Summary
Background
First-line chemotherapy for advanced/metastatic human epidermal growth factor receptor 2 (HER2)-negative gastric/gastroesophageal junction (GEJ) adenocarcinoma results in median overall survival (OS) <1 year. The phase 3 CheckMate 649 study evaluated first-line programmed death (PD)-1 inhibitor-based therapies in gastric/GEJ/oesophageal adenocarcinoma; we report first results for nivolumab-plus-chemotherapy versus chemotherapy.
Methods
We enrolled adults with previously untreated, unresectable, non-HER2-positive gastric/GEJ/oesophageal adenocarcinoma, regardless of PD-ligand (L)1 expression. Patients were randomised to nivolumab (360 mg every 3 weeks or 240 mg every 2 weeks)-plus-chemotherapy (XELOX every 3 weeks or FOLFOX every 2 weeks), nivolumab-plus-ipilimumab, or chemotherapy. Primary endpoints for nivolumab-plus-chemotherapy versus chemotherapy were OS or progression-free survival (PFS) by blinded independent central review, in PD-L1 combined positive score (CPS) ≥5 patients. Safety was assessed in all patients who received at least one dose of the assigned treatment.
Findings
From March 2017 through April 2019, we concurrently randomised 1581 patients to treatment (nivolumab-plus-chemotherapy, 789; chemotherapy, 792). The median follow-up for OS was: nivolumab-plus-chemotherapy, 13·1 months (IQR, 6·7–19·1) and chemotherapy, 11·1 months (5·8–16·1). Nivolumab-plus-chemotherapy resulted in significant improvements in OS (hazard ratio [HR] 0·71 [98·4% CI 0·59–0·86]; p<0·0001) and PFS (HR 0·68 [98% CI 0·56–0·81]; p<0·0001) versus chemotherapy in PD-L1 CPS ≥5 patients (minimum follow-up, 12·1 months). Additional results showed significant improvement in OS, along with PFS benefit, in PD-L1 CPS ≥1 and all-randomised patients. Among all-treated patients, 462 (59%, nivolumab-plus-chemotherapy) and 341 (44%, chemotherapy) patients had grade 3–4 treatment-related adverse events. No new safety signals were identified.
Interpretation
Nivolumab is the first PD-1 inhibitor to demonstrate superior OS, along with PFS benefit, and an acceptable safety profile, in combination with chemotherapy versus chemotherapy alone in previously untreated patients with advanced gastric/GEJ/oesophageal adenocarcinoma. Nivolumab-plus-chemotherapy represents a potential standard first-line treatment for these patients.
Funding
Bristol Myers Squibb (Princeton, NJ, USA), in collaboration with Ono Pharmaceutical Co., Ltd.
ClinicalTrials.gov number, NCT02872116.
Introduction
Gastric cancer, including gastroesophageal junction (GEJ) cancer, is the fourth leading cause of cancer-related deaths worldwide.1 Adenocarcinoma is the most common (>90%) histological type of gastric and GEJ cancer2 and accounts for approximately 65% and 40% of oesophageal cancer in North America and Europe, respectively.3
Fluoropyrimidine plus platinum-based chemotherapy, the standard first-line treatment for unresectable advanced or metastatic human epidermal growth factor receptor 2 (HER2)-negative gastric and GEJ adenocarcinoma, results in poor survival (median overall survival [OS] less than 1 year).4–11 Gastric/GEJ and oesophageal adenocarcinomas share similarities in their molecular profiles12,13 and have comparable clinical outcomes with systemic chemotherapy in an advanced setting.14,15 Although several targeted agents have been evaluated as first-line treatment for HER2-negative gastric and GEJ adenocarcinoma, none has significantly prolonged survival relative to chemotherapy.8–11,16
The programmed death (PD)-1 inhibitor nivolumab provided superior OS versus placebo in heavily pretreated advanced or recurrent gastric or GEJ cancer in the phase 3 ATTRACTION-2 study.17 Chemotherapy, in addition to its direct cytotoxic properties, may contribute to antitumour immune response elicited by nivolumab through induction of immunogenic cell death.18–20 PD-ligand (L)1 expression on tumour cells and tumour-associated immune cells (combined positive score [CPS]) has shown better enrichment for efficacy of checkpoint inhibitors than tumour cell PD-L1 expression in advanced gastric/GEJ/oesophageal adenocarcinoma.21–23
The phase 3 CheckMate 649 study evaluated PD-1 inhibitor-based therapies in previously untreated advanced gastric/GEJ/oesophageal adenocarcinoma; here we report results from the nivolumab plus chemotherapy versus chemotherapy groups.
Methods
Study design and participants
We conducted a multicentre, randomised, open-label, phase 3 study at 175 sites in 29 countries. Eligible patients were 18 years of age or older, with previously untreated, unresectable advanced or metastatic gastric, GEJ, or oesophageal adenocarcinoma, regardless of PD-L1 expression. Other key inclusion criteria were measurable (at least one lesion) or evaluable disease per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1; Eastern Cooperative Oncology Group performance status of 0 or 1; adequate organ function; and availability to provide a fresh or archival tumour sample to evaluate PD-L1. Patients with prior adjuvant or neoadjuvant chemotherapy, radiotherapy, and/or chemoradiotherapy (administered at least 6 months before randomisation) were allowed. Patients with known HER2-positive status; untreated central nervous system metastases peripheral neuropathy (> grade 1); active, known, or suspected autoimmune disease; positive test result for hepatitis B or hepatitis C virus; and known history of positive test for human immunodeficiency virus or known acquired immunodeficiency syndrome were excluded.
During enrolment, the primary population was amended to patients whose tumours expressed PD-L1 CPS ≥5 based on results from the gastroesophageal cohort of CheckMate 032 and other published studies suggesting that PD-L1 CPS may be better associated with anti–PD-1 therapy efficacy than tumour cell PD-L1 expression.21–23 Patients continued to be enrolled regardless of PD-L1 expression.
The trial was conducted according to Good Clinical Practice guidelines developed by the International Council for Harmonisation and in compliance with the trial protocol (appendix protocol). The protocol was approved by the institutional review boards or independent ethics committees at each site. All patients provided written informed consent per Declaration of Helsinki principles. An independent data monitoring committee monitored safety and efficacy data.
Randomisation and masking
Patients were randomly assigned to nivolumab plus chemotherapy (XELOX [capecitabine and oxaliplatin] or FOLFOX [fluorouracil, leucovorin, and oxaliplatin]) or nivolumab plus ipilimumab versus chemotherapy alone at a 1:1:1 ratio after the nivolumab plus chemotherapy group was added and later at a ratio of 1:1 after enrolment in the nivolumab plus ipilimumab group was closed. We report results from patients concurrently randomised to nivolumab plus chemotherapy versus chemotherapy; results for nivolumab plus ipilimumab versus chemotherapy remain blinded and will be reported later.
Randomisation was performed using interactive (voice/web) response technology (block size 6) and stratified according to tumour cell PD-L1 status (≥1% vs <1% or indeterminate), region (Asia vs United States and Canada vs rest of world), Eastern Cooperative Oncology Group performance status (0 vs 1), and type of chemotherapy (XELOX vs FOLFOX). After informed consent was obtained, the patient was enrolled and assigned to treatment, and a treatment allocation list was generated by the sponsor. The web registration system was implemented by a third party, which ensured that the assignment sequence was concealed until the treatment allocation was completed. The study was open label so investigators were not blinded to treatment allocation.
Procedures
Patients were administered nivolumab (360 mg every 3 weeks or 240 mg every 2 weeks) plus investigator’s choice of chemotherapy (XELOX [capecitabine 1000 mg/m2 twice daily, days 1–14 and oxaliplatin 130 mg/m2, day 1, every 3 weeks] or FOLFOX [leucovorin 400 mg/m2, day 1, fluorouracil 400 mg/m2, day 1 and 1200 mg/m2, days 1–2, and oxaliplatin 85 mg/m2, day 1, every 2 weeks]) or chemotherapy alone. All treatments were administered intravenously except for capecitabine, which was administered orally. Treatment continued until documented disease progression, unacceptable toxicity, withdrawal of consent, or study end. Nivolumab was given for a maximum of 2 years. Chemotherapy was given per local standards. Patients were permitted to continue treatment beyond initial disease progression (per RECIST version 1.1) in the nivolumab plus chemotherapy group, based on investigator judgment.
Tumours were assessed using computed tomography or magnetic resonance imaging per RECIST version 1.1, by blinded independent central review (BICR) at baseline, every 6 weeks from the start of cycle 1 for 48 weeks, and every 12 weeks thereafter until disease progression.
PD-L1 immunohistochemistry was performed at two central laboratories using the Dako PD-L1 immunohistochemistry 28–8 pharmDx assay (Dako, an Agilent Technologies company, Santa Clara, CA, USA), which has been analytically validated in gastric/GEJ/oesophageal adenocarcinoma, according to the manufacturer’s instructions with the Dako Autostainer Link-48 system. Tumour cell PD-L1 expression was defined as the percentage of viable tumour cells with partial or complete membrane staining in at least 100 viable tumour cells. CPS was generated by rescoring PD-L1 stained slides using the CPS algorithm, defined as the number of PD-L1–positive tumour cells (partial or complete membrane staining), lymphocytes, and macrophages (membrane staining and/or intracellular staining) divided by the total number of viable tumour cells multiplied by 100.
Treatment-related adverse events (TRAEs) included events reported between first dose and 30 days after last dose of study therapy according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0, and Medical Dictionary for Regulatory Activities, version 23.0, per investigator assessment. Treatment-relatedness in the nivolumab plus chemotherapy group refers to nivolumab, at least one chemotherapy component, or both. Patients were allowed to discontinue individual treatment components and continue on other components in a combination regimen (appendix protocol). TRAEs leading to discontinuation due to any treatment component were recorded in a cumulative manner throughout the duration of treatment and used to calculate the proportion of patients who discontinued treatment due to TRAEs.
