Abstract
Growth factor-dependent kinases, such as phosphatidylinositol 3-kinase (PI 3-kinase) and Raf kinases, have been implicated in the suppression of apoptosis. We have recently established Rat-1 fibroblast cell lines overexpressing B-Raf, leading to activation of the MEK/Erk mitogen-activated protein kinase pathway. Overexpression of B-Raf confers resistance to apoptosis induced by growth factor withdrawal or PI 3-kinase inhibition. This is accompanied by constitutive activation of Erk without effects on the PI 3-kinase/Akt pathway. The activity of MEK is essential for cell survival mediated by B-Raf overexpression, since either treatment with the specific MEK inhibitor PD98059 or expression of a dominant inhibitory MEK mutant blocks the antiapoptotic activity of B-Raf. Activation of MEK is not only necessary but also sufficient for cell survival because overexpression of constitutively activated MEK, Ras, or Raf-1, like B-Raf, prevents apoptosis after growth factor deprivation. Overexpression of B-Raf did not interfere with the release of cytochrome c from mitochondria after growth factor deprivation. However, the addition of cytochrome c to cytosols of cells overexpressing B-Raf failed to induce caspase activation. It thus appears that the B-Raf/MEK/Erk pathway confers protection against apoptosis at the level of cytosolic caspase activation, downstream of the release of cytochrome c from mitochondria.
Many types of mammalian cells are dependent upon growth factors for survival. In a variety of cell types, growth factors prevent apoptosis by stimulation of phosphatidylinositol 3-kinase (PI 3-kinase) (52), which leads to activation of the protein kinase Akt (14, 25–26, 28, 44). Akt directly links growth factor signaling to the central pathways controlling programmed cell death by phosphorylating the Bcl-2 family member BAD (10–11). In its nonphosphorylated state, BAD translocates from the cytosol into mitochondria and promotes apoptotic cell death by inhibiting Bcl-2 or Bcl-xL through protein-protein interactions (51, 55). Phosphorylation by Akt results in the binding of BAD to cytosolic 14-3-3t, allowing Bcl-2 or Bcl-xL to function as inhibitors of apoptosis (10–11, 55). Phosphorylation of glycogen synthase kinase-3 (GSK-3) by Akt has also been reported to contribute to cell survival (36), but the downstream targets of GSK-3 that regulate apoptosis remain to be determined.
Signals that induce apoptosis culminate in the activation of caspases, which are the ultimate effectors of programmed cell death. Activation of the key apoptotic protease caspase-3 during cell death induced by a variety of different stimuli, including growth factor deprivation, is preceded by the release of cytochrome c from mitochondria to the cytosol (32). Cytochrome c then initiates the formation of a ternary complex consisting of cytochrome c, the apoptosis protease-activating factor Apaf-1, and another protease called caspase-9 (31). In this complex, caspase-9 becomes proteolytically activated and eventually activates caspase-3, the major effector protease in apoptosis (19). The release of cytochrome c from mitochondria, as well as activation of caspase-3, is blocked by Bcl-2 or Bcl-xL (3, 7, 16, 27, 50).
As with the PI 3-kinase/Akt pathway, members of the Raf family also function as elements of signaling pathways that are thought to be involved in the regulation of programmed cell death (37, 46, 48), and may provide an alternative growth factor-dependent mechanism of cell survival. In mammals, three members of the Raf family of protein-serine/threonine kinases have been identified (Raf-1, A-Raf, and B-Raf), whose activation is linked to receptor protein-tyrosine kinases by Ras (24). Activation of Raf then leads to activation of a kinase cascade consisting of MEK and Erk1/2. The Erk kinases phosphorylate a diverse group of substrate proteins, including the protein kinase Rsk (pp90 S6 kinase) and transcription factors (41).
Most studies suggest a functional redundancy among the Raf family, since all Raf kinases activate Erk through MEK (39). In Raf knockouts, however, only mice lacking B-Raf, and not mice lacking Raf-1 or A-Raf, showed disturbances in cell survival (40, 47), raising the possibility that B-Raf may possess specific functions in cell death regulation. More recently, a specific Rap-1-dependent activation of B-Raf has been described which is not dependent on Ras (54). In addition, Raf-1 and A-Raf require tyrosine phosphorylation for maximal activation, whereas the appropriate tyrosine residues are missing from B-Raf (18, 33). These data are consistent with the possibility that B-Raf is activated by specific upstream regulators, leading to a unique role for B-Raf in signaling cell survival.
Two alternative mechanisms have been proposed to account for the antiapoptotic activity of Raf-1. In some studies, activation of Erk was found to play a critical role in the prevention of cell death (48). In contrast, other experiments have shown that Raf-1 targeted to mitochondria by Bcl-2 leads to cell survival without Erk activation, probably by phosphorylating substrates other than MEK, such as Bcl-2 family members (46). However, the mechanism of action of B-Raf in preventing apoptosis has not been investigated.
In the present study, we have used Rat-1 fibroblast cell lines overexpressing B-Raf to investigate the role of B-Raf in cell survival. Overexpression of B-Raf conferred resistance to apoptosis induced by either serum deprivation or PI 3-kinase inhibition as a result of constitutive activation of the MEK/Erk signaling pathway. This antiapoptotic activity of B-Raf blocked caspase activation without interfering with the release of cytochrome c from mitochondria, indicating that B-Raf/MEK/Erk signaling can inhibit apoptosis at the level of caspase activation, downstream of the action of Bcl-2 family members in blocking mitochondrial cytochrome c release.
MATERIALS AND METHODS
Cells.
