Abstract
Emerging evidence highlights the several roles that meninges play in relevant brain functions as they are a protective membrane for the brain, produce and release several trophic factors important for neural cell migration and survival, control cerebrospinal fluid dynamics, and embrace numerous immune interactions affecting neural parenchymal functions. Furthermore, different groups have identified subsets of neural progenitors residing in the meninges during development and in the adulthood in different mammalian species, including humans. Interestingly, these immature neural cells are able to migrate from the meninges to the neural parenchyma and differentiate into functional cortical neurons or oligodendrocytes. Immature neural cells residing in the meninges promptly react to brain disease. Injury-induced expansion and migration of meningeal neural progenitors have been observed following experimental demyelination, traumatic spinal cord and brain injury, amygdala lesion, stroke, and progressive ataxia. In this review, we summarize data on the function of meninges as stem cell niche and on the presence of immature neural cells in the meninges, and discuss their roles in brain health and disease. Furthermore, we consider the potential exploitation of meningeal neural progenitors for the regenerative medicine to treat neurological disorders.
Keywords: regenerative medicine, meninges, neural progenitors, stem cell niche, neurogenesis, oligodendrocyte precursor cells
Introduction
Meninges consist of three tissue membranes: the external dura mater, the arachnoid, and the inner pia mater. Meninges host several different cell types and are widely distributed in the central nervous system (CNS). Primitive meninges form as early as the neural tube develops and they are necessary for the development of the whole forebrain (Catala 1998; Etchevers and others 1999; O’Rahilly and Muller 1986) and for the generation of the primitive brain vasculature (Marin-Padilla 2012). Specifically, meninges have been shown to play a fundamental role in the genesis of the cerebral cortex (Radakovits and others 2009), cerebellum (Sievers and others 1986) and hippocampus (Hartmann and others 1992). Meninges greatly influence the biology of ventricular radial glial (RG) cells, which are the neural stem cells (NSCs) involved in cortical development (Gotz and others 1998); indeed, meningeal-derived soluble factors and extracellular matrix (ECM) components provide attachment sites for the RG-endfeet ensuring proper RG survival and neuronal migration (Radakovits and others 2009).
Recently, our knowledge of the involvement of the meninges in the regulation of brain function has expanded. Meninges secrete signaling molecules required for neural progenitor migration and maturation (Barber and others 2018; Borrell and Marin 2006; Choe and others 2012; Davare and others 2014; Fayein and others 1992; Hayashi and others 2008; Lehtinen and others 2011; Raballo and others 2000; Radakovits and others 2009; Reiss and others 2002). They are also involved in the control of the cerebrospinal fluid (CSF) dynamics (Louveau and others 2017); in fact, a glymphatic flux of CSF has been shown to continuously flow from the perivascular space, which is formed by extroflession of the pia mater, to the neural tissue thus clearing metabolites, including Aβ products, from the brain extracellular space (Iliff and others 2012). Furthermore, meninges embrace numerous immune interactions, which can significantly affect neural cell functions (Benakis and others 2018; Duan and others 2018; Hu and others 2020; Schlager and others 2016; Shibata-Germanos and others 2020; Song and others 2020; Van Hove and others 2019).
In this review, we describe the feature of the meninges as a niche for NSCs, the signature of meningeal neural progenitors and their differentiation potentials. Furthermore, we discuss the role of meningeal neural progenitors in health and disease and their potential exploitation for the regenerative medicine of neurological diseases.
Meninges: A Widespread Niche for Neural Progenitors
Different groups have described the presence of neural progenitor cells within the meninges. Meningeal-derived neural progenitors have been shown to migrate to the neural tissue in physiological conditions (Belmadani and others 2015; Bifari and others 2017; Dang and others 2019) and to contribute to the disease-induced neural parenchymal reaction (Dang and others 2019; Decimo and others 2011; Kumar and others 2014; Nakagomi and others 2011; Nakagomi and others 2012; Ninomiya and others 2013), further extending the idea of meninges beyond a mere fibrotic scar-forming tissue. Several studies have described migration of different cell types, mostly immune cells, from the meninges to the brain parenchyma, supporting the view of meninges as a potential relevant route for cellular infiltration to the brain in physiological (Bifari and others 2017; Dang and others 2019) and pathological conditions (Benakis Llovera and Liesz 2018; Duan and others 2018; Schlager and others 2016). In this section, we summarize available evidence describing the meningeal niche feature, including (1) the anatomical distribution of the meninges and their unique relationship with blood, lymphatic vessels, and CSF; (2) the heterogeneity of resident meningeal cell populations; (3) the production and responsiveness to growth, survival, and differentiating factors; and (4) the peculiar ECM organization.
Meningeal Anatomical Distribution
To understand the emerging role of meninges for brain function, dysfunction and regeneration, it is essential to first underline two key properties of this tissue: the extensive distribution and the cellular heterogeneity (Figs. 1 and 2). Overall, the non-neural part of the brain accounts for a relevant part of its wet weight and consists of ECM components, different cell types, and the vasculature. Most of the brain stroma includes meninges where blood vessels, border-associated macrophages, perivascular cells, and fibroblasts are present. In the adult CNS, meninges cover the brain and, in gyrencephalic mammals, follow the cerebral cortex gyri and sulci spreading for a large area (approximately 3 to 4 m2 in humans) (Mota and Herculano-Houzel 2015) (Fig. 1). Meninges deeply penetrate and project between the major brain substructures, including hemispheres, and the hippocampus (Decimo and others 2012b; Mercier and Arikawa-Hirasawa 2012; Mercier and Hatton 2000) (Fig. 1). The pia mater also projects to the stroma of the choroid plexus and its extroflession wraps the choroid plexi and forms the non-neural roof of the third ventricle, a structure known as tela choroidea (Fig. 1). Of note, a pial sheath, which is in direct continuity with the subarachnoid space and is filled with CSF, surrounds all the major brain arteries penetrating the cerebral cortex (Ichimura and others 1991; Jones 1970; Nonaka and others 2003; Reina-De La Torre and others 1998; Rodriguez-Baeza and others 1998; Zhang and others 1990) (Fig. 1). This arrangement provides for a direct communication between perivascular, subpial, and subarachnoid spaces; this allows the perivascular CSF to flow into the brain parenchyma and drain the extracellular metabolites to the venous compartment (glymphatic flux) (Iliff and others 2012). In this context, it is worth highlighting that the pia mater basement membrane and the glia limitans follow the penetrating arterioles that form the blood-meningeal barrier. This barrier is different from the blood-brain barrier (BBB), which is mainly located at the CNS capillary endothelium level. In particular, the perivascular space is beyond the arteriolar endothelium and is separated from the CNS parenchyma by the pia mater basal membrane only (Mercier and Hatton 2000). While the BBB function is determined by different structures, including endothelium, pericytes, astrocytes, and the basal membranes forming the neurovascular unit, pial cells lack of tight junctions and are joined by desmosomes and gap junctions (Castro Dias and others 2019). It is important to note that the perivascular space is endowed with a thin sheath of meningeal cells that forms a network surrounding arterioles all along their longitudinal axis. Most of these perivascular meningeal cells have been identified as meningeal fibroblasts and meningeal macrophages (Mercier and Hatton 2000). Recently, however, many other cell types, including NSCs, have been described at this site (see below and Fig. 2).
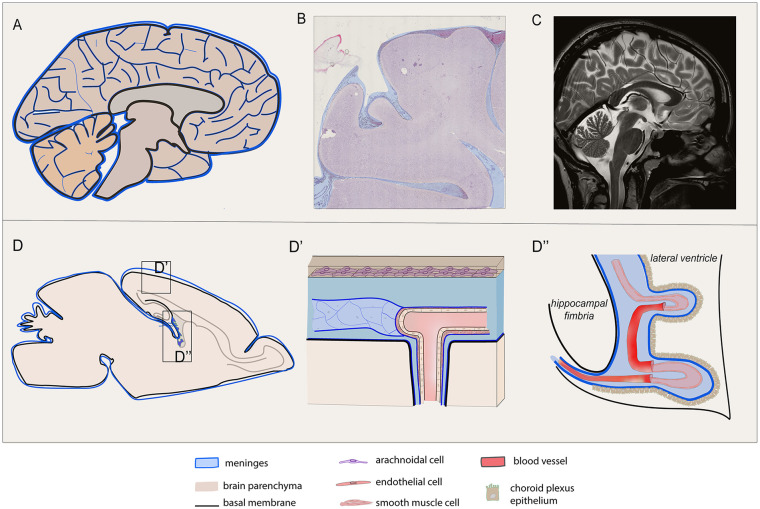
Figure 1.
Meninges are widespread in human and rodent central nervous system (CNS). Meningeal distribution of human (A, B, C) and rodent (D, D′, D′) brain are shown. (A) Sagittal depiction of the human encephalon and (C) the corresponding paramedian T2-weighted magnetic resonance (MR) scan are reported, highlighting the wide distribution of the meningeal layers, excluding the dura mater, as a tissue covering and penetrating inside the cerebral and cerebellar parenchyma, following vessel branches, sulci, and stroma gyration. (B) Coronal section of the human brain stained by hematoxylin and eosin shows meninges penetrating trough the gyri into the sulci. (D) Sagittal graphic view of the rodent brain is reported with enlarged view of the superficial meningeal layer covering the parenchyma at the convexity (D′) and the meningeal substructure penetrating the choroid plexus (D′). (D′) The meningeal arachnoid layer defines the subarachnoid space that is hosting blood vessels, as they deeply penetrate into the sulci and parenchyma in the perivascular spaces projecting through the main brain substructures. The pia mater adheres to the parenchyma and its basal membrane and divides the arteriolar endothelium from the parenchyma. (D′) The pia mater also wraps the choroid plexus (tela choroidea, D′).
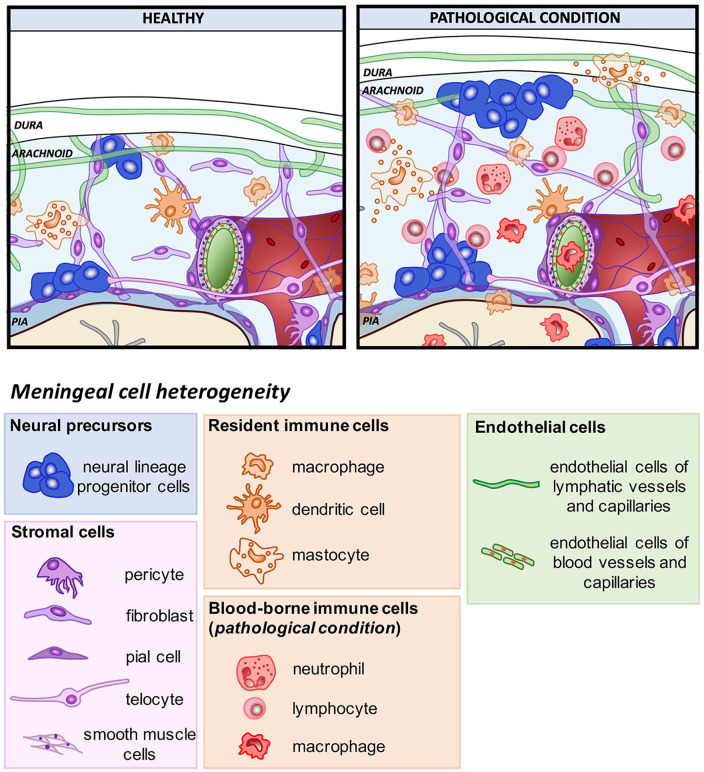
Figure 2.
