Abstract
Background:
Eya2 expression during mouse development has been studied by in situ hybridization and it has been shown to be involved skeletal muscle development and limb formation. Here, we generated Eya2 knockout (Eya2−) and a lacZ knockin reporter (Eya2lacZ) mice and performed a detailed expression analysis for Eya2lacZ at different developmental stages to trace Eya2lacZ-positive cells in Eya2-null mice. We describe that Eya2 is not only expressed in cranial sensory and dorsal root ganglia, retina and olfactory epithelium, and somites as previously reported, but also Eya2 is specifically detected in other organs during mouse development.
Results:
We found that Eya2 is expressed in ocular and trochlear motor neurons. In the inner ear, Eya2lacZ is specifically expressed in differentiating hair cells in both vestibular and cochlear sensory epithelia of the inner ear and Eya2−/− or Eya2lacZ/lacZ mice displayed mild hearing loss. Furthermore, we detected Eya2 expression during both salivary gland and thymus development and Eya2-null mice had a smaller thymus.
Conclusions:
As Eya2 is coexpressed with other members of the Eya family genes, these results together highlight that Eya2 as a potential regulator may act synergistically with other Eya genes to regulate the differentiation of the inner ear sensory hair cells and the formation of the salivary gland and thymus.
Keywords: cranial placodes, cranial sensory ganglia, Eya2, hearing loss, inner ear hair cells, neural crest cells, ocular/trochlear motor neurons, salivary gland, sensory organs, thymus
1 |. INTRODUCTION
Eya2 is a member of the Eyes absent (Eya) family proteins, which are defined by the presence of a highly conserved 271 amino acid carboxyl terminal Eya domain (ED).1 The conserved ED of Eya not only participates in protein-protein interactions but also possesses a catalytic motif belonging to the phosphatase subgroup of the haloacid dehalogenase (HAD) superfamily of enzymes,2–4 while the divergent N-terminus of Eya possesses a transcriptional activation function.5 In Drosophila, Eya has a critical role in eye formation and eya mutation causes progenitor cells in the eye imaginal disc to undergo programmed cell death.6 Eya functions in a regulatory network important to eye formation, including eyeless (Ey), sine oculis (So), and dachshund (Dac), all of which have at least some ability to direct ectopic eye formation and are part of a complex feedback loop that regulates their own expression.7–12
Four different Eya genes (Eya1–4) have been isolated in mouse and human.1,13–16 A number of studies have shown that overexpression of all four human EYA1–4 genes has been associated with various cancers, including colorectal cancer, epithelial ovarian cancer, lung cancer, prostate cancer and breast cancer,17–21 while overexpression studies in transgenic mice have implicated a role for Eya2 in cardiac hypertrophy.22,23 Mutations in the human EYA1 cause branchio-oto-renal (BOR) or branchio-otic (BO) syndrome16 and sporadic cases of congenital cataracts and ocular anterior segment anomalies.24 On the other hand, the majority of EYA4 mutations are associated with autosomal dominant non-syndromic sensorineural hearing loss (DFNA10) and mild cardiac phenotype.25–27 In contrast, EYA2 and EYA3 have not yet been linked to any human syndromes. Loss of function studies in mice have revealed essential function for Eya1 in multiple organ systems including the cranial sensory neurogenesis, inner ear, parathyroid, thymus and kidney28–30 and Eya4 in otitis media.31 In contrast, loss of Eya3 in mice displayed a broad spectrum of minor physiological changes, including decreased bone mineral content and shorter body length.32 We have generated Eya2 knockout mice and Eya2-null mice are viable and fertile without obvious external phenotype. This could be due to a functional redundancy with other members of the Eya family genes, which is supported by the observation that Eya2 has a synergistic role with Eya1 during somatic myogenic precursor cell differentiation.33 Consistent with this view, overlapping expression of both Eya2 and Eya1 was observed in multiple tissues in a mouse embryo, including cranial sensory structures, myogenic precursors and connective precursor cells in the limb.1,5 Interestingly, a recent study reported that deletion of eya2 in axolotl through CRISPR/Cas9 impairs cell cycle progression and results in elevated DNA damage in the limb.34 However, a systematic analysis of the functional role of Eya2 during mouse development and its potential redundancy with other Eya family members is still lacking.
Our previous in situ hybridization detected that at E9.5–10.5, Eya2 is predominantly expressed in sensory structures in the head, including the cranial sensory ganglia and olfactory placode. At E12.5–E14.5, Eya2 expression is maintained in the olfactory epithelium and becomes detectable in the retina, sclera and optic nerve sheath.1 However, it remains unclear whether Eya2-expressing cranial sensory ganglia at E9.5–10.5 in the head is neural crest cell (NCC)- and/or ectodermal placode-derived cells. Furthermore, it is not known whether Eya2 could be a potential regulator of other cranial sensory or developmental processes during mouse organogenesis.
To address this, in addition to the Eya2 knockout (KO) mice, we generated a lacZ knockin (KI) reporter mice and performed β-Gal staining for the Eya2lacZ on whole embryos or sections at different stages of development and traced the β-Gal-positive cells in Eya2lacZ/lacZ homozygotes. Here, we show that Eya2 is exclusively expressed in NCC-derived components of cranial sensory ganglia Vth, VIIth, VIIIth, IXth and IXth, and in other sensory and developmental systems that have not been reported so far: ocular/trochlear motor neurons, inner ear, salivary gland and thymus. We describe that during inner ear development, β-Gal activity is specifically accumulated in differentiating sensory hair cells of all sensory patches and that both Eya2lacZ/lacZ or Eya2−/− mice displayed mild hearing loss. During salivary gland development, β-Gal is expressed in the initial epithelial bud as thickening of the primitive oral epithelium and later during branching morphogenesis, β-Gal is stronger in the lumenized main duct and weaker in the end buds. Moreover, we show that during thymus development, β-Gal is expressed in the parathyroid/thymus primordium of the third pharyngeal pouch endoderm, but later on its expression becomes restricted to the thymus lobes. In the Eya2 homozygotes, the thymic lobes appear smaller in size compared to the heterozygous controls. Altogether, these results complete a study describing the generation of Eya2 KO and Eya2lacZ KI mice and the expression of Eya2 revealed by the Eya2lacZ reporter in both vestibular and cochlear hair cells as well as developing salivary gland and thymus. This work reveals a potential role for Eya2 in these structures. The Eya2lacZ mice generated here can be used as a tool for further systematically investigating the differentiation and maintenance of Eya2-expressing cells in different organ systems.
