Abstract
There is an increase in the incidence of onychomycosis, especially in at-risk populations. Onychomycosis is difficult to treat, as the efficacy of most antifungal agents is relatively low. Nondermatophyte molds (NDMs) and mixed infection (dermatophyte plus NDM) onychomycosis are contributing to growing antifungal resistance, as they are often underestimated and ignored due to incorrect diagnosis. There is a need for a paradigm shift in the management of onychomycosis to a patient-centered, holistic approach with an emphasis on laboratory diagnosis prior to initiating treatment, which enables the rational choice of the antifungal agent. Additionally, in the case of resistant infections, antifungal susceptibility testing is recommended. Strategies for effective management of onychomycosis include disinfection of fungal reservoirs in shoes and socks and prophylaxis posttreatment using topical antifungal agents. These measures may reduce the recurrence of onychomycosis and improve long-term clinical success.
Keywords: Onychomycosis, Treatment, Management, Superficial mycoses, Paradigm, Antifungal resistance
Introduction
Superficial mycoses are becoming a major public health concern as they are one of the most common global infections [1, 2]. In some instances, superficial fungal infections may lead to invasive infections, whose incidence is increasing partly due to a rise in at-risk populations [3, 4, 5]. Systemic antifungal agents are the most effective treatment for onychomycosis; however, they are associated with serious but uncommon side effects such as hepatotoxicity and drug interactions. Therefore, effective topical therapies may be preferred in certain circumstances [6, 7, 8, 9]. Treatment is aimed at the eradication of the fungal pathogen(s) (mycological cure), and, where possible, a return to normal, healthy appearing nails (clinical cure) [6, 7, 10, 11, 12].
Successful treatment options for onychomycosis are limited; treatment failures and disease recurrence are frequently encountered [6, 10, 11, 13]. Onychomycosis patients (10–53%) may experience relapse or reinfection after the initial infection has been eradicated [14, 15, 16, 17, 18, 19]. This may be due to either failure to fully eradicate the fungal pathogen or reinfection with a new causative strain following subsequent exposure [20]. Several factors contribute to the high relapse and/or reinfection rate, including genetic predisposition to onychomycosis; incorrect diagnosis at baseline; infection with nondermatophyte molds (NDMs); mixed infections (dermatophyte and NDM); antifungal pharmacokinetics and pharmacodynamics; comorbidities such as diabetes, HIV, and presence of concurrent tinea pedis; the presence of biofilms; antifungal resistance; and dormant fungal reservoirs (arthroconidia) present in the nail bed that are resistant to antifungals [5, 14, 20, 21, 22, 23, 24].
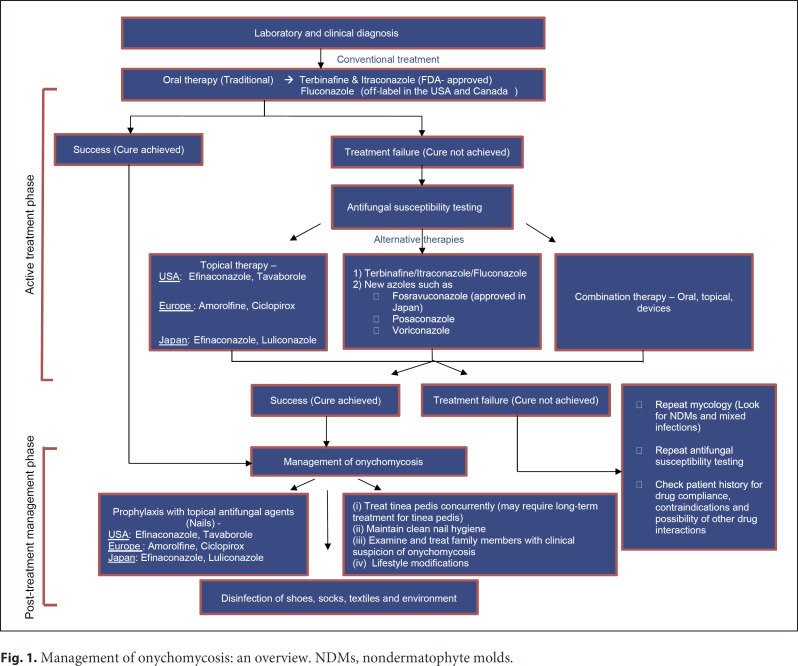
Numerous strategies have been recommended for the management of onychomycosis, most of which focus on antifungal therapy, that is, the choice of antifungal and drug regimen. However, due to the multifactorial nature of onychomycosis, focusing solely on antifungal therapy may not result in the successful eradication of the infection. We propose a paradigm shift in the approach to onychomycosis to a holistic practice (Fig. 1), which includes accurate diagnosis to assist in the optimal choice of antifungal agent, disinfection of fungal reservoirs (shoes, socks, and shower stalls), encouraging lifestyle changes, and prophylaxis posttreatment.
Fig. 1.
Management of onychomycosis: an overview. NDMs, nondermatophyte molds.
Laboratory Diagnosis and the Need for Antifungal Susceptibility Testing
Laboratory diagnosis should be considered wherever possible prior to initiating antifungal therapy. Identification of the fungal pathogen to the species level and its viability can be achieved through appropriate laboratory diagnosis (direct microscopy using KOH, fungal culture, histopathology, or molecular biology techniques such as polymerase chain reaction). To identify and confirm onychomycosis caused by NDMs, clinical diagnosis with serial mycological confirmation (minimum 2 different time points) from patients taken at approximately 4 weeks apart is recommended [25, 26, 27]. Where possible, antifungal susceptibility testing, especially in those patients not responding to primary treatment, should be strongly considered. Minimum inhibitory concentration (MIC) values of the causative organism(s) to the antifungal agent(s) allow for the selection of the most appropriate antifungal agent(s) to manage recalcitrant infections [28].
