Abstract
Background
Androgenetic alopecia (AGA) is the most common hair loss disorder seen in men. It can have an early onset but has also been associated with ageing and senescence. It often induces pronounced psychological impact. ALRV5XR, a new hair loss treatment herein evaluated, was designed to target multiple molecular pathways involved in hair growth and hair follicle stem cell biology. The main objectives of the study were the assessment of safety and efficacy profiles of ALRV5XR in men.
Methods
This 24-week, parallel randomised, placebo-controlled, double-blinded clinical trial was performed in a USA community clinic. Healthy men (age 22–65) with AGA and belonging to the Hamilton-Norwood (HN) classification I–VII and Fitzpatrick skin type (FST) I–VI, were randomly allocated in a 1:1 ratio into ALRV5XR or placebo treatment groups. Dermatologist assessment, phototrichograms, and blood samples were obtained in a blinded fashion at baseline, 12 and 24 weeks. Subjects were given a masked treatment consisting of oral capsules, shampoo, conditioner, and follicle serum, which was intended for daily use. Efficacy was assessed via absolute and per cent changes in terminal hair (TH) density, and response rates. The trial was registered with clinicaltrials.gov (NCT04450589) and is completed.
Findings
Forty-six subjects were enroled in the study, 23 allocated to the ALRV5XR treatment and 23 to the placebo group. Enrolment occurred from April 11 to October 23, 2018. Thirty-six subjects completed the trial (17 ALRV5XR, 19 placebo) and 11 subjects in each group were evaluable for TH outcomes. At 24 weeks, the absolute change in TH density improved by 21·0 THs/cm2 (95% CI: 9·2–32·8; p = 0·0014), and the relative density increased by 16·4% (95% CI: 7·4%–25·5%; p = 0·0012). The odds ratio for being a responder (≥ 0 change) was 87·4. TH density increased linearly and was not affected by HN, FST, ethnicity, age, or body mass index. All subjects in the ALRV5XR group responded to treatment while 81·8% of the placebo group decreased TH density. ALRV5XR induced statistically significant changes in both decrease in vellus hair (VH) density as well as in concomitant increase of the TH/VH ratio when compared to placebo. ALRV5XR was well tolerated, and no adverse events were observed.
Interpretation
ALRV5XR treatment resulted in clinically significant TH regrowth in men with AGA. Furthermore, it appeared to reverse the characteristic hair miniaturisation seen in this condition. When compared to results of published trials of standard therapy, ALRV5XR showed a multi-fold increase both in efficacy and in response rates. In addition, the continuance of TH regrowth from 12 to 24 weeks suggests that the normal structure and function of non-productive telogen follicles is restored and that a normal hair phenotype may be attained by extended ALRV5XR treatment.
Funding
Arbor Life Labs.
Keywords: ALRV5XR, Alopecia, Androgenetic alopecia, Male pattern hair loss, Hair restoration, Minoxidil, Finasteride, Botanical, Vitamin, Supplement, Senescence, Ageing, Wnt/beta-catenin, Stem cell, Hair regeneration, Regenerative medicine, Clinical trial, PRP, Terminal hair, Vellus like hair, Terminal vellus ratio
Research in context.
Evidence before this study
Hair follicle (HF) maintenance and regeneration is a complex process governed by the coherent expression of more than 1000 genes and is dependant on HF stem cell (HFSC) activation and multiple signalling pathways. The Wnt/β-Catenin pathway in HFSCs is an essential component of hair regrowth and maintenance along with modulation of various cytokines, growth factors and hormones. ALRV5XR was developed after studying botanicals, vitamins and minerals that activate Wnt/β-Catenin in HFSCs in vitro and HFs in vivo, and was formulated for oral or topical administration with the aim of influencing more than 20 of the accepted pathways involved in normal HF physiology.
Added value of this study
In men with androgenetic alopecia (AGA), ALRV5XR was found to be clinically effective in preventing HL, regrowing terminal hair (TH) and possibly modifying key pathological features of AGA and senescent alopecia via reversal of hair miniaturisation, all with no side effects. It also displayed a multi-fold increase in response rates and regrowing TH in a shorter time when compared to results of published trials using standard therapies. ALRV5XR's, induction of continuous TH regrowth suggests that its long-term use could restore the normal scalp hair phenotype in men.
Implications of all the available evidence
ALRV5XR may become the first-line standard of care hair regeneration treatment for men with HL. Evaluation of its long-term safety and efficacy profile should be addressed in future research. Similarly, elucidation of the main mechanisms underlying ALRV5XR action might lead to identification of distinct features as well as targets of importance for the regeneration of other tissues.
Alt-text: Unlabelled box
1. Introduction
Alopecia or hair loss (HL) of the scalp and most notably, androgenetic alopecia (AGA) and senescent alopecia (SA) are common clinical signs associated with ageing and cellular senescence [1,2]. Throughout history and to the present, luxuriant hair has been a symbol of health, youthfulness, and virility [3]. The effects of HL on emotional distress and quality of life can have psychological impact, as suggested by studies on self-esteem and body image [3]. HL in men generally presents as AGA, or telogen effluvium (TE) and in men over 60 it also presents as SA [1]. AGA, also called male-pattern hair loss (MPHL), has various commonalities with SA and with the senescence of the hair follicle (HF) [1,2]. While the expression of over 750 genes were found to be common in both AGA and SA, the expression of the Wnt/β-Catenin associated genes has been shown to play a major role in hair generation and maintenance [4,5]. AGA is a heritable, benign, and age-dependent process that displays an appearance-altering trait characterised by a progressive decline in scalp hair density in a sex-dependent pattern [1,3]. While onset of hair thinning begins between the ages of 12 and 40, AGA is present in more than half of men by the age of 50 and in 75% of men over 80 [6,7]. AGA is linked to the metabolic syndrome and its comorbidities, and is an independent predictor of mortality from diabetes mellitus, heart disease and COVID-19 [8,9]. TE is a medical sign related to underlying conditions rather than to a discrete disorder and can resolve spontaneously in otherwise healthy men [1]. Triggering events for TE include acute physical stressors such as surgery, illness, or weight loss, hormonal imbalances, psychological stress, drugs, nutritional deficiencies and scalp inflammation [1]. Miscellaneous and less-common hair disorders and alopeciae have also been associated with deficiencies of minerals such as zinc, selenium, iron and copper as well as of vitamins such as B3, D, and occasionally biotin [10]. Several drugs often interact with absorption or activity of various nutrients relevant to hair health as in the cases of the commonly used metformin which interferes with vitamin B12, statins with selenium and coenzyme Q10, and ACE inhibitors, diuretics and antacids that interfere with zinc [10].
HFs are epithelial skin mini-organs that produce individual hairs by modulating and sustaining hair regrowth over repeated hair cycles [2]. The greatest HF density on the human body is on the scalp, and the normal healthy adult scalp phenotype has a total hair count ranging from 100,000–150,000 hairs. Corresponding density ranges from 118 to 350 HFs per cm2 [1,11]. A normal scalp averages seven terminal hairs (TH) per each vellus hair (VH) [1]. THs are large, pigmented, and have a shaft diameter of 30–150 µm, while diameter of VHs are less than 30 µm, and are short, depigmented and barely visible [1,11].
There is accumulating evidence that AGA is the result of organ-specific premature ageing of HFs [1]. Age-related reduction in hair regrowth and miniaturised hair shaft diameter along with hair cycle abnormalities, including increased time in the resting stage (telogen), and more specifically from the loss of hair (exogen) through the empty follicle phase (kenogen), until the emergence of a replacement hair (neogen) have been noted [12,13]. Similarly, decreased duration of the growth phase (anagen) has been reported [12]. In general, AGA leads to HF miniaturisation resulting in the gradual replacement of THs by vellus-like hairs and the eventual deletion of the HF [1,12]. Furthermore, HF miniaturisation appears indistinguishable between SA and MPHL [1]. In any case, both clinical entities display similar reductions in total hair count and TH/VH ratio [10].
