Abstract
Background
Neuropsychiatric symptoms (NPSs) in early dementia have been suggested to predict a higher risk of dementia progression. However, the literature is not yet clear whether the risk is similar across Alzheimer's dementia (AD) and non-Alzheimer's dementia (non-AD), as well as across different NPSs. This study examined the association between NPSs in early dementia and the risk of progression to severe dementia, specifically in AD and non-AD, as well as across various NPSs.
Method
This cohort study included 7,594 participants who were ≥65 years and had early dementia (global Clinical Dementia Rating [CDR] = 1). Participants completed Neuropsychiatric-Inventory–Questionnaire at baseline and were followed-up almost annually for progression to severe dementia (global CDR = 3) (median follow-up = 3.5 years; interquartile range = 2.1–5.9 years). Cox regression was used to examine progression risk, stratified by AD and non-AD.
Results
The presence of NPSs was associated with risk of progression to severe dementia, but primarily in AD (HR 1.4, 95% confidence interval [CI]: 1.1–1.6) and not in non-AD (HR 0.9, 95% CI: 0.5–1.5). When comparing across various NPSs, seven NPSs in AD were associated with disease progression, and they were depression, anxiety, apathy, delusions, hallucinations, irritability and motor disturbance (HR 1.2–1.6). In contrast, only hallucinations and delusions were associated with disease progression in non-AD (HR 1.7–1.9).
Conclusions
NPSs in early dementia—especially among individuals with AD—can be useful prognostic markers of disease progression. They may inform discussion on advanced care planning and prompt clinical review to incorporate evidence-based interventions that may address disease progression.
Keywords: advanced dementia, Alzheimer’s dementia, behavioural and psychological symptoms of dementia, dementia progression, non-Alzheimer’s dementia, older people
Key Points
Many of the subtypes of NPSs in Alzheimer’s dementia (ad) were associated with higher risks of disease progression.
Only hallucinations and delusions were associated with progression risk in non-ad.
The findings may be related to a more aggressive disease and less optimal dementia care among individuals with NPSs.
NPSs—especially in ad—can be useful prognostic markers of disease progression and may inform advanced care planning.
NPSs in early dementia should prompt evidence-based interventions that may address disease progression.
Introduction
Neuropsychiatric symptoms (NPSs) are common features across all aetiologies of dementia and are experienced by more than 90% of persons with dementia during the course of the disease [1]. NPSs in dementia include symptoms such as agitation, apathy, depression and psychosis [2]. They are often reported as among the greatest challenges in dementia caregiving [1,2], and as such, have often been the focus of clinical interventions to reduce their impact on caregivers [1,2]. Inasmuch as NPSs are relevant to the psychological well-being of caregivers, there is recent evidence to suggest that NPSs can also have direct, biological implications to the persons with dementia, whereby the presence of NPSs has been reported to predict a greater risk of dementia progression [3–5].
However, to date, the literature on NPSs and dementia progression has mostly focused on patients with Alzheimer’s dementia (ad) [3–5]. It is unknown whether the association between NPSs and dementia progression is similarly present among patients with non-AD. Moreover, the literature is also not yet conclusive on whether all NPSs, or only selected NPSs, are associated with the risk of dementia progression. For example, in the Cache County Study (based on residents with incident dementia from Utah, USA) [5], Agitation and Psychosis (but not Affective and Apathy symptoms) predicted dementia progression. Yet, in a subsequent population-based study involving participants in Venezuela [6], none of the NPSs in the earlier stages of dementia were associated with dementia progression. Using a large sample recruited from across USA, this study sought to provide more conclusive evidence on the association between NPSs in early dementia and risk of progression to severe dementia. Specifically, this study examined whether the association was present in both ad and non-ad, as well as whether the association was similar across various NPSs.
Method
Participants and procedures
This study is based on a cohort study-design, involving individuals recruited from ~39 Alzheimer’s Disease Centers across the USA between September 2005 and August 2019 (as available in the National Alzheimer’s Coordinating Center [NACC] database) [7]. It included participants who fulfilled the following criteria at baseline: (i) age ≥ 65 years; (ii) diagnosed with dementia; (iii) no concurrent diagnosis of delirium at baseline; (iv) had global Clinical Dementia Rating (CDR) of 1 (indicating early dementia) and (v) provided information on Neuropsychiatric Inventory–Questionnaire (NPI-Q). Participants were followed-up on an approximately annual basis to evaluate for progression in dementia severity (as measured by CDR). All contributing Alzheimer’s Disease Centers obtained informed consent from their participants, as well as received approval by their local institutional review boards.
Measures
NPI-Q is a clinical measure that screens for the presence of NPSs in the past month. It has 12 items that assess NPSs in 12 domains, namely depression, anxiety, apathy, sleep, appetite, hallucinations, delusions, agitation, irritability, motor disturbance, disinhibition and elation. It was administered to informants by trained healthcare professionals, with each item rated on a 4-point Likert scale: 0 = Not present, 1 = Mild (noticeable, but not a significant change), 2 = Moderate (significant, but not a dramatic change) and 3 = Severe (very marked or prominent; a dramatic change). Mini-Mental State Examination (MMSE) [8] is a widely used cognitive test. It comprises 11 items across cognitive domains such as orientation, memory, concentration, language and constructional praxis.
