Abstract
BACKGROUND
Molecular characterization in pediatric papillary thyroid cancer (PTC), distinct from adult PTC, is important for developing molecularly targeted therapies for progressive radioiodine-refractory (131I-refractory) PTC.
METHODS
PTC samples from 106 pediatric patients (age range: 4.3–19.8 years; n = 84 girls, n = 22 boys) who were admitted to SNUH (January 1983–March 2020) were available for genomic profiling. Previous transcriptomic data from 125 adult PTC samples were used for comparison.
RESULTS
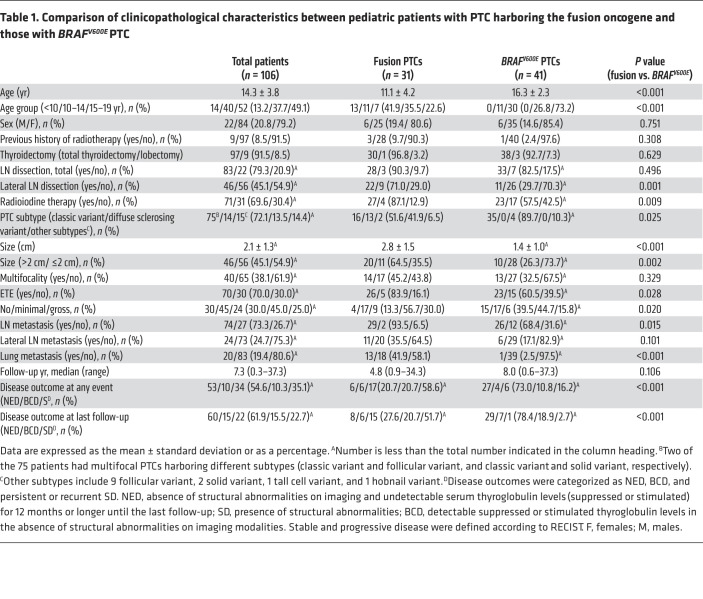
We identified genetic drivers in 80 tumors: 31 with fusion oncogenes (RET in 21 patients, ALK in 6 patients, and NTRK1/3 in 4 patients); 47 with point mutations (BRAFV600E in 41 patients, TERTC228T in 2 patients [1 of whom had a coexisting BRAFV600E], and DICER1 variants in 5 patients); and 2 with amplifications. Fusion oncogene PTCs, which are predominantly detected in younger patients, were at a more advanced stage and showed more recurrent or persistent disease compared with BRAFV600E PTCs, which are detected mostly in adolescents. Pediatric fusion PTCs (in patients <10 years of age) had lower expression of thyroid differentiation genes, including SLC5A5, than did adult fusion PTCs. Two girls with progressive 131I-refractory lung metastases harboring a TPR-NTRK1 or CCDC6-RET fusion oncogene received fusion-targeted therapy; larotrectinib and selpercatinib decreased the size of the tumor and restored 125I radioiodine uptake. The girl with the CCDC6-RET fusion oncogene received 131I therapy combined with selpercatinib, resulting in a tumor response. In vitro 125I uptake and 131I clonogenic assays showed that larotrectinib inhibited tumor growth and restored radioiodine avidity.
CONCLUSIONS
In pediatric patients with fusion oncogene PTC who have 131I-refractory advanced disease, selective fusion-directed therapy may restore radioiodine avidity and lead to a dramatic tumor response, underscoring the importance of molecular testing in pediatric patients with PTC.
FUNDING
The Ministry of Science, ICT and Future Planning (NRF-2016R1A2B4012417 and 2019R1A2C2084332); the Korean Ministry of Health and Welfare (H14C1277); the Ministry of Education (2020R1A6A1A03047972); and the SNUH Research Fund (04-2015-0830).
TRIAL REGISTRATION
Two patients received fusion-targeted therapy with larotrectinib (NCT02576431; NAVIGATE) or selpercatinib (LOXO-RET-18018).
Keywords: Endocrinology, Oncology
Keywords: Cancer, Oncogenes, Thyroid disease
Introduction
Pediatric papillary thyroid cancers (PTCs) have distinct genetic alterations and a higher proportion of gene fusions compared with adult PTCs, in which point mutations predominate (1).
Previous methods to detect BRAFV600E, RAS, or RET/PTC gene alterations identified driver alterations in less than half of pediatric PTCs (2). However, next-generation sequencing (NGS) has increased the detection rate of genetic drivers in sporadic cases by more than 60% (3–7). A significant proportion of fusion oncogenes have been detected (20%–60%), as with radiation-associated thyroid cancers (8). This high frequency of oncogenic fusion may be important in the pathogenesis of pediatric PTCs and could facilitate the selection of patients for recently developed, highly selective and potent fusion-targeted agents (9, 10). Moreover, identification of transcriptomic characteristics may help determine the pathogenesis and biological behavior of pediatric PTCs and differentiate them from adult PTCs. Previous molecular studies of pediatric PTCs have generally been too small to generate age-associated genomic profiles, and transcriptomic information on these tumors is limited.
In this study, we comprehensively characterized age-associated genetic alterations in a large series of pediatric PTCs. Furthermore, we performed transcriptome analyses of pediatric PTCs and compared these profiles with those reported for adult PTCs (11). On the basis of these data, we investigated the role of the NTRK and RET fusion–targeted agents, larotrectinib and selpercatinib, respectively, in the inhibition of tumor growth and restoration of radioiodine uptake in progressive radioiodine-refractory (131I-refractory) PTCs in young children.
Results
Age-associated genetic alterations in pediatric PTCs.