Meningeal cell heterogeneity in healthy and pathological conditions. Schematic representation showing meningeal cell heterogeneity in healthy and pathological conditions. Meninges are formed by three tissue membranes: dura mater, arachnoid, and pia mater. The dura mater contains lymphatic vessels (green) which spread in the arachnoid space with their capillaries. The arachnoid is an epithelial layer filled by cerebrospinal fluid (CSF) (light blue) in which fibroblasts create the typical arachnoid trabeculae. Pia mater (blue) made of a single layer of pial cells, adheres to the surface of the brain parenchyma (astrocytic basal membrane, bordeaux). In physiological conditions, the leptomeninges (arachnoid and pia) host three main different classes of cell populations: neural precursors, resident stromal cells (pericyte, telocyte, smooth muscle cells, fibroblast, and pial cells) and resident immune cells (border-associated macrophages, dendritic cells, and mastocytes). Following disease, meninges increase their thickness and meningeal cell populations react and proliferate increasing their number (stromal cells and neural precursors). Moreover, the pathological condition causes the activation of the resident immune cells and the recruitment of blood-borne immune cells (circulating monocytes, lymphocytes, neutrophils).
The reconsideration of the distribution of meninges in the CNS, as a tissue widely penetrating inside the parenchyma and not limited to the brain external surface, sets the stage for a more extended reappraisal of their role as modulators of CNS function in homeostasis and disease.
Meningeal Cellular Heterogeneity
Meninges consist of highly heterogeneous cell populations as shown in Figure 2. Cells forming the pia mater, arachnoid, and dura mater share location with several other cell types, including arterial, venous, capillary and lymphatic endothelial cells, smooth muscle cells and pericytes, meningeal, choroid plexus, and perivascular macrophages, and different types of blood-borne immune cells. In addition, in meninges have been observed boundary cap cells, which display stem cell properties and participate in the formation of the boundary between the CNS and the peripheral nervous system (Zujovic and others 2011), and telocytes, which are interstitial cells characterized by extremely long cell processes, telopodes, establishing contacts with blood capillaries, nerve fibers, and stem cells (Popescu and others 2012). Furthermore, a surprising discovery was made in the past few years when several groups described the presence of neural precursors in meninges (Belmadani and others 2015; Bifari and others 2009; Bifari and others 2015; Bifari and others 2017; Dang and others 2019; Decimo and others 2011; Dolci and others 2017; Kumar and others 2014; Nakagomi and others 2011; Nakagomi and others 2012; Ninomiya and others 2013). The distribution and the relative abundance of all the different cell types may vary according to the specific meningeal locations, developmental times or physiological versus pathological conditions (Fig. 2).
The characterization of cells residing in the meninges is continuously expanding. Recent data have suggested the presence of a cell population sharing molecular, morphological, and functional characteristics with both lymphatic cells and macrophages, which may represent an evolutionarily conserved cell type with potential roles in homeostasis and immune organization of the meninges (Shibata-Germanos and others 2020). Similarly, a tissue-specific transcriptional signature has been described for the border-associated macrophages residing in the meninges, which changes during development and in pathological conditions (Van Hove and others 2019). Recently, subgroups of meningeal PDGFR-β expressing cells have been identified as early responders to systemic inflammation by rapidly releasing the chemokine CCL2, which in turn increases neuronal excitability and excitatory synaptic transmission in multiple neuronal types (Duan and others 2018). Interestingly, an ingrowth of meningeal lymphatic cells has been shown to occur into the injured parenchyma tissue following brain vascular damage; this reaction may act as a draining network removing excess fluid and providing a scaffold for the growth of new blood vessels into the area (Chen and others 2019).
Similar to its cellular composition, the embryonic origin of meninges may be reconsidered, as far as different cells may have specific embryonic derivation. Immune, endothelial and lymphatic cells possibly originate from the mesoderm, while meningeal fibroblasts and pial cells of the telencephalon likely derived from the neural crest (Catala 1998; Etchevers and others 2001). The embryonic origin of neural precursors in meninges is still not known. In addition, the precise location of where the cells observed in meninges are born is still not clearly defined. It is possible that some meningeal cells derive from the blood, others may come from the CSF or even they may have been migrating from the neural tissue directly.
Such heterogeneous cellular composition confers to the meninges the potential of playing important functions in brain homeostasis.
Meningeal Niche Microenvironment: Signaling Molecules and Growth Factors
Complementary to the cell-intrinsic programs, the stem cell niche microenvironment provides cell-extrinsic signals that regulate survival, self-renewal, proliferation, and differentiation of stem cells (Chau and others 2015; Decimo and others 2012a; Kokovay and others 2012; Lehtinen and others 2011). The nature and origin of the extrinsic signals within the stem cell niche are various and may derive from niche cells, blood, or CSF.
During the postnatal stage, meninges drive for the correct development of calvarian bones by releasing growth factors, osteogenic cytokines, and ECM molecules. Meninges-derived extrinsic cues have been shown to guide also NSC identity, proliferation, and maintenance. Interestingly, many of the meningeal secreting factors, including fibroblast growth factors (FGFs), transforming growth factorβ (TGFβ), and bone morphogenetic proteins (BMPs), that play an instructive role toward calvarial patterning and morphogenesis, also influence NSC biology (Dasgupta and Jeong 2019). These signals can be secreted by meningeal resident cells, or arrive from the blood or be floating in the CSF filling the meninges. Notably, the structure of meningeal capillaries differs from those characterizing the CNS capillaries, where the endothelial cells are sealed by tight junctions thus forming the BBB. Meninges endothelia lack tight junctions and are therefore open to peripheral circulation. Furthermore, the meninges, which form the perivascular space, are beyond the vascular endothelium, and therefore separated from the CNS parenchyma only by the basal lamina (Mercier and Hatton 2000). Therefore, due to both their unique distribution within the parenchyma and their connection with the vasculature, meninges have the potential to provide the brain structures with several growth/trophic factors, which are essential for the development and the function of brain neural progenitors and differentiating cells. Indeed, the presence of primitive meninges during embryonic development is required for the survival and the subsequent growth of neural progenitors both in vitro and in vivo.
Ventricular RG cells receive contact-mediated (i.e., α1/α4 laminins with β1 integrins) and diffusible signals from the meninges, and the absence of these interactions has been shown to decrease the cortical size and to enhance RG cell apoptosis (Radakovits and others 2009). Meninges secreted factors have also been shown in vitro to favor proliferation and differentiation of NSCs as well as of neural cancer cells, including medulloblastoma and glioblastoma cells (Davare and others 2014). Meninges exert direct effects on RG cells also by secreting high levels of retinoic acid, which triggers the switch of RG proliferation pattern from self-renewing to neurogenic divisions, thus regulating cortical neuron generation and anterior hindbrain development (Siegenthaler and others 2009). In fact, the destruction of cerebellar meninges prevents foliation and lamination in the rostral cerebellum (Sievers and others 1986). Similarly, the destruction of meningeal cells over the medial cerebral hemisphere after birth prevents proper formation of the dentate gyrus (Hartmann and others 1992). Moreover, during spinal cord development meninges secrete several axon-guidance molecules for motor and sensory neurons (Suter and others 2017).
Meninges home long-term BrdU (5-bromo-2′-de-oxyuridine) retaining quiescent and proliferating cells as well as neural committed precursors (Bifari and others 2009). Long-term maintenance of stem cells requires their migration to and homing in supportive stem cell niches (Morrison and Spradling 2008). These processes are mediated by recognition and interaction with chemotactic factors (Kokovay and others 2010) and extravascular tissue-specific structures (Kerever and others 2007; Tanentzapf and others 2007). Both during development and in adulthood, meningeal cells express the chemotactic factor stromal-derived factor 1 (SDF1, also known as CXCL12) and its receptor CXC chemokine receptor 4 (CXCR4) (Belmadani and others 2015; Bifari and others 2015; Borrell and Marin 2006; Reiss and others 2002; Stumm and Hollt 2007). SDF1 is a chemokine involved in homing, migration, proliferation and differentiation of different types of stem cells within their niche. In the subventricular zone (SVZ), the NSCs interact with endothelial cells in SDF1- and CXCR4-dependent manner. Similarly, meningeal-derived SDF-1 acts as a chemotactic factor for neural progenitors during corticogenesis (Borrell and Marin 2006) and controls the tangential migration of hem-derived Cajal-Retzius cells (Paredes and others 2006). Interestingly, by using CXCR4 receptor reporter mice, it was possible to identify CXCR4 expressing RG-like NSCs located in the lateral ventricles that navigate through the fimbria-dentate junction to the hippocampal fimbria as a stream of migratory cells ultimately reaching the meninges. These NSCs then migrate along the meninges in the direction of the dentate gyrus in an SDF-1-dependent manner (Belmadani and others 2015). Following spinal cord injury, a modulation of this chemoattractant signaling system has been observed in meninges, possibly facilitating neural precursors to migrate from the meninges and to contribute to the neural parenchymal reaction (Decimo and others 2011).
In addition to the chemotactic action of SDF-1, meninges secrete the signaling factors BMP-4 and -7 and TGFβ family proteins (Choe and others 2014; Choe and others 2012). These factors play a role for the development of the corpus callosum and for the tangential migration of oligodendrocyte precursor cells (OPCs) into the developing cerebral cortex (Choe and others 2012; Choe and others 2014).
Meninges are a source of several other growth and trophic factors that affect proliferation, survival and differentiation of NSCs and play important roles for the stem cell niche function. Among those vascular endothelial growth factors (VEGF), FGFs, and insulin-like growth factors (IGFs) play a fundamental role. VEGFs are important for endothelial and neural cell migration as well as for lymphatic development and function. During cortical development, meningeal VEGF-A guides early-migrating interneurons in the forebrain (Barber and others 2018). Moreover, in adult CNS, VEGF-A provides trophic and survival signals to motor neurons (Lambrechts and others 2003). Meninges are also a source of VEGF-C that is essential for the development of meningeal lymphatic network and plays relevant roles for lymphatic function and for the modulation of immune cells (Song and others 2020).
