Abstract
Stroke is a leading cause of disability, dementia and death worldwide. Approximately 70% of deaths from stroke and 87% of stroke-related disability occur in low-income and middle-income countries. At the turn of the century, the most common diseases in Africa were communicable diseases, whereas non-communicable diseases, including stroke, were considered rare, particularly in sub-Saharan Africa. However, evidence indicates that, today, Africa could have up to 2–3-fold greater rates of stroke incidence and higher stroke prevalence than western Europe and the USA. In Africa, data published within the past decade show that stroke has an annual incidence rate of up to 316 per 100,000, a prevalence of up to 1,460 per 100,000 and a 3-year fatality rate greater than 80%. Moreover, many Africans have a stroke within the fourth to sixth decades of life, with serious implications for the individual, their family and society. This age profile is particularly important as strokes in younger people tend to result in a greater loss of self-worth and socioeconomic productivity than in older individuals. Emerging insights from research into stroke epidemiology, genetics, prevention, care and outcomes offer great prospects for tackling the growing burden of stroke on the continent. In this article, we review the unique profile of stroke in Africa and summarize current knowledge on stroke epidemiology, genetics, prevention, acute care, rehabilitation, outcomes, cost of care and awareness. We also discuss knowledge gaps, emerging priorities and future directions of stroke medicine for the more than 1 billion people who live in Africa.
Subject terms: Stroke, Stroke, Epidemiology
In this Review, Akinyemi and colleagues provide an overview of stroke in Africa, including epidemiology, risk factors, genetics and available stroke services. The authors also discuss the future of stroke care in Africa, highlighting the promise of biobanking and novel leadership initiatives.
Key points
The annual incidence rate of stroke in Africa is up to 316 per 100,000 individuals, which is within the highest incidence rates in the world, and the prevalence rate of 1,460 per 100,000 reported in one region of Nigeria, western Africa, is clearly among the highest in the world.
Hypertension remains the most important modifiable risk factor for stroke in Africa but others include diabetes mellitus, dyslipidaemia, obesity, stress, smoking, alcohol use, physical inactivity and an unhealthy diet.
Africa has a slightly greater preponderance of small vessel disease-related stroke and intracerebral haemorrhagic lesions than elsewhere in the world.
The results of the first African genome-wide association study on stroke are expected soon but genes already known to modify stroke risk in African populations include IL6, APOE, APOL1, CYB11B2 and CDKN2A/2B.
Pragmatic approaches to improving stroke care in Africa include regular monitoring of risk factors and health services, implementation of prevention strategies, improving acute care and rehabilitation services, and encouraging task sharing; the emergence of standalone stroke care and stroke units in some North African and sub-Saharan countries is encouraging.
Numerous challenges face stroke medicine in Africa but awareness and the concerted efforts towards securing support for more stroke research and services via organizations such as the African Stroke Organization, World Stroke Organization and WHO hold much promise.
Introduction
Stroke is a leading cause of disability, dementia and mortality worldwide and is associated with substantial economic costs. Globally, an average of 1 in 4 adults will have a stroke during their lifetime1. Global, age-standardized stroke mortality rates declined substantially between 1990 and 2016, most likely as a result of the increased availability of acute stroke treatments and improved inpatient care in high-income countries; however, the decline in incidence was less steep in Africa2. The 2019 update on global stroke statistics identified a consistent increase in stroke incidence in low-income and middle-income countries (LMICs) on the basis of stroke incidence studies performed between 1971 and 2014 (ref.3). Between 1970 and 2010, 70% of all stroke deaths and 87% of disability owing to stroke occurred in LMICs3,4.
The burden of non-communicable diseases is increasing exponentially in Africa. In addition, infections such as malaria, human immunodeficiency virus (HIV) and tuberculosis persist and are further exacerbated by poverty and conflict5,6. We are also witnessing an increase in the co-morbidity of non-communicable and communicable diseases7,8. Less than a century ago, stroke was relatively uncommon in Africa9,10. However, in recent systematic analyses by the global burden of neurological diseases investigators, stroke was foremost along with migraine and meningitis in terms of disability-adjusted life years (DALYs) in northern, central, western, eastern and southern Africa11. Today, Africa has some of the highest indices of stroke burden in the world4,12–14. The increase in stroke burden in Africa is being driven by multiple factors acting across the lifespan. Key drivers include in utero and early-life undernutrition, which are associated with increased cardiometabolic risk factors in mid-life15–17, increasing exposure to indoor and outdoor particulate air pollution18,19, changes in dietary habits and population ageing20. These relationships are only the tip of the iceberg because, for every case of clinical stroke, there are four other cases of covert or ‘silent strokes’, which can cause cognitive dysfunction and mental health conditions in later years19,21,22.
Stroke medicine in Africa has advanced over the past decade. Our understanding of both traditional and emerging risk factors, including their effect sizes and population attributable risk (PAR), has improved. More information on stroke aetiology and pathophysiological types and subtypes is now available for African populations, including a better understanding of strokes that occur as a consequence of sickle cell disease (SCD), HIV/AIDS or SARS-CoV-2 (COVID-19) infection. Stroke outcomes, rehabilitation, cost of care, quality of life and mortality are receiving more detailed attention, while post-stroke complications, including cognitive impairment and dementia, anxiety and depression, fatigue, and seizure disorders, are increasingly documented in the growing stroke literature from Africa4,12. Candidate-gene studies and ongoing genome-wide association studies (GWAS) have also begun to elucidate the genetic architecture of stroke in Africa23–25.
Despite this progress, substantial gaps remain in our understanding of stroke in Africa as well as in stroke care, practice and policy on the continent. The effective organization of preventative, therapeutic and rehabilitative stroke services also remains a challenge in many African countries. However, emerging insights from research and the introduction of novel stroke leadership initiatives offer huge prospects for tackling the growing burden of stroke on the continent with context-sensitive strategies. In this article, we review the unique epidemiological, aetiological and genetic profiles of stroke in Africa and summarize what we know about stroke prevention, acute care, rehabilitation, cost of care, outcomes and awareness. We also identify knowledge gaps, emerging priorities and future directions for stroke medicine and care and the implications for the people of Africa and the global community.
Stroke epidemiology in Africa
Several epidemiological studies have been undertaken on stroke in Africa with a preponderance of hospital-based over community or population survey studies. Several of the population-based studies have been identified as having methodological shortcomings26,27 and the only study that fulfilled the gold-standard criteria for a stroke epidemiological study was a prevalence study performed in urban Egypt (Al Quseir) by El-Tallawy et al.28.
Incidence
In 1991, a study involving hospital patients estimated the annual stroke incidence in Harare, Zimbabwe, to be 31 per 100,000 individuals29. Other hospital-based incidence studies from Benghazi, Libya (1984 (ref.30) and 1993 (ref.31)), urban Pretoria, South Africa32 (1985) and Maputo, Mozambique (2006)33 reported annual crude incidence rates ranging from 48 to 149 per 100,000 individuals. However, hospital-based studies do not adequately capture the true burden of stroke within a given community because the population at risk is not known, thus interpretation of such studies requires caution. Indeed, a continent-wide meta-analysis34 and a Nigeria-based meta-analysis35 of pooled estimates of stroke incidence both found that hospital-based studies estimated lower incidence rates than community-based studies, suggesting that hospital-derived data under-represent the true incidence of stroke.
The earliest known community-based study of stroke incidence in Africa was derived from the Ibadan Stroke Registry and was conducted from 1973 to 1975 in Ibadan, an urban city in southwestern Nigeria. The study reported an annual crude incidence rate of 26 per 100,000 individuals36. Similar studies from Lagos and Akure, southwestern Nigeria, were conducted in 2007 and 2010 and reported annual crude incidence rates of 25 per 100,000 (ref.37) and 61 per 100,000, respectively38. Other community-based studies have been undertaken in other parts of the continent28,39,40 (Table 1) and have reported crude annual stroke incidence rates ranging from 95 per 100,000 in rural Tanzania, East Africa13, to 260 per 100,000 in urban Egypt, North Africa40. The variation in the reported figures might result from methodological differences, such as completeness of case ascertainment, inclusion of mild or clinically covert strokes, lack of differentiation between first and recurrent strokes, and use of neuroimaging for diagnostic confirmation and subtyping. Other sources of variation include inherent geographical differences in the distribution of risk factors, differences in genetic susceptibility to stroke across the populations studied, and cohort or period effects23,26,41.
Table 1.
Studies of stroke incidence and prevalence in Africa
| Country, region | Study period | Type of study | Case definition | Neuroimaging confirmationa | Stroke subtyping | Crude annual incidence or crude prevalence rateb | Age-adjusted incidence or prevalence rateb | Ref. |
|---|---|---|---|---|---|---|---|---|
| Incidence: annual range 25–260 per 100,000 from 1973 to 2013 | ||||||||
| Nigeria, Ibadan | 1973–1975 | Community | Not stated | No | Yes | 26 | Not stated | 36 |
| Libya, Benghazi | 1983–1984 | Hospital | US national survey of stroke | Yes | Yes | 63 | Not stated | 30 |
| South Africa, Pretoria | 1984–1985 | Hospital | Harvard Cooperative Stroke Registry | Yes | Yes | 101 | Not stated | 32 |
| Zimbabwe, Harare | 1991 | Hospital | WHO criteria | No | No | 31 | 68 | 29 |
| Libya, Benghazi | 1991–1993 | Hospital | Not stated | Yes | Yes | 48 | Not stated | 31 |
| Egypt, Sohag | 1992–1993 | Community | WHO criteria | Yes | Yes | 180 | Not stated | 39 |
| Tanzania, Hai | 2003–2006 | Mixed | WHO criteria | Yes | Yes | 95 | 109 | 13 |
| Tanzania, Dar es Salaam | 2003–2006 | Mixed | WHO criteria | Yes | Yes | 108 | 316 | 13 |
| Mozambique, Maputo | 2005–2006 | Hospital | WHO criteria | Yes | Yes | 149 | 260 | 33 |
| Egypt, Al-Kharga | 2005–2008 | Community | WHO criteria | Yes | No | 260 | 560 | 40 |
| Nigeria, Lagos | 2007 | Community | WHO criteria | Yes | No | 25 | 54 | 37 |
| Egypt, Al-Quseir | 2010–2011 | Community | WHO criteria | Yes | No | 181 | Not stated | 28 |
| Nigeria, Akure | 2010– 2011 | Mixed | Not stated | Yes | Yes | 61 | 61 | 38 |
| Prevalence: crude range 15–1,331 per 100,000 from 1983 to 2016 | ||||||||
| Nigeria, Igbo-Ora, rural | 1983–1984 | Community | WHO criteria | No | No | 56 | Not stated | 42 |
| Tunisia, Kelibia | 1985 | Community | WHO criteria | Yes | Yes | 42 | Not stated | 46 |
| Ethiopia, central and rural | 1988 | Community | WHO criteria | No | No | 15 | Not stated | 44 |
| Egypt, Sohag | 1992–1993 | Community | WHO criteria | Yes | Yes | 508 | Not stated | 39 |
| Tanzania, Hai | 1994–1995 | Community | WHO criteria | No | Yes | 127 | Not stated | 43 |
| South Africac, Limpopo province, rural | 2001 | Community | WHO criteria | No | Yes | 243 | 300 | 47 |
| Nigeria, Lagos, urban | 2007 | Community | WHO criteria | No | No | 114 | 204 | 48 |
| Egypt, Al-Kharga | 2005–2009 | Community | WHO criteria | Yes | Yes | 580 | Not stated | 40 |
| Nigeria, Niger Delta | 2008 | Community | WHO criteria | No | No | 851 | 1,230 | 49 |
| Benin, Cotonou, urban | 2008–2009 | Community | WHO criteria | Yes | Yes | 460 | 771 | 50 |
| Morocco, Rabat | 2008–2009 | Community | WHO criteria | Yes | Yes | 284 | 292 | 51 |
| Nigeria, Kwara, semi-urban | 2009–2010 | Community | WHO criteria | No | No | 1,310 | Not stated | 52 |
| Tanzania, Hai | 2009–2010 | Community | WHO criteria | No | No | 2,420 (>70 years of age) | 2,300 (>70 years) | 53 |
| Egypt, Assiut | 2010 | Community | WHO criteria | Yes | Yes | 963 | 980 | 45 |
| Egypt, Al Quseird | 2010–2011 | Community | WHO criteria | Yes | Yes | 655 | Not stated | 28 |
| Nigeria, SE | 2011 | Community | WHO criteria | No | No | 163 | 163 | 54 |
| Egypt, Qena | 2011–2013 | Community | WHO criteria | Yes | Yes | 922 | 567 | 55 |
| Nigeria, Niger Delta | 2014 | Community | WHO criteria | No | No | 1,331 | 1,460 | 14 |
| Nigeria, Odeda | 2015 | Community | WHO criteria | No | No | 711 | Not stated | 56 |
| Benin, Parakou | 2016 | Community | WHO criteria | No | Yes | 1,156 | 3,223 | 57 |
a‘Yes’ >80%. bPer 100,000 population. cSouth Africa, Agincourt Health and Population Unit. dThe prevalence study performed in urban Egypt (Al Quseir) by El-Tallawy et al. fulfilled the gold-standard criteria for a stroke epidemiological study.
Age-adjusted incidence rates are more representative of the distribution of stroke among the population, especially in Africa, where the population is predominantly <60 years of age26. The most recent and rigorous study of stroke incidence in Africa was performed in Tanzania and published in 2010 (ref.13). The age-standardized stroke incidence rates from the two surveillance sites in Hai (rural Tanzania) and Dar es Salaam (urban Tanzania) were 109 and 316 per 100,000 person-years, respectively13. WHO world population data were used for age-standardization and the researchers used a robust case-ascertainment strategy. If the incidence rates from this study are extrapolated to the rest of Africa, it implies that every minute, six Africans have a stroke. The Dar es Salaam rates were similar to the highest rates in South East Asia and Europe, according to the Global Burden of Disease (GBD) study on global, regional and national burden of stroke, which reported the age-standardized stroke incidence rate as 354 per 100,000 person-years in China and 335 per 100,000 person-years in Latvia2.
Prevalence
The 1982 Igbo-Ora community prevalence study in rural southwestern Nigeria was one of the earliest of such studies to be performed in Africa and found a crude stroke prevalence of 58 per 100,000 (ref.42). The largest community prevalence study on stroke in Africa was performed in 1994 in the rural Hai district of Tanzania and reported an age-standardized prevalence of 154 per 100,000 in men >15 years of age and 114 per 100,000 in women >15 years of age43. Other crude prevalence estimates of stroke from community studies in Africa range from 15 per 100,000 in rural Ethiopia44 to 963 per 100,000 in urban Egypt45, with more recent studies reporting higher stroke prevalence than older studies14,28,39,40,45–57 (Table 1). Age-adjusted stroke prevalence seems to be higher in some parts of Africa than the global average. For example, two studies from rural communities in the Niger Delta region of southern Nigeria reported age-adjusted prevalence rates of 1,230 per 100,000 (ref.49) and 1,460 per 100,000 (ref.14). According to data from the GBD study, global age-adjusted stroke prevalence increased from 1,261 (95% uncertainty interval (UI) 1,208.2–1,318.7) per 100,000 person-years in 1990 to 1,300.6 (UI 1,229–1,374.7) per 100,000 person-years in 2017 (ref.58). Therefore, although stroke prevalence is declining in western Europe and North America, Africa might have some of the highest stroke prevalence rates in the world.
Frequencies in hospitals
In Africa, hospital-based studies on stroke seem to be more common than community-based or population survey-based studies59–68. The results of these hospital-based studies indicate that stroke is the leading cause of adult neurological admissions and medical coma. In one hospital-based case series, stroke accounted for up to 78% of adult neurological admissions69. In another tertiary centre, stroke accounted for up to 57% of individuals admitted with medical coma61. The results of a hospital-based cross-sectional cohort study indicated that stroke constituted ~25% of medical admissions among patients aged ≥60 years in Nigeria, Sudan and Tanzania67. A male preponderance in stroke presentation in African hospitals was generally observed in these studies56–65. A comparative study found ischaemic stroke to be the predominant stroke phenotype in Africa, accounting for up to 73% of stroke admissions; however, the burden of haemorrhagic stroke was higher in Indigenous Africans than in African Americans or Americans of European descent70. Indeed, a separate study, performed in southwestern Nigeria, reported that haemorrhagic strokes constituted ~45% of all stroke admissions65.
Mortality and fatality rates
The results of population-based studies suggest that mortality after stroke is high in Africa — estimates of stroke-related deaths as a proportion of overall mortality range from 5.5%62 to 11%63,71,72. The crude stroke mortality rate in a 1992–1995 study in Agincourt, South Africa, was estimated at 127 per 100,000 in individuals >35 years of age, although a 2016 study from the same site found a mortality rate of 114 per 100,000 (refs47,73). A Tanzanian study on stroke mortality at three sites (one urban, two rural) found yearly age-adjusted stroke mortality of up to 88 per 100,000 for men and 44 per 100,000 for women71. However, the results of stroke mortality studies in Africa require cautionary interpretation, owing to low levels of standardized death registration and the use of verbal autopsies.
Evidence suggests that the case fatality rate for stroke could be higher in parts of Africa than in the rest of the world. In hospital-based studies from Africa, incident case fatality at 30 days ranges from 16.2% to 46%73,74. A systematic review and meta-analysis of stroke case fatality in sub-Saharan Africa was published in 2021 and reported a pooled estimated 1-month case fatality rate of 24.1%, with high heterogeneity and rates up to 83.3%75. These data contrast with the 1-month case fatality rates in high-income countries reported in a 2009 review by Feigin et al., ranging from 8% in France to 49.2% in Estonia76. In Africa, case fatality rates are generally higher for haemorrhagic strokes than ischaemic strokes74,77–82. Studies with a longer follow-up period, such as those performed by Walker et al. in Tanzania, have reported case fatality rates of up to 84.3% at 3 years post-stroke (rural Tanzania) and 82.3% at 7 years post-stroke (rural and urban Tanzania)72,83. Advanced age, stroke severity at presentation, in-hospital complications (especially aspiration pneumonia and sepsis) and poor functional status have been identified as predictors of post-stroke mortality72,83–86. Evidence indicates that early stroke fatality is further accentuated by poor control of metabolic risk factors (blood pressure, blood sugar), delayed stroke recognition and hospital presentation, and difficulties accessing early post-stroke care75,85–87. In addition, the nature and organization of stroke services in Africa present inherent challenges, including limited access to diagnostic and time-dependent interventional services such as reperfusion therapies and stroke surgery, lack of stroke units and difficulty with patient retention during post-stroke rehabilitation88.
Disability-adjusted life years
Data regarding stroke DALYs in Africa are sparse. One systematic review found that the burden of disease owing to stroke in South Africa was 564,000 DALYs in 2008 (ref.89). In the few available studies, estimated DALYs lost due to stroke ranged from 1,070 DALYs per 100,000 person-years in South Africa to 7,738 DALYs per 100,000 person-years in Kenya. Women seem to have more stroke-associated DALYs than men and DALYs were higher for ischaemic stroke than haemorrhagic stroke89,90. Data from the GBD study indicate that, although stroke-associated DALYs decreased in all regions of the world between 1990 and 2016, the region with the smallest reduction was sub-Saharan Africa2. According to the same data, 80% of all incident strokes, 77% of all survivors of stroke, 87% of all deaths from stroke and 89% of all stroke-related DALYs occur in LMICs, including those in Africa91.
