Abstract
Background
Acute kidney injury (AKI) is a common complication amongst people who are critically ill, and it is associated with an increased risk of death. For people with severe AKI, continuous kidney replacement therapy (CKRT), which is delivered over 24 hours, is needed when they become haemodynamically unstable. When CKRT is interrupted due to clotting of the extracorporeal circuit, the delivered dose is decreased and thus leading to undertreatment.
Objectives
This review assessed the efficacy of non‐pharmacological measures to maintain circuit patency in CKRT.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 25 January 2021 which includes records identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal, and ClinicalTrials.gov.
Selection criteria
We included all randomised controlled trials (RCTs) (parallel‐group and cross‐over studies), cluster RCTs and quasi‐RCTs that examined non‐pharmacological interventions to prevent clotting of extracorporeal circuits during CKRT.
Data collection and analysis
Three pairs of review authors independently extracted information including participants, interventions/comparators, outcomes, study methods, and risk of bias. The primary outcomes were circuit lifespan and death due to any cause at day 28. We used a random‐effects model to perform quantitative synthesis (meta‐analysis). We assessed the risk of bias in included studies using the Cochrane Collaboration’s tool for assessing the risk of bias. Summary estimates of effect were obtained using a random‐effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) and 95% CI for continuous outcomes. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
A total of 20 studies involving 1143 randomised participants were included in the review. The methodological quality of the included studies was low, mainly due to the unclear randomisation process and blinding of the intervention. We found evidence on the following 11 comparisons: (i) continuous venovenous haemodialysis (CVVHD) versus continuous venovenous haemofiltration (CVVH) or continuous venovenous haemodiafiltration (CVVHDF); (ii) CVVHDF versus CVVH; (iii) higher blood flow (≥ 250 mL/minute) versus standard blood flow (< 250 mL/minute); (iv) AN69 membrane (AN69ST) versus other membranes; (v) pre‐dilution versus post‐dilution; (vi) a longer catheter (> 20 cm) placing the tip targeting the right atrium versus a shorter catheter (≤ 20 cm) placing the tip in the superior vena cava; (vii) surface‐modified double‐lumen catheter versus standard double‐lumen catheter with identical geometry and flow design; (viii) single‐site infusion anticoagulation versus double‐site infusion anticoagulation; (ix) flat plate filter versus hollow fibre filter of the same membrane type; (x) a filter with a larger membrane surface area versus a smaller one; and (xi) a filter with more and shorter hollow fibre versus a standard filter of the same membrane type.
Circuit lifespan was reported in 9 comparisons. Low certainty evidence indicated that CVVHDF (versus CVVH: MD 10.15 hours, 95% CI 5.15 to 15.15; 1 study, 62 circuits), pre‐dilution haemofiltration (versus post‐dilution haemofiltration: MD 9.34 hours, 95% CI ‐2.60 to 21.29; 2 studies, 47 circuits; I² = 13%), placing the tip of a longer catheter targeting the right atrium (versus placing a shorter catheter targeting the tip in the superior vena cava: MD 6.50 hours, 95% CI 1.48 to 11.52; 1 study, 420 circuits), and surface‐modified double‐lumen catheter (versus standard double‐lumen catheter: MD 16.00 hours, 95% CI 13.49 to 18.51; 1 study, 262 circuits) may prolong circuit lifespan. However, higher blood flow may not increase circuit lifespan (versus standard blood flow: MD 0.64, 95% CI ‐3.37 to 4.64; 2 studies, 499 circuits; I² = 70%). More and shorter hollow fibre filters (versus standard filters: MD ‐5.87 hours, 95% CI ‐10.18 to ‐1.56; 1 study, 6 circuits) may reduce circuit lifespan.
Death from any cause was reported in four comparisons We are uncertain whether CVVHDF versus CVVH, CVVHD versus CVVH or CVVHDF, longer versus a shorter catheter, or surface‐modified double‐lumen catheters versus standard double‐lumen catheters reduced death due to any cause, in very low certainty evidence.
Recovery of kidney function was reported in three comparisons. We are uncertain whether CVVHD versus CVVH or CVVHDF, CVVHDF versus CVVH, or surface‐modified double‐lumen catheters versus standard double‐lumen catheters increased recovery of kidney function.
Vascular access complications were reported in two comparisons. Low certainty evidence indicated using a longer catheter (versus a shorter catheter: RR 0.40, 95% CI 0.22 to 0.74) may reduce vascular access complications, however, the use of surface‐modified double lumen catheters versus standard double‐lumen catheters may make little or no difference to vascular access complications.
Authors' conclusions
The use of CVVHDF as compared with CVVH, pre‐dilution haemofiltration, a longer catheter, and surface‐modified double‐lumen catheter may prolong the circuit lifespan, while higher blood flow and more and shorter hollow fibre filter may reduce circuit life. The overall certainty of the evidence was assessed to be low to very low due to the small sample size of the included studies.
Data from future rigorous and transparent research are much needed in order to fully understand the effects of non‐pharmacological interventions in preventing circuit coagulation amongst people with AKI receiving CKRT.
Plain language summary
Non‐pharmacological interventions for preventing clotting of extracorporeal circuits during continuous kidney replacement therapy
What is the issue?
Acute kidney injury (AKI) is a major problem in people with severe illness. In cases of severe AKI, kidney replacement therapy/dialysis (KRT) using circuits is necessary. Continuous kidney replacement therapy is performed continuously over 24 hours. Clotting of the CKRT circuit can interfere with this treatment. To prevent this, a variety of non‐pharmacological (not using medication) interventions have been studied. We aimed to summarise current evidence regarding the efficacy of non‐pharmacological interventions for preventing clotting of extracorporeal circuits during CKRT.
What did we do?
We searched for available evidence from the Cochrane Kidney and Transplant Specialised Register up to 25 January 2021. Our review summarised the results of 20 randomised studies involving a total of 1143 people.
What did we find?
We found that the quality of the 20 included studies was low, and the number randomised was small. The majority of the included studies did not report death as an outcome. We found that continuous venovenous haemodiafiltration (CVVHDF), as compared with continuous venovenous haemofiltration (CVVH), may prolong circuit lifespan. In addition, pre‐dilution haemofiltration, as compared with post‐dilution haemofiltration, a longer catheter placing the tip at the right atrium, as compared with a shorter catheter placing the tip in the superior vena cava, and surface‐modified double lumen catheter, as compared with standard double lumen catheter, may extend the circuit lifespan. However, higher blood flow compared to standard blood flow rate might not affect circuit lifespan. Overall, the data was limited and of very low certainty.
Conclusions
We found that the effects of non‐pharmacological interventions in people with AKI receiving CKRT remain unclear. There is a need for studies assessing CKRT circuit lifespan as well as other clinically important outcomes.
Summary of findings
Summary of findings 1. CVVHD versus CVVH or CVVHDF for preventing clotting of extracorporeal circuits during CKRT.
| CVVHD compared to CVVH or CVVHDF for preventing clotting of extracorporeal circuits during CKRT | |||||
| Patient or population: AKI patients receiving CKRT Setting: ICU Intervention: CVVHD Comparison: CVVH or CVVHDF | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with CVVH or CVVHDF | Risk with CVVHD | ||||
| Circuit lifespan | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| Death from any cause Follow up: 24 hours to 90 days |
536 per 1,000 | 413 per 1,000 (225 to 751) | RR 0.77 (0.42 to 1.40) | 229 (4) | ⊕⊝⊝⊝ Very low 1,2,3,4 |
| Recovery of kidney function Follow up: ICU discharge |
182 per 1,000 | 165 per 1,000 (67 to 402) | RR 0.91 (0.37 to 2.21) | 93 (2) | ⊕⊝⊝⊝ Very low 1,4 |
| Vascular access complications | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CVVHD: continuous venovenous haemodialysis; CVVH: continuous venovenous haemofiltration; CVVHDF: continuous venovenous haemodiafiltration; CKRT: continuous kidney replacement therapy;AKI: acute kidney injury; ICU: intensive care unit; CI: confidence interval;RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level due to serious risk of bias
2 Downgraded one level due to serious inconsistency
3 Downgraded one level due to serious indirectness (death at the longest follow‐up)
4 Downgraded two levels due to very serious imprecision
Summary of findings 2. CVVHDF versus CVVH for preventing clotting of extracorporeal circuits during CKRT.
| CVVHDF versus CVVH for preventing clotting of extracorporeal circuits during CKRT | |||||
| Patient or population: AKI patients receiving CKRT Setting: ICU Intervention: CVVHDF Comparison: CVVH | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with CVVH | Risk with CVVHDF | ||||
| Circuit lifespana | The mean circuit lifespan with CVVH was 8.55 hours | The mean circuit lifespan with CVVHDF was 10.15 hours higher (5.15 to 15.15 higher) than CVVH | ‐ | 62 (1) | ⊕⊕⊝⊝ Low 1,2 |
| Death from any cause at day 28 of follow‐up | 608 per 1,000 | 413 per 1,000 (316 to 547) | RR 0.68 (0.52 to 0.90) | 206 (1 RCT) | ⊕⊝⊝⊝ Very low 1,2,3 |
| Recovery of kidney function | 714 per 1,000 | 786 per 1,000 (614 to 1,000) | RR 1.10 (0.86 to 1.41) | 96 (1 RCT) | ⊕⊝⊝⊝ Very low 2,4 |
| Vascular access complications | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). a we calculated circuit lifespan per circuit CVVHD: continuous venovenous haemodialysis; CVVH: continuous venovenous haemofiltration; CKRT: continuous kidney replacement therapy; AKI: acute kidney injury; ICU: intensive care unit; CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level due to serious risk of bias
2 Downgraded one level due to serious imprecision
3 Downgraded one level due to serious concern of publication bias
4 Downgraded two levels due to very serious risk of bias (participants were excluded from analyses if died)
Summary of findings 3. Pre‐dilution versus post‐dilution (as defined by study investigators) for preventing clotting of extracorporeal circuits during CKRT.
| Pre‐dilution versus post‐dilution (as defined by study investigators) for preventing clotting of extracorporeal circuits during CKRT | |||||
| Patient or population: AKI patients receiving CKRT Setting: ICU Intervention: pre‐dilution Comparison: post‐dilution | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with post‐dilution | Risk with pre‐dilution | ||||
| Circuit lifespana | The mean circuit lifespan with post‐dilution was 22.5 hours | The mean circuit lifespan with pre‐dilution was 9.34 hours higher (2.60 lower to 21.29 higher) than post‐dilution | ‐‐ | 47 (2) | ⊕⊕⊝⊝ Low 1,2 |
| Death from any cause at day 28 of follow‐up | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| Recovery of kidney function | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| Vascular access complications | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). a we calculated circuit lifespan per circuit CKRT: continuous kidney replacement therapy; AKI: acute kidney injury; ICU: intensive care unit; CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level due to serious risk of bias
2 Downgraded one level due to serious imprecision
Summary of findings 4. Higher blood flow versus standard blood flow for preventing clotting of extracorporeal circuits during CKRT.
| Higher versus standard blood flow for preventing clotting of extracorporeal circuits during CKRT | |||||
| Patient or population: AKI patients receiving CKRT Setting: ICU Intervention: higher blood flow Comparison: standard blood flow | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with standard blood flow | Risk with higher blood flow | ||||
| Circuit lifespana | The mean circuit lifespan with standard blood flow was 17.2 hours | The mean circuit lifespan with higher blood flow was 0.64 hours higher (3.37 lower to 4.64 higher) than standard blood flow | ‐‐ | 499 (2) | ⊕⊕⊝⊝ Low 1,2 |
| Death from any cause at day 28 of follow‐up | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| Recovery of kidney function | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| Vascular access complications | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). a we calculated circuit lifespan per circuit CKRT: continuous kidney replacement therapy; AKI: acute kidney injury; ICU: intensive care unit; CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level due to serious risk of bias
2 Downgraded one level due to serious inconsistency
Summary of findings 5. Long (> 20 cm) versus short (≤ 20 cm) catheter for preventing clotting of extracorporeal circuits during CKRT.
| Long (> 20 cm) versus short (≤ 20 cm) catheter for preventing clotting of extracorporeal circuits during CKRT | |||||
| Patient or population: AKI patients receiving CKRT Setting: ICU Intervention: long catheter (> 20 cm) Comparison: short catheter (≤ 20 cm) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with short catheter (≤ 20cm) | Risk with long catheter (> 20 cm) | ||||
| Circuit lifespana | The mean circuit lifespan with short catheters was 17.5 hours | The mean circuit lifespan with longer catheters was 6.5 hours higher (1.48 to 11.52 higher) than short catheters | ‐‐ | 402 (1) | ⊕⊕⊝⊝ Low 1,4 |
| Death from any cause at day 28 of follow‐up | 170 per 1,000 | 235 per 1,000 (104 to 529) | RR 1.38 (0.61 to 3.11) | 94 (1) | ⊕⊝⊝⊝ Very low 2,3 |
| Recovery of kidney function | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| Vascular access complications | 511 per 1,000 | 194 per 1,000 (102 to 368) | RR 0.38 (0.20 to 0.72) | 94 (1) | ⊕⊕⊝⊝ Low 1,4 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). a we calculated circuit lifespan per circuit CKRT: continuous kidney replacement therapy; AKI: acute kidney injury; ICU: intensive care unit; CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level due to serious risk of bias
2 Downgraded one level due to serious indirectness (used ICU death as a surrogate endpoint)
3 Downgraded two levels due to very serious imprecision
4 Downgraded one level due to serious imprecision
Summary of findings 6. Surface‐modified versus standard double‐lumen catheter with identical geometry and flow design for preventing clotting of extracorporeal circuits during CKRT.
| Surface‐modified versus standard double‐lumen catheter with identical geometry and flow design for preventing clotting of extracorporeal circuits during CKRT | |||||
| Patient or population: AKI patients receiving CKRT Setting: ICU Intervention: surface‐modified double‐lumen catheter Comparison: standard double‐lumen catheter with identical geometry and flow design | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with standard double‐lumen catheter with identical geometry and flow design | Risk with surface‐modified, double‐lumen catheter | ||||
| Circuit lifespan | The mean circuit lifespan with standard double‐lumen catheters was 118 hours | The mean circuit lifespan with surface‐modified double‐lumen catheters was 16 hours higher (13.49 to 18.51 higher) than standard double‐lumen catheters | ‐‐ | 262 (1) | ⊕⊕⊝⊝ Low 1,2 |
| Death from any cause at day 28 of follow‐up | 331 per 1,000 | 357 per 1,000 (251 to 506) | RR 1.08 (0.76 to 1.53) | 236 (1) | ⊕⊕⊝⊝ Very low 1,2,3 |
| Recovery of kidney function | 492 per 1,000 | 447 per 1,000 (344 to 590) | RR 0.91 (0.70 to 1.20) | 236 (1) | ⊕⊕⊝⊝ Low 1,2 |
| Vascular access complications | 76 per 1,000 | 25 per 1,000 (7 to 92) | RR 0.33 (0.09 to 1.20) | 236 (1) | ⊕⊕⊝⊝ Low 1,2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). a we calculated circuit lifespan per circuit CKRT: continuous kidney replacement therapy; AKI: acute kidney injury; ICU: intensive care unit; CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level due to serious risk of bias
2 Downgraded one level due to serious imprecision
3 Downgraded one level due to serious indirectness (used ICU mortality as a surrogate)
Summary of findings 7. Polyethylenimine‐treated membrane (AN69ST) versus other membranes for preventing clotting of extracorporeal circuits during CKRT.
| Polyethylenimine‐treated AN69ST versus other membranes for preventing clotting of extracorporeal circuits during CKRT | |||||
| Patient or population: AKI patients receiving CKRT Setting: ICU Intervention: polyethylenimine‐treated AN69ST Comparison: other membranes | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with other membrane | Risk with polyethylenimine‐treated AN69ST | ||||
| Circuit lifespan | The mean circuit lifespan with other membranes was 14.1 hours | The mean circuit lifespan with polyethylenimine‐treated AN69ST was 1.54 hours higher (4.53 lower to 7.6 higher) than other membranes | ‐‐ | 56 (2) | ⊕⊕⊝⊝ Low 1 |
| Death from any cause at day 28 of follow‐up | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| Recovery of kidney function | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| Vascular access complications | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). a we calculated circuit lifespan per circuit CKRT: continuous kidney replacement therapy; AKI: acute kidney injury; ICU: intensive care unit; CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded two levels due to very serious imprecision
Summary of findings 8. Flat plate versus hollow fibre filter of the same membrane type for preventing clotting of extracorporeal circuits during CKRT.
| Flat plate versus hollow fibre filter of the same membrane type for preventing clotting of extracorporeal circuits during CKRT | |||||
| Patient or population: people with AKI receiving CKRT Setting: ICU Intervention: flat plate filter Comparison: hollow fibre filter (of the same membrane type) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with hollow fibre filter of the same membrane type | Risk with flat plate filter | ||||
| Circuit lifespan | The mean circuit lifespan with hollow fibre filters was 17.1 hours | The mean circuit lifespan with flat plate filters was 1.4 hours lower (12.12 lower to 9.32 higher) than hollow fibre filters | ‐‐ | 38 (1) | ⊕⊝⊝⊝ Very low 1,2 |
| Death from any cause at day 28 of follow‐up | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| Recovery of kidney function | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| Vascular access complications | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CKRT: continuous kidney replacement therapy; AKI: acute kidney injury; ICU: intensive care unit; CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level due to serious risk of bias
2 Downgraded two levels due to very serious imprecision
Summary of findings 9. More and shorter versus standard hollow fibre filter of the same membrane type for preventing clotting of extracorporeal circuits during CKRT.
| More and shorter versus standard hollow fibre filter of the same membrane type for preventing clotting of extracorporeal circuits during CKRT | |||||
| Patient or population: AKI patients receiving CKRT Setting: ICU Intervention: more and shorter hollow fibre filter Comparison: standard hollow fibre filter | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with standard hollow fibre filter of the same membrane type | Risk with more and shorter fibre filters | ||||
| Circuit lifespan | The mean circuit lifespan with standard hollow fibre filters was 21.27 hours | The mean circuit lifespan with more and shorter fibre filters was 5.87 hours lower (10.18 to 1.56 lower) than standard hollow fibre filters | ‐‐ | 6 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Death from any cause at day 28 of follow‐up | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| Recovery of kidney function | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| Vascular access complications | Not reported | Not reported | ‐‐ | ‐‐ | ‐‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CKRT: continuous kidney replacement therapy; AKI: acute kidney injury; ICU: intensive care unit; CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level due to serious risk of bias
2 Downgraded one level due to serious imprecision
Background
Description of the condition
Acute kidney injury (AKI) is defined as an abrupt decline in kidney function with increased serum creatinine or decreased urine output (KDIGO Acute Kidney Injury Work Group 2012). AKI occurs in nearly half of the admitted cases in intensive care units (ICUs) and is related to increased death (Bouchard 2015; Fujii 2018; Hoste 2015; Nisula 2013). For people with severe AKI, kidney replacement therapy (KRT) is required to manage electrolytes, fluid balance, and waste products. Previous epidemiological studies revealed that the need for KRT accounted for 16% to 24% of people with AKI in the ICUs (Fujii 2018; Nisula 2013).
Administration of KRT can be achieved either intermittently or continuously. Although intermittent KRT removes fluids more rapidly, it has been suggested to cause hypotension and further damage to kidney function (Manns 1997; Silversides 2014). Moreover, deranged electrolyte levels, which are frequently observed in people with AKI, are normalised more successfully when KRT is administered continuously (Uchino 2003). Accordingly, continuous kidney replacement therapy (CKRT) is recommended for people with AKI who are haemodynamically unstable, and it is a technique used most frequently in the ICU setting (Bouchard 2015; Fujii 2018; KDIGO Acute Kidney Injury Work Group 2012). A multicentre survey conducted in 33 countries showed that 75.2% of KRT sessions were conducted using the CKRT approach (Hoste 2015).
The choice of CKRT modalities varies across the world. An international observational study in 2007 reported that continuous venovenous haemofiltration (CVVH) had been widely used (Uchino 2007). However, another small observational study showed comparable small and mid‐sized molecular solute removal between CVVH and continuous venovenous haemodialysis (CVVHD) (Ricci 2006). Evidence supporting a clear choice of one CKRT modality over others remains inconclusive although the use of various diffusion‐based techniques, such as CVVHD and continuous venovenous haemodiafiltration (CVVHDF), has been reported in a recent observational study (Fujii 2018).
In clinical practice, CKRT is typically provided through a double‐lumen venous catheter as a continuous 24‐hour therapy; however, CKRT may also operate for 21‐23 hours a day in different clinical settings (Mehta 2001; Uchino 2003; Vesconi 2009). Reasons for the interruption of CKRT could be clotting of the extracorporeal circuits, clogging of the membrane, or the need for transportation to outside of the ICU settings, such as to the operation theatre or the radiology department for imaging tests. Such treatment interruptions decrease the delivered CKRT dose, leading to insufficient uraemic control (Fealy 2002; Mitchell 2003). A small single‐centre study indicated that circuit clotting was the primary reason for the shortened circuit life (Venkataraman 2002). Undoubtedly, exchanging the circuits due to circuit failure leads to increased medical costs and the workload of healthcare professionals (Fealy 2002; Mehta 2001). Thus, treatment interruption is widely considered a key quality indicator for CKRT (Rewa 2017).
Description of the intervention
The primary intervention to maintain the patency of the CKRT circuit is anticoagulation. A previous multicentre trial showed that as many as half of the critically‐ill study subjects were treated with CKRT without the use of any anticoagulation (RENAL 2006). Indeed, anticoagulation therapy is associated with a substantial risk of bleeding, which in itself is a common feature amongst people who are critically ill. Research efforts have recently shifted to identify effective and safe anticoagulants, e.g. citrate, as well as strategies to minimise the risk of clotting without using anticoagulants during CKRT. Since there is an existing Cochrane review exploring the effects of pharmacological interventions in CKRT (Tsujimoto 2020), our present review aimed to focus on non‐pharmacological strategies for circuit survival.
Clotting of CKRT circuits is attributed to stasis or turbulence of blood flow, haemoconcentration, or activation of the intrinsic coagulation system by blood–tube, blood–air, or blood–filter contact (RENAL 2006). Therefore, non‐pharmacological interventions to prevent clotting of the CKRT circuit generally include the strategic selection of a catheter or access site, optimising the blood flow rate, CKRT modalities, and methods of haemodilution.
How the intervention might work
The selection of a catheter and access site may play a significant role in determining circuit life. According to Poiseuille's Law, for fluid/blood flow through an intravenous (IV) catheter a thick and short catheter may be theoretically preferable to avoid stasis. In addition, to avoid kinking or curving of the catheter, which may lead to impaired blood flow, the right jugular venous route may be preferable as it is straight and easily monitored by bedside clinical staff.
In CKRT, blood flow rates are typically set at 100 to 200 mL/minute or based on a filtration ratio. In fact, the pump used in CKRT delivers blood with peristaltic revolutions, and the flow rate is, in effect, the rate of the pump revolution. An observational study revealed a forward and backward blood flow path between the pump and filter, which may cause blood flow stasis (Baldwin 2004). Considering the potential blood flow fluctuations due to the peristaltic roller pump, maintaining the blood flow at a high rate may be useful to prevent CKRT filter clotting.