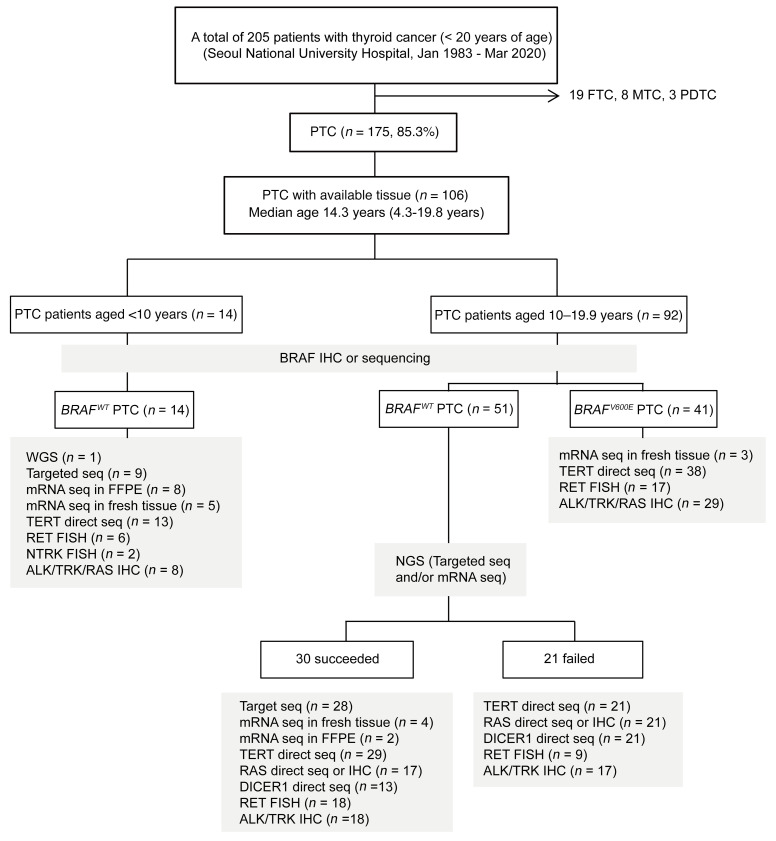
The clinicopathological characteristics and genetic analyses of 106 Korean patients (age range: 4.3–19.8 years; n = 84 girls and n = 22 boys) are summarized in Table 1 and Figure 1. We identified genomic alterations in 80 of these patients, including 31 with oncogenic fusions (NTRK1/3 in 4 patients, RET in 21 patients, and ALK in 6 patients), 47 with point mutations (BRAFV600E in 41 patients, TERTC228T in 2 patients [1 of whom had a coexisting BRAFV600E], and DICER1 variants in 5 patients), and 2 with FGFR1 or EGFR amplifications (Supplemental Tables 1 and 2; supplemental material available online with this article; https://doi.org/10.1172/JCI144847DS1). Detailed information on the fusion partner genes, breakpoints, and methods to detect each fusion oncogene are described in Table 2, Supplemental Table 3, and Supplemental Figure 1. H/K/NRAS mutations were not identified in any of the tested tumors. Among 9 patients who underwent radiotherapy, we identified a genetic driver in 7 of them (2 RET, 1 ALK, 1 BRAFV600E, 1 DICER1D1709G with coexisting loss of heterozygosity at multiple chromosomal loci and 2 amplifications; Supplemental Table 1).
Table 1. Comparison of clinicopathological characteristics between pediatric patients with PTC harboring the fusion oncogene and those with BRAFV600E PTC.
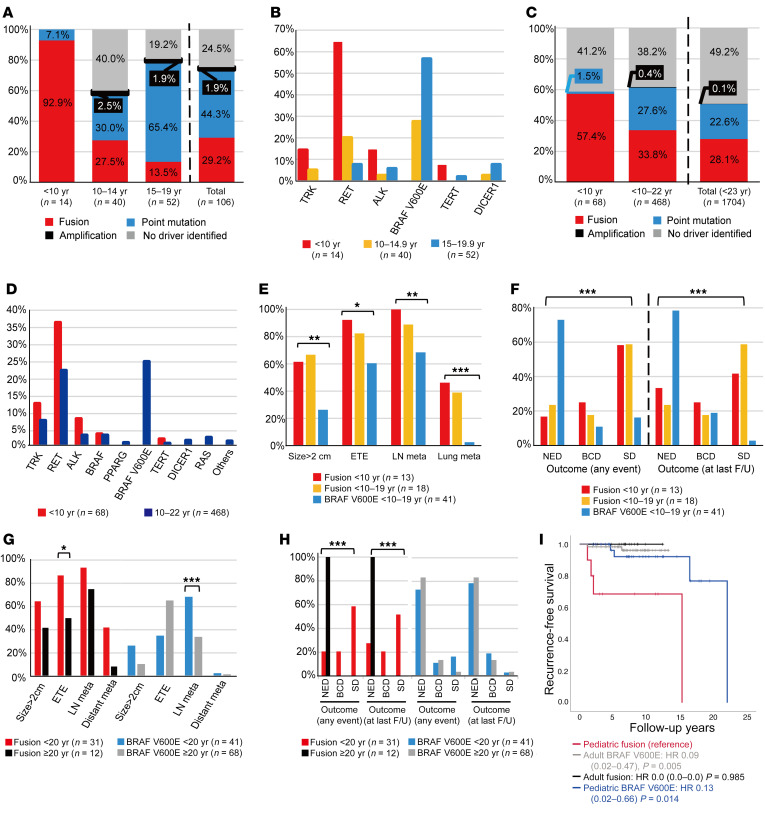
Figure 1. Flow diagram describing comprehensive genomic profiling of pediatric PTC samples.
Tumor tissue samples (n = 106) from pediatric patients with PTC (n = 84 girls, n = 22 boys; median age: 14.3 years; range: 4.3–19.8 years) were analyzed to profile genetic alterations using WGS, targeted sequencing, mRNA sequencing, direct sequencing, FISH, and/or IHC depending on the availability of each tissue. FTC, follicular thyroid cancer; MTC, medullary thyroid cancer; PDTC, poorly differentiated thyroid cancer.
Table 2. Clinicopathological presentation and disease outcomes in pediatric patients with PTC harboring a fusion oncogene.
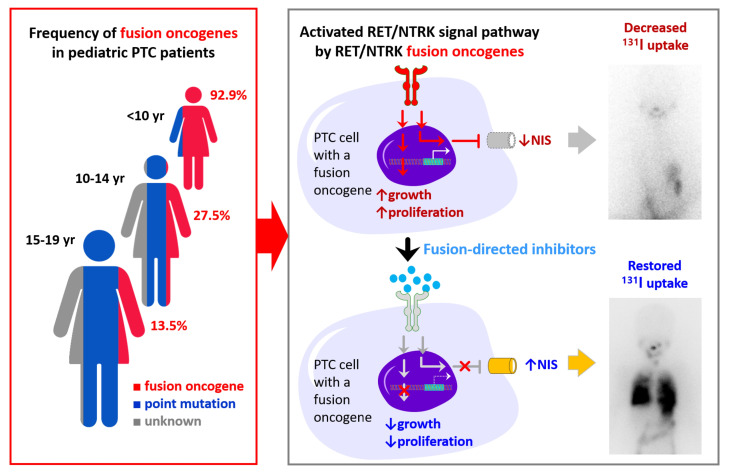
In the 3 groups — patients under 10 years of age (n = 14), patients 10–14 years old (n = 40), and patients 15–19 years old (n = 52) — the proportions of fusion oncogenes were 92.9%, 27.5%, and 13.5%, respectively, whereas the point mutation rates were 7.1%, 30.0%, and 65.4% (Figure 2A), with BRAFV600E rates of 0%, 27.3%, and 57.7%, respectively. The frequency of each gene according to age is shown in Figure 2B and Supplemental Table 2. In particular, among 14 young children below 10 years of age, 13 harbored a fusion oncogene (9 RET, 2 NTRK, and 2 ALK) and 1 had a TERTC228T mutation. The pooled analysis (Supplemental Table 4) revealed similar trends among the patients under 10 years of age and patients 10–22 years of age (Figure 2, C and D).
Figure 2. Age-associated genetic profiles of pediatric PTCs and comparison of the clinicopathological presentation and disease outcomes between fusion oncogene and BRAFV600E PTCs.