Treatment Strategies
Antifungal Resistance
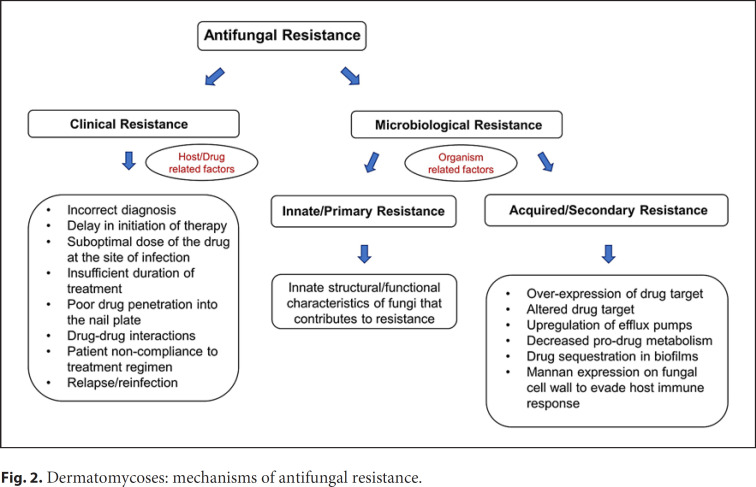
Antifungal resistance is being increasingly reported around the globe [21, 29, 30, 31]. Antifungal resistance can be clinical or microbiological (Fig. 2) [20, 21, 32]. Clinical resistance is attributed to host-, treatment-, or drug-related factors (e.g., incorrect diagnosis, suboptimal dose of the drug, and patient noncompliance) while microbiological resistance is attributed to organism-related factors (e.g., genetic mutations) [32]. These factors may contribute to resistance individually or in combination [5, 21, 32]. A substantial number of the recalcitrant infections (especially Trichophyton isolates) are due to terbinafine resistance [21, 33, 34, 35, 36]. However, not all treatment-failure cases are resistant to initial antifungal therapy. For example, Ghannoum et al. [20] reported that the in vitro susceptibility testing of all isolates from onychomycosis patients who failed treatment with oral terbinafine did not indicate resistance to terbinafine, itraconazole, fluconazole, and/or griseofulvin (based on the MIC values). Instead, the treatment failures were suggested to be due to host or family factors, that is, clinical resistance. Therefore, when antifungal resistance is suspected, the cause behind it (increased MIC, patient noncompliance, or other factors) must be determined to ensure appropriate action is taken (e.g., change of antifungal drug, encouraging patient compliance, and focusing on patient- or family-related factors).
Fig. 2.
Dermatomycoses: mechanisms of antifungal resistance.
Overexposure to the conventional antifungal drugs used to treat systemic and cutaneous fungal infections may have contributed to the terbinafine and azole resistance [5, 33, 34, 37, 38, 39]. Onychomycosis case studies reporting failure to terbinafine therapy are being increasingly documented in the literature [21, 40, 41, 42, 43]. According to a multicenter study, the prevalence of terbinafine-resistant strains (Trichophyton rubrum and Trichophyton mentagrophytes) in India ranges from 16 to 77% [21, 31]. Terbinafine resistance is spreading across countries with isolates reported in Canada, the USA, Iran, Poland, Germany, Switzerland, and Denmark, which may be due to an increase in immigration, travel, and a shift in epidemiology towards more NDMs and mixed infection onychomycosis [21, 36, 44, 45]. Along with clinical terbinafine resistance, mutations in the squalene epoxidase gene are attributed to conferring microbiological terbinafine resistance in T. rubrum and T. mentagrophytes isolates [21, 33, 34, 36, 46].
A potential for developing azole resistance is generally observed in Candida and Aspergillus species and primarily occurs due to mutations in the genes encoding drug target (ERG11) and upregulation of efflux pumps (MDR1, CDR1, and CDR2) [5, 39, 47, 48, 49]. Since azoles are the only class of antifungals used both in agriculture and in the clinic, environmental fungi and opportunistic pathogens such as Aspergillus species may acquire resistance to azoles through exposure to fungicides in agricultural situations, thus are resistant to azole therapeutics even before colonizing the nail unit [5, 50]. Recent studies suggest that azoles also have the potential for developing resistance in dermatophytes through mutations in enzymes in the ergosterol pathways [51]. The global incidence of azole resistance in dermatophytes is roughly 19% [51]. Azole (itraconazole and voriconazole) resistance possibly mediated through multidrug efflux pumps has also been reported in a clinical isolate of T. rubrum [52].
One of the major factors contributing to antifungal resistance is the occurrence of mixed infections, which is often underestimated [53, 54]. Mixed infections account for about 6% of all superficial mycoses [55]. The notion of NDMs as sole etiological agents of onychomycosis, or in combination with a dermatophyte in a mixed infection, is often met with skepticism and dismissed since NDMs are often regarded as common laboratory contaminants or commensals of the nail, skin, and hair [27]. We propose that NDM and mixed infections be investigated as possible etiological agents of onychomycosis especially when there is treatment failure. Broad-spectrum antifungal agents such as itraconazole, efinaconazole, and tavaborole may be considerations in the treatment and management of mixed infection onychomycosis [56, 57]. Terbinafine may now be a less attractive choice if there is a concern for terbinafine-resistant dermatophytes.
Alternative Therapies for Recalcitrant Onychomycosis
Treatment of recalcitrant onychomycosis with azoles such as posaconazole, voriconazole, fosravuconazole, or a combination of these with other oral or topical antifungal agents has been documented [40, 58, 59]. Terbinafine- and itraconazole-resistant onychomycoses due to dermatophytes such as T. rubrum (fingernails), NDMs such as Fusarium species (toenails), or yeasts such as Candida species (fingernails) have been cleared using oral voriconazole (200 mg twice daily for 3 months), oral posaconazole (pulse regimen: 800 mg oral solution/day for 1 week per month for 4 months), or fosravuconazole (100 mg/day for 3 months), respectively [40, 58, 59, 60]. Amphotericin B (once daily application for 12 months), a topical broad-spectrum antifungal agent belonging to the polyene class, has been shown to be effective and safe in treating NDM onychomycosis caused by Fusarium, Acremonium, and Aspergillus species [37, 61].
