Abstract
OBJECTIVES
This study was designed to investigate whether coronary computed tomography angiography assessments of coronary plaque might explain differences in the prognosis of men and women presenting with chest pain.
BACKGROUND
Important sex differences exist in coronary artery disease. Women presenting with chest pain have different risk factors, symptoms, prevalence of coronary artery disease and prognosis compared to men.
METHODS
Within a multicenter randomized controlled trial, we explored sex differences in stenosis, adverse plaque characteristics (positive remodeling, low-attenuation plaque, spotty calcification, or napkin ring sign) and quantitative assessment of total, calcified, noncalcified and low-attenuation plaque burden.
RESULTS
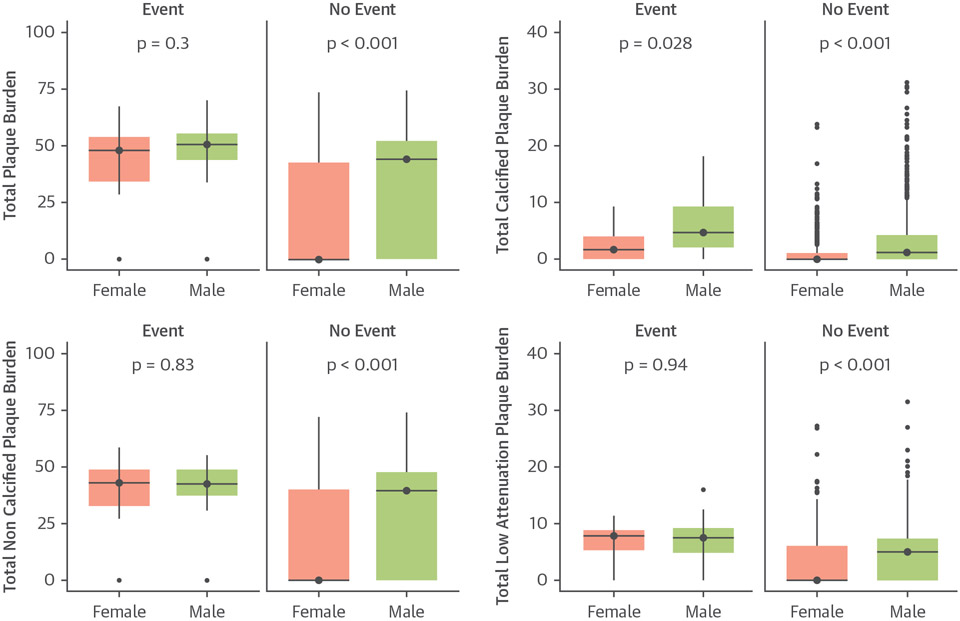
Of the 1,769 participants who underwent coronary computed tomography angiography, 772 (43%) were female. Women were more likely to have normal coronary arteries and less likely to have adverse plaque characteristics (p < 0.001 for all). They had lower total, calcified, noncalcified, and low-attenuation plaque burdens (p < 0.001 for all) and were less likely to have a low-attenuation plaque burden >4% (41% vs. 59%; p < 0.001). Over a median follow-up of 4.7 years, myocardial infarction (MI) occurred in 11 women (1.4%) and 30 men (3%). In those who had MI, women had similar total, noncalcified, and low-attenuation plaque burdens as men, but men had higher calcified plaque burden. Low-attenuation plaque burden predicted MI (hazard ratio: 1.60; 95% confidence interval: 1.10 to 2.34; p = 0.015), independent of calcium score, obstructive disease, cardiovascular risk score, and sex.
CONCLUSIONS
Women presenting with stable chest pain have less atherosclerotic plaque of all subtypes compared to men and a lower risk of subsequent MI. However, quantitative low-attenuation plaque is as strong a predictor of subsequent MI in women as in men. (Scottish Computed Tomography of the HEART Trial [SCOT-HEART]; NCT01149590).
Keywords: computed tomography, computed tomography coronary angiography, coronary artery disease, sex, quantitative plaque analysis
Coronary computed tomography angiography (CCTA) can identify patients at risk of subsequent cardiac events, guide management, and improve outcomes for patients with suspected coronary artery disease (1). Recently we have shown that quantitative atherosclerotic plaque burden on CCTA can identify patients at risk of subsequent events over and above cardiovascular risk score, coronary artery calcification, and presence of obstructive coronary disease. In particular, patients with a low-attenuation plaque burden greater than 4% were nearly 5 times more likely to have a subsequent myocardial infarction (MI) (2). However, whether the burden of different plaque types differs between women and men, and whether these assessments provide equal prognostic information, remains uncertain.
Sex differences in coronary artery disease are well established. Women presenting with chest pain have different risk factor profiles, symptoms, prevalence of coronary artery disease and prognosis compared to men (3). We hypothesized that these differences might be explained by computed tomography (CT) assessments of atherosclerotic plaque. Existing data are limited to small cohorts and short follow-up (4,5). This analysis of the SCOT-HEART (Scottish Computed Tomography of the HEART) study was conducted with the aim of assessing the impact of sex on the burden of different coronary plaque types and whether these quantitative assessments provide similar prognostic information in men and women.
METHODS
STUDY DESIGN.
The SCOT-HEART multicenter randomized controlled trial evaluated the use of CT in patients attending outpatient cardiology clinics with suspected angina due to coronary artery disease (NCT01149590) (6). The primary results and plaque analysis have been published (1,2,7-9). This study assesses sex-specific differences in quantitative plaque and its clinical implications. This study was approved by the regional ethics committee (South East Scotland Research Ethics Committee 02) and participants provided written informed consent.
