Abstract
Obesity is a major risk factor for a wide range of cardiometabolic diseases, however the impact of specific aspects of body morphology remains poorly understood. We combined the GWAS summary statistics of fourteen anthropometric traits from UK Biobank through principal component analysis to reveal four major independent axes: body size, adiposity, predisposition to abdominal fat deposition, and lean mass. Mendelian randomization analysis showed that although body size and adiposity both contribute to the consequences of BMI, many of their effects are distinct, such as body size increasing the risk of cardiac arrhythmia (b = 0.06, p = 4.2 ∗ 10−17) while adiposity instead increased that of ischemic heart disease (b = 0.079, p = 8.2 ∗ 10−21). The body mass-neutral component predisposing to abdominal fat deposition, likely reflecting a shift from subcutaneous to visceral fat, exhibited health effects that were weaker but specifically linked to lipotoxicity, such as ischemic heart disease (b = 0.067, p = 9.4 ∗ 10−14) and diabetes (b = 0.082, p = 5.9 ∗ 10−19). Combining their independent predicted effects significantly improved the prediction of obesity-related diseases (p < 10−10). The presented decomposition approach sheds light on the biological mechanisms underlying the heterogeneity of body morphology and its consequences on health and lifestyle.
Subject terms: Risk factors, Obesity, Obesity
Jonathan Sulc et al. use principal component analysis and Mendelian randomization to conduct a comprehensive analysis of 14 body morphology metrics using data from the UK Biobank. Their results suggest that body size and adiposity have distinct impacts on obesity-related outcomes and highlight a BMI-neutral component affecting body fat distribution, providing further insight into the genetic basis of body shape and its consequences on human health and lifestyle.
Introduction
Obesity is one of the main risk factors for many non-communicable diseases, such as type 2 diabetes (reviewed in ref. 1) and cardiovascular diseases (reviewed in ref. 2). The associated disease risk and underlying biology of obesity are generally studied through the lens of body mass index (BMI, weight [kg]/height2 [m2]), which uses excess body mass as a surrogate for adiposity. This approximation provides a reasonably accurate predictor at the population level3,4 but is blind to many aspects of body shape and composition that may be critical to disease etiology. Indeed, disease risk and progression have been shown to be affected by the location and type of the adipose tissue in which excess calories are stored3–6. Abdominal obesity in particular, usually assessed using waist circumference or waist-to-hip ratio (WHR), is associated with increased disease risk and mortality independent of BMI3.
Although the exact mechanisms underlying the consequences of adiposity on health have not been fully elucidated, the predominant hypothesis is that of adipose tissue expandability: that excess calories are preferentially stored in subcutaneous adipose tissue (SAT), which can expand with little to no deleterious impact on health7,8. When its capacity for expansion is exceeded, adipose cells hypertrophy, causing local inflammation, and fat is increasingly stored as ectopic fat in organs and as visceral adipose tissue (VAT) around organs in a process similar to a mild form of lipodystrophy. Ectopic and visceral fat are thought to play a central role in many of the direct consequences of obesity.
Much remains unknown about the genetic and environmental factors affecting body fat distribution and its contribution to health outcomes. Currently available non-invasive imaging techniques such as magnetic resonance imaging (MRI) and dual-energy X-ray absorptiometry (DXA) allow the accurate measurement of adipose mass in different parts of the body and have considerably improved our understanding of the impact of different subtypes of adiposity on health9,10. However, such techniques remain costly and are therefore generally restricted to smaller sample sizes.
An alternative approach is the concurrent analysis of multiple traits, leveraging the co-occurring changes in multiple phenotypes to understand the underlying causes and mechanisms. Analysis of variance methods, such as principal component analysis (PCA), have been used to investigate the complex architecture underlying body morphology, revealing the main axes of phenotypic variation and increasing the statistical power to detect novel loci affecting body morphology11. While this has improved our understanding of the genetic basis underlying common and distinct components of anthropometric traits, their impact on health and quality of life remains unknown. Other approaches such as clustering and canonical correlation analysis have identified single nucleotide polymorphisms (SNPs) associated with healthier metabolic profiles, despite higher BMI and/or body fat percentage12–15. However, these hypothesis-driven approaches (i.e., identifying clusters of functionally similar SNPs based on both obesity measures and health outcomes) are not suited to determine the causality of these correlated differences or the directionality of potential causal effects because the SNP groups have different health consequences by construction.
Here we followed a hypothesis-free approach to isolate independent axes of variations in body shape and size and investigated their health consequences. We performed a PCA on GWAS summary statistics of 14 anthropometric traits from the UK Biobank16 to extract orthogonal components, each representing different features of body shape (Fig. 1). We show that these measures of body shape can be summarized using four principal components (PCs) affecting body size, adiposity, abdominal fat deposition, and lean mass, respectively. Enrichment analyses highlighted differences in the pathways and tissues involved in these composite traits, providing insight into the underlying biological mechanisms. We then used robust cross-sex Mendelian randomization (MR) to assess the impact of these independent components on health and lifestyle. While many health and lifestyle consequences were shared with individual traits, these orthogonal PCs allowed us to better disentangle the independent contributions of different aspects of body shape. The results can be explored using the shiny app which can be downloaded by following the instructions at http://wp.unil.ch/sgg/pca-mr/. Furthermore, the combination of these PCs improved the prediction accuracy of obesity-related diseases.
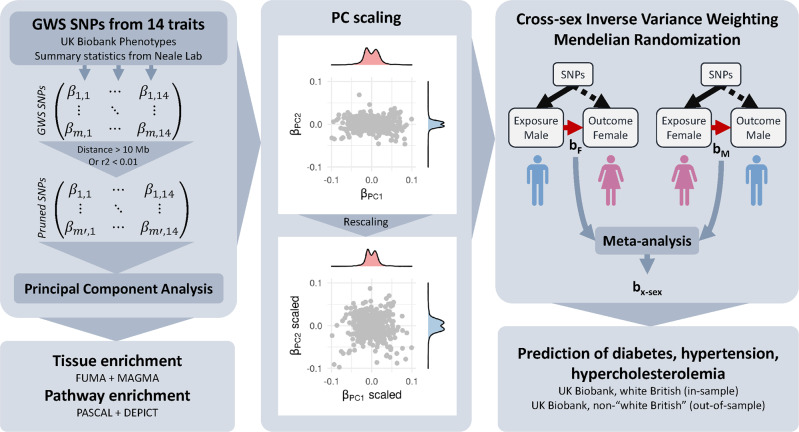
Fig. 1. Overview of the methods.
Summary statistics for anthropometric traits from the UK Biobank were pruned for independence before being subjected to principal component analysis (PCA). Principal component-associated SNPs were tested for enrichment in genes expressed in certain tissues or in pathways. The genetic effects on the resulting components were scaled to obtain effect sizes corresponding to a trait with a variance of 1 (standardized). Mendelian randomization was used to determine the impact of these composite traits on lifestyle and health outcomes. Using these effect estimates, the individual risk was predicted in the UK Biobank and accuracy compared to BMI and WHR.
Results
PCA of genetic effects
A schematic representation of our composite MR analysis framework is shown in Fig. 1. We selected 14 anthropometric and bioimpedance-derived traits (Supplementary Data 1) as the basis for the PCA, 13 of which were available in the UK Biobank16 with genome-wide summary statistics made available by the Neale lab (www.nealelab.is/uk-biobank). Summary statistics for WHR were not available in the UK Biobank, therefore we performed a GWAS in the UK Biobank following the same procedure as that used by the Neale lab. SNPs which were then genome-wide significant (GWS, p < 5 × 10−8) for any trait were pruned to be independent. The resulting SNP x traits matrix of effect estimates was subjected to PCA. The loadings of each PC were then rescaled according to the phenotypic correlation between these traits in the UK Biobank so as to obtain standardized effect sizes, i.e. the resulting PC phenotypes would have a variance of 1. SNP-PC associations were then calculated genome-wide and SNPs for each PC were re-pruned individually.
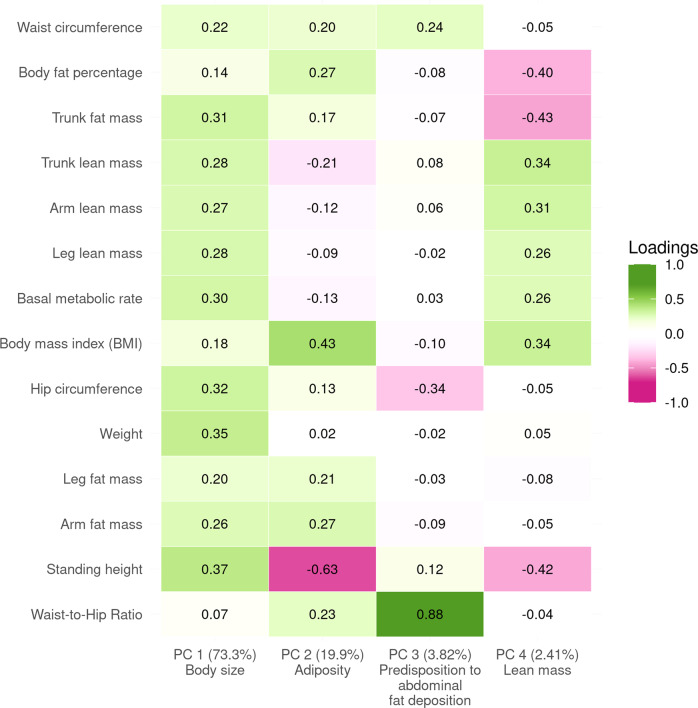
The top four PCs explained more than 99% of the total variance (Fig. 2, Supplementary Data 2). PC1 (73.3% variance) represents an overall increase in body size, with positive weights for all traits indicating a slightly disproportionate increase in body mass compared to height, resulting in higher BMI as well. PC2 (19.9%) shows a decrease in height with an increase in fat mass at the expense of lean mass. PC3 (3.8%) is largely BMI- and body fat mass-neutral, decreasing hip circumference, and increasing waist circumference and hence WHR, reflecting a shift in body fat from hips to the waist. PC4 (2.4%) resulted in decreased height and increased BMI, with an increase in lean mass at the expense of fat mass.
