Traumatic Intrusions | 0.43 (0.13) | 0.31** | T1 Traumatic Intrusions | 0.45 (0.13) | 0.32** |
| T2 General Depression | 0.09 (0.03) | 0.35** | T2 General Depression | 0.09 (0.03) | 0.35** |
| Positive LPP residuals | 0.12 (0.10) | 0.11 | Threatening LPP residuals | 0.12 (0.10) | 0.10 |
| Total PSQ Events | 0.25 (0.11) | 0.25* | Total PSQ Events | 0.27 (0.11) | 0.26* |
| Tot. Events X Pos LPP res. | 0.02 (0.03) | 0.05 | Tot. Events X Threat LPP res. | 0.03 (0.03) | 0.10 |
| Total model R2=0.42 | Total model R2=0.38 |
p<.001,
p<.01,
p<.05,
p<.10
Note: LPP=late-positive potential; PSQ=Pandemic Stress Questionnaire; Int. Events X Pos LPP res.=The interaction between total stressful events and LPP residuals to positive images; Int. Events X Threat LPP res.=The interaction between total stressful events and LPP residuals to threatening images.
Simple slopes revealed the effect of interpersonal events in the prediction of depressive symptom change was significant for positive LPP amplitudes at the mean (b=3.65, SE=1.28, t=2.86, p=.006) and one standard deviation below the mean (b=6.35, SE=1.95, t=3.25, p=.002), but not LPPs one standard deviation above the mean (p=.58). The Johnson-Neyman region of significance indicated the effect of interpersonal stress on depression was significant for LPP amplitudes below 1.25 (observed amplitude range=−7.56–7.79; see Figure 2). The effect of interpersonal events predicting change in traumatic intrusions was significant for threatening LPPs one standard deviation above the mean (b=1.34, SE=0.51, t=2.60, p=.01), but not for LPP amplitudes at the mean or one standard deviation below the mean (ps=.12− .58). The Johnson-Neyman region of significance indicated this effect was significant for LPP amplitudes above 0.75 (observed range=−6.98−6.39; see Figure 2). Given evidence that the LPP is composed of several distinct positivities (29,73), exploratory analyses examining P300/early LPP (300–400ms) and late LPP (900–1000ms) are presented in the Supplemental Information, along with exploratory analyses of early visual processing components (i.e., P1, N1, N2).
Figure 2.
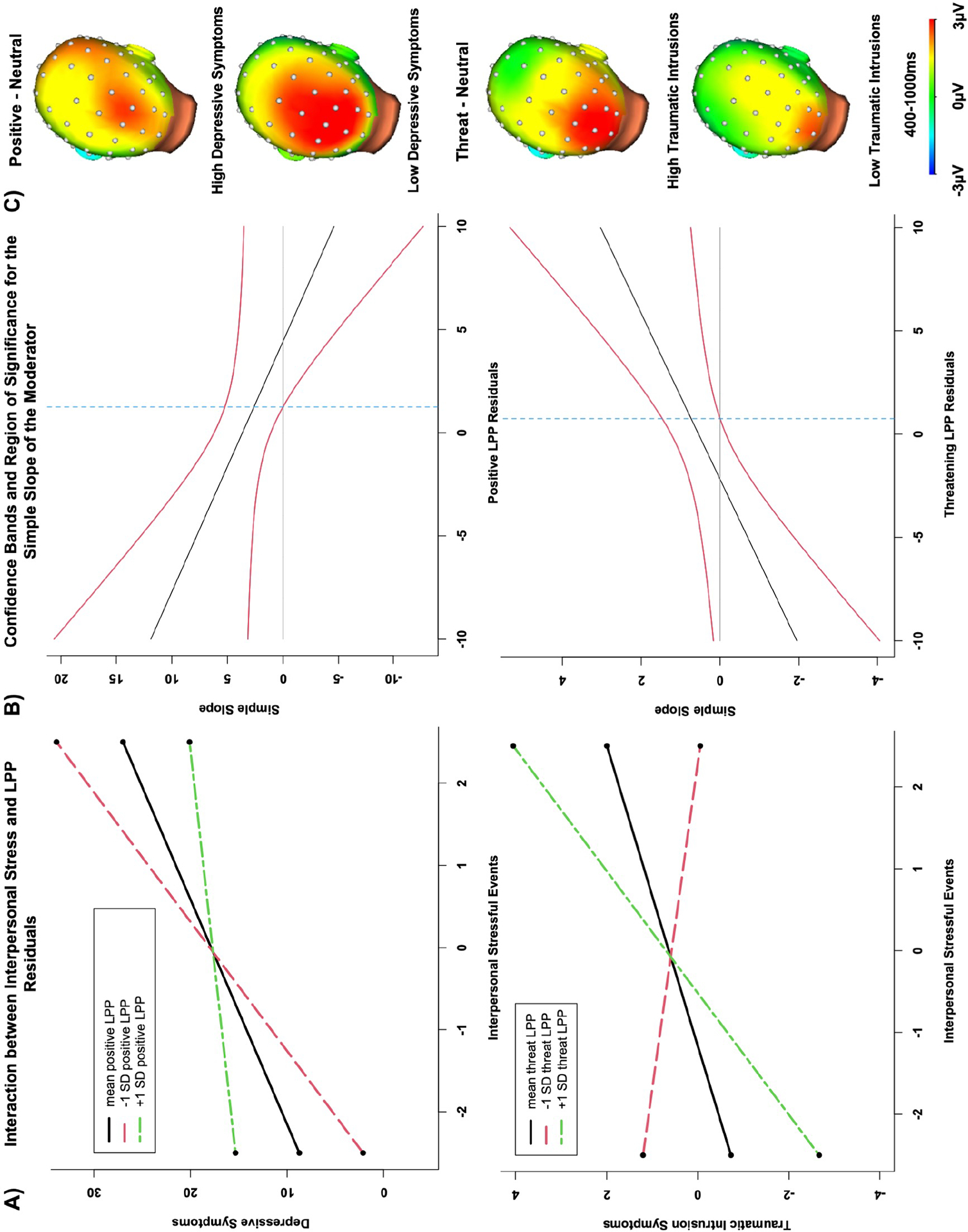
