Abstract
Peroxisome proliferator-activated receptor γ (PPARγ) is a nuclear receptor implicated in adipocyte differentiation and insulin sensitivity. We investigated whether PPARγ expression is dependent on the activity of adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1 (ADD-1/SREBP-1), another transcription factor associated with both adipocyte differentiation and cholesterol homeostasis. Ectopic expression of ADD-1/SREBP-1 in 3T3-L1 and HepG2 cells induced endogenous PPARγ mRNA levels. The related transcription factor SREBP-2 likewise induced PPARγ expression. In addition, cholesterol depletion, a condition known to result in proteolytic activation of transcription factors of the SREBP family, induced PPARγ expression and improved PPRE-driven transcription. The effect of the SREBPs on PPARγ expression was mediated through the PPARγ1 and -3 promoters. Both promoters contain a consensus E-box motif that mediates the regulation of the PPARγ gene by ADD-1/SREBP-1 and SREBP-2. These results suggest that PPARγ expression can be controlled by the SREBP family of transcription factors and demonstrate new interactions between transcription factors that can regulate different pathways of lipid metabolism.
Several transcription factors orchestrate the adipocyte differentiation process (reviewed in references 9, 13, and 43). These include the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) (46, 47), the family of CCAAT enhancer binding proteins (C/EBP) (8, 15, 16, 53–55), and the basic helix-loop-helix leucine zipper transcription factor adipocyte differentiation and determination factor 1 (ADD-1) (21, 48), which was independently cloned as the sterol regulatory element binding protein 1 (SREBP-1), based on its role in cholesterol homeostasis (56). Current data suggest that C/EBPβ and -δ induce the expression of PPARγ (33, 54), which then triggers the adipogenic program. Terminal differentiation appears to require the concerted action of PPARγ, C/EBPα, and ADD-1/SREBP-1 (21, 48). Several arguments support the important role of PPARγ in adipocyte differentiation. First, ectopic expression of PPARγ is sufficient to induce adipocyte conversion of fibroblasts (46, 47). In addition, PPARγ together with C/EBPα can induce transdifferentiation of myoblasts into adipocytes (19). Second, the description of functional peroxisome proliferator-responsive elements (PPREs) in the regulatory sequences of several of the genes which are induced during adipocyte differentiation, such as the genes coding for adipocyte fatty acid binding protein aP2 (46), phosphoenolpyruvate carboxykinase (45), acyl coenzyme A (CoA) synthetase (36, 37), and lipoprotein lipase (35), is consistent with the crucial role attributed to PPARγ in lipid metabolism. Finally, prostaglandin J2 derivatives, certain nonsteroidal anti-inflammatory drugs, and antidiabetic thiazolidinediones, which have been identified as natural and synthetic PPARγ ligands, respectively (5, 14, 23–25, 51), all induce or enhance adipocyte differentiation (2, 3, 7, 14, 23, 24, 29, 47).
The identification of thiazolidinediones as PPARγ ligands together with the central role which adipose tissue plays in the pathogenesis of important metabolic disorders, such as obesity and non-insulin-dependent diabetes mellitus, has generated a major drive to understand the regulation of PPARγ gene expression. Since ADD-1/SREBP-1 and PPARγ both are important during adipocyte differentiation, we analyzed PPARγ expression in cells ectopically expressing ADD-1/SREBP-1. Increased levels of PPARγ mRNA and protein were found under these conditions. SREBP-2 had similar effects on PPARγ expression. It was furthermore shown that PPARγ expression was influenced by cellular cholesterol levels in cells of both hepatic and adipocyte origin, an effect mediated by the SREBP family of transcription factors. The control of PPARγ expression by the SREBP family of transcription factors is mediated through two sequence elements. First, there is a functional E-box in the PPARγ1 promoter. In addition, we also describe a functional E-box element located upstream of the exon A2 of the human PPARγ gene, in the recently described PPARγ3 promoter (12). These observations suggest that regulatory interactions between the SREBPs and PPARγ can coordinate cholesterol and fatty acid metabolism.
MATERIALS AND METHODS
Materials and oligonucleotides.
The oligonucleotides used for various experiments in this report are listed in Table 1. BRL 49,653 and simvastatin were kind gifts of A. Nazdan of Ligand Pharmaceuticals and S. Wright from Merck Research Laboratories, respectively. All other chemicals, unless stated otherwise, were purchased from Sigma (St. Louis, Mo.).
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′→3′) |
|---|---|
| LF-60 | cgttaaaggctgactctcgtttga |
| LF-63 | gtcacatgaatgacgatacctc |
| LF-68 | tcatgtaggtaagactgtgtagaa |
| LF-102 | ctagcgtcattcatgtgacataaa |
| LF-106 | ctagcgtcattcatgcatcataaa |
| LF-107 | ccatcttttatgatgcatgaatga |
| LF-141 | caggaggatcacttgagcccaggag |
| LF-143 | caggaggatgcattgagcccagga |
| LF-144 | tcctgggctcaatgcatcctcctg |
| LF-145 | tggcttgcccttcacacggcgatc |
| LF-146 | ggtcaagcgattctactgcctcag |
| SRE | gatcctgatcacgtgatcgaggag |
Cell culture and retroviral infections.
Standard cell culture conditions were used to maintain 3T3-L1 (obtained from American Type Culture Collection [ATCC]), HeLa (ATCC), RK-13 (ATCC), CCL-39 (a kind gift from Claude Sardet), and HepG2 cells (ATCC). BRL 49,653 and simvastatin were dissolved in dimethyl sulfoxide, and cholesterol and 25-hydroxycholesterol, linoleic acid, and linolenic acid were dissolved in ethanol. Prior to addition to cells, the fatty acids were complexed to bovine serum albumin (37). Control cells received vehicle only. Retroviral infection of 3T3-L1 cells was performed as described previously (21). Briefly, the BOSC23 cell line was transiently transfected with the recombinant retroviral vectors pBabe, ADD-1 403, and ADD-1 (21) by the calcium phosphate method. Viral supernatants were collected 48 h after transfection and titrated. 3T3-L1 cells were incubated with retrovirus for 5 h in the presence of 4 μg of Polybrene per ml. Cells were then subcultured (1:3) for 2 days after infection in medium containing puromycin (2 μg/ml) for selection. Differentiation of 3T3-L1 cells was performed as described previously (26).
RNA isolation and RNase protection assays.
Total cellular RNA was prepared as described previously (32). Human and mouse PPARγ (hPPARγ and mPPARγ, respectively) mRNA levels were determined by RNase protection assay with the templates previously described (12).
Western blot analysis of PPARγ.
Protein extraction, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and electrotransfer were performed as described previously (11). The membranes were blocked overnight in blocking buffer (20 mM Tris, 100 mM NaCl, 1% Tween 20, 10% skim milk). Filters were first incubated for 4 h at 21°C with either a rabbit immunoglobulin G (IgG) anti-mPPARγ (10 mg/ml) (11) or a rabbit IgG anti-mSREBP-1 antibody (Santa Cruz, Biotechnology, Calif.) and then for 1 h at 21°C with a goat anti-rabbit IgG (whole molecule) peroxidase conjugate diluted at 1/5,000. The complex was visualized with 4-chloro-1-naphthol as a reagent.