Fig. 2. The contributions of each trait to the first four genetic principal components (PCs).
The explained variance of each PC is included in parentheses along with the descriptive name used in the main text. The loadings presented here are those typically used in the principal component analysis (PCA), scaled such that the sum of the squared weights is equal to 1 (as opposed to the scaling used to obtain composite traits with a variance of 1). This provides a consistent scale and makes PCs more easily comparable with each other.
Performing this analysis using male- or female-specific summary statistics produced very similar results, though PC3 explained less variance than PC4 in men (Supplementary Data 3–4, Supplementary Note 1). PCs were also robust to changes in the selection of specific traits. For example, excluding WHR yielded effect estimates which were highly correlated with those obtained using the full set of traits (all |r| > 0.99, see Supplementary Note 2), though the altered explained variance also resulted in the reordering of PCs 3 and 4.
PCs 1–3 were highly correlated with weight, BMI, and WHR, respectively, both phenotypically (r ≥ 0.70, Supplementary Fig. 3) and in terms of their causal effects on the tested outcomes. The (genetically) orthogonal nature of PCs nevertheless resulted in much lower phenotypic correlation with each other than between traits (e.g., the phenotypic correlation between PC1 and PC2 was only 0.36 while that between weight and BMI was 0.90, Supplementary Fig. 4, Supplementary Data 5). Note that the phenotypic realization of PCs are not orthogonal, and their correlation is therefore not zero, due to differences between their environmental and genetic correlation. However, the MR-derived causal effect estimates of PCs are independent and therefore additive (though they may still be correlated), which is not the case for individual traits.
For brevity and clarity, we mainly describe the PCs in comparison with BMI and occasionally weight or WHR. Other traits had causal effects similar to or weaker than these traits (e.g., the effects of body fat percentage and BMI on the disease had a correlation coefficient r = 0.95, p = 7.6 × 10−43) or were less relevant to obesity-related health outcomes (e.g., height).
We found 615 independent GWS SNPs associated with PC1 (body size), 641 with PC2 (adiposity), 354 with PC3 (predisposition to abdominal fat deposition), and 610 with PC4 (lean mass). Among these, respectively 3, 83, 137, and 330 SNPs did not reach genome-wide significance for any individual trait. For comparison, BMI had 532 independent GWS SNPs.
Tissue/pathway enrichment
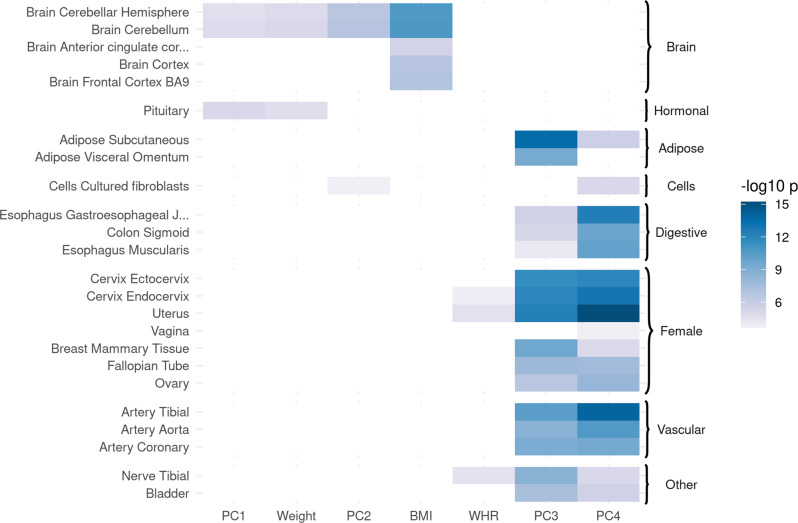
We tested the PCs for the enrichment of genes expressed in specific tissues using the MAGMA method17 through the FUMA interface18 in both GTEx v819 and brain development and aging data from BrainSpan20. We also tested for their enrichment of molecular pathway terms from the DEPICT dataset21 using PASCAL22. The tissue-enrichment results of the four PCs as well as weight, BMI, and WHR are shown in Fig. 3 and listed in Supplementary Data 6–9.
Fig. 3. Body size and accumulation of body fat were mainly enriched for genes expressed in the brain, while the others were enriched for a broader range of tissues.
Enrichment of traits and principal components (PCs) for tissue-specific gene expression (negative log 10 p-values). Genome-wide SNP effect p-values were analyzed using MAGMA on GTEx v8 data (54 tissues). Results not significant after Bonferroni correction are masked in white. Traits with no significant enrichment results are hidden for clarity (full results are available in Supplementary Data 6).
Loci associated with both PC1 (body size) and PC2 (adiposity) were mainly enriched for genes expressed in the cerebellum (p ≤ 2.2 × 10−5) and the pituitary gland (p ≤ 5.7 × 10−4). Using data from BrainSpan20, we found PC2 to be further enriched for genes expressed in the brain specifically during the early to late mid-prenatal phases of development (p ≤ 6.1 × 10−4).
PC3- (predisposition to abdominal fat deposition) associated SNPs were harbored by genes most enriched for expression in SAT (p = 3.0 × 10−14), followed by VAT (p = 4.2 × 10−10), female reproductive tissues (breast mammary tissue, ecto- and endocervix, and uterus, all p ≤ 2.4 × 10−10), nerves (tibial, p = 2 × 10−15), arteries (p ≤ 2.2 × 10−9), and digestive system (p ≤ 8.8 × 10−5). Note that using sex-specific (e.g., male-specific) summary statistics and PC loadings with the same gene expression datasets produced a similar enrichment for female-specific tissues. Data from BrainSpan showed PC3 to be enriched for genes expressed in the late prenatal brain (p = 9.3 × 10−8).
PC4 (lean mass) showed similar enrichment to that of PC3, though stronger for the digestive system (p ≤ 1.6 × 10−10) and some female reproductive tissues (uterus, ecto- and endocervix, all p ≤ 1.3 × 10−12), and weaker for adipose tissue and tibial nerve. PC4 was also enriched for genes expressed in the prostate (p = 2.9 × 10−4). In BrainSpan, PC4 also showed enrichment for genes expressed prenatally in the brain.
Loci associated with BMI showed slightly stronger enrichment than PCs 1 and 2 for genes expressed in the cerebellum, as well as other areas of the adult brain, including the basal ganglia, hippocampus, hypothalamus, amygdala, and frontal cortex. They were also enriched for genes expressed in the mid-prenatal brain, similar to that found for PC2, if slightly weaker.
The analysis of molecular pathways (Supplementary Data 10) showed qualitatively similar enrichment for PCs 1 (341 pathways) and 2 (348), weight (318), and BMI (293). These were mostly terms related to the brain, synapses, behavior, or learning. PC3 had the most numerous enriched terms (628) and the strongest enrichment overall. Most of the enriched terms were not related to brain function, but to embryogenesis and morphology, with many others specifically concerning adiposity, metabolism, and glucose homeostasis. Other terms were related to vascular or heart function, or hormones. PC4 (569) showed some overlap in terms with PC3, mainly in terms related to embryogenesis and morphology. Overall, 1067 pathways were found to be enriched for at least one PC.
Cross-sex MR analysis
We used inverse-variance weighted (IVW) MR to test for causal effects of the 14 anthropometric traits and PCs on disease outcomes, continuous measures of health, lifestyle factors, and diet, as well as the reverse. To avoid bias in the MR estimates due to sample overlap, we used sex-specific effects from opposite sexes for exposures and outcomes (male exposure–female outcome and female exposure–male outcome, using the same combined-sex PC loadings for exposure PCs). This produces unbiased estimates of the sex-specific causal effects in the sex used in the outcome, provided the strength of the instruments’ association with the exposure was not different between sexes. The two cross-sex causal effect estimates (female-to-male and male-to-female) were then meta-analyzed using inverse-variance weighting. Where the strength of the instruments’ association with the exposure differed between sexes, namely for WHR and PC3 (abdominal fat distribution), the meta-analyzed causal effect estimates may be biased (generally towards the null). Although the effect size estimates may be slightly over- or underestimated, this should not affect the type I error rate (see “Methods”).
Effects on disease risk
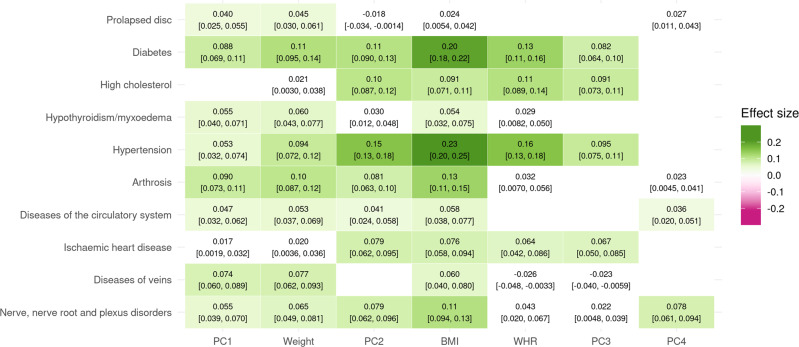
PC1 (body size) increased the risk of many diseases (Fig. 4). An increase of one standard deviation (SD) increased the absolute risk of diabetes by 1.7% (95% CI: 1.3–2.1), that of hypertension by 2.3% (95% CI: 1.4–3.2), as well as many other diseases, such as nerve disorders, diseases of the veins and circulatory system, and prolapsed disc (Supplementary Data 11). Although it also increased the risk of cardiac arrhythmias by 0.93% (95% CI: 0.71–1.1), it did not significantly affect the risk of hypercholesterolemia or heart disease.
