This case-control study evaluates the use, sensitivity, and specificity of the cerebrospinal fluid metagenomic next-generation sequencing assay in identifying aneuploidy and other large copy number variations.
Key Points
Question
Can metagenomic next-generation sequencing (mNGS) of cerebrospinal fluid (CSF), a test designed to diagnose neurological infections, also detect genetic signatures of cancer in patients with presentations that are suggestive of neuroinflammatory disease?
Findings
In this case-control study of 130 patients, many of whom with negative CSF cytologic testing and/or flow cytometry results, CSF mNGS detected genetic evidence for a malignant neoplasm, with a sensitivity of 75% and specificity of 100%.
Meaning
CSF mNGS, an assay with low specimen volume requirements that does not require the preservation of cell integrity, has the potential to be an adjunctive tool for cancer detection even in cases for which CSF cytologic testing and flow cytometry are unrevealing.
Abstract
Importance
Cerebrospinal fluid (CSF) cytologic testing and flow cytometry are insensitive for diagnosing neoplasms of the central nervous system (CNS). Such clinical phenotypes can mimic infectious and autoimmune causes of meningoencephalitis.
Objective
To ascertain whether CSF metagenomic next-generation sequencing (mNGS) can identify aneuploidy, a hallmark of malignant neoplasms, in difficult-to-diagnose cases of CNS malignant neoplasm.
Design, Setting, and Participants
Two case-control studies were performed at the University of California, San Francisco (UCSF). The first study used CSF specimens collected at the UCSF Clinical Laboratories between July 1, 2017, and December 31, 2019, and evaluated test performance in specimens from patients with a CNS malignant neoplasm (positive controls) or without (negative controls). The results were compared with those from CSF cytologic testing and/or flow cytometry. The second study evaluated patients who were enrolled in an ongoing prospective study between April 1, 2014, and July 31, 2019, with presentations that were suggestive of neuroinflammatory disease but who were ultimately diagnosed with a CNS malignant neoplasm. Cases of individuals whose tumors could have been detected earlier without additional invasive testing are discussed.
Main Outcomes and Measures
The primary outcome measures were the sensitivity and specificity of aneuploidy detection by CSF mNGS. Secondary subset analyses included a comparison of CSF and tumor tissue chromosomal abnormalities and the identification of neuroimaging characteristics that were associated with test performance.
Results
Across both studies, 130 participants were included (median [interquartile range] age, 57.5 [43.3-68.0] years; 72 men [55.4%]). The test performance study used 125 residual laboratory CSF specimens from 47 patients with a CNS malignant neoplasm and 56 patients with other neurological diseases. The neuroinflammatory disease study enrolled 12 patients and 17 matched control participants. The sensitivity of the CSF mNGS assay was 75% (95% CI, 63%-85%), and the specificity was 100% (95% CI, 96%-100%). Aneuploidy was detected in 64% (95% CI, 41%-83%) of the patients in the test performance study with nondiagnostic cytologic testing and/or flow cytometry, and in 55% (95% CI, 23%-83%) of patients in the neuroinflammatory disease study who were ultimately diagnosed with a CNS malignant neoplasm. Of the patients in whom aneuploidy was detected, 38 (90.5%) had multiple copy number variations with tumor fractions ranging from 31% to 49%.
Conclusions and Relevance
This case-control study showed that CSF mNGS, which has low specimen volume requirements, does not require the preservation of cell integrity, and was orginally developed to diagnose neurologic infections, can also detect genetic evidence of a CNS malignant neoplasm in patients in whom CSF cytologic testing and/or flow cytometry yielded negative results with a low risk of false-positive results.
Introduction
Identifying the cause of meningoencephalitis is a challenge, with approximately half of the cases being undiagnosed.1,2,3 Major diagnostic considerations include occult infections, autoimmune disorders, and various malignant neoplasms, some of which can manifest as leptomeningeal carcinomatosis.1 However, malignant conditions are notoriously difficult to diagnose even after multiple large-volume cerebrospinal fluid (CSF) examinations. This difficulty may be associated with the paucicellular nature of CSF, including small numbers of malignant cells, or the need to preserve the morphological structure of the cells before they lyse. Because central nervous system (CNS) malignant neoplasms, infections, and inflammatory syndromes can clinically and radiologically overlap, a single test that can detect more than 1 type of hard-to-diagnose condition would have potential clinical utility.
Previous studies have reported on the use of metagenomic next-generation sequencing (mNGS) for detecting a wide array of pathogens in CSF.4,5,6,7,8,9,10,11,12 Metagenomic next-generation sequencing generates sequences from all of the genetic material in a CSF specimen, and most of the sequences in CSF are human. Previous studies have found CNS tumor DNA in CSF.13,14,15,16,17 We hypothesized that CSF mNGS human data could be repurposed to detect aneuploidy as a specific marker for CNS malignant neoplasms (Figure 1A). Aneuploidy and other large copy number variations (CNVs) are prevalent in primary and metastatic CNS tumors, with approximately 90% of all malignant tumors harboring an abnormal number of chromosomes (ie, aneuploidy).18 To ascertain whether CSF mNGS can identify aneuploidy in difficult-to-diagnose cases of CNS malignant neoplasm, we conducted 2 case-control studies using the depth-of-coverage method from noninvasive prenatal testing.19,20
Figure 1. Schematic of the Metagenomic Next-Generation Sequencing (mNGS) Test and Its Overall Performance in 2 Studies.