CDR (CDR® Dementia Staging Instrument) [9] is a well-validated and widely used scale for staging of cognitive impairment [10]. It was initially developed for individuals with ad [9], although in subsequent literature, CDR has also been widely used for staging of non-ad [10–13]. CDR employs a semi-structured interview with both participant and informant to rate performance in six domains (memory, orientation, judgement and problem solving, community affairs, home and hobbies, and personal care), with each domain rated according to one of the five levels of impairment (0 = none, 0.5 = questionable, 1 = mild, 2 = moderate, 3 = severe). Rating from the six domains can be totalled to yield a CDR sum of boxes score which ranges from 0 to 18. Based on the originally published rules [9], responses from the six domains can also be used to assign a global CDR score to indicate the severity of cognitive impairment: 0 = normal cognition (NC), 0.5 = questionable cognitive impairment, 1 = mild dementia, 2 = moderate dementia and 3 = severe dementia. The primary endpoint of severe dementia in this study was based on a global CDR = 3 from the original rules, although an alternate method to define severe dementia—using CDR sum of boxes score—was examined in the sensitivity analysis and is further described in the Statistical analyses section.
The diagnosis of dementia was made based on standardised assessments, which included clinical history, physical examination and detailed neuropsychological testing [7,14,15]. Majority of the diagnoses (81.6%) were made via consensus conference (by two or more clinicians), while the remainder were made by single clinicians. Dementia was diagnosed using McKhann (1984) criteria [16], DSM-IV (Diagnostic and Statistical Manual of Mental Disorders–Fourth Edition) criteria [17] or McKhann (2011) criteria [18]. Each case of dementia was further classified into its primary aetiology based on published criteria [16,18–28], which include those for ad [16,18], vascular dementia [19], frontotemporal lobar degeneration [20–26] and dementia with Lewy Bodies [26–28].
Statistical analyses
Cox proportional hazard regression [29–31] was conducted to evaluate the association between NPSs in early dementia and risk of progression to severe dementia, stratified by ad and non-ad. Time-to-event was defined as the duration from baseline to onset of severe dementia (global CDR = 3). NPSs were included in Cox regression primarily as a binary variable based on the presence or absence of any NPS (1 = Presence of at least one NPS in NPI-Q; and 0 = No reported NPS on all the items in NPI-Q). In addition, NPSs were also examined based on the followings:
severity of NPSs (Mild = at least one item in NPI-Q scored 1 but no items scored ≥2; Moderate = At least one item in NPI-Q scored 2 but no items scored 3; and Severe = At least one item in NPI-Q scored 3).
total score of NPI-Q (by summing the item scores in NPI-Q).
number of NPSs (by counting the number of NPI-Q items with score ≥ 1).
presence of each of the 12 NPSs in NPI-Q.
Cox regression adjusted for potential confounders between NPSs and dementia progression, including the baseline covariates of age, sex, ethnicity, years of education, APOE e4 genotype, MMSE score, CDR sum of boxes score, use of cognitive enhancers, use of antidepressants, use of antipsychotics and use of sedatives. Further details on the conduct of Cox regression are available in Supplementary Material 1.
Additionally, a sensitivity analysis was conducted to evaluate the robustness of the results when the primary endpoint of severe dementia was redefined using CDR sum of boxes scores of 16–18 (instead of global CDR = 3; as determined by the originally published rules of CDR) [9]. The originally published rules of CDR gave greater weightage to the memory domain in dementia staging [9,32] and, arguably, may be more applicable to ad than non-ad. In the literature, an alternate method of staging has been proposed to give equal weightage to the six domains of CDR, which reduces the reliance on memory domain in dementia staging [32]. This method proposes the use of CDR sum of boxes scores instead to define the levels of cognitive impairment, with the total scores of 0 = none, 0.5–4 = questionable, 4.5–9 = mild, 9.5–15.5 = moderate and 16–18 = severe [11,32]. This alternate method of staging was previously shown to be valid in staging both ad and non-ad in NACC database [11]. When examined using item response theory, it was shown in one study to be as good as, and potentially better than, the original staging rules of CDR [32].
All statistical analyses were conducted in Stata (version 16).
Results
A total of 7,594 participants were included in this study. Flow diagram related to participant selection is shown in Figure 1, while participant characteristics are presented in Supplementary Material 2. The participants had a median age of 78 years (interquartile range, IQR 72–83), a median MMSE score of 22 (IQR 19–25) and a median CDR sum of boxes score of 6 (IQR 5–7). Most had the primary aetiology of ad (81.9%), while 2.4% had vascular dementia, 6.8% dementia with Lewy Bodies, 6.5% frontotemporal lobar degeneration and 2.4% other aetiologies of dementia. The most common NPSs were apathy (42.8%) and irritability (40.1%) among participants with ad; and apathy (58.2%) and depression (46.7%) among participants with non-ad. The participants had a median duration of follow-up of 3.5 years (IQR 2.1–5.9 years), with 1,192 (15.7%) progressed to severe dementia during follow-up.