Regarding the choice of CKRT modalities, filtration may shorten the circuit lifetime compared to dialysis by haemoconcentration due to its ultrafiltration process (Ricci 2006). During filtration, haematocrit levels increase and consequently elevate the risk of coagulation in the filter. In CVVH and CVVHDF, substitution fluids can be administered before (pre‐dilution) or after (post‐dilution) filtration. Pre‐dilution CKRT aims to decrease haemoconcentration and improve blood flow. This may improve filter lifespan and efficiency, leading to less treatment interruptions and better clearance. However, a critical aspect of pre‐dilution therapies is the relationship between the blood flow rate and replacement fluid rate (Clark 2017). Pre‐dilution may also reduce molecular clearance when the blood flow is low, and its precise clinical impact upon people who are critically ill is currently unclear.
Why it is important to do this review
Circuit failure during CKRT affects the actual delivery of CKRT, which leads to decreased treatment efficiency. Moreover, it increases the associated medical costs and workload of clinical staff. Thus, maintaining the patency of the CKRT circuit is particularly crucial in ICU settings. This review aimed to provide a clinically relevant and solid body of evidence to assist healthcare decision‐making when delivering CKRT in critical care medicine.
Objectives
This review assessed the efficacy of non‐pharmacological measures to maintain circuit patency during CKRT.
Methods
Criteria for considering studies for this review
Types of studies
We included all parallel‐group and cross‐over randomised controlled trials (RCTs), cluster‐randomised trials and quasi‐randomised trials (studies in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) investigating non‐pharmacological interventions for preventing clotting of extracorporeal circuits during CKRT. We excluded observational studies, case reports, reviews, editorials, and commentaries.
Types of participants
We included people with AKI who received CKRT in the ICU settings regardless of age or sex. In this review, we defined AKI according to the Kidney Disease: Improving Global Outcomes (KDIGO) definition and staging system (KDIGO Acute Kidney Injury Work Group 2012).
Types of interventions
Dialysis modalities
CVVHD versus CVVH or CVVHDF
CVVHDF versus CVVH
Pre‐dilution versus post‐dilution (as defined by study investigators)
Blood flow rate
Higher (≥ 250 mL/minute) blood flow versus standard (< 250 mL/minute) blood flow
Catheter types
Long (> 20 cm) catheter versus short (≤ 20 cm) catheter
Left‐sided catheters versus right‐sided catheter
Surface‐modified double‐lumen catheter versus standard double‐lumen catheter with identical geometry and flow design
Membrane types
Polyethylenimine‐treated polyacrylonitrile membrane (AN69ST) versus other types of membrane
Heparin‐grafted membrane versus other membranes
A flat plate filter versus a hollow fibre filter of the same membrane type
A filter with a larger membrane surface area versus a smaller one
A filter with more and shorter hollow fibres versus a standard filter of the same membrane type
Access sites
Internal jugular access versus femoral access
Subclavian venous versus superior vena cava as the line tip portion in the same access catheter insertion site
Subclavian venous versus right atrium as the line tip portion in the same access catheter insertion site
Superior vena cava versus right atrium as the line tip portion in the same access catheter insertion site
Other types of non‐pharmacological interventions
Single‐site infusion anticoagulation versus double‐site infusion anticoagulation
Infusion anticoagulation from access line versus pre‐filter single site
Saline flushing versus no saline flushing.
We intended to compare the effects of one non‐pharmacological intervention to another. We included studies that used or did not use anticoagulants in both intervention and control groups. We excluded studies comparing non‐pharmacological interventions to pharmacological interventions.
Types of outcome measures
Primary outcomes
Circuit lifespan (commences at the point of starting CKRT and concludes upon circuit cessation for any reason)
Death (any cause) at day 28 of follow‐up or last reported time point
Secondary outcomes
Recovery of kidney function: numbers of participants assessed as free of KRT at day 28, 90, and 180 of follow‐up
Vascular access complications: functions (e.g. ability to use CKRT, uninterrupted use without the need for any intervention, percentage change in access blood flow) or access‐site infections (e.g. those suspected to be catheter‐related) requiring antibiotic therapy
Costs related to health care services (unit: USD, calculated rate 1 USD = 0.93 Euro)
Types and number of dialysis filters, circuits, and catheters
Consumption of dialysate/dialysis fluid
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 25 January 2021 through contact with the Cochrane Kidney and Transplant Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals, and the proceedings and abstracts from major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the WHO International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website under CKT Register of Studies.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
We screened reference lists of narrative review articles, relevant studies, and clinical practice guidelines for additional relevant information. Where appropriate, we contacted experts and professional organisations in the field for information on unpublished or incomplete/ongoing studies.
Due to resource constraints, we did not consider grey literature sources (e.g. conference abstracts, dissertations, and theses) additional to those already included in the Cochrane Kidney and Transplant Register of Studies.
Data collection and analysis
Selection of studies
Two authors (YT and SM) independently screened the titles and abstracts obtained from the literature search in duplicate, and clearly irrelevant records were excluded. Studies that might include relevant data or information were retained initially for subsequent assessment where the two authors independently assessed the full texts of these potentially eligible studies against our prespecified inclusion/exclusion criteria. We arranged for non‐English and non‐Japanese study reports to be translated before full assessment. Disagreements were resolved by discussion with a third author acting as an arbiter (TF).
Data extraction and management
Data extraction was carried out independently by three pairs of review authors (YT and SM, YK and HS, HY and HT) in duplicate, using pre‐standardised data extraction forms. We resolved disagreements by discussion with another author acting as an arbiter (TF). Where more than one record from the same study existed, records were grouped together and the record with the most complete data was identified as the primary reference of the study. We specifically highlighted discrepancies between available study protocols and final published reports where appropriate.
Assessment of risk of bias in included studies
Three pairs of review authors (YT and SM, YK and HS, HY and HT) assessed the risk of bias in included studies using the Cochrane Collaboration’s tool for assessing the risk of bias (Higgins 2011) (see Appendix 2). Any disagreement was resolved by discussion with another author acting as an arbiter (TF).
The Cochrane Collaboration’s tool for assessing the risk of bias considered six aspects of bias: (i) selection bias; (ii) performance bias; (iii) detection bias; (iv) attrition bias; (v) reporting bias; and (vi) other bias.
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Blinding of participants and personnel (performance bias)
Blinding of outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Was the study report free of potential selective outcome reporting (reporting bias)?
For assessing other potential threats to validity ('Other bias'), we assumed that the use of anticoagulation therapy or sedative agents were important co‐interventions in this review. Additionally, we judged how baseline imbalance of anticoagulation affected treatment effect estimates.
Was the study apparently free of other problems that could put it at risk of bias (e.g. co‐intervention of anticoagulation)?
Did baseline imbalance regarding anticoagulation therapy exist?
Measures of treatment effect
For dichotomous outcomes (e.g. death or recovery of kidney function), treatment effect measures were expressed in risk ratios (RRs) with 95% confidence intervals (CIs). Where continuous scales of measurement were used to assess the effects of treatment interventions (e.g. circuit lifespan, or costs), we calculated mean differences (MDs) with their respective 95% CIs.
Unit of analysis issues
We assessed unit of analysis issues in three possible ways in which they might arise.
1. Multiple measurements of the same participants from either individually randomised or cross‐over studies
If there were multiple measurements of the outcomes such as circuit lifespan (e.g. several circuits were used by the same individual due to repeated CKRT using new circuits), unit of analysis issues might arise. We considered addressing these issues by first assessing each included study for any evidence of multiple enrolments (e.g. number of circuits exceeded the number of participants). We then planned to exclude those with multiple measurements by entering the data of only the first circuit, but such information was not available. We, therefore, performed our analysis based on available data as reported, using the total number of circuits as the denominator according to methods employed in a previous study (Tsujimoto 2020).
2. Clustering at the level of the enrolled units in cluster‐randomised studies
In dealing with cluster‐RCTs, for dichotomous data, we would have applied the design effect formula and calculated effective sample size and number of events using the intra‐cluster correlation coefficient (ICC) among each unit and the average cluster size, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the ICC had not been reported, we would have used the ICC of a similar study as a substitute. For continuous data, only the sample size would be reduced; means and standard deviations would remain unchanged (Higgins 2011).
3. Randomised cross‐over studies
We considered only data from the first period (before crossing over) where possible. However, for cross‐over studies that did not report first‐phase data, we decided to include the study and analysed the data as if the trials were parallel trials. We rated its validity according to the Cochrane Collaboration’s tool for assessing the risk of bias as being at 'unclear risk of bias' based on baseline imbalance due to the cross‐over design (Assessment of risk of bias in included studies).
Multiple comparisons
All intervention groups that were assessed to be relevant to this review as per our a priori eligibility criteria were included (Types of interventions).
Dealing with missing data
Where possible, we requested further information from the study corresponding authors via electronic or postal mail and any such requested, relevant information obtained were included in the review. We diligently evaluated important numerical data, such as the numbers of screened/randomised participants as well as intention‐to‐treat, as‐treated and per‐protocol populations were reported. Attrition rates, including drop‐outs, losses to follow‐up and withdrawals, were also investigated. Issues of missing data and imputation methods (for example, use of ‘last observation carried forward’ (LOCF)) employed by study investigators were identified and critically appraised (Higgins 2011).
If studies did not report mean or standard deviation for a continuous outcome, we imputed them from the median and range (Hozo 2005) or from the median and interquartile range (Wan 2014). When there was no information on variability, we imputed the standard deviation from other studies in the same meta‐analysis (Furukawa 2006).
Assessment of heterogeneity
We assessed the presence of statistical heterogeneity by visual inspection of the forest plots. We quantified statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). We followed the guide to the interpretation of I² values as indicated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)/
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi² test, or confidence intervals for I²) (Higgins 2011).
Assessment of reporting biases
In this review, since our analyses did not include more than 10 studies, and we could not perform Egger's test or funnel plot symmetry inspection to explore possible small‐study and publication biases (Egger 1997). Instead, we investigated the scale of ongoing studies in the field based on the search results from the Cochrane Kidney and Transplant Register of Studies, which includes records from trial registries (WHO ICTRP and ClinicalTrials.gov).
Data synthesis
We conducted data synthesis using a random‐effects analytical model.
Subgroup analysis and investigation of heterogeneity
In the protocol, we planned to perform the following subgroup analyses where possible for the primary outcomes to explore possible sources of heterogeneity. However, due to insufficient data, we were unable to pursue the following analyses.
Age of the participants (< 18 years versus ≥ 18 years)
Presence or absence of sepsis (Levi 2013)
Pharmacological co‐intervention (citrates, unfractionated heparins, other types of anticoagulation, no anticoagulation).
Sensitivity analysis
At the protocol stage, we decided to pursue the following sensitivity analyses for the primary outcomes. However, these were not performed for the full review due to limited data.
Repeating the analysis by excluding unpublished studies
Repeating the analysis by taking into account the risk of bias in included studies
Repeating the analysis by excluding large‐scale studies or studies of long duration
Repeating the analysis by excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country
Repeating the analysis by including only studies that used the same dosage of anticoagulants in both arms.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review as 'Summary of findings' tables, created by the GRADEpro GDT software. These tables present key information concerning the quality/certainty of the evidence, the magnitude of the intervention effects, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE approach (Guyatt 2008; Guyatt 2011b), which defines the quality/certainty of a body of evidence as to the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. To assess a body of evidence‐based on the GRADE approach, within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, the precision of effect estimates, and risk of publication bias are important domains for consideration (Schunemann 2011b).
Our 'Summary of findings' tables illustrated evidence relating to the following outcome measures.
Circuit lifespan
Death from any cause at day 28, or last reported time point
Recovery of kidney function
Vascular access complications.
Results
Description of studies
Results of the search
Our study selection process of this review is illustrated as a flow diagram in Figure 1. Our search of the Cochrane Kidney and Transplant Register of Studies identified 69 studies (179 records). After full‐text assessment, 26 studies (37 records) met the eligibility criteria and we excluded 43 studies (142 records). Of these 20 studies were included, five studies are ongoing (Weidhase 2020; NCT01062984; NCT01779635; NCT01790620; NCT03426943), and one study is awaiting classification (ISRCTN02674550). These six studies will be assessed in a future update of this review.
1.
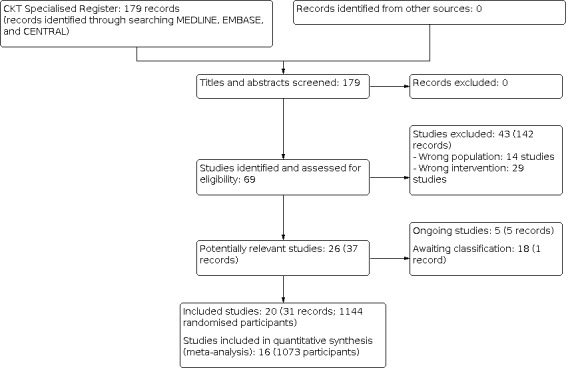
Study flow diagram.
Included studies
Details of our included studies are summarised in the 'Characteristics of included studies' tables. These 20 studies randomised 1143 participants. Of these, four studies (57 participants) did not report any of our prespecified outcome measures (Alamartine 1994a; Broman 2019; Maxvold 2000; Wynckel 2004) and were thus excluded from subsequent quantitative synthesis (meta‐analysis). We, therefore, eventually included 16 studies (1072 participants) in our quantitative synthesis. Of these, one study was published only as a conference abstract (Plata‐Menchaca 2017).
Study design
Of the 20 included studies, nine were parallel‐group RCTs (Alamartine 1994a; Daud 2006; Fealy 2017; Meier 2011a; OMAKI 2012; Plata‐Menchaca 2017; RADICAL 2012; Ramesh Prasad 2000; Saudan 2006); and 11 were cross‐over RCTs (Baldwin 2002; Broman 2019; Davies 2008; de Pont 2006; Dungen 2001; Kellum 1998; Maxvold 2000; Schetz 2012; van der Voort 2005; Wynckel 2004; Yin 2015).
Sample size
Included studies were small in scale, with sample size ranging from 6 to 236 participants, with five studies involving 100 or more participants (Fealy 2017; Meier 2011a; Plata‐Menchaca 2017; RADICAL 2012; Saudan 2006).
Settings
The majority of the included studies were conducted in the USA, Europe, and Australia in the early 2000s. All the reported study settings were ICUs.
Participants
All but one study included adults (mean age 50.5 to 72.7 years) with kidney failure requiring CKRT; Maxvold 2000 included only paediatric participants. In nine studies, APACHE II was used for defining severity score, for which the mean score ranged from 18.5 to 27. The causes of AKI differed between studies, with sepsis being the major cause in 11 studies (Broman 2019; Daud 2006; de Pont 2006; Dungen 2001; Meier 2011a; OMAKI 2012; Plata‐Menchaca 2017; RADICAL 2012; Schetz 2012; van der Voort 2005; Yin 2015); in two studies, the main cause of AKI was surgical procedures (Davies 2008; Fealy 2017).
Interventions and comparators
The comparisons and the corresponding included studies are as follows.
CVVHD versus CVVH or CVVHD: 7 studies (254 participants) (Alamartine 1994a; Daud 2006; Kellum 1998; Maxvold 2000; OMAKI 2012; Plata‐Menchaca 2017; Wynckel 2004).
CVVH versus CVVHDF: 2 studies (251 participants) (Davies 2008; Saudan 2006).
Higher versus standard blood flow: 2 studies (134 participants) (Fealy 2017; Ramesh Prasad 2000).
Pre‐dilution versus post‐dilution: 2 studies (48 participants) (de Pont 2006; van der Voort 2005).
Long (> 20 cm) versus short catheter (≤ 20 cm): 1 study (100 participants) (RADICAL 2012).
Surface‐modified double‐lumen catheter versus standard double‐lumen catheter of identical geometry and flow design: 1 study (236 participants) (Meier 2011a).
Polyethylenimine‐treated AN69 membrane (AN69ST) versus other membranes: 3 studies (76 participants) (Broman 2019; Schetz 2012; Yin 2015).
Filter with more and shorter (21 cm) hollow fibre versus standard (30 cm) hollow fibre: 1 study (6 participants) (Dungen 2001)
Baldwin 2002 (38 participants) included three separate randomised crossover trials and compared the following non‐pharmacological interventions.
Single‐site versus double‐site infusion anticoagulation
Flat plate versus hollow fibre filter of the same membrane type
Filter with a larger membrane surface area versus a smaller one.
We could not identify any evidence regarding the other prespecified comparisons as listed in the section 'Types of interventions'.
Outcomes
The number of studies and participants that assessed our prespecified outcome measures are summarised as follows (Types of outcome measures).
Circuit lifespan: 11 studies (663 participants) (Baldwin 2002; Davies 2008; de Pont 2006; Dungen 2001; Fealy 2017; Meier 2011a; RADICAL 2012; Ramesh Prasad 2000; Schetz 2012; van der Voort 2005; Yin 2015).
-
Death from any cause
One study (206 participants) reported death (any cause) at 28 days (Saudan 2006)
Three studies (357 participants) reported ICU death (Daud 2006; Meier 2011a; RADICAL 2012)
Other death‐related outcome measures reported were death at 24 hours (Kellum 1998), death at 60 days (OMAKI 2012), and death at 90 days (Plata‐Menchaca 2017);
In three cross‐over trials, the numbers of deaths were reported; however, the numbers of deaths in each study arm were unavailable due to the nature of a cross‐over design (Maxvold 2000; Wynckel 2004; Yin 2015).
Recovery of kidney function: 3 studies (304 participants) (Daud 2006; OMAKI 2012; Saudan 2006).
Vascular access complications: 2 studies (336 participants) (Meier 2011a; RADICAL 2012).
Costs to health care services: 2 studies (336 participants) (Meier 2011a; RADICAL 2012).
None of the eligible studies reported the following outcomes: types and number of dialysis filters, circuits and catheters, and consumption of dialysate.
Excluded studies
See Characteristics of excluded studies. Reasons for exclusion were wrong population (14 studies) and wrong intervention (29 studies).
Risk of bias in included studies
See Figure 2 and Figure 3 for findings of our risk of bias assessment in the included studies. Overall, the risk of bias across our 20 included studies was judged to be unclear to high.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Four studies were judged to be at low risk of bias: two used computer‐generated randomisation numbers (RADICAL 2012; Saudan 2006), one study used a web‐based randomisation approach (Fealy 2017), and one study used a random number table (Yin 2015). The remaining studies did not clearly report the method of randomisation and were judged to have an unclear risk of bias.
Allocation concealment
Four studies were judged to be at low risk of bias: three used sealed opaque envelopes for the concealment (Broman 2019; RADICAL 2012; Saudan 2006), and one used a web‐based randomisation approach (Fealy 2017). The remaining included studies did not clearly report their allocation methods to allow for a precise judgment of their risk of selection bias.
Blinding
Two studies blinded the study participants, personnel, and outcome assessors to treatment allocation (Schetz 2012; Yin 2015) and were judged to be at low risk of bias for both performance and detection bias. Five studies were judged to be at high risk of both performance and detection bias (Fealy 2017; Meier 2011a; OMAKI 2012; RADICAL 2012; Ramesh Prasad 2000), and nine studies did not provide sufficient information to determine these risks of bias (Alamartine 1994a; Baldwin 2002; Davies 2008; de Pont 2006; Dungen 2001; Kellum 1998; Maxvold 2000; Plata‐Menchaca 2017).
Broman 2019 blinded study participants and personnel and was judged to be at low risk of performance bias but had unclear detection bias. Three studies were judged to be at high risk of performance bias but at low risk of detection bias (Saudan 2006; van der Voort 2005; Wynckel 2004).
Incomplete outcome data
Sixteen studies followed all the participants from randomisation to end of the study and were judged to be at low risk of attrition bias (Baldwin 2002; Daud 2006; Dungen 2001; Fealy 2017; Kellum 1998; Maxvold 2000; Meier 2011a; OMAKI 2012; Plata‐Menchaca 2017; RADICAL 2012; Ramesh Prasad 2000; Saudan 2006; Schetz 2012; van der Voort 2005; Wynckel 2004; Yin 2015). We judged two studies to be at high risk of attrition bias due to missing data of more than 10% of the study participants (Davies 2008; de Pont 2006), and two studies did not provide sufficient information to determine the risk of bias (Alamartine 1994a; Broman 2019).
Selective reporting
We were able to find the study protocols for only 5 of the 18 included studies (Broman 2019; Fealy 2017; OMAKI 2012; Plata‐Menchaca 2017; RADICAL 2012). We judged Plata‐Menchaca 2017 to be at high risk of reporting bias because the study did not report our prespecified outcome of death.
Other potential sources of bias
For this review, we also defined two potential sources of bias: baseline imbalance and co‐interventions. Ten studies described baseline characteristics and we judged them to be at low risk of other bias regarding baseline imbalance (Alamartine 1994a; Daud 2006; Fealy 2017; Maxvold 2000; Meier 2011a; OMAKI 2012; RADICAL 2012; Ramesh Prasad 2000; Saudan 2006; Schetz 2012).
For co‐interventions, three studies were judged to be at high risk of bias: the amount of heparin used was different between the two groups in Alamartine 1994a and Meier 2011a, and anticoagulant usage and frequency of saline flushing varied between the two groups in Ramesh Prasad 2000. Ten studies were judged to be at low risk of bias (Baldwin 2002; Davies 2008; de Pont 2006; Dungen 2001; Fealy 2017; RADICAL 2012; Schetz 2012; van der Voort 2005; Wynckel 2004; Yin 2015)
For the remaining studies, we judged them to be at unclear risk of bias due to their cross‐over design. We could not determine whether the baseline characteristics were balanced between the intervention and control groups amongst these cross‐over trials since group‐based baseline information was unavailable. Additionally, we planned to include studies that allocated anticoagulants or sedative agents to both intervention and control arms according to a predefined protocol; however, the majority of the included studies did not report the administration procedures of these treatment options. We decided to include these studies in our review but judged their risk of 'other bias' regarding co‐interventions as per the Cochrane Collaboration's tool for assessing the risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Please refer to Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9 for further information on our review findings and corresponding GRADE assessment to assess the certainty of included evidence.
Dialysis modalities
CVVHD versus CVVH or CVVHDF
Four studies were included in this comparison (Daud 2006; Kellum 1998; OMAKI 2012; Plata‐Menchaca 2017).
Death from any cause
None of the included studies reported death at 28 days, therefore we synthesised data from the four studies that reported death at the longest follow‐up period (range 24 hours to 90 days). It is uncertain whether CVVHD reduced the risk of death from any cause at the longest follow‐up because the certainty of the evidence was very low (Analysis 1.1 (4 studies, 216 participants): RR 0.78, 95% CI 0.41 to 1.39; I² = 78%).
1.1. Analysis.

