Abstract
Deltamethrin (DLM) is a Type II pyrethroid pesticide widely used in agriculture, homes, public spaces, and medicine. Epidemiological studies report that increased pyrethroid exposure during development is associated with neurobehavioral disorders. This raises concern about the safety of these chemicals for children. Few animal studies have explored the long-term effects of developmental exposure to DLM on the brain. Here we review the CNS effects of pyrethroids, with emphasis on DLM. Current data on behavioral and cognitive effects after developmental exposure are emphasized. Although, the acute mechanisms of action of DLM are known, how these translate to long-term effects is only beginning to be understood. But existing data clearly show there are lasting effects on locomotor activity, acoustic startle, learning and memory, apoptosis, and dopamine in mice and rats after early exposure. The most consistent neurochemical findings are reductions in the dopamine transporter and the dopamine D1 receptor. The data show that DLM is developmentally neurotoxic but more research on its mechanisms of long-term effects is needed.
Keywords: pyrethroids, deltamethrin, permethrin, developmental neurotoxicity, learning and memory
1. Introduction
Deltamethrin (DLM) is a Type II pyrethroid pesticide used worldwide in agricultural, medical, and household applications. As the use of pyrethroids increases, more people, including children, are exposed. There are epidemiological studies reporting associations between childhood pyrethroid exposure and neurodevelopmental disorders, including attention deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and developmental delay (DD) (Oulhote and Bouchard, 2013; Shelton et al., 2014; Wagner-Schuman, 2015; Xue et al., 2013). Despite the human evidence, there are few studies on long-term pyrethroid effects in preclinical developmental models to identify potential mechanisms (Aziz et al., 2001; Hossain, 2015; Johri et al., 2006; Pitzer, 2019; Richardson, 2015). While much is known about pyrethroid mechanism of poisoning, little is known about possible mechanisms of long-term effects. Of the many pyrethroids, DLM is one of the most investigated and is the principal focus of this review. But before reviewing the animal data on the developmental neurotoxicity of DLM, we summarize the findings in children to help put the animal data in context.
2. Humans
People are exposed to pyrethroids because of their widespread use in agriculture, households, schools, parks, apartments, and work settings to exterminate insects and prevent insect intrusion. Pyrethroids are also applied to children for head lice and to pets for flea and tick control. Most insect sprays and termiticides contain pyrethroids. Pest management companies report using approximately 1.7 million lbs of pyrethroids (~30,000 lbs of DLM) for household pests and over 1.1 million lbs of pyrethroids for lawn and landscape care (EPA, 2020). People are also exposed to pyrethroid residues in food, particularly on fruits and vegetables. In addition, ~200,000 lbs of pyrethroids (10,000 lbs. of DLM) are used for food handling, ~7,600 lbs of DLM to treat crops, and ~1,600 lbs to treat stored grains. The use of pyrethroids in agriculture is widespread and increasing as older compounds are replaced. During the early 2000s the U.S. Environmental Protection Agency (EPA) began phasing out some organophosphate pesticides due to their toxicity. This resulted in increased use of pyrethroids (Oros and Werner, 2005; Williams et al., 2008). Human exposure to pyrethroids is ubiquitous, including to pregnant women and children (Berkowitz et al., 2003; Heudorf et al., 2004; Schettgen et al., 2002; Whyatt et al., 2002). However, an understanding of the developmental effects of pyrethroids is limited, which makes developing a thorough risk assessment more difficult. In regulating pesticide exposures, the EPA uses animal data, such as data from a developmental neurotoxicity study and applies uncertainty factors of 1, 3, or 10-fold below the no observable adverse effect level or the benchmark dose-low set at 2.5, 5, or 10% below the benchmark dose response. Three uncertainty factors are normally used. One for within species variability, one for cross-species variability, and one for human inter-individual differences. In addition, the Food Quality Protection Act (FQPA, 1996) requires an additional 10X uncertainty factor if susceptible subpopulations are identified. The most common reason for this imposed uncertainty factor is when there is evidence of increased prenatal or neonatal susceptibility compared with adults. Here we review evidence that embryos, fetuses, and children are more susceptible to DLM than adults. However, there is insufficient evidence to make similar comparisons for most of the pyrethroids.
Although pesticides are beneficial in controlling agricultural and disease-bearing pests, they pose differential risks to fetuses and children compared with adults. Developing brains are more vulnerable because of the many cellular changes occurring as these processes unfold that can potentially be permanently altered. In addition, children have greater contact with their surroundings than adults. This is from increased contact with environmental surfaces and greater hand-to-mouth behavior than occurs in adults. Children also have less mature blood brain barriers (BBBs) and slower rates of clearance because of immature metabolizing enzymes than adults (Allegaert and van den Anker, 2019; Amaraneni et al., 2017a; Anand, S.S., Kim, K. B., Padilla, S., Muralidhara, S., Kim, H. J., Fisher, J. W., Bruckner, J. V., 2006; Chen et al., 2015; Mortuza et al., 2018). Children also have less body fat to sequester compounds, greater free fractions in circulation because of less plasma protein binding; and ongoing brain development, including proliferation, migration, synaptogenesis, gliogenesis, apoptosis, myelination, and activity-dependent connectivity, processes that are more susceptible to perturbation than in mature brains. This vulnerability begins during organogenesis; extends through the fetal period to birth; and postnatally through childhood, adolescence, and into early adulthood in humans as the brain fully develops. Any of the developmental processes occurring during these stages has the potential to be disrupted by toxic substances (Baloch et al., 2009; Bockhorst et al., 2008; Catalani et al., 2002; Cowan, 1979; Dobbing and Sands, 1979; Gottlieb et al., 1977; Hedner et al., 1986; Jiang and Nardelli, 2016; Miller and Gauthier, 2007; Romijn et al., 1991). Collectively, these factors indicate that prenatal and postnatal brain development are susceptible periods, an idea amply supported by the many studies on the long-term consequences of developmental exposure to polychlorinated biphenyls, lead, manganese, methylmercury, arsenic, organophosphate pesticides, and other environmental agents. Such data lead to the question of how safe are pyrethroids during brain ontogeny?
Epidemiological studies in children use standardized neurodevelopmental and neuropsychological tests in combination with urinary pyrethroid metabolites or other surrogate indices of exposure to compare neurodevelopmental disorders and pyrethroid exposure. A common pyrethroid urinary metabolite is 3-phenoxybenzoic acid (3-PBA). 3-PBA is the most widely used biomarker of pyrethroids but is less than ideal because it is non-specific since it is also a metabolite of Type I pyrethroids such as permethrin. Somewhat more specific metabolites are cis- and trans-DCCA (3-(2,2-dichlorovinyl)-2,2-dimethylycyclopropane carboxylic acid) for permethrin, cypermethrin, and cyfluthrin (Chrustek, Agnieszka et al., 2018; Meeker et al., 2009). A metabolite specific for DLM is urinary cis-DBCA (cis-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane carboxylic acid) (Barr et al., 2010; Chrustek, A. et al., 2018). Another metabolite of DLM and other Type II pyrethroids, cyfluthrin and α-cypermethrin, is 4-F-3-PBA (4-fluoro-3-phenoxybenzoic acid) (Chrustek, Agnieszka et al., 2018). The 4th National Report on Human Exposure to Environmental Chemicals compiles data from the National Health and Nutrition Examination Survey (NHANES). The most recent tables (1999-2010) reported 3-PBA levels in the population to be higher in more recent years than in 1999, 95th percentile and 95% confidence interval (CDC, 2019):
4.33 μg/L (2.62-6.30 μg/L; N=1998) for 1999-2000
3.54 μg/L (2.69-5.25 μg/L; N=3048) for 2001-2002
6.63 μg/L (4.99-8.24 μg/L; N=2454) for 2007-2008
6.50 μg/L (5.57-8.04 μg/L; N=2723) for 2009-2010
In addition, from the same report, 3-PBA levels in urine are higher in children than in adolescents or adults (2009-2010), ages:
6-11 = 8.51 μg/L (4.12-39.4 μg/L; N=383)
12-19 = 3.92 μg/L (2.55-5.85 μg/L; N=398)
20-59 = 6.95 μg/L (5.57-9.31 μg/L: N=1296)
60+ = 5.93 μg/L (3.21-8.95 μg/L: N=646)
The 3-PBA levels recorded from the NHANES differed across cycles. When observing levels of 3-PBA from 2007-2012, levels were the highest in the 2011-2012 NHANES cycle. Geometric mean of 3-PBA levels (Lehmler et al., 2020) are as follows:
Adults:
0.41 μg/L in 2007-2008
0.41 μg/L in 2009-2010
0.66 μg/L in 2011-2012
Children:
0.40 μg/L in 2007-2008
0.46 μg/L in 2009-2010
0.70 μg/L in 2011-2012
These data indicate that exposure to pyrethroids remains a public health concern. It will be critical to observe these metabolite levels as more recent measures from large scale studies, such as the NHANES, become available. However, the non-specificity of commonly used pyrethroid metabolites limits the interpretation of much of the human epidemiological data. Nevertheless, such data suggest that children chronically exposed to pyrethroids have compromised outcomes.
Data from the 1999-2002 NHANES have been used to search for associations between pyrethroid exposures and outcomes in children. A study that used NHANES parental report data that their children have ADHD or from records of prescriptions for ADHD medications, in conjunction with urinary 3-PBA levels showed an association between pyrethroid exposure and ADHD (Richardson et al., 2015). Using a nested case-control analysis for correlations between pyrethroid exposure and ADHD, a modest but significant association between urinary 3-PBA and ADHD prevalence was found. The study was based on 5489 children 6-15 years of age in which 9.2% had ADHD based on parental reports (Bouchard et al., 2010; Braun et al., 2006; Richardson et al., 2015). Of the 5489 ADHD cases, 3-PBA levels were available for 2123 and these concentrations were associated with ADHD with an odds ratio of 2.3 (1.4-3.9) (Richardson et al., 2015). No sex differences in this association were found.
NHANES data were also used in a study by Wagner-Schuman et al. (2015). Based on urinary 3-PBA data and diagnosis of ADHD (Diagnostic Interview Schedule for Children (DISC) from the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition or diagnosis by professionals with experience assessing ADHD), an association was found between pyrethroid exposure and ADHD in 687 children (ages 8-15; 2001-2002 NHANES) (Wagner-Schuman, 2015). Boys with urinary 3-PBA levels above background were twice as likely to be diagnosed with ADHD compared with boys with undetectable 3-PBA levels; no such association was found for girls (Wagner-Schuman, 2015). Wagner-Schuman et al. (2015) reported that for every 10-fold increase in metabolite there was a 50% increase in hyperactivity and impulsivity prevalence but no association with inattention. Overall, the data from the NHANES database reported in these studies indicate an increased likelihood of ADHD associated with elevated pyrethroid exposure.
The Childhood Autism Risks from Genetics and Environment (CHARGE) project is a population based case-control study testing factors associated with ASD (Hertz-Picciotto et al., 2006). Shelton et al. (2014) used data from the CHARGE project, including parental questionnaires about symptoms, exposures, and residential location to search for pesticide associations with ASD. The authors found an association between pesticide application locations, determined from California’s regional pesticide use database, and residential proximity of study participants to where exposed and non-exposed women lived during pregnancy. Using the geographical location, the authors’ determined whether there was an association between proximity to pesticide registrations and a child’s diagnoses with ASD or DD (Shelton et al., 2014). A third of the children lived within 1.5 km of where agricultural pesticides were registered for use, of which pyrethroids were the second most widely used pesticide after organophosphates. Children diagnosed with ASD (N=486; age 36.7 ± 9.7 months) were compared with typically developing children (N= 316; age 36.9 ± 8.9 months) and children with DD (N=168; age 38.3 ± 8.9 months) (Shelton et al., 2014). Children were matched for age, sex, and location. Shelton et al. (2014) found an association between preconception and third trimester pyrethroid and organophosphate exposure and increased prevalence of ASD. They also found an association between third trimester pesticide exposure and DD prevalence (Shelton et al., 2014). The dual association of pyrethroids and organophosphates on ASD prevalence makes it impossible to segregate which pesticide has the larger association beyond the slight trimester relationship, nor could the pyrethroids be separately sorted out. Shelton et al. (2014) did note that the ASD risk was largest after third-trimester exposure for pyrethroid exposure (odds ratio = 2.0; 95% Cl: 1.1, 3.6), but after second trimester it was larger for chlorpyrifos (odds ratio = 3.3; 95% Cl: 1.5, 7.4). The study did not obtain urinary pyrethroid metabolite measurements. Nevertheless, this study, along with those described earlier are consistent in finding that prenatal or postnatal pyrethroid exposure is associated with neurodevelopmental disorders (Richardson, 2015; Shelton et al., 2014; Wagner-Schuman, 2015).
Another dataset used to assess early life pyrethroid exposure and its outcomes was the Canadian Health Measures Survey (CHMS). The CHMS (cycle 1, 2007-2009) used multistage probability sampling and oversampling of selected groups to generate a balanced study sample (Oulhote and Bouchard, 2013). Data were collected from 1,081 children ages 6-11 years. Urine samples were assessed for pyrethroid metabolites. Caregivers completed surveys covering each child’s behavior using the parent version of the Strengths and Difficulties Questionnaire (SDQ) (Goodman, 1997), as well as questions about pesticide use. Cis- and trans-DCCA urinary metabolites were associated with higher scores of difficult behavior on the SDQ (Oulhote and Bouchard, 2013). Type I pyrethroids, such as permethrin, and Type II pyrethroids, such as cypermethrin and cyfluthrin, generate DCCA metabolites (Barr et al., 2010), and implicate pyrethroids in long-term effects on behavior. DCCA is not a metabolite of DLM, therefore, this study implicates non-DLM pyrethroids as compromising children’s behavioral development. However, in an unweighted analysis of the data, 3-PBA levels and girls with high scores for conduct problems resulted in a significant association (adjusted odds ratio = 2.2; 95% CI: 1.0, 4.9) but not for boys (adjusted odds ratio = 0.6; 95% CI: 0.3, 1.4) (Oulhote and Bouchard, 2013). 3-PBA is a general metabolite for many pyrethroids, which limits specificity of this result since both Type I and Type II pyrethroids can be monitored by 3-PBA levels (Barr et al., 2010). Levels of cis-DBCA and 4-F-3-PBA, metabolites of DLM and cyfluthrin, respectively, were below the limit of detection in > 50% of the children in this study (Oulhote and Bouchard, 2013), making tests of association impossible to determine.
A study done in Jiaozuo, China in 2010 examined the effects of pyrethroids on children’s growth and development in infancy after prenatal pyrethroid exposure (Xue et al., 2013). The authors enrolled 497 mother-infant pairs and collected urine once during pregnancy (Xue et al., 2013). At one year of age, infants were assessed for physical, neurological, and mental development using the Development Screen Test (DST) (Xue et al., 2013). The DST assesses movement, social adaptation, inferred intelligence, and a mental development index. Levels of pyrethroid metabolites in maternal urine were negatively correlated with intellectual development (Xue et al., 2013). Other factors, such as the mother’s age, education, place of residence, and disease status, also correlated with lower developmental quotients by multiple linear regression analysis (Xue et al., 2013). This study implicates pyrethroids in lowering early intellectual development but follow up data at older ages will be important for interpreting the early life exposure as a predictor of later outcomes.
These studies were reviewed elsewhere (Burns and Pastoor, 2018). These authors note that the epidemiological studies have limitations. Burns and Pastoor highlight that these studies were all cross-sectional, rather than prospective and longitudinal, and all used non-specific urinary metabolites, including only measuring them once. These limitations prevent any conclusions about the effects of pyrethroids on neurodevelopment. However, the inverse association between pyrethroid metabolites and neurological development, while concerning, hint at neuropsychological short-falls in exposed children that should not be ignored. Until there are more human data, animal data are the best source of information about developmental effects. Before reviewing these data, pyrethroid biology is first reviewed.
3. Classification
Pyrethroids are synthetic derivatives of pyrethrin, which comes from chrysanthemums (Chrysanthemum cinerariaefolium). However, pyrethrins are unstable in light, therefore chemists modified their structures to produce more potent and stable insecticides. Pyrethroids are classified by structure, symptoms, and/or ion channel targets.
I. Classification by structure
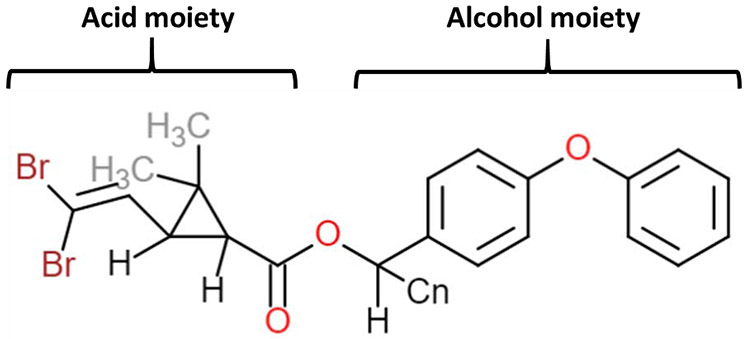
There are two main classes, Type I and Type II. Type I pyrethroids lack a cyano moiety at the alpha-position, whereas Type II pyrethroids contain this group (Gammon et al., 1981; Scott and Matsumura, 1983; Soderlund and Bloomquist, 1989; Soderlund et al., 2002). Pyrethroids share alcohol and acid moieties and a central ester bond (Figure 1). Most pyrethroids are stereoisomers because the acid moiety contains two chiral carbons, and the alcohol group has one chiral carbon. Hence, there can be up to eight stereoisomers for some pyrethroids (Perez-Fernandez et al., 2010) and chirality affects toxicity (Chapman, 1983). In general, cis isomers are more toxic than trans isomers (Rickard and Brodie, 1985). DLM lacks a trans isomer and exists only in a cis configuration (Elliott et al., 1974), suggesting greater toxicity per molecule.
Figure 1:
Chemical structure of the Type II pyrethroid DLM. Pyrethroids contain an alcohol and acid moiety that are connected by an ester bond. Many pyrethroids have various isomeric forms, however, DLM exists only as a cis isomer.
II. Classification by symptoms
At high doses, Type I and II pyrethroids induce different symptoms or syndromes (Kaneko, 2010; Narahashi, 1996; Shafer and Meyer, 2004; Shafer et al., 2005; Soderlund, D. M., 2012; Soderlund et al., 2002). In insects pyrethroids induce uncoordinated movements, convulsions, paralysis, and death (Soderlund and Bloomquist, 1989). In mammals, Type I pyrethroids, such as permethrin, at high doses produce tremors or T syndrome; consisting of fine tremors that become course as dose is increased, along with prostration, and sensitivity to stimuli (Shafer et al., 2005; Soderlund and Bloomquist, 1989). High doses of Type II pyrethroids in mammals produce choreoathetosis and salivation or CS syndrome that includes writhing, salivation, pawing, burrowing, tremors, and clonic seizures (Shafer et al., 2005; Soderlund and Bloomquist, 1989). Pyrethroids with overlapping CS and T symptoms are designated mixed or Type III pyrethroids (Shafer et al., 2005; Soderlund et al., 2002; Verschoyle and Aldridge, 1980).
III. Classification by receptor
i. Sodium channels
A third classification is by receptor binding or target. The principal binding site of pyrethroids is on the α-subunit of voltage-gated sodium channels (VGSC), which makes up the central pore of the channel as well as contains regions for voltage sensing and ion selectivity (Davies et al., 2007; Du et al., 2009; Field et al., 2017; Narahashi, 2000; Silver et al., 2014; Soderlund and Bloomquist, 1989; Wang and Wang, 2003). VGSCs are composed of an α- (220–260 kDa) and one or more β subunits (30–40 kDa) (Aman et al., 2009; Cestele and Catterall, 2000; Isom et al., 1992; Isom et al., 1995; Morgan et al., 2000; Patino and Isom, 2010; Zhang, Fan et al., 2013). The α-subunit of the VGSC shares homology with other voltage dependent channels (i.e., calcium and potassium channels), however, the β-subunits are homologous to neural cell adhesion molecules (Catterall, 2000a). β-subunits are involved in the kinetics and voltage dependence of VGSC, as well as modulating cell adhesion and migration (Patino and Isom, 2010; Zhang, Fan et al., 2013). A schematic representation of the VGSC α and β subunits is shown in Figure 2. Several studies address VGSC structure and its interactions with pyrethroids, such as DLM (Catterall, 2000b; Catterall et al., 2007; Cestele and Catterall, 2000; King, 2011; King et al., 2008; Wang et al., 2011; Zhang, F. et al., 2013).