Quality of life and cost of care
Several studies have examined quality of life after stroke in Africa. The results of these studies consistently indicated that survivors of stroke have poorer quality of life in multiple domains than stroke-free controls92,93. The most commonly reported predictors of quality of life were post-stroke disability, depression and stroke severity. The estimated cost of care per patient with stroke ranges from US$ 145 to US$ 4,860, depending on the care setting94. Cost of stroke care is higher in urban areas than in rural areas and higher in private health facilities than in government health facilities94–98. Data from Africa have also identified a high burden of stroke on the psychological and emotional domains of quality of life of caregivers. Multiple studies have reported that female spouses are the predominant caregivers99–101; in a 2019 study from Lagos, Nigeria, up to 86.7% of caregivers were women101.
Risk factors
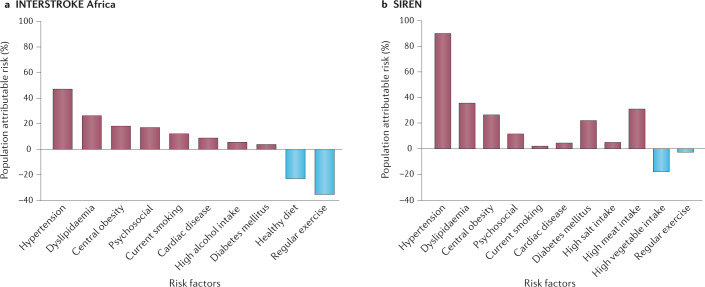
Age is the major non-modifiable risk factor for stroke. A comparative, hospital-based study found mean age at stroke presentation to be at least 10 years lower in Indigenous Africans than in African Americans or Americans of European descent70, suggesting Africans are more likely to be affected by stroke during the theoretical peak of their vocational productivity. Similarly, another study found patients with stroke in Ibadan, Nigeria, to be younger than patients with stroke in Berlin, Germany70,102. Temporal trends from the GBD (1990–2016) study indicate that age-standardized stroke incidence is increasing in southern sub-Saharan Africa, suggesting a persistent burden of underlying stroke risk factors in this region2. Observations from the global INTERSTROKE study, which involved five African countries (Mozambique, Nigeria, South Africa, Sudan and Uganda) suggest that the profile of stroke risk factors is similar between Africa and the rest of the world, although regional differences in effect size exist103. This study identified ten top risk factors (hypertension, dyslipidaemia, diabetes mellitus, central obesity, cardiac causes, current smoking, high alcohol intake, unhealthy diet, physical inactivity and psychosocial factors) that accounted for 82% of the PAR for stroke among Africans and 92% of the PAR for stroke in the rest of the world103. The disparity could have resulted from an underestimation of risk in Africa owing to a small dataset or to unexplained residual factors, such as infectious diseases, contributing to stroke risk. The Stroke Investigative Research and Educational Network Study (SIREN) is the largest multi-site case–control stroke risk factor study to be performed in Africa to date. The study included 2,118 case–control pairs of Indigenous Africans from 15 sites in Nigeria and Ghana104. Using rigorous case ascertainment methodology and a similar case mix as the INTERSTROKE study105,106, the SIREN study found that 98.2% (95% CI 97.2–99.0) of the adjusted PAR of stroke was associated with 11 potentially modifiable risk factors: hypertension, dyslipidaemia, regular meat intake, central obesity, diabetes mellitus, low consumption of green leafy vegetables, stress, added salt at table, cardiac diseases, physical inactivity and current cigarette smoking104 (Fig. 1).
Fig. 1. Risk factors for stroke in Africa.
a | The population attributable risk associated with 10 potentially modifiable risk factors in the INTERSTROKE study (Africa sub-cohort). The study included 973 case–control pairs of Indigenous Africans from sites in Mozambique, Nigeria, Sudan, South Africa and Uganda. Data from ref.103 and ref.105. b | The population attributable risk associated with 11 potentially modifiable risk factors in the Stroke Investigative Research and Educational Network (SIREN) study. The study included 2,118 case–control pairs of Indigenous Africans from multiple sites across Nigeria and Ghana. Data from ref.104.
Hypertension was the major modifiable risk factor identified by the INTERSTROKE study, with an odds ratio (OR) of 4.01 (99% CI 2.59–6.21) and PAR of 34.6 (99% CI 30.4–39.1) for the African countries included in the study103. In the SIREN study, the OR and PAR for hypertension were even higher, at 19.36 (95% CI 12.11–30.93) and 90.8% (95% CI 87.9–93.7), respectively. The INTERSTROKE study had a smaller sample size of Africans and lower statistical power than the SIREN study, which could account for this disparity. Nevertheless, these data suggest that hypertension is the prime modifiable driver of the stroke burden in Africa, although many Africans also have other vascular co-morbidities that act in concert with hypertension to drive stroke burden. Indeed, >45% of people in Africa over 25 years of age are estimated to be hypertensive, which constitutes the highest burden of hypertension in the world, according to the WHO107. Evidence indicates that <50% of individuals with hypertension in Africa are aware that they have the condition or have received a diagnosis and, among those who are diagnosed, at least half are not receiving treatment108. Thus, strategies to reduce the impact of hypertension will greatly reduce the risk of strokes among Africans104,108,109. In contrast to non-African cohorts, atrial fibrillation has not been consistently reported as a substantial risk factor for strokes in Africa (it accounted for 7% of all strokes in the INTERSTROKE study), although this observation could be the result of under-reporting110.
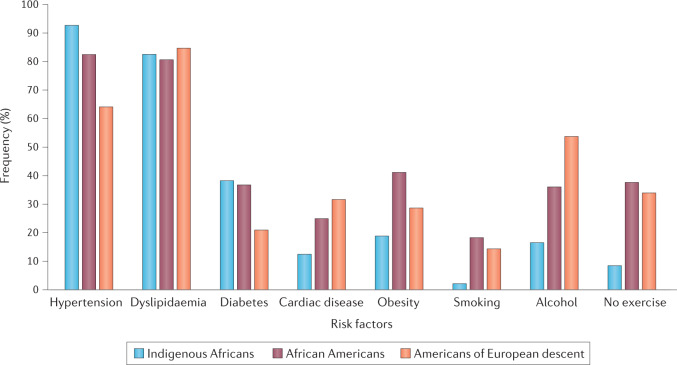
A comparison between data from the SIREN study and data from the population-based Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study of African Americans and Americans of European descent living in the USA found higher frequencies of several cardiometabolic factors (hypertension, diabetes mellitus and dyslipidaemia) among Indigenous Africans and African Americans than in Americans of European descent70 (Fig. 2). However, the comparison also identified a higher frequency of several cardiac and lifestyle factors (smoking, high alcohol intake and physical inactivity) among African Americans and Americans of European descent than among Indigenous Africans. These observations underscore the interaction between ethnic (including genetic) and geographical (lifestyle) factors in the vascular pathophysiological cascade70,102.
Fig. 2. Effect of race and geography on risk factors for stroke.
Graph shows the frequency of eight risk factors in Indigenous Africans, African Americans and Americans of European descent. Study participants were >55 years of age. The data for this analysis came from 1,928 individuals with stroke who met the selection criteria and consisted of 811 Indigenous Africans recruited into the Stroke Investigative Research and Educational Network (SIREN) study, 452 African Americans and 665 Americans of European descent who were participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. The group of participants of African ancestry had a significantly higher prevalence of hypertension and diabetes, similar frequency of dyslipidaemia and lower prevalence of cardiac disorders than Americans of European descent. However, obesity and lifestyle factors, including smoking, alcohol consumption and physical inactivity, were more prevalent among African Americans and Americans of European descent than Indigenous Africans. These data illustrate the complex interaction between racial (genetic) and geographical (environmental, for example, lifestyle) factors in the neurobiology of stroke. Data from ref.70.
Stroke with co-morbid conditions
In Africa, as elsewhere, strokes occur alongside other conditions. In particular, HIV infection increases the risk of stroke111,112. Africa bears the largest global burden of HIV infection — 70% of the 36.8 million people who were living with the virus in 2017 were located in sub-Saharan Africa113. Africans with HIV and stroke generally have a young age at stroke onset (<45 years), severe stroke presentation, preponderance of ischaemic strokes and advanced immunosuppression114–117. The results of a Tanzanian stroke incidence project, which involved community-ascertained controls, found HIV infection to be an independent risk factor for stroke, with an OR of 5.61 (CI 2.41–31.09)53. Several mechanistic links between HIV infection and strokes have been suggested111, including clustering of cardiometabolic factors, inflammation of the brain vascular endothelium, coagulation abnormalities, vasculitis, HIV-associated vasculopathy and dyslipidaemia owing to anti-retroviral therapies and insulin resistance. However, more studies are needed to unravel the epidemiological contribution of HIV infection to the burden of stroke in Africa118.
Africa bears the greatest burden of SCD in the world, as 75% of the >300,000 individuals born with SCD globally every year are born in sub-Saharan Africa119. SCD has been reported to increase stroke risk among children and adults in Africa120,121. There is a bimodal age distribution in the incidence of ischaemic stroke in individuals with SCD: most strokes occur before 20 years and after 30 years of age, with peak incidence at 10–15 years122,123. In a systematic review and meta-analysis involving 18,977 participants with SCD pooled from 23 African studies, the overall prevalence of stroke was 4.2%124. Stroke in individuals with SCD typically presents with transient ischaemic attacks, seizures and focal neurological deficits such as hemiparesis124. Cerebral ischaemia in SCD is thought to result from the occlusion of distal internal carotid arteries, middle cerebral arteries and anterior cerebral arteries120,121,123,124. Haemorrhagic strokes in SCD usually occur in the third decade of life121 and are associated with low steady-state haemoglobin levels and high steady-state leukocyte counts125. Emerging data from Kumasi, Ghana, indicate higher numbers of stroke admissions and stroke mortality in January–June 2020 than in January–June 2019, suggesting a link between stroke risk and SARS-CoV-2 infection126. SARS-CoV-2 infection has been associated with increased fibrin, D-dimers and hypercoagulability, indicating that infection will increase the likelihood of strokes in vulnerable Africans with pre-existing vascular risk factors127.
Drivers of the rising burden of stroke in Africa
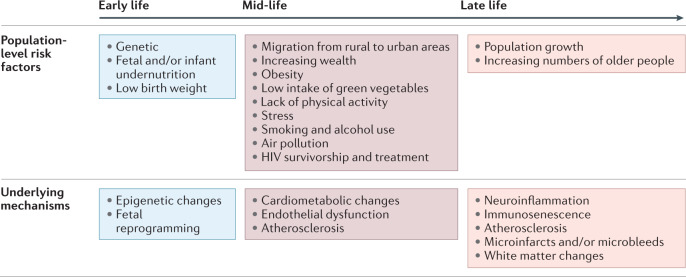
Data accrued over the past two decades and modelling estimates from the GBD study suggest a progressive increase in the burden of stroke in Africa4,128. Indeed, stroke and its risk factors (cardiovascular diseases) are now the leading cause of death of the adult population in the continent129. The drivers of this increasing stroke burden in Africa can be categorized into early-life, mid-life and late-life factors (Fig. 3). Early-life factors, including fetal and/or infant undernutrition and low birth weight, have been associated with cardiometabolic disorders such as high blood pressure, dysglycaemia and dyslipidaemia later in life15,17,130. This link is thought to be the result of fetal reprogramming and epigenetic changes. A good illustration of the delayed effects of early-life undernutrition is the high burden of hypertension, diabetes and overweight status reported among survivors of the Nigerian civil war (the Biafran War), which occurred in the late 1960s16. Many of these individuals were exposed to severe famine in early life as a result of the conflict.
Fig. 3. A life course approach to factors driving stroke burden in Africa.
The population-level risk factors for stroke change across the lifespan. Here, we summarize the risk factors at each stage of the life course that are driving the increasing burden of stroke in Africa. We also highlight the molecular mechanisms and processes involved in stroke risk at each stage of life. HIV, human immunodeficiency virus.
Early-life undernutrition might also partly explain the increased risk of cardiometabolic disorders among individuals who grow up in low-income rural areas but migrate to urban areas131. As these individuals approach mid-life with increasing wealth, they adopt lifestyles associated with urban living. Such lifestyles are often characterized by low levels of physical activity, high caloric intake, smoking, high levels of alcohol consumption, high intake of meat and low consumption of green leafy vegetables. Evidence indicates that exposure to stress and air pollution further exacerbates the course of neurovascular disease through the promotion of neuroinflammation and endothelial dysfunction18,132. Indeed, neither urban nor rural Africa is spared from the emerging risks of stress and air pollution (indoor air pollution from solid fuels and outdoor ambient particulate matter pollution) and both are likely to contribute to the increases in stroke incidence that we are observing in Africa19. The GBD 2013 study identified a substantial contribution of household air pollution to the risk of stroke in LMICs, with the largest household air pollution-associated risk observed in the West African sub-region128.
Late-life factors driving the increasing burden of stroke in Africa include a progressive increase in life expectancy, which is resulting in population ageing133. Multiple cohort and population-based studies in Africa have identified ageing as a key non-modifiable risk factor for stroke, indicating that an increase in the number of older people in the population will result in an increase in stroke burden4,12. Genetic predisposition to stroke and stroke-related epigenetic modifications operate across the life course. For example, an association has been found between IL6rs1800796 and ischaemic stroke among West Africans134. The observation that fine particulate matter air pollution is associated with elevated plasma levels of IL-6 (ref.135) strengthens the possible link between air pollution and the increasing stroke burden.
Another contributor to the increasing burden of stroke in Africa is the limited availability of medication to control the more common risk factors. Drugs for the treatment of hypertension and diabetes are costly and might not be readily available in most health systems in Africa136. Furthermore, the vast majority of African countries do not have health insurance systems that can pay for the medications in full or even in part137,138. As a consequence, adherence to chronic treatment is a substantial economic load on the patient and their family. Poor health literacy can also contribute to a lack of control of vascular risk factors139,140. In the PURE study, which involved >150,000 participants from urban and rural settings around the world, <10% of participants from low-income countries with a previous cardiac or stroke event were on any of the four drugs indicated for secondary prevention, that is, angiotensin-converting enzyme inhibitors, beta blockers, statins or aspirin141. In a study performed in the Southwest region of Cameroon, essential cardiovascular disease medicines were only available in 25.3% of public pharmacies and 49.2% of community pharmacies136. Medicines for heart failure and dyslipidaemia, including beta blockers, angiotensin-converting enzyme inhibitors and statins, cost the equivalent of 2–13 days’ wages for a 1-month supply.
Stroke phenotypes in Africa
Information on the phenotypic characteristics of stroke types and subtypes in Africa was limited until evidence from the SIREN study became available in early 2018. In the initial data from this study, which recruited index stroke cases from 15 sites in Nigeria and Ghana, of 2,118 individuals with neuroradiologically confirmed stroke, 68% had ischaemic stroke, 32% had intracerebral haemorrhage (ICH) and <1% had ischaemic stroke with haemorrhagic transformation104. The SIREN investigators did not recruit participants with sub-arachnoid haemorrhage but it is estimated to contribute to ~5% of all strokes in Africa142.
Ischaemic stroke
In Africa, the most common aetiological subtype of ischaemic stroke is small vessel disease (SVD) (42%), followed by large vessel atherosclerotic disease (17%), cardioembolism (11%) and undetermined (30%)70,104. In a meta-analysis of five different stroke studies involving a total of 2,843 participants with ischaemic stroke from both hospital and community-based cohorts in Ghana, Kenya, Nigeria, Mozambique, South Africa, Sudan and Uganda, we found that SVD accounted for 30% of ischaemic strokes12. By contrast, SVD accounted for only 19% of ischaemic strokes from 13 different studies involving 12,931 patients from hospital and non-hospital or community-based cohorts in western Europe and the USA12. The preponderance of SVD and the relative paucity of cardioembolic strokes among Africans is similar to observations from other LMICs such as China143 and Pakistan144 but in contrast to data from high-income countries. Indeed, studies conducted in mixed ethnic populations in the USA145 and the UK146 strongly suggest that SVD is twice as common among participants of African descent than among participants of European descent. Participants of European descent were more likely than participants of African descent to have a cardioembolic or large-artery atherosclerotic stroke.
The above observations indicate that the relative frequencies of ischaemic stroke subtypes across the globe vary by race, geography and, perhaps, gross national income. Therefore, association studies on the genetics of ischaemic stroke among Indigenous Africans are eagerly awaited. However, it seems likely that the high burden of undiagnosed and uncontrolled asymptomatic risk factors (notably hypertension, dyslipidaemia and dysglycaemia) in sub-Saharan Africa is leading to arteriolosclerosis and microatheroma formation in deep perforating cerebral vessels and thus causing SVD-related ischaemic strokes12. This hypothesis is consistent with the observation that, among Africans, hypertensive disease is more prevalent than atrial fibrillation or atherosclerotic disease (discussed above). Indeed, few studies have researched cervicocephalic atherosclerosis, a marker of epidemiological and stroke transition, among Africans147. Neurovascular ultrasonography detected no substantial carotid artery disease in the Tanzania Stroke Incidence Study148. However, a study in participants with hypertension and stroke in Nigeria found that carotid intima media thickness was a sensitive indicator of cardiovascular phenotype and had a stronger association with stroke than popular cardiovascular risk calculators that are used to estimate the time-related risk of cardiovascular disease149,150. A more recent study of individuals diagnosed with acute stroke-like syndrome in Malawi reported that 39.4% had extracranial atherosclerosis and 19.2% had intracranial atherosclerosis151. Post-mortem studies undertaken ~40 years apart also identified a temporal increase in the frequency and severity of cerebral atherosclerosis among Nigerian Africans in tandem with the overall rise in the burden of cardiovascular disease and stroke occurrence152–154.
Intracerebral haemorrhage
Further evidence of poor blood pressure control in sub-Saharan Africa has been gleaned from the aetiological profile of ICH. In the SIREN study, the aetiology of ICH was classified using the structural lesion, medication, amyloid angiopathy, systemic and/or other disease, hypertension, undetermined (SMASH-U) algorithm155. Using this method, the most common aetiology was hypertension (80.9%); less common were structural vascular anomalies (4.0%), cerebral amyloid angiopathy (0.7%), systemic illnesses (0.5%) and medication-related aetiologies (0.4%)156. Aetiology remained undetermined in 13.7% of participants. Of considerable interest is the observation that, among West Africans, ICH constitutes 53% of all strokes occurring among the young (<50 years of age)156. The six factors most associated with stroke in the young (who constituted 25% of the SIREN cohort) were hypertension, dyslipidaemia, diabetes mellitus, low consumption of green leafy vegetables, psychosocial stress and cardiac disease157. These further underscore the observation that hypertension is more strongly associated with ICH than ischaemic stroke103,104 and thus emphasize that large numbers of strokes, particularly among young Africans, could be avoided by better blood pressure control. The PAR of stroke resulting from hypertension in the SIREN study was 88.7% among young West Africans157, again highlighting the importance of this vascular risk factor. Furthermore, in a recent cross-sectional study in Ghana, the frequency of refractory hypertension (a rare and severe phenotype of treatment-resistant hypertension) among ~1,200 survivors of stroke was 5.8% compared with 0.7% among ~2,800 stroke-free individuals with hypertension158. The prevalence of refractory hypertension was much higher in individuals <60 years of age than in individuals >60 years of age. The odds ratios of refractory hypertension were 13.6 for lacunar stroke, 11.4 for ICH and 3.7 for non-lacunar ischaemic strokes relative to stroke-free hypertension. This further suggests that the phenotypic characteristics of hypertension also exert differential effects on the manifestation of stroke types. Further studies are now required to elucidate the mechanisms underlying these differences and to guide the design of targeted population-level interventions and risk-reduction strategies.