Figure 1 .
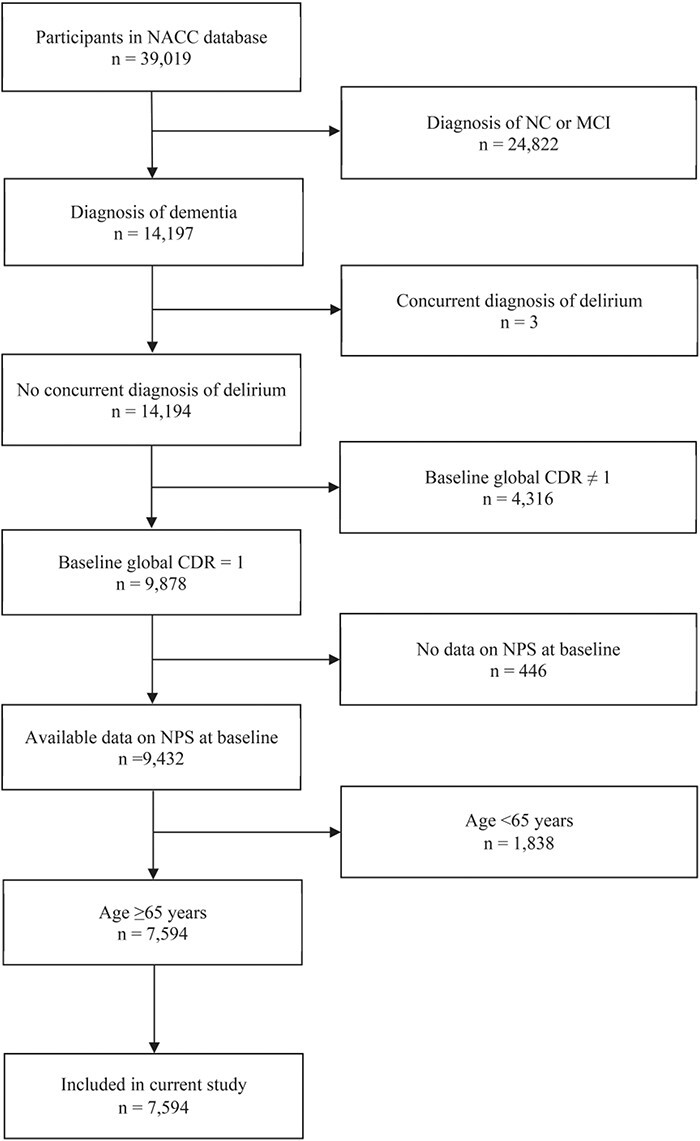
Participant enrolment and exclusion details. MCI, mild cognitive impairment.
NPSs were associated with progression to severe dementia, but primarily among participants with ad and not among those with non-ad. As seen in Table 1, presence of NPSs in ad was associated with 1.4 times higher risk of progression to severe dementia (95% confidence interval [CI] 1.1–1.6), with demonstrable dose–response relationship across the severity and the number of NPSs. Among participants with ad, seven individual NPSs were associated with higher risk of progression, namely depression, anxiety, apathy, delusions, hallucinations, irritability and motor disturbance (hazard ratio, HR 1.2–1.6). In contrast, among participants with non-ad, only two NPSs (delusions and hallucinations) were associated with higher progression risk (HR 1.7–1.9). The findings remained robust in the sensitivity analysis (when the endpoint of severe dementia was redefined using CDR sum of boxes scores of 16–18) and are further presented in Supplementary Material 3.
Table 1 .