2 |. RESULTS AND DISCUSSION
2.1 |. Generation of Eya2 knockout and Eya2lacZ knockin alleles
To inactivate the Eya2 gene, we generated a null allele by replacing exons 11 encoding 55 residues of the conserved 271-amino acid ED domain (Figure 1A, see Experimental Procedures), which mediates protein-protein interactions and possesses intrinsic phosphatase activity. Eya2−/− mice were fertile with normal appearance, suggesting that there may be functional redundancy with other members of the Eya family genes. Consistent with this view, analysis of muscle development found that both Eya1 and Eya2 function synergistically and are necessary for hypaxial somitic myogenesis in the mouse embryo, as Pax3 expression is lost in the ventrolateral (hypaxial) dermomyotomes of the somite in Eya1−/−; Eya2−/− mice.33 In order to follow the fate of the cells after Eya2 depletion, we generated a Eya2lacZ knockin allele by homologous recombination in ES cells to replace exon 2, which contains the endogenous start codon of Eya2, with a promoterless ATG-lacZ-poly(A) cassette and the PGK-neo gene (Figure 1B). Germline transmission of the targeted allele was confirmed by southern blot with a 5′ flanking probe and PCR using a common forward primer in the intron 1 and a reverse primer within the exon2 or within the lacZ gene for detecting Eya2 wild-type or lacZ knockin allele (Figure 1C,D). Similar to Eya2−/− mice, all Eya2lacZlacZ mice are fertile without obvious external phenotype.
Figure 1.

Generation of Eya2 knockout (KO) and Eya2lacZ reporter knockin (KI) mice through gene targeting. A, Schematic diagram illustrating the Eya2 protein and targeted KO deletion of the 54 amino acids within the ED. B, Schematic diagram illustrating the Eya2 locus and targeted Eya2lacZ KI. The endogenous start codon and the exon 1 were replaced by Escherichia coli ATG-lacZ-poly(A) cassette and the neo positive selection cassette. C, Southern blot analysis of ES cell clones. Genomic DNA was digested with EcoRI and XhoI, and probes with the 5′ flanking probe (550-bp XhoI-NotI fragment) and the resulting wild-type band is 9.71-kb and the targeted band is 2.4-kb. D, Heterozygous or homozygous progeny were genotyped by PCR in a same reaction to amplify a 410-bp wild-type band and a 320-bp lacZ knockin band. E and F, β-Gal staining of Eya2lacZ expression in heterozygous and homozygous mouse embryos at E10.5. E′ and F′, Sagittal sections showing β-Gal activity in the motor neurons of the oculomotor (III) and trochlear (IV) nuclei. In panel E′, the bottom panel is the boxed area of the upper panel. G, Whole-mount β-Gal staining of Eya2lacZ in heterozygous embryo at E9.5. Arrow points to cculomotor (III) and trochlear (IV) nerves. G′ and G″, Transverse sections of embryo shown in F. Arrow points to epibranchial placodal ectoderm (outlined by dashed line). V to X, cranial sensory ganglia Vth to Xth; DRG, dorsal root ganglion; Ot, otic vesicle; Ol, invaginating olfactory epithelium; RP, Ratheke’s pouch; Tr, trigeminal placodal ectoderm. Scale bar: 250 μm (E′, F′) and 100 μm (G′, G″, G‴)
As the lacZ reporter gene is driven by the endogenous Eya2 promoter, we next performed β-Gal staining for the expression of Eya2lacZ allele and found β-Gal activity in the nose, eye, ocular (III) and trochlear (IV) motor neurons, Rathke’s pouch, cranial sensory ganglia—the trigeminal (V), facioacoustic (VII–VIII), glossopharyngeal (IX) and vagus (X) ganglia—and the dorsal root ganglion (Figure 1E,E′). As expected, Eya2lacZ/lacZ homozygotes displayed similar pattern of β-Gal expression with stronger β-Gal activity compared to heterozygotes (Figure 1F, F′). This pattern recaptured the pattern of endogenous Eya2 expression detected by in situ hybridization.1 Thus, these mice provide a valuable model for assessing Eya2 expression and tracing the cells after Eya2 depletion.
The sensory ganglia in the vertebrate head are composed of neural crest cell (NCC)-derived neurons and glia and ectodermal placode-derived neurons. The trigeminal and epibranchial (geniculate, petrosal, and nodose) placodes produce neuronal precursor cells that directly delaminate to contribute sensory neurons to the distal part of the Vth (trigeminal) and of the VIIth (geniculate), IXth (petrosal) and Xth (nodose) cranial nerves respectively, whereas the NCCs produce the proximal components and associated glia of these cranial nerves.35,36 The VIIIth (vestibuloacoustic) ganglion is composed of the otic placode-derived neurons and NCC-derived glia and some neurons.37 We observed Eya2 expression in a subset of cranial placodes—the neurogenic olfactory placode1,38 and the non-neurogenic placode-derived Rathke’s pouch (Figure 1E), but not in the non-neurogenic lens1 and neurogenic otic placode-derived otocyst (Figure 1E), which differentiates into all inner ear structures. Although Eya2 is strongly expressed in all cranial ganglia, it remains unclear whether Eya2-expressing cells in the Vth, VIIth, IXth and Xth are derived from NCCs, placodes or both. As the NCCs migrate from the neuroectoderm and Eya2lacZ is expressed in the dorsal tip of the neural tube and migratory NCCs (asterisk in Figure 1E), we thus hypothesized that Eya2 expression may be restricted to or predominantly in NCC-derived parts of the Vth, VIIth, VIIIth, IXth and Xth ganglia. To test this, we performed X-gal staining at E9.5 when the thickened placodes, which are transient structures, are readily detectable. At E9.5, β-Gal+ cells were detected in Vth, VIIth and VIIIth ganglia, while fewer β-Gal+ cells were observed in the XIth and Xth ganglionic regions (Figure 1G). On sections, β-Gal activity was observed in the trigeminal placodal ectoderm (Figure 1G′) and Vth ganglion (Figure 1G″), whereas β-Gal+ cells were not observed in epibranchial placode (arrow, Figure 1G‴) or otocyst (Figure 1G‴). In addition to NCC-derived components of the VIIth and VIIIth ganglia (Figure 1F′), we observed β-Gal+ cells in the mesenchyme surrounding the otocyst or migratory cells in the VIIth ganglionic region. Thus, in addition to the NCC-derived parts of cranial sensory ganglia, Eya2lacZ may be expressed in some differentiating neuronal progenitors that have delaminated from the otocyst or epibranchial placodal ectoderm. The selective expression of Eya2 in the ocular and trochlear motor neurons suggests that Eya2 may have a role in these motor neuron development. Previous studies have shown unique expression of several factors, including Phox2b, Wnt1 and Lmx1b,39 in the ocular and trochlear motor neurons. Further characterization of the Eya family gene expression and their functional redundancy in these motor neurons will reveal the potential relationship of Eya genes with other regulators during ocular and trochlear motor nerve development.
