Abstract
The widespread use of cardiac implantable electronic devices and wearable monitors has led to the detection of subclinical atrial fibrillation in a substantial proportion of patients. There is evidence that these asymptomatic arrhythmias are associated with increased risk of stroke. Thus, detection of subclinical atrial fibrillation may offer an opportunity to reduce stroke risk by initiating anticoagulation. However, it is unknown whether long-term anticoagulation is warranted and in what populations. This scientific statement explores the existing data on the prevalence, clinical significance, and management of subclinical atrial fibrillation and identifies current gaps in knowledge and areas of controversy and consensus.
Keywords: AHA Scientific Statements, ambulatory monitoring, atrial fibrillation, cardiac arrhythmia, cardiac pacing, cerebrovascular stroke, pacemaker
Atrial fibrillation (AF) is a potent risk factor for cardioembolic stroke. Historically, the clinical approach to primary stroke prevention in patients with clinically apparent AF has been relatively straightforward; once AF is documented, regardless of the associated symptoms and regardless of whether the arrhythmia is paroxysmal, persistent, or chronic, anticoagulation is generally recommended if concomitant risk factors for stroke are present. However, this simple approach is upended by the growing recognition of AF detected on implantable and wearable cardiac devices. Such devices have afforded an opportunity to detect often brief, asymptomatic episodes of atrial arrhythmias that are far more common than clinically apparent AF, have less certain clinical significance, and have no well-established, evidence-based approach to management (Figure 1; figures and tables begin on page e950).1
Figure 1. A “tip of the iceberg” analogy describes the spectrum of apparent and subclinical atrial fibrillation in terms of the method/context of detection and clinical significance.
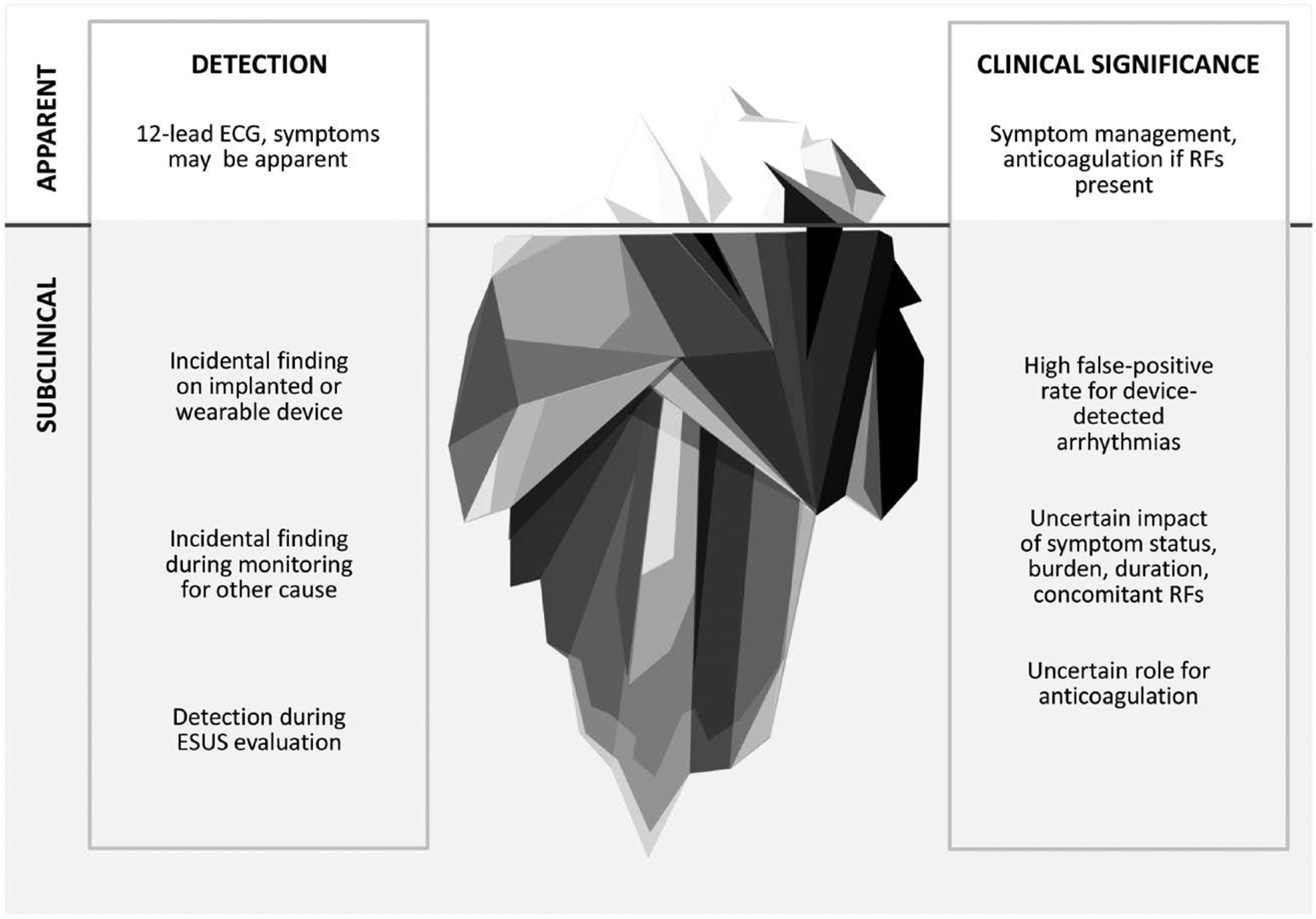
ESUS indicates embolic stroke of undetermined source; and RF, radiofrequency. Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved. Copyright © 2019, Mayo Foundation for Medical Education and Research.
Although the management of subclinical AF (SCAF) is a challenge, recognizing it may offer an opportunity to reduce cardiovascular morbidity and mortality. Each year, 16.9 million people worldwide have a stroke,2 the cause of which remains unexplained in 20% to 40% of cases. Among these unexplained strokes, 10% to 30% may be caused by AF that has eluded detection.3–8 Better recognition and understanding of SCAF could help us determine effective screening and treatment approaches and could potentially prevent many strokes.
SCOPE
This scientific statement explores the existing data on the prevalence, clinical significance, and management of atrial arrhythmias detected on implanted and wearable cardiac devices. We identify current gaps in our knowledge and areas of controversy and consensus.
DEFINITIONS
AF has traditionally been defined by documentation of the arrhythmia on a 12-lead ECG and, in its simplest definition, has been independent of the duration of the arrhythmia or the associated symptoms. However, continuous rhythm monitoring by cardiac implanted electronic devices (CIEDs) with automated atrial rhythm detection has generated a new category of atrial arrhythmias called atrial high-rate episodes (AHREs), which are atrial events, usually tachyarrhythmias, meeting programmed or other specified atrial high-rate criteria (Table 1). Visual confirmation of these recordings is necessary because some AHREs can also be false positives, or artifactual, such as those resulting from far-field signal or noise. SCAF or subclinical atrial tachyarrhythmia (AT) has referred to asymptomatic episodes of AF, atrial flutter, or AT detected and confirmed by intracardiac electrograms and not previously detected by electrocardiographic or ambulatory monitoring.9 By asymptomatic, we mean an absence of palpitations, chest pain, dyspnea, lightheadedness, focal neurological symptoms, or other symptoms commonly attributed to AF.
Table 1.
Definitions of Arrhythmic Events
| Abbreviation | Term | Definition |
|---|---|---|
| AHRE | Atrial high-rate episodes | Device-detected atrial events, usually tachyarrhythmias, meeting programmed or other specified atrial high-rate criteria (usually ranging between 175 and 220 bpm) |
| SCAF | Subclinical atrial fibrillation | Asymptomatic episodes of atrial fibrillation detected and confirmed by intracardiac electrograms and not previously detected by electrocardiographic or ambulatory monitoring |
| SCAT | Subclinical atrial tachyarrhythmia | Asymptomatic episodes of atrial fibrillation, atrial flutter, or atrial tachycardia detected and confirmed by intracardiac electrograms and not previously detected by electrocardiographic or ambulatory monitoring |
However, more recently, detection of SCAF has expanded to detection by implantable cardiac monitors (ICMs) and wearable monitors. For this scientific statement, SCAF is defined as episodes of asymptomatic AF detected by intracardiac, implantable, or wearable monitors and confirmed by intracardiac electrogram or review of the recorded rhythm on the ECG. Duration typically depends on programmed device AT detection criteria, but the duration defined in studies associating SCAF with thromboembolic risk has been variable among studies, with specified durations ranging from 20 seconds to >24 hours.10 Similarly, variability among studies exists with regard to the AHRE specified atrial rate thresholds, ranging between 175 and 220 bpm. Because the threshold duration or rate for thromboembolic risk or need for antithrombotic therapy remains uncertain,11 this scientific statement does not specify duration or specific atrial rate criteria in the definition of SCAF. We also note that SCAF that has been associated with stroke is no longer asymptomatic per se, although there may be no symptoms attributable to the arrhythmia itself.
DETECTION AND DIAGNOSIS
The range of devices that allow detection of SCAF extends from wearables that allow intermittent rhythm assessment to implanted devices that allow continuous surveillance (Figure 2).1 Most data on SCAF are derived from CIEDs with atrial leads, in which intracardiac electrograms enable confirmation of AF. Subcutaneous ICMs yield AF detection, primarily with the use of R-R irregularity algorithms (Figure 3). Longer detection duration may reduce false positives in CIEDs. Development of P-wave detection or other algorithms may reduce the false-positive rate from other irregular rhythms such as frequent atrial ectopy. Optimal products accurately detect AF with fewer false positives, enable days of comfortable continuous monitoring without attachment to electrocardiographic cables, allow continued daily activities (eg, sports and showering), and promptly provide interpretable data that are useful for clinical decision-making.
Figure 2. The devices that allow detection of subclinical atrial fibrillation exist on a spectrum from minimally invasive devices, or wearables, to permanent implanted devices and provide intermittent to continuous surveillance for events.
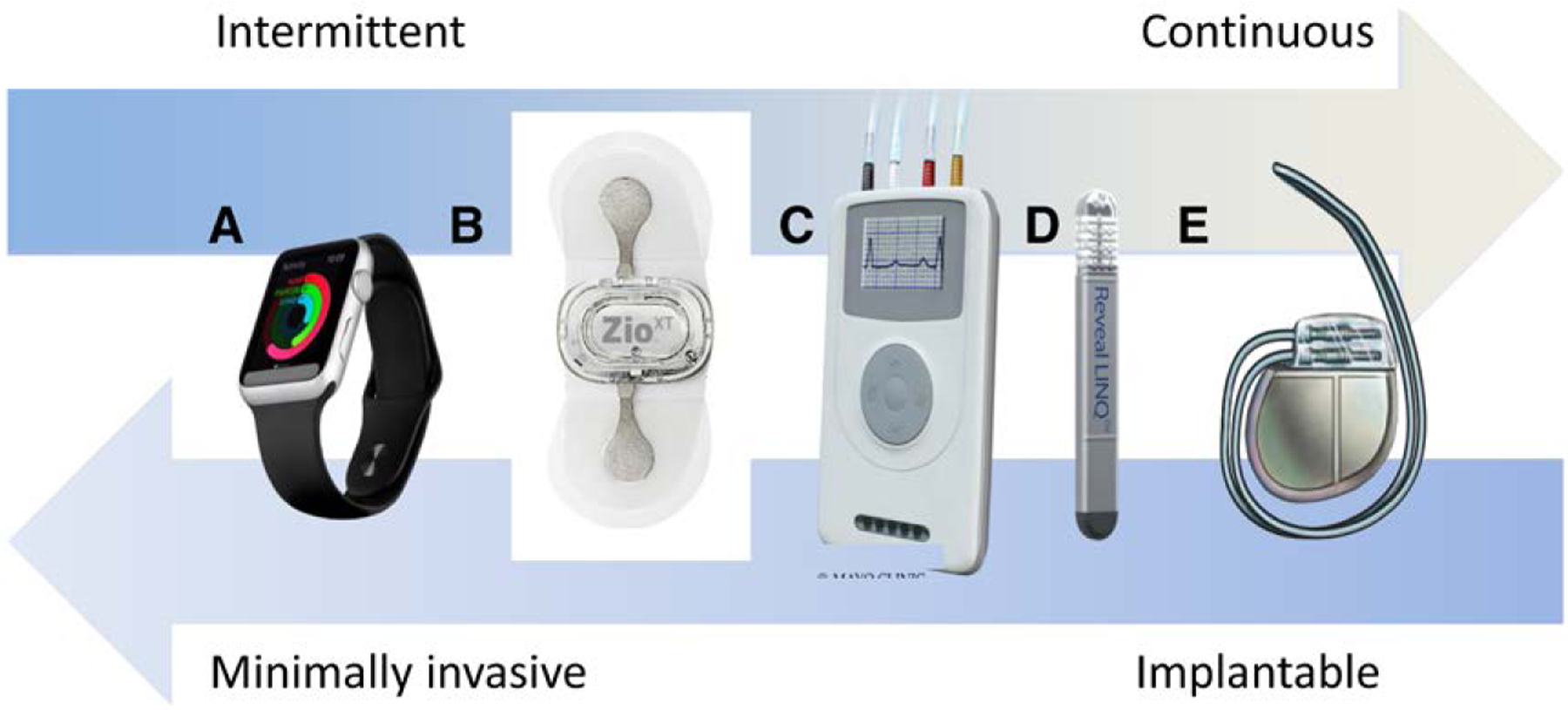
A, Apple Watch. Copyright © Shutterstock/Alexey Boldin. B, ZIO® XT Patch. Used with permission of iRhythm Technologies, Inc. Copyright © iRhythm Technologies, Inc. C, Holter or event monitor. Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved. Copyright © Mayo Clinic. D, Reveal LINQ™. Courtesy of Medtronic. E, Implanted pacemaker or defibrillator. Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved. Copyright © Mayo Clinic.