A) Plots of the simple slopes for the interaction effect between pandemic-related interpersonal events at low, average, and high levels of LPP reactivity to positive stimuli (top) and threatening stimuli (bottom) in the prediction of depressive symptoms (top) and traumatic intrusion symptoms (bottom). B) Plot of the confidence bands and region of significance for the simple slopes. C) Scalp distributions depicting the LPP for participants above the mean for interpersonal stress exposure with high and low levels of symptom changes (note: the median splits for interpersonal stress and high and low residualized internalizing symptom changes were used for illustrative purposes only; analyses examined stress and symptoms as continuous variables).
Discussion
The present study is among the first longitudinal studies to characterize the impact of the COVID-19 pandemic on emerging adults by assessing the frequencies of exposure to pandemic-related events and changes in internalizing symptoms. We additionally examined the predictive utility of neurophysiological reactivity to interpersonal emotional images in conjunction with pandemic-related stressful events on internalizing symptom change during the pandemic. We found overall increases in symptoms of depression and traumatic intrusions, and decreases in social anxiety symptoms during the pandemic, but no change in panic symptoms. Reactivity to emotional interpersonal stimuli before the pandemic, measured by the LPP, moderated the impact of interpersonal events, specifically, on internalizing symptom changes, such that hypo-reactivity to positive stimuli predicted increased depressive symptoms and hyper-reactivity to threatening stimuli predicted increased traumatic intrusion symptoms in combination with greater interpersonal stress exposure.
Emerging adults endorsed many pandemic-related events with high frequency in May 2020, including being unexpectedly separated from close others, unexpected moves, the inability to be with close others, and cancellation of important events and travel. Compared to our prior research validating the PSQ in an online community sample of young adults (9), differences emerged from the present sample. General disruptions and financial items, such as difficulty obtaining supplies, financial strain, and job loss were endorsed at relatively higher rates in the community sample, while interpersonal items, including unexpected separation and conflicts/arguments were endorsed more frequently in the present sample. Although additional research is needed across the lifespan, these comparisons suggest that some types of interpersonal stressors due to COVID-19 may be more common in younger populations, but financial, work, and health-related stressors may be more common in older adults.