Figure 2:
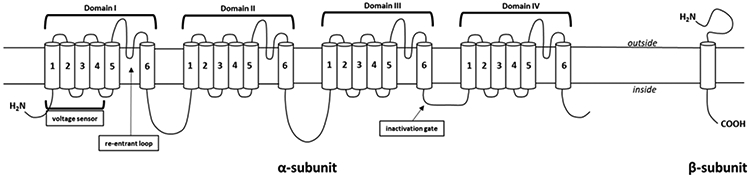
VGSC subunit structure. Both the α- and β-subunit representative images are displayed. The α-subunit is made up of 4 transmembrane domains (denoted by domain I-IV), each of which are made up of 6 transmembrane segments (S1-4 make up the voltage sensor; S5-6 form the pore) and a re-entrant loop (between S5 and 6) that is involved in ion selectivity. An intracellular loop connecting domains 3 and 4 functions as the inactivation gate that can plug the channel pore restricting ion flow. The β-subunit is a single transmembrane domain.
DLM binds to the α-subunit of VGSCs, changing the conformation. Pyrethroids have two proposed binding sites on VGSCs. They bind to an intramembrane site with a long, narrow hydrophobic pocket between domain 2 (transmembrane segments (S)4-S5) and domain 1 (S5/domain 3 S6 helices) (site 1), as well as a sequential region between domain 1 (S4) and domain 2 (S6) (site 2) on the α-subunit (Davies et al., 2007; Du et al., 2013; Silver et al., 2014; Wang and Wang, 2003). These sites are symmetrical but differ functionally. Mutations to the second site have less of an effect on receptor function than mutations to the first binding site (Du et al., 2013). The location of pyrethroid interaction with the VGSC was revealed using site-directed mutagenesis, oocyte expression studies (Du et al., 2009; Silver et al., 2014), and corroborated using computational modeling (Du et al., 2013).
When bound to VGSC, pyrethroids affect channel activation, slowing channel opening and closing (Narahashi, 1996; Soderlund, D. M., 2012). This causes VGSCs to shift the membrane to a more depolarized state making it easier to trigger action potentials (Narahashi, 1996). Pyrethroids hold the channels open longer, allowing more sodium to enter. Type II pyrethroids delay channel inactivation longer than Type I pyrethroids. Because of this, Type II pyrethroids at sufficient doses prolong channel opening and result in depolarization block where no action potentials occur (Shafer et al., 2005). Soderlund (2012) has reviewed pyrethroids actions on VGSC, including isoform and species differences.
VGSC subunits have temporal and regional expression patterns during development, which could have implications on pyrethroid exposure. Embryonic VGSC subunits are replaced with adult α- and β-subunits as development proceeds. Details were reviewed by Shafer et al (2005) for pyrethroids, but in brief the α-subunits Nav 1.1, 1.2, 1.3, and 1.6 and β-subunits (Navβ1-4) are expressed throughout the CNS. In rodents, expression of Nav1.3 is high during embryogenesis and is gradually replaced by Nav1.2 (Albrieux et al., 2004; Felts et al., 1997). Nav1.2 is expressed in immature nodes of Ranvier of retinal ganglia (postnatal day (P)9-10) and are replaced by Nav1.6 as myelination occurs (~P14) (Boiko et al., 2001). The early expression of Navβ3 is replaced by Navβ1 and Navβ2 after P3 in rats. Navβ1 and Navβ2 increase until they reach adult levels (Shah et al., 2001). These developmental patterns may make VGSCs vulnerable to pyrethroids at different ages. Perturbations to these VGSC developmental shifts may make it more likely that they will result in later functional changes. Genetic or pharmacological exposures can alter VGSC development (Adams et al., 1990; Claes et al., 2001; Escayg et al., 2001; Meisler et al., 2001; Noebels, 2002; Shafer et al., 2005; Wallace et al., 2001). VGSC null mutations produce alterations or loss of channel subunits as do those caused by drugs that inhibit VGSCs during channel transitions to adult subtypes (Hatta et al., 1999; Kearney et al., 2001; Meisler et al., 2001; Ohmori et al., 1999; Planells-Cases et al., 2000; Schilling et al., 1999; Shafer et al., 2005; Vorhees et al., 1995). Disruptions to channel composition and function may contribute to the long-term effects from developmental pyrethroid exposure. For example, mice exposed to DLM during gestation and lactation had decreased VGSC mRNA expression as adults, (Magby and Richardson, 2017). It is not yet clear how changes in VGSC composition from developmental pyrethroid exposure results in long-term effects as no direct connection has been made. Nevertheless, this is an idea worth further investigation, due to the understood direct effect pyrethroids have on the VGSC.
ii. Calcium channels
Pyrethroids also affect calcium channels. Voltage gated calcium channels (VGCCs) belong to the same protein superfamily as VGSCs. VGCC are also signal transducers and control calcium influx (King et al., 2008). There are two types and they differ in voltage dependence: low-voltage VGCCs that are activated by small depolarizations but rapidly inactivated; and high-voltage VGCCs that are activated by large depolarizations but are slowly inactivated (King et al., 2008). VGSCs and VGCCs have similar structures, with analogous α subunits (α1 for the VGCCs), however they differ in their regulatory subunits that control the voltage-gated pore, and four repeat domains with six transmembrane segments (S1-S6) containing a loop between S5 and S6 (King et al., 2008) (Catterall et al., 2005). VGCCs differ from VGSCs in regulatory subunits. The high-voltage VGCCs have 4-5 subunits consisting of one pore-forming α1 subunit, an extracellular α2 subunit, a transmembrane δ subunit, an intracellular β subunit, and sometimes a transmembrane γ subunit (Catterall, 2000b; King et al., 2008). The low-voltage VGCCs have the α1 subunit without additional subunits (Catterall et al., 2005; King et al., 2008; Perez-Reyes, 2003).
Despite the overlapping structural similarities between VGSC and VGCCs, pyrethroids have different effects on these channels (Shafer et al., 2005; Soderlund, D. M., 2012; Soderlund et al., 2002). The effects of pyrethroids on VGCCs was reviewed by Soderlund (2012). Tetrodotoxin (TTX) is a potent VGSC inhibitor and attenuates pyrethroid actions. However, TTX only partially abolishes the effects of pyrethroids on VGSCs, indicating that pyrethroids act through other mechanisms (Shafer et al., 2005; Soderlund, D. M., 2012; Soderlund et al., 2002; Symington et al., 2008; Symington et al., 2007). In synaptosomes, Symington et al. (2007b) showed that a combination of TTX and DLM enhanced calcium uptake and release and was blocked by the N-type calcium channel blocker ω-conotoxin MVIIC. Cao et al. (2011), found no effects of pyrethroids on VGCCs in the presence of TTX in cultured mouse cortical neurons. Moreover, the effects of pyrethroids on VGSCs may have implications for NMDA receptor (NMDA-R) function, since pyrethroids and sodium/calcium exchangers modulate NMDA-R mediated depolarization (Cao et al., 2011; Soderlund, D. M., 2012). In addition, pyrethroids may act through VGCCs and that DLM can modify calcium currents under particular conditions (Shafer and Meyer, 2004; Soderlund, D.M., 2012; Soderlund et al., 2002). VGCCs also change during development, with expression increasing with age (McEnery et al., 1998). Therefore, VGCCs may be affected by developmental pyrethroid exposure. Since VGCCs are important in neurotransmitter release, pyrethroids may affect release thereby changing the level of signaling and ultimately behavior. It is possible that doses of DLM that are sufficient to affect VGCCs may induce permanent changes in channel regulation and affect behavior (Soderlund et al., 2002).
iii. Chloride channels
Soderlund et al. (2002) suggested that the CS syndrome could be a product of pyrethroids acting on voltage-gated chloride channels (VGCLCs) (Soderlund et al., 2002). However, only some pyrethroids inhibit VGCLCs only at high doses of Type I pyrethroids and by a few Type II pyrethroids, including DLM, but not by esfenvalerate or λ-cyhalothrin (Burr and Ray, 2004). Therefore, even among Type II pyrethroids there are important differences in how they affect VGCLCs. For Type II pyrethroids that do not affect VGCLCs, they should not contribute to the CS syndrome, and it appears that DLM is an exception because it elicits a CS syndrome and does affect VGCLCs. The effect of pyrethroids on VGCLCs was reviewed in detail by Soderlund (2012). However, the role of developmental DLM exposure on VGCLCs remains an open question.
iv. Chloride-related channels
The γ-aminobutyric acid (GABA) receptor-chloride ionophore is also affected by Type II pyrethroids. Soderlund and Bloomquist (1989) reviewed symptom onset in invertebrate models following pyrethroid exposure, as well as the binding of pyrethroids to sites on the GABA receptor-chloride ionophore. Overall binding of pyrethroids correlated with toxicity (Lawrence and Casida, 1983), without affecting GABA or benzodiazepines binding to the GABA chloride ionophore (Crofton et al., 1987; Lummis et al., 1987), suggesting a different binding site. Morever, the effects of DLM on ligand binding differs in different species. The mammalian GABA receptor-chloride ionophore is affected by pyethroids (Harris and Allan, 1985) but not the homologous receptor in flies (Cohen and Casida, 1986). Moreover, DLM is 1000-fold less potent at inhibiting GABA-dependent chloride uptake than it is at inhibiting VGSCs (Ghiasuddin and Soderlund, 1985), making this observation unlikely to be physiologically relevant.
Pyrethroids also inhibit peripherial-type benzodiazepine receptors (Soderlund and Bloomquist, 1989), a binding site on mitochondria (Papadopoulos, 2004). Pyrethroid interactions with the peripherial benzodiaepine receptors correlate with their proconvulsant effects at doses below those inducing other types of toxicity (Devaud and Murray, 1988; Devaud et al., 1986). The effects of DLM on these receptors during early development remains to be investigated.
In sum, pyrethroids bind to multiple ion channels. They primarily bind to VGSCs, but they have affinity for VGCC, VGCLC, and GABA-ion receptors at increasing doses. The collective effect of pyrethroids on these channels in adult rat and mouse brain are documented (see Shafer et al. (2005) and Soderlund (2012) for details); their effects during development are not yet known. At present there is no clear connection between pyrethroid binding to ion channels and long-term effects on behavior.
4. Neurotransmitters
I. Neurotransmitters: Fast acting
Since it is well understood that pyrethroids act on channels such as VGSC and VGCCs, this effect could lead to alterations in neurotransmission which ultimately can lead to various downstream effects. GABA and glutamate, the main inhibitory and excitatory neurotransmitters, respectively, are affected by DLM exposure. GABA and glutamate release following pyrethroid exposure in hippocampus of adult male rats was investigated using microdialysis. DLM (10, 20, or 60 mg/kg; i.p.) caused dose-dependent increases in glutamate release and decreases in GABA release (Hossain et al., 2008). The Type II pyrethroid cyhalothrin (10, 20, or 60 mg/kg; i.p.), on the other hand, inhibits glutamate release, while the Type I pyrethroid allethrin at 10 or 20 mg/kg i.p. increased release with opposite effects at higher doses (60 mg/kg) (Hossain et al., 2008). GABA levels are decreased after 10 or 20 mg/kg of allethrin, but increased after 60 mg/kg or after 10, 20, or 60 mg/kg of cyhalothrin (Hossain et al., 2008) showing that GABA and glutamate are differentially affected by different pyrethroids at different doses. Changes in GABA and glutamate from DLM, cyhalothrin, or allethrin are blocked by TTX (1 μM) at moderate doses. However, at high doses (60 mg/kg), such changes are blocked by infusion of the L-type Ca2+ channel blocker nimodipine (10 μM) (Hossain et al., 2008), suggesting that DLM acts on VGCCs and VGSCs at high doses, but only on VGSCs at lower doses. Developmental DLM effects on GABA have not been studied (possible long-term DLM effects after developmental exposure on glutamate are discussed below).
II. Neurotransmitters: Cholinergic
Cholinergic neurotransmission was altered following exposure to pyrethroid pesticides. This neurotransmitter class has been examined extensively following pesticide exposure, in part due to many pesticides, such as organophosphates, altering cholinergic neurotransmission. Eriksson and Nordberg (1990) assessed the density of muscarinic and nicotinic acetylcholine receptors with labeled [3H]quinuclidinyl benzilate ([3H]QNB) and [3H]nicotine following DLM exposure in mice. High and low-affinity muscarinic binding was assessed by [3H]QNB/carbachol displacement. Mice exposed to 0.71 mg/kg/day DLM from P10-16 by gavage had increased muscarinic receptor density, increased high-affinity binding sites, and decreased percentage of low-affinity muscarinic receptor binding, as well as increased density of nicotinic receptors in the cerebral cortex 24 h after the last dose, with no effect in the hippocampus (Eriksson and Nordberg, 1990). Additionally, 1.2 mg/kg DLM (P10-16 by gavage), a dose that caused the CS syndrome in mice at these ages, resulted in decreased [3H]QNB binding in the hippocampus and increased [3H]nicotine binding in the cerebral cortex (high vs. low-affinity binding was not distinguished) (Eriksson and Nordberg, 1990). Eriksson and Nordberg (1990) also assessed the effects of developmental bioallethrin exposure (0.72 mg/kg by gavage [bioallethrin is composed of 2 of the 8 stereoisomers of allethrin]), a Type I pyrethroid, on nicotinic and muscarinic receptor binding. Bioallethrin reduced high-affinity and increased low-affinity muscarinic receptor binding. Bioallethrin had no effect on muscarinic or nicotinic binding in the hippocampus or cortex (Eriksson and Nordberg, 1990). Hence, different pyrethroids elicit different effects on cholinergic systems depending on brain region.
III. Neurotransmitters: catecholaminergic
There is mounting evidence implicating catecholamines in the effects of pyrethroids. Embryonic exposure (E6-15) to DLM (0.08 mg/kg by gavage) resulted in increased striatal 3,4-dihydroxyphenylacetic acid (DOPAC) levels in adult male rats, with no change in dopamine (DA) or homovanillic acid (HVA) levels (Lazarini et al., 2001) and an increased DOPAC/DA ratio. No change in DA or its metabolites were observed in the female offspring as adults. However, Lazarini et al. (2001) used a unspecified commercial formulation of DLM and without control for litter effects. Richardson et al. (2015) exposed mice perinatally to DLM (E0-P22; 3 mg/kg DLM/72 h by gavage) and found that DLM increased DA transporter (DAT) levels in the striatum in both sexes, however the magnitude of the effect was larger in males. Using autoradiography, they found increased DAT levels in the nucleus accumbens but not in neostriatum of males (females were not evaluated using autoradiography). The dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) was used to estimate the in vivo consequences of increased DAT levels. MPTP (1 mg/kg, i.p.) induced DA reductions in control mice and larger reductions in DLM-treated mice (Richardson et al., 2015). In addition, DLM exposure decreased extracellular DA release measured by microdialysis, but there was increased DA D1 receptor (DRD1) levels in the n. accumbens in the males, with no change in neostriatum in either sex (Richardson et al., 2015). The functional consequences of increased DRD1 levels were assessed using the DRD1 antagonist SCH23390 (0.025, 0.05, or 0.1 mg/kg), the DRD2 antagonist eticlopride (0.05, 0.1, or 0.2 mg/kg), the DRD2 autoreceptor antagonist quinpirole (0.03 or 0.06 mg/kg quinpirole), the general DA receptor agonist, apomorphine (0.5 mg/kg), the selective DRD1 agonist SKF82958 (1.0 mg/kg), or the indirect DA agonist methylphenidate (1.0 mg/kg). Hyperactivity in DLM-treated male mice was normalized by SCH23390, eticlopride, quinpirole, and methylphenidate (Richardson et al., 2015). Whereas, when DA was first depleted by 5 mg/kg reserpine pretreatment, apomorphine increased locomotor activity in DLM-treated male mice, but not in female mice, compared with controls (Richardson et al., 2015). SKF82958 increased locomotor activity in control and DLM-treated mice, however the increase in DLM-treated mice was greater than in controls (Richardson et al., 2015). Overall, DA alterations from developmental DLM were long-lasting and may partially explain the long-term hyperactivity.
IV. Neurotransmitters: Serotonergic
Data on the effects of pyrethroids on serotonin (5-HT) are limited. Serotonin was measured with microdialysis in the striatum of adult male Sprague-Dawley rats after exposure to DLM, cyhalothrin (Type II pyrethroid), or allethrin (Type I pyrethroid) (10, 20 or 60 mg/kg; i.p.) (Hossain, 2013). DLM dose-dependently decreased basal 5-HT release, whereas it was increased with cyhalothrin (Hossain, 2013). Allethrin had dose-dependent effects, with decreased release of 5-HT after 10 mg/kg but increased release after 20 or 60 mg/kg (Hossain, 2013). These changes on 5-HT release were partially dependent on VGSCs since TTX prevented the effects of allethrin, cyhalothrin, and DLM at some doses (10 and 20 mg/kg) (Hossain, 2013). The 5-HT release effect of DLM at 60 mg/kg was blocked when combined with exposure to TTX and nimodipine (an L-type calcium channel antagonist) (Hossain, 2013). Like the effects observed on GABA and glutamate release, 5-HT release following DLM exposure at higher doses depended on VGCCs and VGSCs, with lower doses being dependent only on VGSCs. The effects on 5-HT release after developmental DLM exposure have not been tested.
The acute effects of DLM on 5-HT and its major metabolite 5-hydroxyindoleacetic acid (5-HIAA) were evaluated in adult male Wistar rats in another study. DLM (20 mg/kg/day, i.p., for 6 days) reduced 5-HT and 5-HIAA in midbrain and striatum, and 5-HIAA in the frontal cortex and hippocampus (Martinez-Larranaga et al., 2003). Reductions in 5-HT and 5-HIAA were also found after exposure to cyfluthrin (14 mg/kg i.p. for 6 days) or λ-cyhalothrin (8 mg/kg i.p. for 6 days). 5-HT was reduced in all regions examined (frontal cortex, hippocampus, midbrain, and striatum) for these Type II pyrethroids (Martinez-Larranaga et al., 2003). 5-HIAA was also reduced following cyfluthrin (frontal cortex and striatum but not hippocampus or midbrain) and λ-cyhalothrin (frontal cortex, midbrain, and striatum but not hippocampus) (Martinez-Larranaga et al., 2003). These data indicate that 5-HT is affected by Type II pyrethroids but whether this also occurs after developmental exposure is unknown.
The above data implicate neurotransmitters in the effects of pyrethroids, including DLM. The effects on DA markers are the most consistent. It is not clear, however, if this is because DA is the most affected neurotransmitter or because it is the most investigated. Little is known about effects of developmental DLM on neurotransmitters other than DA, therefore, there are major gaps that need to be filled. In addition to neurotransmitters, DLM activates microglia via VGSCs in primary microglia culture (Hossain et al., 2016), indicating that more than ion channels and neurotransmitters are involved.
5. Pharmacokinetics and metabolism
To emphasize the importance of further study of developmental pyrethroid exposure, differences in exposure and elimination of these compounds must be considered. Children are estimated to be exposed to pyrethroids at levels of 3.0 μg/kg/day and adults to 0.6 μg/kg/day (EPA, 2015; Mortuza et al., 2018). Agricultural workers and those in urban areas tend to have higher exposures than those in suburban areas. Poisonous exposures that can occur from accidents or improper handling are too sporadic to be of much help in understanding the neurotoxicity of these compounds (Chen et al., 1991; He et al., 1989; Vinayagam, 2017).
