Abstract
Introduction:
Exercise usually results in less weight loss than expected. This suggests increased energy intake and/or deceased expenditure counteract the energy deficit induced by exercise. The aim of this study was to evaluate changes in components of daily energy expenditure (doubly labeled water and room calorimetry) after 24 weeks of exercise training with two doses of aerobic exercise.
Methods:
This was an ancillary study in 42 (29 F, 13 M) sedentary, middle-aged (47.8±12.5 y) individuals with obesity (35±3.7 kg/m2) enrolled in the E-MECHANIC study. Subjects were randomized to three groups: healthy living control group (HL, n=13), aerobic exercise that expended 8 kcal/kg of body weight/week (8 KKW, n=14) or aerobic exercise that expended 20 KKW (n=15). Total daily energy expenditure (TDEE) was measured in free-living condition by doubly labeled water and in sedentary conditions in a metabolic chamber over 24 hours (24EE). Energy intake was calculated over 14-days from TDEE before and after the intervention using the intake-balance method.
Results:
Significant weight loss occurred with 20KKW (−2.1±0.7 kg, p=0.04) but was only half of expected. In the 20KKW group free-living TDEE increased by ~4% (p=0.03), which is attributed to the increased exercise energy expenditure (p=0.001), while 24EE in the chamber decreased by ~4% (p=0.04). Aerobic exercise at 8 KKW did not induce weight change, and there was no significant change in any component of EE. There was no significant change in energy intake for any group (p=0.53).
Conclusion:
Structured aerobic exercise at a dose of 20 KKW, produced less weight loss than expected possibly due to behavioral adaptations leading to reduced 24EE in a metabolic chamber without any change in energy intake.
Keywords: energy expenditure, doubly labeled water, room calorimeter, aerobic exercise, randomized controlled trial
Introduction
An increase in energy expended in physical activity is supposed to increase total daily energy expenditure, thus creating an energy deficit and weight loss. If sustained over time, regular physical activity promises to produce a predictable weight loss unless the energy deficit is counter-balanced by increased energy intake and/or reduced energy expenditure unrelated to the prescribed exercise. Physical activity interventions are commonly proposed to help in losing weight and seem efficacious for weight loss maintenance (1). In the US, 30 minutes of moderate intensity exercise per day for 5 days per week (150 minutes total per week) is recommended for health benefits and possibly improved weight control (2). To support weight loss efforts, the recommended dose increases to 200-300 minutes per week for individuals with overweight/obesity (3).
Physical activity interventions alone, that is without a simultaneous strategy to reduce dietary energy intake, usually result in less weight loss than expected (4, 5). Indeed, exercise energy expenditure increases in proportion to the dose of physical activity, but it has been proposed that the contribution of physical activity to total energy expenditure probably has its limits according to the constrained energy expenditure hypothesis (6). The ‘constrained total energy expenditure model’ posits that increases in total daily energy expenditure due to exercise eventually reaches a plateau due to decreases in other components of daily energy expenditure, thus partly explaining why some individuals fail to lose the expected amount of weight during exercise interventions.
One cannot however ignore the obvious and well-reported explanation that the limited exercise-induced weight loss is more likely due to concomitant increases in energy intake (7, 8). Poor adherence to exercise interventions may be an obvious contributing factor (8), yet well-controlled exercise trials conducted under constant supervision convincingly demonstrate that poor adherence is not the culprit and point to increased energy intake as the central mechanism (9-11), even at high doses (12). There is a large amount of variability in the degree to which people increase energy intake in response to exercise (13). However, the extent to which the energy deficit induced by exercise is also counteracted by compensatory mechanisms (physiological or behavioral) in energy expenditure is not understood.
The Examination of Mechanisms of Exercise-induced Weight Compensation (E-MECHANIC study) was designed to identify mechanisms responsible for weight compensation (14) with increased exercise energy expenditure. Overweight, sedentary individuals (n=171) were randomized to aerobic exercise to expend either 8 kcal per kilogram of weight per week (8 KKW), which is the recommendation for health promotion, 20 kcal per kilogram of weight per week (20 KKW), which is the recommendations for weight loss, or a healthy living control group with no exercise intervention for 24 weeks. As previously reported, both the control (−0.2 kg) and the 8 KKW group (−0.4 kg) maintained weight, whereas exercise at 20 KKW resulted in significant weight loss (−1.6 kg, p=0.02). However, the exercise-induced weight loss was significantly less than expected, indicating a compensation in the form of increased energy intake (91 kcal/day for 8KKW; 124 kcal/day for 20 KKW) or a decrease in energy expenditure outside of the periods of supervised exercise.
E-MECHANIC included doubly labeled water (DLW) measures and additional state-of-the-art energy expenditure measures (whole room indirect calorimetry) were conducted as an ancillary study in a subset of participants before and during the last two weeks of the 24-week intervention. A secondary purpose of this study was to determine if a metabolic adaptation in energy expenditure can explain the lack of weight loss in response to exercise. We hypothesized that metabolic adaptation in non-resting components of energy expenditure would be evident in the 20 KKW group to explain in part the unrealized weight loss.
METHODS
The “Examination of mechanisms of exercise-induced weight compensation (E-MECHANIC)” trial was conducted at Pennington Biomedical Research Center and primary outcomes were recently reported (15). The ancillary study was approved by the Institutional Review Board and participants provided written informed consent for ancillary study testing.
Subjects and study design.