Interpersonal stressors are especially robust predictors of depression and anxiety (3–5,7), and the high rates of interpersonal events due to COVID-19 informed our hypothesis that individual differences in emotional reactivity in the context of interpersonal scenarios, indexed by the LPP, may predict responses to interpersonal stressors, specifically. Consistent with the robust associations between stress and psychopathology (3–5,7), we observed increases in depressive symptoms and trauma-related anxiety symptoms, but decreases in social anxiety symptoms. Considering social distancing mandates and limited in-person interactions, it is possible that reduced exposures to socio-evaluative situations alleviated symptoms of social anxiety.
In examining neurophysiological predictors of symptom change, our results showed that under high exposure to stressful interpersonal events, reduced neural reactivity to positive interpersonal images conferred risk for depressive symptoms, while enhanced reactivity to threatening images predicted increased traumatic intrusions. These findings suggest LPPs to emotional interpersonal stimuli may reflect individual differences in vulnerability to stress or general susceptibility to the environment, and further distinguish risk for depression from trauma-related anxiety. Individuals who have difficulty maintaining attention towards positive interpersonal events may be at risk for depression in an environment in which social rewards are further limited, which is consistent with evidence of stronger effects for reduced reactivity for positive compared to negative stimuli in depression (74,75). It is also possible that individuals with reduced LPPs to interpersonal stimuli pre-pandemic tend to generate dependent interpersonal stressors during the pandemic, thus increasing risk for depressive symptoms (5,6,19).
Interestingly, a distinct pattern of results emerged for traumatic intrusions, such that enhanced LPPs to interpersonal stimuli predicted increased traumatic intrusions in combination with interpersonal events. This is consistent with prior evidence of reductions in emotional reactivity in depression but elevated reactivity in anxiety (33,37,76). These patterns were particularly apparent for LPPs to threatening stimuli, which is consistent with previous LPP and neuroimaging research on responses to stress in youth (47,49), and the broader literature on threat hypervigilance in anxiety (33,37,38). It is important to note that LPP interaction effects were specific to interpersonal rather than total events, which could be explained by the selective use of interpersonal images in our task, with LPPs to interpersonal scenarios eliciting vulnerability primarily in the context of interpersonal stress.
Although we aimed to minimize the number of tests conducted to examine pathways to internalizing psychopathologies, our LPP results did not survive corrections for multiple comparisons, and replication is needed in a larger sample. Though the results showed small to medium effect sizes, there is increasing recognition that small effects are typical in research on complex psychological processes, such as the development of psychopathology (76). The lack of non-social emotional image conditions to directly test the specificity of the effects to interpersonal stimuli is a limitation of the current study. Though, previous ERP research directly comparing LPPs to social and non-social neutral images shows enhanced LPPs to neutral images containing people (56), supporting the potential of this specificity. Other limitations include the susceptibility of the PSQ to subjective interpretations of events compared to stress interview measures (7). However, by asking participants to first report the presence or absence of events prior to rating severity, this subjectivity is mitigated. Further, although the IDAS permits a dimensional approach to assessing risk for internalizing psychopathologies and we did observe high rates for symptoms above established clinical cutoffs, it is unclear whether our results would generalize to clinical samples based on diagnostic categories. Lastly, we focused on ERP components indexing individual differences in attentional processing of emotional images, particularly the LPP, and consideration of the role of ERPs indexing conflict monitoring and other cognitive processes is needed in future work.
The current prospective study is among the first to evaluate neurophysiological predictors of the impact of COVID-19 on internalizing symptoms in emerging adults. Our results support a stress sensitivity model, where the LPP may reflect vulnerabilities for internalizing symptoms when exposure to interpersonal stressors is high. Other research using psychophysiological measures such as pupillometry (77) to assess alterations in emotion further support the possible clinical applications of these methods for identifying those at greatest risk during times of crisis. Notably, our findings also demonstrate the sensitivity of neurophysiological measures of emotion for distinguishing between risk for depression and trauma-related anxiety, with the potential for informing more personalized prevention efforts.
Supplementary Material
Table 2.