Comparison 1: CVVHD versus CVVH or CVVHDF, Outcome 1: Death from any cause
Recovery of kidney function
Two studies reported recovery of kidney function at ICU discharge (Daud 2006; OMAKI 2012). It is uncertain whether CVVHD increased recovery of kidney function because the certainty of the evidence was very low (Analysis 1.2 (2 studies, 93 participants): RR 0.91, 95% CI 0.37 to 2.21; I² = 0%).
1.2. Analysis.

Comparison 1: CVVHD versus CVVH or CVVHDF, Outcome 2: Recovery of kidney function
Other outcomes
The following outcomes were not reported in the four included studies.
Circuit lifespan
Vascular access complications
Costs
Types and number of dialysis filters, circuits, and catheters
Consumption of dialysate/dialysis fluid.
CVVHDF versus CVVH
Two studies were included in this comparison (Davies 2008; Saudan 2006).
Circuit lifespan
CVVHDF may increase circuit lifespan when compared with CVVH (Analysis 2.1 (1 study, 62 circuits): MD 10.15 hours, 95% CI 5.15 to 15.15; low certainty evidence).
2.1. Analysis.

Comparison 2: CVVHDF versus CVVH, Outcome 1: Circuit lifespan
Death from any cause at day 28
It is uncertain whether CVVHDF decreased the risk of death from any cause at 28 days compared to CVVH because the certainty of the evidence was very low (Analysis 2.2 (1 study 206 participants): RR 0.68, 95% CI 0.52 to 0.90).
2.2. Analysis.

Comparison 2: CVVHDF versus CVVH, Outcome 2: Death from any cause at 28 days
Recovery of kidney function
It is uncertain whether CVVHDF improved the recovery of kidney function compared to CVVH because the certainty of the evidence was very low (Analysis 2.3 (1 study, 96 participants): RR 1.10, 95% CI 0.86 to 1.41).
2.3. Analysis.

Comparison 2: CVVHDF versus CVVH, Outcome 3: Recovery of kidney function
Other outcomes
The following outcomes were not reported in the two included studies.
Vascular access complications
Costs
Types and number of dialysis filters, circuits, and catheters
Consumption of dialysate/dialysis fluid.
Pre‐dilution versus post‐dilution (as defined by the study investigators)
Two studies were included in this comparison (de Pont 2006; van der Voort 2005).
Circuit lifespan
Pre‐dilution may lead to an extended circuit lifespan compared to post‐dilution (Analysis 3.1 (2 studies, 47 circuits): MD 9.34 hours, 95% CI ‐2.60 to 21.29; I² = 13%; low certainty evidence).
3.1. Analysis.

Comparison 3: Pre‐dilution versus post dilution (as defined by study investigators), Outcome 1: Circuit lifespan
Costs
van der Voort 2005 reported the costs of one‐week treatment of CVVH using pre‐ or post‐dilution were 1,817 Euros and 2,146 Euros, respectively.
Other outcomes
The following outcomes were not reported in the two included studies.
Death
Vascular access complications
Types and number of dialysis filters, circuits, and catheters
Consumption of dialysate/dialysis fluid.
Blood flow rate
Higher (≥ 250 mL/min) versus standard blood flow (< 250 mL/min)
We included two studies for quantitative synthesis (Fealy 2017; Ramesh Prasad 2000). Ramesh Prasad 2000 compared standard blood flow (125 mL/min) with 100 mL bolus administration once/hour and augmented blood flow (200 to 250 mL/min) with 100 ml fluid bolus administration once every 30 minutes. We considered this comparison to be eligible for inclusion but rated the study as being at high risk of other bias in terms of co‐interventions.
Circuit lifespan
Higher blood flow may make little or no difference to prolonging circuit lifespan compared to standard blood flow (Analysis 4.1 (2 studies, 499 circuits): MD 0.64 hours, 95% CI ‐3.37 to 4.64; I² = 70%; low certainty evidence).
4.1. Analysis.

Comparison 4: Higher blood flow versus standard blood flow, Outcome 1: Circuit lifespan
Other outcomes
The following outcomes were not reported in the two included studies.
Death
Recovery of kidney function
Vascular access complications
Costs
Types and number of dialysis filters, circuits, and catheters
Consumption of dialysate/dialysis fluid.
Catheter types
Longer (> 20 cm) versus shorter (≤ 20 cm) catheter
One study compared the effects for preventing circuit clotting by either placing the tip of a longer catheter targeting the right atrium versus placing a shorter catheter targeting the tip in the superior vena cava (RADICAL 2012).
Circuit lifespan
Compared with a shorter catheter, using a longer catheter may prolong circuit lifespan (Analysis 5.1 (1 study, 402 circuits): MD 6.50 hours, 95% CI 1.48 to 11.52; low certainty evidence).
5.1. Analysis.

Comparison 5: Longer (> 20 cm) versus shorter (≤ 20 cm) catheter, Outcome 1: Circuit lifespan
Death from any cause at day 28
It is uncertain whether a longer catheter reduced the risk of death due to any cause at day 28 compared to a shorter catheter because the certainty of the evidence was very low (Analysis 5.2 (1 study, 94 participants): RR 1.38, 95% CI 0.61 to 3.11).
5.2. Analysis.

Comparison 5: Longer (> 20 cm) versus shorter (≤ 20 cm) catheter, Outcome 2: Death from any cause at 28 days
Vascular access complications
Compared with shorter catheters, the use of a longer catheter may reduce the risk of vascular access complications (Analysis 5.3 (1 study, 94 participants): RR 0.40, 95% CI 0.22 to 0.74; low certainty evidence).
5.3. Analysis.

Comparison 5: Longer (> 20 cm) versus shorter (≤ 20 cm) catheter, Outcome 3: Vascular access complications
Costs
RADICAL 2012 reported that potential cost reduction using a longer catheter targeting the right atrium was 427 USD per person (95% CI 61 to 794) when compared with a shorter catheter targeting the superior vena cava.
Other outcomes
The following outcomes were not reported.
Recovery of kidney function
Types and number of dialysis filters, circuits, and catheters
Consumption of dialysate/dialysis fluid.
Surface‐modified versus standard double‐lumen catheter (based on identical geometry and flow design)
One study compared surface‐modified double‐lumen catheters with standard double‐lumen catheters (Meier 2011a).
Circuit lifespan
Compared with a standard double‐lumen catheter, the use of a surface‐modified double‐lumen catheter may extend circuit lifespan (Analysis 6.1 (1 study, 262 circuits): MD 16.00 hours, 95% CI 13.49 to 18.51; low certainty evidence).
6.1. Analysis.

Comparison 6: Surface‐modified versus standard double‐lumen catheter with identical geometry and flow design, Outcome 1: Circuit lifespan
Death from any cause at day 28
Meier 2011a reported ICU death. It is uncertain whether surface‐modified double‐lumen catheter reduced the risk of ICU deaths compared to standard double‐lumen catheter because the certainty of the evidence was very low (Analysis 6.2 (1 study, 236 participants): RR 1.08, 95% CI 0.76 to 1.53; very low certainty evidence).
6.2. Analysis.

Comparison 6: Surface‐modified versus standard double‐lumen catheter with identical geometry and flow design, Outcome 2: Death from any cause at 28 days
Recovery of kidney function
Compared with a standard double‐lumen catheter, a surface‐modified double‐lumen catheter may not lead to the recovery of kidney function (Analysis 6.3 (1 study, 236 participants): RR 0.91, 95% CI 0.70 to 1.20; low certainty evidence).
6.3. Analysis.

Comparison 6: Surface‐modified versus standard double‐lumen catheter with identical geometry and flow design, Outcome 3: Recovery of kidney function
Vascular access complications
Compared with standard double‐lumen catheters, surface‐modified double‐lumen catheters may make little or no difference to vascular access complications (Analysis 6.4 (1 study, 236 participants): RR 0.32, 95% CI 0.08 to 1.20; low certainty evidence).
6.4. Analysis.