STUDY PARTICIPANTS.
SCOT-HEART recruited 4,146 participants and 2,073 were randomized to CT. Of these, 1,778 underwent CT; 1,769 scans are of suitable quality for analysis. Information on cardiovascular risk factors was obtained from the SCOT-HEART database. The Assessing Cardiovascular Risk Using Scottish Intercollegiate Guidelines Network guideline cardiovascular risk score was used to calculate cardiovascular risk (10). Outcome information on cardiovascular events and mortality was obtained from the electronic Data Research and Innovation Service of the National Health Services Scotland and confirmed by reviewing electronic health records where required. CT was performed as described previously (Supplemental Methods) (6).
QUALITATIVE VISUAL ASSESSMENT OF ATHEROSCLEROTIC PLAQUE.
Information on CT assessment was obtained from the SCOT-HEART database. The Agatston method was used to assess coronary artery calcification (VScore, Vital Images) (11). Visual assessment of coronary stenoses was performed on a 15-segment basis by trained observers with excellent observer agreement (12). Visual assessment classified coronary arteries as normal, nonobstructive (<70% or <50% in the left main stem), or obstructive (≥70% or ≥50% in the left main stem). Obstructive disease was defined as 1 or more major epicardial vessel with a visually assessed obstructive stenosis.
Visual assessment of adverse plaque characteristics was performed on a per-segment basis by 1 of 6 trained observers. Characteristics assessed included positive remodeling, low-attenuation plaque, spotty calcification, and the “napkin ring” sign (Supplemental Table 1 and Supplemental Methods). An individual adverse plaque was defined as having positive remodeling or low-attenuation plaque and patients with one or more adverse plaques were defined as having adverse plaque (13).
QUANTITATIVE ASSESSMENT OF ATHEROSCLEROTIC PLAQUE.
Quantitative assessment of atherosclerotic plaque was performed for all patients with 1 or more segment of nonobstructive or obstructive disease, or an Agatston score greater than zero. Quantitative analysis was performed using semiautomatic software Autoplaque, Version 2.5 (Cedars-Sinai Medical Center, Los Angeles, California) by 1 of 4 trained observers (Figure 1 and Supplemental Methods). Scan-specific thresholds were used to classify plaque constituents (14). Plaque burden was used as it corrects for sex difference in size and length of vessels. Per patient plaque burden was calculated by dividing plaque volume by vessel volume of the assessed region, multiplying by 100, and summing on a per patient basis (15). Plaque burden was calculated for total, calcified, noncalcified, and low-attenuation noncalcified plaque (Supplemental Table 1). Low-attenuation plaque was defined as below 30 HU (16). For patients with visually assessed normal coronary arteries, the plaque burden was set to zero.
FIGURE 1. Low-Attenuation Plaque on CCTA.
Female (58 years of age) with typical chest pain, family history of coronary artery disease and abnormal exercise tolerance test. Coronary computed tomography angiography (CCTA) showed obstructive disease in the left anterior descending (LAD) artery (arrows), confirmed at invasive coronary angiography and treated with a stent. Two years later she experienced a non-ST elevation myocardial infarction. (A) A curved planar reformation with diffuse noncalcified plaque (red) and calcified plaque (yellow). Three-dimensional reformats of the proximal (B) and mid-distal LAD (C) show non calcified (red), low-attenuation (orange), and calcified (yellow) plaque, and coronary lumen (blue).
Area and diameter stenosis were automatically quantified at the narrowest point on the segmented lumen. Lumen volume for was calculated by subtracting plaque volume from vessel volume for segments that were analyzed. Remodeling index was calculated automatically as the ratio of maximum vessel area to the proximal normal reference point (17). Contrast density difference was calculated automatically as the maximum percent difference in contrast density in the diseased segment compared to the proximal normal segment, with contrast density defined as mean luminal attenuation/area automatically computed over 1-mm cross-sections of the segment (17).
STATISTICAL ANALYSIS.
Statistical analysis was performed using R, version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria). Quantitative data are presented as mean and standard deviation or, if not normally distributed, as median and interquartile interval (IQI). Statistical significance was assessed using Pearson chi square test, Fisher exact test, Students t-test, Kruskal-Wallis test, or Mann-Whitney U test as appropriate. Outcome data were analyzed using Cox proportional hazards regression and presented graphically using cumulative incidence plots. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated from the Cox model. Univariable analysis was performed for all quantified plaque burden parameters. Multivariable models were constructed including the individual quantitative plaque burden variables and sex. Further multivariable models were constructed for each quantitative plaque burden variable and sex, visually assessed obstructive disease, and cardiovascular risk score (10). Coronary artery calcium score, plaque burdens, and lumen volume were log transformed for analysis. Receiver operator curve (ROC) analysis was performed to assess the impact of sex on the predictive ability of low-attenuation plaque burden, and ROC curves were compared using Venkatraman’s test. A statistically significant difference was defined as a 2-sided p < 0.05.
RESULTS
STUDY POPULATION.
There were 1,769 participants with CCTAs suitable for analysis, and 772 (43%) were female. Women had a similar age compared to men (age 58 ± 9 vs. 57 ± 9 years; p = 0.063), but had a higher body mass index and serum cholesterol, were more likely to be nonsmokers with a family history of coronary heart disease, and were less likely to have a history of coronary heart disease, peripheral vascular disease, or diabetes mellitus (Table 1). Women were less likely to be taking preventative medication at baseline, 6 weeks, and 5 years (Table 1).
TABLE 1.