The E-MECHANIC parent trial was previously described (16). Briefly, participants were healthy (free of any chronic disease) males and females, aged 18-65 years with a BMI ≥ 25 kg/m2 and ≤ 45 kg/m2, who were not currently exercising >20 minutes on 3 or more days per week as assessed by self-report, and one-week of accelerometry (SenseWear® BodyMedia, Inc., Pittsburgh, PA). Individuals were excluded if they reported engagement in a weight loss program, prior bariatric surgery, smoking in the past 6 months, consumption of more than 14 alcoholic drinks per week, or females who were pregnant or breastfeeding.
E-MECHANIC was a three-arm, 24-week randomized controlled trial with two exercise groups and a control group (16). Group assignment occurred in a 1:1:1 ratio. The two exercise groups differed with respect to exercise doses selected. The exercise doses reflected current recommendations for general health (8 kcal/kg body weight/week; 8 KKW) and weight loss/weight loss maintenance (20 KKW). In a structured aerobic exercise intervention, the 8 KKW dose was designed to expend approximately 800-1000 kcal/week and the 20 KKW 2000-2500 kcal/week. The control group was a non-exercise group who received information for a healthy lifestyle (HL).
Exercise Intervention.
All exercise training occurred in a fitness facility at Pennington Biomedical Research Center under supervision. Participants completed all exercise on a treadmill or stationary bicycle. The exercise intensity was set at a heart rate zone associated with 65-85% of VO2peak. Based on the speed and gradient on the treadmill and watts on the bike, participant weight, and standard ACSM equations (3, 17), exercise energy expenditure was calculated in real-time and the length of each session was adjusted to the prescribed weekly caloric expenditure. The caloric goal of each session was calculated by dividing the weekly caloric expenditure goal by participant selected exercise frequency (two session minimum).
Exercise energy expenditure was measured using a portable metabolic cart (Parvo Medics True Max 2400 Metabolic Measurement Cart, Salt Lake City, Utah) while participants walked on a treadmill at a predetermined speed and grade. The indirect calorimetry data was used to adjust the daily exercise time throughout the intervention to account for changes in biomechanical efficiency that may have occurred with exercise training. This ensured that caloric expenditure carried over from previous weeks remain equivalent throughout the study. Throughout each supervised exercise session, heart rate was continuously monitored using a Polar (Polar Electro, Lake Success, NY, USA) transmitter and perceived exertion recorded every 5 minutes using the Borg scale. Each exercise session consisted of a 3-minute warm-up at a progressively increasing intensity until the prescribed training intensity was reached. Exercise energy expenditure achieved during the intervention divided by exercise energy expenditure prescribed was used to determine intervention adherence.
Participants were instructed not to modify their diet and normal activities of daily living. Participants assigned to the healthy living group received information (e.g., stress management, the benefits of eating fruits and vegetables) via text message, e-mail, postcard, monthly seminars, or quarterly newsletters throughout the study.
Cardiorespiratory Fitness Testing.
Exercise testing was conducted using a standardized graded exercise testing protocol on a treadmill (Trackmaster 425, Newton, KS) while gas exchange was measured with a ParvoMedics True Max 2400 Metabolic Measurement Cart (Salt Lake City, UT). The exercise protocol began at a low intensity (2.8 METS) and progressed (~1.8 METS per stage) every 2 minutes by altering speed, grade, or both.
Free-living Physical Activity.
SenseWear® armbands (BodyMedia, Inc.; Pittsburgh, PA) measured the number of steps taken per day, minutes per day spent in activities of different intensities, and minutes/day spent in physical activity (defined as time ≥3 METS). Measurements spanned 24 hours/day, except during activities involving water, over two-weeks at baseline and one week at weeks 4 and week 24. Armbands detect and record wear time and only full days of data were included in the analyses. A full day of data required that the device be worn 95% of the time, which equates to 22 hours and 48 minutes.
Weight and Body Composition.
Weight was measured twice in the morning following an overnight fast. Body composition (fat mass and fat-free mass) was assessed by dual-energy X-ray absorptiometry (Lunar iDXA with Encore software version 13.60; GE Healthcare, Madison, WI, USA) at baseline (day -14 and day 0) and week 23/24 (day 160 and day 174).
Weight loss is the difference in body weight between baseline and week 24. Expected weight loss was calculated using two methods based upon more recent research which demonstrates that a 7,700 kcal deficit does not always produce 1 kg of weight loss because this formula overestimates weight loss (8, 18, 19). The researchers who produced this body of work developed a more accurate model that accounts for the dynamics of weight change during exercise. This dynamic formula was used to calculate expected weight loss, given the two different doses of exercise, and the accompanying observed weight loss. Weight loss difference was then calculated as observed weight loss minus expected weight loss (4).
Respiratory Chamber Energy Expenditure.
At baseline and at week 24, sedentary twenty-four-hour energy expenditure (24EE) was measured in a whole room indirect calorimeter (20). Volunteers entered the chamber at 8:00 a.m. after an overnight fast and left the chamber at 7:00 a.m. the next morning. No structured exercise was allowed during the chamber stay. Meals prepared by the Metabolic Kitchen were served according to a fixed schedule with a macronutrient breakdown of 55% carbohydrates, 15% protein, and 30% fat. At baseline and week 24, the energy content of the diet was estimated according to a previously developed gender-specific equation of basal energy expenditure taking into account age, body weight and height (21). While in the room calorimeter, subjects were maintained in energy balance by estimating projected 24EE after 3 and 7 hours to adjust the calories provided at lunch and dinner meals when necessary (20). Resting energy expenditure (REE) was calculated in the chamber as the y-intercept of the relationship between EE and % activity (by radar motion detector), multiplied by 1440.
Energy expenditure components.