FGF2 is required for the normal proliferation of cortical progenitor cells and the generation of cortical neurons during neurogenesis, and it is expressed by meninges (Fayein and others 1992; Mercier and Hatton 2001; Raballo and others 2000). FGF2 plays important functions also for meningeal cells as it acts as mitogen (Parr and Tator 2007) and survival molecule (Raballo and others 2000) and mediates nitric oxide–dependent vasodilation of pial arterioles (Rosenblatt and others 1994). Other growth factors are produced by meninges, including the IGF-II, insulin-like growth factor–binding proteins (IGFBP)-2 and -4 (Brar and Chernausek 1993; Khan 2019; Tritos and others 1998). IGFBP-2 is a multifunctional protein that contains IGF- and heparin-binding domains and it is the most abundant IGFs in the CSF. IGFBP-2 promotes neuronal and oligodendrocyte differentiation and survival. Interestingly, transgenic mice bearing the IGFBP-2 lacking a specific heparin-binding domain showed abnormalities in the hippocampus, prefrontal cortex, cerebellum, and olfactory bulb mass, and decrease in myelin expression in the cerebellum (Schindler and others 2017).
Many of the features required to define prototypical stem cell niches have been described in the meninges. In particular, the presence of blood- or meninges-borne molecules modulating NSC survival, proliferation, homing, and identity. Importantly, the wide spatial distribution of meninges and of their interaction allows the transfer of cues and stimuli originating both inside the CNS and outside (the periphery) the CNS, thus potentially modulating the NSC function within the meningeal stem cell niche according to the brain specific needs and body conditions.
Meningeal Niche Extracellular Matrix
The distribution of meningeal-derived morphogens in the brain and in the meningeal niche also depends on the nature of these molecules that can be differently dissolved into the CSF or bound to specific ECM components. Many cytokines, chemokines, growth factors, and trophic factors, express positively charged amino acid consensus sequences for the glycosaminoglycan heparan sulfate molecules (HSPs) (Aviezer and others 1994; Sarrazin and others 2011). Meninges produce different ECM components, including laminin, fibronectin, collagens IV and XV, and several HSPs, such as N-sulfated heparan sulfate, agrin, perlecan, and collagen XVIII (Decimo and others 2012b; Mercier and Arikawa-Hirasawa 2012; Sarrazin and others 2011). Moreover, meninges ECM is organized in fractones, which are specialized extracellular matrix structures rich in laminin and N-sulfated HSPs (Bifari and others 2015). Fractones sequester and concentrate heparin-binding molecules, including FGF2, epidermal growth factor (EGF), IGFs, several chemokines and other morphogens, creating a concentration gradient essential for cell specification, recruitment, and homing (Kerever and others 2007). Interestingly, HSPs enrichments have been found in brain structures where neural progenitors are present, including olfactory bulb, the rostral migratory stream, the SVZ, the subcallosum, and subcapsule zones and the meninges suggesting the existence of a functional ECM network system involved in the regulation of growth factors in neurogenic regions, including meninges (Mercier and Arikawa-Hirasawa 2012). A further indication that meninges are functionally linked to the neural tissue is the presence of gap junction proteins Cx43, Cx30, and Cx26 (Mercier and Hatton 2001). The distribution of these proteins along with a network of cells in the meninges and in their projections into the brain, including meningeal sheaths of blood vessels and stroma of the choroid plexus, suggests the existence of anatomical and functional interactions between meningeal cells, meningeal perivascular cells, ependymocytes, and astrocytes.
Overall, available evidence suggests the presence in meninges of favorable microenvironment competent in hosting and maintaining the NSCs. Many of the signaling molecules, growth factors, and ECM components acting in the meningeal stem cell niche are also operational in the SVZ NSC niche, suggesting that meningeal stem cell niche shares similarities with classical neurogenic niches. The meningeal niche appears to be able to sense signals from outside and inside the brain and to regulate the properties of NSCs accordingly.
Meningeal Neural Progenitors
Several groups have described the presence of neural progenitors in the meninges (Belmadani and others 2015; Bifari and others 2009; Bifari and others 2015; Bifari and others 2017; Dang and others 2019; Decimo and others 2011; Dolci and others 2017; Kumar and others 2014; Nakagomi and others 2011; Nakagomi and others 2012; Ninomiya and others 2013). The distribution of meningeal resident neural progenitors is apparently not restricted to a defined meningeal area of the brain. Indeed, neural progenitors have been observed in the meninges of the spinal cord, cerebellum, ventral and rostral forebrain (Fig. 3). However, potential regional specific features of meningeal resident neural progenitors have never been assessed. Furthermore, the nature and the relative abundance of neural progenitors in the meninges may vary according to the developmental stages, physiological and pharmacological stimuli, or pathological conditions. In the next sections, we will summarize current knowledge on the distribution, fate, function, migratory pathway and regenerative potential of meningeal resident neural progenitors in health and disease.
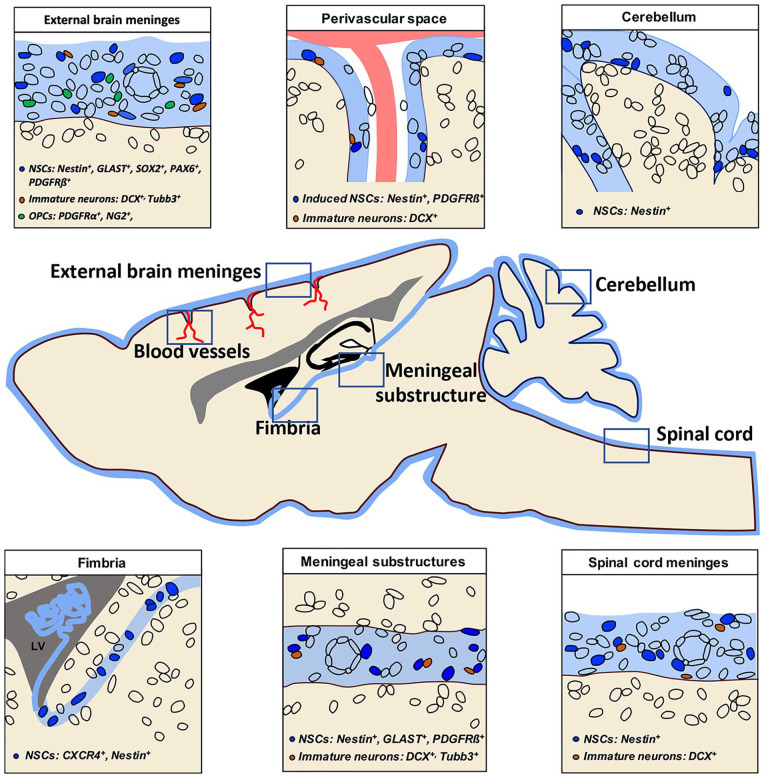
Figure 3.
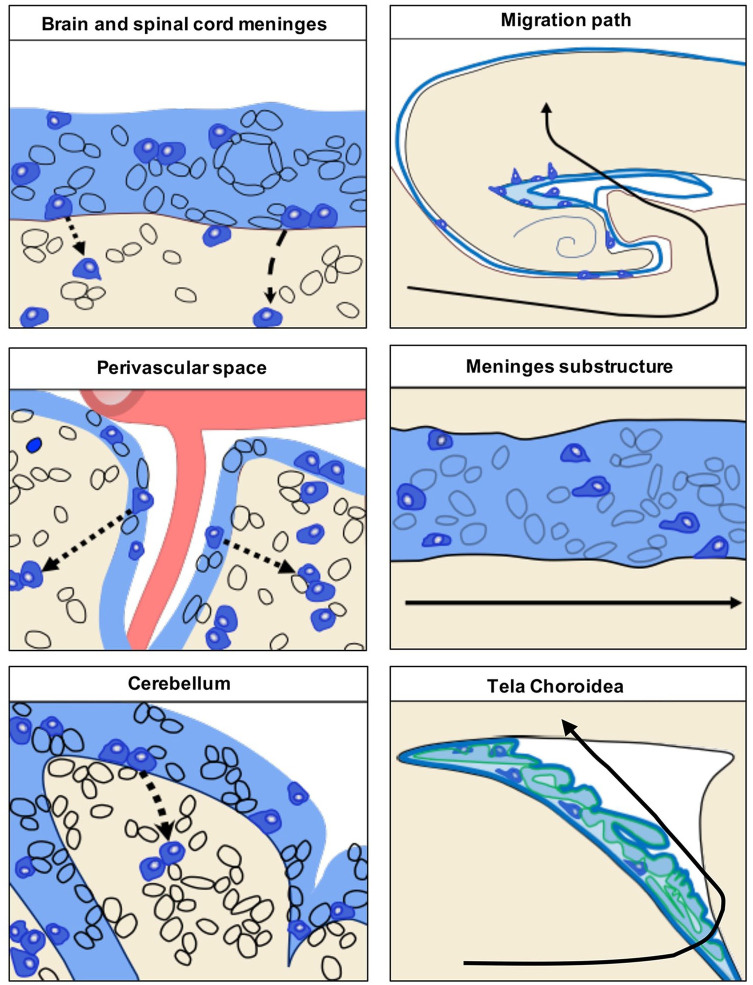
Meningeal neural progenitors are widespread in the central nervous system (CNS). Schematic representation of a sagittal section of rodent brain and spinal cord showing the distribution of neural stem cells (NSCs, blue), immature neurons (brown), and oligodendrocyte precursor cells (OPCs, green) in CNS meninges (light blue). The specific markers expressed by each neural progenitor subclass are shown for each area accordingly with the reviewed literature. As meninges cover the entire CNS (brain and spinal cord) and are widely distributed, also meningeal neural progenitors are not restricted to a defined meningeal area of the brain. Specifically, they have been found in the external brain meninges (upper left panel), in the meninges of perivascular space (upper middle panel), in the cerebellar meninges (upper right panel), along the meninges of hippocampal fimbria (lower left panel), in the meningeal substructures (lower middle panel), and in spinal cord meninges (lower right panel).
Meningeal Neural Progenitors in Health
Although decreasing during development, mouse brain meninges host a subset of cells expressing markers of undifferentiated and differentiating neural precursors and this set of cells persists in adulthood. Meninges may therefore represent a functional niche for neural progenitors during the embryonic development and in adulthood. Importantly, some of these NSC markers have also been identified in embryonic and adult human encephalic (Petricevic and others 2011) and spinal cord meninges (Decimo and others 2011).