Rat-1 cells and transfected Rat-1 cells overexpressing B-Raf (Rat-1/B-Raf-1 and -2 cells) were as previously described (15). Rat-1/control cells were transfected with the B-Raf expression plasmid but after G418 selection failed to overexpress B-Raf. Rat-1 cells overexpressing Bcl-2 (Rat-1/Bcl-2 cells) (35) were a generous gift of J. Yuan. Cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% calf serum.
Assays for apoptosis.
Cells were seeded at 106 cells per 100-mm culture dish (for nuclear morphology assay) or at 2 × 106 cells per 100-mm culture dish (for all other apoptosis assays) in DMEM containing 10% calf serum. After 24 h of incubation, cells were deprived of serum and/or treated with 50 μM LY294002 (inhibitor of PI 3-kinase) or 50 μM PD98059 (inhibitor of MEK) for an additional 16 to 20 h to induce apoptosis. For flow cytometry, DNA fragmentation assay, and caspase-3 cleavage both the adherent and floating cells were then collected for analysis.
For nuclear morphology assay, cells were fixed with formaldehyde, permeabilized with 0.5% Triton X-100, and stained with the DNA dye bisbenzimide (Hoechst 33258), and the number of cells with fragmented nuclei was then scored.
For flow cytometry, cells were fixed in methanol for at least 1 h at −20°C, rehydrated in phosphate-buffered saline for at least an additional hour at 4°C, and then treated with RNase A (50 μg/ml) for 30 min. Propidium iodide (25 μg/ml) was then added to the cells, and samples were analyzed in a flow cytometer (FACScalibur; Becton Dickinson) by recording the propidium iodide staining in the red channel. The percentage of apoptotic cells was determined by calculating the fraction of cells with sub-G1 DNA content.
For DNA fragmentation assays, soluble cytoplasmic DNA was extracted and analyzed by electrophoresis in 1.8% agarose gels containing ethidium bromide as described previously (16).
For assays of caspase-3 cleavage, cytoplasmic lysates were electrophoresed in sodium dodecyl sulfate (SDS)–12% polyacrylamide gels and analyzed by immunoblotting with an anti-caspase-3 antibody (Santa Cruz Biotechnology) as described earlier (16).
Transient transfection and fluorescence microscopy.
Transient transfections were carried out as described previously (36) with minor modifications. Briefly, 3 × 105 Rat-1 or Rat-1/B-Raf cells were plated on poly-l-lysine-coated coverslips 24 h before transfection. Transient transfections were then performed by the Lipofectamine method as suggested by the manufacturer (Life Technologies, Inc.). First, 5 μl of Lipofectamine plus 1 μg of expression vectors (constitutively activated or dominant-negative MEK [9]; v-raf or v-ras [20]) and 0.1 μg of a green fluorescent protein (GFP) expression construct (pEGFP-C1) (Clontech) were incubated with the cells for 3 h. The medium was then replaced with DMEM supplemented with 10% calf serum for 16 h. The cells were then deprived of growth factors by incubation in serum-free medium for an additional 24 h. Cells were then fixed with formaldehyde, permeabilized with 0.5% Triton X-100, and stained with the DNA dye bisbenzimide (Hoechst 33258). Transfected cells were identified by GFP fluorescence and scored for apoptosis by nuclear morphology.
Activation of Erk and Akt.
Cell lysates were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting as previously described (15). The membranes were incubated with one of the following primary antibodies: anti-phospho-Erk (New England Biolabs), anti-Akt (New England Biolabs), anti-phospho-Akt (New England Biolabs), or anti-Erk-1 (Santa Cruz Biotechnology). After incubation with horseradish peroxidase-conjugated secondary antibodies, the proteins were detected by using the ECL system (Amersham Corp.).
Detection of cytosolic cytochrome c.
Isolation of cytosolic fraction for detecting cytochrome c was performed as described previously (6). Briefly, cells were collected and resuspended in a buffer containing 20 mM HEPES (pH 7.5); 1.5 mM MgCl2; 10 mM KCl; 1 mM EDTA; 1 mM EGTA; 1 mM dithiothreitol; 0.1 mM phenylmethyl sulfonyl fluoride; 10 μg of leupeptin, aprotinin, and pepstatin A per ml; and 250 mM sucrose. The cells were homogenized with a Dounce homogenizer and centrifuged at 12,000 × g for 5 min at 4°C. Aliquots of the resulting supernatant containing 10 μg of protein were used as the soluble cytosolic fraction for immunoblot analysis with anti-cytochrome c (Pharmingen) and anti-cytochrome oxidase subunit II (Molecular Probes) antibodies. Approximately equal amounts of the pellet (mitochondrial fraction) were analyzed in parallel as a positive control for detection of mitochondrial proteins.
In vitro assay of PARP cleavage in nuclei.
In vitro assays of poly(ADP-ribose) polymerase (PARP) cleavage in nuclei and preparation of cytosols and nuclei for these assays were performed essentially as described earlier (17). Briefly, 5 μg of cytosolic extracts from untreated cells was combined with intact HeLa cell nuclei in the presence of 1 mM ATP and an ATP regenerating system consisting of 1 mM creatine phosphate and 1 μM creatine phosphokinase. Activation of caspase-3 in control cytosols was induced by the addition of 0.1 μM horse heart cytochrome c (Sigma). Reactions were incubated for 1 h at 37°C, and the cleavage of PARP derived from intact HeLa cell nuclei was analyzed on immunoblots with an anti-PARP monoclonal antibody (obtained from G. G. Poirier, Laval University, Ste-Foy, Quebec, Canada). In some experiments, rather than inducing caspase-3 activation by cytochrome c, active His6-tagged human caspase-3 purified from an Escherichia coli expression system (17) was added to the reaction mixtures.
RESULTS
B-Raf possesses antiapoptotic activity.