Outcomes
Dual primary endpoints for the nivolumab plus chemotherapy versus chemotherapy groups were OS (time from randomisation to death) or progression-free survival (PFS; time from randomisation to the date of first documented tumour progression or death) by BICR per RECIST version 1.1, evaluated in patients with PD-L1 CPS ≥5. Hierarchically tested secondary endpoints were OS in patients with PD-L1 CPS ≥1 and all randomised patients. Additional secondary endpoints that were not formally tested included BICR-assessed PFS and objective response rate at different PD-L1 CPS cutoffs and in all randomised patients. Key prespecified exploratory endpoints included BICR-assessed duration of response; landmark survival rates; biomarkers potentially predictive of efficacy; health-related quality of life (HRQoL); and safety and tolerability.
All randomised patients included all enrolled patients who were randomised concurrently to either nivolumab plus chemotherapy or chemotherapy. Patients with PD-L1 CPS ≥5 included all randomised patients whose tumours expressed PD-L1 CPS ≥5. Objective response was evaluated in all randomised patients who had at least one target/measurable lesion at baseline. Safety analysis was conducted in all treated patients, which included all randomised patients who received at least one dose of study treatment during the trial. Functional Assessment of Cancer Therapy-Gastric (FACT-Ga) analysis was performed for patients with PD-L1 CPS ≥5 and all randomised patients who had an assessment at baseline (day 1, assessment prior to administration of treatment on day of first dose) and at least one subsequent assessment while on treatment. Additional details about HRQoL are in the appendix (p 3). Time to symptom deterioration (TTSD) analysis was conducted in patients with PD-L1 CPS ≥5 and all randomised patients with intent to treat. Biomarker analysis was conducted in all randomised patients with available biomarker data (eg, PD-L1 expression by tumour cell PD-L1 expression and other assays).
Statistical analysis
For the dual primary endpoints, two-sided significance levels of 0·03 and 0·02 (type I error) were allocated to OS and PFS, respectively. Upon superiority of OS in patients with PD-L1 CPS ≥5, it was hierarchically tested in patients with PD-L1 CPS ≥1 with a fraction of alpha (50% α transmitted=0·015), followed by all randomised patients (100% α transmitted=0·015). The study was designed to conduct the final PFS and interim OS analyses at 12-month minimum follow-up and final OS analysis at 24-month minimum follow-up. Lan and DeMets α-spending functions were employed to determine the significance level for the interim analysis of OS.
With an assumed PD-L1 CPS ≥5 prevalence of 35%, based on limited available data,21–23 it was estimated that the primary population would consist of 554 patients. For OS, the hazard ratio (HR) was modelled as a two-piece HR, a delayed effect for the first 6 months followed by a constant HR of 0·65 thereafter, providing an average HR of 0·74. At final analysis, it was expected that 466 events would provide approximately 85% power. The HR for PFS was modelled as a two-piece HR with a delayed effect for the first 3 or 6 months followed by a constant HR of 0·56. At 12-month minimum follow-up, the expected numbers of PFS events were estimated to be 497 for a 3-month delay with approximately 99% power and 506 for a 6-month delay with approximately 60% power.
All patients with PD-L1 CPS ≥5 concurrently randomised to the nivolumab plus chemotherapy or chemotherapy groups were included in the primary OS and PFS analyses. For OS and PFS, the stratified log-rank test was used to compare the treatment groups and the stratified Cox proportional hazards regression model was used to estimate the HR. The proportional hazards assumption was tested using a Cox model with treatment and treatment by time interaction at prespecified significance level of 0·1. For time-to-event endpoints, the median was estimated using the Kaplan-Meier method, and the corresponding two-sided 95% confidence intervals (CIs) were calculated using the log-log transformation method. A post-hoc exploratory analysis was performed to assess a potential treatment effect by baseline characteristics on OS using Cox proportional hazards model with treatment, subgroup, and treatment by subgroup interaction as terms. P values for interaction are provided and there were no adjustments for multiplicity. A prespecified analysis evaluated the treatment effect by biomarker (PD-L1 CPS, tumour cell PD-L1, and microsatellite instability [MSI]) on OS and PFS in all randomised patients using Cox models fitted with the biomarker as categorical variable, the treatment, and the interaction between the biomarker and treatment. The significance level for interaction was predefined at 0·2.
Stratification factors as recorded in interactive (voice/web) response system were used for stratified analyses. The proportion of patients who survived at a given timepoint was derived from the Kaplan-Meier method with corresponding two-sided 95% CIs calculated based on the Greenwood formula for variance derivation based on log-log transformation. The proportion of patients with an objective response and corresponding two-sided 95% CIs were calculated using the Clopper-Pearson method. For subgroup analyses of OS, PFS, and objective response, unstratified HRs and corresponding 95% CIs for nivolumab plus chemotherapy relative to chemotherapy were calculated using a Cox proportional-hazards regression model with treatment as the covariate. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Role of the funding source
The study was sponsored and conducted by Bristol Myers Squibb, in collaboration with Ono Pharmaceutical Co., Ltd. The funder of the study had a role in study design, data collection, data analysis, data interpretation, and writing of the clinical study report. All authors had access to all study data, participated in developing or reviewing the manuscript, and provided final approval to submit the manuscript for publication.
Results
From March 2017 through April 2019, 2687 patients were assessed for eligibility at 175 sites in 29 countries. Of these, 1581 patients were concurrently randomised to receive nivolumab plus chemotherapy (789 patients) or chemotherapy (792 patients); 1549 patients received one or more doses of the assigned treatment (nivolumab plus chemotherapy, 782 patients; chemotherapy, 767 patients; figure 1).
Figure 1: Trial profile.
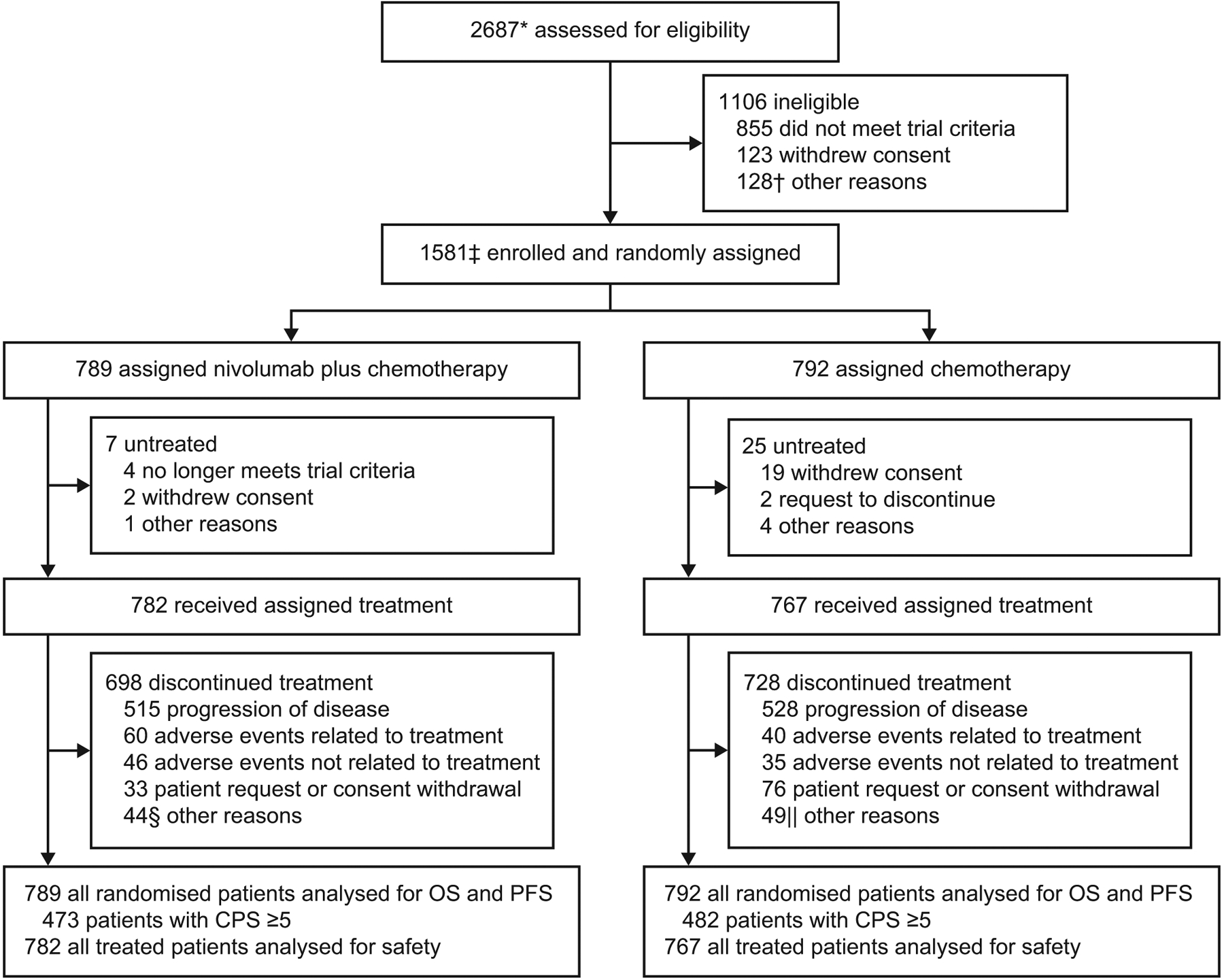
OS=overall survival. PFS=progression-free survival. CPS=combined positive score. *Enrolled patients included all concurrently randomised patients to nivolumab plus chemotherapy or chemotherapy, as well as patients enrolled before the nivolumab plus ipilimumab group was closed and not randomised to any of the treatment groups. The total number of patients randomised to three groups was 2031. The nivolumab plus ipilimumab group will remain blinded until final analysis, and the results will be reported at a later time. †Included death (n=35), adverse events (n=24), poor/noncompliance (n=15), and additional reasons (n=54). ‡Includes patients concurrently randomised to the nivolumab plus chemotherapy and chemotherapy groups. Relevant protocol deviations were noted in 21 (1%) patients. This included usage of prohibited on-treatment anti-cancer therapy (n=12), baseline ECOG PS > 1 (n=5), incorrect cancer diagnosis (n=2), and one case each of prohibited prior anti-cancer therapy (at study entry) and no baseline (measurable or evaluable) disease. §Included completion of treatment (n=20); maximum clinical benefit (n=10); two cases each of death, decline in performance, lost to follow-up, and patient relocation; and one case each of clinical worsening (hand synovitis G2), patient no longer met trial criteria, patient request to receive treatment at home, poor/noncompliance, treatment on hold due to adverse event, and unclear lung and bone lesions. ||Included maximum clinical benefit (n=25); poor/noncompliance (n=4); three cases each of patient no longer met trial criteria and death; two cases each of lost to follow-up and surgery; and one case each of bad performance status, carcinomatoses meninges, clinical progression, disease progression confirmed by central imaging (per blinded independent central review), failure after cranial progression, investigator decision, patient pursuing alternative treatment, treatment delay/discontinuation (per protocol), patient unable to tolerate treatment, and patient request to discontinue.