In addition, to neurotoxicity outcomes, additional data are also needed on exposure. Data on absorption, distribution, metabolism, and excretion (ADME), along with pharmacokinetics (PK) are relevant (Anadon et al., 1996; Anand, S.S., Bruckner, J. V., Haines, W. T., Muralidhara, S., Fisher, J. W., Padilla, S., 2006), including physiologically based pharmacokinetic (PBPK) studies. Understanding how DLM and other pyrethroids are processed in the body can aid in understanding the neurotoxic effects and the differences between adult and developing systems.
I. Physiologically based pharmacokinetics
PBPK studies provide data on age and species differences in dosimetry (Felter et al., 2015). DLM in rats (26 mg/kg by gavage or 1.2 mg/kg I.V. given once) is rapidly absorbed (Anadon et al., 1996) because it is lipophilic. This also means DLM easily enters the CNS and PNS (Anadon et al., 1996). DLM is absorbed in fat in the gut and liver (He et al., 1991; Rehman et al., 2014). Male rats given DLM by gavage (0.4, 2, or 10 mg/kg dissolved in glycerol formal) had rapid but incomplete gastrointestinal absorption, with some entering brain and accumulation in fat, skin, and skeletal muscle (Kim et al., 2008). The BBB restricts passage through the microvasculature (Amaraneni et al., 2017b), however, this is age-dependent. When 1, 10, or 50 μM 14C-DLM are perfused in anesthetized rats, uptake is inversely related to age, such that P15 rats display 2.2-3.7 fold higher uptake of DLM in brain than adults, with P21 rats intermediate (Amaraneni et al., 2017b). This is consistent with the observation that immature rats are more susceptible to the acute toxicity of DLM than adult rats (Cantalamessa, 1993; Sheets et al., 1994). DLM (10 mg/kg) in rats by gavage in glycerol formal at P10, 21, 40, or 90 is lethal at P10 and P21, but not at P40 or P90 Anand, S.S., Kim, K. B., Padilla, S., Muralidhara, S., Kim, H. J., Fisher, J. W., Bruckner, J. V. (2006). The differences in lethality and uptake in relation to age may be due to differences in metabolic enzymes and pharmacodynamics in these two age groups.
DLM in rats is cleared by plasma carboxylesterases (CaEs) and liver cytochrome P450s (CYP450) (Anand et al., 2006). Young and adult rats metabolize DLM efficiently at low doses, e.g., at 2 μM in diluted plasma and in liver microsomes (Anand et al., 2006). In P15, P21, and P90 rats, clearance of 0.1, 0.25, or 0.5 mg/kg DLM by gavage in corn oil, is cleared from plasma within 24 h. Differences in maximum concentrations (Cmax) and 24 h area under the curve (AUC24) are also age-dependent (Anand et al., 2006). Time to eliminate DLM is proportional to dose until enzyme saturation occurs, and this too is age-dependent (Mortuza et al., 2018). At P15, brain, plasma, and liver differences in dosimetry decreased proportionately with dose for Cmax from the highest and middle doses to the lowest dose (Mortuza et al., 2018), but this did not occur at P21 or adult ages. Differences in permeability, CYP450s and CaE activity in younger rats appear to account for this effect.
PBPK models provide data on elimination half-life, Cmax, clearance, and volume of distribution that predict plasma and tissue concentration-time profiles (Jones and Rowland-Yeo, 2013). Mirfazaelian et al. (2006) used a PBPK model for adult male Sprague-Dawley rats using flow-limited and diffusion-limited rate equations. Flow-limited equations account for gastrointestinal, hepatic, brain, and other rapidly perfused tissues, whereas diffusion-limited equations account for slowly perfused tissues, such as blood/plasma and fat. In this way, Mirfazaelian et al. (2006) estimated that 48 h after DLM (2 or 10 mg/kg by gavage) most of the compound is cleared via hepatic biotransformation, with a smaller percentage cleared by plasma CaE catabolism. Concentrations of DLM given by gavage (2 or 10 mg/kg) or by i.v. (1 mg/kg) were determined for blood, plasma, brain, and fat. Adult male rats had much lower DLM levels in brain than young rats (Mirfazaelian et al., 2006), suggesting that immature organisms are more susceptible to the deleterious effects of DLM. Using the same oral route as Mirfazaelian et al. (2006), a subsequent study by Godin and colleagues (2010) extrapolated the data to humans and found threefold greater 48 h AUCs and twofold greater peak DLM concentration will occur in human brain compared to rats at a dose of 1 mg/kg DLM given orally (Godin et al., 2010). Hence, greater brain concentrations are predicted in humans than in rats at equivalent doses.
The Mirfazaelian PBPK model was updated (Tornero-Velez et al., 2010) relative to other models (Amaraneni et al., 2017b; Anand, S.S., Bruckner, J. V., Haines, W. T., Muralidhara, S., Fisher, J. W., Padilla, S., 2006; Scollon et al., 2009). The newer model showed that when P10, 21, 40, and 90 rats were administered DLM at 0.4, 2, or 10 mg/kg DLM by gavage in glycerol formal, younger DLM-treated rats had higher brain concentrations compared with adults and more signs of toxicity (Kim, 2010; Tornero-Velez et al., 2010). Moreover, plasma DLM levels did not reflect brain levels (Kim, 2010). Brain concentrations (brain AUC24) of DLM for P10 rats were 3.8-fold higher than for P90 rats (Tornero-Velez et al., 2010), indicating increased susceptibility of the brain during this early developmental period.
A recent study examined DLM levels in plasma and brain of P15 (0, 1, 2 or 4 mg/kg DLM by gavage) and adult (0, 2, 8, or 25 mg/kg DLM, by gavage) rats 2-8 h following DLM administration (Williams et al., 2019). Regardless of age, dose-dependent increases in DLM were observed in brain and plasma at each dose, however at the 2 mg/kg dose, brain levels were higher at P15 compared with adults. In addition, DLM levels of P15 rats were highest 6 h following 4 mg/kg DLM (~151 ng/g) and remained high for 8 h, whereas adults that were administered a dose > 6 times higher (25 mg/kg DLM) had peak concentrations (~156 ng/g) 2 h after administration that fell by 50% within 4 h. Hence, not only are peak concentrations higher in younger rats they remain higher for longer than in adults. When examining signs of neurotoxicity, adult rats given the 25 mg/kg DLM dose had tremor and salivation for 4 h, whereas P15 rats had tremors after 2 and 4 mg/kg DLM that persisted for 8 h post-treatment. Some mortality was seen in the P15 rats, but not in the adults at these doses (Williams et al., 2019). These enhanced signs of neurotoxicity seen in developing rats follow the age-related concentrations in DLM.
A PBPK model was also used for in vitro to in vivo extrapolation (IVIVE) for age-related differences after DLM (Song et al., 2019). Liver microsomes, cytosol, and plasma from immature and adult rats were scaled to model the in vivo time course of plasma and brain concentrations following oral doses of 0.1, 0.25, 0.5, and 5 mg/kg of DLM (Song et al., 2019). Age-dependent tissue levels were simulated for humans (i.e., brain Cmax) and showed that compartment partitioning and permeability were critical. The model recapitulated developmental changes in clearance as well as BBB maturity effects. The IVIVE-based PBPK model demonstrated an inverse relationship between age and brain DLM uptake, as also found in in situ models (Amaraneni et al., 2017b). The Song et al. (2019) model showed how brain concentrations were affected by body weight, cardiac output, blood volume, hematocrit, blood flow, absorption, metabolism, and age.
II. Metabolism
CaEs, CYP450s, and glutathione-S-transferase (GST) all contribute to DLM metabolism (Lu et al., 2019; Yang et al., 2017). The principal route of DLM metabolism are ester cleavage and oxidation at the 4’ position (Anand, S.S., Bruckner, J. V., Haines, W. T., Muralidhara, S., Fisher, J. W., Padilla, S., 2006; Soderlund and Casida, 1977). Pyrethroids containing the cyano group are less sensitive to oxidation and hydrolysis than non-cyano pyrethroids (Soderlund and Casida, 1977). As noted, studies show trans isomers are readily hydrolyzed by esterases compared with cis isomers (Soderlund and Casida, 1977). DLM is only in the cis isoform (Elliott et al., 1974) and therefore is metabolized more slowly, hence DLM has a longer half-life compared with Type I pyrethroids (Anadon et al., 1996).
CaEs are widely distributed (He et al., 1991; Rehman et al., 2014) and hydrolyze DLM to produce alcohol and acid metabolites (Satoh and Hosokawa, 2006). Hydrolysis creates carboxylic acid ester, amide, and thiol-ester groups (Lu et al., 2019; Rehman et al., 2014; Soderlund and Casida, 1977) that are conjugated and excreted in urine. DLM is also a CaE inhibitor, hence it slows its own hydrolysis (Imai and Ohura, 2010; Lei et al., 2017; Wang et al., 2018; Yoon et al., 2004).
CYP450 enzymes also contribute to DLM metabolism (Lu et al., 2019). CYP450s catalyze aromatic hydroxylation of DLM that is then conjugated for excretion (Lu et al., 2019; Rehman et al., 2014; Soderlund and Casida, 1977). CYP450s exhibit regional differences in brain as well, a little appreciated factor (Yadav, Sanjay et al., 2006). Rat hippocampus have higher levels of Cyp1a1 mRNA expression (Yadav, S. et al., 2006). Also, hippocampus and hypothalamus have higher levels of Cyp2b1 expression than other regions (Yadav, Sanjay et al., 2006). The medulla-pons, cerebellum, and frontal cortex have higher Cyp1a2 and Cyp2b2 mRNA expression than in other regions (Yadav, Sanjay et al., 2006). Both the hippocampus and hypothalamus have longer elimination half-lives of DLM and its metabolite, 4’-OH-DLM, than other regions (Anadon et al., 1996). The higher Cyp1a1 and Cyp2b1 expression in hippocampus and hypothalamus may contribute to the metabolites 2’, 4’ or 5’-OH-DLM found in these regions (Anadon et al., 1996; Yadav, Sanjay et al., 2006). The metabolic capacity of CaEs for DLM is lower than for CYP450s in liver and plasma (Anand et al., 2006), but a key question is how regional brain differences in CYP450 enzymes contribute to how DLM metabolism affects these regions, and as of now there are no data on this subject.
GST in another metabolic enzyme that contributes in the elimination of pyrethroids (Hemingway and Ranson, 2000) by conjugating electrophilic compounds with the thiol group of reduced glutathione allowing for the reduction of antioxidants (Berdikova Bohne et al., 2007; Yang et al., 2017). The nicotinamide adenine dinucleotide phosphate (NADPH)-pathway is also involved; DLM is metabolized in rat liver microsomes via a NADPH-dependent mechanism and in human liver microsomes via a NADPH-independent processes (Godin et al., 2006). The implications of this difference have received little attention.
DLM appears to be metabolized by similar enzymes in humans and rats (Rehman et al., 2014), but there are quantitative differences in the rate of metabolism across species and over the course of development. Humans have low serum CaE levels, whereas rodents have high levels (Crow et al., 2007; Li et al., 2005; Wang et al., 2018). The lower levels in humans result in slower DLM clearance. In addition, the expression of CaE in humans is age dependent, with adults expressing higher levels than children or fetuses (Satoh and Hosokawa, 2006). Microsomes are 4 times more active in adults than in children and 10 times more active than in fetuses (Satoh and Hosokawa, 2006). These differences result in an increased exposure to pyrethroids during development.
For DLM, the parent compound is thought to be the major neurotoxin (Rickard and Brodie, 1985). There are multiple metabolites that can be formed from DLM (Cole et al., 1982). The main metabolites are 2’,4’, and 5’-OH-DLM (Lu et al., 2019; Romero et al., 2012). In SH-SY5Y cells, 2’-OH DLM (0-1000 μM) and 4’-OH-DLM (0-1000 μM) were more toxic than DLM (10-1000 μM) (Lu et al., 2019; Romero et al., 2012), which appears contradictory to the parent compound being the proximate neurotoxin. However, the metabolite comparisons are from cell culture and may not apply in vivo. If metabolites contribute to DLM neurotoxicity, it might help explain differences in DLM toxicity as a function of metabolite levels (Lu et al., 2019). For example, other metabolites of DLM include trans-methyl and ester cleavage groups that form 3-PBA and 4’ and 2’-OH-PBA (Lu et al., 2019). These metabolites are toxic at high doses, but not at concentrations seen in vivo (Henault-Ethier, 2016). Therefore, the relevance of these observations is unclear and in addition, these data are in adults, and similar data in developing animals are not available.
III. Cytotoxicity
Mechanisms of DLM neurotoxicity have primarily been studied in adult rodents or cell culture, with emphasis on apoptosis and oxidative stress. DLM increases apoptosis in cell culture (Enan, E. et al., 1996; Hossain, 2011; Wu et al., 2003) and in vivo (El-Gohary et al., 1999; Hossain, 2019; Wu and Liu, 2000a). Since apoptosis is normal during brain development the question becomes whether DLM induces excess cell death sufficient to explain or contribute to its long-term neurotoxicity.
Oxidative stress is also proposed as a mechanism of DLM-induced neurotoxicity. Oxidative stress is caused by an imbalance of reactive oxygen species and antioxidant enzymes that leads to lipid peroxidation in cells, mitochondria, and nuclear membranes and results in protein degradation or DNA damage (Kumar et al., 2015). DLM-treated male mice (5.6 and 18 mg/kg) exhibit lipid peroxidation and decreased antioxidant enzyme activity in liver and kidney (Rehman et al., 2006). Even relatively low doses of DLM (1.28 mg/kg and 3 mg/kg) increase lipid peroxidation, induce thiobarbituric acid-reactive substances (marker of lipid peroxidation), and decrease levels of GST and antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase) in plasma and lymphoid organs in adult rats (Aydin, 2011; Yousef et al., 2006). PC12 cells exposed to DLM (10 μM/L) exhibit increased nuclear factor erythroid 2-related factor 2 (Nrf2) expression and activity (Li et al., 2007). Nrf2 regulates antioxidant protein levels. These data suggest that DLM-induced oxidative stress is likely to be a contributing factor to DLM-induced neurotoxicity, however how this differs during developmental DLM exposure has yet to be explored.
DLM-induced calcium changes may also play a role in long-term neurotoxicity. Calcium acts as an intracellular signal controlling many functions including neurotransmission (Shafer and Meyer, 2004; Shafer et al., 2005). Transient or oscillating increases in calcium influx will alter cellular activity; sustained calcium elevation leads to apoptosis (Kumar et al., 2015). DLM (5-60 μM), increases phospholipase C (PLC) in glioblastoma cells and increases calcium mobilization that results in reduced rates of calcium/calmodulin dependent protein dephosphorylation in thymocytes (50 μM in vitro; 25 mg/kg in vivo), predisposing the cells to apoptosis (Enan, Essam et al., 1996). PLC generates inositol triphosphate (IP3) and diacylglycerol (DAG), both of which increase intracellular calcium and calcium/calmodulin dependent protein kinase and increase second messengers that promote DNA fragmentation and cell death. Although the effects of PLC and its downstream signaling effects have not been tested in a developmental DLM model to date.
Another mechanism by which DLM may induce calcium-dependent apoptosis is through activation of the endoplasmic reticulum (ER) stress pathway. The ER is a reservoir for calcium, however, excess release within the lumen of the ER can trigger death cascades (Verkhratsky, 2005). DLM activates the ER stress pathway in cell culture (Hossain and Richardson, 2011) and in vivo in mice (Hossain et al., 2015). SK-N-AS cells treated with DLM have increased levels of the transcription factors C/EBP homologous protein/growth arrest and DNA damage 153 (CHOP/GADD153) (Hossain and Richardson, 2011), both of which are ER stress pathway pro-apoptotic factors (Hu et al., 2018; Oslowski and Urano, 2011). CHOP/GADD153 levels are also increased in mice treated with DLM (3 mg/kg every 3 days for 60 days), as are levels of binding immunoglobulin protein/78-kDa glucose-regulated protein (BiP/GRP78) (Hossain et al., 2015). BiP/GRP78 is an ER chaperone that regulates ER stress signaling via protein kinase R (PKR)-like ER kinase (PERK), that at normal levels restores ER stress pathway balance following stress (Wang et al., 2009; Xu et al., 2005). However, when ER stress is prolonged, BiP/GRP78 activates PERK which phosphorylates eukaryotic translation initiation factor 2 subunit 1 (eIF2α), that in turn inhibits the actions of eIF2α by blocking mRNA translation and protein synthesis, thereby exacerbating ER stress mechanisms (Wang et al., 2009; Xu et al., 2005). Thus, BiP/GRP78 is a regulator that reduces ER stress, except when ER stress is high enough to cause BiP/GRP78 to dissociate from the ER stress pathway allowing activation of cell death cascades (Oslowski and Urano, 2011). In addition, when eIF2α has sustained phosphorylation it can lead to CHOP activation through the suppression of B-cell lymphoma 2 (Bcl-2), an anti-apoptotic protein (Kim, 2008; Xu et al., 2005). Hence, eIF2α is a key regulator of the ER stress response. Increased activation of ER stress activates caspases and DNA fragmentation in cell culture assays (Hossain and Richardson, 2011) and in vivo (Hossain, 2019; Hossain et al., 2015) of DLM exposure; and these effects are blocked by the calcium chelator BAPTA-AM (5 μM), by blocking VGSCs with TTX (1 μM), or by salubrinal an eIF2α inhibitor (Hossain, 2019; Hossain and Richardson, 2011).
Another mechanism of DLM-induced neurotoxicity is through the tumor suppressor protein p53, which is also involved in apoptosis (Hughes et al., 1996; Xiang et al., 1998). DLM increases p53 and Bcl-2-associated X protein (Bax; which is regulated by p53) in rat brain (Wu and Liu, 2000a) and in cortical neurons (Wu et al., 2003). These data, along with those above, point to apoptosis as a mechanism of DLM-induced neurotoxicity. However, an understanding of many of these pathways following developmental DLM exposure have yet to be fully explored.
In summary, PBPK models provide information on the ADME of DLM and how these differ as a function of age, especially in relation to brain and cytotoxicity data, suggesting mechanisms of neurotoxicity. Younger animals are more vulnerable to the effects of pyrethroids compared with adults because DLM levels are higher in plasma and brain at younger ages. The limited metabolic capabilities and immature BBB are factors for why CNS levels are higher in young animals, as are limited metabolic capacity at early ages. The vulnerability of younger animals to the effects of pyrethroids highlight the importance of studying the cytotoxicity of DLM during development to determine age-dependent mechanisms.
6. Effects of DLM on brain and behavior
I. Adult effects
The EPA estimates human exposures of pyrethroids at 3.0 μg/kg/day in children and 0.6 μg/kg/day in adults (EPA, 2015). 10 μg/kg/day is the reference dose for DLM for acute dietary exposure (Bennett et al., 2020). These are lower than used in animal studies that find behavioral and cognitive deficits, however, rats metabolize and clear DLM much faster than humans, therefore, administered dose comparisons are not meaningful. Brain concentrations would be ideal but cannot be obtained in people, therefore, direct dose comparisons are not possible.
Studies of pyrethroid behavioral toxicity to 2008 were reviewed previously (Wolansky and Harrill, 2008). The authors noted that adult rodent pyrethroid exposure results in many functional effects that include: altered locomotor activity at doses that do not produce overt neurotoxicity, impaired rotorod performance (DLM and α-cypermethrin), reduced schedule-controlled operant responding (allethrin, permethrin, cis-permethrin, DLM, cypermethrin, and fenvalerate), decreased grip strength (pyrethrum, bifenthrin, S-bioallethrin, permethrin, β-cyfluthrin, cypermethrin, and DLM), and altered startle reflexes, specifically, increased acoustic startle (Type I pyrethroids) or decreased acoustic startle amplitude (Type II pyrethroids) (Wolansky and Harrill, 2008) see also (De Souza Spinosa et al., 1999). Data on cognitive effects are more limited. Adult mice treated with 3 mg/kg DLM (every 3 days for 60 days with testing beginning 3 days following the last dose) had increased latency to find a submerged platform in a Morris water maze (MWM) (Hossain et al., 2015).