Energy expenditure during sleep (SEE) was assessed between 2:00 a.m. and 5:00 a.m. for those minutes during which activity recorded by infrared motion detectors was less than 1%. Spontaneous physical activity (SPA), was determined from radar motion detectors that continuously record movement in the chamber. SPA is expressed as percent of time the participant was active (percent activity) and the energy cost of this activity (22, 23). Percent activity was regressed against EE data for the corresponding time periods. The slope of this regression represents the cost of physical activity per activity unit and is used to calculate the energy cost of activity over 24 hours and is called the energy cost of SPA (kcal/d). The energy cost of arousal is the difference between REE and SEE. The thermic effect of food (TEF) was assumed to be 10% of TDEE for all individuals. Physical activity energy expenditure was calculated by two metrics. First, the physical activity component of TDEE was calculated as TDEE minus all other components (SEE, Arousal, TEF, SPA) and defined as physical activity energy expenditure (PAEE). Second, physical activity level (PAL) was calculated as TDEE/REE.
Free-living Energy Expenditure and Energy Intake with Doubly Labeled Water.
Total daily energy expenditure (TDEE) was measured for a two-week period during baseline testing (days -14 to 0) and week 23/24 (days 160 to 174) by DLW. Briefly, subjects provided two urine samples before being dosed (2.0 g of 10% enriched H218O and 0.12 g of 99.9% enriched 2H2O per kg of estimated total body water), two samples at 4.5 and 6 hours after dosing and a sample day 7 and 14 after dosing which were analyzed for 18O and 2H abundance by isotope ratio mass spectrometry as previously reported (23).
Energy intake was calculated by two methods from TDEE measured by DLW. The first method was objectively measured using the energy intake-balance method using the mean TDEE at baseline and post-intervention plus the change in weight over the two-week DLW period at both of those timepoints (20).
The second method was as described in the main E-MECHANIC trial (14). To quantify change in energy intake for participants who were weight stable or who gained weight during the six-month trial, energy intake was computed as week 24 TDEE minus week 0 TDEE. For participants who lost weight, TDEE was adjusted for change in resting metabolic rate (RMR). The difference between calculated RMR from week 0 to 24 was added to the difference in TDEE to quantify change in energy intake over the 24-week intervention. RMR was quantified with the following equations since measured RMR at week 24 appears to have been elevated, even among those who lost body mass, due to the effects of excess post-exercise oxygen consumption.
Metabolic adaptation.
Metabolic adaptation for sedentary energy expenditure variables (24EE, SEE, REE) was calculated from linear regression models of energy expenditure at baseline (n=53) using fat-free mass, fat mass, age and sex as covariates. Individual data for each covariate measured at week 24 was entered into the baseline model, and the difference between the measured EE variable and EE variable predicted from the model, coined residual EE, was considered a metabolic adaptation to the intervention and the associated changes in weight and body composition as previously described (24-26).
Statistical Analyses.
The power calculations and sample size determination were conducted for the primary endpoint, 24EE) measured in the room calorimeter. The within subject coefficient of variation (CV) of 24EE was measured as previously estimated at 2.4% (27). However, assuming a very conservative 5% within subject CV for repeated measures of sedentary 24EE, only 11 subjects in each group were needed to detect a 10% decrease in 24EE, adjusted for FFM and FM within groups measured in our respiratory chambers, with α=0.05 and β=0.80. Group differences in metabolic adaptation were assessed by two-sample t-tests. Analyses of changes in body composition and energy expenditure were conducted by linear models with a group effect and the baseline value as a covariate in the model. Tukey-Kramer tests with multiple pair-wise group comparisons were used to test between group differences for the primary outcome. All secondary outcomes were assessed in an exploratory nature and no adjustments for multiple group comparisons were made. Baseline data is presented as mean ± SD and change data is presented as LS mean ± SE corrected for the baseline value as the covariate. All analyses were completed using SAS/STAT® software, Version 9.4 of the SAS System for Windows (Cary, NC, USA). All tests were performed with significance level α=0.05.
RESULTS
Subject characteristics.
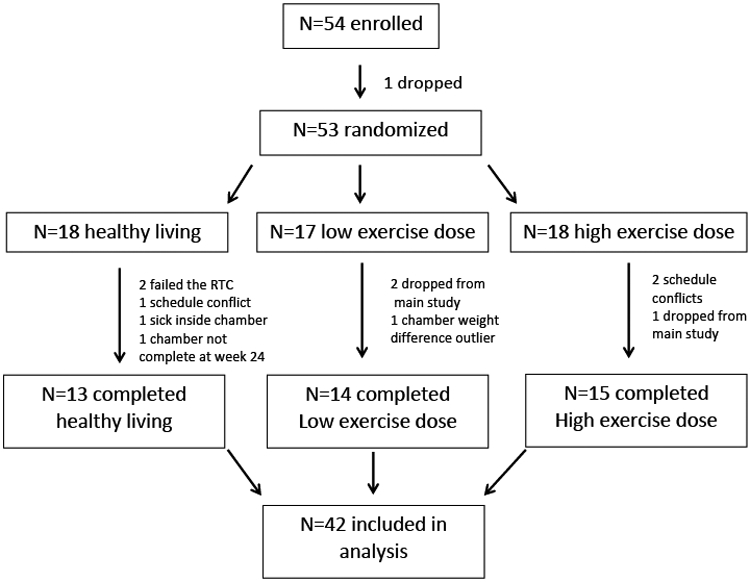
Subject characteristics are presented in Table 1. Fifty-four subjects consented to the ancillary study, 53 were randomized, and 42 (N=13 HL controls, N=14 at 8 KKW exercise, N=15 at 20 KKW exercise) had complete data and were included in the analysis (Figure 1). One subject was removed from just the chamber analyses in the 20 KKW group due to consistent pacing movement through the duration of the day. By design, subjects were middle-aged with obesity with 70% being female, but with similar sex distribution across the three groups.