At the molecular level, hair growth and the physiological cycle of the HF are determined by the complex and rhythmic interaction of multiple pathways [13]. Interestingly, these elements often receive modulating and even apparently opposing influences such as those of cytokines, hormones, prostaglandins and growth factors [13]. Positive and negative forces acting simultaneously on HFs, simply reflect the various aspects of a complex and dynamic phenomenon occurring in a specific physiological period [13], thereby suggesting multiple and miscellaneous pathological causes of HL. In consequence, to improve efficacy, the next generation of HL therapies should include a combination of agents that promote growth and regrowth, along with agents that delay catagen entry while relieving stress on the follicle [14]. Contextually, the canonical Wnt/β-Catenin signalling in HF stem cells is seen as the central component controlling hair cycle induction and maintenance [2]. This cascade is less active in aged vs. young telogen HF stem cells [2]. Furthermore, reduced activity of the Wnt/β-Catenin pathway has been aetiologically linked to hair senescence and AGA [2].
Most available pharmaceutical and non-pharmaceutical hair regrowth treatments target a single, or a limited number of pathways, using high-dose active ingredients. They are effective in only a subset of individuals and, possibly due to the elevated doses needed to attain effective results, are associated with miscellaneous adverse effects [14,15]. Topical minoxidil, at concentrations of 2% and 5%, and oral finasteride (1 mg) were approved for treatment of AGA in men in 1986, 1993 and 1992, respectively [15]. These agents remain as the only FDA approved pharmaceuticals to treat men affected with AGA. Currently, there is no FDA approved treatment for TE or SA. According to a recent meta-analysis, the average improvement due to minoxidil or finasteride vs. placebo in men's hair density was 11·5, 14·9 and 14·8 THs/cm2 after 48 weeks of treatment with 2%, 5% minoxidil or finasteride (1 mg), respectively [7,15,16]. Although 34%, 58% and 52% of male patients with AGA responded to minoxidil 2%, 5% and finasteride (1 mg) treatment, respectively, various adverse events were observed, including sexual dysfunction, suicidality, depression, and anxiety while using finasteride [15,17–19].
Minoxidil is a potassium channel opener that has been associated with miscellaneous mechanisms of action (MOAs) such as increased HGF, PGE and VEGF expression as well as EGF inhibition. Minoxidil is also thought to stimulate the Wnt/β-Catenin pathway [14,15,20]. Finasteride is a type 2 and 3 5α-reductase inhibitor which decreases the conversion of testosterone to dihydrotestosterone (DHT) in younger men by about 65% [15]. It induces similar actions in the prostate gland and scalp [15]. Nevertheless, in men older than 60 years, finasteride is not an effective treatment for MPHL [6]. Despite multiple MOAs of minoxidil, TH density changes in AGA patients tend to peak at 12–16 weeks and decline thereafter [21]. Furthermore, upon treatment discontinuation, there is a rapid regression to a density lower than that seen at baseline [21]. This response to treatment has been explained as a triggering of follicles in the latent part of telogen into anagen without prolongation of anagen or reversal of follicular miniaturisation [21]. There are several studies evaluating terminal hair regrowth with finasteride (1 mg). Most of them were done in men between the ages of 18 and 41 [7,16]. Total hair density peaked at 12 months and was maintained in the second year [6]. After treatment cessation, regression to a density lower than that at baseline was observed, although at a slower rate than observed in minoxidil studies [6]. Fifty-two percent of treated men with early stages of HL had a total hair regrowth response, and the odds ratio (OR) of finasteride vs. placebo was 1·67 [7,19]. Interestingly, insulin-like growth factor-I (rhIGF-1) treatment induced the appearance of normal hair and recovery of hairline in IGF-1 deficient populations, thereby highlighting the wide range of molecules that act on the physiology of hair [22]. Combination therapies of topical minoxidil with finasteride, other pharmaceuticals and or supplements have been studied in randomised placebo-controlled trials (RCTs) in men. However, no statistically significant differences with minoxidil or finasteride monotherapies were found [15]. RCTs using Platelet-Rich Plasma (PRP) for the treatment of AGA showed a significant increase in hair density in men [15]. Combination therapies of PRP supplemented by autologous stem cell transplants, micro-needle wounding and low-level laser therapy (LLLT) have also reported significant improvements in hair regrowth [23]. RCTs with vitamins, minerals, botanical extracts, collagen and various marine extracts have shown limited efficacy of TH regrowth in men [15]. Unfortunately a large fraction of men with AGA do not respond to standard treatments, clearly highlighting an unmet need for safe and effective HL therapies.
Aiming to restore normal HF homoeostasis, ALRV5XR was designed to target various of the multiple underlying physiological causes of HL. The agent is a formulation of standardised botanical extracts, vitamins, and minerals that can be delivered both systemically, as a supplement, or topically, as a shampoo, conditioner, or follicle serum. ALRV5XR is intended to support normal biochemical signalling, thereby contributing to normal HF physiology. ALRV5XR aims to induce neogen in involuted HFs and prolong anagen of HFs. This combination of actions should aid to regenerate the normal scalp hair phenotype. ALRV5XR was also designed to modulate, directly or indirectly, several molecular pathways of the HF associated with the promotion of hair growth and regrowth, as well as those involved in HL inhibition. Amongst them, activation of the Wnt/β-Catenin cascade in HF stem cells and dermal papilla cells appears to be a key driver of new hair formation and growth maintenance [2,4,5,13,14,24]. While activation of the Wnt/β-Catenin signalling pathway with botanical extracts and compounds has been demonstrated [25], inhibition of targets linked to HL is also important as in the case of BMP-4, DHT, or the enzymatic androgen regulator 5α-reductase. In addition, increasing VEGF signalling to enhance blood flow to HFs, which is linked to the promotion of hair growth is relevant for attainment of a healthy hair physiology [13,14]. Similarly the regulation of additional growth factors such as FGF-7, FGF-18, HGF, IGF, KGF, and cytokines such as mTORC1, PKC, PPARγ, Shh, COX, INF-α, INF-γ, Interleukins 1b, 6 and 12, NF-κ β, Prostaglandin D2, TGF-β, and TNF-α [13,26] are likely to be influenced at least indirectly by several of the ingredients of ALRV5XR. We demonstrated that ALRV5XR vs. placebo has a clinically significant TH regrowth effect in women with AGA or TE over 24 weeks, where absolute TH density improved by 30·1 THs/cm2 (p = 0·0002), and relative TH density increased by 19·7% (p = 0·0016) [27]. It acts by regenerating the structure and function of non-productive telogen follicles and by prolonging the anagen phase of TH [27]. Our hypothesis is that the wide molecular targeting approach of ALRV5XR, aimed to modulate miscellaneous pathways, results in a safe and efficacious treatment that achieves regeneration and maintains TH in men.
2. Methods
2.1. Study design
This 24-week parallel, randomised, double-blind, placebo-controlled trial was conducted in San Francisco, California, USA in a single centre community clinic. The study protocol was granted ethics approval on March 22, 2018, by IRB Services, identified with the FDA #IRB00000776. The trial was also registered with ClinicalTrials.gov as NCT04450589 and is completed. A CONSORT checklist has been completed and we declare adherence.
2.2. Sample size calculation
The study design was based on the expected efficacy reported in previous minoxidil studies, and required a sample size of 36 subjects, 18 in each group (before dropouts), to achieve a power of 80% and a significance level of 5%. This sample size was predicted to demonstrate clinically meaningful differences between ALRV5XR and placebo groups, as assessed by total hair counts from baseline to week 24. For sample size calculation, the primary objective of absolute difference in hairs/cm2 took primacy over the second primary objective of relative difference in per cent. Sample size was calculated using a two-sample t-test power calculation for a one-sided hypothesis test based on a mean absolute target difference of 20 total hairs/cm2 ( ± 23). We anticipated a dropout rate of 20% thereby adjusted the sample size to 23 per group, for a total sample size of 46. Using the same power and significance levels, we further estimated a sample size of evaluable subjects based on previous studies of mean absolute TH differences of 14·7 TH/cm2 ( ± 3·1) for 5% minoxidil and 17·5 TH/cm2 ( ± 8·7) finasteride (1 mg) vs placebo [7]. Hence, a sample size of 10 evaluable subjects in each group was suitable to determine primary efficacy outcomes.