II. Developmental effects
Neurobehavioral toxicity data in developing animals is also limited and finding commonalities across studies is challenging because studies vary in design, dependent variables, exposure periods, doses, species, strain within species, routes of exposure, frequency of exposure, vehicles the compounds are dissolved in, sample sizes, and even how the data were analyzed. Here we review the relevant available data.
Locomotor activity is a behavioral measure extensively studied following developmental DLM exposure. NMRI mice were administered 0.7 mg/kg DLM by gavage (dissolved 20% fat emulsion of egg lectin, peanut oil, and water) from P10-16 and did not have changes in locomotor activity when assessed on P17, however when tested as adults (~4 months of age) the mice had increased activity compared with controls (Eriksson and Fredriksson, 1991). Mouse dams were administered 3 mg/kg/72 h DLM (dissolved in corn oil mixed with peanut butter and voluntarily ingested) from E0 to P22, and at 6-week old offspring had increased locomotor activity (Richardson et al., 2015). This effect occurred in males across three days but only at test intervals longer than 30 min with no differences during the first 30 min of testing and no differences in females (Richardson et al., 2015). The increased activity in male DLM-treated mice was normalized by treatment with methylphenidate (1 mg/kg) (Richardson et al., 2015), suggesting to the authors that the change in activity induced by DLM was ADHD-like, due to the use of methylphenidate as a treatment for ADHD as well as its influence of the biochemistry of the disorder. When mice were exposed perinatally to DLM and tested for locomotor activity between 6 to 12 weeks of age, activity decreased with age, but DLM-treated males were more active than control males (Richardson et al., 2015). However, when rats were exposed to DLM only during gestation, locomotor activity was decreased rather than increased in adulthood. Male rats exposed to DLM (0.08 mg/kg, gavage) from E6-15 were less active than vehicle controls at 60 days of age with no change in females (Lazarini et al., 2001). Johri et al. (2006) found reduced activity in male rat offspring exposed prenatally to DLM from E5-21 (oral doses of 0.25, 0.5, or 1.0 mg/kg DLM). The reduction in activity was dose-dependent at 3 weeks of age, but less pronounced when tested at 6 and 9 weeks of age (females were not tested). The effect of DLM on activity, therefore, appears to depend on exposure age, dose, and age at assessment. These rats studies used commercial DLM formulations that contain other ingredients. Additionally, Lazarini et al. (2001) do not report their vehicle formulation and Johri et al. (2006) did not report the use of the vehicle given to controls, adding some uncertainty to the interpretation of these data. There also are limited data on developmental exposure of rats to commercial fenvalerate (Moniz et al., 1999; Moniz et al., 2005).
Richardson et al. (2015), who observed hyperactivity in mice exposed to 3 mg/kg/72 h of DLM prenatally and postnatally, also tested impulsivity and attention using a fixed ratio (FR) wait operant paradigm. Mice received food reinforcement after 25 lever presses. If mice pressed during the wait interval, the wait time was reset, and they had to press the lever an additional 25 times for reinforcement. Male (but not female) DLM-treated mice had reduced wait times and increased FR resets compared with controls, suggesting impulsivity and alterations in attention from developmental DLM exposure.
As in adults, pyrethroids affect the startle response after developmental exposure. In adults, Type I pyrethroids increase and Type II pyrethroids decrease acoustic startle behavior (Crofton and Reiter, 1988; Williams et al., 2018, 2019). Consistent with this, acoustic startle was decreased in P15 rats given DLM (1, 2, or 4 mg/kg dissolved in corn oil and administered by gavage) for 8 h following dosing (Williams et al., 2019). By contrast, this effect lasted only 4 h in adult rats given even larger doses (Williams et al., 2019). The Type I pyrethroid permethrin (60, 90, or 120 mg/kg given by gavage dissolved in corn oil at a dosing volume of 5 mL/kg) induced increased acoustic startle in P15 rats with a delayed onset, becoming significant 6 h following 60 mg/kg permethrin (Williams et al., 2018). By contrast, at 120 mg/kg, startle was decreased 4, 6, and 8 h after dosing, and at 90 mg/kg there was no effect on startle (Williams et al., 2018). Hence, permethrin has a biphasic effect on acoustic startle in P15 rats perhaps through competing physiological processes resulting in facilitated startle at 60 mg/kg but suppression of the faciliatory effect at the higher dose of 90 mg/kg resulting in no effect, and a dominant suppressive effect at 120 mg/kg. In adult rats, the acoustic startle response is only increased by permethrin. Across studies, the effects of pyrethroids on acoustic startle clearly depend on the pyrethroid, age, dose, and time since dosing. Whether these effects on startle occur with other pyrethroids should be determined in future studies.
Developmental DLM exposure also has cognitive effects. In Richardson et al.’s (2015) study, male mice exposed to DLM (3 mg/kg/72 h from E0-P22), had deficits in working memory reflected by decreased spontaneous alternation in a Y-maze. In another study, shock motivated Y-maze visual discrimination learning in rats exposed to 1 mg/kg DLM from E14-20 was impaired in the offspring (Aziz et al., 2001). These rats were first habituated to a Y-maze for 2-3 min, then given 40 trials with foot-shock induced escape to an illuminated safe arm. The following day the safe arm was switched to a different arm in a reversal learning test. DLM-treated offspring at 6 and 12 weeks of age displayed decreased reversal learning but no differences on acquisition (Aziz et al., 2001). Hence, the above data provide some evidence that developmental DLM exposure causes multiple long-term neurobehavioral changes. Specific alterations that were reported following developmental DLM exposure, both behavioral and biochemical, are summarized in Table 1.
TABLE 1.
Developmental DLM Findings
| Organism/ exposure age/ dose/vehicle |
Developmental DLM Effects | Reference |
|---|---|---|
| Mice (NMRI); P10-16; 0.71 or 1.2 mg/kg – gavage; 20% fat emulsion (egg lectin/ peanut oil/water) |
Receptor density: 0.71 mg/kg - ↑ mACh receptor density; ↑ high-affinity binding sites; ↓ percent low-affinity mACh-R binding; ↑ density nACH-R – in cortex 24h after dose, but not hippocampus 1.2 mg/kg - ↓ mACh-R binding in hippocampus; ↑ nACh-R binding in cerebral cortex |
Eriksson & Nordberg, 1990 |
| Mice (NMRI); P10-16; 0.7 mg/kg – gavage; 20% fat emulsion (egg lectin/ peanut oil/water) | Locomotor activity: No change at P17; ↑ activity at ~ 4 mo. of age) | Eriksson & Fredriksson, 1991 |
| Rat (Wistar); E14-20; 1 mg/kg – gavage; corn oil | Y-maze shock avoidance: ↓ reversal learning (6 & 12 weeks of age in offspring) | Aziz et al., 2001 |
| Rat (Wistar); E6-15; 0.08 mg/kg (commercial formulation) – gavage; vehicle formulation not disclosed |
Locomotor activity: Males less active than controls (P60), no change in females Monoamine: ↑ striatal DOPAC (adult males), no change in DA or HVA; ↑ DOPAC/DA ratio |
Lazarini et al., 2001 |
| Rat (Wistar); E5-21; 0.25, 0.5, 1.0 mg/kg (commercial formulation) – gavage; No vehicle control treatment | Locomotor activity: Dose-dependent ↓ at P21 (male); less effect at 6 & 9 weeks of age; females not tested | Johri et al., 2006 |
| Mice (C57BL/6J); E0-P22; 3 mg/kg/72h – oral; voluntary ingestion; corn oil mixed with peanut butter |
Locomotor activity: ↑ activity (P42 offspring), females (unaffected), males normalized by methylphenidate (1 mg/kg), SCH23390 (0.025, 0.05, 0.1 mg/kg), or eticlopride (0.05, 0.1, 0.2 mg/kg); ↑ activity with apormorphine (0.5 mg/kg; when DA is depleted first by reserpine) males but not females; SKF82958 (1.0 mg/kg) ↑ activity; FR wait operant: ↓ wait times; ↑ FR resets (males only) Y maze: ↓ spontaneous alternation DA: ↑ DAT (n. accumbens); MPTP (1 mg/kg) ↓ DA; ↓ extracellular DA release (microdialysis, n. accumbens); ↑ DRD1 (male n. accumbens) |
Richardson et al., 2015 |
| Rat (Sprague-Dawley); P15; 1, 2, 4 mg/kg – gavage; corn oil | Startle: ↓ (up to 8 h following dose) | Williams et al., 2019 |
Neurotoxicity for developmental DLM on behavior and neurotransmitters. Abbreviations: ↑, increase; ↓, decrease; ASR, acoustic startle response; DA, dopamine; DLM, deltamethrin; DRD1, dopamine receptor D1; DAT, dopamine transporter; DOPAC, 3,4-dihydroxyphenylacetic acid; E, embryonic day; FR, fixed ratio; HVA, homovanillic acid; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; P, postnatal day.
III. A Different Developmental Model
We developed a neonatal exposure model with Sprague Dawley rats treated from P3-20 and examined the long-term effects of DLM at doses of 0, 0.25, 0.5, and 1.0 mg/kg dissolved in 5 mL/kg corn oil administered by gavage (Pitzer et al., 2019) to determine if other cognitive and behavioral effects are caused by developmental DLM.
Rats were used because of their greater cognitive ability relative to mice. DLM or corn oil (CO) treated rats were assessed for allocentric hippocampal dependent learning and memory, egocentric striatal dependent learning and memory, conditioned freezing, acoustic and tactile startle, locomotor activity with or without drug challenge, and anxiety-like behavior. Deficits in allocentric reversal learning and memory were observed in the Morris water maze (MWM) (Pitzer et al., 2019) but not during acquisition as Aziz et al. (2001) found in a Y-maze. Thus, DLM-treated rats learned the first phase of the MWM at the same rate as controls, but when the platform was moved to the opposite quadrant (reversal) or moved a second time to an adjacent quadrant (shift) DLM rats had less efficient paths to the goal and increased average heading errors (Pitzer et al., 2019). As the task became harder, only males had deficits, as seen in the shift phase. These findings implicate impairment of cognitive flexibility while sparing basic spatial navigation. By inference, the deficits after the platform was moved suggest impairments in remapping of distal cues (Vorhees and Williams, 2006). Shift trials are a unique procedure used in this lab. We have seen other examples where effects appear on one or two phases but not all three. The precise reason for such patterns is not known, but each phase places different cognitive demands on the spatial abilities of the rats and reveals effects that would not be observed had only acquisition been tested. The deficits on MWM shift trials may be another reflection of flexibility impairment like the reversal deficit, or it may reflect an increase in retroactive interference since during the shift phase, rats must extinguish two previously learned responses, rather than one as during reversal. However, this explanation cannot account for the sex difference that only appeared on the shift trials. Whatever neural circuits mediate shift learning must be impaired in males but spared in females. While the neurophysiological basis of the shift effect is not known, clearly the task demands on the shift phase are unique and unmask effects not otherwise in evidence. This cross-phase unmasking occurs despite that as rats move from one phase to the next their efficiency steadily improves even as the task becomes more difficult given the decreasing size of the goal. It appears that the requirement to extinguish previous responses and acquire new responses places increased demands on the hippocampal-entorhinal processes that DLM exposure compromised. The unmasking effect of shift testing dissociates the learning-to-learn aspects of increasing task proficiency across phases while revealing treatment effects not otherwise known.
Overall it is clear that the hippocampus is critical for spatial learning and flexibility for stimulus-guided behavior (Eichenbaum and Cohen, 2004), yet there are circuitry differences between brain regions that may account for differences between acquisition, reversal, and shift, and require further investigation. However, there may be species related differences as well, with the data in mice yielding a different result for DLM exposure. Hossain et al. (2015) found deficits in MWM acquisition in adult C57BL/6 mice treated with DLM (0 or 3 mg/kg by gavage dissolved in corn oil every 3 days for 60 days). It would be interesting to see what these mice would show during reversal learning. In addition, it is important to note Hossain et al. (2015) observed MWM acquisition deficits in mice exposed to DLM in adulthood. Thus age-dependent or species differences in the exposure models could account for these differences as well.
The hippocampus and its importance in spatial learning has been extensively studied, including how various disruptions to this region can alter different aspects of spatial learning such as cognitive flexibility. Hippocampus-dependent place learning, but not response learning, is required for spatial flexibility, as shown from data conducted in a water-cross maze (Kleinknecht et al., 2012). In the experiment, an intact hippocampus was necessary for learning spatial relationships because mice treated with ibotenic acid to lesion the hippocampus could not learn the water-cross maze (Kleinknecht et al., 2012). Moreover, fornix lesions in rats leads to impaired cognitive flexibility when distal cues or starting positions in a MWM are altered, even as acquisition is spared (Eichenbaum et al., 1990) similar to what we saw after developmental DLM. Morris et al. (1990) showed that hippocampal, subiculum, and combined hippocampal/subiculum lesions result in impaired place navigation in the MWM, but the lesioned groups gradually caught up to controls when given extra trials. Lesioned rats in that study also had impaired spatial matching to place, which involves changing the location of the escape platform daily. On this task, overtraining did not overcome deficits in spatial learning (Morris et al., 1990). Subiculum lesioned rats have milder deficits in a matching to place water maze, with larger deficits after hippocampus or hippocampus/subiculum lesions (Morris et al., 1990). Additionally, Morris et al. (1990) over-trained rats in the MWM, then lesioned them, and retested the rats later. Under these conditions overtraining did not result in catchup effects. The findings show the essential role of the hippocampus in place learning and cognitive flexibility. However, the more unusual finding of spared acquistion and impaired reversal and shift in the MWM, requires further study.
In addition to MWM reversal and shift deficits, the DLM-treated rats had alterations in conditioned freezing (Pitzer et al., 2019). Conditioned freezing assesses two mnemonic processes, one hippocampally dependent (contextual memory) and one amygdala-dependent (cued memory) (Curzon et al., 2009). We found that DLM-treated males had increased freezing during conditioned training and impaired 24 h contextual memory but spared amygdala-related cued memory (Pitzer et al., 2019). Contextual freezing and MWM spatial flexibility rely on the hippocampus and surrounding structures; hence, the two findings appear consistent.
Spatial learning and memory involve place cells in the CA1 region of the hippocampus (Ainge et al., 2007). Rats with hippocampal damage induced by ibotenic acid infused into the dentate gyrus and CA regions and tested in a double-ended Y-maze for position discrimination and alternation, had increased errors when the side reinforced with food was reversed, but not during acquisition (Ainge et al., 2007). Ainge et al. (2007) also found that place fields in the hippocampus encoded both current and projected locations as the task was learned. However, the learned anticipatory information was disrupted when the platform was moved, implying that memory extinction of the previous platform position was impaired after developmental DLM.
In addition to place fields that are activated during spatial learning, hippocampal stabilization of β-catenin and cytoskeleton-associated protein (Arc) are implicated in reversal learning (Mills et al., 2014). Mice with increased β-catenin under a conditional calcium-calmodulin dependent protein kinase II (CaMKII) Cre-lox system have deficits in spatial reversal learning in the MWM, with spared acquisition, as well as impaired flexibility in a second task, delayed nonmatch to place in a T-maze (Mills et al., 2014). In addition, Arc knock-in mice have intact spatial acquisition but deficits in reversal learning in a Barnes maze (Wall et al., 2018). Because Arc is a mediator of synaptic plasticity and relies on proteasome-dependent degradation, changing the timing of Arc activation and thus altering Arc signaling may contribute to disrupted reversal learning. These data converge in showing the importance of the hippocampus in cognitive flexibility. To understand the extent of the effects of DLM on reversal learning, data on signaling pathways such as β-catenin and Arc, as well as others, after developmental DLM are worth investigating.
Given the effects of DLM on spatial learning, the basis of such effects should be traceable to cellular damage and/or apoptosis in the hippocampus and/or related regions. The hippocampus is a region that continues to exhibit neurogenesis after it has ended in other brain regions. The dentate gyrus (DG) specifically, generates new granule cells neonatally (Bayer and Altman, 1974). It is during this phase of development when we administered DLM, suggesting that new neurons in the DG might be inhibited by DLM. Since the DG is important in spatial learning and memory (Okada et al., 2003), an effect of DLM on DG neurogenesis might explain the spatial deficits. Exposures to other toxins, such as lipopolysaccharide (LPS), lead, and vitamin A deficiency, during gestational and/or postnatal development reduces neurogenesis and results in impaired reference memory, impaired novel object recognition, and impaired contextual freezing (Abrous and Wojtowicz, 2015; Bonnet et al., 2008; Graciarena et al., 2010; Jaako-Movits et al., 2005). Stress during these stages of development also reduces hippocampal neurogenesis and leads to impaired spatial memory in a Y-maze and the MWM (Koo et al., 2003; Lemaire et al., 2000), lending credence to the idea that developmental DLM is damaging to the hippocampus.
Although multiple lines of evidence implicating effects on the hippocampus from developmental DLM exposure, there are other aspects of cognition and behavior that are dependent on other regions that are disrupted following developmental DLM. For example, rats have deficits in egocentric learning and memory in the Cincinnati water maze (CWM) following P3-20 DLM exposure (Pitzer et al., 2019). The CWM is a striatal DA dependent task (Braun et al., 2015; Braun et al., 2016; Vorhees and Williams, 2016). DLM-treated males had increased latency and errors in finding their way out of the CWM compared with controls, however, here again, the effect was only in males; females were unaffected (Pitzer et al., 2019).
The sexually dimorphic effect in the DA-dependent CWM task is consistent with male only effects on DA systems influencing locomotor activity in adult mice treated developmentally with DLM from E0-P22 (Richardson et al., 2015). Richardson et al. (2015) observed male-only hyperactivity in an open-field in DLM-treated mice that was dose-dependent and that was attenuated by methylphenidate or DA specific antagonists and exacerbated by DA agonists (as outlined above in III. Neurotransmitters: catecholaminergic). Nevertheless, the role of DA receptors in these changes remains to be elucidated.
It is important to note that although DA is implicated in our study and Richardson’s, the tasks in the two studies were very different. Open field locomotor activity was the only similar test between the studies, yet on this test the findings were opposite. We observed decreased activity in rats (Pitzer, 2019), whereas Richardson et al. (2015) found increased activity in mice. The DLM-induced hypoactivity we observed is consistent with other studies in rats exposed to DLM. For example, rats exposed to DLM from E6-15 (Lazarini et al., 2001) or from E5-21 (Johri et al., 2006) were hypoactive. However, mice treated with DLM from P10-16 had increased activity (Eriksson and Fredriksson, 1991). These data point to mouse vs. rat differences. Richardson et al. (2015) did not test egocentric learning and we did not test impulsivity therefore no direct comparison is possible for these outcomes. In addition, we tested spatial navigation and conditioned freezing, whereas Richardson tested locomotor activity with dopaminergic agonists and antagonists. Reconciling these outcomes is difficult because the test species, exposure periods, doses, and dose frequency are different. Despite this, both studies found sexually dimorphic effects with most of the effects occurring in males.
We also performed drug challenges to probe receptor involvement, using amphetamine (AMPH) and the irreversible NMDA receptor blocker, MK-801 (Pitzer et al., 2019). AMPH is a classic catecholaminergic agonist used to treat ADHD (Bidwell et al., 2011; Miller, 2011). AMPH administration to the rats exposed to DLM or CO increased activity, with the DLM-treated rats having a greater increase than controls, although this effect was not statistically significant. MK-801, on the other hand, increased activity overall, but the increase in the 1.0 and 0.5 mg/kg DLM groups were significantly lower than in controls. This implies glutamatergic dysfunction and represents another potential mechanism of DLM-induced deficits. Since NMDA is important for learning, NMDA dysfunction may be involved in the spatial learning deficits.
Sexually dimorphic changes were also found for startle in DLM-treated rats (Pitzer et al., 2019). Developmental DLM increased acoustic (ASR) and tactile startle responses (TSR) in males, but not females, with no change in pre-pulse inhibition (PPI). These effects after chronic preweaning DLM and a period of no exposure are in marked contrast to DLM decreased startle after an acute dose at P15. Studies are needed to understand mechanistic differences between acute and chronic developmental DLM exposure.