Table 1:
Descriptive statistics of subjects and energy expenditure data before and after 24-weeks of aerobic exercise training
| Control Healthy Living (N=13) |
Low Dose 8 KKW (N=14) |
High Dose 20 KKW (N=15) |
Baseline P-value |
Δ Week 24 P-value |
||||
|---|---|---|---|---|---|---|---|---|
| Subjects’ Characteristics | ||||||||
| Baseline | Δ Week 24 | Baseline | Δ Week 24 | Baseline | Δ Week 24 | |||
| Gender (M/F) | 4/9 | 4/10 | 5/10 | |||||
| Age | 48.2 ± 12.6 | 46.3 ± 11.4 | 48.9 ± 14.2 | 0.80 | ||||
| Weight (kg) | 100.7 ± 10.6 | 0.4 ± 1.2 | 95.8 ± 13.3 | 0.3 ± 0.7 | 94.5 ± 12.2 | −2.1 ± 0.7*+ | 0.39 | 0.04 |
| BMI (kg/m2) | 36.8 ± 4.2 | 0.6 ± 1.0 | 34.6 ± 3.6 | 0.2 ± 0.7 | 34.2 ± 2.8 | −3.0 ± 0.8* | 0.16 | 0.03 |
| Fat Mass (kg) | 47.8 ± 12.0 | 0.6 ± 1.1 | 43.7 ± 7.3 | 0.4 ± 0.6 | 42.3 ± 8.0 | −2.0 ± 0.6*#+ | 0.28 | 0.01 |
| Fat-free Mass (kg) | 52.9 ± 9.2 | −0.1 ± 0.3 | 52.2 ± 9.1 | −0.2 ± 0.4 | 52.3 ± 11.4 | −0.2 ± 0.3 | 0.98 | 0.99 |
| (ml/kg/min) | 21.6 ± 5.8 | −2.0 ± 0.7* | 21.7 ± 3.2 | 1.1 ± 0.6# | 21.8 ± 3.3 | 4.0 ± 0.7*#+ | 0.99 | <.0001 |
| Energy Expenditure Data | ||||||||
| TDEE from DLW (kcal/day) | 2510 ± 385 | 33 ± 60 | 2680 ± 550 | 63 ± 54 | 2676 ± 449 | 144 ± 62* | 0.56 | 0.42 |
| Chamber 24hrEE (kcal/day) | 1991 ± 269 | 28 ± 26 | 1978 ± 263 | 39 ± 41 | 2026 ± 252 | −75 ± 35* | 0.88 | 0.05 |
| Chamber SEE (kcal/day) | 1581 ± 209 | 16 ± 31 | 1576 ± 197 | 8 ± 37 | 1572 ± 221 | −50 ± 37 | 0.99 | 0.37 |
| Chamber REE (kcal/day) | 1910 ± 233 | 18 ± 27 | 1892 ± 235 | 40 ± 43 | 1878 ± 279 | −37 ± 36 | 0.95 | 0.34 |
| Chamber SPA (kcal/day) | 185 ± 77 | 10 ± 17 | 186 ± 94 | 4 ± 8 | 248 ± 77+ | −31 ± 19 | 0.08 | 0.22 |
| % Activity | 13.2 ± 4.9 | 1.7± 1.0 | 14.6 ± 4.9 | −0.8 ± 1.1 | 18.4 ± 5.6# | −2.3 ± 1.2# | 0.04 | 0.04 |
Baseline data presented as mean ± SD, Change scores (Δ) are shown adjusted with the baseline value as the covariate and presented as mean ± SE.
p<0.05 from baseline
p<0.05 difference from healthy living
p<0.05 difference from low dose. TDEE = total daily energy expenditure from doubly labeled water (DLW), 24hrEE = 24-hour energy expenditure from the metabolic chamber, SEE = sleep energy expenditure, REE = resting energy expenditure, AREE = activity related energy expenditure, SPA = spontaneous physical activity, KKW = kcal/kg/week
Figure 1: Consort diagram of subject recruitment, enrollment, and final analysis.
Exercise intervention.
The prescribed exercise dose was 33.3±1.0 minutes per day for the 8 KKW group compared to 55.5±1.6 minutes per day for the 20 KKW group. Overall adherence to the assigned exercise intervention was excellent at 93 ± 7% of minutes completed for individuals in the 8 KKW group and 92 ± 4% of minutes completed for individuals in the 20 KKW group. Relative VO2max increased only in the 20 KKW group (p=<.0001) while it decreased in the HL control group.
Changes in body weight and body composition with the intervention.
There was no significant change in body weight in neither the HL control group nor the 8 KKW group. The 20 KKW group lost 2.1±0.7 kg (p=0.04). The weight loss in the 20 KKW group was primarily from a reduction in fat mass (−1.9±0.6 kg, p=0.002).
Respiratory Chamber Energy Expenditure.
The three groups did not differ with respect to sedentary energy expenditures (24EE, SEE, REE) at baseline (Table 1). There were no changes in sedentary energy expenditures within and between groups after the intervention except for a decreased 24EE within the 20 KKW group by 75 ± 35 kcal (p=0.04). The between group significant difference in 24EE is lost when adjusting for multiple comparisons between the groups (p=0.06). There were also no changes in energy expenditure from SPA in the HL group (p=0.54). Similarly, no changes in EE related to SPA or percent activity were present in the 8 KKW group (p=0.66 and p=0.45, respectively). Finally, in the 20 KKW group, the contribution of energy from SPA was also not significantly changed (p=0.11) although activity in the chamber had a trend to decrease (p=0.06).