Baseline Characteristics of Female and Male Participants
| Overall (N = 1,769) |
Female (n = 772) |
Male (n = 997) |
p Value | |
|---|---|---|---|---|
| Characteristics | ||||
| Age, yrs | 58 ± 9 | 58 ± 9 | 57 ± 9 | 0.063 |
| Body mass index, kg/m2 | 30 ± 6 | 30 ± 6 | 29 ± 5 | 0.002 |
| Atrial fibrillation | 34 (1.9) | 9 (1.2) | 25 (2.5) | 0.062 |
| History of CHD | 178 (10) | 45 (6) | 133 (13) | <0.001 |
| History of CVD | 79 (4.5) | 28 (3.6) | 51 (5.1) | 0.162 |
| History of PVD | 31 (1.8) | 7 (0.9) | 24 (2.4) | 0.028 |
| Smoking status | 0.007 | |||
| Current smoker | 330 (19) | 153 (20) | 177 (18) | |
| Ex-smoker | 593 (34) | 228 (30) | 365 (37) | |
| Nonsmoker | 845 (48) | 391 (51) | 454 (46) | |
| Hypertension | 608 (35) | 254 (33) | 354 (36) | 0.245 |
| Diabetes mellitus | 196 (11) | 64 (8) | 132 (13) | 0.001 |
| Family history of CHD | 765 (44) | 364 (48) | 401 (41) | 0.003 |
| Total cholesterol, mg/dl | 205 (162-235) | 208 (174-243) | 197 (158-228) | <0.001 |
| Chest pain symptoms | <0.001 | |||
| Non-anginal | 683 (39) | 298 (39) | 385 (39) | |
| Atypical angina | 432 (24) | 222 (2) | 210 (21) | |
| Typical angina | 654 (37) | 252 (33) | 402 (40) | |
| Cardiovascular risk score | 16 (10-23) | 14 (8-21) | 17 (12-25) | <0.001 |
| Preventative medication | ||||
| Baseline | 1,049 (59) | 431 (56) | 618 (62) | 0.01 |
| 6 weeks | 1,215 (69) | 479 (62) | 736 (74) | <0.001 |
| 5 years | 1,162 (66) | 459 (60) | 703 (71) | <0.001 |
Values are mean ± SD, n (%), or median (interquartile interval). Bold indicates p < 0.05.
CHD = coronary heart disease; CVD = cerebrovascular disease; PVD = peripheral vascular disease.
VISUAL ASSESSMENT OF PLAQUE.
Women had a lower coronary artery calcium score compared to men (0 [IQI: 0 to 54] vs. 80 [IQI: 0 to 447]; p < 0.001) and were more likely to have zero or low (<100 Agatston units) coronary artery calcium score (Table 2). They were also more likely to have visually assessed normal coronary arteries (50% vs. 26%; p < 0.001) and had fewer vessels with visually assessed obstructive disease (Table 2). A similar proportion of men and women had visually assessed nonobstructive coronary disease (37% vs. 39%; p < 0.001). Visually assessed adverse plaques were less frequent among women (20% vs. 45%; p < 0.001), and the frequency of visually assessed positive remodeling, low-attenuation plaque, spotty calcification, and the napkin-ring sign were all lower in women (p < 0.001) (Table 2).
TABLE 2.
Coronary Artery Calcium Score and Coronary Artery Disease on Computed Tomography
| Overall (N = 1,769) |
Female (n = 772) |
Male (n = 997) |
p Value | |
|---|---|---|---|---|
| Coronary artery calcium score | 21 (0-230) | 0 (0-54) | 80 (0-447) | <0.001 |
| Coronary artery calcium score group | <0.001 | |||
| 0 | 642 (36) | 392 (51) | 250 (25) | |
| 1–99 AU | 509 (29) | 233 (30) | 276 (28) | |
| 100–399 AU | 303 (17) | 93 (12) | 210 (21) | |
| 400–999 AU | 169 (10) | 37 (5) | 132 (13) | |
| ≥1,000 AU | 146 (8) | 17 (2) | 129 (13) | |
| CCTA | <0.001 | |||
| Normal | 646 (37) | 383 (50) | 263 (26) | |
| Non-obstructive plaque | 671 (38) | 284 (37) | 387 (39) | |
| Obstructive plaque | 452 (26) | 105 (14) | 347 (35) | |
| Obstructive disease | <0.001 | |||
| 1-vessel disease | 207 (12) | 60 (8) | 147 (15) | |
| 2-vessel disease | 128 (7) | 31 (4) | 97 (10) | |
| ≥3-vessel disease | 117 (7) | 14 (2) | 103 (10) | |
| Visually assessed adverse plaques | 608 (34) | 156 (20) | 452 (45) | <0.001 |
| Positive remodeling | 603 (34) | 154 (20) | 449 (45) | <0.001 |
| Low-attenuation plaque | 168 (10) | 36 (5) | 132 (13) | <0.001 |
| Spotty calcification | 299 (17) | 85 (11) | 214 (22) | <0.001 |
| Napkin ring sign | 76 (4) | 17 (2) | 59 (6) | <0.001 |
Values are median (interquartile interval) or n (%). Bold indicates p < 0.05.
AU = Agatston units.
QUANTITATIVE ASSESSMENT OF PLAQUE.
Quantitatively assessed total plaque burden (22.7% vs. 44.3%; p < 0.001) as well as the burden of all plaque subtypes were lower in women (Table 3), independent of cardiovascular risk score (Supplemental Table 2). Women were less likely to have a quantitatively assessed low-attenuation plaque burden >4% (n = 315, 41% vs. n = 591, 59%; p < 0.001). Per-patient quantitatively assessed area stenosis, diameter stenosis, remodeling index, plaque length, lumen volume, and contrast density drop were also lower in women. However, among patients with visually assessed nonobstructive and obstructive coronary artery disease, there was no difference in the quantitatively assessed burden of noncalcified plaque and low-attenuation plaque between women and men (Supplementary Table 3).