Nestin, an intermediate filament of neuroepithelial derivation that has been detected in stem/progenitor cells of neural and non-neural tissues (Lendahl and others 1990; Wiese and others 2004), was found to be expressed by rodent and human meninges during embryonic stages up to adulthood (Bifari and others 2009; Bifari and others 2015; Dang and others 2019; Decimo and others 2011; Kumar and others 2014; Nakagomi and others 2011; Ninomiya and others 2013; Petricevic and others 2011). Similarly, both gene and protein expression analysis revealed glutamate/aspartate transporter (GLAST) expressing cells in meninges (Bifari and others 2017). At the embryonic day (E) 14, when there is the peak of cortical neurogenesis, nestin and GLAST are RG specific markers (Pino and others 2017). To permanently trace RG cells and their descendants, lineage tracing experiments were performed by intercrossing the transgenic GLAST-CreERT2 mice (Mori and others 2006) and Nestin-CreERT2 mice (Lagace and others 2007) with the Rosa26-lox-stop-lox-YFP reporter line, yielding GLAST-YFP or Nestin-YFP mice, respectively (Mori and others 2006). Upon tamoxifen injection of GLAST-YFP or Nestin-YFP mice at E14, a fraction of meningeal cells (nestin-derived 1%, GLAST-derived 7.90%) was labelled in postnatal day (P) o meninges (Bifari and others 2017). Other RG specific markers have been found in meningeal cells. The transcription factor PAX6 is expressed in the ventricular zone of the developing cortex by RG cells (Gotz and others 1998). RG-like PAX6 gene expressing cells were found in the perinatal mouse (Bifari and others 2017) and PAX6 protein expression was also found in adult meningeal cells (see Zeisel and colleagues, Figure 2C) (Zeisel and others 2015). Similarly, the HMG-Box transcription factor SOX2 is expressed in the neural tube during development and in postnatal RG cells (Zappone and others 2000). Sox2 gene and protein expression were found in embryonic, early postnatal and rarely also in adult brain meninges (Bifari and others 2015; Nakagomi and others 2011; Qin and others 2008).
The assessment of the whole transcriptome of the cells composing the brain meninges at P0 confirmed the presence of RG-like cells in meninges (Bifari and others 2017). Single-cell RNA sequencing (scRNAseq) analysis identified a small fraction of the sorted meningeal cells with a signature corresponding to RG-like cells, based on the expression of the RG markers Slc1a3 (also known as GLAST), Fabp7 (Blbp), and Ptprz1 (Llorens-Bobadilla and others 2015). In addition to RG-like cells, a population with a neuroblast signature identified on the basis of Tubb3, Cd24a, and Sox11 gene expression was found in meninges (Bifari and others 2017). Intriguingly, the scRNAseq analysis also revealed a small population of cells with an intermediate signature, possibly indicating a transitional state between the RG-like and neuronal cell types. The protein expression of the neuroblast marker beta-3-tubulin and doublecortin were also confirmed by histology and western blot analysis of developing and adult rodent meninges (Bifari and others 2015; Decimo and others 2011; Nakagomi and others 2012).
Remarkably, gene transfer, lineage tracer and birth dating experiments further indicate the neurogenic potential of meningeal RG-like cells in vivo (Fig. 4). In the meninges of perinatal mice, the neurogenic progenitors have been shown to migrate from the meninges to the cortical layers II–IV of the retrosplenial visual-motor cortex and differentiate into Satb2+ neurons (Bifari and others 2017). The resulting neurons acquired an intrinsic excitable electrical phenotype in vivo (see section on functional properties of meningeal-derived neurons for further details and Figure 5).
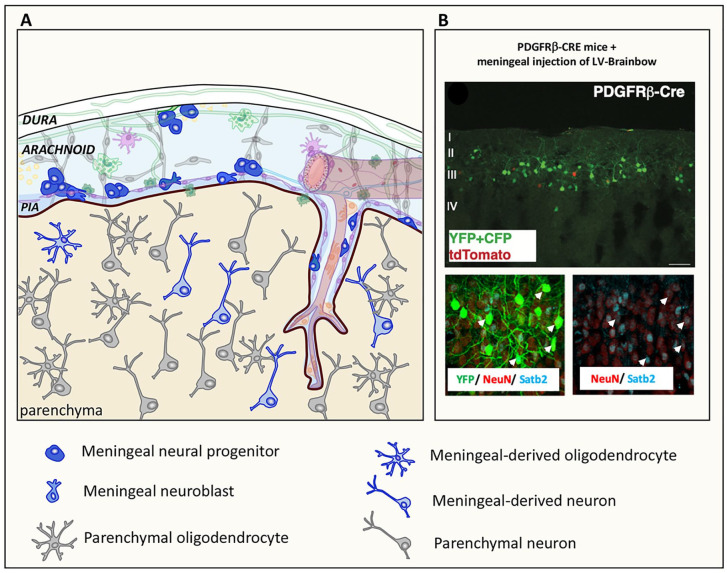
Figure 4.
Meningeal neural progenitors generate parenchymal neurons and oligodendrocytes in physiological condition. (A) Schematic representation showing that immature neural progenitor cells and neuroblasts in meninges generate meningeal-derived neurons or rare oligodendrocytes in the brain parenchyma. In (B) meningeal-derived neurons (YFP+/CFP+, green and tdTomato, red) in the brain cortex of a postnatal day 30 (P30) PDGFRβ-Cre mouse (upper panel) expressing the neuronal markers NeuN and Satb2 (lower panel, arrowhead) are shown. Meningeal cells were labelled by injecting PDGFRβ-Cre P0 mice with a lentiviral vector expressing the Brainbow 1.0(L) reporter in the meninges allowing to trace the Cre expressing PDGFRβ meningeal cells (YFP+/CFP+ cells, green). tdTomato cells (red) are meningeal derived cells that do not express PDGFRβ. The upper panel shows that the meningeal cells migrated into cortical layers II to IV were mostly PDGFRβ-Cre-derived YFP+/CFP+ cells (green). In the lower panel, YFP/CFP meningeal-derived cells (green), NeuN (red), and Satb2 (blue), showing that the PDGFRβ-Cre-derived YFP+/CFP+ cells were NeuN+/Satb2+ neurons (arrows). Modified from Bifari and others (2017).
Figure 5.
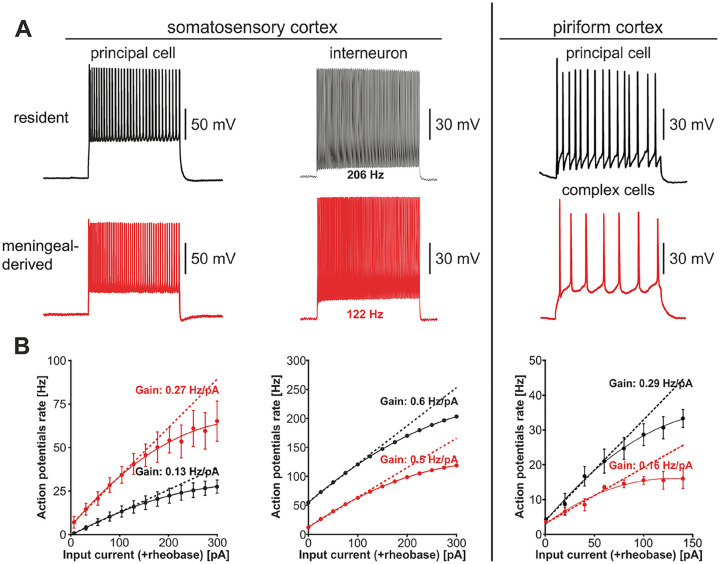
Functional features of meningeal derived neurons. Electrical phenotype of resident and meningeal-derived neurons in somatosensory cortex (A, left panel). Patch current-clamp recordings of action potentials evoked by direct positive current injection in a resident principal cell and interneuron (black) and in cells of glutamatergic and GABAergic phenotype of meningeal origin (red). In B (left panel) the traces are the frequency-current curves obtained by direct positive current injection above rheobase. Meningeal-derived glutamatergic cells exhibit a higher action potential rate and a higher frequency gain as compared to their resident counterparts. The frequency gain of resident and meningeal-derived interneurons is similar, although the meningeal-derived interneuron generates action potentials at a lower rate as compared to its resident counterpart. Modified with permission from Bifari and others (2017). For comparison, the patch current-clamp recordings of action potentials evoked by direct positive current injection in a mature resident principal cell (black) and a postnatal differentiated mature complex cell (red) of the piriform cortex are shown (A, right panel). In B (right panel) the traces are the frequency-current curves obtained by direct positive current injection above rheobase. Complex cells exhibit different action potential rate and frequency gain as compared to their resident counterparts, similarly to meningeal-derived neuronal cells. Patch-clamp traces modified with permission from Benedetti and others (2020). Frequency-current curves obtained from the original dataset from Benedetti and others (2020) kindly provided by the authors.
Meningeal-derived neurons differentiate from non-proliferating embryonic derived quiescent progenitors. Birth-dating experiments indicated that the meningeal neurogenic cells were born during embryo development (E13.5-E16.5), and remained quiescent until birth (Bifari and others 2017). After birth, meningeal neurogenic cells migrated from the meninges to the cortex and, without proliferation, differentiated into cortical neurons. This is consistent with the literature indicating the absence of newly generated neural cells originating from a pool proliferating after birth (Rakic 2002).
Lineage tracing experiments also revealed that immature neural precursors residing in the meninges of healthy adult mice migrated into the brain parenchyma to differentiate into OPCs (Dang and others 2019) (Fig. 4). Interestingly, meningeal-derived OPCs in the healthy brain also originated from non-proliferating meningeal progenitors (Dang and others 2019).
A further indication of the presence of a neural progenitor population in meninges is the potential of cells extracted from meninges to be cultured in vitro as NSCs. The signature of cultured neurospheres derived from meningeal cells and from ventricular prominin-positive RG cells was compared by whole transcriptomics analysis (Bifari and others 2017). Heatmap and metric multidimensional scaling analysis revealed that the meninges-derived cells shared expression of numerous NSC genes (Fabp7, Sox9, Sox2, and Nes) with VZ/SVZ-derived (Bifari and others 2017). Moreover, in vitro cultured meningeal-derived NSCs can differentiate into electrically functional neuronal cells in vitro (Decimo and others 2011; Nakagomi and others 2011) and, following transplantation into the adult hippocampus, differentiated into neurons in vivo (Bifari and others 2009). In addition to neuronal differentiation, NSCs obtained from the adult brain and spinal cord meninges can be cultured and differentiated into mature oligodendrocytes (Dang and others 2019; Decimo and others 2011; Dolci and others 2017) with in vivo myelinating potential (Dolci and others 2017). Although meningeal-derived NSCs share comparable global trascriptome with the VZ/SVZ-derived NSCs, meningeal-derived neurospheres show some peculiarity, including the lower expression of the astrocytic gene GFAP (Bifari and others 2009). Similarly, in vivo astrocyte differentiation of meningeal-derived NSCs appears to be much lower than that observed for the VZ/SVZ-derived NSCs. Seldom meningeal-derived astrocytes have been observed both following transplantation of in vitro cultured meningeal-derived NSCs in the brain (Bifari and others 2009), and after in vivo fate mapping of migrated NSCs from meninges to the cortex (Bifari and others 2017). This observation highlights potentially relevant differences between meningeal-derived and VZ/SVZ NSCs in terms of differentiation potential, function, and ontogenesis.