(A–D) Age-associated proportions of fusion oncogenes, point mutations, and genetic drivers among the pediatric patients in this study (A and B; patients aged <10 years, n = 14; 10–14 years; n = 40, and 15–19 years, n = 52) and a pooled analysis of 1704 patients under 23 years of age (C and D; patients aged <10 years, n = 68 and patients aged 10–22 years, n = 468, plus other patients without detailed age information). Comparison of the clinicopathological presentation (E) and disease outcomes (F) among the 3 pediatric groups: fusion PTCs in patients <10 years of age (n = 13), fusion PTCs in patients 10–19 years of age (n = 18), and BRAFV600E PTCs in all patients 10–19 years of age (n = 41). Comparison of the clinicopathological presentation (G) and disease outcomes (H) between the pediatric fusion (n = 31) and adult fusion (n = 12) groups, and between the pediatric BRAFV600E (n = 41) and adult BRAFV600E (n = 68) groups. Categorical variables were compared between the 2 groups using the χ2 or Fisher’s exact test, whereas the χ2 test for trend or logistic regression was used for comparisons among the 3 groups (*P < 0.05, **P < 0.01, and ***P < 0.001). (I) Recurrence-free survival was compared among these 4 groups with reference to the pediatric fusion group. Recurrence-free survival plots were constructed using the Kaplan-Meier method, and groups were compared using the Cox proportional hazards model. The HRs, 95% CIs, and P values are reported in the figure. F/U, follow-up; meta, metastasis.
Clinicopathological characteristics and outcomes according to the genetic alterations in pediatric PTCs.
We found that oncogenic fusion PTCs were associated with a higher proportion of large tumors (>2 cm), extrathyroidal extension (ETE), and lymph node (LN) and lung metastasis compared with BRAFV600E PTCs (Tables 1 and 2 and Supplemental Table 5). RET fusion PTC was predominantly a diffuse sclerosing variant (DSV) (55.0%), whereas BRAFV600E PTC was mostly a classic variant (89.7%). The oncogenic fusion PTCs were categorized into 2 age groups: fusion PTCs in patients under 10 years of age (n = 13) and fusion PTCs in patients 10–19 years of age (n = 18), and BRAFV600E PTCs were all in the 10–19 years age group (n = 41). The proportion of large tumors, ETE, LN or lung metastasis (Figure 2E), and biochemical disease (BCD) or structural disease (SD) (Figure 2F) significantly decreased from the group of patients under 10 years of age with fusion PTC, to the 10–19 years age groups with fusion PTC, and to the 10–19 years age groups with BRAFV600E PTC. Among the 13 patients with persistent lung metastasis despite 131I treatment (2 patients with NTRK1, 7 patients with RET, 2 patients with ALK, 1 patient with DICER1, and 1 patient with no driver identified), 10 patients maintained stable status, while 3 young children (P1 with a TPR-NTRK1, P8 with a CCDC6-RET, and P11 with an ERC1-RET fusion) had 131I-refractory progressive disease (Table 2). P1 and P8 were exposed to the fusion-targeted kinase inhibitor described below. The other child, a 9-year-old boy (P11) with an ERC1-RET fusion, showed mixed responses resulting in a progressive decrease in radioiodine uptake uptake during repeated high-dose 131I therapy (cumulative dose = 520 mCi, 5 times) (Supplemental Figure 2). He is currently planning to participate in a phase III clinical trial of fusion-targeted therapy.
Comparison of clinicopathological characteristics and outcomes between pediatric and adult PTCs.
We compared the clinicopathological presentation and outcomes between pediatric and adult patients at Seoul National University Hospital (11) according to whether the genetic driver was a fusion oncogene (NTRK1/3, RET, or ALK) or BRAFV600E. The pediatric fusion oncogen group (n = 31) had higher rates of ETE (Figure 2G) and BCD or SD (Figure 2H) than did the adult fusion oncogene group (n = 12). Although the adult BRAFV600E group (n = 68) had a higher rate of LN metastasis than the pediatric BRAFV600E group (n = 41), disease outcomes did not differ between the pediatric and adult BRAFV600E groups (Supplemental Table 6 and Figure 2, G and H). Recurrence-free survival was significantly higher in the pediatric BRAFV600E and adult BRAFV600E groups compared with the pediatric fusion group (P <0.05 for both, Figure 2I) When our pediatric data were compared with those in The Cancer Genome Atlas (TCGA) database, including the adult fusion group (n = 42) and BRAFV600E group (n = 241), we obtained similar results (Supplemental Table 7).
Comparison of gene expression levels between pediatric and adult PTCs.
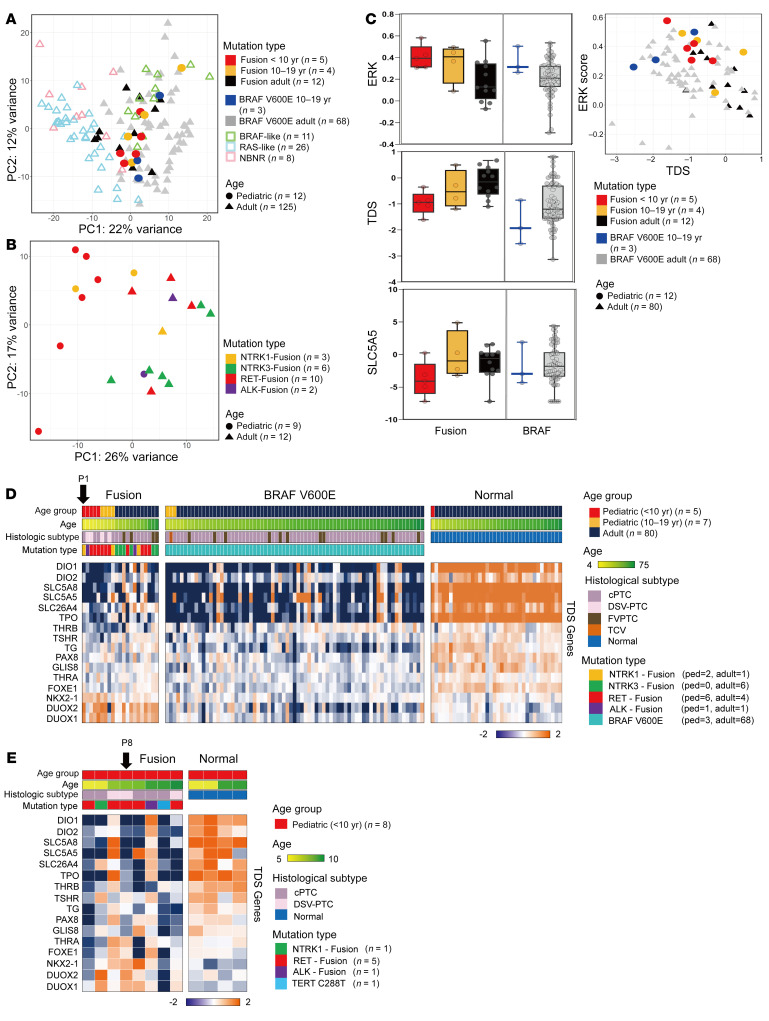
A more advanced presentation and worse outcome of oncogenic fusion PTCs and their predominance among younger patients imply distinct molecular characteristics that differ between pediatric and adult PTCs (12). The gene expression profiles of 9 oncogenic fusion PTCs from children clustered closer to those of the adult BRAF-like group, whereas adult PTCs harboring fusion oncogenes were scattered (Figure 3A) (11). Figure 3B shows age-associated clustering of oncogenic fusion PTCs. As indicated in Figure 3C and Supplemental Figure 3, pediatric oncogenic fusion PTC patients, particularly those in the group under 10 years of age, showed higher expression of MAPK signaling pathway genes (ERK score) and lower expression of genes related to thyroid differentiation (thyroid differentiation score [TDS]) than did the adult fusion PTC groups (11, 13). We also found higher ERK scores and lower TDSs in the pediatric BRAFV600E group than in the adult BRAFV600E group (Figure 3C). Transcriptomic expression analysis of individual TDS genes demonstrated that several genes, including SLC5A5, SLC26A4, SLC5A8, DIO1, and DIO2, tended to have lower expression levels in pediatric fusion PTCs (<10 years of age) compared with adult fusion PTCs (Figure 3D). Notably, the expression of SLC5A5 (sodium-iodide symporter [NIS]), which is an important determinant of 131I avidity, also decreased in childhood fusion PTCs. However, the difference was not significant, given the limited number of fresh tissues; therefore, we also explored the lower TDSs and lower expression levels of the SLC5A5 gene in pediatric fusion tumors compared with normal tissues by analyzing the formalin-fixed, paraffin-embedded (FFPE) samples of 8 fusion PTCs from young children (Figure 3E). Remarkably, the two 131I-refractory progressive PTC patients had very low expression of SLC5A5 in their tumor tissues (P1 in Figure 3D, and P8 in Figure 3E).