Sequential or combined oral antifungal therapy with terbinafine and itraconazole used off-label is a consideration for those patients who fail therapy or show poor response to initial monotherapy [62]. Alternatively, oral terbinafine and itraconazole may be combined off-label with topical amorolfine, ciclopirox, efinaconazole, or tavaborole. The different routes of administration allow for complementary drug penetration to the infection site in effective concentrations [62]. Additionally, combination antifungal therapy may be effective in treating difficult NDM/Candida onychomycosis and mixed infections due to the synergistic action of the drugs involved, broad-spectrum activity, and increased efficacy [63, 64, 65].
It is important to examine the patient for the presence of tinea pedis, as onychomycosis often coexists with tinea pedis [11, 66, 67]. The surrounding skin may act as a fungal reservoir and predispose the nail to a cycle of reinfection and recurrence [14, 66, 68]. Concurrent tinea pedis can be effectively treated with topical therapy [69, 70].
Management of Onychomycosis: Beyond the Nail
Relapse and reinfection rates of onychomycosis (10–53%) [14, 15, 16, 19] are high, with recurrence likely to occur within 30 months of cure following treatment with systemic antifungal agents [14]. Several nontreatment factors may contribute to recurrence, including family history, underlying physiology, lifestyle, occupation, physical trauma, and environmental conditions [14, 71]. Strategies to reduce recurrence should be encouraged.
Sanitization Techniques
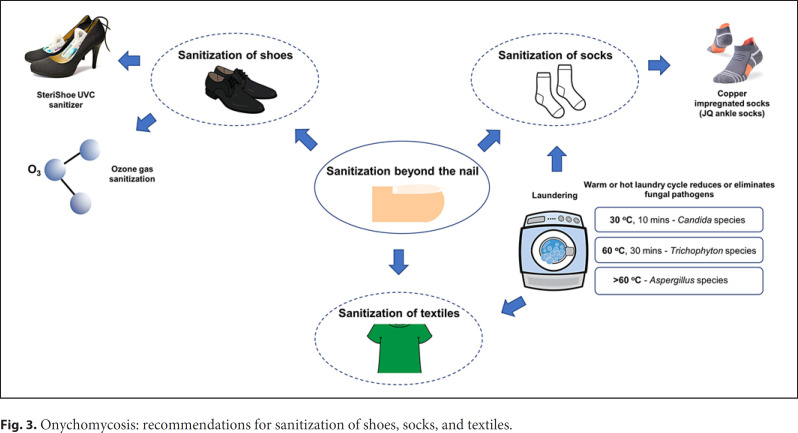
Continued or re-exposure to fungal reservoirs, such as infected shoes, socks, or textiles, can contribute to reinfection (Fig. 3) [72, 73, 74, 75, 76, 77]. Dermatophytes are known to colonize and survive for a long duration in footwear worn by patients with onychomycosis and tinea pedis [78]. Fungi may use sweat and skin cells trapped in footwear as a source of nutrients to create and maintain fungal reservoirs [72, 79, 80]. Textiles such as towels, sheets, and blankets are also potential fungal reservoirs [72, 79]. Contaminated socks and shoes can act as a source of reinfection when worn by patients after achieving cure with antifungal therapy [81]. Footwear and textiles can possibly contaminate the sterile laundry of patients when stored in the same laundry basket or when washed in the same washing machine [72, 82]. The basic sanitization recommendations for patients include replacing heavily contaminated footwear with a new pair, changing socks to an absorbent material with once or twice daily replacement, and storing contaminated clothing separately from sterile wear to minimize transmission and contamination [72].
Fig. 3.
Onychomycosis: recommendations for sanitization of shoes, socks, and textiles.
Textiles represent an important link in the chain of dermatophyte infections [82, 83]. The use of functionalized, antimicrobial fabrics is recommended as a measure to reduce the spreading of dermatophyte infections [82]. For example, copper-impregnated socks may be effective against dermatophytes as copper has fungicidal and antibacterial properties [73, 84, 85].
Laundering contaminated clothing plays a role in reducing the fungal burden. Washing at high temperatures reduces or eliminates dermatophytes and Candida species [13, 75, 76, 86, 87]. Hammer and colleagues [75] observed that washing at 30°C for 10 min eliminated C. albicans, while washing at higher temperatures (60°C) for 30 min was required to completely eradicate T. rubrum. However, Aspergillus species still persisted in some of the socks washed at 60°C, which may require higher temperature laundering and drying [76]. It is suggested that if laundry machines are not properly sanitized, they may act as a fungal reservoir, contaminating other sterile textiles. This is believed to be the cause of concurrent tinea cruris (jock itch) in a certain percentage of tinea pedis patients [72, 75, 87, 88].
Multiple devices have been developed to sterilize infected footwear. Ozone gas disinfection is another novel technique recommended for sanitization of contaminated footwear, exhibiting fungicidal activity against T. rubrum and T. mentagrophytes colonies [72, 74, 89, 90]. Ultraviolet shoe sanitizers have been developed to irradiate the inner soles of shoes and reduce fungal burden, as fungi absorb UV light at a wavelength of 200–300 nm with germicidal effects [72, 77, 91, 92, 93, 94, 95].
Microwave irradiation has been investigated for its inhibitory effect on dermatophytes such as T. rubrum, T. interdigitale, and Microsporum canis [96]. Complete inhibition of fungal growth occurred on dermatophyte-infected cork and polyethylene sponge insoles after a 30-s exposure to microwaves at 560 W and a maximum temperature of 60°C, without damaging the insoles [96]. The mechanism of microwave irradiation to disinfect contaminated footwear is not yet fully understood [96].
Environmental and Lifestyle Changes
Wearing shoes or sandals in high-risk areas such as swimming pools and baths, wearing properly fitting footwear, drying feet following a bath or shower, and maintaining personal and nail hygiene by cutting nails short and keeping them clean may help reduce the spread of fungal organisms. Examining and treating infected family members and housemates for onychomycosis and tinea pedis is also important, as they may act as a source of fungal reservoirs [13, 97].
