Abstract
This study wants to investigate the effects of kombucha tea based on seagrapes on blood glucose levels, total cholesterol, and PGC-1α in Swiss albino mice that were given cholesterol- and fat-enriched diets (CFED). Anti-glycation, tyrosinase inhibitory, and α-glucosidase inhibitory activity were also determined. Forty male swiss webster albino mice weighing between 20 g–30 g were used for this study. Animals were distributed in random into 4 groups of 10 animals each; group A served as normal control (received standard dry pellet diet), group B were fed on CFED for 4 weeks, and groups C and D were fed on CFED and were administered 150 and 300 mg/kg of kombucha tea from seagrapes (Caulerpa racemosa) (p.o.). In vitro study show that the activity of anti-glycation, L-Tyrosine, L-Dopa, α-glucosidase, and α-amylase inhibition were 62.79 ± 0.78, 9.05 ± 0.16, 27.14 ± 1.62, 90.42 ± 0.77, and 80.44 ± 1.00, respectively. Group C has a better activity in increasing PGC-1-alpha serum in mice than group D (p < 0.05). There were no meaningful differences between group C and D in blood cholesterol and blood glucose reduction (p = 0.222), both groups have the same effect in lowering total cholesterol and blood glucose in mice. In conclusion, kombucha tea from seagrapes has potential as an anti-ageing functional food.
Keywords: Functional food, Anti-ageing, PGC1α, Anti-glycation, Tyrosinase inhibitory, Blood glucose, Total cholesterol, α-glucosidase, Caulerpa racemosa
Functional food; Anti-ageing; PGC1α; Anti-glycation; Tyrosinase inhibitory; Blood glucose; Total cholesterol; α-glucosidase; Caulerpa racemosa.
1. Introduction
Ageing is a process by which structural and functional changes are accumulated in an organism as a result of many factors, such as changes due to oxidative damage caused by reactive oxygen species (ROS) [1]. ROS is defined as a reactive molecule that contains oxygen from molecular oxygen reduction in the cell [2]. A good balance between ROS production and detoxification may result in better longevity [3]. A high level of ROS can cause cellular damage, have a role in neurodegenerative diseases [4], cancer [5], impaired host defense, and innate immune response [6], and may lead to metabolic dysfunction [7] by oxidizing DNA, RNA, carbohydrates, proteins, and lipids; resulting in cell death [8]. Recently, high antioxidant functional foods is popular due to its effect on reactive oxygen species (ROS) and many diseases related to ageing and chronic diseases [9, 10]. ROS can accelerate the ageing process of the skin through oxidative stress due to UV rays, while the consumption of high functional foods with high levels of antioxidants can inhibit the acceleration process [11, 12]. Antioxidant majorly belongs phenolics and flavonoids (phenolic derivatives), which contain hydroxyl groups that act as hydrogen donors to stabilize free radicals and stop the formation of new free radicals [13].
Premature ageing is one of the skin damage and high melanin production (hyperpigmentation) caused by high exposure to UV rays and makes the skin darker (depigmentation) [14]. Inhibiting tyrosinase is the most useful way to avoid depigmentation. This enzyme converts tyrosine into 3,4-dihydroxyphenylalanine (DOPA), then into dopaquinone, and at the end of the process produces melanin [15]. External factors such as UV, smoking, pollutants, unhealthy diet, and lifestyle contribute to free radicals and ROS production [16]. This stimulates inflammation of the skin triggering a series of biochemical reactions on the skin causing damage to dermis collagen tissue and premature skin ageing [17].
PGC-1α is a transcriptional co-activator, known for its role in regulating the body's heat production, mitochondrial synthesis, glucose and lipid metabolism, and skeletal muscle fiber type conversion [18]. PGC-1α is mainly found in tissues with an abundance of mitochondria, such as brown fat, liver, and skeletal muscle [19]. Recent work also proves PGC-1α potential in attenuating ROS-oxidative stress in diabetic cardiomyopathy patients [20].
Seagrapes (Caulerpa racemosa) or lawi-lawi (local terminology) is popular for their nutritional benefits since seagrapes have many bioactives, such as protein, polysaccharides, polyphenol, flavonoids, and antioxidants [21, 22] along with rich content of essential amino acids, micronutrient, dietary fibers, and polyunsaturated fatty acids (PUFA) [23]. Moreover, C. racemosa has a high antioxidant level and is advised to be functional food or nutraceuticals [22, 24]. The extract of C. racemosa can reduce glucose level, aspartate aminotransferase (AST), alanine aminotransferase as well as have a hepatoprotective activity [23], and nephroprotective activity [25] in diabetic rats.
Kombucha is a fermented beverage created from sugared tea fermentation, with a starter culture from bacteria and yeast [26]. Kombucha contains glucuronic acid with hepatoprotective effect as the result of its fermentation [26], has antioxidant activity, anti-inflammatory effect [27], may reduce blood pressure, inhibit cancer growth [28, 29], improve the immune system, liver, and gastrointestinal function [30].
Seeing that seagrapes have many bioactive compounds such as antioxidants and polyphenols that have the potential to fight free radicals that can cause aging from several factors (such as dietary factors, lifestyle, oxidative stress). Therefore, this study aims to evaluate the effects of kombucha tea based on seagrapes on blood glucose levels, total cholesterol, and PGC-1α in Mus musculus Swiss albino mice fed cholesterol- and fat-enriched diets (CFED). Anti-glycation, tyrosinase inhibitory, α-glucosidase, and α-amylase inhibitory activity were also determined to see its potential as functional anti-ageing food in vitro and in vivo.
2. Materials and methods
This study includes in vitro and in vivo experimental studies using animal models, conducted at the Pharmacological Laboratory, Faculty of Mathematics and Natural Sciences, Sam Ratulangi University.
2.1. Sample preparation
Seagrapes were obtained in North Sulawesi, around 10–20 m above sea level. Fresh seagrapes were drained at 25 °C for about 5 h to reduce the water content. Next, the drained seagrapes were blended using a blender. The main formulation for all kombucha tea samples are 25 g of seagrapes, 50 mL of water, and 10 g of SCOBY gel (Symbiotic Culture of Bacteria and Yeast), with a diameter of 16 cm and with the addition of 100 g of Trigona sapiens honey 20% v/v SCOBY starter solution. This formulation was chosen for in vitro and in vivo tests because previously it had shown high levels of polyphenols and antioxidants in the formulation with the addition of 100 g Trigona sapiens honey 20% v/v SCOBY starter solution. The formulation also has ash, water level, alcohol, and pH level of 7.07 ± 0.15%, 44.85 ± 0.96%, 0.62 ± 0.50%, and 4.78%, respectively. The results of the antioxidant and polyphenol test in the formulation, have also been entered to be presented on the "Irish Section Conference 2021: Nutrition, Health, and Ageing - Translating Science into Practice" and publications at Proceedings of the Nutrition Society [31].
2.2. Determination of anti-glycation activity
This anti-glycation test refers to the method proposed by Povichit, 2010 [32], with the preparation of several test solution as shown in Table 1. All of the test solutions were incubated for 40 h at 60 °C. After incubation, 100 μL of the solution was pipetted into a 96-well plate. The relative amount of glycated BSA (Bovine Serum Albumin) was measured using a fluorometer at an excitation wavelength of 370 nm and emission of 440 nm.
Table 1.