The median follow-up for OS (time from concurrent randomisation of the last patient to last known date alive or death) was 13·1 months (IQR, 6·7–19·1) in the nivolumab plus chemotherapy group and 11·1 months (5·8–16·1) in the chemotherapy group. A total of 698 and 728 patients discontinued treatment with nivolumab plus chemotherapy and chemotherapy, respectively; the most common reason for treatment discontinuation in both groups was disease progression (515 patients [66%] and 528 patients [69%], respectively; figure 1).
Baseline characteristics were balanced across the treatment groups in the primary population (PD-L1 CPS ≥5) and all randomised patients (table 1 and appendix p 4). A total of 473 (60%) of 789 patients in the nivolumab plus chemotherapy group and 482 (61%) of 792 patients in the chemotherapy group had tumours expressing PD-L1 CPS ≥5. Most patients were non-Asian (1206 [76%] of 1581) and most had gastric cancer (1110 [70%] of 1581), while 260 (16%) had GEJ cancer and 211 (13%) had oesophageal adenocarcinoma.
Table 1:
Demographics and baseline clinical characteristics
| Patients with PD-L1 CPS ≥5 | All randomised patients | |||
|---|---|---|---|---|
| Nivolumab plus chemotherapy (n=473) | Chemotherapy (n=482) | Nivolumab plus chemotherapy (n=789) | Chemotherapy (n=792) | |
| Median age (IQR), years | 63 (54–69) | 62 (54–68) | 62 (54–69) | 61 (53–68) |
| <65 | 266 (56%) | 286 (59%) | 473 (60%) | 488 (62%) |
| ≥65 | 207 (44%) | 196 (41%) | 316 (40%) | 304 (38%) |
| Sex | ||||
| Male | 331 (70%) | 349 (72%) | 540 (68%) | 560 (71%) |
| Female | 142 (30%) | 133 (28%) | 249 (32%) | 232 (29%) |
| Race | ||||
| Asian | 119 (25%) | 117 (24%) | 186 (24%) | 189 (24%) |
| Non-Asian | 354 (75%) | 365 (76%) | 603 (76%) | 603 (76%) |
| Region | ||||
| Asia | 117 (25%) | 111 (23%) | 178 (23%) | 178 (22%) |
| United States and Canada | 67 (14%) | 70 (15%) | 131 (17%) | 132 (17%) |
| Rest of world | 289 (61%) | 301 (62%) | 480 (61%) | 482 (61%) |
| ECOG performance status * | ||||
| 0 | 194 (41%) | 203 (42%) | 326 (41%) | 336 (42%) |
| 1 | 279 (59%) | 278 (58%) | 462 (59%) | 452 (57%) |
| 2 | 0 | 0 | 1 (<1%) | 3 (<1%) |
| Not reported | 0 | 1 (<1%) | 0 | 1 (<1%) |
| Primary tumour location at initial diagnosis | ||||
| GC | 333 (70%) | 334 (69%) | 554 (70%) | 556 (70%) |
| GEJC | 84 (18%) | 86 (18%) | 132 (17%) | 128 (16%) |
| EAC | 56 (12%) | 62 (13%) | 103 (13%) | 108 (14%) |
| Tumour cell PD-L1 expression | ||||
| <1%† | 363 (77%) | 362 (75%) | 663 (84%) | 664 (84%) |
| ≥1% | 110 (23%) | 120 (25%) | 126 (16%) | 127 (16%) |
| Prior surgery | ||||
| Yes | 97 (21%) | 105 (22%) | 160 (20%) | 176 (22%) |
| No | 376 (79%) | 377 (78%) | 629 (80%) | 616 (78%) |
| Disease stage | ||||
| Metastatic | 454 (96%) | 461 (96%) | 757 (96%) | 756 (95%) |
| Locally advanced | 16 (3%) | 20 (4%) | 27 (3%) | 34 (4%) |
| Locally recurrent | 3 (<1%) | 1 (<1%) | 5 (<1%) | 2 (<1%) |
| Organs with metastases | ||||
| 1 | 98 (21%) | 105 (22%) | 164 (21%) | 183 (23%) |
| ≥2 | 361 (76%) | 362 (75%) | 602 (76%) | 583 (74%) |
| Site of metastases | ||||
| Liver | 191 (40%) | 217 (45%) | 301 (38%) | 314 (40%) |
| Peritoneum | 101 (21%) | 96 (20%) | 188 (24%) | 188 (24%) |
| CNS | 1 (<1%) | 0 | 1 (<1%) | 0 |
| Signet ring cell carcinoma ‡ | ||||
| Yes | 72 (15%) | 69 (14%) | 145 (18%) | 136 (17%) |
| No | 401 (85%) | 413 (86%) | 644 (82%) | 656 (83%) |
| Lauren classification | ||||
| Intestinal type | 171 (36%) | 176 (37%) | 272 (34%) | 267 (34%) |
| Diffuse type | 137 (29%) | 141 (29%) | 254 (32%) | 273 (34%) |
| Mixed | 37 (8%) | 30 (6%) | 58 (7%) | 48 (6%) |
| Unknown | 128 (27%) | 135 (28%) | 205 (26%) | 204 (26%) |
| MSI status | ||||
| MSS | 423 (89%) | 423 (88%) | 695 (88%) | 682 (86%) |
| MSI-H | 18 (4%) | 16 (3%) | 23 (3%) | 21 (3%) |
| Not reported or invalid | 32 (7%) | 43 (9%) | 71 (9%) | 89 (11%) |
| Chemotherapy regimen § | ||||
| FOLFOX | 237 (51%) | 242 (52%) | 422 (54%) | 406 (53%) |
| XELOX | 231 (49%) | 223 (48%) | 360 (46%) | 361 (47%) |
Data are n (%), unless otherwise stated. PD-L1=programmed death ligand 1. CPS=combined positive score. IQR=interquartile range. ECOG=Eastern Cooperative Oncology Group. GC=gastric cancer. GEJC=gastroesophageal junction cancer. EAC=oesophageal adenocarcinoma. CNS=central nervous system. MSI=microsatellite instability. MSS=microsatellite stable. MSI-H=microsatellite instability-high. FOLFOX=5-fluorouracil plus leucovorin plus oxaliplatin. XELOX=capecitabine plus oxaliplatin.
Based on case report form. All randomised patients had ECOG performance status of 0 or 1 based on interactive response technology.
Includes indeterminate tumour cell PD-L1 expression.
Per World Health Organization histologic classification.
Patients who received at least one dose of the assigned treatment.
Both primary endpoints were met. At a minimum follow-up (time from concurrent randomisation of the last patient to data cutoff [May 27, 2020]) of 12·1 months, nivolumab plus chemotherapy demonstrated superior OS, with a 29% reduction in the risk of death compared with chemotherapy (HR 0·71 [98·4% CI 0·59–0·86]; p<0·0001) and a 3·3-month improvement in median OS (14·4 months [95% CI 13·1–16·2] vs 11·1 months [10·0–12·1], respectively) in patients with PD-L1 CPS ≥5 (figure 2A). The proportion of patients alive at 12 months was numerically higher with nivolumab plus chemotherapy (57% [95% CI 53–62]) than with chemotherapy (46% [95% CI 42–51]). Nivolumab plus chemotherapy also provided superior PFS in patients with PD-L1 CPS ≥5, with a 32% reduction in the risk of progression or death versus chemotherapy (HR 0·68 [98% CI 0·56–0·81]; p<0·0001) (figure 3A). Median PFS was 7·7 months (95% CI 7·0–9·2) with nivolumab plus chemotherapy versus 6·05 months (5·6–6·9) with chemotherapy. The 12-month PFS estimate was 36% (95% CI 32–41) with nivolumab plus chemotherapy versus 22% (95% CI 18–26) with chemotherapy.
Figure 2: Overall survival.