Stroke complications and sequelae
Stroke complications include a range of neurological, psychiatric or other systemic manifestations that occur in the acute, subacute or chronic phases of a stroke. Most data on stroke complications in Africa come from hospital-based studies and suggest that some form of complication occurs in >80% of inpatients with stroke (Table 2). Stroke complications in Africa include delirium159–161, post-stroke aspiration pneumonia85,86,162–166, bacteriuria and urinary tract infection167–169, aphasia and deglutition disorders170, anxiety99,171–173, fatigue174–176, sexual dysfunction177–179, pain (central post-stroke pain syndrome)180–183, depressive illness184–193 and cognitive impairment that often leads to dementia194–197.
Table 2.
Stroke complications and sequelae in Africa
| Post-stroke complication | Features | Reported prevalence (%)a | Predictors | Countries | Refs |
|---|---|---|---|---|---|
| Delirium | Onset 1 week after stroke; confusional states | 32–33 | Age, NIHSS score | Nigeria | 159–161 |
| Aspiration pneumonia | Cause of death after stroke | 64–79 | Age, stroke severity, consciousness | Benin, Burkina Faso, Ethiopia, Mozambique, Tanzania | 85,86,162–166 |
| Bacteriuria and urinary tract infection | Defined as >105 CFU/ml | 9.3–12.3 | Infections | Ghana, Nigeria | 167–169 |
| Aphasia and deglutition disorders | Aphasia can last for up to 60 months after stroke | ~50 | Age, left-hemispheric stroke, cognitive impairment | Tanzania | 170 |
| Anxiety | Accumulative over 12 months | 10–34 | Female sex, low socioeconomic status, haemorrhagic lesions, depression | Nigeria, Tanzania | 99,171–173 |
| Fatigue | Peak at 6 months after stroke | 60 | Poor quality of life | Ghana, Nigeria | 174–176 |
| Sexual dysfunction | Most common: erectile dysfunction and low libido | >80 | Age, male sex | Nigeria | 177–179 |
| Pain | Usually musculoskeletal pain or central post-stroke pain syndrome | 5–80 | Background history of pain, differential thresholds, age at stroke onset | Nigeria, Zimbabwe | 180–183 |
| Depression | Most common: mood disorders, suicidality and tedium vitae | 16–80; median 30 | Age | Democratic Republic of Congo, Gabon, Ghana, Nigeria | 184–193 |
| Cognitive impairment | Vascular cognitive impairment, dementia | 30–50 | Age, low literacy, vascular risk factors, Black race, recent infection, MTLA, WMH | Ghana, Nigeria, South Africa | 194–197 |
On the basis of data from hospital-based studies. For details on post-stroke epilepsy and functional disability, see Tables 3 and 4. CFU, colony-forming unit; MTLA, medial temporal lobe atrophy; NIHSS, National Institutes of Health Stroke Scale; WMH, white matter hyperintensities. aPercentage of individuals with stroke who go on to develop these complications.
On average, 40–50% of survivors of stroke in Africa develop some form of cognitive dysfunction194,196, suggesting that vascular cognitive impairment could become the most common precursor to dementia in Africa. Several hospital-based studies have investigated the prevalence of post-stroke seizures in Africa. The prevalence of early (≤7 days of stroke onset) post-stroke seizures were 9.3%198, 9.6%199 and 13%200 in the three studies that recorded this data. The prevalence of epilepsy among survivors of stroke in Africa was 1.98–16.9%201–204 (Table 3). Factors associated with an increased risk of post-stroke epilepsy included male sex, cortical infarcts and poor access to neurological services. However, the results of these studies need to be interpreted in the context of variation in study methods and case definition and the potential inaccuracy of patient reports owing to the cultural stigma around epilepsy in Africa.
Table 3.
Estimated prevalence of post-stroke epilepsy in Africa
| Country | Year | Study type | Sample size | Diagnostic criteria | Prevalence (%)a | Ref. |
|---|---|---|---|---|---|---|
| Burkina Faso | 2006–2014 | Hospital based; retrospective | 1,616 | Clinical, EEG, brain CT | 1.98 | 201 |
| Sudan | 2006–2008 | Hospital based | 165 | Clinical, eye-witness reports, EEG, brain CT | 16.90 | 202 |
| Benin | 2015–2016 | Hospital based; retrospective | 1,703 | Clinical, EEG, brain CT | 2.00 | 203 |
| Ghana | 2018–2020 | Hospital based; cross-sectional | 1,101 | Clinical, brain CT, EEG | 11.40 | 204 |
aPercentage of individuals with stroke who go on to develop post-stroke epilepsy.
In several studies, the functional outcomes in patients with stroke in Africa were carefully monitored using established scales43,50,205–214 (Table 4). The results of these studies suggest that there is a substantial post-stroke disease burden that needs attention. Evidence from the few available studies in Africa suggests that survivors of stroke are likely to return to work after the first year215,216. Shorter post-stroke duration, lower functional independence and worse post-stroke cognitive dysfunction were associated with a lower likelihood of a return to work.
Table 4.
Estimated frequency of functional disability after stroke in Africa
| Country (City) | Year | Type of study | Study population | Sample size | Tools used | Percentage with disability | Ref. |
|---|---|---|---|---|---|---|---|
| Gambia (Banjul) | 1990–1991 | Hospital | Not stated | 106 | Barthel Index | 91.5 at 1 month after stroke | 205 |
| Tanzania (Hai) | 1994 | Community and census | 85,152 (aged >15 years) | 108 | Barthel Index | 60 | 43 |
| South Africa (Cape Town) | 2004–2006 | Hospital | 4,524,335 | 196 | MRS, Barthel Index | 48 (moderate to severe disability) | 206 |
| Benin (Cotonou) | 2009 | Community | 69,869 | 14,969 | MRS, FIM, MADRS | 60 | 50 |
| South Africa (Johannesburg) | Not known | Hospital and community | Not stated | 68 | Barthel Index | 47 at discharge; 93 at 6 weeks after discharge | 207 |
| South Africa (Cape Town) | 2010 | Hospital | Not stated | 67 | Barthel Index | 81.82 at discharge | 208 |
| Nigeria (Ibadan) | 2013 | Hospital | Not stated | 128 | MRS | 60.9 | 209 |
| Benin (Parakou) | 2013 | Hospital | Not stated | 85 | MRS | 53 at 1 month after stroke | 210 |
| Nigeria (Benin) | Not known | Hospital and outpatient | Not stated | 102 | MRS | 71.6 | 211 |
| Uganda (Kampala) | 2014 | Hospital | Not stated | 127 | Barthel Index | 46.1 at 1 month after stroke | 212 |
| Egypt (Cairo) | 2015–2016 | Hospital | Not stated | 397 | MRS | 18 | 213 |
| Egypt (Cairo) | 2018–2019 | Hospital | Not stated | 61 (posterior circulation stroke) | MRS | 72.13 at 3 months after stroke | 214 |
FIM, functional independence measure; MADRS, Montgomery–Asberg depression rating scale; MRS, modified Rankin score.
Genetics of stroke in Africa
Evidence indicates that, compared with populations of European descent, stroke in the Indigenous African population has an earlier age of onset and is more likely to be haemorrhagic or SVD-related ischaemic4,23,70,102. Differences in stroke severity and outcomes have also been observed between Indigenous Africans and populations of European descent. Broad differences in the demographic structures of the African population and populations in Europe and the USA as well as differences in the availability of health services offer plausible explanations for these disparities217. Nevertheless, a possible role of genetic and/or epigenetic factors warrants further exploration23,24. For example, differences between chronological age and biological age (which is determined on the basis of epigenetic changes) have been documented among individuals of European ancestry with stroke218 and whether a similar phenomenon occurs among African individuals needs to be explored219. Higher stroke heritability among individuals of African ancestry than among individuals of European ancestry has been reported in the South London Stroke Registry220 but the exact effect of this heritability requires in-depth studies in Indigenous African populations.
Genomic diversity in Africa
Africa is regarded as the cradle of modern humans, Homo sapiens, and African genomes are more diverse than those from any other continent221–223; however, only a fraction of the genetic diversity among African individuals has been surveyed — to date, <2% of GWAS were performed on African data224. In a high-depth study of African genomes, whole-genome sequencing was performed on samples from 426 individuals from 50 ethnolinguistic groups; the genetic makeup of many of these groups had not been previously studied225. The results of this study, published in 2020, identified more than 3 million previously undescribed genetic variants. The implications of the identification of these variants for our understanding of the genetics of the stroke phenome are immense and are relevant to both Africans and the global population25,226. In particular, the Human Heredity and Health in Africa Initiative222 is facilitating the genetic exploration of African populations, including the SIREN study cohort, by use of approaches including candidate gene studies and GWAS23,24. The overall aim is that inclusion of Indigenous Africans in multi-ethnic genomic studies, combined with the use of trans-omics approaches, will facilitate genetic fine mapping, trans-ancestry meta-analysis and development of ancestry-sensitive polygenic risk scores as well as the discovery of new risk loci, pathophysiological pathways, biomarkers and drug targets for the enhancement of precision stroke medicine24,25,226,227.
Candidate gene studies and GWAS
To date, the majority of genetic studies of stroke in Africa have adopted a candidate gene approach (Table 5). Candidate gene studies and GWAS are two methods that are used to detect genetic susceptibility to diseases. The main difference between the two approaches is that candidate gene studies investigate genetic variation within a small number of pre-identified genes of interest, whereas in GWAS, the entire genome is investigated for common genetic variations that are associated with the disease of interest. In northern Africa, candidate gene studies have reported associations between stroke, particularly ischaemic stroke, and polymorphisms in multiple genes, including those encoding apolipoprotein E (APOE), IL-1, IL-1β, peroxisome proliferator-activated receptor-δ (PPARD), apolipoprotein A5 (APOA5) and aldosterone synthase (CYB11B2)228–242. In addition, a polymorphism in the gene encoding angiotensinogen (AGT) was associated with stroke in an Egyptian cohort consisting of adolescents with SCD243. A candidate gene study performed in Zambia identified an association between APOEε2/ε4 genotype and an increased risk of both haemorrhagic and ischaemic stroke244. Findings from the West African SIREN study included a significant association of variants on IL6 and CDKN2A/2B with risk of ischaemic stroke among men134 as well as a significant association of variants on APOL1, CDKN2A/2B and HDAC9 with risk of SVD ischaemic stroke245. This study was the first to identify a stroke-related variant on APOL1, although the gene had previously been associated with chronic kidney disease in individuals of African descent246,247. A particularly intriguing observation is that, whereas APOE alleles influenced the risk of stroke in northern and southern Africa, an APOLI risk variant was associated with stroke in western sub-Saharan Africa. Further studies are needed to explore the potential geographical disparity in the effect of these two prominent apolipoprotein genes; the results of such studies could also be relevant to other brain disorders such as Alzheimer disease. The first stroke GWAS in Africa is currently under way and is anticipated to provide further insight into the genetic architecture of stroke in Indigenous Africans by identifying novel stroke-associated variants.
Table 5.
Stroke candidate gene studies in Africa
| Study | Gene name | Study population | Salient findings |
|---|---|---|---|
| Saidi et al. (2007)234 | Plasminogen activator inhibitor 1 (PAI1); tissue plasminogen activator (tPA) | Tunisian | PAI1 4G/5G polymorphism associated with increased PAI1 expression, decreased tPA expression and increased risk of stroke |
| Saidi et al. (2007)233 | Apolipoprotein E (APOE) | Tunisian | APOE ε4 associated with increased risk of ischaemic stroke, small vessel disease and statin use |
| Saidi et al. (2008)230 | Human platelet alloantigen 1–5 (HPA1–5) | Tunisian | Lower HPA1a frequency and higher HPA1b frequency in the stroke group than in controls |
| Saidi et al. (2008)229 | HPA1–5 | Tunisian | HPA1a/b and HPA5a/b alleles associated with increased risk of stroke; HPA1b/b and HPA5b/b associated with extent of symptoms and risk of recurrence |
| Saidi et al. (2009)235 | APOE; angiotensin-converting enzyme (ACE) | Tunisian | Higher APOEε4 frequency and lower APOEε3 frequency in the stroke group than in controls; APOε4 ACE Del/Del genotype was associated with large vessel stroke and was detected in higher proportions in older (>50 years) patients |
| Saidi et al. (2009)231 | Angiotensinogen (AGT) | Tunisian | Multiple AGT polymorphisms were significantly associated with an increased risk of stroke |
| Saidi et al. (2010)228 | Aldosterone synthase (CYP11B2) | Tunisian | CYP11B2 C-344T polymorphisms (T allele bearing) were associated with risk of ischaemic stroke |
| Chehaibi et al. (2013)236 | Peroxisome proliferator-activated receptor-δ (PPARD) | Tunisian | PPARD +294T/C polymorphism was associated with increased risk of ischaemic stroke |
| Fekih-Mrissa et al. (2013)238 | Methylenetetrahydrofolate reductase (MTHFR) | Tunisian | MTHFR C677T and A1298 polymorphisms were associated with an increased risk of ischaemic stroke |
| Atadzhanov et al. (2013)244 | APOE | Zambian | APOEε2/ε4 genotype associated with increased risk for both haemorrhagic stroke and ischaemic stroke |
| Chehaibi et al. (2014)237 | Matrix metalloproteinase 1 (MMP1) and MMP12 | Tunisian | MMP12 polymorphisms were associated with an increased risk of ischaemic stroke in individuals with diabetes |
| Diakite et al. (2015)239 | C2491T | Moroccan | C2491T FV mutation was associated with risk of ischaemic stroke |
| Diakite et al. (2014)241 | eNOS | Moroccan | eNOS G894T polymorphism was associated with risk of ischaemic stroke |
| Rezk et al. (2015)242 | IL-1 cluster genes: IL1B, IL1A and IL1RN | Egyptian | IL1B −511 and IL1A −889 polymorphisms were associated with increased risk of acute stroke |
| Diakite et al. (2016)240 | APOA5; arachidonate 5-lipoxygenase-activating protein (ALOX5AP) | Moroccan | APOA5 T1131C and ALOX5AP SG13S114 polymorphisms were associated with risk of ischaemic stroke |
| Akinyemi et al. (2017)134 | IL6; cyclin-dependent kinase inhibitor 2A/2B (CDKN2A/2B) | West African (Nigeria and Ghana) | IL6rs1800796 and CDKN2A/2Brs2383207 are associated with risk of ischaemic stroke in men |
| Akinyemi et al. (2018)245 | Apolipoprotein 1 (APOL1); CDKN2A/2B; histone deacetylase 9 (HDAC9) | West African (Nigeria and Ghana) | APOL1rs73885319, CDKN2A/2Brs2383207 and HDAC9rs2107595 are associated with risk of small vessel disease ischaemic stroke |
Stroke services in Africa
Stroke services are organized systems and structures for delivering preventive, therapeutic and rehabilitative stroke care at an institutional, regional or national level. The World Bank’s Disease Control Priorities 3rd edition248 identified five major platforms for the provision of essential universal health coverage in LMICs: population-level platforms, community-level platforms, health centres, first-level hospitals and referral hospitals. These platforms provide a model for building chains of stroke care that can be divided into three categories: pre-hospital, hospital and post-hospital services. Pre-hospital stroke services consist of elemental and primary prevention policies, surveillance, pre-hospital emergency care, and outreach and awareness services. Hospital-based stroke services include emergency medical services, acute stroke treatments, secondary prevention and acute rehabilitation. Post-hospital stroke services consist of community rehabilitation, social and family support, and follow-up.
Population-based services
Stroke shares a common risk-factor profile with many cardiovascular diseases; thus, population-based approaches to stroke prevention in Africa have conventionally focused on non-communicable diseases as a whole. In 2015, non-communicable diseases were incorporated into the United Nations Sustainable Development Goals: target 3.4 is “By 2030, to reduce by one third premature mortality from non-communicable diseases through prevention and treatment and to promote mental health and well-being”249. However, progress reports from Africa published in 2017–2020 suggest low levels of progress towards this target as a result of a lack of political will, poor intersectoral collaboration, absence of universal health coverage, widespread poverty, the challenges involved in making changes to existing health systems and low levels of public spending on health250–252. The joint World Stroke Organization (WSO)–WHO–Lancet Neurology Commission for Stroke is advocating pragmatic, context-sensitive approaches that involve multiple stakeholders, including healthcare providers, physicians, patients, the general population, policy-makers and payers, with the aim of identifying solutions that will enhance stroke prevention and surveillance services across a range of income levels253.
Pre-hospital acute stroke care
Pre-hospital acute stroke services conventionally function within existing emergency medical services, although they can also be standalone services. Emergency medical services are poorly developed in Africa, except in some areas in South Africa and northern Africa that have a relatively higher income. In a recent survey, established emergency medical services were identified in only 16 African countries; most of the identified services were government operated, had fee-for-service payment structures and poor uptake254. Organizations such as the African Federation of Emergency Medicine have developed processes and guidelines for emergency medicine training in Africa, raising hopes of improving access to quality and effective emergency care; however, organizational, fiscal and regulatory challenges remain255,256. Few studies have examined the specific factors responsible for pre-hospital delays in stroke presentation in Africa; however, the available evidence suggests that poor stroke knowledge is a major cause of pre-hospital delays in presentation and poor outcomes257. Other contributing factors include stroke onset during normal sleeping hours, transportation-related delays, poor availability or a lack of ambulance services, absence of trained first responders and a paucity of neuroimaging services88,258–260. Evidence from a 2015 geospatial analytic study indicated that, for 71% of Africans, travel time to the nearest hospital is >2 hours261, highlighting a dire need for broad intersectoral strategies to improve access to healthcare.
Onset-to-door or onset-to-needle time is often used as an indicator of timeliness of stroke triage and efficiency of acute reperfusion therapy. Evidence from the European Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS MOST) and Safe Implementation of Treatments in Stroke–Eastern Europe (SITS EAST)262 study indicates a median onset-to-door time of 140 minutes. Similarly, data from the American Get With The Guidelines – Stroke registry (GWTG-Stroke)263 indicate a median onset-to-door time of 144 minutes. Indeed, the paradigm of acute stroke care in high-income countries currently emphasizes ultra-early pre-hospital stroke diagnosis, treatment using mobile stroke units and telemedicine for stroke assessments264,265. By contrast, a systematic review of studies from Africa found a median time from stroke onset to hospital admission of 31 hours266. In a single hospital study from Burkina Faso, median stroke onset to hospital admission time was ~7 hours but only 19% of patients presented within 4.5 hours of stroke onset and were thus eligible for thrombolysis267.
Hospital-based acute stroke care
The profile, pattern and quality of hospital-based acute stroke care in Africa is heterogeneous. Services for stroke often exist as part of general medical services, although stroke units have now been reported in at least ten countries, including Morocco, Tunisia, Algeria, Egypt, Mauritania, Ghana, Nigeria, Guinea, Central African Republic and South Africa3,268,269. In addition, acute stroke services do exist in private hospitals in some parts of Africa.
The WSO has produced the global stroke services guidelines and action plan270 as a framework to guide the development of stroke services around the world. The framework proposes three tiers of stroke services (minimal, essential and advanced), depending on the availability of multidisciplinary expertise, diagnostic infrastructure and capacity for acute reperfusion therapy. The applicability and effectiveness of this framework in low-resource African settings were recently demonstrated in Conakry, Guinea, where a minimal stroke unit was set up. This unit consisted of a dedicated space equipped with three acute beds, heart rate, blood pressure and blood oxygen saturation monitoring equipment, and a portable oxygen concentrator. Patient outcomes 1 year after establishment of the unit were better than patient outcomes 1 year before the unit was established; introduction of the stroke unit was associated with lower mortality (7.2 versus 22.3%; P < 0.0001), fewer medical complications (4.1 versus 27.7%; P < 0.001) and a lower rate of pneumonia (3.3 versus 14.5%; P < 0.001)268.
