ABSTRACT
With the progress of COVID-19 vaccination programs worldwide, some new adverse events associated with the available vaccines may unfold, especially in subpopulations, representatives of whom were not included in phase I, II, and III clinical trials of these vaccines, such as patients with autoimmune diseases, including multiple sclerosis (MS). A 34-year-old woman presented with severe right hemiplegia and ataxia. She was diagnosed with relapsing-remitting MS (RRMS) 13 years ago and treated with rituximab (an anti-CD20 monoclonal antibody) during the last 15 months. She had received her first dose of adenovirus-vectored COVID-19 vaccine Gam-COVID-Vac (Sputnik V) three months after her last infusion of rituximab and three days before experiencing her latest MS relapse episode, preceded by mild symptoms (fatigue, myalgia, generalized weakness, etc.). Magnetic resonance imaging revealed several new periventricular, juxtacortical, brainstem, and cerebellar peduncle lesions. She received corticosteroid therapy for five consecutive days, and her neurological deficits slightly improved. Twenty-one days after receiving the first dose of the vaccine, her anti-SARS-CoV-2 antibodies were below the lower detection limit. However, a decision was made to adhere to the vaccination schedule and not risk the patient’s safety against an unfortunate COVID-19 contraction, and thus, she was advised to receive the second Gam-COVID-Vac dose after discontinuation of oral steroid taper. The safety of adenovirus-based vaccines in patients with autoimmune diseases requires further investigation. Meanwhile, clinicians should raise awareness among their patients regarding the potentially limited efficacy of COVID-19 vaccination in those treated with anti-CD20 treatments. After careful, individualized risk-benefit assessments, planning a delay/pause in such treatments to create a time window for patients to receive the vaccine and develop anti-SARS-CoV-2 immunity may be recommended.
KEYWORDS: COVID-19, SARS-CoV-2, multiple sclerosis, vaccines, COVID-19 vaccines
Introduction
Since the outbreak of the COVID-19 pandemic, extensive global efforts have been put into producing and administering vaccines on a large scale to generate herd immunity against the responsible viral agent, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). With the currently available vaccines and the national vaccination programs in many countries, several questions are to be answered regarding the efficacy, safety, and probable post-vaccination adverse events in immunocompromised patients, such as multiple sclerosis (MS) patients being treated with disease-modifying therapies (DMTs). A successful COVID-19 vaccination should bring about protective levels of neutralizing antiviral antibodies, specifically those targeting the receptor-binding domain of SARS-CoV-2 spike protein.1 However, many MS patients receive anti-CD20 treatments (e.g., rituximab and ocrelizumab) that induce CD20+ B-cell apoptosis and are suspected of hindering an effective immunization against SARS-CoV-2 after vaccination.
Considering that phase I, II, and III clinical trials of the available vaccines are designed to evaluate their safety and efficacy in the general healthy population and not in those with deviated or suppressed immune systems,2–4 case studies and preliminary observations may hint toward the subpopulations in whom the safety and efficacy of these vaccines require further evaluations. We describe the case of a female relapsing-remitting MS (RRMS) patient receiving rituximab who developed an episode of neurological exacerbation shortly after receiving the first dose of the Gam-COVID-Vac (Sputnik V) as part of the ongoing national vaccination program in Iran.
Case presentation
A 34-year-old female MS patient was referred to the neurology department with severe right hemiplegia and ataxia. She was a nurse working in a tertiary healthcare center and had received her first dose of the COVID-19 vaccine (Gam-COVID-Vac, Sputnik V) three days before her symptoms presented.
She was diagnosed with MS thirteen years ago during the clinical workup and magnetic resonance imaging (MRI) evaluations after an episode of left hemiparesis. Her treatment with interferon-β 1a was then initiated. The patient’s symptoms were well under control until nine years later, when she had two episodes of relapse, namely, optic neuritis and bilateral lower limb paresthesia separated by approximately 30 days. Three years later, in 2019, after experiencing another acute MS attack (paraparesis), her treatment plan was switched to rituximab. Two initial doses, each containing 1000 mg, were administered in two weeks, followed by 1000 mg every six months. She had received her last dose of rituximab (the fourth dose) three months before receiving the vaccine.
