Abstract
Objectives
To examine whether British South Asian children differ in insulin resistance, adiposity, and cardiovascular risk profile from white children.
Design
Cross sectional study.
Setting
Primary schools in 10 British towns.
Participants
British children aged 8 to 11 years (227 South Asian and 3415 white); 73 South Asian and 1287 white children aged 10 and 11 years provided blood samples (half fasting, half after glucose load).
Main outcome measures
Insulin concentrations, anthropometric measures, established cardiovascular risk factors.
Results
Mean ponderal index was lower in South Asian children than in white children (mean difference −0.43 kg/m3, 95% confidence interval −0.13 kg/m3 to −0.73 kg/m3). Mean waist circumferences and waist:hip ratios were similar. Mean insulin concentrations were higher in South Asian children (percentage difference was 53%, 14% to 106%, after fasting and 54%, 19% to 99%, after glucose load), though glucose concentrations were similar. Mean heart rate and triglyceride and fibrinogen concentrations were higher among South Asian children; serum total, low density lipoprotein, and high density lipoprotein cholesterol concentrations were similar in the two groups. Differences in insulin concentrations remained after adjustment for adiposity and other potential confounders. However, the relations between adiposity and insulin concentrations (particularly fasting insulin) were much stronger among South Asian children than among white children.
Conclusions
The tendency to insulin resistance observed in British South Asian adults is apparent in children, in whom it may reflect an increased sensitivity to adiposity. Action to prevent non-insulin dependent diabetes in South Asian adults may need to begin during childhood.
What is already known on this topic
Compared with white people British South Asians are at increased risk of coronary heart disease, stroke, and non-insulin dependent diabetes
There is evidence that these conditions originate in early life
What this study adds
British South Asian children show higher average levels of insulin and insulin resistance than white children
These ethnic differences in insulin resistance in childhood are not associated with corresponding differences in adiposity, particularly central adiposity
Insulin metabolism seems to be more sensitive to a given degree of adiposity among the South Asian children compared with white children
The prevention of insulin resistance and its consequences may need to begin during childhood, particularly in South Asians
Introduction
In the United Kingdom men and women from many parts of the Indian subcontinent (including India, Pakistan, and Bangladesh) have markedly higher mortality from coronary heart disease than is seen in the general population.1,2 The greater prevalence of non-insulin dependent (type II) diabetes, impaired glucose tolerance, and insulin resistance observed in South Asian men may be important contributory factors,3–12 though South Asian men tend to have lower blood cholesterol concentrations and smoke less than white people.3–6,9–12 While genetic factors probably play a part in these differences, the expression of insulin resistance differs between environmental settings, and there may be a strong environmental component.13,14
Although cardiovascular disease and non-insulin dependent diabetes may originate early in life,15,16 there has been little attempt to study whether differences in cardiovascular risk profiles (particularly in insulin resistance) in South Asian and white people are apparent in childhood. We compared such profiles in British South Asian and white children.
Participants and methods
The “ten towns heart health studies” are based in 10 towns in England and Wales with widely differing adult cardiovascular mortality. Of these, Burnley and Rochdale include a substantial proportion of children of South Asian origin. Details of the 1994 study have been reported elsewhere.17,18 The study took place in a stratified random sample of 10 primary schools in each town. In each school we invited 50 children aged 8-11 years to take part and asked the 22 oldest (aged 10-11 years) to provide additional measurements including a blood sample. We obtained ethical approval from all relevant local research ethics committees and informed written consent from parents.
Two research teams visited towns in sequence between April and November 1994. They measured height, weight, and blood pressure (two seated measurements with the Dinamap 1846SX oscillometric blood pressure recorder, which also recorded heart rate) in all children. The older pupils fasted overnight before their assessments, which also included measurements of waist and hip circumference, a simplified three level assessment of Tanner staging for breast development among girls19 with Tanner grades 2-3 and 4-5 combined, and the collection of a blood sample. In half the children this was collected after fasting and in half it was collected 30 minutes after a standard oral glucose load (1.75 g/kg). Blood samples were separated and frozen at −20oC within four hours of collection, with snap freezing of samples for haemostatic measurements. Serum insulin concentration was measured by an ELISA (enzyme linked immunosorbent assay) method which does not cross react with proinsulin.20 Plasma glucose concentration (fluoride oxalate sample) was measured with the Glucose-Technicon Axon system (method No SM4-2143F90). Fibrinogen concentration was measured by the Clauss method21 and factor VIIC by a one stage semiautomated bioassay.22 Serum lipid measurements have been described elsewhere.17
Ethnicity and social class
We classified ethnicity into four main groups on the basis of the child's appearance (white, Asian, other, mixed race) and cross checked with surname and with questionnaire information on parents' place of birth, religion, and first language. To determine response rates by ethnic group we defined the ethnic group of non-participants on the basis of surname and checked with schools in cases of doubt. Parents provided information on their longest held occupation, which we classified using the registrar general's 1980 classification of occupations.