Similarly, the introduction of multidisciplinary stroke care in a South African secondary-level hospital was associated with a substantial reduction in in-hospital mortality and improved uptake of neuroimaging271. Neuroimaging services (CT or MRI) are essential for the accurate diagnosis of stroke but the availability, accessibility and affordability of these services are limited in many African settings88,259,260. The SIREN Study Team developed a novel computer software application — the AIM on ClearCanvas Enriched Stroke phenotyping Software (ACCESS) — that enables the creation of simple, standardized annotations for the reporting of brain images of all stroke types. The software enabled concordant and reproducible classification of stroke subtypes by multiple investigators, making it suitable for routine clinical use and multicentre research272.
The availability of and access to acute reperfusion therapies in Africa are currently limited but growing. These therapies are now available in Morocco, Tunisia, Algeria, Egypt, Central African Republic and South Africa88,269,273–276. Barriers to access include all causes of late hospital presentation, diagnostic delays, the high cost of thrombolytic agents and insufficient numbers of clinicians with the appropriate skills88,275,277,278. Early reports from the countries listed above have consistently shown that intravenous thrombolysis is associated with improved patient outcomes269,273,274,276. Door-to-needle times were between 54 and 160 minutes and ≤5% of patients developed spontaneous ICH complications269,273,276. Two prospective observational studies conducted at the Ain Shams University hospitals in Egypt reported remarkable success in overcoming barriers to thrombolysis. Implementation of a comprehensive action plan — including government funding of alteplase (tPA) treatment and the introduction of needs-based training programmes for stroke care nurses, doctors and emergency physicians — was associated with an increase in the proportion of patients eligible for intravenous thrombolysis from 13.2% to 94.3%276.
Strong leadership, improved governmental funding, enhanced stroke awareness, training and partnerships have been reported as factors that have remarkably transformed acute stroke care in the northern African and Middle East region269,279. Over a 5-year period, the region recorded a dramatic increase in the number of stroke units, multidisciplinary stroke specialists and centres with capacity for acute reperfusion therapy279. Widespread uptake of the Safe Implementation of Treatments in Stroke (SITS) registry and partnership with the Angels Initiative of the European Stroke Organization has resulted in a remarkable transformation of the acute stroke care landscape in North Africa269. The lessons from this impressive success story can be deployed and adapted across other African regions.
Stroke rehabilitation services
Stroke rehabilitation is an important component of stroke care. However, the layout, referral pathways, extent of integration into stroke units or stroke multidisciplinary teams, and the time spent on rehabilitation varies widely across Africa. Ideally, stroke rehabilitation should start as soon as possible after a stroke, preferably within a stroke unit, with the aim of achieving early mobilization in order to reduce complications. However, studies of rehabilitation in African stroke units are scarce. Furthermore, the numbers of stroke rehabilitation professionals, including physiotherapists, occupational therapists, and speech and language therapists, seem to be insufficient280. In 2017, the WHO launched ‘Rehabilitation 2030: a call for action’281, which highlights the need for all countries to scale up rehabilitation services and support the use of health information systems to improve patient follow-up for rehabilitation both within hospitals and in the community.
Daily experience from our stroke care practice in Africa suggests that rehabilitation commonly begins soon after admission and is usually supervised by physiotherapists, who are supported by occupational therapists, speech therapists and social workers. Stroke rehabilitation in Africa often occurs in the general medical setting and is provided by professionals who also see patients with other conditions. A review published in 2020 identified poor physician knowledge of the role of rehabilitation, lack of rehabilitation components in the standard of care, long interval from stroke onset to rehabilitation, short duration of rehabilitation and poor financial support of rehabilitation services as inherent problems in the rehabilitation landscape in Africa280. Given these challenges, the responsibility of post-discharge rehabilitative care of patients with stroke falls on family members and caregivers. Task shifting and tele-health approaches have been suggested as strategies to mitigate the current scarcity of rehabilitation personnel and improve access to stroke rehabilitation in Africa281–283. A study of family-led rehabilitation after stroke (the ATTEND trial) was performed in India and this option could also be explored in Africa277,284. In a prospective, single-arm, pre–post study, 20 survivors of stroke recruited from a tertiary medical centre in Ghana received a smartphone with the 9zest Stroke Rehabilitation Therapy app (9zest) to deliver an individualized, goal-targeted exercise programme 5 days a week. Rehabilitation was remotely supervised by a tele-therapist for 12 weeks. The study demonstrated the feasibility of administering a mobile health-delivered physical therapy intervention in sub-Saharan Africa, with high user satisfaction285. Ongoing studies include the MAMBO (Measuring Ambulation, Motor, and Behavioural Outcomes with post-stroke fluoxetine in Tanzania), which aims to determine the safety and efficacy of fluoxetine among Africans with acute stroke and to establish whether the treatment is associated with any improvement in motor outcomes286.
Stroke support and community outreach
Stroke support and community outreach can be achieved through direct and indirect services. In Africa, direct stroke support and community outreach are primarily offered by non-governmental organizations (NGOs) or agencies. National departments of health are yet to invest sufficiently in the detection, treatment and care of non-communicable diseases (including strokes). NGOs face funding challenges that affect their capacity to support patients with stroke and their families. Although stroke support services and community outreach seem to have attained some visibility across Africa as a whole within the past decade, in large parts of the continent, these services do not meet the criteria for universal health coverage. The type of stroke support available varies across Africa. Direct services can include home-based care, patient-focused groups and some form of rehabilitation. Indirect services can include telephone support, including emotional support, for the patient with stroke and concerned family members. In addition, many NGOs conduct community-based health-risk assessments to identify individuals with a level of cumulative risk that qualifies them for referral to medical facilities. Hypertension, in particular, is the focus of many stroke-prevention community outreach programmes108.
Stroke knowledge and perception
A number of quantitative surveys and qualitative studies have explored stroke knowledge and perception among diverse groups of people in Africa and have revealed largely suboptimal awareness and perceptions that are influenced by religious and cultural beliefs287–289. Therefore, community leaders and leaders of faith-based organizations have a vital role in disseminating stroke knowledge across Africa. The percentage of participants who did not recognize the brain as the organ in which stroke occurs varied between 16.9% in a sample of hospital workers and 85.9% in another sample of urban dwellers287,289,290. Hypertension and stress were most commonly identified as stroke risk factors and educational attainment was the most common determinant of stroke knowledge287,291. Among non-neurologist health workers292 and adolescent students293, stroke-based educational interventions were associated with substantial improvement in stroke literacy. We have also previously shown the feasibility of an educational mobile phone short messaging service (SMS) including information on stroke for the control of blood pressure among West African survivors of stroke294,295. Across multiple sites in Nigeria and Ghana, treatment choices were found to be influenced by beliefs about stroke causation, and the development of culturally sensitive and acceptable community-based educational interventions was necessary for the reduction of stroke burden291.
Expert-consensus practice guidelines
High-quality, pragmatic guidelines for the management of stroke risk factors and treatment of stroke in LMICs, including Africa, are lacking. The implementation of contextually appropriate, evidence-based, expert-recommended stroke prevention guidelines is particularly important in LMICs. A systematic review of 22 published stroke prevention guidelines found 8 from LMICs (36%) and 14 from high-income countries (64%) but only 1 from Africa (South Africa)296. LMIC-issued guidelines were less likely to provide clear recommendations (62% versus 100%; P = 0.03), utilize high-quality systematic reviews (21% versus 79%; P = 0.006), engage in good dissemination avenues (12% versus 71%; P = 0.02) or invite input from an external reviewer (12% versus 57%; P = 0.07)296. Thus, the quality and quantity of expert consensus stroke management guidelines in Africa clearly need to improve.
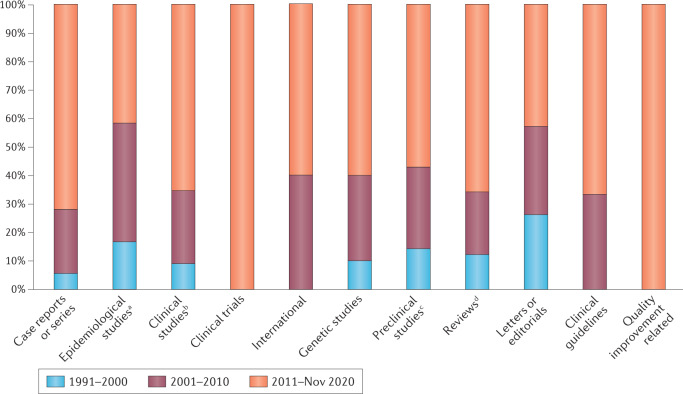
Clinical trials
Increasing numbers of stroke-related clinical trials are being performed in Africa295,297–301 (Fig. 4). The dominant effect of hypertension among African survivors of stroke means that evidence-based interventions to control this risk factor are an important area of research. The first randomized controlled trials of hypertension control among survivors of stroke in Africa involved multi-modal interventions that aimed to address barriers to hypertension control at the level of the patient, provider and practice. For example, the Phone-based Interventions under Nurse Guidance after Stroke (PINGS) study in Ghana assessed the feasibility and preliminary efficacy of a domiciliary blood pressure-monitoring intervention that was mobile health technology-based and nurse led295,302. A cohort of 60 recent survivors of stroke took part in the trial, which had a cluster randomized controlled design; 30 participants received the intervention and 30 participants received usual care using a cluster randomized controlled trial design. The intervention lasted for 3 months and participants were followed-up for a further 3 months. The results indicated that the intervention is feasible and identified a possible efficacy signal in terms of blood pressure control. Specifically, an intention-to-treat analysis found that, at month 3, 67% of participants in the intervention group had systolic blood pressure of <140 mmHg compared with just 47% of participants in the control group (P = 0.12). At month 9, 73% of participants in the intervention group had systolic blood pressure of <140 mmHg compared with 43% of the participants in the control group (P = 0.035). Furthermore, medication possession ratio scores (measure of medication adherence) at month 3 and month 9 were higher in the intervention group than in the control group.
Fig. 4. Studies on stroke in Africa.
Here, we summarize the published literature on stroke in Africa, from 1999 to November 2020, and including 107 case reports or series, 29 epidemiological studies, 562 clinical studies, 5 clinical trials, 4 international studies, 30 genetic studies, 21 preclinical studies, 136 reviews, 52 letters or editorials, 6 clinical guidelines and 4 quality improvement-related publications. aIncludes community-based prevalence and incidence studies on stroke in Africa. bIncludes hospital-based studies, whether of observational or interventional, retrospective or prospective, longitudinal or case–control designs. cRefers to studies that report the testing of a drug, procedure or other medical treatment in animals, where the disease of interest in the study was a stroke. dIncludes narrative reviews, scoping reviews, systematic reviews and meta-analyses.
The Tailored Hospital-based Risk reduction to Impede Vascular Events after Stroke (THRIVES) study in Nigeria was much larger than the PINGS study and randomized 400 survivors of stroke to two treatment groups. One group received usual care and the other received a bundled intervention that included patient report cards, phone text messaging, an educational video and co-ordination of post-hospitalization care303. After 12 months of administration, systolic blood pressure in the group of participants receiving the THRIVES was not significantly lower than in the group of participants receiving usual care. However, in a sub-group of participants with uncontrolled blood pressure, both usual care and the THRIVES intervention were associated with a reduction in blood pressure, in both arms, compared with the pre-treatment baseline294. An ongoing secondary prevention trial — the Stroke Minimization through Additive Anti-atherosclerotic Agents in Routine Treatment (SMAART) trial — is a phase II, open-label, evaluator-blinded trial involving 120 participants from Ghana who have recently survived a stroke. Participants were randomly allocated in a 1:1 ratio to either receive the intervention or usual care. Participants in the intervention arm receive a polypill (Polycap DS, Cadila Pharmaceuticals) that contains 100 mg aspirin, 50 mg atenolol, 5 mg ramipril, 12.5 mg thiazide and 20 mg simvastatin per capsule and taken as two capsules once daily. Participants in the usual care group will receive separate, individual secondary preventive medications prescribed at the physician’s discretion. Both groups will be followed for 12 months to assess changes in carotid intima media thickness regression300,301 and results are expected in late 2021. PINGS-2 is a randomized controlled trial of a theoretical model-based, mobile health technology-centred, nurse-led, multi-level integrated approach to improve longer-term blood pressure control. The trial includes 500 patients who have recently had a stroke recruited from 10 hospitals in Ghana and is currently ongoing304.
Evidence for an association between prevalence of tobacco smoking and prevalence and incidence of stroke in Africa is lacking. However, overall epidemiological trends of cardiovascular disease can be used as a proxy for the burden of stroke. For example, in South Africa, despite the use of tobacco taxation measures to discourage smoking, cardiovascular disease is still one of the highest sources of non-communicable disease burden. Although selected countries in Africa are using such fiscal measures to reduce tobacco use, large-scale multi-pronged efforts that include behavioural interventions to reduce the burden of tobacco smoking are lacking. The reality in Africa is that there are well-known inequalities and inefficiencies, both among and within countries, that make it difficult to follow through on the WHO’s framework for tobacco control305.
Biobanking and precision stroke medicine
The stroke research landscape in Africa has witnessed progress and diversification in the past decade. The increasing burden and poor outcomes of stroke on the African continent require an aggressive research agenda to identify, characterize and confront risk factors and to implement evidence-based strategies. Indeed, basic, clinical, translational and health systems research are required to reduce the burden of stroke. In particular, precision medicine-focused trans-omics stroke research approaches are gaining increasing attention while not abandoning the traditional public health-driven approaches25,306,307.
The past decade heralded an explosion of genomic research and biobanking in Africa, largely driven by funds provided by the Wellcome Trust and the NIH. The NIH-funded SIREN study is exploring the genetic architecture of stroke among Indigenous Africans. More than 4,000 case–control pairs have already been recruited to the study and several publications on stroke phenomics and preliminary candidate gene analyses have been generated. The SIREN study has also undertaken the first-ever GWAS to unravel the genetic architecture of stroke in Indigenous Africans and the results are eagerly awaited. Stroke neurobanking resources consisting of blood fractions, extracted DNA, neuroimages and databases of clinical information are also being built in Africa and could facilitate data science-driven trans-omics research (including epigenomics, transcriptomics, proteomics and metabolomics) as well as the development of precision medicine products such as Afrocentric risk calculators, polygenic risk scores, biomarkers and drug targets23–25,227,307,308. The SIREN neurobiobank comprises a group of constantly monitored ultra-low-temperature (–86 °C) freezers located in Ibadan, Nigeria, constantly powered –20 °C chest freezers located in Ibadan and other recruitment sites, barcode scanners and printers, a laboratory information management system, a secure multi-terabyte server, user-friendly software for archiving neuroimages, and solar-powered inverter systems to provide uninterrupted electric power back-up272,309. The neurobiobank contains >3,800 brain images and 160,000 blood samples including serum, plasma, red cell concentrates and extracted DNA. The ethical, legal and societal implications of stroke neurobiobanking and genomics in the complex African sociocultural landscape are also being explored310.
Future directions
Stroke care in Africa faces numerous challenges related to epidemiological surveillance, health promotion and disease prevention, acute care and rehabilitation: the four domains of the stroke quadrangle311 (Fig. 5). However, various pragmatic solutions to these challenges exist (Table 6). First, epidemiological surveillance can be improved by the establishment of a framework for regular monitoring and evaluation of stroke (including burden and risk factors) and health services at the national level. This framework could involve a combination of community-based surveys and surveillance systems. Second, the implementation of integrated population-wide and individual prevention strategies could reduce exposure to and improve control of modifiable risk factors. Third, the effective planning of acute stroke care services, including workforce training and capacity-building, can improve acute stroke care. Last, the promotion of access to interdisciplinary care, including task sharing approaches, can improve post-stroke rehabilitation services. To coordinate these activities, the newly launched African Stroke Control Observatory Risk reduction Ecosystem (A-SCORE), which will be a major part of the global SCORE (G-SCORE) under the auspices of the WSO–WHO–Lancet Neurology Commission on Stroke, should be enhanced253. A-SCORE involves policy-makers, patients, payers, providers, the populace and implementation partners. Through surveillance and research, stroke in Africa will become quantifiable and understandable; through prevention, stroke will become avoidable; through provision of acute care, stroke will become treatable; and through rehabilitation, stroke will be surmountable.
Fig. 5. The stroke quadrangle.
The four pillars of the stroke quadrangle are surveillance, prevention, acute care and rehabilitation. Together, these pillars can lead to the reduction of stroke incidence, prevalence, disability and mortality. DALYs, disability-adjusted life years.
Table 6.
Stroke care in Africa: challenges and solutions
| Aspect of stroke care | Challenges | Pragmatic solutions |
|---|---|---|
| Surveillance | Valid, reliable data on stroke incidence, prevalence, mortality and disability in Africa are extremely limited; no surveillance system is in place to track trends in the burden of stroke at continental, regional and country levels | Establish stroke surveillance systems to measure and monitor the burden of stroke |
| Prevention | No robust systems for detection and control of major stroke risk factors such as hypertension, diabetes mellitus and dyslipidaemia; high rates (93%) of uncontrolled hypertension | Increase awareness, screening and control of hypertension, dyslipidaemia, diabetes mellitus and other major stroke risk factors at the primary health-care level in synergy with programmes for NCDs; implement sensitization programme to involve the entire population across the lifespan |
| Acute care | Scarcity of high-quality hyperacute and acute care services; very low rates of thrombolysis and thrombectomy; few multidisciplinary stroke units | Synergistic action by all stakeholders, including pharmaceutical companies and stroke experts, to improve the availability of services and increase the number of stroke units |
| Rehabilitation | Few multidisciplinary stroke rehabilitation centres | Increase the number of centres and settings that offer multidisciplinary care; promote recovery and re-integration |
NCDs, non-communicable diseases.
Novel stroke leadership initiatives are springing up in Africa and hold great promise for tackling the growing burden of stroke on the continent. For example, the African Stroke Organization is a new pan-African, multidisciplinary coalition of stroke professionals, stroke support organizations and national stroke societies with the vision “to reduce the burden of stroke in Africa”312 (Fig. 6). The organization aims to achieve this vision through multidisciplinary research and capacity-building, promoting the development of effective stroke prevention and intervention services, enhancing stroke awareness, advocating for survivors of stroke, their families and caregivers, and driving the formulation of stroke-friendly policies312. Multiple non-governmental initiatives working as stroke support organizations are springing up across the continent. These organizations are working with survivors of stroke and their caregivers to enhance rehabilitation and life after stroke as well as engaging policy-makers and synergizing with extant stroke prevention initiatives313. Furthermore, the African Stroke Organization is launching an annual pan-African scientific conference on stroke to regularly examine gaps in evidence and practice, accelerate translational research, guide priorities for stroke care practice and policy, build future stroke leaders, and strengthen regional collaboration and research output. The first African Stroke Organization Conference is expected to be held in autumn 2021 (refs314,315).
Fig. 6. Conceptual framework of the African Stroke Organization.
The aim of the African Stroke Organization (ASO) is to reduce the burden of stroke in Africa. This figure illustrates the framework through which the ASO plans to meet this goal. The colourful network represents the rich genetic, cultural and geographical diversity in Africa, and the neuronal network of the brain. The interconnected individuals represent the African Ubuntu Philosophy of inclusiveness, cooperation and collaboration. The pale green layer depicts the four core pillars of ASO activities: research; capacity-building programmes; development of stroke services; promotion of stroke awareness, and advocacy and empowerment of survivors of stroke, their families and their caregivers. The dark green layer represents the broader core values of the ASO: working with partners to involve people and positively influence practice and policy. Adapted with permission from ref.312, SAGE.