Comparison 6: Surface‐modified versus standard double‐lumen catheter with identical geometry and flow design, Outcome 4: Vascular access complications
Costs
Compared with standard double‐lumen catheters, surface‐modified double‐lumen catheters may lead to reduced medical costs (exchange costs of temporary catheters) (Analysis 6.5 (1 study, 236 participants): MD ‐5508.00 USD, 95% CI ‐5612.98 to ‐5403.02; low certainty evidence)
6.5. Analysis.

Comparison 6: Surface‐modified versus standard double‐lumen catheter with identical geometry and flow design, Outcome 5: Costs
Other outcomes
The following outcomes were not reported.
Types and number of dialysis filters, circuits, and catheters
Consumption of dialysate/dialysis fluid.
Membrane types
Polyethylenimine‐treated AN69 membrane (AN69ST) versus other membranes
Three studies compared AN69ST with other types of membranes as a non‐pharmacological intervention for preventing circuit clotting during CKRT (Broman 2019; Schetz 2012; Yin 2015). Broman 2019 but did not examine the prespecified outcome measures of this review.
Circuit lifespan
AN69ST may make little or no difference to circuit lifespan compared to other membranes (Analysis 7.1 (2 studies, 56 circuits): MD 1.54 hours, 95% CI ‐4.53 to 7.60; I² = 0%; low certainty evidence).
7.1. Analysis.

Comparison 7: Polyethylenimine‐treated AN69 membrane (AN69ST) versus other membrane, Outcome 1: Circuit lifespan
Other outcomes
The following outcomes were not reported in the three included studies.
Death
Recovery of kidney function
Vascular access complications
Costs
Types and number of dialysis filters, circuits, and catheters
Consumption of dialysate/dialysis fluid.
Flat plate versus hollow fibre filters of the same membrane type
One study compared flat plate and hollow fibre filters for preventing circuit clotting during CKRT (Baldwin 2002).
Circuit lifespan
It is uncertain whether the use of a flat plate haemofilter extended circuit lifespan compared to hollow fibre filters as the certainty of the evidence was very low (Analysis 8.1 (1 study, 38 participants): MD ‐1.40 hours, 95% CI ‐12.12 to 9.32).
8.1. Analysis.

Comparison 8: Flat plate versus hollow fibre filter of the same membrane type, Outcome 1: Circuit lifespan
The following outcomes were not reported.
Death
Recovery of kidney function
Vascular access complications
Costs
Types and number of dialysis filters, circuits, and catheters
Consumption of dialysate/dialysis fluid
More and shorter hollow fibre filters versus standard filters
One study compared more and shorter hollow fibre filters with standard filters (Dungen 2001).
Circuit lifespan
Compared with standard filters, the use of a filter with more and shorter hollow fibres may reduce circuit lifespan (Analysis 9.1 (1 study, 6 participants): MD ‐5.87 hours, 95% CI ‐10.18 to ‐1.56; low certainty evidence).
9.1. Analysis.