Anxiety-like behavioral effects are associated with DLM exposure. Adult rats treated with an acute dose of DLM had increased anxiety-like behavior in an elevated plus maze 30-125 min following exposure, with fewer center crossings, reduced arm entries, and reduced time in open arms (Ricci et al., 2013). In addition, zebrafish larvae exhibit increased thigmotaxis following DLM exposure; an effect indicative of anxiety-like behavior in fish (Li et al., 2019). However, after our chronic developmental DLM exposure, we did not observe changes in an elevated zero-maze test of anxiety-like behavior (Pitzer et al. 2019).
IV. Developmental effects on monoamines
Since several studies that have linked changes in dopamine to DLM exposure, we sought to verify these effects. We observed that DLM-treated male rats had decreased AMPH-stimulated extracellular DA release in the n. accumbens by microdialysis (Pitzer et al., 2019). Mice exposed to DLM from E0-P22 similarly had reduced DA release in the n. accumbens by the same method (Richardson et al., 2015). This reduced DA release may contribute to the egocentric learning and memory deficits in CWM, since 6-OHDA induced dopaminergic lesions in the n. accumbens cause CWM deficits (Braun et al., 2016). This would be consistent with data that neurotoxic doses of methamphetamine or AMPH during development cause striatal DA depletion and CWM impairments (Vorhees et al., 2011). In addition, 6-OHDA induced DA depletion in subregions of the striatum resulted in deficits in the CWM (Braun et al., 2015; Braun et al., 2016; Braun et al., 2012).
To determine if the reduction in DA release after developmental DLM also changed DA levels, monoamines were assessed via HPLC-ECD (Pitzer et al., 2019). No changes in hippocampus or neostriatum DA, 5-HT, or their major metabolites DOPAC, 5-HIAA, and HVA were found. In mice developmentally exposed to DLM there were also no changes in DA, DOPAC, or HVA levels (Richardson et al., 2015). The only monoamine effect we found was a decrease in hippocampal norepinephrine (NE) in the DLM rats (Pitzer et al., 2019). NE plays a role in synaptic plasticity, including in long-term potentiation (LTP) (Ikegaya et al., 1997). NE is also involved in Arc expression associated with emotional memory (McIntyre et al., 2005). Application of NMDA in rat hippocampal superfused mini-slices induces [3H]-NE uptake in CA1-CA3 and DG, showing a connection between NE and NMDA in this region (Andres et al., 1993). NE also influences phosphorylation of the GluR1 subunit of the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor and facilitates trafficking of AMPA receptors to the synapse during LTP induction (Hu et al., 2007). Wild-type mice administered epinephrine have enhanced contextual freezing and LTP, whereas mice with phosphorylated GluR1 when administered epinephrine to elevate NE have no effect on contextual freezing nor LTP (Hu et al., 2007). Therefore, the increase in hippocampal NE may contribute to the contextual memory deficits we found after DLM treatment.
In the neostriatum, male rats developmentally treated with DLM, had Drd1 mRNA decreased compared with controls (Pitzer et al., 2019). The decrease could be related to the changes in n. accumbens DA release that we observed. No effect of developmental DLM was found on striatal DRD1, DRD2, or DAT by western blot (unpublished data). This differs from what was observed in mice following DLM exposure from E0 to P22 in which DAT was increased in both sexes, but DRD1 was increased in males only in the n. accumbens. DA receptor expression, including for DRD1, increases during embryogenesis, reaching a peak during adolescence before declining to adult levels in rats (Caille et al., 1995), mice (Araki et al., 2007), and humans (Brana et al., 1996). Perturbations during the embryonic period, when expression is not yet at adult levels, may cause greater effects than when treatment is later in development. Further research to clarify the impact of DLM during different exposure periods is needed.
V. Developmental effects on LTP
The effect of DLM from P3-20 described above, was evaluated for LTP, a cellular mechanism associated with spatial learning and memory (Dringenberg, 2020). We used hippocampal CA1 slices at P25-35. DLM-treated males and females had increased LTP compared with controls (Pitzer et al., 2019). We then tested an identically treated group of rats when they were older (> P60). Only male DLM-treated rats had increased LTP in adulthood, the female effect was no longer significant (unpublished data). Even in DLM-treated males, the LTP effect in adults was not as large as it was at P25-35. Decreased LTP is associated with deficits in learning and memory (Lynch, 2004) but our finding was of increased LTP that were also associated with deficits in learning and memory. Like many processes, too much or too little can be detrimental. Our hypothesis is that the elevated LTP in DLM-treated rats is such a case, an over-response has adverse effects. However, further research is needed to elucidate this mechanism.
Mice engineered to lack the AMPA receptor GluR2 also have increased CA1 hippocampal LTP and decreased exploration of a novel object compared with wild-type mice (Jia et al., 1996). A similar outcome is reported in mice deficient in postsynaptic density protein 95 (PSD-95), which exhibit increased LTP and deficits in MWM learning (Migaud et al., 1998), just as we found after DLM exposure. Future experiments could test LTP in rats after MWM testing and see how closely correlated the two effects are. We did not assess long-term depression (LTD), which is associated with forgetting. Experiments to assess LTD after developmental DLM might be valuable by providing a more complete picture of how hippocampus-mediated spatial learning and memory are altered by DLM.
LTP is involved in synaptic plasticity. It requires increased calcium levels within the postsynaptic neuron, which acts through NMDA receptors, VGCCs, and release of intracellular calcium (Baudry et al., 2015; Blackwell and Jedrzejewska-Szmek, 2013; Fino et al., 2010; Herring and Nicoll, 2016). Calmodulin is a calcium binding partner, which binds to CaMKII, leading to downstream signals that facilitate synaptic activity (Faas et al., 2011; Lisman et al., 2012). Calcium activates calcineurin (protein phosphatase 2B) leading to LTD (Mulkey et al., 1994). The amount of calcium influx determines which pathway is activated, with lower concentrations activating calcineurin and higher concentrations activating CaMKII (Blackwell and Jedrzejewska-Szmek, 2013; Bliss and Collingridge, 1993; Malenka and Bear, 2004). Activating CaMKII phosphorylates AMPA receptors (McGlade-McCulloh et al., 1993; Roche et al., 1996; Tan et al., 1994), which increases synaptic strength. CaMKII can translocate to the synapse, bind NMDA receptors, and cause synaptic potentiation (Lisman et al., 2012). Additional mechanisms of LTP include activation of protein kinase A (PKA) and protein kinase C (PKC), both of which are activated by second messenger G protein coupled receptors (GPCRs), cyclic adenosine monophosphate (cAMP) and diacylglycerol (DAG), respectively (Blackwell and Jedrzejewska-Szmek, 2013). PKA phosphorylates multiple targets, including glutamate receptors, as well as regulating its own activity through the phosphorylation of phosphodiesterases. PKA can activate nuclear targets too, including cyclic AMP response element–binding protein (CREB), leading to LTP (Impey et al., 1996; Sassone-Corsi, 1995). PKC and PKA activate mitogen-activated protein kinase cascades (Deisseroth et al., 1996; Hirono et al., 2001; Sassone-Corsi, 1995; Tanaka and Augustine, 2008; Xing et al., 1996) that modulate protein synthesis to alter synaptic efficiency for periods longer than LTP lasts. LTP is a complex process. It regulates spatial learning and retrieval and the data, although limited, suggest that developmental DLM exposure disrupts these LTP-related signaling pathways. Readers are referred to reviews that focus on LTP, its mechanisms, and its relationship to learning and memory (Baudry et al., 2015; Dringenberg, 2020; Herring and Nicoll, 2016; Kandel, 2001; Kumar, 2011).
VI. Glutamatergic effects
We also measured AMPA and NMDA receptor levels by western blots, due to their importance in learning and memory. GluR1, GluR2, and NMDA-NR1 were not altered in the hippocampus after P3-20 DLM (unpublished data). However, in the hippocampus of DLM-treated males, but not females, NR2A levels were increased and NR2B levels were decreased (unpublished data). NMDA receptors contain one or two NR2 subunits (NR2A, NR2B, NR2C) (Flores-Soto et al., 2012; Vyklicky et al., 2014), and the composition of NR2 subunits influences neuroplasticity. For example, NR2A activation favors LTD and NR2B activation favors LTP (Yashiro and Philpot, 2008). The expression of NR2A and NR2B undergo changes during development. NR2A increases postnatally to adult levels where it remains elevated; NR2B is expressed embryonically, increases postnatally up to P21, then decreases to adult levels (Wenzel et al., 1997). This developmental switch in NR2 expression occurs during the period we treated rats with DLM (P3-20) and therefore this may have altered the NR2 subunit composition leading to changes in function. Examiniation of NR2 insertion into synapses during development in relation to LTP vs. LTD, might shed light on how spatial nagivation is affected by DLM exposure. For example, mice with the Nmdar1 gene knockout in CA1, have normal DG LTP but lack LTP in CA1 (Tsien et al., 1996). These mice have spatial memory deficits but normal non-spatial learning, showing the importance of NMDA-Rs for LTP and spatial navigation.
Greater NMDA-NR2A levels are reported to favor LTD, which is unlike the effects elicited by developmental DLM. We measured field excitatory postsynaptic potentials (fEPSPs), which are the sum of many synapses and therefore do not differentiate among synapses. Sharp electrode methods might be useful to identify affected neurons after DLM treatment since these receptors are heterogeneous and the effects of DLM may be cell specific. Western blots, as we used, use protein from whole brain regions providing only limited spatial resolution. Immunohistochemistry would permit better localization.
Although NR2A and NR2B are involved in LTP and LTD, channel kinetics of the NR2 subunits also affect the favorability for one form of plasticity over the other. NR2A has a lower affinity for CaMKII compared with NMDA-NR2B (Leonard et al., 1999; Strack and Colbran, 1998; Strack et al., 2000; Yashiro and Philpot, 2008). In addition, NR2A-containing NMDA-Rs limit the flow of inward calcium flux (Erreger et al., 2005), which influences the induction of LTP vs LTD on a calcium concentration-dependent basis. It is possible that LTD is a mechanism by which DLM alters learning and memory based on increased NR2A/NR2B ratios. However, the observed effects on LTP in DLM-treated rats is not consistent with this idea, hence experiments to clarify this are needed. Additional tests are also needed to understand the impact of developmental DLM on channel kinetics. NR2A channels open more briefly and deactivate faster than NR2B channels, but they also have a higher probability of opening (Erreger et al., 2005; Santucci and Raghavachari, 2008). Under conditions where a stimulus is applied repetitively, increased NR2A levels could lead to increased CA1 LTP.
To further elucidate effects on learning we explored changes in glutamatergic signaling. Given the effects we saw with the MK-801 challenge on activity, we examined stimulated hippocampal glutamate release by microdialysis. No change was seen after potassium stimulated release in DLM-treated rats compared with controls (unpublished data). However, DLM administered to adult rats increases extracellular glutamate and decreases GABA release by microdialysis at high doses when tested shortly after treatment (Hossain et al., 2008). This effect should be explored after developmental exposure. Overall, the effects on LTP and NMDA-NR2 expression are promising areas for further study of DLM-induced alterations in allocentric learning and memory and cognitive flexibility.
VII. Inflammatory cytokines and apoptosis
We used in situ terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) to test for cells undergoing apoptosis from DLM exposure. Developmentally DLM-treated males had increased cell death in the striatum and hippocampus when assessed at P21 (unpublished data; females were not included initially). We then examined cell death in the DG by cell counting. We observed increased TUNEL positive cells in the DG of DLM-treated males compared with controls (unpublished data). The observed increase in apoptotic cells in DLM-treated rats may contribute to the alterations in learning and memory since the DG is involved in spatial learning and reference memory.
DLM also leads to increased cell death in culture (Elwan et al., 2006; Hossain and Richardson, 2011; Wu et al., 2003) and in vivo in adult animals (Hossain, 2019; Wu and Liu, 2000a, b) at higher doses than we used. Caspase-3 is increased in DLM-treated SK-N-AS cells (Hossain and Richardson, 2011) and in adult DLM-treated mice (Hossain, 2019; Hossain et al., 2015). However, in rats treated developmentally with DLM, we found no alterations in caspase-3 expression by western blot (unpublished data). It is important to note that caspase-3 expression is low during early postnatal development thereby limiting the detection sensitivity at these ages with this method.
We also assessed changes in proinflammatory cytokines. Cytokines are secreted by immune cells to initate defense against injury, infection, or toxicity (Turner et al., 2014; Zhang and An, 2007). Few studies have examined the effect of DLM on cytokine expression. One study observed increased TNFα levels in primary microglia cultures following DLM treatment (Hossain et al., 2016). TNF-α and IL-1β induce neurotoxicity by increasing glutamate excitotoxicity (Ye et al., 2013). Preliminary data after exposure to 1 mg/kg DLM or CO from P3-20 treated rats with tissue collected on P20 had several trends (p<0.10 but not p<0.5 and should be viewed with caution) (Table S1). Three of the four trends were found in the hippocampus and two in the striatum. In the hippocampus, trends were observed for DLM-induced increased IL-13 (F(1,31.6) = 2.93, p<0.10), IL-4 (F(1,36.3) = 3.02, p<0.09), and decreased Kcgro (F(1,21.2) = 3.93, p=0.06). In the striatum, a trend was observed for DLM-induced decrease in IL-6 (F(1,42.8) = 3.81, p<0.06) and in TNFα (F(1,34.7) = 2.89, p < 0.10). The meaning of these trends is not yet known. Oxidative stress is associated with DLM exposure (Kumar et al., 2015) and can affect cytokines. IL-1β, for example, plays a role in oxidative stress (He et al., 2015; Hewett et al., 2012) and is important in neuronal survival (Albrecht et al., 2002; Hewett et al., 2012; John et al., 2005; Saavedra et al., 2007). The pattern in the hippocampus suggests both proinflammatory changes reflected by increases in IL-4 and IL-13 and anti-inflammatory effects of decreased KC/GRO (also known as CXCL1). IL-4 and IL-13 are closely related to each another. Increases in IL-4 and IL-13 activate Th2 cells and stimulate B and T cells, resulting in increased IgE and MHC-II cells, whereas reduced CXCL1 decreases binding to its receptor, CXLR2, and results in reduced inflammation. In striatum, the trend was for a decrease in IL-6. Increases in IL-6 have proinflammatory effects, therefore, decreases are anti-inflammatory. IL-6 crosses the blood brain barrier and when increased in brain, increases the synthesis of prostaglandin E2 (PGE2) in the hypothalamus and activates glucagon-like peptide (GLP1), a short-acting regulator of blood glucose. Research is needed to determine if these interleukin changes are sufficient to be part of the mechanism of the developmental neurotoxicity of DLM.
VIII. Sexually dimorphic effects
Several male-specific effects of developmental DLM exposure were observed in our experiments and in those of Hossain and Richardson. Although some tests had changes in both sexes, many were only in males. This was also seen in other animal studies (Lazarini et al., 2001) (Richardson, 2015) and in epidemiological studies (Wagner-Schuman et al., 2015). However, the basis of these differential effects is unknown. Future studies should test hormones and their receptors. Pyrethroids are suspected endocrine-disruptors. DLM in vitro, as well as the metabolite 3-PBA, exhibit antagonistic effects for androgen receptors (Du et al., 2010). DLM also has weak estrogenic effects, and 3-PBA has anti-estrogenic effects (Du et al., 2010). DLM is antagonistic for the thyroid hormone receptor (Du et al., 2010). These data indicate that DLM has hormonal effects that may underlie the sexual dimorphism after DLM exposure. Estrogens are known to be CNS protectants and may explain why developmental DLM has few effects on female rats (Gillies and McArthur, 2010; Wise et al., 2001; Zárate et al., 2017).
7. Conclusions
In rodents, DLM has multiple developmental effects, with long-lasting influences on cognition and behavior. The mechanism by which these behaviors are changed is not yet known, but there is mounting evidence that dopaminergic and glutamatergic pathways are involved (Table 2). Recent studies in rats and mice show that DLM has lasting effects after early exposure, however, differences between studies in experimental conditions make only limited generalizations possible. Once the mechanisms are elucidated there will remain the task of determining the best way to extrapolate animal findings to children. Nevertheless, recent experiments provide leads worth pursuing, including the reduced release of DA in the n. accumbens, reduced Drd1 mRNA expression in striatum, increased CA1 LTP, changes in NMDA-NR2 subunits, cell death, and possibly changes in cytokines. These effects are region specific, showing the need to avoid studies in whole brain. If pyrethroids adversely affect children’s neurodevelopment, finding out how this occurs and at what exposure levels is required. Experiments reviewed here represent the beginning of the process of finding the developmental mechanism, but more studies are needed and are important given the widespread use of DLM.
TABLE 2.
Summary of Results
| Test | Dependent variable | Age (P) | Result |
|---|---|---|---|
| Open-Field a | Locomotor activity | 60 | Decreased at 0.25, 0.5, and 1 mg/kg DLM |
| EZM a | Anxiety-like behavior | 61 | No effects |
| Straight Channel a | Latency | 62 | No effects |
| MWM a | Latency, path efficiency, swim speed | 63-85 | Impaired cognitive flexibility (reversal) at 0.5 and 1 mg/kg DLM box sexes; impaired shift at 1 mg/kg DLM males |
| CWM a | Latency, errors | 86-103 | Increased errors and latency at 1 mg/kg DLM |
| ASR/TSR a | Peak startle response | 104 | Increased ASR and TSR at 0.5 and 1 mg/kg DLM males |
| PPI a | Sensorimotor gating | 105 | No effects |
| Conditioned Freezing a | Contextual and cued memory | 106-108 | Increased freezing during conditioning at 0.5 and 1 mg/kg DLM males; Impaired contextual memory at 0.25 and 1 mg/kg DLM |
| Open-field with AMPH a | AMPH-induced hyperactivity | 109 | No differential effects |
| Open-field with MK-801 a | MK-801-induced hyperactivity | 116 | Under response to MK-801 at 1 mg/kg DLM |
| Monoamines a | DA, NE, 5-HT, DOPAC, 5-HIAA, HVA): neostriatum and hippocampus | 123-130 | Decreased hippocampal NE at 0.5 and 1 mg/kg DLM |
| qPCR a | Gene expression: neostriatum and hippocampus | 123-130 | Decreased Drd1 mRNA in neostriatum at 1 mg/kg DLM |
| Juvenile LTP a | CA1 | 25-35 | Increased LTP at 1 mg/kg DLM |
| AMPH-stimulated DA release a | Microdialysis, n. accumbens | ≥60 | Decreased DA release * |
| Adult LTP b | fEPSPs in CA1 | ≥60 | Increased LTP males at 1 mg/kg DLM |
| Western blot b | DRD1, DRD2, DAT), AMPAR (GluR1 and GluR2), NMDAR (NR1, NR2A, and NR2B): hippocampus, n. accumbens, and neostriatum | ≥60 | Increased NR2A, decreased NR2B in hippocampus, 1 mg/kg DLM males |
| Stimulated Glu release b | Microdialysis: K+ stimulated Glu release in hippocampus | ≥ 60 | No effects * |
| Caspase-3 b | Pro- and cleaved caspase-3 in hippocampus, n. accumbens, and neostriatum | 3-21 | No effects |
| TUNEL b | Apoptosis in hippocampus and striatum | 21 | Increased TUNEL positive cells in Dentate gyrus |
| Cytokines b | IFN-γ, IL-1β, IL-4, IL5, IL6, IL-10, IL-13, KC/GRO, TNF-α) in hippocampus, n. accumbens, and neostriatum | 20 | Preliminary Data: Not significant (See Table S1) |
1 mg/kg DLM compared with controls
1 mg/kg DLM males compared with controls.
Unpublished. Abbreviations: See Table 1 and AMPH, Amphetamine; ASR/TSR, Acoustic and tactile startle response; CWM, Cincinnati water maze; DRD2, dopamine receptor subunit D2; DOPAC, 3,4-dihydroxyphenylacetic acid; fEPSP, field excitatory postsynaptic potential; EZM, elevated zero maze; GluR1, AMPA receptor subunit R1; GluR2, AMPA receptor subunit R2; HPLC, high performance liquid chromatograph; IFN-γ, interferon gamma; IL-1β, interleukin 1β; IL-4, interleukin 4; IL-5, interleukin 5; IL-6, interleukin 6; IL-10, interleukin 10, IL-13, interleukin 13; KC/GRO, keratinocyte chemoattractant/growth-regulated oncogene; LTP, long-term potentiation; MWM, Morris water maze; NE, norepinephrine; NR1, NMDA receptor subunit NR1; NR2A, NMDA receptor subunit NR2A; NR2B, NMDA receptor subunit NR2B; PPI, prepulse inhibition of startle; qPCR, quantitative polymerase chain reaction; TNF-α, Tumor Necrosis Factor alpha; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling.