Analysis of promoter activity and transactivation assays.
The PAC clone P-8856 (11), containing the full-length PPARγ gene, was sequenced with the oligonucleotides LF-60 and LF-63 pointing upstream of exon A2. An 800-bp fragment of the PAC clone 8856 was isolated by PCR with the amplimers LF-60 (binding to the antisense strand in exon A2) and LF-68 (binding sense at position −800 of the PPARγ3 promoter). This PCR fragment was sequenced, inserted into the EcoRV site of pBluescript SK(+) (Stratagene, La Jolla, Calif.), and, after SpeI and KpnI restriction, subcloned into pGL3 (Promega, Madison, Wis.), creating the reporter vector pGL3γ3p800. For the construction of the reporter vector pGL3γ1p2000, the previously described pGL3γ1p3000 (11) was shortened by 1 kb at its 5′ end by digestion with KpnI and PmlI. The reporter pGL3γ2p1000 was described previously (11). Site-directed mutagenesis of the E-box in the PPARγ3 promoter and the E-box in the PPARγ1 promoter was performed by splicing overlapping ends PCR (18), with the oligonucleotide pairs LF-106/LF-60 and LF-107/LF-68, to generate the plasmid pGL3γ3p800-E-boxmut, and the oligonucleotide pairs LF-145/LF-143 and LF-146/LF-144, to generate the plasmid pGL3γ1p2000-E-boxmut. This changed the three bases underlined in the sequence of the γ3 promoter 5′-ATTCATGTGACAT-3′ to 5′-ATTCATGCATCAT-3′ and the bases underlined in the sequence of the γ1 promoter 5′AGGATCACTTGAGCCC3′ to 5′AGGATGCATTGAGCCC3′. The J3-TK-Luc (49) and ACO-TK-Luc (30) luciferase reporter vectors and the expression vectors encoding for ADD-1, a dominant-negative form of ADD-1, and SREBP-1a (48, 56) were described before. Transfections, luciferase, and β-galactosidase assays were performed as described previously (37). To analyze the effect of cholesterol depletion in transfection experiments, the cells were divided into two pools after transfection. Half of the transfected cells were incubated with delipidated medium, whereas the other half of the cells were incubated with the same medium supplemented with a mixture of cholesterol (10 μM) and 25-hydroxycholesterol (1 μM).
Electrophoretic mobility shift assays (EMSAs) and oligonucleotide sequences.
SREBP-1a protein was produced in a baculovirus system, and ADD-1 was produced by in vitro transcription. The quality of the proteins was verified by SDS-PAGE. Proteins were incubated for 15 min on ice in a total volume of 20 μl with 2.5 μg of poly(dI-dC), 1 μg of herring sperm DNA, and 1 ng of T4-polynucleotide kinase end-labelled double-stranded oligonucleotide corresponding either to the PPARγ1-E-box (LF-141) or the PPARγ3-E-box (LF-102) in binding buffer (10 mM Tris-HCl [pH 7.9], 40 mM KCl, 10% glycerol, 0.05% Nonidet P-40, 1 mM dithiothreitol). For competition experiments, increasing amounts of cold double-stranded oligonucleotides (10-, 50-, and 100-fold molar excess) corresponding to the PPARγ1-E-box, the PPARγ3-E-box, the consensus 3-hydroxy-3-methylglutaryl (HMG)-CoA synthase sterol response element (SRE) site (42), or the mutated PPARγ1-E-box (LF-143) and PPARγ3-E-box (LF-106) were included just before addition of labelled oligonucleotide. DNA-protein complexes were separated by electrophoresis on a 4% polyacrylamide gel in 0.25 × Tris-borate-EDTA buffer at 4°C (17).
RESULTS
Ectopic expression of ADD-1/SREBP-1 or SREBP-2 induces PPARγ mRNA expression.
In view of the adipogenic effects of ADD-1/SREBP-1, we investigated a potential role of ADD-1/SREBP-1 in the expression of the PPARγ gene. For that purpose, HepG2 cells were electroporated with vectors expressing either SREBP-1a (56), ADD-1 (48), or SREBP-2 (20). RNA was extracted 48 h after transfection and analyzed by RNase protection assay for the presence of the PPARγ1 and PPARγ3 mRNAs. PPARγ1 mRNA levels were, as expected, the most abundant and were eight-, six-, and eightfold higher in the cells transfected with SREBP-1a, ADD-1, or SREBP-2, respectively (Fig. 1A, lanes 2 to 4). The same degree of induction was observed when the PPARγ3 mRNA levels were quantified. No induction of either PPARγ1 or -3 mRNAs was detected in cells transfected with an empty expression vector (Fig. 1A, lane 1). When a separate probe, designed to specifically detect PPARγ2 mRNA, was used in RNase protection assays, no changes in its mRNA levels were detected after transfection with either ADD-1/SREBP-1 or SREBP-2 (data not shown).
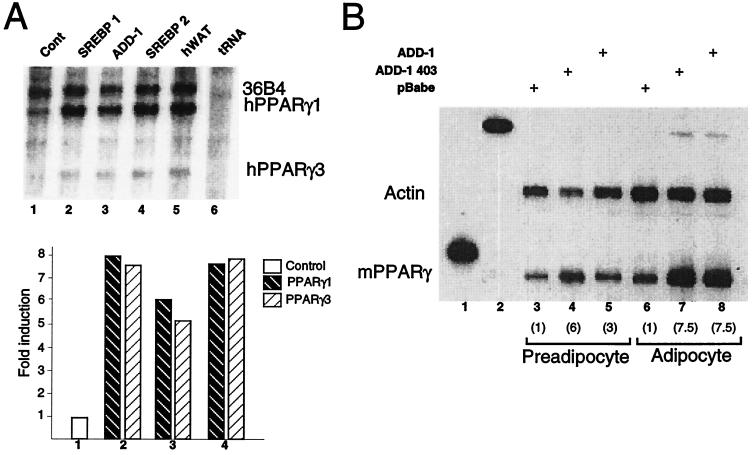
FIG. 1.
Increased expression of ADD-1, SREBP-1, or SREBP-2 induces PPARγ mRNA expression. (A) RNase protection assay of total RNA from HepG2 cells transfected with either an empty vector (control [cont]; lane 1), an SREBP-1a expression vector (lane 2), an ADD-1 expression vector (lane 3), or an SREBP-2 expression vector (lane 4) or from human white adipose tissue (hWAT [as a positive control]; lane 5). Protected fragments corresponding to PPARγ1 and 3 mRNAs are indicated. Results were normalized with a 36B4 probe. Densitometric quantification of the results is shown. (B) RNase protection assay of total RNA from 3T3-L1 preadipocytes (lanes 3 to 5) or differentiated 3T3-L1 adipocytes (lanes 6 to 8) infected with an empty retroviral vector (lanes 3 and 6) or a retrovirus encoding ADD1-403 (lanes 4 and 7) or the full-length form of ADD-1 (lanes 5 and 8) as indicated. Lanes 1 and 2 show the undigested probes used to analyze PPARγ and actin mRNA. An actin probe was used for normalization in this RNase protection assay. The fold induction of PPARγ mRNA as determined by densitometric quantification of the results is shown in parentheses underneath the number of the lane.