Descriptive statistics and bivariate correlations among primary study variables.
| M (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Total PSQ Events | 7.39 (3.38) | - | ||||||||||
| 2 | Interpersonal PSQ Events | 2.24 (0.98) | .57** | - | |||||||||
| 3 | T1 General Depression | 40.64 (12.39) | .18 | .08 | - | ||||||||
| 4 | T1 Traumatic Intrusions | 6.29 (2.50) | −.17 | .00 | .47** | - | |||||||
| 5 | T1 Social Anxiety | 10.36 (4.50) | −.06 | .06 | .54** | .36** | - | ||||||
| 6 | T1 Panic | 11.83 (4.68) | .16 | .12 | .54** | .46** | .50** | - | |||||
| 7 | T2 General Depression | 47.53 (13.94) | .50** | .39** | .38** | .15 | .30** | .27* | - | ||||
| 8 | T2 Traumatic Intrusions | 8.09 (3.46) | .38** | .31** | .13 | .33** | .02 | .31** | .53** | - | |||
| 9 | T2 Social Anxiety | 8.63 (3.63) | .18 | .14 | .18 | .18 | .28* | .19 | .44** | .40** | - | ||
| 10 | T2 Panic | 12.17 (4.94) | .33** | .31** | .31** | .26* | .24* | .66** | .56** | .50** | .45** | - | |
| 11 | Positive LPP residuals | 0.00 (3.28) | .05 | .14 | .05 | .03 | .12 | .22^ | .06 | .16 | .22^ | .33** | - |
| 12 | Threat LPP residuals | 0.00 (2.88) | −.02 | .20 | −.02 | .02 | .06 | .04 | .07 | .14 | .23 ^ | .08 | .57** |
p<.001,
p<.01,
p<.05,
p<.10
Table 4.
Multiple regression analyses testing the main and interactive effect of pandemic-related interpersonal stressful events and LPP to emotional interpersonal stimuli in the prediction of traumatic intrusion symptom changes from pre- to during the pandemic.
| b(SE) | β | b(SE) | β | ||
|---|---|---|---|---|---|
| T1 Traumatic Intrusions | 0.31 (0.13) | 0.23* | T1 Traumatic Intrusions | 0.33 (0.13) | 0.25** |
| T2 General Depression | 0.11 (0.03) | 0.45*** | T2 General Depression | 0.11 (0.02) | 0.44*** |
| Positive LPP residuals | 0.07 (0.10) | 0.06 | Threatening LPP residuals | 0.02 (0.11) | 0.02 |
| Interpersonal PSQ Events | 0.32 (0.35) | 0.09 | Interpersonal PSQ Events | 0.55 (0.35) | 0.16 |
| Int. Events X Pos LPP res. | 0.19 (0.11) | 0.17^ | Int. Events X Threat LPP res. | 0.25 (0.10) | 0.23* |
| Total model R2=0.39 | Total model R2=0.38 |
p<.001,
p<.01,
p<.05,
p<.10
Note: LPP=late-positive potential; PSQ=Pandemic Stress Questionnaire; Int. Events X Pos LPP res.=The interaction between interpersonal stressful events and LPP residuals to positive images; Int. Events X Threat LPP res.=The interaction between interpersonal stressful events and LPP residuals to threatening images.
Table 5.
Multiple regression analyses testing the main and interactive effect of total pandemic-related stressful events and LPP to emotional interpersonal stimuli in the prediction of depressive symptom changes from pre- to during the pandemic.
| b(SE) | β | b(SE) | β | ||
|---|---|---|---|---|---|
| T1 General Depression | 0.32 (0.10) | 0.29** | T1 General Depression | 0.33 (0.10) | 0.29** |
| T2 Traumatic Intrusions | 1.56 (0.38) | 0.39*** | T2 Traumatic Intrusions | 1.54 (0.37) | 0.38*** |
| Positive LPP residuals | −0.09 (0.37) | −0.02 | Threatening LPP residuals | 0.16 (0.38) | 0.04 |
| Total PSQ Events | 1.30 (0.38) | 0.31** | Total PSQ Events | 1.26 (0.38) | 0.30** |
| Tot. Events X Pos LPP res. | −0.09 (0.13) | −0.06 | Tot. Events X Threat LPP res. | −0.18 (0.10) | −0.15^ |
| Total model R2=0.46 | Total model R2=0.49 |
p<.001,
p<.01,
p<.05,
p<.10
Note: LPP=late-positive potential; PSQ=Pandemic Stress Questionnaire; Tot. Events X Pos LPP res.=The interaction between total stressful events and LPP residuals to positive images; Tot. Events X Threat LPP res.=The interaction between total stressful events and LPP residuals to threatening images.
