Abstract
Background
The COVID‐19 pandemic has created unprecedented challenges in all fields of society with social, economic, and health‐related consequences worldwide.
In this context, gastroenterology patients and healthcare systems and professionals have seen their routines changed and were forced to adapt, adopting measures to minimize the risk of infection while guaranteeing continuous medical care to chronic patients.
Objective
At this point, it is important to evaluate the impact of the pandemic on this field to further improve the quality of the services provided in this context.
Methods/Results/Conclusion
We performed a literature review that summarizes the main aspects to consider in gastroenterology, during the pandemic crisis, and includes a deep discussion on the main changes affecting gastroenterology patients and healthcare systems, anticipating the pandemic recovery scenario with future practices and policies.
Keywords: COVID‐19, endoscopy, gastroenterology, inflammatory bowel disease, SARS‐CoV‐2, vaccination
INTRODUCTION
Since the emergence of the SARS‐CoV‐2 virus in December 2019,1 the COVID‐19 pandemic has spread globally with far‐reaching consequences on every echelon of society. As of 20 April 2021, COVID‐19 has infected over 140 million people and claimed at least 3 million lives.2 Controlling the pandemic has been at the forefront of the World Health Organization (WHO) and international communities. As countries implement public health reforms which reverberate through healthcare, social, education, travel, and economic sectors,3, 4, 5, 6 the gastroenterology community has also been forced to accommodate sweeping adaptations.
Specific to gastroenterology, stakeholders affected by COVID‐19 include patients, healthcare professionals (HCPs), researchers, societies, and health policy makers.7, 8, 9 On this setting, the susceptibility, monitoring, diagnosis, and treatment of patients with chronic gastrointestinal (GI) diseases are major concerns.10, 11, 12 Regarding diagnosis, endoscopy is one of the most affected procedures and the impact of the decrease of procedures is yet to be determined.13, 14
Gastroenterologists have seen their clinical routine disturbed by the pandemic with adaptations in patients' management and evidence of burnout and mental health among HCP.10, 15 Telemedicine became a reality,16, 17 and team and scientific meetings were adapted to virtual format,18 as well as medical training and learning.15
The vaccination process is now a priority, and the conditions in which GI patients shall be managed must be clarified. This article intends to provide a global perspective on the major changes that have been affecting gastroenterology during the pandemic, while providing a deep discussion on their impact on patients, healthcare systems, and professionals, considering all the lessons learned and the management plan for the pandemic, in the next years.
COVID‐19 AND THE ALIMENTARY TRACT—PATHOPHYSIOLOGY
SARS‐CoV‐2 infection is dependent on cell entry; this occurs via the binding of the viral spike protein to the angiotensin‐converting enzyme 2 (ACE‐2) receptor, and cleavage of S‐protein by transmembrane serine protease 2 (TMPRSS2). Although expressed in the respiratory tract, 19 ACE‐2 and TMPRSS2 are highly expressed in the brush border of enterocytes and the evidence of SARS‐CoV‐2 intestinal infections highlights the potential influence of the gut inflammatory response.20, 21 Indeed, multiple in vitro and in vivo animal studies showed that SARS‐CoV‐2 can enter and replicate in enterocytes.22, 23 This has been confirmed in several human studies through detection of viral RNA, subgenomic RNA, antigens, and virions in intestinal tissue samples. The prolonged detection of viral RNA in fecal samples, in about half of patients with COVID‐19 21, 23, 24 provides further evidence for the relation between SARS‐CoV‐2 and enterocytes. Viral RNA is detectable in stools, for a median time of 28 days, persisting for a mean of 11 days, after negative nasopharyngeal swab PCR testing.21, 25, 26, 27 In some cases, peak concentrations were higher than those in pharyngeal swabs. The analysis of human excrements in sewage content are being considered as a strategy to estimate the prevalence of COVID‐19 and evaluate emerging virus strains.28 These evidences of the presence of SARS‐CoV‐2 in intestinal tissues and fecal samples have raised concerns about a potential fecal‐oral transmission.21, 29, 30, 31 According to histological analyses, the replication of SARS‐CoV‐2 in enterocytes causes tissue inflammatory infiltration, usually without major injury.21, 23, 32 The possibility of gut inflammatory response is supported by the occurrence of diarrhea and increased concentrations of calprotectin in stools of patients with COVID‐19,33 and high concentrations of enterocyte‐specific cytokines (IL‐18), in severe COVID‐19 patients.23, 34 Fecal microbiota analyses have shown a significant and prolonged effect of SARS‐CoV‐2 infection on dysbiosis, including depletion of commensals and selection of opportunistic pathogens, which correlate with inflammatory markers and disease severity.35, 36
Multiple largescale meta‐analysis have reported GI symptoms and elevation of liver enzymes, in patients with COVID‐19. This arises either as a result of SARS‐CoV‐2 infection or of adverse events caused by drugs.37, 38 Diarrhea is the most common GI symptom (2%–16.5%); it can persist for 1–9 days and has been associated to viral RNA detection in stools.21, 23, 29 The mechanism of diarrhea is unknown and may involve changes in gut microbiota, gut epithelial inflammation, and release of virulent antigens.23, 39 Other common GI symptoms include nausea, vomiting, anorexia, and abdominal pain, whereas rare cases of GI bleeding and ischemic injury have been reported.29, 33, 40 In a propensity‐score matched study, COVID‐19 patients had higher rates of GI complications including mesenteric ischemia, suggesting a different phenotype for COVID‐19 when compared with conventional acute respiratory distress syndrome (ARDS).41 However, it remains inconclusive whether GI symptoms are related to severity of COVID‐19.21, 23, 42, 43 As GI signs may be present at the onset of the disease, COVID‐19 may be considered as a differential diagnosis, even in the absence of respiratory symptoms.44
Subacute or chronic diarrhea have been observed in 0.9%–10.5% of patients suffering from post‐acute COVID‐19.24, 45, 46 The subacute and long‐term consequences of COVID‐19 on the GI system, including post‐infectious irritable bowel syndrome (IBS), are still being studied (NCT04691895). A population‐based survey, including 2704 people from 33 countries, revealed that 5% of respondents developed IBS‐like symptoms during the first 3 months of the COVID‐19 pandemic. Patients with IBS prior to the COVID‐19 pandemic (11%) reported significantly worse emotional, social, and psychological well‐being, compared with non‐IBS respondents.47
GI symptoms can also be a consequence of the socio‐cultural changes that emerged during the COVID‐19 pandemic. In fact, lockdowns and social distancing are modifying behaviors that are being associated with unhealthy eating habits, decreased physical exercise, decreased patient interactions with medical services, increased anxiety and alcohol consumption (or relapse in abstinent patients). All these events may have negative impact on GI and liver health48, 49, 50 and are important to re‐emphasize to our patients.
COVID‐19 AND GASTROENTEROLOGY PRACTICE
The COVID‐19 pandemic has disrupted our medical routines and impacted a wide variety of medical activities, resulting in an exponential increase of telemedicine.51, 52 A US study revealed that, during the pandemic, 94% of GI/hepatology visits were virtual via telemedicine, compared to only 5% 2 weeks before the onset of COVID‐19.52
Overall, the pandemic is affecting general Gastroenterology services with impact on patients, HCPs, and policy makers.53 Outpatient care has evolved, and patients have seen their appointments delayed and their visiting rights restricted.
Healthcare systems were forced to implement measures to minimize the risk of virus spread: the services were reconfigured with changes affecting patients' triaging, healthcare personnel (redeployment, “shielding” of vulnerable HCP), medical and technical training, and Protective Personal Equipment (PPE). All this in a changing policy environment with tremendous pressures on policy makers to issue guidance on the pandemic and to manage the vaccination process.
COVID‐19 IN IBD
The increased susceptibility of IBD patients, per se, to infections was a matter of debate even before the pandemic crisis.54, 55 This increased risk can be modulated by many factors including medications such as steroids, immunosuppressive, or biologic therapies.56
Despite previous evidence, both physicians and patients have been facing challenges while unveiling how to adapt to COVID‐19.7 Reassuringly, IBD patients do not appear to be at increased risk of SARS‐CoV‐2 infection compared to the general population.57, 58, 59, 60, 61 The discussion has been focused on the overexpression of the ACE‐2 receptor in the colonic mucosa and its downregulation in the small bowel.62, 63 Available data seem to indicate that factors such as IBD phenotype, disease location, or the degree of mucosal inflammation do not influence the risk of infection. Although IBD does not increase risk of transmission, certain classes of treatments seem to be associated with increased severity and mortality from COVID‐19.64, 65
The outcome of the infection and factors affecting the risk of infection with SARS‐CoV‐2 are somehow conflicting. Data from the SECURE registry, a prospective, international, and collaborative database, showed that the sex‐standardized mortality ratio was similar to that of the general population,66 but updated data suggested that mortality in IBD might be higher (data not published).67 However, a separate multicenter analysis of 232 patients did not find differences in hospitalization or mortality risk.68, 69 In this context, the risk of severe COVID‐19 in patients with IBD (defined as ICU admission, use of mechanical ventilation, or death) seems to be driven by the same risk factors as in the general population. While age appears to confer additional prognostic risk as in the general population, this risk is not increased in IBD per se, 64, 66, 70, 71 as observed in subjects with concomitant non‐IBD comorbidities.58, 64, 66, 70
However, some factors demand particular attention. It has been demonstrated, across different cohorts, that steroids increase the risk of infection and that active disease should be considered as a risk factor.66, 68, 70 In addition, thiopurines have been identified as the major responsible for the increased risk of viral infections in IBD patients.54, 55, 56, 72 The SECURE‐IBD registry found that thiopurines either as monotherapy or in combination with anti‐TNF inhibitors were associated with more severe disease.73 Some authors have also reported an increased risk of severe disease among patients with ulcerative colitis (UC) 60, 70, 74 and in those receiving aminosalicylates,66 but these observations need further validation in population‐based studies. No further concerns have been reported with the remaining drugs, including small molecules like tofacitinib and biologics like ustekinumab and vedolizumab.73, 75, 76, 77
In spite of available evidence, management of IBD during COVID‐19 remains heterogenous.78, 79To harmonize management, societies, including European Crohn's and Colitis Organisation, the International Organisation for the study of Inflammatory Bowel Disease, and the American Gastroenterological Association, have published best practice recommendations for managing IBD during COVID‐19.8, 80, 81The use of biologics can be optimized with the following recommendations: (i) consider subcutaneous administration on new patients to reduce burden and contacts; (ii) avoid elective switching from infliximab infusions to subcutaneous anti‐TNF formulations, as it may increase the risk of relapse; (iii) consider withholding immunomodulator therapy to reduce infection risk in patients on combination therapy and deep remission in older patients; (iv) adopt therapeutic drug monitoring to guide decisions; (v) consider withholding anti‐TNF therapies for 2 weeks in patients in contact with a COVID‐19 patient; and (vi) consider withholding biologics in SARS‐CoV‐2 positive and/or COVID‐19 patients.