2.2 |. Expression of Eya2 in olfactory epithelium, eye, dorsal root ganglion and somite
Consistent with our previous observation obtained by in situ hybridization,1,38 strong β-Gal activity was observed in the olfactory placode and invaginating olfactory epithelium at E9.5–10.5 (Figure 1E,F,G,G′). At E12.5, Eya2lacZ was strongly expressed in the olfactory system (Figure 2A). The olfactory placode is a neurogenic placode and it differentiates into neuronal and non-neuronal cells in the olfactory system. The mammalian olfactory epithelium (OE) is composed of sensory neurons, which are generated in the basal region and extend apically to the nasal cavity, and an apical layer of glial-like sustentacular cells (Figure 2B).40,41 X-gal staining on OE sections from E14.5 revealed that β-Gal activity is restricted to the neuronal progenitors in the basal layer but not in the sustentacular cells in the apical layer of the OE (Figure 2B). β-Gal activity was also detected in the vomeronasal organ (VNO) (Figure 2C). There was no obvious difference in the expression levels of Eya2lacZ in the OE between Eya2lacZ/+ and Eya2lacZ/lacZ. As in mammals, initial detection of olfactory stimuli is mediated by sensory neurons in the OE and VNO, the expression of Eya2 in the olfactory sensory neurons suggests that Eya2 may be involved in olfactory sensory neurogenesis.
Figure 2.
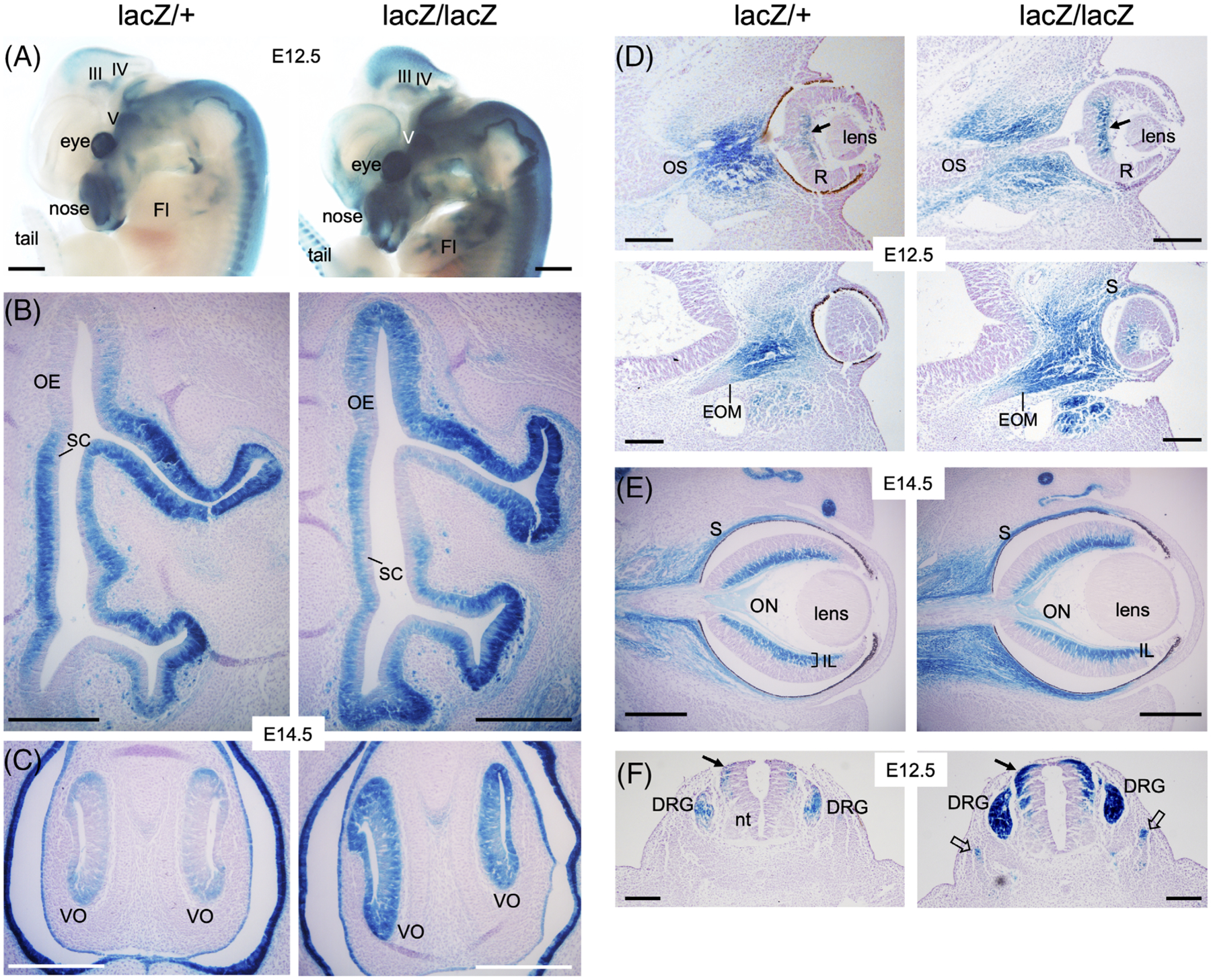
Eya2lacZ reporter is expressed in differentiating olfactory epithelium, retina, dorsal root ganglion and somite. A, Whole-mount X-gal staining of E12.5 embryos showing Eya2lacZ expression in the cranial ganglia and nerves, olfactory system, eye and dorsal root ganglia and forelimb (Fl) in Eya2lacZ/+ and Eya2lacZ/lacZ. III/IV, oculomotor/trochlear nuclei; V, Vth ganglion. B and C, X-gal staining on transverse sections revealing Eya2lacZ expression in the basal neuronal progenitors but not in the apical layer of sustentacular cells (SC) of the olfactory epithelium (OE) and vomeronasal organ (VNO) in Eya2lacZ/+ and Eya2lacZ/lacZ at E14.5. D, X-gal staining at E12.5 showing β-Gal activity in mesenchymal cells surrounding optic stalk (OS), in extraocular muscles (EOM), in sclera (S) and inner layer (arrow) in the center of retina (R) in both heterozygotes and homozygotes. E, At E14.5, β-Gal activity was similarly detected in the sclera and inner layer (IL) of the retina and weakly in the optic nerve (ON) in both heterozygotes and homozygotes. F, X-gal staining showing β-Gal activity in dorsal root ganglion (DRG) and dorsal neural tube (arrows) in Eya2lacZ/+ and with stronger intensity in Eya2lacZ/lacZ, in which β-Gal activity also became detectable in somite (open arrows). Scale bar: 500 μm (A) and 150 μm (B–F)
The lens is non-neurogenic and differentiates into both lens fiber and lens epithelial cells.42,43 Eya2 expression was not detected in the lens placode at E9.5–10.5.1 Consistent with this, Eya2lacZ expression was also not observed in lens development at any stages, while whole mount revealed Eya2lacZ expression in the mesenchyme around the eye (Figure 1E). In the retina, X-gal staining of Eya2lacZ at E12.5 showed β-Gal activity in Eya2lacZ/+ and Eya2lacZ/lacZ (Figure 2D). By E14.5, β-Gal activity is restricted to the inner nuclear layer of the retina with similar intensity between Eya2lacZ/+ and Eya2lacZ/lacZ (Figure 2E). β-Gal activity was also observed in the sclera, extraocular muscles, and nerve fibers within the optic stalk, which will develop into optic (II) nerve (Figure 2D,E). This is consistent with our previous observation detected by in situ hybridization.1
In addition, β-Gal activity was observed in limb tendons (Figure 2A), dorsal root ganglion and somite with stronger intensity in the homozygotes compared to the heterozygotes (Figure 2F). Altogether these data support that Eya2 may have a role in olfactory and retinal neuronal differentiation, dorsal root ganglion, limb and somite.