Fig. 4. Single and composite traits increase the risk of multiple diseases.
Mendelian randomization causal effects of traits and principal components (PCs) on a selection of diseases on a standardized scale. The 95% confidence interval of the effect is indicated in brackets. Effects that were not significant at the Bonferroni-corrected threshold (p < 4.3 × 10−5) are colored in white. The full list of effects can be found in Supplementary Data 11.
PC2 (adiposity) had much stronger effects on many obesity-related diseases (Fig. 4), where a 1 SD increase also increased the absolute risk of diabetes by 2.1% (95% CI: 1.7–2.5), hypertension by 6.8% (95% CI: 5.8–7.7), as well as hypercholesterolemia by 3.4% (95% CI: 2.8–4.0) and ischemic heart disease (IHD) by 1.8% (95% CI: 1.5–2.2). The risk of many other diseases, such as arthrosis and diseases of the nervous system were also increased (Supplementary Data 11).
PC3 (predisposition to abdominal fat deposition), despite being weight- and BMI-neutral, was a risk factor for many of the same obesity-related diseases as PC2 (Fig. 4, Supplementary Data 11). A 1 SD increase in PC3 increased the absolute risk of diabetes by 1.6% (95% CI: 1.2–1.9), hypertension by 4.2% (95% CI: 3.3–5.0), hypercholesterolemia by 3.0% (95% CI: 2.4–3.6), and IHD by 1.6% (95% CI: 1.2–2.0).
PC4 (lean mass) had few consequences on health, only significantly increasing the risk of nerve disorders and diseases, carpal tunnel syndrome, and joint disorders (Fig. 4, Supplementary Data 11).
For comparison, a 1 SD increase in BMI (5.1 kg/m2 in women, 4.2 kg/m2 in men) increased the risk of diabetes by 3.8% (95% CI: 3.4–4.2), hypertension by 9.9% (95% CI: 8.8–11.0), hypercholesterolemia by 3.0% (95% CI: 2.3–3.7), and IHD by 1.8% (95% CI: 1.4–2.2). Weight and WHR had similar, if somewhat weaker, effects (see Supplementary Data 11). Although in many cases these effects exceed those of individual PCs, they remain less than the cumulative (summed) effects of the four PCs (Supplementary Figs. 5–25).
Effects on continuous health outcomes
Many continuous health indicators were affected by both PCs and traits, in a manner largely consistent with expectations (Supplementary Data 12).
Consistent with the increased risk of diabetes, PCs 1–3 all increased the levels of glycated hemoglobin in blood, though the effect was strongest for PC2 (b = 0.13, 95% CI: 0.11–0.15). Glucose levels were similarly affected, though the effects were weaker. All three PCs also increased triglyceride levels (b between 0.095 and 0.24) and decreased HDL cholesterol (b between −0.22 and −0.16), but only PC1 decreased LDL cholesterol (b = −0.076, 95% CI: −0.094 to −0.059) and total cholesterol. They all increased blood pressure (systolic and/or diastolic), with the strongest effects from PC2 (b > 0.15). Plasma concentrations of several liver enzymes, such as γ-glutamyltransferase (GGT) and alanine aminotransferase (ALT), were found to be increased as well, possibly indicating liver damage.
PCs 1 and 2 also increased levels of cystatin C and decreased albumin, possibly indicative of impaired kidney function23, whereas the effects of PC3 were in the opposite direction (decreasing cystatin C and increasing albumin). C-reactive protein (CRP) was also increased by PCs 1 and 2 (b1 = 0.17, 95% CI 0.15–0.20; b2 = 0.25, 95% CI 0.22–0.27) but unaffected by PC3. PCs 1–3 all strongly decreased levels of sex hormone-binding globulin (SHBG) with the strongest effects from PC2 (b = −0.19, 95% CI: −0.21 to −0.16), as well as testosterone where the effects of PCs 1 and 3 were stronger (b1 = −0.065, 95% CI −0.086 to −0.043; b3 = −0.058, 95% CI −0.082 to −0.034).
PC4 mainly increased creatinine levels while decreasing CRP (b = −0.090, 95% CI: −0.11 to −0.067) and the maximum heart rate during fitness test. The levels of triglycerides and the liver function markers GGT and ALT were slightly decreased but only alkaline phosphatase reached Bonferroni-corrected significance (b = −0.068, 95% CI: −0.09 to −0.046).
The effects of BMI were comparable to a combination of PCs 1 and 2, increasing levels of glycated hemoglobin (b = 0.2, 95% CI 0.17–0.22) and glucose (b = 0.12, 95% CI 0.10–0.15), as well as increasing the levels of triglycerides while decreasing both HDL (b = −0.29, 95% CI −0.32 to −0.26) and LDL (b = −0.059, 95% CI −0.082 to −0.037) cholesterol. Systolic and diastolic blood pressure was also increased, as were levels of CRP (b = 0.33, 95% CI 0.3–0.36) and liver function markers such as ALT and GGT. SHBG (b = −0.24, 95% CI −0.26 to −0.21) and testosterone (b = −0.11, 95% CI −0.13 to −0.084) were also decreased.
Effects on lifestyle factors
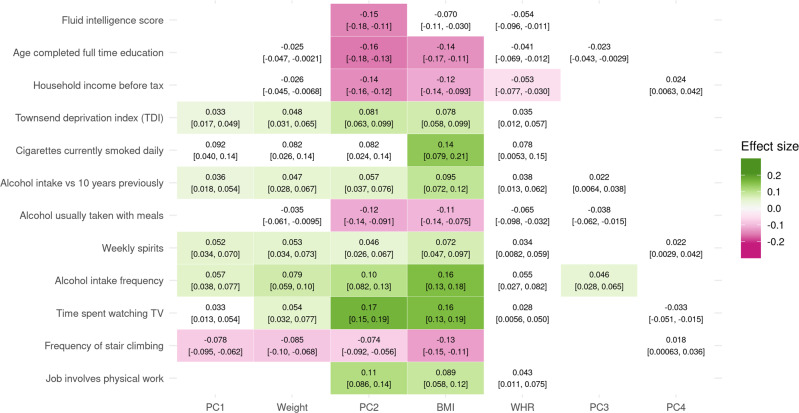
PC1 slightly reduced socio-economic status (SES), as shown by increased Townsend deprivation index (b = 0.033, 95% CI: 0.017–0.049). This was accompanied by a longer working week, as well as an increase in smoking and alcohol consumption, particularly spirits (Fig. 5, Supplementary Data 13). The duration and frequency of physical activity, as well as walking pace, were all decreased in favor of increased time spent using the computer. PC1 also increased daytime dozing and napping but decreased snoring.
Fig. 5. Single and composite traits affect many aspects of lifestyle.
Mendelian randomization causal effects of traits and principal components (PCs) on a selection of lifestyle factors on a standardized scale. The 95% confidence interval of the effect is indicated in brackets. Effects that were not significant at the Bonferroni-corrected threshold (p < 7.1 × 10−5) are colored in white. The full list of effects can be found in Supplementary Data 13.
The effects of PC2 on SES were similar but much more pronounced (Fig. 5, Supplementary Data 13), not only associated with increased Townsend deprivation index (b = 0.057, 95% CI: 0.037–0.076) and the likelihood of having a job involving heavy physical work (b = 0.11, 95% CI: 0.086–0.14), but strongly linked to decreased income, fluid intelligence score, and education (all b < −0.14). Accompanying these were lifestyle changes similar to those of PC1, increasing smoking and the frequency of alcohol consumption, with a decrease in wine in favor of spirits and alcohol being taken more often outside of meals. Although PC2 increased the duration of walks (b = 0.05, 95% CI: 0.031–0.069) and vigorous activity, the duration of walking for pleasure was decreased (b = −0.044, 95% CI: −0.062 to −0.025), as were several other measures of physical activity, namely the frequency of stair climbing and the usual walking pace. Unlike PC1, PC2 decreased the time spent using the computer in favor of time spent watching TV.
PC3 increased alcohol intake frequency (b = 0.046, 95% CI: 0.028– 0.065) and napping during the day (b = 0.035, 95% CI: 0.018–0.053) (Fig. 5, Supplementary Data 13).
PC4 only associated with an increased length of the working week (b = 0.043, 95% CI: 0.024–0.062) (Supplementary Data 13).
The effects of BMI on lifestyle were most similar to those of PC2, linked to decreased SES (Townsend deprivation index b = 0.078, 95% CI 0.058–0.099) and physical activity while increasing smoking and alcohol consumption. Additional comparisons of overall effects on lifestyle can be seen in Supplementary Figs. 26–46.
Effects on diet
PC1 was associated with greater reported variation in diet (b = 0.052, 95% CI: 0.034–0.069) and increased consumption of healthy foods such as fresh fruit, vegetables, and water (Supplementary Data 14). Consumption of coffee was also increased (b = 0.087, 95% CI: 0.068–0.11).
PC2 was similarly associated with reportedly increased variation in diet (b = 0.084, 95% CI: 0.065–0.10), as well as salt added to food (b = 0.045, 95% CI: 0.026–0.064). Other changes in diet reflect increased consumption of cheaper meats, namely pork and poultry, while decreasing intake of grain products (bread and cereal), cheese, and dried fruits (Supplementary Data 14).
PC3 was associated with increased bread consumption (b = 0.035, 95% CI: 0.017–0.053).