Implementation science is a distinct research priority for stroke in Africa312. Existing evidence for the primordial, primary and secondary prevention of stroke needs to be contextualized to target Africa. Barriers to and facilitators of vascular risk factor control, both traditional and novel, need to be unmasked. Accurate, rigorous and detailed epidemiological studies to track the trends of incidence, prevalence, case fatality and mortality, and DALYs will be required to inform detailed and pragmatic planning of population-based strategies to control the escalating stroke burden in Africa. The ongoing African Rigorous Innovative Stroke Epidemiological Surveillance (ARISES) study was designed to address the methodological limitations of previous epidemiological studies26. This new study is also using technological innovations, including geographical information systems and mobile health-driven stroke information systems, to gather accurate data on incident strokes in the demographic sites where the study is being conducted. The mobile health-driven stroke information system will be deployed to improve local stroke literacy and enhance near-total case detection. Results are expected in 2025.
Translational interdisciplinary science, including trans-omics technology and data science, will be needed to gain deeper insight into novel stroke-associated genetic loci and pathophysiological pathways. These approaches will also enable the discovery of new, population-specific risk calculators, biomarkers and drug targets with the aim of achieving personalized, or precision, stroke treatment24,227. New treatments and novel preventive approaches are needed to inform clinical trials and the development of multicentre and multinational networks to tackle the increasing stroke burden.
The WSO–WHO–Lancet Neurology Commission on Stroke253 conducted a survey of stroke services in five African nations to assess needs and offer pragmatic solutions; however, this effort needs to be scaled up to cover other African countries316. Public education is needed to fill the unique gaps in stroke knowledge present in different African populations and to address the influence of cultural and religious beliefs and practices relating to stroke289,291. To fill the critical gaps in the availability of stroke professionals in Africa, focused training in stroke medicine needs to be incorporated into the curricula of postgraduate medical, nursing and allied professional education. Short courses and conference teachings should also be provided. Online resources that are sensitive to the unique epidemiological, cultural and socioeconomic environment in the continent will be very useful for filling knowledge gaps. Large multinational initiatives, such as the Angels Initiative and SITS Registry, have scaled up acute stroke services in Europe, the Middle East and North Africa269. We suggest that organizations within Africa develop partnerships with such initiatives to improve stroke services in Africa. African governments and regional organizations need to formulate policies that advance stroke science and care in Africa in tandem with their expressed commitments to actualize Sustainable Development Goal 3.4, which aims to ensure healthy lives and promote well-being and reduce premature death from non-communicable diseases, including stroke, by one-third by the year 2030.
Conclusions
Alarmingly, as the current epidemiological evidence shows, the scourge of stroke is on the increase in Africa. Modifiable risk factors such as hypertension are clearly key primary targets for treatment and prevention. As is evident from the experience of higher-income countries, reducing the burden of stroke incidence in Africa will undoubtedly reduce mortality, morbidity, disability and the neurological as well as cognitive sequelae associated with stroke. Fortunately, welcome progress has been made in stroke medicine, awareness, prioritization, care, education, research and policy-making. If such progress continues, the anticipated future toll of stroke on the continent and its over 1 billion people could be substantially mitigated. However, successfully building on current efforts is by no means guaranteed. Patients, providers, payers, policy-makers and the public, in concert with scientists and funders, will need to maintain prospective vigilance of the continental stroke burden, apply vigour to unravelling the unique determinants of stroke in the region, and prioritize the development of contextual preventive and therapeutic solutions to avert and minimize the burden of stroke316.
Acknowledgements
R.O.A. is supported by the UK Royal Society/African Academy of Sciences FLAIR Grants FLR/R1/191813 and FCG/R1/201034, and a GCRF Networking Grant from the UK Academy of Medical Sciences. R.O.A., M.O.O., B.O. and F.S.S. are also supported by grants U54HG007479 and U01HG010273 from the US National Institutes of Health (NIH) as part of the H3Africa Consortium. M.O.O., B.O., R.O.A. and F.S.S. are further supported by NIH grant R01NS107900. R.N.K.’s research on elderly survivors of stroke has been supported by the Medical Research Council, RCUK Newcastle Centre for Brain Ageing and Vitality (MRC G0500247), Alzheimer’s Research UK, the Dunhill Medical Trust, UK, and the Newcastle National Institute for Health Research Biomedical Research Centre in Ageing and Age-Related Diseases, Newcastle upon Tyne Hospitals National Health Service Foundation Trust.
Author contributions
R.O.A., B.O., O.A.A., F.S.S., R.N.K. and M.O.O. researched data for the article, made a substantial contribution to discussion of content, wrote the article, and reviewed and edited the manuscript before submission. F.A.-A., T.A., O.S.O., A.D., R.W.W. and A.O. made a substantial contribution to discussion of content and reviewed and edited the manuscript before submission. P.N. made a substantial contribution to discussion of content, wrote the article, and reviewed and edited the manuscript before submission.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Neurology thanks A. Rhoda, C. Wolfe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rajesh N. Kalaria, Mayowa O. Owolabi.
References
- 1.GBD Lifetime Risk of Stroke Collaborators et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N. Engl. J. Med. 2018;379:2429–2437. doi: 10.1056/NEJMoa1804492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD Stroke Collaborators Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J, et al. Global stroke statistics 2019. Int. J. Stroke. 2020;15:819–838. doi: 10.1177/1747493020909545. [DOI] [PubMed] [Google Scholar]
- 4.Owolabi MO, et al. The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc. J. Afr. 2015;26(Suppl. 1):S27–S38. doi: 10.5830/CVJA-2015-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mufunda J, et al. Emerging non-communicable disease epidemic in Africa: preventive measures from the WHO Regional Office for Africa. Ethn. Dis. 2006;16:521–526. [PubMed] [Google Scholar]
- 6.Bain LE, Kum AP, Ekukwe NC, Clovis NC, Enowbeyang TE. HIV, cardiovascular disease, and stroke in sub-Saharan Africa. Lancet HIV. 2016;3:e341–e342. doi: 10.1016/S2352-3018(16)30092-3. [DOI] [PubMed] [Google Scholar]
- 7.Kagaruki GB, et al. Magnitude and risk factors of non-communicable diseases among people living with HIV in Tanzania: a cross sectional study from Mbeya and Dar es Salaam regions. BMC Public Health. 2014;14:904. doi: 10.1186/1471-2458-14-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciccacci F, et al. Noncommunicable diseases burden and risk factors in a cohort of HIV+ elderly patients in Malawi. AIDS Res. Hum. Retroviruses. 2019;35:1106–1111. doi: 10.1089/aid.2019.0125. [DOI] [PubMed] [Google Scholar]
- 9.Osuntokun BO, Odeku EL, Adeloye RB. Cerebrovascular accidents in Nigerians: a study of 348 patients. West Afr. Med. J. Niger. Pract. 1969;18:160–173. [PubMed] [Google Scholar]
- 10.Osuntokun BO. Stroke in the Africans. Afr. J. Med. Med Sci. 1977;6:39–53. [PubMed] [Google Scholar]
- 11.GBD Neurology Collaborators Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akinyemi RO, et al. Stroke, cerebrovascular diseases and vascular cognitive impairment in Africa. Brain Res. Bull. 2019;145:97–108. doi: 10.1016/j.brainresbull.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker R, et al. Stroke incidence in rural and urban Tanzania: a prospective, community-based study. Lancet Neurol. 2010;9:786–792. doi: 10.1016/S1474-4422(10)70144-7. [DOI] [PubMed] [Google Scholar]
- 14.Ezejimofor MC, et al. Stroke survivors in Nigeria: a door-to-door prevalence survey from the Niger Delta region. J. Neurol. Sci. 2017;372:262–269. doi: 10.1016/j.jns.2016.11.059. [DOI] [PubMed] [Google Scholar]
- 15.Barker DJ. The intrauterine origins of cardiovascular disease. Acta Paediatr. Suppl. 1993;82(Suppl. 391):93–99. doi: 10.1111/j.1651-2227.1993.tb12938.x. [DOI] [PubMed] [Google Scholar]
- 16.Hult M, et al. Hypertension, diabetes and overweight: looming legacies of the Biafran famine. PLoS ONE. 2010;5:e13582. doi: 10.1371/journal.pone.0013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker DJ. The intrauterine environment and adult cardiovascular disease. Ciba Found. Symp. 1991;156:3–10. doi: 10.1002/9780470514047.ch2. [DOI] [PubMed] [Google Scholar]
- 18.Wichmann J, Voyi K. Ambient air pollution exposure and respiratory, cardiovascular and cerebrovascular mortality in Cape Town, South Africa: 2001-2006. Int. J. Env. Res. Public Health. 2012;9:3978–4016. doi: 10.3390/ijerph9113978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azarpazhooh MR, Hachinski V. Air pollution: a silent common killer for stroke and dementia. Int. J. Stroke. 2018;13:667–668. doi: 10.1177/1747493018784476. [DOI] [PubMed] [Google Scholar]
- 20.Keates AK, Mocumbi AO, Ntsekhe M, Sliwa K, Stewart S. Cardiovascular disease in Africa: epidemiological profile and challenges. Nat. Rev. Cardiol. 2017;14:273–293. doi: 10.1038/nrcardio.2017.19. [DOI] [PubMed] [Google Scholar]
- 21.Kalaria RN, Akinyemi R, Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim. Biophys. Acta. 2016;1862:915–925. doi: 10.1016/j.bbadis.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hachinski V, et al. Implementing the proclamation of stroke and potentially preventable dementias. Int. J. Stroke. 2018;13:780–786. doi: 10.1177/1747493018799965. [DOI] [PubMed] [Google Scholar]
- 23.Akinyemi RO, et al. Stroke genomics in people of African ancestry: charting new paths. Cardiovasc. J. Afr. 2015;26(Suppl. 1):S39–S49. doi: 10.5830/CVJA-2015-039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owolabi M, et al. Advancing stroke genomic research in the age of trans-omics big data science: emerging priorities and opportunities. J. Neurol. Sci. 2017;382:18–28. doi: 10.1016/j.jns.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akinyemi RO, et al. Neurogenomics in Africa: perspectives, progress, possibilities and priorities. J. Neurol. Sci. 2016;366:213–223. doi: 10.1016/j.jns.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owolabi M, et al. The epidemiology of stroke in Africa: a systematic review of existing methods and new approaches. J. Clin. Hypertens. 2018;20:47–55. doi: 10.1111/jch.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feigin V, Hoorn SV. How to study stroke incidence. Lancet. 2004;363:1920. doi: 10.1016/S0140-6736(04)16436-2. [DOI] [PubMed] [Google Scholar]
- 28.El-Tallawy HN, et al. Epidemiology of non-fatal cerebrovascular stroke and transient ischemic attacks in Al Quseir, Egypt. Clin. Interv. Aging. 2013;8:1547–1551. doi: 10.2147/CIA.S48785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matenga J. Stroke incidence rates among black residents of Harare — a prospective community-based study. S. Afr. Med. J. 1997;87:606–609. [PubMed] [Google Scholar]
- 30.Ashok PP, Radhakrishnan K, Sridharan R, El-Mangoush MA. Incidence and pattern of cerebrovascular diseases in Benghazi, Libya. J. Neurol. Neurosurg. Psychiatry. 1986;49:519–523. doi: 10.1136/jnnp.49.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Zunni S, Ahmed M, Prakash PS, Hassan KM. Stroke: incidence and pattern in Benghazi, Libya. Ann. Saudi Med. 1995;15:367–369. doi: 10.5144/0256-4947.1995.367. [DOI] [PubMed] [Google Scholar]
- 32.Rosman KD. The epidemiology of stroke in an urban black population. Stroke. 1986;17:667–669. doi: 10.1161/01.STR.17.4.667. [DOI] [PubMed] [Google Scholar]
- 33.Damasceno A, et al. An epidemiological study of stroke hospitalizations in Maputo, Mozambique: a high burden of disease in a resource-poor country. Stroke. 2010;41:2463–2469. doi: 10.1161/STROKEAHA.110.594275. [DOI] [PubMed] [Google Scholar]
- 34.Adeloye D. An estimate of the incidence and prevalence of stroke in Africa: a systematic review and meta-analysis. PLoS ONE. 2014;9:e100724. doi: 10.1371/journal.pone.0100724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adeloye D, et al. Estimating morbidity due to stroke in Nigeria: a systematic review and meta-analysis. J. Neurol. Sci. 2019;402:136–144. doi: 10.1016/j.jns.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osuntokun BO, Bademosi O, Akinkugbe OO, Oyediran AB, Carlisle R. Incidence of stroke in an African city: results from the Stroke Registry at Ibadan, Nigeria, 1973-1975. Stroke. 1979;10:205–207. doi: 10.1161/01.STR.10.2.205. [DOI] [PubMed] [Google Scholar]
- 37.Danesi MA, Okubadejo NU, Ojini FI, Ojo OO. Incidence and 30-day case fatality rate of first-ever stroke in urban Nigeria: the prospective community based Epidemiology of Stroke in Lagos (EPISIL) phase II results. J. Neurol. Sci. 2013;331:43–47. doi: 10.1016/j.jns.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 38.Okon M, et al. Stroke incidence and case fatality rate in an urban population. J. Stroke Cerebrovasc. Dis. 2015;24:771–777. doi: 10.1016/j.jstrokecerebrovasdis.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Kandil MR, El-Tallawy HN, Farawez HM, Khalifa G, Ahmed M. Epidemiology of cerebrovascular stroke and TIA in upper Egypt (Sohag) — relative frequency of stroke in Assiut University Hospital. Egypt. J. Neurol. Psychiat. Neurosurg. 2006;43:593–602. [Google Scholar]
- 40.Farghaly WM, et al. Epidemiology of nonfatal stroke and transient ischemic attack in Al-Kharga District, New Valley, Egypt. Neuropsychiatr. Dis. Treat. 2013;9:1785–1790. doi: 10.2147/NDT.S48322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanchard RD, Bunker JB, Wachs M. Distinguishing aging, period and cohort effects in longitudinal studies of elderly populations. Socioecon. Plann. Sci. 1977;3:137–146. doi: 10.1016/0038-0121(77)90032-5. [DOI] [Google Scholar]
- 42.Osuntokun BO, et al. Neurological disorders in Nigerian Africans: a community-based study. Acta Neurol. Scand. 1987;75:13–21. doi: 10.1111/j.1600-0404.1987.tb07883.x. [DOI] [PubMed] [Google Scholar]
- 43.Walker RW, et al. Age specific prevalence of impairment and disability relating to hemiplegic stroke in the Hai District of northern Tanzania. Adult Morbidity and Mortality Project. J. Neurol. Neurosurg. Psychiatry. 2000;68:744–749. doi: 10.1136/jnnp.68.6.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tekle-Haimanot R, et al. Community-based study of neurological disorders in rural central Ethiopia. Neuroepidemiology. 1990;9:263–277. doi: 10.1159/000110783. [DOI] [PubMed] [Google Scholar]
- 45.Khedr EM, et al. Epidemiological study and risk factors of stroke in Assiut Governorate, Egypt: community-based study. Neuroepidemiology. 2013;40:288–294. doi: 10.1159/000346270. [DOI] [PubMed] [Google Scholar]
- 46.Attia Romdhane N, et al. Prevalence study of neurologic disorders in Kelibia (Tunisia) Neuroepidemiology. 1993;12:285–299. doi: 10.1159/000110330. [DOI] [PubMed] [Google Scholar]
- 47.Connor MD, et al. Prevalence of stroke survivors in rural South Africa: results from the Southern Africa Stroke Prevention Initiative (SASPI) Agincourt field site. Stroke. 2004;35:627–632. doi: 10.1161/01.STR.0000117096.61838.C7. [DOI] [PubMed] [Google Scholar]
- 48.Danesi M, Okubadejo N, Ojini F. Prevalence of stroke in an urban, mixed-income community in Lagos, Nigeria. Neuroepidemiology. 2007;28:216–223. doi: 10.1159/000108114. [DOI] [PubMed] [Google Scholar]
- 49.Onwuchekwa AC, Tobin-West C, Babatunde S. Prevalence and risk factors for stroke in an adult population in a rural community in the Niger Delta, south-south Nigeria. J. Stroke Cerebrovasc. Dis. 2014;23:505–510. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Cossi MJ, et al. Stroke: prevalence and disability in Cotonou, Benin. Cerebrovasc. Dis. 2012;33:166–172. doi: 10.1159/000334195. [DOI] [PubMed] [Google Scholar]
- 51.Engels T, et al. Socioeconomic status and stroke prevalence in Morocco: results from the Rabat-Casablanca study. PLoS ONE. 2014;9:e89271. doi: 10.1371/journal.pone.0089271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanya EO, et al. Prevalence of stroke in three semi-urban communities in middle-belt region of Nigeria: a door to door survey. Pan Afr. Med. J. 2015;20:33. doi: 10.11604/pamj.2015.20.33.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dewhurst F, et al. The prevalence of neurological disorders in older people in Tanzania. Acta Neurol. Scand. 2013;127:198–207. doi: 10.1111/j.1600-0404.2012.01709.x. [DOI] [PubMed] [Google Scholar]
- 54.Enwereji KO, et al. Epidemiology of stroke in a rural community in southeastern Nigeria. Vasc. Health Risk Manag. 2014;10:375–388. doi: 10.2147/VHRM.S57623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khedr EM, et al. Prevalence of ischemic and hemorrhagic strokes in Qena Governorate, Egypt: community-based study. J. Stroke Cerebrovasc. Dis. 2014;23:1843–1848. doi: 10.1016/j.jstrokecerebrovasdis.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Otubogun FM, Akinyemi R, Ogunniyi S. Burden of adult neurological diseases in Odeda Area, Southwest Nigeria. BMJ Neurol. Open. 2020;2:e000062. doi: 10.1136/bmjno-2020-000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adoukonou T, et al. Prevalence of stroke survivors in Parakou in northern Benin: a door-to-door community survey. Rev. Neurol. 2020;176:839–845. doi: 10.1016/j.neurol.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Avan A, et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019;17:191. doi: 10.1186/s12916-019-1397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eze CO, Kalu UA. Pattern of neurological admissions in the tropics: experience at Abakaliki south-eastern Nigeria. Niger. J. Med. 2014;23:302–305. [PubMed] [Google Scholar]
- 60.Ekenze O, Onwuekwe I, Ezeala B. Profile of neurological admissions at the University of Nigeria Teaching Hospital Enugu. Niger. J. Med. 2010;19:419–422. doi: 10.4314/njm.v19i4.61967. [DOI] [PubMed] [Google Scholar]
- 61.Kayode-Iyasere EO, Obasohan AO, Odiase FE. Medical coma in a secondary health centre in Benin City, Nigeria: a 3-year review. Port. Harcourt Med. J. 2019;13:58–62. [Google Scholar]
- 62.Obiako O, Oparah S, Ogunniyi A. Causes of medical coma in adult patients at the University College Hospital, Ibadan Nigeria. Niger. Postgrad. Med. J. 2011;18:1–7. [PubMed] [Google Scholar]
- 63.Ojini F, Danesi M. The pattern of neurological admissions at the Lagos University Teaching Hospital. Niger. J. Clin. Pract. 2003;6:38–41. [Google Scholar]
- 64.Siddiqi OK, Atadzhanov M, Birbeck GL, Koralnik IJ. The spectrum of neurological disorders in a Zambian tertiary care hospital. J. Neurol. Sci. 2010;290:1–5. doi: 10.1016/j.jns.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogun S, Ojini F, Ogungbo B, Kolapo K, Danesi M. Stroke in South West Nigeria: a 10-year review. Stroke. 2005;36:1120–1122. doi: 10.1161/01.STR.0000166182.50840.31. [DOI] [PubMed] [Google Scholar]
- 66.Talabi O. A 3-year review of neurologic admissions in University College Hospital Ibadan, Nigeria. West Afr. J. Med. 2003;22:150–151. doi: 10.4314/wajm.v22i2.27937. [DOI] [PubMed] [Google Scholar]
