Abstract
BACKGROUND:
Maternal anemia is a common pregnancy complication and often leads to a requirement for additional treatments and interventions. Identifying the frequency at which women with antenatally diagnosed anemia experience severe morbidity at the time of admission to the labor and delivery unit will guide future recommendations regarding screening and interventions for anemia during pregnancy.
OBJECTIVE:
The objective of this study was to evaluate the association between antenatally diagnosed anemia and severe maternal morbidity as defined by the Centers for Disease Control and Prevention in a large, contemporary, US cohort. Neonatal outcomes were also examined.
STUDY DESIGN:
This was a secondary analysis of the Consortium on Safe Labor database from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, which collected data on 228,438 deliveries in 19 United States hospitals from 2002 to 2008. This analysis included women with viable, singleton gestations and excluded stillbirths and gestations with severe congenital anomalies. Women with a diagnosis of antenatal anemia were compared with those without. Identification of diagnoses of antenatal anemia was obtained via electronic medical record abstraction and International Classification of Diseases coding according to each hospital protocol within the Consortium on Safe Labor. The primary maternal outcome consisted of a composite of severe maternal morbidity as defined by the Centers for Disease Control and Prevention and included maternal death, eclampsia, thrombosis, transfusion, hysterectomy, and maternal intensive care unit admission. The primary neonatal outcome was a composite that included a 5-minute Apgar score of <7, hypoxic ischemic encephalopathy, respiratory distress syndrome, necrotizing enterocolitis, seizures, intracranial hemorrhage, periventricular or intraventricular hemorrhage, neonatal sepsis, neonatal intensive care unit admission, and neonatal death. Each outcome within the composites was assessed individually along with other additional secondary outcomes, including a composite of severe maternal morbidity not including transfusion morbidity. All statistical analyses were performed with Stata version 14.2 (StataCorp LLC, College Station, TX) using Student’s t test, chi-square test, Fisher’s exact test, and Wilcoxon rank-sum (Mann-Whitney U) test, as appropriate. A multivariable logistic regression was performed with potential confounding variables entered into the regression equation if they differed between groups at a significance level of P<.05.
RESULTS:
A total of 166,566 women met the inclusion criteria. From the original cohort, 56,734 women could not be included because of an unknown diagnosis of anemia. Of those included, 10,217 (6.1%) were diagnosed with anemia during the pregnancy. Women with anemia were more likely to be younger, non-Hispanic Black, single, multiparous, and have a higher prepregnancy body mass index than those without anemia. The frequency of the primary maternal composite outcome, the neonatal composite outcome, and other secondary outcomes including the severe maternal morbidity composite not including transfusion, maternal death, transfusion during labor and the postpartum period, hysterectomy, postpartum hemorrhage, infectious morbidity, cesarean delivery, and preterm delivery were more common in women with anemia (P<.05). After multivariable logistic regression analysis adjusting for confounders, higher rates of severe maternal morbidity remained persistently associated with anemia (adjusted odds ratio, 2.04; 95% confidence interval, 1.86–2.23) in addition to the association of anemia with the severe maternal morbidity composite not including transfusion, maternal death, thrombosis, transfusion, hysterectomy, intensive care unit admission, postpartum hemorrhage, hypertensive disorders of pregnancy, cesarean delivery, and infectious morbidity. The composite neonatal outcome also remained associated with anemia after adjusting for confounders (adjusted odds ratio, 1.14; 95% confidence interval, 1.06–1.23).
CONCLUSION:
Women with antepartum anemia experienced increased rates of severe maternal morbidity and other serious adverse outcomes. Diagnosis and treatment of anemia during the antepartum period may lead to the identification and treatment of women at higher risk for maternal morbidity and mortality.
Keywords: anemia, maternal death, maternal morbidity, pregnancy
Anemia affects approximately 56 million pregnant women globally, including more than 50% of women in some countries,1–7 and contributes considerably to maternal morbidity and mortality throughout the world.2,3,7–9 Although a physiological reduction in hemoglobin occurs during pregnancy because of the disproportionate increase in intravascular volume compared with the increase in red blood cell production,6,10 pathologic states of anemia are associated with more frequent adverse pregnancy outcomes including higher rates of blood product transfusion, cesarean delivery, hysterectomy, preterm delivery, small for gestational age (SGA) neonates, and other adverse maternal and neonatal outcomes.1–3,7–10
Pathologic anemia during pregnancy, defined by the Centers for Disease Control and Prevention (CDC) as hemoglobin and hematocrit levels of less than 11 g/dL and 33% in the first and third trimesters and less than 10.5 g/dL and 32% in the second trimester,10 is an important gauge identified by The American College of Obstetricians and Gynecologists (ACOG) to identify those who require additional work-up and treatment.6 Iron deficiency anemia accounts for 75% of all anemias during pregnancy and is caused by the increased iron demand required to support the 25% increase in total red blood cell mass.6,11,12 The incidence of megaloblastic anemia, which is caused by folate or vitamin B12 deficiency, is more prevalent among women with inadequate dietary intake and those who are not receiving appropriate prenatal supplementation.6,10–12 Other causes of anemia among pregnant women are similar to those among nonpregnant women and include acquired and hereditary causes.6,10–12
Several studies, both from developed and developing countries, and a recent expert review highlighted the negative impact of anemia during pregnancy on maternal and neonatal outcomes, including the life-long effects of iron-deficiency on the neonate.1–4,7–10,13,14 However, data examining the contribution of anemia to severe maternal morbidity (SMM) in the United States, as defined specifically by the CDC,15 is lacking.9,16 Utilized by the CDC for targeting interventions to mitigate rising maternal mortality, prolonged hospital stays, and increasing medical costs, SMM is an informative pregnancy morbidity index.15 Furthermore, despite the prevalence of anemia in some parts of the United States,6 there has not been a large-scale examination on the impact of anemia on maternal and neonatal outcomes.
Finally, in reviewing national guidelines regarding the timing and frequency of testing for anemia during pregnancy, recommendations remain vague because of insufficient data.6,10,17 The need for additional research in this area is further demonstrated by the results of a current practice survey conducted by Marcewicz et al,18 which highlighted the vast variability in anemia diagnosis and management practices among ACOG pregnancy care providers across the United States.18 Additional information regarding pregnancy outcomes in women with anemia will allow providers to assess whether a practice change is warranted in screening for and intervening in this potentially modifiable disease.
Therefore, our primary objective was to evaluate the association of anemia with SMM in a large US-based cohort. We in addition examined the impact of maternal anemia on adverse maternal and neonatal complications identified during the labor and delivery course. We hypothesized that women with anemia would have higher rates of SMM and adverse neonatal outcomes, particularly SGA neonates and preterm delivery.