Figure 3. A Lorenz plot depicts variability in RR intervals as the δRR(n) on the “n beat” on the y axis and the δRR(n-1) on the “N-1 beat” on the x axis.
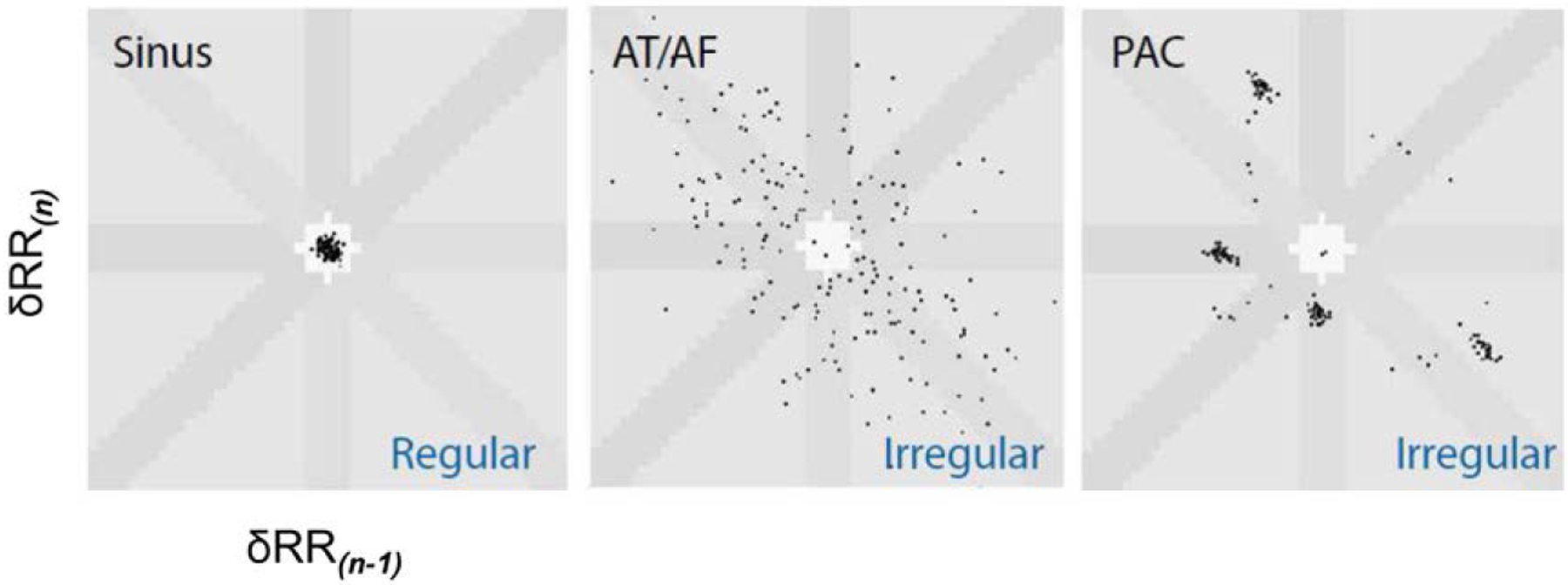
Regular rhythms cluster around the origin; atrial fibrillation (AF) is marked by scatter of the RR intervals; and premature atrial contractions (PACs) are represented as clusters of points on the Lorenz plot. AT indicates atrial tachycardia.
Wearable devices encompass adhesive patches with sensors worn on the chest, as well as wristbands, watches, bras, and shirts with imbedded leads and sensors. Detection may use photoplethysmography to detect an irregular rhythm, single-lead or multilead ECGs, or a combination of these approaches. Wearable heart rate devices that detect high or irregular rates require additional electrocardiographic monitoring to diagnose AF. For continuous monitoring devices, data are processed by algorithms stored in the device or cloud and downloaded by the manufacturer to produce a report. Currently, of wearable devices, adhesive patches have the most widespread use for detecting AF in embolic stroke of undetermined source (ESUS) or evaluating AF burden after invasive interventions. Although consumer-grade devices hold some promise for AF detection outside the traditional medical settings, the actionability of these AF detections is currently uncertain, and the best practices for managing, interpreting, and treating patients on the basis of such data have not yet been established. This is particularly true when AF is detected with a photoplethysmography sensor rather than a direct electric recording. However, studies are underway to determine the value and acceptability of monitoring with watches or garments. Table 212–21 presents the characteristics of selected wearable products to continuously monitor for AF.
Table 2.
Characteristics of Selected Wearable Devices for Continuous Monitoring to Detect AF
| Device/Manufacturer | Underlying Technology | Performance to Detect AF or to Differentiate Between AF and SR | Comment |
|---|---|---|---|
| Adhesive devices with sensor | |||
| ZIO patch, iRhythm12,13 | Single-lead ECG | Concordance of AF burden between Holter and patch, r=0.96 Concordance of AF burden between pacemaker and patch, r=0.99 |
Used in 15 trials for AF detection 14-d wear time Patient may shower |
| MCOT/ePatch, Biotel14,15 | 3-Lead ECG (MCOT) | MCOT: 100% sensitivity, 100% specificity for AF lasting >30 s | MCOT: 128% higher diagnostic yield compared with loop recorder Used in >30 trials to detect AF or to evaluate burden Up to 30 d of monitoring MCOT requires monitor to be within 30 ft of patient at all times; sensor transmits via Bluetooth ePatch is nontelemetry version with 14-d wear time Patient may shower |
| BodyGuardian Heart, BodyGuardian Mini, Preventice16,17 | Single-lead ECG Deep learning BeatLogic platform |
Body Guardian Heart: Episode detection for AF lasting >60 s: 99% sensitivity, 89% specificity |
BodyGuardian Mini: Released 2018 16-d use Claimed as the smallest monitor Claimed to have superior P-wave visibility Patient may submerse in up to 3 ft of water |
| Carnation Ambulatory Monitor, Bardy Diagnostics12 | Single-lead ECG | AF burden concordance with pacemaker electrograms=0.96 High precision for detecting AF >30 s: 0.99 (0.98–1.1) |
7-d monitoring P-wave centric Claims superior P-wave visibility |
| Bands, wrist, or chest | |||
| Kardiaband by AliveCor with Apple Watch18 | PPG to detect irregular rhythm Single-lead ECG |
Differentiation between AF and SR: 93% sensitivity, 83% specificity | Requires paired iPhone Wearer may prompt recording of ECG, but ECG is not continuous Susceptible to error because of noise Use of this device may be limited because of availability of Apple Watch ECG Commercially available |
| Apple Watch ECG19 | PPG to detect irregular rhythm using deep learning algorithm Single-lead ECG |
C statistic=0.97 for differentiating AF from SR | Proof-of-concept study Follow-up study in progress Wearer may prompt recording of ECG, but recording is not continuous Susceptible to error because of noise Population who purchase device may not benefit the most because AF if present may not require treatment Commercially available |
| Simband, Samsung20 | Multiwaveform PPG to detect irregular rhythm Single-channel ECG Accelerometer |
Differentiation between AF and SR: 97% sensitivity, 94% specificity | Testing of an algorithm designed to reduce interference/noise Study participants were not engaged in usual activity Further study needed to evaluate device during daily activities Commercially available |
| Qardiocore chest band, Qardio21 | 3-Lead ECG transmits via Bluetooth real time to iPhone Qardio MD platform |
Not reported in scientific literature | FDA approved 2017 Water resistant Measures HR variability, respiratory rate, activity tracking Commercially available |
This list is not exhaustive, and inclusion in this table is not an endorsement of a given product. The list is a representative sample of the technologies currently in clinical and consumer use.
AF indicates atrial fibrillation; FDA, US Food and Drug Administration; HR, heart rate; MCOT, mobile cardiac outpatient telemetry; PPG, photoplethysmography; and SR, sinus rhythm.
PREVALENCE OF SCAF
As demonstrated in Table 3,14,22–31 a number of studies have shown a high incidence of CIED-detected AHREs. However, these studies showed great variability with regard to the defined rate and duration for detection of AT. In addition, not all AHRE recordings obtained from CIEDs are AF; some are false positives caused by far-field R-wave oversensing, lead noise, or atrial arrhythmias other than AF. For example, in ASSERT (Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial), 82.7% of AHREs were true AT/AF, and 17.3% were false positives.32 It is also evident that the longer the duration is and the higher the risk of the population is, the higher the rate of detection is, keeping also in mind that patients with CIEDs tend to be older and often have significant structural heart disease, which are risk factors for AF.
Table 3.
Prevalence of Newly Detected AF in Nonstroke Populations With Implanted Permanent Pacemakers or Cardioverter-Defibrillators
| Study/Year | Device Type and Indication | Patient Profile | Definition of AF | Follow-Up Duration | Prevalence of AT/AF, n (%) |
|---|---|---|---|---|---|
| Gillis and Morck,22
2002 |
PPMs for all indications | All | 180 bpm, number of beats for atrial tachycardia detection of 200 | 718±383 d | 126/231 (55) |
| MOST,23 2003 | PPMs for SND | All | Atrial rate >220 bpm for >5 min | 27 mo | 156/312 (50) |
| Tse and Lau,24 2006 | PPMs for SND | All | Not defined | 84 mo | 99/226 (44) with at least 1 episode, and 30/139 (22) patients without a history of AF |
| SAFE Registry,25 2008 | PPMs for all indications | No prior AF | AT with an atrial rate ≥180 bpm lasting ≥5 min | 6 mo | 150/1501 (10) 5–60 min in 26 (76) |
| TRENDS,26 2009 | PPMs, ICDs, or CRT for all indications | ≥1 Stroke risk factor (CHF, hypertension, age >65 y, DM, or prior TE) | AT/AF defined atrial rate >175 bpm for >20 s AT/AF burden over prior 30-d period subsets: zero, low (<5.5 h), and high (≥5.5 h) |
1.4 y | PPM: 1234/2486 (49.6) ICD: 781/2486 (31.4) CRT: 471/2486 (19.0) |
| Botto et al,27 2009 | PPMs for tachy-brady syndrome | Prior atrial arrhythmias documented | >5 min of AF | 1 y | AF for 24 h: (39.2) AF for 5 min: (31.5) AF-free: (29.2) |
| ASSERT,14 2012 | PPMs and ICDs for all indications | Age >65 y, history of hypertension, no history of AF | Atrial rate >190 bpm for >6 min | 2.5 y | At 3 mo: 261/2580 (10.1) An additional 633 patients (24.5) during 2.5-y follow-up: 894/2580 (34.7) |
| Healey et al,28 2013 | PPMs for all indications | All | Not defined | 51.5 ± 39.7 mo | AF was detected by the pacemaker in 246/445 patients (55.3), 65.8% of the 111 patients with a clinical history of AF before PPM implantation |
| Gonzalez et al,29 2014 | PPMs for all indications | No history of AF | AHREs >178 bpm | 6 mo | 39/224 (17.4) |
| Benezet-Mazuecos et al,30 2015 | PPMs and ICDs for all indications | All | Atrial rate ≥225 bpm with a minimum duration of 5 min | 12 mo | 28/109 (25.7) |
| Lima et al,31 2016 | PPMs for all indications | Age >60 y | AF was the designation for AHREs >2 min in AT histogram >250 bpm | Average follow-up of 15.7±7.7 mo | 63/300 (21) |
AF indicates atrial fibrillation; AHRE, atrial high-rate episode; ASSERT, Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial; AT, atrial tachyarrhythmia; CHF, congestive heart failure; CRT, cardiac resynchronization therapy; DM, diabetes mellitus; ICD, implantable cardioverter-defibrillator; MOST, Mode Selection Trial; PPM, permanent pacemaker; SAFE, Silent Atrial Fibrillation Detection With Stored EGMs; SND, sinus node dysfunction; TE, thromboembolic event; and TRENDS, A Prospective Study of the Clinical Significance of Atrial Arrhythmias Detected by Implanted Device Diagnostics.