Comparison 9: More and shorter versus standard hollow fibre filter of the same membrane type, Outcome 1: Circuit lifespan
The following outcomes were not reported.
Death
Recovery of kidney function
Vascular access complications
Costs
Types and number of dialysis filters, circuits, and catheters
Consumption of dialysate/dialysis fluid
Filters with a larger membrane surface area versus a smaller membrane surface area
Baldwin 2002 reported circuit lifespan did not differ between larger versus smaller membrane surface areas (15.8 hours (SE 14.3) versus 16.8 hours (SE 13.1), respectively). However, the precise numbers of participants involved in these two analyses were not reported. We contacted the corresponding author in an attempt to obtain additional information for further clarification, but the respective data were not available.
Discussion
Summary of main results
This review included 20 studies involving 1143 randomised participants receiving CKRT that reported the effects of a variety of non‐pharmacological interventions on circuit clotting‐related outcomes. Most of the included studies were judged to be at unclear risk of bias. All but one study involved adult participants. The causes of AKI varied across studies but the major cause (as indicated in 11 included studies) was sepsis. According to the GRADE approach, we found that the certainty of the available evidence regarding non‐pharmacological interventions for preventing clotting of extracorporeal circuits during CKRT was low to very low. CVVHDF (as compared with CVVH), pre‐dilution haemofiltration (as compared with post‐dilution haemofiltration), placing the tip of a longer catheter targeting the right atrium (as compared with placing a shorter catheter targeting the tip in the superior vena cava, and surface‐modified double‐lumen catheter (as compared with a standard catheter) may extend circuit lifespan. It is worth noting that the majority of the included studies reported findings on circuit lifespan but not death.
Overall completeness and applicability of evidence
We conducted the present review using standard Cochrane review methodology in order to present a comprehensive assessment of available evidence indicating the effects of non‐pharmacological interventions for preventing circuit clotting during CKRT; we included eligible studies irrespective of participants' age, sex, or underlying/primary conditions. Our approach yielded evidence on two primary outcomes and five secondary outcomes across 10 treatment comparisons. However, as our prespecified outcomes of interest were often not reported, we were only able to present limited evidence for a few outcomes in each comparison. Furthermore, only one RCT contributed to each estimate of circuit lifespan in the following comparisons: CVVHDF versus CVVH (Davies 2008), long versus short catheter (RADICAL 2012), surface‐modified versus standard double‐lumen catheter with identical geometry and flow design (Meier 2011a), flat plate versus hollow fibre filter of the same membrane type (Baldwin 2002), and more and shorter versus standard hollow fibre filter (Dungen 2001). For a number of comparisons, we found only one eligible study reporting the outcome measures of interest. Cross‐over studies, accounting for more than half of the included studies in this review, did not provide data from the first phase i.e. before crossing over. We thus decided instead to use the results of within‐group comparisons. Due to limited data, we could not perform predetermined subgroup or sensitivity analyses, nor did we identify sufficient numbers of studies to assess publication bias. We identified five ongoing studies (NCT01062984; NCT01779635; NCT01790620; NCT03426943; Weidhase 2020); of these, three planned to compare the modes of haemodialysis: CVVH, CVVHD, and CVVHDF (Weidhase 2020; NCT01062984; NCT01790620), but we were unable to identify any of our prespecified outcome measures in the study registers. NCT01779635 and NCT03426943 plan to examine the effects of AN69ST membrane, which falls into the scope of our review, on the circuit life and death. This information will be incorporated in future updates.
We are aware that the applicability of our review evidence is limited because the majority of the included studies were performed more than 10 years ago and, understandably, clinical practice in this field has since changed. As an example, a number of the included studies did not use specific treatment criteria but instead relied on physicians' judgment for the initiation of CKRT (Davies 2008; Fealy 2017; Schetz 2012). Moreover, the appropriate timing of CKRT initiation is yet to be fully investigated (Gaudry 2020; Wald 2014). Regional citrate anticoagulation has been widely used and it was recommended in international guidelines (KDIGO Acute Kidney Injury Work Group 2012). The choice of anticoagulants should have an impact on the filter life in the current era. It should be highlighted that the evaluation of pharmacological interventions to prevent clotting is covered by a separate Cochrane review, as such, the findings from this review should be read in combination with the review on pharmacological interventions to prevent clotting of extracorporeal circuits during CKRT (Tsujimoto 2020).
Quality of the evidence
We assessed the certainty of included randomised evidence using the GRADE approach (Guyatt 2008). Overall, the certainty of our review evidence was judged to be low to very low due to the high risk of bias and small sample size amongst the included studies. For the majority of these studies, random sequence generation, allocation concealment, and blinding domains were assessed to be at unclear or high risk of bias (Figure 3). The total numbers of participants in all meta‐analyses were less than the optimal information size of 400, as recommended by the GRADE framework (Guyatt 2011a). With regards to the thresholds for appreciable treatment benefits and harms, we used a relative risk reduction of 0.25 for binary outcomes, and a standard deviation of 0.2 for continuous outcomes (Cohen 1988; Guyatt 2011a). Regarding the timing of mortality measurements, only one study in this review reported the prespecified 28‐day mortality and the others were downgraded for indirectness. As aforementioned, identified ongoing studies did not plan to include our review outcomes of interest and we judged that publication bias was highly unlikely.
Potential biases in the review process
We used the effect estimates from within‐group comparisons in the included cross‐over trials since outcome data from the first phase (before crossing over) were unavailable. However, it is unlikely that participant status before and after crossing over was similar since health conditions, in general, often change drastically within a short period of time in the ICU setting (Leloup 2020). Additionally, one could foresee the circuit lifespan during the second phase (after crossing over) given that the study interventions were not blinded to study personnel or participants. This, in effect, might lead to the use of co‐interventions, such as saline flushes, in the second phase. Therefore, we explicitly considered baseline imbalance and co‐interventions as potential sources of 'Other risk of bias' as per the Cochrane Collaboration's tool for assessing the risk of bias (Higgins 2011). As a result, half of the included studies were judged to be at high or unclear risk of co‐interventions. The lack of adjustment for anticoagulation methods should also be acknowledged as a limitation of this review. Additionally, we did not consider censoring effect on circuit life as this often needs individual participant data. The robustness of our findings should be further confirmed by comparing survival censoring for not clotting or for catheter reasons. We were unable to locate study plans/protocols for numerous included studies and consequently rated the risk of reporting bias as unclear.
Agreements and disagreements with other studies or reviews
A published non‐Cochrane systematic review included both RCTs and observational studies assessing the effects of non‐pharmacological interventions (Brain 2017). The review authors reported results similar to ours, where longer catheter or CVVHDF were superior in terms of prolonging circuit lifespan. On the other hand, as opposed to findings reported in Brain 2017, we found that higher blood flow exerted no effects on circuit lifespan. This might be due to the inclusion of non‐randomised evidence in Brain 2017. It should be noted that the included trials examined higher blood flow versus standard blood flow. As such, lowering the blood flow rate below the target, which is based on filtration fraction when filtration is used is not recommended for improving circuit life in this review. However, as mentioned above, our review outcomes, including death due to any cause and kidney function recovery, were often not reported.
Brain 2017 assessed the effects of several non‐pharmacological interventions that intended to prolong circuit life on several outcomes, such as different access sites and types of catheters however, the evidence came from observational studies and it was not our intention to consider observational studies in this review. It is worth highlighting some novel findings presented in this Cochrane review, where the surface‐modified double‐lumen catheter (as compared with standard double‐lumen catheter and the use of a long catheter (as compared with a short catheter) are generally cheaper and may prolong circuit lifespan.
As for the other included non‐pharmacological interventions, the currently available evidence is insufficient to allow for comprehensive assessment on the effects of a non‐pharmacological approach on circuit clotting as well as prognosis for people with AKI undergoing CKRT. We would like to highlight that a major strength of our review is adopting the robust Cochrane methodology during the entire review development process, in particular the use of a prespecified protocol as well as the GRADE approach to assess of certainty of evidence.
Authors' conclusions
Implications for practice.
Current evidence shows that the following non‐pharmacological strategies: CVVHDF, pre‐dilution haemofiltration, longer catheter, and surface‐modified double‐lumen catheter, may extend circuit lifespan during CKRT. On the other hand, a filter with more and shorter hollow fibres may reduce circuit lifespan. Analyses revealed that applying a higher blood flow may not alter circuit life. The available evidence is limited and thus we are unable to draw any solid conclusions in regards to the role and impact of the other non‐pharmacological interventions for preventing circuit clotting amongst people receiving CKRT.
Implications for research.
Recent clinical practice guidelines did not address the use of non‐pharmacological interventions for maintaining circuit patency (KDIGO Acute Kidney Injury Work Group 2012). Current evidence is limited and thus further high‐quality RCTs are needed to provide a more comprehensive assessment before anyone particular non‐pharmacological intervention is to be recommended for clinical practice. Our review illustrates that any research into non‐pharmacological methods should be prospective, randomised, adequately powered, and transparently reported. Furthermore, future studies should not only consider circuit lifespan as an outcome measure but also other patient‐important outcomes including death. We remain cautious as to the appropriateness of a cross‐over study design for future studies in this field due to systematic differences between the two periods of the cross‐over studies are highly likely, which may lead to a higher risk of bias; a potentially higher rate of drop‐outs/losses to follow‐up after the first period is also likely due to high death rates in the critically ill patients in the ICU settings.
What's new
| Date | Event | Description |
|---|---|---|
| 8 November 2021 | Amended | Addition of third comparison for recovery of kidney function to Abstract results |
History
Protocol first published: Issue 5, 2019 Review first published: Issue 9, 2021
| Date | Event | Description |
|---|---|---|
| 17 September 2021 | Amended | Minor edit to PLS |
| 15 September 2021 | Amended | Author affiliation updated |
Acknowledgements
We wish to thank the Cochrane Kidney and Transplant Group for their support, especially Fiona Russell and Gail Higgins. We wish to thank Joey Kwong and Cochrane Japan for linguistic support.
We wish to thank Prof. Rinaldo Bellomo for answering our query.
The authors are grateful to the following peer reviewers for their time and comments: Chris Kirwan, Stuart Goldstein, and also to the one reviewer who wishes to remain anonymous.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Cochrane Collaboration's tool for assessing risk of bias
| Potential sources of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s prespecified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not prespecified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. (e.g. co‐intervention of anticoagulation) |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. CVVHD versus CVVH or CVVHDF.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Death from any cause | 4 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.41, 1.39] |
| 1.1.1 Death at 24 hours | 1 | 13 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.05, 3.64] |
| 1.1.2 Death at 60 days | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.66, 1.46] |
| 1.1.3 Death at 90 days | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.23, 0.68] |
| 1.1.4 Death in ICU | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.79, 1.74] |
| 1.2 Recovery of kidney function | 2 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.37, 2.21] |
Comparison 2. CVVHDF versus CVVH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Circuit lifespan | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 10.15 [5.15, 15.15] |
| 2.2 Death from any cause at 28 days | 1 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.52, 0.90] |
| 2.3 Recovery of kidney function | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.86, 1.41] |
Comparison 3. Pre‐dilution versus post dilution (as defined by study investigators).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Circuit lifespan | 2 | 47 | Mean Difference (IV, Random, 95% CI) | 9.34 [‐2.60, 21.29] |
Comparison 4. Higher blood flow versus standard blood flow.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Circuit lifespan | 2 | 499 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐3.37, 4.64] |
Comparison 5. Longer (> 20 cm) versus shorter (≤ 20 cm) catheter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Circuit lifespan | 1 | 402 | Mean Difference (IV, Random, 95% CI) | 6.50 [1.48, 11.52] |
| 5.2 Death from any cause at 28 days | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.61, 3.11] |
| 5.3 Vascular access complications | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.20, 0.72] |
Comparison 6. Surface‐modified versus standard double‐lumen catheter with identical geometry and flow design.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Circuit lifespan | 1 | 262 | Mean Difference (IV, Random, 95% CI) | 16.00 [13.49, 18.51] |
| 6.2 Death from any cause at 28 days | 1 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.76, 1.53] |
| 6.3 Recovery of kidney function | 1 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.70, 1.20] |
| 6.4 Vascular access complications | 1 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.09, 1.20] |
| 6.5 Costs | 1 | 236 | Mean Difference (IV, Random, 95% CI) | ‐5.51 [‐5.61, ‐5.40] |
Comparison 7. Polyethylenimine‐treated AN69 membrane (AN69ST) versus other membrane.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Circuit lifespan | 2 | 56 | Mean Difference (IV, Random, 95% CI) | 1.54 [‐4.53, 7.60] |
Comparison 8. Flat plate versus hollow fibre filter of the same membrane type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Circuit lifespan | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐12.12, 9.32] |
Comparison 9. More and shorter versus standard hollow fibre filter of the same membrane type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Circuit lifespan | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐5.87 [‐10.18, ‐1.56] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alamartine 1994a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available; none of our prespecified outcomes were reported |
| Other bias: baseline imbalance | Low risk | We did not identify any substantial differences between groups |
| Other bias: co‐interventions | High risk | The amount of heparin use in the two groups was substantially different |
Baldwin 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comparison 1
Comparison 2
Comparison 3
|
| Selective reporting (reporting bias) | Unclear risk | Trial protocol was not available |
| Other bias: baseline imbalance | Unclear risk | Cross‐over RCT; unable to assess baseline imbalance |
| Other bias: co‐interventions | Low risk | Study investigators predefined the treatment protocol for heparin during CVVH |
Broman 2019.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | simple sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The treating physician and staff as well as the participant were blinded to the type of filter by covering the brand marks on the front and the bar code on the back using non‐transparent tape |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement; no outcome of interest was reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement; no outcome of interest was reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; no outcome of interest was reported |
| Other bias: baseline imbalance | Unclear risk | Cross‐over design; could not assess baseline imbalance |
| Other bias: co‐interventions | Unclear risk | Insufficient information to permit judgement |
Daud 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data identified |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; study protocol was not available |
| Other bias: baseline imbalance | Low risk | No substantial differences between the two groups |
| Other bias: co‐interventions | Unclear risk | Insufficient information to permit judgement |
Davies 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 14 participants who did not cross‐over were eventually excluded from study analysis without further information of the intervention they received in the first phase |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; trial protocol was not available |
| Other bias: baseline imbalance | Unclear risk | Cross‐over design; could not assess baseline imbalance |
| Other bias: co‐interventions | Low risk | Study investigators predefined the protocol for administering heparin during CVVH |
de Pont 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In one participant, kidney function recovered after the first haemofiltration run, obviating the need for a second run |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; study protocol was not available |
| Other bias: baseline imbalance | Unclear risk | Cross‐over trial; could not assess baseline imbalance |
| Other bias: co‐interventions | Low risk | Heparin was equally administered to both arms. Before starting CVVH, a loading dose of 2850 IU nadroparin was administered intravenously, followed by a continuous prefilter infusion of 456 IU hourly |
Dungen 2001.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; study protocol was not available |
| Other bias: baseline imbalance | Unclear risk | Cross‐over trial; we could not assess baseline imbalance |
| Other bias: co‐interventions | Low risk | No difference in anticoagulants |
Fealy 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based central randomisation service |
| Allocation concealment (selection bias) | Low risk | Web‐based central randomisation service |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Protocol stated: open (masking not used) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Protocol stated: open (masking not used) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data as reported was minimal (4 of 100) and the reasons for such missing data were well balanced (randomised but CKRT not performed) |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were reported |
| Other bias: baseline imbalance | Low risk | No substantial difference between the 2 groups |
| Other bias: co‐interventions | Low risk | Anticoagulation was provided according to a predefined ICU protocol, which stated that no anticoagulation in participants at risk of bleeding from coagulopathy or thrombocytopenia |
Kellum 1998.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; study protocol was not available |
| Other bias: baseline imbalance | Unclear risk | Cross‐over trial; we could not assess baseline imbalance |
| Other bias: co‐interventions | Unclear risk | Insufficient information to permit judgement |
Maxvold 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; study protocol was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; study protocol was not available |
| Other bias: baseline imbalance | Low risk | Cross‐over study; we could not assess baseline imbalance |
| Other bias: co‐interventions | Unclear risk | insufficient information to permit judgement |
Meier 2011a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Only single‐blinded (participants) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; study protocol was not available |
| Other bias: baseline imbalance | Low risk | No substantial baseline imbalances were detected. |
| Other bias: co‐interventions | High risk | Continuous heparin dose was different between two groups |
OMAKI 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of study participants with missing data was < 10% |
| Selective reporting (reporting bias) | Low risk | All reported outcomes in the protocol were subsequently reported in the full report |
| Other bias: baseline imbalance | Low risk | No substantial differences between groups |
| Other bias: co‐interventions | Unclear risk | Insufficient information to permit judgement |
Plata‐Menchaca 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double blind" written in protocol, but "single blind" written in abstract of full study report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double blind" written in protocol, but "single blind" written in abstract of full study report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The rate of missing data was less than 10% |
| Selective reporting (reporting bias) | High risk | The results of mortality was not shown in the publication |
| Other bias: baseline imbalance | Unclear risk | Insufficient information to permit judgement |
| Other bias: co‐interventions | Unclear risk | Insufficient information to permit judgement |
RADICAL 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE "...used a randomization table generated by a computer; sequential numbered and sealed in envelopes to achieve randomization and allocation concealment, respectively" |
| Allocation concealment (selection bias) | Low risk | QUOTE "...used a randomization table generated by a computer; sequential numbered and sealed in envelopes to achieve randomization and allocation concealment, respectively" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data was less than 10% |
| Selective reporting (reporting bias) | Low risk | All outcomes were as reported in the protocol |
| Other bias: baseline imbalance | Low risk | No substantial difference between groups |
| Other bias: co‐interventions | Low risk | No difference in anticoagulation |
Ramesh Prasad 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Thrombosis was diagnosed by unblinded nursing staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; study protocol was not available |
| Other bias: baseline imbalance | Low risk | No substantial differences between groups |
| Other bias: co‐interventions | High risk | Use of anticoagulants was left to the discretion of the attending physician, and the reported frequency of saline flush was different between groups. |
Saudan 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE "...computer‐generated randomization list, in random blocks of four and six participants" |
| Allocation concealment (selection bias) | Low risk | QUOTE "...corresponding treatment allocation cards were placed in consecutively numbered opaque envelopes. Each time a participant was enrolled in the study, the next available envelope was opened by the nephrologist on call, and the allocated treatment option was communicated to the treatment team" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was impossible for "logistic reasons" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were objective by nature |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; study protocol was not available |