One day after her COVID-19 vaccination, she had mild symptoms, including fatigue, myalgia, and generalized weakness. Unfortunately, after three days, she developed severe right hemiplegia and ataxia. The attack had started 10 hours before she visited our department. She reported no other symptoms, and her vital signs were stable. Muscle force was 2/5 and 3/5 in her right lower and upper limbs, respectively. She had severe imbalance and an ataxic gait; her knee and biceps reflexes were 2+, bilaterally. No sensory deficits were present upon examination. The patient did not take any drugs, had no other comorbid conditions besides MS, and reported no history of contracting COVID-19. She did not report the use of alcohol, tobacco, and illicit drugs.
Results from the lab studies were normal upon presentation. MRI scans showed several new periventricular, juxtacortical, and brainstem lesions on T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences (Figure 1), without gadolinium enhancement on T1-weighted sequences. Intravenous methylprednisolone (500 mg/day) was administered for five consecutive days. Her neurological deficits slightly improved after the acute phase management. Of note, serum anti-SARS-CoV-2 IgG and IgM levels, 21 days after receiving the first dose of the vaccine, were not detectable (IgG:0.29, IgM:0.01; >1.1 = positive) using enzyme-linked immunosorbent assay (ELISA). Despite this unsuccessful immunization, she was advised to adhere to the vaccination schedule and take her second dose of the vaccine shortly after complete discontinuation of tapering oral methylprednisolone treatment (for three weeks), given the risk of potentially severe outcomes following COVID-19 contraction. The patient’s neurological deficits resolved substantially after the completion of her oral steroid taper. Informed consent was obtained from the patient regarding the publication of her case.
Figure 1.
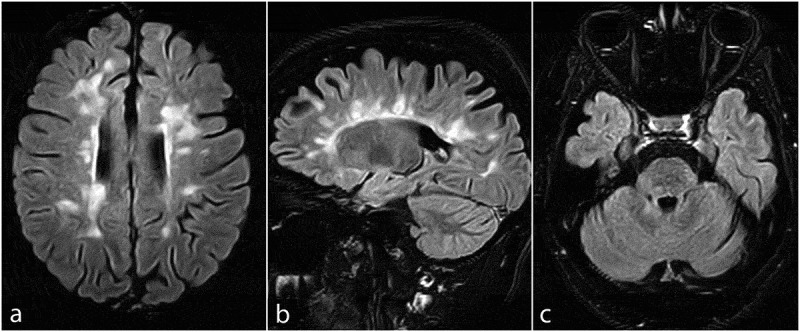
Axial and sagittal fluid-attenuated inversion recovery (FLAIR) MRI sequences of the brain, showing several new periventricular, Juxtacortical (a and b), brainstem, and cerebellar peduncle (c) lesions
Discussion
Gam-COVID-Vac (Sputnik V), ChAdOx1 nCoV-19 (AZD1222), and Ad26.COV2.S (by Johnson and Johnson) are replication-deficient adenovirus-vectored vaccines, carrying the gene for the full-length glycoprotein S of SARS-CoV-2.2–4 One rare yet severe adverse event associated with adenovirus-based vaccines is transverse myelitis.5 Indeed, two individuals in phase III clinical trial of ChAdOx1 nCoV-19 reportedly developed transverse myelitis (TM).2 The first case of TM was reported 14 days after the second dose administration, and it was considered “possibly related to vaccination”. The second case had developed TM 10 days after receiving the first dose, but further investigations showed preexisting, but unnoticed MS, and thus, this episode of TM was considered “unlikely to be related to vaccination”.2 In addition to our case, these reports show that adenovirus-based SARS-CoV-2 vaccines may be associated with neurological exacerbation in a small fraction of MS patients. However, given the limited available data on the currently available COVID-19 vaccines and the different types of adenoviral vectors utilized, and a lack of experimental data, hypothesizing concerning the probable mechanisms that could explain such adverse events may not be wise yet.