PC4 increased fruit intake, both fresh and dried (b > 0.041), while decreasing processed meat intake (Supplementary Data 14).
Effects of BMI on diet were similar to those of PC2, such as increased variation in diet (b = 0.12, 95% CI: 0.097–0.14) and decreased intake of grain products and cheese (Supplementary Data 14). In addition, some effects were similar to those of PC1, including increased vegetable and fruit consumption, as well as coffee intake (b = 0.11, 95% CI: 0.080–0.13).
Sex-specific effects
The genetic effects of the selected IVs on PCs 1, 2, and 4, as well as BMI and weight, were not significantly different between men and women, which made it possible to obtain unbiased sex-specific causal effects (see “Methods”). Briefly, if the IV effects on the exposure do not differ between sexes, summary statistics for the opposite sex can be used for the exposure while using those of the sex of interest as outcome. Those of PC3 (and WHR) were stronger in women (pPC3 = 6.9 × 10−46, pWHR = 1.4 × 10−159) and were unsuitable for this purpose. Full results are available in Supplementary Data 15–22 and shown in Supplementary Note 5.
None of the PCs had significantly different effects on diet and lifestyle in men and women, though PC2 did have a tendency for stronger effects on disease risk in men (TLS slope = 1.27), mainly driven by diabetes (bm = 0.15, 95% CI 0.12–0.17; bf = 0.080, 95% CI 0.052–0.11; pdiff = 1.8 × 10−3) and heart diseases such as IHD (bm = 0.11, 95% CI 0.081–0.013; bf = 0.059, 95% CI 0.037–0.08; pdiff = 4.7 × 10−3). However, many blood molecular traits and other continuous health outcomes were differently affected by PCs in men and women.
Testosterone in particular was differently affected between sexes, strongly decreased in men by both PC1 (b = −0.15, 95% CI −0.18 to −0.12) and PC2 (b = −0.18, 95% CI −0.21 to −0.14), whereas in women it was unaffected by PC1 and increased by PC2 (b = 0.082, 95% CI 0.05–0.11). SHBG was similarly affected by PC2 in both sexes, but the decrease caused by PC1 was twice as strong in women (bm = −0.084, 95% CI −0.12 to −0.051; bf = −0.17, 95% CI −0.21 to −0.14; pdiff = 1.5 × 10−4).
Other differences include PC1 causing a stronger decrease in albumin in women (bm = −0.095, 95% CI −0.12 to −0.068; bf = −0.17, 95% CI −0.20 to −0.15; pdiff = 6.0 × 10−5) and increasing glycated hemoglobin only in men (b = 0.12, 95% CI 0.089–0.15). The effects of PC2 in men were stronger for ALT, GGT, and pulse rate, and an increase in total protein was only seen in men.
The effects of BMI were again similar to those of PCs 1 and 2. The increase in glycated hemoglobin was also stronger in men and was accompanied a larger increase in glucose as well (bm = 0.16, 95% CI 0.13–0.20; bf = 0.083, 95% CI 0.05–0.12; pdiff = 5.2 ∗ 10−4). LDL was decreased only in men (b = −0.099, 95% CI −0.13 to −0.067). The opposite effects on testosterone levels were also observed for BMI (bm = −0.24, 95% CI −0.27 to −0.21; bf = 0.072, 95% CI 0.035–0.11; pdiff = 7.9 × 10−36).
Bi-directional MR
In addition to determining which environmental factors may affect body shape, we were interested in feedback loops. The stringency of the selection criteria for IVs, namely that they be GWS for the sex used as exposure, resulted in few exposures significantly affecting the body traits or PCs. Several exposures had unrealistically large estimated effect sizes (>0.4), likely due to confounding, and are not considered here. For example, forced vital capacity (FVC), which is dependent on lung—and therefore body—size, showed strong bi-directional associations with most body traits. The main results are summarized here, full results are listed in Supplementary Data 23–26.
While PC1 reduced cholesterol, it was in turn reduced by LDL and total cholesterol (b ≤ −0.068), as well as apolipoprotein A and diagnosis of hypercholesterolemia. PC1 also showed a positive feedback loop with cystatin C but a negative one with IGF-1, reducing IGF-1 levels in the blood (b = −0.13, 95% CI: −0.16 to −0.11) but being increased by it (b = 0.042, 95% CI: 0.024–0.061).
PC2 showed a positive feedback loop with CRP and mutual negative loop with creatinine. PC2 was also reduced by IGF-1 (b = −0.071, 95% CI: −0.089 to −0.052).
PC3 was decreased by hypertension (b = −0.07, 95% CI: −0.11 to −0.035), forming a negative feedback loop. It also formed a positive feedback loop with diabetes (as did WHR), although this seems to be due to a negative effect of diabetes on hip circumference (HC, b = −0.16, 95% CI: −0.26 to −0.067) rather than an increase in waist circumference (WC). PC3 also had a positive feedback loop with triglycerides (from decreased HC), and mutual negative effects on apolipoprotein A and HDL cholesterol (from decreased WC). We also found the negative effects of PC3 on testosterone and SHBG to be reciprocal (b ≤ −0.058).
PC4 had a positive feedback loop with creatinine and was also increased by IGF-1 (b = 0.12, 95% CI: 0.11–0.14). SHBG and testosterone both decreased PC4, though for testosterone this appears to have been driven by an effect in women only (b = −0.09, 95% CI: −0.11 to −0.065).
BMI increased CRP in a positive feedback loop, similar to that of PC2, and reciprocal negative effects with LDL and total cholesterol (b ≤ −0.035), similar to PC1.
DXA-based measures
DXA and MRI technologies provide increased accuracy in measurements of body composition and have been suggested to provide a clearer picture of obesity and its consequences on health24. We were able to impute many such traits based on the subset of ~5000 UK Biobank participants with these phenotypes (Supplementary Data 27). Several of these, including both trunk and android tissue fat percentages (both predicted with r2 above 0.75), had effects on disease comparable to BMI and PC2 (correlation r ≥ 0.97) but with a tendency for slightly larger effects (TLS slopes of 1.17 and 1.16, respectively). However, with fewer GWS SNPs (~28% fewer than BMI), these phenotypes revealed no additional diseases affected by obesity (see Supplementary Data 28).
Prediction of disease risk
To further emphasize the biological relevance of PCs over single traits, we compared the accuracy of prediction of obesity-related disease, namely diabetes, high cholesterol, and hypertension, in the UK Biobank. Given the independence of estimated PC effects, these could be combined in a weighted linear fashion to obtain the 4-PC prediction and were compared with the single trait prediction from BMI and WHR using receiver operating characteristic (ROC) curves, in a subsample of the UK Biobank similar to that used by the Neale lab.
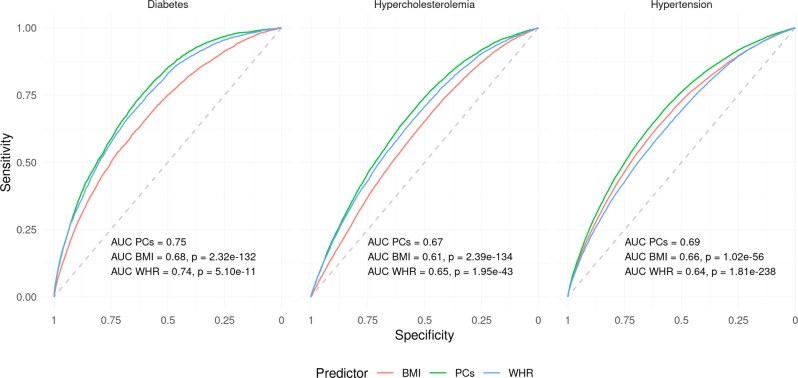
As expected, both BMI and WHR were effective in predicting these obesity-related diseases, as indicated by areas under the ROC curve (AUC) ranging from 0.62 to 0.74 “within-sample.” BMI was more accurate in predicting hypertension (0.66 vs. 0.64, p = 1.3 × 10−72), while WHR was more accurate for high cholesterol (0.65 vs. 0.62, p = 4.9 × 10−101) and slightly more for diabetes (0.74 vs. 0.73, p = 3.0 × 10−5). In all cases, the PC-based predictors significantly outperformed both BMI and WHR with AUCs of 0.68 for hypertension, 0.67 for high cholesterol, and 0.78 for diabetes (all p < 2.3 × 10−183, Supplementary Figs. 67–68, Supplementary Data 29).
To explore the transferability of PCs, we replicated these results out-of-sample in 76,756 non-”white British” UK Biobank participants, who had therefore been excluded from the original summary statistics. Despite the considerable potential for disparities between these samples, the AUCs for high cholesterol and hypertension were almost exactly identical to those obtained above (Fig. 6, Supplementary Fig. 69, Supplementary Data 29), with PC-based predictors significantly outperforming either trait (p ≤ 2.0 × 10−43). The accuracy of PCs and BMI in predicting diabetes out-of-sample decreased to 0.75 and 0.68, respectively, and although WHR was no less accurate out-of-sample (AUC = 0.74) it remained less accurate than PCs (p = 5.1 × 10−11).
Fig. 6. Principal components (PCs) improve prediction of obesity-related diseases out-of-population.
Receiver operating characteristic (ROC) curves for PC-, BMI-, and WHR-based prediction of diabetes, hypercholesterolemia, and hypertension out-of-sample/-population. The indicated p-values for the difference between the PC- and single trait-based curves were obtained using the DeLong method. AUC area under the curve.
We also tested whether a weighted linear combination of BMI and WHR (analogous to that of the 4-PC predictor) would improve the prediction, and found that in most (5 out of 6) cases the AUC of the combined predictor performed worse than the better of the two traits alone. In all cases, this predictor did not exceed the accuracy of the PCs (p < 3.1 × 10−12). This is likely due to the fact that these risk factors are not independent and hence their conferred risk is not simply additive.