Acknowledgments
The authors would like to thank all of the lab members who contributed to data collection.
Funding sources
This work was supported in part by UL1 TR000445 from NCATS/NIH and the Brain and Behavior Research Foundation Katherine Deschner Family Young Investigator Grant awarded to AK. SP was supported by NIH/NIMH T32-MH18921 during the completion of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Biomedical Financial Interests and Potential Conflicts of Interest
Dr. Kujawa, Lindsay Dickey, Michael West, Samantha Pegg, and Haley Green reported no biomedical financial interests or potential conflicts of interest.
Open Practices Statement
The task images and valence and arousal ratings are available upon request from the corresponding author.
References
- 1.Kessler RC, Wai TC, Demler O, Walters EE (2005): Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Wang PS (2008): The descriptive epidemiology of commonly occurring mental disorders in the United States. Annual Review of Public Health. 10.1146/annurev.publhealth.29.020907.090847 [DOI] [PubMed] [Google Scholar]
- 3.Hammen C (2005): Stress and depression. Annual Review of Clinical Psychology. 10.1146/annurev.clinpsy.1.102803.143938 [DOI] [PubMed] [Google Scholar]
- 4.Rapee RM (1991): Generalized anxiety disorder: A review of clinical features and theoretical concepts. Clin Psychol Rev. 10.1016/0272-7358(91)90116-C [DOI] [Google Scholar]
- 5.Vrshek-Schallhorn S, Stroud CB, Mineka S, Hammen C, Zinbarg RE, Wolitzky-Taylor K, Craske MG (2015): Chronic and episodic interpersonal stress as statistically unique predictors of depression in two samples of emerging adults. J Abnorm Psychol. 10.1037/abn0000088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uliaszek AA, Zinbarg RE, Mineka S, Craske MG, Griffith JW, Sutton JM, et al. (2012): A longitudinal examination of stress generation in depressive and anxiety disorders. J Abnorm Psychol 121: 4–15. [DOI] [PubMed] [Google Scholar]
- 7.Harkness KL, Monroe SM (2016): The assessment and measurement of adult life stress: Basic premises, operational principles, and design requirements. J Abnorm Psychol. 10.1037/abn0000178 [DOI] [PubMed] [Google Scholar]
- 8.Hyland P, Shevlin M, McBride O, Murphy J, Karatzias T, Bentall RP, et al. (2020): Anxiety and depression in the Republic of Ireland during the COVID-19 pandemic. Acta Psychiatr Scand. 10.1111/acps.13219 [DOI] [PubMed] [Google Scholar]
- 9.Kujawa A, Green H, Compas BE, Dickey L, Pegg S (2020): Exposure to COVID-19 pandemic stress: Associations with depression and anxiety in emerging adults in the United States. Depress Anxiety. 10.1002/da.23109 [DOI] [PubMed] [Google Scholar]
- 10.Odriozola-González P, Planchuelo-Gómez Á, Irurtia MJ, de Luis-García R (2020): Psychological effects of the COVID-19 outbreak and lockdown among students and workers of a Spanish university. Psychiatry Res. 10.1016/j.psychres.2020.113108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starr LR, Hammen C, Connolly NP, Brennan PA (2014): Does relational dysfunction mediate the association between anxiety disorders and later depression? Testing an interpersonal model of comorbidity. Depress Anxiety 31: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawkley LC, Cacioppo JT (2010): Loneliness matters: A theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 10.1007/s12160-010-9210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul KI, Moser K (2009): Unemployment impairs mental health: Meta-analyses. J Vocat Behav. 10.1016/j.jvb.2009.01.001 [DOI] [Google Scholar]
- 14.Swartz JR, Knodt AR, Radtke SR, Hariri AR (2015): A neural biomarker of psychological vulnerability to future life stress. Neuron. 10.1016/j.neuron.2014.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maier SF, Seligman MEP (2016): Learned helplessness at fifty: Insights from neuroscience. Psychol Rev 123: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson EE, Jarcho JM, Guyer AE (2016): Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience. 10.1016/j.dcn.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005): Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- 18.Kessler RC, Merikangas KR, Wang PS (2007): Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annual Review of Clinical Psychology. 10.1146/annurev.clinpsy.3.022806.091444 [DOI] [PubMed] [Google Scholar]