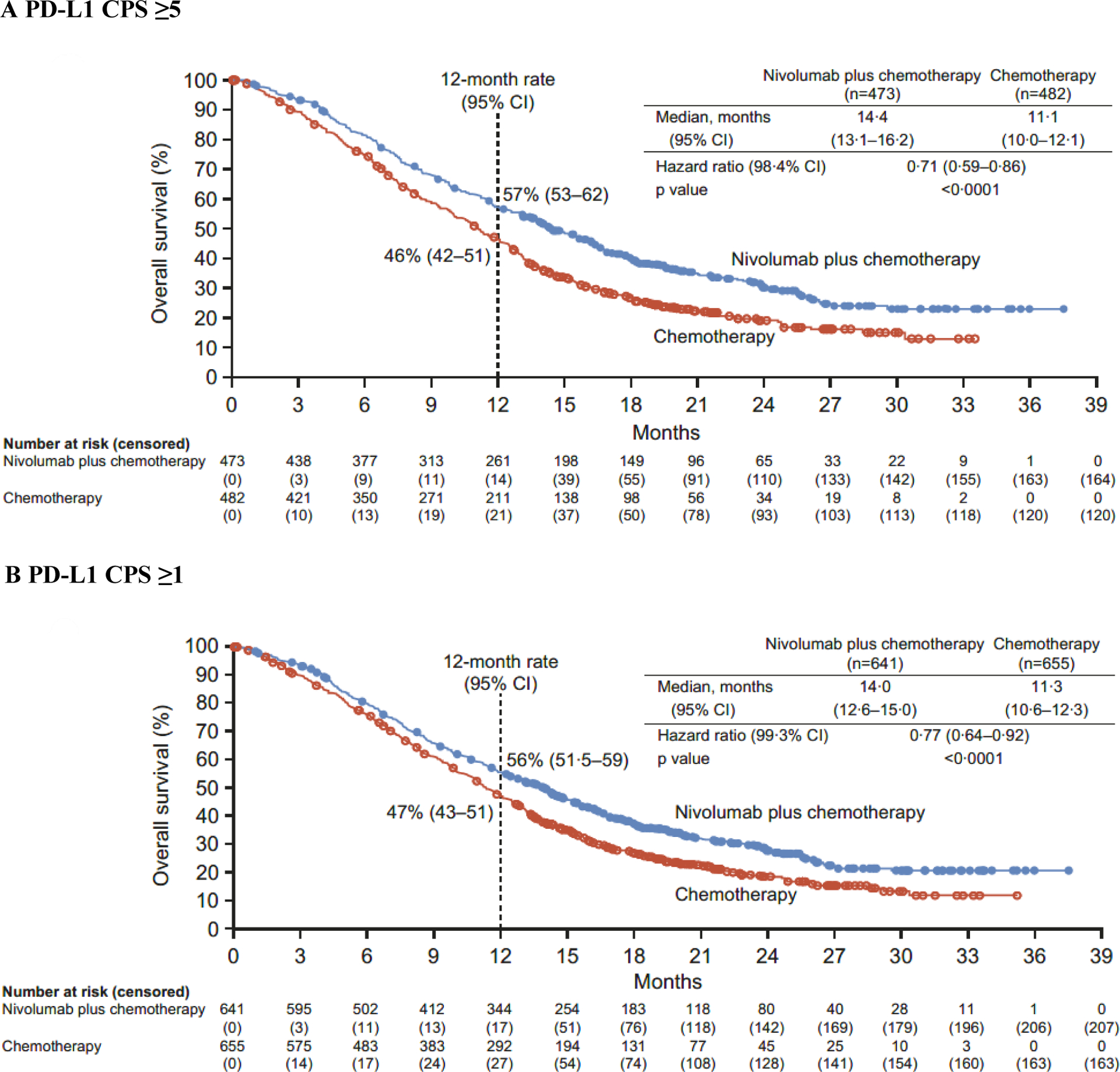
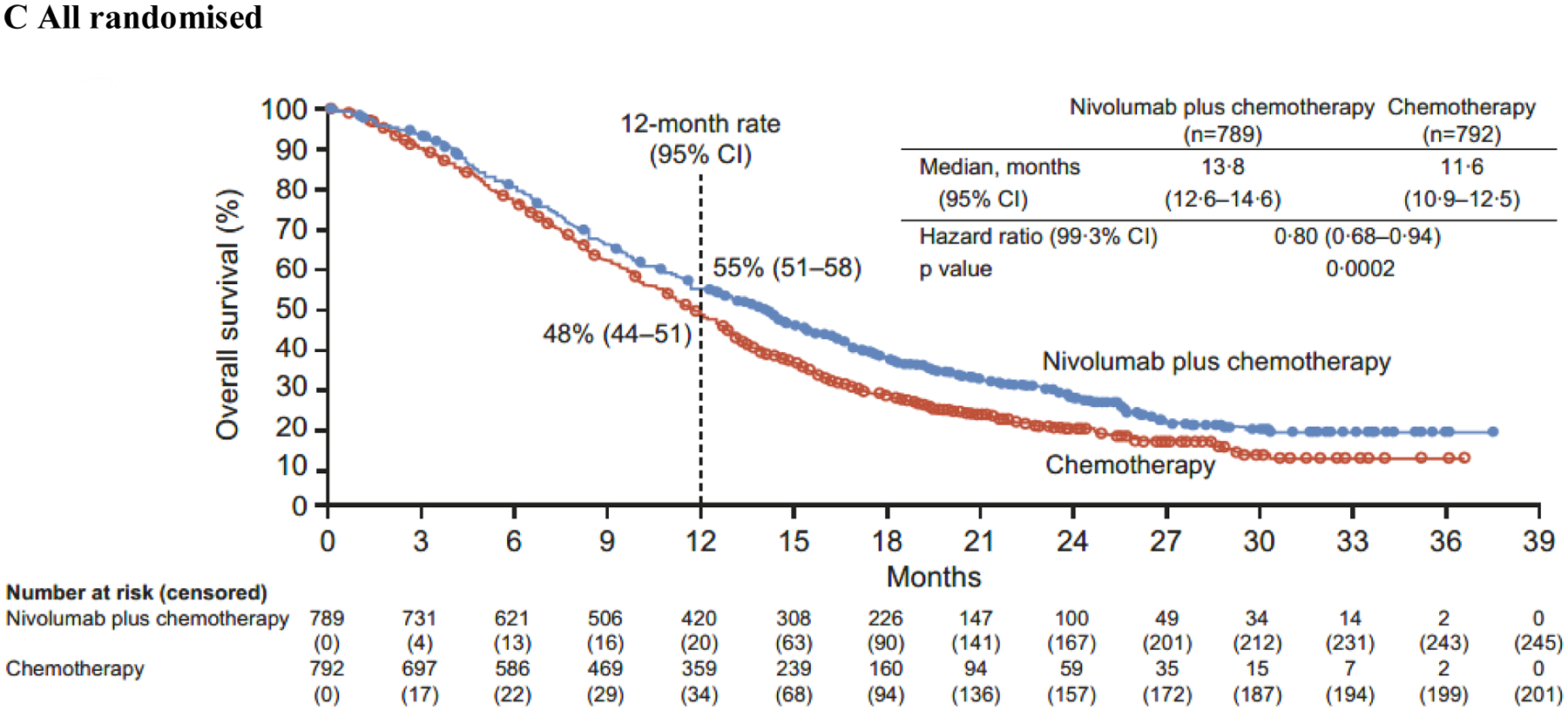
PD-L1=programmed death ligand 1. CPS=combined positive score. CI=confidence interval.
Figure 3: Progression-free survival.