The composition of the solution in the anti-glycation activity test.
| Materials | Solution A (Glycation Control) (μL) |
Solution B (Control Corrector) (μL) |
Solution C (Sample) (μL) |
Solution D (Sample Corrector) (μL) |
|---|---|---|---|---|
| Phosphate Buffer 200 mM pH 7.4 (KH2PO4 0.2M + K2HPO4 0.2 M in Distilled Water) | 200 | 200 | 200 | 200 |
| BSA 20 mg/mL | 80 | 80 | 80 | 80 |
| Glucose 235 mM | 40 | - | 40 | - |
| Fructose 235 mM | 40 | - | 40 | - |
| Extract/Aminoguanidin | - | - | 80 | 80 |
2.3. Determination of tyrosinase inhibitory activity
The determination of the tyrosinase enzyme inhibitory activity refers to Batubara, 2015 [33]. In testing the inhibitory activity of the tyrosinase enzyme, L-tyrosine and L-DOPA were used as substrates and kojic acid as positive controls. Samples were dissolved with dimethyl sulfoxide (DMSO) as stock solution. The concentration variant was created by dissolving collagen using a phosphate buffer with a pH of 6.5. A total of 70 μL of the solution was pipetted into the 96 well plates, then 30 μL of tyrosinase enzyme (Sigma, 333 units mL-1 in phosphate buffer solution was added) and the mixture was incubated for 5 min. After that, 110 μL of the substrate (L-tyrosine 2 mM) was added and incubated at 37 °C for 30 min. The absorbance was measured at a wavelength of 492 nm using the Microplate Reader Spectrophotometer.
2.4. Assay for α-glucosidase inhibitory activity
Inhibitory activity of α-glucosidase was determined using the method introduced from literature [34]. α-glucosidase solution (1.52 UI/ml) was obtained by mixing 1 mg powder (76 UI) with 50 mL phosphate buffer (pH 6.9). Then the solution was kept at -20 °C. 0.1 mL of gradient concentrations (3 mg/mL) of kombucha tea then was mixed with 0.35 ml of sucrose (65 mM) and maltose solution (65 mM), respectively. After preheated (37 °C, 5 min), 0.2 mL of alpha-glucosidase solution was added into the preheated system and then reacted at 37 °C for 15 min. The reaction was done by heating the system in a 100 °C water bath for 2 min. Acarbose was used in this experiment as the positive control. The treatment of control was the same as the treatment of kombucha tea. The activity of alpha-glucosidase was expressed as the glucose production level in the experiment. 0.2 mL of testing solution was combined with the solution got from the alpha-glucosidase inhibitory test then 3 mL color reagent was added into the reactive system. Later the system was heated at 37 °C for 5 min and the absorption of the solution was checked at 505 nm.
2.5. α-Amylase activity assay
500 μL of dilution samples and 500 μL of 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) containing 0.5 mg/mL porcine pancreatic α-amylase (effective concentration 3.2.1.1) were incubated at 25 °C for 10 min. After that, 500 μL of 1% starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) was added to each mixture. The mixture was incubated (25 °C) for 10 min and then stopped with 1.0 mL of 3,5-Dinitrosalicylic acid color reagent. The mixture was incubated in a 100 °C water bath for 5 min and rested to room temperature. The mixture was then diluted by adding 10 mL of distilled water, and the absorbance was measured at 540 nm. The reference sample includes all other reagents and enzymes except the sample. The percentage of enzyme inhibitory activity of the kombucha tea was then calculated according to Worthington (1993) [47], Acarbose was used as a positive control.
2.6. Animal handling and ethical approval
All experimental mice had a standardized free access of feed and ad libitum of water. The study was conducted in the Laboratory of Pharmacology, Faculty of Mathematics and Natural Sciences, Sam Ratulangi University, Manado, Indonesia. Forty male mus musculus Swiss albino mice (3–5 weeks) weighing between 20 g–30 g were obtained from the Laboratory Animals Farming Makassar, Indonesia. The animals were grouped and housed in cages and maintained under standard laboratory conditions (temperature: 27 ± 2 °C) with light and dark cycles (12/12 h). The mice were acclimatized to laboratory conditions for 10 days before the commencement of the experiment. Food and water intake are influenced by ambient temperature, therefore we strongly maintain the environment to stay at room temperature. The average food and water intake of mice are 4.58 ± 0.59 g and 4.95 ± 0.55 mL. This range is in accordance with normal conditions (not under pressure or stress) referring to Heldrich et al, (2004) [48]. The research protocol (use of experimental animals) used was based on the Declaration of Helsinki, The Council for International Organizations of Medical Sciences (CIOMS), and has been approved for the application of ethical health research protocols RSUP Prof. Dr. RD. Kandou, Manado with Ethical Approval No. 085/EC/KEPK-KANDOU/VI/2021.
2.7. In vivo studies of Kombucha tea seagrapes against blood glucose levels, total cholesterol, and PGC-1α
2.7.1. CFED production
The CFED production refers to the previous study [35]. The CFED consists of standard mouse food supplemented with 1% colic acid, 2% pure cholesterol powder, 20% fat (animal source/pork oil), and 2% corn oil. All the components are mixed until homogeneous, added 1000 mL of distilled water, and formed into small pellets. Then the pellets are left to dry at room temperature in sterile conditions and stored at 4 °C to reduce CFED oxidation by air. CFED consists of carbohydrate 43.57%, protein 12.38%, fiber 4.73%, fat 3.17%, cholesterol 2%, colic acid 1%, animal fat 20%, corn oil 2%, total ash 4.3%, and moisture 6.85%. A normal diet contains 58.1% carbohydrates, 16.51% crude protein, 0% animal fat, corn oil, cholesterol, and folic acid.
2.7.2. Kombucha tea seagrapes administration scheme
Mice were randomly distributed into four groups, each group consisting of 10 mice. Group A as normal control received a standard diet, then groups B, C, and D received CFED for four weeks. Groups C and D were treated with 150 and 300 mg/kg BW (respectively) of Kombucha Tea for four weeks. CFED and kombucha tea were administered by oral.
2.7.3. Sample collection
Sample collection was performed after the 4th week of experimental feeding; the mice fasted overnight before that. Anesthesia was performed with ketamine. Blood was collected from muscle tissue, placed in dry and clean tubes without anticoagulants, and allowed to clot at room temperature. For serum collection, the blood was centrifuged at 3000 rpm for 20 min. Then the serum was used for the blood glucose, total cholesterol, and PGC-1α analysis.
2.7.4. Biomedical analysis of blood sample
We analyze blood glucose and cholesterol levels using COBAS Integra® 400 plus analyzer (Roche). For PGC-1α, samples were cleared with Phosphate Buffered Saline (PBS, pH 7.4) 1% until the laundry liquid is clear. Then, PBS 1% of the sample at 3000 rpm is centrifuged for 20 min to obtain pellets and supernatant. Finally, the supernatant was analyzed with PGC Mouse 1α ELISA Kit Sunlong Biotech Co., Ltd.
2.8. Data management and analysis
The data obtained from the results of the study were analyzed statistically using the ANOVA multivariate test. Levene test is used to determine which posthoc tests to be used. If the p-value of the Levene Test <0.05 then the posthoc test uses Least Significant Difference (LSD) (equal variances not assumed) but if the Levene Test results show insignificant results (p > 0.05) then the posthoc test uses the Bonferroni Test with equal variances assumed. SPPS 26.0 for the Windows version is used to perform statistical analyses.