Alternatively, external cardiac monitors and ICMs, which do not have an atrial electrode, detect AF by analyzing the irregularity and incoherence of successive R-R intervals in a minimum required amount of time (Figure 3). In patients with ESUS, for example, the longer the monitoring period is, the higher the yield is for AF detection. In the EMBRACE trial (30-Day Event Monitoring Belt for Recording Atrial Fibrillation After a Cerebral Ischemic Event)8 (30-day external monitors) and the CRYSTAL AF trial (Cryptogenic Stroke and Underlying Atrial Fibrillation)4 (ICMs), the yield of AF detection was significantly higher than that of a 24-hour Holter, and a longer monitoring duration resulted in a higher yield (Table 4).34 A meta-analysis of 32 trials that used either external cardiac monitors or ICMs for AF detection after ESUS documented a detection rate of 11.5%.33 ASSERT 2 studied a high-risk population without prior AF; the yield of SCAF was significant.35
Table 4.
Studies of Prolonged Monitoring After ESUS
| Study/Year | Device | Patient Profile | Patients, n | Monitoring Strategy/Duration | AF Detection Yield (vs Control) |
|---|---|---|---|---|---|
| EMBRACE8 | External recorder | Mean age 55 y, 75 d after stroke or TIA | 572 | 30 d compared with 24-h Holter | 16.1 vs 3.2% for 24-h Holter |
| CRYSTAL AF4 | ICM | 38 d after CS, age >40 y | 441 | Prolonged monitoring vs 24-h Holter | 8.9% vs 1.4% at 6 mo; 12.4% vs 2.0% at 12 mo |
| Kishore et al33 | Any cardiac monitoring | NA | Meta-analysis: 32 studies and 5038 patients | >12 h | 11.5% |
| Sposato et al34 | Any monitoring | NA | Meta-analysis: 50 studies and 11 658 patients | Phase 1 (emergency department) consisted of admission ECG; phase 2 (in hospital) comprised serial ECG, continuous inpatient monitoring with ECG, continuous inpatient cardiac telemetry, and in-hospital Holter monitoring; phase 3 (first ambulatory period) consisted of ambulatory Holter; and phase 4 (second ambulatory period) consisted of mobile cardiac outpatient telemetry, external loop recording, and ILR | Overall AF detection yield after all phases of sequential cardiac monitoring was 23.7%; higher yield with longer monitoring |
| ASSERT 235 | ILR | Patients ≥65 y of age attending cardiovascular or neurology outpatient clinics with no history of AF but with any of the following: CHA2DS2-VASc score ≥2, sleep apnea, or body mass index >30 kg/m2; plus either left atrial enlargement (≥4.4 cm or volume ≥58 mL) or increased (≥290 pg/mL) serum NT-proBNP | 256 | SCAF lasting ≥5 min; follow-up 16.3±3.8 mo | Yield of SCAF in those with a history of stroke, systemic embolism, or TIA was 39.4%/y vs 30.3%/y without |
AF indicates atrial fibrillation; ASSERT 2, Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial 2; CRYSTAL AF, Cryptogenic Stroke and Underlying Atrial Fibrillation; CS, cryptogenic stroke; EMBRACE, 30-Day Event Monitoring Belt for Recording Atrial Fibrillation After a Cerebral Ischemic Event; ESUS, embolic stroke of undetermined source; ICM, implantable cardiac monitor; ILR, implantable loop recorder; NA, not available; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SCAF, subclinical atrial fibrillation; and TIA, transient ischemic attack.
PREDICTORS OF SCAF
Various predictors of SCAF have been identified among patients with CIEDs. Cheung et al36 found that, among patients with pacemakers implanted for sinus node dysfunction, a high proportion of right ventricular pacing predicted SCAF, whereas in those with pacemakers for atrioventricular block, hypertension was the only predictor.36 In TRENDS (A Prospective Study of the Clinical Significance of Atrial Arrhythmias Detected by Implanted Device Diagnostics), patients with history of clinical AF or with new SCAF were older, had higher diastolic blood pressure, and were less likely to have diabetes mellitus than patients without AF or SCAF.26 In ASSERT, sinus node dysfunction and a lower resting heart rate predicted SCAF; patients with SCAF were less likely to have diabetes mellitus or prior myocardial infarction.14 Gonzalez et al29 found that only history of heart failure (HF) predicted SCAF. A recent pooled analysis including patients from TRENDS found that those with SCAF were older, more likely to be male, and less likely to have diabetes mellitus. In a study of 880 patients, Kim et al37 found that prior HF, sinus node disease, and increased left atrial volume index independently predicted SCAF. Finally, a meta-analysis including almost 25 000 patients from 28 dual-chamber device studies reported that, in the 10 studies that reported baseline characteristics, only history of HF predicted SCAF; hypertension, diabetes mellitus, age, thromboembolic events, coronary disease, and CHADS2 score did not.38
Additional studies have examined predictors of SCAF detected by ICMs in high-risk populations. Pedersen et al39 implanted ICMs in 105 patients with transient ischemic attack (TIA); 7 had AF detected by the implantable loop recorder. Predictors were history of recurrent TIA and HF. In a study of patients with ESUS, Israel et al40 implanted 123 patients with loop recorders and detected AF in 29. Predictors were older age, higher CHA2DS2-VASc score, and cerebral microangiopathy.40 Healey et al35 implanted implantable loop recorders in 256 patients with age ≥65 years and 2 additional high-risk features (CHA2DS2-VASc score of at least 2 or sleep apnea or elevated body mass index and elevation of left atrial size or pro-B-type natriuretic peptide). Independent predictors of AF were increased age, increased left atrial size, and lower systolic blood pressure.41
RELATIONSHIP WITH STROKE
Association With Incident Stroke
In a meta-analysis that evaluated 7 studies and 15 353 patients, SCAF was associated with a 2.4-fold increase in stroke risk (95% CI, 1.8–3.3; P<0.001), with an absolute annual rate of 1.89 per 100 person-years (95% CI, 1.02–3.52).42 On initial analysis, the definition of episodes that predicted stroke varied significantly between different studies; in MOST (Mode Selection Trial) and ASSERT, the duration of AHREs was 5 and 6 minutes, respectively, whereas in TRENDS, increased risk was observed in those with >5.5 hours of AF within the past 30 days.14,23,26 However, when further stratified, the ASSERT trial showed that the risk of stroke was significant only when episodes were >17.72 hours,14 and subsequent analyses have shown that the bulk of events occurred in patients with >24 hours of SCAF (Figure 4).43 Another recent study showed that short AT/AF episodes (<15–20 seconds) were not associated with clinical events,44 suggesting that there is a gradient of risk depending on AF duration and frequency (burden). The totality of the evidence suggests that there is a dose relationship between AF duration and stroke risk (Figure 5).46
Figure 4. In ASSERT (Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial), the cumulative event rate for cardioembolic events was highest in patients with subclinical atrial fibrillation (SCAF) >24 hours in duration as demonstrated by these extended Kaplan-Meier curves of ischemic stroke/systemic embolism stratified by time-dependent durations of SCAF.
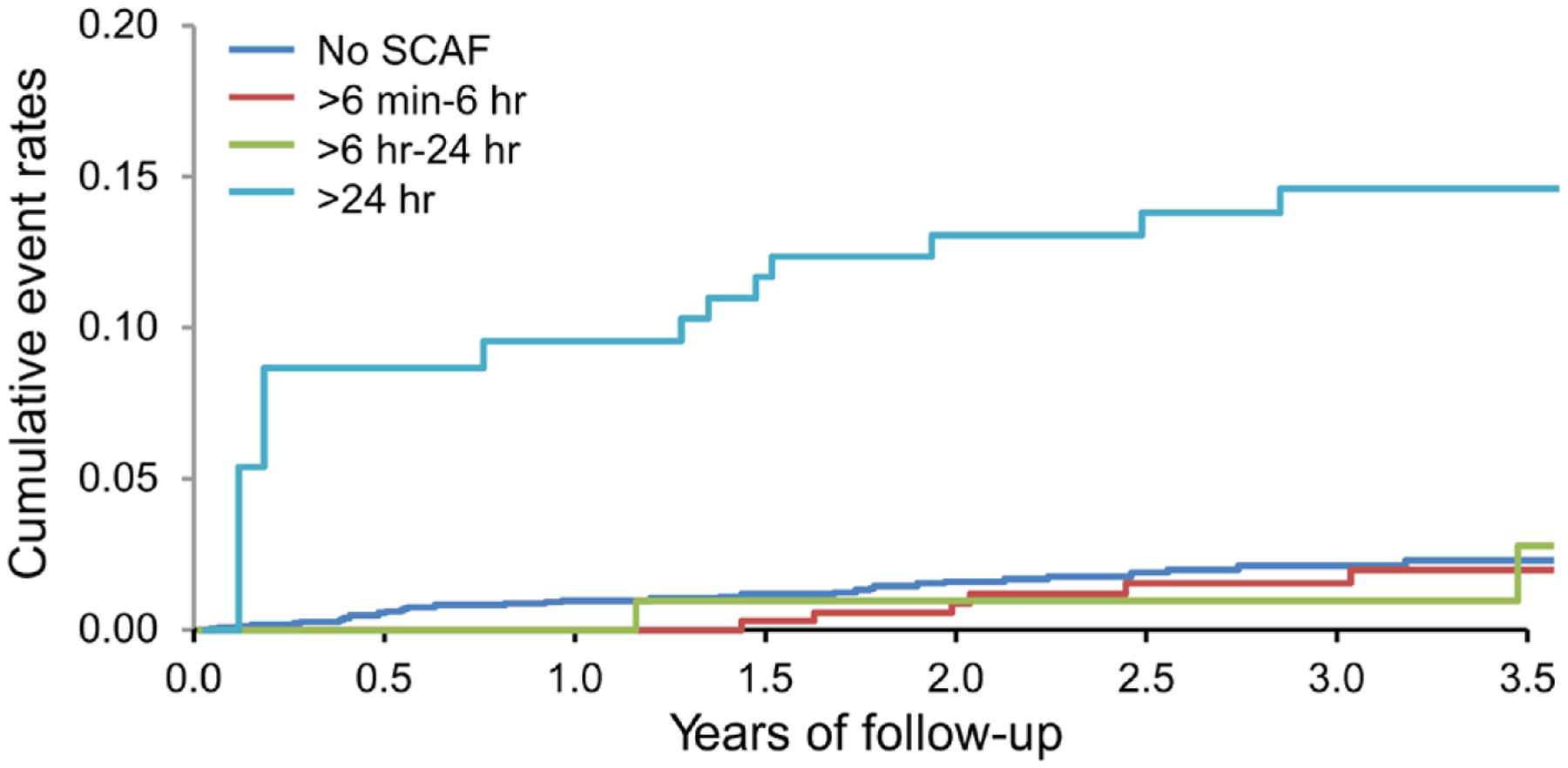
Adapted from Van Gelder et al43 by permission of the European Society of Cardiology. Copyright © 2017, The Author. Published on behalf of the European Society of Cardiology.