Functional stem cell niche quickly senses and responds to stimuli from the CNS and from the periphery. Pharmacological administration of FGF-2 and NGF in meninges results in a strong proliferation of meningeal cells and in hyperplastic changes within the meninges of the rat and monkey (Day-Lollini and others 1997; Parr and Tator 2007). Similarly, the administration of EGF and FGF2 to organotypic brain cultures induced the proliferation of meningeal nestin-positive cells (Nakagomi and others 2011). Depending on the micro-environmental conditions, meningeal neural progenitors may be highly responsive to principal mitogens or stay quiescent (long-retaining BrdU) for a long period of time (Day-Lollini and others 1997; Decimo and others 2011; Decimo and others 2012b; Parr and Tator 2007). However, meningeal NSC response to well-known specific neurogenic stimuli such as environmental enrichment (EE) tasks (Kempermann and others 1997) or drugs (i.e., fluoxetine) (Zhou and others 2016) and their potential role in CNS plasticity has yet to be determined.
Overall, data available suggest the presence of neural progenitors in meninges able to contribute to neuro-glia parenchyma during postnatal and adult stages.
Functional Features of Meningeal-Derived Neurons
The functional role of newly born neurons in the adult brain has been proposed according to the location where the new neurons established connection with the preexisting neural circuitry (Kempermann and others 2015). In light of the great interest of the scientific community on adult hippocampal neurogenesis, and on its implications in health and disease, the developmental functional features and biophysical properties of the subgranular zone (SGZ)-derived dentate gyrus (DG) granule cells (GCs) have been extensively characterized (Kempermann and others 2015; Pedroni and others 2014; van Praag and others 2002). At variance, despite the evidence supporting the active contribution of meningeal NSCs as reservoir for migrating functional neurons, only one study has so far addressed the functionality of meningeal-NSCs-derived cells (Bifari and others 2017). In this study, mice were engineered to enable tracing the migratory pathway of meningeal NSCs-derived neurons and identify them by epifluorescent tags to perform targeted patch-clamp recordings (Fig. 5). The study demonstrated that meningeal-derived cells migrate into the superficial layers of the somatosensory cortex, where they differentiate into neuronal cells, which functionally integrate with the preexisting circuitry formed by resident neurons through excitatory and inhibitory synaptic connections. Meningeal-derived neurons can acquire the electrical phenotype of both glutamatergic principal neurons and GABAergic interneurons, they exhibit decreased action potential half-width and increased action potential repolarizing velocity (Fig. 5 and Table 1) and their electrically evoked GABAergic and glutamatergic postsynaptic potentials are similar to the adult phenotype. Interestingly, glutamatergic neurons of meningeal origin can fire action potentials at a higher frequency than their endogenous counterparts, whereas the meningeal-derived interneurons fire at a lower frequency.
Table 1.
Passive and Active Properties of Resident and Adult-Differentiated Neurons in the Dentate Gyrus, Piriform Cortex, and Somatosensory Neocortex.a
| Brain Region | Cell Type (Source) | Passive Membrane Properties | Action Potential Properties | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cm (pF) | Rin (Mohm) | τm (ms) | Vmr (mV) | Threshold (mV) | Amplitude (mV) | Half-width (ms) | Frequency (Hz) | |||
| Dentate gyrus | DG GC immature resident |
14 ± 1 | 1400 ± 100 | 283 ± 23 | −38 ± 1 | −26 ± 1 | 13 ± 1 | 6.1 ± 0.6 | N/A | Pedroni and others 2014 |
| DG GC mature newly generated (local niche) |
42.3 ± 10.2 | 350 ± 37 | 16.6 ± 4 | −69.7 ± 1.7 | −45.8 ± 2.9 | — | — | 27.5 ± 2.6 | van Praag and others 2002 | |
| DG GC mature resident |
99.2 ± 12.7 | 388 ± 37 | 33.7 ± 3.7 | −74.8 ± 1.1 | −39.6 ± 1.5 | — | — | 29.9 ± 4.8 | ||
| Piriform cortex | Neuronal precursor | 24 ± 20 | 1950 ± 1160 | 23 ± 17 | −46.5 ± 8.8 | N/A | N/A | N/A | N/A | Benedetti and others 2020 |
| Immature non-newly
generated adult-differentiated (local niche) |
64 ± 42 | 560 ± 250 | 45 ± 11 | −57.3 ± 4.3 | — | — | — | 12 ± 5.2 | ||
| Immature resident | 110 ± 33 | 480 ± 180 | 36 ± 17 | −68.3 ± 7.6 | — | — | — | 38.4 ± 10.8 | ||
| Mature non-newly generated adult-differentiated (local niche) |
118 ± 53 | 310 ± 240 | 45 ± 17 | −65.0 ± 10.9 | 35 ± 7 | — | — | 17.1 ± 6.7 | ||
| Mature resident |
108 ± 30 | 420 ± 100 | 31 ± 8 | −64.5 ± 7.6 | 38 ± 7 | — | — | 34.2 ± 12.7 | ||
| Somatosensory neocortex | Principal neuron non-newly generated adult-differentiated (meninges) |
152.2 ± 18.3 | 172.1 ± 18.9 | 24.5 ± 2.2 | −67.3 ± 1.8 | −35.6 ± 2.5 | 60.8 ± 4.7 | 0.9 ± 0.1 | 54.8 ± 7 | Bifari and others 2017 |
| Mature principal neuron resident |
181.1 ± 14.4 | 135.8 ± 7.4 | 24.7 ± 2.5 | −69.8 ± 2.8 | 34.01 ± 1.5 | 70.2 ± 3.2 | 1.4 ± 0.1 | 27 ± 2.9 | ||
| Interneuron non-newly generated adult-differentiated (meninges) |
— | — | — | −77.4 | −35.1 | 51.2 | 0.4 | 122 | ||
| Interneuron resident |
— | — | — | −79.4 | −44.5 | 45.4 | 0.4 | 206 | ||
Passive and active properties of endogenous and adult-differentiated neurons in dentate gyrus (DG, orange scale), piriform cortex (green scale), and somatosensory cortex (blue scale). Distinct color hues indicate different stages of neuronal maturation (lighter color, immature neurons and darker color, mature neurons). Resident neurons: neurons found in the region of the analysis, which do not derive from differentiation of precursor cells in adulthood. Newly generated neurons: neurons derived from precursor cells that underwent cell division and subsequent differentiation in adulthood. Non newly generated and adult-differentiated neurons: neurons differentiated in adulthood from quiescent progenitor cells that underwent cell division before birth. Cm = membrane capacitance; Rin = input resistance; τm =membrane time constant; Vmr = resting membrane potential; N/A, not applicable.
Remarkably, immature SGZ-derived GCs of adult brain display increased excitability of synaptic origin, such as reduced inhibitory GABAergic drive and enhanced plasticity due to the lower long term potentiation (LTP) induction threshold (Ge and others 2007; Wang and others 2000) although their glutamatergic innervation is paradoxically lower as compared to that of resident adult DG GCs (Dieni and others 2016; Mongiat and others 2009). Such hyperexcitability is reminiscent of meningeal-derived glutamatergic neurons (Table 1).
Overall, these findings suggest that the NSC-derived neuronal population may express a slightly different biophysical machinery than resident cells. Although this may be ascribed to an earlier maturation stage of NSC-derived neurons versus neighboring neocortical cells, it is also possible that the NSC-derived lineage represents a distinct population that actively contributes to neocortical function; if this is the case, their role going beyond the replenishment of the neocortical neuronal pool.
A similar hypothesis has been more recently formulated regarding an immature neuronal cell population residing in layer II of the piriform cortex that expresses the immature markers DCX and the polysialylated-neural cell adhesion molecule (Benedetti and others 2020) (Fig. 5). Similar to the meningeal-derived neurons, immature neurons found in layer II of the piriform cortex are generated prenatally, remain quiescent and differentiate in the postnatal cortex. These immature neurons appear to have a distinct neuronal functional phenotype. However, the functional properties of these cells differ from meningeal-derived neocortical neurons, as they appear to be exclusively glutamatergic and they fire at a lower frequency than endogenous resident principal cells. In addition, although the dendritic spines express glutamatergic terminals, these immature neurons of the piriform cortex primarily receive GABAergic inputs (Fig. 5 and Table 1).
It remains to be clarified whether the specific electrical properties of meningeal-derived neurons represent the biophysical phenotype of a selected stage of neuronal differentiation, similar to DG GCs, or that of a distinct cellular entity, as hypothesized in the piriform cortex. Selective cellular depletion experiments or a direct functional readout obtained with optogenetic/chemogenetic manipulations will be needed to demonstrate the physiological role for the non-newly generated neurons differentiated in the adulthood from these two neurogenic niches. Furthermore, what determines the settlement zone, the neuronal lineage differentiation, and the biophysical phenotype of migrating meningeal NSCs still remains an open question.
Meningeal Neural Progenitors in Disease
Following diseases, NSC niche is activated and precursor cells migrate and participate to the parenchymal reaction (Decimo and others 2012a; Morrison and Spradling 2008) (Fig. 6). NSC niche activation by diseases can be induced by different signals that include tissue damage, vascular and blood perfusion impairment, cell death, and inflammatory signals (Decimo and others 2012a). Modifications may occur following diseases within the niche: (1) invasion by inflammatory molecules, chemokines, and immune cells; (2) niche cell proliferation; (3) change of the molecular signature and nature of neural progenitors; and (4) remodelling in ECM composition.
Figure 6.