Figure 3. Comparison of expression signatures between pediatric and adult PTCs.
(A and B) Results of K-means clustering (obtained via principal component analysis). (A) Comparison between 12 pediatric PTCs (9 fusion oncogenes and 3 BRAFV600E PTCs) and 125 adult PTCs, including BRAF-like, RAS-like, and non-BRAF–non-RAS (NBNR). (B) Comparison between pediatric (n = 9) and adult (n = 12) PTCs with fusion oncogenes. The patients’ ages and mutation types are represented by shapes and colors, respectively. (C) Box plots (left) show the ERK score, TDS, and SLC5A5 (NIS) analysis results. Scatterplot (right) shows the results of the TDS and ERK score analysis. (D) Heatmap shows the expression levels of 16 TDS genes associated with thyroid function and metabolism. Comparison of TDS gene expression levels between the pediatric (ped) and adult fusion groups and between the pediatric and adult BRAFV600E groups using fresh-frozen tissue samples. cPTC, classic variant PTC; DSV-PTC, diffuse sclerosing variant PTC; FVPTC, follicular variant PTC; TCV, tall cell variant PTC. (E) Comparison of TDS genes between pediatric PTCs and normal thyroid tissues based on an analysis of FFPE samples. Two young girls (P1 and P8) with progressive 131I-refractory lung metastasis had low expression of SCL5A5 in their tumor tissues.
Larotrectinib decreases the tumor size and restores radioiodine uptake in 131I-refractory progressive metastatic TPR-NTRK1 fusion–positive pediatric PTCs.
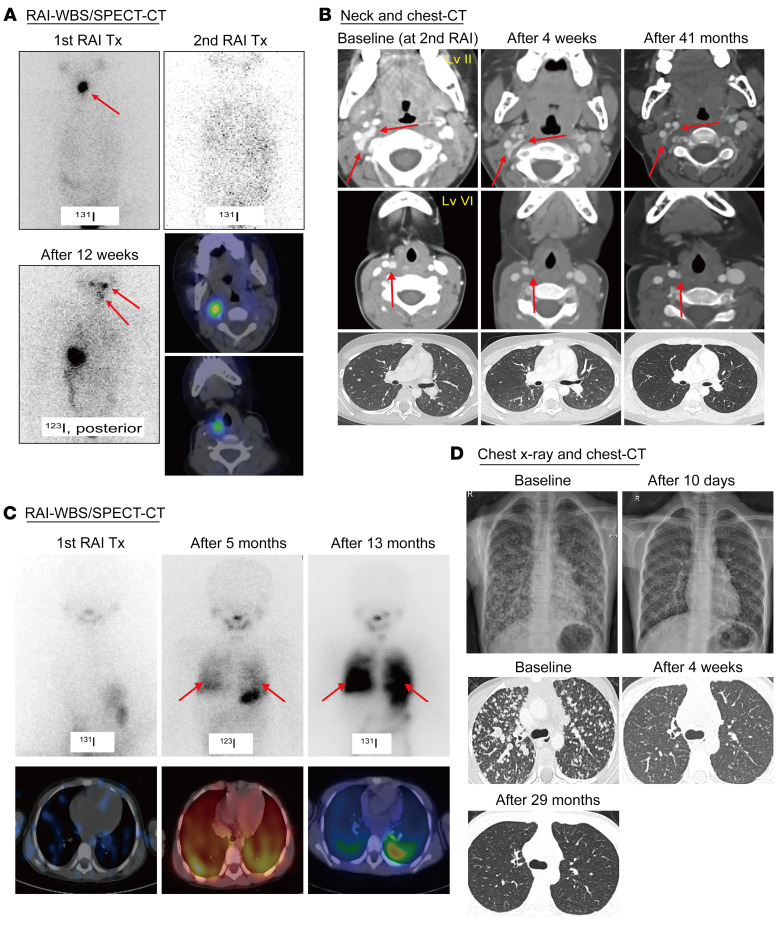
A 4.3-year-old girl (P1 in Table 2) was diagnosed with a 3.6 cm classic variant PTC with extensive LN involvement and lung metastases. She underwent a total thyroidectomy and neck dissection, followed by administration of 30 mCi (0.06 GBq/kg) 131I. The post-treatment whole-body scan (WBS) revealed remnant thyroid uptake only (Figure 4A, upper left). No 131I uptake was identified on the post-treatment scan after the second dose of 30 mCi (Figure 4A, upper right), despite locoregional recurrence and progressive lung disease (Figure 4B, baseline). The patient’s thyrotropin-stimulating hormone (TSH-stimulated) serum thyroglobulin level was 1150 ng/mL. We identified a TPR-NTRK1 rearrangement and initiated larotrectinib at 100 mg orally, twice daily (per the NAVIGATE trial protocol; NCT02576431). CT revealed a dramatic improvement in the LN and lung metastases after 4 weeks (Figure 4B) and a complete response at 21 months, according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. After 12 weeks of therapy, radioiodine uptake was shown to be restored in the neck and lungs by a diagnostic 123I scan (Figure 4A, lower left and right). The patient did not undergo 131I therapy because of her participation in the clinical trial and has remained responsive, without dose-limiting toxicity at 41 months (Figure 4B).