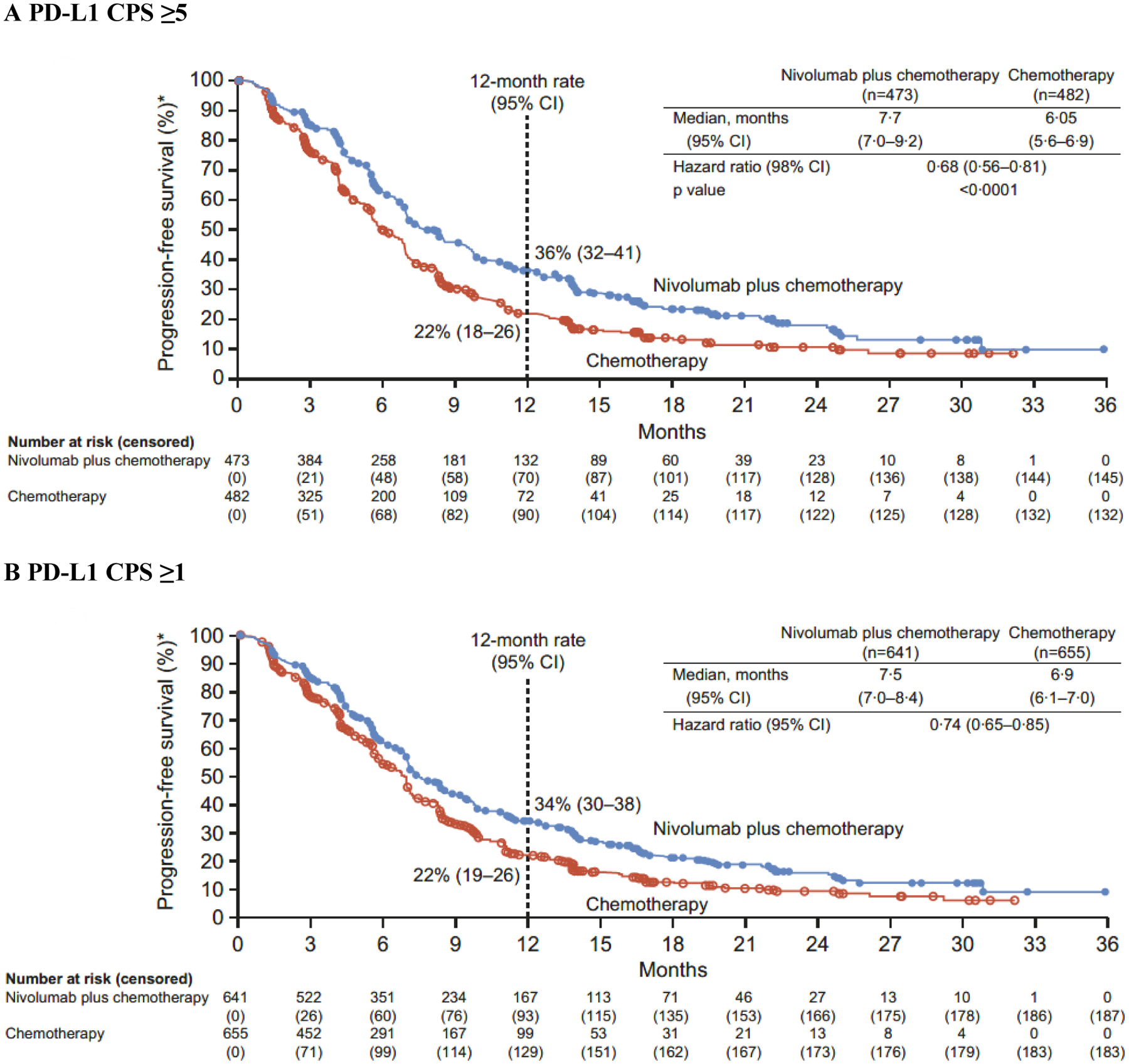
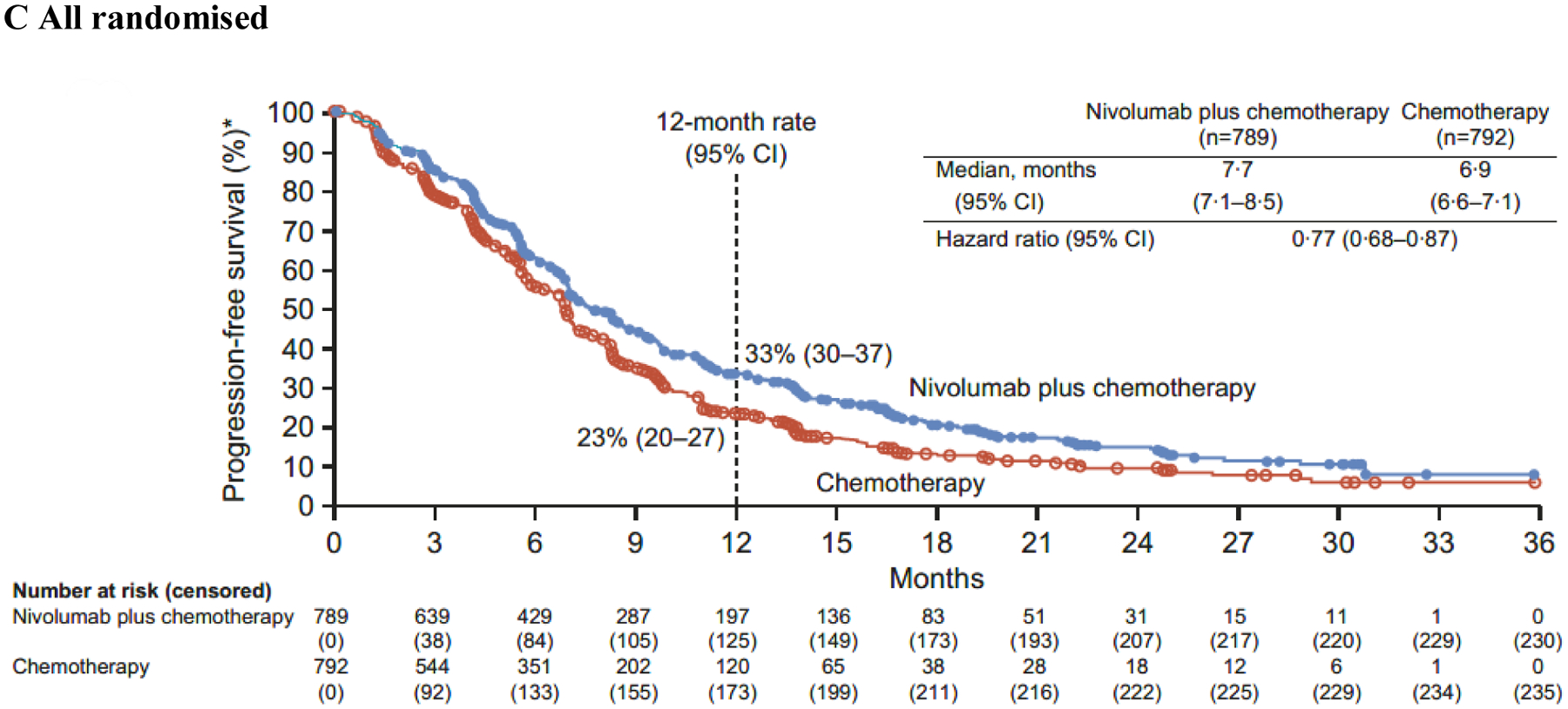
PD-L1=programmed death ligand 1. CPS=combined positive score. CI=confidence interval. *Per blinded independent central review assessment.
In addition to the primary population, nivolumab plus chemotherapy demonstrated a significant improvement in OS in patients with PD-L1 CPS ≥1 and all randomised patients versus chemotherapy (HR 0·77 [99·3% CI 0·64–0·92]; p<0·0001 and 0·80 [0·68–0·94]; p=0·0002, respectively) (figure 2B–C). The interaction p value between treatment and time when testing the proportional hazards assumption was non-significant based on the predefined level (0·1) for all endpoints prespecified in the statistical hierarchy, supporting that the proportional assumptions were not violated (appendix p 5). While not formally tested, HRs of 0·74 (95% CI 0·65–0·85) and 0·77 (0·68–0·87) indicated that PFS benefit was also observed with nivolumab plus chemotherapy versus chemotherapy in patients with PD-L1 CPS ≥1 and all randomised patients, respectively (figure 3B–C).
The unstratified HRs for OS with nivolumab plus chemotherapy versus chemotherapy in patients with PD-L1 CPS <1 and <5 were 0·92 (95% CI 0·70–1·23) and 0·94 (0·78–1·13), respectively; unstratified HRs for PFS were 0·93 (0·69–1·26) and 0·93 (0·76–1·12), respectively (appendix p 11). Interaction analysis of OS by PD-L1 CPS cutoffs showed significant interaction by PD-L1 CPS at the cutoff of 5 (p=0·0107) but not at the cutoff of 1 (p=0·2041) (appendix p 11).
The HRs for OS favoured nivolumab plus chemotherapy over chemotherapy across multiple prespecified subgroups in patients with PD-L1 CPS ≥5 and in all randomised patients (appendix p 12 and 13). The unstratified HRs for OS with nivolumab plus chemotherapy versus chemotherapy among patients with MSI-high and microsatellite stable tumours were 0·33 (95% CI 0·12–0·87) and 0·73 (0·62–0·85), respectively, in patients with PD-L1 CPS ≥5 and 0·37 (0·16–0·87) and 0·80 (0·71–0·91), respectively, in all randomised patients. Post-hoc interaction analyses indicated no evident interaction of treatment effect on OS by the majority of the baseline demographics and disease characteristics subgroups.
In the primary population, 226 (60% [95% CI 55–65]) of 378 patients in the nivolumab plus chemotherapy group and 177 (45% [40–50]) of 391 patients in the chemotherapy group achieved an objective response (per BICR assessment). The proportion of patients with a complete response was 12% and 7%, respectively, and median duration of response was 9·5 months (95% CI 8·0–11·4) versus 7·0 months (5·7–7·9), respectively (appendix p 6 and 15). Consistent results were observed in all randomised patients (appendix p 6 and 15). The proportion of patients with PD-L1 CPS <1 and <5 who achieved objective response was 51% (47 of 93 patients) and 55% (121 of 219 patients) in the nivolumab plus chemotherapy group and 41% (35 of 85 patients) and 46% (97 of 209 patients) in the chemotherapy group, respectively (appendix p 11).
Among all randomised patients, 297 (38%) of 789 patients in the nivolumab plus chemotherapy group and 326 (41%) of 792 patients in the chemotherapy group received at least one subsequent therapy for advanced gastric/GEJ/oesophageal adenocarcinoma. The most common subsequent therapy in both groups was systemic anticancer therapy (268 [34%] of 789 and 311 [39%] of 792 patients, respectively); 12 (2%) and 64 (8%) patients received subsequent immunotherapy, respectively (appendix p 7).
Among all treated patients, the median (IQR) treatment duration was 6·8 months (3·7–13·3) with nivolumab plus chemotherapy and 4·9 months (2·5–8·4) with chemotherapy (appendix p 8). The most common TRAEs were nausea, diarrhoea, and peripheral neuropathy across both groups (table 2). Grade 3–4 treatment-related adverse events (TRAEs) occurred in 462 of 782 (59%) and 341 of 767 (44%) patients, respectively, and any-grade TRAEs leading to discontinuation were reported in 284 (36%) and 181 (24%) patients, respectively (table 2). Any-grade serious TRAEs were reported in 172 (22%) of 782 patients treated with nivolumab plus chemotherapy (grade 3–4, 131 patients [17%], four grade 5 events), and in 93 (12%) of 767 patients treated with chemotherapy (grade 3–4, 77 patients [10%], no grade 5 events). Sixteen deaths (2%) in the nivolumab plus chemotherapy group and four deaths (<1%) in the chemotherapy group were considered treatment related (table 2). Of the 16 deaths in the nivolumab plus chemotherapy group, four were deemed to be related to nivolumab, five to nivolumab plus chemotherapy, and seven to chemotherapy (appendix p 9). Dose delays due to any-grade TRAEs were observed in 524 of 782 (67%) and 447 of 767 (58%) patients in the nivolumab plus chemotherapy and chemotherapy groups, respectively. Modifications in chemotherapy doses were comparable across groups (appendix p 8). The majority of TRAEs with potential immunologic aetiology were grade 1 or 2; grade 3–4 events occurred in ≤5% of patients (appendix p 10).