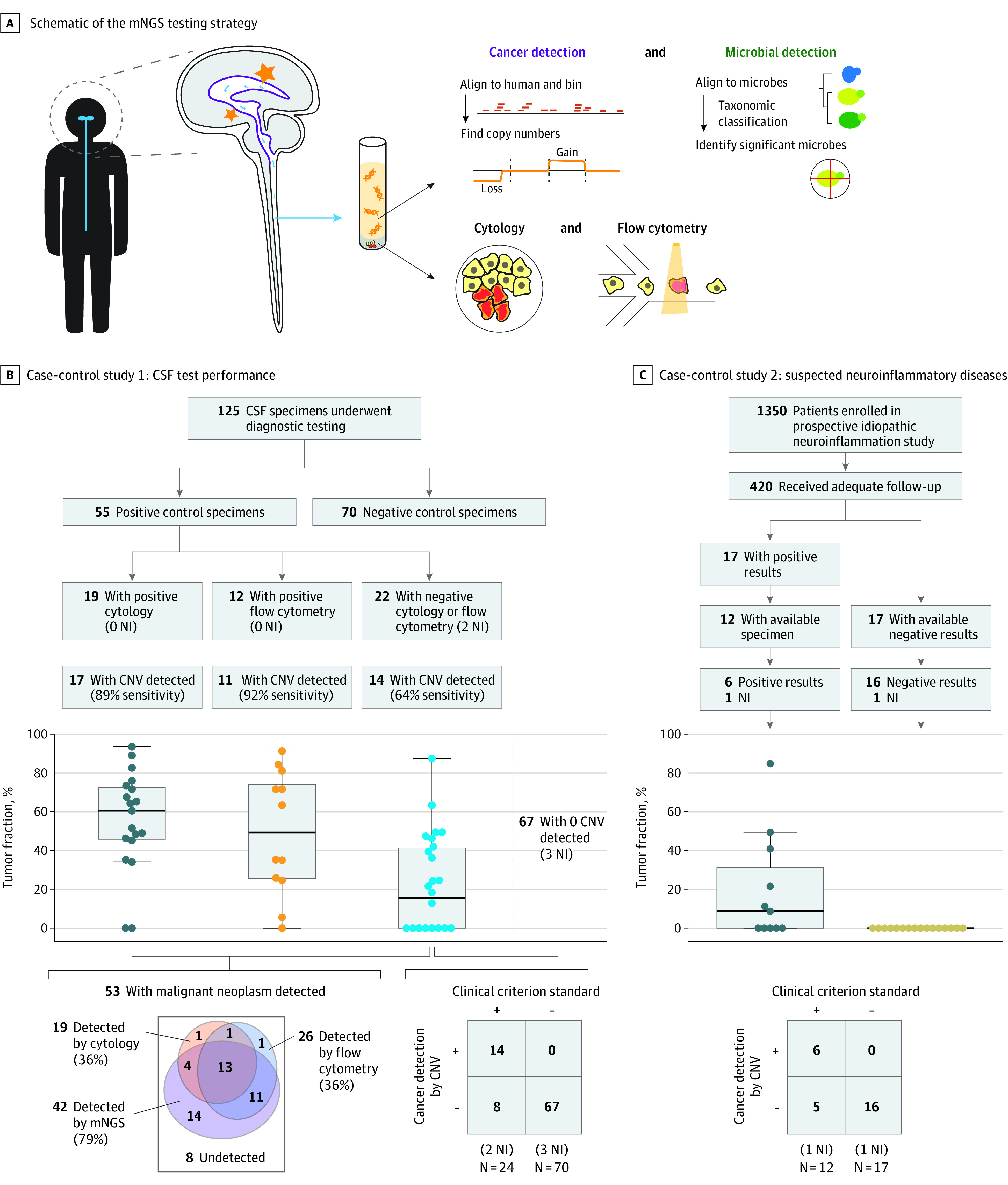
A, Brain lesions release pathogen or cancer cell–free DNA into the cerebrospinal fluid (CSF). An mNGS test performed on all specimens assessed for aneuploidy and pathogens. B, CSF specimens were obtained after flow cytometry and/or cytologic testing. Of these, 55 were positive controls (ie, from patients with central nervous system [CNS] malignant neoplasm) and 70 were negative controls from patients with an ultimate diagnosis of infectious or autoimmune disease. Tumor fractions are based on the amplitude of the copy number change. Cerebrospinal fluid mNGS was 64% sensitive and 100% specific for detecting the subset of malignant neoplasms not detected by flow cytometry and/or cytologic testing. C, Cases and control patients were from the idiopathic neuroinflammatory disease study. Cerebrospinal fluid mNGS had 55% sensitivity and 100% specificity for CNS malignant neoplasm detection. CNV indicates copy number variations; NI, not interpretable.
First, we characterized test performance using a pilot set of CSF specimens that were obtained from patients who underwent clinical evaluation for a CNS malignant neoplasm (primarily CNS lymphoma) and other neurological diseases. Second, we performed the same analyses using CSF specimens from participants with presentations that were suggestive of neuroinflammatory disease who were enrolled in an ongoing, prospective study.5,7
Methods
For the test performance case-control study, CSF specimens were originally collected at the University of California, San Francisco (UCSF) Clinical Laboratories between July 1, 2017, and December 31, 2019, as part of routine clinical testing and were retrospectively included in the study. The protocols for this first study were approved by the UCSF Institutional Review Board, and written consent was given to obtain CSF for clinical purposes. For the neuroinflammatory disease case-control study, written informed consent was obtained from UCSF patients with presentations that were suggestive of neuroinflammatory disease (or from their surrogate representatives) who were enrolled in a UCSF Institutional Review Board–approved prospective study between April 1, 2014, and July 31, 2019.
Specimen Selection
In the test performance case-control study, specimens from patients with a CNS malignant neoplasm (positive control specimens) or without a CNS malignant neoplasm (negative control specimens) were retrospectively identified through continuous screening using inclusion and exclusion criteria.
Positive control specimens were from patients with either a primary or metastatic CNS malignant neoplasm, excluding leukemia. The positivity of CSF specimens was ascertained by either (1) CSF cytologic test showing malignant cells or (2) CSF flow cytometry showing results that were consistent with a clonal, malignant cell population. Cerebrospinal fluid specimens with negative cytologic test and/or flow cytometry results were placed into a separate positive control category if a tumor was eventually confirmed by either (1) a positive CSF cytologic test or flow cytometry result in another CSF specimen, (2) a tissue diagnosis, or (3) a diagnostic consensus among hospital clinicians with an intention to treat. Cerebrospinal fluid specimens were excluded if they came from patients who were already undergoing active chemotherapy or radiotherapy, unless the cytologic test and flow cytometry of the CSF had a positive result.
Negative control specimens were from patients who ultimately received a clinical diagnosis of a CNS infection or autoimmune disease but had no known malignant cancer diagnosis, unless they had a paraneoplastic disease or had been in remission for more than 10 years. Cases without a definitive diagnosis on follow-up were excluded because they did not fit into the positive or negative categories.
Most specimens (80% of the 105 CSF specimens with records) were continuously received from flow cytometry testing. The rest of the specimens were from the hematology and microbiology laboratories and were screened because of positive results from cytologic or microbiological testing in a previous study enrollment.12 The number of positive and negative control specimens that were ultimately chosen was a convenience sample based on the maximum number of available CSF specimens and not based on a prespecified power calculation.
In the neuroinflammatory disease case-control study, cases with adult cancer and control patients were identified from a prospective study of CSF mNGS for presentations that were suggestive of neuroinflammatory disease. These participants were referred to neurologists between April 1, 2014, and July 31, 2019. Cases were identified by retrospective medical record review. Patients for whom a definitive diagnosis was never obtained or who had a benign tumor were excluded. Patients with an eventual diagnosis of an autoimmune disorder were included as negative control participants. Patients who were clinically determined (and confirmed with long-term follow-up) to have a paraneoplastic neurologic disease were not excluded.
Neuroimaging Analysis
To draw an association between CSF cancer detection and the brain magnetic resonance imaging (MRI) findings of the participants in the test performance and neuroinflammatory disease studies, one of us (A.M.R.), a board-certified neuroradiologist who was blinded to the mNGS results, reviewed all available MRI scans and recorded the number of lesions, location of the lesions, presence or absence of leptomeningeal involvement, and whether at least 1 lesion abutted the CSF space.
CSF and Tumor Tissue DNA Extraction
For the test-performance study, residual CSF specimens were collected from the UCSF Clinical Laboratories (hematology and flow cytometry, chemistry, and microbiology). The archival material was kept at 4 °C for up to 2 weeks and then processed before freezing. For the neuroinflammatory disease study, CSF specimens were collected directly from patients, or residual CSF was collected from the clinical laboratory. DNA from the specimens was either immediately extracted or after the CSF had been frozen at −80 °C either undiluted or in storage buffer (20% glycerol, 20 mM HEPES, and 0.02% sodium azide in phosphate buffer saline) at a 1:1 sample to buffer volumetric ratio.
Original or thawed CSF was centrifuged at 3000g to 16 000g for 10 minutes, and the supernatant was stored at −80 °C. Total nucleic acid extraction was performed by the EZ1 Advanced XL BioRobot using the EZ1 Virus Mini Kit, version 2.0 (QIAGEN), with 400 μL CSF input and 60 μL extracted DNA output.
Formalin-fixed paraffin blocks were used to obtain associated CNV data from the cancer tissue of the same participant. All archival tissue was no longer needed for clinical care. One of us (T.T.), a neuropathologist, identified regions of high tumor content on associated tissue sections. A disposable dermal punch was used to either punch out or scrape tissue from regions of interest. DNA was extracted from the fixed tissue using the Quick-DNA FFPE Miniprep kit (Zymo Research). Each specimen was sheared using focused acoustics to approximately 250 bp in a microTUBE (Covaris) and quantified on a spectrometer (Nanodrop; Thermo Fisher). Up to 50 ng of sheared DNA was used for whole genome sequencing library preparation.