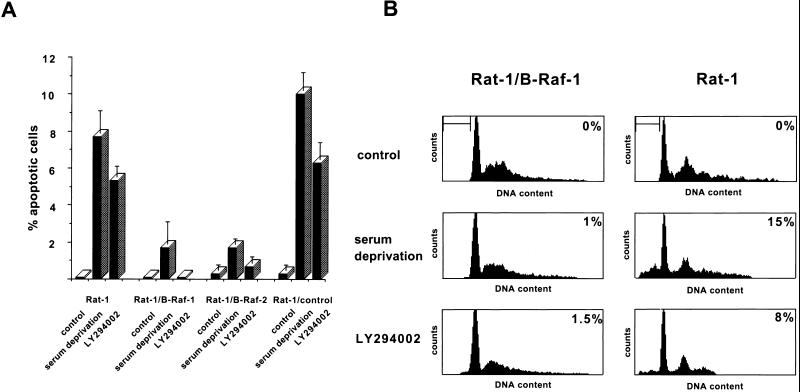
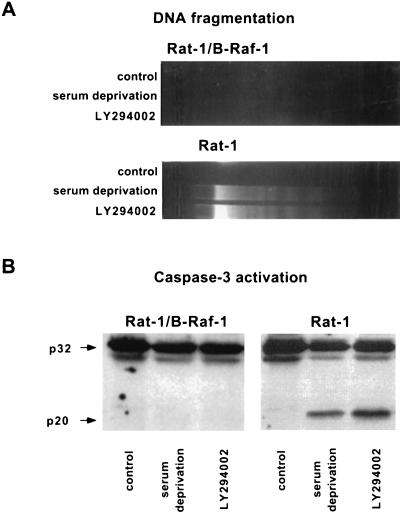
To test whether B-Raf promotes cell survival, Rat-1 fibroblast cell lines overexpressing a wild-type 90-kDa isoform of human B-Raf (Rat-1/B-Raf cells) (15) were deprived of growth factors, and apoptosis was quantified by determining the fraction of cells with fragmented nuclei by Hoechst stain fluorescence. After 16 h of growth factor deprivation, two independent clones overexpressing B-Raf were resistant to cell death, whereas approximately 10% of wild-type Rat-1 cells underwent apoptosis (Fig. 1A). Like wild-type Rat-1 cells, a control line of transfected Rat-1 cells without detectable B-Raf overexpression (Rat-1/control) remained sensitive to growth factor deprivation-induced apoptosis. We have confirmed with additional apoptosis assays that overexpression of B-Raf blocks other characteristic events of programmed cell death, including increase in sub-G1 DNA content with flow cytometry, oligonucleosomal fragmentation of DNA, and caspase-3 activation (Fig. 1B and 2).
FIG. 1.
Induction of apoptosis by growth factor deprivation and PI 3-kinase inhibition. (A) Wild-type Rat-1 cells (Rat-1), transfected Rat-1 cells overexpressing B-Raf (Rat-1/B-Raf-1 and -2), and transfected Rat-1 cells that fail to overexpress B-Raf (Rat-1/control) were maintained in normal growth medium with or without the addition of 50 μM LY294002 or were incubated in serum-free medium for 16 h. Cells were then fixed, permeabilized, and stained with the DNA dye bisbenzimide (Hoechst 33258), and the apoptotic nuclei were scored on the basis of nuclear morphology. Data were averaged from three experiments, and at least 300 cells were counted per experiment. The error bars are standard errors of the mean. (B) Rat-1/B-Raf-1 and Rat-1 cells were maintained and treated as described in the legend to Fig. 1A. They were then collected and stained with propidium iodide, and the DNA content was analyzed by flow cytometry. The bar indicates cells with sub-G1 DNA content, a characteristic of apoptosis. The percentage of cells with sub-G1 DNA content is indicated in the upper right corner of each panel. The results are representative of at least two similar experiments.
FIG. 2.
Induction of oligonucleosomal DNA fragmentation and processing of caspase-3 by growth factor deprivation and PI 3-kinase inhibition. Rat-1/B-Raf-1 and Rat-1 cells were maintained and treated as described in the legend to Fig. 1. (A) Oligonucleosomal fragmentation of DNA. (B) Immunoblot analysis of caspase-3. The positions of the 32-kDa proenzyme and the 20-kDa active subunit are indicated by arrows.
Since the PI 3-kinase/Akt pathway is thought to be the major survival pathway activated by serum growth factors (52–53), we also treated Rat-1/B-Raf and Rat-1 cells with the PI 3-kinase inhibitor LY294002 in the presence of serum rather than inducing apoptosis by serum deprivation. Consistent with the results obtained in growth factor-deprived cells, overexpressed B-Raf blocked cell death induced by PI 3-kinase inhibitors (Fig. 1 and 2). These data indicate that cells overexpressing B-Raf lose growth factor dependence for survival and that the antiapoptotic activity of B-Raf is not mediated by PI 3-kinase.
Activation of MEK is essential for cell survival conferred by B-Raf.
Our previous studies have demonstrated that in Rat-1/B-Raf cell lines B-Raf is a catalytically active kinase that activates MEK and Erk (15). We therefore sought to determine whether MEK is a component of the B-Raf-initiated survival pathway. To this end, Rat-1 and Rat-1/B-Raf cells were treated with the specific MEK inhibitor PD98059, either alone or in combination with inducers of apoptosis. Apoptosis was quantified by flow cytometry and by analysis of the proteolytic processing of caspase-3 on immunoblots.