2.3 |. Eya2 is expressed in differentiating sensory hair cells of all inner ear sensory organs
While Eya2 expression was not observed in the otic ectoderm at earlier stages its expression during inner ear development at later stages has not been examined. To analyze whether Eya2 could be a potential regulator in the inner ear or other developmental processes, we performed detailed X-gal staining to detect Eya2lacZ expression during mouse embryogenesis. During inner ear development, the sensory progenitors are specified within the ventral region of the otocyst at E10.5 and proliferate to expand. After reaching a defined number, they undergo differentiation in the vestibule around E12.5 and in cochlea around E14.5 to form sensory hair cells or underlying supporting cells within each sensory epithelium in the inner ear. X-gal staining of Eya2lacZ at E9.5–12.5 failed to detect β-Gal activity in the otic epithelium of either heterozygotes or homozygotes (Figure 3A). In contrast, Eya2lacZ expression persists in the dorsal neural tube and the sensory ganglia of Vth, VIIth, VIIIth, IXth, Xth nerves and cranial and spinal part of the accessory (XI) nerve with weaker β-Gal activity in the heterozygotes and stronger activity in homozygotes (Figure 3A). By E14.5–15.5, β-Gal activity was readily detected in the differentiating sensory hair cells of all five vestibular sensory organs, the macula of utricle and saccule and the crista ampullaris of each of the semicircular canals in both Eya2lacZ/+ and Eya2lacZ/lacZ (Figure 3B,C). The differentiation of the sensory organ for hearing (the organ of Corti) in the cochlea occurs E14.5 in mid-basal region toward base and apex and medial-to-lateral (inner-to-outer hair cell) directions. β-Gal activity became detectable in the organ of Corti of the basal cochlea (bracket, Figure 3B,D). As differentiation proceeds, β-Gal activity became progressively detectable toward apex and reached apex by E18.5 in both inner and outer hair cells (Figure 3D).
Figure 3.
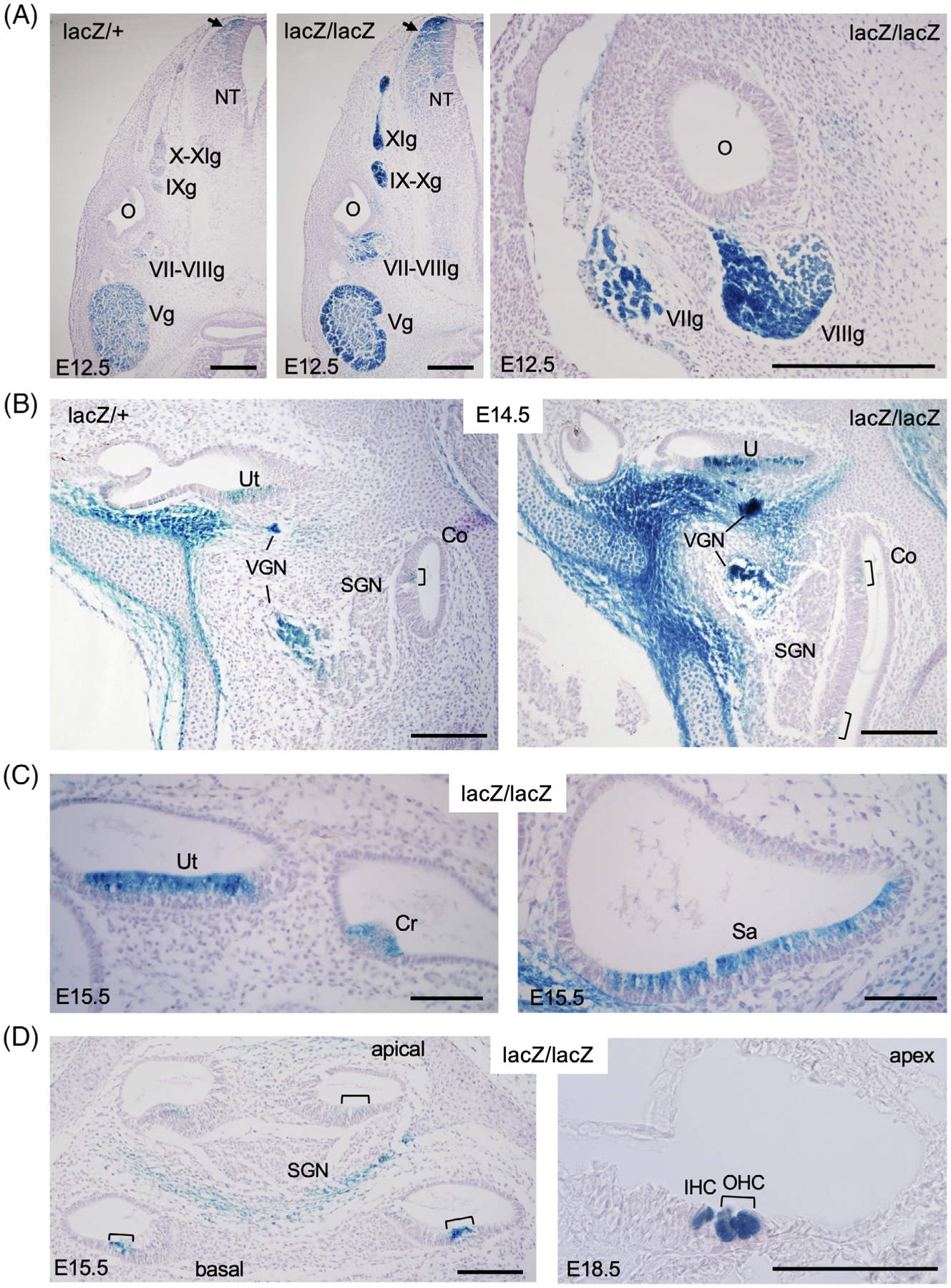
Eya2lacZ reporter is expressed in differentiating sensory hair cells in all six sensory epithelia of the inner ear. A, X-gal staining revealing β-Gal activity in Vth–XIth ganglia (Vg-XIg and dorsal neural tube (arrow) at E12.5. O, otic/inner ear; NT, neural tube. B, At E14.5, β-Gal activity was detected in vestibular ganglion neurons (VGN) and differentiating hair cells in utricle (U) and faintly in the hair cells of the organ of Corti (brackets) of the basal cochlea (Co) in both Eya2lacZ/+ and Eya2lacZ/lacZ. C, At E15.5, β-Gal activity was detected differentiating hair cells in all vestibular sensory epithelia, including utriclar macula (Ut), cristae ampullaris (Cr), sacullar macula (Sa). D, In the cochlea, stronger β-Gal activity was observed in the basal turn but weaker in the apical turns of the organ of Corit (brackets) at E15.5 and by E18.5, stronger β-Gal activity was observed in both inner (IHC) and outer hair cells (OHC) throughout the cochlear duct. Scale bar: 300 μm (A), 150 μm (B), 75 μm (C), and 100 μm (D)
The vestibuloacoustic VIIIth ganglion differentiates into vestibular and spiral ganglion. We observed β-Gal activity in the vestibular ganglion but not in the spiral ganglion neurons (Figure 3B,D). Some β-Gal activity was observed in cells surrounding the spiral ganglion neurons in the cochlea (Figure 3D). Thus, it is possible that Eya2 is specifically turned on in a subset of differentiating VIIIth ganglion neuronal progenitors that will differentiate into vestibular ganglion neurons. Our results also indicate that Eya2 expression is specifically turned on in the differentiating hair cells of all six sensory epithelia in the inner ear.