2.3. Study participants
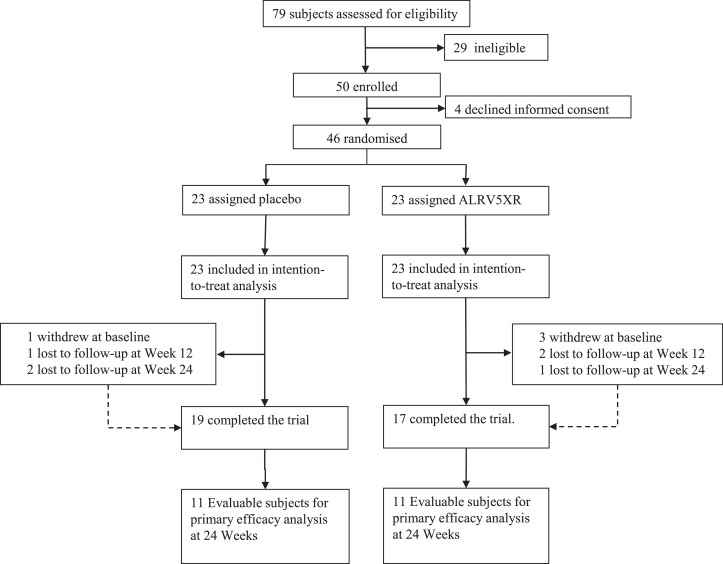
Eligible subjects were healthy 18–65-year-old males with self-reported hair thinning, AGA, TE, or hair loss occurring for more than 3 months prior to screening. Hair loss was confirmed by physical examination done by a certified dermatologist who excluded ineligible types of alopecia, hair, or scalp disease, and assigned a clinical diagnosis of AGA or TE, later confirmed by phototrichometry. AGA was determined clinically by observing hair thinning and miniaturisation in the region of the scalp that was consistent with the Hamilton-Norwood (HN) classification I–VII. AGA diagnosis was confirmed by phototrichogram analysis of diversity of TH diameters and then graded with the anisotrichosis score (the ratio of one standard deviation of TH diameters to the mean TH diameter) of more than 20% and a TH/VH ratio of < 4:1. TE was diagnosed by subject history including reports of self-reported thinning hair and shedding, by evaluating signs of general thinning or diffuse hair loss of the scalp, and by directly ruling out underlying treatable causes. Phototrichogram analysis of TH telogen rate greater than 20% was used to confirm the TE diagnosis. HN classification was assigned by the clinical dermatologist and verified from global photographs. For this trial, a primary diagnosis of AGA or TE was a prerequisite before subjects were randomised to a given study arm. As seen in Fig. 1, seventy-nine subjects were assessed; 50 deemed as eligible for enrolment in the trial with a primary diagnosis of AGA, and 46 subjects were further enroled and randomised.
Fig. 1.
Profile of Randomised Controlled Trial. 79 subjects were assessed for eligibility which resulted in 19 subjects in the placebo group and 17 subjects in the ALRV5XR (treatment) group completing the trial and were included in the safety analysis. Evaluable subjects for primary efficacy analysis at 24 weeks resulted in 11 from the placebo group and 11 from the ALRV5XR group.
Exclusion criteria included scarring types of alopecia, chemical or physical burns, and traumatic lesions. Reasons for exclusion also included psoriasis, scaling, and follicular dermatitis, fungal or bacterial infection, lice, and flea infestation of the scalp. Additional reasons for exclusion included subjects with unusual thinning patches, traction alopecia or trichokryptomania, trichothiodystrophy, pili annulati, monilethrix or clear signs of trichodysmorphia, undernutrition or poor hygiene. Subjects with abnormalities in or around assessment areas, and scalp hair loss on treatment surfaces due to disease, injury, skin damage or medical therapy as well as those with history of hair transplants, surgical correction of scalp hair loss, and hair weave were also excluded. Men with a known history of HIV/AIDS, or autoimmune diseases such as systemic lupus erythematosus, inflammatory bowel disease, alopecia areata, and alopecia totalis, were not included.
Participating men were not allowed to use razors, depilatories, or wax on the scalp if, in the opinion of the investigators, it might interfere with study assessments. Subjects diagnosed with neurological diseases such as Parkinson's disease, stroke, and traumatic brain injury and those with diabetes, endocrine, cardiovascular disease, or hypertension were excluded. Evidence of hepatic or renal dysfunction were additional reasons for exclusion. Subjects with history of malignancy in the past 5 years or undergoing chemo or radiation therapy were also not included. Men who were on hormone replacement therapy within the past six months were also excluded. Subjects with uncontrolled thyroid disease or any other disorders that may interfere with the treatment were additional reasons for exclusion. Participants were not allowed to use any medications within 30 days prior to the baseline visit, including natural health products, if these were known to affect hair growth.
Forty-six eligible subjects with a diagnosis of AGA (FST I-VI, HN hair loss classification I–VII), as clinically established by a certified dermatologist, were enroled if willing to follow study requirements and procedures, and after signing an IRB approved informed consent form.
2.4. Randomisation and masking
Randomisation and masked allocation into two blinded groups were conducted as follows: All screened subjects were allocated a sequential screening number by a dermatologist. A second dermatologist assigned the screening numbers of the eligible subjects sequentially to a biostatistician generated simple randomisation table. This resulted in subjects being randomised in a 1:1 ratio into one of two parallel blinded treatment groups: Group A or B. Both dermatologists were masked from subject identity, characteristics, and diagnosis, to avoid potential bias during the randomisation process.
Study materials and label codes were masked for study subjects, site dermatologists, site personnel, tricho-analyst, and biostatistician. Upon completion of the biostatistician's report, the trial was unblinded and group A became the Placebo group and Group B the ALRV5XR group. An assessment of randomisation and masking success was evaluated by various factors including baseline characteristics, global photographs, test material dispensing records and inventory, and by observing positive responders in the placebo group.
2.5. Procedures
Similar looking test materials for both groups included oral capsules, shampoo, conditioner, and follicle serum. The ALRV5XR treatment contained botanical extracts, vitamins, and minerals (BVM) as listed in Table S2 of the supplementary materials. Each of the BVMs was dosed well within their safe limits or recommended daily allowance. Placebo test preparations contained identical vehicle compounds as those in the ALRV5XR group materials. Arbor life labs provided the ALRV5XR and placebo study materials.
All subjects were required to ingest one supplement capsule (842 mg) twice per day, along with daily use of a shampoo (3–7 ml), conditioner (3–7 ml), and follicle serum (1 ml) for a period of 24 weeks. Subjects were required to maintain a diary of the study materials used and to report adverse events. Study duration encompassed 28 weeks, including a screening period designed for eligibility assessment, a baseline visit, and visits at 12 and 24 weeks during the 24 week comparison period.
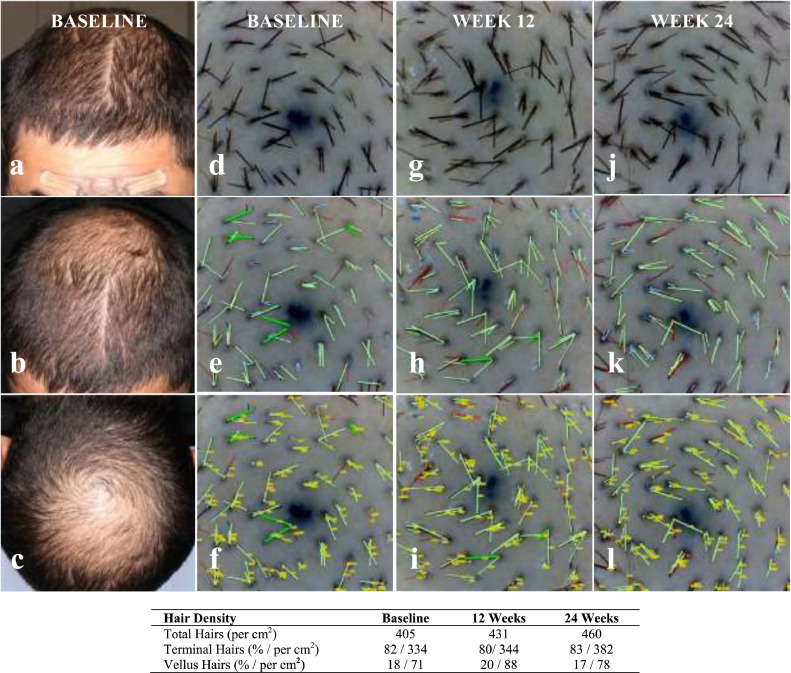
Subjects were evaluated by a certified dermatologist for eligibility, hair loss diagnosis, and hair loss pattern classification. At weeks 12 and 24, subjects were assessed for changes in safety parameters. A 35 mm Nikon digital camera mounted on a Canfield Scientific stereotactic device was used to take 8MP resolution global photographs of the frontal, mid-scalp, and vertex of the subject's head, at 0, 45 and 90° (See Fig. 2).