To study the effects of ADD-1 on PPARγ expression in more detail and in the context of adipocyte differentiation, 3T3-L1 cells were infected with either an empty retroviral vector, pBabe, or the same vector encoding full-length ADD-1 or the superactive ADD-1 403. Northern blot analysis showed that retroviral infection, by the virus encoding ADD-1, resulted in a twofold higher level of ADD-1 expression (data not shown). Infected cells were then cultured to confluence and consecutively treated with differentiation medium. Total RNA was isolated at confluence (preadipocytes) and at day 6 after confluence (adipocytes). The RNase protection assay indicated that the expression of PPARγ mRNA was induced in both 3T3-L1 preadipocytes (threefold) and adipocytes (sevenfold) which ectopically express ADD-1 relative to cells which express the empty pBabe vector (Fig. 1B). Interestingly, a truncated form, ADD-1 403, equivalent to the proteolytically activated protein, which lacks the membrane-anchoring domain, was twofold more active in inducing PPARγ expression in undifferentiated preadipocytes (Fig. 1B). These results suggest that the adipogenic effects of ADD-1/SREBP-1 previously demonstrated are at least in part due to an up-regulation of the PPARγ gene expression.
PPARγ protein expression is induced in cells grown under conditions which stimulate the activation of the SREBPs.
In order to evaluate the possibility that PPARγ was induced under more physiological conditions, associated with activation of the activation of SREBPs, we quantitated the relative expression of PPARγ protein by Western blot analysis in undifferentiated 3T3-L1 cells (Fig. 2A) and HepG2 cells (Fig. 2B) grown in medium containing different cholesterol concentrations (50). In both cell lines, PPARγ protein was induced at least ninefold upon cholesterol depletion during 24 h, a condition known to enhance the production of mature and active ADD-1/SREBP-1 (31, 50) (Fig. 2C). Interestingly, PPARγ protein levels were decreased acutely by readdition of cholesterol (10 μM) and 25-hydroxycholesterol (1 μM) to the culture medium for 6 h (Fig. 2A and B, lane 3).
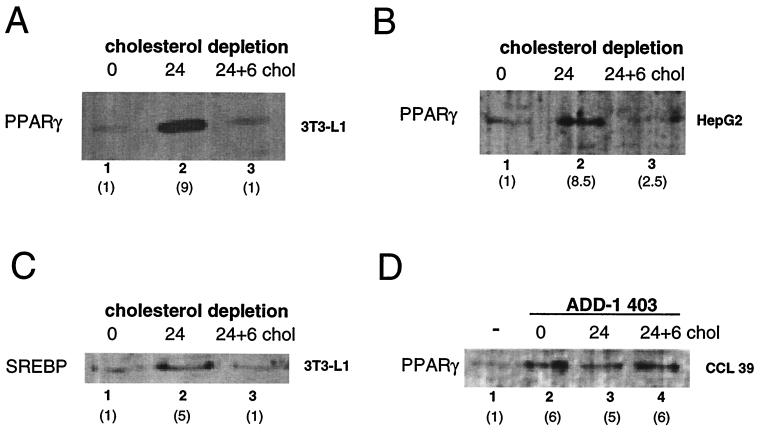
FIG. 2.
Cholesterol depletion induces PPARγ expression. (A) Western blot analysis of nuclear extracts of 3T3-L1 preadipocytes with an anti-PPARγ antibody. Preconfluent cells (lane 1) were incubated for 24 h (lane 2) in cholesterol-depleted medium. After 24 h of incubation in cholesterol-depleted medium, a mixture containing 10 μM cholesterol and 1 μM 25-OH-cholesterol was added to the medium for 6 additional h (24 + 6 chol) (lane 3). The fold induction of PPARγ or SREBP as determined by densitometric quantification of the results is shown in parentheses underneath the number of the lane. (B) Similar Western blot experiments as described for panel A, but with HepG2 nuclear extracts instead of 3T3-L1 nuclear extracts. (C) Expression of SREBP-1 protein as detected after Western blot analysis of the 3T3-L1 nuclear extracts used in panel A. Western blotting was performed with an anti-SREBP-1 antiserum. (D) Western blot analysis of nuclear extracts of CCL-39 cells transfected with the constitutively active form of ADD-1, ADD-1 403. Cells were exposed to the same cholesterol depletion as specified for panel A.
In order to elucidate if the observed effects of cholesterol depletion on PPARγ expression were mediated by ADD-1/SREBP-1, the same experiment was performed with the hamster lung cell line CCL-39 transfected with the constitutively active form of ADD-1, ADD-1 403. As expected, PPARγ expression was induced sixfold in the cells transfected with ADD-1 403 (Fig. 2D, lane 2). No further changes in the expression of PPARγ could be observed when cells were exposed to cholesterol-depleted medium (Fig. 2D, lane 3). As expected, in view of the cotransfection of ADD-1 403, no further reduction in PPARγ levels was observed upon readdition of cholesterol to the medium. PPARγ expression hence seems subject to a tight and fast control by alterations in intracellular cholesterol levels, and this effect is mediated by the SREBP family of transcription factors.
PPARγ protein expression is induced in cells treated with HMG-CoA reductase inhibitors and is not affected by fatty acids.
Treatment with HMG-CoA reductase inhibitors, which block the enzyme responsible for the rate-limiting step of cholesterol synthesis, provide another way to modify cellular cholesterol levels. Upon treatment with compounds such as compactin (mevastatin) or simvastatin, cells will become cholesterol depleted and the production of the active forms of ADD-1/SREBP-1 will increase (31, 40). Therefore, the expression of PPARγ protein was evaluated in HepG2 cells before and after treatment with the potent HMG-CoA reductase inhibitor simvastatin. Treatment of the cells with simvastatin (5 × 10−7 M) for 6 h resulted in a robust and fast induction of PPARγ protein levels (eightfold), which was sustained 12 h after addition (Fig. 3A), further supporting the notion that cellular cholesterol levels influence the expression of PPARγ.
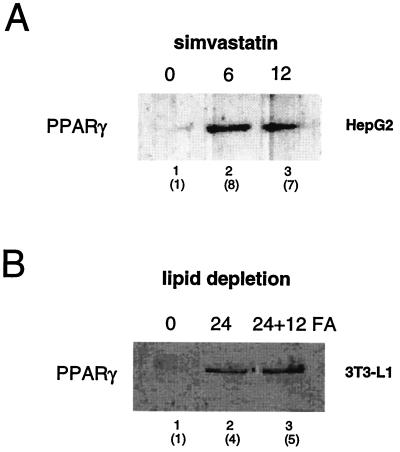
FIG. 3.
Inhibition of de novo cholesterol synthesis by statins induces PPARγ expression. (A) Analysis of the PPARγ protein by Western blotting of cell extracts from HepG2 cells incubated with medium supplemented with simvastatin (0.5 μM) for either 6 or 12 h. Fold induction of PPARγ protein levels is shown in parentheses. (B) Quantification of PPARγ protein levels in nuclear extracts from 3T3-L1 preadipocytes. Cells were lipid starved as in Fig. 2, and a mixture of linoleic acid (150 μM) and linolenic acid (150 μM) was added to the medium for a period of 12 h. An anti-PPARγ specific antibody was used for Western blot analysis. Fold induction of PPARγ protein levels is shown in parentheses.