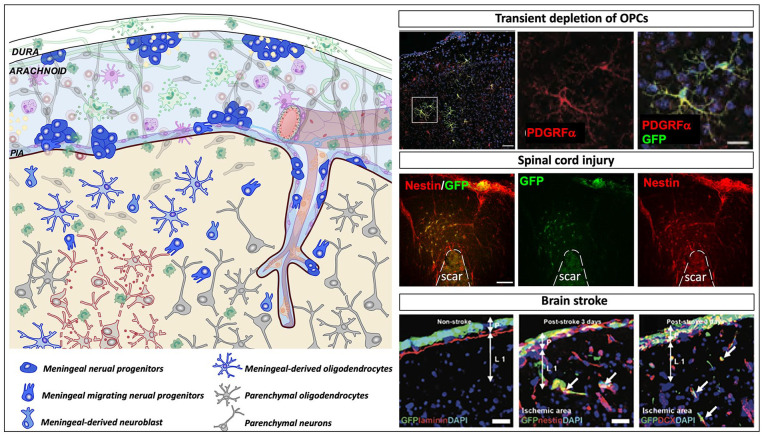
Meningeal neural progenitors in diseases. Schematic representation showing the meningeal environment in the central nervous system pathological conditions (left panel). Meninges are formed by three tissue membranes: dura mater, arachnoid and pia mater. Following diseases, different signals that include tissue damage, vascular and blood perfusion impairment, cell death, and inflammatory signals activate the meningeal niche. Meningeal progenitor cells, promptly react, proliferate, and migrate from the meninges to the brain parenchyma and differentiate into immature neurons and functional cortical oligodendrocytes. In the right panels, the injury induced meningeal-derived neural cell contribution to three different pathological conditions is shown. In the upper right panel, following transient depletion of oligodendrocyte precursor cells (OPCs), meningeal derived OPCs migrate to the injured parenchyma and differentiate into oligodendrocytes; modified from Dang and others (2019). In the middle right panel, following spinal cord injury, meningeal neural precursors (nestin+, red) migrate to the glial scar site; modified from Decimo and others (2011). In the lower right panel, after brain stroke, meninges increase the expression of nestin- and DCX-positive cells, which migrate to the injured cortex and potentially contribute to cortical regeneration/repair; modified from Nakagomi and others (2012).
Lineage tracing and in vivo labeling as well as histological, ex vivo and in vitro approaches have been used to describe disease-induced meningeal NSC niche activation. Several and different animal models of diseases showed the injury-induced activation of neural progenitors in meninges, including stroke, progressive ataxia, epilepsy, and spinal cord injury (Dang and others 2019; Decimo and others 2011; Kumar and others 2014; Nakagomi and others 2011; Nakagomi and others 2012; Ninomiya and others 2013; Tatebayashi and others 2017) (Fig. 6).
Cerebral ischemia is followed by activation of endogenous NSCs (Lin and others 2015). Interestingly, cells expressing Nestin, PDGFRβ, and SOX2 appear in the poststroke meninges and in the perivascular space of infiltrating pial vessels (Nakagomi and others 2011) (Fig. 6). Further supporting the NSC nature of these cells, ex vivo organotypic brain culture experiments showed that ischemia-induced meningeal NSCs (iNSCs) formed neurosphere-like cell clusters with self-renewal activity and neural differentiation potential. In addition to the increase in stem cell potential, in vivo labeling techniques were used to show that ischemia induced in meninges an increased expression of DCX-positive cells, which migrate to the poststroke cortex and potentially contribute to cortical regeneration/repair (Nakagomi and others 2012). However, the number of meninges-derived DCX-positive cells in the poststroke cortex gradually decreased with time, and no meningeal-derived mature neurons were present in these regions 60 days after stroke in vivo (Nakagomi and others 2012).
The contribution of meningeal cells to the CNS injured parenchyma was also confirmed in other pathological settings. Following spinal cord injury, nestin-positive cells increased their self-renewal and proliferative properties, and DCX-positive cells were observed in adult spinal cord meninges (Decimo and others 2011) (Fig. 6). Cells extracted from spinal cord injured meninges formed in vitro neurospheres, which can differentiate into functional neurons and mature oligodendrocytes (Decimo and others 2011). By an in vivo labeling approach, it was shown that following spinal cord injury, meningeal NSCs proliferate, increase in number, and migrate in the neural parenchyma where they contribute to the neural parenchymal reaction (Decimo and others 2011). Interestingly, meninges-derived cells present in the parenchyma, outside and far from the fibrotic scar, expressed the NSC and neuroblast markers nestin and DCX. Some meningeal-derived nestin- and seldom DCX-positive cells were also observed in the injured spinal cord parenchyma one month after the injury. These cells did not express either the glia reactive marker GFAP or the oligodendrocyte precursor marker NG2 (Decimo and others 2011).
The neurogenic potential of meningeal NSCs was nicely shown in a model of adult progressive cerebellar degeneration with early-onset microgliosis (Kumar and others 2014). Here, the transplantation into the cerebellum of human cerebellar granule neuron precursors (GNP) triggered the proliferation of endogenous nestin-positive precursors in the meninges. Remarkably, although transplanted GNPs did not survive more than a few weeks, meningeal endogenous NSCs were activated, crossed the outermost molecular layer and differentiated into mature neurons. These phenomena were accompanied by the preservation of the granule and Purkinje cell layers and delayed ataxic changes (Kumar and others 2014). The neurogenic potential of cerebellar meninges in this mouse model transplanted with exogenous human GNPs, was also confirmed by in vitro cultures of neurospheres and their subsequent neuronal differentiation.
In amygdala kindling animal model of epilepsy, a strong activation of nestin-expressing cells was shown in the meninges (Ninomiya and others 2013). Interestingly, genetic ablation of nestin expression resulted in a high susceptibility to kindling, suggesting that the nestin-positive cells activated by amygdala kindling may exert an anti-epileptogenic role (Ninomiya and others 2013).
In addition to the neurogenic potential of meninges NSCs, results obtained in the adult brain suggest that these cells also have the potential to generate oligodendrocytes (Fig. 6). Following transient ablation of OPCs in the adult brain, a strong activation of NSCs residing in cortical meninges could be observed (Dang and others 2019). OPCs expressing platelet-derived growth factor receptor-alpha (PDGFRα) were ablated by using an inducible transgenic mouse where the PDGFRα expression was knocked out in all the cells following tamoxifen administration. In this model, OPCs were fully eliminated after 5 days of tamoxifen treatment. Interestingly, already three days from the tamoxifen treatment, repopulating PDGFRα+ OPCs were seen as small clusters randomly distributed in meninges and in perivascular localizations as well as within the brain parenchyma. These cells were highly proliferating and displayed the typical immature OPC morphology, including short studded cytoplasmic processes (Dang and others 2019). By selectively labeling the proliferating OPCs of meninges with retroviral GFP transduction, it was possible to observe that the meninges-derived GFP+/PDGFRα+ cells with the typical immature OPC morphology had migrated in the cerebral cortex near the meningeal labelling site. Interestingly, after 14 days from the tamoxifen treatment, meninges-derived GFP+/PDGFRα+ cells had increased in number and were distributed into the deeper layers of the cortex, showing a highly ramified morphology characteristic of differentiating OPCs. At a later stage, some of the meninges-derived cells also expressed myelin proteins suggesting a contribution of these cells to the myelin-forming cell pool in the brain: thus, participation of the meningeal pool of cells can be considered an alternative and complementary pathway (other than the classical OPC-self renewal pathway) available for the regeneration of a severely damaged oligodendroglial lineage (Bergles and Richardson 2015) (Fig. 6). These meningeal-derived OPCs apparently originate from PDGFRα-negative cells. In particular, by using the transgenic reporter mice harboring a Nestin-promoter and enhancer-driven, Nestin-Cre, intercrossed with Rosa26-mCherry reporter, yielding Nestin:Cherry mouse, it was possible to show that most of the meninges-derived OPCs were originated from nestin expressing cells. Quite remarkably, this reporter mice have the enhancer encoded in the second intron, a condition whereby the nestin gene is specifically induced in neural stem cells of the CNS, and not in other mesodermal stem cells (Zimmerman and others 1994), further suggesting the NSC origin of the meningeal cells.
Overall, the in vivo data suggest the presence of a reservoir of neural precursor in the meninges in physiological condition that promptly respond to neural pathological states. In vivo neurogenic and oligodendrogenic differentiation potential of meninges NSCs has been shown both in health and diseases (Figs. 4 and 6). Accumulated disease-induced meningeal neural precursors migrate to the neural parenchyma and contribute to the neural regeneration of the CNS.
Meningeal Neural Progenitor Migratory Pathway
The migratory pathways of meningeal neural progenitors to the parenchyma, the precise mechanisms of accessing to the brain and the chemotactic guidance signaling regulating this migration have not yet been fully described. They may vary depending on the developmental stage, on the selected meningeal progenitor phenotype and on specific meningeal region (e.g., brain, spinal cord). In mice, during neonatal stage, meningeal neural progenitors have been described to follow, as track route, the major meningeal substructure underneath the hippocampus, which allow them to migrate from the posterior meningeal cortex to the ventricles (tela choroidea) (Bifari and others 2017) (Fig. 7). From the ventricles, they infiltrate into the parenchyma reaching their terminal destination in the layer II-III of the cortex where they differentiate into neurons. In line with this migratory pathway, postnatal and adult CXCR4-expressing neural progenitors of ventricular derivation were observed migrating along the meningeal substructure in direction of the hippocampal dentate gyrus (Belmadani and others 2015).
Figure 7.
Schematic representation of the migratory pathway of meningeal neural progenitors to the parenchyma. In the left panel proposed mechanisms of the meningeal neural progenitor cell migration to the underlying parenchyma through the pial basal lamina (dashed line). Meningeal neural progenitors migrate from specific meningeal areas, including brain and spinal cord meninges, meninges of the perivascular space and of the cerebellum. The precise mechanisms of accessing to the brain and the chemotactic guidance signaling regulating this migration have not yet been clearly described.
In the right upper panel, the migration path of meningeal progenitor cells to the cortex during perinatal stage (black line). Specifically, meningeal neural progenitors migrate to the cortex via the meningeal substructure (right middle panel) and tela choroidea (lower right panel). Modified from Bifari and others (2017).
At variance, in adult mice, meningeal OPCs have been proposed to migrate to the cortex from the surrounding meninges suggesting they may infiltrate directly into the parenchyma through the pial basal lamina (Dang and others 2019).
In pathological conditions, neural progenitors have been observed to migrate from the brain, spinal cord, and cerebellar meninges to the injured parenchyma (Decimo and others 2011; Kumar and others 2014; Nakagomi and others 2011). Although their migratory pathway has not been fully described yet, they seem to reach the injured parenchyma directly form the neighboring meninges. Parenchymal infiltration through the basal lamina from the adjacent meninges and meningeal perivascular space has been described also for brain reactive immune cells, which became able to reach the area of damaged tissue (Schlager and others 2016) (Fig. 7). In adult animal model of progressive cerebellar degeneration, meningeal NSCs have been observed to enter the parenchyma from the nearby leptomeninges crossing the outermost molecular layer of the cerebellum (Kumar and others 2014). In spinal cord injury, demyelination, and stroke, meningeal progenitors were frequently observed in perivascular locations suggesting that they may use vessels as track for their migration to the parenchyma (Dang and others 2019; Decimo and others 2011; Nakagomi and others 2011) (Fig. 7). What are the attractive molecules guiding meningeal progenitors to the damaged parenchyma tissue still remains unknown; however, it can be speculated that the increase of chemokines such as SDF1 at the injured site may drive meningeal NSCs response and attract them to the injured parenchyma. Insights on the traveling mechanisms of NSCs may derive from many studies on the biology of the SVZ-derived NSCs that migrate within the rostral migratory stream and generate olfactory bulb interneurons (Lois and others 1996). SVZ-derived NSCs also migrate to ischemic regions following perivascular tracks (Kojima and others 2010).