Fig. 2.
Sample Global Photographs and Corresponding Phototrichoscopy Computer Image Analysis at baseline, week 12 and week 24. Phototrichoscopy images for Subject: M013 were analysed with the TrichoSciencePro software (version 1.6): a: Baseline (BL) Global Frontal; b: BL Global Top; c: BL Global Vertex; d: BL Phototrichoscopy (PT); e: BL PT Processed (PR); f: BL PT PR & Tagged (TG); g: Week 12 (W12) PT Raw; h: W12 PT PR; i: W12 PT PR & TG; j: Week 24 (W24) PT Raw; k: W24 PT PRD; l: W24 PT PR & TG. This analysis resulted in the following sample measurements: Subject: M013; Sex: Male; Age: 34; Ethnicity: Hispanic; Ethnic Hair Type: Latin/Indian/Semitic; Fitzpatrick Skin Type: IV; Diagnosis: Androgenetic Alopecia; Hamilton-Norwood Class: IIIV; Trichoscopy Area of View: 42·19 mm2; Trichoscopy Magnification: 40x.
Target area hair counts (TAHC) were performed at baseline, and at week 12 and 24. At baseline, a circular area at the centre of the trichoglyph located on the vertex region of the scalp was chosen for the TAHC. A permanent ink dot tattoo was placed for precise localisation of the target area on subsequent evaluations. Hairs in the target area were clipped to less than 0·5 mm and then dyed. Mineral oil was applied to the target area to enhance the image contrast between hair and scalp.
Phototrichoscopic images of the target area were taken at each visit, using a Firefly DE330T Trichoscope with 2MP resolution at 40x magnification, and a 42·19 mm2 field of view. A cover glass was fixed to the lens of the trichoscope to protect the lens and to orientate hair shafts in the target area at 90° to the lens. Evaluable phototrichoscopies for outcomes analysis required that all the procedures be executed, and that the field of view was in focus and the target area was uncontaminated of undyed hair, fluorescence, excess mineral oil, hair clippings or confounding artefacts.
Phototrichoscopy images were evaluated by a blinded tricho-analyst using the TrichoSciencePro version 1.6 computer software. Terminal (non-vellus) hair density was determined by counting hairs in the field of view and by measuring their thickness. It was required that hairs be fully visible from the trimmed end to the exiting position of a scalp pore. THs were identified by a hair diameter greater than or equal to 40 µm, while VHs were those with a diameter of less than 40 µm. Automatic computer analysis of digital images was used to determine hair count and to measure hair shaft diameter. This was repeated and compared using semi-automatic and manual detection, and by diameter measurement performed by a tricho-analyst. TH density was calculated from the number of THs in the TAHC per cm2. (See Fig. 2).
2.6. Outcomes measures
The purpose of this study was to evaluate the efficacy and safety of ALRV5XR used for hair regrowth in men with AGA and TE. The primary efficacy objective was the quantification of terminal (non-vellus) hair density change from baseline to week 24, as measured by TH count (THC), and evaluation of TH regrowth by per cent change. Secondary objectives included evaluation of the TH density change from baseline to week 12 and ALRV5XR response rates, as measured in graduated intervals of TH regrowth changes at weeks 12 and 24. Individuals with no TH loss were classified as responders and were grouped by graduated levels of changes in TH density at weeks 12 and 24. A quality of life (QoL) questionnaire was completed by subjects at each visit.
2.7. Safety outcomes
Safety of ALRV5XR was evaluated based on dermatologist assessments and subject reporting. Safety analysis also included physical examinations and blood and urine laboratory tests performed on subjects completing each visit.
2.8. Statistical analysis
Comparisons of treatment and placebo groups were performed using Welch's two-sample t-test which allows comparison of group means with differing variances. This method was also used to compare changes within each treatment group from baseline to week 12 and from baseline to week 24, and then confirmed with analysis of variance (ANOVA) using Fisher's F-test. For comparison of categorical variables between treatment groups at baseline, Pearson's chi-squared test (with simulated p-value) was used [28,29]. Statistical significance was determined as p values to be less than or equal to 0.05.
Longitudinal hair variations over time were assessed using a linear mixed-effects model which examines the change in THs from baseline to week 24. All covariates were adjusted, and demographic and physical subject characteristics such as age, weight, ethnicity, skin type, diagnosis and Hamilton-Norwood classification were considered [[30], [31], [32]]. See Appendix S1 in the Supplementary materials for further details. All statistical analyses were performed using the R software version 4.0.5 (2021–3–31) [33].
2.9. Role of the funding source
The funder of the study had no role in the recruitment of subjects or data collection. The funder had a role in the study design, data analysis and interpretation, as well as writing the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit the report for publication.
3. Results
3.1. Study participants
A total of 46 men with an age range of 22 to 65 years and a primary diagnosis of AGA were enroled in the study. Twenty-three men were randomly assigned to each group between April 11 and October 23, 2018, and were included in the intent-to-treat population (ITT). After baseline assessment, one subject in the placebo and three in the ALRV5XR group withdrew. The placebo group had one subject who missed the week 12 visit and two subjects the week 24 visit, and were therefore excluded. Two subjects in the ALRV5XR group were lost to follow-up at the week 12 visit and one subject at the week 24 visit, and were also excluded. In summary, 19 subjects receiving placebo and 17 receiving ALRV5XR completed the trial. All subjects completing each visit were compliant with study procedures and with proper use of the materials provided. Missing data or non-evaluable trichoscopic images required to determine a difference from baseline were excluded from the outcomes analysis. Phototrichoscopies from 11 subjects in each group were evaluable at 24 weeks to determine primary efficacy outcomes (see Fig. 1). Baseline demographic and physical characteristics of the ALRV5XR and placebo groups including sex, age, height, weight, body mass index (BMI), blood pressure, TH density, ethnicity, FST, diagnosis, or HN classification can be seen in Table 1.
Table 1.
Baseline Characteristics by Treatment Group (ITT Population).
| Placebo | ALRV5XR | |
|---|---|---|
| (N = 23) | (N = 23) | |
| Sex | ||
| Male | 23 (100%) | 23 (100%) |
| Female | 0 (0%) | 0 (0%) |
| Age (years) | ||
| Mean ( ± SD) | 45·7 ( ± 12·9) | 48·3 ( ± 7·9) |
| Range | 22–65 | 32–63 |
| Anthropometrics | ||
| Height (cm)† | 172·7 (170·3–185·5) | 175·3 (170·5–177·8) |
| Weight (kg)† | 80·2 (69·3–90·8) | 81·4 (73·7–93·6) |
| BMI (kg/m2)† | 25·1 (22·6–31·2) | 27·1 (23·7–31·8) |
| BP (mmHg) | 128·3/84·1 ( ± 13·5/9·9) | 128·1/84·4 ( ± 21·5/17·9) |
| Trichometry | ||
| Terminal hair density (per cm2) | 141·6 ( ± 66·2) | 139·1 ( ± 68·6) |
| Vellus hair density (per cm2) | 139·2 ( ± 62·2) | 123·7 ( ± 66·2) |
| Ethnicity | ||
| African American | 4 (17·4%) | 4 (17·4%) |
| Caucasian | 10 (43·5%) | 7 (30·4%) |
| Chinese | 5 (21·7%) | 6 (26·1%) |
| Hispanic | 1 (4·3%) | 3 (13%) |
| Indian | 1 (4·3%) | 0 (0%) |
| Filipino | 0 (0%) | 1 (4·3%) |
| Korean | 1 (4·3%) | 0 (0%) |
| Mongolian | 0 (0%) | 1 (4·3%) |
| Thai | 0 (0%) | 1 (4·3%) |
| Vietnamese | 1 (4·3%) | 0 (0%) |
| Fitzpatrick Skin Type | ||
| I | 3 (13%) | 1 (4·3%) |
| II | 3 (13%) | 2 (8·7%) |
| III | 10 (43·5%) | 15 (65·2%) |
| IV | 2 (8·7%) | 1 (4·3%) |
| V | 2 (8·7%) | 1 (4·3%) |
| VI | 2 (8·7%) | 3 (13%) |
| Unknown | 1 (4·3%) | 0 (0%) |
| Diagnosis | ||
| AGA | 23 (100%) | 23 (100%) |
| TE | 0 (0%) | 0 (0%) |
| Hamilton–Norwood Classification | ||
| I | 4 (17·4%) | 0 (0%) |
| II | 2 (8·7%) | 1 (4·3%) |
| IIA | 2 (8·7%) | 1 (4·3%) |
| III | 1 (4·3%) | (0%) |
| IIIA | 0 (0%) | 1 (4·3%) |
| IIIV | 5 (21·7%) | 6 (26·1%) |
| IV | 1 (4·3%) | 4 (17·4%) |
| V | 0 (0%) | 1 (4·3%) |
| VA | 3 (13%) | 2 (8·7%) |
| VI | 1 (4·3%) | 0 (0%) |
| VII | 2 (8·7%) | 3 (13%) |
| Missing | 2 (8·7%) | 4 (17·4%) |
Abbreviations: ± or SD=standard deviation; † Height, Weight and BMI are reported as Median (IQR).