TABLE 3.
Quantitative Plaque Assessment on CCTA in all Patients and in Patients Who Had a Subsequent Myocardial Infarction
| Female | Male | p Value | |
|---|---|---|---|
| All patients | |||
| Total plaque burden, % | 22.7 (0.0–42.7) | 44.3 (0.0–52.5) | <0.001 |
| Total calcified plaque burden | 0.0 (0.0–1.1) | 1.2 (0.0–4.5) | <0.001 |
| Total noncalcified plaque burden | 20.6 (0.0–40.7) | 39.8 (0.0–47.8) | <0.001 |
| Total low-attenuation noncalcified plaque burden | 1.4 (0.0–6.1) | 5.0 (0.0–7.5) | <0.001 |
| Area stenosis, % | 10.1 (0.0–62.3) | 66.5 (0.0–87.6) | <0.001 |
| Diameter stenosis, % | 37.5 (28.4–54.3) | 51.0 (36.4–78.7) | <0.001 |
| Remodeling index | 1.0 (1.0–1.3) | 1.3 (1.0–1.6) | <0.001 |
| Plaque length, mm | 11.8 (0.0–60.1) | 66.8 (0.0–138.0) | <0.001 |
| Lumen volume, mm3 | 499 (264–909) | 890 (471–1,449) | <0.001 |
| Contrast density difference, % | 0.0 (0.0–29.1) | 23.2 (0.0–36.4) | <0.001 |
| Patients who had myocardial infarction | (n = 11) | (n = 30) | |
| Total plaque burden, % | 48.1 (34.3–53.7) | 50.5 (43.8–55.4) | 0.289 |
| Total calcified plaque burden | 1.6 (0.0–4.0) | 4.6 (2.1–9.2) | 0.027 |
| Total noncalcified plaque burden | 42.9 (33.0–48.7) | 42.4 (37.4–48.9) | 0.814 |
| Total low-attenuation noncalcified plaque burden | 7.9 (5.2–8.8) | 7.4 (4.8–9.3) | 0.930 |
| Area stenosis, % | 79.2 (58.5–84.6) | 80.4 (66.2–100.0) | 0.366 |
| Diameter stenosis, % | 52.8 (40.9–62.5) | 62.0 (41.3–100.0) | 0.394 |
| Remodeling index | 1.6 (1.3–1.8) | 1.5 (1.2–1.7) | 0.713 |
| Plaque length, mm | 124.6 (59.5–150.7) | 116.9 (70.6–161.1) | 0.702 |
| Lumen volume, mm3 | 839 (560–1,058) | 1,048 (578–1472) | 0.320 |
| Contrast density difference | 28.0 (24.8–39.8) | 33.8 (21.4–40.4) | 0.837 |
Values are median (interquartile interval). Bold indicates p < 0.05.
CLINICAL OUTCOMES.
Over a median follow-up of 4.7 (IQI: 4.0 to 5.7) years fatal or nonfatal MI occurred in 11 women (1.4%) and 30 men (3%). Both women and men who had a subsequent fatal or nonfatal MI had a higher quantitatively assessed total plaque burden and all subtypes of plaque burden compared to those without events. Irrespective of these differences, women who had an MI had the same quantitatively assessed burden of total, noncalcific, low-attenuation plaque and other plaque parameters as men who had an event (Figure 2, Table 3).