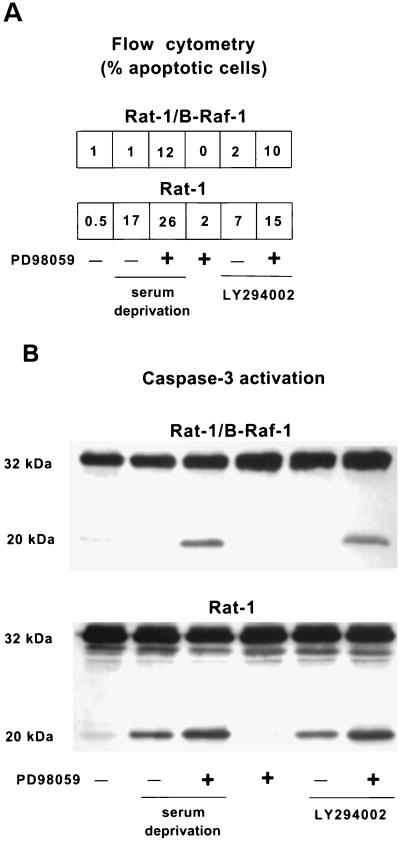
Inhibition of MEK in the presence of serum did not result in significant cell death in either Rat-1 or Rat-1/B-Raf cells (Fig. 3), suggesting that MEK is not required for survival in the presence of serum growth factors. In contrast, serum-starved Rat-1/B-Raf cells underwent apoptosis if MEK was inhibited by treatment with PD98059, although neither serum starvation nor MEK inhibition alone induced apoptosis in these cells (Fig. 3). Similar results were obtained with the nuclear morphology assay by scoring the fragmented apoptotic nuclei in two independent clones overexpressing B-Raf (data not shown). Results obtained with PD98059 were also confirmed with 10 μM UO126 (Promega), a structurally different MEK inhibitor (not shown). It thus appears that MEK is required for cell survival mediated by overexpression of B-Raf but not for cell survival in the presence of growth factors.
FIG. 3.
Induction of apoptosis by inhibiting MEK with PD98059. Cells were maintained in normal growth medium or in serum-free medium and treated with 50 μM LY294002 or 50 μM PD98059, as indicated, for 16 h. (A) Fraction of apoptotic cells with sub-G1 DNA content as determined by flow cytometry. (B) Immunoblot analysis of caspase-3. The positions of the 32-kDa proenzyme and the 20-kDa active subunits are indicated. The results are representative of at least two similar experiments.
In contrast to Rat-1/B-Raf cells, growth factor deprivation alone effectively induced apoptosis of normal Rat-1 cells. This effect was enhanced by treatment with PD98059, leading to a further increase in both the number of apoptotic cells with sub-G1 DNA content and the formation of active caspase-3 subunits (Fig. 3). This additional increase in cell death may reflect a residual MEK activity persisting in serum-starved cells which is eliminated by the MEK inhibitor treatment.
Similar results were obtained in both Rat-1 and Rat-1/B-Raf cells when apoptosis was induced by treatment with the PI 3-kinase inhibitor LY294002 in the presence of serum instead of by serum deprivation (Fig. 3). This is consistent with the view that serum growth factors promote survival primarily by activating PI 3-kinase (52–53) but that activation of MEK can provide an alternative cell survival pathway.
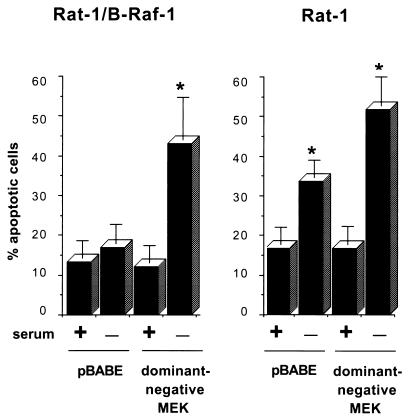
To further confirm that survival signaling by B-Raf is mediated through MEK, Rat-1 and Rat-1/B-Raf cells were transiently transfected with a catalytically inactive MEK mutant, which acts as a dominant inhibitor of MEK activity, and a construct expressing GFP. Transfected cells were identified by fluorescence microscopy to detect GFP expression, and apoptotic cells were scored by nuclear morphology after being stained with Hoechst dye. In control cells transfected with an empty vector, ca. 15% of the nuclei showed apoptotic morphology, a result apparently due to the Lipofectamine treatment (Fig. 4). In Rat-1/B-Raf cells, expression of B-Raf prevented cell death induced by growth factor deprivation, but B-Raf was unable to protect cells from apoptosis when growth factor deprivation was accompanied by expression of the dominant inhibitory MEK (Fig. 4). In contrast, in wild-type Rat-1 cells the background level of apoptosis was increased up to 40% upon serum deprivation, and approximately 20% more cells underwent apoptosis when the cells were also transfected with dominant inhibitory MEK (Fig. 4).
FIG. 4.
Induction of apoptosis by ectopic expression of dominant-negative MEK. Cells were cotransfected with an expression vector for dominant-negative MEK or an empty vector control (pBABE), together with an expression construct for GFP. Cells were maintained in normal growth medium or in serum-free medium for an additional 24 h. Transfected cells were then identified by fluorescence microscopy and scored for apoptosis on the basis of nuclear morphology. Data are presented as the percentage of GFP-positive cells with apoptotic nuclei. Data were averaged from three experiments, and 100 cells transfected with each vector were counted per experiment. The error bars are standard errors of the mean. Asterisks indicate a significant difference (P < 0.05) from controls maintained in serum (analysis of variance test).
Constitutively expressed active MEK is sufficient to inhibit cell death.