Statistical methods
We used the SAS statistical package version 6.12 (SAS Institute, Cary, NC, USA) for all statistical analyses. Serum insulin and triglyceride concentrations were markedly skewed and were log transformed. For these variables we have presented geometric mean values and percentage differences (with 95% confidence limits). We used ponderal index (weight (kg)/height (m)3)—independent of age and height—as an index of weight for height. We calculated estimates of insulin resistance (insulin × glucose/22.5) from fasting values in accordance with “homeostasis model assessment.”23 Physical measurements were adjusted for observer (four levels). We adjusted blood glucose and insulin concentrations for time of day and for variation in the interval between glucose load and venepuncture.18 We adjusted all the main analyses for town, sex, and age. When appropriate we also adjusted for height and ponderal index. We determined differences between ethnic groups in slope between adiposity and insulin concentration by regressing log (insulin) on each adiposity measure using PROC GLM and fitting an interaction term for adiposity measure*ethnic group.
Results
Overall, 3415 white and 227 South Asian children took part (response rates 73% and 80% respectively). We excluded 18 children of mixed race from analyses. We took blood samples from 1287 white and 73 South Asian children (response rates 64% and 61% respectively). Demographic and social characteristics are shown in table 1. South Asian children were slightly younger on average than white children, but a similar proportion were girls. Most of the South Asian children had been born in the United Kingdom, and a high proportion were measured in Burnley and Rochdale. More white children had parents with non-manual occupations. Of the 120 South Asian mothers who provided information, 82 (68%) were born in Pakistan, 15 (13%) in Bangladesh, and 5 (4%) in India. Results were similar for fathers. Almost all (95%) were Muslim. These characteristics were similar in the subset of children who provided blood samples.
Table 1.
Characteristics of study participants
| South Asian children (n=227*) | White children (n=3415*) | P value (no ethnic difference) | |
|---|---|---|---|
| Mean (SD) age (years) | 10.2 (0.54) | 10.5 (0.66) | <0.0001 |
| No (%) of girls | 107 (47) | 1639 (48) | 0.66 |
| No (%) born in United Kingdom | 117/141 (83) | 2789/2817 (99) | <0.0001 |
| No (%) measured in Burnley or Rochdale | 202 (89) | 546 (16) | <0.0001 |
| No (%) with parents in non-manual occupation | 27/93 (29) | 1334/2723 (49) | <0.0001 |
Base number except where shown otherwise.
Ethnic group and cardiovascular risk factors
Body build, blood pressure, and heart rate
White children were heavier and slightly taller on average, with a greater mean ponderal index than their South Asian counterparts (table 2). However, mean waist and hip circumferences and waist:hip ratios were similar in the two groups. Mean diastolic blood pressure and heart rate were higher in the South Asian children, particularly after adjustment for height and ponderal index. The difference in mean diastolic blood pressure was greatly reduced after we also adjusted for heart rate (from 1.4 to 0.7 mm Hg, 95% confidence interval −0.3 mm Hg to 1.7 mm Hg, P=0.19).
Table 2.