3.2. Outcomes
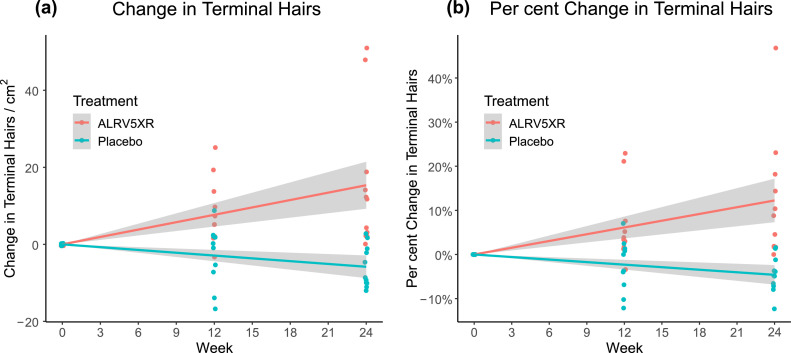
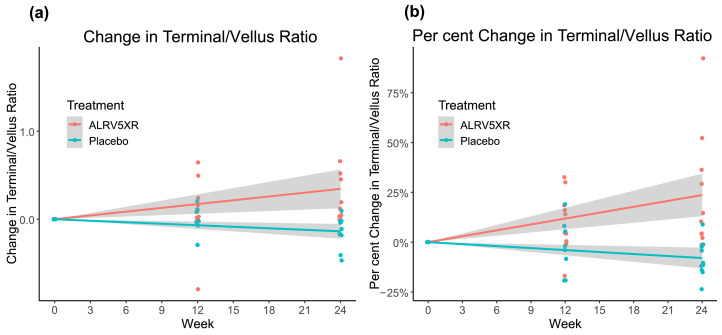
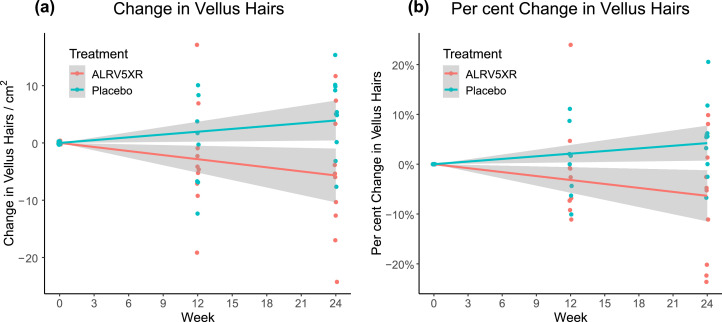
Results of hair density analysis were determined per protocol from evaluable phototrichoscopies and are summarised in Tables 2 and 3. They depict the mean changes in THs/cm2, and by per cent changes, and are stratified by visit, treatment group, and response rates. The main results are shown in Figs. 3–5. Fig. 3 depicts a strong and significant correlation between treatment duration and ALRV5XR efficacy, measured as increases in TH density. Also, during the trial, there was a statistically significant decrease in VH density when comparing ALRV5XR to placebo (Fig. 4). It was accompanied by a statistically significant increase in the TH to VH ratio (see Fig. 5). After adjusting for categorical variables (age, weight, ethnicity, diagnosis, HN classification and FST), none of the covariates affected the outcome in a statistically significant manner. Differences in QoL changes at 24 weeks between the ALRV5XR and placebo groups were not statistically significant. There is no multiplicity for secondary outcomes.
Table 2.
Summary of Efficacy: Mean Changes in Hair. This table shows statistically significant efficacy in primary outcomes of the ALRV5XR group measured by increases in absolute and per cent Terminal Hair at week 24. Secondary outcomes all show statistically significant changes in favour of the ALRV5XR group at 24 weeks and in absolute and per cent Terminal Hair at week 12. The effect size shows highly favourable odds ratios in favour of ALRV5XR at 12 weeks, with multi-fold increases from 12 to 24 weeks.
| Placebo |
ALRV5XR |
Efficacy |
Effect size | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Min | Max | N | Mean | SD | Min | Max | ∆-Mean | (95% CI) | p-value | Odds Ratio | |
| Terminal hairs/cm2 | ||||||||||||||
| Week 0–12 | 10 | −2·9 | ± 8·0 | −17 | 9 | 11 | 8·0 | ± 8·4 | −3 | 25 | 10·9 | 3·4 – 18·4 | 0·0065 | 10·0 |
| Week 0–24 (Primary) | 11 | −5·8 | ± 5·5 | −12 | 3 | 11 | 15·2 | ± 18·0 | 0 | 51 | 21·0 | 9·2 – 32·8 | 0·0014 | 87·4 |
| Terminal Hairs (%) | ||||||||||||||
| Week 0–12 | 10 | −2·5% | ± 6·0% | −12·1% | 7·0% | 11 | 6·7% | ± 8·2% | −3·4% | 22·9% | 9·3% | 2·7 – 15·9% | 0·0084 | 10·0 |
| Week 0–24 (Primary) | 11 | −4·5% | ± 4·1% | −12·3% | 1·6% | 11 | 11·9% | ± 13·8% | 0 | 46·8% | 16·4% | 7·4 – 25·5% | 0·0012 | 87·4 |
| Vellus Hairs/cm2 | ||||||||||||||
| Week 0–12 | 8 | −0·3 | ± 7·8 | −12 | −8 | 10 | −2·8 | ± 9·6 | −19 | 17 | −2·6 | −11·4 – 6·3 | 0·5511 | 0·2 |
| Week 0–24 | 10 | 4·8 | ± 6·9 | −8 | 15 | 10 | −5·7 | ± 11·0 | −24 | 12 | −10·5 | −19·1 – 1·9 | 0·0196 | 0·1 |
| Vellus Hairs (%) | ||||||||||||||
| Week 0–12 | 8 | −0·3% | ± 7·2% | −10·1% | 11·1% | 10 | −1·7% | ± 10·1% | −11·1% | 23·9% | −2·1% | −11·2 – 7·0% | 0·6314 | 0·2 |
| Week 0–24 | 10 | −4·9% | ±7·5% | −6·7% | 20·5% | 10 | −7·1% | ± 12·2% | −23·6% | 9·9% | −12·0% | −21·5 – 2·5% | 0·0158 | 0·1 |
| Terminal to Vellus Ratio | ||||||||||||||
| Week 0–12 | 8 | −0·0 | ± 0·2 | 0·3 | 0·2 | 10 | 0·1 | ± 0·4 | −0·8 | 0·65 | 0·1 | −0·2 – 0·4 | 0·3955 | 6·7 |
| Week 0–24 | 10 | 0·2 | ± 0·2 | 0·5 | 0·1 | 10 | 0·4 | ± 0·6 | −0·0 | 1·8 | 0·5 | 0·1 – 0·9 | 0·0097 | 81·0 |
| Terminal to Vellus Ratio (%) | ||||||||||||||
| Week 0–12 | 8 | −2·5% | ± 13·3% | −19·2% | 19·0% | 10 | 10·3% | ± 15·0% | −16·9% | 32·6% | 12·8% | −1·6 – 27·2% | 0·0769 | 6·7 |
| Week 0–24 | 10 | −8·6% | ± 9·0% | −23·6% | 8·9% | 10 | 24·4% | ± 29·4% | −1·3% | 92·2% | 33·0% | 12·5 – 53·4% | 0·0033 | 81·0 |
Abbreviations: Primary = Primary Outcomes; Week = Baseline; N = Per Protocol evaluable population paired with baseline; SD = standard deviation; Mean = Mean of Differences between Week 12 or 24 and Baseline; ∆-Mean = Difference of Means between ALRV5XR and Placebo.
Table 3.