- 67.Akinyemi RO, et al. Contribution of noncommunicable diseases to medical admissions of elderly adults in Africa: a prospective, cross-sectional study in Nigeria, Sudan, and Tanzania. J. Am. Geriatr. Soc. 2014;62:1460–1466. doi: 10.1111/jgs.12940. [DOI] [PubMed] [Google Scholar]
- 68.Garbusinski JM, et al. Stroke presentation and outcome in developing countries: a prospective study in the Gambia. Stroke. 2005;36:1388–1393. doi: 10.1161/01.STR.0000170717.91591.7d. [DOI] [PubMed] [Google Scholar]
- 69.Owolabi LF, Shehu MY, Shehu MN, Fadare J. Pattern of neurological admissions in the tropics: experience at Kano, northwestern Nigeria. Ann. Indian Acad. Neurol. 2010;13:167–170. doi: 10.4103/0972-2327.70875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Owolabi M, et al. Stroke in indigenous Africans, African Americans, and European Americans: interplay of racial and geographic factors. Stroke. 2017;48:1169–1175. doi: 10.1161/STROKEAHA.116.015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker RW, et al. Stroke mortality in urban and rural Tanzania. Adult Morbidity and Mortality Project. Lancet. 2000;355:1684–1687. doi: 10.1016/S0140-6736(00)02240-6. [DOI] [PubMed] [Google Scholar]
- 72.Walker RW, et al. Post-stroke case fatality within an incident population in rural Tanzania. J. Neurol. Neurosurg. Psychiatry. 2011;82:1001–1005. doi: 10.1136/jnnp.2010.231944. [DOI] [PubMed] [Google Scholar]
- 73.Maredza M, Bertram MY, Tollman SM. Disease burden of stroke in rural South Africa: an estimate of incidence, mortality and disability adjusted life years. BMC Neurol. 2015;15:54. doi: 10.1186/s12883-015-0311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lekoubou A, Nkoke C, Dzudie A, Kengne AP. Stroke admission and case-fatality in an urban medical unit in sub-Saharan Africa: a fourteen year trend study from 1999 to 2012. J. Neurol. Sci. 2015;350:24–32. doi: 10.1016/j.jns.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Adoukonou T, et al. Stroke case fatality in sub-Saharan Africa: systematic review and meta-analysis. Int. J. Stroke. 2021 doi: 10.1177/1747493021990945. [DOI] [PubMed] [Google Scholar]
- 76.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 77.Ojini F, Ogun SA, Danesi MA. Thirty-day case fatality of stroke at the Lagos University Teaching Hospital. Nig. Q. J. Hosp. Med. 2004;14:64–66. [Google Scholar]
- 78.Sagui E, et al. Ischemic and hemorrhagic strokes in Dakar, Senegal: a hospital-based study. Stroke. 2005;36:1844–1847. doi: 10.1161/01.STR.0000177864.08516.47. [DOI] [PubMed] [Google Scholar]
- 79.Longo-Mbenza B, Lelo Tshinkwela M, Mbuilu Pukuta J. Rates and predictors of stroke-associated case fatality in black Central African patients. Cardiovasc. J. Afr. 2008;19:72–76. [PMC free article] [PubMed] [Google Scholar]
- 80.Mudzi W, Stewart A, Musenge E. Case fatality of patients with stroke over a 12-month period post stroke. S. Afr. Med. J. 2012;102:765–767. doi: 10.7196/SAMJ.5742. [DOI] [PubMed] [Google Scholar]
- 81.Nkoke C, Lekoubou A, Balti E, Kengne AP. Stroke mortality and its determinants in a resource-limited setting: a prospective cohort study in Yaounde, Cameroon. J. Neurol. Sci. 2015;358:113–117. doi: 10.1016/j.jns.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 82.Lekoubou A, Nkoke C, Dzudie A, Kengne AP. Recurrent stroke and early mortality in an urban medical unit in Cameroon. J. Stroke Cerebrovasc. Dis. 2017;26:1689–1694. doi: 10.1016/j.jstrokecerebrovasdis.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 83.Walker RW, et al. Case-fatality and disability in the Tanzanian Stroke Incidence Project cohort. Acta Neurol. Scand. 2016;133:49–54. doi: 10.1111/ane.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walker RW, et al. Correlates of short- and long-term case fatality within an incident stroke population in Tanzania. S. Afr. Med. J. 2013;103:107–112. doi: 10.7196/SAMJ.5793. [DOI] [PubMed] [Google Scholar]
- 85.Okeng’o K, Chillo P, Gray WK, Walker RW, Matuja W. Early mortality and associated factors among patients with stroke admitted to a large teaching hospital in Tanzania. J. Stroke Cerebrovasc. Dis. 2017;26:871–878. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 86.Gomes J, et al. Determinants of early case-fatality among stroke patients in Maputo, Mozambique and impact of in-hospital complications. Int. J. Stroke. 2013;8:69–75. doi: 10.1111/j.1747-4949.2012.00957.x. [DOI] [PubMed] [Google Scholar]
- 87.Russell JBW, Charles E, Conteh V, Lisk DR. Risk factors, clinical outcomes and predictors of stroke mortality in Sierra Leoneans: a retrospective hospital cohort study. Ann. Med. Surg. 2020;60:293–300. doi: 10.1016/j.amsu.2020.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akinyemi RO, Adeniji OA. Stroke care services in Africa: a systematic review. J. Stroke Med. 2018;1:55–64. doi: 10.1177/2516608518775233. [DOI] [Google Scholar]
- 89.Bertram MY, Katzenellenbogen J, Vos T, Bradshaw D, Hofman KJ. The disability adjusted life years due to stroke in South Africa in 2008. Int. J. Stroke. 2013;8(Suppl. A100):76–80. doi: 10.1111/j.1747-4949.2012.00955.x. [DOI] [PubMed] [Google Scholar]
- 90.Kaduka L, et al. Disability-adjusted life-years due to stroke in Kenya. Neuroepidemiology. 2019;53:48–54. doi: 10.1159/000498970. [DOI] [PubMed] [Google Scholar]
- 91.Krishnamurthi RV, Ikeda T, Feigin VL. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology. 2020;54:171–179. doi: 10.1159/000506396. [DOI] [PubMed] [Google Scholar]
- 92.Bello UM, et al. Quality of life of stroke survivors in Africa: a systematic review and meta-analysis. Qual. Life Res. 2021;30:1–19. doi: 10.1007/s11136-020-02591-6. [DOI] [PubMed] [Google Scholar]
- 93.Owolabi MO. Impact of stroke on health-related quality of life in diverse cultures: the Berlin-Ibadan multicenter international study. Health Qual. Life Outcomes. 2011;9:81. doi: 10.1186/1477-7525-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Birabi BN, Oke KI, Dienye PO, Okafor UC. Cost burden of post stroke condition in Nigeria: a pilot study. Glob. J. Health Sci. 2012;4:17–22. doi: 10.5539/gjhs.v4n6p17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Touré K, et al. Evaluation of the cost of stroke management in Dakar, Senegal. Med. Trop. 2005;65:458–464. [PubMed] [Google Scholar]
- 96.Kabadi GS, Walker R, Donaldson C, Shackley P. The cost of treating stroke in urban and rural Tanzania: a 6-month pilot study. Afr. J. Neurol. Sci. 2013;32:45–53. [Google Scholar]
- 97.Guinhouya KM, et al. Cost of stroke in Lomé (Togo) Sante. 2010 doi: 10.1684/san.2010.0192. [DOI] [PubMed] [Google Scholar]
- 98.Maredza M, Chola L. Economic burden of stroke in a rural South African setting. eNeurologicalSci. 2016;3:26–32. doi: 10.1016/j.ensci.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones MP, et al. Anxiety and depression in incident stroke survivors and their carers in rural Tanzania: a case-control follow-up study over five years. Neurol. Psychiatry Brain Res. 2012;18:122–128. doi: 10.1016/j.npbr.2012.01.003. [DOI] [Google Scholar]
- 100.Badaru UM, Ogwumike OO, Adeniyi AF, Nelson EE. Determinants of caregiving burden and quality of life of informal caregivers of African stroke survivors: literature review. Int. J. Disabil. Hum. Dev. 2017;16:249–258. doi: 10.1515/ijdhd-2016-0041. [DOI] [Google Scholar]
- 101.Oni OD, Olagunju AT, Okpataku CI, Erinfolami AR, Adeyemi JD. Predictors of caregiver burden after stroke in Nigeria: effect on psychosocial well-being. Indian J. Psychiatry. 2019;61:457–464. doi: 10.4103/psychiatry.IndianJPsychiatry_395_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Owolabi M, Ugoya S, Platz T. Racial disparity in stroke risk factors: the Berlin-Ibadan experience; a retrospective study. Acta Neurol. Scand. 2009;119:81–87. doi: 10.1111/j.1600-0404.2008.01077.x. [DOI] [PubMed] [Google Scholar]
- 103.O’Donnell MJ, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 104.Owolabi MO, et al. Dominant modifiable risk factors for stroke in Ghana and Nigeria (SIREN): a case-control study. Lancet Glob. Health. 2018;6:e436–e446. doi: 10.1016/S2214-109X(18)30002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O’Donnell M, et al. Rationale and design of INTERSTROKE: a global case-control study of risk factors for stroke. Neuroepidemiology. 2010;35:36–44. doi: 10.1159/000306058. [DOI] [PubMed] [Google Scholar]
- 106.Akpalu A, et al. Phenotyping stroke in sub-Saharan Africa: Stroke Investigative Research and Education Network (SIREN) phenomics protocol. Neuroepidemiology. 2015;45:73–82. doi: 10.1159/000437372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.WHO. A global brief on hypertension: silent killer, global public health crisis: World Health Day 2013. WHOhttps://apps.who.int/iris/handle/10665/79059 (2013).
- 108.Geldsetzer P, et al. The state of hypertension care in 44 low-income and middle-income countries: a cross-sectional study of nationally representative individual-level data from 1.1 million adults. Lancet. 2019;394:652–662. doi: 10.1016/S0140-6736(19)30955-9. [DOI] [PubMed] [Google Scholar]
- 109.Kengne AP, Mayosi BM. Modifiable stroke risk factors in Africa: lessons from SIREN. Lancet Glob. Health. 2018;6:e363–e364. doi: 10.1016/S2214-109X(18)30030-5. [DOI] [PubMed] [Google Scholar]
- 110.Jacobs MS, et al. Atrial fibrillation in Africa — an under-reported and unrecognized risk factor for stroke: a systematic review. Glob. Heart. 2019;14:269–279. doi: 10.1016/j.gheart.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 111.Benjamin LA, et al. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 2012;11:878–890. doi: 10.1016/S1474-4422(12)70205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abdallah A, et al. Stroke in human immunodeficiency virus-infected individuals in sub-Saharan Africa (SSA): a systematic review. J. Stroke Cerebrovasc. Dis. 2018;27:1828–1836. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.GBD HIV Collaborators Global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV. 2019;6:e831–e859. doi: 10.1016/S2352-3018(19)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ortiz G, Koch S, Romano J, Forteza A, Rabinstein A. Mechanisms of ischemic stroke in HIV-infected patients. Neurology. 2007;68:1257–1261. doi: 10.1212/01.wnl.0000259515.45579.1e. [DOI] [PubMed] [Google Scholar]
- 115.Mochan A, Modi M, Modi G. Stroke in black South African HIV-positive patients: a prospective analysis. Stroke. 2003;34:10–15. doi: 10.1161/01.STR.0000043821.35051.FA. [DOI] [PubMed] [Google Scholar]
- 116.Hoffmann M, Berger JR, Nath A, Rayens M. Cerebrovascular disease in young, HIV-infected, black Africans in the KwaZulu Natal province of South Africa. J. Neurovirol. 2000;6:229–236. doi: 10.3109/13550280009015825. [DOI] [PubMed] [Google Scholar]
- 117.Benjamin LA, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology. 2016;86:324–333. doi: 10.1212/WNL.0000000000002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sarfo FS, et al. Risk factors for stroke occurrence in a low HIV endemic West African country: a case-control study. J. Neurol. Sci. 2018;395:8–16. doi: 10.1016/j.jns.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wonkam A, Makani J. Sickle cell disease in Africa: an urgent need for longitudinal cohort studies. Lancet Glob. Health. 2019;7:e1310–e1311. doi: 10.1016/S2214-109X(19)30364-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fatunde O, Adamson F, Ogunseyinde O, Sodeinde O, Familusi J. Stroke in Nigerian children with sickle cell disease. Afr. J. Med. Med. Sci. 2005;34:157–160. [PubMed] [Google Scholar]
- 121.Isa H, et al. Sickle cell disease clinical phenotypes in Nigeria: a preliminary analysis of the Sickle Pan Africa Research Consortium Nigeria database. Blood Cell Mol. Dis. 2020;84:102438. doi: 10.1016/j.bcmd.2020.102438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Adewoyin AS. Management of sickle cell disease: a review for physician education in Nigeria (sub-Saharan Africa) Anemia. 2015;2015:791498. doi: 10.1155/2015/791498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Strouse JJ, Lanzkron S, Urrutia V. The epidemiology, evaluation and treatment of stroke in adults with sickle cell disease. Expert Rev. Hematol. 2011;1:597–606. doi: 10.1586/ehm.11.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Noubiap JJ, Mengnjo MK, Nicastro N, Kamtchum-Tatuene J. Neurologic complications of sickle cell disease in Africa: a systematic review and meta-analysis. Neurology. 2017;3:1516–1524. doi: 10.1212/WNL.0000000000004537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ohene-Frempong K, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 126.Sarfo FS, et al. COVID-19 and stroke: experience in a Ghanaian healthcare system. J. Neurol. Sci. 2020;416:117044. doi: 10.1016/j.jns.2020.117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Altable M, de la Serna JM. Cerebrovascular disease in COVID-19: is there a higher risk of stroke? Brain Behav. Immun. Health. 2020;6:100092. doi: 10.1016/j.bbih.2020.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Feigin VL, et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 129.GBD Demographics Collaborators Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1160–1203. doi: 10.1016/S0140-6736(20)30977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sliwa K, Acquah L, Gersh BJ, Mocumbi AO. Impact of socioeconomic status, ethnicity, and urbanization on risk factor profiles of cardiovascular disease in Africa. Circulation. 2016;133:1199–1208. doi: 10.1161/CIRCULATIONAHA.114.008730. [DOI] [PubMed] [Google Scholar]
- 132.Yammine L, Kang DH, Baun MM, Meininger JC. Endothelin-1 and psychosocial risk factors for cardiovascular disease: a systematic review. Psychosom. Med. 2014;76:109–121. doi: 10.1097/PSY.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 133.Velkoff, V. A. & Kowal, P. R. in Aging in Sub-Saharan Africa: Recommendation for Furthering Research (eds Cohen, B. & Menken, J.) (National Academies Press, 2006). [PubMed]
- 134.Akinyemi R, et al. Interleukin-6 (IL-6) rs1800796 and cyclin dependent kinase inhibitor (CDKN2A/CDKN2B) rs2383207 are associated with ischemic stroke in indigenous West African Men. J. Neurol. Sci. 2017;379:229–235. doi: 10.1016/j.jns.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Quay JL, Reed W, Samet J, Devlin RB. Air pollution particles induce IL-6 gene expression in human airway epithelial cells via NF-kappaB activation. Am. J. Respir. Cell Mol. Biol. 1998;19:98–106. doi: 10.1165/ajrcmb.19.1.3132. [DOI] [PubMed] [Google Scholar]
- 136.Dzudie A, et al. Availability, cost and affordability of essential cardiovascular disease medicines in the south west region of Cameroon: preliminary findings from the Cameroon Science for Disease Study. PLoS ONE. 2020;15:e0229307. doi: 10.1371/journal.pone.0229307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Carapinha JL, Ross-Degnan D, Desta AT, Wagner AK. Health insurance systems in five sub-Saharan African countries: medicine benefits and data for decision making. Health Policy. 2011;99:193–202. doi: 10.1016/j.healthpol.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 138.Fenny AP, Yates R, Thompson R. Social health insurance schemes in Africa leave out the poor. Int. Health. 2018;10:1–3. doi: 10.1093/inthealth/ihx046. [DOI] [PubMed] [Google Scholar]
- 139.Safeer RS, Cooke CE, Keenan J. The impact of health literacy on cardiovascular disease. Vasc. Health Risk Manag. 2006;2:457–464. doi: 10.2147/vhrm.2006.2.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nyaaba GN, Masana L, Aikins AD, Stronks K, Agyemang C. Lay community perceptions and treatment options for hypertension in rural northern Ghana: a qualitative analysis. BMJ Open. 2018;8:e023451. doi: 10.1136/bmjopen-2018-023451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yusuf S, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378:1231–1243. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 142.Ogungbo B, Mendelow AD, Walker R. The epidemiology, diagnosis and treatment of subarachnoid haemorrhage in Nigeria: what do we know and what do we need to know? Br. J. Neurosurg. 2004;18:362–366. doi: 10.1080/02688690400005057. [DOI] [PubMed] [Google Scholar]
- 143.Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology. 2013;81:264–272. doi: 10.1212/WNL.0b013e31829bfde3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Syed NA, et al. Ischemic stroke subtypes in Pakistan: the Aga Khan University Stroke Data Bank. J. Pak. Med. Assoc. 2003;53:584–588. [PubMed] [Google Scholar]
- 145.White H, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 146.Stewart JA, Dundas R, Howard RS, Rudd AG, Wolfe CD. Ethnic differences in incidence of stroke: prospective study with stroke register. BMJ. 1999;318:967–971. doi: 10.1136/bmj.318.7189.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Connor MD. Carotid artery disease in sub-Saharan Africa: a marker of epidemiological and stroke transition. Neuroepidemiology. 2012;38:120–121. doi: 10.1159/000336110. [DOI] [PubMed] [Google Scholar]
- 148.Jusabani A, Gray WK, Swai M, Walker R. Post-stroke carotid ultrasound findings from an incident Tanzanian population. Neuroepidemiology. 2011;37:245–248. doi: 10.1159/000334610. [DOI] [PubMed] [Google Scholar]
- 149.Owolabi MO, Agunloye AM, Umeh EO, Akpa OM. Can common carotid intima media thickness serve as an indicator of both cardiovascular phenotype and risk among black Africans? Eur. J. Prev. Cardiol. 2015;22:1442–1451. doi: 10.1177/2047487314547656. [DOI] [PubMed] [Google Scholar]
- 150.Owolabi MO, Akpa OM, Agunloye AM. Carotid IMT is more associated with stroke than risk calculators. Acta Neurol. Scand. 2016;133:442–450. doi: 10.1111/ane.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kamtchum-Tatuene J, et al. A cross-sectional feasibility study of neurovascular ultrasound in Malawian adults with acute stroke-like syndrome. PLoS ONE. 2020;15:e0229033. doi: 10.1371/journal.pone.0229033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oladapo OO, Olusakin J, Ogun GO, Akang EE. Atherosclerosis of the intracranial carotid arteries in Nigerians: a pilot autopsy study. Nig. J. Cardiol. 2013;10:62–67. doi: 10.4103/0189-7969.127002. [DOI] [Google Scholar]
- 153.Erete EI, Ogun OG, Oladapo OO, Akang EE. Prevalence and severity of atherosclerosis in extra cranial carotid arteries in Nigeria: an autopsy study. BMC Cardiovasc. Disord. 2012;12:106. doi: 10.1186/1471-2261-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Williams AO, Loewenson RB, Lippert DM, Resch JA. Cerebral atherosclerosis and its relationship to selected diseases in Nigerians: a pathological study. Stroke. 1975;6:395–401. doi: 10.1161/01.STR.6.4.395. [DOI] [PubMed] [Google Scholar]
- 155.Meretoja A, et al. SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke. 2012;43:2592–2597. doi: 10.1161/STROKEAHA.112.661603. [DOI] [PubMed] [Google Scholar]
- 156.Sarfo FS, et al. Unraveling the risk factors for spontaneous intracerebral hemorrhage among West Africans. Neurology. 2020;94:e998–e1012. doi: 10.1212/WNL.0000000000009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sarfo FS, et al. Stroke among young West Africans: evidence from the SIREN (Stroke Investigative Research and Educational Network) large multisite case-control study. Stroke. 2018;49:1116–1122. doi: 10.1161/STROKEAHA.118.020783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sarfo FS, et al. Frequency and factors linked to refractory hypertension among stroke survivors in Ghana. J. Neurol. Sci. 2020;415:116976. doi: 10.1016/j.jns.2020.116976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ojagbemi A, Owolabi M, Bello T, Baiyewu O. Stroke severity predicts poststroke delirium and its association with dementia: longitudinal observation from a low income setting. J. Neurol. Sci. 2017;375:376–381. doi: 10.1016/j.jns.2017.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shi Q, Presutti R, Selchen D, Saposnik G. Delirium in acute stroke: a systematic review and meta-analysis. Stroke. 2012;43:645–649. doi: 10.1161/STROKEAHA.111.643726. [DOI] [PubMed] [Google Scholar]