Free-Living Activity.
Over two-week periods at baseline and week 23/24, accelerometry did not change in minutes lying down for HL (4620 ± 1587 min and 4359 ± 1587 min, p=0.33), 8 KKW (4583 ± 1144 min and 4836 ± 1313 min, p=0.89), or 20 KKW participants (4438 ± 1239 min and 4273 ± 1301 min, p=0.98). Likewise, there were no changes in total step counts (not including structured exercise) per week during the last 2 weeks of the intervention for either group (HL: 46896 ± 14735 and 46268 ± 23402, p=0.53), 8 KKW (54800 ± 28128 and 47107 ± 23580, p=0.18), or 20 KKW (54281 ± 13762 and 51067 ± 25032, p=0.88). Accelerometry data also showed no changes in time spent lying down for any of the groups (p=0.66).
Free-Living Energy Expenditure and Energy Intake.
In comparison to baseline, absolute TDEE (Table 1) did not change in the HL (33 ± 60 kcal/d) and 8 KKW (63 ± 54 kcal/d) groups but increased significantly in the 20 KKW group by 144 ± 62 kcal/d (p=0.03) after the intervention. There were no between group differences in TDEE post-intervention. When adjusted for the changes in fat mass and fat-free mass, TDEE was not changed from baseline in any group nor when adjusted for body weight change (data not shown). The changes in energy intake as calculated by the intake-balance method was ~50% higher for the 20 KKW group compared to HL and 8 KKW however it was not significantly different (HL: 53 ± 65 kcal/d, 8 KKW:54 ± 62 kcal/d, 20 KKW: 114 ± 62 kcal/d, p=0.73). Similarly, the change in energy intake as calculated in the main trial also was not significantly different between the three groups (HL:79 ± 63 kcal/d, 8 KKW:65 ± 61 kcal/d, 20 KKW: 156 ± 61 kcal/d, p=0.53).
Physical Activity Energy Expenditure (PAEE).
When extrapolated to energy expenditure per week, PAEE amounted to 869 ± 1801 kcal/week (~4.5% of TDEE) in the 8 KKW group and 1854 ± 1220 kcal/week (~9.4% of TDEE) in the 20 KKW group. PAL did not change in the HL group (1.31 ± 0.05 vs. 1.33 ± 0.03, p=0.46) or 8 KKW group (1.41 ± 0.05 vs. 1.42 ± 0.04, p=0.58), but increased significantly from baseline in the 20 KKW group (1.43 ± 0.04 vs. 1.52 ±0.05, p=0.0003). PAL was also significantly higher in the 20 KKW group compared to the 8 KKW group (p=0.05).
Components of Energy Expenditure.
At baseline, TDEE in the HL group was comprised of 63% of SEE, 13% to support the energy cost of arousal, 10% TEF, 7% for SPA, and the remaining 7% for the contribution of physical activity (TDEE minus all other components). The components of 24-hour sedentary energy expenditure in the exercise groups were similar at baseline (8 KKW: 59% SEE, 12% arousal, 10% TEF, 7% SPA, and 12% physical activity; 20 KKW 59% SEE, 11% arousal, 10% TEF, 9% SPA, and 11% physical activity). The absolute change in overall TDEE and energy expenditure components following the 24-week intervention are shown in Figure 2. In the HL and 8 KKW group, there was no significant change in any of the components of energy. The contribution of physical activity was maintained in the HL (−44 ± 66 kcal/d (p=0.51) and 8 KKW (36 ± 56 kcal/d; p=0.51) groups, but increased significantly (202 ± 56 kcal/d) in the 20 KKW group (p=0.001).
Figure 2: Changes from baseline in the components of total daily energy expenditure (TDEE) after a 24-week aerobic exercise intervention in a healthy living control (HL), a low dose of exercise (8 kcal/kg/week, KKW) and a high dose of exercise (20 kcal/kg/week, KKW).
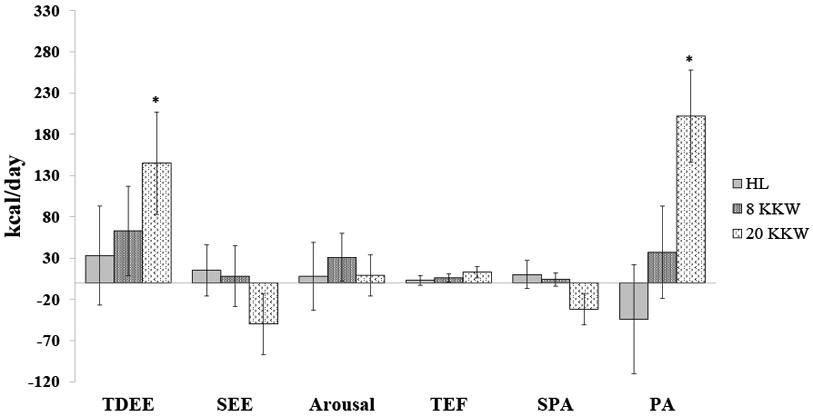
All components are calculated from TDEE measured by doubly labeled water except for sleep energy expenditure, which was calculated in a whole-room respiratory chamber. SEE = sleep energy expenditure, TEF = thermic effect of food, arousal = SEE – resting energy expenditure in the chamber, SPA = spontaneous physical activity (measured in whole-room chamber), PA = physical activity (calculated as TDEE minus all other components). Changes are presented with the baseline value adjusted as the covariate in the model. *=p<0.05
Metabolic adaptation in Sedentary Energy Expenditure.