FIGURE 2. Quantitative Plaque Burden in Women and Men.
Sex-based difference in plaque burden in patients who did or did not have a fatal or nonfatal myocardial infarction.
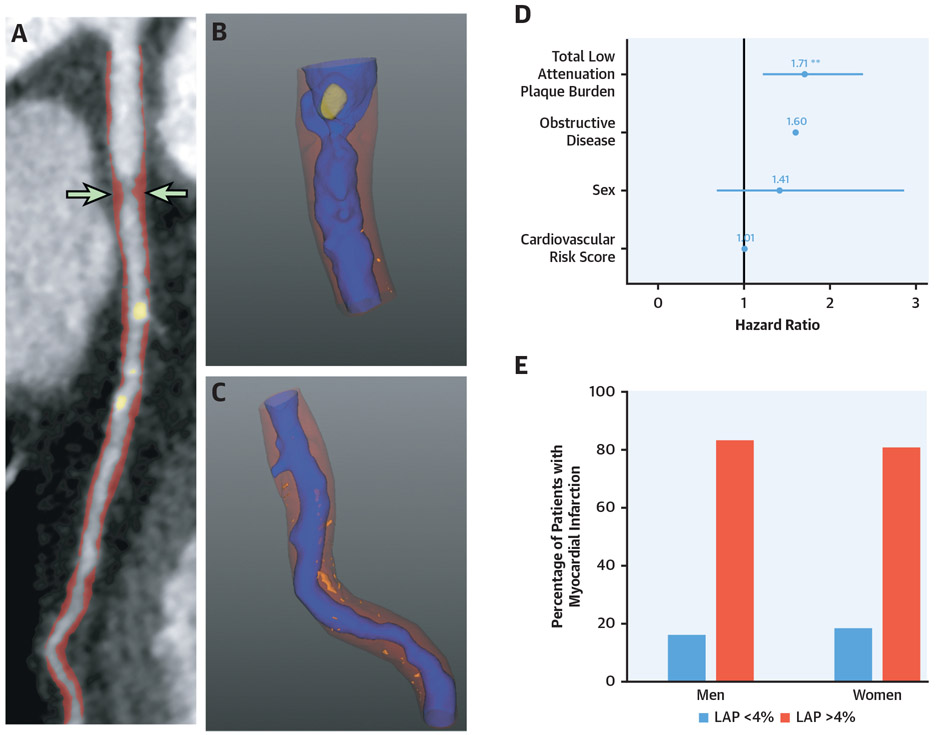
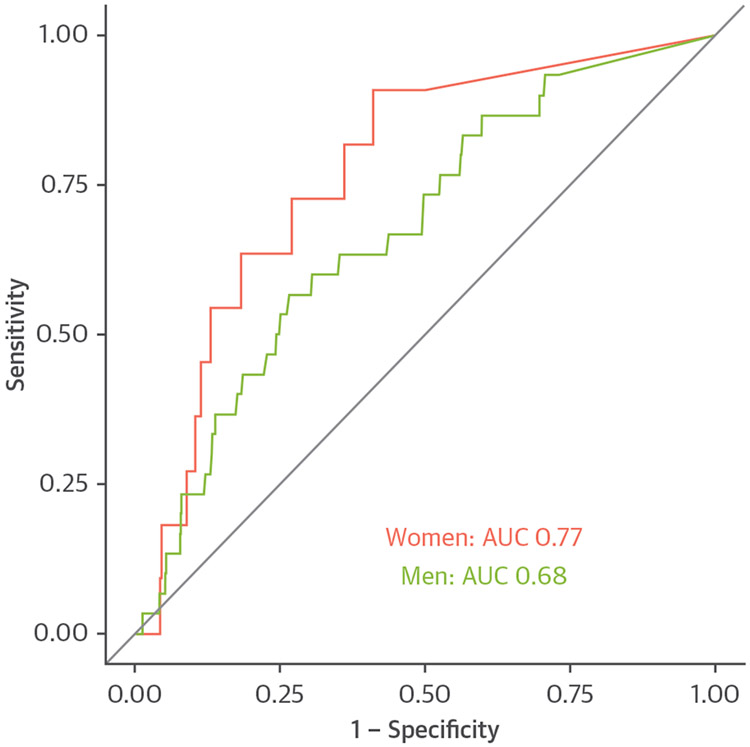
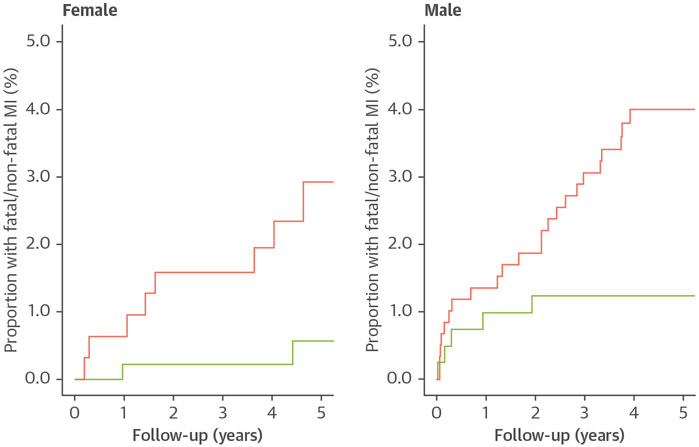
Men were twice as likely to undergo fatal or nonfatal MI as women (HR: 2.12; 95% CI: 1.07 to 4.24; p = 0.033). Quantitatively assessed total, calcified, noncalcified, and low-density calcified plaque burdens were all predictive of subsequent fatal or nonfatal MI independent of sex (Table 4, Supplemental Table 4). Quantitatively assessed low-attenuation plaque burden was an independent predictor of fatal or nonfatal MI in multivariable analysis (HR: 1.71; 95% CI: 1.22 to 2.39; p = 0.002), independent of sex (HR: 1.41; 95% CI: 0.69 to 2.87; p = 0.339), visually assessed obstructive disease (HR: 1.60, 95% CI: 0.82 to 3.14; p = 0.169), and cardiovascular risk scores (HR: 1.01: 95% CI: 0.98 to 1.04; p = 0.502) (Central Illustration). Similar results were found in models adjusted for quantitatively assessed diameter stenosis and lumen volume (Supplemental Table 5). Quantitatively assessed low-attenuation plaque was similarly predictive of subsequent outcomes in women (area under the curve [AUC]: 0.77; 95% CI: 0.65 to 0.90) as compared to men (AUC: 0.68; 95% CI: 0.58 to 0.77; p = 0.346) (Figure 3, Supplemental Figure 1). Quantitatively assessed low-attenuation plaque burden >4% was associated with a 6-fold increased risk of fatal or nonfatal MI in women (HR: 6.57; 95% CI: 1.42 to 30.41; p = 0.016; men HR: 3.44; 95% CI: 1.32 to 8.98; p = 0.012) (Figure 4).
TABLE 4.
Multivariable Analysis Adjusted for Sex
| Multivariable Analysis 1* |
Multivariable Analysis 2† |
|||
|---|---|---|---|---|
| Hazard Ratio (95% CI) |
p Value | Hazard Ratio (95% CI) |
p Value | |
| Total plaque burden‡ | 1.43 (1.15–1.78) | 0.001 | 1.34 (1.07–1.69) | 0.011 |
| Calcified plaque burden‡ | 1.70 (1.36–2.14) | <0.001 | 1.57 (1.20–2.05) | <0.001 |
| Noncalcified plaque burden‡ | 1.86 (1.37–2.52) | <0.001 | 1.31 (1.06–1.64) | 0.015 |
| Low-attenuation plaque burden‡ | 1.40 (1.14–1.72) | 0.001 | 1.71 (1.22–2.39) | 0.002 |
Multivariable analysis 1 including the individual plaque parameter adjusted for sex.