Supplementary Material
HIGHLIGHTS.
Pyrethroid insecticides are used worldwide may adversely affect children.
Pyrethroids mechanisms and effects are reviewed with emphasis on deltamethrin.
Deltamethrin can cause acute and chronic effects on brain and behavior.
Deltamethrin is developmentally neurotoxic to dopamine systems.
Developmental deltamethrin causes cognitive impairments in rats and mice.
Acknowledgement
This review was made possible with the support of training grant T32 ES007051 (E.M.P.) and NIH grant R01 ES032270 (C.V.V. & M.T.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interest regarding this work.
References
- Abrous DN, Wojtowicz JM, 2015. Interaction between Neurogenesis and Hippocampal Memory System: New Vistas. Cold Spring Harbor perspectives in biology 7(6), a018952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J, Vorhees CV, Middaugh LD, 1990. Developmental neurotoxicity of anticonvulsants: human and animal evidence on phenytoin. Neurotoxicology and teratology 12(3), 203–214. [DOI] [PubMed] [Google Scholar]
- Ainge JA, Tamosiunaite M, Woergoetter F, Dudchenko PA, 2007. Hippocampal CA1 place cells encode intended destination on a maze with multiple choice points. The Journal of neuroscience : the official journal of the Society for Neuroscience 27(36), 9769–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht PJ, Dahl JP, Stoltzfus OK, Levenson R, Levison SW, 2002. Ciliary neurotrophic factor activates spinal cord astrocytes, stimulating their production and release of fibroblast growth factor-2, to increase motor neuron survival. Exp. Neurol 173(1), 46–62. [DOI] [PubMed] [Google Scholar]
- Albrieux M, Platel J-C, Dupuis A, Villaz M, Moody WJ, 2004. Early expression of sodium channel transcripts and sodium current by cajal-retzius cells in the preplate of the embryonic mouse neocortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 24(7), 1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegaert K, van den Anker J, 2019. Ontogeny of Phase I Metabolism of Drugs. J. Clin. Pharmacol 59Suppl 1, S33–S41. [DOI] [PubMed] [Google Scholar]
- Aman TK, Grieco-Calub TM, Chen C, Rusconi R, Slat EA, Isom LL, Raman IM, 2009. Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels. The Journal of neuroscience : the official journal of the Society for Neuroscience 29(7), 2027–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaraneni M, Pang J, Bruckner JV, Muralidhara S, Mortuza TB, Gullick D, Hooshfar S, White CA, Cummings BS, 2017a. Influence of Maturation on In Vivo Tissue to Plasma Partition Coefficients for Cis- and Trans-Permethrin. J. Pharm. Sci 106(8), 2144–2151. [DOI] [PubMed] [Google Scholar]
- Amaraneni M, Pang J, Mortuza TB, Muralidhara S, Cummings BS, White CA, Vorhees CV, Zastre J, Bruckner JV, 2017b. Brain uptake of deltamethrin in rats as a function of plasma protein binding and blood-brain barrier maturation. Neurotoxicology 62, 24–29. [DOI] [PubMed] [Google Scholar]
- Anadon A, Martinez-Larranaga MR, Fernandez-Cruz ML, Diaz MJ, Fernandez MC, Martinez MA, 1996. Toxicokinetics of deltamethrin and its 4'-HO-metabolite in the rat. Toxicol. Appl. Pharmacol 141(1), 8–16. [DOI] [PubMed] [Google Scholar]
- Anand SS, Bruckner JV, Haines WT, Muralidhara S, Fisher JW, Padilla S, 2006. Characterization of deltamethrin metabolism by rat plasma and liver microsomes. Toxicol. Appl. Pharmacol 212(2), 156–166. [DOI] [PubMed] [Google Scholar]
- Anand SS, Kim KB, Padilla S, Muralidhara S, Kim HJ, Fisher JW, Bruckner JV, 2006. Ontogeny of hepatic and plasma metabolism of deltamethrin in vitro: role in age-dependent acute neurotoxicity. Drug Metab. Dispos 34(3), 389–397. [DOI] [PubMed] [Google Scholar]
- Anand SS, Kim KB, Padilla S, Muralidhara S, Kim HJ, Fisher JW, Bruckner JV, 2006. Ontogeny of hepatic and plasma metabolism of deltamethrin in vitro: role in age-dependent acute neurotoxicity. Drug Metab. Dispos 34(3), 389–397. [DOI] [PubMed] [Google Scholar]
- Andres ME, Bustos G, Gysling K, 1993. Regulation of [3H]norepinephrine release by N-methyl-D-aspartate receptors in minislices from the dentate gyrus and the CA1-CA3 area of the rat hippocampus. Biochem. Pharmacol 46(11), 1983–1987. [DOI] [PubMed] [Google Scholar]
- Araki KY, Sims JR, Bhide PG, 2007. Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and postnatal development. Brain Res. 1156, 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin B, 2011. Effects of thiacloprid, deltamethrin and their combination on oxidative stress in lymphoid organs, polymorphonuclear leukocytes and plasma of rats. Pesticide Biochemistry and Physiology 100(2), 165–171. [Google Scholar]
- Aziz MH, Agrawal AK, Adhami VM, Shukla Y, Seth PK, 2001. Neurodevelopmental consequences of gestational exposure (GD14-GD20) to low dose deltamethrin in rats. Neurosci. Lett 300(3), 161–165. [DOI] [PubMed] [Google Scholar]
- Baloch S, Verma R, Huang H, Khurd P, Clark S, Yarowsky P, Abel T, Mori S, Davatzikos C, 2009. Quantification of brain maturation and growth patterns in C57BL/6J mice via computational neuroanatomy of diffusion tensor images. Cereb. Cortex 19(3), 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Olsson AO, Wong LY, Udunka S, Baker SE, Whitehead RD, Magsumbol MS, Williams BL, Needham LL, 2010. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: National Health and Nutrition Examination Survey 1999–2002. Environ. Health Perspect 118(6), 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M, Zhu G, Liu Y, Wang Y, Briz V, Bi X, 2015. Multiple cellular cascades participate in long-term potentiation and in hippocampus-dependent learning. Brain Res. 1621, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J, 1974. Hippocampal development in the rat: cytogenesis and morphogenesis examined with autoradiography and low-level X-irradiation. J. Comp. Neurol 158(1), 55–79. [DOI] [PubMed] [Google Scholar]
- Bennett B, Workman T, Smith MN, Griffith WC, Thompson B, Faustman EM, 2020. Characterizing the Neurodevelopmental Pesticide Exposome in a Children's Agricultural Cohort. Int. J. Environ. Res. Public Health 17(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdikova Bohne VJ, Hamre K, Arukwe A, 2007. Hepatic metabolism, phase I and II biotransformation enzymes in Atlantic salmon (Salmo Salar, L) during a 12 week feeding period with graded levels of the synthetic antioxidant, ethoxyquin. Food Chem. Toxicol 45(5), 733–746. [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, Landrigan PJ, Wolff MS, 2003. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environmental health perspectives 111(1), 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell LC, McClernon FJ, Kollins SH, 2011. Cognitive enhancers for the treatment of ADHD. Pharmacology, biochemistry, and behavior 99(2), 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell KT, Jedrzejewska-Szmek J, 2013. Molecular mechanisms underlying neuronal synaptic plasticity: systems biology meets computational neuroscience in the wilds of synaptic plasticity. Wiley Interdiscip Rev Syst Biol Med 5(6), 717–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL, 1993. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31. [DOI] [PubMed] [Google Scholar]
- Bockhorst KH, Narayana PA, Liu R, Ahobila-Vijjula P, Ramu J, Kamel M, Wosik J, Bockhorst T, Hahn K, Hasan KM, Perez-Polo JR, 2008. Early postnatal development of rat brain: in vivo diffusion tensor imaging. J. Neurosci. Res 86(7), 1520–1528. [DOI] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G, 2001. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron 30(1), 91–104. [DOI] [PubMed] [Google Scholar]
- Bonnet E, Touyarot K, Alfos S, Pallet V, Higueret P, Abrous DN, 2008. Retinoic acid restores adult hippocampal neurogenesis and reverses spatial memory deficit in vitamin A deprived rats. PLoS One 3(10), e3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG, 2010. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics 125(6), e1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brana C, Caille I, Pellevoisin C, Charron G, Aubert I, Caron MG, Carles D, Vital C, Bloch B, 1996. Ontogeny of the striatal neurons expressing the D1 dopamine receptor in humans. J. Comp. Neurol 370(1), 23–34. [DOI] [PubMed] [Google Scholar]
- Braun AA, Amos-Kroohs RM, Gutierrez A, Lundgren KH, Seroogy KB, Skelton MR, Vorhees CV, Williams MT, 2015. Dopamine depletion in either the dorsomedial or dorsolateral striatum impairs egocentric Cincinnati water maze performance while sparing allocentric Morris water maze learning. Neurobiology of learning and memory 118, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AA, Amos-Kroohs RM, Gutierrez A, Lundgren KH, Seroogy KB, Vorhees CV, Williams MT, 2016. 6-Hydroxydopamine-Induced Dopamine Reductions in the Nucleus Accumbens, but not the Medial Prefrontal Cortex, Impair Cincinnati Water Maze Egocentric and Morris Water Maze Allocentric Navigation in Male Sprague-Dawley Rats. Neurotox. Res 30(2), 199–212. [DOI] [PubMed] [Google Scholar]
- Braun AA, Graham DL, Schaefer TL, Vorhees CV, Williams MT, 2012. Dorsal striatal dopamine depletion impairs both allocentric and egocentric navigation in rats. Neurobiology of learning and memory 97(4), 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP, 2006. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environmental health perspectives 114(12), 1904–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CJ, Pastoor TP, 2018. Pyrethroid epidemiology: a quality-based review. Critical reviews in toxicology 48(4), 297–311. [DOI] [PubMed] [Google Scholar]
- Burr SA, Ray DE, 2004. Structure-activity and interaction effects of 14 different pyrethroids on voltage-gated chloride ion channels. Toxicol. Sci 77(2), 341–346. [DOI] [PubMed] [Google Scholar]
- Caille I, Dumartin B, Le Moine C, Begueret J, Bloch B, 1995. Ontogeny of the D1 Dopamine Receptor in the Rat Striatonigral System: an lmmunohistochemical Study. European Journal of Neuroscience 7(4), 714–722. [DOI] [PubMed] [Google Scholar]
- Cantalamessa F, 1993. Acute toxicity of two pyrethroids, permethrin, and cypermethrin in neonatal and adult rats. Arch. Toxicol 67(7), 510–513. [DOI] [PubMed] [Google Scholar]
- Cao Z, Shafer TJ, Murray TF, 2011. Mechanisms of pyrethroid insecticide-induced stimulation of calcium influx in neocortical neurons. The Journal of pharmacology and experimental therapeutics 336(1), 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalani A, Sabbatini M, Consoli C, Cinque C, Tomassoni D, Azmitia E, Angelucci L, Amenta F, 2002. Glial fibrillary acidic protein immunoreactive astrocytes in developing rat hippocampus. Mech. Ageing Dev 123(5), 481–490. [DOI] [PubMed] [Google Scholar]
- Catterall WA, 2000a. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26(1), 13–25. [DOI] [PubMed] [Google Scholar]
- Catterall WA, 2000b. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol 16, 521–555. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Cestele S, Yarov-Yarovoy V, Yu FH, Konoki K, Scheuer T, 2007. Voltage-gated ion channels and gating modifier toxins. Toxicon 49(2), 124–141. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J, 2005. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev 57(4), 411–425. [DOI] [PubMed] [Google Scholar]
- CDC, 2019. Centers for Disease Control and Prevention: Fouth Report on Human Exposure to Environmental Chemicals: Updated Tables (January 2019). Centers for Disease Control and Prevention, Atlanta, GA, pp. 1–866 and 861–994. [Google Scholar]
- Cestele S, Catterall WA, 2000. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie 82(9–10), 883–892. [DOI] [PubMed] [Google Scholar]
- Chapman RA, 1983. Chiral-phase high-performance liquid chromatographic separation of enantiomers of pyrethroid insecticide esters derived from α-cyano-3-phenoxybenzyl alcohol. Journal of Chromatography A 258, 175–182. [Google Scholar]
- Chen SY, Zhang ZW, He FS, Yao PP, Wu YQ, Sun JX, Liu LH, Li QG, 1991. An epidemiological study on occupational acute pyrethroid poisoning in cotton farmers. British journal of industrial medicine 48(2), 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Trzoss L, Yang D, Yan B, 2015. Ontogenic expression of human carboxylesterase-2 and cytochrome P450 3A4 in liver and duodenum: postnatal surge and organ-dependent regulation. Toxicology 330, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrustek A, Holynska-Iwan I, Dziembowska I, Bogusiewicz J, Wróblewski M, Cwynar A, Olszewska-Slonina D, 2018. Current Research on the Safety of Pyrethroids Used as Insecticides. Medicina (Kaunas) 54(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrustek A, Hołyńska-Iwan I, Dziembowska I, Bogusiewicz J, Wróblewski M, Cwynar A, Olszewska-Słonina D, 2018. Current Research on the Safety of Pyrethroids Used as Insecticides. Medicina (Kaunas, Lithuania) 54(4), 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P, 2001. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet 68(6), 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Casida JE, 1986. Effects of insecticides and GABAergic agents on a house fly [35S]t-butylbicyclophosphorothionate binding site. Pesticide Biochemistry and Physiology 25(1), 63–72. [Google Scholar]
- Cole LM, Ruzo LO, Wood EJ, Casida JE, 1982. Pyrethroid metabolism: comparative fate in rats of tralomethrin, tralocythrin, deltamethrin, and (1R,.alpha.S)-cis-cypermethrin. Journal of Agricultural and Food Chemistry 30(4), 631–636. [DOI] [PubMed] [Google Scholar]
- Cowan WM, 1979. The development of the brain. Sci. Am 241(3), 113–133. [PubMed] [Google Scholar]
- Crofton KM, Reiter LW, 1988. The effects of type I and II pyrethroids on motor activity and the acoustic startle response in the rat. Fundam Appl Toxicol 10(4), 624–634. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Reiter LW, Mailman RB, 1987. Pyrethroid insecticides and radioligand displacement from the gaba receptor chloride ionophore complex. Toxicology Letters 35(2), 183–190. [DOI] [PubMed] [Google Scholar]
- Crow JA, Borazjani A, Potter PM, Ross MK, 2007. Hydrolysis of pyrethroids by human and rat tissues: examination of intestinal, liver and serum carboxylesterases. Toxicol. Appl. Pharmacol 221(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon P, Rustay NR, Browman KE, 2009. Cued and Contextual Fear Conditioning for Rodents, in: J.J. B (Ed.) Methods of Behavior Analysis in Neuroscience. CRC Press; /Taylor & Francis, Boca Raton, FL. [PubMed] [Google Scholar]
- Davies TG, Field LM, Usherwood PN, Williamson MS, 2007. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB life 59(3), 151–162. [DOI] [PubMed] [Google Scholar]
- De Souza Spinosa H, Silva YM, Nicolau AA, Bernardi MM, Lucisano A, 1999. Possible anxiogenic effects of fenvalerate, a type II pyrethroid pesticide, in rats. Physiol. Behav 67(4), 611–615. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW, 1996. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron 16(1), 89–101. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Murray TF, 1988. Involvement of peripheral-type benzodiazepine receptors in the proconvulsant actions of pyrethroid insecticides. J. Pharmacol. Exp. Ther 247(1), 14–22. [PubMed] [Google Scholar]
- Devaud LL, Szot P, Murray TF, 1986. PK 11195 antagonism of pyrethroid-induced proconvulsant activity. European journal of pharmacology 121(2), 269–273. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J, 1979. Comparative aspects of the brain growth spurt. Early Hum. Dev 3, 79–83. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, 2020. The history of long-term potentiation as a memory mechanism: Controversies, confirmation, and some lessons to remember. Hippocampus 30(9), 987–1012. [DOI] [PubMed] [Google Scholar]
- Du G, Shen O, Sun H, Fei J, Lu C, Song L, Xia Y, Wang S, Wang X, 2010. Assessing hormone receptor activities of pyrethroid insecticides and their metabolites in reporter gene assays. Toxicol. Sci 116(1), 58–66. [DOI] [PubMed] [Google Scholar]
- Du Y, Lee J-E, Nomura Y, Zhang T, Zhorov BS, Dong K, 2009. Identification of a cluster of residues in transmembrane segment 6 of domain III of the cockroach sodium channel essential for the action of pyrethroid insecticides. The Biochemical journal 419(2), 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Nomura Y, Satar G, Hu Z, Nauen R, He SY, Zhorov BS, Dong K, 2013. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc. Natl. Acad. Sci. U. S. A 110(29), 11785–11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ, 2004. From Conditioning to Conscious Recollection: Memory Systems of the Brain. Oxford University Press. [Google Scholar]
- Eichenbaum H, Stewart C, Morris RG, 1990. Hippocampal representation in place learning. J. Neurosci 10(11), 3531–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gohary M, Awara WM, Nassar S, Hawas S, 1999. Deltamethrin-induced testicular apoptosis in rats: the protective effect of nitric oxide synthase inhibitor. Toxicology 132(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Elliott M, Farnham AW, Janes NF, Needham PH, Pulman DA, 1974. Synthetic insecticide with a new order of activity. Nature 248(5450), 710–711. [DOI] [PubMed] [Google Scholar]
- Elwan MA, Richardson JR, Guillot TS, Caudle WM, Miller GW, 2006. Pyrethroid pesticide-induced alterations in dopamine transporter function. Toxicol. Appl. Pharmacol 211(3), 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enan E, Pinkerton KE, Peake J, Matsumura F, 1996. Deltamethrin-induced thymus atrophy in male Balb/c mice. Biochem. Pharmacol 51(4), 447–454. [DOI] [PubMed] [Google Scholar]
- Enan E, Pinkerton KE, Peake J, Matsumura F, 1996. Deltamethrin-induced thymus atrophy in male Balb/c mice. Biochemical pharmacology 51(4), 447–454. [DOI] [PubMed] [Google Scholar]
- EPA, 2015. Deltamethrin: Human Health Risk Assessment for the Proposed Use of Deltamethrin as a Mosquito Adulticide Over Agricultural Crops. DP No. D417556. , in: Prevention, O.o.C.S.a.P (Ed.). Washington, DC. [Google Scholar]
- EPA, 2020. Deltamethrin: Proposed Interim Registration Review Decision, Case Number 7414. U.S. Environmental Protection Agency, Washington, D.C., pp. 1–33. [Google Scholar]
- Eriksson P, Fredriksson A, 1991. Neurotoxic effects of two different pyrethroids, bioallethrin and deltamethrin, on immature and adult mice: changes in behavioral and muscarinic receptor variables. Toxicol. Appl. Pharmacol 108(1), 78–85. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Nordberg A, 1990. Effects of two pyrethroids, bioallethrin and deltamethrin, on subpopulations of muscarinic and nicotinic receptors in the neonatal mouse brain. Toxicol. Appl. Pharmacol 102(3), 456–463. [DOI] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF, 2005. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J. Physiol 563(Pt 2), 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escayg A, Heils A, MacDonald BT, Haug K, Sander T, Meisler MH, 2001. A novel SCN1A mutation associated with generalized epilepsy with febrile seizures plus--and prevalence of variants in patients with epilepsy. Am J Hum Genet 68(4), 866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas GC, Raghavachari S, Lisman JE, Mody I, 2011. Calmodulin as a direct detector of Ca2+ signals. Nat. Neurosci 14(3), 301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felter SP, Daston GP, Euling SY, Piersma AH, Tassinari MS, 2015. Assessment of health risks resulting from early-life exposures: Are current chemical toxicity testing protocols and risk assessment methods adequate? Crit. Rev. Toxicol 45(3), 219–244. [DOI] [PubMed] [Google Scholar]
- Felts PA, Yokoyama S, Dib-Hajj S, Black JA, Waxman SG, 1997. Sodium channel alpha-subunit mRNAs I, II, III, NaG, Na6 and hNE (PN1): different expression patterns in developing rat nervous system. Brain Res. Mol. Brain Res 45(1), 71–82. [DOI] [PubMed] [Google Scholar]
- Field LM, Emyr Davies TG, O'Reilly AO, Williamson MS, Wallace BA, 2017. Voltage-gated sodium channels as targets for pyrethroid insecticides. Eur Biophys J 46(7), 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Paille V, Cui Y, Morera-Herreras T, Deniau JM, Venance L, 2010. Distinct coincidence detectors govern the corticostriatal spike timing-dependent plasticity. J. Physiol 588(Pt 16), 3045–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Soto ME, Chaparro-Huerta V, Escoto-Delgadillo M, Vazquez-Valls E, Gonzalez-Castaneda RE, Beas-Zarate C, 2012. [Structure and function of NMDA-type glutamate receptor subunits]. Neurologia 27(5), 301–310. [DOI] [PubMed] [Google Scholar]
- FQPA, F.Q.P.A.o., 1996. Public Law 104–170.