Sequencing Library Preparation
Whole genome sequencing library preparation was performed using the NEBNext Ultra II DNA Library Preparation Kit (New England Biolabs Inc) on an epMotion 5075 liquid handler (Eppendorf) according to the manufacturer’s protocol, unless otherwise stated. All reagent volumes were halved, and the input was also halved to 25 μL of extracted DNA. For bead purification, we used AMPure XP beads (Beckman Coulter). Polymerase chain reaction amplification of the adapter-ligated DNA was up to 26 cycles, according to the manufacturer’s protocol, and the cDNA libraries were dual-indexed before pooling. Sequencing was performed on a HiSeq 1500 or 2500 system or Nextseq 550 system (Illumina Inc) in either the single end or optionally paired end for 140 bp.
Bioinformatics
Raw data were demultiplexed to raw FASTQ files and the adapter was trimmed with Cutadapt, version 1.16 (Marcel Martin). The mNGS analysis pipeline used SURPI8,21 to make microbial identifications. The CNVs were called by aligning with the human genome hg38 and de-duplicating reads with Burrows-Wheeler Alignment tool, version 0.7.12.22 CNVkit, version 0.9.1,19 was used to segment the genome into bins, display a log2 copy number across all bins, and calculate a median line (orange in plots). Sequencing data from CSF specimens were normalized to a reference from an asymptomatic, healthy male plasma control participant. Sequencing data derived from the tumor blocks were normalized to a resected tonsil tissue from an otherwise healthy male participant with an oropharyngeal infection.
All CNV calls and calculated tumor fractions were made according to blinded interpretation of the log2 copy ratio plots by one of us (W.G.), a board-certified molecular pathologist. In keeping with the interpretation of aneuploidy from copy ratio plots, clinically and from past experience with plasma and other body fluids, we called only large (>10 Mbp) CNVs or whole chromosome arms that were larger than that size and elevated or depressed at an even level of copy ratio. Frequent, low-level, and small deviations from the baseline of log2 copy ratio of 0 were interpreted as artifacts on the basis of noise. Sex chromosomes and areas near telomeres and centromeres that are known for routine copy number changes were not used. Chromosome 19, which is known for high GC content and high degrees of noise,20,23 was not used to call CNVs but was used as a metric for the degree of background noise.
Statistical Analysis
After the mNGS assay was assessed for its ability to identify CNVs in CSF, and these results were compared with the clinical and laboratory evidence for each patient, we calculated test performance characteristics, including sensitivity and specificity along with 95% CIs. Sensitivity and specificity CIs were calculated using the Clopper-Pearson exact method.
Results
A total of 130 participants across 2 studies were included (median [interquartile range (IQR)] age, 57.5 [43.3-68.0] years; 58 women [44.6%] and 72 men [55.4%]). A total of 155 CSF specimens from these patients were examined.
Test Performance Study
From the residual specimens in the clinical laboratory, we identified 125 CSF specimens from 103 patients (Figure 1B, Table; eTable in Supplement 1; eTable in Supplement 2), including 55 specimens from 47 patients with a CNS malignant neoplasm. Positive control specimens were obtained from lumbar punctures (n = 46), drains (n = 4), shunts (n = 1), and Ommaya reservoirs (n = 4). Most of the malignant neoplasms were lymphomas (36 of 55 [65.5%]). A subset of the positive control specimens was identified by CSF cytologic test and/or flow cytometry results (n = 31 specimens from 23 patients). The rest of the patients with a CNS malignant neoplasm (n = 24 specimens from 24 patients) had negative or indeterminate results on CSF cytologic test and/or flow cytometry but were eventually diagnosed with a malignant neoplasm using a combination of tissue biopsy (n = 22 specimens from 24 patients), repeat CSF cytologic test and/or flow cytometry, and diagnostic consensus among hospital clinicians with an intention to treat.
Table. Patient and Specimen Characteristics in the Test Performance and Neuroinflammatory Disease Case-Control Studies.
| Characteristic | No. (%) | |
|---|---|---|
| Positive mNGS resultsa | Negative mNGS results | |
| Test performance study | ||
| Patient demographics | ||
| No. of patients | 55 | 70 |
| Age, median (IQR) [range], y | 62 (54-71) [27-84] | 53 (38-66) [0-86] |
| Female sex | 23 (41.8) | 31 (44.3) |
| Male sex | 32 (58.2) | 39 (55.7) |
| History of malignant CNS neoplasmb | 30 (54.5) | 0 |
| Specimen characteristics | ||
| Cytologic testing | ||
| Positive result | 18 (34.5) | 0 |
| Atypical cells present | 10 (18.2) | 4 (5.7) |
| Benign | 21 (38.2) | 37 (52.9) |
| Not performed or unavailable | 5 (9.1) | 29 (41.4) |
| Flow cytometry | ||
| Aberrant clone | 26 (47.3) | 2 (2.9) |
| Atypical clone | 4 (7.3) | 0 |
| No evidence of lymphoproliferative disorder | 22 (40.0) | 39 (55.7) |
| Not performed or unavailable | 3 (5.5) | 29 (41.4) |
| Culture performed | 20 (38.5) | 47 (69.1) |
| WBC count, median (IQR) [range], cells/μL | 5 (1-106.5) [0-495]c | 16 (2-111) [0-13 000]d |
| Fluid protein, median (IQR) [range], mg/dL | 58.5 (37.5-142) [<10-920] | 79 (38.3-168) [13-5525] |
| Imaging | ||
| Leptomeningeal | 18 (10.5) | NA |
| Lesion abuts CSF space | 42 (75.0) | NA |
| Unknown | 1 (1.8) | NA |
| Neuroinflammatory disease study | ||
| Patient demographics | ||
| No. of patients | 12 | 17 |
| Age, median (IQR) [range], y | 71.5 (63.5-74.8) [47-83] | 44 (33-62) [5-71] |
| Female sex | 3 (25.0) | 9 (52.9) |
| Male sex | 9 (75.0) | 8 (47.1) |
| Specimen characteristics | ||
| Cytologic testing | ||
| Positive result | 0 | 0 |
| Atypical cells present | 2 (16.7) | 0 |
| Benign | 9 (75.0) | 7 (41.2) |
| Not performed or unavailable | 1 (8.3) | 17 (58.8) |
| Flow cytometry | ||
| Aberrant or atypical clone | 0 | 0 |
| No evidence of lymphoproliferation | 8 (66.7) | 9 (52.9) |
| Not performed or unavailable | 4 (33.3) | 8 (47.1) |
| Culture performed (data available for n = 10) | 9 (90.0) | 7 (41.2) |
| History of cancer | 2 (16.7) | 2 (11.8) |
| WBC count, median (IQR) [range], cells/μL | 12 (4.75-18.0) [0-128] | 7 (2-14.3) [0-325]e |
| Fluid protein, median (IQR) [range], mg/dL | 73.5 (40.8-302) [23-832] | 48.5 (38.8-72.8) [14-224] |
| Imaging | ||
| Leptomeningeal | 4 (36.0) | NA |
| Lesion abuts CSF space | 7 (64.0) | NA |
| Unknown | 1 (9.1) | NA |
Abbreviations: CNS, central nervous system; CSF, cerebrospinal fluid; IQR, interquartile range; mNGS, metagenomic next-generation sequencing; NA, not applicable; WBC, white blood cell.