Associations between the NPSs in early dementia and the risk of progression to severe dementia, stratified by those with AD (n = 6,221) and those with non-AD (n = 1,373)
| NPSs in mild dementia | Risk of progression to severe dementia | |||
|---|---|---|---|---|
| Participants with AD | Participants with non-AD | |||
| HR (95% CI)a | P-valuea | HR (95% CI)a | P-valuea | |
| Presence of any NPS | ||||
| No | 1.0 (Ref) | Ref | 1.0 (Ref) | Ref |
| Yes | 1.4 (1.1–1.6) | 0.001 | 0.9 (0.5–1.5) | 0.661 |
| Severity of NPSsb | ||||
| No NPS | 1.0 (Ref) | Ref | 1.0 (Ref) | Ref |
| Mild | 1.2 (1.0–1.5) | 0.049 | 0.8 (0.5–1.5) | 0.526 |
| Moderate | 1.4 (1.1–1.7) | 0.001 | 1.0 (0.6–1.7) | 0.916 |
| Severe | 1.6 (1.2–2.0) | <0.001 | 0.8 (0.4–1.5) | 0.467 |
| NPI–Q total scorec | ||||
| 0–2 | 1.0 (Ref) | Ref | 1.0 (Ref) | Ref |
| 3–4 | 1.2 (1.0–1.4) | 0.100 | 1.2 (0.8–1.9) | 0.449 |
| 5–7 | 1.3 (1.1–1.6) | 0.001 | 1.1 (0.7–1.7) | 0.749 |
| ≥8 | 1.4 (1.1–1.7) | 0.001 | 1.0 (0.6–1.6) | 0.968 |
| Number of NPSsd | ||||
| 0–1 | 1.0 (Ref) | Ref | 1.0 (Ref) | Ref |
| 2–3 | 1.2 (1.0–1.5) | 0.013 | 0.8 (0.5–1.3) | 0.410 |
| 4–5 | 1.3 (1.0–1.5) | 0.020 | 0.9 (0.6–1.4) | 0.617 |
| ≥6 | 1.5 (1.2–1.9) | <0.001 | 0.9 (0.6–1.5) | 0.718 |
| Presence of individual NPS | ||||
| Depression | 1.2 (1.1–1.4) | 0.004 | 1.0 (0.7–1.4) | 0.987 |
| Anxiety | 1.2 (1.1–1.4) | 0.005 | 0.9 (0.7–1.3) | 0.711 |
| Apathy | 1.2 (1.0–1.3) | 0.020 | 0.9 (0.7–1.3) | 0.634 |
| Sleep | 1.1 (0.9–1.2) | 0.500 | 1.2 (0.8–1.6) | 0.333 |
| Appetite | 1.1 (1.0–1.3) | 0.172 | 1.0 (0.7–1.5) | 0.800 |
| Delusions | 1.4 (1.1–1.7) | 0.002 | 1.7 (1.1–2.5) | 0.008 |
| Hallucinations | 1.6 (1.2–2.0) | 0.002 | 1.9 (1.3–2.7) | 0.002 |
| Agitation | 1.1 (0.9–1.3) | 0.241 | 1.1 (0.8–1.5) | 0.590 |
| Irritability | 1.2 (1.0–1.3) | 0.037 | 0.6 (0.5–0.9) | 0.005 |
| Motor disturbance | 1.4 (1.1–1.6) | <0.001 | 1.1 (0.8–1.6) | 0.580 |
| Disinhibition | 1.1 (0.9–1.2) | 0.490 | 0.8 (0.6–1.1) | 0.097 |
| Elation | 1.0 (0.7–1.3) | 0.860 | 0.4 (0.2–0.8) | 0.014 |
Ref, reference group.
aModel adjusted for baseline covariates of age, sex, ethnicity, years of education, APOE e4 genotype, MMSE score, CDR sum of boxes score, use of cognitive enhancers, use of antidepressants, use of antipsychotics and use of sedatives. Significant risk-estimates (with P ≤ 0.05) are highlighted in bold.
bThe NPSs were split into 4 levels of severity based on responses on the 12 items in NPI-Q: No NPS was defined if all items in NPI-Q were scored 0, Mild NPS was defined if at least one item in NPI-Q was scored 1 but no items scored ≥2, Moderate NPS was defined if at least one item in NPI-Q was scored 2 but no items scored 3, and Severe NPS was defined if at least one item in NPI-Q was scored 3.
cThe NPI-Q total score was split into 4 quartiles.
dThe number of NPSs was split into 4 quartiles.
The progression risk was further examined by focusing only on the individual NPSs that had been identified to be significant, namely the seven NPSs in ad (depression, anxiety, apathy, delusions, hallucinations, irritability and motor disturbance) and the two NPSs in non-ad (delusions and hallucinations). As seen in Table 2, across the seven significant NPSs in ad, progression risk rose incrementally corresponding to the number of NPSs that were endorsed, with HR 1.3–1.4 among those who endorsed 1–3 NPSs, HR 1.9 among those who endorse 4 NPSs and HR 2.3 among those who endorsed 5–7 NPSs. In the absence of the seven significant NPSs, half of the participants developed severe dementia within 6.0 years of follow-up. This duration shortened to 5.2–5.4 years in the presence of 1–3 significant NPSs, 4.8 years in the presence of 4 NPSs, and 4.3 years in the presence of 5–7 NPSs. Similarly, for participants with non-ad, the risk rose incrementally across the two significant NPSs. In the absence of the two significant NPSs, half of the participants developed severe dementia within 5.0 years of follow-up. This duration shortened to 4.2 years in the presence of 1 NPS and 3.2 years in the presence of 2 NPSs. The differential risks across the number of significant NPSs are further visible in the Kaplan–Meier curves in Figure 2.
Table 2 .