- 19.Hammen C (2006): Stress generation in depression: Reflections on origins, research, and future directions. Journal of Clinical Psychology. 10.1002/jclp.20293 [DOI] [PubMed] [Google Scholar]
- 20.Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ (2000): Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biol Psychol. 10.1016/S0301-0511(99)00044-7 [DOI] [PubMed] [Google Scholar]
- 21.Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Tiffany I, Lang PJ (2000): Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 10.1017/S0048577200001530 [DOI] [PubMed] [Google Scholar]
- 22.Hajcak G, Macnamara A, Olvet DM (2010): Event-related potentials, emotion, and emotion regulation: An integrative review. Dev Neuropsychol 35: 129–155. [DOI] [PubMed] [Google Scholar]
- 23.Bunford N, Kujawa A, Fitzgerald KD, Monk CS, Phan KL (2018): Convergence of BOLD and ERP measures of neural reactivity to emotional faces in children and adolescents with and without anxiety disorders. Biol Psychol. 10.1016/j.biopsycho.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Huang H, McGinnis-Deweese M, Keil A, Ding M (2012): Neural substrate of the late positive potential in emotional processing. J Neurosci. 10.1523/JNEUROSCI.3109-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabatinelli D, Lang PJ, Keil A, Bradley MM (2007): Emotional perception: Correlation of functional MRI and event-related potentials. Cereb Cortex. 10.1093/cercor/bhl017 [DOI] [PubMed] [Google Scholar]
- 26.Sabatinelli D, Keil A, Frank DW, Lang PJ (2013): Emotional perception: Correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biol Psychol. 10.1016/j.biopsycho.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bondy E, Stewart JG, Hajcak G, Weinberg A, Tarlow N, Mittal VA, Auerbach RP (2018): Emotion processing in female youth: Testing the stability of the late positive potential. Psychophysiology. 10.1111/psyp.12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassidy SM, Robertson IH, O’Connell RG (2012): Retest reliability of event-related potentials: Evidence from a variety of paradigms. Psychophysiology. 10.1111/j.1469-8986.2011.01349.x [DOI] [PubMed] [Google Scholar]
- 29.Pegg S, Dickey L, Mumper E, Kessel E, Klein DN, Kujawa A (2019): Stability and change in emotional processing across development: A 6-year longitudinal investigation using event-related potentials. Psychophysiology 56. 10.1111/psyp.13438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kujawa A, Burkhouse KL (2017): Vulnerability to Depression in Youth: Advances from Affective Neuroscience HHS Public Access. Biol Psychiatry Cogn Neurosci Neuroimaging 2: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foti D, Olvet DM, Klein DN, Hajcak G (2010): Reduced electrocortical response to threatening faces in major depressive disorder. Depress Anxiety 27: 813–820. [DOI] [PubMed] [Google Scholar]
- 32.Kujawa A, Hajcak G, Torpey D, Kim J, Klein DN (2012): Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. J Child Psychol Psychiatry Allied Discip. 10.1111/j.1469-7610.2011.02461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kujawa A, Macnamara A, Fitzgerald KD, Monk CS, Luan Phan K, Phan KL (2015): Enhanced Neural Reactivity to Threatening Faces in Anxious Youth: Evidence from Event-Related Potentials. J Abnorm Child Psychol 43: 1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacNamara A, Jackson TB, Fitzgerald JM, Hajcak G, Phan KL (2019): Working Memory Load and Negative Picture Processing: Neural and Behavioral Associations With Panic, Social Anxiety, and Positive Affect. Biol Psychiatry Cogn Neurosci Neuroimaging. 10.1016/j.bpsc.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moser JS, Huppert JD, Duval E, Simons RF (2008): Face processing biases in social anxiety: An electrophysiological study. Biol Psychol. 10.1016/j.biopsycho.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 36.Solomon B, Decicco JM, Dennis TA (2012): Emotional picture processing in children: An ERP study. Dev Cogn Neurosci. 10.1016/j.dcn.