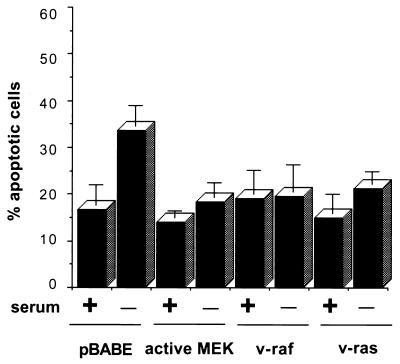
Since MEK was required for the antiapoptotic activity of B-Raf, we next examined whether activation of MEK alone was sufficient for cell survival. Rat-1 cells were transfected with a constitutively active MEK mutant by using the GFP cotransfection assay to identify transfected cells. Expression of activated MEK completely blocked apoptosis induced by serum withdrawal (Fig. 5). In addition, the expression of active Ras and Raf-1, which also activate MEK, similarly inhibited cell death induced by growth factor deprivation (Fig. 5). These results are consistent with data obtained in other cell types (2, 8, 22, 30, 34, 48) and indicate that MEK activation is sufficient to prevent apoptosis resulting from growth factor deprivation.
FIG. 5.
Inhibition of apoptosis by expression of activated MEK, Raf, or Ras in Rat-1 cells. Rat-1 cells were cotransfected with the indicated expression vectors and a GFP expression construct. Transfected cells were maintained in normal growth medium or in serum-free medium for an additional 24 h. Data are averaged from three experiments, as in Fig. 4. The extent of apoptosis in pBABE-transfected cells deprived of serum was significantly different (P < 0.05) from controls maintained in serum, whereas apoptosis in serum-deprived cells transfected with MEK, Raf, or Ras expression constructs did not differ significantly from controls.
Overexpression of B-Raf results in increased Erk activity without effects on the PI 3-kinase pathway.
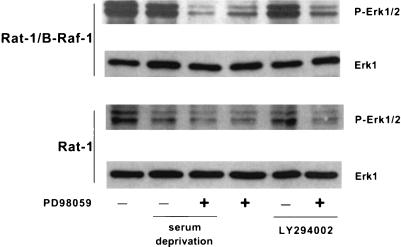
Since the antiapoptotic activity of B-Raf was mediated by MEK, we investigated the activity of the MEK target Erk in Rat-1 and Rat-1/B-Raf cells in the presence or absence of serum. Activating phosphorylations of Erk (38) were assayed by immunoblotting with an anti-phospho-Erk(202-Thr/204-Tyr) antibody. Overexpression of B-Raf in Rat-1/B-Raf cells resulted in a constitutively high Erk activity, even if the cells were deprived of growth factors or treated with PI 3-kinase inhibitors (Fig. 6). In normal Rat-1 cells, however, Erk was weakly phosphorylated in the presence of serum; this was further inhibited by growth factor withdrawal but not by PI-3 kinase inhibitors (Fig. 6). As expected, activation of Erk was effectively blocked by treatment with the MEK inhibitor PD98059 in both Rat-1 and Rat-1/B-Raf cells (Fig. 6). It thus appears that overexpression of B-Raf in Rat-1/B-Raf cells results in an elevated level of Erk activity which persists after growth factor deprivation or inhibition of PI 3-kinase and may therefore be responsible for the antiapoptotic effect of B-Raf.
FIG. 6.
Overexpression of B-Raf results in increased Erk activity. Cells were maintained in normal growth medium or in serum-free medium and were treated with either 50 μM LY294002 or 50 μM PD98059, as indicated, for 16 h. Collected cell lysates were analyzed by immunoblotting with antibodies to phospho-Erk1/2 or Erk1.
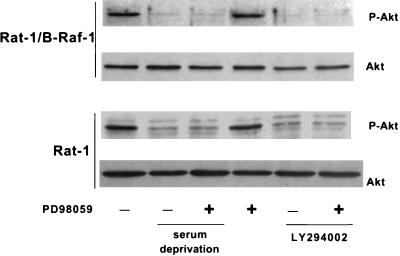
To test whether PI 3-kinase is activated by B-Raf, we measured the phosphorylation of the PI 3-kinase target Akt on Ser-473, which is thought to reflect Akt activation (1). In the absence of growth factors or in cells treated with LY294002, this phosphorylation of Akt was strongly diminished in both Rat-1 and Rat-1/B-Raf cells (Fig. 7). In contrast, inhibition of MEK did not affect Akt phosphorylation in either cell type (Fig. 7), indicating that the B-Raf/MEK pathway does not have a direct effect on PI 3-kinase/Akt signaling.
FIG. 7.
Overexpression of B-Raf does not affect activation of Akt. Cells were maintained in normal growth medium or in serum-free medium and were treated with either 50 μM LY294002 or 50 μM PD98059, as indicated, for 16 h. Collected cell lysates were analyzed by immunoblotting with antibodies to phospho-Akt or Akt.
The B-Raf/MEK/Erk pathway inhibits apoptosis downstream of cytochrome c release from mitochondria.
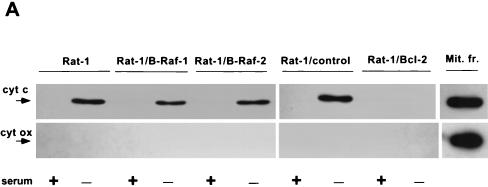
To position the interaction between the B-Raf survival pathway and the central apoptotic machinery, we first sought to determine whether the release of cytochrome c from the mitochondria was inhibited by B-Raf overexpression in Rat-1/B-Raf cells. Cytosols prepared from control and growth factor-deprived Rat-1 and Rat-1/B-Raf cells were analyzed by immunoblotting to detect cytochrome c release. In parallel blots, cytochrome oxidase served as a marker of mitochondrial contamination of the extracts. Growth factor deprivation led to a similar increase in cytosolic cytochrome c, without detectable release of cytochrome oxidase, both in two independent Rat-1 clones overexpressing B-Raf and in normal Rat-1 cells, as well as in transfected Rat-1 cells without B-Raf overexpression (Fig. 8A). In contrast, overexpression of Bcl-2, as shown earlier (27, 50), prevented the release of cytochrome c from the mitochondria. Therefore, it appeared that overexpression of B-Raf inhibited apoptosis at a later step than did Bcl-2, downstream of mitochondrial cytochrome c release.