SI conversion factor: To convert WBC to ×109 per liter, multiply by 0.001.
Two patients overlapped between the 2 studies: cases 54 and 137 and cases 37 and 138.
History of CNS neoplasm before the presentation period from each specimen was obtained.
WBC counts from 3 cases with positive results were not available.
WBC counts from 6 cases with negative results were not available.
WBC counts from 3 cases with negative results were not available.
The negative control specimens (n = 70 specimens from 56 patients with other neurological diseases) had an alternative diagnosis (eg, infection and autoimmune disease) that explained the neurological presentation. Negative control specimens were obtained from lumbar punctures (n = 53), drains (n = 12), and shunts (n = 5). Infectious disease testing results for a subset of these patients, including pathogens identified by mNGS, have been previously reported.12
The median (IQR) depth of sequencing was 7 (5.0-9.4) million reads. All but 4 specimens underwent paired-end sequencing of more than 125 base pairs on an Illumina sequencer. The remaining 4 specimens underwent single-end sequencing. Large (>10 Mbp) CNVs across the genome were characterized by the bioinformatics pipeline and by blinded interpretation of the copy ratio plots. Four percent of the results (2 positive control specimens and 3 negative control specimens) did not generate interpretable copy ratio plots, presumably because of low DNA input, and were not included in the performance calculations.
The sensitivity for detecting at least 1 large CNV in all positive control specimens was 79% (95% CI, 66%-89%), excluding 2 cases that were not interpretable (Figure 1B). Specifically, in the positive control specimens with positive CSF cytologic test (n = 19) or flow cytometry (n = 12) results, sensitivity was 89% (95% CI, 67%-99%) for the cytologic test and 92% (95% CI, 62%-100%) for flow cytometry. In the 22 positive control specimens with cytologic test and/or flow cytometry results that were negative (benign) or inconclusive (eg, atypical cells), 64% (95% CI, 41%-83%; n = 14) were found by mNGS to have positive results for aneuploidy or other large CNVs above 10 Mbp (Figure 1B). In the 44 positive specimens obtained by lumbar puncture rather than a drain or Ommaya reservoir, the sensitivity was 75% (95% CI, 60%-87%). The specificity was 100% (95% CI, 95%-100%) in the negative control specimens (n = 70); that is, no false-positives were identified.
We calculated tumor fractions according to maximal deviations from the copy number baseline and assumptions about the tumor copy numbers in that region. The estimated tumor fractions of all mNGS positive results (n = 55) were a median (IQR) of 49% (35%-72%). In the subset of positive specimens in which the traditional methods (cytologic test and flow cytometry) failed to detect the malignant neoplasm (n = 24), the tumor fraction of mNGS positive cases was still surprisingly high at a median (IQR) of 41% (24%-49%). Of the detectable cases, 38 (90.5%) had multiple CNVs, 34 (81.0%) had 4 or more CNVs, and 12 (28.6%) had 10 or more CNVs. Given that most cases had a positive result for lymphoma, we inspected the nonlymphoma cases (n = 12), and the overall tumor fraction was also high at a median (IQR) of 48% (32%-61%).
Neuroinflammatory Disease Study
After gathering preliminary data on the ability of the mNGS assay to detect aneuploidy in positive and negative control CSF specimens, we tested mNGS performance in clinically relevant cases and control participants who were retrospectively identified from an ongoing prospective study that enrolled patients with presentations that were suggestive of neuroinflammatory diseases. These patients had conditions that can be hard to differentiate clinically as infectious, autoimmune, or neoplastic. Thus, a CSF mNGS assay might be ordered to look broadly for a neurologic infection.
After the first 1350 patients were screened, 420 (31.1%) received adequate follow-up and an eventual clinical diagnosis. Of these patients, we identified 12 (2.9%) with residual CSF who had a clinical diagnosis of either a primary CNS tumor (n = 9) or a systemic tumor that had metastasized to the CNS (n = 3) as documented by previous sites of involvement (5 additional patients lacked residual CSF). All CSF specimens were obtained by lumbar puncture. A CSF cytologic test and flow cytometry showed benign results in 10 cases and atypical cells in 2 cases. The cases were matched with 17 negative control participants (who had an autoimmune diagnosis but not a CNS malignant neoplasm) from the same study (Figure 1C; eTable in Supplement 1).
Copy number variations were detected by CSF mNGS in 6 of the 12 cases with malignant neoplasm, and 1 case was not interpretable (presumably because of insufficient DNA input), for a sensitivity of 55% (95% CI, 23%-83%) (Figure 1C; eTable in Supplement 1). Three of the cases with malignant neoplasm had more than 10 large (>10 Mbp) CNVs, and another 3 cases had 1 CNV, the next 3 cases had 8 CNVs, and 3 other cases had 8 CNVs. The median tumor fraction of the positive results was 31% (95% CI, 14%-47%). Similar to the test performance study, the neuroinflammatory disease study found that no CNVs were detected in the negative control participants (n = 16; 1 case was not interpretable presumably because of insufficient DNA input), for a specificity of 100% (95% CI, 79%-100%). Positive NGS findings from the neuroinflammatory disease case-control study included 2 cases of primary CNS lymphoma, 2 cases of intravascular lymphoma, and 2 cases of metastatic melanoma (Figure 2 and Figure 3).
Figure 2. Undiagnosed Primary CNS Lymphoma Cases.