Prophylaxis
Topical antifungal agents such as amorolfine, efinaconazole, and tavaborole should be considered as prophylaxis posttreatment to delay or avoid recurrence [14, 98]. With high concentrations of efinaconazole (well above the MIC of the causative dermatophytes) in the nail for up to 2 weeks posttreatment, twice a week application of efinaconazole as a prophylactic agent may prevent reinfection [14, 99]. Prophylaxis with efinaconazole for the affected nail(s) and a topical antifungal for tinea pedis should be maintained for 2–3 years, or even longer, especially in those patients with diabetes and poor peripheral circulation.
A Holistic Approach with a Laboratory-Directed Focus towards Treatment and Management of Onychomycosis: A Proposed Paradigm Shift
Treating onychomycosis should not be confined to the nail. The proposed paradigm shift in the treatment of onychomycosis from the conventional drug-centered approach to a patient-centered approach is a holistic practice with a laboratory-directed focus. It is important to have mycological confirmation and clinical diagnosis of onychomycosis. In cases of treatment failures and suspected antifungal resistance, antifungal susceptibility testing and investigation into environmental and patient-related factors will assist in determining the most appropriate antifungal agent(s) for targeted therapy. Disinfection of shoes and socks and prophylactic antifungal therapy for the affected nail(s) and surrounding skin may reduce the spread of fungal organisms, which will help to achieve successful long-term management of onychomycosis.
Conflict of Interest Statement
A.K.G. reports grants from Bausch Health and Ortho-Dermatologics outside the submitted work. M.V. and H.J.R. report personal fees from Mediprobe Research Inc. during the conduct of the study. R.C.S. has nothing to disclose. N.H.S. reports personal fees from Alpha Laboratories outside the submitted work. V.P. reports personal fees from Pfizer, Abbvie, Janssen, UCB, Novartis, Almirall, and Celgene and grants from AbbVie, Bausch Health, Celgene, Janssen, LEO Pharma, Lilly, NAOS, Novartis, Pfizer, Pierre-Fabre, Sanofi, and Kyowa Kirin Co., Ltd. outside the submitted work.
Funding Sources
The authors have no funding to declare.
Author Contributions
Aditya K. Gupta contributed to the conceptualization and writing of the manuscript. Maanasa Venkataraman and Helen Renaud contributed to the writing of the manuscript. Richard Summerbell, Neil Shear, and Vincent Piguet provided their critical review of the manuscript.
References
- 1.Hazarika D, Jahan N, Sharma A. Changing trend of superficial mycoses with increasing nondermatophyte mold infection: a clinicomycological study at a tertiary referral center in Assam. Indian J Dermatol. 2019 Jul–Aug;64((4)):261–5. doi: 10.4103/ijd.IJD_579_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warnock DW. Fungal diseases: an evolving public health challenge. Med Mycol. 2006 Dec;44((8)):697–705. doi: 10.1080/13693780601009493. [DOI] [PubMed] [Google Scholar]
- 3.Baran R, McLoone N, Hay RJ. Could proximal white subungual onychomycosis be a complication of systemic spread? The lessons to be learned from Maladie Dermatophytique and other deep infections. Br J Dermatol. 2005 Nov;153((5)):1023–5. doi: 10.1111/j.1365-2133.2005.06838.x. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Rubio R, Cuenca-Estrella M, Mellado E. Triazole resistance in Aspergillus species: an emerging problem. Drugs. 2017 Apr;77((6)):599–613. doi: 10.1007/s40265-017-0714-4. [DOI] [PubMed] [Google Scholar]
- 5.Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, Krause R, et al. COVID-19 associated pulmonary aspergillosis (CAPA)-from immunology to treatment. J Fungi. 2020;6((2)):91. doi: 10.3390/jof6020091. Published 2020 Jun 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta AK, Stec N, Summerbell RC, Shear NH, Piguet V, Tosti A, et al. Onychomycosis: a review. J Eur Acad Derm Venereol. 2020 Jun;34:1972–90. doi: 10.1111/jdv.16394. [DOI] [PubMed] [Google Scholar]
- 7.Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019 Apr 1;80((4)):853–67. doi: 10.1016/j.jaad.2018.05.1260. [DOI] [PubMed] [Google Scholar]
- 8.Gupta AK, Ryder JE, Johnson AM. Cumulative meta-analysis of systemic antifungal agents for the treatment of onychomycosis. Br J Dermatol. 2004;150((3)):537–44. doi: 10.1046/j.1365-2133.2003.05728.x. [DOI] [PubMed] [Google Scholar]
- 9.Gupta AK, Paquet M. Systemic antifungals to treat onychomycosis in children: a systematic review. Pediatr Dermatol. 2013 Jun;30((3)):294–302. doi: 10.1111/pde.12048. [DOI] [PubMed] [Google Scholar]
- 10.Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998 Jul;11((3)):415–29. doi: 10.1128/cmr.11.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta AK, Mays RR, Versteeg SG, Piraccini BM, Takwale A, Shemer A, et al. Global perspectives for the management of onychomycosis. Int J Dermatol. 2019;58((10)):1118–29. doi: 10.1111/ijd.14346. [DOI] [PubMed] [Google Scholar]
- 12.Westerberg DP, Voyack MJ. Onychomycosis: current trends in diagnosis and treatment. Am Fam Physician. 2013 Dec 1;88((11)):762–70. [PubMed] [Google Scholar]