3. Results
3.1. Results of anti-glycation, tyrosinase inhibition, and α-glucosidase inhibition activity
Table 2 shows the results of in vitro study. The activity of anti-glycation, L-Tyrosine, L-Dopa, α-glucosidase, and α-amylase inhibition were 62.79 ± 0.78, 9.05 ± 0.16, 27.14 ± 1.62, 90.42 ± 0.77, and 80.44 ± 1.00, respectively. Glycation inhibition activity in kombucha tea from seagrapes is better when compared to commercial anti-glycation of aminoguanidine with an IC50 of 80 ppm. Inhibition activity of tyrosine enzymes using the L-Tyrosine and L-Dopa substrates shows that this kombucha tea is also better when compared to a positive control (in this case Kojic Acid with an IC50 of 8.90 ppm). Kombucha tea α-glucosidase and α-amylase inhibition value are close to the inhibition value of α-glucosidase and α-amylase from acarbose (an anti-diabetic drug as positive control) which can inhibit up to 90% and 80%.
Table 2.
Results of Anti-Glycation, Tyrosinase Inhibition, α-Glucosidase, and α-Amylase Inhibition Activity.
| ∗Anti-Glycation (%) |
∗∗Tyrosinase Inhibition (%) |
Kombucha Tea α-Glucosidase Inhibition (%) | Acarbose α-Glucosidase Inhibition (%) | Kombucha Tea α-Amylase Inhibition (%) |
Acarbose α-Amylase Inhibition (%) |
||
|---|---|---|---|---|---|---|---|
| L-Tyrosine | L-Dopa | ||||||
| 1 | 62.88 | 8.92 | 25.7 | 91.3 | 97.8 | 81.54 | 87.53 |
| 2 | 63.52 | 9.23 | 28.9 | 90.02 | 99.6 | 80.20 | 89.60 |
| 3 | 61.96 | 9.01 | 26.83 | 89.93 | 98.01 | 79.58 | 88.65 |
| Average | 62.79 ± 0.78 | 9.05 ± 0.16 | 27.14 ± 1.62 | 90.42 ± 0.77 | 98.47 ± 0.98 | 80.44 ± 1.00 | 88.59 ± 1.03 |
Glycation at 2000 ppm, IC50 Aminoguanidine = 80 ppm.
Tyrosinase at 1000 ppm, Kojic Acid IC50: 8.90 ppm.
3.2. Glucose, total cholesterol, and PGC-1α across all groups
Results of glucose, total cholesterol, and PGC-1α are shown in Table 3. These data will be further analyzed by using Multivariate Analysis and Levene's Homogeneity Test.
Table 3.
Glucose, total cholesterol, and PGC-1α across all groups.
| Treatment | Mean | Standard Deviation | N | |
|---|---|---|---|---|
| Glucose Level (mg/dL) | Normal | 71.9500 | 3.76128 | 10 |
| CFED | 86.1580 | 3.28821 | 10 | |
| CFED and Kombucha Tea (150) | 64.7500 | 1.64401 | 10 | |
| CFED and Kombucha Tea (300) | 67.2300 | 1.65399 | 10 | |
| Total | 75.5220 | 8.80028 | 40 | |
| Total Cholesterol Level (mg/dL) | Normal | 42.4600 | 4.57267 | 10 |
| CFED | 66.3600 | 3.65671 | 10 | |
| CFED and Kombucha Tea (150) | 31.6700 | 2.20104 | 10 | |
| CFED and Kombucha Tea (300) | 35.2900 | 3.72542 | 10 | |
| Total | 43.9450 | 14.1232 | 40 | |
| PGC-1α (pg/mL) | Normal | 101.5600 | 3.42514 | 10 |
| CFED | 80.4400 | 2.45139 | 10 | |
| CFED and Kombucha Tea (150) | 121.1500 | 1.42926 | 10 | |
| CFED and Kombucha Tea (300) | 116.7300 | 2.33859 | 10 | |
| Total | 104.9700 | 16.29981 | 40 |
3.3. Multivariate analysis
Table 4 states that each of the multivariate tests has a p-value <0.05. There is a significant effect from every treatment on all variables in a 95% CI.
Table 4.
Multivariate analysis.
| Effect | Value | F | Hypothesis Df | Error Df | Sig. | |
|---|---|---|---|---|---|---|
| Treatment | Pillai's Trace | 1.000 | 23630.870 | 3.000 | 34.000 | 0.000 |
| Wilks' Lambda | 0.000 | 23630.870 | 3.000 | 34.000 | 0.000 | |
| Hotelling's Trace | 2085.077 | 23630.870 | 3.000 | 34.000 | 0.000 | |
| Roy's Largest Root | 2085.077 | 23630.870 | 3.000 | 34.000 | 0.000 | |
| Group | Pillai's Trace | 1.274 | 8.861 | 9.000 | 108.000 | 0.000 |
| Wilks' Lambda | 0.007 | 62.978 | 9.000 | 82.898 | 0.000 | |
| Hotelling's Trace | 106.914 | 388.060 | 9.000 | 98.000 | 0.000 | |
| Roy's Largest Root | 106.519 | 1278.232 | 3.000 | 36.000 | 0.000 |
3.4. Levene's homogeneity test
It was shown in Table 5 that Glucose and PGC-1α were homogenous (p < 0.05). Therefore, these data will be interpreted using LSD (equal variance not assumed), while cholesterol with p-value >0.05 will be defined by post-hoc test using Bonferroni (equal variances assumed).
Table 5.
Levene's homogeneity test.