Using the 53 individuals randomized at baseline, the linear regression equation of this cohort for relating 24EE to FFM, FM, sex, and age was:
The residual energy expenditure for 24EE and SEE (data not shown) was not significantly different from zero at follow up for all groups indicating that the intervention did not induce a metabolic adaptation in sedentary expenditures.
DISCUSSION
Decades of research have shown a large amount of variance in weight loss in response to exercise interventions (4, 5, 9). It is not entirely clear what is driving this variance. It has been postulated that it could be due to a combination of low doses of exercise leading to insufficient increases in energy expenditure (negative energy balance), increased compensatory energy intake, compensatory decrease in energy expenditure during periods outside of the intervention (e.g., less spontaneous physical activity or NEAT, more sleep), or poor adherence to exercise interventions (8). The potential role of compensations in energy expenditure to these phenomena is poorly understood. With simultaneous measurements of energy expenditure in a metabolic chamber and in conjunction with stable isotopes and accelerometry, we were able to differentiate the cost of the various components of energy expenditure at different doses of physical activity. A subset of sedentary individuals randomized to two doses of exercise, one for general health purposes, the other recommended for weight loss, or a control group, had all components of daily free-living energy expenditure measured by combining the doubly labeled water method with indirect calorimetry measured in a respiratory chamber. At a low dose of exercise, no weight loss was observed, and energy expenditure was maintained from baseline. At a high dose of exercise that expended on average ~1800 kcal/week, individuals lost weight (~2.1 kg), but the weight loss represented only half of that expected if there was no compensation. These individuals did not have evidence of a metabolic adaptation but a lower 24EE (on average ~75 kcal/day) in the metabolic chamber in the 20 KKW group. This may be due to a lower trend for activity, as measured in the metabolic chamber.
The 24-hour stay in the metabolic chamber demonstrated no metabolic adaptation despite a within group decrease in 24EE in the 20 KKW group, but no between group differences. However, the direction and magnitude of the estimates of group differences (20 KKW v HL: 103.12, 20 KKW v 8 KKW: 113.99) indicate evidence of a benefit in the high-dose exercise group and the lack of statistical significance is more likely due to limited sample sizes than lack of an actual exercise effect. This is interesting because we and others have observed a significant metabolic adaptation in SEE with weight loss, albeit induced by dietary energy restriction and not by exercise as in this study (28). Metabolic adaptation has also been observed with combined diet and exercise interventions that have resulted in modest (28, 29) and substantial weight loss with large amounts of physical activity (30). It is hypothesized that weight loss-induced metabolic adaptation occurs in order to preserve the current body weight (25). This so-called body weight set point theory has been a mechanism to explain the inability for weight loss to be maintained long-term (31, 32). The molecular and physiological underpinnings of a decline in metabolism that is disproportionate to the metabolic mass loss are largely unknown.
It has been postulated that the human body may regulate the capacity to expend excess calories as a defensive mechanism to maintain weight, particularly in cultures where physical activity is required to maintain food supply (6, 33, 34). Interestingly, despite a clear difference in the exercise energy expenditure and PAL between the two exercise groups, between group differences in free-living TDEE were not apparent (absolute or when adjusted for changes in metabolic body mass). This study is the first structured and supervised exercise intervention with different doses providing data in support of the constrained energy expenditure model (33). It is of importance to characterize how energy expenditure is partitioned during periods of increased physical activity levels to evaluate whether the body is attempting to ‘conserve’ the energy necessary to support basal metabolism. Although the 20 KKW group significantly increased total energy expenditure, the TDEE was only 81 kcal/d higher than the 8 KKW group. There was also no change in SPA after the intervention for any of the groups. SPA, or what is also referred to as non-exercise activity thermogenesis, is the energy that is expended in activities of daily living. That is all activities other than structured exercise or sustained bouts of continuous movement (35, 36). We did find a significant inverse relationship with the change in fitness () and change in SPA (R2 = 0.14, p=0.02, data not shown), which is also supported by a previous study (32). Our study indicated that higher doses of structured exercise reduced sedentary activity inside the metabolic chamber. Similar findings were reported in other exercise studies although in groups comprised of women (32,33) or elderly individuals (37). Whether or not this trend in a reduction in activity in the chamber is occurring consciously or unconsciously and therefore a physiological or behavioral compensation mechanism cannot be determined in our study. The possible reduction in activity could simply reflect a reduced amount of time in non-exercise activities, however, we observed no differences in steps or minutes spent lying down before and after the intervention with accelerometry.
The obvious compensatory mechanism that could explain the unachieved weight loss is an increase in energy intake. Changes in appetite and increased food consumption have long been considered as a limiting factor in weight loss following exercise (38, 39). The increase in energy intake in the ancillary study cohort did not reach significance, which is in contrast to the findings of the main study, yet the difference is small (~30 kcal/d) in both the 8 KKW (65 kcal/d versus 91 kcal/d) and the 20 KKW (156 kcal/d versus 124 kcal/d) groups respectively. As described in the methods section, the ancillary study was powered on 24EE in the metabolic chamber and not on energy intake as was primary outcome for the parent trial. The effect size (Cohen’s D) between the control group and experimental group for the high exercise dose is 0.57 in the main paper and 0.34 in this present manuscript. The subject characteristics between our ancillary cohort and the main trial were also similar but we caution that the discrepancy between energy intake in the two studies may not be entirely explained by small sample size (lack of statistical power) in the ancillary study. We should not ignore that individuals with higher physical activity levels may better be able to adjust energy intake in response to disturbances in energy balance (40). Both exercise groups, but in particular, the 20 KKW group, could have been able to adapt their energy intake needs to that of the high dose of exercise.