Since polyunsaturated fatty acids have been reported to decrease the expression of promoters under the control of SREBP (44, 52), we analyzed whether the induction of PPARγ protein expression upon cholesterol depletion was affected by the presence of fatty acids in the culture medium. As expected, when 3T3-L1 cells were incubated under lipid-free conditions, a significant induction of the levels of PPARγ protein was observed (Fig. 3B, lane 2). Surprisingly, and in contrast to previous reports in the literature (44, 52), PPARγ protein levels were not down-regulated when a mixture of linoleic and linolenic acids (150 μM each) was added to the lipid-depleted medium (Fig. 3B, lane 3).
Regulatory effect of ADD-1/SREBP-1 and SREBP-2 on the hPPARγ1 and -3 promoters.
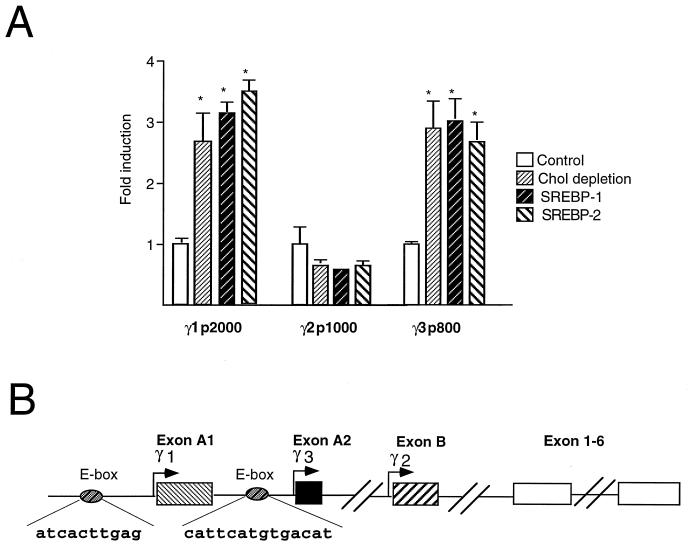
To investigate the possibility of a direct transcriptional effect of the SREBPs on PPARγ expression, we analyzed the 5′ upstream regulatory sequences of the human PPARγ gene which we have previously determined (11). Therefore, we transfected CCL-39 cells with the pGL3γ1p2000, pGL3γ2p1000, and pGL3γ3p800 reporter plasmids (11), which contain, respectively 2 kb, 1 kb, and 800 bp of the human PPARγ1, -2, and -3 promoters. The activity of the pGL3γ1p2000 and pGL3γ3p800 reporter constructs was induced at least threefold when the ADD-1/SREBP-1 expression vector was cotransfected, suggesting that the increase in PPARγ mRNA levels mentioned above was mediated by an effect on the proximal PPARγ1 and -3 promoters (Fig. 4A). Interestingly, whereas the activity of the pGL3γ1p2000 plasmid was significantly induced by ADD-1/SREBP-1, no such induction was observed with pGL3γ1p3000, which contains an additional 1,000 bp at it’s 5′ end, which suggests the presence of an inhibitory element in this region (data not shown). The activities of the PPARγ1 and -3 promoters were induced to a similar extent (at least threefold) when an expression vector for SREBP-2 was cotransfected instead of ADD-1/SREBP-1 or when cells were exposed to cholesterol-depleted medium (Fig. 4A). Consistent with our mRNA data, no effect of either cotransfection of ADD-1/SREBP-1 or SREBP-2 or cholesterol depletion could be observed on PPARγ2 promoter activity.
FIG. 4.
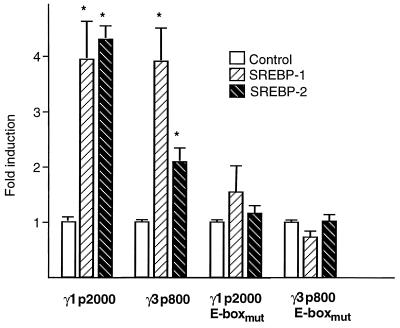
ADD-1/SREBP-1 and SREBP-2 transactivate the PPARγ1 and -3 promoters. (A) ADD-1/SREBP-1 and SREBP-2 transactivate the PPARγ1 and -3 promoters, but not the PPARγ2 promoter. Relative luciferase activity as determined after transfection of CCL-39 cells with the reporter constructs pGL3γ1p2000, pGL3γ2p1000, and pGL3γ3p800. Cells were either cotransfected with an empty expression vector (control) and exposed to medium containing cholesterol (10 μM) and 25-hydroxycholesterol (1 μM), cotransfected with an empty expression vector and maintained in cholesterol-depleted medium (Chol depletion), or cotransfected with an expression plasmid for SREBP-1 (SREBP-1) or SREBP-2 (SREBP-2) in medium containing cholesterol (10 μm) and 25-hydroxycholesterol (1 μM). Results are expressed as fold induction and represent the mean ± standard deviation of three independent experiments. Statistically significant differences by Student’s t test (P < 0.05) are indicated. (B) A scheme of the genomic structure of the 5′ end of the human PPARγ gene and of the approximate location of the response elements. Exons 1 to 6 are shared by all three subtypes. PPARγ1 contains in addition the untranslated exons A1 and A2; PPARγ2 contains exon B, which is translated; and PPARγ3 contains only the untranslated exon A2. The respective hPPARγ promoters are indicated by arrows. The approximate locations of the E-boxes are indicated.
ADD-1/SREBP-1 controls the hPPARγ expression through E-box motifs in the γ1 and γ3 promoters.
In order to investigate whether the induction of PPARγ1 and -3 expression was the consequence of direct binding of the SREBPs to the PPARγ1 and -3 promoters, a detailed computer-assisted sequence homology analysis was performed. Potential binding sites for the SREBP transcription factor family, corresponding to putative E-box motifs, were detected in both the PPARγ1 and PPARγ3 promoters (see Fig. 4B for the scheme). In order to demonstrate direct binding of ADD-1/SREBP-1 to the putative PPARγ1-E-box (at position −1535 from the transcription initiation site 5′ of the exon A1) and the PPARγ3-E-box (at position −341 from the transcription initiation site 5′ of the A2 exon), we used double-stranded oligonucleotides corresponding to the PPARγ1-E-box (LF-141) and PPARγ3-E-box (LF-102) as probes in EMSAs. Baculovirus-produced and partially purified SREBP-1a, a different splice variant of the ADD-1/SREBP-1 gene, is capable of binding to both sites. Competition gel shift assays using increasing amounts of cold double-stranded oligonucleotides containing either the sites mentioned above (PPARγ1-E-box [Fig. 5A] or PPARγ3-E-box [Fig. 5C]), the consensus SRE of the HMG-CoA synthase gene (42), or the mutated PPARγ1-E-boxmut (from AGGATCACTTGAGCCC to AGGATGCATTGAGCCC) and PPARγ3-E-boxmut (from ATTCATGTGACAT to ATTCATGCATCAT), were performed next in order to demonstrate the specificity of the binding (Fig. 5A and C). Binding of SREBP-1a to the PPARγ1-E-box is competed by both the cold PPARγ1-E-box (Fig. 5A, lanes 2 to 3) and by the consensus SRE oligonucleotides (Fig. 5A, lanes 5 and 6), whereas the mutated PPARγ1-E-boxmut oligonucleotide was unable to compete with the PPARγ1-E-box for binding of SREBP-1a (Fig. 5A, lanes 8 and 9). Similarly, cold PPARγ3-E-box and the consensus SRE oligonucleotides were able to compete for the binding of SREBP-1a to the PPARγ3-E-box probe (Fig. 5C, lanes 2 to 4 and 5 to 7), whereas the mutated PPARγ3-E-boxmut was not (Fig. 5C, lanes 8 to 10). Similar EMSA results were obtained when SREBP-2 was used (data not shown).