| Other bias: baseline imbalance | Low risk | No substantial differences between groups |
| Other bias: co‐interventions | Unclear risk | Insufficient information to permit judgement |
Schetz 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for generating random sequence were unclear. Study authors only reported that the sequence was "determined by randomization (numbered and sealed allocation)" |
| Allocation concealment (selection bias) | Unclear risk | Methods regarding allocation concealment were unclear. Study authors only reported that the sequence was "determined by randomization (numbered and sealed allocation)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinicians and investigators were blinded to the nature of the membrane |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinicians and investigators were blinded to the nature of the membrane |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Rate of missing data was < 10% |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; study protocol was not available |
| Other bias: baseline imbalance | Low risk | No substantial differences between groups |
| Other bias: co‐interventions | Low risk | Co‐interventions were consistent amongst the two groups |
van der Voort 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Study 1
Study 2
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were objective by nature |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias: baseline imbalance | Unclear risk | Cross‐over trial; we could not assess baseline imbalance |
| Other bias: co‐interventions | Low risk | Standardised protocol in terms of dialysis machine, ultrafiltration rate, blood flow, filter, anticoagulation, substitution fluid, and catheter was used, |
Wynckel 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to conduct blinding due to the nature of the study intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were objective by nature |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; study protocol was not available |
| Other bias: baseline imbalance | Unclear risk | Cross‐over trial; unable to assess baseline imbalance |
| Other bias: co‐interventions | Low risk | Study investigators predefined the treatment protocol for anti‐coagulation |
Yin 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table was used |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinicians and participants were blinded to the nature of the membrane |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were objective by nature |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; study protocol was not available |
| Other bias: baseline imbalance | Unclear risk | Due to the cross‐over study design, we were unable to assess baseline imbalance |
| Other bias: co‐interventions | Low risk | Study investigators predefined the protocol for using anticoagulants |
ACEi ‐ angiotensin‐converting enzyme inhibitor; AKI ‐ acute kidney injury; APACHE ‐ Acute Physiology And Chronic Health Evaluation; APPT ‐ activated partial thromboplastin time; BFR ‐ blood flow rate; BUN ‐ blood urea nitrogen; CKRT ‐ continuous kidney replacement therapy; CrCl ‐ creatinine clearance; CVVH ‐ continuous venovenous haemofiltration; CVVHD ‐ continuous venovenous haemodialysis; CVVHDF ‐ continuous venovenous haemodiafiltration; ESKD ‐ end‐stage kidney disease; HD ‐ haemodialysis; HIV ‐ human immunodeficiency virus; ICU ‐ intensive care unit; IQR ‐ interquartile range; KRT ‐ kidney replacement therapy; LMWH ‐ low molecular weight heparin; M/F ‐ male/female; MAP ‐ mean arterial pressure; PT ‐ prothrombin time; RCT ‐ randomised controlled trial; RIFLE ‐ Risk Injury Failure Loss ESKD; SAP ‐ simplified acute physiology; SBP ‐ systolic blood pressure; SCr ‐ serum creatinine; SD ‐ standard deviation; SIRS ‐ systemic immune response syndrome; SOFA ‐ sequential organ failure assessment; WCC ‐ white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abe 2010c | Wrong intervention: sustained HDF versus CVVHDF |
| Abe 2011d | Wrong intervention: sustained HDF versus CVVHDF |
| Albino 2014 | Wrong intervention: prolonged intermittent KRT sessions (10 versus 6 hours) |
| Allen 2005 | Wrong population: included participants without AKI |
| Anstey 2016 | Wrong intervention: dilute citrate solutions (15 versus 18 mmol/L) |
| Atan 2014 | Wrong intervention: high cut‐off versus standard HF |
| Atapour 2006 | Wrong population: included participants without AKI |
| ATN 2005 | Wrong population: included intermittent HD |
| Augustine 2004 | Wrong intervention: CVVHD versus intermittent HD |
| Badawy 2013 | Wrong intervention: extended daily dialysis versus CVVHDF |
| Baldwin 2007 | Wrong intervention: CVVH and extended daily dialysis with filtration |
| CATHEDIA 2008 | Wrong population: included intermittent HD |
| CONVINT 2014 | Wrong population: included intermittent HD |
| Davenport 1993a | Wrong intervention: intermittent versus continuous HF |
| ENDoX 2013 | Wrong population: included participants without AKI |
| Farkas 1992 | Wrong population: included participants without AKI |
| Feliciani 2007 | Wrong population: included intermittent HD |
| George 2011 | Wrong intervention: CVVHDF versus PD |
| Haase 2007b | Wrong intervention: 9 hours of HF |
| Hakim 1994 | Wrong intervention: biocompatible versus bioincompatible membrane |
| Hassan 2013 | Wrong intervention: coupled plasma filtration and adsorption plus CVVH or CVVFH alone |
| Humes 2010 | Wrong intervention: immunomodulatory membranes |
| ISRCTN01121161 | Wrong intervention: compared two different heparinisation strategies |
| Jeffrey 1994 | Wrong intervention: compared HF and HD for 30 minutes |
| Kielstein 2004 | Wrong intervention: extended dialysis versus CVVH |
| Klouche 2007 | Wrong population: included intermittent HD |
| Kumar 2004 | Wrong intervention: extended daily dialysis versus continuous HD |
| Leslie 1996 | Wrong intervention: diluted heparin with different injection points |
| Libetta 2013 | Wrong population: participants with AKI who did not undergo CKRT |
| Mishra 2017 | Wrong intervention: sustained low‐efficiency dialysis (SLED) versus CKRT |
| Nand 2010 | Wrong intervention: CAVHDF versus CVVHDF |
| NCT03496935 | Wrong population: included intermittent HD |
| Opatrny 2002b | Wrong intervention: pharmacological intervention |
| Oudemans‐van Straaten 2009 | Wrong intervention: filtrate flow of 4 L/hour and converted to 2 L/hour after 60 min in group 1, and vice versa in group 2 |
| Park 2016 | Wrong intervention: conventional (40 mL/kg/hour) versus high (80 mL/kg/hour) doses of CVVHDF |
| Pedrini 2009 | Wrong population: included intermittent HD |
| RENAL 2006 | Wrong intervention: conventional (25 mL/kg/hour) versus high (40 mL/kg/hour) doses of CVVHDF |
| RESCUE 2012 | Wrong population: not CKRT but SLED |
| Su 2017 | Wrong population: included intermittent HD |
| Tolwani 2008 | Wrong intervention: standard (20 mL/kg/hour) versus high‐dose (35 mL/kg/hour) CVVHDF |
| Uehlinger 2005 | Wrong intervention: CVVHDF versus intermittent HD |
| Weidhase 2019 | Wrong intervention: high‐flux dialyser versus high‐cut‐off dialyser |
| Zhang 2004a | Wrong intervention: CVVH versus high‐volume HF; only 8 hours of treatment |
AKI ‐ acute kidney injury; CKRT ‐ continuous kidney replacement therapy; CAVHDF ‐ continuous arteriovenous haemodiafiltration; CVVHDF ‐ continuous venovenous haemodiafiltration; CVVH ‐ continuous venovenous haemofiltration; ICU ‐ intensive care unit; KRT ‐ kidney replacement therapy; HD ‐ haemodialysis; HDF ‐ haemodiafiltration; HF ‐ haemofiltration; PD ‐ peritoneal dialysis; SLED ‐ sustained low‐efficiency dialysis
Characteristics of studies awaiting classification [ordered by study ID]
ISRCTN02674550.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
CKRT ‐ continuous kidney replacement therapy
Characteristics of ongoing studies [ordered by study ID]
NCT01062984.
| Study name | Continuous venovenous hemofiltration versus continuous venovenuous hemodialysis |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | Jan 2009 |
| Contact information | Jay Koyner, MD University of Chicago Chicago, Illinois, United States, 60637 |
| Notes | Funding sources: NxStage Medical |
NCT01779635.
| Study name | Efficacy and safety of heparin‐grafted membrane for CRRT (CARROM) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | None of the prespecified outcomes of interest were reported |
| Starting date | August 2013 to December 2016 |
| Contact information | Horng‐Ruey Chua, MBBS National University Health System |
| Notes | Funding sources: unclear |
NCT01790620.
| Study name | Impact of CVVHD with adsorption capacity membranes in septic acute kidney injury |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | May 2013 to Oct 2017 |
| Contact information | Joan Sabater Riera, MD Hospital Universitari de Bellvitge |
| Notes | Funding sources: unclear |
NCT03426943.
| Study name | Endotoxins and cytokines removal during continuous hemofiltration with oXiris™ (ECRO) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | December 21, 2018 |
| Contact information | Thomas RIMMELE, MD, PhD Hospices Civils de Lyon |
| Notes | Funding source: Baxter Healthcare Corporation, Hospices Civils de Lyon |
Weidhase 2020.
| Study name | Myoglobin clearance in CVVHD and CVVHDF with regional citrate anticoagulation |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | None of the prespecified outcomes of interest were reported |
| Starting date | 2017/05/02 |
| Contact information | Universitätsklinikum Leipzig AöR Interdisziplinäre Internistische Intensivmedizin Leiter Prof. Dr. med. Sirak Petros Dr. med. Lorenz Weidhase Liebigstraße 20 04103 Leipzig Germany |
| Notes | Funding: Fresenius Medical Care Deutschland GmbH |
AKI ‐ acute kidney injury; BFR ‐ blood flow rate; CCU ‐ coronary care unit; CKRT ‐ continuous kidney replacement therapy; CVVHD ‐ continuous venovenous haemodialysis; CVVHDF ‐ continuous venovenous haemodiafiltration; CVVH ‐ continuous venovenous haemofiltration; ESKD ‐ end‐stage kidney disease; DVT ‐ deep vein thrombosis; Hb ‐ haemoglobin; ICU ‐ intensive care unit
Differences between protocol and review
We planned to include only participants with AKI but we decided to expand the scope to include all participants who received CKRT. This decision was derived from the fact that some studies did not report the reasons behind administering CKRT.
We included the following comparisons that were not predefined in the published Cochrane protocol.
Surface‐modified versus standard double‐lumen catheter with identical geometry and flow design
Flat plate versus a hollow fibre filter of the same membrane type
Filter with a larger membrane surface area versus a smaller one
Filter with more and shorter hollow fibres versus a standard filter.
We planned to include studies that allocated anticoagulant or sedative agents to both intervention and control arms according to a predefined protocol; however, the majority of the included studies did not report the administration procedures of these treatment options. We decided to include these studies in our review but judged their risk of 'other bias' regarding co‐interventions as per the Cochrane Collaboration's tool for assessing the risk of bias.
We did not consider 'unit of analysis' issues regarding multiple measurements of outcome measures in our published protocol. We added our approach on handling these issues in the current full review (Unit of analysis issues).
Contributions of authors
Drafting the protocol: SM, HT, YT, HS, HY, YK, TF
Study selection: YT, SM, HT, HS, HY, YK, TF
Extracting data from studies: YT, SM, HT, HS, HY, YK
Entering data into RevMan: YT
Carrying out the analysis: YT
Interpreting the analysis: YT, SM, HT, HS, HY, YK, TF
Drafting the final review: YT, SM, HT, HS, HY, YK, TF
Disagreement resolution: TF
Updating the review: YT, SM, HT, HS, HY, YK, TF
Sources of support
Internal sources
-
Kyoritsu Hospital, Japan
Salary to YT
-
Sumitomo Hospital, Japan
Salary to SM
-
Hyogo Prefectural Amagasaki General Medical Center, Japan
Salary to HS and YK
-
Kameda Medical Center, Japan
Salary to HY
-
Australian and New Zealand Intensive Care Research Centre, Department of Epidemiology and Preventive Medicine, Monash University, Australia
Salary to TF in 2019
-
Jikei University Hospital, Jikei University School of Medicine, Japan
Salary to TF in 2020
External sources
No sources of support provided
Declarations of interest
Yasushi Tsujimoto has declared that they have no conflict of interest
Sho Miki has declared that they have no conflict of interest
Hiraku Tsujimoto has declared that they have no conflict of interest
Hiroki Shimada has declared that they have no conflict of interest
Hideto Yasuda has declared that they have no conflict of interest
Yuki Kataoka has declared that they have no conflict of interest
Tomoko Fujii: supported by Japan Society for the Promotion of Science (JSPS) and has received a grant from JSPS. JSPS played no role in the review design, collection, management, analysis, and interpretation of data, writing of the review or the decision to submit the review for editorial approval.
Edited (no change to conclusions)
References
References to studies included in this review
Alamartine 1994a {published data only}
- Alamartine E, Filippis JP, Toulon J, Berthoux F. On-line continuous venovenous hemodiafiltration: a technique for the control of ultrafiltration and convection during continuous renal replacement therapy. Renal Failure 1994;16(6):707-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baldwin 2002 {published data only}
- Baldwin I, Tan HK, Bridge N, Bellomo R. Possible strategies to prolong circuit life during hemofiltration: three controlled studies. Renal Failure 2002;24(6):839-48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Broman 2019 {published data only}
- Broman ME, Hansson F, Vincent JL, Bodelsson M. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: a randomized crossover double-blind study. PLoS ONE [Electronic Resource] 2019;14(8):e0220444. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daud 2006 {published data only}
- Daud KM, Leong GB, Visvanathan R. Acute dialytic support for the critically ill: continuous venovenous haemodialysis versus continuous venovenous haemofiltration. International Medical Journal 2006;13(1):37-42. [EMBASE: 43569217] [Google Scholar]
Davies 2008 {published data only}
- Davies HT, Leslie G, Pereira SM, Webb SA. A randomized comparative crossover study to assess the affect on circuit life of varying pre-dilution volume associated with CVVH and CVVHDF. International Journal of Artificial Organs 2008;31(3):221-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Pont 2006 {published data only}38768865
- Pont AC, Bouman CS, Bakhtiari K, Schaap MC, Nieuwland R, Sturk A, et al. Predilution versus postdilution during continuous venovenous hemofiltration: a comparison of circuit thrombogenesis. ASAIO Journal 2006;52(4):416-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dungen 2001 {published data only}
- Dungen HD, Heymann C, Ronco C, Kox WJ, Spies CD. Renal replacement therapy: physical properties of hollow fibers influence efficiency. International Journal of Artificial Organs 2001;24(6):357-66. [MEDLINE: ] [PubMed] [Google Scholar]
Fealy 2017 {published data only}
- Fealy N, Aitken L, du Toit E, Bailey M, Baldwin I. Evaluation of urea and creatinine change during continuous renal replacement therapy: effect of blood flow rate. Critical Care & Resuscitation 2018;20(1):41-7. [MEDLINE: ] [PubMed] [Google Scholar]
- Fealy N, Aitken L, DuToit E, Baldwin I. Blood flow rate and circuit life in continuous renal replacement therapy (CRRT): a pilot randomised controlled trial [abstract]. Anaesthesia & Intensive Care 2016;44(2):305. [EMBASE: 72325668] [Google Scholar]
- Fealy N, Aitken L, du Toit E, Lo S, Baldwin I. Faster blood flow rate does not improve circuit life in continuous renal replacement therapy: a randomized controlled trial. Critical Care Medicine 2017;45(10):e1018-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kellum 1998 {published data only}
- Kellum JA, Johnson JP, Kramer D, Palevsky P, Brady JJ, Pinsky MR. Diffusive vs. convective therapy: effects on mediators of inflammation in patient with severe systemic inflammatory response syndrome. Critical Care Medicine 1998;26(12):1995-2000. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maxvold 2000 {published data only}
- Maxvold NJ, Smoyer WE, Custer JR, Bunchman TE. Amino acid loss and nitrogen balance in critically ill children with acute renal failure: a prospective comparison between classic hemofiltration and hemofiltration with dialysis. Critical Care Medicine 2000;28(4):1161-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meier 2011a {published data only}
- Meier P, Meier R, Turini P, Friolet R, Blanc E. Prolonged catheter survival in patients with acute kidney injury on continuous renal replacement therapy using a less thrombogenic micropatterned polymer modification. Nephrology Dialysis Transplantation 2011;26(2):628-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Meier P. Prolonged catheter survival in patients with acute kidney insufficiency on continuous renal replacement therapy using a less thrombogenic micropatterned polymer modification [abstract]. Hemodialysis International 2009;13(3):416. [EMBASE: 70213431] [Google Scholar]
OMAKI 2012 {published data only}
- Wald R, Friedrich JO, Bagshaw SM, Burns KE, Garg AX, Hladunewich MA, et al. Optimal Mode of clearance in critically ill patients with Acute Kidney Injury (OMAKI) - A pilot randomized controlled trial of hemofiltration versus hemodialysis: A Canadian Critical Care Trials Group project. Critical Care (London, England) 2012;16(5):R205. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald R, Friedrich JO, Bagshaw SM, Burns KE, Garg AX, Hladunewich MA, et al. The optimal mode of renal replacement therapy in acute kidney injury (OMAKI): a pilot randomized controlled trial of CVVH vs CVVHD [abstract no: LB-PO3173]. Journal of the American Society of Nephrology 2011;22(Abstracts):10B. [Google Scholar]
Plata‐Menchaca 2017 {published data only}
- Plata-Menchaca EP, Cardenas-Campos P, Moreno-Gonzalez G, Corral-Velez V, Vazquez-Rebellon JM, Pinseaun-Castillo JP, et al. Comparison of two continuous renal replacement techniques associated with an increased absorption membrane: a randomized control trial [abstract no: 0030]. Intensive Care Medicine Experimental 2017;5(Suppl 2):18. [EMBASE: 619044415] [Google Scholar]
- Plata-Menchaca EP, Perez-Fernandez XL, Estruch-Alrich M, Betbese-Roig A, Cardenas Campos P, Rojas-Lora M, et al. Impact of continuous veno-venous hemodialyisis with an increased adsorption membrane in septic acute kidney injury [abstract no: A325]. Intensive Care Medicine Experimental 2016;4(Suppl 1):171. [EMBASE: 617955433] [Google Scholar]
RADICAL 2012 {published data only}
- Ho KM, Morgan DJ. Patient factors associated with frequent clotting of dialysers during haemodiafiltration in critically ill patients: a post hoc analysis of a randomised controlled study. Anaesthesia & Intensive Care 2014;42(1):59-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morgan D, Ho K, Murray C, Davies H, Louw J. A randomized trial of catheters of different lengths to achieve right atrium versus superior vena cava placement for continuous renal replacement therapy. American Journal of Kidney Diseases 2012;60(2):272-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ramesh Prasad 2000 {published data only}
- Ramesh Prasad GV, Palevsky PM, Burr R, Lesko JM, Gupta B, Greenberg A. Factors affecting system clotting in continuous renal replacement therapy: results of a randomized, controlled trial. Clinical Nephrology 2000;53(1):55-60. [MEDLINE: ] [PubMed] [Google Scholar]
Saudan 2006 {published data only}
- Saudan P, Niederberger M, De Seigneux S, Romand J, Pugin J, Perneger T, et al. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney International 2006;70(7):1312-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Saudan P, Niederberger M, De Seigneux S, Romand J, Pugin J, Perneger T, et al. A prospective randomized trial comparing continuous hemodiafiltration versus hemofiltration in critically ill patients with acute renal failure [abstract no: PUB003]. Journal of the American Society of Nephrology 2004;15(Oct):763A. [CENTRAL: CN-00724917] [Google Scholar]
- Saudan P, Niederberger M, Sellweger M, Pugin J, Romand J, Perneger T, et al. Continuous hemofiltration versus continuous hemodiafiltration in critically ill patients with acute renal failure [abstract no: PUB003]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):666. [CENTRAL: CN-00447595] [Google Scholar]
- Saudan P, Ponte B, Pugin J, Martin P. Improving survival with a greater dialysis dose in patients with AKI: does it depend on early onset of treatment? [abstract no: SU121]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009. [CENTRAL: CN-01658135]
- Saudan P, Triverio PA, Romand JA, Pugin J, Martin PY. Long-term prognosis in critically ill patients with acute renal failure treated by continuous renal replacement therapy [abstract no: TH-PO822]. Journal of the American Society of Nephrology 2006;17(Abstracts):282A. [CENTRAL: CN-00724918] [Google Scholar]
- Triverio PA, Martin PY, Romand J, Pugin J, Perneger T, Saudan P. Long-term prognosis after acute kidney injury requiring renal replacement therapy. Nephrology Dialysis Transplantation 2009;24(7):2186-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schetz 2012 {published data only}58520610
- Schetz M, Van Cromphaut S, Dubois J, Van den Berghe G. Does the surface-treated AN69 membrane prolong filter survival in CRRT without anticoagulation? Intensive Care Medicine 2012;38(11):1818-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van der Voort 2005 {published data only}
- Voort PH, Gerritsen RT, Kuiper MA, Egbers PH, Kingma WP, Boerma EC. Filter run time in CVVH: pre- versus post-dilution and nadroparin versus regional heparin-protamine anticoagulation. Blood Purification 2005;23(3):175-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wynckel 2004 {published data only}
- Wynckel A, Cornillet J, Bene B, Stolz A, Lepouse C, Paris B, et al. Improved removal of small proteins using continuous venovenous hemofiltration to treat acute renal failure. ASAIO Journal 2004;50(1):81-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yin 2015 {published data only}
- Yin Y, Zhao C, Hu Z, Wei S, Huo Y. The effect of AN69 ST membrane on filter lifetime in continuous renal replacement therapy without anticoagulation in patients with high risk of bleeding. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015;27(5):343-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abe 2010c {published data only}
- Abe M, Okada K, Suzuki M, Nagura C, Ishihara Y, Fujii Y, et al. Comparison of sustained hemodiafiltration with continuous venovenous hemodiafiltration for the treatment of critically ill patients with acute kidney injury. Artificial Organs 2010;34(4):331-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Abe 2011d {published data only}
- Abe M, Maruyama N, Matsumoto S, Okada K, Fujita T, Matsumoto K, et al. Comparison of sustained hemodiafiltration with acetate-free dialysate and continuous venovenous hemodiafiltration for the treatment of critically ill patients with acute kidney injury. International Journal of Nephrology 2011;2011:432094. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Albino 2014 {published data only}33774458
- Albino BB, Balbi AL, Abrao JM, Ponce D. Dialysis complications in acute kidney injury patients treated with prolonged intermittent renal replacement therapy sessions lasting 10 versus 6 hours: results of a randomized clinical trial. Artificial Organs 2015;39(5):423-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Albino BB, Balbi AL, Ponce D. Dialysis complications in AKI patients treated with extended daily dialysis: is the duration of therapy important? BioMed Research International 2014;2014:153626. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarin Albino B, Gobo-Oliveira M, Balbi AL, Ponce D. Mortality and recovery of renal function in acute kidney injury patients treated with prolonged intermittent hemodialysis sessions lasting 10 versus 6 hours: results of a randomized clinical trial. International Journal of Nephrology 2018;2018:4097864. [EMBASE: 30186631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2005 {published data only}