Table 2:
Summary of treatment-related adverse events in all treated patients
| Nivolumab plus chemotherapy (n=782)* | Chemotherapy (n=767)* | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 5† | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | |
| All events | 272 (35%) | 358 (46%) | 104 (13%) | 4 (<1%) | 338 (44%) | 285 (37%) | 56 (7%) | 0 |
| Serious events | 37 (5%) | 97 (12%) | 34 (4%) | 4 (<1%) | 16 (2%) | 63 (8%) | 14 (2%) | 0 |
| Events leading to discontinuation | 148 (19%) | 109 (14%) | 23 (3%) | 4 (<1%) | 114 (15%) | 58 (8%) | 9 (1%) | 0 |
| Events leading to death ‡ | 16§ (2%) | 4|| (<1%) | ||||||
| Any-grade events in 10% or more of treated patients in either group | ||||||||
| Nausea | 303 (39%) | 20 (3%) | 0 | 0 | 273 (36%) | 19 (2%) | 0 | 0 |
| Diarrhoea | 218 (28%) | 33 (4%) | 2 (<1%) | 0 | 182 (24%) | 23 (3%) | 1 (<1%) | 0 |
| Peripheral neuropathy | 190 (24%) | 29 (4%) | 2 (<1%) | 0 | 168 (22%) | 22 (3%) | 0 | 0 |
| Vomiting | 178 (23%) | 17 (2%) | 0 | 0 | 142 (19%) | 24 (3%) | 0 | 0 |
| Fatigue | 172 (22%) | 30 (4%) | 0 | 0 | 156 (20%) | 16 (2%) | 1 (<1%) | 0 |
| Anaemia | 156 (20%) | 44 (6%) | 3 (<1%) | 0 | 150 (20%) | 20 (3%) | 1 (<1%) | 0 |
| Decreased appetite | 143 (18%) | 14 (2%) | 0 | 0 | 126 (16%) | 12 (2%) | 1 (<1%) | 0 |
| Thrombocytopenia | 138 (18%) | 15 (2%) | 4 (<1%) | 0 | 132 (17%) | 12 (2%) | 1 (<1%) | 0 |
| Platelet count decreased | 136 (17%) | 17 (2%) | 3 (<1%) | 0 | 96 (13%) | 15 (2%) | 4 (<1%) | 0 |
| Peripheral sensory neuropathy | 121 (15%) | 16 (2%) | 0 | 0 | 105 (14%) | 14 (2%) | 0 | 0 |
| Aspartate aminotransferase increased | 110 (14%) | 12 (2%) | 0 | 0 | 64 (8%) | 5 (<1%) | 0 | 0 |
| White blood cell count decreased | 89 (11%) | 20 (3%) | 3 (<1%) | 0 | 64 (8%) | 12 (2%) | 1 (<1%) | 0 |
| Alanine aminotransferase increased | 83 (11%) | 6 (<1%) | 0 | 0 | 45 (6%) | 5 (<1%) | 0 | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 83 (11%) | 11 (1%) | 0 | 0 | 75 (10%) | 6 (<1%) | 0 | 0 |
| Neutrophil count decreased | 75 (10%) | 60 (8%) | 23 (3%) | 0 | 51 (7%) | 50 (7%) | 17 (2%) | 0 |
| Neutropenia | 73 (9%) | 87 (11%) | 31 (4%) | 0 | 88 (11%) | 70 (9%) | 23 (3%) | 0 |
| Asthenia | 66 (8%) | 7 (<1%) | 0 | 0 | 71 (9%) | 9 (1%) | 1 (<1%) | 0 |
| Lipase increased | 44 (6%) | 34 (4%) | 11 (1%) | 0 | 18 (2%) | 14 (2%) | 2 (<1%) | 0 |
Data are n (%).
Patients who received at least one dose of the assigned treatment. Includes events reported between first dose and 30 days after last dose of trial therapy. Treatment-relatedness in the nivolumab plus chemotherapy group refers to nivolumab, at least one chemotherapy component, or both. National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0, and Medical Dictionary for Regulatory Activities, version 23.0.
There were four grade 5 events in the nivolumab plus chemotherapy group, one case each of cerebrovascular accident, febrile neutropenia, gastrointestinal inflammation, and pneumonia. There were no grade 5 events in the chemotherapy group.
Preferred terms for cause of death were per investigator assessment.
Twelve treatment-related deaths in the nivolumab plus chemotherapy group were due to three cases of pneumonitis, two cases of febrile neutropenia/neutropenic fever, and one case each of gastrointestinal bleeding, gastrointestinal toxicity, infection, intestinal mucositis, pneumonia, septic shock, and stroke. An additional four deaths due to “Other” reasons were specified as related to treatment by the investigator. These included one case each of acute cerebral infarction, mesenteric thrombosis, disseminated intravascular coagulation, and pneumonitis.
Treatment-related deaths in the chemotherapy group (n=4; one for each event) were due to diarrhoea, asthenia and severe loss of appetite, pulmonary thromboembolism, and pneumonitis.
The proportion of patients with PD-L1 CPS ≥5 and all randomised patients completing the FACT-Ga questionnaire was ≥90% at baseline and ≥80% at most subsequent assessments for which at least ten patients responded (until week 109). Baseline FACT-Ga total scores were similar between the nivolumab plus chemotherapy and chemotherapy groups for patients with PD-L1 CPS ≥5 (127·6 [standard deviation, 27·4] and 127·6 [26·4], respectively) and all randomised patients (126·6 [28·3] and 126·8 [26·8], respectively), with an improvement from baseline in FACT-Ga total score at all on-treatment assessments. In patients with PD-L1 CPS ≥5 and all randomised patients, the least squares mean difference between treatment groups favoured nivolumab plus chemotherapy versus chemotherapy (at timepoints with ≥50 patients in each group). However, this was less than the minimally important difference of 15·1 points (appendix p 16). Patients in the nivolumab plus chemotherapy group had decreased risk of symptom deterioration compared with the chemotherapy group while on treatment (patients with PD-L1 CPS ≥5, HR 0·64 [95% CI 0·49–0·83] and all randomised patients, HR 0·77 [0·63–0·95]) (appendix p 17).
Discussion
CheckMate 649 met both primary endpoints and all formally tested secondary endpoints. This is the first global study to demonstrate superior OS in a randomised controlled trial with a median OS exceeding 1 year in the first-line setting for patients with non–HER2-positive gastric/GEJ/oesophageal adenocarcinoma, where treatment options are limited and no advances have been made in recent years. With over 1580 patients randomised, nivolumab plus chemotherapy provided a significant and clinically meaningful OS benefit in patients with PD-L1 CPS ≥5 and ≥1, as well as in all randomised patients. PFS benefit was also observed in these populations, including when statistically tested in patients with PD-L1 CPS ≥5.
The proportion of patients with an objective response was numerically higher, with more complete and durable responses with nivolumab plus chemotherapy versus chemotherapy, in patients with PD-L1 CPS ≥5 and all randomised patients. The numerically higher 12-month OS and PFS estimates versus chemotherapy with sustained separation of Kaplan-Meier curves also suggest durable benefit with nivolumab plus chemotherapy in these populations.
The efficacy results demonstrate significant survival advantage with nivolumab plus chemotherapy for each endpoint prespecified by statistical hierarchical testing (PD-L1 CPS ≥5 and ≥1, and all randomised patients). The relatively large proportion of patients whose tumours express PD-L1 CPS ≥5 in the overall study population impacts the magnitude of the benefit observed in patients with PD-L1 CPS ≥1 and all randomised patients. In an exploratory analysis, the unstratified HRs for OS with nivolumab plus chemotherapy versus chemotherapy in patients with PD-L1 CPS <1 and <5 were higher than in all randomised patients. The observed HRs indicate enrichment of OS and PFS benefit with higher PD-L1 CPS cutoffs, along with a significant interaction of OS by PD-L1 CPS at the cutoff of 5 but not at the cutoff of 1. However, the higher objective response observed with nivolumab plus chemotherapy relative to chemotherapy across PD-L1 CPS cutoffs, including CPS <1 and <5, coupled with the potential for delayed treatment effect often seen with immuno-oncology therapy, suggests the magnitude of survival benefit may improve in these patients with longer follow-up.
CheckMate 649 enrolled patients regardless of PD-L1 CPS expression and to date is the most robust dataset to report CPS prevalence using a validated assay in advanced gastric, GEJ, or oesophageal adenocarcinoma. At the time of study amendment, a conservative PD-L1 CPS ≥5 prevalence of 35% was assumed based on limited available data in this disease setting.21–23 The prevalence of PD-L1 CPS ≥5 (60% of all randomised patients) observed in this large, randomised controlled trial was numerically higher than that reported in previous studies in gastric, GEJ, or oesophageal adenocarcinoma (17–50%).22,24,25 This variation in prevalence of PD-L1 CPS ≥5 may be attributed to several factors, including tumour heterogeneity, differences in patient population, and methodology.26,27 Future studies are needed to explore the analytical concordance of the assays and the factors that influence the prevalence of PD-L1 CPS expression in gastric/GEJ/oesophageal adenocarcinoma.