FIG. 5.
Binding of ADD-1/SREBP-1 to the PPARγ1 and -3 promoters. (A) EMSA with a partially purified baculovirus-produced SREBP-1a protein, a different splice variant of ADD-1, and a labelled double-stranded oligonucleotide representing the PPARγ1-E-box. Competition experiments were performed with cold oligonucleotides representing either the PPARγ1-E-box (γ1-E-box; LF-141), the consensus HMG-CoA synthase SRE site (SREcons) (42), or the mutated PPARγ1-E-box (γ1-E-boxmut; LF-143) at either 10-, 50-, or 100-fold molar excess. (B) Sequence of the wild-type and mutated PPARγ1-E-box. The consensus E-box is indicated in bold characters. The three bases which are mutated are indicated underneath the original bases. (C) EMSA performed under exactly the same conditions as described for panel A with the PPARγ3-E-box (LF-102) as a labelled double-stranded oligonucleotide and the mutated PPARγ3-E-box (γ3 E-boxmut; LF-106) as a competitor instead of the PPARγ1-E-box and γ1-E-boxmut, respectively. (D) Sequence of the wild-type and mutated PPARγ3 E-boxes. The consensus E-box is underlined, whereas a potential SRE is indicated in boldface. The three bases mutated are indicated underneath the original bases.
To unequivocally demonstrate that it is through binding to the PPARγ1-E-box and PPARγ3-E-box that ADD-1/SREBP-1 and SREBP-2 stimulate the activity of the hPPARγ1 and -3 promoters, we substituted, respectively, three bases in the PPARγ1-E-box (Fig. 5B) and in the PPARγ3-E-box (Fig. 5D) in the context of the native PPARγ1 and -3 promoters to generate the pGL3γ1p2000-E-boxmut and pGL3γ3p800-E-boxmut reporter plasmids. In contrast to the wild-type reporter vectors (Fig. 6A), cotransfected ADD-1/SREBP-1 or SREBP-2 was unable to stimulate the activity of the mutated pGL3γ1p2000-E-boxmut and pGL3γ3p800-E-boxmut reporter vectors in the CCL-39 lung-derived cell line (Fig. 6B).
FIG. 6.
The PPARγ1-E-box and the PPARγ3-E-box mediate the induction of the PPARγ gene by ADD-1/SREBP-1 and SREBP-2. Relative luciferase activity as determined after transfection of CCL39 cells with the reporter constructs pGL3γ1p2000, pGL3γ1p2000-E-boxmut, pGL3γ3p800, and pGL3γ3p800-E-boxmut. Cells were cotransfected with either an empty expression vector (control) or an expression plasmid for SREBP-1 or SREBP-2. Values are the mean ± standard deviation of three independent experiments. Statistically significant differences (P < 0.05) by Student’s t test are indicated by asterisks.
PPARγ activity is stimulated by activation of ADD-1/SREBP-1.
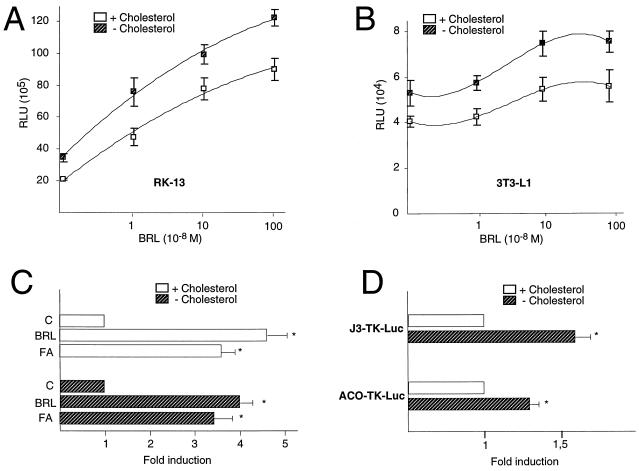
Next we assessed whether the changes in endogenous PPARγ expression mentioned above, induced by modulating the cholesterol concentration in the medium, were associated with altered expression of a PPRE-driven reporter gene. We transfected the J3-TK-Luc luciferase reporter gene, which contains three copies of the PPRE of the apolipoprotein A-II gene J site (49), into rabbit kidney-derived RK-13 cells and maintained half of the cells in cholesterol-depleted medium, whereas the other half were grown in the same medium supplemented with a mixture of cholesterol and 25-hydroxycholesterol. Under both conditions, increasing amounts of the synthetic PPARγ ligand BRL 49,653 were added to the medium, resulting in a dose-dependent activation of promoter activity by BRL 49,653 (Fig. 7A). Under conditions of cholesterol depletion, the reporter gene was, however, activated to a significantly higher level. In fact, the BRL 49,653 dose-response curve was shifted proportionally, keeping the slope constant and suggesting that the observed effect of cholesterol depletion was the result of increased expression of the PPARγ protein. Similar results were obtained when 3T3-L1 (Fig. 7B) and ob-1771 preadipocyte cells were used (data not shown). Consistent with the effect of synthetic PPARγ agonists, addition of the fatty acid linolenic acid (C18:3 at 400 μM) to the medium resulted in a roughly similar fold of induction of the PPRE-driven reporter gene in cholesterol-depleted or cholesterol-containing medium (Fig. 7C). In order to exclude the possibility that the observed effect was specific for the apolipoprotein A-II PPRE, we performed a cotransfection experiment with a different luciferase reporter driven by a single copy of the PPRE from the acyl CoA oxidase (ACO) gene (ACO-TK-Luc [30]). Also, the activity of the ACO-TK-Luc reporter was significantly induced by cholesterol depletion (Fig. 7D).
FIG. 7.