Discussion
By combining multiple traits through PCA, we found that more than 99% of genetically determined variation in body shape and size (as defined by the 14 selected traits) can be summarized using four PCs affecting (1) body size, (2) adiposity, (3) predisposition to abdominal fat deposition, and (4) lean mass. PCs 1–3 showed some similarity with weight, BMI, and WHR, respectively, and the latter two especially have been widely studied in the context of obesity. However, these traits are highly correlated and partially redundant, making it difficult to understand their individual contributions to health outcomes, whereas PCs are orthogonal by design and their effects additive. The PCs obtained here also share some similarities with the average PCs (avPCs) obtained by Ried et al.11 by meta-analyzing PCs from individual-level phenotypic data. Specifically, avPC1 increased all body measures (though the impact of height was weaker than for PC1); avPC2 showed opposing directions for height and BMI/WHR (though WHR had much more importance than BMI); avPC3 was dominated by WHR (however both height and BMI contributed as well); and loadings for avPC4 showed an increase in weight and BMI despite decreased waist and hip circumference, suggesting increased density of body mass and consistent with decreased body fat percentage. The robustness of the obtained PCs to changes in the trait selection or the use of male- or female-specific data supports the hypothesis that these represent true biological mechanisms underlying the shared variance across these anthropometric traits. Furthermore, the molecular basis of PCs 3 and 4 in particular appear to be more homogenous, as shown by enrichment for genes expressed in many tissues, such as SAT and VAT but also in the digestive, reproductive, and vascular systems, which were not picked up by individual traits. This is also reflected in the much broader spectrum of pathways enriched only for these PCs.
Leveraging the orthogonality of PCs, we can dissect the etiology of obesity-related health and lifestyle consequences by comparing the effects of different PCs. Both PCs 1 and 2 embody the excessive accumulation of body mass and their enrichment for genes expressed in the brain and involved in neuronal signaling is consistent with findings from BMI25 suggesting that behavioral changes are likely one of the major factors underpinning heritable susceptibility to obesity (reviewed in ref. 26). Shared effects between PC1 and PC2 highlight diseases whose etiology involves elements common to both, such as metabolic overexertion in the case of diabetes27 or the physical burden of a larger body in the case of arthrosis28. Differences between their effects can provide insight into disease etiology which single traits cannot. For example, Hyppönen et al.29 performed a phenome-wide MR analysis to assess the effects of BMI on a number of diseases and found that BMI increased the risk of IHD, cardiac ar-/dysrhythmia, and diseases of the veins (phlebitis and thrombophlebitis), among others. However, our results show that these do not all arise through the same mechanisms, IHD being increased by PCs 2 and 3 but not PC1, consistent with the predominant role of adipose tissue-related dyslipidemia in this disease8, whereas cardiac arrhythmia and diseases of the veins were only increased by PC1. BMI is also a known risk factor for prolapsed disc30, though it is unknown whether this occurs due to mechanical overstraining, dysregulation of the metabolic and immune systems, or a combination of these31. That PC1 alone increased the risk of prolapsed disc strongly supports the role of mechanical stress over any form of lipotoxicity.
The decreased SES associated with PC2 is consistent with what has been reported for BMI32,33, although recent results suggest that this is due to residual population stratification and non-genetic familial effects34,35. Many other differences in lifestyle can be considered as concurrent with this change in SES (e.g., reduced education and income) whereas others are likely secondary to these (e.g., alcohol taken outside of meals36 and increased consumption of cheaper meats). Despite a moderate association with lower SES, PC1 was not associated with any of these secondary/concurrent changes, though the increased time spent using a computer may be indicative of white-collar occupations rather than the physical jobs associated with PC2. The similarity of the effects of both PCs on smoking and alcohol consumption suggests mechanisms independent of SES. For example, increased smoking may reflect the use of smoking as a strategy for weight loss, leveraging the appetite suppressant effects of nicotine37, and is consistent with the observational correlation found between the number of cigarettes smoked and the risk of obesity38. Although we cannot exclude residual population stratification, the increased alcohol consumption found for both PC1 and PC2 suggests a directionality of causal effects which is rarely considered, as obesity is typically viewed as a consequence of alcohol consumption rather than the cause39. The lack of robust genetic instruments to explore the distinct effect of different alcoholic beverages render MR analysis suboptimal to resolve such reverse causations. The increased variation in diet and salt added to food may reflect a greater desire for palatable foods, possibly due to altered or reduced activation of the reward system which has been shown to occur in obese individuals (reviewed in ref. 40). The healthy aspects of the dietary shift from PC1, e.g., greater consumption of fruits and vegetables, could possibly represent attempts to eat healthier, however, this is likely to be highly confounded by biased reporting41. The weak to non-existent effects of cholesterol and other health risks or diseases on these measures of body size suggests that any lifestyle changes upon disease diagnosis are generally minimal and likely insufficient to have a real impact.
PC3, i.e., predisposition to abdominal fat deposition, was of particular interest as a hypothesis-free emergent component affecting body shape while remaining independent of its size or composition. This is in contrast to WHR which is highly correlated with BMI and body fat percentage, particularly in men42. The increased waist circumference at the expense of that of the hips suggests a change in body fat distribution, with a shift from subcutaneous (contributing to both hips and waist) to VAT (contributing to waist alone). This is supported by the enrichment for terms related to energy homeostasis and adipose tissue function, similar to those previously reported for WHR adjusted for BMI43, and the impacts on health which appear specific to lipotoxicity and are similar to what has been found for genetic variants affecting subcutaneous-to-visceral adipose tissue ratio12,13. The implication of genes expressed in adipose tissues, particularly subcutaneous, is in line with the hypothesis that adipose tissue function, in particular its expandability, may be a critical factor in determining the distribution and impact of excess adiposity7 and its study may provide pharmaceutical avenues which may reduce health risks associated with obesity. The enrichment of both PCs 3 and 4 (as well as WHR) for genes expressed in several tissues of the digestive tract is not entirely surprising44, however, their strong enrichment for all female-specific tissues tested was unexpected. It is possible that these tissues, and the genes expressed therein or associated sexual hormones, contribute to this phenotype, which could explain the increased variance of PC3 (and more generally WHR) observed in women compared to men. Additional studies will be required to determine how these genes and tissues are involved in the regulation of body fat distribution.
The additivity implied by the independence of the PCs’ causal effects provides a straightforward estimation of disease risk through linear combination, which proved more accurate than single trait-based prediction. Although causal effects are not ideally suited for within-sample outcome prediction, since they do not take advantage of non-causal correlates, their basis in causality proved reliable even out-of-population. The reason for the drop in accuracy seen for out-of-population PC- and BMI-based prediction of diabetes is unclear, though a number of factors may contribute, such as ethnicity-dependent differences in susceptibility, lifestyle, or ascertainment bias45, which exceed the scope of this study.
The systematic application of MR over a broad range of phenotypes carries the risk of violating its assumptions. Despite the steps taken to avoid any such violations or mitigate their impact, some sources of bias may remain inherent to the methods employed. GWAS effect estimates themselves have been shown to be biased due to population effects, such as parental effects, population stratification, and assortative mating46. Although such biases may be present in the exposure, they would only bias MR estimates if they affect the outcome in a similar fashion34,35, which is difficult to test in a real setting. This study is also subject to other limitations of the MR approach, such as the estimated univariable causal effects summarizing the consequences of a lifetime exposure of a difference in phenotype at the population level. For example, the effect of 1 SD difference in weight (~14 kg) in the population is not equivalent to a sole gain/loss of 14 kg, since this difference in the population distribution will be accompanied by a difference in average height as well (as illustrated by PC1 loadings). The causal effect being modeled linearly, it also averages what may be different effects in different strata of the population, i.e. if the causal effect is non-linear. For example, the effects of BMI on all cause mortality have been shown to be non-linear47, although the linearity of effects on specific diseases such as diabetes remains unclear48. In the case of non-linear effects, the MR causal effect estimates can be interpreted as an average effect at the population mean.
In summary, we established a scale-preserving method for the linear combination of traits at the summary-statistics level, mimicking a GWAS performed on a standardized composite trait but circumventing the need for individual-level data. Furthermore, this allows the combination of traits across different cohorts, since the covariance of effect size estimates between different traits can be estimated from summary statistics via cross-trait LD score regression intercept49. We also showed that cross-sex MR can reduce the bias by avoiding sample overlap, while preserving much of the statistical power from a potential one-sample MR using the full sample, still relying only on publicly available summary statistics. Applying these to the PCs of anthropometric traits, body size, adiposity, abdominal fat distribution, and lean mass, we showed that their distinct effects on health- and lifestyle-related outcomes can aid in understanding the etiology of the consequences of obesity. These components can be visualized and their effects on hundreds of outcomes compared through the shiny app which can be downloaded following the instructions at http://wp.unil.ch/sgg/pca-mr/. Finally, we showed their effectiveness in predicting obesity-related diseases, confirming the additivity of the conferred risks and emphasizing their relevance to disease etiology. Although we identified four PCs here, larger sample sizes or more detailed anthropometric traits may achieve a finer scale identification of obesity subtypes.