Multivariable analysis 2 including the individual plaque parameter adjusted for sex, presence of visually observed obstructive coronary artery disease, and cardiovascular risk score.
Per doubling.
CI = confidence interval.
CENTRAL ILLUSTRATION. Low-Attenuation Plaque Is an Important Predictor of Events in Both Women and Men.
(A,B) Images from a 58-year-old woman with nonobstructive disease, but a heavy burden of low-attenuation plaque. Two years later she suffered a non-ST-segment elevation myocardial infarction. (C) Results of the multivariable model where low-attenuation plaque burden was a predictor of subsequent fatal or non-fatal myocardial infarction, independent of gender, presence of obstructive disease on CCTA and cardiovascular risk score. (D) The percentage of women or men who suffered myocardial infarction that had a low-attenuation plaque burden (LAP) above or below 4%. CCTA = coronary computed tomography angiography.
FIGURE 3. Predictive Ability of Low-Attenuation Plaque in Women and Men.
Receiver operating curve (ROC) showing ability of low-attenuation plaque to predict fatal or nonfatal myocardial infarction for men (green) and women (red). AUC = area under the curve.
FIGURE 4. Myocardial Infarction and Low-Attenuation Plaque in Women and Men.
Cumulative incidence plots of fatal or nonfatal myocardial infarction in women and men showing the difference in events between those with (red) and without (green) low-attenuation plaque above 4%.
DISCUSSION
We have confirmed that, compared to men, women with stable chest pain have less coronary artery plaque burden and fewer subsequent MI. However, in those who subsequently had MI, women had a similar quantitatively assessed burden of total, noncalcified, and low-attenuation plaque as men. The quantitatively assessed low-attenuation plaque burden was predictive of subsequent cardiac events, over and above sex, cardiovascular risk score, coronary artery calcification, and the presence of obstructive coronary artery disease. These important observations show that quantitative plaque analysis is a robust predictor of cardiac endpoints, irrespective of sex.
Cardiovascular disease remains the leading cause of mortality in women (18). Quantitative assessment of atherosclerotic plaque provides additive value to traditional CCTA assessment, and we have shown that this is valuable for both women and men (2). Quantitative assessment of atherosclerotic plaque is a robust technique with good observer and interscan agreement, which improves upon the poor observer agreement that has hampered visual plaque assessments (16,19). In particular, low-attenuation plaque provides important information on the burden of plaque most closely associated with plaque rupture and MI: those with a lipid-rich necrotic core. Whereas most individual plaques with these features will heal without causing events, patients who tend to form such adverse plaques will also form multiple similar lesions over time, one of which may ultimately cause an event. Assessments of plaque type are therefore helpful in identifying vulnerable patients. Measures of the adverse plaque burden extend this concept further: the more adverse plaques a patient has, the higher their risk of events.
Among patients with stable chest pain, we have confirmed that women have less coronary artery calcification, less obstructive coronary disease, and less frequent adverse plaque compared to men (20-23). Women have a lower plaque burden and higher proportion of noncalcified or mixed plaques compared to men in previous studies (21,24-27). However, in patients with obstructive disease the proportion of different plaque types is similar in men and women (28). The CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry also showed that there were no sex-based differences in the predictive value of coronary stenoses for major adverse cardiac events (22). In our study, the burden of total, noncalcific, and low-attenuation plaque in those who sustained an MI was similar in women and men. This supports other recent data indicating that among patients presenting with acute coronary syndromes, there is no sex difference in quantitative plaque characteristics in culprit lesions (4).
The ICONIC (Incident Coronary Events Identified by Computed Tomography) study also performed quantitative plaque analysis on CCTA in patients with a subsequent acute coronary syndrome. They showed that women had lower total and fibrous/fibrofatty plaque volume compared to men, but no difference in necrotic core volume or visually assessed high-risk plaque features (4). Intravascular ultrasound in patients with acute coronary syndromes shows that women have similar plaque burden per lesion compared to men and sex-based differences in nonculprit plaque composition are present in those younger than, but not older than, 65 years of age (29,30). Similarly, no sex-based differences were identified on optical coherence tomography in culprit lesions of patients presenting with acute coronary syndromes, but sex-based differences have been identified in nonculprit lesions (31,32).
A recent analysis of the PARADIGM (Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography Imaging) study found that compared to men, women had greater progression of calcified plaque, slower progression of noncalcified plaque, and less frequent development of visually assessed high-risk plaques (5). However, at present, the clinical implications of the increased progression of calcified plaque in women is uncertain. A systematic review of studies involving serial intravascular ultrasound showed no sex-based differences in plaque progression (33). Thus, although women with stable coronary artery disease have different plaque characteristics and plaque progression, the culprit lesions in MI appear to be similar irrespective of sex.
The only sex-based difference on quantitative plaque analysis in those who suffered an MI in our study was in the burden of calcified plaque, which was 3 times higher in men than in women. This reflects a pattern of increased risk for women at lower coronary artery calcium levels compared to men. In the 63,215 patients in the coronary artery calcium (CAC) consortium, a CAC score above zero was associated with a 1.3 higher HR for cardiovascular death in women compared to men (20). However, in the ICONIC study there was no difference in calcified plaque volume between men and women who had cardiac events (4). Women had a lower burden of calcified plaque in all subgroups in our study. It is well established that women have lower CAC scores compared to men (34). Recently, women have been shown to have a smaller number, but larger size and density, of calcified plaques compared to men, but with similar radiomic characteristics (20,35). These new noninvasive imaging parameters advance our understanding of high-risk plaque subtypes. However, despite the difference in burden of calcified plaque between men and women who underwent MI, only low-attenuation plaque burden was an independent predictor of outcomes in this study.