Body build, blood pressure, and pulse rate in South Asian and white children. Figures are means (SE)
| South Asian§(n=227) | White (n=3415)§ |
Difference (95% CI)
|
||
|---|---|---|---|---|
| Adjustment 1§ | Adjustment 2¶ | |||
| Height (cm) | 140.5 (0.5) | 140.9 (0.1) | −0.4 (−1.4 to 0.6) | — |
| Weight (kg) | 34.5 (0.6) | 35.8 (0.1) | −1.3 (−2.5 to −0.1)* | — |
| Ponderal index (kg/m3) | 12.27 (0.15) | 12.70 (0.03) | −0.43 (−0.73 to −0.13)† | — |
| Systolic blood pressure (mm Hg) | 111.7 (0.9) | 112.4 (0.2) | −0.6 (−2.4 to 1.1) | 0.3 (−1.3 to 1.9) |
| Diastolic blood pressure (mm Hg) | 66.6 (0.5) | 65.5 (0.1) | 1.1 (0.0 to 2.2) | 1.4 (0.3 to 2.5)* |
| Heart rate (beats/min) | 84.9 (0.9) | 81.5 (0.2) | 3.4 (1.6 to 5.2)‡ | 3.7 (1.9 to 5.5)‡ |
| Waist circumference (cm)** | 61.2 (1.0) | 61.0 (0.2) | 0.3 (−1.7 to 2.3) | — |
| Hip circumference (cm)** | 75.6 (0.9) | 76.3 (0.2) | −0.7 (−2.5 to 1.2) | — |
| Waist:hip ratio** | 0.807 (0.006) | 0.799 (0.001) | 0.008 (−0.005 to 0.021) | — |
0.01⩽P<0.05.
0.005⩽P<0.01.
P<0.005.
Adjusted for age, sex, and town.
Adjusted for age, sex, town, childhood height, and ponderal index (not carried out for body build measures).
No=86 for South Asian children and 1415 for white children.
Blood lipids and coagulation factors
Mean concentrations of total, low density lipoprotein, and high density lipoprotein cholesterol were slightly but not significantly lower in South Asian children (table 3). Mean triglyceride concentration was significantly higher in South Asian children than white children. Mean fibrinogen concentrations were markedly higher in South Asian children. Factor VII concentrations were similar in the two groups.
Table 3.
Blood lipids and haemostatic factors in South Asian and white children
|
South Asian§
|
White§
|
Difference (95% CI)
|
|||||
|---|---|---|---|---|---|---|---|
| No | Mean (SE) | No | Mean (SE) | Adjustment 1§ | Adjustment 2¶ | ||
| Cholesterol (mmol/l): | |||||||
| Total | 73 | 4.56 (0.10) | 1287 | 4.66 (0.02) | −0.10 (−0.30 to 0.11) | −0.10 (−0.31 to 0.10) | |
| Low density lipoprotein | 73 | 2.70 (0.09) | 1286 | 2.78 (0.02) | −0.09 (−0.27 to 0.10) | −0.09 (−0.28 to 0.09) | |
| High density lipoprotein | 73 | 1.38 (0.04) | 1286 | 1.43 (0.01) | −0.05 (−0.13 to 0.03) | −0.05 (−0.13 to 0.03) | |
| Triglyceride (mmol/l)** | 73 | 1.01 | 1287 | 0.91 | 11.7% (0.0 to 24.8%)* | 12.1% (0.9 to 24.5%)* | |
| Fibrinogen (g/l) | 55 | 2.80 (0.08) | 667 | 2.52 (0.02) | 0.28 (0.10 to 0.45)‡ | 0.22 (0.06 to 0.39)† | |
| Factor VII (%) | 55 | 105.9 (4.3) | 667 | 104.9 (1.1) | 1.0 (−7.9 to 10.0) | −0.9 (−9.7 to 7.9) | |
0.01⩽P<0.05.
0.005⩽P<0.01.
P<0.005.
Adjusted for age, sex, and town.
Adjusted for age, sex, town, childhood height, and ponderal index.
Geometric mean.
Glucose and insulin concentration
Mean glucose concentrations were similar in South Asian and white children, but insulin concentration both after fasting and after a glucose load were markedly higher in South Asian children (table 4). These differences in insulin concentration persisted after we adjusted for height and ponderal index. The mean difference in insulin resistance between South Asian and white children with homeostasis model assessment23 was similar to the difference in fasting insulin concentration: 54% (14% to 108%) after adjustment for age and sex and 69% (29% to 121%) after additional adjustment for height and ponderal index. The percentage differences in insulin concentrations between South Asian and white children were slightly higher among girls (fasting 74%, 5% to 189%; after glucose load 57%, 6% to 130%) than boys (fasting 36%, −9% to 102%; after glucose load 49%, −2% to 125%), but there was no evidence of sex interaction for either measurement (fasting P=0.4; after glucose load P=0.9). Breast development was less pronounced among South Asian girls than white girls (45% v 35% still at Tanner stage 1). However, adjustment for pubertal status tended to increase rather than diminish ethnic differences in insulin concentration.