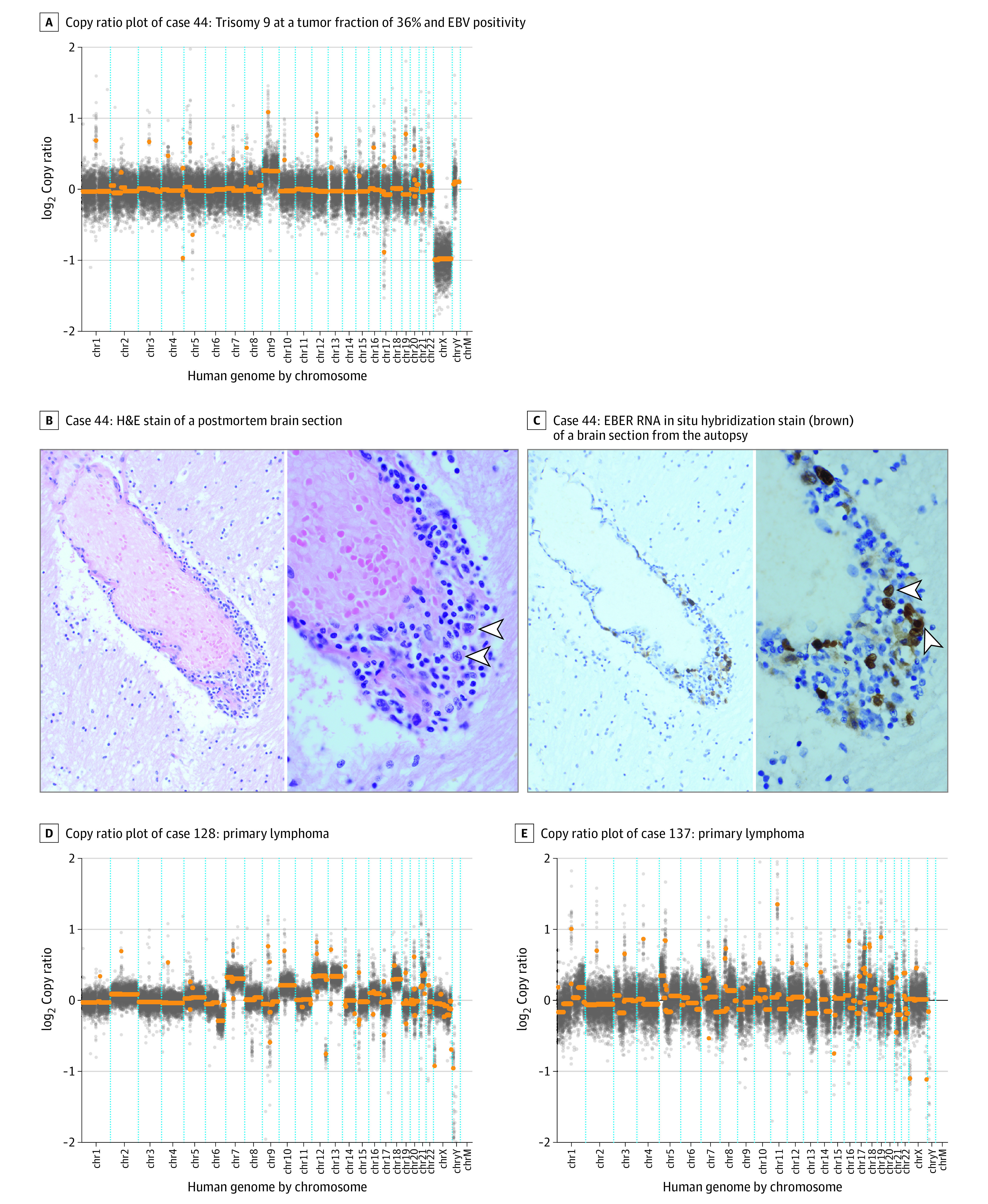
A, Conventional methods of brain biopsy and cytologic testing of the cerebrospinal fluid (CSF) were nondiagnostic in case 44. Flow cytometry showed atypical cells and was not definitive. Diagnosis was confirmed by autopsy 2 weeks after the CSF sampling. B, Arrowheads highlight large, perivascular B cells that have large, irregular nuclei with loose chromatin; hematoxylin-eosin (H&E) stain was used, with original magnification ×200 (left image) and ×1000 (right image). C, The large cells were stained positive (brown) for Epstein-Barr encoding region (EBER) in situ hybridization as evidence of Epstein-Barr virus (EBV) RNA expression in malignant lymphoma. D and E, Copy ratio plots are from 2 patients enrolled in the neuroinflammatory disease case-control study.
Figure 3. Other Undiagnosed Cases.
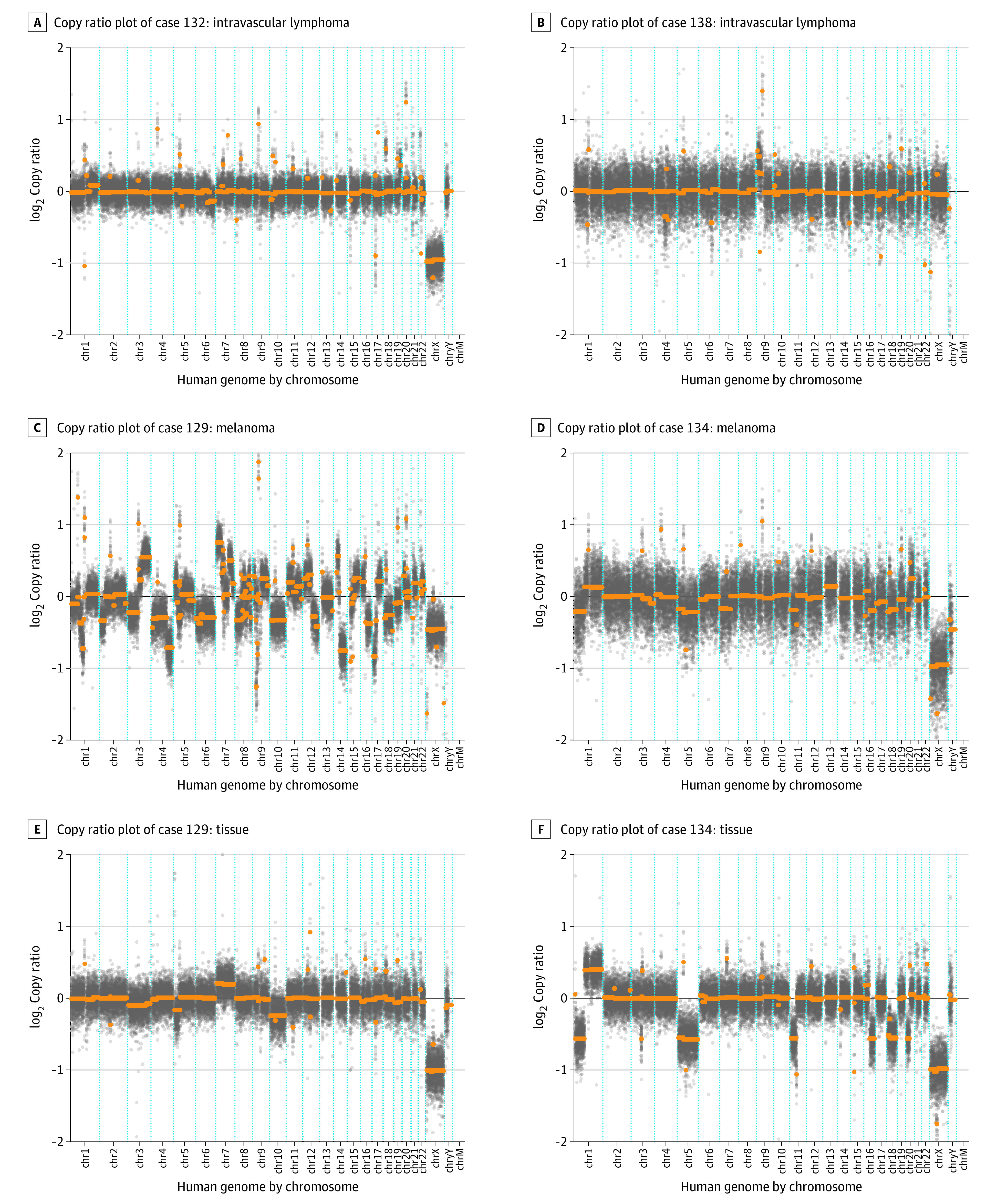
For cases 129 and 134, the comparable copy ratio plots derived from directly sequencing the neoplasm are shown.
When all interpretable CSF specimens from both studies were combined, the sensitivity of CNV detection by mNGS was 75% (95% CI, 63%-85%) and the specificity was 100% (95% CI, 96%-100%). Given that some patients had multiple CSF specimens and there was an overlap of 2 cases between the 2 studies, we recalculated an accuracy for unique patients across all studies by using the earliest CSF specimens. On a unique patient basis, the sensitivity was 71% (95% CI, 58%-83%) and the specificity was 100% (95% CI, 95%-100%). For all unique cytologic tests with benign results, the sensitivity was 59% (13 of 22 CSF specimens); for all cytologic tests with atypical results (negative or atypical results on flow cytometry), the sensitivity was 50% (2 of 4 CSF specimens).
Associated Tissue Correlation and Radiographic Correlation
The characteristics of CSF CNVs reflected the CNVs that were detected in primary tumor tissue. Across both studies, there were 13 patients diagnosed with a CNS malignant neoplasm who had residual tumor tissue or had undergone a molecular or cytogenetic analysis. Of these 13 cases, 4 (30.8%) did not have CNVs that were detected by CSF mNGS. The remaining 9 cases (69.2%) demonstrated matching CNVs between the associated cancer tissue, although exact matches were precluded presumably by tumor genome evolution and subsequent genetic heterogeneity (Figure 2D and E; eTable in Supplement 1).