Materials and Methods
This was a secondary analysis of deidentified data from the Consortium on Safe Labor (CSL) database obtained from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (https://dash.nichd.nih.gov/). The original study included 228,438 pregnancies of women who delivered at 23 weeks’ gestation or later at 19 hospitals in the United States between 2002 and 2008. Data about maternal and neonatal outcomes were collected retrospectively via abstraction from electronic medical records and International Classification of Diseases, Ninth Revision, (ICD-9) codes. The original study aimed to obtain a better understanding of labor and delivery practices throughout the United States to assess risk factors for cesarean delivery.19 All participating sites in the CSL study received institutional review board (IRB) approval at the time of the original data collection, and further approval was obtained for our secondary analysis from the Medical College of Wisconsin IRB.
The inclusion criteria for the study were women with a viable, singleton gestation who delivered at or later than 23 weeks’ gestation. Exclusion criteria were women with an unknown diagnosis of anemia, a stillbirth, multiple gestation, and fetuses with severe congenital anomalies. The latter fetus-related exclusion criteria were applied to allow the results to accurately reflect the relationship between anemia and the timing of delivery and rates of SGA because women with stillbirths, multiple gestations, and fetuses with congenital anomalies often experience preterm birth and have SGA neonates. The primary exposure in our study was a diagnosis of antenatal anemia before delivery. A diagnosis of antenatal anemia was assigned if a diagnosis of anemia was noted either during electronic medical record abstraction or ICD coding from prenatal records according to each hospital protocol within the CSL. Of note, the original publication described the process of data maintenance and accuracy as follows: “Data transferred from the clinical centers were mapped to predefined common codes for each variable at the data coordinating center. Data inquiries, cleaning, recoding and logic checking were performed. We also conducted validation studies for four key outcome diagnoses.…” This validation process found the data to be “highly accurate” with most variables showing >95% accuracy.19 Baseline characteristics, background health and pregnancy information, and pregnancy outcomes were included in the analyses.
The primary maternal outcome was a composite of SMM, which included maternal death, eclampsia, thrombosis, transfusion, hysterectomy, and maternal intensive care unit (ICU) admission. Of note, the original data collected by the CSL did not include all SMM indicators as listed by the CDC, which is likely because the CDC SMM criteria were not established until 2012, which is after the completion of the CSL data collection period,20 however, we utilized ICU admission as a surrogate for the variables not collected (Appendix 1), acknowledging that any of these diagnoses would typically warrant an ICU stay. Additional secondary maternal outcomes included all individual outcomes in the composite outcome, these are additional outcomes beyond the composite preterm delivery; blood loss; postpartum hemorrhage (PPH); preeclampsia; this is one of the individual composite outcomes; hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome; cesarean delivery; a composite of infectious morbidity (including chorioamnionitis, endometritis, and wound infection); a separate SMM composite excluding transfusion; and gestational age at delivery. The primary neonatal outcome was a composite that included 5-minute Apgar score of <7, hypoxic ischemic encephalopathy, respiratory distress syndrome (RDS), necrotizing enterocolitis, seizures, intracranial hemorrhage, periventricular or intraventricular hemorrhage, neonatal sepsis, neonatal intensive care unit (NICU) admission, and neonatal death. Additional secondary neonatal outcomes included each individual outcome of the composite, birthweight less than 2500 g, fetal distress during labor, neonatal anemia, and need for transfusion.
All statistical analyses were performed using Student’s t test, chi-square test, Fisher’s exact test, and Wilcoxon rank-sum (Mann-Whitney U) test, as appropriate. Multivariable logistic regression analysis was performed with potential confounding variables entered into the regression equation if they differed between groups at a level of P<.05. These potential confounders included maternal age, body mass index (BMI), nulliparity, maternal race, marital status, insurance, and history of diabetes, asthma, previous cesarean delivery, and alcohol use. A P value of <.05 was considered statistically significant. All statistical analyses were conducted using Stata version 14.2 (StataCorp LLC, College Station, TX).
Results
A total of 166,566 women were eligible for our analysis after exclusion of 56,734 women owing to an unknown diagnosis of antepartum anemia, 3938 women owing to multiple gestations, 302 women owing to congenital anomalies, and 1052 women owing to stillbirth (Figure). Of the remaining women, 10,217 (6.1%) were diagnosed with antepartum anemia. Women with anemia were more likely to be younger (26.2±6.2 vs 27.7±6.2 years; P<.001), non-Hispanic Black (33.7% vs 24.9%; P<.001), single (51.3% vs 37.2%; P<.001), multiparous (61.3% vs 59.6%; P=.001), and have a higher prepregnancy BMI (25.9±6.5 vs 25.4±6.2 kg/m2; P<.001) than women without anemia (Table 1). In contrast, women without anemia were more likely to be privately insured (58.8% vs 48.1%; P<.001), of Hispanic ethnicity (19.5% vs 12.8%; P<.001), and have a history of pregestational diabetes (2.5% vs 1.5%; P<.001) (Table 1).
FIGURE. Flowchart.