In addition, the British Society of Gastroenterology adapted the guidelines for acute severe UC, by means of a RAND panel, to face the challenges of the pandemic.82 The panel recommended that: (i) patients should be isolated during hospital stays; (ii) intravenous hydrocortisone shall be used with caution in patients with COVID‐19 pneumonia; (iii) colectomy shall not be delayed; and (iv) prophylactic anticoagulation post‐discharge is appropriate in patients with a positive SARS‐CoV‐2 swab.
Regarding the daily care of these patients, telemedicine has now arrived in the field of IBD, with new tools and devices that will enable the development of the forthcoming models of patients care.83, 84 The reduction of endoscopic procedures resulted in a maximum decrease of 46.3% in new diagnoses and in a decrease of 25.5% in indefinite and low‐grade dysplasia diagnoses.85
In terms of monitoring, noninvasive biomarkers have been included as targets of IBD management, in the recent STRIDE‐II recommendations.86 In this setting, it is expected that the implementation of remote monitoring, with PROMS and point of care tests, will become more widely utilized in the upcoming years87 (Figure 1).
FIGURE 1.
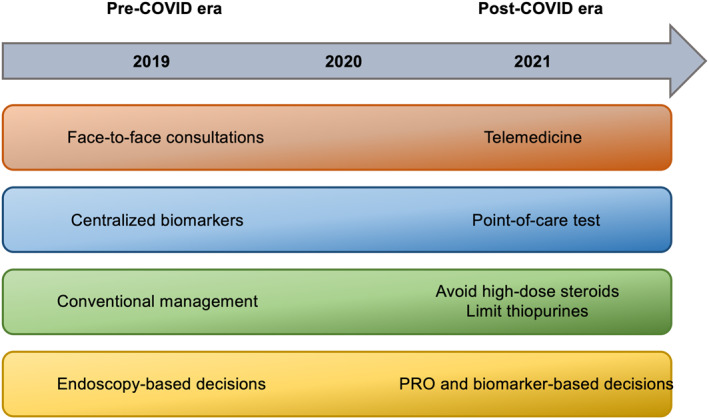
Main changes on the management of IBD patients during the COVID‐19 pandemic. IBD, inflammatory bowel disease; PRO, patient reported outcomes
COVID‐19 IN LIVER AND PANCREAS
COVID‐19 related liver injury
Within the liver, ACE‐2 is expressed predominantly in cholangiocytes (59.7% of cells) and to a lesser extent, hepatocytes (2.6% of cells).88 Liver injury associated with COVID‐19 is typically hepatocellular in nature with transaminitis.89, 90 Possible causative mechanisms include: direct hepatocytopathic effect of SARS‐CoV‐2, liver engorgement from increased pulmonary pressure, drug‐induced liver injury, or ischemic hepatitis.91 Two recent meta‐analyses showed that the prevalence of “COVID‐19 acute liver injury” in hospitalized patients was about 24%–27%, and that 2% of patients developed chronic liver disease (CLD). Acute liver injury was associated with poor outcomes and was found in 45% of patients with severe COVID‐19 and in 20% of non‐severe COVID‐19 patients.89, 90 An important sidenote here is that the definitions of “severe COVID‐19” or “acute liver injury” were heterogeneous across studies. Of note is also the increase of mortality and liver disease severity associated with the decrease of liver transplantation procedures due to patient's vulnerability, scarcity of deceased donor organs, and to imposed restrictions to decrease virus transmission.92, 93
Chronic liver disease
The overall mortality rate for COVID‐19 is estimated at 0%–2% in CLD patients,89, 90 with risk factors comprising cirrhosis, alcohol‐related liver disease (ALD), increasing age, obesity, and diabetes. Patients with metabolic‐associated fatty liver disease (MAFLD) may also be at higher risk.94 As expected, rates of acute‐on‐chronic liver failure (ACLF) and severe COVID‐19 disease course increase with the stage of liver disease, according to the Child–Pugh classification.94
In the setting of the pandemic, the lack of physical activity, mental health issues, and increased alcohol consumption can contribute to the increase of ALD and MAFLD burdens.95
Liver transplant recipients and autoimmune hepatitis
Immunosuppression is associated with increased risk of acquiring SARS‐CoV‐2. A recent study showed a hospital admission rate of 84% in liver transplant recipients, and a mortality rate of 20%, with respiratory failure as the most prevalent cause of death.96 However, studies highlighted the possible protective effect of calcineurin inhibitors and potential deleterious effects of mycophenolate mofetil.96, 97 The protective effect of immunosuppressants may be due to mitigation of the cytokine storm. In a European/American retrospective study of 110 patients with autoimmune hepatitis (AIH), patients with COVID‐19 were not at increased risk for worse outcomes with an overall all‐cause mortality rate of 10%, and 22% for hospitalized patients.98 In this study, 92% of patients were on immunosuppressants. The authors concluded that immunosuppression was protective for liver injury and did not predispose to a more severe disease course.98
COVID‐19 and the pancreas
Even though causality cannot be confirmed, pathophysiological findings seem to indicate that the pancreas is affected by COVID‐19.99 In fact, ACE‐2 receptor is expressed in the exocrine and endocrine pancreas100, 101 and SARS‐CoV‐2 infects and replicates in pancreatic cells.102
In the early stages of the pandemic, a few studies reported increased levels of lipase and amylase, in COVID‐19 patients (9 of 52 patients) with severe pneumonia.103This evidence led to the hypotheses that COVID‐19 infection could directly result in acute pancreatitis (AP). The publication of reports on cases of COVID‐induced pancreatitis corroborated that theory.104, 105, 106However, in most cases, lipase levels were less than three times higher than the upper limit‐of‐normal (ULN), and patients showed no typical symptoms of pancreatitis. Thus, these reports lacked specificity for the diagnosis of AP.107, 108Also on this setting, a US multicenter study reported hyperlipasemia in 12.1% of hospitalized COVID‐19 patients; in this study, 2.2% of patients presented lipase levels three times higher than the ULN and no patient developed AP.109 Comparable data were reported in Asian110 and German patients,111 and in other US study.112 However, a large retrospective study analyzed 48,012 patients who were admitted during the COVID‐19 pandemic. Some 189 had evidence for AP and, 32 from this cohort were COVID‐19 positive. In patients with COVID‐19, the cause of AP was more often undetermined.113 Moreover, in a prospective study from China, 12.6% of patients with COVID‐19 pneumonia developed AP, which was a risk factor for severe illness and mortality.114 These findings were confirmed by a large prospective UK study that determined COVID‐19 as a risk factor for severe AP, with worse clinical outcome.115
To conclude, increased amylase or lipase levels might not be associated with AP in COVID‐19 and may be a consequence of concurrent clinical conditions. There is no evidence for a COVID‐19‐induced AP.99, 116, 117
COVID‐19 AND ENDOSCOPY
The largest challenge during the first wave of the COVID‐19 pandemic was the high asymptomatic carrier rate, along with the lack of effective means to detect the virus.
Even though the incidence of asymptomatic cases varies across studies (from 1.6% to 56.5%), it has been early recognized that these patients are potentially infective.118 It became clear that, although endoscopy is a high‐risk aerosol generating procedure, the adoption of protective measures reduces infective transmission.119, 120, 121 This improved with the availability of nasopharyngeal antigen testing, followed by rapid point of care tests, although false negative rates remain high.
Anyway, the first wave led to a marked decrease in endoscopy activity as elective procedures were curbed to minimize footfall and hospital transmission. The redistribution of HCP and lack of PPE were initial contributory factors. Several societies worldwide were quick to issue guidance on prioritizing activity and patient risk stratification for procedures.119, 120, 121 This marked reduction in activity (to 10%–15% of pre‐COVID‐19) included also cancer screening procedures.13 The selective control of indications for GI endoscopy led to an increase in cancer detection rate per procedure and to a concerning decrease in colorectal cancer diagnosis (of 72% in the United Kingdom and 50% in the Unitesd States).13, 122 In the United Kingdom, colorectal and esophageal cancer deaths will increase 15% and 6%, respectively, in the next 5 years.123
After the first wave, endoscopy departments faced the challenges of reconfiguring services to adapt, revert to pre‐pandemic levels of activity, and address waiting list backlogs (Table 1). Patients were discouraged to attend hospitals and started avoiding healthcare contacts (and/or having access difficulties), given that the risk of contracting COVID‐19 was perceived as high, outweighing the risk of a delay in cancer diagnosis.124 Since the start of the pandemic, overall cancer diagnoses decreased in the United States, not meaning that the actual incidence of cancer has dropped. Undiagnosed cancers summed up with those that where deprioritized to preserve clinical capacity for COVID‐19 patients, with delayed surgeries and less frequent chemotherapy and/or radiotherapy, are matter of serious concern.125 The impact of these tendencies is predictable if we acknowledge that even a slight 3‐month delay in cancer diagnosis (especially T2‐T3) may have significant impact on survival.126 For instance, model predictions indicate an excess of 10,000 deaths from breast and colorectal cancer, in the next decades.125
TABLE 1.