| Levene Statistic | Df1 | Df2 | Sig. | |
|---|---|---|---|---|
| Glucose Level | 5.578 | 3 | 36 | 0.003 |
| Total Cholesterol Level | 0.601 | 3 | 36 | 0.619 |
| PGC-1α | 3.632 | 3 | 36 | 0.022 |
3.5. Glucose multivariate test results
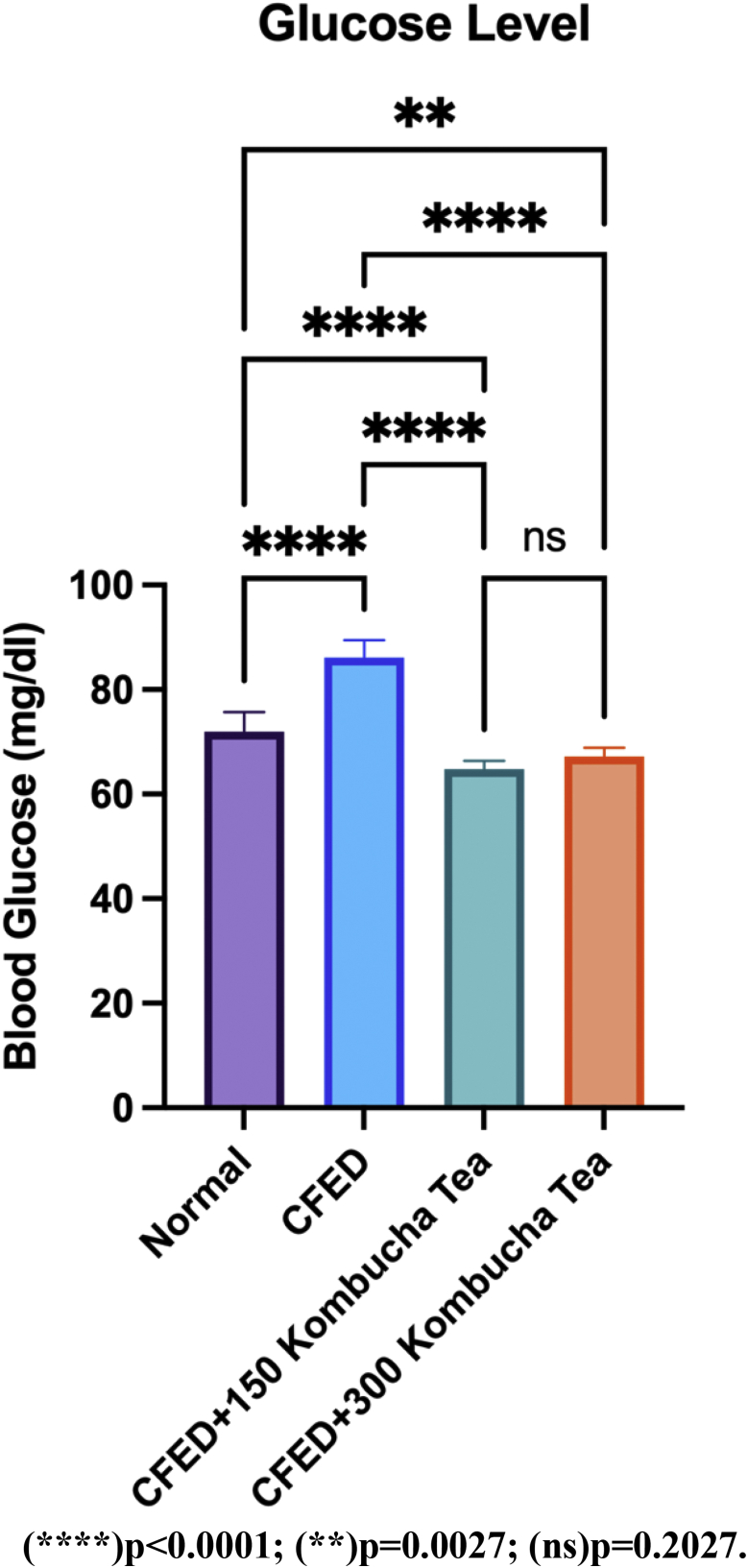
Figure 1 shows that blood glucose increases significantly than control group when given a CFED diet (p < 0.05). Blood glucose decreases significantly in both the control group and the treatment group when given CFED treatment + 150 mg/kgBW kombucha tea as well as CFED treatment + 300 mg/kgBW kombucha tea (p < 0.05). The effect of kombucha tea administration of 150 mg/kgBW was more effective than the administration of 300 mg/kgBW of kombucha tea in decreasing the blood glucose of mice, but the result is not significant (p > 0.05). A detailed table of these test results is available in (Table 6).
Figure 1.
Both doses of seagrapes kombucha tea significantly reduce blood glucose.
Table 6.
Glucose multivariate test results.
| Test details | Mean 1 | Mean 2 | Mean Diff. | SE of diff. | n1 | n2 | q | DF |
|---|---|---|---|---|---|---|---|---|
| Normal vs. CFED | 71.95 | 86.16 | -14.21 | 1.233 | 10 | 10 | 16.3 | 36 |
| Normal vs. CFED+150 Kombucha Tea | 71.95 | 64.75 | 7.2 | 1.233 | 10 | 10 | 8.259 | 36 |
| Normal vs. CFED+300 Kombucha Tea | 71.95 | 67.23 | 4.72 | 1.233 | 10 | 10 | 5.414 | 36 |
| CFED vs. CFED+150 Kombucha Tea | 86.16 | 64.75 | 21.41 | 1.233 | 10 | 10 | 24.56 | 36 |
| CFED vs. CFED+300 Kombucha Tea | 86.16 | 67.23 | 18.93 | 1.233 | 10 | 10 | 21.71 | 36 |
| CFED+150 Kombucha Tea vs. CFED+300 Kombucha Tea | 64.75 | 67.23 | -2.48 | 1.233 | 10 | 10 | 2.845 | 36 |
3.6. Total cholesterol multivariate test results
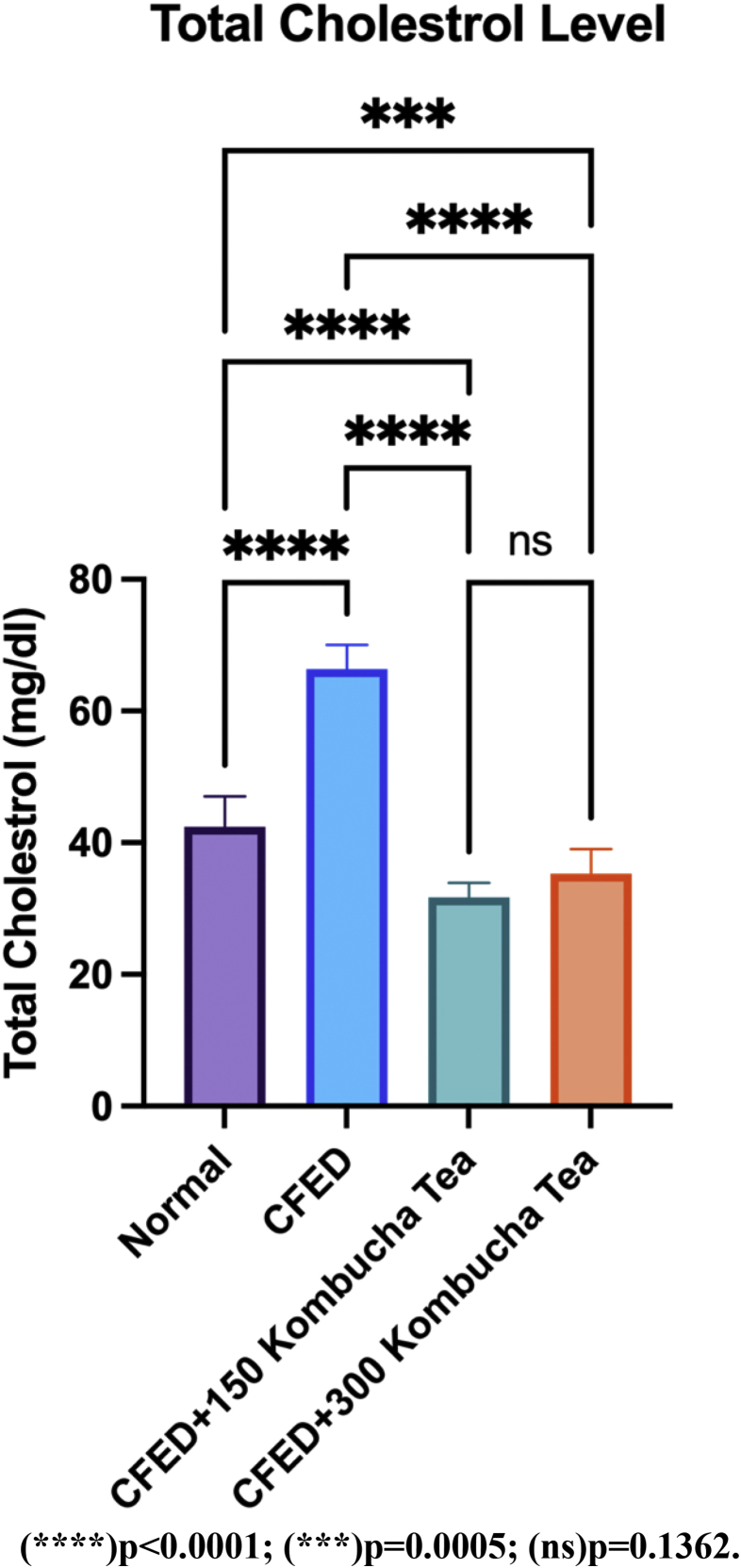
Figure 2 presents the significant increase in the blood cholesterol of mice when given a CFED diet only compared to the control group (p < 0.05). Blood cholesterol decreased significantly in both control and treatment group when given CFED treatment + kombucha tea 150 mg/kgBW and CFED treatment + kombucha tea 300 mg/kgBW (p < 0.05). The effect of kombucha tea administration of 150 mg/kgBW was more effective than the administration of 300 mg/kgBW of kombucha tea in decreasing the blood cholesterol of mice, but the result is not significant (p > 0.05). A detailed table of these test results is available in (Table 7).