Risk of progression to severe dementia, focusing only on the individual NPSs that had been identified to be significant in the current study a
| Number of significant NPSs a | HR (95% CI) b | P-value | Median time to severe dementia, year (95% CI)c |
|---|---|---|---|
| (A) Participants with AD | |||
| 0 | 1.0 (Ref) | Ref | 6.0 (5.6–6.5) |
| 1 | 1.3 (1.0–1.6) | 0.023 | 5.3 (4.7–5.9) |
| 2 | 1.4 (1.1–1.7) | 0.003 | 5.2 (5.0–5.5) |
| 3 | 1.4 (1.1–1.8) | 0.002 | 5.4 (4.9–5.9) |
| 4 | 1.9 (1.5–2.4) | <0.001 | 4.8 (4.3–5.4) |
| 5–7 d | 2.3 (1.7–3.2) | <0.001 | 4.3 (3.7–4.9) |
| (B) Participants with non-AD | |||
| 0 | 1.0 (Ref) | Ref | 5.0 (4.2–5.9) |
| 1 | 1.5 (1.1–2.2) | 0.020 | 4.2 (2.7–5.7) |
| 2 | 2.3 (1.4–3.9) | 0.002 | 3.2 (1.7–4.8) |
Ref, reference group.
aBased on the individual NPSs that were significantly associated with progression risk as identified in the current study. For AD, this is based on the presence of seven significant NPSs (namely, depression, anxiety, apathy, delusions, hallucinations, irritability and motor disturbance). For non-AD, this is based on the presence of two significant NPSs (namely, delusions and hallucinations).
bModel adjusted for baseline covariates of age, sex, ethnicity, years of education, APOE e4 genotype, MMSE score, CDR sum of boxes score, use of cognitive enhancers, use of antidepressants, use of antipsychotics and use of sedatives.
cThe estimated time that is needed for half of the participants to develop severe dementia. The 95% CI was computed with 1,000 bootstrap sampling.
dParticipants with 5–7 significant NPSs were combined into one category due to limited sample size in each group.
Figure 2 .
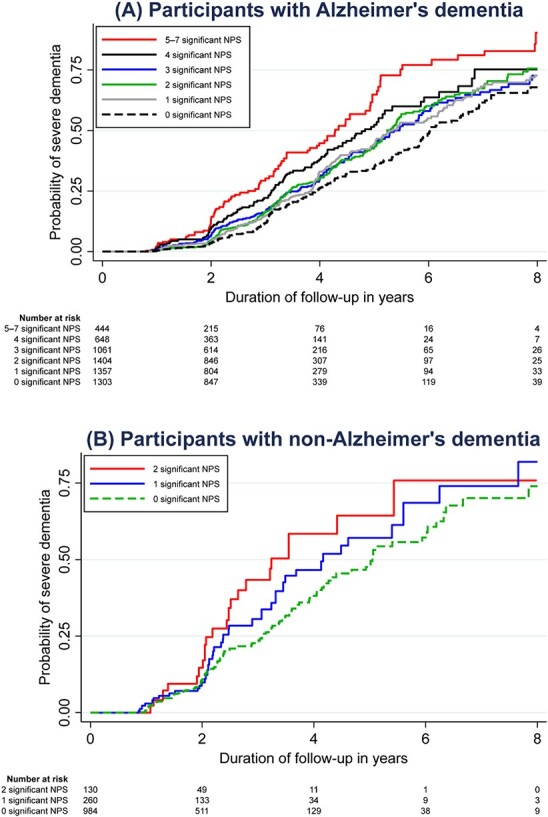
Kaplan–Meier curves on the risk of progression to severe dementia, focusing only on the individual NPSs that had been identified to be significant in the current study. For AD, this is based on the presence of seven significant NPSs (namely, depression, anxiety, apathy, delusions, hallucinations, irritability and motor disturbance). For non-AD, this is based on the presence of two significant NPSs (namely, delusions and hallucinations).
Discussion
NPSs in early dementia were associated with the risk of progression to severe dementia, with demonstratable dose–response relationships across the severity and the number of NPSs. However, progression risk was primarily present among participants with ad and not among those with non-ad. When comparing across various NPSs, seven NPSs in ad were associated with progression risk, and they were depression, anxiety, apathy, delusions, hallucinations, irritability and motor disturbance. In contrast, only two NPSs (delusions and hallucinations) were associated with progression risk in non-ad.
The findings are not inconsistent with those reported in extant literature. Several studies have demonstrated the association between NPSs in early dementia and progression risk [3–5]. However, these prior studies only focused on individuals with ad. Two prior studies also attempted to identify the specific NPSs that was associated with dementia progression [5,6]. However, both studies had relatively small sample (n = 97–335), which resulted in inconclusive findings—one study [5] showed significant association of agitation and psychosis (but not affective and apathy symptoms), while the other study [6] reported non-significant association across all NPSs. In contrast, the current study has a larger sample and possibly may afford a clearer answer on the association between NPSs and dementia progression, across ad and non-ad as well as across various NPSs.