An improved metabolic efficiency has been proposed by several investigators, yet this hypothesis remains to be appropriately tested with in vivo methods such as phosphorous magnetic resonance spectroscopy (41, 42). Indeed, in vitro studies support an increase in mitochondrial biogenesis with weight loss and aerobic exercise training (43-45) and the lack of muscle biopsies in our study prevent us from exploring this in our cohort. Other mechanisms include adaptive changes in sympathetic nervous system activity (26), thyroid axis hormones and catecholamine excretion (46), and leptin (47). Although we do not have data on urinary catecholamine excretion or thyroid hormones, we did see a significant decrease in leptin (data not shown) only in the 20 KKW group (42.2 ± 23.0 and 35.1 ± 20.8 ng/mL, p=0.01), but not in the 8 KKW (45.7 ± 20.4 and 47.9 ± 20.3 ng/mL, p=0.72) or HL (67.5 ± 45.6 and 58.1 ± 35.2 ng/mL, p=0.06) groups. Interestingly, leptin has been the major correlate of metabolic adaptations in our previous studies (30, 48).
A strength of this study is that we tested whether two distinct doses of aerobic exercise under highly controlled and supervised conditions as study subjects were directly monitored for compliance to their weekly assignments. Unlike previous studies that argue poor adherence is why individuals do not lose weight in an exercise intervention (8), our intervention was highly structured with all exercise sessions supervised, rending the lack of adherence an unlikely cause of poor weight loss efficacy. It is important to point out limitations of studies conducted in a metabolic chamber and extrapolation of data from the chamber to free-living conditions. Namely in the chamber, participants are confined to only spontaneous activities and therefore activity patterns in the chamber might not be truly representative of the individual in the free-living state. Despite the small volume of the chamber (10’ x 13’ x 8’), individuals do have the liberty to get up and move around, look out the window, use the lavatory and wash basin, etc. The chambers are furnished with a bed and desk and individuals have freedom to explore as they choose. Although to account for free-living conditions, accelerometers were also used over a period of 2-weeks before and after the intervention.
In conclusion, exercise that expended ~1800 kcal/week produced some weight loss, but less than expected. This blunted exercise-induced weight loss was not attributed to a metabolic adaptation in sedentary energy expenditures or reduced energy intake, but possibly to a reduction in 24EE and activity inside of a metabolic chamber. Since this was only evident in the metabolic chamber and not accelerometry (steps per day), we speculate that aerobic exercise induces an increase in metabolic efficiency in non-exercise activities, which leads to less overall energy expenditure. Nevertheless, the energy expenditure changes during structured physical activity interventions do not fully explain the poor weight loss efficacy. This study also shows that compensatory mechanisms, either conscious or unconscious, to decrease energy expended in non-exercise activities are also implicated. Future studies are warranted to explore this complex area and to test whether improved exercise efficiency, including mitochondrial adaptations, may explain the constrained energy expenditure model in humans and why individuals may be protected from exercise-induced weight loss.
ACKNOWLEDGMENTS
This study was supported by NHLBI grant R01 HL102166, the Nutrition Obesity Research Center (P30DK072476), and the Louisiana Clinical and Translational Science Center (U54 GM104940). Thanks to Angela Eldridge in coordinating this trial, technical support of Dr. Jennifer Rood in the DLW analysis and study participants who committed time and effort above the parent trial for this important ancillary study. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. Results of the present study do not constitute endorsement by ACSM.
Footnotes
CONFLICT OF INTEREST
The authors having nothing to disclose.
REFERENCES
- 1.Donnelly JE, Blair SN, Jakicic JM et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71. [DOI] [PubMed] [Google Scholar]
- 2.Piercy KL, Troiano RP, Ballard RM et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Sports Medicine., Pescatello LS, Arena R, Riebe D, Thompson PD. ACSM's guidelines for exercise testing and prescription. 9th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2013, xxiv, 465 p. p. [DOI] [PubMed] [Google Scholar]
- 4.Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PloS one. 2009;4(2):e4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Medicine and science in sports and exercise. 2001;33(6 Suppl):S521–7; discussion S8-9. [DOI] [PubMed] [Google Scholar]
- 6.Pontzer H, Durazo-Arvizu R, Dugas LR et al. Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Current biology : CB. 2016;26(3):410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doucet E, McInis K, Mahmoodianfard S. Compensation in response to energy deficits induced by exercise or diet. Obes Rev. 2018;19Suppl 1:36–46. [DOI] [PubMed] [Google Scholar]
- 8.Thomas DM, Bouchard C, Church T et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012;13(10):835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly JE, Hill JO, Jacobsen DJ et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Archives of internal medicine. 2003;163(11):1343–50. [DOI] [PubMed] [Google Scholar]
- 10.Finlayson G, Bryant E, Blundell JE, King NA. Acute compensatory eating following exercise is associated with implicit hedonic wanting for food. Physiology & behavior. 2009;97(1):62–7. [DOI] [PubMed] [Google Scholar]
- 11.Whybrow S, Hughes DA, Ritz P et al. The effect of an incremental increase in exercise on appetite, eating behaviour and energy balance in lean men and women feeding ad libitum. The British journal of nutrition. 2008;100(5):1109–15. [DOI] [PubMed] [Google Scholar]
- 12.Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA. Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Medicine and science in sports and exercise. 2013;45(8):1600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blundell JE, Stubbs RJ, Hughes DA, Whybrow S, King NA. Cross talk between physical activity and appetite control: does physical activity stimulate appetite? The Proceedings of the Nutrition Society. 2003;62(3):651–61. [DOI] [PubMed] [Google Scholar]