- Gammon DW, Brown MA, Casida JE, 1981. Two classes of pyrethroid action in the cockroach. Pesticide Biochemistry and Physiology 15(2), 181–191. [Google Scholar]
- Ghiasuddin SM, Soderlund DM, 1985. Pyrethroid insecticides: Potent, stereospecific enhancers of mouse brain sodium channel activation. Pesticide Biochemistry and Physiology 24(2), 200–206. [Google Scholar]
- Gillies GE, McArthur S, 2010. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacological reviews 62(2), 155–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin SJ, DeVito MJ, Hughes MF, Ross DG, Scollon EJ, Starr JM, Setzer RW, Conolly RB, Tornero-Velez R, 2010. Physiologically based pharmacokinetic modeling of deltamethrin: development of a rat and human diffusion-limited model. Toxicological sciences : an official journal of the Society of Toxicology 115(2), 330–343. [DOI] [PubMed] [Google Scholar]
- Godin SJ, Scollon EJ, Hughes MF, Potter PM, DeVito MJ, Ross MK, 2006. Species differences in the in vitro metabolism of deltamethrin and esfenvalerate: differential oxidative and hydrolytic metabolism by humans and rats. Drug Metab. Dispos 34(10), 1764–1771. [DOI] [PubMed] [Google Scholar]
- Goodman R, 1997. The Strengths and Difficulties Questionnaire: a research note. J. Child Psychol. Psychiatry 38(5), 581–586. [DOI] [PubMed] [Google Scholar]
- Gottlieb A, Keydar I, Epstein HT, 1977. Rodent brain growth stages: an analytical review. Biol. Neonate 32(3–4), 166–176. [DOI] [PubMed] [Google Scholar]
- Graciarena M, Depino AM, Pitossi FJ, 2010. Prenatal inflammation impairs adult neurogenesis and memory related behavior through persistent hippocampal TGFbeta1 downregulation. Brain. Behav. Immun 24(8), 1301–1309. [DOI] [PubMed] [Google Scholar]
- Harris RA, Allan AM, 1985. Functional coupling of gamma-aminobutyric acid receptors to chloride channels in brain membranes. Science 228(4703), 1108–1110. [DOI] [PubMed] [Google Scholar]
- Hatta T, Ohmori H, Murakami T, Takano M, Yamashita K, Yasuda M, 1999. Neurotoxic effects of phenytoin on postnatal mouse brain development following neonatal administration. Neurotoxicol. Teratol 21(1), 21–28. [DOI] [PubMed] [Google Scholar]
- He F, Wang S, Liu L, Chen S, Zhang Z, Sun J, 1989. Clinical manifestations and diagnosis of acute pyrethroid poisoning. Arch. Toxicol 63(1), 54–58. [DOI] [PubMed] [Google Scholar]
- He FS, Deng H, Ji X, Zhang ZW, Sun JX, Yao PP, 1991. Changes of nerve excitability and urinary deltamethrin in sprayers. Int. Arch. Occup. Environ. Health 62(8), 587–590. [DOI] [PubMed] [Google Scholar]
- He Y, Jackman NA, Thorn TL, Vought VE, Hewett SJ, 2015. Interleukin-1beta protects astrocytes against oxidant-induced injury via an NF-kappaB-dependent upregulation of glutathione synthesis. Glia 63(9), 1568–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner J, Lundell KH, Breese GR, Mueller RA, Hedner T, 1986. Developmental variations in CSF monoamine metabolites during childhood. Biol. Neonate 49(4), 190–197. [DOI] [PubMed] [Google Scholar]
- Hemingway J, Ranson H, 2000. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol 45, 371–391. [DOI] [PubMed] [Google Scholar]
- Henault-Ethier L, Soumis N, Bouchard M, 2016. Health and environmental impact of pyrethroid insecticides: What we know, what we don't know and what we should do about it: Executive summary and scientific review. Health Canada, Montreal, p. 66. [Google Scholar]
- Herring BE, Nicoll RA, 2016. Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu. Rev. Physiol 78, 351–365. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN, 2006. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ. Health Perspect 114(7), 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heudorf U, Angerer J, Drexler H, 2004. Current internal exposure to pesticides in children and adolescents in Germany: urinary levels of metabolites of pyrethroid and organophosphorus insecticides. Int. Arch. Occup. Environ. Health 77(1), 67–72. [DOI] [PubMed] [Google Scholar]
- Hewett SJ, Jackman NA, Claycomb RJ, 2012. Interleukin-1β in Central Nervous System Injury and Repair. Eur J Neurodegener Dis 1(2), 195–211. [PMC free article] [PubMed] [Google Scholar]
- Hirono M, Sugiyama T, Kishimoto Y, Sakai I, Miyazawa T, Kishio M, Inoue H, Nakao K, Ikeda M, Kawahara S, Kirino Y, Katsuki M, Horie H, Ishikawa Y, Yoshioka T, 2001. Phospholipase Cbeta4 and protein kinase Calpha and/or protein kinase CbetaI are involved in the induction of long term depression in cerebellar Purkinje cells. J. Biol. Chem 276(48), 45236–45242. [DOI] [PubMed] [Google Scholar]
- Hossain MM, DiCicco-Bloom E, Richardson JR, 2015. Hippocampal ER stress and learning deficits following repeated pyrethroid exposure. Toxicological sciences : an official journal of the Society of Toxicology 143(1), 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MM, DiCicco-Bloom E, Richardson JR, 2015. Hippocampal ER stress and learning deficits following repeated pyrethroid exposure. Toxicol. Sci 143(1), 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MM, Liu J, Richardson JR, 2016. Pyrethroid Insecticides Directly Activate Microglia Through Interaction With Voltage-Gated Sodium Channels. Toxicological Sciences 155(1), 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MM, Richardson JR, 2011. Mechanism of pyrethroid pesticide-induced apoptosis: role of calpain and the ER stress pathway. Toxicol Sci 122(2), 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MM, Richardson JR, 2011. Mechanism of pyrethroid pesticide-induced apoptosis: role of calpain and the ER stress pathway. Toxicol. Sci 122(2), 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MM, Sivaram G, Richardson JR, 2019. Regional Susceptibility to ER Stress and Protection by Salubrinal Following a Single Exposure to Deltamethrin. Toxicol. Sci 167(1), 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MM, Suzuki T, Unno T, Komori S, Kobayashi H, 2008. Differential presynaptic actions of pyrethroid insecticides on glutamatergic and GABAergic neurons in the hippocampus. Toxicology 243(1), 155–163. [DOI] [PubMed] [Google Scholar]
- Hossain MM, Suzuki T, Richardson JR, Kobayashi H, 2013. Acute effects of pyrethroids on serotonin release in the striatum of awake rats: an in vivo microdialysis study. J. Biochem. Mol. Toxicol 27(2), 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R, 2007. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell 131(1), 160–173. [DOI] [PubMed] [Google Scholar]
- Hu H, Tian M, Ding C, Yu S, 2018. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol 9, 3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PE, Alexi T, Yoshida T, Schreiber SS, Knusel B, 1996. Excitotoxic lesion of rat brain with quinolinic acid induces expression of p53 messenger RNA and protein and p53-inducible genes Bax and Gadd-45 in brain areas showing DNA fragmentation. Neuroscience 74(4), 1143–1160. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Nakanishi K, Saito H, Abe K, 1997. Amygdala beta-noradrenergic influence on hippocampal long-term potentiation in vivo. Neuroreport 8(14), 3143–3146. [DOI] [PubMed] [Google Scholar]
- Imai T, Ohura K, 2010. The role of intestinal carboxylesterase in the oral absorption of prodrugs. Curr Drug Metab 11(9), 793–805. [DOI] [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR, 1996. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron 16(5), 973–982. [DOI] [PubMed] [Google Scholar]
- Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA, 1992. Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science 256(5058), 839–842. [DOI] [PubMed] [Google Scholar]
- Isom LL, Ragsdale DS, De Jongh KS, Westenbroek RE, Reber BF, Scheuer T, Catterall WA, 1995. Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell 83(3), 433–442. [DOI] [PubMed] [Google Scholar]
- Jaako-Movits K, Zharkovsky T, Romantchik O, Jurgenson M, Merisalu E, Heidmets LT, Zharkovsky A, 2005. Developmental lead exposure impairs contextual fear conditioning and reduces adult hippocampal neurogenesis in the rat brain. Int. J. Dev. Neurosci 23(7), 627–635. [DOI] [PubMed] [Google Scholar]
- Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna FA, Velumian A, MacDonald J, Carlen P, Abramow-Newerly W, Roder J, 1996. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron 17(5), 945–956. [DOI] [PubMed] [Google Scholar]
- Jiang X, Nardelli J, 2016. Cellular and molecular introduction to brain development. Neurobiol. Dis 92(Pt A), 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Lee SC, Song X, Rivieccio M, Brosnan CF, 2005. IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia 49(2), 161–176. [DOI] [PubMed] [Google Scholar]
- Johri A, Yadav S, Singh RL, Dhawan A, Ali M, Parmar D, 2006. Long lasting effects of prenatal exposure to deltamethrin on cerebral and hepatic cytochrome P450s and behavioral activity in rat offspring. Eur. J. Pharmacol 544(1–3), 58–68. [DOI] [PubMed] [Google Scholar]
- Jones H, Rowland-Yeo K, 2013. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacometrics Syst Pharmacol 2, e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, 2001. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294(5544), 1030–1038. [DOI] [PubMed] [Google Scholar]
- Kaneko H, 2010. Chapter 76 - Pyrethroid Chemistry and Metabolism, in: Krieger R (Ed.) Hayes' Handbook of Pesticide Toxicology (Third Edition). Academic Press, New York, pp. 1635–1663. [Google Scholar]
- Kearney JA, Plummer NW, Smith MR, Kapur J, Cummins TR, Waxman SG, Goldin AL, Meisler MH, 2001. A gain-of-function mutation in the sodium channel gene Scn2a results in seizures and behavioral abnormalities. Neuroscience 102(2), 307–317. [DOI] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC, 2008. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat/ Rev. Drug Discov 7(12), 1013–1030. [DOI] [PubMed] [Google Scholar]
- Kim KB, Anand SS, Kim HJ, White CA, Bruckner JV, 2008. Toxicokinetics and tissue distribution of deltamethrin in adult Sprague-Dawley rats. Toxicological sciences : an official journal of the Society of Toxicology 101(2), 197–205. [DOI] [PubMed] [Google Scholar]
- Kim KB, Anand SS, Kim HJ, White CA, Fisher JW, Tornero-Velez R, Bruckner JV, 2010. Age, dose, and time-dependency of plasma and tissue distribution of deltamethrin in immature rats. Toxicol. Sci 115(2), 354–368. [DOI] [PubMed] [Google Scholar]
- King GF, 2011. Venoms as a platform for human drugs: translating toxins into therapeutics. Expert Opin. Biol. Ther 11(11), 1469–1484. [DOI] [PubMed] [Google Scholar]
- King GF, Escoubas P, Nicholson GM, 2008. Peptide toxins that selectively target insect Na(V) and Ca(V) channels. Channels (Austin) 2(2), 100–116. [DOI] [PubMed] [Google Scholar]
- Kleinknecht K, Bedenk B, Kaltwasser S, Gruenecker B, Yen Y-C, Czisch M, Wotjak C, 2012. Hippocampus-dependent place learning enables spatial flexibility in C57BL6/N mice. Frontiers in Behavioral Neuroscience 6(87). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Park CH, Choi SH, Kim NJ, Kim HS, Choe JC, Suh YH, 2003. The postnatal environment can counteract prenatal effects on cognitive ability, cell proliferation, and synaptic protein expression. FASEB J. 17(11), 1556–1558. [DOI] [PubMed] [Google Scholar]
- Kumar A, 2011. Long-Term Potentiation at CA3-CA1 Hippocampal Synapses with Special Emphasis on Aging, Disease, and Stress. Front. Aging Neurosci 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Sasmal D, Sharma N, 2015. An insight into deltamethrin induced apoptotic calcium, p53 and oxidative stress signalling pathways. Toxicology and Environmental Health Sciences 7(1), 25–34. [Google Scholar]
- Lawrence LJ, Casida JE, 1983. Stereospecific action of pyrethroid insecticides on the gamma-aminobutyric acid receptor-ionophore complex. Science 221(4618), 1399–1401. [DOI] [PubMed] [Google Scholar]
- Lazarini CA, Florio JC, Lemonica IP, Bernardi MM, 2001. Effects of prenatal exposure to deltamethrin on forced swimming behavior, motor activity, and striatal dopamine levels in male and female rats. Neurotoxicol. Teratol 23(6), 665–673. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, Simonsen D, Liu B, Bao W, 2020. Environmental exposure to pyrethroid pesticides in a nationally representative sample of U.S. adults and children: The National Health and Nutrition Examination Survey 2007–2012. Environ. Pollut 267, 115489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Wang DD, Dou TY, Hou J, Feng L, Yin H, Luo Q, Sun J, Ge GB, Yang L, 2017. Assessment of the inhibitory effects of pyrethroids against human carboxylesterases. Toxicol. Appl. Pharmacol 321, 48–56. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN, 2000. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl. Acad. Sci. U. S. A 97(20), 11032–11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW, 1999. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. U. S. A 96(6), 3239–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Sedlacek M, Manoharan I, Boopathy R, Duysen EG, Masson P, Lockridge O, 2005. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem. Pharmacol 70(11), 1673–1684. [DOI] [PubMed] [Google Scholar]
- Li HY, Wu SY, Shi N, 2007. Transcription factor Nrf2 activation by deltamethrin in PC12 cells: Involvement of ROS. Toxicol. Lett 171(1–2), 87–98. [DOI] [PubMed] [Google Scholar]
- Li M, Liu X, Feng X, 2019. Cardiovascular toxicity and anxiety-like behavior induced by deltamethrin in zebrafish (Danio rerio) larvae. Chemosphere 219, 155–164. [DOI] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S, 2012. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci 13(3), 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Sun Y, Ares I, Anadón A, Martínez M, Martínez-Larrañaga M-R, Yuan Z, Wang X, Martínez M-A, 2019. Deltamethrin toxicity: A review of oxidative stress and metabolism. Environmental research 170, 260–281. [DOI] [PubMed] [Google Scholar]
- Lummis SCR, Chow SC, Holan G, Johnston GAR, 1987. γ-Aminobutyric Acid Receptor Ionophore Complexes: Differential Effects of Deltamethrin, Dichlorodiphenyltrichloroethane, and Some Novel Insecticides in a Rat Brain Membrane Preparation. Journal of neurochemistry 48(3), 689–694. [DOI] [PubMed] [Google Scholar]
- Lynch MA, 2004. Long-Term Potentiation and Memory. Physiological reviews 84(1), 87–136. [DOI] [PubMed] [Google Scholar]
- Magby JP, Richardson JR, 2017. Developmental pyrethroid exposure causes long-term decreases of neuronal sodium channel expression. Neurotoxicology 60, 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF, 2004. LTP and LTD: an embarrassment of riches. Neuron 44(1), 5–21. [DOI] [PubMed] [Google Scholar]
- Martinez-Larranaga MR, Anadon A, Martinez MA, Martinez M, Castellano VJ, Diaz MJ, 2003. 5-HT loss in rat brain by type II pyrethroid insecticides. Toxicol. Ind. Health 19(7–10), 147–155. [DOI] [PubMed] [Google Scholar]
- McEnery MW, Vance CL, Begg CM, Lee W-L, Choi Y, Dubel SJ, 1998. Differential Expression and Association of Calcium Channel Subunits in Development and Disease. Journal of Bioenergetics and Biomembranes 30(4), 409–418. [DOI] [PubMed] [Google Scholar]
- McGlade-McCulloh E, Yamamoto H, Tan SE, Brickey DA, Soderling TR, 1993. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature 362(6421), 640–642. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL, 2005. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc. Natl. Acad. Sci. U. S. A 102(30), 10718–10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Hauser R, 2009. Pyrethroid insecticide metabolites are associated with serum hormone levels in adult men. Reprod. Toxicol. 27(2), 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler MH, Kearney J, Ottman R, Escayg A, 2001. Identification of epilepsy genes in human and mouse. Annu Rev Genet 35, 567–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O'Dell TJ, Grant SG, 1998. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396(6710), 433–439. [DOI] [PubMed] [Google Scholar]
- Miller FD, Gauthier AS, 2007. Timing is everything: making neurons versus glia in the developing cortex. Neuron 54(3), 357–369. [DOI] [PubMed] [Google Scholar]
- Miller GM, 2011. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. Journal of neurochemistry 116(2), 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills F, Bartlett TE, Dissing-Olesen L, Wisniewska MB, Kuznicki J, Macvicar BA, Wang YT, Bamji SX, 2014. Cognitive flexibility and long-term depression (LTD) are impaired following β-catenin stabilization in vivo. Proceedings of the National Academy of Sciences 111(23), 8631–8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirfazaelian A, Kim KB, Anand SS, Kim HJ, Tornero-Velez R, Bruckner JV, Fisher JW, 2006. Development of a physiologically based pharmacokinetic model for deltamethrin in the adult male Sprague-Dawley rat. Toxicol. Sci 93(2), 432–442. [DOI] [PubMed] [Google Scholar]
- Moniz AC, Cruz-Casallas PE, Oliveira CA, Lucisano A, Florio JC, Nicolau AA, Spinosa HS, Bernardi MM, 1999. Perinatal fenvalerate exposure: behavioral and endocrinology changes in male rats. Neurotoxicol. Teratol 21(5), 611–618. [DOI] [PubMed] [Google Scholar]
- Moniz AC, Cruz-Casallas PE, Salzgeber SA, Varoli FM, Spinosa HS, Bernardi MM, 2005. Behavioral and endocrine changes induced by perinatal fenvalerate exposure in female rats. Neurotoxicol. Teratol 27(4), 609–614. [DOI] [PubMed] [Google Scholar]
- Morgan K, Stevens EB, Shah B, Cox PJ, Dixon AK, Lee K, Pinnock RD, Hughes J, Richardson PJ, Mizuguchi K, Jackson AP, 2000. beta 3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proceedings of the National Academy of Sciences of the United States of America 97(5), 2308–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Schenk F, Tweedie F, Jarrard LE, 1990. Ibotenate Lesions of Hippocampus and/or Subiculum: Dissociating Components of Allocentric Spatial Learning. Eur. J. Neurosci 2(12), 1016–1028. [DOI] [PubMed] [Google Scholar]
- Mortuza T, Chen C, White CA, Cummings BS, Muralidhara S, Gullick D, Bruckner JV, 2018. Toxicokinetics of Deltamethrin: Dosage Dependency, Vehicle Effects, and Low-Dose Age-Equivalent Dosimetry in Rats. Toxicol. Sci 162(1), 327–336. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC, 1994. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369(6480), 486–488. [DOI] [PubMed] [Google Scholar]
- Narahashi T, 1996. Neuronal ion channels as the target sites of insecticides. Pharmacol. Toxicol 79(1), 1–14. [DOI] [PubMed] [Google Scholar]
- Narahashi T, 2000. Neuroreceptors and ion channels as the basis for drug action: past, present, and future. J. Pharmacol. Exp. Ther 294(1), 1–26. [PubMed] [Google Scholar]
- Noebels JL, 2002. Sodium channel gene expression and epilepsy. Novartis Found. Symp 241, 109–120; discussion 120–103, 226–132. [PubMed] [Google Scholar]
- Ohmori H, Ogura H, Yasuda M, Nakamura S, Hatta T, Kawano K, Michikawa T, Yamashita K, Mikoshiba K, 1999. Developmental neurotoxicity of phenytoin on granule cells and Purkinje cells in mouse cerebellum. J. Neurochem 72(4), 1497–1506. [DOI] [PubMed] [Google Scholar]