Based on available literature, the association between NPSs and dementia progression has been explained by at least 2 postulations, both of which are summarised in Figure 3. NPSs may be the symptoms of a more aggressive disease [4,5], and hence, those with NPSs may have faster rates of dementia progression. This postulation has some support from recent evidence, where the presence of NPSs—even among those without dementia—predicted greater cognitive decline [33,34]. At the same time, the association between NPSs and dementia progression may also be mediated by less optimal dementia care (Figure 3). NPSs can cause great distress and burden to caregivers [1,2,35–38]. Inadvertently, this may lead to care environments that are less than optimal, as well as more conducive for dementia progression [5,39]. For example, in the face of NPSs and caregiver burden, caregivers may be less willing to engage persons with dementia in activities with social stimulation and may become less proactive in discussing the appropriate care options with healthcare providers [39]. In the presence of psychotic symptoms (i.e. hallucinations and delusions), clinicians may also be more inclined to prescribe antipsychotic medications [1,2], which have been shown to cause greater cognitive decline in recent literature (e.g. in the CATIE-ad trial) [40]. Further research is still needed to better understand the mechanisms by which NPSs in early dementia can be associated with disease progression, and how there can be differential risks across ad and non-ad. Such research may improve our understanding on the neurobiological underpinnings of NPSs as well as identify specific aspects of dementia care that may moderate NPSs and disease progression.
Figure 3 .
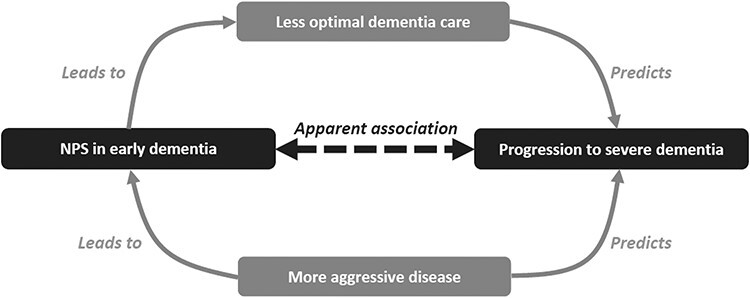
A directed acyclic graph to explain the apparent association between NPSs in early dementia and progression to severe dementia.
From the clinical perspective, the findings demonstrate the potential usefulness of NPSs as prognostic markers of dementia progression. Using the results in Table 2 by way of example, among patients with early ad, those who displayed 0 out of the 7 significant NPSs have ~6.0 years before they progress to severe dementia, while those with 5–7 NPSs have much shorter time to severe dementia (4.3 years). Such information can be useful in disease counselling and may facilitate discussion on advanced care planning. Given the prognostic utility of NPSs, their presence in early dementia should also prompt clinical review to incorporate evidence-based interventions that may address disease progression [1,2,4,35,40–48]. Plausibly, the clinical review may be guided by the postulated diagram in Figure 3—with a focus on addressing aggressive disease and optimising dementia care—and are further described in the following paragraph.
To address aggressive disease in the presence of NPSs, cognitive enhancers should be considered if they are indicated but have not been used. This is consistent with prior literature on the prominent treatment effects of cognitive enhancers among patients with ad with rapid cognitive decline [4]. In particular, patients’ cognitive function should be closely monitored for evidence of disease progression (e.g. steeper decline in MMSE or Montreal Cognitive Assessment scores) [15,49–51], with further consideration for high-dose cholinesterase inhibitors [41,42] or add-on memantine when indicated [43]. To optimise dementia care in the presence of NPSs, clinicians should review the use of psychiatric medications and consider the various non-pharmacological interventions. Psychiatric medications may sometimes be needed to manage more severe NPSs [1,2], but they should be used sparingly and deprescribed when no longer indicated, especially given that some psychiatric medications (e.g. antipsychotics and valproate) may lead to greater cognitive decline [40,44]. Non-pharmacological interventions that may be considered include caregiver training (to improve caregiving competency in managing NPSs) [1,2,35,45], case management (to identify care needs) [35,45] and tailored cognitive and physical activities, given prior meta-analytic evidence on the effectiveness of these interventions in improving cognition among patients with dementia [46–48].
Several limitations should be considered. First, participants in the study involved those who volunteered at Alzheimer’s Disease Centers. They may be more representative of patients who voluntarily present to healthcare settings than those in the community. Second, a small number of participants (n = 189) had the diagnosis of mixed Alzheimer’s/vascular dementia, of which 107 had primary aetiology of ad (with contributing cerebrovascular disease) and 82 had primary aetiology of vascular dementia (with contributing Alzheimer’s disease). Although these participants could still be classified by the primary aetiology of dementia (i.e. either Alzheimer’s or vascular dementia), the presence of mixed pathology may confound the findings across ad and non-ad. Third, global CDR score was used to define the primary endpoint of severe dementia. Arguably, global CDR score was primarily developed for the staging of ad [9]–with heavy weightage on the memory domain [9,32]–and hence may not be as accurate for staging of non-ad. This limitation was addressed in the sensitivity analysis, with results remaining robust even when the endpoint of severe dementia was redefined using an alternate method [11,32] that has less reliance on memory domain in dementia staging [32].