We have shown that visually assessed high-risk plaques are less frequent in women compared to men. A substudy of the PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trial identified similar findings, with 2 feature-positive high-risk plaques occurring in 11% of women and 20% of men (23). They also identified a stronger association between high-risk plaque and major adverse cardiac events in women compared to men (23). However, in both PROMISE and SCOT-HEART, there was a low frequency of subsequent events in patients with visually assessed high-risk plaques (9,23).
STUDY LIMITATIONS.
Important limitations of this study are the number of patients and the small number of events (1.4% in women, 3% in men) reported. As such, a limited number of factors can be included in multivariable analysis and the models may be over-fitted. The patients who underwent CT had their treatment appropriately optimized; therefore, effects identified in this study are a conservative estimate. Women were less likely to be taking preventative medication which may affect the impact of plaque features on outcomes. Quantitative plaque analysis is an emerging field and further validation is required in other cohorts. Differences in vessel size between women and men will affect assessment of plaque volume, but using plaque burden in the analysis largely takes account of these differences. This was a post hoc analysis using data from the SCOT-HEART trial and prospective studies are required to assess the impact of these findings on management.
CONCLUSIONS
This study shows that quantitative plaque analysis is a robust predictor of cardiac endpoints, irrespective of sex. Where present, low-attenuation plaque is as powerful predictor in women as men, with a low-attenuation plaque >4% associated with 6-fold increase in the risk of MI.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Women presenting with stable chest pain have lower quantitatively assessed plaque burdens compared to men. However, women who develop subsequent MI have less calcified plaque, but similar quantities of other plaque subtypes. Low-attenuation plaque burden is as strong a predictor of MI in women and men.
TRANSLATIONAL OUTLOOK:
Future studies will assess these findings in patients presenting in other clinical situations, such as acute chest pain and asymptomatic patients. In addition, studies are required to assess whether changes in plaque type with medication use lead to improved clinical outcomes.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
Drs. Dey, Slomka, and Berman and Mr. Cadet may receive software royalties from Cedars-Sinai Medical Center; and Drs. Dey, Slomka, and Berman have a patent. This trial was funded by The Chief Scientist Office of the Scottish Government Health and Social Care Directorates (CZH/4/588), with supplementary awards from Edinburgh and Lothian’s Health Foundation Trust and the Heart Diseases Research Fund. Drs. Williams, Mills, Newby, and Dweck are supported by the British Heart Foundation (FS/ICRF/20/26002, CH/09/002, FS/11/014, FS/16/14/32023, RG/20/10/34966, RE/18/5/34216, RG/16/10/32375, FS/14/78/31020). Dr. Williams was supported by The Chief Scientist Office of the Scottish Government Health (PCL/17/04). Dr. Newby is the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA). Dr. van Beek is supported by Scottish Imaging Network: A Platform of Scientific Excellence (SINAPSE). Dr. Adamson is supported by a National Heart Foundation of New Zealand Senior Fellowship (1844). Dr. Dweck is supported by the Sir Jules Thorn Biomedical Research Award 2015 (15/JTA). The Royal Bank of Scotland supported the provision of 320-multidetector CT for NHS Lothian and the University of Edinburgh. The Edinburgh Imaging facility QMRI (Edinburgh) is supported by the National Health Service Research Scotland (NRS) through National Health Service Lothian Health Board. The Clinical Research Facility Glasgow and Clinical Research Facility Tayside are supported by National Health Service Research Scotland (NRS). Ms. McElhinney and Dr. Dey are supported by National Institute of Health/National Heart, Lung, and Blood Institute grants (1R01HL148787-01A1 and 1R01HL151266). Mr. Cadet is supported by the Miriam and Sheldon G. Adelson Medical Research Foundation. All other authors have no reported that they have norelationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CCTA
computed tomography coronary angiography
- CI
confidence interval
- CT
computed tomography
- HR
hazard ratio
- HU
Hounsfield unit
- MI
myocardial infarction
- ROC
receiver operator curve
- SCOT-HEART
Scottish Computed Tomography of the HEART
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For supplemental Methods, tables, and a figure, please see the online version of this paper.