- 13.Gupta AK, Versteeg SG, Shear NH, Piguet V, Tosti A, Piraccini BM. A practical guide to curing onychomycosis: how to maximize cure at the patient, organism, treatment, and environmental level. Am J Clin Dermatol. 2019 Feb;20((1)):123–33. doi: 10.1007/s40257-018-0403-4. [DOI] [PubMed] [Google Scholar]
- 14.Gupta AK, Elewski BE, Rosen T, Caldwell B, Pariser DM, Kircik LH, et al. Onychomycosis: strategies to minimize recurrence. J Drugs Dermatol. 2016 Mar;15((3)):279–82. [PubMed] [Google Scholar]
- 15.Gupta AK, Cooper EA, Paquet M. Recurrences of dermatophyte toenail onychomycosis during long-term follow-up after successful treatments with mono- and combined therapy of terbinafine and itraconazole. J Cutan Med Surg. 2013 Jun;17((3)):201–6. doi: 10.2310/7750.2013.12088. [DOI] [PubMed] [Google Scholar]
- 16.Tosti A, Piraccini BM, Stinchi C, Colombo MD. Relapses of onychomycosis after successful treatment with systemic antifungals: a three-year follow-up. Dermatology. 1998;197((2)):162–6. doi: 10.1159/000017990. [DOI] [PubMed] [Google Scholar]
- 17.Drake LA, Shear NH, Arlette JP, Cloutier R, Danby FW, Elewski BE, et al. Oral terbinafine in the treatment of toenail onychomycosis: North American multicenter trial. J Am Acad Dermatol. 1997 Nov;37((5 Pt 1)):740–5. doi: 10.1016/s0190-9622(97)70111-7. [DOI] [PubMed] [Google Scholar]
- 18.Shemer A, Gupta AK, Kamshov A, Babaev M, Farhi R, Daniel CR, et al. Topical antifungal treatment prevents recurrence of toenail onychomycosis following cure. Dermatol Ther. 2017 Sept;30((5)) doi: 10.1111/dth.12545. [DOI] [PubMed] [Google Scholar]
- 19.Sigurgeirsson B, Olafsson JH, Steinsson JB, Paul C, Billstein S, Evans EG. Long-term effectiveness of treatment with terbinafine vs itraconazole in onychomycosis: a 5-year blinded prospective follow-up study. Arch Dermatol. 2002 Mar;138((3)):353–7. doi: 10.1001/archderm.138.3.353. [DOI] [PubMed] [Google Scholar]
- 20.Ghannoum M, Isham N. Fungal nail infections (onychomycosis): a never-ending story? PLoS Pathog. 2014;10((6)):e1004105. doi: 10.1371/journal.ppat.1004105. Published 2014 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta AK, Renaud HJ, Quinlan EM, Shear NH, Piguet V. The growing problem of antifungal resistance in onychomycosis and other superficial mycoses. Am J Clin Dermatol. 2020 Dec 22;22:149–57. doi: 10.1007/s40257-020-00580-6. [DOI] [PubMed] [Google Scholar]
- 22.Pereira LD, Vila T, Borba-Santos LP, de Souza W, Navarro M, Rozental S. Activity of metal-azole complexes against biofilms of Candida albicans and Candida glabrata. Curr Pharm Des. 2020;26((14)):1524–31. doi: 10.2174/1381612826666200217120321. [DOI] [PubMed] [Google Scholar]
- 23.Gupta AK, Daigle D, Carviel JL. The role of biofilms in onychomycosis. J Am Acad Dermatol. 2016 Jun;74((6)):1241–6. doi: 10.1016/j.jaad.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Córdova-Alcántara IM, Venegas-Cortés DL, Martínez-Rivera MÁ, Pérez NO, Rodriguez-Tovar AV. Biofilm characterization of Fusarium solani keratitis isolate: increased resistance to antifungals and UV light. J Microbiol. 2019 Jun;57((6)):485–97. doi: 10.1007/s12275-019-8637-2. [DOI] [PubMed] [Google Scholar]
- 25.Gupta AK, Drummond-Main C, Cooper EA, Brintnell W, Piraccini BM, Tosti A. Systematic review of nondermatophyte mold onychomycosis: diagnosis, clinical types, epidemiology, and treatment. J Am Acad Dermatol. 2012 Mar;66((3)):494–502. doi: 10.1016/j.jaad.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 26.Gupta AK, Cooper EA, MacDonald P, Summerbell RC. Utility of inoculum counting (Walshe and English criteria) in clinical diagnosis of onychomycosis caused by nondermatophytic filamentous fungi. J Clin Microbiol. 2001 Jun;39((6)):2115–21. doi: 10.1128/JCM.39.6.2115-2121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Summerbell RC. Epidemiology and ecology of onychomycosis. Dermatology. 1997;194((Suppl 1)):32–6. doi: 10.1159/000246182. [DOI] [PubMed] [Google Scholar]
- 28.Rex JH, Pfaller MA, Walsh TJ, Chaturvedi V, Espinel-Ingroff A, Ghannoum MA, et al. Antifungal susceptibility testing: practical aspects and current challenges. Clin Microbiol Rev. 2001 Oct;14((4)):643–58. doi: 10.1128/CMR.14.4.643-658.2001. Table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandeputte P, Ferrari S, Coste AT. Antifungal resistance and new strategies to control fungal infections. Int J Microbiol. 2012;2012:713687. doi: 10.1155/2012/713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh S, Chandra U, Anchan VN, Verma P, Tilak R. Limited effectiveness of four oral antifungal drugs (fluconazole, griseofulvin, itraconazole and terbinafine) in the current epidemic of altered dermatophytosis in India: results of a randomized pragmatic trial. Br J Dermatol. 2020 Jun 15;183:840–6. doi: 10.1111/bjd.19146. [DOI] [PubMed] [Google Scholar]
- 31.Ebert A, Monod M, Salamin K, Burmester A, Uhrlaß S, Wiegand C, et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: a multicentre study. Mycoses. 2020 Jul;63((7)):717–28. doi: 10.1111/myc.13091. [DOI] [PubMed] [Google Scholar]
- 32.Pai V, Ganavalli A, Kikkeri NN. Antifungal resistance in dermatology. Indian J Dermatol. 2018 Sept–Oct;63((5)):361–8. doi: 10.4103/ijd.IJD_131_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh A, Masih A, Khurana A, Singh PK, Gupta M, Hagen F, et al. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses. 2018 Jul;61((7)):477–84. doi: 10.1111/myc.12772. [DOI] [PubMed] [Google Scholar]