Methods
Data and phenotype selection
We used GWAS summary statistics data derived from the UK Biobank16, a cohort of ~500,000 participants aged 37–73 (median 58), recruited between 2006 and 2010. UK Biobank obtained ethics approval from the North West Multi-centre Research Ethics Committee (MREC) and obtained informed consent from all participants. Phenome-wide GWAS in UK Biobank data were performed and the summary statistics made available by the Neale Lab (http://www.nealelab.is/uk-biobank) for three different sex groups: men, women, and both sexes combined. This includes effect estimates for 13.7 million SNPs which were tested for association with 4203 unique phenotypes across 361,194 unrelated, white British individuals (167,020 men, 194,174 women). All were adjusted for age, age2, sex, age ∗ sex, age2 ∗ sex, and the top 20 genetic PCs to correct for population stratification. We excluded SNPs from the HLA region (chr6: 28,477,797–33,448,354, www.ncbi.nlm.nih.gov/grc/human/regions/MHC?asm=GRCh37). For continuous phenotypes, we used effect estimates for the inverse rank-normalized trait. Summary statistics for WHR were calculated in the UK Biobank across a similar sample of 378,139 unrelated, white British individuals (175,155 men, 202,984 women) and correcting for the same covariates after inverse rank-normalization. For binary traits, we divided the effect estimates and standard errors by the square root of the variance of the trait (that of the analyzed sample where provided, otherwise across a similar subset of the UK Biobank). As such, all effects are expressed on the SD scale and comparable with continuous traits. The linear models used to estimate the SNP-binary trait association provide well-calibrated p-values, as long as rare SNPs (MAF < 0.1%) are not evaluated for a trait with a highly imbalanced case fraction (<10%) or as long as the product of MAF and disease prevalence exceeds 0.000150. Since we analyzed diseases only with >1% prevalence, any SNPs with MAF > 1% are safe to use as instruments. The largest fraction of low-frequency (MAF < 1%) IVs were observed for height (88/1704 SNPs, i.e., 5.2% vs. max 2.8% for other traits/PCs) and the lowest disease prevalence were between 1 and 2%. Still the resulting causal effects from MR analysis between height and these diseases did not change appreciably upon the exclusion of IVs below 1% MAF (Supplementary Note 7).
In total, 232 phenotypes that had at least one GWS SNP (p < 5 × 10−8) were divided into five mutually exclusive categories: body measures (14 phenotypes), continuous measures of health (37 phenotypes), dietary habits (19 phenotypes), diseases (108 phenotypes), and lifestyle factors (54 phenotypes), which are briefly described below. The full list of selected phenotypes can be found in Supplementary Data 1.
Body measures included BMI, height, weight, hip and waist circumference, and WHR, as well as bioimpedance-derived fat and lean mass estimates in arms, legs, trunk, and overall body fat percentage. For arms and legs, summary statistics were available for left and right sides. As these were almost identical, the statistics for the left side alone were used. Basal metabolic rate was also included in body measurements as it is derived from bioimpedance measures.
Continuous health outcomes included the biomarker panel of the UK Biobank, including 34 biomarkers measured in either blood or urine, as well as systolic and diastolic blood pressure (BP), heart rate at rest and during effort, and FVC.
Dietary habits were obtained from a food frequency questionnaire (FFQ). UK Biobank also includes more specific questions such as 24-h recall (food consumed on the last day) which is more reliable but less representative, as well as specific questions such as the type of bread or milk typically consumed, however, these were not included in the present analysis.
Disease summary statistics in the UK Biobank were available for self-reported disease status, ICD-10-classified hospital diagnoses, and diseases curated by the Neale Lab in collaboration with the FinnGen consortium (www.finngen.fi). We included data from both self-reported answers and the FinnGen curated diseases, excluding the raw ICD10 diagnoses which were considered less informative. We included any diseases which had a prevalence of at least 1% in the analyzed sample.
Lifestyle included both environmental factors and lifestyle choices, mainly relating to physical activity, alcohol consumption, smoking, sleep, work, and SES.
We restricted SNPs to those in common between the summary statistics from Neale and the UK10K reference panel used for LD pruning. We also removed SNPs with a minor allele frequency below 0.001, resulting in 9,675,947 SNPs. For each trait, SNPs were pruned separately using plink v1.90b6 with the UK10K European LD panel to obtain independent MR instruments. SNPs were considered independent if separated by more than 10 Mb or the linkage disequilibrium was r2 < 0.01.
High-accuracy body composition measurements
In addition to bioimpedance measurements, the UK Biobank provides other body composition phenotypes derived from more accurate methods, namely DXA and MRI. Unfortunately, the sample sizes for these phenotypes were too low (~5000 individuals) for their inclusion as body phenotypes in the PCA. We did, however, investigate whether approximating these traits through linear combination of other available traits (with regression weights calculated in the UK Biobank) could provide a hypothesis-driven alternative to the hypothesis-free PCA approach. We were able to estimate 57 body composition measurements using the same 14 anthropometric and bioimpedance-based traits with varying accuracy (see Supplementary Data 27). 18 out of 57 had an r2 above 80%, including abdominal SAT, and another 18 had r2 above 70%, including VAT. Others, such as bone mass and liver fat percentage could not be accurately approximated using the included traits.
Principal component analysis
PCA was performed on a matrix of effect estimates of independent SNPs on the 14 body traits described above. We selected all SNPs with a GWS effect (p < 5 × 10−8) on at least one of the body traits and pruned them using the same procedure as for single traits (distance > 10 Mb or r2 < 0.01). SNPs were prioritized according to the highest rank within any significantly associated trait, i.e., for each trait, all SNPs significantly associated with it were ranked by p-value; then each SNP has attributed a priority based on the highest rank obtained with any significantly associated trait. This was done to avoid the well-powered traits (e.g., height) overshadowing other traits and driving the SNP selection. Any missing effect estimates in the resulting matrix were set to 0. Data were neither centered nor scaled prior to the PCA, as the effect estimates were standardized and should therefore have zero mean and their variance for a given trait is informative (distantly related to trait heritability).
PCA was then performed on the resulting matrix of genetic effects to obtain the PC loadings, which were then used to calculate SNP-PC associations across the entire genome. These were then individually pruned to obtain the final set of SNPs for each PC.
Although the SNP-phenotype associations are standardized (effects are on an SD/SD scale), a linear combination of effects would yield non-standard effects since the resulting traits do not have a variance of 1. To maintain comparable effects between PCs and traits, the PC loadings should be scaled such that the resulting βs would represent SNP effects on composite traits with unit variance, which would typically involve performing the rotation on the phenotypes, standardizing, and rerunning a GWAS on the resulting composite trait with variance 1. Instead, we can consider the linear regression coefficients for trait j calculated as:
| 1 |
where yj is the vector of values for trait j and G is the genotype matrix, and a weighted sum of n variables:
| 2 |
where wj is the weight for trait j. The effect size for the composite trait can then be calculated as:
| 3 |
The expected variance of variable yC can be written as51
| 4 |
where K is the covariance matrix of the n traits composing yC, which in the case of standardized trait variables, is the correlation matrix. Knowing the variance of the composite trait, we can simply rescale the vector of weights such that the expected variance in the composite output variable yC is equal to one, resulting in standardized effect sizes. Applying this to the PC loadings, we rescaled the weights as follows:
| 5 |
where wi and vi are the unadjusted and adjusted (trait) loading vectors for PC i, respectively, and K is the pairwise phenotypic correlation matrix of body trait phenotypes, which was calculated on a subset of the UK Biobank similar to that used for the summary statistics (i.e. unrelated white British individuals) and the phenotypes were corrected for the same covariates as used by the Neale lab prior to calculating the correlation. The effect estimates were then calculated using these adjusted loadings, i.e.
| 6 |
where is the matrix of genetic effects on the 14 anthropometric traits. The corresponding standard errors were calculated following the formula for a weighted sum of random variables51:
| 7 |
where is the standard error for the association of SNP j with PC i and Σj is a diagonal matrix with the standard error of the association of SNP j with each trait. Since we use only UK Biobank summary statistics, the correlation between effect estimates simplifies to the phenotypic correlation between the traits. The advantage of this approach is that we do not need to calculate the composite trait and run a GWAS but can directly compute the association summary statistics.
Tissue specificity and pathway enrichment
We tested PC- and trait-associated SNPs for the enrichment of tissue-specific genes using the MAGMA tool for gene set analysis17 through the FUMA v 1.3.5e interface18 using the default parameters, with a Bonferroni-adjusted p-value threshold. We used gene expression data in 54 tissues from GTEx v819 as well as brain development and age data from BrainSpan20.
We used the molecular pathway gene sets defined by DEPICT21 and tested for enrichment using PASCAL22. As with most gene-based pathway enrichment methods, we assume that most regulatory variants for a given gene are in close (50 kb) physical proximity to the gene body52, hence we may ignore more distant regulatory variants.
Mendelian randomization
We used IVW MR to estimate causal effects of body traits and PCs on all non-body traits, as well as the reverse. MR mimics a randomized controlled trial (RCT) where the treatment corresponds to the random allocation of an exposure-associated allele, called an instrumental variable (IV)53. MR relies on three key assumptions to infer causality: (1) the IV is associated with a change in the exposure; (2) the IV is independent of the outcome, except through its association with the exposure; (3) the IV is independent of any confounders of the exposure-outcome association. If these assumptions are verified, MR provides an unbiased estimate of the causal effect of the exposure on the outcome and can be done using summary statistics data alone. We used IVW MR as the default method for all our analyses, but we compared IVW causal effect estimates to those obtained from weighted median-based MR to ensure robustness.