Adaptative measures in endoscopic units
| Adaptative measure | Comment |
|---|---|
| Pre‐procedure | |
| Change in patient indications | Emergent and urgent indications in first wave; return to pre‐COVID‐19 activity, with re‐triage and prioritization of patients |
| Triage of symptoms/signs of infection, high‐risk contacts, and travel to high‐risk areas | Universal; 2–3 days before endoscopy and at admission |
| Limitation of family members at the hospital | Possible difficult communication. Phone contact policy with relatives is useful |
| Pre‐procedural swab testing | Significant healthcare burden and costs; not universally adopted, but may be useful depending on the local phase of the pandemic and resources |
| Linear flow of patients throughout units | Strict social distancing rules; minimization of time spent in departments |
| Procedure | |
| Limitation of staff members in the endoscopy suit | Impact in training |
| Protective personal equipment use; appropriate donning and doffing | According to local policy/guidelines |
| Barrier protection | Not universally adopted but in development (transparent aerosol boxes, plastic shields); questionable benefit if other protective measures are strictly followed |
| Negative pressure rooms | For procedures in COVID‐19 positive patients/high‐suspicion patients pending results |
| Post‐procedure | |
| Enhanced cleaning procedures | According to local policy/guidelines |
| Routine high‐level disinfection | Minimal/null risk of transmission through endoscopes after high‐level disinfection |
| Procedural room downtime | Depending on patient COVID‐19 status, room volume, changes per hour |
| Post‐procedure patient tracking/contact | Tracking of contacts |
At this point, with all the lessons learned, and with vaccination under way with good results in most countries, gastroenterologists and health providers shall assure that:
-
(a)
Cancelled and delayed procedures are resumed, through review of waiting lists and adequate prioritization
-
(b)
Individuals perceive the risks of postponing cancer screening/diagnostic procedures
-
(c)
Screening programs are resumed, at least by non‐invasive methods, if endoscopic capability is low
-
(d)
Training programs for physicians and technicians are resumed with minimum impact to trainees
The impact of these measures will be further improved by proper patient education programs that are being adapted to the digital format in many hospitals.127
The pandemic had also a negative impact on endoscopy training worldwide.128, 129 The decrement of case volume, PPE shortage, exclusion from endoscopy procedures, or redeployment to another clinical area were the main challenges that endoscopy trainees had to face.128, 130 The substantial reduction of hands‐on opportunities disrupted further endoscopy skills development. Additional concerns stemmed from the potential endoscopy training prolongation and from the lack of institutional support for trainees' emotional health care.128, 131, 132 All this has been translated into growing frustration, anxiety (52.4%), and even burnout (18.8%), among endoscopy trainees.128, 131 These conclusions became a call for prompt reorganization of the training path by involving societies, endoscopy units, and course directors.133
So far, the visible changes regarding endoscopy training are strongly related to the translocation of endoscopic education to online platforms, shifting the focus to cognitive skills development.133 Learning resources were developed and released on the websites of the major GI and endoscopy societies. In addition, trainees can have close contact with experts and access to public discussions, during interactive webinars or conferences, which also became a new virtual reality. Also, podcast series created by journals (Endoscopy, GIE) are gaining popularity. Social media platforms (Twitter and LinkedIn) opened new learning and sharing opportunities, including international collaboration and experience sharing.134
However, patient‐based endoscopy exposure for technical skills development remained the greatest concern for endoscopy trainees.135 The emergence of international and national position statements on GI endoscopy, during COVID‐19 pandemic, led to the adaptation of endoscopy units, providing safety along with high‐quality procedures performance.136 The increment of endoscopy case volume, with prior‐to‐procedure testing, along with vaccination and adequate PPE, may allow incorporating advanced fellows back into the endoscopy room.133 Adaptative strategies have included: simulation‐based teaching programs,137 non‐technical skills teaching, resilience training and emotional support for staff and trainees, distance mentorship, proposals to move away from emphasizing minimum procedure numbers toward competency‐based curricula backed by competency assessment tools.137 As examples of simulators, we highlight Endoscopic Retrograde Cholangiopancreatography and Endoscopic ultrasound, that are being used as alternatives for upper and lower GI endoscopy and advanced procedure.
COVID‐19 VACCINES: WHERE ARE WE NOW?
SARS‐CoV‐2 vaccines are the key for pandemic control. The vaccines approved by US Food and Drug Administration and European Medicines Agency are based on two new platforms: mRNA vaccines and adenovirus vector‐based vaccines (Table 2). At least three other vaccines are under evaluation: a protein subunit‐based vaccine, an adenovirus‐based vaccine and other mRNA vaccine (Table 2). Data from Phase 3 clinical trials (BNT162b2, mRNA‐1273, and ChAdOx1 nCoV‐19), that included almost 100,000 adults, showed that mild local injection site reactions (pain, swelling, redness) and systemic features (fatigue, headache, chills) were common, but not serious, for most vaccines.138, 139, 146 So far, except for rare thrombotic events associated with adenovirus AstraZeneca vaccine,147, 148 rare reports of cerebral venous sinus thrombosis, and low level of platelets associated with Johnson & Johnson vaccine,149 no major side effects were reported for these vaccines. Even though, additional data and new statements from the regulatory agencies, concerning thrombotic events and vectorial vaccines, are expected to be published soon. Meanwhile, the Johnson & Johnson vaccine is temporarily suspended in the United States, South Africa, and European Union, following a recommendation of the US Centers for Disease Control and Prevention,150 and several European countries suspended the administration of the AstraZeneca vaccine, in some groups of the general population, such as people below 60 or 55 years of age.
TABLE 2.
Commercialized and under evaluation SARS‐CoV‐2 vaccines (EMA)
| Manufacter/Vaccine | BioNTech/Pfizer BNT162b2 (US)138 | Moderna mRNA‐1273 (US)138 | Oxford/AstraZeneca ChAdOx1 Vaxzevria (UK)139 | Johnson & Johnson Ad26.CoV2.S (US)140 | Sputnik‐V (JNJ‐784436735) GamCovid‐vac (Russia)141 | CureVac/CvnCoV (Germany, US) | Novavax NVX‐CoV2373 (US)142 |
|---|---|---|---|---|---|---|---|
| Plataform | mRNA; encoding a genetically modified SARS‐CoV‐2 spike protein (lipid nanoparticle) | mRNA; encoding a genetically modified SARS‐CoV‐2 spike protein (lipid nanoparticle) | Non‐replicating; defective chimpanzee adenovirus vector, Ad5 containing SARS‐CoV‐2 spike protein | Non‐replicating; incompetent adenovirus vector, Ad26, encoding a full‐length SARS‐CoV‐2 spike protein | Heterologous; recombinant adenovirus‐based vaccine (rAd): rAd type26 (first shot), rAd type5 (second shot) | mRNA; encoding a genetically modified SARS‐CoV‐2 spike protein; | Protein subunit; recombinant nanoparticle vaccine |
| Storage conditions | −80°C to −60°C; 2–8°C for 5 days; Room temperature 6 h after reconstitution | −25°C to −15° up to 6 months; 2–8°C for 30 days; Room temperature: for 24 h and 6 h after reconstitution | +2°C to 8°C for 6 months | 2–8°C for 3 months; 6 h refrigeration after reconstitution | −18°C (liquid form) for up to 6 months; 2–8°C (freeze dried) for up to 6 months | 2–8°C for 3 months; Room temperature for 24 h | 2–8°C for 6 months; 24 h at room temperature |
| Dose | 30 µg | 100 µg | 5 × 1010 viral particles | 5 × 1010 viral particles | 1011 viral particles per dose for each recombinant adenoviruses | 12 µg | 5 µg of protein and 50 µg of Matrix‐M adjuvant |
| Dosage | Two doses, 3 weeks apart (from 3–12 weeks apart) | Two doses, 4 weeks apart | Two doses, 4 weeks apart; (12 weeks apart great efficacy) | One dose versus two doses: 0,28 days | Two doses, 3 weeks apart | Two doses, 30 days apart | Two doses, 3 weeks apart |
| Age | >16 | ≥18 | ≥18 | >18 | >18 | ‐ | 18‐59 |
| Efficacy | 95% against symptomatic Covid‐19 after two doses | 94.1% against symptomatic COVID‐19, ≥14 days after second dose | 66.7% against virologically confirmed symptomatic Covid‐19 disease ≥14 days after two dose; when the two doses ≥12 weeks apart efficacy 81.3% (standard dose) and 80.0% (low dose plus standard) | 72% in the United States; 64% in South Africa—neutralizing antibody responses | 91.6% PCR—Covid‐19 confirmed ≥21 days of first dose | Ongoing study (HERALD phase 2b/3 trial in Europe and Latin America | Ongoing study PREVENT Phase 3 trial on United States and Mexico |
| Efficacy against severe disease/hospitalisation | RCT–not reported; Israeli real‐world data143: 92%/87% | 100% | 100%/100% (>21 days after the second dose)144 | NA | 100% against moderate to severe COVID‐19145 | NA | NA |
| Trial phase published | 3 | 3 | 3 | 1,2 | 3 | ‐ | 1,2 |
| Approval EU | Yes | Yes | Yes | Yes | Under evaluation EMA | Under evaluation EMA | Under evaluation EMA |
Abbreviation: EMA, European Medicines Agency.