FIG. 8.
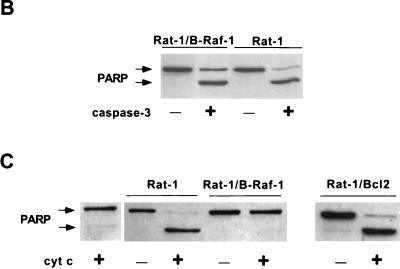
Effect of B-Raf overexpression on cytochrome c-dependent caspase activation. (A) Wild-type Rat-1 cells (Rat-1), transfected Rat-1 cells overexpressing B-Raf (Rat-1/B-Raf-1 and -2) or Bcl-2 (Rat-1/Bcl-2), and transfected Rat-1 cells that fail to overexpress B-Raf (Rat-1/control) were maintained in the presence of serum or deprived of growth factors for 24 h. Equal amounts of cytosolic proteins were then immunoblotted for cytochrome c (cyt c) and cytochrome oxidase subunit II (cyt ox), along with similar amounts of mitochondrial fraction (Mit. fr.) of Rat-1 cells maintained in serum as a positive control. The positions of cytochrome c and cytochrome oxidase are indicated by arrows. (B) Cytosols from Rat-1 and Rat-1/B-Raf cells were combined with purified, active His6-tagged human caspase-3 and intact HeLa cell nuclei and then incubated for 1 h at 37°C. The reaction mixture was used for immunoblot analysis to measure the cleavage of the caspase-3 substrate PARP. Intact PARP (116 kDa) and the cleaved fragment (85 kDa) are indicated by arrows. (C) Activation of caspase-3 was induced by the addition of cytochrome c to cytosols from nonapoptotic Rat-1, Rat-1/B-Raf, and Rat-1/Bcl-2 cells maintained in the presence of serum. Reaction mixtures were combined with intact HeLa cell nuclei, and the cleavage of PARP was assayed by immunoblot analysis. Lane 1 is a control in which cytochrome c was added to nuclei without cytosolic extract. The results are representative of at least three similar experiments.
To further test this possibility, we used in vitro assays of nuclear PARP cleavage to determine whether cytosols of cells overexpressing B-Raf contained inhibitors of caspase activation. HeLa cell nuclei were added to cytosols of Rat-1 or Rat-1/B-Raf cells, and the activity of caspases was assayed by analyzing the appearance of a PARP cleavage fragment by immunoblotting. First, we determined the effect of Rat-1/B-Raf and Rat-1 cytosols on the activity of already activated caspase-3 purified from an E. coli expression system. Active caspase-3 added to cytosols prepared from either Rat-1/B-Raf or Rat-1 cells induced a similar level of nuclear PARP cleavage (Fig. 8B), indicating that cytosols of Rat-1/B-Raf cells did not contain inhibitors that were effective against the activated caspase.
We next tested the possibility that cytosols of Rat-1/B-Raf cells contained inhibitors of caspase activation mediated by cytochrome c released from mitochondria. Cytochrome c was added to cytosols of either Rat-1/B-Raf cells or normal Rat-1 cells, and activation of caspase-3 was monitored by PARP cleavage in HeLa cell nuclei. The addition of cytochrome c effectively induced caspase-3 activation and PARP cleavage in cytosols of Rat-1 cells but not in cytosols of Rat-1/B-Raf cells (Fig. 8C). This inhibition of caspase activation in cytosols of Rat-1/B-Raf cells contrasted with Rat-1 cells protected from apoptosis by overexpression of Bcl-2. Consistent with the action of Bcl-2 in preventing apoptosis by blocking the release of cytochrome c from mitochondria, the addition of cytochrome c to cytosols of Rat-1/Bcl-2 cells resulted in effective caspase-3 activation (Fig. 8C). It thus appeared that cytochrome c-mediated caspase activation was blocked in cytosols of Rat1/B-Raf cells, indicating that the B-Raf signaling pathway confers protection against apoptosis at the level of caspase activation, downstream of cytochrome c release from mitochondria.
DISCUSSION
Growth factor-dependent kinases prevent programmed cell death by blocking activation of the apoptotic machinery. Thus, upon overexpression of constitutively active growth factor dependent kinases, such as PI 3-kinase/Akt and Raf-1, programmed cell death is suppressed in a variety of different cell systems (8, 14, 25–26, 28, 44, 46). In the present study, we have demonstrated that overexpression of B-Raf, another member of the Raf kinase family, is also a potent inhibitor of apoptosis. This protection by B-Raf was mediated through MEK and did not involve activation of the PI 3-kinase/Akt pathway.