Nevertheless, the most significant concern is the effective immunization of patients against SARS-CoV-2. The depletion of CD20+B-cells (e.g., naïve B-cells in the blood, lymphoid tissues, and to some extent, the bone marrow) tackles the efficient and timely production of anti-SARS-CoV-2 IgG.6,7 Furthermore, this blunted seroconversion and weakened humoral immune response have previously been shown to diminish the efficacy of several vaccines in patients treated with rituximab and ocrelizumab.6 This was the case in our patient as well. Her serum anti-SARS-CoV-2 IgG level, 21 days after receiving the first dose of Gam-COVID-Vac, were below the lower limit of detection, although the results from the phase III clinical trial of the vaccine suggested that at least a partial protective effect would be achieved even before the second dose administration.3
There are two approaches to overcome this hurdle, 1- administering more doses of the vaccines than suggested by the manufacturers, or 2- delaying/temporarily pausing the anti-CD20 treatment and creating a time window for patients to receive regular vaccine doses in a way that keeps the risk of autoreactive memory B-cells expansion and clinical exacerbation at its lowest. Given the rare yet probable adverse events associated with adenovirus-based vaccines in patients with autoimmune diseases, the first approach may not be the safest strategy in regions receiving this class of vaccines. However, with the second approach, owing to the differential repopulation kinetics of naïve B-cells (necessary to generate post-vaccination immunity against SARS-CoV-2) and autoreactive memory B-cells, there may be a chance to generate anti-SARS-CoV-2 immunity while preventing MS exacerbations in patients for whom anti-CD20 treatments are paused. The limiting effect of rituximab on peripheral B-cell counts often persists for 6–9 months, and the naïve B-cell populations typically recover after 12 months. Memory B-cells can remain depleted even up to five years.8 It has recently been stated that vaccine administration three months after the last infusion of anti-CD20 drugs may also be reasonable.9 Fortunately, resuming the anti-CD20 treatment is not suggested to endanger the vaccine-acquired immunity against SARS-CoV-2.6
In conclusion, although vaccine-associated adverse events such as neurological exacerbations are rare, future research is warranted to investigate this phenomenon in more depth. More importantly, clinicians are advised to raise awareness among their patients about the anti-CD20 treatment-induced hypogammaglobulinemia and the limited efficacy of vaccination in those treated with anti-CD20 agents. Individualized evaluations and cost-benefit assessments should be made to plan a delay/pause in the anti-CD20 treatment schedule to create a time window for early and efficient immunization with the currently available COVID-19 vaccines.
Acknowledgments
We thank the patient for cooperating and allowing the use of her data in this paper.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Suthar MS, Zimmerman MG, Kauffman RC, Mantus G, Linderman SL, Hudson WH, Wrammert J, Nyhoff L, Davis CW, Adekunle O.. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med. 2020;1(3):100040. doi: 10.1016/j.xcrm.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, … Bijker E.. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS; Gam-COVID-Vac Vaccine Trial Group . Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–81. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kremer EJ. Pros and cons of adenovirus-based SARS-CoV-2 vaccines. Mol Ther. 2020;28(11):2303–04. doi: 10.1016/j.ymthe.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephenson KE, Le Gars M, Sadoff J, De Groot AM, Heerwegh D, Truyers C, Barouch DH, Loos C, Chandrashekar A, McMahan K. Immunogenicity of the Ad26. COV2. S vaccine for COVID-19. JAMA. 2021;325(15):1535. doi: 10.1001/jama.2021.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker D, Roberts CA, Pryce G, Kang AS, Marta M, Reyes S, Amor S, Giovannoni G, Amor S. COVID‐19 vaccine‐readiness for anti‐CD20‐depleting therapy in autoimmune diseases. Clin Exp Immunol. 2020;202(2):149–61. doi: 10.1111/cei.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maillart E, Papeix C, Lubetzki C, Roux T, Pourcher V, Louapre C. Beyond COVID-19: DO MS/NMO-SD patients treated with anti-CD20 therapies develop SARS-CoV2 antibodies? Mult Scler Relat Disord. 2020;46:102482. doi: 10.1016/j.msard.2020.102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chisari CG, Sgarlata E, Arena S, Toscano S, Luca M, Patti F. Rituximab for the treatment of multiple sclerosis: a review. J Neurol. 2021;1–25. doi: 10.1007/s00415-020-10362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centonze D, Rocca MA, Gasperini C, Kappos L, Hartung HP, Magyari M, Oreja-Guevara C, Trojano M, Wiendl H, Filippi M. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: an expert consensus. J Neurol. 2021;1–8. doi: 10.1007/s00415-021-10545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