Despite these concerns, vaccine effectiveness seems to be the key concern, rather than safety. All the entities and experts recommend COVID‐19 vaccination for IBD patients,151, 152, 153, 154, 155 including those who had anaphylaxis following biologic treatment.151
In addition, the British Society of Gastroenterology and the British Association for the Study of the Liver recommend that patients with CLD, AIH, and those with liver transplants shall be vaccinated for COVID‐19, with one of the available vacines.156
COVID‐19 vaccination data in special populations, such as patients with IBD, pregnant and breastfeeding women, and immunosuppressed patients, are scarce and consist mainly of experts' opinions, and position statements from regulatory agencies and safety surveillance reports.
At this point, it is vital to understand if immunosuppressive agents mitigate or even prevent side effects related to vaccine immunogenicity, in IBD patients.157 In fact, COVID‐19 vaccines efficacy might be reduced in IBD patients treated with immunosuppressants, biologicals, or corticosteroids. Several studies found that patients with COVID‐19 infection, treated with infliximab, have a blunted anti‐SARS‐CoV‐2 response,158, 159 that is further reduced with concomitant immunomodulator use.158 However, a blunted response does not equate to vaccine failure. This effect was not observed with vedolizumab. 160 Another concern is the possibility of an accelerated wanning of protective antibody titers, in patients treated with immunosuppressants, as verified with common vaccines (hepatitis B, measles, pertussis).161, 162, 163 In this context, the International Organization for the study of Inflammatory Bowel Disease advises that maintenance therapies should not be withheld.9 Thus, patients shall be vaccinated as soon as possible 155 and, whenever possible, the vaccine should be administered to stable patients, before the start of immunosuppression and under a dose of corticosteroids lower than 20 mg of prednisolone a day (or equivalent), as systemic corticosteroids are known to have immunosuppressive effect above this dosage.164 This does not exclude the need to consider comorbidities, age, health condition, and risk exposition to COVID‐19, prior to vaccine administration. With the objective of guiding physicians worldwide, we, herein, propose a flow‐chart for SARS‐CoV‐2 vaccination, in IBD adult patients (Figure 2).
FIGURE 2.
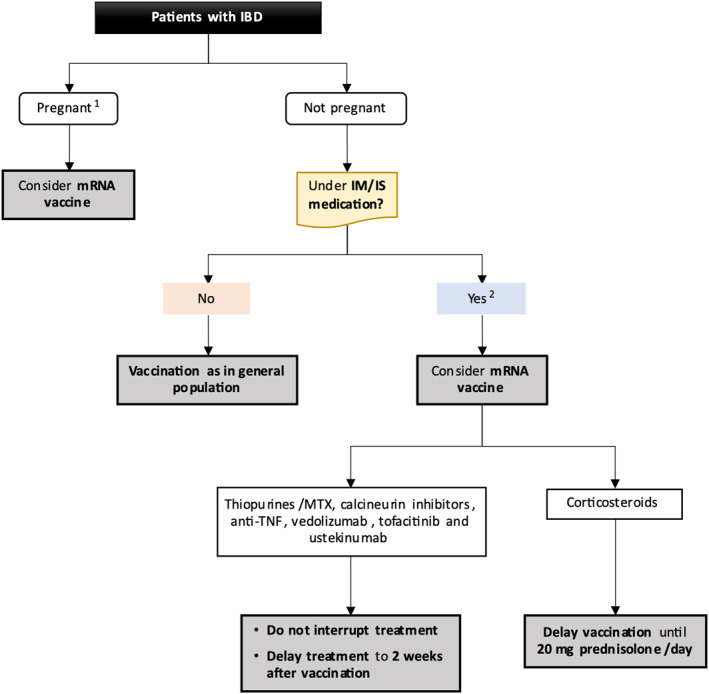
COVID‐19 vaccination in inflammatory bowel disease patients. 1Case‐by‐case decision according to comorbidities and risk exposure. 2Within this group of patients: (1) possibility of reduced protection, (2) vaccination not precluded, (3) vaccine booster may be needed, and (4) consider check antibodies after vaccine
Regarding other GI diseases, the European Association for the Study of the Liver considered that patients with CLD, significant fibrosis, hepatobiliary cancer, and those who have had or await liver transplantation are prime candidates for receiving the COVID‐19 vaccines, as all other highly vulnerable people.165
Recent concern has been raised about variants of SARS‐CoV‐2 that may escape current vaccines, as changes in SARS‐CoV‐2 spike can alter neutralization sensitivity and reduce vaccine efficacy.166, 167 New SARS‐CoV‐2 variants are emerging rapidly, such as B.1.1.7, B.1.351, and P.1 lineages, and it is critical to understand if antibody responses induced by current vaccines remain effective. Despite all the uncertainty, in real‐world, COVID‐19 vaccines seem to be effective when the process is carried out with efficiency. For instance, Pfizer and BioNtech announced a reduction of 94% of symptomatic and asymptomatic infections, in Israel.168
DISCUSSION
This article presents an overview of COVID‐19 in Gastroenterology, the lessons learned so far, in the scope of this specialty, as well as implications to the future169 (Figure 3).
FIGURE 3.
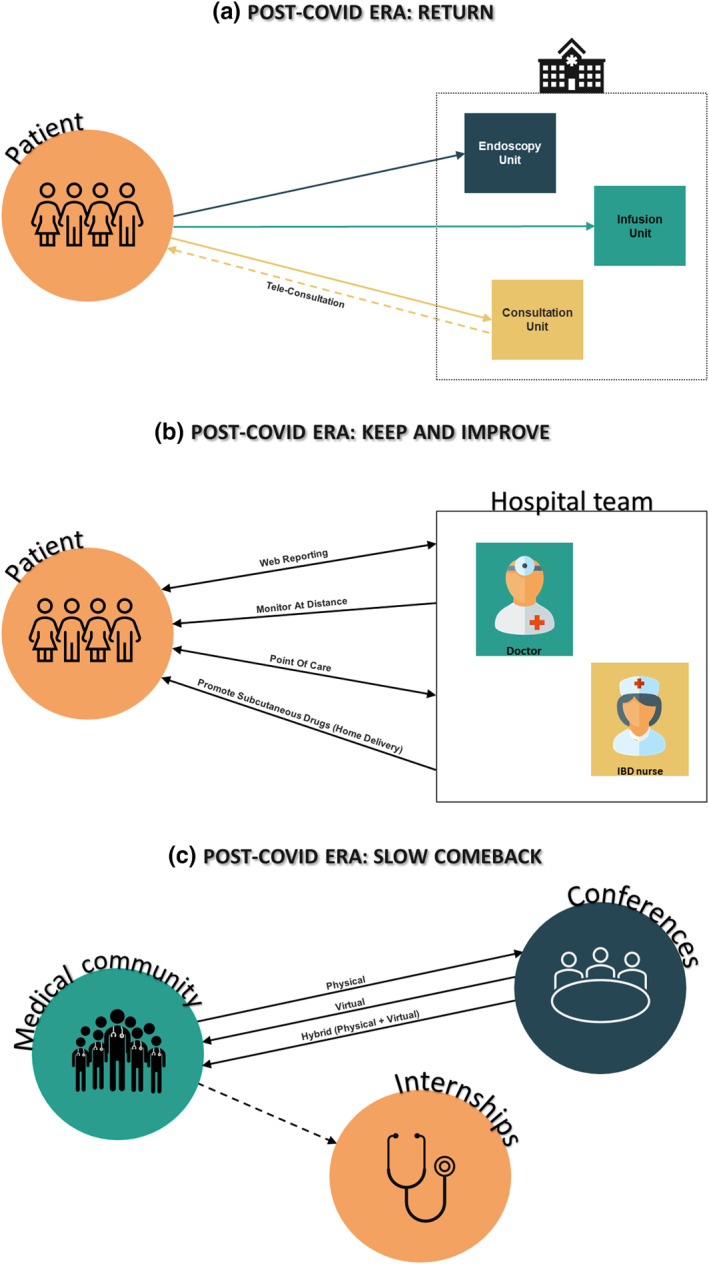
Gastroenterology in the post‐COVID‐19 era
It is now clear that GI manifestations are common in COVID‐19 patients, but without established relation with disease severity.20 These manifestations can be further aggravated by the reduction of patients' contact with medical services and by the sedentary lifestyle adopted by the majority of the population, during lockdowns.
GI patients, as others, have been also affected by the reduction of the frequency of medical attendances, with a wide range of consequences, such as progression or decompensation of chronic diseases, late diagnosis of complications, and failure in monitoring medical treatments. In this setting, we highlight, with great concern, the difficulties concerning viral hepatitis control as defined by WHO, that aimed at a reduction of newly infected persons and related mortality by 90% and 65% respectively, by 2030. The pandemic crisis is affecting the achievement of this goal mainly by decreasing diagnosis, access to treatment and harm reduction programs.170
In this scenario, telemedicine was explored to mitigate the effects of the pandemic on the care provided to chronic GI patients and allowed medical monitoring in a remote format.17 We predict that, considering its recent developments and indicators, such as reduction of costs and administrative burdens, telemedicine can remain a valid strategy for IBD patients, in combination with conventional visits, both for continuous care and procedure's monitoring (Figure 3).16 However, it is mandatory to observe how telemedicine will evolve and impact the whole management, while guaranteeing individual accessibility.83 An important aspect to consider, in this transition phase, is patient's satisfaction. In fact, virtual appointments are still viewed as distant contacts and may not fulfill the needs of older and less favored patients.
One year after the beginning of the COVID‐19 pandemic, it is clear that IBD does not confer an increased risk for COVID‐19, per se. However, we can identify risk factors, including medications such as thiopurines, that should be considered in the risk stratification. As caregivers, we must adapt and individualize our clinical practice and treatment strategies based on best available evidence, careful appraisal of risk and benefit and acceptability to patients.