- Allen S, McBride WT, Young IS, MacGowan SW, McMurray TJ, Prabhu S, et al. A clinical, renal and immunological assessment of surface modifying additive treated (SMART) cardiopulmonary bypass circuits. Perfusion 2005;20(5):255-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Anstey 2016 {published data only}
- Anstey C, Campbell V, Richardson A. A comparison between two dilute citrate solutions (15 vs. 18 mmol/l) in continuous renal replacement therapy: The Base Excess and Renal Substitution Solution study. Blood Purification 2016;42(3):194-201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Atan 2014 {published data only}
- Atan R, May C, Bailey SR, Tanudji M, Visvanathan K, Skinner N, et al. Nucleosome levels and toll-like receptor expression during high cut-off haemofiltration: a pilot assessment. Critical Care & Resuscitation 2015;17(4):239-43. [MEDLINE: ] [PubMed] [Google Scholar]
- Atan R, Peck L, Prowle J, Licari E, Eastwood GM, Storr M, et al. A double-blind randomized controlled trial of high cutoff versus standard hemofiltration in critically Ill patients with acute kidney injury. Critical Care Medicine 2018;46(10):e988-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Atan R, Peck L, Visvanathan K, Skinner N, Eastwood G, Bellomo R, et al. High cut-off hemofiltration versus standard hemofiltration: effect on plasma cytokines. International Journal of Artificial Organs 2016;39(9):479-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Atan R, Virzi GM, Peck L, Ramadas A, Brocca A, Eastwood G, et al. High cut-off hemofiltration versus standard hemofiltration: a pilot assessment of effects on indices of apoptosis. Blood Purification 2014;37(4):296-303. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Atapour 2006 {published data only}
- Atapour A, Shahidi S. Does tunneling the temporary vascular access extend its lifetime? Journal of Research in Medical Sciences 2006;11(1):41-7. [EMBASE: 44335890] [Google Scholar]
ATN 2005 {published data only}
- Afshinnia F, Belanger K, Palevsky PM, Young EW. Effect of ionized serum calcium on outcomes in acute kidney injury needing renal replacement therapy: secondary analysis of the acute renal failure trial network study. Renal Failure 2013;35(10):1310-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley S, Schein R, Dev D, Finkel K, Vijayan A, Paganini E, et al. Dialysis catheter complications in the VA/NIH ATN study [abstract no: SA-PO557]. Journal of the American Society of Nephrology 2007;18(Abstracts):463A. [Google Scholar]
- Crowley S, Schein R, Dev D, Finkel K, Vijayan A, Paganini E, et al. Lessons for successful study enrollment from the VA/NIH ATN study [abstract no: SA-PO932]. Journal of the American Society of Nephrology 2006;17(Abstracts):770A. [CENTRAL: CN-00601950] [Google Scholar]
- Crowley ST, Chertow GM, Vitale J, O'Connor T, Zhang J, Schein RM, et al. Lessons for successful study enrollment from the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study. Clinical Journal of the American Society of Nephrology: CJASN 2008;3(4):955-61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delos Santos RB, Li T, Palevsky PM, Vijayan A. Intermittent hemodialysis in acute kidney injury: results from the VA/NIH ATN trial [abstract no:TH-OR037]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):9A. [Google Scholar]
- Demirjian S, Paganini EP, Zhang JH, O'Connor TZ, Vitale J, Palevsky PM. Severity of illness does not modify the effect of intensity of renal replacement therapy (RRT) on outcome in critically ill patients with AKI: results from the VA/NIH acute renal failure trial network (ATN) study [abstract no: SA-PO2997]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):791A. [Google Scholar]
- Demirjian S, Paginini EP, Zhang JH, O'Connor TZ, Vitale J, Palevsky PM, et al. Predictive scoring systems perform poorly in critically ill patients with AKI requiring renal replacement: data from the VA/NIH acute renal failure trial network (ATN) study [abstract no: SA-PO3010]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):794A. [Google Scholar]
- Ganta K, Ng Y, Davis HT, Unruh ML. Vascular access in acute kidney injury: results from the ATN study [abstract no: TH-PO847]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):286a. [Google Scholar]
- Griffin BR, Jovanovich A, You Z, Palevsky P, Faubel S, Jalal D. Effects of baseline thrombocytopenia and platelet decrease following renal replacement therapy initiation in patients with severe acute kidney injury. Critical Care Medicine 2019;47(4):e325-31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen KL, Smith MW, Unruh ML, Siroka AM, O'Connor T, Palevsky PM. Predictors of health utility among 60-day survivors of AKI in the VA/NIH Acute Renal Failure Network Study [abstract no: TH-FC086]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):21A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen KL, Smith MW, Unruh ML, Siroka AM, O'Connor TZ, Palevsky PM, et al. Predictors of health utility among 60-day survivors of acute kidney injury in the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(8):1366-72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce VR, Smith MW, Johansen KL, Unruh ML, Siroka AM, O'Connor TZ, et al. Health-related quality of life as a predictor of mortality among survivors of AKI. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(7):1063-70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KV, Zhang JH, Palevsky PM. Urea reduction ratio may be a simpler approach for measurement of adequacy of intermittent hemodialysis in acute kidney injury. BMC Nephrology 2019;20(1):82. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCausland FR, Asafu-Adjei J, Betensky RA, Palevsky PM, Waikar SS. Comparison of urine output among patients treated with more intensive versus less intensive RRT: results from the Acute Renal Failure Trial Network Study. Clinical Journal of the American Society of Nephrology: CJASN 2016;11(8):1335-42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCausland FR, Asafuadjei JK, Betensky RA, Palevsky PM, Waikar SS. Renal replacement intensity and urine volume in critically Ill patients-results from the ATN study [abstract no: SA-PO003]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):633A. [Google Scholar]
- Murugan R, Wen X, Keener C, Pike F, Palevsky PM, Unruh M, et al. Associations between intensity of RRT, inflammatory mediators, and outcomes. Clinical Journal of the American Society of Nephrology: CJASN 2015;10(6):926-33. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng YH, Ganta K, Davis H, Pankratz VS, Unruh M. Vascular access site for renal replacement therapy in acute kidney injury: a post hoc analysis of the ATN Study. Frontiers in Medicine 2017;4:40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palevsky PM, Franchini R, O'Connor TZ, Zhang JH, VA/NIH Acute Renal Failure Trial Network. Recovery of kidney function in critically ill patients with acute kidney injury (AKI) treated with intensive versus less-intensive renal replacement therapy (RRT) [abstract no: SA-PO2994]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):790A. [Google Scholar]
- Palevsky PM, O'Connor T, Zhang JH, Star RA, Smith MW. Design of the VA/NIH Acute Renal Failure Trial Network (ATN) Study: intensive versus conventional renal support in acute renal failure. Clinical Trials 2005;2(5):423-35. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palevsky PM, O'Connor TZ, Chertow GM, Crowley ST, Zhang JH, Kellum JA, et al. Intensity of renal replacement therapy in acute kidney injury: perspective from within the Acute Renal Failure Trial Network Study. Critical Care (London, England) 2010;13(4):310. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palevsky PM, O'Connor TZ, Zhang JH, Star R. VA/NIH Acute Renal Failure Trial: Study design [abstract no: SA-PO970]. Journal of the American Society of Nephrology 2003;14(Program & abstracts):512A. [Google Scholar]
- Palevsky PM, Overberger P, Franchini R, O'Connor TZ, Zhang JH, VA/NIH Acute Renal Failure Trial Network. One-year outcomes in critically ill patients with acute kidney injury (AKI) treated with intensive versus less-intensive renal replacement therapy (RRT) [abstract no: SA-FC414]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):93A. [Google Scholar]
- Palevsky PM, Zhang J, O'Connor T. Intensive versus non-intensive renal replacement therapy (RRT) in critically ill patients with acute kidney injury (AKI) [abstract]. In: American Thoracic Society International Conference; 2008 May 16-21; Toronto, Canada. 2008:A767.
- Pankratz VS, Argyropoulos C, Abdel-Kader K, Liang KV, Palevsky PM, Unruh ML. SOFA scores as predictors of mortality and dialysis dependency in acute kidney injury [abstract no: FR-PO488]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):470A. [Google Scholar]
- Pankratz VS, Argyropoulos C, Myers O, Unruh ML. Prediction of mortality or the need for continued renal replacement therapy following acute kidney injury requiring dialysis [abstract no: TH-PO709]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):257A. [Google Scholar]
- Pesacreta M, Overberger P, Palevsky PM, VA/NIH Acute Renal Failure Trial Network. Management of renal replacement therapy in acute renal failure: a survey of practitioner prescribing practices [abstract no: SA-PO227]. Journal of the American Society of Nephrology 2004;15(Oct):350A. [CENTRAL: CN-00601951] [DOI] [PMC free article] [PubMed] [Google Scholar]
- VA/NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, et al. Intensity of renal support in critically ill patients with acute kidney injury [Erratum in: N Engl J Med. 2009 Dec 10;361(24):2391]. New England Journal of Medicine 2008;359(1):7-20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, O'Connor T, Palevsky PM, VA/NIH Acute Renal Failure Trial Network. Evaluation of treatment separation in the VA/NIH Acute Renal Failure Trial Network (ATN) Study [abstract no: A63]. Clinical Trials 2009;6(5):523-4. [CENTRAL: CN-00783257] [Google Scholar]
- Zhang JH, O'Connor T, Swanson K, Palevsky PM, VA/NIH Acute Renal Failure Trial Network. Evaluation of trial safety in an ICU trial: experience from the VA/NIH Acute Renal Failure Trial Network (ATN) Study [abstract no: P71]. Clinical Trials 2009;6(5):560. [CENTRAL: CN-00783258] [Google Scholar]
- Zhang JH, Palevsky PM, Chertow GM, Hartigan J, O'Connor TZ, Guarino P, et al. Piecewise analysis of patient survival after onset of AKI. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(10):1679-84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Augustine 2004 {published data only}
- Augustine JJ, Sandy D, Lee J, Paganini EP. Hemodynamic stability in a prospective randomized trial of intermittent versus continuous dialysis in acute renal failure [abstract no: SU-PO893]. Journal of the American Society of Nephrology 2003;14(Nov):731A. [CENTRAL: CN-00583467] [Google Scholar]
- Augustine JJ, Sandy D, Seifert TH, Paganini EP. A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. American Journal of Kidney Diseases 2004;44(6):1000-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sandy D, Moreno L, Lee JC, Paganini EP. A randomized, stratified, dose equivalent comparison of continuous veno-venous hemodialysis (CVVHD) vs intermittent hemodialysis (IHD) support in ICU acute renal failure patients (ARF) [abstract no: S253]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):225A. [CENTRAL: CN-00447576] [Google Scholar]
Badawy 2013 {published data only}
- Badawy SS, Hassan AR, Samir EM. A prospective randomized comparative pilot trial on extended daily dialysis versus continuous venovenous hemodiafiltration in acute kidney injury after cardiac surgery. Egyptian Journal of Cardiothoracic Anesthesia 2013;7:69-73. [Google Scholar]
Baldwin 2007 {published data only}
- Baldwin I, Bellomo R, Naka T, Koch B, Fealy N. A pilot randomized controlled comparison of extended daily dialysis with filtration and continuous veno-venous hemofiltration: fluid removal and hemodynamics. International Journal of Artificial Organs 2007;30(12):1083-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baldwin I, Naka T, Koch B, Fealy N, Bellomo R. A pilot randomised controlled comparison of continuous veno-venous haemofiltration and extended daily dialysis with filtration: effect on small solutes and acid-base balance. Intensive Care Medicine 2007;33(5):830-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chua HR, Baldwin I, Fealy N, Naka T, Bellomo R. Amino acid balance with extended daily diafiltration in acute kidney injury. Blood Purification 2012;33(4):292-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CATHEDIA 2008 {published data only}
- Dugue AE, Levesque SP, Fischer MO, Souweine B, Mira JP, Megarbane B, et al. Vascular access sites for acute renal replacement in intensive care units. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(1):70-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parienti JJ, Dugue AE, Daurel C, Mira JP, Megarbane B, Mermel LA, et al. Continuous renal replacement therapy may increase the risk of catheter infection. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(8):1489-96. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parienti JJ, Megarbane B, Fischer MO, Lautrette A, Gazui N, Marin N, et al. Catheter dysfunction and dialysis performance according to vascular access among 736 critically ill adults requiring renal replacement therapy: a randomized controlled study. Critical Care Medicine 2010;38(4):1118-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Parienti JJ, Thirion M, Megarbane B, Souweine B, Ouchikhe A, Polito A, et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: a randomized controlled trial. JAMA 2008;299(20):2413-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CONVINT 2014 {published data only}
- Pschowski R, Briegel S, Haehling S, Doehner W, Bender TO, Pape UF, et al. Effects of dialysis modality on blood loss, bleeding complications and transfusion requirements in critically ill patients with dialysis-dependent acute renal failure. Anaesthesia & Intensive Care 2015;43(6):764-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schefold JC, Haehling S, Pschowski R, Bender T, Berkmann C, Briegel S, et al. The effect of continuous versus intermittent renal replacement therapy on the outcome of critically ill patients with acute renal failure (CONVINT): a prospective randomized controlled trial. Critical Care (London, England) 2014;18(1):R11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davenport 1993a {published data only}
- Davenport A, Will EJ, Davidson AM. Improved cardiovascular stability during continuous modes of renal replacement therapy in critically ill patients with acute hepatic and renal failure. Critical Care Medicine 1993;21(3):328-38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Davenport A, Will EJ, Davison AM. Effect of renal replacement therapy on patients with combined acute renal and fulminant hepatic failure. Kidney International - Supplement 1993;41:S245-51. [MEDLINE: ] [PubMed] [Google Scholar]
ENDoX 2013 {published data only}
- Maggiorini M. The effects of a polyethyleneimine-coated membrane (oXiris™) for hemofiltration versus polymyxin B- immobilized fibre column (Toraymyxin™) for hemoperfusion on endotoxin activity and inflammatory conditions in septic shock- a randomized controlled pilot study (ENDoX-study). clinicaltrials.gov/ct2/show/NCT01948778 (first received 24 September 2013).
Farkas 1992 {published data only}
- Farkas JC, Liu N, Bleriot JP, Chevret S, Goldstein FW, Carlet J. Single- versus triple-lumen central catheter-related sepsis: a prospective randomized study in a critically ill population. American Journal of Medicine 1992;93(3):277-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Feliciani 2007 {published data only}
- Feliciani A, Riva MA, Zerbi S, Ruggiero P, Plati AR, Cozzi G, et al. New strategies in haemodiafiltration (HDF): prospective comparative analysis between on-line mixed HDF and mid-dilution HDF. Nephrology Dialysis Transplantation 2007;22(6):1672-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pedrini LA, Feliciani A, Riva A, Zerbi S, Cozzi G. Middle molecular uremic toxins removal. Prospective comparative study between two new on-line haemodiafiltration (HDF) techniques: mixed HDF and mid-dilution HDF [abstract no: SP695]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv249. [CENTRAL: CN-00615864] [Google Scholar]
George 2011 {published data only}
- George J, Pisharody R, Upendran B, Varma S, Leelakumari M, Palliyil S. Clinical trial of continuous veno venous hemodiafiltration and peritoneal dialysis in critically ill acute renal failure [abstract no: SaP218]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi305. [CENTRAL: CN-01912345] [Google Scholar]
- George J, Varma S, Kumar S, Thomas J, Gopi S, Pisharody R. Comparing continuous venovenous hemodiafiltration and peritoneal dialysis in critically ill patients with acute kidney injury: a pilot study. Peritoneal Dialysis International 2011;31(4):422-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haase 2007b {published data only}
- Haase M, Silvester W, Uchino S, Goldsmith D, Davenport P, Tipping P, et al. A pilot study of high-adsorption hemofiltration in human septic shock. International Journal of Artificial Organs 2007;30(2):108-17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hakim 1994 {published data only}
- Hakim RM, Tolkoff-Rubin N, Himmelfarb J, Wingard RL, Parker RA. A multicenter comparison of bioincompatible (BICM) and biocompatible (BCM) membranes in the treatment of acute renal failure (ARF) [abstract]. Journal of the American Society of Nephrology 1994;5(3):394. [CENTRAL: CN-00583872] [DOI] [PubMed] [Google Scholar]
- Hakim RM, Wingard RL, Lawrence P, Parker RA, Schulman G. Use of biocompatible membranes (BCM) improves outcome and recovery from acute renal failure (ARF) [abstract]. Journal of the American Society of Nephrology 1992;3(3):367. [CENTRAL: CN-00460887] [Google Scholar]
- Hakim RM, Wingard RL, Parker RA. Effect of the dialysis membrane in the treatment of patients with acute renal failure. New England Journal of Medicine 1994;331(20):1338-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Himmelfarb J, Tolkoff-Rubin N, Chandran P, Parker RA, Wingard RL, Hakim R. A multicenter comparison of dialysis membranes in the treatment of acute renal failure requiring dialysis. Journal of the American Society of Nephrology 1998;9(2):257-66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Parker RA, Himmelfarb J, Tolkoff-Rubin N, Chandran P, Wingard RL, Hakim RM. Prognosis of patients with acute renal failure requiring dialysis: results of a multicenter study. American Journal of Kidney Diseases 1998;32(3):432-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hassan 2013 {published data only}
- Hassan J, Cader RA, Kong NC, Mohd M, Rahman AR, Hod R. Coupled plasma filtration adsorption (CPFA) plus continuous veno-venous haemofiltration (CVVH) versus CVVH alone as an adjunctive therapy in the treatment of sepsis. EXCLI Journal 2013;12:681-92. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Humes 2010 {published data only}
- Humes HD, Sobota JT, Ding F, Song JH, RAD Investigator Group. A selective cytopheretic inhibitory device to treat the immunological dysregulation of acute and chronic renal failure. Blood Purification 2010;29(2):183-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ISRCTN01121161 {published data only}01121161
- Henriksson B. Evaluation of lifespan in AN69ST with two different heparinization strategies. www.isrctn.com/ISRCTN01121161 (first received 13 March 2013).
Jeffrey 1994 {published data only}
- Jeffrey RF, Khan AA, Prabhu P, Todd N, Goutcher E, Will EJ, et al. A comparison of molecular clearance rates during continuous hemofiltration and hemodialysis with a novel volumetric continuous renal replacement system. Artificial Organs 1994;18(6):425-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kielstein 2004 {published data only}
- Kielstein JT, Kretschmer U, Ernst T, Hafer C, Bahr MJ, Haller H, et al. Efficacy and cardiovascular tolerability of extended dialysis in critically ill patients: a randomized controlled study. American Journal of Kidney Diseases 2004;43(2):342-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Kretschmer U, Ernst T, Hafer C, Haller H, Fliser D. Extended dialysis for treatment of critically patients with renal failure in the intensive care unit [abstract]. In: ISN - ERA/EDTA World Congress of Nephrology Satellite Symposium on ARF; 2003 Jun 13-15; Ghent, Belgium. 2003:O20. [CENTRAL: CN-00461064]
- Kielstein JT, Kretschmer U, Ernst T, Hafer C, Haller H, Fliser D. Randomized controlled study on efficacy and cardiovascular tolerability of slow low-efficient daily dialysis (SLEDD) vs. CVVH in critically ill patients with acute renal failure [abstract no: SU-PO897]. Journal of the American Society of Nephrology 2003;14(Nov):732A. [CENTRAL: CN-00653751] [Google Scholar]
- Kielstein JT, Kretschmer U, Ernst T, Hafer C, Haller H, Fliser D. Slow low-efficient daily dialysis (SLED) as renal replacement therapy for acute renal failure in the intensive care unit: combination of superior detoxification and excellent cardiovascular tolerability in severely ill patients [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):667. [CENTRAL: CN-00446081] [Google Scholar]
Klouche 2007 {published data only}
- Klouche K, Amigues L, Deleuze S, Beraud JJ, Canaud B. Complications, effects on dialysis dose, and survival of tunneled femoral dialysis catheters in acute renal failure. American Journal of Kidney Diseases 2007;49(1):99-108. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klouche K, Amigues L, Massanet P, Deleuze S, Canaud B, Beraud JJ. Vascular access for renal replacement therapy in ICU-acute renal failure: a comparison of tunnelled and non tunnelled femoral catheters [abstract no: T-PO40007]. Nephrology 2005;10(Suppl):A176-A7. [Google Scholar]
Kumar 2004 {published data only}
- Kumar V, Tseng R, Depner T. Extended daily dialysis (EDD) controls azotemia in intensive care unit (ICU) patients with acute renal failure [abstract no: 42]. American Journal of Kidney Diseases 2002;39(4):A21. [CENTRAL: CN-00401569] [Google Scholar]
- Kumar VA, Yeun JY, Depner TA, Don BR. Extended daily dialysis vs. continuous hemodialysis for ICU patients with acute renal failure: a two-year single center report. International Journal of Artificial Organs 2004;27(5):371-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leslie 1996 {published data only}
- Leslie GD, Jacobs IG, Clarke GM. Proximally delivered dilute heparin does not improve circuit life in continuous venovenous haemodiafiltration. Intensive Care Medicine 1996;22(11):1261-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Libetta 2013 {published data only}
- Libetta C, Esposito P, Sepe V, Rampino T, Zucchi M, Canevari M, et al. Acute kidney injury: effect of hemodialysis membrane on Hgf and recovery of renal function. Clinical Biochemistry 2013;46(1-2):103-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mishra 2017 {published data only}
- Mishra SB, Azim A, Prasad N, Singh RK, Poddar B, Gurjar M, et al. A pilot randomized controlled trial of comparison between extended daily hemodialysis and continuous veno-venous hemodialysis in patients of acute kidney injury with septic shock. Indian Journal of Critical Care Medicine 2017;21(5):262-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nand 2010 {published data only}
- Nand N, Yadav RK, Bharti K, Sharma M. Evaluation of efficacy and safety of continuous hemodiafiltration in cases of acute renal failure. Journal International Medical Sciences Academy 2010;23(4):223-5. [EMBASE: 362008284] [Google Scholar]
NCT03496935 {published data only}
- Mendu M, Kelly Y. Tunneled dialysis catheters versus non-tunneled dialysis catheters as first-line for renal replacement therapy in the ICU (BACKDOOR) [A randomized controlled trial of tunneled dialysis catheters versus non-tunneled dialysis catheters for renal replacement therapy in the ICU]. clinicaltrials.gov/ct2/show/NCT03496935 (first received 12 April 2018).
Opatrny 2002b {published data only}
- Opatrny K Jr, Polanska K, Krouzecky A, Vit L, Novak I, Kasal E. The effect of heparin rinse on the biocompatibility of continuous veno-venous hemodiafiltration. International Journal of Artificial Organs 2002;25(6):520-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oudemans‐van Straaten 2009 {published data only}