Response Rates: Change in Terminal Hairs from Baseline. This table shows the post hoc stratified response by treatment group with a substantial increase in response in hair growth in the ALRV5XR group over time, while the placebo group's hair loss continues to progress. The ALRV5XR group separates into a trimodal responder pattern of low, moderate, and high responders when measured by absolute changes in Terminal Hair density however, these response rates are more uniform when stratified by per cent changes in Terminal Hair density. 54·5% of the ALRV5XR group increased their TH density by 10 or more THs/cm2 by week 24 and 18·2% of the group increased density by more than 25 THs/cm2.
| Placebo |
ALRV5XR |
|||
|---|---|---|---|---|
| Week 12 | Week 24 | Week 12 | Week 24 | |
| Positive responders | 50·0% | 18·2% | 91·9% | 100% |
| Terminal hairs/cm2 | ||||
| <0 | 50·0% | 81·8% | 9·1% | – |
| 0–5 | 40·0% | 18·2% | 27·3% | 45·5% |
| 5–10 | 10·0% | – | 27·3% | – |
| 10–15 | – | – | 18·2% | 27·3% |
| 15–20 | – | – | 9·1% | 9·1% |
| 20–25 | – | – | – | – |
| >25 | – | – | 9·1% | 18·2% |
| Max regrowth | 9 | 3 | 25 | 51 |
| Min regrowth | −17 | −12 | −3 | 0 |
| Terminal hairs (%) | ||||
| <0% | 50·0% | 81·8% | 9·1% | 0·0% |
| 0–5% | 40·0% | 18·2% | 45·5% | 45·5% |
| 5–10% | 10·0% | – | 27·3% | 9·1% |
| 10–15% | – | – | – | 18·2% |
| 15–20% | – | – | – | 9·1% |
| 20–25% | – | – | 18·2% | 9·1% |
| > 25% | – | – | – | 9·1% |
| Max regrowth | 7·0% | 1·6% | 22·9% | 46·8% |
| Min regrowth | −12·1% | −12·3% | 3·4% | 0% |
Fig. 3.
Changes in Terminal Hair Density from Baseline Over 24 Weeks. Treatment group ALRV5XR was statistically significantly superior to the placebo group for both (a) Absolute Change in Terminal Hairs: 21·0 THs/cm2 with an Odds Ratio (OR) of 87·4 [95% CI: 9·2–32·8; (p = 0·0014)] and (b) Per Cent Change in Terminal Hairs: 16·4% with an OR of 87·4 [95% CI: 7·4% – 25·5%; (p = 0·0012)]. Linear regressions are shown as lines with 95% confidence intervals shown in grey.
Fig. 5.
Changes in Terminal to Vellus Hair Ratio from Baseline Over 24 Weeks. Treatment group ALRV5XR increased statistically significantly to the placebo group for both (a) Absolute Change in Terminal to Vellus Hair Ratio: 0·5 compared to placebo with OR of 81·0 [95% CI: 0·2–0·9; (p = 0·0097)] and (b) Per Cent Change in Terminal to Vellus Hair Ratio: 33·0% with an OR of 81·0 [95% CI: 12·5%–53·4%; (p = 0·0033)]. Linear regressions are shown as lines with 95% confidence intervals shown in grey.
Fig. 4.
Changes in Vellus Hair Density from Baseline Over 24 Weeks. Treatment group ALRV5XR decreased statistically significantly to placebo group for both (a) Absolute Change in Vellus Hairs: −10·5 VHs/cm2 with OR 0·1 [95% CI: −1·9–−19·1; (p = 0·0196)] and (b) Per Cent Change in Terminal Hairs: −12·0% with OR 0·1 [95% CI: −2·5%–21·5%; (p = 0·0158)]. Linear regressions are shown as lines with 95% confidence intervals shown in grey.
3.3. Primary outcomes
For the primary outcomes at 24 weeks, ALRV5XR induced a statistically significant TH regrowth of 21·0 THs/cm2 more than placebo with an Odds Ratio (OR) of 87·4 [95% CI: 9·2–32·8; (p = 0·0014)], and 16·4% more than placebo with an OR of 87·4 [95% CI: 7·4%–25·5%; (p = 0·0012)]. Notably, 11 (100%) of evaluable subjects responded favourably in the ALRV5XR group in comparison with two (18·2%) of 11 evaluable subjects in the placebo group (see Table 2 and Fig. 3). An ANOVA analysis of the primary efficacy for terminal hair changes for the overall trial of ALRV5XR vs. placebo found a statistically significant Fisher's F-test statistic of F = 13·48 with p < 0·0001. Treatment with ALRV5XR was safe, well-tolerated and no adverse effects were observed. Concordantly, no statistically significant changes were observed in physical examinations or urine laboratory parameters. Any statistically significant changes in blood laboratory parameters were not clinically relevant (see Tables S3a, S3b and S3c in the supplemental materials).
3.4. Secondary outcomes
At 12 weeks the efficacy of ALRV5XR over placebo was 10·9 THs/cm2 with an OR of 10·0 [95% CI: 3·4–18·4; (p = 0·0065)], and 9·3% with an OR of 10·0 [95% CI: 2·7%–15·9%; (p = 0·0084)]. Ten (90·9%) of 11 evaluable subjects in the ALRV5XR group responded favourably in comparison to five (50·0%) of ten evaluable subjects in the placebo group (see Table 2 and Fig. 3).
3.5. Vellus hair density outcomes
At 24 weeks the average VH density of the ALRV5XR group when compared to placebo changed by −10·5 VHs/cm2 with OR 0·1 [95% CI: −1·9–−19·1; (p = 0·0196)], and −12·0% with OR 0·1 [95% CI: −2·5% – 21·5%; (p = 0·0158)]. Only three (30·0%) of ten evaluable subjects in the ALRV5XR group had increasing VH density compared to eight (80·0%) of ten evaluable subjects in the placebo group (see Table 2 and Fig. 4).
3.6. Terminal to vellus hair ratio outcomes
At 24 weeks, the average TH/VH ratio in the ALRV5XR group increased significantly by 0·5 compared to placebo, with an OR of 81·0 [95% CI: 0·2–0·9; (p = 0·0097)], and 33·0% with an OR of 81·0 [95% CI: 12·5%–53·4%; (p = 0·0033)] (see Table 2 and Fig. 5).
3.7. Total hair density outcomes
As described above, when compared to placebo, ALRV5XR induced statistically significant increases in TH density, reductions in VH density, and a higher TH/VH ratio. In addition, there was a non-statistically significant increase of 13·4 hairs/cm2 or 5·0% in total hair density.
3.8. Responders
At 24 weeks, a post-hoc analysis showed 100% of the ALRV5XR group vs 18·2% of the placebo responded favourably in TH outcomes. At 12 weeks, 36·4% of the ALRV5XR group increased TH density by more than 10 THs/cm2, further increasing to 54·5% at 24 weeks with no subjects losing THs. In contrast, the placebo group reported no increases in TH density at 12 weeks above the 10 THs/cm2 level and at 24 weeks, the maximum gain reported, was within the 0–5 THs/cm2 segment, and 81·8% of the placebo subjects lost THs. When comparing results measured as the per cent change in TH density, at 12 weeks 18·2% of the ALRV5XR group increased their TH density by more than 10%, further increasing to 45·5% of the group attaining these results at 24 weeks. The placebo group had no increased TH density beyond the levels of 10% at 12 weeks and 5% at 24 weeks. Moreover, at 12 weeks, the greatest increases in TH density in the ALRV5XR group was 25·0 THs/cm2 and 22·9% vs. 9·0 THs/cm2 and 7% in the placebo group. At 24 weeks, the greatest increases seen in the ALRV5XR group were 51·0 THs/cm2 and 46·8% vs 3·0 THs/cm2 and 1·6% in the placebo group. (More details are presented in Table 3, Supplementary Fig. S1 and Supplementary Table S4).
4. Discussion
During this 24 week clinical trial, ALRV5XR was well-tolerated by all subjects and displayed a comparable safety profile to that of placebo. Overall, ALRV5XR demonstrated superiority vs. placebo in the treatment of AGA. The net efficacy effect, as reflected by odds ratio determination, was favourable to the ALRV5XR treatment and increased by a factor of 9 from 12 to 24 weeks. Outcomes were not affected by covariates such as age, weight, ethnicity, FST, diagnosis or HN classification.