Because of their high anatomical complexity, widespread distribution and intimate connection with the CNS parenchyma and vasculature, meninges may provide a net of potential trails properly suited to allow migration of neural precursors toward any site within the CNS. This consideration raises the question of whether the cells observed in meninges, including neural precursors, have originated in meninges or are just travelling through them. Moreover, the possibility of a long-distance migration of neural precursors trough the meningeal net may suggest the existence of a functional network of NSC niches in the CNS that may use the threads of the meningeal net as tracks. In this scenario, proliferation of NSCs is not a mandatory requirement for the CNS plasticity obtained by addition of new cells, as far as quiescent neural or glial progenitors may migrate long distances and, without proliferation, reach and integrate into the neural tissue (Decimo and others 2012a).
Further studies using in vivo migration and motility assays as well as in vitro tracing by two-photon intravital microscopy will be important to identify the specific chemotactic signals and the migration pathways of meningeal neural progenitors.
Meningeal Neural Progenitors and Cancer
Expression of NSCs and neuroblast markers, including DCX, have been observed in cases of meningeal tumors (Ide and others 2011; Petricevic and others 2011). As expected from a niche for immature stem cells, meninges host metastasis of nearly every malignancy, with the highest incidence in hematologic, melanoma, lung, breast, and brain cancers. The precise role of endogenous NSCs and meningeal NSC niche in the brain cancer development is an overlooked topic.
The relevance of the meningeal niche in neuro-oncology is being revisited with particular relevance for two distinct domains: the tumor microenvironment, where the meningeal niche contributes to the regulation of the interstitial fluid flow (Stine and Munson 2019) and of the intracranial lymphatic drainage; and the immunosurveillance of primary brain tumors, such as gliomas. Gliomas are endowed with a remarkable infiltrative nature (Boye and others 2017; Giese and Westphal 1996; Kingsmore and others 2016; Qazi Shi and Tarbell 2011), and they are likelihood of progression and recurrence, and ultimate resistance to available surgical and adjuvant therapies, including immunotherapeutic approaches (Lim and others 2018). As for the role of microenvironment, gliomas induce derangement of normal local extracellular matrix and of non-neuronal cell populations (Geer and Grossman 1997), supporting differential flow of the interstitial fluid. Gliomas spread through perivascular spaces (Cuddapah and others 2014), along white matter tracts (Geer and Grossman 1997), in perineuronal spaces, and along the meningeal layers lining the brain, as demonstrated by the pattern of local progression and recurrence of the tumors away from the primary CNS site of origin (Fukuya and others 2019; Konishi and others 2012). As for the lymphatic drainage, it should be considered that the meningeal lymphatic system also drains interstitial fluid tumor components, thus representing a pathway for dissemination of tumor cells (Hu and others 2020), as demonstrated in mice models where glioma and melanoma cells were implanted intracranially (Hu and others 2020).
Dendritic cells play a pivotal role in immunosurveillance of primary glioma. The dendritic cell trafficking was demonstrated to occur through the meningeal lymphatic system of mice models of gliomas and melanoma (Hu and others 2020). More recent data further support the argument that a relevant contribution of immunosurveillance of brain tumors could come from a morpho-functional reshaping of the meningeal lymphatic system (Song and others 2020). Here, the VEGF-C and its receptor partner have a role in enhancing the trafficking of CD8 T cells to the deep cervical lymph nodes and ameliorating a synergistic effect with checkpoint immunotherapeutic agents (Song and others 2020). The microenvironment of glioblastoma is deprived of lymphangiogenic signals, thus contributing to increasing its protection from T cells (Song and others 2020).
A deeper knowledge of the contribution of the meningeal niche to tumor spread and resilience to available treatments is thus needed to improve the understanding the pathogenic mechanisms of these lesions and, more relevantly, to disclosed new potential pathways for delivering therapies and/or for identifying new targets to enhance immunosurveillance of both primary and metastatic brain tumors.
Furthermore, whether meningeal NSCs influence brain tumors formation and progression is still completely unexplored.
Meningeal Neural Progenitor Regenerative Potential
The evidence described so far converges toward the view that the regenerative potential of meningeal neural progenitors is an emerging frontier of investigation to explore the plastic properties of the brain and its ability to self-repair. The reaction of the endogenous meningeal neural progenitors to CNS disorders may provide new therapeutic targets to be exploited for CNS disorders treatment. A better understanding of the molecular signals activating endogenous meningeal progenitors and inducing their expansion, migration, and neural differentiation potential, will set the stage for potential relevant new approaches for regenerative medicine of the CNS.
Meninges can be involved in CNS regenerative medicine also from another perspective that considers this structure as a relevant route of cell infiltration to the brain in physiological condition. Following CNS pathological states, there may be discontinuity in meningeal barrier or increased fenestration of the pia basal lamina, which in turn, may increase the migration of meningeal cells to the CNS parenchyma. Furthermore, meninges are closely associated with the blood vessels forming the perivascular space. From here, neural progenitors may migrate for long distance and supply new neural cells to the damaged CNS area. Disease-induced meningeal activation also involves several other cell types in addition to neural progenitors. Immune cells have been described to accumulate in the meninges at an early stage of CNS disorders and invade the neural parenchyma from the meninges (Schlager and others 2016). The increased cellular migration through the meninges has been well documented in multiple sclerosis, where meninges represent a checkpoint at which activated brain-reactive T cells are licensed to enter the CNS parenchyma and subsequently to damage neural tissue (Russi and Brown 2015; Walker-Caulfield and others 2015). Moreover, other meningeal cells, including fibroblasts, pericytes and lymphatic cells, can be activated by pathological conditions and contribute to secrete diffusible morphogens, chemokines and ECM molecules (Aviezer and others 1994; Benakis and others 2018; Chen and others 2019; Davare and others 2014; Decimo and others 2012b; Duan and others 2018; Lehtinen and others 2011; Paredes and others 2006; Schlager and others 2016; Song and others 2020). As a whole, the disease-induced meningeal reaction involves many different cell types and molecules. Therefore, multiple therapies aimed at modulating this response can be employed to further increase the neural regenerative potential of meninges.
Another very important consideration is the potential to obtain NSC-like cultures from NSCs extracted from the meninges (Fig. 8). In vitro cultured meningeal NSCs have been obtained in different animal models at several developmental stages including the adulthood (Bifari and others 2009; Decimo and others 2011; Dolci and others 2017). Moreover, NSC-like cultures have been obtained from different meningeal locations, suggesting that meningeal NSCs are not restricted to small selected meningeal areas. Meningeal NSCs can be expanded in vitro and subsequently differentiated into mature neurons and myelinating oligodendrocytes (Bifari and others 2009; Bifari and others 2020; Dang and others 2019; Decimo and others 2011; Dolci and others 2017; Kumar and others 2014; Martano and others 2019; Nakagomi and others 2011; Ninomiya and others 2013) (Fig. 8). Importantly, the in vitro cultured meningeal NSCs can undergo neuronal differentiation following transplantation in the hippocampus (Bifari and others 2009) and express myelinating potential following transplantation in the spinal cord (Dolci and others 2017). The feasibility of obtaining NSC-like culture from the meninges is remarkable for the following reasons:
Figure 8.
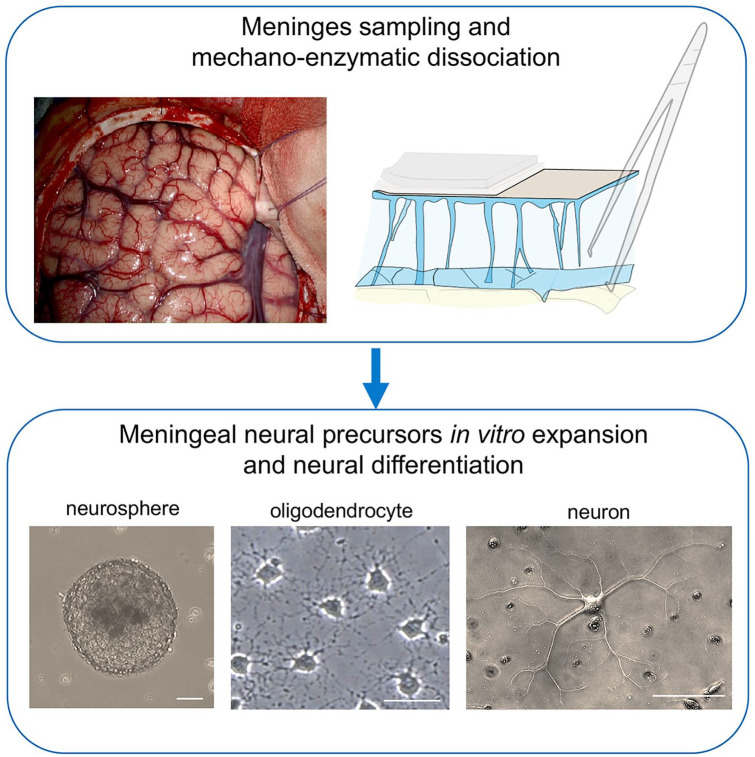
Regenerative potential of meningeal neural progenitors. In the upper panel image, the human brain meninges exposed during neurosurgery. Meningeal neural progenitors can be isolated from meningeal samples by mechano-enzymatic dissociation and in vitro cultured. Meningeal neural stem cells (NSCs) can be expanded in vitro and subsequently differentiated into oligodendrocytes and neurons (light microscope images in the lower panel). Meningeal NSCs can potentially be used for regenerative medicine in autologous graft setting. Scale bars are 20 µm.
Somatic meningeal NSCs are present in the adult mammalian CNS, including the human CNS (Beppu and others 2019; Tatebayashi and others 2017).
Meningeal NSCs are located at the CNS surface, therefore being potentially more accessible for tissue sampling.
Meningeal NSCs can potentially be used in autologous graft setting. The NSCs extracted from a subject, may be cultured and expanded in vitro and subsequently transplanted in the damaged CNS area of the same patient (Fig. 8).
Indeed, from a small biopsy of spinal cord meninges it has been possible to obtain in vitro high yield of myelinating oligodendrocytes (Dolci and others 2017). Though most of our knowledge on meninges and NSCs comes from animal studies, it is noteworthy that NSC-like cultures have been obtained from autoptic sample of poststroke human brain and cerebellum (Beppu and others 2019; Tatebayashi and others 2017). Although with some specificities in gene expression, suggesting some region-specific traits, these in vitro cultured cells showed neuronal differentiation potential.
Autologous meningeal NSCs transplantation may be achievable for brain regenerative medicine in human. However, the access to meninges implies an invasive intervention and the number of available meningeal progenitors per meningeal volume present in the adult human meninges may be a constraint. Further studies elucidating the feasibility of the use of human NSCs resident in meninges for regenerative medicine are needed.