Figure 2.
Both doses of seagrapes kombucha tea significantly reduce blood cholesterol.
Table 7.
Total cholesterol multivariate test results.
| Test details | Mean 1 | Mean 2 | Mean Diff. | SE of diff. | n1 | n2 | q | DF |
|---|---|---|---|---|---|---|---|---|
| Normal vs. CFED | 42.46 | 66.36 | -23.9 | 1.628 | 10 | 10 | 20.76 | 36 |
| Normal vs. CFED+150 Kombucha Tea | 42.46 | 31.67 | 10.79 | 1.628 | 10 | 10 | 9.373 | 36 |
| Normal vs. CFED+300 Kombucha Tea | 42.46 | 35.29 | 7.17 | 1.628 | 10 | 10 | 6.229 | 36 |
| CFED vs. CFED+150 Kombucha Tea | 66.36 | 31.67 | 34.69 | 1.628 | 10 | 10 | 30.14 | 36 |
| CFED vs. CFED+300 Kombucha Tea | 66.36 | 35.29 | 31.07 | 1.628 | 10 | 10 | 26.99 | 36 |
| CFED+150 Kombucha Tea vs. CFED+300 Kombucha Tea | 31.67 | 35.29 | -3.62 | 1.628 | 10 | 10 | 3.145 | 36 |
3.7. PGC-1α multivariate test results
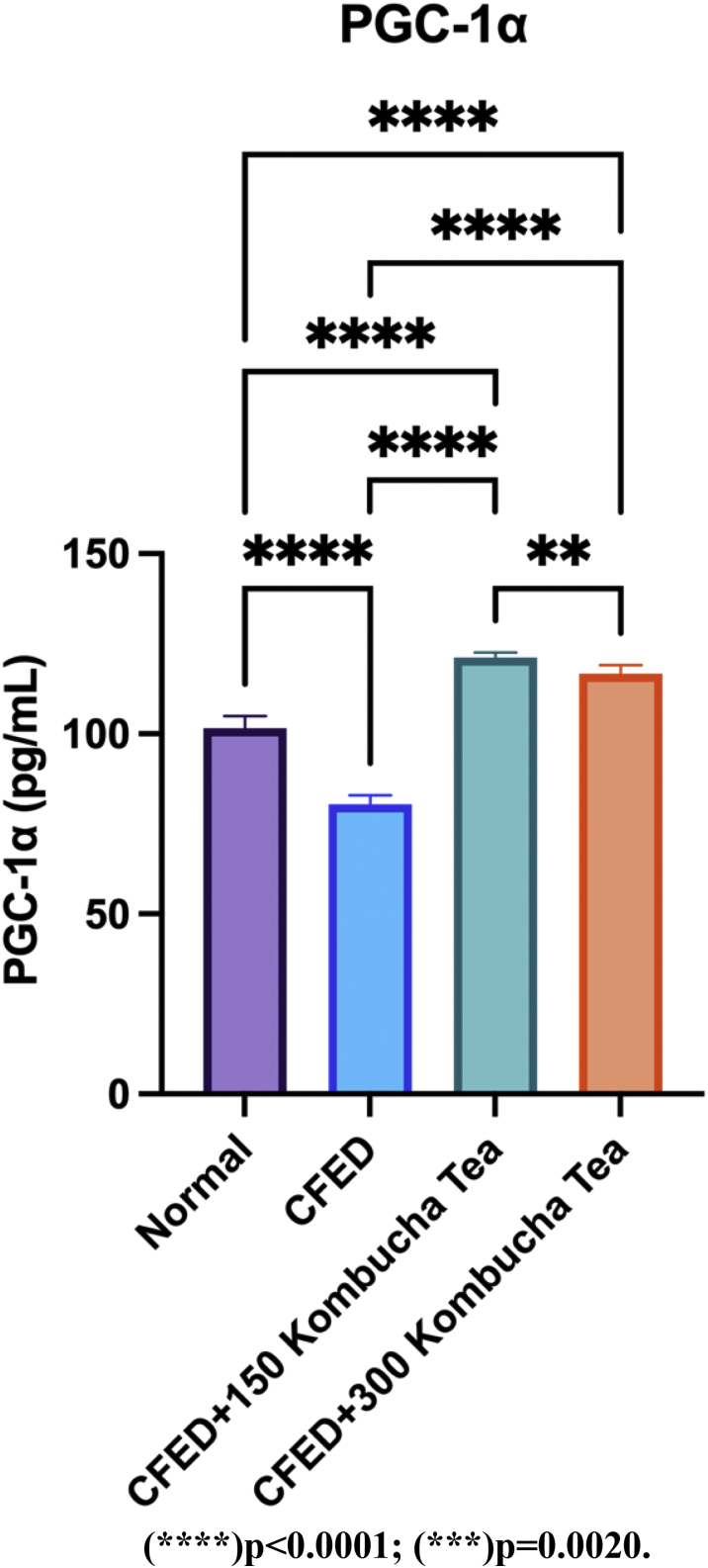
Figure 3 shows that PGC-1α serum decreased significantly after being given CFED treatment only (p < 0.05). PGC-1α serum increased significantly in both the control group and treatment group after being treated CFED + 150 mg/kgBW kombucha tea as well as CFED + 300 mg/kgBW kombucha tea. The effect of the administration of kombucha tea of 150 mg/kgBW is more effective than the administration of 300 mg/kgBW kombucha tea in increasing the PGC-1α serum level in mice, significantly (p < 0.05). A detailed table of these test results is available in (Table 8).
Figure 3.
The low dose of seagrapes kombucha tea is more effective in significantly increasing PGC-1α.
Table 8.