Table 4.
Plasma glucose and serum insulin concentrations in South Asian and white children
|
South Asian*
|
White*
|
Difference (95% CI)
|
||||||
|---|---|---|---|---|---|---|---|---|
| No | Mean (SE) | No | Mean (SE) | Adjustment 1* | Adjustment 2† | |||
| Fasting | ||||||||
| Glucose (mmol/l) | 41 | 4.85 (0.07) | 625 | 4.85 (0.01) | 0.00 (−0.14 to 0.14) | 0.02 (−0.12 to 0.15) | ||
| Insulin (pmol/l)‡ | 40 | 47.8 | 622 | 31.2 | 53.4% (14.4% to 105.6%)§ | 68.0% (29.1% to 118.5%)§ | ||
| After glucose load | ||||||||
| Glucose (mmol/l) | 31 | 7.16 (0.32) | 587 | 7.01 (0.06) | 0.15 (−0.49 to 0.79) | 0.18 (−0.46 to 0.82) | ||
| Insulin (pmol/l)‡ | 31 | 459.8 | 581 | 298.7 | 54.0% (19.1% to 99.0%)§ | 42.7% (12.8% to 80.3%)§ | ||
Adjusted for age, sex, and town.
Adjusted for age, sex, town, childhood height, and ponderal index.
Geometric mean.
P<0.005.
When we restricted analyses to children from Burnley and Rochdale we found similar ethnic differences in insulin concentration, though confidence intervals were larger (fasting 43%, –6% to 120%; after glucose load 53%, 16% to 103%). When we restricted analyses to children of Pakistani origin the results were similar to those for the whole study population. Adjustment for waist circumference or waist:hip ratio, social class, heart rate (as a proxy for physical fitness), and physical activity (based on parents' assessment of child's usual level of physical activity) had no important effect on estimated ethnic differences in insulin. Adjustment for birth weight reduced the fasting difference by a quarter but did not affect the difference after glucose load.
Body build and insulin concentration: relation in different ethnic groups
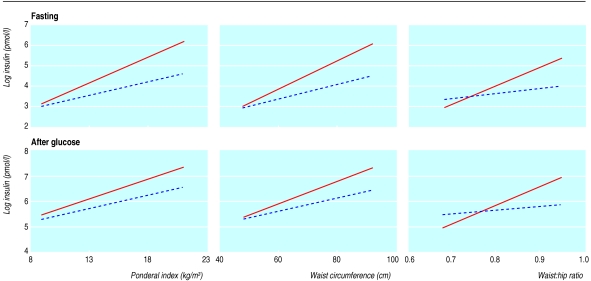
Ponderal index, waist circumference, and to a lesser extent waist:hip ratio were related to fasting insulin concentration (r=0.36, 0.35, 0.05, respectively with log insulin concentration) and to insulin concentration after glucose load (r=0.35, 0.35, 0.05, respectively with log insulin concentration). The relations between body build and insulin concentration were examined separately in white and South Asian children (table 5). The slopes regressing log insulin concentration on each of the three measures of body build were markedly steeper in South Asian children than in white children (figure). For fasting insulin concentration the differences in slope between ethnic groups (tests for interaction) were significant for all three measures. For insulin concentration after a glucose load only the difference for waist:hip ratio was significant (P=0.05).
Table 5.
Percentage difference (95% confidence interval) in insulin concentration per one unit increase in adiposity measure in each ethnic group. All analyses adjusted for age, sex, and town
| Measure | White | South Asian | P value (no ethnic difference) |
|---|---|---|---|
| Ponderal index (kg/m3) | |||
| Fasting | 14.3 (11.0 to 17.8) | 28.8 (18.4 to 40.1) | 0.009 |
| After glucose load | 10.7 (8.1 to 13.5) | 16.3 (8.2 to 25.1) | 0.21 |
| Waist circumference (cm) | |||
| Fasting | 3.4 (2.6 to 4.3) | 6.9 (4.7 to 9.2) | 0.003 |
| After glucose load | 2.6 (1.9 to 3.3) | 4.3 (2.5 to 6.1) | 0.08 |
| Waist:hip ratio* | |||
| Fasting | 2.6 (1.5 to 3.8) | 9.5 (5.5 to 13.7) | 0.001 |
| After glucose load | 1.5 (0.6 to 2.5) | 7.4 (3.1 to 11.9) | 0.008 |
One unit change in waist:hip ratio taken as 0.01.