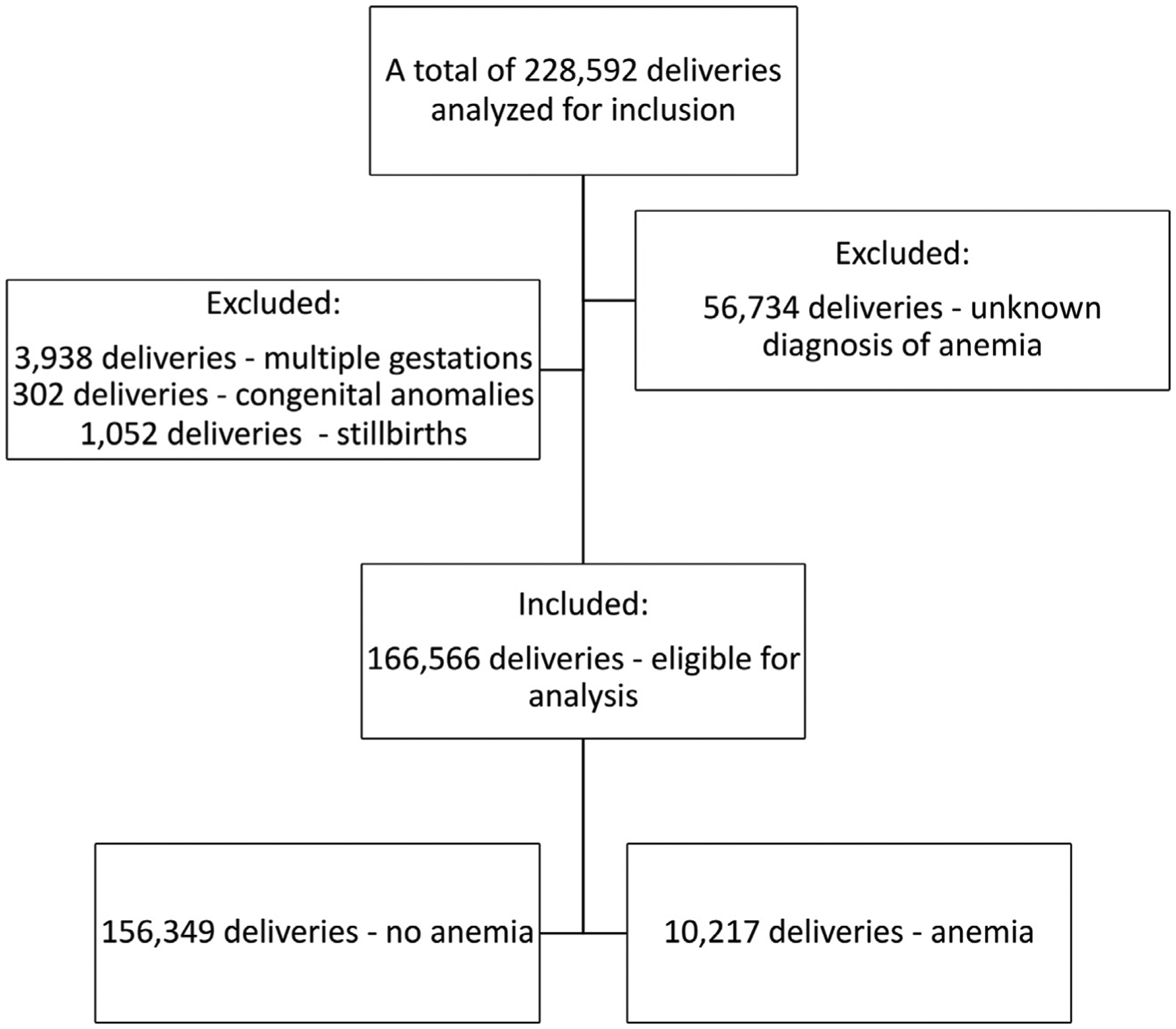
Eligibility criteria for subject selection.
TABLE 1.
Baseline characteristics of women with and without anemia
| Maternal characteristics | No anemia (n=156,349) | Anemia (n=10,217) | P value |
|---|---|---|---|
| Maternal age (y) (n=166,467) | 27.7±6.2a | 26.2±6.2a | <.001a |
| Prepregnancy BMI (kg/m2) (n=121,460) | 25.4±6.2a | 25.9±6.5a | <.001a |
| Nulliparity (n=166,566) | 63,082 (40.4)a | 3957 (38.7)a | .001a |
| Race and ethnicity (n=166,565) | <.001a | ||
| White | 71,231 (45.6)a | 4724 (46.2)a | |
| Non-Hispanic Black | 38,989 (24.9)a | 3444 (33.7)a | |
| Hispanic | 30,441 (19.5)a | 1312 (12.8)a | |
| Other | 15,687 (10.0)a | 737 (7.2)a | |
| Marital status (n=163,190) | <.001a | ||
| Married | 93,850 (61.2)a | 4673 (46.4)a | |
| Divorced | 2379 (1.6)a | 240 (2.4)a | |
| Single | 56,884 (37.2)a | 5164 (51.3)a | |
| Education status (n=68,716) | .001a | ||
| More than HS diploma | 32,493 (49.8)a | 1603 (46.6)a | |
| Less than HS diploma | 12,781 (19.6)a | 722 (21.0)a | |
| HS diploma | 20,000 (30.6)a | 1117 (32.5)a | |
| Insurance (n=143,834) | <.001a | ||
| Private | 78,732 (58.8)a | 4780 (48.1)a | |
| Public | 53,182 (39.7)a | 4982 (50.2)a | |
| Self-pay | 1992 (1.5)a | 166 (1.7)a | |
| History of cesarean delivery (n=154,917) | 21,503 (14.5)a | 1706 (18.4)a | <.001a |
| Pregestational diabetes (n=166,565) | 3927 (2.5)a | 152 (1.5)a | <.001a |
| Chronic hypertension (n=152,293) | 4825 (3.4) | 301 (3.1) | .173 |
| Asthma (n=166,565) | 9756 (6.2)a | 972 (9.5)a | <.001a |
| Gestational diabetes (n=148,848) | 4728 (3.4)a | 302 (3.0)a | .016a |
| Tobacco use (n=166,566) | 8906 (5.7) | 552 (5.4) | .214 |
| Alcohol use (n=166,566) | 3244 (2.1)a | 343 (3.4)a | <.001a |
Data are presented as number (percentage) or mean±standard deviation.
BMI, body mass index; HS, high school.
Denotes statistical significance.
Maternal anemia was significantly associated with several adverse maternal outcomes in the univariable analysis including the SMM composite outcome (7.5% vs 4.0%; P<.001), maternal death (0.04% vs 0.01%; P<.001), requirement for transfusion during labor and the postpartum period (2.9% vs 1.0%; P<.001 and 6.7% vs 3.7%; P<.001, respectively), hysterectomy (0.3% vs 0.1%; P<.001), PPH (13.8% vs 6.9%; P<.001), infectious morbidity (3.7% vs 3.2%; P<.001), cesarean delivery (36.7% vs 28.4%; P<.001), SMM composite excluding transfusion (1.8% vs 0.7%; P<.001), and preterm delivery (12.9% vs 12.0%; P=.005) among other adverse outcomes as listed in Table 2.
TABLE 2.