Figure 5. Hazard ratio (HR) for thromboembolism reported in previous studies plotted as a function of the log of the duration of the longest episode of atrial fibrillation (AF) detected (with a trend line).
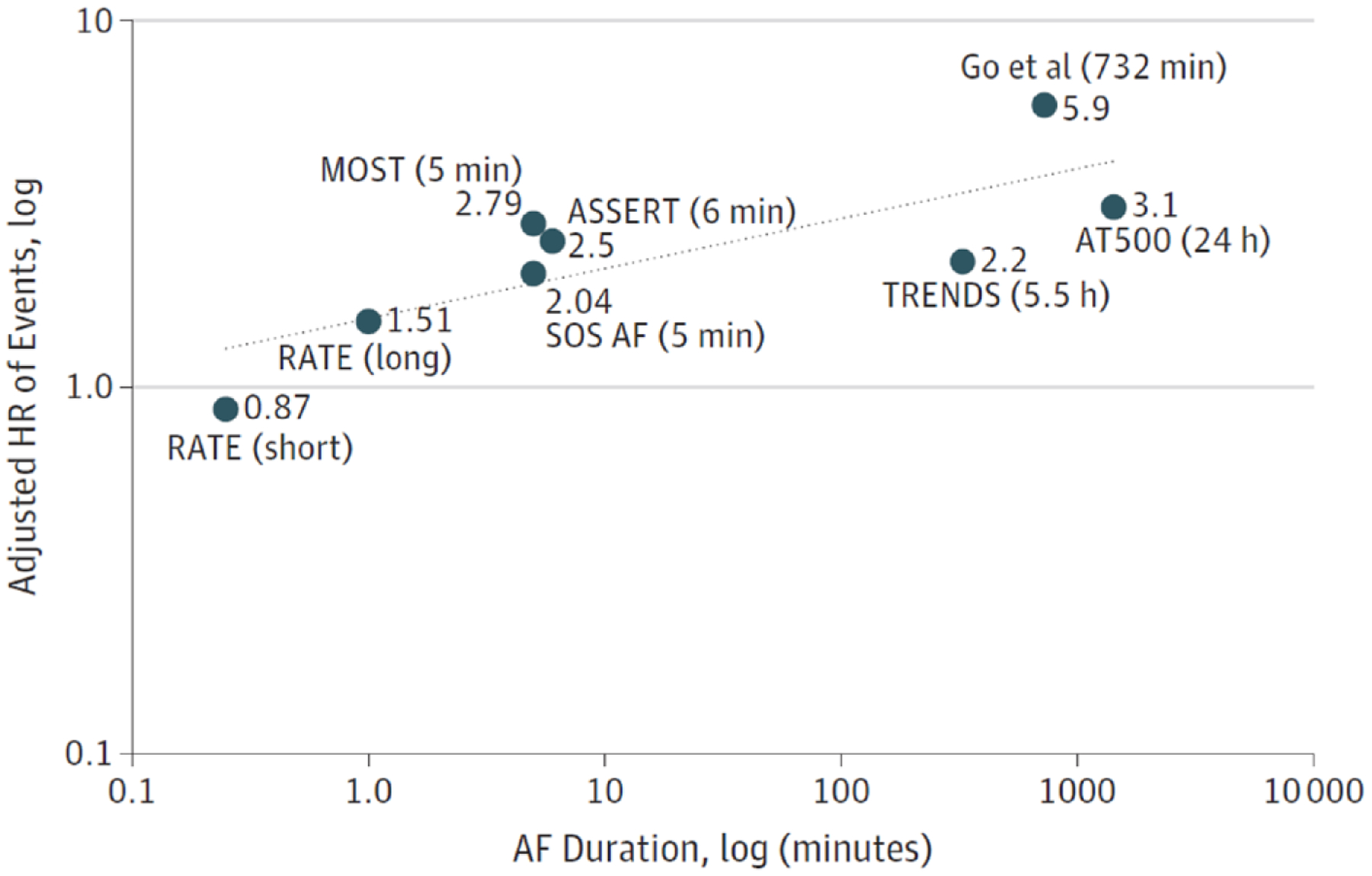
ASSERT indicates Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial; MOST, Mode Selection Trial; RATE, Registry of Atrial Tachycardia and Atrial Fibrillation Episodes; SOS AF, Stroke Prevention Strategies Based on Atrial Fibrillation Information From Implanted Devices; and TRENDS, A Prospective Study of the Clinical Significance of Atrial Arrhythmias Detected by Implanted Device Diagnostics. Reproduced with permission from Steinberg and Piccini.45 Copyright © 2018, American Medical Association. All rights reserved.
Stroke risk also seems to depend on traditional risk factors. For example, in ASSERT, absolute stroke risk increased with increasing CHADS2 score, reaching a rate of 3.78 per year in those with a score >2.14 Capucci and colleagues47 showed increased risk with an AF duration >24 hours, as well as with traditional risk factors such as diabetes mellitus, hypertension, and coronary artery disease. Botto et al27 stratified risk according to AF duration and CHADS2 score, with a CHADS2 score of 1 increasing the risk only if the AF duration was >24 hours, whereas for CHADS2 scores ≥2, episodes lasing >5 minutes increased risk. This latter point illustrates the fact that, among patients with stroke, even very brief episodes of AF are likely to be associated with increased risk because all patients with stroke have a CHADS2 score ≥2.
There is likely a complex interplay among baseline stroke risk factors, AF duration/burden, and stroke risk. The observation that in ASSERT many strokes were not immediately preceded by AF episodes48 (Figure 648) has led some to argue that AF should be thought of as a risk marker rather than a direct cause of stroke (Figure 7). However, although not all strokes are cardioembolic and not all thrombi are directly related to AF, other studies have demonstrated a temporal relationship between AF epodes and embolic events.49
Figure 6. In ASSERT (Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial), many stroke/systemic embolism events were not temporally associated with subclinical atrial fibrillation (SCAF) episodes.
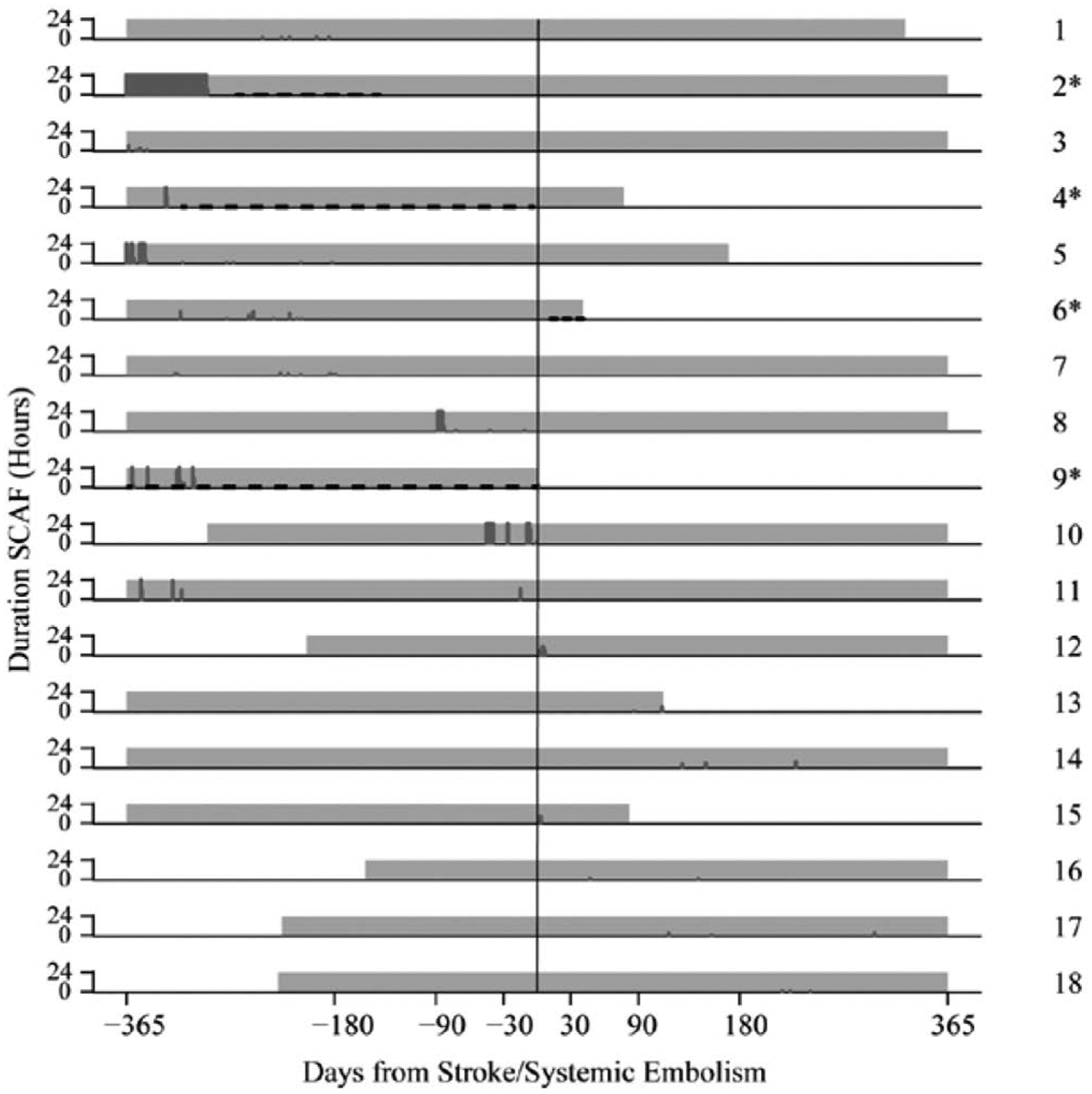
Each row represents data collected from each of 18 patients who had SCAF within 1 year before or after the event. Total hours of atrial episodes per day are denoted by the height of each dark gray vertical line. Gray-shaded areas correspond to the period of continuous monitoring with cardiac device. Asterisks and black dashed lines denote use and period of oral anticoagulation therapy. Modified from Brambatti et al.48 Copyright © 2014, American Heart Association, Inc.
Figure 7. The association between atrial fibrillation (AF) and stroke can be conceptualized by 2 models: AF as a risk marker or AF as a direct cause of stroke.