Discussion
This cross sectional study has shown that the tendency among British South Asian adults to develop insulin resistance is apparent in childhood, though it is not associated with overt glucose intolerance at that stage. The proportional difference in fasting insulin concentration between South Asian and white children was similar to that seen in British adults.6,7 However, the difference in insulin concentration after glucose load in children was smaller than that seen in most adult studies.4,6,9 It is unlikely that the results are an artefact of sampling or differential response rates (only marginally different in the two groups) or the result of ethnic differences in pubertal status. In this study South Asian children were younger, slightly shorter, and, in the case of girls, showed less pubertal development—all factors that tend to reduce insulin concentrations.
Previous studies
Previous studies have suggested that ethnic differences in insulin resistance develop before adulthood. Among young adult relatives of patients with coronary artery disease, South Asian people had higher insulin concentrations than white people.24 Among South African schoolchildren aged 10-12 years, those of Indian origin had higher insulin concentrations after glucose load than white children.25 In populations at exceptionally high risk for non-insulin dependent diabetes (for example, Pima Indians) overt insulin resistance and diabetes is seen in childhood.26
The increased mean concentrations of triglyceride and somewhat lower concentrations of high density lipoprotein cholesterol in South Asian children are consistent both with the higher degree of insulin resistance observed27 and with earlier reports in adults.4,6,11 The higher mean fibrinogen concentration seen among the South Asian children is unexpected. Fibrinogen concentrations in South Asian adults have generally been similar to or lower than those in white people,4,5,12,28 except for those seen in one recent US study.29 Our finding is not explained by active cigarette smoking, which is less common among South Asian children, and awaits confirmation. The higher mean heart rate observed in South Asian children could reflect lower levels of physical fitness or increased sympathoadrenal activity in this group.30
Explanation for findings
The causes of the increased insulin resistance in these South Asian children remain unclear. Adiposity (particularly central adiposity) is a prominent correlate of insulin resistance in South Asian adults.6 However, even though our measures of adiposity (including central adiposity) are limited, our results suggest that the ethnic difference in insulin concentrations is not accompanied by a concomitant difference in adiposity. Hyperinsulinaemia may precede adiposity in the early stages of the pathogenesis of insulin resistance,31 and insulin metabolism of South Asian people may be more sensitive to a given degree of adiposity, general or central. Similar findings have been described in one recent study in adults,32 though not in earlier ones.3,6 The cause of the ethnic difference in the sensitivity of insulin metabolism to obesity is difficult to establish within the present small study. Both environmental and genetic influences are likely to be important. The roles of fetal nutrition, physical fitness, and physical activity, for which we could make only crude adjustments, require further investigation. Childhood nutrition might also be important. When we used a food frequency questionnaire we found that South Asian children consumed less fresh fruit and vegetables than white children did. Infective or inflammatory factors could also play a part.33
Implications
Our results imply that the primary prevention of insulin resistance, non-insulin dependent diabetes, and cardiovascular disease in high risk populations (including British South Asians) may need to begin before adult life. Given that South Asian people may be particularly sensitive to the metabolic consequences of obesity (currently increasing in prevalence among British children34), the prevention of obesity in childhood and adolescence among South Asian people, with a combination of dietary measures and increased physical activity,35 is a strong priority while other influences on the development of ethnic differences in insulin resistance are assessed.
Figure.
Regressions of log insulin concentration (fasting and after glucose load) on three measures of adiposity (ponderal index, waist circumference, waist:hip ratio) in South Asian (continuous line) and white (broken line) children
Acknowledgments
We are grateful to the research team members (Drs Fiona Adshead and Stephanie Taylor, Valerie Wilson, Sally Gassor, Angela Murphy, Catherine Stuart, Louise Went) and to participating schools, pupils, and parents. Insulin measurements were carried out at the Department of Diabetes, University of Newcastle upon Tyne, and fibrinogen and factor VII measurements at the MRC Epidemiology and Medical Care Unit, London. All other biochemical measurements were carried out at the Department of Clinical Biochemistry, St George's Hospital, London.