Maternal outcomes
| Outcome | No anemia (n=156,349) | Anemia (n=10,217) | P value |
|---|---|---|---|
| SMM composite outcomea (n=166,566) | 6269 (4.0)b | 761 (7.5)b | <.001b |
| Maternal death (n=134,620) | 7 (0.01)b | 4 (0.04)b | <.001b |
| Eclampsia (n=166,566) | 142 (0.1)b | 19 (0.2)b | .003b |
| Postpartum thrombosis (n=110,635) | 66 (0.1)b | 18 (0.2)b | <.001b |
| Antepartum thrombosis (n=134,576) | 329 (0.3)b | 58 (0.6)b | <.001b |
| Transfusion during labor (n=110,635) | 977 (1.0)b | 246 (2.9)b | <.001b |
| Transfusion postpartum (n=130,427) | 4557 (3.7)b | 563 (6.7)b | <.001b |
| Hysterectomy (n=166,566) | 79 (0.1)b | 30 (0.3)b | <.001b |
| Maternal ICU admission (n=166,565) | 868 (0.6) | 64 (0.6) | .350 |
| SMM composite without transfusion (n=110,635) | 697 (0.7)b | 156 (1.8)b | <.001b |
| Blood loss (mL) (n=118,081) | 451.1±257.0b | 617.0±10,531.0b | <.001b |
| Postpartum hemorrhage (n=166,566) | 10,785 (6.9)b | 1405 (13.8)b | <.001b |
| Preeclampsia or eclampsia or HELLP (n=166,566) | 5856 (3.8)b | 565 (5.5)b | <.001b |
| Cesarean delivery (n=166,566) | 44,416 (28.4)b | 3754 (36.7)b | <.001b |
| Composite infectious morbidityc (n=166,566) | 5027 (3.2)b | 378 (3.7)b | .007b |
| Wound infection (n=110,635) | 564 (0.6)b | 90 (1.1)b | <.001b |
| Antepartum chorioamnionitis (n=93,431) | 1052 (1.2)b | 144 (2.3)b | <.001b |
| Intrapartum chorioamnionitis (n=166,565) | 4169 (2.7) | 261 (2.6) | .496 |
| Endometritis (n=67,754) | 498 (0.8) | 49 (0.8) | .801 |
| GA at delivery (n=166,566) | 38.5±2.3 | 38.5±2.4 | .358 |
| Preterm delivery (n=166,566) | 18,716 (12.0)b | 1318 (12.9)b | .005b |
Data are presented as number (percentage) or mean±standard deviation.
GA, gestational age; HELLP, hemolysis, elevated liver enzymes, low platelet count; ICU, intensive care unit; SMM, severe maternal morbidity.
Composite includes maternal death, eclampsia, thrombosis, transfusion, hysterectomy, and ICU admission;
Denotes statistical significance;
Composite includes wound infection, chorioamnionitis, and endometritits.
Regarding our primary composite neonatal outcome, 15.5% (vs 13.3%; P<.001) of infants born to mothers with anemia experienced an adverse neonatal outcome, most commonly NICU admission (14.9% vs 12.6%; P<.001). Additional adverse neonatal outcomes that were noted more frequently in infants of mothers with anemia included antenatal and antepartum fetal distress (8.0% vs 5.2%; P<.001 and 7.4% vs 6.3%; P<.001 respectively) and birthweight less than 2500 g (9.8% vs 9.0%; P=.005) (Table 3). Anemia in mothers led to lower rates of RDS (2.9% vs 3.3%; P=.028) and neonatal transfusions (0.1% vs 0.2%; P=.018) (Table 3).
TABLE 3.
Neonatal outcomes
| Neonatal Outcome | No anemia (n=156,349) | Anemia (n=10,217) | P value |
|---|---|---|---|
| Composite neonatal outcomea (n=166,566) | 20,716 (13.3)b | 1579 (15.5)b | <.001b |
| 5-min Apgar score <7 (n=166,566) | 2816 (1.8)b | 227 (2.2)b | .002b |
| HIE (n=165,666) | 25 (0.02) | 2 (0.02) | .777 |
| RDS (n=166,566) | 5153 (3.3)b | 296 (2.9)b | .028b |
| NEC (n=164,382) | 218 (0.2) | 24 (0.2) | .216 |
| Seizures (n=164,382) | 305 (0.2) | 24 (0.2) | .395 |
| ICH (n=166,566) | 463 (0.3) | 26 (0.3) | .451 |
| Neonatal sepsis (n=166,566) | 4146 (2.7) | 287 (2.8) | .339 |
| PVH or IVH (n=166,566) | 877 (0.6) | 60 (0.6) | .730 |
| Neonatal death (n=150,739) | 466 (0.3) | 22 (0.2) | .065 |
| NICU admission (n=166,566) | 19,676 (12.6)b | 1525 (14.9)b | <.001b |
| Antenatal fetal distress (n=166,566) | 8088 (5.2)b | 819 (8.0)b | <.001b |
| Intrapartum fetal distress (n=166,565) | 9861 (6.3)b | 769 (7.4)b | <.001b |
| Birthweight (g) (n=165,412) | 3236.5±601.1 | 3237.8±613.9 | .825 |
| Birthweight <2500 g (n=166,566) | 14,045 (9.0)b | 1001 (9.8)b | .005b |
| Neonatal anemia or transfusion (n=166,566) | 3231 (2.1) | 225 (2.2) | .351 |
| Neonatal anemia (n=166,566) | 3116 (2.0) | 223 (2.2) | .185 |
| Neonatal transfusion (n=166,566) | 236 (0.2)b | 6 (0.1)b | .018b |
Data are presented as number (percentage) or mean±standard deviation.
HIE, hypoxic ischemic encephalopathy; ICH, intracranial hemorrhage; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; PVH, periventricular hemorrhage; RDS, respiratory distress syndrome.
Composite includes 5-minute Apgar score of <7, HIE, RDS, NEC, seizures, ICH, neonatal sepsis, PVH/IVH, neonatal death, NICU admission;
Denotes statistical significance.
In the multivariable logistic regression, after adjusting for confounders, the following remained persistently and independently associated with maternal anemia: SMM (adjusted odds ratio [aOR], 2.44; 95% confidence interval [CI], 1.86–2.23), maternal death (aOR, 18.10; 95% CI, 2.48–131.61), antepartum and postpartum thrombosis (aOR, 2.95; 95% CI, 2.09–4.16 and aOR, 2.59; 95% CI, 1.26–5.33), transfusion during labor and the postpartum period (aOR, 3.16; 95% CI, 2.61–3.83 and aOR, 1.84; 95% CI, 1.66–2.05, respectively), hysterectomy (aOR, 7.66; 95% CI, 4.57–12.85), ICU admission (aOR, 4.07; 95% CI, 2.87–12.85), PPH (aOR, 4.07; 95% CI, 2.87–5.75), preeclampsia, eclampsia, or HELLP syndrome (aOR, 1.33; 95% CI, 1.18–1.50), cesarean delivery (aOR, 1.47; 95% CI, 1.37–1.57), infectious morbidity (aOR, 1.42; 95% CI, 1.24–1.61), and SMM composite excluding transfusion (aOR, 3.40; 95% CI, 2.73–4.22) (Table 4). The composite neonatal outcome, NICU admission, and antenatal fetal distress also remained significantly associated with maternal anemia during gestation after controlling for confounders (aOR, 1.14; 95% CI, 1.06–1.23 and aOR, 1.16; 95% CI, 1.07–1.25 and aOR, 1.15; 95% CI, 1.02–1.30, respectively) (Table 5).
TABLE 4.