Consistent with recent results obtained in several different cell types, such as hematopoietic cells, PC12 rat pheochromocytoma cells, and fibroblasts (5, 29, 42–43, 49, 52–53), our data with inhibitors of MEK and PI 3-kinase indicate distinct roles for B-Raf/MEK and PI 3-kinase/Akt signaling in cell survival. Growth factors maintain a significant steady state level of PI 3-kinase activity, providing protection against cell death, whereas the basal activity of MEK is not sufficient to prevent apoptosis. In contrast, a constitutively high MEK activity induced by overexpression of B-Raf leads to survival even in the absence of PI 3-kinase activity. This implies that overexpression of any oncogene leading to MEK activation will result in protection from apoptosis, including all three Raf kinases, Ras, and Rap-1. In agreement with this, we have demonstrated that overexpression of activated Raf-1 and Ras, as well as activated MEK, blocks apoptosis resulting from growth factor deprivation. Similarly, the activated forms of Ras, Raf-1, and MEK have been reported to prevent cell death in a variety of different cell types, including PC12 cells, hematopoietic cells, T cells, and fibroblasts (2, 8, 21–22, 30, 34, 48). Activation of MEK by B-Raf results in constitutive Erk activity, and the role of this pathway in cell survival is further supported by results obtained from PC12 and HeLa cells, where Erk activity appeared to be necessary for survival (5, 21, 37, 48).
Growth factor deprivation induces a mitochondrion-dependent apoptosis pathway, ultimately leading to cytochrome c release from mitochondria and cytochrome c-dependent activation of caspases (23). The primary site of action of Bcl-2 and Bcl-xL appears to be in the mitochondria because their overexpression inhibits the release of cytochrome c (23, 27, 50) but cannot block caspase activation by microinjected cytochrome c (13). Since the PI 3-kinase/Akt pathway acts at least in part by phosphorylating BAD and allowing Bcl-2 and Bcl-xL to function as inhibitors of apoptosis (10–11), PI 3-kinase/Akt signaling is also expected to inhibit mitochondrial cytochrome c release.
In contrast, our data indicate that the B-Raf/MEK/Erk pathway interferes with apoptosis at the level of cytosolic caspase activation, downstream of the release of cytochrome c from mitochondria. First, cytochrome c accumulated in the cytosol of growth factor-deprived Rat-1/B-Raf cells without significant activation of caspase-3 and cell death. In addition, cytochrome c failed to induce caspase activation in cytosols prepared from Rat-1/B-Raf cells, suggesting that cytosols from cells overexpressing B-Raf contained an inhibitor of caspase activation. This action of B-Raf is also distinct from prevention of apoptosis by Raf-1 targeted to mitochondria, which was reported to inhibit apoptosis through Bcl-2 family members without the involvement of Erk (46).
Such postmitochondrial regulation of apoptosis at the level of caspase activation has already been described with inhibitors of caspases, including the human IAP-like proteins (12), raising the possibility that B-Raf/MEK/Erk signaling may affect the expression or activity of such caspase inhibitors. Moreover, the Drosophila gene hid, which interacts with IAPs (45) and controls caspase activation, has recently been identified as a direct target of Ras-dependent survival signaling mediated by Erk (4). Although there are no mammalian equivalents of Drosophila hid known to date, the existence of a functional homolog would provide a potential mechanism by which the B-Raf/MEK/Erk pathway promotes survival in mammalian cells.
ACKNOWLEDGMENTS
This research was supported by Massachusetts Breast Cancer Research grant 34088PP1010 (to P.E.), American Cancer Society grant IRG-72-001-24-IRG (to P.E.), and NIH grant RO1 CA18689 (to G.M.C.).
REFERENCES
- 1.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Arends M J, McGregor A H, Toft N J, Brown E J, Wyllie A H. Susceptibility to apoptosis is differentially regulated by c-myc and mutated Ha-ras oncogenes and is associated with endonuclease availability. Br J Cancer. 1993;68:1127–1133. doi: 10.1038/bjc.1993.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong R C, Aja T, Xiang J, Gaur S, Krebs J F, Hoang K, Bai X, Korsmeyer S J, Karanewsky D S, Fritz L F, Tomaselli K J. Fas-induced activation of the cell death-related protease CPP32 is inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem. 1996;271:16850–16855. doi: 10.1074/jbc.271.28.16850. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann A, Agapite J, McCall K, Steller H. The Drosophila gene hid is a direct molecular target of ras-dependent survival signaling. Cell. 1998;95:331–341. doi: 10.1016/s0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- 5.Berra E, Diaz-Meco M T, Moscat J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem. 1998;273:10792–10797. doi: 10.1074/jbc.273.17.10792. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan D, Pandey P, Ogata A, Teoh G, Krett N, Halgren R, Rosen S, Kufe D, Kharbanda S, Anderson K. Cytochrome c-dependent and -independent induction of apoptosis in multiple myeloma cells. J Biol Chem. 1997;272:29995–29997. doi: 10.1074/jbc.272.48.29995. [DOI] [PubMed] [Google Scholar]
- 7.Chinnaiyan A M, Orth K, O’Rourke K, Duan H, Poirier G G, Dixit V M. Molecular ordering of the cell death pathway. Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 8.Cleveland J L, Troppmair J, Packham G, Askew D S, Lloyd P, Gonzalez-Garcia M, Nunez G, Ihle J N, Rapp U R. v-raf suppresses apoptosis and promotes growth of interleukin-3-dependent myeloid cells. Oncogene. 1994;9:2217–2226. [PubMed] [Google Scholar]
- 9.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 10.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 11.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 12.Deveraux Q L, Roy N, Stennicke H R, Van Arsdale T, Zhou Q, Srinivasula S M, Alnemri E S, Salvesen G S, Reed J C. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duckett C S, Li F, Wang Y, Tomaselli K J, Thompson C B, Armstrong R C. Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome c. Mol Cell Biol. 1998;18:608–615. doi: 10.1128/mcb.18.1.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R S, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 15.Erhardt P, Troppmair J, Rapp U R, Cooper G M. Differential regulation of Raf-1 and B-Raf and Ras-dependent activation of mitogen-activated protein kinase by cyclic AMP in PC12 cells. Mol Cell Biol. 1995;15:5524–5530. doi: 10.1128/mcb.15.10.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erhardt P, Cooper G M. Activation of the CPP32 apoptotic protease by distinct signaling pathways with differential sensitivity to Bcl-xL. J Biol Chem. 1996;271:17601–17604. doi: 10.1074/jbc.271.30.17601. [DOI] [PubMed] [Google Scholar]