- 34.Yamada T, Maeda M, Alshahni MM, Tanaka R, Yaguchi T, Bontems O, et al. Terbinafine resistance of Trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob Agents Chemother. 2017 Jun 27;61((7)):e00115–17. doi: 10.1128/AAC.00115-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudramurthy SM, Shankarnarayan SA, Dogra S, Shaw D, Mushtaq K, Paul RA, et al. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother. 2018 May;62((5)) doi: 10.1128/AAC.02522-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saunte DML, Hare RK, Jørgensen KM, Jørgensen R, Deleuran M, Zachariae CO, et al. Emerging terbinafine resistance in Trichophyton: clinical characteristics, squalene epoxidase gene mutations, and a reliable EUCAST method for detection. Antimicrob Agents Chemother. 2019 Sept 23;63((10)):e01126–19. doi: 10.1128/AAC.01126-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monod M, Méhul B. Recent findings in onychomycosis and their application for appropriate treatment. J Fungi. 2019 Feb 22;5((1)):20. doi: 10.3390/jof5010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Digby SS, Hald M, Arendrup MC, Hjort SV, Kofoed K. Darier disease complicated by terbinafine-resistant Trichophyton rubrum: a case report. Acta Derm Venereol. 2017 Apr;97((1)):139–40. doi: 10.2340/00015555-2455. [DOI] [PubMed] [Google Scholar]
- 39.Gaur M, Puri N, Manoharlal R, Rai V, Mukhopadhayay G, Choudhury D, et al. MFS transportome of the human pathogenic yeast Candida albicans. BMC Genomics. 2008 Dec 3;9:579. doi: 10.1186/1471-2164-9-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nofal A, Fawzy MM, El-Hawary EE. Successful treatment of resistant onychomycosis with voriconazole in a liver transplant patient. Dermatol Ther. 2020 Jul 15;33((6)):e14014. doi: 10.1111/dth.14014. [DOI] [PubMed] [Google Scholar]
- 41.Ahmadi B, Hashemi SJ, Zaini F, Shidfar MR, Moazeni M, Mousavi B, et al. A case of onychomycosis caused by Aspergillus candidus. Med Mycol Case Rep. 2012 Jul 3;1((1)):45–8. doi: 10.1016/j.mmcr.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura U, Hiruma M, Kano R, Matsumoto T, Takamori K, Suga Y. Onychomycosis caused by Scopulariopsis brevicaulis: the third documented case in Japan. J Dermatol. 2019 May;46((5)):e167–8. doi: 10.1111/1346-8138.14677. [DOI] [PubMed] [Google Scholar]
- 43.Mulvaney PM, Telang GH, Jellinek N. Trichophytum rubrum endonyx onychomycosis resistant to standard oral and topical therapies. Dermatol Online J. 2015 Sept 17;21((9)):21. [PubMed] [Google Scholar]
- 44.Gallo JG, Woods M, Graham RM, Jennison AV. A severe transmissible Majocchi's granuloma in an immunocompetent returned traveler. Med Mycol Case Rep. 2017 Jul 6;18:5–7. doi: 10.1016/j.mmcr.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nenoff P, Verma SB, Ebert A, Süß A, Fischer E, Auerswald E, et al. Spread of terbinafine-resistant Trichophyton mentagrophytes type VIII (India) in Germany-“the tip of the iceberg?”. J Fungi. 2020 Oct 5;6((4)):207. doi: 10.3390/jof6040207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta AK, Nakrieko KA. Trichophyton rubrum DNA strain switching increases in patients with onychomycosis failing antifungal treatments. Br J Dermatol. 2015 Jan;172((1)):74–80. doi: 10.1111/bjd.13165. [DOI] [PubMed] [Google Scholar]
- 47.Ksiezopolska E, Gabaldón T. Evolutionary emergence of drug resistance in Candida opportunistic pathogens. Genes. 2018 Sept 19;9((9)):461. doi: 10.3390/genes9090461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morio F, Jensen RH, Le Pape P, Arendrup MC. Molecular basis of antifungal drug resistance in yeasts. Int J Antimicrob Agents. 2017 Nov;50((5)):599–606. doi: 10.1016/j.ijantimicag.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Abastabar M, Hosseini T, Valadan R, Lagzian M, Haghani I, Aslani N, et al. Novel point mutations in cyp51A and cyp51B genes associated with itraconazole and posaconazole resistance in Aspergillus clavatus isolates. Microb Drug Resist. 2019 Jun;25((5)):652–62. doi: 10.1089/mdr.2018.0300. [DOI] [PubMed] [Google Scholar]
- 50.Meneau I, Sanglard D. Azole and fungicide resistance in clinical and environmental Aspergillus fumigatus isolates. Med Mycol. 2005 May;43((Suppl 1)):S307–11. doi: 10.1080/13693780500090826. [DOI] [PubMed] [Google Scholar]
- 51.Ghannoum M. Azole resistance in dermatophytes: prevalence and mechanism of action. J Am Podiatr Med Assoc. 2016 Feb;106((1)):79–86. doi: 10.7547/14-109. [DOI] [PubMed] [Google Scholar]
- 52.Monod M, Feuermann M, Salamin K, Fratti M, Makino M, Alshahni MM, et al. Trichophyton rubrum azole resistance mediated by a new ABC transporter, TruMDR3. Antimicrob Agents Chemother. 2019 Oct 22;63((11)):e00863–19. doi: 10.1128/AAC.00863-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta AK, Taborda VBA, Taborda PRO, Shemer A, Summerbell RC, Nakrieko KA. High prevalence of mixed infections in global onychomycosis. PLoS One. 2020;15((9)):e0239648. doi: 10.1371/journal.pone.0239648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Idris NFB, Huang G, Jia Q, Yuan L, Li Y, Tu Z. Mixed infection of toe nail caused by Trichosporon asahii and Rhodotorula mucilaginosa. Mycopathologia. 2020 Apr;185((2)):373–6. doi: 10.1007/s11046-019-00406-y. [DOI] [PubMed] [Google Scholar]