Association of maternal anemia with maternal outcomes
| Maternal outcome | Unadjusted OR | 95% CI | Adjusted ORa | 95% CI |
|---|---|---|---|---|
| SMM composite outcomeb | 1.93c | 1.78–2.08c | 2.04c | 1.86–2.23c |
| Maternal death | 7.71c | 2.26–26.35c | 18.10c | 2.48–131.61c |
| Eclampsia | 2.05c | 1.27–3.31c | 1.74 | 0.89–3.40 |
| Antepartum thrombosis | 2.30c | 1.74–3.04c | 2.95c | 2.09–4.16c |
| Postpartum thrombosis | 3.30c | 1.96–5.55c | 2.59c | 1.26–5.33c |
| Transfusion in labor | 3.10c | 2.69–3.57c | 3.16c | 2.61–3.83c |
| Transfusion postpartum | 1.85c | 1.69–2.02c | 1.84c | 1.66–2.05c |
| Hysterectomy | 5.83c | 3.82–8.87c | 7.66c | 4.57–12.85c |
| Maternal ICU admission | 1.13 | 0.88–1.46 | 4.07c | 2.87–5.75c |
| SMM composite without transfusion | 2.73c | 2.29–3.25c | 3.40c | 2.73–4.22c |
| Postpartum hemorrhage | 2.15c | 2.03–2.28c | 2.08c | 1.94–2.24c |
| Preeclampsia or eclampsia or HELLP | 1.50c | 1.38–1.64c | 1.33c | 1.18–1.50c |
| Cesarean delivery | 1.49c | 1.40–1.53c | 1.47c | 1.37–1.57c |
| Composite infectious morbidityd | 1.16c | 1.04–1.29c | 1.42c | 1.24–1.61c |
| Wound infection | 1.94c | 1.55–2.42c | 2.17c | 1.71–2.76c |
| Intrapartum chorioamnionitis | 0.96 | 0.84–1.09 | 1.18c | 1.01–1.38c |
| Antepartum chorioamnionitis | 1.88c | 1.57–2.24c | 1.68c | 1.26–2.23c |
| Endometritis | 0.96 | 0.72–1.29 | 1.10 | 0.74–1.65 |
| Preterm delivery | 1.09c | 1.03–1.16c | 1.05 | 0.97–1.14 |
CI, confidence interval; HELLP, hemolysis, elevated liver enzymes, and low platelet counts; ICU, intensive care unit; OR, odds ratio; SMM, severe maternal morbidity.
Confounders controlled for were maternal age, body mass index, nulliparity, maternal race, marital status, insurance, diabetes, asthma, history of cesarean delivery, and alcohol use;
Composite includes maternal death, eclampsia, thrombosis, transfusion, hysterectomy, ICU admission;
Denotes statistical significance;
Composite includes wound infection, chorioamnionitis, endometritits.
TABLE 5.
Association of maternal anemia with neonatal outcomes
| Neonatal outcome | Unadjusted OR | 95% CI | Adjusted ORa | 95% CI |
|---|---|---|---|---|
| Composite neonatal outcomeb | 1.20c | 1.13–1.27c | 1.14c | 1.06–1.23c |
| 5-min Apgar score of <7 | 1.24c | 1.08–1.42c | 1.21 | 1.00–1.47 |
| HIE | 1.23 | 0.29–5.20 | 1 | –– |
| RDS | 0.88c | 0.78–0.99c | 0.97 | 0.83–1.12 |
| NEC | 1.30 | 0.86–1.97 | 1.14 | 0.65–2.03 |
| Seizures | 1.19 | 0.79–1.82 | 1.13 | 0.64–1.99 |
| ICH | 0.86 | 0.58–1.28 | 0.88 | 0.52–1.49 |
| Neonatal sepsis | 1.06 | 0.94–1.20 | 1.14 | 0.97–1.33 |
| PVH or IVH | 1.05 | 0.81–1.36 | 1.20 | 0.84–1.69 |
| Neonatal death | 0.67 | 0.44–1.03 | 0.70 | 0.39–1.26 |
| NICU admission | 1.22c | 1.15–1.29c | 1.16c | 1.07–1.25c |
| Antenatal fetal distress | 1.60c | 1.48–1.72c | 1.15c | 1.02–1.30c |
| Intrapartum fetal distress | 1.21c | 1.12–1.30c | 0.98 | 0.88–1.09 |
| Birthweight <2500 g | 1.10c | 1.03–1.18c | 1.03 | 0.94–1.14 |
| Neonatal anemia or transfusion | 1.07 | 0.93–1.22 | 1.10 | 0.91–1.31 |
| Neonatal anemia | 1.10 | 0.96–1.26 | 1.12 | 0.93–1.34 |
| Neonatal transfusion | 0.39c | 0.17–0.87c | 0.50 | 0.18–1.36 |
CI, confidence interval; HIE, hypoxic ischemic encephalopathy; ICH, intracranial hemorrhage; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; OR, odds ratio; PVH or IVH, periventricular or intraventricular hemorrhage; RDS, respiratory distress syndrome.
Controlled for body mass index, nulliparity, maternal age, maternal race, marital status, insurance, diabetes, asthma, history of cesarean delivery, alcohol use;
Composite includes 5-minute Apgar score of <7, HIE, RDS, NEC, seizures, ICH, neonatal sepsis, PVH or IVH, neonatal death, and NICU admission;
Denotes statistical significance.
Comment
Principal findings
Our study demonstrated an increase in the rates of several adverse maternal outcomes in women with antenatally diagnosed anemia, including notably higher rates of SMM and maternal death. Rates of SGA infants and preterm delivery were not increased.
Results
Anemia was independently associated with SMM and maternal death after controlling for confounders. The direct and indirect contribution of anemia to maternal death or near-misses has been demonstrated previously in low-resource settings, but also in large data set analyses in high-resource settings such as Canada, Scotland, and France, which showed similar findings.16,21–30 Our study further demonstrated the role of anemia as an independent risk factor for higher rates of blood transfusions, PPH, need for hysterectomy, preeclampsia, cesarean delivery, and infectious morbidity, which is consistent with previous publications.3,4,9,16,23,26,29,31
Women with anemia are less tolerant of greater volumes of blood loss than women without anemia because of their lower reserves of red blood cells, thus increasing their risks for transfusion, hysterectomy, and ICU admission as seen in our study and previous studies.3,9,16,30 However, the independent association of antenatally diagnosed maternal anemia with PPH, hypertensive disorders of pregnancy, and infectious morbidity warrant further attention, because these comprise 3 of the top causes of pregnancy-related deaths throughout the world in both high- and low-resource settings and are not frequently considered in conjunction with anemia.20,32,33 Our study found a 2-fold greater risk for PPH in women with anemia, consistent with previous studies.9,23,30,31 This phenomenon is explained by impaired uterine muscle strength secondary to a decrease in uterine perfusion seen in patients with decreased iron stores.34 Regarding hypertensive disorders of pregnancy, a 0.33-fold increase in these diagnoses was seen in women with anemia, which is also similar to findings of previous studies, and is most likely related to decreased uterine and placental perfusion.7,9,34 Because of this finding, an additional logistic regression was performed to evaluate the impact of chronic hypertension, particularly with regard to eclampsia and other hypertensive disorders of pregnancy by including it in the model, and the results were essentially unchanged from those seen in Table 4. Finally, the higher rates of infectious morbidity observed in women with anemia in our study are supported by previous studies9,30 and likely explained by inadequate blood flow and oxygen delivery necessary for wound healing and infection control.35
Regarding neonatal outcomes, we found that infants born to women with anemia experienced mildly increased rates of adverse outcomes including NICU admission and antenatal fetal distress after controlling for confounders. Other adverse neonatal outcomes that were previously reported as associated with anemia were not higher in our population and include SGA infants, preterm delivery, or birthweight <2500 g.1–4,9,13,36
Clinical implications
It is well recognized that maternal mortality is rising in the United States32,33,37 prompting nationwide investigations into potentially modifiable risk factors. Our study sought to ascertain whether anemia should be considered as a specific target for maternal mortality prevention based on its association with SMM. When considering the independent relationship identified in our study between anemia and increased rates of PPH, hypertensive disorders of pregnancy, and infectious morbidity, plus the significantly increased rates of maternal death and SMM,15,38 the impact of anemia on maternal mortality cannot be overlooked. Importantly, based on these findings, it is consequently encouraging that the identification and treatment of anemia may serve as a promising preventative measure for seemingly distinct causes of maternal death.