Figure 4. Selective fusion-targeted therapy decreased the tumor size and restored radioiodine uptake in 131I-refractory progressive metastatic pediatric PTCs.
Radioactive iodine (RAI) WBS and single-photon emission computed tomography (SPECT) CT scans of a 4.3-year-old girl with TPR-NTRK1 fusion–positive PTC (A and B) and a 7.4-year-old girl with CCDC6-RET fusion–positive PTC (C and D). (A) The post-treatment WBS showed remnant thyroid uptake only. Radioiodine uptake was restored in the cervical LN and lung lesions after 12 weeks of larotrectinib therapy. (B) A CT scan revealed a dramatic improvement in the LN and lung target lesions (decreased to 35% of baseline) after 4 weeks. The patient achieved complete remission after 21 months and remained responsive, with no dose-limiting toxicity seen during 41 months of larotrectinib therapy. (C) The post-treatment WBS revealed minimal uptake of radioiodine in the lungs. Radioiodine uptake was restored in the entire lung field after 5 months of selpercatinib treatment (Tx). The addition of 131I (60 mCi) 13 months after starting selpercatinib treatment resulted in remarkable radioiodine uptake in the lung field. (D) Lung lesions were markedly improved according to a chest radiograph done 10 days later and decreased to 42.9% of baseline on a CT scan after 4 weeks. The patient achieved partial remission after 4 weeks and remained responsive, with no dose-limiting toxicity seen during 29 months of selpercatinib therapy.
Selpercatinib decreases the tumor size and restores radioiodine uptake in 131I-refractory progressive metastatic CCDC6-RET fusion–positive pediatric PTC.
A 7.4-year-old girl (P8 in Table 2) was diagnosed with a 2.8 cm DSV PTC with LN involvement and lung metastases. She underwent total thyroidectomy and neck dissection, followed by the administration of 50 mCi (0.11 GBq/kg) 131I. The post-treatment WBS identified minimal lung uptake (Figure 4C, left). Her TSH-stimulated serum thyroglobulin level was 5990 ng/mL. After 4 months, locoregional recurrence and progressive lung metastases were detected (Figure 4D, baseline). We identified a CCDC6-RET rearrangement and initiated selpercatinib at 80 mg orally, twice daily (LOXO-RET-18018). The lung lesions were markedly decreased in size according to a chest radiograph on day 10 (Figure 4D, upper right). Since achieving a partial response after 4 weeks according to RECIST, version 1.1 (Figure 4D, lower middle), the patient has remained responsive, with no dose-limiting toxicity. Radioiodine uptake was restored in the lung on a diagnostic 123I scan at 5 months (Figure 4C, middle), which enabled administration of 60 mCi (0.11GBq/kg) 131I combined with selpercatinib, leading to remarkable radioiodine uptake in the entire lung field at 13 months (Figure 4C, right) and a TSH-stimulated serum thyroglobulin level of 1930 ng/mL. 131I therapy of 60 mCi (0.11 GBq/kg) was additionally administered after 19 months of the selpercatinib therapy, leading to persistent radioiodine uptake in the lung field with a TSH-stimulated serum thyroglobulin level of 855 ng/mL. CT revealed stable lung disease at 29 months (Figure 4D, lower right).
In vitro effects of larotrectinib on tumor growth and radioiodine uptake capacity.
The restoration of radioiodine uptake in 131I non-avid lesions after larotrectinib and selpercatinib treatment implies that these selective inhibitors not only abrogate cellular proliferation but also induce the restoration of iodine uptake and processing in these cancers, similar to what was previously reported on MAPK inhibitors (14–16).
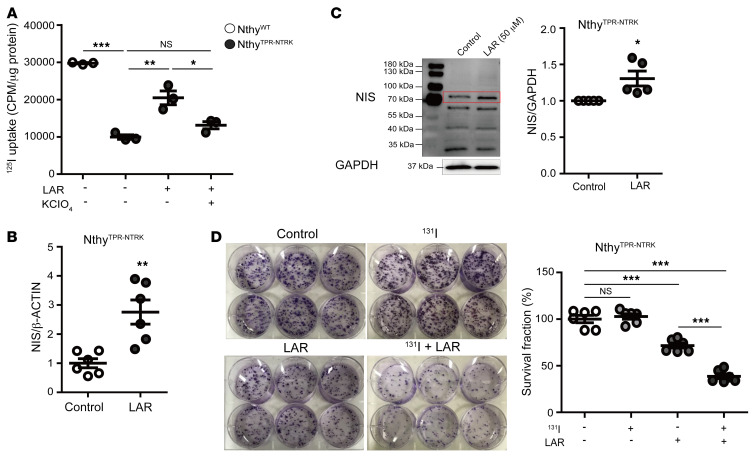
In vitro experiments showed that basal 125I uptake was markedly decreased in NthyTPR-NTRK cells compared with control NthyWT cells, but was restored by larotrectinib treatment (Figure 5A and Supplemental Figure 4, A and B). This larotrectinib-induced restoration was mediated by the NIS, as indicated by the effects being blocked by potassium perchlorate (KCIO4), a competitive inhibitor of iodide transport through the NIS (Figure 5A and Supplemental Figure 4C). A trend toward increased expression of the NIS at the mRNA and protein levels was associated with larotrectinib treatment in NthyTPR-NTRK cells (Figure 5, B and C, and Supplemental Figure 4D), but not in NthyWT cells (Supplemental Figure 4D). To evaluate whether larotrectinib treatment enhances the therapeutic effect of 131I, we pretreated NthyTPR-NTRK cells with larotrectinib followed by 100 μCi of 131I. Although 131I alone did not suppress the colony-forming ability in NthyTPR-NTRK cells, larotrectinib alone inhibited colony formation. Moreover, the combination of 131I and larotrectinib further enhanced the inhibition of colony forming (Figure 5D and Supplemental Figure 4E).
Figure 5. In vitro effects of larotrectinib on radioiodine uptake capacity and cell growth.
(A) Baseline 125I uptake decreased in NthyTPR-NTRK cells compared with NthyWT cells but was restored by larotrectinib treatment mediated by the NIS; this was demonstrated by blocking the effects with KCIO4. Expression of the NIS at the mRNA (B) and protein (C) levels tended to increase in NthyTPR-NTRK cells with larotrectinib (50 μM) treatment. (D) The colony-forming ability of NthyTPR-NTRK cells did not change after 131I therapy alone but decreased after larotrectinib treatment, and then further decreased after combined 131I and larotrectinib therapy. *P < 0.05, **P < 0.01, and ***P < 0.001, by Student’s t test or 1-way ANOVA with Bonferroni’s multiple-comparison test. All data represent the mean ± standard deviation. LAR, larotrectinib (50 μM).