2.4 |. Eya2lacZ/lacZ mice exhibit hearing loss
Eya2lacZ/lacZ or Eya2−/− mice do not exhibit vestibular dysfunction such as headtossing or circling behavior, but some homozygotes showed a weaker response to Preyer reflex test (Figure 4A). We therefore used measurements of auditory brainstem response (ABR) thresholds to assess hearing deficiency of Eya2lacZ/lacZ mice and the control heterozygous littermates at 8 weeks of age. Compared to the baseline, ABR thresholds were significantly elevated in frequency stimuli ranging from 5.6 to 45.2 kHz in Eya2lacZ/lacZ mice (n = 7) compared with control littermates (n = 5) (Figure 4B), while 3 mice (one male and two females) had no ABR response. There were no statistically significant differences between the right and left ears or male and female animals.
Figure 4.

Eya2lacZ/lacZ mice show mild hearing loss. A, Gross and behavioral abnormalities of Eya2lacZ/+ and Eya2lacZ/lacZ mice. B, ABR threshold measurements of 8 weeks old mice. Three Eya2lacZ/lacZ mice (one male and two females) that had no ABR response were not included. Average threshold ± s.e.m. for Eya2lacZ/+ (n = 5) and Eya2lacZ/lacZ (n = 7) ears at 8 weeks of age (threshold shift by 20 to 31 dB in right ear and 20 to 25 dB in left ear of homozygotes)
We have previously reported that no inner ear structure forms in Eya1−/− mice28 and no inner ear hair cell formation in Eya1 conditional mutant mice when Eya1 was deleted at later stages.44 Thus, while Eya1 has an early role in inner ear development, Eya2 may have a redundant role with Eya1 specifically in differentiating hair cells.
2.5 |. Eya2 is expressed in salivary gland development
Interestingly, we also detected β-Gal activity in the salivary gland (Figure 5), which was also not reported previously. The salivary gland contains three major types—submandibular (SMG) secreting seromucous saliva, sublingual (SLG) secreting mucous saliva, and parotid (PG) secreting serous saliva, all of which arose as epithelial buds in the oral cavity. Among them, the SMG is the most commonly studied and the SMG bud forms around E12.5 in mice.45 At this stage, very faint β-Gal activity was only observed in the PG bud region in Eya2lacZ/+ (Figure 5A). In Eya2lacZ/lacZ, β-Gal activity appeared stronger in the PG bud region, and weaker in the SMG bud (Figure 5A). The bud undergoes branching to produce a cluster of branches and buds, which continue branching to produce a multi-lobed gland by E14.5. The main duct begins to lumenize, while the end buds undergo reorganization and begin to form acini—the main secretory units of the salivary gland. At E14.5, β-Gal activity was observed in all three types of the salivary glands, with stronger intensity in the initial duct (Figure 5B–D). These data suggest that Eya2 may have a role in salivary gland development or function.
Figure 5.
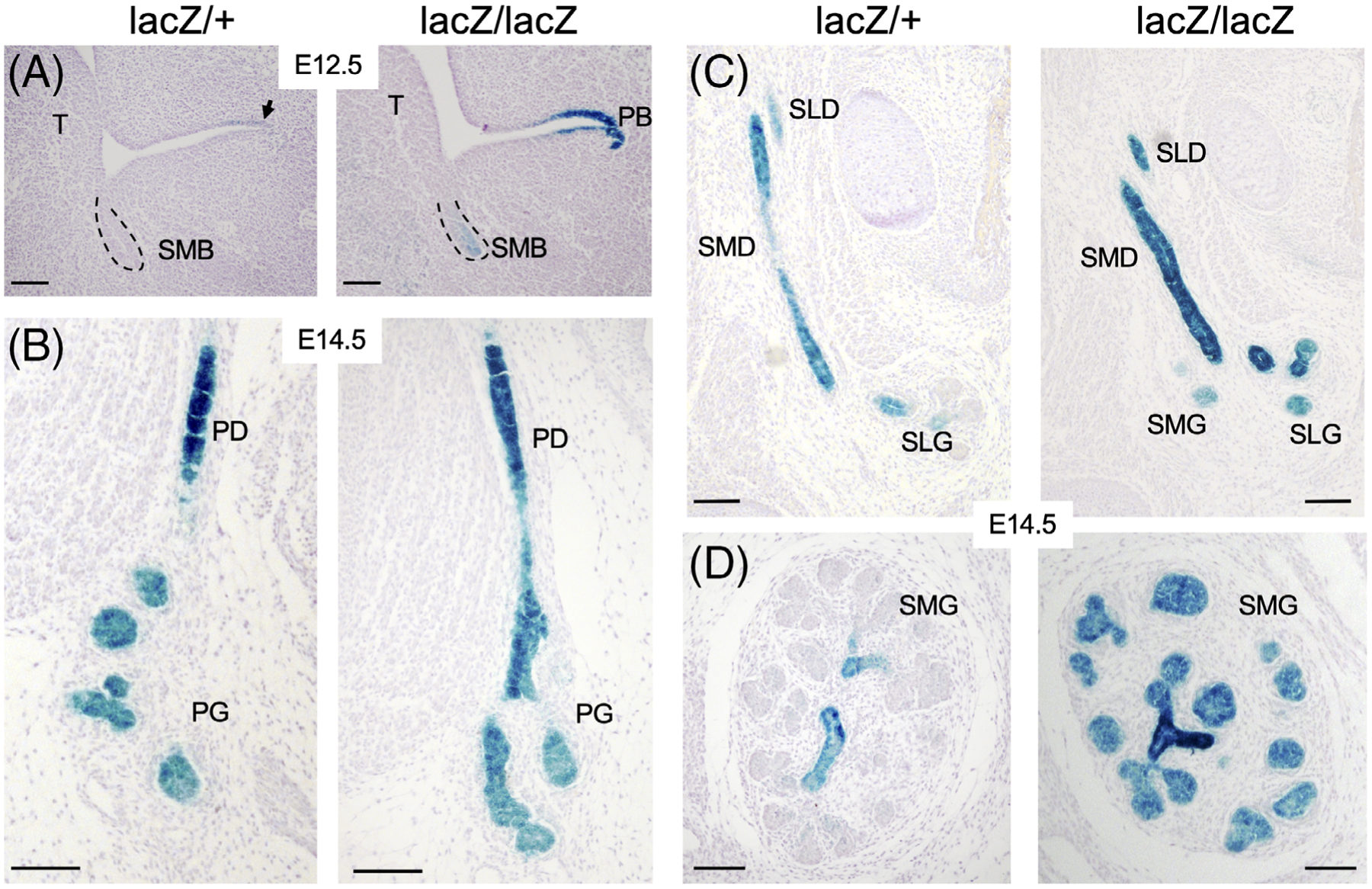
Eya2lacZ reporter gene expression in salivary gland. A, X-gal staining on sections at E12.5 showing β-Gal-activity weaker in submandibular bud (SMB) and stronger in parotid bud (PB) in Eya2lacZ/lacZ but only faint β-Gal-activity in parotid bud in Eya2lacZ/+. B, At E14.5, stronger β-Gal activity in parotid duct (PD) and weaker in branching parotid gland (PD) in both Eya2lacZ/+ and Eya2lacZ/lacZ. C and D, Similarly, stronger β-Gal activity in submandibular (SMD) and sublingual duct (SLD) and relatively weaker in branching submandibular (SMG) and sublingual gland (SLG). Scale bar: 100 μm
Detailed analyses of expression of other members of the Eya gene family and loss of function studies in compound mutant during salivary gland development and branching morphogenesis will provide molecular basis of salivary gland branching morphogenesis. The Eya2lacZ mice may serve as a tool for a systematic level understanding of salivary gland development.
2.6 |. Eya2 is expressed during thymus development and Eya2lacZ/lacZ mice exhibit smaller thymus