OS consistently favoured nivolumab plus chemotherapy versus chemotherapy across multiple prespecified baseline demographics and disease characteristics in the primary population and all randomised patients. Particularly, survival benefit with nivolumab plus chemotherapy occurred regardless of MSI status, although the 3% of patients with MSI-high tumours had greater reduction in the risk of death than those with microsatellite stable tumours. Post-hoc interaction analyses confirmed that the majority of the baseline demographics and disease characteristics were not determinant of the OS benefit. While the prespecified interaction p value for tumour cell PD-L1 status was less than 0·2, the HRs for OS were less than 1 in both subgroups of patients with tumour cell PD-L1 expression ≥1% and <1%, suggesting a difference in magnitude of effect but no change in the direction of the treatment effect. Further research is needed to characterise patients with advanced gastric/GEJ/oesophageal adenocarcinoma who may derive the greatest clinical benefit from immunotherapies.
Pembrolizumab plus chemotherapy did not result in significant improvement in OS versus chemotherapy in patients with advanced or recurrent gastric/GEJ adenocarcinoma and PD-L1 CPS ≥1 (HR 0·85; p=0·05) and ≥10 (HR 0·85; p=0·16) in the smaller KEYNOTE-062 study. In the phase 3 part of the Asian ATTRACTION-4 study of previously untreated advanced gastric/GEJ cancer, nivolumab plus chemotherapy significantly improved PFS (HR 0·68; p<0·001) but not OS (HR 0·90; p=0·26) in the all randomised population.28 The differences in efficacy observed among these three studies could be due to differences in study design (including statistical considerations, biomarker selection, patient population, geography, and treatment regimens including chemotherapy backbone) and subsequent therapies. A large proportion of patients (66%) received subsequent therapy in ATTRACTION-4.28 The proportion (39%) of patients receiving subsequent therapy observed in CheckMate 649 was consistent with the global phase 3 KEYNOTE-062 study and may reflect the practice patterns and limited therapeutic options in some countries.
The safety profile of nivolumab plus chemotherapy was consistent with the known safety profiles of the individual treatments and no new safety signals were identified.11,17,28–30 Duration of chemotherapy was similar among the treatment groups when comparing the same chemotherapy backbone, suggesting that the addition of nivolumab did not negatively impact chemotherapy administration. Treatment-related deaths were more common in the nivolumab plus chemotherapy group versus chemotherapy. However, seven out of 16 deaths in the nivolumab plus chemotherapy group were related to chemotherapy alone and the overall proportion of treatment-related deaths was low (2%), consistent with that observed in other first-line PD-1–inhibitor-chemotherapy regimens in gastric/GEJ adenocarcinoma.16,28 Despite more frequent grade 3–4 TRAEs and events leading to discontinuation with nivolumab plus chemotherapy versus chemotherapy, grade 3–4 TRAEs with potential immunologic aetiology occurred in ≤5% of patients, and the overall safety profile was acceptable. There was a trend towards improved HRQoL with nivolumab plus chemotherapy, although not clinically meaningful per the predefined threshold, along with a decreased risk of TTSD while on treatment, suggesting that the addition of nivolumab maintains HRQoL. The acceptable safety profile combined with significant improvement in OS, along with PFS benefit, improved and durable objective responses, and maintained HRQoL, indicate a favourable benefit-risk profile for nivolumab plus chemotherapy.
Our study had some limitations. Based on data available at the time of study design, tumour cell PD-L1 expression was chosen as a stratification factor for CheckMate 649. Following reports which indicated that PD-L1 CPS had better enrichment for efficacy than tumour cell PD-L1 expression in advanced gastric/GEJ/oesophageal adenocarcinoma,21–23 the protocol was amended to use PD-L1 CPS ≥5 to define the primary population. Although tumour cell PD-L1 expression remained a stratification factor, patients whose tumours expressed PD-L1 CPS ≥5 were balanced between the two treatment groups. In addition, demographics and baseline disease characteristics were balanced between treatment groups in the PD-L1 CPS ≥5 population. Patients with known HER2-positive status were excluded from CheckMate 649. However, because HER2 testing may not have been performed routinely at all study sites, patients with unknown HER2 status were permitted to be enrolled. Importantly, the proportion of these patients (~40%) was balanced across the treatment groups. Based on the known incidence of HER2-overexpressing tumours in gastric/GEJ cancer (~20%),31–35 it is expected that the majority of patients with not reported HER2 status in this study were HER2-negative. Another limitation of CheckMate 649 is its open-label study design, which may have potentially influenced patient responses in the HRQoL questionnaires and adverse event causality assessment. However, an open-label design was considered appropriate due to the inclusion of multiple treatments with different dosing regimens. Centrally assessed primary endpoints and adverse event management using standard treatment algorithms were not expected to be impacted by bias.
In conclusion, nivolumab is the first PD-1 inhibitor to demonstrate superior OS, along with clinically meaningful PFS benefit, improved and durable objective responses, maintained HRQoL, and an acceptable safety profile, in combination with chemotherapy versus chemotherapy alone in previously untreated patients with advanced gastric/GEJ/oesophageal adenocarcinoma and represents a potential standard first-line treatment for these patients.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed in November 2020 for English language articles, using the terms “Gastric OR Gastroesophageal Junction OR Esophagogastric Junction OR Esophageal adenocarcinoma OR Oesophageal adenocarcinoma,” and “PD-1 OR PD-L1,” and “First-line OR Previously untreated OR Treatment naive” in the title or abstract, with no time limits. To identify results from clinical trials that were not yet published in peer-reviewed journals, we also searched the American Society of Clinical Oncology and European Society for Medical Oncology congress websites for publications between September 1, 2018, and December 1, 2020, using the same key words. Our search identified 259 abstracts, from which we selected primary publications from randomised phase 3 studies of PD-1 or PD-L1 inhibitors in previously untreated patients with advanced gastric cancer and/or gastroesophageal junction cancer and/or oesophageal adenocarcinoma (GC/GEJC/EAC). Using these criteria, four studies with efficacy and safety data were identified: ATTRACTION-4, KEYNOTE-062, KEYNOTE-590, and JAVELIN Gastric 100. In the phase 3 ATTRACTION-4 study of previously untreated Asian patients with advanced GC/GEJC, nivolumab plus chemotherapy significantly improved progression-free survival (PFS) but not overall survival (OS) in the all randomised population with a manageable safety profile. Similarly, in the global, phase 3 KEYNOTE-062 study, which enrolled patients with PD-L1 combined positive score (CPS) ≥1, pembrolizumab plus chemotherapy did not provide superior OS benefit but provided a modest improvement in PFS and objective response with a manageable safety profile in patients with advanced or recurrent GC/GEJC with PD-L1 CPS ≥1 or ≥10. In the phase 3 KEYNOTE-590 study of oesophageal/GEJC (mainly squamous cell carcinoma histology), first-line pembrolizumab plus chemotherapy provided improved OS and PFS in advanced unresectable or metastatic gastroesophageal junction (Siewert type 1) or oesophageal adenocarcinoma in a subgroup analysis and a manageable safety profile. In the phase 3 JAVELIN Gastric 100 study of advanced GC/GEJC, avelumab maintenance after first-line chemotherapy did not demonstrate superior OS versus continued chemotherapy in the primary population of all randomised patients or in patients with tumour cell PD-L1 expression ≥1%.
Added value of this study
With nearly 1600 patients randomised in the CheckMate 649 trial, nivolumab in combination with chemotherapy demonstrated superior OS, along with PFS benefit, versus chemotherapy alone in previously untreated patients with advanced GC/GEJC/EAC. To our knowledge, CheckMate 649 is the first global study to demonstrate superior OS with a median OS exceeding 1 year in the first-line setting for patients with non–human epidermal growth factor receptor 2-positive GC/GEJC/EAC. The safety profile of nivolumab plus chemotherapy was consistent with the known safety profiles of the individual treatment components and no new safety signals were identified. Although grade 3–4 treatment-related adverse events and events leading to discontinuation were more frequent with nivolumab plus chemotherapy versus chemotherapy, the safety profile was acceptable in the context of the significant improvement in OS, along with PFS benefit, improved and durable objective responses, and maintained health-related quality of life.
Implications of all the available evidence
The CheckMate 649 trial addresses a high unmet need in previously untreated patients with GC/GEJC/EAC, where no advances have been made in recent years. Nivolumab is the first PD-1 inhibitor to demonstrate superior OS, along with PFS benefit and an acceptable safety profile, in combination with chemotherapy versus chemotherapy alone and represents a potential standard first-line treatment in patients with advanced GC/GEJC/EAC.
Acknowledgments
This study was supported by Bristol Myers Squibb (BMS) and Ono Pharmaceutical Co., Ltd. We thank the patients and their families for making the study possible; the investigators and the clinical study teams at BMS (Princeton, NJ, USA) and Ono Pharmaceutical Co., Ltd. (Osaka, Japan). From BMS we thank protocol managers Maria Kistkina and Charlotte Corbisier. Analysis of patient-reported outcomes was supported by Steven Blum (BMS, Princeton, NJ, USA), and Lawrence Rasouliyan and David McSorley (RTI Health Solutions, Research Triangle Park, NC, USA). Dako (an Agilent Technologies company, Santa Clara, CA, USA) participated in collaborative development of the PD-L1 IHC 28-8 pharmDx assay. Professional medical writing assistance was provided by Tanmayi Mankame of Parexel International, funded by BMS.