Discussion
Our comprehensive genomic analysis revealed age-associated driver profiles of pediatric PTC. Oncogenic fusions predominated in children with PTCs under 10 years of age, after which the frequency decreased to levels similar to those seen in adults. Furthermore, the incidence of driver point mutations increased with age and became common in adolescents aged 15–19 years, as in adults. Pediatric patients with oncogenic fusion PTCs presented with more advanced disease and had worse outcomes than did pediatric patients with BRAFV600E PTCs. The transcriptomic data showed that pediatric oncogenic fusion PTCs in young children under 10 years of age had a lower TDS (including NIS expression) than did adult fusion PTCs. NTRK and RET fusion–targeted therapy with larotrectinib or selpercatinib yielded a remarkable tumor response and restored radioiodine uptake in 2 pediatric patients with 131I-refractory progressive PTCs harboring TPR-NTRK1 and CCDC6-RET fusion oncogenes, respectively.
The detection rate of genetic alterations was 75.5% in our pediatric PTC population with the use of NGS, FISH, and IHC. To our knowledge, this was the largest pediatric study to date showing age-associated genetic alterations, consistent with a pooled analysis of previously reported cases, including a large recent pediatric study of 93 patients that used DNA and RNA-Seq (Supplemental Table 4) (2, 17). Oncogenic fusions accounted for the majority of cases among children under 10 years of age, while BRAFV600E was the most common driver in adolescents, with a frequency similar to that seen in adults (13, 18). DICER1 was the second most common point mutation, a finding consistent with a recent pediatric study (19). However, TERT promoter and RAS mutations were uncommon, in line with previous pediatric reports (Supplemental Table 4) (2–7, 17, 20–22).
The etiology of age-associated genetic alterations remains unexplained, although chromosomal rearrangements have a strong association with exposure to ionizing radiation, whereas BRAFV600E point mutations may be linked to excess dietary iodine intake or exposure to chemical elements in volcanic areas (23). DNA fragility and repair defects have been suggested as mechanisms for radiation-induced genetic changes or spontaneous oncogenic fusion (4, 5, 24). The thyroid cells of young children may be more susceptible to the effects of ionizing radiation and/or lose key factors in the DNA repair machinery, leading to uncoupled double-stranded breaks and translocation with partner genes (24). Analysis of post- analysis of post-Chernobyl thyroid cancers showed that the mean age at radiation exposure was lower in patients with tumors harboring oncogenic fusion genes (7.1 years) than in those with tumors harboring point mutations (10.9 years) (25). As almost all patients under 10 years of age with sporadic PTC also harbored oncogenic fusions in this study, further analysis to determine as-yet unknown risk factors for the development of fusions is needed.
Children with oncogenic fusion PTCs presented with more advanced-stage disease; 42% of the children had lung metastasis and a higher risk for recurrence or persistence than did those with BRAFV600E PTC. In particular, among 13 patients with persistent lung metastasis, the disease was stable in 10 patients, while it progressed in 3, despite 131I therapy. Consistent with previous reports (4, 21), the lower TDSs and higher ERK scores demonstrate the aggressiveness of oncogenic fusion PTCs in young children. Although the influence of the BRAFV600E mutation alone on tumor aggressiveness remains controversial (21), synergistic effects of TERTC228T/C250T and BRAFV600E mutations have been shown to lead to a worse prognosis in patients with PTC (26). Therefore, the very low frequency of TERTC228T/C250T mutations in pediatric PTCs (2, 3, 5, 7, 17, 20) may explain the less aggressive behavior of pediatric BRAFV600E PTCs (21). The reason for the more aggressive nature of pediatric oncogenic fusion PTCs remains unclear.
The low expression of SLC5A5 (NIS) could explain the radioiodine refractoriness of these tumors. A similar downregulation of thyroid differentiation genes, including SLC5A5, has been reported in cases of post-Chernobyl oncogenic fusion PTC (8), although the reported effects of oncogenic fusions on thyroid cancer dedifferentiation are inconsistent (27). It is important to elucidate the genetic alterations and corresponding targeted drugs that most affect the response to 131I therapy depending on NIS expression. In this study, 2 young girls with 131I-refractory progressive PTC and markedly decreased NIS expression exhibited dramatic responses to oncogenic fusion–targeted therapy, which not only decreased tumor size but also restored radioiodine uptake.
The tumor responses in this study were consistent with previous reports of patients with TRK fusion–positive thyroid cancer treated with larotrectinib (9, 28) and a recent report on patients with RET-altered medullary thyroid cancer treated with selpercatinib (29). Surprisingly, however, the combination of selpercatinib and 131I therapy enhanced radioiodine uptake and yielded a remarkable tumor response in a girl with PTC harboring a CCDC6-RET fusion, implying that selpercatinib could be an effective redifferentiation therapy in 131I-refractory advanced tumors harboring the RET fusion oncogene. The treatment response decreased after the third 131I treatment in a 9-year-old boy (P11) recruited for a clinical trial of fusion-targeted therapy. Assuming restoration of 131I avidity in the girl harboring the RET fusion oncogene, it would have been helpful if the 9-year-old boy had received selpercatinib in combination with the third 131I treatment. In vitro experiments also support the efficacy of larotrectinib for restoring NIS expression and radioiodine avidity, in addition to inhibiting tumor growth. Therefore, this study supports further investigation of fusion-targeted therapy for redifferentiation of 131I-refractory progressive thyroid cancer (14–16). Our pediatric cases are in agreement with a recent adult case report on larotrectinib-enhanced 131I uptake in advanced PTC (30). Considering the predominance of oncogenic fusions in pediatric patients with PTC and their association with tumor aggressiveness, recently developed, potent, and specific kinase inhibitors targeting oncogenic fusions in PTC could be the optimal therapeutic option for 131I-refractory advanced PTCs in children. Furthermore, reactivation of radioiodine uptake indicates that 131I therapy combined with fusion-targeted therapy can be effective in previously 131I-refractory PTC patients (30). Targeted oncogene therapies before surgery may induce tumor regression in cases of invasive thyroid cancer (31). Diagnostic molecular testing to detect driver oncogenic fusions and point mutations is becoming imperative in such cases.
This study was limited by the small number of fresh pediatric tissue samples, so the difference in gene expression between pediatric and adult PTCs needs to be further replicated in a large-sample study. To date, there are no available published data on pediatric PTCs allowing comparison of gene expression with that in adult PTCs. In addition, various methods were applied to identify the genetic alterations, due to issues with tissue availability and sample quality, particularly in the NGS-failed FFPE tissue samples. Although the use of FISH, IHC, and direct sequencing is beneficial for detecting RET or ALK fusions and the DICER1 variant (Supplemental Table 1), there may have been unidentified genetic drivers in the NGS-failed samples (n = 15), leading to an underestimation of the detection rate in our study. Nonetheless, to our knowledge, this is the first pediatric study to show that fusion-targeted therapy reactivated radioiodine uptake and inhibited tumor growth in patients with 131I-refractory PTC. In addition, we performed an in vitro experiment to demonstrate the role of fusion-targeted therapy in restoring NIS expression and radioiodine uptake.