In addition to the salivary gland, we also observed Eya2 expression in thymus development for the first time (Figure 6). The thymus is the primary organ responsible for generating functional T cells in vertebrates and it develops from the third pharyngeal pouch primordium. The third pouch evaginates at E10.5 to form the primordia of thymus/parathyroid at around E12.5 and β-Gal activity was detectable in the primordium at these stages in both Eya2lacZ/+ and Eya2lacZ/lacZ embryos (Figure 6A and data not shown). At E13.5 and E14.5, the parathyroid have separated from thymus lobes, which continue to descend toward midline, Eya2 showed thymus-restricted expression at these stages (Figure 6B). In Eya2lacZ/lacZ embryos, the thymic primordia formed, detached from the pharynx and migrated to their normal position above the heart (Figure 6B). However, from the E15.5 sections we noted that the two thymus lobes in Eya2lacZ/lacZ embryos were smaller compared to Eya2lacZ/+ littermates (Figure 6C). To confirm the size reduction, we dissected out the whole thymus lobes at P0 and found that Eya2lacZ/lacZ thymus was apparently smaller compared to heterozygous thymus (Figure 6D), suggesting that Eya2 may have a role during thymus differentiation.
Figure 6.

Eya2lacZ reporter gene expression in thymus and reduced size of thymus lobes associated with Eya2-deficincy. A and B, Transverse sections showing that in Eya2lacZ/+ embryos, the third pouches evaginate and then separate as buds to form the primordia of thymus/parathyroid (th/pt) at around E12.5. Eya2 was detected in the th/pt at E12.5 (A), E14.5 thymus lobes (th), trachea (tr) and esophagus (es) (B). C, At E15.5, Eya2 expression was detected in the two thymus lobes, esophagus and trachea in Eya2lacZ/+ and Eya2lacZ/lacZ embryos. D, Whole thymus lobes in Eya2lacZ/+ embryos (G) and Eya2lacZ/lacZ embryos (H) at P0. Scale bar: 150 μm (A–C) and 500 μm (D)
We have previously reported that Eya1 is expressed in the pharyngeal endoderm from as early as E9.5 and in the primordium of and later in both parathyroid and thymus.30 Eya1 is required early for both parathyroid and thymus development as the primordium of parathyroid/thymus failed to form in Eya1−/− mice.30 Eya2 may have a synergistic role with Eya1 to specifically regulate thymus development and differentiation. Analyzing whether different lineages of thymic cells develop normally in the absence of Eya2 or in Eya1;Eya2 compound mutant mice will shed light on the functional roles of Eya2 and Eya1 in thymus differentiation and function.
3 |. CONCLUSIONS
In this study, we report the generation of targeted knockout and lacZ reporter knockin alleles for the mouse Eya2 gene and performed a detailed analysis of Eya2 expression and its potential role during mouse development. Eya2lacZ expression recaptured Eya2 mRNA expression in the cranial sensory ganglia, olfactory epithelium, dorsal root ganglion, limb tendons, and somatic myogenic precursors previously detected by in situ hybridization. We report here our novel finding that Eya2-expressing cells in the sensory ganglia (Vth, VIIth, VIIIth, IXth, and Xth) are predominately derived from NCCs and that only small portion of them are neuronal progenitors delaminated from a subset of ectoderm placodes. Furthermore, we found that Eya2 is expressed in the ocular and trochlear motor neurons, differentiating hair cells of both vestibular and cochlear sensory organs, and developing salivary gland and thymus. While Eya2lacZ expression in the homozygous organs depicted no difference with the heterozygous littermates, we found mild phenotypes in thymus and hearing due to Eya2 depletion. As the Eya family proteins are widely expressed in embryogenesis, there is likely a redundancy with other Eya genes, which can substitute for the loss of Eya2. The Eya2lacZ allele generated here could assist further investigation of the potential functions of Eya2 and its redundancy with other Eya genes by sorting the Eya2lacZ-positive cells for transcriptome profiling to identify differentially expressed genes and transcriptomic pathways regulated by Eya2 alone or combination with other Eya genes.