- Oudemans-van Straaten HM, Schilfgaarde M, Molenaar PJ, Wester JP, Leyte A. Hemostasis during low molecular weight heparin anticoagulation for continuous venovenous hemofiltration: a randomized cross-over trial comparing two hemofiltration rates. Critical Care (London, England) 2009;13(6):R193. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2016 {published data only}
- Park JT, Lee H, Kee YK, Park S, Oh HJ, Han SH, et al. High-dose versus conventional-dose continuous venovenous hemodiafiltration and patient and kidney survival and cytokine removal in sepsis-associated acute kidney injury: a randomized controlled trial. American Journal of Kidney Diseases 2016;68(4):599-608. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pedrini 2009 {published data only}
- Feliciani A. Reverse mid-dilution HDF: technical optimization for clearance optimization [abstract no: SP255]. In: XLV ERA-EDTA Congress; 2008 May 10-13; Stockholm, Sweden. 2008. [CENTRAL: CN-00757929]
- Pedrini LA, Feliciani A, Zerbi S, Cozzi G, Ruggiero P. Optimization of mid-dilution haemodiafiltration: technique and performance. Nephrology Dialysis Transplantation 2009;24(9):2816-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RENAL 2006 {published data only}
- Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Kim I, et al. The relationship between hypophosphataemia and outcomes during low-intensity and high-intensity continuous renal replacement therapy [Erratum in: Crit Care Resusc. 2014 Jun;16(2):139]. Critical Care & Resuscitation 2014;16(1):34-41. [MEDLINE: ] [PubMed] [Google Scholar]
- Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lee J, et al. Calorie intake and patient outcomes in severe acute kidney injury: findings from The Randomized Evaluation of Normal vs. Augmented Level of Replacement Therapy (RENAL) study trial. Critical Care (London, England) 2014;18(2):R45. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lee J, et al. Daily protein intake and patient outcomes in severe acute kidney injury: findings of the randomized Evaluation of Normal versus Augmented Level of Replacement Therapy (RENAL) trial. Blood Purification 2014;37(4):325-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bellomo R, Lipcsey M, Calzavacca P, Haase M, Haase-Fielitz A, Licari E, et al. Early acid-base and blood pressure effects of continuous renal replacement therapy intensity in patients with metabolic acidosis. Intensive Care Medicine 2013;39(3):429-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bellomo R, Martensson J, Kaukonen KM, Lo S, Gallagher M, Cass A, et al. Epidemiology of RBC transfusions in patients with severe acute kidney injury: analysis from the Randomized Evaluation of Normal Versus Augmented Level Study. Critical Care Medicine 2016;44(5):892-900. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bellomo R, Martensson J, Lo S, Kaukonen KM, Cass A, Gallagher M, et al. Femoral access and delivery of continuous renal replacement therapy dose. Blood Purification 2016;41(1-3):11-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bellomo R. Do we know the optimal dose for renal replacement therapy in the intensive care unit? Kidney International 2006;70(7):1202-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cass A, Bellomo R, Cole L, Finfer S, Gallagher M, Lo S, et al. Intensity of continuous renal replacement therapy in critically ill patients with severe acute kidney injury [abstract no: O78]. Nephrology 2009;14(Suppl 1):A21. [Google Scholar]
- Finfer S, Cass A, Gallagher M, Lee J, Su S, Bellomo R, et al. The RENAL (Randomised Evaluation of Normal vs. Augmented Level of Replacement Therapy) study: statistical analysis plan. Critical Care & Resuscitation 2009;11(1):58-66. [MEDLINE: ] [PubMed] [Google Scholar]
- Gallagher M, Bellomo R, Cass A, Cole L, Finfer S, Lee J, et al. Fluid balance and patient outcomes in AKI: analysis of the RENAL study participants [abstract no: 128]. Nephrology 2011;16(Suppl 1):57. [EMBASE: 70532476] [Google Scholar]
- Gallagher M, Bellomo R, Cass A, Finfer S, Gattas D, Lee J, et al. Long term outcomes of severe AKI: results of the post-RENAL study [abstract no: 071]. Nephrology 2012;17(Suppl 2):45. [EMBASE: 71377309] [Google Scholar]
- Gallagher M, Cass A, Bellomo R, Finfer S, Gattas D, Lee J, et al. Long-term survival and dialysis dependency following acute kidney injury in intensive care: extended follow-up of a randomized controlled trial. PLoS Medicine 2014;11(2):e1001601. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher MP, Bellomo R, Cass A, Cole L, Lee JY, Lo SN, et al. Fluid balance and patient outcomes in AKI: analysis of the RENAL study participants [abstract no: SA-PO2521]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):698A. [Google Scholar]
- Gallagher MP, Bellomo R, Cass A, Gattas D, Lee JY, Lo SN, et al. Long term outcomes of severe AKI: results of the post-RENAL study [abstract no: SA-OR008]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):67A. [Google Scholar]
- Jun M, Bellomo R, Cass A, Gallagher M, Lo S, Lee J, et al. Timing of renal replacement therapy and patient outcomes in the randomized evaluation of normal versus augmented level of replacement therapy study. Critical Care Medicine 2014;42(8):1756-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jun M, Bellomo R, Cass A, Gallagher M, Lo S. Timing of renal replacement therapy and patient outcomes in the randomized evaluation of normal vs augmented level of replacement therapy trial [abstract no: 008]. Nephrology 2012;17(Suppl 2):29. [EMBASE: 71377246] [Google Scholar]
- Jun M, Bellomo R, Cass A, Gallagher MP, Lo SN. Timing of renal replacement therapy and outcomes in critically III patients with acute kidney injury in the randomized evaluation of normal versus augmented level of replacement therapy therapy trial [abstract no: TH-OR038]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):8A. [Google Scholar]
- Lin J, Gallagher M, Bellomo R, Duan M, Trongtrakul K, Wang AY, et al. SOFA coagulation score and changes in platelet counts in severe acute kidney injury: analysis from the randomized evaluation of normal versus augmented level (RENAL) study. Nephrology 2019;24(5):518-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lin J, Wang Y, Bellomo R, Duan ML, Gallagher MP. SOFA coagulation score and patient outcomes in severe acute kidney injury: analysis from the randomized evaluation of normal versus augmented level (RENAL) study [abstract]. Nephrology 2017;22(Suppl 3):51. [EMBASE: 618236384] [DOI] [PubMed] [Google Scholar]
- Lin J, Wang Y, Bellomo R, Duan ML, Gallagher MP. SOFA coagulation score and patient outcomes in severe AKI: analysis from the Randomised Evaluation of Normal versus Augmented Level (RENAL) study [abstract no: PUB049]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):976-7. [EMBASE: 633701676] [Google Scholar]
- Murugan R, Kerti SJ, Chang CH, Gallagher M, Clermont G, Palevsky PM, et al. Association of net ultrafiltration rate with mortality among critically ill adults with acute kidney injury receiving continuous venovenous hemodiafiltration: a secondary analysis of the Randomized Evaluation of Normal vs Augmented Level (RENAL) of Renal Replacement Therapy trial. JAMA Network Open 2019;2(6):e195418. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien Z, Cass A, Cole L, Finfer S, Gallagher M, McArthur C, et al. Higher versus lower continuous renal replacement therapy intensity in critically ill patients with liver dysfunction. Blood Purification 2018;45(1-3):36-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- O'Brien Z, Cass A, Cole L, Finfer S, Gallagher M, McArthur C, et al. Sex and mortality in septic severe acute kidney injury. Journal of Critical Care 2019;49:70-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, et al. Intensity of continuous renal-replacement therapy in critically ill patients. New England Journal of Medicine 2009;361(17):1627-38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. An observational study fluid balance and patient outcomes in the Randomized Evaluation of Normal vs. Augmented Level of Replacement Therapy trial. Critical Care Medicine 2012;40(6):1753-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- RENAL Replacement Therapy Trial Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. Screening and study enrolment in the Randomized Evaluation of Normal vs. Augmented Level (RENAL) Replacement Therapy Trial. Blood Purification 2009;27(2):199-205. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- RENAL Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. Design and challenges of the Randomized Evaluation of Normal versus Augmented Level Replacement Therapy (RENAL) Trial: high-dose versus standard-dose hemofiltration in acute renal failure. Blood Purification 2008;26(5):407-16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Roberts D, Roberts M, Liu X, Roberts J, Lipman J, Bellomo R. Clearance of antibiotics by high and low intensity continuous renal replacement therapy in critically ill patients [abstract no: 232]. Nephrology 2010;15(Suppl 4):87. [EMBASE: 70467236] [Google Scholar]
- Roberts DM, Liu X, Roberts JA, Nair P, Cole L, Roberts MS, et al. A multicenter study on the effect of continuous hemodiafiltration intensity on antibiotic pharmacokinetics. Critical Care (London, England) 2015;19(1):84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Lo S, Bellomo R, Cass A, Gallagher M. Ace inhibitor use and AKI outcomes: an analysis of the randomised evaluation of normal vs augmented level of replacement therapy (RENAL) trial [abstract no: 104]. Nephrology 2013;18(Suppl 1):41. [EMBASE: 71357086] [Google Scholar]
- Wang AY, Bellomo R, Cass A, Finfer S, Gattas D, Myburgh J, et al. Health-related quality of life in survivors of acute kidney injury: The Prolonged Outcomes Study of the Randomized Evaluation of Normal versus Augmented Level Replacement Therapy study outcomes. Nephrology 2015;20(7):492-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wang AY, Bellomo R, Cass A, Myburgh J, Finfer S, Gatta D, et al. Predictors of health-related quality of life in survivors following acute kidney injury: a secondary analysis of post-RENAL study outcomes [abstract no: SP117]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii107-8. [EMBASE: 71491744] [Google Scholar]
- Wang AY, Bellomo R, Ninomiya T, Lo S, Cass A, Jardine M, et al. Angiotensin-converting enzyme inhibitor usage and acute kidney injury: a secondary analysis of RENAL study outcomes. Nephrology 2014;19(10):617-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wang AY, Trongtrakul K, Bellomo R, Li Q, Cass A, Gallagher M, et al. HMG-CoA reductase inhibitors (statins) and acute kidney injury: a secondary analysis of renal study outcomes. Nephrology 2019;24(9):912-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wang Y, Lo SN, Gallagher MP, Li Q, Cass A, Myburgh JA, et al. Renal replacement therapy intensity for acute kidney injury and recovery to dialysis independence [abstract no: SA-OR105]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):86A. [Google Scholar]
- Xie Y, Lin J, Gallagher M, Bellomo R, Wang X, Jardine M, et al. Prognostic significance of low level of blood glucose in severe acute kidney injury: a secondary analysis from the RENAL study [abstract no: SP243]. Nephrology Dialysis Transplantation 2018;33(Suppl 1):i425. [EMBASE: 622604949] [Google Scholar]
RESCUE 2012 {published data only}
- Doig GS. Concerns regarding use of one-tailed tests in the SLED-BD vs. CVVH trial. Critical Care (London, England) 2012;16(5):448. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenger V, Weigand MA, Hoffmann O, Dikow R, Kihm LP, Seckinger J, et al. Sustained low efficiency dialysis using a single-pass batch system in acute kidney injury - a randomized interventional trial: the REnal Replacement Therapy Study in Intensive Care Unit PatiEnts [Erratum in: Crit Care. 2012;16(5):451]. Critical Care (London, England) 2012;16(4):R140. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2017 {published data only}
- Su T, Jin Q, Liu Z. Polyethyleneimine-treated polyacrylonitrile membrane hemofilter for critically ill patients receiving anticoagulant-free prolonged intermittent renal replacement therapy: a single-center, prospective, self-controlled pilot study. BMC Nephrology 2017;18(1):208. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tolwani 2008 {published data only}
- Lyndon W, Wille K, Tolwani A. Solute clearance in CRRT: comparing measured effluent volume to actual delivered dose [abstract no:177]. American Journal of Kidney Diseases 2011;57(4):A61. [EMBASE: 70379736] [Google Scholar]
- Lyndon WD, Wille KM, Tolwani AJ. Solute clearance in CRRT: comparing measured effluent volume to actual delivered dose [abstract no: 26]. In: 16th International Conference on CRRT; 2011 Feb 22-25; San Diego (CA). 2011:127.
- Lyndon WD, Wille KM, Tolwani AJ. Solute clearance in CRRT: prescribed dose versus actual delivered dose. Nephrology Dialysis Transplantation 2012;27(3):952-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM. Standard versus high-dose CVVHDF for ICU-related acute renal failure. Journal of the American Society of Nephrology 2008;19(6):1233-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwani AJ, Speer R, Stofan B, Lai KR, Wille KM. A randomized prospective study comparing high dose continuous venovenous hemodiafiltration (CVVHDF) to standard CVVHDF in critically ill patients with acute renal injury [abstract no: 22]. Blood Purification 2007;25:193. [Google Scholar]
Uehlinger 2005 {published data only}
- Farese S, Jakob SM, Frey FJ, Uehlinger DE. More than 50% lower costs by IHD than by CVVHDF to treat acute renal failure in the ICU [abstract no: SA-PO939]. Journal of the American Society of Nephrology 2006;17(Abstracts):772A. [CENTRAL: CN-00740528] [Google Scholar]
- Farese S, Jakob SM, Kalicki R, Frey FJ, Uehlinger DE. Treatment of acute renal failure in the intensive care unit: lower costs by intermittent dialysis than continuous venovenous hemodiafiltration. Artificial Organs 2009;33(8):634-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ferrari P, Uehlinger DE, Jakob SM, Eichelberger M, Huynh-Do U, Marti HP, et al. A comparison of continuous and intermittent renal replacement therapy for acute renal failure [abstract no: 47]. Nephrology 2003;8(Suppl 3):A66. [CENTRAL: CN-00644145] [DOI] [PubMed] [Google Scholar]
- Uehlinger DE, Jakob SM, Eichelberger M, Ferrari P, Huynh-Do U, Marti HP, et al. A randomized, controlled single-center study for the comparison of continuous renal replacement therapy (CVVHDF) with intermittent hemodialysis (IHD) in critically ill patients with acute renal failure [abstract no: A1425]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):278A. [CENTRAL: CN-00448092] [Google Scholar]
- Uehlinger DE, Jakob SM, Ferrari P, Eichelberger M, Huynh-Do U, Marti HP, et al. Comparison of continuous and intermittent renal replacement therapy for acute renal failure. Nephrology Dialysis Transplantation 2005;20(8):1630-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weidhase 2019 {published data only}
- Weidhase L, Haussig E, Haussig S, Kaiser T, Fallois J, Petros S. Middle molecule clearance with high cut-off dialyzer versus high-flux dialyzer using continuous veno-venous hemodialysis with regional citrate anticoagulation: a prospective randomized controlled trial. PLoS ONE [Electronic Resource] 2019;14(4):e0215823. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2004a {published data only}
- Zhang J, Tao LJ, Ning JP, Xu H, Ai YH, Zhao SP. Effects of high-volume hemofiltration on serum levels of tumor necrosis factor and its receptors in patients with multiple organ dysfunction syndromes. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue [Chinese Critical Care Medicine] 2004;16(2):81-4. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
ISRCTN02674550 {published data only}02674550
- Allaouchiche B. Pilot, prospective, multicentric, open study: use of a new Prismaflex filter (coated with heparin) without any addition of heparin in the extracorporeal circuit. Study with direct individual benefit. https://doi.org/10.1186/ISRCTN02674550 2006.
References to ongoing studies
NCT01062984 {published data only (unpublished sought but not used)}
- Koyner J. Continuous venovenous hemofiltration versus continuous venovenuous hemodialysis [A comparison of the efficacy of continuous venovenous hemofiltration versus continuous venovenous hemodialysis for renal replacement therapy in acute kidney injury]. clinicaltrials.gov/ct2/show/NCT01062984 (first received 4 February 2010).
NCT01779635 {published data only (unpublished sought but not used)}
- Chua HR. Efficacy and safety of heparin-grafted membrane for CRRT (CARROM) [Continuous renal replacement therapy with anticoagulation-free regimen in bleeding-risk patients using oXiris membrane - CARROM Study]. clinicaltrials.gov/ct2/show/NCT01779635 (first received 30 January 2013).
NCT01790620 {published data only (unpublished sought but not used)}
- Riera JS. Impact of CVVHD with adsorption capacity membranes in septic acute kidney injury [Impact of a continuous dialysis technique associated with adsorption capacity membranes in patients with sepsis associated - acute kidney injury]. clinicaltrials.gov/ct2/show/NCT01790620 (first received 13 February 2013).
NCT03426943 {published data only}
- Rimmele T. Endotoxins and cytokines removal during continuous hemofiltration with oXiris (ECRO). clinicaltrials.gov/ct2/show/NCT03426943 (first received 8 February 2018).
Weidhase 2020 {published data only (unpublished sought but not used)}
- Weidhase L, Fallois J, Hausig E, Kaiser T, Mende M, Petros S. Myoglobin clearance with continuous veno-venous hemodialysis using high cutoff dialyzer versus continuous veno-venous hemodiafiltration using high-flux dialyzer: a prospective randomized controlled trial. Critical Care (London, England) 2020;24(1):644. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Baldwin 2004
- Baldwin I, Bellomo R, Koch B. Blood flow reductions during continuous renal replacement therapy and circuit life. Intensive Care Medicine 2004;30(11):2074-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bouchard 2015
- Bouchard J, Acharya A, Cerda J, Maccariello ER, Madarasu RC, Tolwani AJ, et al. A prospective international multicenter study of AKI in the intensive care unit. Clinical Journal of the American Society of Nephrology: CJASN 2015;10(8):1324-31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brain 2017
- Brain M, Winson E, Roodenburg O, McNeil J. Non anti-coagulant factors associated with filter life in continuous renal replacement therapy (CRRT): a systematic review and meta-analysis. BMC Nephrology 2017;18(1):69. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2017
- Clark WR, Leblanc M, Ricci Z, Ronco C. Quantification and dosing of renal replacement therapy in acute kidney injury: areappraisal. Blood Purification 2017;44(2):140-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd edition. Hillsdale, NJ: Erlbaum, 1988. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fealy 2002
- Fealy N, Baldwin I, Bellomo R. The effect of circuit “down-time” on uraemic control during continuous veno-venous haemofiltration. Critical Care & Resuscitation 2002;4(4):266-70. [MEDLINE: ] [PubMed] [Google Scholar]
Fujii 2018
- Fujii T, Uchino S, Doi K, Sato T, Kawamura T, JAKID study group. Diagnosis, management, and prognosis of patients with acute kidney injury in Japanese intensive care units: the JAKID Study. Journal of Critical Care 2018;47:185-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa T A, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 2006;59(1):7-10. [DOI] [PubMed] [Google Scholar]
Gaudry 2020
- Gaudry S, Hajage D, Benichou N, Chaïbi K, Barbar S, Zarbock A, et al. Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet 2020;395(10235):1506-15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 6 May 2020. Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence - Imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hoste 2015
- Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Medicine 2015;41(8):1411-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hozo 2005
- Hozo S P, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
KDIGO Acute Kidney Injury Work Group 2012
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney International Supplements 2012;2(2):1-138. [DOI: 10.1038/kisup.2012.1] [DOI] [Google Scholar]
Leloup 2020
- Leloup M, Briatte I, Langlois A, Cariou A, Lesieur O, ACIR study group. Unexpected cardiac arrests occurring inside the ICU: outcomes of a French prospective multicenter study. Intensive Care Medicine 2020;46(5):1005-15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levi 2013
- Levi M, Schultz M, Poll T. Sepsis and thrombosis. Seminars in Thrombosis & Hemostasis 2013;39(5):559-66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Manns 1997
- Manns M, Sigler MH, Teehan BP. Intradialytic renal haemodynamics - potential consequences for the management of the patient with acute renal failure. Nephrology Dialysis Transplantation 1997;12(5):870-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mehta 2001
- Mehta RL, McDonald B, Gabbai FB, Pahl M, Pascual MT, Farkas A, et al. A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney International 2001;60(3):1154-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mitchell 2003
- Mitchell A, Daul AE, Beiderlinden M, Schafers RF, Heemann U, Kribben A, et al. A new system for regional citrate anticoagulation in continuous venovenous hemodialysis (CVVHD). Clinical Nephrology 2003;59(2):106-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nisula 2013
- Nisula S, Kaukonen KM, Vaara ST, Korhonen AM, Poukkanen M, Karlsson S, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Medicine 2013;39(3):420-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rewa 2017
- Rewa OG, Villeneuve PM, Lachance P, Eurich DT, Stelfox HT, Gibney RT, et al. Quality indicators of continuous renal replacement therapy (CRRT) care in critically ill patients: a systematic review. Intensive Care Medicine 2017;43(6):750-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ricci 2006
- Ricci Z, Ronco C, Bachetoni A, D'amico G, Rossi S, Alessandri E, et al. Solute removal during continuous renal replacement therapy in critically ill patients: convection versus diffusion. Critical Care (London, England) 2006;10(2):R67. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Silversides 2014
- Silversides JA, Pinto R, Kuint R, Wald R, Hladunewich MA, Lapinsky SE, et al. Fluid balance, intradialytic hypotension, and outcomes in critically ill patients undergoing renal replacement therapy: a cohort study. Critical Care (London, England) 2014;18(6):624. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsujimoto 2020
- Tsujimoto H, Tsujimoto Y, Nakata Y, Fujii T, Takahashi S, Akazawa M, et al. Pharmacological interventions for preventing clotting of extracorporeal circuits during continuous renal replacement therapy. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD012467. [DOI: 10.1002/14651858.CD012467.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uchino 2003
- Uchino S, Fealy N, Baldwin I, Morimatsu H, Bellomo R. Continuous is not continuous: the incidence and impact of circuit "down-time" on uraemic control during continuous veno-venous haemofiltration. Intensive Care Medicine 2003;29(4):575-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Uchino 2007
- Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, et al. Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Medicine 2007;33(9):1563-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Venkataraman 2002
- Venkataraman R, Kellum JA, Palevsky P. Dosing patterns for continuous renal replacement therapy at a large academic medical center in the United States. Journal of Critical Care 2002;17(4):246-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vesconi 2009
- Vesconi S, Cruz DN, Fumagalli R, Kindgen-Milles D, Monti G, Marinho A, et al. Delivered dose of renal replacement therapy and mortality in critically ill patients with acute kidney injury. Critical Care (London, England) 2009;13(2):R57. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wald 2014
- Wald R, Bagshaw SM. The timing of renal replacement therapy initiation in acute kidney injury: is earlier truly better?*. Critical Care Medicine 2014;42(8):1933-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Miki 2019
- Miki S, Tsujimoto Y, Shimada H, Tsujimoto H, Yasuda H, Kataoka Y, et al. Non‐pharmacological interventions for preventing clotting of extracorporeal circuits during continuous renal replacement therapy. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD013330. [DOI: 10.1002/14651858.CD013330] [DOI] [PMC free article] [PubMed] [Google Scholar]