- 161.Ojagbemi A, Bello T, Owolabi M, Baiyewu O. Cognitive, functional, and mortality outcomes of attenuated delirium syndrome in stroke survivors. J. Geriatr. Psychiatry Neurol. 2020 doi: 10.1177/0891988720944234. [DOI] [PubMed] [Google Scholar]
- 162.Greffie ES, Mitiku T, Getahun S. Risk factors, clinical pattern and outcome of stroke in a referral hospital, Northwest Ethiopia. Clin. Med. Res. 2015;4:182–188. doi: 10.11648/j.cmr.20150406.13. [DOI] [Google Scholar]
- 163.Diendéré J, et al. Post-stroke complications and mortality in burkinabè hospitals: relationships with deglutition disorders and nutritional status. Dysphagia. 2020;36:85–95. doi: 10.1007/s00455-020-10111-4. [DOI] [PubMed] [Google Scholar]
- 164.Labodi LD, et al. Impact of medical and neurological complications on intra-hospital mortality of stroke in a reference hospital in Ouagadougou (Burkina Faso) J. Adv. Med. Med. Res. 2018;26:1–13. [Google Scholar]
- 165.Gnonlonfoun DD, et al. Stroke: medium and long-term mortality and associated factors in French-speaking West Africa, case of Benin. World J. Neurosci. 2014;4:68–74. doi: 10.4236/wjns.2014.41008. [DOI] [Google Scholar]
- 166.Gebremariam SA, Yang HS. Types, risk profiles, and outcomes of stroke patients in a tertiary teaching hospital in northern Ethiopia. eNeurologicalSci. 2016;3:41–47. doi: 10.1016/j.ensci.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Donkor ES, et al. Post-stroke bacteriuria among stroke patients attending a physiotherapy clinic in Ghana: a cross-sectional study. Ther. Clin. Risk Manag. 2016;12:457–462. doi: 10.2147/TCRM.S90474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Donkor ES, Darkwah S, Akpalu A. Post-stroke bacteriuria: a longitudinal study among stroke outpatients and inpatients at the Korle-Bu teaching hospital in Ghana. Med. Sci. 2017;5:11. doi: 10.3390/medsci5020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jombo GT, Egah DZ, Banwat EB, Ayeni JA. Nosocomial and community acquired urinary tract infections at a teaching hospital in north central Nigeria: findings from a study of 12,458 urine samples. Niger. J. Med. 2006;15:230–236. doi: 10.4314/njm.v15i3.37219. [DOI] [PubMed] [Google Scholar]
- 170.Miller N, et al. Aphasia and swallowing problems in subjects with incident stroke in rural northern Tanzania: a case-control study. Top. Stroke Rehabil. 2014;21:52–62. doi: 10.1310/tsr12-86R1. [DOI] [PubMed] [Google Scholar]
- 171.Oni OD, Olagunju AT, Ogunnubi PO, Aina OF, Ojini FI. Poststroke anxiety disorders in a Nigerian hospital: prevalence, associated factors, and impacts on quality of life. J. Clin. Sci. 2017;1:106–112. doi: 10.4103/jcls.jcls_68_16. [DOI] [Google Scholar]
- 172.Ojagbemi A, et al. Prevalence and predictors of anxiety in an African sample of recent stroke survivors. Acta Neurol. Scand. 2017;136:617–623. doi: 10.1111/ane.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ojagbemi A, et al. Predictors and prognoses of new onset post-stroke anxiety at one year in black Africans. J. Stroke Cerebrovasc. Dis. 2020;29:105082. doi: 10.1016/j.jstrokecerebrovasdis.2020.105082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sarfo FS, et al. Prevalence, trajectory, and predictors of poststroke fatigue among Ghanaians. J. Stroke Cerebrovasc. Dis. 2019;28:1353–1361. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vincent-Onabajo G, Adamu A. Impact of poststroke fatigue on health-related quality of life of Nigerian stroke survivors. J. Stroke. 2014;16:195–201. doi: 10.5853/jos.2014.16.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vincent-Onabajo G, Adamu A. Determinants of poststroke fatigue among stroke survivors undergoing rehabilitation in Nigeria. Middle East J. Rehabilitation Health Stud. 2017;4:e57478. [Google Scholar]
- 177.Oyewole OO, Ogunlana MO, Gbiri CAO, Oritogun KS. Prevalence and impact of disability and sexual dysfunction on health-related quality of life of Nigerian stroke survivors. Disabil. Rehabil. 2017;39:2081–2086. doi: 10.1080/09638288.2016.1219395. [DOI] [PubMed] [Google Scholar]
- 178.Oyewole OO, Ogunlana MO, Gbiri CAO, Oritogun KS. Sexual dysfunction in a Nigerian stroke cohort: a comparative cross-sectional study. Sex. Disabil. 2017;35:341–351. doi: 10.1007/s11195-017-9488-6. [DOI] [Google Scholar]
- 179.Akinpelu AO, Osose AA, Odole AC, Odunaiya NA. Sexual dysfunction in Nigerian stroke survivors. Afr. Health Sci. 2013;13:639–645. doi: 10.4314/ahs.v13i3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hamzat TK, Osundiya OC. Musculoskeletal pain and its impact on motor performance among stroke survivors. Hong Kong Physiother. J. 2010;28:11–15. doi: 10.1016/j.hkpj.2010.11.001. [DOI] [Google Scholar]
- 181.Bashir AH, Abdullahi A, Abba MA, Mukhtar NB. Central poststroke pain: its profile among stroke survivors in Kano, Nigeria. Behav. Neurol. 2017;2017:9318597. doi: 10.1155/2017/9318597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ntsiea V. The prevalence and management of central post-stroke pain at a hospital in Zimbabwe. Malawi Med. J. 2020;32:132–138. doi: 10.4314/mmj.v32i3.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Osundiya OC, Owolabi MO, Hamzat TK. Sensitivity and responsiveness of Ibadan stroke-specific pain scale. Afr. J. Physiother. Rehabil. Sci. 2016;8:17–20. [Google Scholar]
- 184.Ezema CI, et al. Influence of post-stroke depression on functional independence in activities of daily living. Ethiop. J. Health Sci. 2019;29:841–846. doi: 10.4314/ejhs.v29i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Olibamoyo O, Adewuya A, Ola B, Coker O, Atilola O. Prevalence and correlates of depression among Nigerian stroke survivors. S. Afr. J. Psychiatr. 2019;25:1–7. doi: 10.4102/sajpsychiatry.v25i0.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sarfo FS, et al. Post-stroke depression in Ghana: characteristics and correlates. J. Neurol. Sci. 2017;379:261–265. doi: 10.1016/j.jns.2017.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Camara IA. Post-stroke depression at teaching hospital center of Libreville. Open Access Library J. 2018;5:1. [Google Scholar]
- 188.Ibeneme SC, et al. Symptoms of poststroke depression among stroke survivors: an appraisal of psychiatry needs and care during physiotherapy rehabilitation. Scientifica. 2016;2016:5646052. doi: 10.1155/2016/5646052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mpembi MN, et al. Sociodemographic profile and social support for post-stroke depression in Kinshasa: a rehabilitation based cross-sectional study. Open J. Epidemiol. 2013;3:111–117. doi: 10.4236/ojepi.2013.3318. [DOI] [Google Scholar]
- 190.Ojegbemi A, Owolabi M, Baiyewu O. Stroke lesions and post-stroke depression among survivors in Ibadn, Nigeria. Afr. J. Med. Sci. 2013;42:245–251. [PubMed] [Google Scholar]
- 191.Ojagbemi A, Akpa O, Elugbadebo F, Owolabi M, Ovbiagele B. Depression after stroke in sub-Saharan Africa: a systematic review and meta-analysis. Behav. Neurol. 2017;2017:4160259. doi: 10.1155/2017/4160259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ojagbemi A, Bello T. Tedium vitae in stroke survivors: a comparative cross-sectional study. Top. Stroke Rehabil. 2019;26:195–200. doi: 10.1080/10749357.2019.1590971. [DOI] [PubMed] [Google Scholar]
- 193.Ojagbemi A, Bello T, Elugbadebo F. Suicidal thoughts and contexts in Black African stroke survivors. J. Geriatr. Psychiatry Neurol. 2019;32:74–80. doi: 10.1177/0891988718824035. [DOI] [PubMed] [Google Scholar]
- 194.Akinyemi RO, et al. Profile and determinants of vascular cognitive impairment in African stroke survivors: the CogFAST Nigeria Study. J. Neurol. Sci. 2014;346:241–249. doi: 10.1016/j.jns.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 195.Akinyemi RO, et al. Medial temporal lobe atrophy, white matter hyperintensities and cognitive impairment among Nigerian African stroke survivors. BMC Res. Notes. 2015;8:625. doi: 10.1186/s13104-015-1552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sarfo FS, Akassi J, Adamu S, Obese V, Ovbiagele B. Burden and predictors of poststroke cognitive impairment in a sample of Ghanaian stroke survivors. J. Stroke Cerebrovasc. Dis. 2017;26:2553–2562. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hoffmann M. Stroke in the young in South Africa — an analysis of 320 patients. S. Afr. Med. J. 2000;90:1226–1237. [PubMed] [Google Scholar]
- 198.Shehta N, Fahmi RM, Ramadan BM, Emad EM, Elsaid AF. Early post-stroke seizures in a sample of Egyptian patients with first-ever stroke. Neurol. India. 2018;66:1031–1035. doi: 10.4103/0028-3886.236973. [DOI] [PubMed] [Google Scholar]
- 199.Aiwansoba IF, Chukwuyem OW. Early post-acute stroke seizures: clinical profile and outcome in a Nigerian stroke unit. Ann. Afr. Med. 2014;13:11–15. doi: 10.4103/1596-3519.126936. [DOI] [PubMed] [Google Scholar]
- 200.Mohamed C, Kissani N. Early seizures in acute stroke. Pan Afr. Med. J. 2015;20:136. doi: 10.11604/pamj.2015.20.136.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Napon C, Dabilgou A, Kyelem J, Kabore J. Post-stroke epilepsy in Burkina Faso (West Africa) J. Neurol. Sci. 2016;368:47–48. doi: 10.1016/j.jns.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 202.Abdulrahman A, et al. Post stroke epilepsy in Sudan. Sudan Med. J. 2009;45:30–35. [Google Scholar]
- 203.Gnonlonfoun DD, et al. Post-stroke epilepsy within a teaching hospital in Cotonou, Benin. Neurosci. Med. 2017;8:47–52. doi: 10.4236/nm.2017.84007. [DOI] [Google Scholar]
- 204.Sarfo FS, et al. Prevalence and predictors of post-stroke epilepsy among Ghanaian stroke survivors. J. Neurol. Sci. 2020;418:117138. doi: 10.1016/j.jns.2020.117138. [DOI] [PubMed] [Google Scholar]
- 205.Walker RW, Rolfe M, Kelly PJ, George MO, James OFW. Mortality and recovery after stroke in the Gambia. Stroke. 2003;34:1604–1609. doi: 10.1161/01.STR.0000077943.63718.67. [DOI] [PubMed] [Google Scholar]
- 206.De Villiers L, Badri M, Ferreira M, Bryer A. Stroke outcomes in a socio-economically disadvantaged urban community. S. Afr. Med. J. 2011;101:345–348. doi: 10.7196/SAMJ.4588. [DOI] [PubMed] [Google Scholar]
- 207.Mamabolo MV, Mudzi W, Stewart AS, Olorunju S, Singh A. A study to determine post discharge functional improvements in patients with stroke. S. Afr. J. Occup. Ther. 2009;39:15–18. [Google Scholar]
- 208.Joseph C, Rhoda A. Activity limitations and factors influencing functional outcome of patients with stroke following rehabilitation at a specialised facility in the Western Cape. Afr. Health Sci. 2013;13:646–654. doi: 10.4314/ahs.v13i3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ojagbemi A, Owolabi M. Predictors of functional dependency after stroke in Nigeria. J. Stroke Cerebrovasc. Dis. 2013;22:e381–e387. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 210.Adoukonou T, et al. Short term (3 months) prognosis of stroke in Parakou. Neurosci. Med. 2018;9:81–93. doi: 10.4236/nm.2018.92009. [DOI] [Google Scholar]
- 211.Imarhiagbe FA, Abidakun A. Functional motor recovery in stroke survivors-determinants in a sub-Saharan African stroke unit. East Afr. Med. J. 2014;91:119–124. [PubMed] [Google Scholar]
- 212.Nakibuuka J, et al. Early mortality and functional outcome after acute stroke in Uganda: prospective study with 30 day follow-up. Springerplus. 2015;4:450. doi: 10.1186/s40064-015-1252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fouad MM, Farag SM, Hegazy MI, Aziz MAE. Prediction of functional outcome in ischemic stroke patients: an observational Study on Egyptian population. Cureus. 2017;9:e1392. doi: 10.7759/cureus.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nasra FMA, Ali AH, Hassan AM, Alzainy YA. Prediction of the functional outcome in a group of Egyptian patients with posterior circulation stroke. Egypt J. Hosp. Med. 2019;77:4727–4732. doi: 10.21608/ejhm.2019.46752. [DOI] [Google Scholar]
- 215.Ntsiea MV, van Aswegen HOS. Factors which are predictive of return work after stroke. S. Afr. J. Physiother. 2013;16:42–47. [Google Scholar]
- 216.Peters GO, Buni SG, Oyeyemi AY, Hamzat TK. Determinants of return to work among Nigerian stroke survivors. Disabil. Rehabil. 2013;1:455–459. doi: 10.3109/09638288.2012.697251. [DOI] [PubMed] [Google Scholar]
- 217.Norrving B, Kissela B. The global burden of stroke and need for a continuum of care. Neurology. 2013;80(Suppl. 2):S5–S12. doi: 10.1212/WNL.0b013e3182762397. [DOI] [PubMed] [Google Scholar]
- 218.Soriano-Tarraga C, et al. Ischemic stroke patients are biologically older than their chronological age. Aging. 2016;8:2655–2666. doi: 10.18632/aging.101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hobbs A, Ramsay M. Epigenetics and the burden of noncommunicable disease: a paucity of research in Africa. Epigenomics. 2015;7:627–639. doi: 10.2217/epi.15.17. [DOI] [PubMed] [Google Scholar]
- 220.Traylor M, et al. Genetics of stroke in a UK African ancestry case-control study: South London Ethnicity and Stroke Study. Neurol. Genet. 2017;3:e142. doi: 10.1212/NXG.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tishkoff SA, et al. The genetic structure and history of Africans and African Americans. Science. 2009;324:1035–1044. doi: 10.1126/science.1172257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.H3Africa Consortium et al. Research capacity. Enabling the genomic revolution in Africa. Science. 2014;344:1346–1348. doi: 10.1126/science.1251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rotimi CN, et al. The genomic landscape of African populations in health and disease. Hum. Mol. Genet. 2017;26:R225–R236. doi: 10.1093/hmg/ddx253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sirugo G, Williams SM, Tishkoff SA. The missing diversity in human genetic studies. Cell. 2019;177:26–31. doi: 10.1016/j.cell.2019.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Choudhury A, et al. High-depth African genomes inform human migration and health. Nature. 2020;586:741–748. doi: 10.1038/s41586-020-2859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bentley AR, Callier SL, Rotimi CN. Evaluating the promise of inclusion of African ancestry populations in genomics. NPJ Genom. Med. 2020;5:5. doi: 10.1038/s41525-019-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hinman JD, et al. Principles of precision medicine in stroke. J. Neurol. Neurosurg. Psychiatry. 2017;88:54–61. doi: 10.1136/jnnp-2016-314587. [DOI] [PubMed] [Google Scholar]
- 228.Saidi S, Mahjoub T, Almawi WY. Aldosterone synthase gene (CYP11B2) promoter polymorphism as a risk factor for ischaemic stroke in Tunisian Arabs. J. Renin Angiotensin Aldosterone Syst. 2010;11:180–186. doi: 10.1177/1470320309360816. [DOI] [PubMed] [Google Scholar]
- 229.Saidi S, et al. Association of human platelet alloantigen 1 through 5 polymorphisms with ischemic stroke. Cerebrovasc. Dis. 2008;25:81–86. doi: 10.1159/000111995. [DOI] [PubMed] [Google Scholar]
- 230.Saidi S, et al. Polymorphisms of the human platelet alloantigens HPA-1, HPA-2, HPA-3, and HPA-4 in ischemic stroke. Am. J. Hematol. 2008;83:570–573. doi: 10.1002/ajh.21171. [DOI] [PubMed] [Google Scholar]
- 231.Saidi S, Mallat SG, Almawi WY, Mahjoub T. Association between renin-angiotensin-aldosterone system genotypes and haplotypes and risk of ischemic stroke of atherosclerotic etiology. Acta Neurol. Scand. 2009;119:356–363. doi: 10.1111/j.1600-0404.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 232.Saidi S, Mallat SG, Almawi WY, Mahjoub T. Endothelial nitric oxide synthase Glu298Asp, 4b/a, and -786T>C gene polymorphisms and the risk of ischemic stroke. Acta Neurol. Scand. 2010;121:114–119. doi: 10.1111/j.1600-0404.2009.01192.x. [DOI] [PubMed] [Google Scholar]
- 233.Saidi S, Slamia LB, Ammou SB, Mahjoub T, Almawi WY. Association of apolipoprotein E gene polymorphism with ischemic stroke involving large-vessel disease and its relation to serum lipid levels. J. Stroke Cerebrovasc. Dis. 2007;16:160–166. doi: 10.1016/j.jstrokecerebrovasdis.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 234.Saidi S, Slamia LB, Mahjoub T, Ammou SB, Almawi WY. Association of PAI-1 4G/5G and -844G/A gene polymorphism and changes in PAI-1/tPA levels in stroke: a case-control study. J. Stroke Cerebrovasc. Dis. 2007;16:153–159. doi: 10.1016/j.jstrokecerebrovasdis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 235.Saidi S, et al. Interaction of angiotensin-converting enzyme and apolipoprotein E gene polymorphisms in ischemic stroke involving large-vessel disease. J. Thromb. Thrombolysis. 2009;27:68–74. doi: 10.1007/s11239-007-0165-y. [DOI] [PubMed] [Google Scholar]
- 236.Chehaibi K, et al. Effect of genetic polymorphism +294T/C in peroxisome proliferator-activated receptor delta on the risk of ischemic stroke in a Tunisian population. J. Mol. Neurosci. 2013;50:360–367. doi: 10.1007/s12031-013-9997-4. [DOI] [PubMed] [Google Scholar]
- 237.Chehaibi K, et al. Matrix metalloproteinase-1 and matrix metalloproteinase-12 gene polymorphisms and the risk of ischemic stroke in a Tunisian population. J. Neurol. Sci. 2014;342:107–113. doi: 10.1016/j.jns.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 238.Fekih-Mrissa N, et al. Methylenetetrahydrofolate reductase (C677T and A1298C) polymorphisms, hyperhomocysteinemia, and ischemic stroke in Tunisian patients. J. Stroke Cerebrovasc. Dis. 2013;22:465–469. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 239.Diakite B, Hamzi K, Hmimech W, Nadifi S, GMRAVC First study of C2491T FV mutation with ischaemic stroke risk in Morocco. J. Genet. 2015;94:313–315. doi: 10.1007/s12041-015-0525-x. [DOI] [PubMed] [Google Scholar]
- 240.Diakite B, Hamzi K, Hmimech W, Nadifi S, GMRAVC. Genetic polymorphisms of T-1131C APOA5 and ALOX5AP SG13S114 with the susceptibility of ischaemic stroke in Morocco. J. Genet. 2016;95:303–309. doi: 10.1007/s12041-016-0635-0. [DOI] [PubMed] [Google Scholar]
- 241.Diakite B, et al. G894T endothelial nitric oxide synthase polymorphism and ischemic stroke in Morocco. Meta Gene. 2014;2:349–357. doi: 10.1016/j.mgene.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rezk NA, Mohamad HS. Influence of interleukin-1 gene cluster polymorphisms on the susceptibility and outcomes of acute stroke in Egyptian patients. Cell Biochem. Biophys. 2015;71:637–647. doi: 10.1007/s12013-014-0243-7. [DOI] [PubMed] [Google Scholar]
- 243.ElAlfy MS, et al. Angiotensinogen M235T gene polymorphism is a genetic determinant of cerebrovascular and cardiopulmonary morbidity in adolescents with sickle cell disease. J. Stroke Cerebrovasc. Dis. 2019;28:441–449. doi: 10.1016/j.jstrokecerebrovasdis.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 244.Atadzhanov M, et al. Association of the APOE, MTHFR and ACE genes polymorphisms and stroke in Zambian patients. Neurol. Int. 2013;5:e20. doi: 10.4081/ni.2013.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Akinyemi R, et al. APOL1, CDKN2A/CDKN2B, and HDAC9 polymorphisms and small vessel ischemic stroke. Acta Neurol. Scand. 2018;137:133–141. doi: 10.1111/ane.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Gutierrez OM, et al. APOL1 nephropathy risk variants and incident cardiovascular disease events in community-dwelling black adults. Circ. Genom. Precis. Med. 2018;11:e002098. doi: 10.1161/CIRCGEN.117.002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tayo BO, et al. Genetic variation in APOL1 and MYH9 genes is associated with chronic kidney disease among Nigerians. Int. Urol. Nephrol. 2013;45:485–494. doi: 10.1007/s11255-012-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jamison, D. T. et al. Disease Control Priorities: Improving Health and Reducing Poverty 3rd Edn Vol. 9 (World Bank, 2017). [PubMed]
- 249.WHO. Towards a global action plan for healthy lives and well-being for all. Uniting to accelerate progress towards the health-related SDGs. WHOhttps://apps.who.int/iris/handle/10665/311667 (2018).