Cross-sex MR
In our case, the use of summary data from the UK Biobank for both the exposure and outcome effect sizes (i.e., full sample overlap) would lead to a bias in MR causal effect estimate in the direction of the observed correlation of the phenotypes54. To circumvent this, we used the existing summary statistics for two non-overlapping samples from the Neale Lab, those for men and women separately. Each sex was used as exposure on the other as outcome and then both causal effect estimates were meta-analyzed (using inverse variance weighting). This removed the correlation between the error terms of the effect estimates of IVs on exposure and outcome, considerably reducing the bias from sample overlap, while minimizing loss of power. This would lead to a slight bias away from zero even when there is no true difference in the genetic effects on the exposure in men and women, given that:
| 8 |
where α is the common causal effect (for men and women), βm and βf are the coefficients of association for a given IV with the exposure for men and women, respectively. This bias increases if there is a difference between sexes in the strength of association with the exposure, but its magnitude remains small. For example, if the SNP effects on the exposure were consistently 1.37 times stronger in one sex, the relative theoretical bias would only be 5%. In practice, however, the IVs were filtered for genome-wide significance in each sex prior to being used as exposure, resulting in fewer IVs being selected in the sex with weaker effects (on the exposure). This reduced the power and increased the variance in the overestimated causal effect, which was correspondingly down-weighted by the IVW meta-analysis. For example, we found PC3 to have a strong sex-specificity, with effects on exposure tending to be ~2.2 times stronger in women, which would theoretically lead to a 34% bias away from the null. However, this yielded 242 GWS IVs in women but only 33 in men. This increased the variance in the male exposure–female outcome causal effect estimate by a median of 6.8-fold, resulting in a corresponding down-weighting of the overestimated causal effect. In this example, we expect a meta-analyzed estimate which is biased towards the null by ~32% (since the IVW causal effect estimate is expected to be (((6.1/7.1) ∗ (1/1.7) + (1/7.1) ∗ 1.7) ∗ = 0.68 ∗ ). Importantly, since the bias is multiplicative, this introduces no bias in the absence of causal effect, indicating it will not affect the type I error rate. In practice, the causal effect estimates from cross-sex MR were closer to those obtained from a two-sample setting, whereas the standard IVW MR effect estimates were generally overestimated (see Supplementary Notes 8–9).
Although the PCs were constructed using combined-sex summary statistics, the selected IVs were filtered for genome-wide significance in the sex-specific summary statistics prior to MR analysis to avoid weak instrument bias inflating causal effect estimates. This may slightly exacerbate Winner’s curse, inflating the SNP-exposure association, which would result in a small bias towards the null. In the presence of a true causal effect, however, the SNP-outcome association (being assessed in the same sample) may be proportionally increased, which would mitigate this bias. Such biases are difficult to correct for without using three independent samples.
For each sex, the GWS IVs associated with the exposure formed the initial set of IVs. Those with significantly larger effects on the outcome than on the exposure were removed, as these would indicate a violation of MR assumptions (likely reverse causality and/or confounding). The effect sizes being on a standardized scale, they were compared directly using a one-sided t-test and removed if the magnitude of the effects in the outcome were significantly greater (p < 0.05). To avoid unreliable causal effect estimates, MR was only performed if at least 10 IVs remained. This was done using the TwoSampleMR R package v0.5.455.
The MR causal effect estimates from individual IVs were tested for heterogeneity using Cochran’s Q test:
| 9 |
where βiexp and βiout are the coefficients of association of SNP i with the exposure and outcome, respectively, with σiexp and σiout the corresponding standard errors, and βMR and σMR are the MR estimate and standard error of the causal effect of the exposure on the outcome. The test statistic Qi follows a χ2 distribution with 1 degree of freedom. If any of the IVs used had an associated p < 10−3, the most heterogeneous one (with the lowest p-value) was removed. If at least 5 IVs remained, the MR was then repeated with the remaining IVs. In practice, few SNPs were filtered as outliers (on average <1%), though we also explored alternative methods for dealing with heterogeneity, namely performing no filtering, using weighted median MR with or without filtering, or using exact Q statistics56 instead of Cochran’s Q (see Supplementary Note 10). All of these produced similar effect estimates, though those from weighted median MR tended to be ~6% lower and lacked the statistical power to find many effects.
Due to the standardized scales of IV effect estimates, the resulting causal effects are on a scale representing the SD change in the outcome for a change of 1 SD in the exposure.
To account for multiple testing, we adjusted the p-value threshold with Bonferroni correction for the number of tests within the exposure-outcome category pair. For example, the p-value threshold for the effect of BMI (body phenotype) on type 2 diabetes (disease) is 0.05/(number of body phenotypes ∗ number of diseases).
Although the forward MR analyses using anthropometric traits (whether individual or composite) as exposures were well-powered to find many associations, the reverse/bi-directional MR analyses often lacked sufficient GWS IVs. This can be seen in Supplementary Data 30, showing the total explained variance by all GWS SNPs for each phenotype in each sex group. Only continuous health outcomes had IVs with a reasonably large (combined) contribution to the phenotypic variance. Those of phenotypes from other categories explained at most 3.5% in the combined-sex group (hypertension), though frequently much less, which was further reduced by the restriction for genome-wide significance within the sex used as exposure (to a maximum explained variance of 2.4% for hypothyroidism in women).
Sex-specific MR
The simplest method to test for sex-specific effects would be to use sex-specific summary statistics for the sex of interest for both exposure and outcome, however, this would bias results away from the null due to correlated errors in the exposure and outcome. In most cases, we were instead able to obtain unbiased causal effect estimates using the exposure summary statistics from the opposite sex on those of the sex of interest in the outcome. This relies on the strength of the association between the IVs and the exposure to be identical (or at least not systematically different) between sexes. We tested this using both paired Wilcoxon signed-rank test on the absolute genetic effects of the IVs and total least squares (TLS) regression. Exposures with non-significant results for both tests were considered identical in both sexes for this purpose and the summary statistics for the opposite sex were used for the exposure.
Using the GIANT consortium as a separate sample25, we were able to confirm that the use of opposite-sex summary statistics for the exposure produced effect estimates which were highly consistent with those using two-sample same-sex MR (see Supplementary Note 11).
Comparison of causal effects
To test the significance of a difference in the effects of two exposures on a given outcome, we used a two-sided Z-test57. We accounted for multiple testing using Bonferroni correction, adjusting for the total number of tests, i.e. the number of significant effects of either exposure on the category outcomes. For example, to test whether the effects of BMI on type 2 diabetes were different from those of WHR, the significance threshold would be 0.05/total number of diseases significantly affected by either BMI or WHR.
In some cases, it was useful to compare the causal effects of two exposures on all outcomes of a category (e.g., the effects of weight on disease risk compared to those of PC1) or the same exposure in different experimental settings (comparison of methods or sex-stratified analyses). In these cases, we obtained the slope estimate using TLS regression with no intercept, which considers error in both axes rather than ordinary least squares (OLS) which minimizes only the vertical offset. The standard error on the angle of the regression line (rather than the slope, which is not symmetrical and dependent on the phenotype placed on the y-axis) was computed using a jackknife procedure, which was then used to compare the obtained estimate with the null hypothesis that the true causal effects were identical (i.e., an angle of 45° or a slope of 1). The TLS procedure was performed using the deming R package v1.458.
Prediction of disease risk
The accuracy of disease prediction was assessed in-sample in the UK Biobank across 371,523 unrelated, white British individuals (199,699 women, 171,824 men) for diabetes, high cholesterol, and hypertension. PCs were calculated based on the 14 scaled and centered anthropometric traits for each individual. These were then combined in a disease-specific linear combination based on the estimated causal effect on the disease. Rather than ignoring PCs with non-significant effects, the effect estimates were IVW to account for the uncertainty of the effect:
| 10 |
where γi and σi are the estimated causal effect of PC i on the disease of interest and its standard error, respectively, and pi is the individual’s phenotypic realization of that PC. The combined predictor for BMI+WHR was obtained in an analogous manner using the estimated effects of the respective traits on the outcome of interest.
The accuracy of this predictor was assessed using ROC curves by comparing the AUC with those of BMI and WHR. The significance of the difference between the AUCs for each predictor was determined using DeLong’s test in the pROC R package (version 1.16.2)59.
Out-of-sample prediction accuracy was assessed in a sample of 76,756 UK Biobank participants (42,407 women, 34,349 men) who were not flagged as “in.white.British.ancestry.subset” in the sample QC file. This includes individuals who either did not self-report ‘white British’ or whose genetic ancestry (as determined by the genomic PCs) was dissimilar from other white British individuals. This sample thereby excluded all the individuals included in the original analysis and any relatives in the UK Biobank. The phenotypes were scaled according to the original distribution, i.e. the mean and SD of the original sample were used to scale individuals without relying on the distribution in the second sample. Using the values of the second distribution for scaling changed little (the AUC increased by at most 4.4 × 10−4).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This research has been conducted using the UK Biobank resource (#16389), which has been approved by the National Research Ethics Service Committee. The computations have been carried out on the HPC server of the Lausanne University Hospital. L.D. was calculated based on the UK10K data resource (EGAD00001000740, EGAD00001000741). Z.K. was funded by the Swiss National Science Foundation (31003A-143914 and 310030-189147). B.D. is supported by the Swiss National Science Foundation (NCCR Synapsy, project grant Nr. 32003B_135679, 32003B_159780, 324730_192755, and CRSK-3_190185) and the Leenaards Foundation. LREN is very grateful to the Roger De Spoelberch and Partridge Foundations for their generous financial support. T.O.K. was funded by the Novo Nordisk Foundation (NNF17OC0026848, NNF18CC0034900).
Author contributions
A.S. and J.S. preprocessed the data and performed the initial analyses. N.M. assisted with initial data preprocessing. B.D. contributed to the initial design. J.S. performed the remaining analyses. Z.K. and J.S. developed the methods. Z.K. designed and supervised the project. J.S. wrote the initial manuscript. A.S., N.M., C.A., E.M., L.D., B.D., T.O., P.J., R.F., and Z.K. provided critical feedback, helped interpret the findings and revise the manuscript.