Declaration of interests
YYJ reports receiving consulting/advisory board fees from AstraZeneca, Daiichi Sankyo, Imugene, Jounce Therapeutics, Merck Serono, Michael J. Hennessy Associates, Paradigm Medical Communications, LLC, Pfizer, Seattle, Genetics, Zymeworks Inc.; receiving consulting/advisory and research funding from Eli Lilly, Bristol Myers Squibb, Merck & Co, Inc; receiving research funding from Bayer, Boehringer Ingelheim, Genentech/Roche, MSK Cancer Center Support Grant/Core Grant (P30 CA008748), and Ono Pharmaceutical Company; receiving speaker’s bureau fees from the American Society of Clinical Oncology; and receiving stock options from Rgenix, outside the submitted work. KS reports receiving personal fees for advisory roles from AbbVie, Inc., Bristol Myers Squibb, GlaxoSmithKline, Novartis, Ono Pharmaceutical Company, Pfizer Inc, and Takeda; receiving advisory role/research funding from Astellas Pharma, Eli Lilly, Merck Pharmaceutical, and Taiho Pharmaceutical; receiving honoraria (lecture fee) from AbbVie Inc, Novartis, and Yakult Honsha; receiving research funding from Chugai Pharma, Daiichi Sankyo, Dainippon Sumitomo Pharma, and Medi Science, outside the submitted work. MM reports receiving research grants from AIO, Amgen, German Federal Ministry of Education and Research, Bristol Myers Squibb, European Organisation for Research and Treatment of Cancer, German Cancer Aid, Merck Serono, Merck Sharpe & Dohme, and Pfizer; receiving personal fees from Bristol Myers Squibb, Falk Foundation, Lilly, MCI Group, Merck Serono, Merck Sharpe & Dohme, Pfizer, and Roche; and receiving non-financial support from AIO, Amgen, Bristol Myers Squibb, German Federal Ministry of Education and Research, European Organisation for Research and Treatment of Cancer, and German Cancer Aid, outside the submitted work. MG reports receiving grants and personal fees from Bristol Myers Squibb and Novartis; and receiving personal fees from Merck Sharpe & Dohme and Roche, outside the submitted work. KY reports receiving grants and personal fees from Daiichi-Sankyo, Ono Pharmaceutical Company, Taiho Pharmaceutical, and Yakult Honsha; receiving personal fees from Chugai, Lilly, and Takeda; receiving grants from Sanofi; and receiving personal fees from Bristol Myers Squibb, Merck Serono, and Takeda, outside the submitted work. MS reports receiving personal fees for clinical research from Bristol Myers Squibb, during the conduct of the study; and receiving personal fees for clinical research from Astellas, AstraZeneca, Eli Lilly, GlaxoSmithKline, Merck Serono, Merck Sharpe & Dohme, Novartis, Pfizer, Regeneron, and Roche, outside the submitted work. MT reports receiving research funding from Bristol Myers Squibb, during the conduct of the study. RK reports receiving grants from Amgen, AstraZeneca, Athenex, Eli Lilly, Nektar, and Sanofi; receiving grants and non-financial support from Roche; grants and personal fees from Astellas and Novartis; grants, personal fees, and non-financial support from Bristol Myers Squibb, Merck Sharpe & Dohme, and Pfizer; personal fees from Gador, outside the submitted work. MVK reports serving in an advisory role for Bristol Myers Squibb, Merck Sharpe & Dohme, Ipsen, Roche, Sandoz, Sanofi, and Servier, outside the submitted work. RB reports serving as a medical advisor for Merck Serono and Novartis; receiving clinical research funding from Novartis; serving as a medical advisor and speaker for AstraZeneca, Bristol Myers Squibb, and Pfizer; receiving clinical research funding from Bristol Myers Squibb, Merck Sharpe & Dohme, and Roche; and serving as a speaker for Merck, during the conduct of the study. TZ reports other relationships with AstraZeneca, Bristol Myers Squibb, Lilly, Merck Sharpe & Dohme, Novartis, Roche, and Sanofi, outside the submitted work. JC reports receiving personal fees and consulting and travel support from Bristol Myers Squibb; and receiving research funding from AstraZeneca, Esperas Pharma, Merck, and Tesaro, outside the submitted work. VP reports employment with Bristol Myers Squibb and ownership of stock in Bristol Myers Squibb. DC reports employment with Bristol Myers Squibb and ownership of stock in Bristol Myers Squibb. M Lei reports employment with Bristol Myers Squibb and ownership of stock in Bristol Myers Squibb. HX reports employment with Bristol Myers Squibb and ownership of stock in Bristol Myers Squibb. KK reports employment with Bristol Myers Squibb and ownership of stock in Bristol Myers Squibb. M Li reports employment with Bristol Myers Squibb and ownership of stock in Bristol Myers Squibb. JAA reports receiving clinical research grants and receiving personal advisory board fees from Bristol Myers Squibb, during the conduct of the study. All other authors declare no competing interests.
Footnotes
Data sharing
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
- 1.GLOBOCAN 2020. Cancer fact sheets, stomach. 2020.
- 2.Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis Primers 2017; 3: 17036. [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 2020. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Gastric Cancer. Version 2.2021. https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed March 17, 2021). [Google Scholar]
- 5.Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27: v38–v49. [DOI] [PubMed] [Google Scholar]
- 6.Wang FH, Shen L, Li J, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond) 2019; 39: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18: 1467–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs CS, Shitara K, Di Bartolomeo M, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20: 420–35. [DOI] [PubMed] [Google Scholar]
- 10.Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013; 14: 490–9. [DOI] [PubMed] [Google Scholar]
- 11.Shah MA, Bang YJ, Lordick F, et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The METGastric Randomized Clinical Trial. JAMA Oncol 2017; 3: 620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network, Analysis Working Group: Asan University, B. C. Cancer Agency, et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017; 541: 169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salem ME, Puccini A, Xiu J, et al. Comparative Molecular Analyses of Esophageal Squamous Cell Carcinoma, Esophageal Adenocarcinoma, and Gastric Adenocarcinoma. Oncologist 2018; 23: 1319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pape M, Vissers PAJ, Bertwistle D, McDonald L, Laarhoven HWMV, Verhoeven RHA. A nationwide population-based study comparing survival in unresectable advanced or synchronous metastatic esophageal and gastric adenocarcinoma. Journal of Clinical Oncology 2020; 38: 308-. [Google Scholar]
- 15.Chau I, Norman AR, Cunningham D, et al. The impact of primary tumour origins in patients with advanced oesophageal, oesophago-gastric junction and gastric adenocarcinoma--individual patient data from 1775 patients in four randomised controlled trials. Ann Oncol 2009; 20: 885–91. [DOI] [PubMed] [Google Scholar]
- 16.Shitara K, Van Cutsem E, Bang Y-J, et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538–12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 2461–71. [DOI] [PubMed] [Google Scholar]
- 18.Park SJ, Ye W, Xiao R, et al. Cisplatin and oxaliplatin induce similar immunogenic changes in preclinical models of head and neck cancer. Oral Oncol 2019; 95: 127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Wu L, Zhang J, Wu H, Han E, Guo Q. Chemoimmunotherapy by combining oxaliplatin with immune checkpoint blockades reduced tumor burden in colorectal cancer animal model. Biochem Biophys Res Commun 2017; 487: 1–7. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2014; 2: 846–56. [DOI] [PubMed] [Google Scholar]
- 21.Kulangara K, Guerrero L, Posch A, et al. Investigation of PD-L1 expression and response to pembrolizumab (pembro) in gastric cancer (GC) and cervical cancer (CC) using combined positive score (CPS) and tumor proportion score (TPS). Journal of Clinical Oncology 2018; 36: 4065-. [Google Scholar]
- 22.Lei M, Siemers N, Pandya D, et al. Abstract 2673: Association of PD-L1 combined positive score and immune gene signatures with efficacy of nivolumab (NIVO) ± ipilimumab (IPI) in patients with metastatic gastroesophageal cancer (mGEC). Cancer Research 2019; 79: 2673-. [Google Scholar]
- 23.Shitara K, Özgüroğlu M, Bang Y et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;6736(18):31257–1. [DOI] [PubMed] [Google Scholar]
- 24.Fassan M, Brignola S, Pennelli G, et al. PD-L1 expression in gastroesophageal dysplastic lesions. Virchows Arch 2020; 477: 151–6. [DOI] [PubMed] [Google Scholar]
- 25.Hagi T, Kurokawa Y, Kawabata R, et al. Multicentre biomarker cohort study on the efficacy of nivolumab treatment for gastric cancer. Br J Cancer 2020; 123: 965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suda K, Mitsudomi T. Inter-tumor heterogeneity of PD-L1 status: is it important in clinical decision making? J Thorac Dis 2020; 12: 1770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye M, Huang D, Zhang Q, et al. Heterogeneous programmed death-ligand 1 expression in gastric cancer: comparison of tissue microarrays and whole sections. Cancer Cell Int 2020; 20: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boku N, Ryu MH, Oh D-Y, et al. , Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538–37) study. Oral presentation at the European Society for Medical Oncology. LBA7_PR. 2020. [Google Scholar]
- 29.Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 2008; 26: 1435–42. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008; 358: 36–46. [DOI] [PubMed] [Google Scholar]
- 31.Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes--a systematic review. Int J Cancer 2012; 130: 2845–56. [DOI] [PubMed] [Google Scholar]
- 32.Hsu JT, Chen TC, Tseng JH, et al. Impact of HER-2 overexpression/amplification on the prognosis of gastric cancer patients undergoing resection: a single-center study of 1,036 patients. Oncologist 2011; 16: 1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janjigian YY, Werner D, Pauligk C, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol 2012; 23: 2656–62. [DOI] [PubMed] [Google Scholar]
- 34.Sheng WQ, Huang D, Ying JM, et al. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol 2013; 24: 2360–4. [DOI] [PubMed] [Google Scholar]
- 35.Van Cutsem E, Bang YJ, Feng-Yi F, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015; 18: 476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