- 55.Gawaz A, Weisel G. Mixed infections are a critical factor in the treatment of superficial mycoses. Mycoses. 2018 Oct;61((10)):731–5. doi: 10.1111/myc.12794. [DOI] [PubMed] [Google Scholar]
- 56.De Doncker PR, Scher RK, Baran RL, Decroix J, Degreef HJ, Roseeuw DI, et al. Itraconazole therapy is effective for pedal onychomycosis caused by some nondermatophyte molds and in mixed infection with dermatophytes and molds: a multicenter study with 36 patients. J Am Acad Dermatol. 1997 Feb;36((2 Pt 1)):173–7. doi: 10.1016/s0190-9622(97)70275-5. [DOI] [PubMed] [Google Scholar]
- 57.Gupta AK, Paquet M. Management of onychomycosis in Canada in 2014. J Cutan Med Surg. 2015 Jun;19((3)):260–73. doi: 10.2310/7750.2014.14090. [DOI] [PubMed] [Google Scholar]
- 58.Al-Hatmi AM, Bonifaz A, Calderón L, Curfs-Breuker I, Meis JF, van Diepeningen AD, et al. Proximal subungual onychomycosis caused by Fusarium falciforme successfully cured with posaconazole. Br J Dermatol. 2015 Jul;173((1)):253–5. doi: 10.1111/bjd.13589. [DOI] [PubMed] [Google Scholar]
- 59.Noguchi H, Matsumoto T, Kimura U, Hiruma M, Kano R, Yaguchi T, et al. Fungal melanonychia caused by Candida parapsilosis successfully treated with oral fosravuconazole. J Dermatol. 2019;46((10)):911–3. doi: 10.1111/1346-8138.15024. [DOI] [PubMed] [Google Scholar]
- 60.Noguchi H, Matsumoto T, Hiruma M, Kimura U, Kano R, Yaguchi T, et al. Tinea unguium caused by terbinafine-resistant Trichophyton rubrum successfully treated with fosravuconazole. J Dermatol. 2019 Dec;46((12)):e446–7. doi: 10.1111/1346-8138.15033. [DOI] [PubMed] [Google Scholar]
- 61.Lurati M, Baudraz-Rosselet F, Vernez M, Spring P, Bontems O, Fratti M, et al. Efficacious treatment of non-dermatophyte mould onychomycosis with topical amphotericin B. Dermatology. 2011;223((4)):289–92. doi: 10.1159/000335093. [DOI] [PubMed] [Google Scholar]
- 62.Olafsson JH, Sigurgeirsson B, Baran R. Combination therapy for onychomycosis. Br J Dermatol. 2003;149((Suppl 65)):15–8. doi: 10.1046/j.1365-2133.149.s65.2.x. [DOI] [PubMed] [Google Scholar]
- 63.Gupta AK, Cernea M, Foley KA. Improving cure rates in onychomycosis. J Cutan Med Surg. 2016 Nov;20((6)):517–31. doi: 10.1177/1203475416653734. [DOI] [PubMed] [Google Scholar]
- 64.Bristow IR, Baran R. Topical and oral combination therapy for toenail onychomycosis: an updated review. J Am Podiatr Med Assoc. 2006 Apr;96((2)):116–9. doi: 10.7547/0960116. [DOI] [PubMed] [Google Scholar]
- 65.Gupta AK, Versteeg SG, Shear NH. Onychomycosis in the 21st century: an update on diagnosis, epidemiology, and treatment. J Cutan Med Surg. 2017 Dec;21((6)):525–39. doi: 10.1177/1203475417716362. [DOI] [PubMed] [Google Scholar]
- 66.Lipner SR, Scher RK. Management of onychomycosis and co-existing tinea pedis. J Drugs Dermatol. 2015 May;14((5)):492–4. [PubMed] [Google Scholar]
- 67.Szepietowski JC, Reich A, Garlowska E, Kulig M, Baran E. Factors influencing coexistence of toenail onychomycosis with tinea pedis and other dermatomycoses: a survey of 2761 patients. Arch Dermatol. 2006 Oct;142((10)):1279–84. doi: 10.1001/archderm.142.10.1279. [DOI] [PubMed] [Google Scholar]
- 68.Sigurgeirsson B, Steingrímsson O. Risk factors associated with onychomycosis. J Eur Acad Dermatol Venereol. 2004 Jan;18((1)):48–51. doi: 10.1111/j.1468-3083.2004.00851.x. [DOI] [PubMed] [Google Scholar]
- 69.Gupta AK, Foley K, Versteeg S, Mays R, Villanueva E, John D. Topical and device-based treatments for fungal infections of the toenails. Cochrane Database Syst Rev. 2020;1((1)):CD012093. doi: 10.1002/14651858.CD012093.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Markinson BC, Caldwell BD. Efinaconazole topical solution, 10%: efficacy in patients with onychomycosis and coexisting tinea pedis. J Am Podiatr Med Assoc. 2015 Apr 13;105:407–11. doi: 10.7547/14-088. [DOI] [PubMed] [Google Scholar]
- 71.Tosti A, Elewski BE. Onychomycosis: practical approaches to minimize relapse and recurrence. Skin Appendage Disord. 2016 Sept;2((1–2)):83–7. doi: 10.1159/000448056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta AK, Versteeg SG. The role of shoe and sock sanitization in the management of superficial fungal infections of the feet. J Am Podiatr Med Assoc. 2019 Mar;109((2)):141–9. doi: 10.7547/17-043. [DOI] [PubMed] [Google Scholar]
- 73.Zatcoff RC, Smith MS, Borkow G. Treatment of tinea pedis with socks containing copper-oxide impregnated fibers. Foot. 2008 Sept;18((3)):136–41. doi: 10.1016/j.foot.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 74.Gupta AK, Brintnell WC. Sanitization of contaminated footwear from onychomycosis patients using ozone gas: a novel adjunct therapy for treating onychomycosis and tinea pedis? J Cutan Med Surg. 2013 Aug;17((4)):243–9. doi: 10.2310/7750.2012.12068. [DOI] [PubMed] [Google Scholar]
- 75.Hammer TR, Mucha H, Hoefer D. Infection risk by dermatophytes during storage and after domestic laundry and their temperature-dependent inactivation. Mycopathologia. 2011 Jan;171((1)):43–9. doi: 10.1007/s11046-010-9347-9. [DOI] [PubMed] [Google Scholar]