Footnotes
Editorial by Bhopal
Funding: Wellcome Trust (project grant 038976/Z/93/Z).
Competing interests: None declared.
References
- 1.Wild S, McKeigue P. Cross sectional analysis of mortality by country of birth in England and Wales, 1970-92. BMJ. 1997;314:705–710. doi: 10.1136/bmj.314.7082.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balarajan R. Ethnicity and variations in mortality from coronary heart disease. Health Trends. 1996;28:45–51. [Google Scholar]
- 3.McKeigue PM, Marmot MG, Adelstein AM, Hunt SP, Shipley MJ, Butler SM, et al. Diet and risk factors for coronary heart disease in Asians in north-west London. Lancet. 1985;ii:1086–1090. doi: 10.1016/s0140-6736(85)90684-1. [DOI] [PubMed] [Google Scholar]
- 4.McKeigue PM, Marmot MG, Syndercombe Court YD, Cottier DE, Rahman S, Riemersma RA. Diabetes, hyperinsulinaemia and coronary risk factors in Bangladeshis in east London. Br Heart J. 1988;60:390–396. doi: 10.1136/hrt.60.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller GJ, Kotecha S, Wilkinson WH, Wilkes H, Stirling Y, Sanders TA, et al. Dietary and other characteristics relevant for coronary heart disease in men of Indian, West Indian and European descent in London. Atherosclerosis. 1988;70:63–72. doi: 10.1016/0021-9150(88)90100-1. [DOI] [PubMed] [Google Scholar]
- 6.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337:382–386. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 7.Cruickshank JK, Cooper J, Burnett M, MacDuff J, Drubra U. Ethnic differences in fasting plasma C-peptide and insulin in relation to glucose tolerance and blood pressure. Lancet. 1991;338:842–847. doi: 10.1016/0140-6736(91)91501-k. [DOI] [PubMed] [Google Scholar]
- 8.Simmons D, Williams DRR, Powell MJ. The Coventry diabetes study: prevalence of diabetes and impaired glucose tolerance in Europids and Asians. Q J Med. 1991;81:1021–1030. doi: 10.1093/qjmed/81.3.1021. [DOI] [PubMed] [Google Scholar]
- 9.Knight TM, Smith Z, Whittles A, Sahota P, Lockton JA, Hogg G, et al. Insulin resistance, diabetes and risk markers for ischaemic heart disease in Asian men and non-Asian men in Bradford. Br Heart J. 1992;67:343–350. doi: 10.1136/hrt.67.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappuccio FP, Cook DG, Atkinson RW, Strazzullo P. Prevalence, detection and management of cardiovascular risk factors in different ethnic groups in South London. Heart. 1997;78:555–563. doi: 10.1136/hrt.78.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitty CJ, Brunner EJ, Shipley MJ, Hemingway H, Marmot MG. Differences in biological risk factors for cardiovascular disease between three ethnic groups in the Whitehall II Study. Atherosclerosis. 1999;142:279–286. doi: 10.1016/s0021-9150(98)00239-1. [DOI] [PubMed] [Google Scholar]
- 12.Bhopal R, Unwin N, White M, Yallop J, Walker L, Alberti KG, et al. Heterogeneity of coronary heart disease risk factors in Indian, Pakistani, Bangladeshi, and European origin populations: cross sectional study. BMJ. 1999;319:215–220. doi: 10.1136/bmj.319.7204.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams B. Westernized Asians and cardiovascular disease: nature or nurture? Lancet. 1995;345:401–402. doi: 10.1016/s0140-6736(95)90394-1. [DOI] [PubMed] [Google Scholar]
- 14.Bhatanagar D, Anand IS, Durrington PN, Patel DJ, Wander GS, Mackness MI, et al. Coronary risk factors in people from the Indian subcontinent living in West London and their siblings in India. Lancet. 1995;345:405–409. doi: 10.1016/s0140-6736(95)90398-4. [DOI] [PubMed] [Google Scholar]
- 15.Berenson GS, Srinivasan SR. Prevention of atherosclerosis in childhood. Lancet. 1999;354:1223–1224. doi: 10.1016/S0140-6736(99)00274-3. [DOI] [PubMed] [Google Scholar]