Data availability
UK Biobank summary statistics for WHR can be obtained at wp.unil.ch/sgg/pca-mr/, the others were downloaded from www.nealelab.is/uk-biobank. The external validation summary statistics were downloaded from https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files25. Cross-trait phenotypic correlation and disease risk prediction were performed using data from the UK Biobank (application #16389). L.D. was calculated based on the UK10K data resource (EGAD00001000740, EGAD00001000741).
Code availability
The code for the shiny app and an example pipeline using this method (along with required data files) can be obtained from wp.unil.ch/sgg/pca-mr/.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review informationCommunications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: George Inglis. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-02550-y.
References
- 1.Boles A, Kandimalla R, Reddy PH. Dynamics of diabetes and obesity: epidemiological perspective. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:1026–1036. doi: 10.1016/j.bbadis.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ. Res. 2016;118:1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 3.Pischon T, et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 4.Kaess BM, et al. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55:2622–2630. doi: 10.1007/s00125-012-2639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenquist KJ, et al. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc. Imaging. 2013;6:762–771. doi: 10.1016/j.jcmg.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132:1639–1647. doi: 10.1161/CIRCULATIONAHA.114.015000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome−an allostatic perspective. Biochim. Biophys. Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 2017;376:1492. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Hu T, Zhang S, Zhou L. Associations of different adipose tissue depots with insulin resistance: a systematic review and meta-analysis of observational studies. Sci. Rep. 2015;5:18495. doi: 10.1038/srep18495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter SA, et al. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ried JS, et al. A principal component meta-analysis on multiple anthropometric traits identifies novel loci for body shape. Nat. Commun. 2016;7:13357. doi: 10.1038/ncomms13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaghootkar H, et al. Genetic evidence for a normal-weight ‘metabolically obese’ phenotype linking insulin resistance, hypertension, coronary artery disease, and type 2 diabetes. Diabetes. 2014;63:4369–4377. doi: 10.2337/db14-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaghootkar H, et al. Genetic evidence for a link between favorable adiposity and lower risk of type 2 diabetes, hypertension, and heart disease. Diabetes. 2016;65:2448–2460. doi: 10.2337/db15-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotta LA, et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 2017;49:17–26. doi: 10.1038/ng.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji Y, et al. Genome-wide and abdominal MRI data provide evidence that a genetically determined favorable adiposity phenotype is characterized by lower ectopic liver fat and lower risk of type 2 Diabetes, heart disease, and hypertension. Diabetes. 2019;68:207–219. doi: 10.2337/db18-0708. [DOI] [PubMed] [Google Scholar]
- 16.Sudlow C, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 2015;11:e1004219. doi: 10.1371/journal.pcbi.1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 2017;8:1826. doi: 10.1038/s41467-017-01261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguet F, et al. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369:1318–1330. doi: 10.1126/science.aaz1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JA, et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pers, T. H. et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat. Commun.6, 5820 (2015). [DOI] [PMC free article] [PubMed]
- 22.Lamparter D, Marbach D, Rueedi R, Kutalik Z, Bergmann S. Fast and rigorous computation of gene and pathway scores from SNP-based summary statistics. PLoS Comput. Biol. 2016;12:e1004714. doi: 10.1371/journal.pcbi.1004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang J, et al. Association of serum albumin levels with kidney function decline and incident chronic kidney disease in elders. Nephrol. Dial. Transpl. 2018;33:986–992. doi: 10.1093/ndt/gfx229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marra M, et al. Assessment of body composition in health and disease using bioelectrical impedance analysis (BIA) and dual energy x-ray absorptiometry (DXA): a critical overview. Contrast Media Mol. Imaging. 2019;2019:3548284. doi: 10.1155/2019/3548284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locke AE, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Klaauw AA, Farooqi IS. The hunger genes: pathways to obesity. Cell. 2015;161:119–132. doi: 10.1016/j.cell.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Halban PA, et al. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37:1751–1758. doi: 10.2337/dc14-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duclos M. Osteoarthritis, obesity and type 2 diabetes: the weight of waist circumference. Ann. Phys. Rehabil. Med. 2016;59:157–160. doi: 10.1016/j.rehab.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Hyppönen E, Mulugeta A, Zhou A, Santhanakrishnan VK. A data-driven approach for studying the role of body mass in multiple diseases: a phenome-wide registry-based case-control study in the UK Biobank. Lancet Digit Health. 2019;1:e116–e126. doi: 10.1016/S2589-7500(19)30028-7. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder GD, Guyre CA, Vaccaro AR. The epidemiology and pathophysiology of lumbar disc herniations. Semin. Spine Surg. 2016;28:2–7. doi: 10.1053/j.semss.2015.08.003. [DOI] [Google Scholar]
- 31.Weiler C, et al. Histological analysis of surgical lumbar intervertebral disc tissue provides evidence for an association between disc degeneration and increased body mass index. BMC Res. Notes. 2011;4:497. doi: 10.1186/1756-0500-4-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Beydoun MA. The obesity epidemic in the united states gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol. Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 33.Howe, L. D. et al. Effects of body mass index on relationship status, social contact and socio-economic position: Mendelian randomization and within-sibling study in UK Biobank. Int. J. Epidemiol. 10.1093/ije/dyz240 (2019). [DOI] [PMC free article] [PubMed]
- 34.Darrous, L., Mounier, N. & Kutalik, Z. Simultaneous estimation of bi-directional causal effects and heritable confounding from GWAS summary statistics. Preprint at medRxiv10.1101/2020.01.27.20018929 (2020). [DOI] [PMC free article] [PubMed]
- 35.Brumpton B, et al. Avoiding dynastic, assortative mating, and population stratification biases in Mendelian randomization through within-family analyses. Nat. Commun. 2020;11:3519. doi: 10.1038/s41467-020-17117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oksanen A, Kokkonen H. Consumption of wine with meals and subjective well-being: a finnish population-based study. Alcohol Alcohol. 2016;51:716–722. doi: 10.1093/alcalc/agw016. [DOI] [PubMed] [Google Scholar]
- 37.Mineur YS, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dare S, Mackay DF, Pell JP. Relationship between smoking and obesity: a cross-sectional study of 499,504 middle-aged adults in the UK general population. PLoS ONE. 2015;10:e0123579. doi: 10.1371/journal.pone.0123579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traversy G, Chaput J-P. Alcohol consumption and obesity: an update. Curr. Obes. Rep. 2015;4:122–130. doi: 10.1007/s13679-014-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol. Behav. 2009;97:551–560. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pirastu, N. et al. Using genetics to disentangle the complex relationship between food choices and health status. In PLoS Genetics10.1101/829952 (2019).
- 42.Sulc J, Winkler TW, Heid IM, Kutalik Z. Heterogeneity in obesity: genetic basis and metabolic consequences. Curr. Diab. Rep. 2020;20:1. doi: 10.1007/s11892-020-1285-4. [DOI] [PubMed] [Google Scholar]
- 43.Shungin D, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winkler TW, et al. A joint view on genetic variants for adiposity differentiates subtypes with distinct metabolic implications. Nat. Commun. 2018;9:1946. doi: 10.1038/s41467-018-04124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goff LM. Ethnicity and type 2 diabetes in the UK. Diabet. Med. 2019;36:927–938. doi: 10.1111/dme.13895. [DOI] [PubMed] [Google Scholar]
- 46.Young, A. I. et al. Mendelian imputation of parental genotypes for genome-wide estimation of direct and indirect genetic effects. Preprint at bioRxiv10.1101/2020.07.02.185199 (2020).
- 47.Sun Y-Q, et al. Body mass index and all cause mortality in HUNT and UK Biobank studies: linear and non-linear mendelian randomisation analyses. BMJ. 2019;364:l1042. doi: 10.1136/bmj.l1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wainberg M, et al. Homogeneity in the association of body mass index with type 2 diabetes across the UK Biobank: a Mendelian randomization study. PLoS Med. 2019;16:e1002982. doi: 10.1371/journal.pmed.1002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bulik-Sullivan B, et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 2015;47:1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loh P-R, Kichaev G, Gazal S, Schoech AP, Price AL. Mixed-model association for biobank-scale datasets. Nat. Genet. 2018;50:906–908. doi: 10.1038/s41588-018-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson AR, Wichern DW. Applied multivariate statistical analysis. Biometrics. 1988;44:920. doi: 10.2307/2531616. [DOI] [Google Scholar]
- 52.Boix CA, James BT, Park YP, Meuleman W, Kellis M. Regulatory genomic circuitry of human disease loci by integrative epigenomics. Nature. 2021;590:300–307. doi: 10.1038/s41586-020-03145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013;37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 2016;40:597–608. doi: 10.1002/gepi.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife7, e34408 (2018). [DOI] [PMC free article] [PubMed]
- 56.Bowden J, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int. J. Epidemiol. 2019;48:728–742. doi: 10.1093/ije/dyy258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paternoster R, Brame R, Mazerolle P, Piquero A. Using the correct statistical test for the equality of regression coefficients. Criminology. 1998;36:859–866. doi: 10.1111/j.1745-9125.1998.tb01268.x. [DOI] [Google Scholar]
- 58.Therneau, T. deming: Deming, Theil-Sen, Passing-Bablock and Total Least Squares Regression, R package version 1.4. https://CRAN.R-project.org/package=deming (2018).
- 59.Robin X, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinforma. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
UK Biobank summary statistics for WHR can be obtained at wp.unil.ch/sgg/pca-mr/, the others were downloaded from www.nealelab.is/uk-biobank. The external validation summary statistics were downloaded from https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files25. Cross-trait phenotypic correlation and disease risk prediction were performed using data from the UK Biobank (application #16389). L.D. was calculated based on the UK10K data resource (EGAD00001000740, EGAD00001000741).
The code for the shiny app and an example pipeline using this method (along with required data files) can be obtained from wp.unil.ch/sgg/pca-mr/.