- 250.WHO. Noncommunicable diseases progress monitor 2020. WHOhttps://www.who.int/publications/i/item/ncd-progress-monitor-2020 (2020).
- 251.Nyaaba GN, Stronks K, Aikins AD-G, Kengne AP, Agyemang C. Tracing Africa’s progress towards implementing the Non-Communicable Diseases Global action plan 2013–2020: a synthesis of WHO country profile reports. BMC Public Health. 2017;17:297. doi: 10.1186/s12889-017-4199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tesema AG, et al. How well are non-communicable disease services being integrated into primary health care in Africa: a review of progress against World Health Organization’s African regional targets. PLoS ONE. 2020;15:e0240984. doi: 10.1371/journal.pone.0240984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Owolabi M, Johnson W, Khan T, Feigin V. Effectively combating stroke in low- and middle-income countries: placing proof in pragmatism — the Lancet Neurology Commission. J. Stroke Med. 2018;1:65–67. doi: 10.1177/2516608518776706. [DOI] [Google Scholar]
- 254.Mould-Millman N-K, et al. The state of emergency medical services (EMS) systems in Africa. Prehosp. Disaster Med. 2017;32:273–283. doi: 10.1017/S1049023X17000061. [DOI] [PubMed] [Google Scholar]
- 255.Mould-Millman N-K, et al. Developing emergency medical dispatch systems in Africa — recommendations of the African Federation for Emergency Medicine/International Academies of Emergency Dispatch Working Group. Afr. J. Emerg. Med. 2015;5:141–147. doi: 10.1016/j.afjem.2015.06.005. [DOI] [Google Scholar]
- 256.Reynolds TA, et al. AFEM consensus conference 2013 summary: emergency care in Africa — where are we now? Afr. J. Emerg. Med. 2014;4:158–163. doi: 10.1016/j.afjem.2014.07.004. [DOI] [Google Scholar]
- 257.Philip-Ephraim EE, et al. Factors associated with prehospital delay among stroke patients in a developing African country. Int. J. Stroke. 2015;10:E39. doi: 10.1111/ijs.12469. [DOI] [PubMed] [Google Scholar]
- 258.Bahnasy WS, Ragab OAA, Elhassanien ME. Stroke onset to needle delay: where these golden hours are lost? An Egyptian center experience. eNeurologicalSci. 2019;14:68–71. doi: 10.1016/j.ensci.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Adejoh T, et al. Computed tomography scanner census and adult head dose in Nigeria. Egypt. J. Radiol. Nucl. Med. 2018;49:66–70. doi: 10.1016/j.ejrnm.2017.09.001. [DOI] [Google Scholar]
- 260.Ogbole GI, Adeyomoye AO, Badu-Peprah A, Mensah Y, Nzeh DA. Survey of magnetic resonance imaging availability in West Africa. Pan Afr. Med. J. 2018;30:240. doi: 10.11604/pamj.2018.30.240.14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ouma PO, et al. Access to emergency hospital care provided by the public sector in sub-Saharan Africa in 2015: a geocoded inventory and spatial analysis. Lancet Glob. Health. 2018;6:e342–e350. doi: 10.1016/S2214-109X(17)30488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Norrving B, et al. Action plan for stroke in Europe 2018-2030. Eur. Stroke J. 2018;3:309–336. doi: 10.1177/2396987318808719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Saver JL, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–2488. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 264.Fassbender K, et al. Mobile stroke units for prehospital thrombolysis, triage, and beyond: benefits and challenges. Lancet Neurol. 2017;16:227–237. doi: 10.1016/S1474-4422(17)30008-X. [DOI] [PubMed] [Google Scholar]
- 265.Mathur S, et al. Improving prehospital stroke services in rural and underserved settings with mobile stroke units. Front. Neurol. 2019;10:159. doi: 10.3389/fneur.2019.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Urimubenshi G, Cadilhac DA, Kagwiza JN, Wu O, Langhorne P. Stroke care in Africa: a systematic review of the literature. Int. J. Stroke. 2018;13:797–805. doi: 10.1177/1747493018772747. [DOI] [PubMed] [Google Scholar]
- 267.Napon C, Dabilgou A, Kyelem J, Bonkoungou P, Kabore J. Therapeutic route of patients at the acute phase of their stroke in Burkina Faso. J. Neurol. Sci. 2017;372:75–77. doi: 10.1016/j.jns.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 268.Cisse FA, et al. Minimal setting stroke unit in a sub-Saharan African Public Hospital. Front. Neurol. 2019;10:856. doi: 10.3389/fneur.2019.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Al-Rukn S, et al. Stroke in the Middle-East and North Africa: a 2-year prospective observational study of intravenous thrombolysis treatment in the region. Results from the SITS-MENA registry. Int. J. Stroke. 2020;15:980–987. doi: 10.1177/1747493019874729. [DOI] [PubMed] [Google Scholar]
- 270.Lindsay P, Furie KL, Davis SM, Donnan GA, Norrving B. World Stroke Organization global stroke services guidelines and action plan. Int. J. Stroke. 2014;9(Suppl. A100):4–13. doi: 10.1111/ijs.12371. [DOI] [PubMed] [Google Scholar]
- 271.de Villiers L, Kalula SZ, Burch VC. Does multidisciplinary stroke care improve outcome in a secondary-level hospital in South Africa? Int. J. Stroke. 2009;4:89–93. doi: 10.1111/j.1747-4949.2009.00254.x. [DOI] [PubMed] [Google Scholar]
- 272.Owolabi M, et al. Development and reliability of a user-friendly multicenter phenotyping application for hemorrhagic and ischemic stroke. J. Stroke Cerebrovasc. Dis. 2017;26:2662–2670. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wasserman S, Bryer A. Early outcomes of thrombolysis for acute ischaemic stroke in a South African tertiary care centre. S. Afr. Med. J. 2012;102:541–544. doi: 10.7196/SAMJ.5403. [DOI] [PubMed] [Google Scholar]
- 274.Chtaou N, et al. Intravenous thrombolysis with rt-PA in stroke: experience of the Moroccan stroke unit. Pan Afr. Med. J. 2016;24:207. doi: 10.11604/pamj.2016.24.207.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Baatiema L, Chan CKY, Sav A, Somerset S. Interventions for acute stroke management in Africa: a systematic review of the evidence. Syst. Rev. 2017;6:213. doi: 10.1186/s13643-017-0594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zakaria MF, et al. Egyptian experience in increasing utilization of reperfusion therapies in acute ischemic stroke. Int. J. Stroke. 2018;13:525–529. doi: 10.1177/1747493017711949. [DOI] [PubMed] [Google Scholar]
- 277.Pandian JD, et al. Stroke systems of care in low-income and middle-income countries: challenges and opportunities. Lancet. 2020;396:1443–1451. doi: 10.1016/S0140-6736(20)31374-X. [DOI] [PubMed] [Google Scholar]
- 278.Meretoja A, et al. Stroke doctors: who are we? A world stroke organization survey. Int. J. Stroke. 2017;12:858–868. doi: 10.1177/1747493017701150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Khan M, et al. Changing the face of stroke care in the Middle East North Africa region. J. Neurol. Sci. 2020;412:116727. doi: 10.1016/j.jns.2020.116727. [DOI] [PubMed] [Google Scholar]
- 280.Bernhardt J, Urimubenshi G, Gandhi DBC, Eng JJ. Stroke rehabilitation in low-income and middle-income countries: a call to action. Lancet. 2020;396:1452–1462. doi: 10.1016/S0140-6736(20)31313-1. [DOI] [PubMed] [Google Scholar]
- 281.Chimatiro GL, Rhoda AJ. Scoping review of acute stroke care management and rehabilitation in low and middle-income countries. BMC Health Serv. Res. 2019;1:789. doi: 10.1186/s12913-019-4654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Grimmer K, et al. A South African experience in applying the Adopt-Contextualise-Adapt framework to stroke rehabilitation clinical practice guidelines. Health Res. Policy Syst. 2019;17:56. doi: 10.1186/s12961-019-0454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Sarfo FS, Adamu S, Awuah D, Sarfo-Kantanka O, Ovbiagele B. Potential role of tele-rehabilitation to address barriers to implementation of physical therapy among West African stroke survivors: a cross-sectional survey. J. Neurol. Sci. 2017;381:203–208. doi: 10.1016/j.jns.2017.08.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.ATTEND Collaborative Group Family-led rehabilitation after stroke in India (ATTEND): a randomised controlled trial. Lancet. 2017;390:588–599. doi: 10.1016/S0140-6736(17)31447-2. [DOI] [PubMed] [Google Scholar]
- 285.Sarfo FS, Adusei N, Ampofo M, Kpeme FK, Ovbiagele B. Pilot trial of a tele-rehab intervention to improve outcomes after stroke in Ghana: a feasibility and user satisfaction study. J. Neurol. Sci. 2018;387:94–97. doi: 10.1016/j.jns.2018.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Vogel AC, et al. MAMBO: measuring ambulation, motor, and behavioral outcomes with post-stroke fluoxetine in Tanzania: protocol of a phase II clinical trial. J. Neurol. Sci. 2020;408:116563. doi: 10.1016/j.jns.2019.116563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Boateng D, et al. Knowledge and awareness of and perception towards cardiovascular disease risk in sub-Saharan Africa: a systematic review. PLoS ONE. 2017;12:e0189264. doi: 10.1371/journal.pone.0189264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Farrag MA, et al. Public stroke knowledge, awareness, and response to acute stroke: multi-center study from 4 Egyptian governorates. J. Neurol. Sci. 2018;384:46–49. doi: 10.1016/j.jns.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 289.Akinyemi RO, et al. Knowledge and perception of stroke amongst hospital workers in an African community. Eur. J. Neurol. 2009;16:998–1003. doi: 10.1111/j.1468-1331.2009.02666.x. [DOI] [PubMed] [Google Scholar]
- 290.Cossi MJ, Preux PM, Chabriat H, Gobron C, Houinato D. Knowledge of stroke among an urban population in Cotonou (Benin) Neuroepidemiology. 2012;38:172–178. doi: 10.1159/000336862. [DOI] [PubMed] [Google Scholar]
- 291.Jenkins C, et al. Knowledge, attitudes and practices related to stroke in Ghana and Nigeria: a SIREN call to action. PLoS ONE. 2018;13:e0206548. doi: 10.1371/journal.pone.0206548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Akinyemi RO, et al. Task-shifting training improves stroke knowledge among Nigerian non-neurologist health workers. J. Neurol. Sci. 2015;359:112–116. doi: 10.1016/j.jns.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Komolafe MA, Olorunmoteni OE, Fehintola FO. Effect of health education on level of awareness and knowledge of Nigerian in-school adolescents on stroke and its risk factors. J. Stroke Cerebrovasc. Dis. 2020;29:104757. doi: 10.1016/j.jstrokecerebrovasdis.2020.104757. [DOI] [PubMed] [Google Scholar]
- 294.Owolabi MO, et al. Randomized trial of an intervention to improve blood pressure control in stroke survivors. Circ. Cardiovasc. Qual. Outcomes. 2019;12:e005904. doi: 10.1161/CIRCOUTCOMES.119.005904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sarfo F, et al. PINGS (Phone-Based Intervention Under Nurse Guidance After Stroke): interim results of a pilot randomized controlled trial. Stroke. 2018;49:236–239. doi: 10.1161/STROKEAHA.117.019591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bayona H, et al. A systematic comparison of key features of ischemic stroke prevention guidelines in low- and middle-income vs. high-income countries. J. Neurol. Sci. 2017;375:360–366. doi: 10.1016/j.jns.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Owolabi MO, et al. Randomized controlled trial of a multipronged intervention to improve blood pressure control among stroke survivors in Nigeria. Int. J. Stroke. 2014;9:1109–1116. doi: 10.1111/ijs.12331. [DOI] [PubMed] [Google Scholar]
- 298.Owolabi MO, et al. Tailored hospital-based risk reduction to impede vascular events after stroke (THRIVES) study: qualitative phase protocol. Crit. Pathw. Cardiol. 2014;13:29–35. doi: 10.1097/HPC.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sarfo FS, et al. Phone-based Intervention under Nurse Guidance after Stroke (PINGS): study protocol for a randomized controlled trial. Trials. 2016;17:436. doi: 10.1186/s13063-016-1557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sarfo FS, Ovbiagele B. Stroke minimization through additive anti-atherosclerotic agents in routine treatment (SMAART): a pilot trial concept for improving stroke outcomes in sub-Saharan Africa. J. Neurol. Sci. 2017;377:167–173. doi: 10.1016/j.jns.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 301.Sarfo FS, et al. Stroke Minimization through Additive Anti-atherosclerotic Agents in Routine Treatment (SMAART): study protocol for a randomized controlled trial. Trials. 2018;19:181. doi: 10.1186/s13063-018-2564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Sarfo FS, et al. Phone-based intervention for blood pressure control among Ghanaian stroke survivors: a pilot randomized controlled trial. Int. J. Stroke. 2019;14:630–638. doi: 10.1177/1747493018816423. [DOI] [PubMed] [Google Scholar]
- 303.Agunloye AM, Owolabi MO. Exploring carotid sonographic parameters associated with stroke risk among hypertensive stroke patients compared to hypertensive controls. J. Ultrasound Med. 2014;33:975–983. doi: 10.7863/ultra.33.6.975. [DOI] [PubMed] [Google Scholar]
- 304.Ogbole GI, Owolabi MO, Yusuf BP. White matter changes on magnetic resonance imaging: a risk factor for stroke in an African population? J. Stroke Cerebrovasc. Dis. 2013;22:e227–e233. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 305.WHO. WHO framework convention on tobacco control (WHO Press, 2003).
- 306.Pereira L, Mutesa L, Tindana P, Ramsay M. African genetic diversity and adaptation inform a precision medicine agenda. Nat. Rev. Genet. 2021;22:284–306. doi: 10.1038/s41576-020-00306-8. [DOI] [PubMed] [Google Scholar]
- 307.Akinyemi RO, et al. Knowledge, attitudes and practices of West Africans on genetic studies of stroke: evidence from the SIREN Study. Int. J. Stroke. 2019;14:69–79. doi: 10.1177/1747493018790059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ekoru K, et al. Genetic risk scores for cardiometabolic traits in sub-Saharan African populations. Int. J. Epidemiol. 2021 doi: 10.1093/ije/dyab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Akinyemi RO, et al. Biobanking in a challenging african environment: unique experience from the SIREN project. Biopreserv. Biobank. 2018;16:217–232. doi: 10.1089/bio.2017.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Akinyemi RO, et al. Unraveling the ethical, legal, and social implications of neurobiobanking and stroke genomic research in Africa: a study protocol of the African Neurobiobank for Precision Stroke Medicine ELSI Project. Int. J. Qual. Methods. 2020;19:1–13. doi: 10.1177/1609406920923194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Owolabi MO. Taming the burgeoning stroke epidemic in Africa: stroke quadrangle to the rescue. West Indian Med. J. 2011;60:412–421. [PubMed] [Google Scholar]
- 312.Akinyemi R, et al. Conceptual framework for establishing the African Stroke Organization. Int. J. Stroke. 2021;16:93–99. doi: 10.1177/1747493019897871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Burton A. Trying to change the stroke landscape in Nigeria. Lancet Neurol. 2017;16:869–870. doi: 10.1016/S1474-4422(17)30339-3. [DOI] [PubMed] [Google Scholar]
- 314.Owolabi M, Sarfo FS, Akinyemi R, Gebreyohanns M, Ovbiagele B. The sub-Saharan Africa Conference on Stroke (SSACS): an idea whose time has come. J. Neurol. Sci. 2019;400:194–198. doi: 10.1016/j.jns.2019.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Gebreyohanns M, et al. The inaugural African Stroke Organization conference. J. Neurol. Sci. 2020;418:117089. doi: 10.1016/j.jns.2020.117089. [DOI] [PubMed] [Google Scholar]
- 316.Akinyemi RO, Brainin M. The African Stroke Organization — a new dawn for stroke in Africa. Nat. Rev. Neurol. 2021;17:127–128. doi: 10.1038/s41582-021-00456-1. [DOI] [PubMed] [Google Scholar]