Transactivation of PPARγ is enhanced under conditions of cholesterol depletion. (A and B) Promoter activity of the PPRE-driven luciferase reporter vector J3-TK-Luc after addition of different doses of BRL 49,653 in cells maintained in medium with (dashed squares) or without added cholesterol (10 μM) and 25-hydroxycholesterol (1 μM) (open squares). The results in RK-13 cells (A) and in 3T3-L1 preadipocytes (B) are shown. The results represent the mean ± standard deviation of three independent experiments. Differences between the two conditions were statistically significant. RLU, relative light units. (C) Activity of the J3-TK-Luc reporter gene is stimulated by linolenic acid (C18:3 [400 μM]) in RK-13 cells. Cells were transfected with J3-TK-Luc reporter constructs and maintained for an additional 16 h in medium with (open bars; upper part of the graph) or without added cholesterol (10 μM) and 25-hydroxycholesterol (1 μM) (hatched bars; lower part of the panel). Cells grown under these basal conditions (control cells [C]) were compared with cells treated with BRL 49653 (1 μM [BRL]) or linolenic acid (400 μM [FA]). The results represent the mean ± standard deviation of three independent experiments. The asterisks are indicative of significant differences between the stimulated cells and controls by Student’s t test (P < 0.05). (D) Activity of both the J3-TK-Luc and ACO-TK-Luc reporter genes is stimulated by cholesterol depletion in undifferentiated 3T3-L1 cells. Cells were transfected with J3-TK-Luc or ACO-TK-Luc reporter constructs and incubated under the same conditions than in panel C. The results represent the mean ± standard deviation of three independent experiments. The asterisks are indicative of significant differences by Student’s t test (P < 0.05).
DISCUSSION
PPARγ has been identified as one of the key factors controlling adipocyte differentiation (6, 13). Full differentiation of preadipocytes into adipocytes is regulated by a complex interplay of the C/EBP family, the PPARγ proteins, and ADD-1/SREBP-1 (13). Although it has been reported that C/EBPα, ADD-1/SREBP-1, and PPARγ by themselves can promote adipocyte differentiation, an orchestrated action of all these factors is most likely required to trigger adipocyte differentiation effectively. We demonstrated here that ADD-1/SREBP-1 and SREBP-2 directly control the expression of the human PPARγ gene at a transcriptional level.
ADD-1/SREBP-1 and SREBP-2 have a dual specificity in DNA binding and have been shown to be capable of interacting both with E-box sequences and SREs (21). EMSAs and cotransfection assays demonstrated that the ADD-1/SREBP-1 family of transcription factors can stimulate the expression of the PPARγ1 promoter and the expression of the recently cloned PPARγ3 promoter through binding to E-box motives which are present in both promoters. Previously it has been shown that ectopically expressed ADD-1/SREBP-1 can increase the number of fibroblasts undergoing adipocyte differentiation (reference 21 and unpublished data). Our data suggest that one of the mechanisms by which ADD-1/SREBP-1 might exert its adipogenic action is through the induction of PPARγ expression, which in its turn will induce the expression of downstream adipocyte target genes (Fig. 7). This hence suggests that the ADD-1/SREBP-1 family might function as proximal regulatory factors relative to PPARγ in the induction of adipocyte differentiation. Furthermore, the induction of PPARγ expression and consequent stimulation of lipogenesis could contribute to the massive cholesterol and fatty acid accumulation seen in the livers of animals overexpressing the mature form of SREBP-1a (38) and the more moderate fatty acid accumulation observed in animals overexpressing SREBP-1c (39). Interestingly, the observation that transgenic mice overexpressing SREBP-1c, under the control of the adipose tissue-specific aP2 promoter, are lipodystrophic (41) appears at odds with the general proadipogenic effect of ADD-1/SREBP-1 (21, 38, 39) and suggests that SREBP-1c under certain conditions could negatively influence adipogenesis. The differences between this last study (41) and previous work (21, 38, 39), as well as our present data, are most likely explained by differential effects SREBP-1c might have at different steps during the development of adipose tissue (11a).
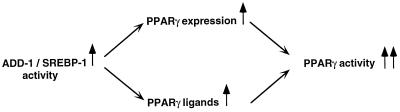
In addition to the transcriptional induction of PPARγ, ADD-1/SREBP-1 induces the expression of several important genes involved in lipogenesis in the adipocyte, such as fatty acid synthase (4, 21, 38), acetyl CoA carboxylase (27, 38), glycerol-3-phosphate acyltransferase (10), and the lipoprotein lipase gene (21, 33a, 38). These ADD-1/SREBP-1 target genes control important steps in fatty acid metabolism, which may lead to the production of natural fatty acid-derived PPAR ligands and activators, suggesting a second more indirect pathway by which ADD-1/SREBP-1 regulates adipocyte differentiation (i.e., by controlling the production of natural activators of PPARγ) (22) (Fig. 8). Besides this important regulatory effect of cholesterol and the ADD-1/SREBP-1 family of transcription factors on fatty acid metabolism, fatty acids were recently also reported to inhibit the maturation of ADD-1/SREBP-1 and decrease the expression of promoters driven by sterol regulatory elements (44, 52). Interestingly, we did not observe an effect of unsaturated fatty acids on the induction of PPARγ expression by cholesterol depletion (Fig. 3B). Furthermore, fatty acids were like thiazolidinediones capable of further inducing expression of a PPRE-driven reporter gene to a similar level in medium with or without sterols (Fig. 7C). All of this suggests that in the case of PPARγ, the inhibitory effects of fatty acids might be insufficient to overcome the potent stimulatory effects of cholesterol depletion on PPARγ expression or, alternatively, that the addition of fatty acids might have an independent and direct stimulatory effect on PPARγ expression. In addition, the absence of PPARγ expression in medium with cholesterol (Fig. 2A and B, lanes 1 and 3) would obscure any further inhibitory effect fatty acids might have on this regulation.
FIG. 8.
Scheme summarizing the different links between ADD-1/SREBP activation and PPARγ activity. The present report provides evidence that PPARγ expression is induced by ADD-1/SREBP, whereas the role of ADD-1/SREBP in inducing PPARγ ligands was described before (22).
Interestingly, the implications of the control of PPARγ expression by the ADD-1/SREBP-1 family of transcription factors and cholesterol may extend beyond the control of adipogenesis and affect total body lipid and glucose metabolism. First, in view of the important insulin sensitization which accompanies PPARγ activation in vivo, the regulation of PPARγ expression and activity by changes in cellular cholesterol concentration suggests that cholesterol homeostasis could have an impact on whole-body glucose homeostasis. Further in vivo studies exploring this issue are definitely needed. Second, the regulation of the expression of PPARγ, a nuclear receptor that is activated by fatty acid metabolites, by the cholesterol-regulated transcription factors of the ADD-1/SREBP-1 family, links transcriptional control by these two important classes of lipids. Changes in intracellular cholesterol levels will, via modulation of ADD-1/SREBP-1 and/or SREBP-2 activity (31, 40, 50), profoundly affect fatty acid and triglyceride metabolism, which is controlled by PPARγ activity. One interesting example of such an interrelationship between cholesterol and fatty acid metabolism, is the observation that powerful HMG-CoA reductase inhibitors, such as simvastatin (28) or atorvastatin (1), not only reduce circulating cholesterol but also reduce triglyceride levels. Whereas the reduction in cholesterol levels could be explained by their inhibitory effect on the key enzyme controlling cholesterol biosynthesis, HMG-CoA reductase, no explanation is available for their beneficial effect on triglyceride levels. Cholesterol depletion induced by these agents, however, leads to proteolytic activation of the ADD-1/SREBP family (31, 40). If this causes an induction in PPARγ levels, as shown here, the increased PPARγ transcriptional activity would be expected to induce the expression of several genes involved in triglyceride clearance (for review, see reference 34). Hence, this mode of interaction between transcription factors controlling different lipid pathways may provide an explanation for both the somewhat unexpected triglyceride-lowering effects that have been observed when these cholesterol-lowering agents have been used in this therapeutic context and for the pronounced beneficial effects of the statins in patients with diabetic hyperlipidemia. This new knowledge could provide a basis for development of agents which have a broader or more specific ability to regulate different aspects of lipid metabolism.