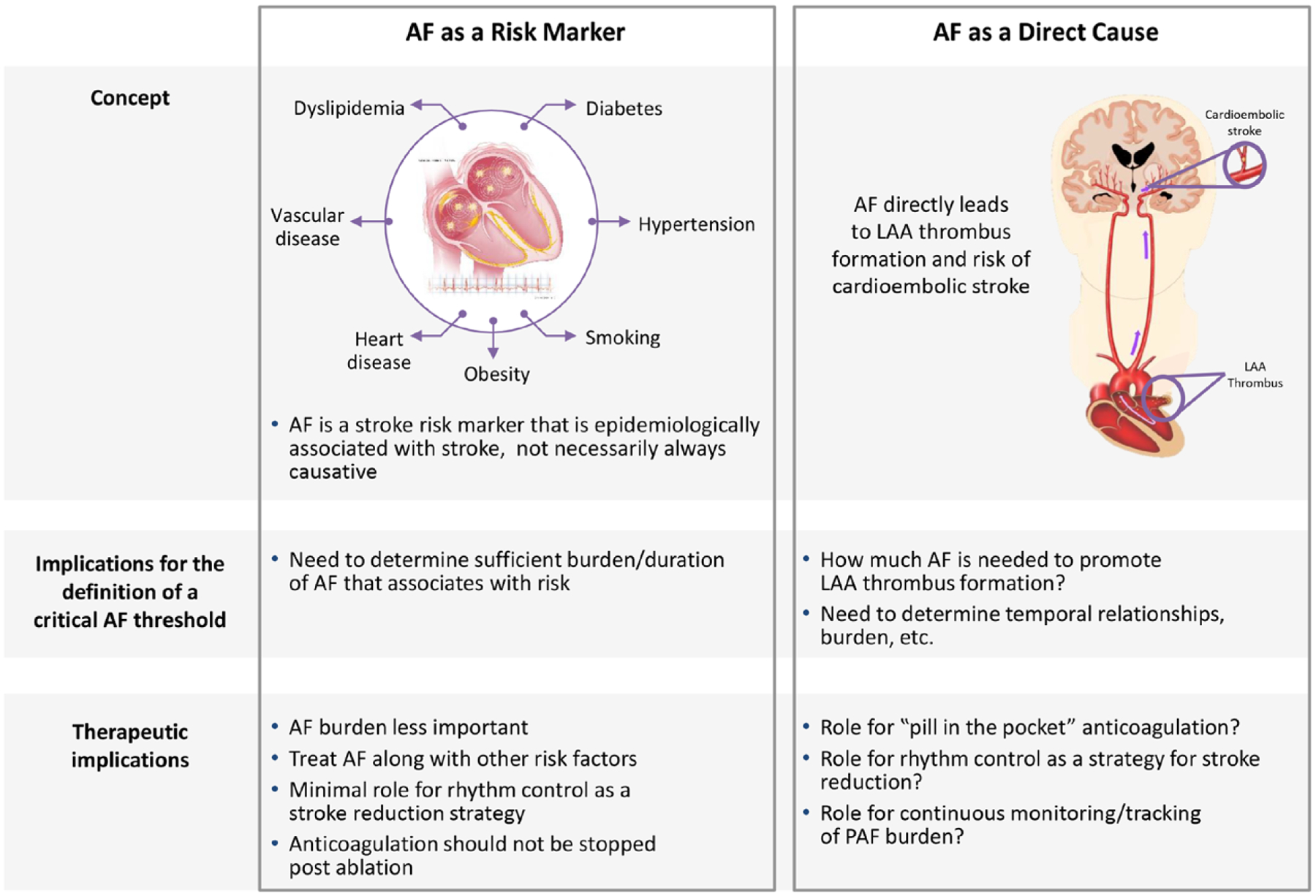
These concepts have implications for the treatment and definition of a critical clinically relevant threshold of AF burden or duration. LAA indicates left atrial appendage; and PAF, paroxysmal atrial fibrillation.
Association With ESUS
SCAF is also commonly detected in patients after a stroke (Table 4). In a meta-analysis of 50 studies, SCAF was detected during follow-up in ≈24% (95% CI, 17–31) of patients with ESUS, depending on the duration of monitoring and device used.34 In the EMBRACE trial, 572 patients with ESUS or TIA with previously negative 24-hour Holter monitor were randomized to monitoring with a 30-day event-triggered loop recorder or repeat 24-hour Holter monitoring. Detection rates of SCAF (duration ≥30 seconds) were significantly higher with monitoring (16.1%) compared with repeat Holter (3.2%).8 The CRYSTAL AF trial found significantly elevated rates of SCAF (duration of ≥30 seconds) among 441 patients with ESUS using an ICM compared with a strategy of monitoring for only 24 hours: 9% at 6 months, 12% at 12 months, and 30% at 36 months among the ICM group.4 Most AF was asymptomatic, and 97% of patients received anticoagulation after AF detection. AF duration was ≤6 minutes in 8% of patients. Patients in EMBRACE were older than those in CRYSTAL AF (mean age, 73 years versus 61 years), and they had not already undergone transesophageal echocardiography to exclude thrombi, which may explain the higher SCAF detection rates in EMBRACE.4,8 SCAF may also be detected among patients with stroke of known causes, including small vessel and atherosclerotic strokes, and the ongoing STROKE-AF randomized trial (Stroke of Known Cause and Underlying Atrial Fibrillation; NCT 02700945) will seek to determine whether monitoring for AF with an ICM is more likely to find AF among these patients than standard detection.50
There are several predictors of detection of SCAF among patients with ESUS. Among 237 participants in EMBRACE randomized to 30 days of monitoring, the median 24-hour baseline Holter atrial premature beat count was significantly higher among those who subsequently had AF detected compared with those who did not (629 versus 45; P<0.001).51 The AF detection rate was >25% for those with ≥500 atrial premature beats in 24 hours. Runs of AT, age, and left atrial enlargement were not independently predictive of AF. In CRYSTAL AF, among 221 patients randomized to ICM, age (hazard ratio [HR] per decade, 1.9 [95% CI, 1.3–2.8]) and PR interval (HR per 10 milliseconds, 1.3 [95% CI, 1.2–1.4]) were predictive of AF at 12 and 36 months after stroke.52 A risk score that included 7 clinical variables (age, obesity, congestive HF, hypertension, coronary artery disease, peripheral vascular disease, and valvular disease) was predictive of detection of AF among patients with unexplained stroke or TIA in an academic medical center record database and permitted discrimination of patients into low-, medium-, and high-risk groups.53
PROGRESSION TO CLINICAL AF
Shorter episodes of SCAF have been associated with a higher likelihood of subsequent longer episodes of SCAF,44 including a 5.7 times higher risk of clinical AF.42 Data from ASSERT (n=2580) showed that SCAF in the first 3 months (n=216) was associated with a 5.6-fold higher hazard of clinical AF during the 2.5-year mean follow-up.14 Similar results were seen in the subset of patients (n=312) from MOST (n=2010) who had SCAF during the 2.25-year median follow-up; SCAF was associated with a 5.9-fold higher hazard of clinical AF.23
Among 415 patients from ASSERT with 6 minutes to 24 hours of AF during the first year, 16% progressed to clinical AF or SCAF lasting >24 hours over the subsequent 2-year median follow-up.54 Older age (HR, 1.59 per 10-year increase), higher body mass index (HR, 1.83 per 10–kg/m2 increase), and longer episodes of SCAF (HR, 1.13 per 1-hour increase in longest episode length) were associated with AF progression.54 A pooled analysis of 3 studies (TRENDS, PANORAMA [Study of the Efficacy and Safety of Intravitreal (IVT) Aflibercept for the Improvement of Moderately Severe to Severe Nonproliferative Diabetic Retinopathy], and SOS AF [Stroke Prevention Strategies Based on Atrial Fibrillation Information From Implanted Devices]) analyzed 6580 patients with CIEDs and found that longer initial episodes of SCAF were associated with subsequent episodes ≥23 hours (Figure 8).56 The time between the first detected episode and the first episode lasing ≥23 hours decreased from a median of 6.9 to 2.9 to 0.07 to 0.03 months for initial episodes of 5 to <60 minutes, 1 to <6 hours, 6 to <12 hours, and 12 to <23 hours, respectively.56
Figure 8. A pooled analysis of 3 prospective studies (TRENDS [A Prospective Study of the Clinical Significance of Atrial Arrhythmias Detected by Implanted Device Diagnostics], PANORAMA [Study of the Efficacy and Safety of Intravitreal (IVT) Aflibercept for the Improvement of Moderately Severe to Severe Nonproliferative Diabetic Retinopathy], and SOS AF [Stroke Prevention Strategies Based on Atrial Fibrillation Information From Implanted Devices]) found that longer initial episodes of subclinical atrial fibrillation (AF) were associated with the development of AF lasting ≥23 hours.
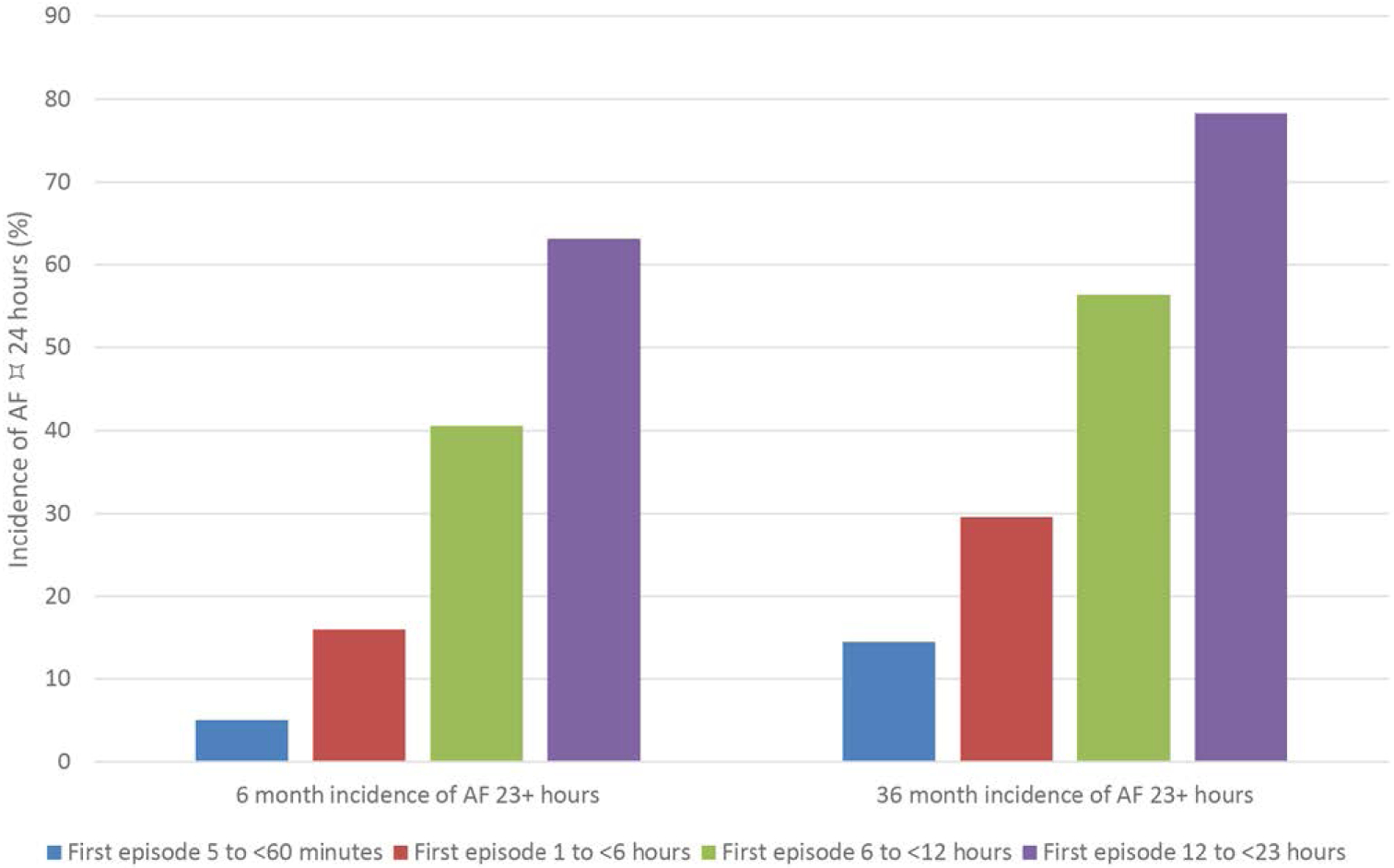
Data derived from Boriani et al.55
RELATIONSHIP WITH HF
The association between AF and HF has been recognized and studied for almost a century.1 Paul Dudley White once observed that “auricular fibrillation often complicates very serious heart disease…. Its occurrence may precipitate heart failure or even death, unless successful therapy is quickly instituted.”2
Several studies have highlighted the prevalence of AF in patients with HF, with estimates in the 13% to 50% range, a significant increase compared with the general population.3–7 As an example, the Framingham Heart Study registered 1470 participants who developed new-onset AF or HF between 1948 and 1995. A total of 383 of those individuals (26%) developed both AF and HF.8 In addition, the prevalence of AF in patients with HF appeared to correlate with the severity of the disease, ranging from 5% in patients with mild HF to 10% to 26% among patients with moderate disease to 50% in patients with severe forms of HF.9
A direct cause-effect relationship between these 2 prevalent conditions has been the focus of basic and clinical investigation. At a minimum, this strong association can be at least partially explained by common risk factors such as age, hypertension, diabetes mellitus, and obesity, as well as valvular, ischemic, and non-ischemic structural heart disease. These factors are associated with electrophysiological and neurohormonal changes at the myocardial cellular and extracellular levels, allowing an environment that predisposes the heart to both myocardial failure and AT.10
There is no defined AF burden threshold beyond which HF becomes more prevalent, and this association appears relevant for both paroxysmal and persistent forms of AF.8–11 However, device-detected AF lasting for at least 24 hours was associated with HF hospitalization in ASSERT.54
PRACTICE UNCERTAINTY, GAPS IN EVIDENCE, AND NEXT STEPS
Physician Attitudes and Practice Patterns
The lack of clearly defined thresholds of AF burden at which to initiate oral anticoagulation has resulted in substantial variation in physician attitudes and practice patterns. Practice variation is illustrated by a retrospective cohort study that used data from the Veterans Health Administration linked to remote monitoring data, which included day-level AF burden.57 Among 10 212 patients who received CIEDs in 2011 to 2014, 4570 (45%), 3969 (39%), 3263 (32%), and 2469 (24%) patients had SCAF lasting ≥6 minutes, >1 hour, >6 hours, and >24 hours, respectively. The proportion who received oral anticoagulation within 90 days of SCAF varied according to AF burden, increasing with higher AF burden (≥6 minutes, 13%; >1 hour, 16%; >6 hours, 21%; >24 hours, 27%). Of note, the average time to oral anticoagulation prescription after device-detected AF was >30 days for all AF burdens, and warfarin was the most commonly used agent (>92%).