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacNamara A, Kotov R, Hajcak G (2016): Diagnostic and Symptom-Based Predictors of Emotional Processing in Generalized Anxiety Disorder and Major Depressive Disorder: An Event-Related Potential Study. Cognit Ther Res 40: 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson BD, Perlman G, Hajcak G, Klein DN, Kotov R (2015): Familial risk for distress and fear disorders and emotional reactivity in adolescence: An event-related potential investigation. Psychol Med. 10.1017/S0033291715000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auerbach RP, Stanton CH, Proudfit GH, Pizzagalli and DA (2015): Self-Referential Processing in Depressed Adolescents: A High- Density ERP Study Randy. J Abnorm Psychol. 10.1007/s12671-013-0269-8.Moving [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Speed BC, Nelson BD, Auerbach RP, Klein DN, Hajcak G (2016): Depression Risk and Electrocortical Reactivity During Self-Referential Emotional Processing in 8 to 14 Year-Old Girls. J Abnorm Psychol. 10.1037/abn0000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burkhouse KL, Owens M, Feurer C, Sosoo E, Kudinova A, Gibb BE (2017): Increased neural and pupillary reactivity to emotional faces in adolescents with current and remitted major depressive disorder. Soc Cogn Affect Neurosci. 10.1093/scan/nsw184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rottenberg J, Gross JJ, Gotlib IH (2005): Emotion context insensitivity in major depressive disorder. J Abnorm Psychol. 10.1037/0021-843X.114.4.627 [DOI] [PubMed] [Google Scholar]
- 43.Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, et al. (2009): Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci U S A. 10.1073/pnas.0910651106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bylsma LM, Morris BH, Rottenberg J (2008): A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 10.1016/j.cpr.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 45.Armstrong T, Olatunji BO (2012): Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical Psychology Review. 10.1016/j.cpr.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richards HJ, Benson V, Donnelly N, Hadwin JA (2014): Exploring the function of selective attention and hypervigilance for threat in anxiety. Clinical Psychology Review. 10.1016/j.cpr.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 47.Kujawa A, Hajcak G, Danzig AP, Black SR, Bromet EJ, Carlson GA, et al. (2016): Neural Reactivity to Emotional Stimuli Prospectively Predicts the Impact of a Natural Disaster on Psychiatric Symptoms in Children. Biol Psychiatry. 10.1016/j.biopsych.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein BL, Kessel EM, Kujawa A, Finsaas MC, Davila J, Hajcak G, Klein DN (2020): Stressful life events moderate the effect of neural reward responsiveness in childhood on depressive symptoms in adolescence. Psychol Med. 10.1017/S0033291719001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan MA (2014): Amygdala response to negative stimuli predicts ptsd symptom onset following a terrorist attack. Depression and Anxiety. 10.1002/da.22284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woody ML, Burkhouse KL, Siegle GJ, Kudinova AY, Meadows SP, Gibb BE (2017): Pupillary Response to Emotional Stimuli as a Risk Factor for Depressive Symptoms Following a Natural Disaster: The 2011 Binghamton Flood. Clin Psychol Sci. 10.1177/2167702617699932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lang PJ, Bradley MM, Cuthbert BN (1997): International affective picture system (IAPS): Technical manual and affective ratings. NIMH Cent Study Emot Atten. [Google Scholar]
- 52.Kennedy DP, Adolphs R (2012): The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences. 10.1016/j.tics.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olofsson JK, Nordin S, Sequeira H, Polich J (n.d.): Affective Picture Processing: An Integrative Review of ERP Findings. [DOI] [PMC free article] [PubMed]
- 54.Thom N, Knight J, Dishman R, Sabatinelli D, Johnson DC, Clementz B (2014): Emotional scenes elicit more pronounced self-reported emotional experience and greater EPN and LPP modulation when compared to emotional faces. Cogn Affect Behav Neurosci 14: 849–860. [DOI] [PubMed] [Google Scholar]
- 55.Weinberg A, Hajcak G (2010): Beyond Good and Evil: The Time-Course of Neural Activity Elicited by Specific Picture Content. Emotion. 10.1037/a0020242 [DOI] [PubMed] [Google Scholar]
- 56.Ferri J, Weinberg A, Hajcak G (2012): I see people: The presence of human faces impacts the processing of complex emotional stimuli. Soc Neurosci. 10.1080/17470919.2012.680492 [DOI] [PubMed] [Google Scholar]