PGC-1α multivariate test results.
| Test details | Mean 1 | Mean 2 | Mean Diff. | SE of diff. | n1 | n2 | q | DF |
|---|---|---|---|---|---|---|---|---|
| Normal vs. CFED | 101.6 | 80.44 | 21.12 | 1.124 | 10 | 10 | 26.58 | 36 |
| Normal vs. CFED+150 Kombucha Tea | 101.6 | 121.2 | -19.59 | 1.124 | 10 | 10 | 24.66 | 36 |
| Normal vs. CFED+300 Kombucha Tea | 101.6 | 116.7 | -15.17 | 1.124 | 10 | 10 | 19.09 | 36 |
| CFED vs. CFED+150 Kombucha Tea | 80.44 | 121.2 | -40.71 | 1.124 | 10 | 10 | 51.24 | 36 |
| CFED vs. CFED+300 Kombucha Tea | 80.44 | 116.7 | -36.29 | 1.124 | 10 | 10 | 45.67 | 36 |
| CFED+150 Kombucha Tea vs. CFED+300 Kombucha Tea | 121.2 | 116.7 | 4.42 | 1.124 | 10 | 10 | 5.563 | 36 |
4. Discussion
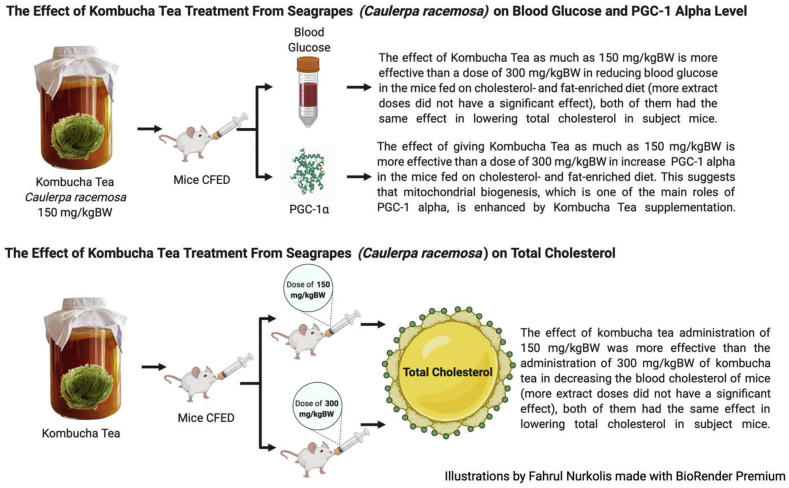
This study shows the significant blood glucose and cholesterol-lowering effects of kombucha tea from seagrapes (Caulerpa racemosa) in mice that were given CFED (Figure 4). Kombucha tea treatment also increases PGC-1α in mice that regulate glucose and lipid metabolism [18].
Figure 4.
Effects of Kombucha tea from Caulerpa racemosa on subject mice.
Chronic hyperglycemia may result in elevated oxidative stress levels and damage to proteins [36] while excess oxidative stress may lead to metabolic dysfunction [7] and result in ageing because of damaged cells. In this study, blood glucose decreases significantly in both the CFED + 150 mg/kgBW kombucha tea as well as CFED + 300 mg/kgBW kombucha tea treatment group (p < 0.05). Blood glucose and cholesterol-lowering effects of kombucha tea from seagrapes in this study also correspond to other studies [23, 25], providing better evidence that seagrapes have an antidiabetic and antihyperglycemic activity that is beneficial for health, specifically minimizing ageing and chronic diseases.
A significant hypolipidemic effect from kombucha tea was also observed in the study. However, the treatment of 150 mg/kgBW kombucha tea gave a better result in lowering cholesterol levels than the 300 mg/kgBW dose of kombucha tea. Palmitic acid content, which is a saturated fatty acid, constitutes about 80% of fatty acids in seagrapes [23] may contribute to this result. In addition, in this formulation of kombucha tea there is also the addition of honey from Trigona sapiens which clinically, the honey has a good effect in fighting metabolic syndrome and aging [37].
PGC-1α levels increased meaningfully in mice receiving kombucha tea administration compared to the control group. This proves that kombucha tea from seagrapes may give beneficial effects on health since an increase in PGC-1α is correlated with an increase of antioxidant activity, inhibit ROS-induced oxidative stress [20], and improved the condition of metabolism and ageing [38]. Polyphenols, antioxidants and flavonoids in seagrapes may also contribute to these results [21, 22]. Moreover, flavonoid supplementation is also reported to improve endurance performance via an elevation in the “master regulator” PGC-1α expression, which regulates biogenesis and angiogenesis of skeletal muscle [39].
Mice fed with CFED will have an elevated level of blood glucose and cholesterol. However, kombucha tea treatment will reduce the glucose and cholesterol level while increasing the PGC-1α level, along with an increase in the activity of α-glucosidase inhibitors. Inhibitors of α-glucosidase slow down the carbohydrate digestion process and minimize glucose entering into circulation, therefore lowering the glucose level [40]. Kombucha tea treatment also exhibits α-glucosidase and α-amylase inhibition activity of 90.42 ± 0.77% and 80.44 ± 1.00% compared to acarbose, an approved α-glucosidase and α-amylase inhibitor with 98.47 ± 0.98% and 88.59 ± 1.03% inhibition activity. However, the result of α-glucosidase inhibition activity from kombucha tea treatment in this study was higher than acarbose α-glucosidase inhibitory activity of 80% in other studies [41, 42], which shows a good α-glucosidase. These results were in line with other studies, showing that natural products from sea have a potential a-amylase inhibition activity and antidiabetes [23].
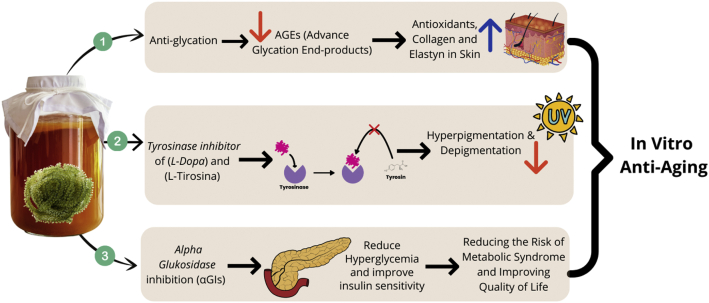
High melanin production or hyperpigmentation caused by excessive exposure to UV rays can cause the skin to become dark or depigmented [14], and it stimulates inflammation of the skin which triggers a series of biochemical reactions to the skin that cause damage to the skin. skin collagen tissue and premature skin aging [17]. Therefore, skin collagen tissue damage and premature aging can be prevented and minimized by reducing excess melanin production. Excessive melanin production can be prevented through the inhibition of tyrosinase. The inhibitory activity of tyrosinase at a concentration of 1000 ppm is presented in Table 2 and Figure 5. The inhibition activity of tyrosinase enzymes using the substrates L-Tyrosine and L-DOPA shows that kombucha tea from seagrapes has an inhibitory activity of tyrosine enzymes that potentially inhibit melanin production so that hyperpigmentation and depigmentation that can cause the skin to become dark and damaged can be minimized.
Figure 5.
In vitro effects of seagrapes Kombucha tea in ageing.