REFERENCES
- 1.SCOT-HEART Investigators. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379:924–33. [DOI] [PubMed] [Google Scholar]
- 2.Williams MC, Kwiecinski J, Doris M, et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART trial (Scottish Computed Tomography of the HEART). Circulation 2020;141:1452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bairey Merz CN, Shaw LJ, et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol 2006;47:S21–9. [DOI] [PubMed] [Google Scholar]
- 4.Conte E, Dwivedi A, Mushtaq S, et al. Age- and sex-related features of atherosclerosis from coronary computed tomography angiography in patients prior to acute coronary syndrome: results from the ICONIC study. Eur Heart J Cardiovasc Imaging 2020;22:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SE, Sung JM, Andreini D, et al. Sex differences in compositional plaque volume progression in patients with coronary artery disease. J Am Coll Cardiol Img 2020;13:2386–96. [DOI] [PubMed] [Google Scholar]
- 6.Newby DE, Williams MC, Flapan AD, et al. Role of multidetector computed tomography in the diagnosis and management of patients attending the rapid access chest pain clinic. The Scottish Computed Tomography of the HEART (SCOT-HEART) trial: study protocol for randomized controlled trial. Trials 2012;13:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–91. [DOI] [PubMed] [Google Scholar]
- 8.Williams MC, Hunter A, Shah ASV, et al. Use of coronary computed tomographic angiography to guide management of patients with coronary disease. J Am Coll Cardiol 2016;67:1759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams MC, Moss AJ, Dweck M, et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol 2019;73:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodward M, Brindle P, Tunstall-Pedoe H, et al. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart 2007;93:172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15: 827–32. [DOI] [PubMed] [Google Scholar]
- 12.Williams MC, Golay SK, Hunter A, et al. Observer variability in the assessment of CT coronary angiography and coronary artery calcium score: substudy of the Scottish Computed Tomography of the HEART (SCOT-HEART) trial. Open Heart 2015;2:e000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49–57. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto H, Watanabe S, Kyo E, et al. Effect of tube potential and luminal contrast attenuation on atherosclerotic plaque attenuation by coronary CT angiography: in vivo comparison with intravascular ultrasound. J Cardiovasc Comput Tomogr 2019;13:219–25. [DOI] [PubMed] [Google Scholar]
- 15.Dey D, Diaz Zamudio M, Schuhbaeck A, et al. Relationship between quantitative adverse plaque features from coronary computed tomography angiography and downstream impaired myocardial flow reserve by 13n-ammonia positron emission tomography: a pilot study. Circ Cardiovasc Imaging 2015;8:e003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dey D, Cheng VY, Slomka PJ, et al. Automated 3-dimensional quantification of noncalcified and calcified coronary plaque from coronary CT angiography. J Cardiovasc Comput Tomogr 2009;3: 372–82. [DOI] [PubMed] [Google Scholar]
- 17.Diaz-Zamudio M, Fuchs TA, Slomka P, et al. Quantitative plaque features from coronary computed tomography angiography to identify regional ischemia by myocardial perfusion imaging. Eur Heart J Cardiovasc Imaging 2017;18: 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020;141:e139–596. [DOI] [PubMed] [Google Scholar]
- 19.Maroules CD, Hamilton-Craig C, Branch K, et al. Coronary artery disease reporting and data system (CAD-RADS(TM)): inter-observer agreement for assessment categories and modifiers. J Cardiovasc Comput Tomogr 2018;12:125–30. [DOI] [PubMed] [Google Scholar]
- 20.Shaw LJ, Min JK, Nasir K, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J 2018;39:3727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plank F, Beyer C, Friedrich G, Wildauer M, Feuchtner G. Sex differences in coronary artery plaque composition detected by coronary computed tomography: quantitative and qualitative analysis. Neth Heart J 2019;27:272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulman-Marcus J, Hartaigh BO, Gransar H, et al. Sex-specific associations between coronary artery plaque extent and risk of major adverse cardiovascular events: the CONFIRM long-term registry. J Am Coll Cardiol Img 2016; 9:364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferencik M, Mayrhofer T, Bittner DO, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol 2018;3:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qureshi W, Blaha MJ, Nasir K, Al-Mallah MH. Gender differences in coronary plaque composition and burden detected in symptomatic patients referred for coronary computed tomographic angiography. Int J Cardiovasc Imaging 2013;29: 463–9. [DOI] [PubMed] [Google Scholar]
- 25.Blaha MJ, Nasir K, Rivera JJ, et al. Gender differences in coronary plaque composition by coronary computed tomography angiography. Coron Artery Dis 2009;20:506–12. [DOI] [PubMed] [Google Scholar]
- 26.Han SH, Bae JH, Holmes DR Jr., et al. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J 2008;29:1359–69. [DOI] [PubMed] [Google Scholar]
- 27.Bharadwaj AS, Vengrenyuk Y, Yoshimura T, et al. Multimodality intravascular imaging to evaluate sex differences in plaque morphology in stable CAD. J Am Coll Cardiol Img 2016;9:400–7. [DOI] [PubMed] [Google Scholar]
- 28.Khosa F, Khan AN, Nasir K, et al. Comparison of coronary plaque subtypes in male and female patients using 320-row MDCTA. Atherosclerosis 2013;226:428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. J Am Coll Cardiol Img 2012;5: S62–72. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Garcia J, Lerman A, Weisz G, et al. Age- and gender-related changes in plaque composition in patients with acute coronary syndrome: the PROSPECT study. EuroIntervention 2012;8:929–38. [DOI] [PubMed] [Google Scholar]
- 31.Chia S, Christopher Raffel O, Takano M, Tearney GJ, Bouma BE, Jang IK. In-vivo comparison of coronary plaque characteristics using optical coherence tomography in women vs. men with acute coronary syndrome. Coron Artery Dis 2007; 18:423–7. [DOI] [PubMed] [Google Scholar]
- 32.Kataoka Y, Puri R, Hammadah M, et al. Sex differences in nonculprit coronary plaque microstructures on frequency-domain optical coherence tomography in acute coronary syndromes and stable coronary artery disease. Circ Cardiovasc Imaging 2016;9:e004506. [DOI] [PubMed] [Google Scholar]
- 33.Nicholls SJ, Wolski K, Sipahi I, et al. Rate of progression of coronary atherosclerotic plaque in women. J Am Coll Cardiol 2007;49:1546–51. [DOI] [PubMed] [Google Scholar]
- 34.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2006;113:30–7. [DOI] [PubMed] [Google Scholar]
- 35.Eslami P, Parmar C, Foldyna B, et al. Radiomics of coronary artery calcium in the Framingham Heart Study. Radiol Cardiothorac Imaging 2020;2: e190119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.