- 57.Pegg S, Kujawa A (2020): The effects of a brief motivation manipulation on reward responsiveness: A multi-method study with implications for depression. Int J Psychophysiol. 10.1016/j.ijpsycho.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 58.Kujawa A, Klein DN, Hajcak G (2012): Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Dev Cogn Neurosci 2: 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurdi B, Lozano S, Banaji MR (2017): Introducing the Open Affective Standardized Image Set (OASIS). Behav Res Methods. 10.3758/s13428-016-0715-3 [DOI] [PubMed] [Google Scholar]
- 60.Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, et al. (2007): Development and Validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychol Assess. 10.1037/1040-3590.19.3.253 [DOI] [PubMed] [Google Scholar]
- 61.Moran TP, Jendrusina AA, Moser JS (2013): The psychometric properties of the late positive potential during emotion processing and regulation. Brain Res. 10.1016/j.brainres.2013.04.018 [DOI] [PubMed] [Google Scholar]
- 62.Foti D, Hajcak G (2008): Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. J Cogn Neurosci. 10.1162/jocn.2008.20066 [DOI] [PubMed] [Google Scholar]
- 63.Stange JP, MacNamara A, Barnas O, Kennedy AE, Hajcak G, Phan KL, Klumpp H (2017): Neural markers of attention to aversive pictures predict response to cognitive behavioral therapy in anxiety and depression. Biol Psychol 123: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Codispoti M, De Cesarei A, Ferrari V (2012): The influence of color on emotional perception of natural scenes. Psychophysiology. 10.1111/j.1469-8986.2011.01284.x [DOI] [PubMed] [Google Scholar]
- 65.Van Dongen NNN, Van Strien JW, Dijkstra K (2016): Implicit emotion regulation in the context of viewing artworks: ERP evidence in response to pleasant and unpleasant pictures. Brain Cogn. 10.1016/j.bandc.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 66.Bates D, Mächler M, Bolker BM, Walker SC (2015): Fitting linear mixed-effects models using lme4. J Stat Softw. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 67.Meyer A, Lerner MD, De Los Reyes A, Laird RD, Hajcak G (2017): Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology 54: 114–122. [DOI] [PubMed] [Google Scholar]
- 68.Rosseel Y (2012): Lavaan: An R package for structural equation modeling. J Stat Softw. 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- 69.Johnson PO, Neyman J (1936): Test of certain linear hypotheses and the application to some statistical problems. Stat Res Mem. [Google Scholar]
- 70.Preacher KJ, Curran PJ, Bauer DJ (2006): Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 10.3102/10769986031004437 [DOI] [Google Scholar]
- 71.Benjamini Y, Hochberg Y (1995): Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 72.Stasik-O’Brien SM, Brock RL, Chmielewski M, Naragon-Gainey K, Koffel E, McDade-Montez E, et al. (2019): Clinical Utility of the Inventory of Depression and Anxiety Symptoms (IDAS). Assessment. 10.1177/1073191118790036 [DOI] [PubMed] [Google Scholar]
- 73.Foti D, Hajcak G, Dien J (2009): Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology. 10.1111/j.1469-8986.2009.00796.x [DOI] [PubMed] [Google Scholar]
- 74.Bylsma LM, Rottenberg J, Morris BH (2008): A meta-analysis of emotional reactivity in major depressive disorder Neurobehavioral predictors of youth at high and low familial risk for depression (MH104325) View project A meta-analysis of emotional reactivity in major depressive disorder ☆. Artic Clin Psychol Rev. 10.1016/j.cpr.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 75.Hill K, South S, Foti D, Egan R (2019): Abnormal emotional reactivity in depression: Contrasting theoretical models using neurophysiological data. Biol Psychol 141. 10.1016/j.biopsycho.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 76.Klein F, Schindler S, Neuner F, Rosner R, Renneberg B, Steil R, Iffland B (2019): Processing of affective words in adolescent PTSD—Attentional bias toward social threat. Psychophysiology 56: 1–14. [DOI] [PubMed] [Google Scholar]
- 77.Graur S, Siegle G (2013): Pupillary motility: Bringing neuroscience to the psychiatry clinic of the future. Curr Neurol Neurosci Rep. 10.1007/s11910-013-0365-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