Recent evidence shows that liver injury is associated with severe COVID‐19 and poor outcomes. However, clear definitions or cut‐offs for liver biomarkers, to determine the prognosis of these COVID‐19 patients, have not been defined yet. Patients with cirrhosis have higher risk of poor outcome, which increases with the stage of liver disease. Liver transplant patients are more frequently admitted to the hospital; however, the course of COVID‐19 disease seems to be mild. The current recommendation for AIH patients is to maintain immunosuppression. In the case of liver transplant recipients, calcineurin inhibitors seem to protect against severe COVID‐19, while it may be advisable to taper or withhold mycophenolate mofetil.
Despite all the concerns around endoscopy, current evidence shows that the negative impact of the reduction of procedures during the pandemic surpasses the risk of contracting COVID‐19.126 After some readjustments, it seems that endoscopy is back on track, to levels similar to those of the pre‐COVID‐19 era, with new (and perhaps better) habits, allowing the continued provision of safe and valuable procedures (Figure 3).171 Considering that endoscopy is a core diagnosis and treatment modality for GI pathology, these readjustments are vital. Even though, the widespread use of point‐of‐care testing to cohort patients, may obviate the need for aerosol generating procedure PPE and room turnover precautions.
Overall, the scientific and medical communities are also concerned about the impact of COVID‐19 on medical education and training.15, 130 Several tools have been developed and implemented to provide long distance classes and training, and despite all the associated advantages, the lack of hands‐on training will impair skills development. Moreover, this loses the element of social interactivity, which is not only important for feedback and learning, but also emotional support, which can affect emotional health.130, 131 It is hence important to maximize hands‐on training opportunities and apply evidence‐based interventions that optimize the endoscopy learning curve. Hybrid learning models may be the solution over the next years (Figure 3).
Researchers and scientists are also facing constraints regarding the discussion of research results. Conferences all around the world were adapted to virtual meetings with advantages in terms of cost, flexibility and accessibility.18 For instance, more than 1800 researchers, from 64 countries, attended the e‐symposium “Vaccinology in the age of pandemics”. This is a good example of the importance of digital media technologies for scientific discussion, in this period of social and traveling constraints. We believe, from our experience in the GI area, that, after the pandemic, most scientific meetings will keep a virtual component (Figure 3). Although, we admit that virtual events cannot fully simulate the networking that is provided by regular science conferences, in which colleagues can discuss all the aspects of their research, in person. The pandemic has also fostered international research collaborations and the establishment of prospective databases, like SECURE‐IBD, SECURE‐Liver, COVID‐HEP that are sources of important information for HCPs, policy makers, and patients.
At this point of the pandemic, researchers, physicians, and governments are focused on vaccination. Evidence shows that a careful evaluation of chronic GI patients regarding corticosteroids and immunosuppressants will guarantee safety and efficacy, during the vaccination process.155 However, even if group immunity is achieved in some regions, general population shall be aware of the need to keep sanitary (hands washing and masks) and social distance rules, to further reduce the risk of SARS‐CoV‐2 dissemination. Anyway, the COVID‐19 “vaccine passport” is being discussed worldwide with the objective of allowing for citizens who were vaccinated or who tested negative, or recovered from the virus, to travel between countries with minimum risks.
Future research will further increase the knowledge on SARS‐CoV‐2 and COVID‐19 and guide patients' management. We highlight the need to clarify the role of the GI tract on severe COVID‐19 forms and on virus multiplication, as well as post‐COVID complications such as IBS and dysbiosis.
In conclusion, the pandemic crisis has created unprecedent challenges for gastroenterologists and GI patients. One year after the first lockdowns worldwide, the impact of COVID‐19 on healthcare systems, disease's courses and diagnosis and on education and training were evaluated, and are herein discussed in detail, enabling supported decisions. We believe that we have gathered enough knowledge to assume that some of the adopted measures presented evident benefits, such as those related with telemedicine and online learning, while others showed to have negative impact in patients such as those related with endoscopic procedures and excessive reduction of medical attendances (Figure 3). Thoughtful decisions shall be now made regarding the transition to normality, in order to guarantee the best care possible for chronic GI patients, while taking advantage of the technological tools that can reduce disease burdens for patients and HCP and systems.
CONFLICT OF INTEREST
IR‐L has received financial support for traveling and educational activities from or has served as an advisory board member for MSD, Pfizer, Abbvie, Takeda, Janssen, Tillotts Pharma, Shire Pharmaceuticals, Roche, Celltrion, Faes Farma, Ferring, Dr. Falk Pharma, Otsuka Pharmaceutical, and Adacyte. Financial support for research from Tillotts Pharma.
Supporting information
Supporting Information 1
ACKNOWLEDGMENTS
The authors thank Paula Pinto, PharmD, PhD (PMA—Pharmaceutical Medicine Academy) for providing medical writing and editorial assistance. IRL‐L is supported by a research grant from Biocruces Bizkaia Health Research Institute (Grant No INT‐BC‐2018‐007) and Gobierno Vasco‐Eusko Jaurlaritza (Grant No 2020222004).
Magro F, Nuzzo A, Abreu C, Libânio D, Rodriguez‐Lago I, Pawlak K, et al. COVID‐19 in gastroenterology: where are we now? Current evidence on the impact of COVID‐19 in gastroenterology. United European Gastroenterol J. 2021;9(7):750–765. 10.1002/ueg2.12115
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1.Cui J, Li F, Shi Z‐L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17 (3):181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. COVID‐19 weekly epidemiological update on COVID‐19 ‐ 20 April 2021. 2021. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐on‐covid‐19‐‐‐20‐april‐2021
- 3.Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al‐Jabir A, Iosifidis C, et al. The socio‐economic implications of the coronavirus pandemic (COVID‐19): a review. Int J Surg. 2020;78:185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montemurro N. The emotional impact of COVID‐19: from medical staff to common people. Brain Behav Immun. 2020;87:23–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali I, Alharbi OML. COVID‐19: disease, management, treatment, and social impact. Sci Total Environ. 2020;728:138861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah S, Diwan S, Kohan L, Rosenblum D, Gharibo C, Soin A, et al. The technological impact of COVID‐19 on the future of education and health care delivery. Pain Physician. 2020;23 (4S):S367–80. [PubMed] [Google Scholar]
- 7.Magro F, Abreu C, Rahier J‐F. The daily impact of COVID‐19 in gastroenterology. United Eur Gastroenterol J. 2020;8:520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magro F, Rahier JF, Abreu C, MacMahon E, Hart A, van der Woude CJ, et al. Inflammatory bowel disease management during the COVID‐19 outbreak: the ten do's and don'ts from the ECCO‐COVID taskforce. J Crohns Colitis. 2020;14 (Suppl 3):S798–S806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin DT, Abreu MT, Rai V, Siegel CA. Management of patients with Crohn's disease and ulcerative colitis during the COVID‐19 pandemic: results of an international meeting. Gastroenterology; 2020;159:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danese S, Cecconi M, Spinelli A. Management of IBD during the COVID‐19 outbreak: resetting clinical priorities. Nat Rev Gastroenterol Hepatol. 2020;17 (5):253–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J, Aghemo A, Forner A, Valenti L. COVID‐19 and liver disease. Liver Int. 2020;40 (6):1278–81. [DOI] [PubMed] [Google Scholar]
- 12.Patel KP, Patel PA, Vunnam RR, Hewlett AT, Jain R, Jing R, et al. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID‐19. J Clin Virol. 2020;128:104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutter MD, Brookes M, Lee TJ, Rogers P, Sharp L. Impact of the COVID‐19 pandemic on UK endoscopic activity and cancer detection: a National Endoscopy Database Analysis. Gut. 2021;70 (3):537–43. [DOI] [PubMed] [Google Scholar]
- 14.Lantinga MA, Theunissen F, Ter Borg PCJ, Bruno MJ, Ouwendijk RJT, Siersema PD, et al. Impact of the COVID‐19 pandemic on gastrointestinal endoscopy in The Netherlands: analysis of a prospective endoscopy database. Endoscopy. 2021;53 (2):166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal H, Gajendran M, Boregowda U, Perisetti A, Aziz M, Bansal P, et al. Current and future implications of COVID‐19 on gastroenterology training and clinical practice. Int J Clin Pract. 2020;74 (12):e13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguas M, Del Hoyo J, Faubel R, Valdivieso B, Nos P. Telemedicine in the treatment of patients with inflammatory bowel disease. Gastroenterol Hepatol. 2017;40 (9):641–7. [DOI] [PubMed] [Google Scholar]
- 17.George LA, Cross RK. Telemedicine in gastroenterology in the wake of COVID‐19. Expert Rev Gastroenterol Hepatol. 2020;14 (11):1013–5. [DOI] [PubMed] [Google Scholar]
- 18.Jarvis T, Weiman S, Johnson D. Reimagining scientific conferences during the pandemic and beyond. Sci Adv. 2020;6 (38):eabe5815. 10.1126/sciadv.abe5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature. 2020;581 (7807):215–20. [DOI] [PubMed] [Google Scholar]
- 20.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526 (1):135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, et al. Gastrointestinal manifestations of SARS‐CoV‐2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta‐analysis. Gastroenterology. 2020;159 (1):81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, et al. Breugem TI., et al. SARS‐CoV‐2 productively infects human gut enterocytes. Science. 2020;369 (6499):50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo M, Tao W, Flavell RA, Zhu S. Potential intestinal infection and faecal‐oral transmission of SARS‐CoV‐2. Nat Rev Gastroenterol Hepatol. 2021;18 (4):269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27 (4):601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, et al. Prolonged presence of SARS‐CoV‐2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5 (5):434–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology. 2020;158 (6):1831–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, et al. Characteristics of pediatric SARS‐CoV‐2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26 (4):502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medema G, Heijnen L, Elsinga G, Italiaander R, Brouwer A. Presence of SARS‐coronavirus‐2 RNA in sewage and correlation with reported COVID‐19 prevalence in the early stage of the epidemic in The Netherlands. Environ Sci Technol Lett. 2020;7 (7):511–6. [DOI] [PubMed] [Google Scholar]
- 29.Jin X, Lian J‐S, Hu J‐H, Gao J, Zheng L, Zhang Y‐M, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus‐infected disease 2019 (COVID‐19) with gastrointestinal symptoms. Gut. 2020;69 (6):1002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581 (7809):465–9. [DOI] [PubMed] [Google Scholar]