In summary, oncogenic fusions are the main genetic drivers of PTCs identified in young children. Selective fusion-targeted therapy may restore radioiodine avidity, as well as produce a tumor response in pediatric fusion oncogene PTCs with 131I-refractory advanced characteristics, making molecular testing imperative for pediatric patients presenting with advanced PTC.
Methods
Patients and tissue samples.
In total, we analyzed 106 tumor tissue samples from pediatric patients with PTC, obtained at the SNUH between January 1983 and March 2020 (Figure 1). Fresh-frozen tumor tissue samples were obtained from 12 pediatric patients under the age of 20 years. The transcriptomic data on adult patients aged 20 years or older (125 cases at SNUH) were analyzed to compare gene expression profiles (11). Detailed information on the treatment and follow-up strategies was obtained (12), and disease outcomes were categorized as no evidence of disease (NED), BCD, or persistent or recurrent SD (32), as described in the Supplemental Methods.
Genomic profiling by NGS, direct sequencing, FISH, and/or IHC.
Genetic analysis was performed using whole-genome sequencing (WGS), targeted sequencing, RNA-Seq, direct sequencing, FISH, and/or IHC according to the tissue availability (Figure 1 and Supplemental Table 1). RNA-Seq libraries of fresh-frozen and FFPE tissues were constructed using the TruSeq RNA Library Preparation Kit, version 2, and the TruSeq RNA Access Library Preparation Kit (both from Illumina), respectively. The TruSeq Nano DNA Preparation Kit (Illumina) was used to construct the library for WGS. The subsequent NGS analysis is described in the Supplemental Methods. The BRAF exon 15, TERT promoter (C228T and C250T) region, H/K/NRAS codons 12, 13, and 61, and the sequence encoding the DICER1 RNase IIIb domain were amplified by PCR using the appropriate primers for direct sequencing of the BRAF, RAS, DICER1, and TERT genes (Supplemental Table 8) (18). Supplemental Table 9 lists the target genes for the SNUH FIRST cancer panel (version 3). The FISH probe for the NTRK and RET rearrangements and the antibodies for IHC of BRAFV600E, NRAS Q61R, ALK, and pan-Trk are described in the Supplemental Methods.
Cell culturing and in vitro assays.
The human TPR-NTRK1 expression vector was constructed by subcloning the corresponding cDNAs into the pcDNA6/V5-His A expression vector (Thermo Fisher Scientific) (Supplemental Figure 5 and Supplemental Table 5). The pcDNA6/V5-His A-TPR-NTRK1 fusion construct NthyTPR-NTRK and the unmodified vector control pcDNA6/V5-His A (NthyWT) were transfected into N-thyroid cells (European Collection of Authenticated Cell Cultures [ECACC]). The degree of overexpression of NthyTPR-NTRK cells was similar to that seen in NTRK fusion cancer, based on comparison of the NTRK mRNA levels in NthyWT cells, normal thyroid tissue, NthyTPR-NTRK cells, and thyroid cancer tissues with a TPR-NTRK fusion (Supplemental Figure 6).
After incubation with larotrectinib (provided by Bayer AG), mRNA and protein expression levels and 125I uptake were analyzed. 131I clonogenic assays were performed in NthyTPR-NTRK or NthyWT transfected cells, as described previously (33). NIS mRNA and protein expression was analyzed by reverse transcriptase PCR (RT-PCR) (using the appropriate primers; see Supplemental Table 8) and immunoblotting with an anti-NIS antibody (Thermo Fisher Scientific), respectively.
Statistics.
All analyses were performed using SPSS software for Windows (version 25.0). Differences in continuous variables were compared between 2 groups using a 2-tailed Student’s t test or a Mann–Whitney U test. Categorical variables were compared between the 2 groups using the χ2 or Fisher’s exact test, whereas the χ2 test for trend or logistic regression was used for comparisons among 3 groups. Recurrence-free survival plots were constructed using the Kaplan-Meier method, and groups were compared using the Cox proportional hazards model. The HRs, 95% CIs, and P values are reported in the figures and legends. A P value of less than 0.05 was considered significant.
Data availability.
The RNA-Seq data set produced in this study was deposited in the NCBI’s Sequence Read Archive (SRA PRJNA701374).
Study approval.
Written informed consent for participation in this study was obtained from patients or their parents or guardians, as required by the SNUH IRB (approval IDs: H-1307-034-501, 1505-023-670).
Author contributions
YAL, JIK, and YJP conceived and designed the study. HL, SWI, and JC analyzed NGS data under the supervision of JIK. YAL, DYO, HJK, JKW, KCJ, DK, EJC, JHH, JCP, JHK, CHS, and YJP delivered multidisciplinary therapy to pediatric patients with thyroid cancer, prepared tissue samples, explored the pathologies in play, and collected imaging data. In particular, DYO and HJK delivered fusion-directed targeted therapy. OHK, JMO, and BCA performed the in vitro experiment. YAL, HL, SWI, YSS, JIK, and YJP drafted the manuscript. LJW contributed to data interpretation and manuscript revision. All authors contributed to multiple revisions of the manuscript and approved the final version. The corresponding authors, JIK and YJP, had full access to all the data in the study and had final responsibility for the decision to submit it for publication.
Supplementary Material
Acknowledgments
All research, except the clinical trials for the 2 patients with progressive 131I-refractory lung metastases harboring a TPR-NTRK1 or CCDC6-RET fusion who received fusion-targeted therapy with larotrectinib (NCT02576431; NAVIGATE) or selpercatinib (LOXO-RET-18018), was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning (NRF-2016R1A2B4012417 and 2019R1A2C2084332); a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (H14C1277); and the Basic Science Research Program through the NRF, funded by the Ministry of Education (2020R1A6A1A03047972). This study was also supported by the SNUH Research Fund (grant no. 04-2015-0830). The authors thank Bayer AG and Eli Lilly and Company for medical monitoring of the patients treated with larotrectinib and selpercatinib, respectively. The authors especially thank Jeong Seon Lee, Hwan Hee Kim, Gyeongseo Jung, and Sookyung Kim for sample preparation and molecular analyses.
Version 1. 07/08/2021
In-Press Preview
Version 2. 09/15/2021
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(18):e144847. https://doi.org/10.1172/JCI144847.
See the related Commentary at Oncogene-specific inhibition in the treatment of advanced pediatric thyroid cancer.
Contributor Information
Young Ah Lee, Email: nina337@snu.ac.kr.
Hyunjung Lee, Email: hyunjunglee@snu.ac.kr.
Sun-Wha Im, Email: first0061@hanmail.net.
Young Shin Song, Email: yssongmd@gmail.com.
Do-Youn Oh, Email: ohdoyoun@snu.ac.kr.
Hyoung Jin Kang, Email: kanghj@snu.ac.kr.
Jae-Kyung Won, Email: jkyung.won@gmail.com.
Kyeong Cheon Jung, Email: jungkc66@snu.ac.kr.
Dohee Kwon, Email: kwondh11@gmail.com.
Eun-Jae Chung, Email: voicechung@snu.ac.kr.