Conclusion
Many subtypes of NPSs in ad were associated with higher risks of disease progression. In contrast, only hallucinations and delusions were associated with disease progression in non-ad. The findings may be related to a more aggressive disease and less optimal dementia care among individuals with NPSs. NPSs—especially in ad—can be useful prognostic markers of disease progression and may inform advanced care planning. They should prompt clinical review to incorporate evidence-based interventions that may address disease progression, such as prescribing higher dose cognitive enhancers when indicated, deprescribing antipsychotics and valproate when not indicated, and optimising non-pharmacological interventions.
Supplementary Material
Acknowledgements
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Declaration of Sources of Funding
T.M.L. was supported by research grants under the Singapore Ministry of Health’s National Medical Research Council (Grant No.: NMRC/Fellowship/0030/2016 and NMRC/CSSSP/0014/2017). The funding sources had no involvement in any part of the project.
Declaration of Conflicts of Interest
None.
References
- 1.Kales HC, Lyketsos CG, Miller EM, Ballard C. Management of behavioral and psychological symptoms in people with Alzheimer's disease: an international Delphi consensus. Int Psychogeriatr 2019; 31: 83–90. [DOI] [PubMed] [Google Scholar]
- 2.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ 2015; 350: h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarmeas N, Brandt J, Blacker Det al. . Disruptive behavior as a predictor in Alzheimer disease. Arch Neurol 2007; 64: 1755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez OL, Schwam E, Cummings Jet al. . Predicting cognitive decline in Alzheimer's disease: an integrated analysis. Alzheimers Dement 2010; 6: 431–9. [DOI] [PubMed] [Google Scholar]
- 5.Peters ME, Schwartz S, Han Det al. . Neuropsychiatric symptoms as predictors of progression to severe Alzheimer's dementia and death: the Cache County dementia progression study. Am J Psychiatry 2015; 172: 460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro FA, Melgarejo JD, Lee JH, Maestre GE. Neuropsychiatric symptoms and their relationship with progression to severe dementia and death: findings of the Maracaibo aging study (MAS). Alzheimers Dement 2016; 12: P495–6. [Google Scholar]
- 7.Beekly DL, Ramos EM, Belle Get al. . The National Alzheimer's coordinating Center (NACC) database: an Alzheimer disease database. Alzheimer Dis Assoc Disord 2004; 18: 270–7. [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]
- 9.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412–4. [DOI] [PubMed] [Google Scholar]
- 10.Rikkert MG, Tona KD, Janssen Let al. . Validity, reliability, and feasibility of clinical staging scales in dementia: a systematic review. Am J Alzheimers Dis Other Demen 2011; 26: 357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Bryant SE, Lacritz LH, Hall Jet al. . Validation of the new interpretive guidelines for the clinical dementia rating scale sum of boxes score in the national Alzheimer's coordinating center database. Arch Neurol 2010; 67: 746–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka H, Nagata Y, Ishimaru D, Ogawa Y, Fukuhara K, Nishikawa T. Clinical utility of the cognitive test for severe dementia: factor analysis, minimal detectable change. and Longitudinal Changes Dement Geriatr Cogn Dis Extra 2018; 8: 214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang HC, Tseng YM, Chen YC, Chen PY, Chiu HY. Diagnostic accuracy of the clinical dementia rating scale for detecting mild cognitive impairment and dementia: a bivariate meta-analysis. Int J Geriatr Psychiatry 2021; 36:239–51. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub S, Besser L, Dodge HHet al. . Version 3 of the Alzheimer Disease Centers' neuropsychological test battery in the uniform data set (UDS). Alzheimer Dis Assoc Disord 2018; 32: 10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liew TM. Developing a brief neuropsychological battery for early diagnosis of cognitive impairment. J Am Med Dir Assoc 2019; 201054: e11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease. Report of the NINCDS-ADRDA work group* under the auspices of Department of Health and Human Services Task. Force on Alzheimer's Disease 1984; 34: 939. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington: American Psychiatric Association, 2000. [Google Scholar]
- 18.McKhann GM, Knopman DS, Chertkow Het al. . The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Román GC, Tatemichi TK, Erkinjuntti Tet al. . Vascular dementia. Diagnostic criteria for research studies: Report of the NINDS-AIREN International Workshop*, vol. 43, 1993; 250. [DOI] [PubMed] [Google Scholar]
- 20.Rascovsky K, Hodges JR, Knopman Det al. . Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134: 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, Leigh PN. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain 2009; 132: 156–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong MJ, Litvan I, Lang AEet al. . Criteria for the diagnosis of corticobasal degeneration. Neurology 2013; 80: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000; 1: 293–9. [DOI] [PubMed] [Google Scholar]
- 24.Neary D, Snowden JS, Gustafson Let al. . Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51: 1546–54. [DOI] [PubMed] [Google Scholar]
- 25.Litvan I, Agid Y, Calne Det al. . Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996; 47: 1–9. [DOI] [PubMed] [Google Scholar]