- Okada T, Yamada N, Tsuzuki K, Horikawa HP, Tanaka K, Ozawa S, 2003. Long-term potentiation in the hippocampal CA1 area and dentate gyrus plays different roles in spatial learning. Eur. J. Neurosci 17(2), 341–349. [DOI] [PubMed] [Google Scholar]
- Oros DR, Werner I, 2005. Pyrethroid Insecticides: An Analysis of Use Patterns, Distributions, Potential Toxicity and Fate in the Sacramento-San Joaquin Delta and Central Valley. [Google Scholar]
- Oslowski CM, Urano F, 2011. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 490, 71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Bouchard MF, 2013. Urinary metabolites of organophosphate and pyrethroid pesticides and behavioral problems in Canadian children. Environmental health perspectives 121(11–12), 1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, 2004. In search of the function of the peripheral-type benzodiazepine receptor. Endocrin. Res 30(4), 677–684. [DOI] [PubMed] [Google Scholar]
- Patino GA, Isom LL, 2010. Electrophysiology and beyond: multiple roles of Na+ channel beta subunits in development and disease. Neurosci. Lett 486(2), 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Fernandez V, Garcia MA, Marina ML, 2010. Characteristics and enantiomeric analysis of chiral pyrethroids. J. Chromatogr. A 1217(7), 968–989. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, 2003. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol. Rev 83(1), 117–161. [DOI] [PubMed] [Google Scholar]
- Pitzer EM, Sugimoto C, Gudelsky GA, Huff Adams CL, Williams MT, Vorhees CV, 2019. Deltamethrin Exposure Daily From Postnatal Day 3–20 in Sprague-Dawley Rats Causes Long-term Cognitive and Behavioral Deficits. Toxicol. Sci 169(2), 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzer EM, Sugimoto C, Gudelsky GA, Huff Adams CL, Williams MT, Vorhees CV, 2019. Deltamethrin exposure daily from postnatal day 3–20 in Sprague-Dawley rats causes long-term cognitive and behavioral deficits. Toxicol. Sci 169(2), 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planells-Cases R, Caprini M, Zhang J, Rockenstein EM, Rivera RR, Murre C, Masliah E, Montal M, 2000. Neuronal death and perinatal lethality in voltage-gated sodium channel alpha(II)-deficient mice. Biophys. J 78(6), 2878–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman H, Ali M, Atif F, Kaur M, Bhatia K, Raisuddin S, 2006. The modulatory effect of deltamethrin on antioxidants in mice. Clin. Chim. Acta 369(1), 61–65. [DOI] [PubMed] [Google Scholar]
- Rehman H, Althbyani A, Saggu S, Zahid A, Mohan D, Ansari A, 2014. Systematic review on pyrethroid toxicity with special reference to deltamethrin. Journal of Entomology and Zoology Studies 2, 60–70. [Google Scholar]
- Ricci E, Jr V, Habr SF, Macrini D, Bernardi M, Spinosa H, 2013. Behavioral and neurochemical evidence of deltamethrin anxiogenic-like effects in rats. Brazilian Journal of Veterinary Research and Animal Science 50, 33–42. [Google Scholar]
- Richardson JR, Taylor MM, Shalat SL, Guillot TS 3rd, Caudle WM, Hossain MM, Mathews TA, Jones SR, Cory-Slechta DA, Miller GW, 2015. Developmental pesticide exposure reproduces features of attention deficit hyperactivity disorder. Faseb j 29(5), 1960–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Taylor MM, Shalat SL, Guillot TS III, Caudle WM, Hossain MM, Mathews TA, Jones SR, Cory-Slechta DA, Miller GW, 2015. Developmental pesticide exposure reproduces features of attention deficit hyperactivity disorder. FASEB J. 29(5), 1960–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard J, Brodie ME, 1985. Correlation of blood and brain levels of the neurotoxic pyrethroid deltamethrin with the onset of symptoms in rats. Pesticide Biochemistry and Physiology 23(2), 143–156. [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL, 1996. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16(6), 1179–1188. [DOI] [PubMed] [Google Scholar]
- Romero A, Ramos E, Castellano V, Martinez MA, Ares I, Martinez M, Martinez-Larranaga MR, Anadon A, 2012. Cytotoxicity induced by deltamethrin and its metabolites in SH-SY5Y cells can be differentially prevented by selected antioxidants. Toxicol. In Vitro 26(6), 823–830. [DOI] [PubMed] [Google Scholar]
- Romijn HJ, Hofman MA, Gramsbergen A, 1991. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum. Dev 26(1), 61–67. [DOI] [PubMed] [Google Scholar]
- Saavedra A, Baltazar G, Duarte EP, 2007. Interleukin-1beta mediates GDNF up-regulation upon dopaminergic injury in ventral midbrain cell cultures. Neurobiol. Dis 25(1), 92–104. [DOI] [PubMed] [Google Scholar]
- Santucci DM, Raghavachari S, 2008. The effects of NR2 subunit-dependent NMDA receptor kinetics on synaptic transmission and CaMKII activation. PLoS Comput. Biol 4(10), e1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P, 1995. Transcription factors responsive to cAMP. Annu. Rev. Cell Dev. Biol 11, 355–377. [DOI] [PubMed] [Google Scholar]
- Satoh T, Hosokawa M, 2006. Structure, function and regulation of carboxylesterases. Chem. Biol. Interact 162(3), 195–211. [DOI] [PubMed] [Google Scholar]
- Schettgen T, Heudorf U, Drexler H, Angerer J, 2002. Pyrethroid exposure of the general population-is this due to diet. Toxicol. Lett 134(1–3), 141–145. [DOI] [PubMed] [Google Scholar]
- Schilling MA, Inman SL, Morford LL, Moran MS, Vorhees CV, 1999. Prenatal phenytoin exposure and spatial navigation in offspring: effects on reference and working memory and on discrimination learning. Neurotoxicology and teratology 21(5), 567–578. [DOI] [PubMed] [Google Scholar]
- Scollon EJ, Starr JM, Godin SJ, DeVito MJ, Hughes MF, 2009. In vitro metabolism of pyrethroid pesticides by rat and human hepatic microsomes and cytochrome p450 isoforms. Drug Metab. Dispos 37(1), 221–228. [DOI] [PubMed] [Google Scholar]
- Scott JG, Matsumura F, 1983. Evidence for two types of toxic actions of pyrethroids on susceptible and DDT-resistant german cockroaches. Pesticide Biochemistry and Physiology 19(2), 141–150. [Google Scholar]
- Shafer TJ, Meyer DA, 2004. Effects of pyrethroids on voltage-sensitive calcium channels: a critical evaluation of strengths, weaknesses, data needs, and relationship to assessment of cumulative neurotoxicity. Toxicol. Appl. Pharmacol 196(2), 303–318. [DOI] [PubMed] [Google Scholar]
- Shafer TJ, Meyer DA, Crofton KM, 2005. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environmental health perspectives 113(2), 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah BS, Stevens EB, Pinnock RD, Dixon AK, Lee K, 2001. Developmental expression of the novel voltage-gated sodium channel auxiliary subunit beta3, in rat CNS. The Journal of physiology 534(Pt 3), 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets LP, Doherty JD, Law MW, Reiter LW, Crofton KM, 1994. Age-dependent differences in the susceptibility of rats to deltamethrin. Toxicol Appl Pharmacol 126(1), 186–190. [DOI] [PubMed] [Google Scholar]
- Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, Hansen RL, Hertz-Picciotto I, 2014. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ. Health Perspect 122(10), 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver KS, Du Y, Nomura Y, Oliveira EE, Salgado VL, Zhorov BS, Dong K, 2014. Voltage-Gated Sodium Channels as Insecticide Targets. Adv. In Insect Phys. 46, 389–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM, 2012. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch Toxicol 86(2), 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM, 2012. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch. Toxicol 86(2), 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM, Bloomquist JR, 1989. Neurotoxic actions of pyrethroid insecticides. Ann. Rev. Entomol 34, 77–96. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Casida JE, 1977. Effects of pyrethroid structure on rates of hydrolysis and oxidation by mouse liver microsomal enzymes. Pesticide Biochemistry and Physiology 7(4), 391–401. [Google Scholar]
- Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML, 2002. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology 171(1), 3–59. [DOI] [PubMed] [Google Scholar]
- Song G, Moreau M, Efremenko A, Lake BG, Wu H, Bruckner JV, White CA, Osimitz TG, Creek MR, Hinderliter PM, Clewell HJ, Yoon M, 2019. Evaluation of Age-Related Pyrethroid Pharmacokinetic Differences in Rats: Physiologically-Based Pharmacokinetic Model Development Using In Vitro Data and In Vitro to In Vivo Extrapolation. Toxicol. Sci 169(2), 365–379. [DOI] [PubMed] [Google Scholar]
- Strack S, Colbran RJ, 1998. Autophosphorylation-dependent targeting of calcium/ calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl- D-aspartate receptor. J. Biol. Chem 273(33), 20689–20692. [DOI] [PubMed] [Google Scholar]
- Strack S, McNeill RB, Colbran RJ, 2000. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem 275(31), 23798–23806. [DOI] [PubMed] [Google Scholar]
- Symington SB, Frisbie RK, Clark JM, 2008. Characterization of 11 commercial pyrethroids on the functional attributes of rat brain synaptosomes. Pesticide Biochemistry and Physiology 92(2), 61–69. [Google Scholar]
- Symington SB, Frisbie RK, Lu KD, Marshall Clark J, 2007. Action of cismethrin and deltamethrin on functional attributes of isolated presynaptic nerve terminals from rat brain. Pesticide Biochemistry and Physiology 87(2), 172–181. [Google Scholar]
- Tan SE, Wenthold RJ, Soderling TR, 1994. Phosphorylation of AMPA-type glutamate receptors by calcium/calmodulin-dependent protein kinase II and protein kinase C in cultured hippocampal neurons. J. Neurosci 14(3 Pt 1), 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Augustine GJ, 2008. A positive feedback signal transduction loop determines timing of cerebellar long-term depression. Neuron 59(4), 608–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero-Velez R, Mirfazaelian A, Kim KB, Anand SS, Kim HJ, Haines WT, Bruckner JV, Fisher JW, 2010. Evaluation of deltamethrin kinetics and dosimetry in the maturing rat using a PBPK model. Toxicol. Appl. Pharmacol 244(2), 208–217. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S, 1996. The Essential Role of Hippocampal CA1 NMDA Receptor–Dependent Synaptic Plasticity in Spatial Memory. Cell 87(7), 1327–1338. [DOI] [PubMed] [Google Scholar]
- Turner MD, Nedjai B, Hurst T, Pennington DJ, 2014. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1843(11), 2563–2582. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, 2005. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiological reviews 85(1), 201–279. [DOI] [PubMed] [Google Scholar]
- Verschoyle RD, Aldridge WN, 1980. Structure-activity relationships of some pyrethroids in rats. Arch. Toxicol 45(4), 325–329. [DOI] [PubMed] [Google Scholar]
- Vinayagam MM, 2017. Molecular Mechanism of Neurodevelopmental Toxicity Risks of Occupational Exposure of Pyrethroid Pesticide with Reference to Deltamethrin - A Critical Review. BAOJ Pathol, an open access journal 1, 1–14. [Google Scholar]
- Vorhees CV, Acuff-Smith KD, Schilling MA, Moran MS, 1995. Prenatal exposure to sodium phenytoin in rats induces complex maze learning deficits comparable to those induced by exposure to phenytoin acid at half the dose. Neurotoxicology and teratology 17(6), 627–632. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, He E, Skelton MR, Graham DL, Schaefer TL, Grace CE, Braun AA, Amos-Kroohs R, Williams MT, 2011. Comparison of (+)-methamphetamine, +/−−methylenedioxymethamphetamine, (+)-amphetamine and +/−−fenfluramine in rats on egocentric learning in the Cincinnati water maze. Synapse (New York, N.Y.) 65(5), 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT, 2006. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature protocols 1(2), 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT, 2016. Cincinnati water maze: A review of the development, methods, and evidence as a test of egocentric learning and memory. Neurotoxicol. Teratol 57, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, Lichnerova K, Černý J, Krusek J, Dittert I, Horak M, Vyklicky L, 2014. Structure, Function, and Pharmacology of NMDA Receptor Channels. Physiological research / Academia Scientiarum Bohemoslovaca 63 Suppl 1, S191–203. [DOI] [PubMed] [Google Scholar]
- Wagner-Schuman M, Richardson JR, Auinger P, Braun JM, Lanphear BP, Epstein JN, Yolton K, Froehlich TE, 2015. Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of U.S. children. Environmental health : a global access science source 14, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner-Schuman M, Richardson JR, Auinger P, Braun JM, Lanphear BP, Epstein JN, Yolton K, Froehlich TE, 2015. Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of U.S. children. Environ. Health 14, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Collins DR, Chery SL, Allen ZD, Pastuzyn ED, George AJ, Nikolova VD, Moy SS, Philpot BD, Shepherd JD, Müller J, Ehlers MD, Mabb AM, Corrêa SAL, 2018. The Temporal Dynamics of Arc Expression Regulate Cognitive Flexibility. Neuron 98(6), 1124–1132.e1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RH, Scheffer IE, Barnett S, Richards M, Dibbens L, Desai RR, Lerman-Sagie T, Lev D, Mazarib A, Brand N, Ben-Zeev B, Goikhman I, Singh R, Kremmidiotis G, Gardner A, Sutherland GR, George AL Jr., Mulley JC, Berkovic SF, 2001. Neuronal sodium-channel alpha1-subunit mutations in generalized epilepsy with febrile seizures plus. Am J Hum Genet 68(4), 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zou L, Jin Q, Hou J, Ge G, Yang L, 2018. Human carboxylesterases: a comprehensive review. Acta Pharmaceutica Sinica B 8(5), 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yarov-Yarovoy V, Kahn R, Gordon D, Gurevitz M, Scheuer T, Catterall WA, 2011. Mapping the receptor site for alpha-scorpion toxins on a Na+ channel voltage sensor. Proc. Natl. Acad. Sci. U. S. A 108(37), 15426–15431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wey S, Zhang Y, Ye R, Lee AS, 2009. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal 11(9), 2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, Wang GK, 2003. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell. Signal 15(2), 151–159. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D, 1997. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J. Neurochem 68(2), 469–478. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Camann DE, Kinney PL, Reyes A, Ramirez J, Dietrich J, Diaz D, Holmes D, Perera FP, 2002. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ. Health Perspect 110(5), 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MK, Rundle A, Holmes D, Reyes M, Hoepner LA, Barr DB, Camann DE, Perera FP, Whyatt RM, 2008. Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000–2001 U.S. Environmental Protection Agency restriction of organophosphates. Environ. Health Perspect 116(12), 1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Gutierrez A, Vorhees CV, 2018. Effects of Acute Exposure of Permethrin in Adult and Developing Sprague-Dawley Rats on Acoustic Startle Response and Brain and Plasma Concentrations. Toxicol. Sci 165(2), 361–371. [DOI] [PubMed] [Google Scholar]
- Williams MT, Gutierrez A, Vorhees CV, 2019. Effects of Acute Deltamethrin Exposure in Adult and Developing Sprague Dawley Rats on Acoustic Startle Response in Relation to Deltamethrin Brain and Plasma Concentrations. Toxicol. Sci 168(1), 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Böttner M, 2001. Minireview: Neuroprotective Effects of Estrogen—New Insights into Mechanisms of Action. Endocrinology 142(3), 969–973. [DOI] [PubMed] [Google Scholar]
- Wolansky MJ, Harrill JA, 2008. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol. Teratol 30(2), 55–78. [DOI] [PubMed] [Google Scholar]
- Wu A, Li L, Liu Y, 2003. Deltamethrin induces apoptotic cell death in cultured cerebral cortical neurons. Toxicol. Appl. Pharmacol 187(1), 50–57. [DOI] [PubMed] [Google Scholar]
- Wu A, Liu Y, 2000a. Apoptotic cell death in rat brain following deltamethrin treatment. Neurosci. Lett 279(2), 85–88. [DOI] [PubMed] [Google Scholar]
- Wu A, Liu Y, 2000b. Deltamethrin induces delayed apoptosis and altered expression of p53 and bax in rat brain. Environ. Toxicol. Pharmacol 8(3), 183–189. [DOI] [PubMed] [Google Scholar]
- Xiang H, Kinoshita Y, Knudson CM, Korsmeyer SJ, Schwartzkroin PA, Morrison RS, 1998. Bax involvement in p53-mediated neuronal cell death. J. Neurosci 18(4), 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Ginty DD, Greenberg ME, 1996. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273(5277), 959–963. [DOI] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC, 2005. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest 115(10), 2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z, Li X, Su Q, Xu L, Zhang P, Kong Z, Xu J, Teng J, 2013. Effect of synthetic pyrethroid pesticide exposure during pregnancy on the growth and development of infants. Asia Pac. J. Public Health 25(4 Suppl), 72S–79S. [DOI] [PubMed] [Google Scholar]
- Yadav S, Johri A, Dhawan A, Seth PK, Parmar D, 2006. Regional specificity in deltamethrin induced cytochrome P450 expression in rat brain. Toxicol. Appl. Pharmacol 217(1), 15–24. [DOI] [PubMed] [Google Scholar]
- Yadav S, Johri A, Dhawan A, Seth PK, Parmar D, 2006. Regional specificity in deltamethrin induced cytochrome P450 expression in rat brain. Toxicology and applied pharmacology 217(1), 15–24. [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhang Y, Jiang Y, Zhu F, Zeng L, Wang Y, Lei X, Yao Y, Hou Y, Xu L, Xiong C, Yang X, Hu K, 2017. Transcriptional responses in the hepatopancreas of Eriocheir sinensis exposed to deltamethrin. PLoS One 12(9), e0184581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD, 2008. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 55(7), 1081–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Huang Y, Zhao L, Li Y, Sun L, Zhou Y, Qian G, Zheng JC, 2013. IL-1beta and TNF-alpha induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J. Neurochem 125(6), 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KJ, Hyatt JL, Morton CL, Lee RE, Potter PM, Danks MK, 2004. Characterization of inhibitors of specific carboxylesterases: development of carboxylesterase inhibitors for translational application. Mol. Cancer Ther 3(8), 903–909. [PubMed] [Google Scholar]
- Yousef MI, Awad TI, Mohamed EH, 2006. Deltamethrin-induced oxidative damage and biochemical alterations in rat and its attenuation by Vitamin E. Toxicology 227(3), 240–247. [DOI] [PubMed] [Google Scholar]
- Zárate S, Stevnsner T, Gredilla R, 2017. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front Aging Neurosci 9, 430–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Xu X, Li T, Liu Z, 2013. Shellfish toxins targeting voltage-gated sodium channels. Mar. Drugs 11(12), 4698–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Xu X, Li T, Liu Z, 2013. Shellfish toxins targeting voltage-gated sodium channels. Mar Drugs 11(12), 4698–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-M, An J, 2007. Cytokines, inflammation, and pain. Int Anesthesiol Clin 45(2), 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.