4 |. EXPERIMENTAL PROCEDURES
4.1 |. Gene targeting and animals
We isolated Eya2 genomic clones encoding the Eya domain from a 129/SvJ BAC genomic library and generated the targeting vector by ligation of a 3′ arm (3.8-kb XbaI fragment) and a 5′ arm (3.1-kb KpnI-HindII fragment) flanking a neo cassette into the vector pPNT. In the resulting plasmid (pPNT-Eya2), neo and tk are in opposite orientation with regard to Eya2. Correct targeting resulted in deletion of a 1.3-kb HindIII-XbaI fragment from 292 bp upstream to 850 bp downstream of exon 11 in the Eya domain region and replacement with pgk-neo. The targeting construct was linearized with NotI, electroporated into R1 ES cells and selected with G418 and FIAU. We identified two independent homologous recombinant ES lines by Southern blot using a 5′ external probe (0.5-kb EcoRI-Kpn1 fragment) and obtained four chimaeras by blastocyst injection that yielded germline transmission. Heterozygous or homozygous progeny were genotyped by PCR using a common forward primer (5′-TTAGCTTCAGGTAGCTGCTC-3′) and either of two allele-specific reverse primers (wild type, 5′-ACACATCTTGTCCCAGAACG-3′; Eya2neo, 5′-CAAGCAAAACCAAATTAAGGG-3′) in same or separate reactions to generate 230- and 250-bp amplicons, respectively.
For Eya2lacZ knockin, the same targeting vector was used and ES transfection, generation of chimeric mice and germline transmission were similarly performed. Heterozygous or homozygous progeny were genotyped by PCR using forward and reverse primers: WT forward primer: 5′-CTGAGTGACAGGGAAGGTAGG 3′; WT reverse primer: 5′-CTTCCTTGACTTCAACCCAAC-3′; mutant forward primer: 5′-TTGGGAATAGGTAATCAGCTT-3′; mutant reverse primer: 5′-TCTTCGCTATTACGCCAGCTG-3′. Eya2+/−, Eya2−/− or Eya2lacZ mice were maintained on a 129/Sv and C57BL/6J mixed background. All animal protocols were approved by Animal Care and Use Committee of the Icahn School of Medicine at Mount Sinai (protocols #06–0807 and #06–0822).
4.2 |. β-Gal staining
Mice were anesthetized with isoflurane and transcardially perfused with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde (PFA). Embryos or tissue organs were fixed with 4% PFA for 0.5 hour, cryoprotected in 30% sucrose overnight, embedded in OCT compound (Tissue-Tek; Sakura Finetek USA), and sectioned on a cryotome at 10 μm thickness. Sections were air dried, rinsed in PBS, and stained in 0.8 mg/mL X-gal in 35 mM K3Fe(CN)6, 35 mMK4Fe(CN)6, and 2 mM MgCl2 in PBS at room temperature until the blue staining appears. Sections were dehydrated through serial graded ethanol, cleared in xylenes, and cover slipped with permount. For whole mount staining, the embryos were fixed with 4% PFA for 0.5 hour, rinsed in PBS, and stained with the chemicals listed above.
4.3 |. Preyer reflex test using a click box
The click-box hearing test elicits a Preyer reflex in hearing mice and provides a convenient, fast, low-cost phenotypic screen. The click box produces an 18.9-kHz burst of 106-dB sound pressure levels (SPLs) at a distance of 10 cm (Institute of Hearing Research, Nottingham, UK).
4.4 |. Auditory-evoked brainstem response (ABR) testing
Eya2lacZ/lacZ and control littermates were tested for hearing thresholds via ABR. A computer-assisted evoked potential system (Tucker-Davis technologies) was used to obtain ABR thresholds for tone pips at frequencies of 5, 8, 11, 16, 22.6, 32, and 45.2 kHz (tone pip duration 5 ms; repetition rate 30/s) and averaged responses to 512 pips of alternating polarity as described before.46
ACKNOWLEDGMENTS
We thank past and present lab members for their assistance in the maintenance of Eya2 KO and Eya2lacZ KI mouse lines. This work is supported by NIH RO1DC014718 and DK064640 (PXX).
Funding information
National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: DK064640; National Institute on Deafness and Other Communication Disorders, Grant/Award Number: RO1DC014718
REFERENCES
- 1.Xu PX, Woo I, Her H, Beier DR, Maas RL. Mouse Eya homologues of the drosophila eyes absent gene require Pax6 for expression in lens and nasal placode. Development. 1997;124(1):219–231. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Oghi KA, Zhang J, et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426(6964):247–254. 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- 3.Rayapureddi JP, Kattamuri C, Steinmetz BD, et al. Eyes absent represents a class of protein tyrosine phosphatases. Nature. 2003;426(6964):295–298. 10.1038/nature02093. [DOI] [PubMed] [Google Scholar]
- 4.Tootle TL, Silver SJ, Davies EL, et al. The transcription factor eyes absent is a protein tyrosine phosphatase. Nature. 2003;426 (6964):299–302. 10.1038/nature02097. [DOI] [PubMed] [Google Scholar]
- 5.Xu PX, Cheng J, Epstein JA, Maas RL. Mouse Eya genes are expressed during limb tendon development and encode a transcriptional activation function. Proc Natl Acad Sci U S A. 1997; 94(22):11974–11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonini NM, Leiserson WM, Benzer S. The eyes absent gene: genetic control of cell survival and differentiation in the developing drosophila eye. Cell. 1993;72(3):379–395. [DOI] [PubMed] [Google Scholar]
- 7.Bonini NM, Bui QT, Gray-Board GL, Warrick JM. The drosophila eyes absent gene directs ectopic eye formation in a pathway conserved between flies and vertebrates. Development. 1997;124(23):4819–4826. [DOI] [PubMed] [Google Scholar]
- 8.Chen R, Amoui M, Zhang Z, Mardon G. Dachshund and eyes absent proteins form a complex and function synergistically to induce ectopic eye development in drosophila. Cell. 1997;91(7): 893–903. [DOI] [PubMed] [Google Scholar]
- 9.Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins so and Eya form a complex and regulate multiple steps in drosophila eye development. Cell. 1997;91(7):881–891. [DOI] [PubMed] [Google Scholar]
- 10.Shen W, Mardon G. Ectopic eye development in drosophila induced by directed dachshund expression. Development. 1997; 124(1):45–52. [DOI] [PubMed] [Google Scholar]
- 11.Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring WJ. Eyeless initiates the expression of both sine oculis and eyes absent during drosophila compound eye development. Development. 1998;125(12):2181–2191. [DOI] [PubMed] [Google Scholar]
- 12.Heanue TA, Reshef R, Davis RJ, et al. Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for drosophila eye formation. Genes Dev. 1999;13(24):3231–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerman JE, Bui QT, Steingrimsson E, et al. Cloning and characterization of two vertebrate homologs of the drosophila eyes absent gene. Genome Res. 1997;7(2):128–141. [DOI] [PubMed] [Google Scholar]
- 14.Duncan MK, Kos L, Jenkins NA, Gilbert DJ, Copeland NG, Tomarev SI. Eyes absent: a gene family found in several metazoan phyla. Mamm Genome. 1997;8(7):479–485. 10.1007/s003359900480. [DOI] [PubMed] [Google Scholar]