ACKNOWLEDGMENTS
The technical help of D. Cayet and C. Haby and the support of and/or discussion with A. Negro-Villar, R. Heyman, M. Leibowitz, D. Moller, S. Wright, and D. De Chaffoy are kindly acknowledged. We acknowledge the gift of materials from Samuel Wright and Alex Nadzan.
This work was supported by grants from INSERM, Région Nord-Pas-de-Calais, Institut Pasteur, Université de Lille II, ARC (no. 6403), and Ligand pharmaceuticals. J.A. is a research director with the CNRS, and K.S. is a research assistant with INSERM. L.F. was supported by the Janssen Research Foundation.
REFERENCES
- 1.Alaupovic P, Heinonen T, Shurzinske L, Black D M. Effect of a new HMG-CoA reductase inhibitor, atorvastatin, on lipids, apolipoproteins and lipoprotein particles in patients with elevated serum cholesterol and triglyceride levels. Atherosclerosis. 1997;133:123–133. doi: 10.1016/s0021-9150(97)00119-6. [DOI] [PubMed] [Google Scholar]
- 2.Amri E-Z, Bertrand B, Ailhaud G, Grimaldi P. Regulation of adipose cell differentiation. I. Fatty acids are inducers of the aP2 gene expression. J Lipid Res. 1991;32:1449–1456. [PubMed] [Google Scholar]
- 3.Aubert J, Ailhaud G, Negrel R. Evidence for a novel regulatory pathway activated by (carba)prostacyclin in preadipose and adipose cells. FEBS Lett. 1996;397:117–121. doi: 10.1016/s0014-5793(96)01152-0. [DOI] [PubMed] [Google Scholar]
- 4.Bennet M K, Lopez J M, Sanchez H B, Osborne T F. Sterol regulation of fatty acid synthase promoter:coordinate feedback regulation of two major lipid pathways. J Biol Chem. 1995;270:25578–25583. doi: 10.1074/jbc.270.43.25578. [DOI] [PubMed] [Google Scholar]
- 5.Berger J, Bailey P, Biswas C, Cullinan C A, Doebber T W, Hayes N S, Saperstein R, Smith R G, Leibowitz M D. Thiazolidinediones produce a conformational change in peroxisomal proliferator-activated receptor-γ: binding and activation correlate with antidiabetic actions in db/db mice. Endocrinology. 1996;137:4189–4195. doi: 10.1210/endo.137.10.8828476. [DOI] [PubMed] [Google Scholar]
- 6.Brun R P, Kim J B, Hu E, Spiegelman B M. Peroxisome proliferator-activated receptor gamma and the control of adipogenesis. Curr Opin Lipidol. 1997;8:212–218. doi: 10.1097/00041433-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Chawla A, Lazar M A. Peroxisome proliferator and retinoid signaling pathways co-regulate preadipocyte phenotype and survival. Proc Natl Acad Sci USA. 1994;91:1786–1790. doi: 10.1073/pnas.91.5.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christy R J, Yang V W, Ntambi J M, Geiman D E, Landschulz W H, Friedman A D, Nakabeppu Y, Kelly T J, Lane M D. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989;3:1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- 9.Cornelius P, MacDougald O A, Lane M D. Regulation of adipocyte development. Annu Rev Nutr. 1994;14:99–129. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- 10.Ericsson J, Jackson S M, Kim J B, Spiegelman B M, Edwards P A. Identification of glycerol-3-phosphate acyltransferase as an adipocyte determination and differentiation factor 1- and sterol regulatory element-binding protein-responsive gene. J Biol Chem. 1997;272:7298–7305. doi: 10.1074/jbc.272.11.7298. [DOI] [PubMed] [Google Scholar]
- 11.Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre A M, Saladin R, Najib J, Laville M, Fruchart J C, Deeb S, Vidal-Puig A, Flier J, Briggs M R, Staels B, Vidal H, Auwerx J. Organization, promoter analysis and expression of the human PPARγ gene. J Biol Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 11a.Fajas, L., and J. Auwerx. Unpublished results.
- 12.Fajas L, Fruchart J C, Auwerx J. PPARγ3 mRNA: a distinct PPARγ mRNA subtype transcribed from an independent promoter. FEBS Lett. 1998;438:55–60. doi: 10.1016/s0014-5793(98)01273-3. [DOI] [PubMed] [Google Scholar]
- 13.Fajas L, Fruchart J C, Auwerx J. Transcriptional control of adipogenesis. Curr Opin Cell Biol. 1998;10:165–173. doi: 10.1016/s0955-0674(98)80138-5. [DOI] [PubMed] [Google Scholar]
- 14.Forman B M, Tontonoz P, Chen J, Brun R P, Spiegelman B M, Evans R M. 15-Deoxy-Δ12,14 prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 15.Freytag S O, Geddes T J. Reciprocal regulation of adipogenesis by Myc and C/EBPα. Science. 1992;256:379–382. doi: 10.1126/science.256.5055.379. [DOI] [PubMed] [Google Scholar]
- 16.Freytag S O, Paielli D L, Gilbert J D. Ectopic expression of the CCAAT/enhancer-binding protein α promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 1994;8:1654–1663. doi: 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- 17.Fried M G, Crothers D M. CAP and RNA polymerase interactions with the lac promoter: binding stoichiometry and long range effects. Nucleic Acids Res. 1983;11:141–158. doi: 10.1093/nar/11.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho S N, Hunt H D, Horton N M, Pullen J K, Pease L R. Side directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 19.Hu E, Tontonoz P, Spiegelman B M. Transdifferentiation of myoblasts by the adipogenic transcription factors PPARγ and C/EBPα. Proc Natl Acad Sci USA. 1995;92:9856–9860. doi: 10.1073/pnas.92.21.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua X, Yokoyama C, Wu J, Briggs M R, Brown M S, Goldstein J L, Wang X. SREBP-2, a second basic-helix-loop-helix leucine zipper protein that stimulates transcription by binding to a sterol regulatory element. Proc Natl Acad Sci USA. 1993;90:11603–11607. doi: 10.1073/pnas.90.24.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J B, Spiegelman B M. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10:1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- 22.Kim J B, Wright H M, Wright M, Spiegelman B M. ADD1/SREBP1 activates PPARγ through the production of endogenous ligand. Proc Natl Acad Sci USA. 1998;95:4333–4337. doi: 10.1073/pnas.95.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kliewer S A, Lenhard J M, Willson T M, Patel I, Morris D C, Lehman J M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann J M, Lenhard J M, Oliver B B, Ringold G M, Kliewer S A. Peroxisome proliferator-activated receptors α and γ are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 25.Lehmann J M, Moore L B, Smith-Oliver T A, Wilkison W O, Willson T M, Kliewer S A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 26.Lin F T, Lane M D. Antisense CCAAT/enhancer binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 adipocytes. Genes Dev. 1992;6:533–544. doi: 10.1101/gad.6.4.533. [DOI] [PubMed] [Google Scholar]
- 27.Lopez J M, Bennett M K, Sanchez H B, Rosenfeld J M, Osborne T F. Sterol regulation of acetyl coenzyme A carboxylase: a mechanism for coordinate control of cellular lipid. Proc Natl Acad Sci USA. 1996;93:1049–1053. doi: 10.1073/pnas.93.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mol M J T M, Erkelens D W, Gevers Leuven J A, Schouten J A, Stalenhoef A F H. Lancet:936–939. 1986. Effects of synvinolin (MK-733) on plasma lipids in familial hypercholesterolemia. [DOI] [PubMed] [Google Scholar]
- 29.Negrel R, Gaillard D, Ailhaud G. Prostacyclin as a potent effector of adipose-cell differentiation. Biochem J. 1989;257:399–405. doi: 10.1042/bj2570399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osumi T, Wen J K, Hashimoto T. Two cis-acting regulatory elements in the peroxisome proliferator-responsive element enhancer region of rat acyl-CoA oxidase gene. Biochem Biophys Res Commun. 1991;175:866–871. doi: 10.1016/0006-291x(91)91645-s. [DOI] [PubMed] [Google Scholar]
- 31.Sakai J, Duncan E A, Rawson R B, Hua X, Brown M S, Goldstein J L. Sterol-regulated release of SREBP-2 from cell membranes requires two sequential cleavages, one within a transmembrane segment. Cell. 1996;85:1037–1046. doi: 10.1016/s0092-8674(00)81304-5. [DOI] [PubMed] [Google Scholar]
- 32.Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J. Transient increase in obese gene expression after food intake and insulin administration. Nature. 1995;377:527–529. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- 33.Saladin R, Fajas L, Dana S, Halvorsen Y D, Auwerx J, Briggs M. Differential regulation of peroxisome proliferator activated receptor γ1 (PPARγ1) and PPARγ2 mRNA expression in early stages of adipogenesis. Cell Growth Differ. 1999;10:43–48. [PubMed] [Google Scholar]
- 33a.Schoonjans, K. Unpublished data.