- 31.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, et al. Viral load dynamics and disease severity in patients infected with SARS‐CoV‐2 in Zhejiang province, China, January‐March 2020: retrospective cohort study. BMJ. 2020;369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian Q, Fan L, Liu W, Li J, Yue J, Wang M, et al. Direct evidence of active SARS‐CoV‐2 replication in the intestine. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Effenberger M, Grabherr F, Mayr L, Schwaerzler J, Nairz M, Seifert M, et al. Faecal calprotectin indicates intestinal inflammation in COVID‐19. Gut. 2020;69 (8):1543–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, et al. Longitudinal analyses reveal immunological misfiring in severe COVID‐19. Nature. 2020;584 (7821):463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, et al. Alterations in gut microbiota of patients with COVID‐19 during time of hospitalization. Gastroenterology. 2020;159 (3):944–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeoh YK, Zuo T, Lui GC‐Y, Zhang F, Liu Q, Li AY, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID‐19. Gut. 2021;70 (4):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alqahtani SA, Schattenberg JM. Liver injury in COVID‐19: the current evidence. United Eur Gastroenterol J. 2020;8 (5):509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schattenberg JM, Labenz C, Wörns M‐A, Menge P, Weinmann A, Galle PR, et al. Patterns of liver injury in COVID‐19—a German case series. United Eur Gastroenterol J. 2020;8 (7):814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massironi S, Viganò C, Dioscoridi L, Filippi E, Pagliarulo M, Manfredi G, et al. Endoscopic findings in patients infected with 2019 novel coronavirus in Lombardy, Italy. Clin Gastroenterol Hepatol. 2020;18 (10):2375–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng F, Liao C, Fan Q‐H, Chen H‐B, Zhao X‐G, Xie Z‐G, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020;40 (2):275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Moheb M, Naar L, Christensen MA, Kapoen C, Maurer LR, Farhat M, et al. Gastrointestinal complications in critically ill patients with and without COVID‐19. J Am Med Assoc. 2020;324 (18):1899–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hajifathalian K, Krisko T, Mehta A, Kumar S, Schwartz R, Fortune B, et al. Gastrointestinal and hepatic manifestations of 2019 novel coronavirus disease in a large cohort of infected patients from New York: clinical implications. Gastroenterology. 2020;159 (3):1137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao R, Rieder F, Ben‐Horin S, Kaplan GG, Ng SC, Wong GL, et al. Implications of COVID‐19 for patients with pre‐existing digestive diseases: an update. Lancet Gastroenterol Hepatol. 2021;6 (4):258–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID‐19. Nat Med. 2020;26 (7):1017–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arnold DT, Hamilton FW, Milne A, Morley AJ, Viner J, Attwood M, et al. Patient outcomes after hospitalisation with COVID‐19 and implications for follow‐up: results from a prospective UK cohort. Thorax. 2020;76 (4):399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreno‐Pérez O, Merino E, Leon‐Ramirez J‐M, Andres M, Ramos JM, Arenas‐Jiménez J, et al. Post‐acute COVID‐19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82 (3):378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quek SXZ, Loo EXL, Demutska A, Chua CE, Kew GS, Wong S, et al. Impact of the coronavirus disease 2019 pandemic on irritable bowel syndrome. J Gastroenterol Hepatol. 2021. 10.1111/jgh.15466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marjot T, Webb GJ, Barritt AS, Moon AM, Stamataki Z, Wong VW, et al. COVID‐19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18 (5):348–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JU, Majid A, Judge R, Crook P, Nathwani R, Selvapatt N, et al. Effect of COVID‐19 lockdown on alcohol consumption in patients with pre‐existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5 (10):886–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamp KJ, Levy RL, Munson SA, Heitkemper MM. Impact of COVID‐19 on individuals with irritable bowel syndrome and comorbid anxiety and/or depression. J Clin Gastroenterol. 2021. 10.1097/MCG.0000000000001515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perisetti A, Goyal H. Successful distancing: telemedicine in gastroenterology and hepatology during the COVID‐19 pandemic. Dig Dis Sci. 2021;66 (4):945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serper M, Nunes F, Ahmad N, Roberts D, Metz DC, Mehta SJ. Positive early patient and clinician experience with telemedicine in an academic gastroenterology practice during the COVID‐19 pandemic. Gastroenterology. 2020;159 (4):1589–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maida M, Sferrazza S, Savarino E, Ricciardiello L, Repici A, Morisco F, et al. Impact of the COVID‐19 pandemic on Gastroenterology Divisions in Italy: a national survey. Dig Liver Dis. 2020;52 (8):808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zabana Y, Rodriguez L, Lobaton T, Gordillo J, Montserrat A, Mena R, et al. Relevant infections in inflammatory bowel disease, and their relationship with immunosuppressive therapy and their effects on disease mortality. J Crohns Colitis. 2019;13 (7):828–37. [DOI] [PubMed] [Google Scholar]
- 55.Wisniewski A, Kirchgesner J, Seksik P, Landman C, Bourrier A, Nion‐Larmurier I, et al. Increased incidence of systemic serious viral infections in patients with inflammatory bowel disease associates with active disease and use of thiopurines. United Eur Gastroenterol J. 2020;8 (3):303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray‐Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155 (2):337–46. [DOI] [PubMed] [Google Scholar]
- 57.Khan N, Patel D, Xie D, Pernes T, Lewis J, Yang YX. Are patients with inflammatory bowel disease at an increased risk of developing SARS‐CoV‐2 than patients without inflammatory bowel disease? Results from a nationwide veterans’ affairs cohort study. Am J Gastroenterol. 2020:jjaa061. [DOI] [PubMed] [Google Scholar]
- 58.Derikx L, Lantinga MA, de Jong DJ, van Dop WA, Creemers RH, Romkens TEH, et al. Clinical outcomes of covid‐19 in patients with inflammatory bowel disease: a nationwide cohort study. J Crohns Colitis. 2020;15 (4):529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Attauabi M, Poulsen A, Theede K, Pedersen N, Larsen L, Jess T, et al. Prevalence and outcomes of COVID‐19 among patients with inflammatory bowel disease ‐ a Danish prospective population‐based cohort study. J Crohns Colitis. 2020;15(4):540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taxonera C, Sagastagoitia I, Alba C, Mañas N, Olivares D, Rey E. Novel coronavirus disease (COVID‐19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2019;14(12):1187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh AK, Jena A, Kumar MP, Sharma V, Sebastian S. Risk and outcomes of coronavirus disease (COVID‐19) in patients with inflammatory bowel disease: a systematic review and meta‐analysis. United Eur Gastroenterol J. 2020;9 (2):159–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nowak JK, Lindstrom JC, Kalla R, Ricanek P, Halfvarson J, Satsangi J. Age, inflammation, and disease location are critical determinants of intestinal expression of SARS‐CoV‐2 receptor ACE2 and TMPRSS2 in inflammatory bowel disease. Gastroenterology. 2020;159 (3):1151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suarez‐Farinas M, Tokuyama M, Wei G, Huang R, Livanos A, Jha D, et al. Intestinal inflammation modulates the expression of ACE2 and TMPRSS2 and potentially overlaps with the pathogenesis of SARS‐CoV‐2‐related disease. Gastroenterology. 2021;160 (1):287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burke KE, Kochar B, Allegretti JR, Winter RW, Lochhead P, Khalili H, et al. Immunosuppressive therapy and risk of COVID‐19 infection in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2021;27 (2):155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khan N, Patel D, Xie D, Lewis J, Trivedi C, Yang YX. Impact of anti‐tumor necrosis factor and thiopurine medications on the development of COVID‐19 in patients with inflammatory bowel disease: a nationwide veterans administration cohort study. Gastroenterology. 2020;159 (4):1545–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous‐Hunt M, Lewis JD, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID‐19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159 (2):481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ungaro RC, Kappelman MD, Rubin DT, Colombel JF. COVID‐19 and inflammatory bowel disease: lessons learned, practical recommendations, and unanswered questions. Gastroenterology. 2020;160(5):1447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh S, Khan A, Chowdhry M, Bilal M, Kochhar GS, Clarke K. Risk of severe coronavirus disease 2019 in patients with inflammatory bowel disease in the United States: a multicenter research network study. Gastroenterology. 2020;159 (4):1575–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ludvigsson JF, Axelrad J, Halfvarson J, Khalili H, Larsson E, Lochhead P, et al. Inflammatory bowel disease and risk of severe COVID‐19: a nationwide population‐based cohort study in Sweden. United Eur Gastroenterol J. 2021;9 (2):177–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bezzio C, Saibeni S, Variola A, Allocca M, Massari A, Gerardi V, et al. Outcomes of COVID‐19 in 79 patients with IBD in Italy: an IG‐IBD study. Gut. 2020;69 (7):1213–7. [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez‐Lago I, Ramirez de la Piscina P, Elorza A, Merino O, Ortiz de Zarate J, Cabriada JL. Characteristics and prognosis of patients with inflammatory bowel disease during the SARS‐CoV‐2 pandemic in the Basque country (Spain). Gastroenterology. 2020;159 (2):781–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh S, Heien HC, Herrin J, Dulai PS, Sangaralingham L, Shah ND, et al. Comparative risk of serious infections with tumor necrosis factor‐alpha antagonists vs. Vedolizumab in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous‐Hunt M, Lewis JD, et al. Effect of IBD medications on COVID‐19 outcomes: results from an international registry. Gut. 2021;70 (4):725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodriguez‐Lago I, Alonso‐Galan H, Cabriada JL. Cytokine storm in IBD: balancing the risks of IBD medical therapy. Gastroenterology. 2021;160(5):1878–80. [DOI] [PubMed] [Google Scholar]
- 75.Al‐Ani AH, Prentice RE, Rentsch CA, Johnson D, Ardalan Z, Heerasing N, et al. Review article: prevention, diagnosis and management of COVID‐19 in the IBD patient. Aliment Pharmacol Ther. 2020;52 (1):54–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Agrawal M, Brenner EJ, Zhang X, Modesto I, Woolcott J, Ungaro RC, et al. Characteristics and outcomes of IBD patients with COVID‐19 on tofacitinib therapy in the SECURE‐IBD registry. Inflamm Bowel Dis. 2021;27 (4):585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bossa F, Carparelli S, Latiano A, Palmieri O, Tavano F, Panza A, et al. Impact of the COVID‐19 outbreak and the serum prevalence of SARS‐CoV‐2 antibodies in patients with inflammatory bowel disease treated with biologic drugs. Dig Liver Dis. 2021;53 (3):277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agrawal M, Brenner EJ, Zhang X, Colombel JF, Kappelman MD, Ungaro RC. Physician practice patterns on holding inflammatory bowel disease medications due to COVID‐19 in the SECURE‐IBD registry. J Crohns Colitis. 2020;15(5):860–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin Arranz E, Suarez Ferrer C, Garcia Ramirez L, Rueda Garcia JL, Sanchez‐Azofra M, Poza Cordon J, et al. Management of COVID‐19 pandemic in Spanish inflammatory bowel disease units: results from a national survey. Inflamm Bowel Dis. 2020;26 (8):1149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubin DT, Abreu MT, Rai V, Siegel CA. International Organization for the study of inflammatory bowel D. Management of patients with Crohn's disease and ulcerative colitis during the coronavirus disease‐2019 pandemic: results of an international meeting. Gastroenterology. 2020;159 (1):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rubin DT, Feuerstein JD, Wang AY, Cohen RD. AGA clinical practice update on management of inflammatory bowel disease during the COVID‐19 pandemic: expert commentary. Gastroenterology. 2020;159 (1):350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.British Society of Gastroenterology . Adaptations to the BSG guidelines on the management of acute severe ulcerative colitis in the context of the COVID‐19 pandemic: a RAND appropriateness panel. https://www.bsg.org.uk/covid‐19‐advice/adaptation‐of‐the‐bsg‐guidelines‐on‐the‐management‐of‐acute‐severe‐ulcerative‐colitis‐in‐the‐context‐of‐the‐covid‐19‐pandemic‐a‐rand‐appropriateness‐panel/. Accessed 18 May 2021.