- 16.Barker DJP. Mothers, babies and health in later life. 2nd ed. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- 17.Whincup PH, Cook DG, Adshead FA, Taylor S, Papacosta O, Walker M, et al. Cardiovascular risk factors in British children in towns with widely differing adult cardiovascular mortality. BMJ. 1996;313:79–84. doi: 10.1136/bmj.313.7049.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whincup PH, Cook DG, Adshead F, Taylor SJC, Walker M, Papacosta O, et al. Childhood size is more strongly related than size at birth to glucose and insulin levels in 10-11 year-old children. Diabetologia. 1997;40:319–326. doi: 10.1007/s001250050681. [DOI] [PubMed] [Google Scholar]
- 19.Tanner JM. Growth at adolescence. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- 20.Andersen L, Dinesen B, Jorgensen PN, Poulsen F, Roder ME. Enzyme immunoassay for intact human insulin in serum or plasma. Clin Chem. 1993;39:578–582. [PubMed] [Google Scholar]
- 21.Clauss A. Gerinnungsphysiologische schnellmethode zur bestimmung des fibrinogens. Acta Haematol. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 22.Miller GJ, Stirling Y, Esnouf MP, Heinrich J, van de Loo J, Kienast J, et al. Factor VII-deficient substrate plasmas depleted of protein C raise the sensitivity of the factor VII bioassay to activated factor VII: an international study. Thromb Haemost. 1994;71:38–48. [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Shaukat N, Douglas JT, Bennett JL, de Bono DP. Can physical activity explain the differences in insulin levels and fibrinolytic activity between young Indo-origin and European relatives of patients with coronary artery disease? Fibrinolysis. 1995;9:55–63. [Google Scholar]
- 25.Walker ARP, Bernstein RE, du Plessis I. Hyperinsulinaemia from glucose dose in South African Indian children. S Afr Med J. 1972;46:1916. [PubMed] [Google Scholar]
- 26.Fagot-Campagna A, Pettitt D, Engelgau MM, Burrows NR, Geiss LS, Valdez R, et al. Type 2 diabetes among North American children and adolescents; an epidemiological review and a public health perspective. J Pediatr. 2000;136:664–672. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- 27.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 28.Cook DG, Cappuccio FP, Atkinson RW, Wicks PD, Chitolie A, Nakandakare ER, et al. Ethnic differences in fibrinogen levels: the role of environmental factors and the beta-fibrinogen gene. Am J Epidemiol. 2001;153:799–806. doi: 10.1093/aje/153.8.799. [DOI] [PubMed] [Google Scholar]
- 29.Markovitz JH, Kulkarni K, Goldschmidt-Clermont P, Kiefe CI, Rustagi P, Sekar P, et al. Increased platelet activation and fibrinogen in Asian Indians. Potential implication for coronary risk. Eur Heart J. 1998;19:720–726. doi: 10.1053/euhj.1997.0800. [DOI] [PubMed] [Google Scholar]
- 30.Phillips DI, Barker DJ. Association between low birthweight and high resting pulse rate in adult life: is the sympathetic nervous system involved in programming the insulin resistance syndrome? Diabet Med. 1997;14:673–677. doi: 10.1002/(SICI)1096-9136(199708)14:8<673::AID-DIA458>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X) Diabetes. 1992;41:715–722. doi: 10.2337/diab.41.6.715. [DOI] [PubMed] [Google Scholar]
- 32.Forouhi NG. The relationship between body fat distribution, insulin sensitivity and postprandial lipids in Europeans and South Asians: a cross-sectional study. London: London University; 2000. (PhD Thesis). [Google Scholar]
- 33.Cook DG, Mendall MA, Whincup PH, Carey IM, Ballam L, Morris JE, et al. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149:139–150. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- 34.Chinn S, Rona RJ. Prevalence and trends in overweight and obesity in three cross sectional studies of British children, 1974-94. BMJ. 2001;322:24–26. doi: 10.1136/bmj.322.7277.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKeigue PM. Coronary heart disease in Indians, Pakistanis, and Bangladeshis: aetiology and possibilities for prevention. Br Heart J. 1992;67:341–342. doi: 10.1136/hrt.67.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]