- 34.Schoonjans K, Martin G, Staels B, Auwerx J. Peroxisome proliferator-activated receptors, orphans with ligands and functions. Curr Opin Lipidol. 1997;8:159–166. doi: 10.1097/00041433-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Schoonjans K, Peinado-Onsurbe J, Lefebvre A M, Heyman R, Briggs M, Deeb S, Staels B, Auwerx J. PPARα and PPARγ activators direct a tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 36.Schoonjans K, Staels B, Grimaldi P, Auwerx J. Acyl-CoA synthetase mRNA expression is controlled by fibric-acid derivatives, feeding and liver proliferation. Eur J Biochem. 1993;216:615–622. doi: 10.1111/j.1432-1033.1993.tb18181.x. [DOI] [PubMed] [Google Scholar]
- 37.Schoonjans K, Watanabe M, Suzuki H, Mahfoudi A, Krey G, Wahli W, Grimaldi P, Staels B, Yamamoto T, Auwerx J. Induction of the acyl-coenzyme A synthetase gene by fibrates and fatty acids is mediated by a peroxisome proliferator response element in the C promoter. J Biol Chem. 1995;270:19269–19276. doi: 10.1074/jbc.270.33.19269. [DOI] [PubMed] [Google Scholar]
- 38.Shimano H, Horton J D, Hammer R E, Shimomura I, Brown M S, Goldstein J L. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Investig. 1996;98:1575–1584. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimano H, Horton J D, Shimomura I, Hammer R E, Brown M S, Goldstein J L. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Investig. 1997;99:846–854. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimomura I, Bashmakov Y, Shimano H, Horton J D, Goldstein J L, Brown M S. Cholesterol feeding reduces nuclear forms of sterol regulatory element binding proteins in hamster liver. Proc Natl Acad Sci USA. 1997;94:12345–12359. doi: 10.1073/pnas.94.23.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimomura I, Hammer R E, Richardson J A, Ikemoto S, Bashmakov Y, Goldstein J L, Brown M S. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: a model for congenital generalized lipodystrophy. Genes Dev. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith J R, Osborne T F, Brown M S, Goldstein J L, Gil G. Multiple sterol regulatory elements in the promoter for hamster 3-hydroxy-3-methylglutaryl coenzyme A synthase. J Biol Chem. 1988;263:18480–18487. [PubMed] [Google Scholar]
- 43.Spiegelman B M, Flier J S. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 44.Thewke D P, Panini S R, Sinensky M. Oleate potentiates oxysterol inhibition of transcription from sterol regulatory element-1-regulated promoters and maturation of sterol regulatory element-binding proteins. J Cell Biochem. 1998;273:21402–21407. doi: 10.1074/jbc.273.33.21402. [DOI] [PubMed] [Google Scholar]
- 45.Tontonoz P, Hu E, Devine J, Beale E G, Spiegelman B M. PPARγ2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1995;15:351–357. doi: 10.1128/mcb.15.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tontonoz P, Hu E, Graves R A, Budavari A I, Spiegelman B M. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 47.Tontonoz P, Hu E, Spiegelman B M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 48.Tontonoz P, Kim J B, Graves R A, Spiegelman B M. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol. 1993;13:4753–4759. doi: 10.1128/mcb.13.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vu-Dac N, Schoonjans K, Kosykh V, Dallongeville J, Fruchart J-C, Staels B, Auwerx J. Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. J Clin Investig. 1995;96:741–750. doi: 10.1172/JCI118118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Sato R, Brown M S, Hua X, Goldstein J L. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77:53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 51.Willson T M, Cobb J E, Cowan D J, Wiethe R W, Correa I D, Prakash S R, Beck K D, Moore L B, Kliewer S A, Lehmann J M. The structure activity relationship between peroxisome proliferator activated receptor γ agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem. 1996;39:665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- 52.Worgall T S, Sturley S L, Seo T, Osborne T F, Deckelbaum R J. Polyunsaturated fatty acids decrease expression of promoters with sterol regulatory elements by decreasing levels of mature sterol regulatory element-binding protein. J Biol Chem. 1998;273:25537–25540. doi: 10.1074/jbc.273.40.25537. [DOI] [PubMed] [Google Scholar]
- 53.Wu Z, Bucher N L R, Farmer S R. Induction of peroxisome proliferator-activated receptor γ during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol Cell Biol. 1996;16:4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Z, Xie Y, Bucher N L R, Farmer S R. Conditional ectopic expression of C/EBPβ in NIH-3T3 cells induces PPARγ and stimulates adipogenesis. Genes Dev. 1995;9:2350–2363. doi: 10.1101/gad.9.19.2350. [DOI] [PubMed] [Google Scholar]
- 55.Yeh W C, Cao Z, Classon M, McKnight S. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- 56.Yokoyama C, Wang X, Briggs M R, Admon A, Wu J, Hua X, Goldstein J L, Brown M S. SREBP-1, a basic helix-loop-helix-leucine zipper protein that controls transcription of the LDL receptor gene. Cell. 1993;75:187–197. [PubMed] [Google Scholar]