Stroke Prevention
The optimal approach to primary stroke prevention remains uncertain for many patients with SCAF. Questions remain about the net clinical benefit of anticoagulation for patients at low to intermediate risk, risk factor management to prevent AF progression, and novel ways to risk-stratify these patients. These gaps are compounded by the fact that there is currently inadequate standardization of the definitions and metrics used for diagnosis and treatment. Future efforts at standardization of these definitions could be beneficial.
Two ongoing clinical trials among patients with CIED-detected SCAF will likely inform management: ARTESiA (Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Subclinical Atrial Fibrillation; NCT 01938248)41 and NOAH (Non–Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodes; NCT 02618577).58
In the absence of definitive evidence, it is reasonable for clinicians to consider the duration/burden of SCAF in combination with the CHA2DS2-VASc score (Figure 9).10 However, this approach has inherent limitations. First, although the CHA2DS2-VASc is the current gold standard for clinical stroke risk assessment, few studies have examined its performance in SCAF. Second, most risk factors in the CHA2DS2-VASc score are also risk factors for bleeding; thus, patients with a high CHA2DS2-VASc score are at high risks of both stroke and bleeding. It is unclear whether CHA2DS2-VASc is the best tool to determine the net clinical benefit from anticoagulation in this population. Ultimately, the benefit of anticoagulation in these patients boils down to the balance of stroke and bleeding risks with and without anticoagulation (Figure 10). Among patients who have already experienced a stroke, moreover, the finding of any duration or burden of AF is generally considered a reason to anticoagulate, especially in the era of the direct-acting oral anticoagulants. In CRYSTAL AF, for example, by 12 months, 97% of patients in whom AF had been detected were receiving oral anticoagulants, although the median value for the mean time in AF per day was 4.3 minutes.4
Figure 9. A potential approach to patients with subclinical atrial fibrillation (SCAF) could consider both patient risk (as gauged by the CHA2DS2-VASc score) and SCAF burden/duration.
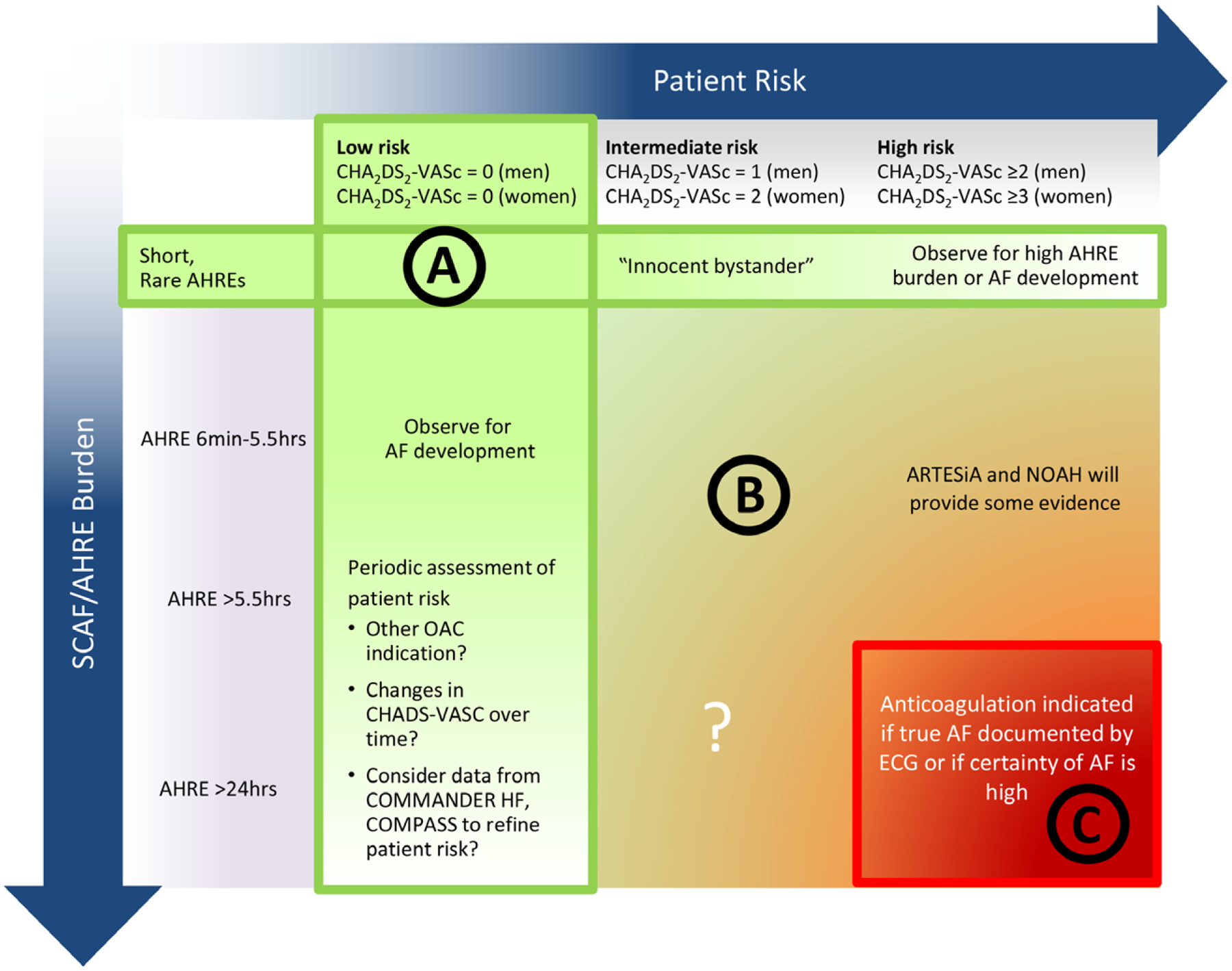
A, Patients at low risk or with short and infrequent atrial high-rate episodes (AHREs) do not require anticoagulation. B, Patients with intermediate risk and AHREs lasting >6 minutes to 24 hours are an uncertain population but are currently under study in 2 prospective randomized controlled trials. C, Patients at high risk with longer episodes could be considered reasonable candidates for anticoagulation, although the precise threshold for SCAF duration remains uncertain. AF indicates atrial fibrillation; ARTESiA, Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Subclinical Atrial Fibrillation trial; COMMANDER HF, A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants With Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure; COMPASS, Cardiovascular Outcomes for People Using Anticoagulation Strategies; NOAH, Non–Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodes Trial; and OAC, oral anticoagulation. Modified from Freedman et al10 with permission. Copyright © 2017, Springer Nature.
Figure 10. The benefit of anticoagulation can be conceptualized as a balance between stroke and bleeding risks with and without anticoagulation.
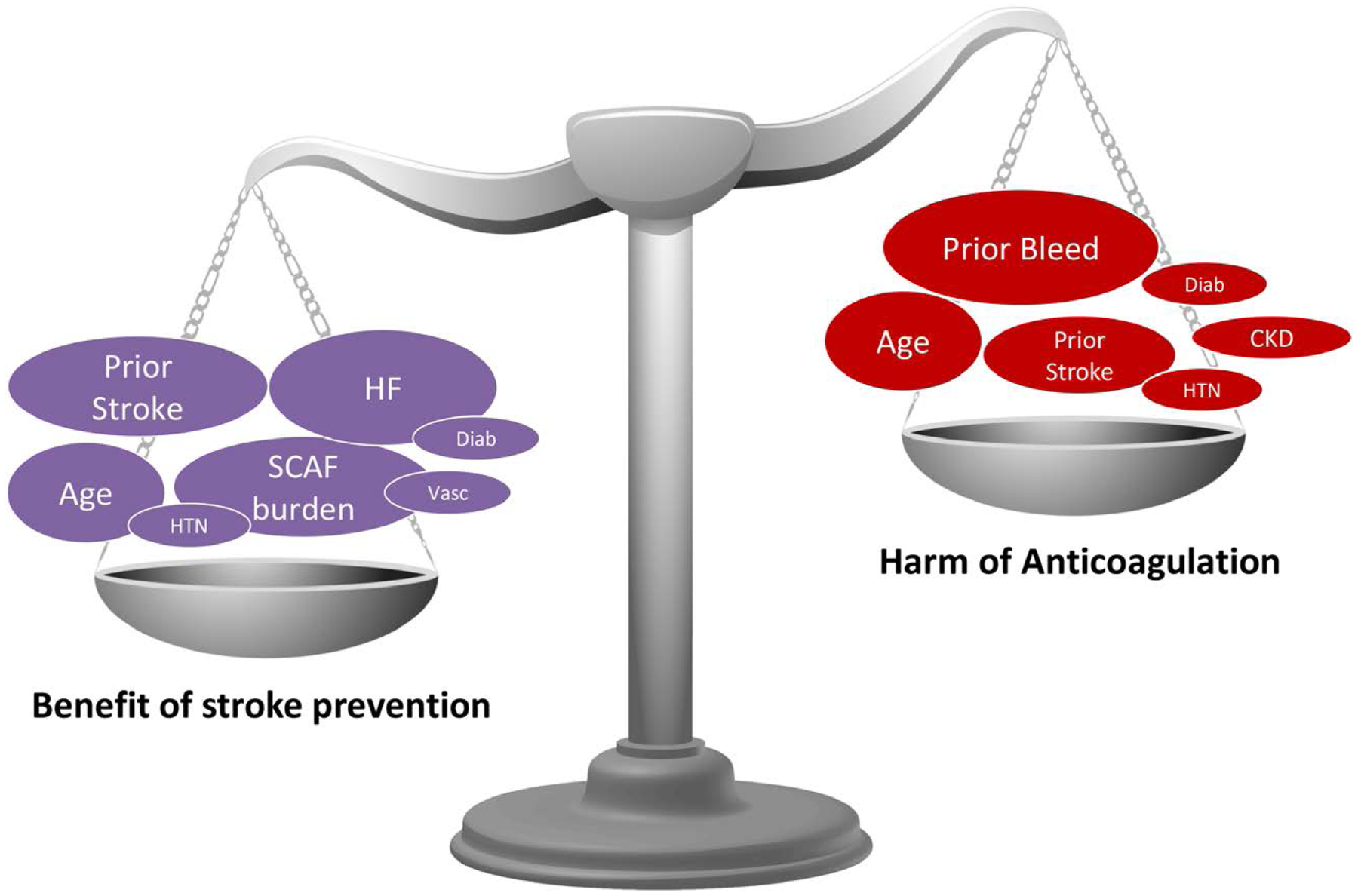
Non-vitamin K antagonist oral coagulants randomized controlled trial data in the non-atrial fibrillation population (eg, COMPASS [Cardiovascular Outcomes for People Using Anticoagulation Strategies], COMMANDER HF [A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants With Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure], NAVIGATEESUS [New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial Versus ASA to Prevent Embolism in Embolic Stroke of Undetermined Source]) could help estimate the balance of risk in SCAF. CKD indicates chronic kidney disease; Diab, diabetes mellitus; HF, heart failure; HTN, hypertension; SCAF, subclinical atrial fibrillation, and Vasc, vascular disease.