A 2015 Cochrane review that evaluated the impact of iron supplementation on maternal and neonatal outcomes in women with anemia stated that although there is a reduction in the risk for adverse outcomes with iron supplementation, the quality of evidence is low.39 Importantly, iron supplementation was found to be effective in reducing anemia and iron deficiency at term when identified antenatally.40 Our large-scale assessment of a US-based population serves as further evidence of the importance of anemia identification and treatment, particularly acknowledging that iron deficiency, which comprises 75% of anemia in pregnancy, can be appropriately treated. With this information, consideration should be taken into updating national guidelines regarding anemia identification and treatment during pregnancy. For example, a third trimester hemoglobin level could be obtained at the time of routine glucola screening to allow for adequate time to improve hemoglobin levels before delivery. This practice approach is currently inconsistent throughout the United States with regional variation.18
Finally, vitally important to the prevention of maternal mortality and SMM is the importance of highlighting higher rates of these unfavorable outcomes in women identifying as non-Hispanic Black.33,37,41,42 Our study found non-Hispanic Black women were more likely to have anemia than any other racial group (34.8% of women with anemia identified as non-Hispanic Black vs 26.0% of women without anemia; P<.001). Although maternal race was controlled for in the regression analysis, additional contributions of sickle cell disease, biases in anemia identification and treatment, and access to care, among other concerns related to maternal race that were not identified and may have affected outcomes, cannot be discounted and should be further explored.
Research implications
This is the first large-scale examination of the effect of anemia specifically focused on adverse maternal outcomes in a US-based population. Unfortunately, the majority of studies on this topic are retrospective. Therefore, future researchers should consider a prospective trial evaluating the impact of widespread identification and proper treatment of anemia on pregnancy outcomes. Furthermore, additional research is needed to examine whether antenatal anemia that presents on admission for delivery contributes more strongly to adverse outcomes or whether women diagnosed antenatally have additional unidentified risk factors that lead to these adverse outcomes. Finally, from a public health perspective, isolating the impact of inadequate access to iron supplements or IV iron and reasons for noncompliance to iron therapy would allow for targeted interventions.
Strengths and limitations
Strengths of this study include the large sample size from the CSL database, which allowed analysis of multiple hospital systems across the United States. A limitation of our study is the large number of women (56,734; 24.8% of the data set) who were excluded from the analysis secondary to an unknown antepartum anemia status, meaning that in the CSL data file under the heading “antenatal anemia,” the value was labeled as “unknown” or “missing.” To assess bias based on this limitation, we evaluated the baseline characteristics of those with information regarding antenatal anemia diagnosis and those without (Appendix 2). Of the 12 hospital systems that contributed data to the CSL, 4 did not collect information on antenatal anemia, leading to differences between groups as shown in Appendix 2. A further limitation is that the CSL does not contain data on all 21 SMM indicators, becuase data collection was completed before publication of the original SMM description.20 Therefore, the variable of “ICU admission” was used as a surrogate for these diagnoses (listed in Appendix 1) because these would typically warrant ICU admission in a pregnant patient. In addition, we were unable to stratify our data based on the type of anemia (microcytic, normocytic, macrocytic), on whether patients had a history of anemia of chronic disease, on chronic kidney disease status, or sickle cell anemia, which are significant contributors to poor obstetrical outcomes, and on whether or not women received treatment for anemia. These limitations reduced our ability to extrapolate how these disease states may be modified at a population level.
In addition, we did not find clinically significant increased rates of SGA neonates, preterm delivery, or low birthweight as seen in similar previous studies.1–4,9,13,36 We suspect that these dissimilar findings are related to the inclusion of stillbirths, multiple gestations, and/or congenital anomalies in previous similar studies, which were excluded in our study. Although this does not allow for direct comparison, our results are unique in that we have identified that the risks for preterm delivery, SGA, and a birthweight <2500 g may be diminished in pregnancies with anemia unaffected by stillbirth, multiple gestations, and/or congenital anomalies.
In comparison with similar studies, our study did not evaluate separate cohorts based on the severity of anemia because there were no available hemoglobin or hematocrit levels in this database.9,23,30 Although this permitted for a larger sample size, it limited the applicability of the findings to specific patients and their respective degrees of anemia severity. However, despite this difference, we identified similar adverse outcomes as previous studies that stratified by severity,9,23,30 demonstrating the likely negative impact of anemia at all hemoglobin levels. In fact, Ray et al16 previously identified increasing rates of SMM starting at the lower end of normal hemoglobin levels. Also, the gestational age at which anemia was diagnosed was not available within the CSL data set, which would assist in clarifying recommendations for timing of screening. Nevertheless, previous studies have demonstrated negative impacts of anemia regardless of trimester at diagnosis.1,13,14,23,30
Conclusion
Women with antepartum anemia experienced higher rates of SMM, maternal death, and other adverse maternal outcomes known to contribute to maternal mortality. Diagnosis and treatment of anemia during the antepartum period is essential to optimize maternal health and may contribute to a decrease in maternal morbidity and mortality.
Supplementary Material
AJOG MFM at a Glance.
Why was this study conducted?
The study was conducted to evaluate the impact of maternal anemia on severe maternal morbidity in a large US population.
Key findings
Women diagnosed with anemia during pregnancy were more likely to experience a severe complication at the time of delivery, including higher rates of postpartum hemorrhage, thrombosis, transfusion, hysterectomy, intensive care unit admission, infection, hypertensive disorders of pregnancy, cesarean delivery, and maternal death.