Tumor detection was not limited to radiographic lesions that abutted the CSF space. Brain MRI scans of patients who were ultimately diagnosed with a CNS malignant neoplasm (64 of 66 cases over the 2 studies) were reviewed by a neuroradiologist (A.M.R.) who was blinded to the CSF mNGS CNV results (Figure 4). Magnetic resonance imaging scans were performed within 2 days of CSF collection in 50% of cases and within 1 week in 80% of the cases. In cases for whom aneuploidy was detected by CSF mNGS and imaging was available, 37 of 46 cases (80.4%) had either leptomeningeal involvement or were abutting the CSF space. However, the few remaining cases detected by mNGS either had lesions that did not abut the CSF space (6 of 46 [13.0%]) or did not have any radiographic lesions within 7 days of collection (3 of 46 [6.5%]). This finding is in contrast to results of a previous smaller study that reported that all 5 CNS tumors that did not abut the CSF space were not detected.14 The detection rate (sensitivity) of all unique CSF specimens with positive results and MRI data was 81% (95% CI, 58%-95%) for those that showed leptomeningeal disease and 79% (95% CI, 58%-93%) for those that showed a lesion abutting the CSF space. The sensitivity was 53% (95% CI, 27%-79%) for lesions that were radiologically occult or not abutting the CSF space.
Figure 4. Images of Abnormalities in 4 Cases With Presentations Suggestive of Neuroinflammatory Disease at or Near the Time of Brain Biopsy.
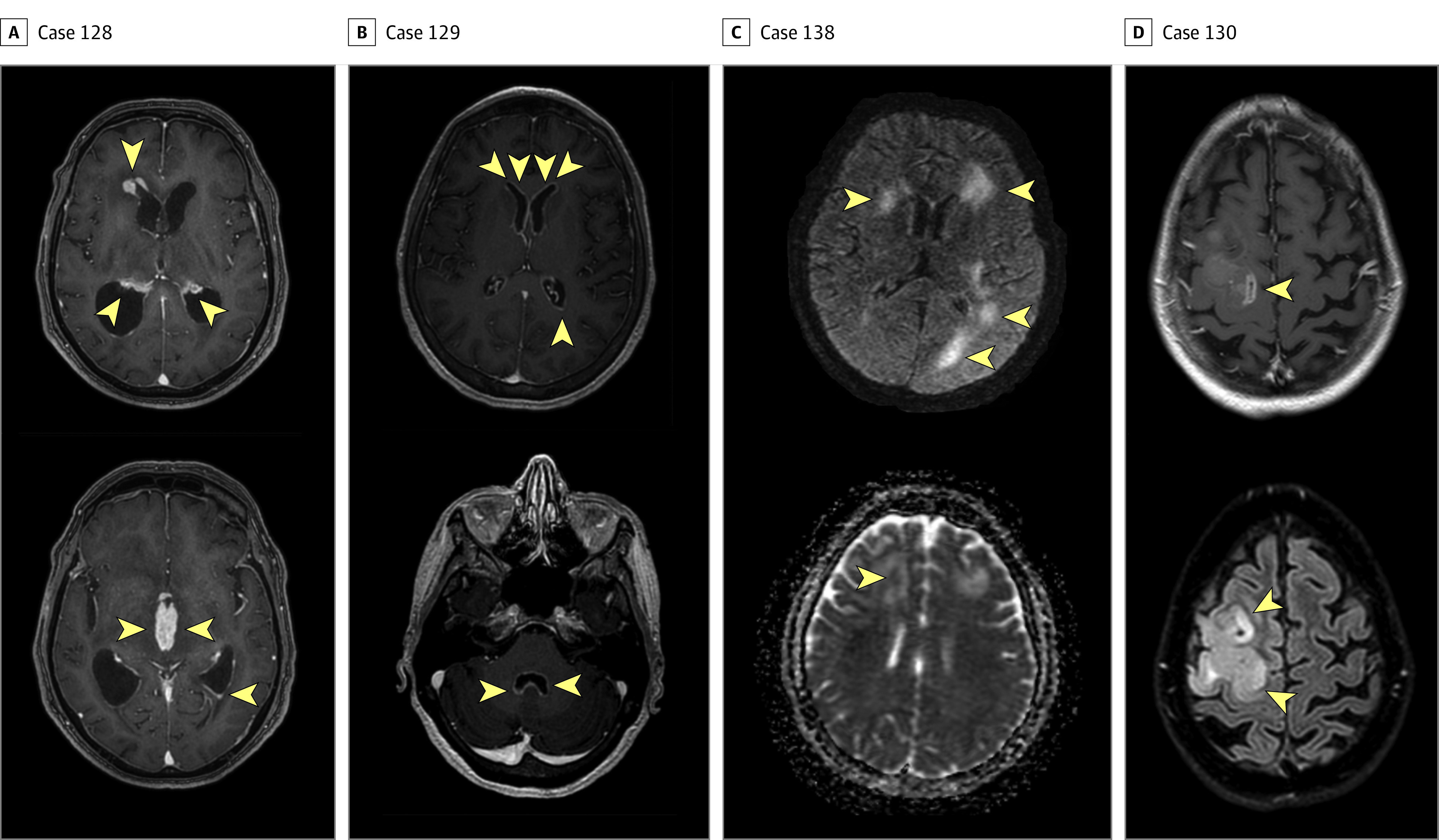
A, In case 128, postcontrast T1-weighted images demonstrate thick areas of enhancement along nearly all ependymal surfaces, including the lateral ventricles (top image), third ventricle (bottom image), and fourth ventricle, resulting in acute hydrocephalus. The imaging appearance was believed to be consistent with disseminated tuberculosis and less likely to be lymphoma because of limited restricted diffusion. The final diagnosis was primary central nervous system (CNS) lymphoma. B, In case 129, postcontrast T1-weighted images demonstrate extensive, thin ependymal enhancement along the lateral ventricles (top image) and fourth ventricle (bottom image), with minimal adjacent signal abnormality in the parenchyma. The imaging appearance was believed to be most consistent with infectious ventriculitis and less likely to be carcinomatosis. The final diagnosis was melanoma. C, In case 138, motion-degraded T2-weighted fluid attenuated inversion recovery (T2/FLAIR; top image) image and apparent diffusion coefficient (bottom image) demonstrate asymmetric white matter signal abnormality with variable foci of mildly restricted diffusion. In this immunocompromised patient, images were believed to be potentially consistent with progressive multifocal leukoencephalopathy. The final diagnosis was intravascular lymphoma. D, In case 130, postcontrast T1-weighted (top image) and T2/FLAIR (bottom image) sequences demonstrate expansile signal abnormality with areas of heterogeneous enhancement. Glioblastoma was diagnosed using brain biopsy. Arrowheads indicate areas of abnormalities.
Case Vignettes
Case 44 was a man with a history of HIV (CD4 count, <35 cells/mm3) who was not receiving antiretroviral therapy. He presented with gait instability and was found to have multifocal (frontal and cerebellar) enhancing lesions on a brain MRI scan that were primarily suggestive of CNS lymphoma, toxoplasmosis, or tuberculosis. Despite extensive CSF testing, including cytologic test (benign results), flow cytometry (atypical B cells), and a nondiagnostic brain biopsy (ie, gliosis with mild chronic inflammation), a definitive diagnosis was not reached before he died a few weeks later. An autopsy confirmed a diagnosis of Epstein-Barr virus (EBV)–positive primary CNS lymphoma. We performed mNGS on the CSF that was aspirated from a drain for several weeks, and we detected trisomy 9 in all 3 specimens with a tumor fraction ranging from 36% to 51% (Figure 2A). We detected EBV by CSF mNGS and by plasma EBV polymerase chain reaction test, which was confirmed by EBV immunohistochemistry in the autopsy brain tissue (Figure 2B and C).