- 83.Lees CW, Regueiro M, Mahadevan U. International Organization for the Study of Inflammatory Bowel D. Innovation in inflammatory bowel disease care during the COVID‐19 pandemic: results of a global telemedicine survey by the International Organization for the Study of Inflammatory Bowel Disease. Gastroenterology. 2020;159 (3):805–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewin S, Lees C, Regueiro M, Hart A, Mahadevan U. International Organization for the Study of Inflammatory Bowel Disease: global strategies for telemedicine and inflammatory bowel diseases. J Crohns Colitis. 2020;14 (Suppl 3):S780–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Te Groen M, Derks MEW, Kuijpers CCHJ, Nagtegaal ID, Hoentjen F. Reduction in inflammatory bowel disease healthcare during the coronavirus disease 2019 pandemic: a nationwide retrospective cohort study. Gastroenterology. 2021;160 (3):935–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE‐II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat‐to‐target strategies in IBD. Gastroenterology. 2021;160(5):1570–83. [DOI] [PubMed] [Google Scholar]
- 87.Scaldaferri F, Pugliese D, Privitera G, Onali S, Lopetuso LR, Rizzatti G, et al. Impact of COVID‐19 pandemic on the daily management of biotechnological therapy in inflammatory bowel disease patients: reorganisational response in a high‐volume Italian inflammatory bowel disease centre. United Eur Gastroenterol J. 2020;8 (7):775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. BioRxiv. 2020. [Google Scholar]
- 89.Kumar ‐MP, Mishra S, Jha DK, Shukla J, Choudhury A, Mohindra R, et al. Coronavirus disease (COVID‐19) and the liver: a comprehensive systematic review and meta‐analysis. Hepatol Int. 2020;14 (5):711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, et al. Liver disease and outcomes among COVID‐19 hospitalized patients ‐ a systematic review and meta‐analysis. Ann Hepatol. 2021;21:100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cha MH, Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID‐19: a comprehensive review. World J Gastroenterol. 2020;26 (19):2323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Di Maira T, Berenguer M. COVID‐19 and liver transplantation. Nat Rev Gastroenterol Hepatol. 2020;17 (9):526–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sahin TT, Akbulut S, Yilmaz S. COVID‐19 pandemic: its impact on liver disease and liver transplantation. World J Gastroenterol. 2020;26 (22):2987–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marjot T, Moon AM, Cook JA, Abd‐Elsalam S, Aloman C, Armstrong MJ, et al. Outcomes following SARS‐CoV‐2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74 (3):567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Williams R, Alessi C, Alexander G, Allison M, Aspinall R, Batterham RL, et al. New dimensions for hospital services and early detection of disease: a Review from the Lancet Commission into liver disease in the UK. London, England: Lancet; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Belli LS, Fondevila C, Cortesi PA, Conti S, Karam V, Adam R, et al. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with covid‐19: results from the ELITA/ELTR multi‐center European study. Gastroenterology. 2021;160 (4):1151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Colmenero J, Rodríguez‐Perálvarez M, Salcedo M, Arias‐Milla A, Muñoz‐Serrano A, Graus J, et al. Epidemiological pattern, incidence, and outcomes of COVID‐19 in liver transplant patients. J Hepatol. 2021;74 (1):148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Efe C, Dhanasekaran R, Lammert C, Ebi B, Higuera‐de la Tijera F, Aloman C, et al. Outcome of COVID‐19 in patients with autoimmune hepatitis: an international multi‐centre study. Hepatology. 2021;73 (6):2099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de‐Madaria E, Capurso G. COVID‐19 and acute pancreatitis: examining the causality. Nat Rev Gastroenterol Hepatol. 2021;18 (1):3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS‐CoV‐2 infection. Clin Gastroenterol Hepatol. 2020;18 (9):2128–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fignani D, Licata G, Brusco N, Nigi L, Grieco GE, Marselli L, et al. SARS‐CoV‐2 receptor angiotensin I‐converting enzyme type 2 (ACE2) is expressed in human pancreatic β‐cells and in the human pancreas microvasculature. Front Endocrinol (Lausanne). 2020;11:596898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, et al. SARS‐CoV‐2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3 (2):149–65. [DOI] [PubMed] [Google Scholar]
- 103.Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology. 2020;159 (1):367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wifi M‐N, Nabil A, Awad A, Eltatawy R. COVID‐induced pancreatitis: case report. Egypt J Intern Med. 2021;33 (1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hadi A, Werge M, Kristiansen KT, Pedersen UG, Karstensen JG, Novovic S, et al. Coronavirus Disease‐19 (COVID‐19) associated with severe acute pancreatitis: case report on three family members. Pancreatology. 2020;20 (4):665–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumaran NK, Karmakar BK, Taylor OM. Coronavirus disease‐19 (COVID‐19) associated with acute necrotising pancreatitis (ANP). BMJ Case Rep. 2020;13 (9):e237903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de‐Madaria E, Siau K, Cárdenas‐Jaén K. Increased amylase and lipase in patients with COVID‐19 pneumonia: don't blame the pancreas just yet! Gastroenterology. 2021;160 (5):1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Juhász MF, Ocskay K, Kiss S, Hegyi P, Párniczky A. Insufficient etiological workup of COVID‐19‐associated acute pancreatitis: a systematic review. World J Gastroenterol. 2020;26 (40):6270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McNabb‐Baltar J, Jin DX, Grover AS, Redd WD, Zhou JC, Hathorn KE, et al. Lipase elevation in patients with COVID‐19. Am J Gastroenterol. 2020;115 (8):1286–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bansal P, Margekar SL, Suman V, Sud R, Meena S, Sharma AK, et al. Pancreatic injury in COVID‐19 patients. J Assoc Phys India. 2020;68 (12):58–60. [PubMed] [Google Scholar]
- 111.Rasch S, Herner A, Schmid RM, Huber W, Lahmer T. High lipasemia is frequent in Covid‐19 associated acute respiratory distress syndrome. Pancreatology. 2021;21 (1):306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barlass U, Wiliams B, Dhana K, Adnan D, Khan SR, Mahdavinia M, et al. Marked elevation of lipase in COVID‐19 disease: a cohort study. Clin Transl Gastroenterol. 2020;11 (7):e00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Inamdar S, Benias PC, Liu Y, Sejpal DV, Satapathy SK, Trindade AJ, et al. Prevalence, risk factors, and outcomes of hospitalized patients with coronavirus disease 2019 presenting as acute pancreatitis. Gastroenterology. 2020;159 (6):2226–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Akarsu C, Karabulut M, Aydin H, Sahbaz NA, Dural AC, Yegul D, et al. Association between acute pancreatitis and COVID‐19: could pancreatitis Be the missing piece of the puzzle about increased mortality rates? J Invest Surg. 2020:1–7. [DOI] [PubMed] [Google Scholar]
- 115.Pandanaboyana S, Moir J, Leeds JS, Oppong K, Kanwar A, Marzouk A, et al. SARS‐CoV‐2 infection in acute pancreatitis increases disease severity and 30‐day mortality: COVID PAN collaborative study. Gut. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thaweerat W. Current evidence on pancreatic involvement in SARS‐CoV‐2 infection. Pancreatology. 2020;20 (5):1013–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang J‐K, Lin S‐S, Ji X‐J, Guo L‐M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47 (3):193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S, et al. A systematic review of asymptomatic infections with COVID‐19. J Microbiol Immunol Infect. 2021;54 (1):12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Castro Filho EC, Castro R, Fernandes FF, Pereira G, Perazzo H. Gastrointestinal endoscopy during the COVID‐19 pandemic: an updated review of guidelines and statements from international and national societies. Gastrointest Endosc. 2020;92 (2):440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Libanio D, Bastos P, Pimentel‐Nunes P. Safe and valuable endoscopy in the COVID era. GE Port J Gastroenterol. 2020;27 (4):219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chai N, Mei Z, Zhang W, Du C, Wang X, Li L, et al. Endoscopy works during the pandemic of coronavirus COVID‐19: recommendations by the Chinese Society of Digestive Endoscopy. United Eur Gastroenterol J. 2020;8 (7):798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID‐19) pandemic. JAMA Netw Open. 2020;3 (8):e2017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‐based, modelling study. Lancet Oncol. 2020;21 (8):1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rees CJ, Rutter MD, Sharp L, Hayee B, East JE, Bhandari P, et al. COVID‐19 as a barrier to attending for gastrointestinal endoscopy: weighing up the risks. Lancet Gastroenterol Hepatol. 2020;5 (11):960–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sharpless NE. COVID‐19 and cancer. Science. 2020;368 (6497):1290. [DOI] [PubMed] [Google Scholar]