J. Hun Hah, Email: jhunhah@gmail.com.
Jin Chul Paeng, Email: paengjc@snu.ac.kr.
Ji-hoon Kim, Email: jihnkim@gmail.com.
Jaeyong Choi, Email: mesnger@nate.com.
Ok-Hee Kim, Email: ohkim323@gmail.com.
Ji Min Oh, Email: ojm0366@naver.com.
Byeong-Cheol Ahn, Email: abc2000@knu.ac.kr.
Lori J. Wirth, Email: LWIRTH@mgh.harvard.edu.
Choong Ho Shin, Email: chshinpd@snu.ac.kr.
Jong-Il Kim, Email: jongil@snu.ac.kr.
Young Joo Park, Email: yjparkmd@snu.ac.kr.
References
- 1.Bauer AJ. Molecular genetics of thyroid cancer in children and adolescents. Endocrinol Metab Clin North Am. 2017;46(2):389–403. doi: 10.1016/j.ecl.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Paulson VA, et al. Thyroid cancer in the pediatric population. Genes (Basel) 2019;10(9):E723. doi: 10.3390/genes10090723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pozdeyev N, et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res. 2018;24(13):3059–3068. doi: 10.1158/1078-0432.CCR-18-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prasad ML, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer. 2016;122(7):1097–1107. doi: 10.1002/cncr.29887. [DOI] [PubMed] [Google Scholar]
- 5.Picarsic JL, et al. Molecular characterization of sporadic pediatric thyroid carcinoma with the DNA/RNA ThyroSeq v2 next-generation sequencing assay. Pediatr Dev Pathol. 2016;19(2):115–122. doi: 10.2350/15-07-1667-OA.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballester LY, et al. Integrating molecular testing in the diagnosis and management of children with thyroid lesions. Pediatr Dev Pathol. 2016;19(2):94–100. doi: 10.2350/15-05-1638-OA.1. [DOI] [PubMed] [Google Scholar]
- 7.Pekova B, et al. Somatic genetic alterations in a large cohort of pediatric thyroid nodules. Endocr Connect. 2019;8(6):796–805. doi: 10.1530/EC-19-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricarte-Filho JC, et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest. 2013;123(11):4935–4944. doi: 10.1172/JCI69766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drilon A, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drilon A, et al. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 2018;15(3):150. doi: 10.1038/nrclinonc.2017.188. [DOI] [PubMed] [Google Scholar]
- 11.Yoo SK, et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016;12(8):e1006239. doi: 10.1371/journal.pgen.1006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YA, et al. Pediatric patients with multifocal papillary thyroid cancer have higher recurrence rates than adult patients: a retrospective analysis of a large pediatric thyroid cancer cohort over 33 years. J Clin Endocrinol Metab. 2015;100(4):1619–1629. doi: 10.1210/jc.2014-3647. [DOI] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–90. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho AL, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368(7):623–632. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothenberg SM, et al. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015;21(5):1028–1035. doi: 10.1158/1078-0432.CCR-14-2915. [DOI] [PubMed] [Google Scholar]
- 16.Dunn LA, et al. Vemurafenib redifferentiation of BRAF mutant, RAI-refractory thyroid cancers. J Clin Endocrinol Metab. 2019;104(5):1417–1428. doi: 10.1210/jc.2018-01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pekova B, et al. RET, NTRK, ALK, BRAF and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid. 2020;30(12):1771–1780. doi: 10.1089/thy.2019.0802. [DOI] [PubMed] [Google Scholar]
- 18.Song YS, et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer. 2016;122(9):1370–1379. doi: 10.1002/cncr.29934. [DOI] [PubMed] [Google Scholar]
- 19.Wasserman JD, et al. DICER1 mutations are frequent in adolescent-onset papillary thyroid carcinoma. J Clin Endocrinol Metab. 2018;103(5):2009–2015. doi: 10.1210/jc.2017-02698. [DOI] [PubMed] [Google Scholar]
- 20.Alzahrani AS, et al. Uncommon TERT promoter mutations in pediatric thyroid cancer. Thyroid. 2016;26(2):235–241. doi: 10.1089/thy.2015.0510. [DOI] [PubMed] [Google Scholar]
- 21.Alzahrani AS, et al. Single point mutations in pediatric differentiated thyroid cancer. Thyroid. 2017;27(2):189–196. doi: 10.1089/thy.2016.0339. [DOI] [PubMed] [Google Scholar]
- 22.Cordioli MI, et al. Fusion oncogenes are the main genetic events found in sporadic papillary thyroid carcinomas from children. Thyroid. 2017;27(2):182–188. doi: 10.1089/thy.2016.0387. [DOI] [PubMed] [Google Scholar]
- 23.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7(10):569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 24.Roukos V, Misteli T. The biogenesis of chromosome translocations. Nat Cell Biol. 2014;16(4):293–300. doi: 10.1038/ncb2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Efanov AA, et al. Investigation of the relationship between radiation dose and gene mutations and fusions in post-Chernobyl thyroid cancer. J Natl Cancer Inst. 2018;110(4):371–378. doi: 10.1093/jnci/djx209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing M, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32(25):2718–2726. doi: 10.1200/JCO.2014.55.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romei C, et al. BRAFV600E mutation, but not RET/PTC rearrangements, is correlated with a lower expression of both thyroperoxidase and sodium iodide symporter genes in papillary thyroid cancer. Endocr Relat Cancer. 2008;15(2):511–520. doi: 10.1677/ERC-07-0130. [DOI] [PubMed] [Google Scholar]
- 28.Hong DS, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21(4):531–540. doi: 10.1016/S1470-2045(19)30856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wirth LJ, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383(9):825–835. doi: 10.1056/NEJMoa2005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groussin L, et al. Larotrectinib-enhanced radioactive iodine uptake in advanced thyroid cancer. N Engl J Med. 2020;383(17):1686–1687. doi: 10.1056/NEJMc2023094. [DOI] [PubMed] [Google Scholar]
- 31.Kazahaya K, et al. Targeted oncogene therapy before surgery in pediatric patients with advanced invasive thyroid cancer at initial presentation: is it time for a paradigm shift? JAMA Otolaryngol Head Neck Surg. 2020;146(8):748–753. doi: 10.1001/jamaoto.2020.1340. [DOI] [PubMed] [Google Scholar]
- 32.Jeon MJ, et al. Practical initial risk stratification based on lymph node metastases in pediatric and adolescent differentiated thyroid cancer. Thyroid. 2018;28(2):193–200. doi: 10.1089/thy.2017.0214. [DOI] [PubMed] [Google Scholar]
- 33.Oh JM, et al. Reverting iodine avidity of radioactive-iodine refractory thyroid cancer with a new tyrosine kinase inhibitor (K905-0266) excavated by high-throughput NIS (sodium iodide symporter) enhancer screening platform using dual reporter gene system. Oncotarget. 2018;9(6):7075–7087. doi: 10.18632/oncotarget.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-Seq data set produced in this study was deposited in the NCBI’s Sequence Read Archive (SRA PRJNA701374).