Unhealthy diets and lifestyles can worsen premature aging. When glucose is at high levels and insulin in the body is limited, the glycation process begins [43]. In this process, glucose binds to proteins in the skin, resulting in stiff and irregular skin [44]. In anti-glycation tests conducted (Table 2 and Figure 5), kombucha tea from seagrapes had an anti-glycation activity of 62.79 ± 0.78%. Anti-glycation activity is better compared to the results of antiglycation research of collagen in Shita's dissertation in 2018 [45], which is 17.74% at a concentration of 3000 ppm. Glycation produces Advanced Glycation End-products (AGEs) that can disable antioxidants, attack collagen, and elastin on the skin. As a result, the skin loses moisture and is no longer supple, easily damaged, wrinkled, dry, and dull, as well as premature aging [46]. This study is an in vitro and in vivo study. It does not necessarily represent the results in human study, but it can be used as a basic reference for clinical trials to further research the beneficial effects of kombucha tea from seagrapes for human consumption. However, it is also necessary to do the same research (Effect of Kombucha tea from Seagrapes) with parameters other than blood sugar, cholesterol and PGC-1a, in order to expand the scope of its metabolism.
5. Conclusions
Kombucha tea from Caulerpa racemosa is proved to improve blood glucose level, total cholesterol level, and PGC-1α on mice fed with CFED. Moreover, anti-glycation, tyrosinase inhibition, α-glucosidase, α-amylase inhibition properties of kombucha tea were also observed. Results prove that kombucha tea from seagrapes has a good potential and activity as an anti-ageing functional food. Further evidence is required for determining the functional potential of segrapes kombucha tea consumption in humans and the biomechanism underlying this result.
Declarations
Author contribution statement
Happy Kurnia Permatasari, Fahrul Nurkolis, Piko Satria Augusta: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Nelly Mayulu, Nurpudji Astuti Taslim, Defny Silvia Wewengkang: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mury Kuswari: Performed the experiments; Analyzed and interpreted the data.
Siti Chairiyah Batubara, William Ben Gunawan: Performed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank all contributors for their amazing work in creating the paper. We would also like to express our gratitude to Prof. Hardinsyah, MS., Ph.D. as President of the Federations of Asian Nutrition Societies; President of the Food and Nutrition Society of Indonesia (PERGIZI PANGAN Indonesia); and Chair of Southeast Asia Probiotics Scientific and Regulatory Experts Network (SEA PROBIOTICS SREN), who has provided inputs in the research and writing process of this paper as well as the motivation he gave us to keep the passion for research during a pandemic.
References
- 1.Shields H.J., Traa A., Van Raamsdonk J.M. Beneficial and detrimental effects of reactive oxygen species on lifespan: a comprehensive review of comparative and experimental studies. Front. Cell Dev. Biol. 2021;9(February):1–27. doi: 10.3389/fcell.2021.628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos A.L., Sinha S., Lindner A.B. The good, the bad, and the ugly of ROS: new insights on aging and aging-related diseases from eukaryotic and prokaryotic model organisms. Oxid. Med. Cell Longev. 2018;2018 doi: 10.1155/2018/1941285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munro D., Baldy C., Pamenter M.E., Treberg J.R. The exceptional longevity of the naked mole-rat may be explained by mitochondrial antioxidant defenses. Aging Cell. 2019;18(3):1–13. doi: 10.1111/acel.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nissanka N., Moraes C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018;592(5):728–742. doi: 10.1002/1873-3468.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa A., Scholer-Dahirel A., Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin. Cancer Biol. 2014;25:23–32. doi: 10.1016/j.semcancer.2013.12.007. (2014) [DOI] [PubMed] [Google Scholar]
- 6.Vatner S.F., Zhang J., Oydanich M., Berkman T., Naftalovich R., Vatner D.E. Vol. 64. Ageing Res Rev; 2020. (Healthful Aging Mediated by Inhibition of Oxidative Stress). (June):101194. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Kathy K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122(6):877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh N., Das A., Chaffee S., Roy S., Sen C.K. Immunity and Inflammation in Health and Disease: Emerging Roles of Nutraceuticals and Functional Foods in Immune Support. Elsevier; 2017. Reactive oxygen species, oxidative damage and cell death; pp. 45–55. Available from: [Google Scholar]
- 9.Park S. The effects of high concentrations of vitamin C on cancer cells. Nutrients. 2013;5(9):3496–3505. doi: 10.3390/nu5093496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S., Han S.S., Park C.H., Hahm E.R., Lee S.J., Park H.K. L-Ascorbic acid induces apoptosis in acute myeloid leukemia cells via hydrogen peroxide-mediated mechanisms. Int. J. Biochem. Cell Biol. 2004;36(11):2180–2195. doi: 10.1016/j.biocel.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Eren B., Tuncay Tanrıverdi S., Aydın Köse F., Özer Ö. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J. Cosmet. Dermatol. 2019;18(1):242–250. doi: 10.1111/jocd.12665. [DOI] [PubMed] [Google Scholar]
- 12.Schumacker P.T. Reactive oxygen species in cancer: a dance with the devil. Canc. Cell. 2015;27(2):156–157. doi: 10.1016/j.ccell.2015.01.007. Available from: [DOI] [PubMed] [Google Scholar]
- 13.Pereira D.M., Valentão P., Pereira J.A., Andrade P.B. Phenolics: from chemistry to biology. Molecules. 2009;14(6):2202–2211. [Google Scholar]
- 14.Saeedi M., Eslamifar M., Khezri K. Vol. 110. Biomed Pharmacother; 2019. Kojic Acid Applications in Cosmetic and Pharmaceutical Preparations; pp. 582–593. (November 2018) [DOI] [PubMed] [Google Scholar]
- 15.Pillaiyar T., Manickam M., Namasivayam V. Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017;32(1):403–425. doi: 10.1080/14756366.2016.1256882. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aseervatham G.S.B., Sivasudha T., Jeyadevi R., Arul Ananth D. Environmental factors and unhealthy lifestyle influence oxidative stress in humans--an overview. Environ. Sci. Pollut. Res. Int. 2013;20(7):4356–4369. doi: 10.1007/s11356-013-1748-0. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S., Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738. doi: 10.1177/0963689717725755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrzejewski S., Klimcakova E., Johnson R.M., Tabariès S., Annis M.G., McGuirk S. PGC-1α promotes breast cancer metastasis and confers bioenergetic flexibility against metabolic drugs. Cell Metabol. 2017;26(5):778–787. doi: 10.1016/j.cmet.2017.09.006. e5. [DOI] [PubMed] [Google Scholar]