Case 128 was a man who presented with progressive cognitive decline and idiopathic hydrocephalus complicated by worsening encephalopathy. A brain MRI scan showed multifocal, thick mass-like ependymal and subependymal enhancement with extensive surrounding T2 hyperintensity involving the deep and periventricular white matter (Figure 4A). An examination of the CSF that was obtained from a lumbar puncture revealed an opening pressure of 9.5 cm and a white blood cell (WBC) count of 33/μL that comprised 24% lymphocytes, 1% neutrophils, and 75% monocytes. A CSF cytologic test documented atypical cells on 3 separate CSF specimens, but a bone marrow biopsy and peripheral flow cytometry showed negative results for malignant neoplasm. The consensus diagnosis was CNS lymphoma, which was based on the atypical cells that were seen on the CSF cytologic test, the clinical presentation, the radiographic findings, and the initial dramatic therapeutic response to corticosteroids that was followed by the development of septic shock and death 1 month after presentation. Cerebrospinal fluid mNGS detected a tumor fraction of 85% and more than 10 chromosomal-level CNVs (Figure 2D).
Case 129 was a woman with a history of metastatic melanoma that was treated with surgery, radiation, and chemotherapy. She presented with headache and diplopia, nausea, and vomiting. She was treated empirically for bacterial and fungal meningitis after a CSF examination revealed a WBC count of 332/μL that comprised 74% neutrophils, 11% monocytes, and 15% lymphocytes. An MRI scan of the spinal cord showed smooth, diffuse leptomeningeal enhancement predominating in the lower thoracic cord and around the conus medullaris, favoring a nonmalignant meningitis with carcinomatosis that was considered to be less likely. Similarly, a brain MRI showed subependymal enhancement along the ventricular surfaces and the tentorium (Figure 4B). The CSF cytologic test results in 2 specimens were benign, and flow cytometry showed no evidence of a lymphoproliferative disorder. Brain biopsy results indicated metastatic melanoma. Cerebrospinal fluid mNGS detected a tumor fraction of 41% with more than 20 CNVs, including 4 CNVs found in the patient’s lymph node biopsy in which melanoma had been found (Figure 3C).
Case 132 was a man with a history of diabetes. He presented with asymmetrical weakness and was found to have longitudinally extensive transverse myelitis and enhancing brain lesions on an MRI scan. He was initially empirically treated with glucocorticoids for a presumed inflammatory demyelinating condition. An examination of his CSF revealed a WBC count of 7/μL that comprised 75% lymphocytes, 3% neutrophils, and 22% monocytes. Three separate CSF specimens that were sent for cytologic testing had benign results, and results of a bone marrow biopsy with flow cytometry showed no evidence of a lymphoproliferative disorder. Intravascular large B-cell lymphoma was diagnosed by brain biopsy. Cerebrospinal fluid mNGS detected 3 CNVs and a tumor fraction of 8.7% (Figure 3A).
Case 134 was a man with a medical history of cardiomyopathy status after defibrillator placement, chronic obstructive pulmonary disease, diabetes, and gait difficulty and communicating hydrocephalus that required placement of an extraventricular drain. He presented with progressive short-term memory loss and worsening gait, which prompted an evaluation for an underlying neurodegenerative process. A CSF examination revealed a WBC count of 168/μL that comprised 80% lymphocytes, 1% neutrophils, 15% monocytes, and 4% pigment-laden histiocytes. Results of a CSF cytologic test showed atypical cells, but flow cytometry showed no evidence of a lymphoproliferative disorder. A computed tomography scan of the brain and spinal cord showed possible leptomeningeal enhancement over the surface of the medulla and spinal cord and a focus of nodular enhancement in the interhemispheric fissure that abutted the left pregenual cingulate. The diagnosis of melanoma was ultimately confirmed by a brain biopsy. Cerebrospinal fluid mNGS detected a tumor fraction of 11% with more than 10 CNVs. Seven of the CSF mNGS CNVs overlapped with the CNVs that were detected in the brain biopsy specimen (Figure 3D and F).
Case 137 was a man with a history of atrial fibrillation and use of anticoagulation and treated syphilis who developed gait difficulty. He was found to have multifocal parenchymal lesions with ependymal involvement of unclear etiology on a brain MRI scan. He benefited from empirical corticosteroids after no diagnosis could be made with CSF testing (no safe brain biopsy target initially). When the patient clinically worsened as the corticosteroids were tapered, an examination of his CSF revealed a WBC count of 17/μL that comprised 80% lymphocytes and 20% monocytes. Results of a CSF cytologic test were benign, and flow cytometry showed no evidence of a lymphoproliferative disorder. He was ultimately diagnosed with large B-cell lymphoma by brain biopsy. Cerebrospinal fluid mNGS detected more than 20 CNVs at a tumor fraction of 22%.
Case 138 was a woman with a history of hypertension, depression, ulcerative colitis, and rheumatoid arthritis who was receiving azathioprine. She presented with cognitive decline and gait instability and was found to have multifocal, T2-hyperintense, bifrontal left posterior temporal and parietooccipital lesions with restricted diffusion on an MRI scan (Figure 4C). An examination of her CSF revealed a WBC count of 18/μL that comprised 85% lymphocytes and 15% monocytes. Results of a CSF cytologic test were benign, and flow cytometry was not performed. Intravascular large B-cell lymphoma was diagnosed by brain biopsy. Cerebrospinal fluid mNGS detected 1 CNV and a tumor fraction of 49%.
Discussion
Transformative advances in NGS and bioinformatics allow us to generate and parse through genomic data from patients to find evidence of inherited diseases, cancers, and infections. Because various medical fields take advantage of similar technological platforms, data that were generated to make 1 type of diagnosis (eg, noninvasive prenatal testing to identify fetal chromosomal abnormalities) can be reanalyzed to detect other disease entities (eg, maternal cancers24,25). The present study showed that data that were generated by an mNGS assay that was developed to diagnose infections can be reanalyzed to detect malignant aneuploidy in CSF with moderate sensitivity and high specificity, including in patients with clinical phenotypes that were initially suggestive of CNS infections. We identified imaging characteristics of tumors (ie, leptomeningeal involvement and abutting the CSF space) with patterns of increased sensitivity of the CSF mNGS assay but not statistical significance. However, we also found cases without radiological evidence of CSF involvement that still had positive assay results, a contrast to a finding in a past study.14
The detection of solid CNS tumors and CNS lymphomas can be challenging, with CSF cytologic test sensitivity ranging from 2% to 32% in the first CSF specimen.26 In the 2 case-control studies we performed, we detected aneuploidy when cytologic test and flow cytometry of the CSF did not show positive results or had ambiguous findings. Although the detection of aneuploidy in CSF does not render a specific cancer diagnosis, the high specificity we observed suggests that a positive result could motivate more focused diagnostic testing for a CNS neoplasm in the right clinical context.