- 17.Erhardt P, Tomaselli K J, Cooper G M. Identification of the MDM2 oncoprotein as a substrate for CPP32-like proteases. J Biol Chem. 1997;272:15049–15052. doi: 10.1074/jbc.272.24.15049. [DOI] [PubMed] [Google Scholar]
- 18.Fabian J R, Daar I O, Morrison D K. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol Cell Biol. 1993;13:7170–7179. doi: 10.1128/mcb.13.11.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faleiro L, Kobayashi R, Fearnhead H, Lazebnik Y. Multiple species of CPP32 and Mch2 are the major active caspases present in apoptotic cells. EMBO J. 1997;16:2271–2281. doi: 10.1093/emboj/16.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feig L A, Cooper G M. Inhibition of NIH 3T3 cell proliferation by a mutant Ras protein with preferential affinity for GDP. Mol Cell Biol. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardner A M, Johnson G L. Fibroblast growth factor-2 suppression of tumor necrosis factor alpha-mediated apoptosis requires Ras and the activation of mitogen-activated protein kinase. J Biol Chem. 1996;271:14560–14566. doi: 10.1074/jbc.271.24.14560. [DOI] [PubMed] [Google Scholar]
- 22.Gomez J, Martinez A C, Fernandez B, Garcia A, Rebollo A. Critical role of Ras in the proliferation and prevention of apoptosis mediated by IL-2. J Immunol. 1996;157:2272–2281. [PubMed] [Google Scholar]
- 23.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 24.Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 25.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature (London) 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 27.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 28.Kulik G, Klippel A, Weber M J. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kummer J L, Rao P K, Heidenreich K A. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]
- 30.Lebowitz P F, Sakamuro D, Prendergast G C. Farnesyl transferase inhibitors induce apoptosis of Ras-transformed cells denied substratum attachment. Cancer Res. 1997;57:708–713. [PubMed] [Google Scholar]
- 31.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 33.Marais R, Light Y, Paterson H F, Mason C S, Marshall C J. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272:4378–4383. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- 34.McKenna W G, Bernhard E J, Markiewicz D A, Rudoltz M S, Maity A, Muschel R J. Regulation of radiation-induced apoptosis in oncogene-transfected fibroblasts: influence of H-ras on the G2 delay. Oncogene. 1996;12:237–245. [PubMed] [Google Scholar]
- 35.Miura M, Zhu H, Rotello R, Hartwieg E A, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 36.Pap M, Cooper G M. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 37.Parrizas M, Saltiel A R, LeRoith D. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J Biol Chem. 1997;272:154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- 38.Payne D M, Rossomando A J, Martino P, Erickson A K, Her J H, Shabanowitz J, Hunt D F, Weber M J, Sturgill T W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase) EMBO J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pritchard C A, Samuels M L, Bosch E, McMahon M. Conditionally oncogenic forms of the A-Raf and B-Raf protein kinases display different biological and biochemical properties in NIH 3T3 cells. Mol Cell Biol. 1995;15:6430–6442. doi: 10.1128/mcb.15.11.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pritchard C A, Bolin L, Slattery R, Murray R, McMahon M. Post-natal lethality and neurological and gastrointestinal defects in mice with targeted disruption of the A-Raf protein kinase gene. Curr Biol. 1996;6:614–617. doi: 10.1016/s0960-9822(02)00548-1. [DOI] [PubMed] [Google Scholar]
- 41.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 42.Roulston A, Reinhard C, Amiri P, Williams L T. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor alpha. J Biol Chem. 1998;273:10232–10239. doi: 10.1074/jbc.273.17.10232. [DOI] [PubMed] [Google Scholar]
- 43.Scheid M P, Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/Akt: involvement of MEK upstream of Bad phosphorylation. Proc Natl Acad Sci USA. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Songyang Z, Baltimore D, Cantley L C, Kaplan D R, Franke T F. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vucic D, Kaiser W J, Miller L K. Inhibitor of apoptosis proteins physically interact with and block apoptosis induced by Drosophila proteins HID and GRIM. Mol Cell Biol. 1998;18:3300–3309. doi: 10.1128/mcb.18.6.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H G, Rapp U R, Reed J C. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 47.Wojnowski L, Zimmer A M, Beck T W, Hahn H, Bernal R, Rapp U R, Zimmer A. Endothelial apoptosis in B-Raf deficient mice. Nat Genet. 1997;16:293–297. doi: 10.1038/ng0797-293. [DOI] [PubMed] [Google Scholar]
- 48.Xia Z, Dickens M, Raingeud J, Davis R J, Greenberg M E. Opposing effects of Erk and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 49.Yan C Y I, Greene L A. Prevention of PC12 cell death by N-acetylcysteine requires activation of the Ras pathway. J Neurosci. 1998;18:4042–4049. doi: 10.1523/JNEUROSCI.18-11-04042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 51.Yang E, Zha J, Jockel J, Boise L H, Thompson C B, Korsmeyer S J. Bad, a heterodimeric partner for Bcl-xL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 52.Yao R, Cooper G M. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 53.Yao R, Cooper G M. Growth factor-dependent survival of rodent fibroblasts requires phosphatidylinositol 3-kinase but is independent of pp70S6K activity. Oncogene. 1996;13:343–351. [PubMed] [Google Scholar]
- 54.York R D, Yao H, Dillon T, Ellig C L, Eckert S P, McClesey E W, Stork P J S. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature (London) 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 55.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not Bcl-xL. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]