- 76.Amichai B, Grunwald MH, Davidovici B, Farhi R, Shemer A. The effect of domestic laundry processes on fungal contamination of socks. Int J Dermatol. 2013 Nov;52((11)):1392–4. doi: 10.1111/ijd.12167. [DOI] [PubMed] [Google Scholar]
- 77.Ghannoum MA, Isham N, Long L. Optimization of an infected shoe model for the evaluation of an ultraviolet shoe sanitizer device. J Am Podiatr Med Assoc. 2012;102((4)):309–13. doi: 10.7547/1020309. [DOI] [PubMed] [Google Scholar]
- 78.Dixon HA. The study of fungi in diseases of the skin. Can Med Assoc J. 1924 Nov;14((11)):1097–9. [PMC free article] [PubMed] [Google Scholar]
- 79.Broughton R. Reinfection from socks and shoes in tinea pedis. Br J Dermatol. 1955;67((7)):249. doi: 10.1111/j.1365-2133.1955.tb12730.x. [DOI] [PubMed] [Google Scholar]
- 80.Ara K, Hama M, Akiba S, Koike K, Okisaka K, Hagura T, et al. Foot odor due to microbial metabolism and its control. Can J Microbiol. 2006 Apr;52((4)):357–64. doi: 10.1139/w05-130. [DOI] [PubMed] [Google Scholar]
- 81.Sasagawa Y. Internal environment of footwear is a risk factor for tinea pedis. J Dermatol. 2019 Nov;46((11)):940–6. doi: 10.1111/1346-8138.15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hammer TR, Mucha H, Hoefer D. Dermatophyte susceptibility varies towards antimicrobial textiles. Mycoses. 2012 Jul;55((4)):344–51. doi: 10.1111/j.1439-0507.2011.02121.x. [DOI] [PubMed] [Google Scholar]
- 83.Neely AN, Orloff MM. Survival of some medically important fungi on hospital fabrics and plastics. J Clin Microbiol. 2001 Sept;39((9)):3360–1. doi: 10.1128/JCM.39.9.3360-3361.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Borkow G. Using copper to improve the well-being of the skin. Curr Chem Biol. 2014 Aug;8((2)):89–102. doi: 10.2174/2212796809666150227223857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borkow G, Kanmukhla V, Monk A. Improvement to foot and skin appearance by using copper oxide impregnated socks: a report of a large scale internet based user survey. J Cosmetol Trichol. 2017 Jan 1;:03. [Google Scholar]
- 86.Ossowski B, Duchmann U. [Effect of domestic laundry processes on mycotic contamination of textiles] Hautarzt. 1997;48((6)):397. doi: 10.1007/s001050050600. [DOI] [PubMed] [Google Scholar]
- 87.Fijan S, Sostar-Turk S, Cencic A. Implementing hygiene monitoring systems in hospital laundries in order to reduce microbial contamination of hospital textiles. J Hosp Infect. 2005;61((1)):30–8. doi: 10.1016/j.jhin.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 88.Brunton WA. Infection and hospital laundry. Lancet. 1995 Jun 17;345((8964)):1574–5. doi: 10.1016/s0140-6736(95)91124-3. [DOI] [PubMed] [Google Scholar]
- 89.Ouf SA, Moussa TA, Abd-Elmegeed AM, Eltahlawy SR. Anti-fungal potential of ozone against some dermatophytes. Braz J Microbiol. 2016 Sept;47((3)):697–702. doi: 10.1016/j.bjm.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gupta AK, Brintnell W. Ozone gas effectively kills laboratory strains of Trichophyton rubrum and Trichophyton mentagrophytes using an in vitro test system. J Dermatolog Treat. 2014 Jun;25((3)):251–5. doi: 10.3109/09546634.2012.714456. [DOI] [PubMed] [Google Scholar]
- 91.Reed NG. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010 Jan–Feb;125((1)):15–27. doi: 10.1177/003335491012500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Katara G, Hemvani N, Chitnis S, Chitnis V, Chitnis DS. Surface disinfection by exposure to germicidal UV light. Indian J Med Microbiol. 2008 Sept;26((3)):241–2. doi: 10.4103/0255-0857.42034. [DOI] [PubMed] [Google Scholar]
- 93.Dai T, Tegos GP, Rolz-Cruz G, Cumbie WE, Hamblin MR. Ultraviolet C inactivation of dermatophytes: implications for treatment of onychomycosis. Br J Dermatol. 2008 Jun;158((6)):1239–46. doi: 10.1111/j.1365-2133.2008.08549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nematollahi AR, Badiee P, Nournia E. The Efficacy of ultraviolet irradiation on Trichophyton species isolated from nails. Jundishapur J Microbiol. 2015 Jun;8((6)):e18158. doi: 10.5812/jjm.18158v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dai T, Vrahas MS, Murray CK, Hamblin MR. Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev Anti Infect Ther. 2012 Feb;10((2)):185–95. doi: 10.1586/eri.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Budihardja D, Mayser P. The effect of microwave irradiation on the vitality of various dermatophytes. Mycoses. 2014 Apr;57((4)):209–13. doi: 10.1111/myc.12144. [DOI] [PubMed] [Google Scholar]
- 97.Gupta AK, Ryder JE. How to improve cure rates for the management of onychomycosis. Dermatol Clin. 2003 Jul;21((3)):499–vii. doi: 10.1016/s0733-8635(03)00026-3. vii. [DOI] [PubMed] [Google Scholar]
- 98.Sigurgeirsson B. Prognostic factors for cure following treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2010 Jun;24((6)):679–84. doi: 10.1111/j.1468-3083.2009.03487.x. [DOI] [PubMed] [Google Scholar]
- 99.Sakamoto M, Sugimoto N, Kawabata H, Yamakawa E, Kodera N, Pillai R, et al. Transungual delivery of efinaconazole: its deposition in the nail of onychomycosis patients and in vitro fungicidal activity in human nails. J Drugs Dermatol. 2014 Nov 1;13((11)):1388–92. [PubMed] [Google Scholar]