- 15.Borsani G, DeGrandi A, Ballabio A, et al. EYA4, a novel vertebrate gene related to drosophila eyes absent. Hum Mol Genet. 1999;8(1):11–23. [DOI] [PubMed] [Google Scholar]
- 16.Abdelhak S, Kalatzis V, Heilig R, et al. A human homologue of the drosophila eyes absent gene underlies branchio-Oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet. 1997;15(2):157–164. 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- 17.Zou HZ, Harrington JJ, Shire AM, et al. Highly methylated genes in colorectal neoplasia: implications for screening. Cancer Epidemol Biomark. 2007;16(12):2686–2696. 10.1158/1055-9965.Epi-07-0518. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Yang N, Huang J, et al. Transcriptional coactivator drosophila eyes absent homologue 2 is up-regulated in epithelial ovarian cancer and promotes tumor growth. Cancer Res. 2005;65(3):925–932. [PubMed] [Google Scholar]
- 19.Liu ZY, Zhao L, Song YS. Eya2 is overexpressed in human prostate cancer and regulates docetaxel sensitivity and mitochondrial membrane potential through AKT/Bcl-2 signaling. Biomed Res Int. 2019;2019:3808432. 10.1155/2019/3808432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anantharajan J, Zhou HB, Zhang LD, et al. Structural and functional analyses of an allosteric EYA2 phosphatase inhibitor that has on-target effects in human lung cancer cells. Mol Cancer Ther. 2019;18(9):1484–1496. 10.1158/1535-7163.Mct-18-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu PX. The EYA-SO/SIX complex in development and disease. Pediatr Nephrol. 2013;28(6):843–854. 10.1007/s00467-012-2246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SH, Yang DK, Choi BY, et al. The transcription factor Eya2 prevents pressure overload-induced adverse cardiac remodeling. J Mol Cell Cardiol. 2009;46(4):596–605. 10.1016/j.yjmcc.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Kim J, Ryu JY, et al. Transcription coactivator Eya2 is a critical regulator of physiological hypertrophy. J Mol Cell Cardiol. 2012;52(3):718–726. 10.1016/j.yjmcc.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Azuma N, Hirakiyama A, Inoue T, Asaka A, Yamada M. Mutations of a human homologue of the drosophila eyes absent gene (EYA1) detected in patients with congenital cataracts and ocular anterior segment anomalies. Hum Mol Genet. 2000;9(3): 363–366. [DOI] [PubMed] [Google Scholar]
- 25.Abe S, Takeda H, Nishio SY, Usami SI. Sensorineural hearing loss and mild cardiac phenotype caused by an EYA4 mutation. Hum Genome Variat. 2018;5:23;5:23. 10.1038/s41439-018-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morin M, Borreguero L, Booth KT, et al. Insights into the pathophysiology of DFNA10 hearing loss associated with novel EYA4 variants. Sci Rep. 2020;10(1):6213. 10.1038/s41598-020-63256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makishima T, Madeo AC, Brewer CC, et al. Nonsyndromic hearing loss DFNA10 and a novel mutation of EYA4: evidence for correlation of normal cardiac phenotype with truncating mutations of the Eya domain. Am J Med Genet A. 2007;143A(14):1592–1598. 10.1002/ajmg.a.31793. [DOI] [PubMed] [Google Scholar]
- 28.Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23(1):113–117. 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- 29.Zou D, Silvius D, Fritzsch B, Xu PX. Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development. 2004;131(22):5561–5572. 10.1242/dev.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu PX, Zheng W, Laclef C, et al. Eya1 is required for the morphogenesis of mammalian thymus, parathyroid and thyroid. Development. 2002;129(13):3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Depreux FF, Darrow K, Conner DA, et al. Eya4-deficient mice are a model for heritable otitis media. J Clin Invest. 2008;118(2):651–658. 10.1172/JCI32899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soker T, Dalke C, Puk O, et al. Pleiotropic effects in Eya3 knockout mice. BMC Dev Biol. 2008;8:118. 10.1186/1471-213X-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grifone R, Demignon J, Giordani J, et al. Eya1 and Eya2 proteins are required for hypaxial somitic myogenesis in the mouse embryo. Dev Biol. 2007;302(2):602–616. 10.1016/j.ydbio.2006.08.059. [DOI] [PubMed] [Google Scholar]
- 34.Sousounis K, Bryant DM, Fernandez JM, et al. Eya2 promotes cell cycle progression by regulating DNA damage response during vertebrate limb regeneration. Elife. 2020;9:e51217. 10.7554/elife.51217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayer-Le Lievre CS, Le Douarin NM. The early development of cranial sensory ganglia and the potentialities of their component cells studied in quail-chick chimeras. Dev Biol. 1982;94(2):291–310. 10.1016/0012-1606(82)90349-9. [DOI] [PubMed] [Google Scholar]
- 36.D’Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat. 1983;166(4):445–468. 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- 37.Freyer L, Aggarwal V, Morrow BE. Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development. 2011;138(24):5403–5414. 10.1242/dev.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen B, Kim EH, Xu PX. Initiation of olfactory placode development and neurogenesis is blocked in mice lacking both Six1 and Six4. Dev Biol. 2009;326(1):75–85. 10.1016/j.ydbio.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jahan I, Kersigo J, Elliott KL, Fritzsch B. Smoothened overexpression causes trochlear motoneurons to reroute and innervate ipsilateral eyes. Cell Tissue Res. 2021. 10.1007/s00441-020-03352-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holbrook EH, Szumowski KEM, Schwob JE. An immune-chemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J Comp Neurol. 1995;363(1):129–146. 10.1002/cne.903630111. [DOI] [PubMed] [Google Scholar]
- 41.Whitby-Logan GK, Weech M, Walters E. Zonal expression and activity of glutathione S-transferase enzymes in the mouse olfactory mucosa. Brain Res. 2004;995(2):151–157. 10.1016/j.brainres.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Cvekl A, Duncan MK. Genetic and epigenetic mechanisms of gene regulation during lens development. Prog Retin Eye Res. 2007;26(6):555–597. 10.1016/j.preteyeres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang RA. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48(8–9):783–791. 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed M, Wong EY, Sun J, Xu J, Wang F, Xu PX. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev Cell. 2012;22(2):377–390. 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tucker AS. Salivary gland development. Semin Cell Dev Biol. 2007;18(2):237–244. 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130(17):3989–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]