Ongoing trials, including NOAH and ARTESiA, may help to clarify the balance of these risks. Small trials have evaluated the role for “pill in the pocket” anticoagulation guided by continuous AF monitoring.59,60 Although controversial, this strategy may minimize a patient’s bleeding risk by reducing lifetime exposure to anticoagulation while still providing thrombo-prophylaxis during periods of AF, but the efficacy of this approach requires further study. This strategy hinges on the unresolved concept of whether AF is a stroke risk marker or cause (Figure 8).
Role for Shared Decision-Making in Navigating Uncertainty
Treatment choices are best made in collaboration with patients with thorough exploration of the current knowledge and knowledge gaps, as well as careful consideration of an individual patient’s goals and preferences. However, shared decision-making is often challenging in the context of busy clinical practice and when the data themselves are uncertain or incomplete. A comprehensive decision or conversation aid could be beneficial to synthesize available data, to elicit patient’s goals and preferences, to explore treatment options and ramifications, to ease clinicians’ cognitive burden, and to improve patients’ comprehension and engagement.
Role for Pragmatic Trials and Real-World Data
Given the proliferation of convenient tools to monitor and detect AF and the large amount of electronic data generated from routine practice, it is becoming increasingly attractive to conduct pragmatic trials with such data. Prescribing patterns, medication adherence, and patient comorbidity and complexity can differ between practice and trials, and understanding realized outcomes in practice can inform some of our most challenging treatment decisions.
SUMMARY AND CONCLUSIONS
Discovery of SCAF with CIEDs and wearable monitors is common, especially in populations known to have an increased risk of stroke (or recurrent stroke). Although detection of SCAF provides an opportunity to reduce the risk of embolic stroke, a number of factors should be considered before the patient is exposed to the risks of long-term anticoagulation. First, the accuracy of SCAF detection should be confirmed by a review of electrograms to exclude false positives. Second, the longest continuous episode and highest daily burden should be quantified because stroke risk increases with longer durations and higher overall burden of SCAF, particularly those lasting >24 hours. Third, clinicians should assess the traditional stroke risk factors such as age, diabetes mellitus, hypertension, and HF.
However, the precise combination of SCAF duration/burden and patient risk factors that warrants long-term anticoagulation is unknown, and the current practice variation reflects this uncertainty. According to the current, incomplete evidence, it appears reasonable to defer anticoagulation for patients with no stroke risk factors or those who have only very brief AHREs but to consider anticoagulation for longer episodes in patients with stroke, TIA, or other stroke risk factors. The exact threshold remains controversial, however, especially because stroke risk factors are also associated with bleeding risk. We anticipate that 2 ongoing studies, ARTESiA and NOAH, will help to clarify the clinical relevance of SCAF and to guide therapy decisions for patients with shorterduration episodes.
Disclosures
Writing Group Disclosures
| Writing Group Member | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Peter A. Noseworthy | Mayo Clinic | None | None | None | None | None | None | None |
| Elizabeth S. Kaufman | MetroHealth Campus/Case Western Reserve University Heart & Vascular Research Center | None | None | None | None | None | None | None |
| Lin Y. Chen | University of Minnesota | NIH (PI on 2 R01 grants from NHLBI)† | None | None | None | None | None | None |
| Mina K. Chung | Cleveland Clinic | NIH†; AHA† | None | None | None | None | Amarin Corp; data monitoring committee for REDUCE-IT clinical trial (unpaid)*; Biotronik Steering Committee for EPIC Alliance, a program formulated to support careers and global networking of women electrophysiologists; inactive over the past year (unpaid)* | American Board of Internal Medicine (Cardiovascular Disease Board Writing Committee)†; Heart Rhythm Society (Board of Trustees; Research Committee member [unpaid])*; AHA (chair, ECG & Arrhythmias Committee; member Clinical Cardiology Leadership Committee, Committee for Scientific Sessions Programming; various writing committees [unpaid])*; American College of Cardiology (previous member of the EP Leadership Committee [unpaid])*; Elsevier Publishers (book author)*; UpToDate (author)*; AHA (Circulation: Arrhythmia and Electrophysiology associate editor)†; NIH (ad hoc study section reviewer)*; France ANR (grant review panel member)* |
| Mitchell S.V. Elkind | Columbia University Neurological Institute | BMS-Pfizer Alliance for Eliquis (receives study medication in kind but no personal compensation for a federally funded trial of apixaban for cryptogenic stroke)†; Roche (receives support but no personal compensation for a federally funded trial of apixaban for cryptogenic stroke)† | Medtronic (his institution receives support for his participation in analyses of data from clinical studies related to monitoring for atrial fibrillation)* | None | LivaNova†; UpToDate* | None | None | None |
| José A. Joglar | UT Southwestern Medical Center | None | None | None | None | None | None | None |
| Miguel A. Leal | University of Wisconsin | None | Medtronic (Fellowship Support Grant)* | None | None | None | None | None |
| Pamela J. McCabe | Mayo Clinic | None | None | None | None | None | None | None |
| Sean D. Pokorney | Duke University Medical Center | Bristol-Myers Squibb*; Pfizer* (both RENALAFT trial funding)*; Janssen (QUANTUMAF trial funding)* | None | None | None | None | Boston Scientific*; Bristol-Myers Squibb* Pfizer* Medtronic* Janssen*; Portola* | None |
| Xiaoxi Yao | Mayo Clinic | None | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Reviewer Disclosures
| Reviewer | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Taya V. Glotzer | Hackensack University Medical Center, Electrophysiology Associates of Northern New Jersey | None | None | None | None | None | None | None |
| Jonathan P. Piccini | Duke University | AHA (grant to institution; I serve as PI)†; Abbott (grant to institution; I serve as PI)†; Boston Scientific (grant to institution; I serve as PI)†; Gilead (grant to institution; I serve as PI)*; Johnson & Johnson (grant to institution; I serve as PI)†; NHLBI (grant to institution; I serve as PI)†; Philips (grant to institution; I serve as PI)† | None | None | None | None | Abbott†; Allergan*; Biotronik*; ARCA biopharma*; Boston Scientific†; Johnson & Johnson*; LivaNova*; Philips†; Medtronic†; Sanofi†; Milesone* | None |
| Benjamin A. Steinberg | University of Utah | None | None | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Footnotes
The devices listed here serve only to illustrate examples of these types of devices. This is not intended to be an endorsement of any commercial product, process, service, or enterprise by the American Heart Association.
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on September 19, 2019, and the American Heart Association Executive Committee on October 22, 2019. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 843-216-2533 or meredith.edelman@wolterskluwer.com.
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
REFERENCES
- 1.Noseworthy PA. Rochester, MN: Department of Cardiovascular Medicine, Mayo Clinic; 2019.
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, et al. ; Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rojo-Martinez E, Sandín-Fuentes M, Calleja-Sanz AI, Cortijo-García E, García-Bermejo P, Ruiz-Piñero M, Rubio-Sanz J, Arenillas-Lara JF. High performance of an implantable Holter monitor in the detection of concealed paroxysmal atrial fibrillation in patients with cryptogenic stroke and a suspected embolic mechanism [in Spanish]. Rev Neurol. 2013;57:251–257. [PubMed] [Google Scholar]
- 4.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, et al. ; CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600 [DOI] [PubMed] [Google Scholar]
- 5.Flint AC, Banki NM, Ren X, Rao VA, Go AS. Detection of paroxysmal atrial fibrillation by 30-day event monitoring in cryptogenic ischemic stroke: the Stroke and Monitoring for PAF in Real Time (SMART) Registry. Stroke. 2012;43:2788–2790. doi: 10.1161/STROKEAHA.112.665844 [DOI] [PubMed] [Google Scholar]
- 6.Brachmann J, Morillo CA, Sanna T, Di Lazzaro V, Diener HC, Bernstein RA, Rymer M, Ziegler PD, Liu S, Passman RS. Uncovering atrial fibrillation beyond short-term monitoring in cryptogenic stroke patients: three-year results from the cryptogenic stroke and underlying atrial fibrillation trial. Circ Arrhythm Electrophysiol. 2016;9:e003333. doi: 10.1161/CIRCEP.115.003333 [DOI] [PubMed] [Google Scholar]
- 7.Tayal AH, Tian M, Kelly KM, Jones SC, Wright DG, Singh D, Jarouse J, Brillman J, Murali S, Gupta R. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology. 2008;71:1696–1701. doi: 10.1212/01.wnl.0000325059.86313.31 [DOI] [PubMed] [Google Scholar]
- 8.Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, Vaid H, O’Donnell M, Laupacis A, Côté R, et al. ; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. doi: 10.1056/NEJMoa1311376 [DOI] [PubMed] [Google Scholar]
- 9.Gorenek B, Bax J, Boriani G, Chen SA, Dagres N, Glotzer TV, Healey JS, Israel CW, Kudaiberdieva G, Levin LÅ, et al. ; ESC Scientific Document Group. Device-detected subclinical atrial tachyarrhythmias: definition, implications and management: an European Heart Rhythm Association (EHRA) consensus document. Europace. 2017;19:1556–1578. doi: 10.1093/europace/eux163 [DOI] [PubMed] [Google Scholar]
- 10.Freedman B, Boriani G, Glotzer TV, Healey JS, Kirchhof P, Potpara TS. Management of atrial high-rate episodes detected by cardiac implanted electronic devices. Nat Rev Cardiol. 2017;14:701–714. doi: 10.1038/nrcardio.2017.94 [DOI] [PubMed] [Google Scholar]
- 11.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 12.Eysenck W, Freemantle N, Sulke N. A randomized trial evaluating the accuracy of AF detection by four external ambulatory ECG monitors compared to permanent pacemaker AF detection [published online ahead of print February 11, 2019]. J Interv Card Electrophysiol. doi: 10.1007/s10840-019-00515-0.https://link.springer.com/article/10.1007%2Fs10840-019-00515-0. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg MA, Samuel M, Thosani A, Zimetbaum PJ. Use of a noninvasive continuous monitoring device in the management of atrial fibrillation: a pilot study. Pacing Clin Electrophysiol. 2013;36:328–333. doi: 10.1111/pace.12053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, et al. ; ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575 [DOI] [PubMed] [Google Scholar]
- 15.Derkac WM, Finkelmeier JR, Horgan DJ, Hutchinson MD. Diagnostic yield of asymptomatic arrhythmias detected by mobile cardiac outpatient telemetry and autotrigger looping event cardiac monitors. J Cardiovasc Electrophysiol. 2017;28:1475–1478. doi: 10.1111/jce.13342 [DOI] [PubMed] [Google Scholar]
- 16.Preventice Solutions. BodyGuardian® Heart. https://www.preventicesolutions.com/patients/body-guardian-heart.html.AccessedOctober 7, 2019.
- 17.Adam B, Teplitsky M, Mehta P, Ghanbari H. Real-world performance of atrial fibrillation detection from wearable patient ECG monitoring using deep learning. Heart Rhythm. 2019;16:S–215. Abstract. [Google Scholar]
- 18.Bumgarner JM, Lambert CT, Hussein AA, Cantillon DJ, Baranowski B, Wolski K, Lindsay BD, Wazni OM, Tarakji KG. Smartwatch algorithm for automated detection of atrial fibrillation. J Am Coll Cardiol. 2018;71:2381–2388. doi: 10.1016/j.jacc.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 19.Tison GH, Sanchez JM, Ballinger B, Singh A, Olgin JE, Pletcher MJ, Vittinghoff E, Lee ES, Fan SM, Gladstone RA, et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 2018;3:409–416. doi: 10.1001/jamacardio.2018.0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemati S, Ghassemi MM, Ambai V, Isakadze N, Levantsevych O, Shah A, Clifford GD. Monitoring and detecting atrial fibrillation using wearable technology. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:3394–3397. doi: 10.1109/EMBC.2016.7591456 [DOI] [PubMed] [Google Scholar]
- 21.Qardiocore. https://www.getqardio.com/qardiocore-wearable-ecg-ekg-monitor-iphone/.AccessedOctober 7, 2019.