- 126.Sud A, Jones ME, Broggio J, Loveday C, Torr B, Garrett A, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID‐19 pandemic. Ann Oncol. 2020;31 (8):1065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Giuliani M, Papadakos T, Papadakos J. Propelling a new era of patient education into practice—cancer care post–COVID‐19. Int J Radiat Oncol. 2020;108 (2):404–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pawlak KM, Kral J, Khan R, Amin S, Bilal M, Lui RN, et al. Impact of COVID‐19 on endoscopy trainees: an international survey. Gastrointest Endosc. 2020;92 (4):925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shah R, Satyavada S, Ismail M, Kurin M, Smith ZL, Cooper GS, et al. COVID‐19 pandemic through the lens of a gastroenterology fellow: looking for the silver lining. Gastrointest Endosc. 2020;92 (2):394–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Siau K, Iacucci M, Dunckley P, Penman I, EndoTrain Survey C, Kral J, et al. The impact of COVID‐19 on gastrointestinal endoscopy training in the United Kingdom. Gastroenterology. 2020;159 (4):1582–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ong AM. Outrunning burnout in a GI fellowship program during the COVID‐19 pandemic. Dig Dis Sci. 2020;65 (8):2161–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ciacci C, Siniscalchi M. Tips from the battlefront: psychological support of patients with a chronic illness during the COVID‐19 lockdown in four steps. United Eur Gastroenterol J. 2020;8 (6):741–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Siddiqui UD, Aslanian HR. The new virtual reality: advanced endoscopy education in the COVID‐19 era. Dig Dis Sci. 2020;65 (7):1888–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Keswani RN, Sethi A, Repici A, Messmann H, Chiu PW. How to maximize trainee education during the coronavirus disease‐2019 pandemic: perspectives from around the world. Gastroenterology. 2020;159 (1):26–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boškoski I, Costamagna G. Gastrointestinal endoscopy and the COVID‐19 pandemic: urgent issues in endoscopic retrograde cholangio‐pancreatography and endoscopic training. United Eur Gastroenterol J. 2020;8 (6):743–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gralnek IM, Hassan C, Beilenhoff U, Antonelli G, Ebigbo A, Pellise M, et al. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and COVID‐19: an update on guidance during the post‐lockdown phase and selected results from a membership survey. Endoscopy. 2020;52 (10):891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Soetikno R, Teoh AYB, Kaltenbach T, Lau JYW, Asokkumar R, Cabral‐Prodigalidad P, et al. Considerations in performing endoscopy during the COVID‐19 pandemic. Gastrointest Endosc. 2020;92 (1):176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine. N Engl J Med. 2020;383 (27):2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397 (10269):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim results of a phase 1‐2a trial of Ad26.COV2.S covid‐19 vaccine. N Engl J Med. 2021;384 (19):1824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatulin AI, Shcheblyakov DV, Dzharullaeva AS, et al. Safety and immunogenicity of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine in two formulations: two open, non‐randomised phase 1/2 studies from Russia. Lancet (London, England). 2020;396 (10255):887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1–2 trial of a SARS‐CoV‐2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383 (24):2320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA covid‐19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384 (15):1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single‐dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397 (10277):881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet (London, England). 2021;397 (10275):671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384 (5):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021;384 (22):2092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund‐Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384 (22):2124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sadoff J, Davis K, Douoguih M. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination — response from the manufacturer. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.CDC and FDA. Joint CDC and FDA statement on Johnson & Johnson COVID‐19 vaccine. https://www.cdc.gov/media/releases/2021/s0413‐JJ‐vaccine.html. Accessed 27 Apr 2021.
- 151.Alexander JL, Moran GW, Gaya DR, Raine T, Hart A, Kennedy NA, et al. SARS‐CoV‐2 vaccination for patients with inflammatory bowel disease: a British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement. Lancet Gastroenterol Hepatol. 2021;6 (3):218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Melmed GY, Rubin DT, McGovern DPB. Winter is coming! Clinical, immunologic, and practical considerations for vaccinating patients with inflammatory bowel disease during the coronavirus disease‐2019 pandemic. Gastroenterology. 2021;160 (3):639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Queiroz NSF, Teixeira FV, Freire CCF, Motta MP, Vasconcellos MAM, Chebli LA, et al. Brazilian IBD study group position statement on SARS‐CoV2 vaccination. Arq Gastroenterol. 2021. [DOI] [PubMed] [Google Scholar]
- 154.Wellens J, Colombel JF, Satsangi JJ, Wong SY. SARS‐CoV‐2 vaccination in IBD: past lessons, current evidence and future challenges. J Crohns Colitis. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Siegel CA, Melmed GY, McGovern DP, Rai V, Krammer F, Rubin DT, et al. SARS‐CoV‐2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021;70 (4):635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.British Society of Gastroenterology . A joint statement on vaccination for Sars‐CoV2 in patients with liver disease. https://www.bsg.org.uk/covid‐19‐advice/a‐joint‐statement‐on‐vaccination‐for‐sars‐cov2‐in‐patients‐with‐liver‐disease/. Accessed 18 May 2021.
- 157.Talotta R. Do COVID‐19 RNA‐based vaccines put at risk of immune‐mediated diseases? In reply to “potential antigenic cross‐reactivity between SARS‐CoV‐2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2021;224:108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kennedy NA, Goodhand JR, Bewshea C, Nice R, Chee D, Lin S, et al. Anti‐SARS‐CoV‐2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021. [DOI] [PubMed] [Google Scholar]
- 159.Ray K. Antibody responses to SARS‐CoV‐2 infection are attenuated in infliximab‐treated patients with IBD. Nat Rev Gastroenterol Hepatol. 2021:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kennedy NA, Lin S, Goodhand JR, Chanchlani N, Hamilton B, Bewshea C, et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV‐19 SARS‐CoV‐2 vaccines in patients with IBD. Gut. 2021. [DOI] [PubMed] [Google Scholar]
- 161.Gisbert JP, Villagrasa JR, Rodríguez‐Nogueiras A, Chaparro M. Kinetics of anti–hepatitis B surface antigen titers after hepatitis B vaccination in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19 (3):554–8. [DOI] [PubMed] [Google Scholar]
- 162.Cleveland NK, Rodriquez D, Wichman A, Pan I, Melmed GY, Rubin DT. Many inflammatory bowel disease patients are not immune to measles or pertussis. Dig Dis Sci. 2016;61 (10):2972–6. [DOI] [PubMed] [Google Scholar]
- 163.Ting S‐W, Chen Y‐C, Huang Y‐H. Risk of hepatitis B reactivation in patients with psoriasis on ustekinumab. Clin Drug Invest. 2018;38 (9):873–80. [DOI] [PubMed] [Google Scholar]
- 164.de León‐Rendón JL, Hurtado‐Salazar C, Yamamoto‐Furusho JK. Aspects of inflammatory bowel disease during the COVID‐19 pandemic and general considerations. Rev Gastroenterol México (English). 2020;85 (3):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID‐19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74 (4):944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weisblum Y, Schmidt F, Zhang F, DaSilva J, Poston D, Lorenzi JC, et al. Escape from neutralizing antibodies by SARS‐CoV‐2 spike protein variants. Elife. 2020;9:e61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rees‐Spear C, Muir L, Griffith SA, Heaney J, Aldon Y, Snitselaar JL, et al. The effect of spike mutations on SARS‐CoV‐2 neutralization. Cell Rep. 2021;34 (12):108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wise J. Covid‐19: Pfizer BioNTech vaccine reduced cases by 94% in Israel, shows peer reviewed study. BMJ. 2021;372:n567. [DOI] [PubMed] [Google Scholar]
- 169.Ma C, Cong Y, Zhang H. COVID‐19 and the digestive system. Am J Gastroenterol. 2020;115 (7):1003–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Karimi‐Sari H, Rezaee‐Zavareh MS. COVID‐19 and viral hepatitis elimination programs: are we stepping backward? Liver Int. 2020;40 (8):2042. [DOI] [PubMed] [Google Scholar]
- 171.Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh Q‐D. Cancer screening tests and cancer diagnoses during the COVID‐19 pandemic. JAMA Oncol. 2021;7 (3):458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