Tumor fractions of cases with positive results were high (median of 31%-41%) and were occasionally higher than brain tissue (eg, case 44; Figure 2), which is consistent with previously reported high tumor fractions in CSF.17 These results suggest that further molecular testing, such as with cancer panels or epigenetic classification,27,28 has the theoretical potential to distinguish between tumor types using CSF. In contrast to cancer somatic mutation panels, the increasingly rapid turnaround time for mNGS, its low DNA input requirements, and the lack of a required enrichment protocol makes mNGS a potentially useful adjunctive tool for cancer detection in CSF that, unlike cytologic test and flow cytometry, is not dependent on preserving cell integrity. This hypothesis is especially true in patients with a CNS malignant neoplasm whose clinical presentations were initially suggestive of infectious meningitis or meningoencephalitis.
We believe that these case-control studies, which included cases in which tumor detection could have been accomplished earlier without additional invasive testing, motivate further research in this area.
Limitations
This study has several limitations. The case-control studies we conducted were retrospective. In addition, we could not directly assess the positive and negative predictive value of the CSF mNGS assay because the prevalence of a CNS malignant neoplasm in this study was artificial and not reflective of a relevant clinical population. For the test performance study, the source of residual CSF specimens was mainly through flow cytometry testing, which introduced a selection bias toward patients who may have lymphoma. Excluding patients with acute leukemia and most cases of systematic cancers limited our ability to assess the false-positive rate in patients with coincident systemic malignant neoplasm; hence, the test specificity may be lower for those patients. We also did not assess benign tumors or precancers. Thus, the real-world accuracy of the CSF mNGS assay needs to be assessed in a larger, prospective cohort study of patients who are referred by their physicians for CSF mNGS with more systematic and long-term follow-up. The clinical utility of the assay could be evaluated in a study that randomizes participants to CNV analysis of their mNGS data, especially if no pathogen is identified.
Conclusions
The CSF mNGS assay, which has low specimen volume requirements and does not require the preservation of cell integrity, can be an adjunctive tool for cancer detection. It has a low risk of false-positive results and can be especially useful in cases in which CSF cytologic test and/or flow cytometry results are negative. These 2 preliminary studies, which included patients whose tumor could have been detected earlier without additional invasive testing, warrant further research in this area.
eTable. Study 1 Samples: Correlated Body Fluid and Cancer Tissue Copy Ratio Plots
eTable. Excel File of Data
References
- 1.Glaser CA, Honarmand S, Anderson LJ, et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43(12):1565-1577. doi: 10.1086/509330 [DOI] [PubMed] [Google Scholar]
- 2.Granerod J, Tam CC, Crowcroft NS, Davies NWS, Borchert M, Thomas SL. Challenge of the unknown: a systematic review of acute encephalitis in non-outbreak situations. Neurology. 2010;75(10):924-932. doi: 10.1212/WNL.0b013e3181f11d65 [DOI] [PubMed] [Google Scholar]
- 3.Granerod J, Ambrose HE, Davies NW, et al. ; UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group . Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835-844. doi: 10.1016/S1473-3099(10)70222-X [DOI] [PubMed] [Google Scholar]
- 4.Wilson MR, Naccache SN, Samayoa E, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408-2417. doi: 10.1056/NEJMoa1401268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson MR, O’Donovan BD, Gelfand JM, et al. Chronic meningitis investigated via metagenomic next-generation sequencing. JAMA Neurol. 2018;75(8):947-955. doi: 10.1001/jamaneurol.2018.0463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson MR, Shanbhag NM, Reid MJ, et al. Diagnosing Balamuthia mandrillaris encephalitis with metagenomic deep sequencing. Ann Neurol. 2015;78(5):722-730. doi: 10.1002/ana.24499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson MR, Sample HA, Zorn KC, et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med. 2019;380(24):2327-2340. doi: 10.1056/NEJMoa1803396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller S, Naccache SN, Samayoa E, et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. Genome Res. 2019;29(5):831-842. doi: 10.1101/gr.238170.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simner PJ, Miller HB, Breitwieser FP, et al. Development and optimization of metagenomic next-generation sequencing methods for cerebrospinal fluid diagnostics. J Clin Microbiol. 2018;56(9):e00472-18. doi: 10.1128/JCM.00472-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Chen Y, Wang D, et al. The feasibility of metagenomic next-generation sequencing to identify pathogens causing tuberculous meningitis in cerebrospinal fluid. Front Microbiol. 2019;10:1993. doi: 10.3389/fmicb.2019.01993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piantadosi A, Kanjilal S, Ganesh V, et al. Rapid detection of powassan virus in a patient with encephalitis by metagenomic sequencing. Clin Infect Dis. 2018;66(5):789-792. doi: 10.1093/cid/cix792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu W, Deng X, Lee M, et al. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat Med. 2021;27(1):115-124. doi: 10.1038/s41591-020-1105-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan W, Gu W, Nagpal S, Gephart MH, Quake SR. Brain tumor mutations detected in cerebral spinal fluid. Clin Chem. 2015;61(3):514-522. doi: 10.1373/clinchem.2014.235457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Springer S, Zhang M, et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci U S A. 2015;112(31):9704-9709. doi: 10.1073/pnas.1511694112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Mattos-Arruda L, Mayor R, Ng CKY, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839. doi: 10.1038/ncomms9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pentsova EI, Shah RH, Tang J, et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol. 2016;34(20):2404-2415. doi: 10.1200/JCO.2016.66.6487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seoane J, De Mattos-Arruda L, Le Rhun E, Bardelli A, Weller M. Cerebrospinal fluid cell-free tumour DNA as a liquid biopsy for primary brain tumours and central nervous system metastases. Ann Oncol. 2019;30(2):211-218. doi: 10.1093/annonc/mdy544 [DOI] [PubMed] [Google Scholar]
- 18.Taylor AM, Shih J, Ha G, et al. ; Cancer Genome Atlas Research Network . Genomic and functional approaches to understanding cancer aneuploidy. Cancer Cell. 2018;33(4):676-689.e3. doi: 10.1016/j.ccell.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talevich E, Shain AH, Botton T, Bastian BC. CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput Biol. 2016;12(4):e1004873. doi: 10.1371/journal.pcbi.1004873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc Natl Acad Sci U S A. 2008;105(42):16266-16271. doi: 10.1073/pnas.0808319105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naccache SN, Federman S, Veeraraghavan N, et al. A cloud-compatible bioinformatics pipeline for ultrarapid pathogen identification from next-generation sequencing of clinical samples. Genome Res. 2014;24(7):1180-1192. doi: 10.1101/gr.171934.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754-1760. doi: 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimwood J, Gordon LA, Olsen A, et al. The DNA sequence and biology of human chromosome 19. Nature. 2004;428(6982):529-535. doi: 10.1038/nature02399 [DOI] [PubMed] [Google Scholar]
- 24.Dharajiya NG, Grosu DS, Farkas DH, et al. Incidental detection of maternal neoplasia in noninvasive prenatal testing. Clin Chem. 2018;64(2):329-335. doi: 10.1373/clinchem.2017.277517 [DOI] [PubMed] [Google Scholar]
- 25.Ji X, Li J, Huang Y, et al. Identifying occult maternal malignancies from 1.93 million pregnant women undergoing noninvasive prenatal screening tests. Genet Med. 2019;21(10):2293-2302. doi: 10.1038/s41436-019-0510-5 [DOI] [PubMed] [Google Scholar]
- 26.Scott BJ, Douglas VC, Tihan T, Rubenstein JL, Josephson SA. A systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol. 2013;70(3):311-319. doi: 10.1001/jamaneurol.2013.606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller AM, Shah RH, Pentsova EI, et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565(7741):654-658. doi: 10.1038/s41586-019-0882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469-474. doi: 10.1038/nature26000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Study 1 Samples: Correlated Body Fluid and Cancer Tissue Copy Ratio Plots
eTable. Excel File of Data