- 26.Litvan I, Bhatia KP, Burn DJet al. . Movement disorders society scientific issues committee report: SIC task force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 2003; 18: 467–86. [DOI] [PubMed] [Google Scholar]
- 27.McKeith IG, Boeve BF, Dickson DWet al. . Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology 2017; 89: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKeith IG, Dickson DW, Lowe Jet al. . Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 2005; 65: 1863–72. [DOI] [PubMed] [Google Scholar]
- 29.Liew TM. Depression, subjective cognitive decline, and the risk of neurocognitive disorders. Alzheimers Res Ther 2019; 11: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liew TM. Subjective cognitive decline, anxiety symptoms, and the risk of mild cognitive impairment and dementia. Alzheimers Res Ther 2020; 12: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liew TM. Trajectories of subjective cognitive decline, and the risk of mild cognitive impairment and dementia. Alzheimers Res Ther 2020; 12: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balsis S, Miller TM, Benge JF, Doody RS. Dementia staging across three different methods. Dement Geriatr Cogn Disord 2011; 31: 328–33. [DOI] [PubMed] [Google Scholar]
- 33.Liew TM. Symptom clusters of neuropsychiatric symptoms in mild cognitive impairment and their comparative risks of dementia: a cohort study of 8530 older persons. J Am Med Dir Assoc 2019; 201054: e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liew TM. Neuropsychiatric symptoms in cognitively normal older persons, and the association with Alzheimer's and non-Alzheimer's dementia. Alzheimers Res Ther 2020; 12: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ying J, Yap P, Gandhi M, Liew TM. Iterating a framework for the prevention of caregiver depression in dementia: a multi-method approach. Int Psychogeriatr 2018; 30: 1119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liew TM, Tai BC, Yap P, Koh GC. Contrasting the risk factors of grief and burden in caregivers of persons with dementia: multivariate analysis. Int J Geriatr Psychiatry 2019; 34: 258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying J, Yap P, Gandhi M, Validity LTM. Utility of the Center for Epidemiological Studies Depression Scale for detecting depression in family caregivers of persons with dementia. Dement Geriatr Cogn Disord 2019; 47: 323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liew TM, Yap P. A 3-item screening scale for caregiver burden in dementia caregiving: scale development and score mapping to the 22-item Zarit burden interview. J Am Med Dir Assoc 2019; 20: 629, e12–33. [DOI] [PubMed] [Google Scholar]
- 39.Norton MC, Clark C, Fauth EBet al. . Caregiver personality predicts rate of cognitive decline in a community sample of persons with Alzheimer's disease. The Cache County dementia progression study. Int Psychogeriatr 2013; 25: 1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vigen CL, Mack WJ, Keefe RSet al. . Cognitive effects of atypical antipsychotic medications in patients with Alzheimer's disease: outcomes from CATIE-AD. Am J Psychiatry 2011; 168: 831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cummings J, Froelich L, Black SEet al. . Randomized, double-blind, parallel-group, 48-week study for efficacy and safety of a higher-dose rivastigmine patch (15 vs. 10 cm(2)) in Alzheimer's disease. Dement Geriatr Cogn Disord 2012; 33: 341–53. [DOI] [PubMed] [Google Scholar]
- 42.Cummings JL, Geldmacher D, Farlow M, Sabbagh M, Christensen D, Betz P. High-dose donepezil (23 mg/day) for the treatment of moderate and severe Alzheimer's disease: drug profile and clinical guidelines. CNS Neurosci Ther 2013; 19: 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McShane R, Westby MJ, Roberts Eet al. . Memantine for dementia. Cochrane Database Syst Rev 2019; 3: Cd003154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleisher AS, Truran D, Mai JTet al. . Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology 2011; 77: 1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liew TM, Lee CS. Reappraising the efficacy and acceptability of multicomponent interventions for caregiver depression in dementia: the utility of network meta-analysis. Gerontologist 2019; 59: e380–92. [DOI] [PubMed] [Google Scholar]
- 46.Duan Y, Lu L, Chen Jet al. . Psychosocial interventions for Alzheimer's disease cognitive symptoms: a Bayesian network meta-analysis. BMC Geriatr 2018; 18: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia RX, Liang JH, Xu Y, Wang YQ. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr 2019; 19: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang JH, Xu Y, Lin L, Jia RX, Zhang HB, Hang L. Comparison of multiple interventions for older adults with Alzheimer disease or mild cognitive impairment: a PRISMA-compliant network meta-analysis. Medicine (Baltimore) 2018; 97: e10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liew TM, Feng L, Gao Q, Ng TP, Yap P. Diagnostic utility of Montreal cognitive assessment in the fifth edition of diagnostic and statistical manual of mental disorders: major and mild neurocognitive disorders. J Am Med Dir Assoc 2015; 16: 144–8. [DOI] [PubMed] [Google Scholar]
- 50.Liew TM. The optimal short version of Montreal cognitive assessment in diagnosing mild cognitive impairment and dementia. J Am Med Dir Assoc 2019; 20: e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liew TM. A 4-item case-finding tool to detect dementia in older persons. J Am Med Dir Assoc 2019; 20: 1529, e6–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