- 22.Gillis AM, Morck M. Atrial fibrillation after DDDR pacemaker implantation. J Cardiovasc Electrophysiol. 2002;13:542–547. doi: 10.1046/j.1540-8167.2002.00542.x [DOI] [PubMed] [Google Scholar]
- 23.Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, Cook J, Paraschos A, Love J, Radoslovich G, et al. ; MOST Investigators. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107:1614–1619. doi: 10.1161/01.CIR.0000057981.70380.45 [DOI] [PubMed] [Google Scholar]
- 24.Tse HF, Lau CP. Prevalence and clinical implications of atrial fibrillation episodes detected by pacemaker in patients with sick sinus syndrome. Heart. 2005;91:362–364. doi: 10.1136/hrt.2003.027219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mittal S, Stein K, Gilliam FR 3rd, Kraus SM, Meyer TE, Christman SA. Frequency, duration, and predictors of newly-diagnosed atrial fibrillation following dual-chamber pacemaker implantation in patients without a previous history of atrial fibrillation. Am J Cardiol. 2008;102:450–453. doi: 10.1016/j.amjcard.2008.03.080 [DOI] [PubMed] [Google Scholar]
- 26.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, Miller C, Qi D, Ziegler PD. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. doi: 10.1161/CIRCEP.109.849638 [DOI] [PubMed] [Google Scholar]
- 27.Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, Zolezzi F, Favale S, Molon G, Ricci R, Biffi M, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20:241–248. doi: 10.1111/j.1540-8167.2008.01320.x [DOI] [PubMed] [Google Scholar]
- 28.Healey JS, Martin JL, Duncan A, Connolly SJ, Ha AH, Morillo CA, Nair GM, Eikelboom J, Divakaramenon S, Dokainish H. Pacemaker-detected atrial fibrillation in patients with pacemakers: prevalence, predictors, and current use of oral anticoagulation. Can J Cardiol. 2013;29:224–228. doi: 10.1016/j.cjca.2012.08.019 [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez M, Keating RJ, Markowitz SM, Liu CF, Thomas G, Ip JE, Lerman BB, Cheung JW. Newly detected atrial high rate episodes predict long-term mortality outcomes in patients with permanent pacemakers. Heart Rhythm. 2014;11:2214–2221. doi: 10.1016/j.hrthm.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 30.Benezet-Mazuecos J, Rubio JM, Cortés M, Iglesias JA, Calle S, de la Vieja JJ, Quiñones MA, Sanchez-Borque P, de la Cruz E, Espejo A, et al. Silent ischaemic brain lesions related to atrial high rate episodes in patients with cardiac implantable electronic devices. Europace. 2015;17:364–369. doi: 10.1093/europace/euu267 [DOI] [PubMed] [Google Scholar]
- 31.Lima C, Martinelli M, Peixoto GL, Siqueira SF, Wajngarten M, Silva RT, Costa R, Filho R, Ramires JA. Silent atrial fibrillation in elderly pacemaker users: a randomized trial using home monitoring. Ann Noninvasive Electrocardiol. 2016;21:246–255. doi: 10.1111/anec.12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman ES, Israel CW, Nair GM, Armaganijan L, Divakaramenon S, Mairesse GH, Brandes A, Crystal E, Costantini O, Sandhu RK, et al. ; ASSERT Steering Committee and Investigators. Positive predictive value of device-detected atrial high-rate episodes at different rates and durations: an analysis from ASSERT. Heart Rhythm. 2012;9:1241–1246. doi: 10.1016/j.hrthm.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 33.Kishore A, Vail A, Majid A, Dawson J, Lees KR, Tyrrell PJ, Smith CJ. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke. 2014;45:520–526. doi: 10.1161/STROKEAHA.113.003433 [DOI] [PubMed] [Google Scholar]
- 34.Sposato LA, Cipriano LE, Saposnik G, Ruíz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14:377–387. doi: 10.1016/S1474-4422(15)70027-X [DOI] [PubMed] [Google Scholar]
- 35.Healey JS, Alings M, Ha A, Leong-Sit P, Birnie DH, de Graaf JJ, Freericks M, Verma A, Wang J, Leong D, et al. ; ASSERT-II Investigators. Subclinical atrial fibrillation in older patients. Circulation. 2017;136:1276–1283. doi: 10.1161/CIRCULATIONAHA.117.028845 [DOI] [PubMed] [Google Scholar]
- 36.Cheung JW, Keating RJ, Stein KM, Markowitz SM, Iwai S, Shah BK, Lerman BB, Mittal S. Newly detected atrial fibrillation following dual chamber pacemaker implantation. J Cardiovasc Electrophysiol. 2006;17:1323–1328. doi: 10.1111/j.1540-8167.2006.00648.x [DOI] [PubMed] [Google Scholar]
- 37.Kim BS, Chun KJ, Hwang Jk, Park S-J, Park K-M, Kim JS, On YK. Predictors and long-term clinical outcomes of newly developed atrial fibrillation in patients with cardiac implantable electronic devices. Medicine (Baltimore). 2016;95:e4181. doi: 10.1097/MD.0000000000004181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belkin MN, Soria CE, Waldo AL, Borleffs CJW, Hayes DL, Tung R, Singh JP, Upadhyay GA. Incidence and clinical significance of new-onset device-detected atrial tachyarrhythmia: a meta-analysis. Circ Arrhythm Electrophysiol. 2018;11:e005393. doi: 10.1161/CIRCEP.117.005393 [DOI] [PubMed] [Google Scholar]
- 39.Pedersen KB, Madsen C, Sandgaard NCF, Diederichsen ACP, Bak S, Brandes A. Subclinical atrial fibrillation in patients with recent transient ischemic attack. J Cardiovasc Electrophysiol. 2018;29:707–714. doi: 10.1111/jce.13470 [DOI] [PubMed] [Google Scholar]
- 40.Israel C, Kitsiou A, Kalyani M, Deelawar S, Ejangue LE, Rogalewski A, Hagemeister C, Minnerup J, Schäbitz WR. Detection of atrial fibrillation in patients with embolic stroke of undetermined source by prolonged monitoring with implantable loop recorders. Thromb Haemost. 2017;117:1962–1969. doi: 10.1160/TH17-02-0072 [DOI] [PubMed] [Google Scholar]
- 41.Lopes RD, Alings M, Connolly SJ, Beresh H, Granger CB, Mazuecos JB, Boriani G, Nielsen JC, Conen D, Hohnloser SH, et al. Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am Heart J. 2017;189:137–145. doi: 10.1016/j.ahj.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 42.Mahajan R, Perera T, Elliott AD, Twomey DJ, Kumar S, Munwar DA, Khokhar KB, Thiyagarajah A, Middeldorp ME, Nalliah CJ, et al. Sub-clinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018;39:1407–1415. doi: 10.1093/eurheartj/ehx731 [DOI] [PubMed] [Google Scholar]
- 43.Van Gelder IC, Healey JS, Crijns HJGM, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau CP, Morillo CA, Hobbelt AH, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344. doi: 10.1093/eurheartj/ehx042 [DOI] [PubMed] [Google Scholar]
- 44.Swiryn S, Orlov MV, Benditt DG, DiMarco JP, Lloyd-Jones DM, Karst E, Qu F, Slawsky MT, Turkel M, Waldo AL; RATE Registry Investigators. Clinical implications of brief device-detected atrial tachyarrhythmias in a cardiac rhythm management device population: results from the Registry of Atrial Tachycardia and Atrial Fibrillation Episodes. Circulation. 2016;134:1130–1140. doi: 10.1161/CIRCULATIONAHA.115.020252 [DOI] [PubMed] [Google Scholar]
- 45.Steinberg BA, Piccini JP. When low-risk atrial fibrillation is not so low risk: beast of burden. JAMA Cardiol. 2018;3:558–560. doi: 10.1001/jamacardio.2018.1205 [DOI] [PubMed] [Google Scholar]
- 46.Go AS, Reynolds K, Yang J, Gupta N, Lenane J, Sung SH, Harrison TN, Liu TI, Solomon MD. Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: the KP-RHYTHM Study. JAMA Cardiol. 2018;3:601–608. doi: 10.1001/jamacardio.2018.1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capucci A, Santini M, Padeletti L, Gulizia M, Botto G, Boriani G, Ricci R, Favale S, Zolezzi F, Di Belardino N, et al. ; Italian AT500 Registry Investigators. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46:1913–1920. doi: 10.1016/j.jacc.2005.07.044 [DOI] [PubMed] [Google Scholar]
- 48.Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, Lau CP, Van Gelder IC, Hohnloser SH, Carlson M, et al. ; ASSERT Investigators. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825 [DOI] [PubMed] [Google Scholar]
- 49.Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, Keung EK, Singer DE. Atrial fibrillation burden and short-term risk of stroke: casecrossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015;8:1040–1047. doi: 10.1161/CIRCEP.114.003057 [DOI] [PubMed] [Google Scholar]
- 50.Bernstein RA, Kamel H, Granger CB, Kowal RC, Ziegler PD, Schwamm LH. Stroke of Known Cause and Underlying Atrial Fibrillation (STROKE-AF) randomized trial: design and rationale. Am Heart J. 2017;190:19–24. doi: 10.1016/j.ahj.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 51.Gladstone DJ, Dorian P, Spring M, Panzov V, Mamdani M, Healey JS, Thorpe KE; EMBRACE Steering Committee and Investigators. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke. 2015;46:936–941. doi: 10.1161/STROKEAHA.115.008714 [DOI] [PubMed] [Google Scholar]
- 52.Thijs VN, Brachmann J, Morillo CA, Passman RS, Sanna T, Bernstein RA, Diener HC, Di Lazzaro V, Rymer MM, Hogge L, et al. Predictors for atrial fibrillation detection after cryptogenic stroke: results from CRYSTAL AF. Neurology. 2016;86:261–269. doi: 10.1212/WNL.0000000000002282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwong C, Ling AY, Crawford MH, Zhao SX, Shah NH. A clinical score for predicting atrial fibrillation in patients with cryptogenic stroke or transient ischemic attack. Cardiology. 2017;138:133–140. doi: 10.1159/000476030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong JA, Conen D, Van Gelder IC, McIntyre WF, Crijns HJ, Wang J, Gold MR, Hohnloser SH, Lau CP, Capucci A, et al. Progression of device-detected subclinical atrial fibrillation and the risk of heart failure. J Am Coll Cardiol. 2018;71:2603–2611. doi: 10.1016/j.jacc.2018.03.519 [DOI] [PubMed] [Google Scholar]
- 55.Boriani G, Glotzer TV, Ziegler PD, De Melis M, Mangoni di S Stefano L, Sepsi M, Landolina M, Lunati M, Lewalter T, Camm AJ. Detection of new atrial fibrillation in patients with cardiac implanted electronic devices and factors associated with transition to higher device-detected atrial fibrillation burden. Heart Rhythm. 2018;15:376–383. doi: 10.1016/j.hrthm.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 56.Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, Gasparini M, Lewalter T, Camm JA, Singer DE. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014;35:508–516. doi: 10.1093/eurheartj/eht491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perino AC, Fan J, Askari M, Heidenreich PA, Keung E, Raitt MH, Piccini JP, Ziegler PD, Turakhia MP. Practice variation in anticoagulation prescription and outcomes after device-detected atrial fibrillation. Circulation. 2019;139:2502–2512. doi: 10.1161/CIRCULATIONAHA.118.038988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirchhof P, Blank BF, Calvert M, Camm AJ, Chlouverakis G, Diener HC, Goette A, Huening A, Lip GYH, Simantirakis E, et al. Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH-AFNET 6) trial. Am Heart J. 2017;190:12–18. doi: 10.1016/j.ahj.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Passman R, Leong-Sit P, Andrei AC, Huskin A, Tomson TT, Bernstein R, Ellis E, Waks JW, Zimetbaum P. Targeted anticoagulation for atrial fibrillation guided by continuous rhythm assessment with an insertable cardiac monitor: the Rhythm Evaluation for Anticoagulation With Continuous Monitoring (REACT.COM) Pilot Study. J Cardiovasc Electrophysiol. 2016;27:264–270. doi: 10.1111/jce.12864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, Lip GY, Ip J, Holcomb R, Akar JG, Halperin JL; IMPACT Investigators. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J. 2015;36:1660–1668. doi: 10.1093/eurheartj/ehv115 [DOI] [PubMed] [Google Scholar]