What does this add to what is known?
This study highlights the breadth of maternal complications associated with anemia during pregnancy in the United States, particularly maternal mortality and severe maternal morbidity. Furthermore, it highlights a potential area of intervention for reducing maternal morbidity and mortality.
ACKNOWLEDGMENTS
We would like to acknowledge the following institutions that were involved in the Consortium for Safe Labor, in alphabetical order: Baystate Medical Center, Springfield, Massachusetts; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, California; Christiana Care Health System, Newark, Delaware; The EMMES Corporation, Rockville, Maryland (Data Coordinating Center); Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, Indiana; Intermountain Healthcare and the University of Utah, Salt Lake City, Utah; Maimonides Medical Center, Brooklyn, New York; Metro Health Medical Center, Cleveland, Ohio; Summa Health System, Akron City Hospital, Akron, Ohio; University of Illinois at Chicago, Chicago, Illinois; University of Miami, Miami, Florida; and University of Texas Health Science Center at Houston, Houston, Texas.
This study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K08HL150340 (J.J.M.).
Appendix 1
**ICU admission functioned as a surrogate for the following:
Acute myocardial infarction
Aneurysm
Acute renal failure
Adult respiratory distress syndrome
Amniotic fluid embolism
Cardiac arrest/ventricular fibrillation/Conversion of cardiac rhythm
Disseminated intravascular coagulation
Heart failure/arrest during surgery
Peurperal cerebrovascular disorders
Pulmonary edema/acute heart failure
Severe anesthesia complications
Sepsis
Shock
Sickle cell disease with crisis
Temporary tracheostomy
Ventilation
Appendix 2
Baseline Characteristics of Women with and without Anemia Information
| Maternal Characteristic | No Anemia Information (N=56,734) | Anemia Information (N=171,704) | p-value |
|---|---|---|---|
| Maternal age (years) (N=228,115) | 27.5 ± 6.2 | 27.7 ± 6.2 | <0.001 |
| Prepregnancy BMI (kg/m2) (N=151,620) | 25.6 ± 6.3 | 25.4 ± 6.2 | <0.001 |
| Nulliparity (N=228,438) | 22,065 (38.9%) | 69,206 (40.3%) | <0.001 |
| Race (N=228,437) | <0.001 | ||
| White Non-Hispanic | 34,834 (61.4%) | 78,390 (45.7%) | |
| Non-Hispanic Black | 7,528 (13.3%) | 43,864 (25.5%) | |
| Hispanic | 7,138 (12.6%) | 32,578 (19.0%) | |
| Other | 7.234 (12.7%) | 16,871 (9.8%) | |
| Marital status (N=220,987) | <0.001 | ||
| 1 Married | 32,651 (61.8%) | 101,795 (60.5%) | |
| 2 Divorced | 923 (1.8%) | 2,683 (1.6%) | |
| 3 Single | 19,226 (36.4%) | 63,709 (37.9%) | |
| Education status (N=71,837) | <0.001 | ||
| Less than HS diploma | 616 (45.4%) | 13,808 (19.6%) | |
| HS diploma | 462 (34.0%) | 21,604 (30.7%) | |
| More than HS diploma | 280 (20.6%) | 35,067 (49.7%) | |
| Insurance (N=204,392) | <0.001 | ||
| Private | 41,707 (74.4%) | 86,326 (58.2%) | |
| Public | 13,819 (24.7%) | 59,821 (40.3%) | |
| Self-pay | 500 (0.9%) | 2,219 (1.5%) | |
| History of cesarean (N=215,219) | 7,350 (13.3%) | 23,979 (15.0%) | <0.001 |
| Pregestational diabetes (N=220,560) | 514 (1.1%) | 4,263 (2.5%) | <0.001 |
| Chronic hypertension (N=205,893) | 759 (1.6%) | 5,369 (3.4%) | <0.001 |
| Asthma (N=220,560) | 4,270 (8.7%) | 11,124 (6.5%) | <0.001 |
| Gestational diabetes (N=202,169) | 2,503 (5.1%) | 5,220 (3.4%) | <0.001 |
| Tobacco use (N=228,438) | 5,500 (9.7%) | 9,747 (5.7%) | <0.001 |
| Alcohol use (N=228,438) | 466 (0.8%) | 3,714 (2.2%) | <0.001 |
BMI=body mass index, HS=high school
Data displayed as number (percentage) or mean ± SD
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajogmf.2021.100395.
The authors report no conflict of interest.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The findings of this study were accepted for presentation at the 67th annual scientific meeting of the Society for Reproductive Investigation, Vancouver, British Columbia, Canada, March 11–14, 2020.
References
- 1.Ren A, Wang J, Ye RW, Li S, Liu JM, Li Z. Low first-trimester hemoglobin and low birth weight, preterm birth and small for gestational age newborns. Int J Gynaecol Obstet 2007; 98:124–8. [DOI] [PubMed] [Google Scholar]
- 2.Vural T, Toz E, Ozcan A, Biler A, Ileri A, Inan AH. Can anemia predict perinatal outcomes in different stages of pregnancy? Pak J Med Sci 2016;32:1354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drukker L, Hants Y, Farkash R, Ruchlemer R, Samueloff A, Grisaru-Granovsky S. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for cesarean section and adverse maternal and neonatal outcomes. Transfusion 2015;55:2799–806. [DOI] [PubMed] [Google Scholar]
- 4.Levy A, Fraser D, Katz M, Mazor M, Sheiner E. Maternal anemia during pregnancy is an independent risk factor for low birthweight and preterm delivery. Eur J Obstet Gynecol Reprod Biol 2005;122:182–6. [DOI] [PubMed] [Google Scholar]
- 5.Agbozo F, Abubakari A, Der J, Jahn A. Maternal dietary intakes, red blood cell indices and risk for anemia in the first, second and third trimesters of pregnancy and at predelivery. Nutrients 2020;12:777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ACOG Practice Bulletin No. 105: bariatric surgery and pregnancy. Obstet Gynecol 2009;113:1405–13. [DOI] [PubMed] [Google Scholar]
- 7.Ali AA, Rayis DA, Abdallah TM, Elbashir MI, Adam I. Severe anaemia is associated with a higher risk for preeclampsia and poor perinatal outcomes in Kassala hospital, eastern Sudan. BMC research notes. 2011;4:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geelhoed D, Agadzi F, Visser L, et al. Maternal and fetal outcome after severe anemia in pregnancy in rural Ghana. Acta Obstet Gynecol Scand 2006;85:49–55. [DOI] [PubMed] [Google Scholar]
- 9.Smith C, Teng F, Branch E, Chu S, Joseph KS. Maternal and perinatal morbidity and mortality associated with anemia in pregnancy. Obstet Gynecol 2019;134:1234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep 1998;47:1–29. [PubMed] [Google Scholar]
- 11.Sifakis S, Pharmakides G. Anemia in pregnancy. Ann N Y Acad Sci 2000;900:125–36. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz KM, Ingardia CJ, Borgida AF. Anemia in pregnancy. Clin Lab Med 2013;33: 281–91. [DOI] [PubMed] [Google Scholar]
- 13.Menon KC, Ferguson EL, Thomson CD, et al. Effects of anemia at different stages of gestation on infant outcomes. Nutrition 2016; 32:61–5. [DOI] [PubMed] [Google Scholar]
- 14.Georgieff MK. Iron deficiency in pregnancy. Am J Obstet Gynecol 2020;223:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. 2021. Available at: https://www.cdc.gov/reproductivehealth/MaternalInfantHealth/SevereMaternalMorbidity.html.AccessedAugust 5, 2020.