Baseline trichoscopic and characteristic differences between study groups were not statistically or clinically relevant and did not affect study outcome, especially considering that the balance of the differences had no influence on the results of either group. When compared to placebo, the use of ALRV5XR in subjects affected with AGA increased TH density and TH per cent change from baseline to week 12 with statistical significance. The larger effect observed at week 24, with no plateauing or flattening, suggest actions of the ALRV5XR treatment that should be considered as cumulative (See Fig. 3).
The entire ALRV5XR group responded favourably at 24 weeks and 90·9% of the group increased their TH density at 12 weeks. At 12 weeks of treatment, there was a high responder subgroup within the ALRV5XR group. This circumstance generated a bimodal effect that could be appreciated within the responders. This bimodality was not dependant on absolute TH or% TH density changes. By week 24, the TH density response profile of the ALRV5XR treated group displayed a trimodal distribution of hair regrowth response, with 45·5% of the group behaving as low responders (0–5 THs/cm2), 36·4% as moderate responders (10–20 THs/cm2), and 18·2% as high responders (> 25 THs/cm2). Nevertheless, when evaluated on a percentage basis at 24 weeks, all ALRV5XR subjects were more uniformly distributed. This response pattern suggests that approximately 45% of the ALRV5XR subjects will at least maintain their baseline TH while mildly improving their TH density. Of note, approximately 55% of men will have important favourable responses, regardless of individual characteristics. In clear contrast, 50% and 81·8% of the placebo group subjects decreased their TH density at weeks 12 and 24, respectively. (See Table 3, Supplementary Table S4 and Supplementary Fig. S1).
In addition to statistically significant improvements in TH density, ALRV5XR's effect on vellus hair at 24 weeks was remarkable. Indeed, a statistically significant decrease in VH density and a simultaneous statistically significant increase in the ratio of TH to VH occurred. This effect suggests that ALRV5XR treatment is not only inducing anagen in non-productive telogen hair follicles, but also that it is possibly reversing the miniaturisation of hair, a feature that is a characteristic hallmark of AGA. This transformation of VH to TH occurred from baseline to 24 weeks. (See Figs. 4 and 5). The reversal of the miniaturisation of hair herein reported, while not seen with minoxidil use [21], was observed during the second year of treatment with finasteride in men under 40 years of age [6]. Of note, since AGA and SA have TH miniaturisation as a common feature, and also share parallel derangements in gene expression [1,4], future research on ALRV5XR-induced miniaturisation reversal in SA may show similar results.
The uniform and statistically significant increase in TH in the ALRV5XR group at weeks 12 and 24 was associated with a nine-fold increase, from 10·0 to 87·4 in the OR, from 12 to 24 weeks. This suggests that prolonged treatment with ALRV5XR will probably result in significant TH regrowth well beyond 24 weeks. This finding implies that sustained exposure to ALRV5XR for longer periods may continuously induce anagen in involuted (or telogen) HFs and that it might result in substantial regeneration leading to a healthy and normal scalp hair phenotype.
Results from published clinical trials and meta-analyses of current standard therapy showed that 48 weeks of treatment resulted in average gains of hair density of 11·5 THs/cm2 for 2% minoxidil, 14·9 THs/cm2 for 5% minoxidil, and 14·8 THs/cm2 for 1 mg finasteride [7,15,16]. The efficacy of ALRV5XR reported in this study displays clear superiority over those results. Indeed, after 12 weeks, ALRV5XR's efficacy of 10·9 THs/cm2 reaches 73 to 94% of the 48 week efficacy of standard therapy. At 24 weeks and with an efficacy of 21·0 THs/cm2, ALRV5XR reached gains that are 141 to 182% of those corresponding to the efficacy established for standard therapies at 48 weeks. Longer-term studies are needed to further confirm the apparent linear progression of the linear effect of ALRV5XR treatment. The current results suggest that TH regrowth to more than 40 THs/cm2 over 48 weeks may be possible. This would be a 2·8 to 3·6 fold efficacy improvement of ALRV5XR over standard therapies. Furthermore, the 100% response rate seen with ALRV5XR is clearly superior to the approximately 34%, 58% and 52% reported for 2%, 5% minoxidil and finasteride (1 mg), respectively [18,19]. Furthermore, ALRV5XR treatment has a clearly improved safety profile when compared to standard therapies, which have been aetiologically associated with a range of miscellaneous adverse events [15].
Attempts to improve hair regrowth in men have included research in various combination therapies using various topical or oral agents. Results showed minimal improvements if any when compared to individual treatments [15], and positive effects are attributed to the addition of either minoxidil or finasteride to such agents. ALRV5XR, while in accord with the combination approach, focuses on multi-molecular targeting designed to directly, or indirectly, modulate various of the known pathways involved in healthy HF biology. Results of its use suggest a synergistic effect that leads to a superior outcome.
An expert opinion on the effects of PRP and autologous stem cell therapy noted that hair regeneration and wound healing share many common molecular pathways, especially the stimulation of Wnt/β-catenin signalling [26], that are similarly influenced by ALRV5XR. A meta-analysis of clinical trials of PRP in men and women found a mean efficacy of change in total hair count of 23.5 (95%CI: 9.9–37.1) hairs/cm2, however within 9–12 months of completing PRP or stem cell therapy, the gains in hair density decline by approximately 50% [16,26]. When considering the initial rapid hair regrowth effect followed by a decline in hair density after PRP and stem cell therapy [23,26], studying the use of ALRV5XR as an adjuvant for pre and post PRP or stem cell therapy, may offer a potentiating effect to enhance or sustain the hair density improvement. Furthermore, this commonality of growth factors and cytokines suggests that ALRV5XR should be investigated for its wound healing and skin maintenance effects.
Due to ALRV5XR's inherent widespread actions, and as hypothesised, it was proven to be a highly effective treatment in regenerating and maintaining TH in men. The statistically significant clinical effect without side effects and the consistent increase in TH density from week 12–24, is likely due to induction of anagen in involuted hair follicles and prolongation of anagen while reversing miniaturisation of hair follicles. These effects can be regarded as regenerative and homoeostatic physiological effects on the HF, and have been linked to the activation of hair follicle stem cells via the Wnt/β-catenin pathway as well as to modulation of multiple cytokines, growth factors and hormone mediators, possibly relieving stress on the HF [2,13,14,22,24]. Besides exploring the events occurring in hair physiology, future research should also focus on defining the main mechanisms of action of ALRV5XR for the potential development of applications in other tissues.
ALRV5XR promises to fill an unmet need for an effective, safe, and well-tolerated treatment for men impacted by HL and within all HN degrees and classifications, regardless of age and ethnicities as well as in all FSTs with premature ageing or senescent hair loss associated with AGA. The multi-molecular targeting approach of ALRV5XR for hair regrowth increased TH density with clinical significance and likely induced normal structure and function of hair follicles via regeneration of non-productive telogen hair follicles, by prolonging anagen and by reversing hair follicle miniaturisation. Besides exhibiting an improved safety profile over the FDA approved standard of care drugs, minoxidil and finasteride, ALRV5XR achieved improvement in efficacy and a 100% response rate in half the time (24 weeks). While longer, larger and multi-centred studies are needed to demonstrate the complete potential and generalisable safety of ALRV5XR in men, the progressive increase of TH regrowth over 24 weeks suggests that ALRV5XR will be an effective long-term treatment for hair loss in men, and that its prolonged use may restore a normal hair phenotype.
Contributors
PRF and DB contributed to the study concept and design. PRF obtained funding. BMM contributed to recruitment of subjects, investigation, supervision, data acquisition and data curation. PRF, KMF and BMM accessed and were responsible for the raw data associated with the study. PRF, KMF, CP, BMM and JG contributed to data analysis and interpretation. PRF, KMF and CP contributed to the validation, statistical analysis, and visualisation. CP and KMF drafted the manuscript. PRF, KMF, CP, BMM, DB, DJC and JG critically revised the manuscript for important intellectual content.
Declaration of Competing Interest
PRF is the director of Arbor Life Labs (ALL), PRF, DJC and DB are coinventors of patent pending PCT/US2020/067585 titled Botanicals as Wnt/β-Catenin Activators, Molecular Pathway Regulators, Tissue Regenerators and Health Biomarker Regulators, filed for ALRV5XR and have a share in ALL. PRF, KMF, CP, BMM, DJC and JG, received honoraria from ALL and proposed submission for publication. DJC is an employee of ALL All authors had full access to the data in the study.