- 19.Bost F., Kaminski L. The metabolic modulator PGC-1α in cancer. Am. J. Cancer Res. 2019;9(2):198–211. http://www.ncbi.nlm.nih.gov/pubmed/30906622%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC6405967 Available from: [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu N., Yan X., Li H. Clinical significance of serum pgc-1 alpha levels in diabetes mellitus with myocardial infarction patients and reduced ros-oxidative stress in diabetes mellitus with myocardial infarction model. Diabetes, Metab. Syndrome Obes. Targets Ther. 2020;13:4041–4049. doi: 10.2147/DMSO.S276163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang P., Liu D.Q., Liang T.J., Li J., Zhang H.Y., Liu A.H. Bioactive constituents from the green alga Caulerpa racemosa. Bioorg. Med. Chem. 2015;23(1):38–45. doi: 10.1016/j.bmc.2014.11.031. Available from: [DOI] [PubMed] [Google Scholar]
- 22.Yap W.F., Tay V., Tan S.H., Yow Y.Y., Chew J. Decoding antioxidant and antibacterial potentials of Malaysian green seaweeds: caulerpa racemosa and caulerpa lentillifera. Antibiotics. 2019;8(3) doi: 10.3390/antibiotics8030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aroyehun A.Q.B., Razak S.A., Palaniveloo K., Nagappan T., Rahmah N.S.N., Jin G.W. Bioprospecting cultivated tropical green algae, caulerpa racemosa: a perspective on nutritional properties, antioxidative capacity and anti-diabetic potential. Foods. 2020;9(9) doi: 10.3390/foods9091313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanna B., Yadav S., Mishra A. Anti-proliferative and ROS-inhibitory activities reveal the anticancer potential of Caulerpa species. Mol. Biol. Rep. 2020;47(10):7403–7411. doi: 10.1007/s11033-020-05795-8. Available from: [DOI] [PubMed] [Google Scholar]
- 25.Cao M., Li Y., Famurewa A.C., Olatunji O.J. Antidiabetic and nephroprotective effects of polysaccharide extract from the seaweed caulerpa racemosa in high fructose-streptozotocin induced diabetic nephropathy. Diabetes, Metab. Syndrome Obes. Targets Ther. 2021;14:2121–2131. doi: 10.2147/DMSO.S302748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez-Leal J., Ponce-García N., Escalante-Aburto A. Recent evidence of the beneficial effects associated with glucuronic acid contained in kombucha beverages. Curr. Nutr. Rep. 2020;9(3):163–170. doi: 10.1007/s13668-020-00312-6. [DOI] [PubMed] [Google Scholar]
- 27.Villarreal-Soto S.A., Beaufort S., Bouajila J., Souchard J.P., Taillandier P. Understanding kombucha tea fermentation: a review. J. Food Sci. 2018;83(3):580–588. doi: 10.1111/1750-3841.14068. [DOI] [PubMed] [Google Scholar]
- 28.Applegate K.B., Cheek P.R., Inlow J.K. Analysis of kombucha to teach biochemical concepts and techniques to undergraduate students. Biochem. Mol. Biol. Educ. 2019;47(4):459–467. doi: 10.1002/bmb.21240. [DOI] [PubMed] [Google Scholar]
- 29.Chakravorty S., Bhattacharya S., Bhattacharya D., Sarkar S., Gachhui R. The Science of Beverages. Vol. 6. Elsevier; 2019. Kombucha: a promising functional beverage prepared from tea [Internet]. Non-alcoholic Beverages; pp. 285–327. Available from: [Google Scholar]
- 30.Leal J.M., Suárez L.V., Jayabalan R., Oros J.H., Escalante-Aburto A. A review on health benefits of kombucha nutritional compounds and metabolites. CYTA - J. Food. 2018;16(1):390–399. Available from: [Google Scholar]
- 31.Augusta P., Nurkolis F., Noor S., Permatasari H., Hardinsyah, Taslim N.…Rotinsulu H. Probiotic beverage: the potential of anti-diabetes within kombucha tea made from sea grapes (Ceulerpa racemosa) containing high antioxidant and polyphenol total. Proc. Nutr. Soc. 2021;80(OCE3):E149. [Google Scholar]
- 32.Povichit N., Phrutivorapongkul A., Suttajit M., Leelapornpisid P. Antiglycation and antioxidant activities of oxyresveratrol extracted from the heartwood of artocarpus lakoocha roxb. Maejo Int. J. Sci. Technol. 2010;4(3):454–461. [Google Scholar]
- 33.Batubara I., Julita I., Darusman L.K., Muddathir A.M., Mitsunaga T. Flower bracts of temulawak (curcuma xanthorrhiza) for skin care: anti-acne and whitening agents. Proc. Chem. 2015;14:216–224. Available from: [Google Scholar]
- 34.Rodrigues B., Cam M.C., Kong J., Goyal R.K., McNeill J.H. Strain differences in susceptibility to streptozotocin-induced diabetes: effects on hypertriglyceridemia and cardiomyopathy. Cardiovasc. Res. 1997;34(1):199–205. doi: 10.1016/s0008-6363(97)00045-x. [DOI] [PubMed] [Google Scholar]
- 35.Harb A.A., Bustanji Y.K., Abdalla S.S. Hypocholesterolemic effect of β-caryophyllene in rats fed cholesterol and fat enriched diet. J. Clin. Biochem. Nutr. 2018;62(3):230–237. doi: 10.3164/jcbn.17-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turpin C., Catan A., Guerin-Dubourg A., Debussche X., Bravo S.B., Álvarez E. Enhanced oxidative stress and damage in glycated erythrocytes. PloS One. 2020;15(7 July):1–19. doi: 10.1371/journal.pone.0235335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramli N.Z., Chin K.Y., Zarkasi K.A., Ahmad F. A review on the protective effects of honey against metabolic syndrome. Nutrients. 2018;10(8):1–21. doi: 10.3390/nu10081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chambers J.M., Wingert R.A. PGC-1α in disease: recent renal insights into a versatile metabolic regulator. Cells. 2020;9(10):1–18. doi: 10.3390/cells9102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khani M., Motamedi P., Dehkhoda M.R., Dabagh Nikukheslat S., Karimi P. Effect of thyme extract supplementation on lipid peroxidation, antioxidant capacity, PGC-1α content and endurance exercise performance in rats. J. Int. Soc. Sports Nutr. 2017;14(1):1–8. doi: 10.1186/s12970-017-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong L., Feng D., Wang T., Ren Y., Liu Y., Wang J. Inhibitors of α-amylase and α-glucosidase: potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020;8(12):6320–6337. doi: 10.1002/fsn3.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dej-Adisai S., Pitakbut T., Wattanapiromsakul C. Alpha-glucosidase inhibitory activity and phytochemical investigation of Borassus flabellifer Linn. Afr. J. Pharm. Pharmacol. 2017;11(3):45–52. [Google Scholar]
- 42.Dej-Adisai S., Pitakbut T. Determination of α-glucosidase inhibitory activity from selected Fabaceae plants. Pak. J. Pharm. Sci. 2015;28(5):1679–1683. [PubMed] [Google Scholar]
- 43.Hantzidiamantis P.J., Lappin S.L. StatPearls; 2020. Physiology, Glucose.https://www.ncbi.nlm.nih.gov/books/NBK545201/ Available from: [PubMed] [Google Scholar]
- 44.Kim C.-S., Park S., Kim J. The role of glycation in the pathogenesis of aging and its prevention through herbal products and physical exercise. J. Exerc. Nutr. Biochem. 2017;21(3):55–61. doi: 10.20463/jenb.2017.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shita F., Irmanida B., Mala N. Institut Pertanian Bogor; 2018. Hidrolisat Kolagen Kulit Ikan Tuna Sirip Kuning (Thunnus albacares) Sebagai Anti Penuaan.http://repository.ipb.ac.id/handle/123456789/92399 Available from: [Google Scholar]
- 46.Gill V., Kumar V., Singh K., Kumar A., Kim J.J. Advanced glycation end products (AGEs) may be a striking link between modern diet and health. Biomolecules. 2019;9(12):1–21. doi: 10.3390/biom9120888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Worthington K. Worthington Biochemical Corporation; Lakewood, NJ: 1993. Manual Enzim Alfa Amilase Worthington (Hlm. 36–41. [Google Scholar]
- 48.Hedrich H.J., Mossmann H., Nicklas W. In: Hedrich H.J., Bullock G., editors. Vol. 1. Elsevier; London: 2004. Housing and maintenance; pp. 395–408. (The Laboratory Mouse). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.