- 16.Ray JG, Davidson AJF, Berger H, Dayan N, Park AL. Haemoglobin levels in early pregnancy and severe maternal morbidity: population-based cohort study. BJOG 2020;127:1154–64. [DOI] [PubMed] [Google Scholar]
- 17.Siu AL. U.S. Preventive Services Task Force. Screening for iron deficiency anemia and iron supplementation in pregnant women to improve maternal health and birth outcomes: US Preventive Services Task Force recommendation statement. Ann Intern Med 2015;163:529–36. [DOI] [PubMed] [Google Scholar]
- 18.Marcewicz LH, Anderson BL, Byams VR, Grant AM, Schulkin J. Screening and treatment for Iron deficiency Anemia in women: results of a survey of obstetrician-gynecologists. Matern child health j. 2017;21:1627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Troendle J, Reddy UM, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol 2010;203. 326.e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol 2012;120:1029–36. [DOI] [PubMed] [Google Scholar]
- 21.Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutri. 2001;131:604S–15S. [DOI] [PubMed] [Google Scholar]
- 22.Alemu FM, Fuchs MC, Martin Vitale T, Abdalla Mohamed Salih M. Severe maternal morbidity (near-miss) and its correlates in the world’s newest nation: South Sudan. Int J Womens Health 2019;11:177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guignard J, Deneux-Tharaux C, Seco A, et al. Gestational anaemia and severe acute maternal morbidity: a population-based study. Anaesthesia 2021;76:61–71. [DOI] [PubMed] [Google Scholar]
- 24.Motlagh ME, Nasrollahpour Shirvani SD, Torkestani F, et al. The frequency of anemia and underlying factors among Iranian pregnant women from provinces with different maternal mortality rate. Iran J Public Health 2019;48:338–44. [PMC free article] [PubMed] [Google Scholar]
- 25.Zamané H, Sow HE, Kain DP, et al. Maternal mortality at the Dori Regional Hospital in Northern Burkina Faso, 2014–2016. Int J MCH AIDS 2019;7:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daru J, Zamora J, Fernández-Félix BM, et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. Lancet Glob Health 2018;6:e548–54. [DOI] [PubMed] [Google Scholar]
- 27.Bwana VM, Id SFR, Mremi IR, Lyimo EP, Mboera LEG. Patterns and causes of hospital maternal mortality in. PLoS One 2006;2019:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rathod AD, Chavan RP, Bhagat V, Pajai S, Padmawar A, Thool P. Analysis of near-miss and maternal mortality at tertiary referral centre of rural India. J Obstet Gynaecol India 2016; 66:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parks S, Hoffman MK, Goudar SS, et al. Maternal anaemia and maternal, fetal, and neonatal outcomes in a prospective cohort study in India and Pakistan. BJOG 2019; 126:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rukuni R, Bhattacharya S, Murphy MF, Roberts D, Stanworth SJ, Knight M. Maternal and neonatal outcomes of antenatal anemia in a Scottish population: a retrospective cohort study. Acta Obstet Gynecol Scand 2016;95: 555–64. [DOI] [PubMed] [Google Scholar]
- 31.Kavle JA, Stoltzfus RJ, Witter F, Tielsch JM, Khalfan SS, Caulfield LE. Association between anaemia during pregnancy and blood loss at and after delivery among women with vaginal births in Pemba Island, Zanzibar, Tanzania. J Health Popul Nutr 2008;26:232–40. [PMC free article] [PubMed] [Google Scholar]
- 32.Davis NL, Smoots AN, Goodman DA. Pregnancy-related deaths: data from 14 U.S. Maternal Mortality Review Committees, 2008–2017. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. Available at: https://www.cdc.gov/reproductivehealth/maternal-mortality/erase-mm/MMR-Data-Brief_2019-h.pdf.AccessedAugust 5, 2020. [Google Scholar]
- 33.Hill WC, Lindsay MK, Green VL. Executive summary and call to action: maternal mortality summit at National Medical Association meeting, July 28, 2019 in Honolulu, Hawaii Obstetrics and Gynecology Section of the National Medical Association. J Natl Med Assoc 2020;112:402–10. [DOI] [PubMed] [Google Scholar]
- 34.Soltan MH, Ibrahim EM, Tawfek M, Hassan H, Farag F. Raised nitric oxide levels may cause atonic postpartum hemorrhage in women with anemia during pregnancy. Int J Gynaecol Obstet 2012;116:143–7. [DOI] [PubMed] [Google Scholar]
- 35.Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malhotra M, Sharma JB, Batra S, Sharma S, Murthy NS, Arora R. Maternal and perinatal outcome in varying degrees of anemia. Int J Gynaecol Obstet 2002;79:93–100. [DOI] [PubMed] [Google Scholar]
- 37.Moaddab A, Dildy GA, Brown HL, et al. Health care disparity and pregnancy-related mortality in the United States, 2005–2014. Obstet Gynecol 2018;131:707–12. [DOI] [PubMed] [Google Scholar]
- 38.Say L, Pattinson RC, Gülmezoglu AM. WHO systematic review of maternal morbidity and mortality: the prevalence of severe acute maternal morbidity (near miss). Reprod Health 2004;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peña-rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2015:CD004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yakoob MY, Bhutta ZA. Effect of routine iron supplementation with or without folic acid on anemia during pregnancy. BMC Public Health 2011;11(Suppl3):S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen EE, Davis NL, Goodman D, et al. Racial/ethnic disparities in pregnancy-related deaths—United States, 2007–2016. MMWR Morb Mortal Wkly Rep 2019;68:762–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howell EA, Egorova N, Balbierz A, Zeitlin J, Hebert PL. Black-white differences in severe maternal morbidity and site of care. Am J Obstet Gynecol 2016;214. 122.e1–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