Acknowledgments
Data sharing
Data collected for the study, including individual participant data and a data dictionary, will be made available. Data available includes de-identified participant data, study protocol and informed consent form. These data will be available beginning 6 months and ending 3 years after publication. Data will be shared with investigator support to researchers who provide a methodologically sound proposal with a signed data access agreement. Contact corresponding author.
Funding
This clinical trial was funded by Arbor Life Labs, Toronto, Ontario, Canada.
Acknowledgments
The authors express their profound appreciation and acknowledgement to Dr Luciano Eugenio Marra, PhD for his contribution to developing ALRV5XR–we dedicate this publication to Dr Marra, who died shortly after the commencement of this trial; Dr Kurt Stenn MD for reviewing the data and his guidance on reporting and interpreting results; Dr Elizabeth Renouf, Ph.D for Biostatistical analysis and Deborah Cahan, without whom ALRV5XR would not have been developed due to her persistent and complex alopecia which dramatically changed her life. This trial was funded by Arbor Life Labs.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101124.
Contributor Information
Peter R Feldman, Email: petefeldman@gmail.com.
Klaus M Fiebig, Email: klausfiebig@gmail.com.
Appendix. Supplementary materials
References
- 1.McMichael A.J., Hordinsky M.K. 2nd ed. CRC Press; Boca Raton: 2018. Hair and scalp disorders: medical, surgical, and cosmetic treatments. [Google Scholar]
- 2.Matsumura H., Mohri Y., Binh N.T. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science. 2016;351(6273):aad4395. doi: 10.1126/science.aad4395. [DOI] [PubMed] [Google Scholar]
- 3.Cash T.F. The psychosocial consequences of androgenetic alopecia: a review of the research literature. Br J Dermatol. 1999;141(3):398–405. doi: 10.1046/j.1365-2133.1999.03030.x. [DOI] [PubMed] [Google Scholar]
- 4.Karnik P., Shah S., Dvorkin-Wininger Y., Oshtory S., Mirmirani P. Microarray analysis of androgenetic and senescent alopecia: comparison of gene expression shows two distinct profiles. J Dermatol Sci. 2013;72(2):183–186. doi: 10.1016/j.jdermsci.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heilmann S., Kiefer A.K., Fricker N. Androgenetic alopecia: identification of four genetic risk loci and evidence for the contribution of WNT signalling to its etiology. J Invest Dermatol. 2013;133(6):1489–1496. doi: 10.1038/jid.2013.43. [DOI] [PubMed] [Google Scholar]
- 6.Price V.H. Treatment of hair loss. N Engl J Med. 1999;341(13):964–973. doi: 10.1056/NEJM199909233411307. [DOI] [PubMed] [Google Scholar]
- 7.Adil A., Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(1):136–141. doi: 10.1016/j.jaad.2017.02.054. e135. [DOI] [PubMed] [Google Scholar]
- 8.Su L.H., Chen L.S., Lin S.C., Chen H.H. Association of androgenetic alopecia with mortality from diabetes mellitus and heart disease. JAMA Dermatol. 2013;149(5):601–606. doi: 10.1001/jamadermatol.2013.130. [DOI] [PubMed] [Google Scholar]
- 9.Wambier C.G., Vano-Galvan S., McCoy J., Pai S., Dhurat R., Goren A. Androgenetic alopecia in COVID-19: compared to age-matched epidemiologic studies and hospital outcomes with or without the Gabrin sign. J Am Acad Dermatol. 2020;83(6):e453–e454. doi: 10.1016/j.jaad.2020.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trüeb R.M. Springer Nature; Switzerland: 2020. Nutrition for healthy hair: guide to understanding and proper practice. [Google Scholar]
- 11.Whiting D.A. Fairfield; NY Canfield: 2004. The structure of the human hair follicle: light microscopy of vertical and horizontal sections of scalp biopsies. [Google Scholar]
- 12.Courtois M., Loussouarn G., Hourseau C., Grollier J.F. Ageing and hair cycles. Br J Dermatol. 1995;132(1):86–93. doi: 10.1111/j.1365-2133.1995.tb08630.x. [DOI] [PubMed] [Google Scholar]
- 13.Bernard B.A. Advances in understanding hair growth. F1000Res. 2016;5 doi: 10.12688/f1000research.7520.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasserot A.P., Geyfman M., Poloso N.J. Androgenetic alopecia: combing the hair follicle signalling pathways for new therapeutic targets and more effective treatment options. Expert Opin Ther Targets. 2019;23(9):755–771. doi: 10.1080/14728222.2019.1659779. [DOI] [PubMed] [Google Scholar]
- 15.Kanti V., Messenger A., Dobos G. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32(1):11–22. doi: 10.1111/jdv.14624. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A.K., Bamimore M.A., Foley K.A. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyzes, and an assessment of evidence quality. J Dermatolog Treat. 2020:1–11. doi: 10.1080/09546634.2020.1749547. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen D.D., Marchese M., Cone E.B. Investigation of suicidality and psychological adverse events in patients treated with finasteride. JAMA Dermatol. 2021;157(1):35–42. doi: 10.1001/jamadermatol.2020.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen E.A., Dunlap F.E., Funicella T. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47(3):377–385. doi: 10.1067/mjd.2002.124088. [DOI] [PubMed] [Google Scholar]
- 19.Leyden J., Dunlap F., Miller B. Finasteride in the treatment of men with frontal male pattern hair loss. J Am Acad Dermatol. 1999;40(6):930–937. doi: 10.1016/s0190-9622(99)70081-2. Pt 1. [DOI] [PubMed] [Google Scholar]
- 20.Kwack M.H., Kang B.M., Kim M.K., Kim J.C., Sung Y.K. Minoxidil activates beta-catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation effect. J Dermatol Sci. 2011;62(3):154–159. doi: 10.1016/j.jdermsci.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Messenger A.G., Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150(2):186–194. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- 22.Guevara-Aguirre J., Torres C., Pena G. IGF-I deficiency and enhanced insulin sensitivity due to a mutated growth hormone receptor gene in humans. Mol Cell Endocrinol. 2021;519 doi: 10.1016/j.mce.2020.111044. [DOI] [PubMed] [Google Scholar]
- 23.Gentile P., Garcovich S. Systematic review of platelet-rich plasma use in androgenetic alopecia compared with minoxidil(R), finasteride(R), and adult stem cell-based therapy. Int J Mol Sci. 2020;21(8):2702. doi: 10.3390/ijms21082702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman A., Herman A.P. Mechanism of action of herbs and their active constituents used in hair loss treatment. Fitoterapia. 2016;114:18–25. doi: 10.1016/j.fitote.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Choi B.Y. Targeting Wnt/beta-catenin pathway for developing therapies for hair loss. Int J Mol Sci. 2020;21(14) doi: 10.3390/ijms21144915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gentile P., Alves R., Cole J.P. AIRMESS – academy of international regenerative medicine & surgery societies: recommendations in the use of platelet-rich plasma (PRP), autologous stem cell-based therapy (ASC-BT) in androgenetic alopecia and wound healing. Expert Opin Biol Ther. 2021:1–7. doi: 10.1080/14712598.2021.1908995. [DOI] [PubMed] [Google Scholar]
- 27.Feldman P., Fiebig K., Piwko C. Safety and efficacy of ALRV5XR in women with androgenetic alopecia or telogen effluvium: a randomized, double-blinded, placebo-controlled clinical trial. EClinicalMedicine. 2021;37 doi: 10.1016/j.eclinm.2021.100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dupont W.D. Cambridge Press; New York: 2002. Statistical modeling for biomedical researchers: a simple introduction to the analysis of complex data. [Google Scholar]
- 29.Venables W.N., Ripley B.D. 4th ed. Springer; Verlag, New York: 2002. Modern applied statistics with s-plus. [Google Scholar]
- 30.Fitzmaurice G.M., Laird N., Ware J.H. 2nd ed. Wiley; New Jersey: 2011. Applied longitudinal analysis. [Google Scholar]
- 31.Lang W. CRC Press; New York: 2010. Mixed effects models for complex data. [Google Scholar]
- 32.Hackl S., Koch A., Lasch F. Empirical evaluation of the implementation of the EMA guideline on missing data in confirmatory clinical trials: specification of mixed models for longitudinal data in study protocols. Pharm Stat. 2019;18(6):636–644. doi: 10.1002/pst.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Core Team. A language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing. 2012; available at: http://cran.r-project.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.