- 14.Martin CK, Johnson WD, Myers CA et al. Effect of different doses of supervised exercise on food intake, metabolism, and non-exercise physical activity: The E-MECHANIC randomized controlled trial. Am J Clin Nutr. 2019; 110(3):583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin CK, Johnson WD, Myers CA et al. Effect of different doses of supervised exercise on food intake, metabolism, and non-exercise physical activity: The E-MECHANIC randomized controlled trial. Am J Clin Nutr. 2019;110(3):583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers CA, Johnson WD, Earnest CP et al. Examination of mechanisms (E-MECHANIC) of exercise-induced weight compensation: study protocol for a randomized controlled trial. Trials. 2014;15:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute of Medicine (U.S.). Panel on Macronutrients., Institute of Medicine (U.S.). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, D.C.: National Academies Press; 2005, xxv, 1–972, 1259–331 p. p. [Google Scholar]
- 18.Heymsfield SB, Thomas D, Nguyen AM et al. Voluntary weight loss: systematic review of early phase body composition changes. Obes Rev. 2011;12(5):e348–61. [DOI] [PubMed] [Google Scholar]
- 19.Thomas DM, Martin CK, Lettieri S et al. Can a weight loss of one pound a week be achieved with a 3500-kcal deficit? Commentary on a commonly accepted rule. Int J Obes (Lond). 2013;37(12):1611–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam YY, Redman LM, Smith SR et al. Determinants of sedentary 24-h energy expenditure: equations for energy prescription and adjustment in a respiratory chamber. The American journal of clinical nutrition. 2014;99(4):834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.USDA Food and Nutrient Database for Dietary Studies, 3.0. Beltsville, MD: Agricultural Research Service, Food Surveys Research Group; 2008. [Google Scholar]
- 22.Lam YY, Ravussin E. Analysis of energy metabolism in humans: A review of methodologies. Mol Metab. 2016;5(11):1057–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin CK, Heilbronn LK, de Jonge L et al. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity. 2007;15(12):2964–73. [DOI] [PubMed] [Google Scholar]
- 24.Galgani JE, Santos JL. Insights about weight loss-induced metabolic adaptation. Obesity (Silver Spring). 2016;24(2):277–8. [DOI] [PubMed] [Google Scholar]
- 25.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. The New England journal of medicine. 1995;332(10):621–8. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. International journal of obesity. 2010;34Suppl 1:S47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. The Journal of clinical investigation. 1986;78(6):1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heilbronn LK, de Jonge L, Frisard MI et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295(13):1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss EP, Albert SG, Reeds DN et al. Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: a randomized intervention trial. The American journal of clinical nutrition. 2016;104(3):576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knuth ND, Johannsen DL, Tamboli RA et al. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity. 2014;22(12):2563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garn SM. Role of set-point theory in regulation of body weight. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1992;6(2):794. [DOI] [PubMed] [Google Scholar]
- 32.Weinsier RL, Nagy TR, Hunter GR, Darnell BE, Hensrud DD, Weiss HL. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. The American journal of clinical nutrition. 2000;72(5):1088–94. [DOI] [PubMed] [Google Scholar]
- 33.Pontzer H Constrained Total Energy Expenditure and the Evolutionary Biology of Energy Balance. Exercise and sport sciences reviews. 2015;43(3):110–6. [DOI] [PubMed] [Google Scholar]
- 34.Ravussin E, Peterson CM. Physical Activity and the Missing Calories. Exercise and sport sciences reviews. 2015;43(3):107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johannsen DL, Ravussin E. Spontaneous physical activity: relationship between fidgeting and body weight control. Current opinion in endocrinology, diabetes, and obesity. 2008;15(5):409–15. [DOI] [PubMed] [Google Scholar]
- 36.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283(5399):212–4. [DOI] [PubMed] [Google Scholar]
- 37.Goran MI, Poehlman ET. Endurance training does not enhance total energy expenditure in healthy elderly persons. The American journal of physiology. 1992;263(5 Pt 1):E950–7. [DOI] [PubMed] [Google Scholar]
- 38.Blundell JE, Gibbons C, Caudwell P, Finlayson G, Hopkins M. Appetite control and energy balance: impact of exercise. Obes Rev. 2015;16Suppl 1:67–76. [DOI] [PubMed] [Google Scholar]
- 39.King NA, Caudwell PP, Hopkins M, Stubbs JR, Naslund E, Blundell JE. Dual-process action of exercise on appetite control: increase in orexigenic drive but improvement in meal-induced satiety. Am J Clin Nutr. 2009;90(4):921–7. [DOI] [PubMed] [Google Scholar]
- 40.Dorling J, Broom DR, Burns SF et al. Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity. Nutrients. 2018;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldsmith R, Joanisse DR, Gallagher D et al. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. American journal of physiology. Regulatory, integrative and comparative physiology. 2010;298(1):R79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenbaum M, Vandenborne K, Goldsmith R et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. American journal of physiology. Regulatory, integrative and comparative physiology. 2003;285(1):R183–92. [DOI] [PubMed] [Google Scholar]
- 43.Civitarese AE, Carling S, Heilbronn LK et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS medicine. 2007;4(3):e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menshikova EV, Ritov VB, Ferrell RE, Azuma K, Goodpaster BH, Kelley DE. Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. Journal of applied physiology. 2007;103(1):21–7. [DOI] [PubMed] [Google Scholar]
- 45.Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53(8):1714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. The American journal of clinical nutrition. 2000;71(6):1421–32. [DOI] [PubMed] [Google Scholar]
- 47.Rosenbaum M, Goldsmith R, Bloomfield D et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. The Journal of clinical investigation. 2005;115(12):3579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lecoultre V, Ravussin E, Redman LM. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J Clin Endocrinol Metab. 2011;96(9):E1512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