Several data accumulated over the last decades suggest that major regenerative properties of transplanted NSC reside in their so-called non-neurogenic functions, which include the trophic and immune modulatory activity. Cultured meninges NSCs support T, B, and NK cell survival (Di Trapani and others 2013). Once primed by inflammatory stimuli (INF-γ and TNFα), meningeal NSCs drastically modify their properties and up-regulate molecules that are important for immune cell interactions such as CD40, PDL-1, CD112, CD115, and the adhesion molecules ICAM-I and VCAM (Di Trapani and others 2013). The in vivo relevance of these immune modulatory properties has to be addressed. Several pathological conditions involve meninges inflammatory activation, which further supports immune cell attachment and migration into the CNS parenchyma. Whether the impairment of meningeal resident NSC immune modulatory activity may also contribute to meningeal inflammation remains to be determined. Similarly, whether the pharmacological modulation of the meningeal resident NSC immune modulatory activity may represent an additional target to improve brain regeneration has never been assessed.
Conclusion and Future Perspectives
Data from literature describe meninges as a niche for NSCs during development and in the adulthood. These immature neural cells are able to migrate from the meninges to the brain parenchyma and differentiate into functional cortical neurons or oligodendrocytes (Bifari and others 2017; Dang and others 2019). Immature neural cells residing in the meninges promptly react to brain disease. Injury-induced expansion and migration of meningeal neural progenitors have been observed following experimental demyelination, traumatic spinal cord and brain injury, amygdala lesion, stroke, and progressive ataxia (Dang and others 2019; Decimo and others 2011; Kumar and others 2014; Nakagomi and others 2011; Ninomiya and others 2013).
As a whole, the meninges appear as an overlooked pharmacological target for regenerative medicine of the CNS. Drugs acting by modulating the meningeal endogenous neural progenitors may provide new and effective therapies for CNS disorders. Moreover, the meninges are a potential source of adult, somatic, autologous NSCs that can be expanded in vitro and endowed with neural and oligodendrocyte differentiation potential following transplantation in vivo. Cultured meninges NSCs can also be a valuable somatic alternative to the induced pluripotent stem cells, to study and better understand the pathophysiology of genetic neurodegenerative diseases as well as a drug screening platform to test and identify therapeutic leads for neuronal and myelin cell survival and differentiation.
Acknowledgments
We acknowledge Marzia Di Chio for the help with the images of human brain samples.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Cariverona Foundation (Grant Number 2017-0604), Fondazione Italiana Sclerosi multipla (FISM) (Grant Number 2017/R/11) and financed or co-financed with 5 per mille public funding; Italian patient association la Colonna and GALM and University of Verona (Grant Number DDSP-FUR-6616) to I.D. and G.F.; University of Milan (Grant Number BIOMETRA15-6-3003005-1 and PSR2018_RIVA_BIFARI) to M.R. and F.B; and Fondazione Telethon–Italy (Grant Number GGP19250) to F.B.
ORCID iDs: Marco Riva  https://orcid.org/0000-0003-4643-6451
https://orcid.org/0000-0003-4643-6451
Francesco Bifari  https://orcid.org/0000-0003-2028-3012
https://orcid.org/0000-0003-2028-3012
References
- Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A.1994. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell 79(6):1005–13. [DOI] [PubMed] [Google Scholar]
- Barber M, Andrews WD, Memi F, Gardener P, Ciantar D, Tata M, and others. 2018. Vascular-derived vegfa promotes cortical interneuron migration and proximity to the vasculature in the developing forebrain. Cereb Cortex 28(7):2577–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani A, Ren D, Bhattacharyya BJ, Rothwangl KB, Hope TJ, Perlman H, and others. 2015. Identification of a sustained neurogenic zone at the dorsal surface of the adult mouse hippocampus and its regulation by the chemokine SDF-1. Hippocampus 25(11):1224–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C, Llovera G, Liesz A.2018. The meningeal and choroidal infiltration routes for leukocytes in stroke. Ther Adv Neurol Disord 11:1756286418783708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti B, Dannehl D, Konig R, Coviello S, Kreutzer C, Zaunmair P, and others. 2020. Functional integration of neuronal precursors in the adult murine piriform cortex. Cereb Cortex 30(3):1499–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beppu M, Nakagomi T, Takagi T, Nakano-Doi A, Sakuma R, Kuramoto Y, and others. 2019. Isolation and characterization of cerebellum-derived stem cells in poststroke human brain. Stem Cells Dev 28(8):528–42. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Richardson WD. 2015. Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol 8(2):a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifari F, Berton V, Pino A, Kusalo M, Malpeli G, Di Chio M, and others. 2015. Meninges harbor cells expressing neural precursor markers during development and adulthood. Front Cell Neurosci 9:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifari F, Decimo I, Chiamulera C, Bersan E, Malpeli G, Johansson J, and others. 2009. Novel stem/progenitor cells with neuronal differentiation potential reside in the leptomeningeal niche. J Cell Mol Med 13(9B):3195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifari F, Decimo I, Pino A, Llorens-Bobadilla E, Zhao S, Lange C, and others. 2017. Neurogenic radial glia-like cells in meninges migrate and differentiate into functionally integrated Neurons in the Neonatal Cortex. Cell Stem Cell 20(3):360–373.e7. [DOI] [PubMed] [Google Scholar]
- Bifari F, Dolci S, Bottani E, Pino A, Di Chio M, Zorzin S, and others. 2020. Complete neural stem cell (NSC) neuronal differentiation requires a branched chain amino acids-induced persistent metabolic shift towards energy metabolism. Pharmacol Res 158:104863. [DOI] [PubMed] [Google Scholar]
- Borrell V, Marin O.2006. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci 9(10):1284–93. [DOI] [PubMed] [Google Scholar]
- Boye K, Pujol N, I DA, Chen YP, Daubon T, Lee YZ, and others. 2017. The role of CXCR3/LRP1 cross-talk in the invasion of primary brain tumors. Nat Commun 8(1):1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar AK, Chernausek SD. 1993. Localization of insulin-like growth factor binding protein-4 expression in the developing and adult rat brain: analysis by in situ hybridization. J Neurosci Res 35(1):103–14. [DOI] [PubMed] [Google Scholar]
- Castro Dias M, Mapunda JA, Vladymyrov M, Engelhardt B.2019. Structure and junctional complexes of endothelial, epithelial and glial brain barriers. Int J Mol Sci 20(21):5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala M. 1998. Embryonic and fetal development of structures associated with the cerebro-spinal fluid in man and other species: Part I. The ventricular system, meninges and choroid plexuses. Arch Anat Cytol Pathol 46(3):153–69. [PubMed] [Google Scholar]
- Chau KF, Springel MW, Broadbelt KG, Park HY, Topal S, Lun MP, and others. 2015. Progressive differentiation and instructive capacities of amniotic fluid and cerebrospinal fluid proteomes following neural tube closure. Dev Cell 35(6):789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, He J, Ni R, Yang Q, Zhang Y, Luo L.2019. Cerebrovascular injuries induce lymphatic invasion into brain parenchyma to guide vascular regeneration in zebrafish. Dev Cell 49(5):697–710.e5. [DOI] [PubMed] [Google Scholar]
- Choe Y, Huynh T, Pleasure SJ. 2014. Migration of oligodendrocyte progenitor cells is controlled by transforming growth factor β family proteins during corticogenesis. J Neurosci 34(45):14973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe Y, Siegenthaler JA, Pleasure SJ. 2012. A cascade of morphogenic signaling initiated by the meninges controls corpus callosum formation. Neuron 73(4):698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddapah VA, Robel S, Watkins S, Sontheimer H.2014. A neurocentric perspective on glioma invasion. Nat Rev Neurosci 15(7):455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang TC, Ishii Y, Nguyen V, Yamamoto S, Hamashima T, Okuno N, and others. 2019. Powerful homeostatic control of oligodendroglial lineage by pdgfralpha in adult brain. Cell Rep 27(4):1073–1089.e5. [DOI] [PubMed] [Google Scholar]
- Dasgupta K, Jeong J.2019. Developmental biology of the meninges. Genesis 57(5):e23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare MA, Lal S, Peckham JL, Prajapati SI, Gultekin SH, Rubin BP, and others. 2014. Secreted meningeal chemokines, but not VEGFA, modulate the migratory properties of medulloblastoma cells. Biochem Biophys Res Commun 450(1):555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day-Lollini PA, Stewart GR, Taylor MJ, Johnson RM, Chellman GJ. 1997. Hyperplastic changes within the leptomeninges of the rat and monkey in response to chronic intracerebroventricular infusion of nerve growth factor. Exp Neurol 145(1):24–37. [DOI] [PubMed] [Google Scholar]
- Decimo I, Bifari F, Krampera M, Fumagalli G.2012. a. Neural stem cell niches in health and diseases. Curr Pharm Des 18(13):1755–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decimo I, Bifari F, Rodriguez FJ, Malpeli G, Dolci S, Lavarini V, and others. 2011. Nestin- and doublecortin-positive cells reside in adult spinal cord meninges and participate in injury-induced parenchymal reaction. Stem Cells 29(12):2062–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decimo I, Fumagalli G, Berton V, Krampera M, Bifari F.2012. b. Meninges: from protective membrane to stem cell niche. Am J Stem Cells 1(2):92–105. [PMC free article] [PubMed] [Google Scholar]
- Di Trapani M, Bassi G, Ricciardi M, Fontana E, Bifari F, Pacelli L, and others. 2013. Comparative study of immune regulatory properties of stem cells derived from different tissues. Stem Cells Dev 22(22):2990–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieni CV, Panichi R, Aimone JB, Kuo CT, Wadiche JI, Overstreet-Wadiche L.2016. Low excitatory innervation balances high intrinsic excitability of immature dentate neurons. Nat Commun 7:11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolci S, Pino A, Berton V, Gonzalez P, Braga A, Fumagalli M, and others. 2017. High yield of adult oligodendrocyte lineage cells obtained from meningeal biopsy. Front Pharmacol 8:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Zhang XD, Miao WY, Sun YJ, Xiong G, Wu Q, and others. 2018. PDGFRβ cells rapidly relay inflammatory signal from the circulatory system to neurons via chemokine CCL2. Neuron 100(1):183–200.e8. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Couly G, Vincent C, Le Douarin NM. 1999. Anterior cephalic neural crest is required for forebrain viability. Development 126(16):3533–43. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF. 2001. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 128(7):1059–68. [DOI] [PubMed] [Google Scholar]
- Fayein NA, Courtois Y, Jeanny JC. 1992. Basic fibroblast growth factor high and low affinity binding sites in developing mouse brain, hippocampus and cerebellum. Biol Cell 76(1):1–13. [DOI] [PubMed] [Google Scholar]
- Fukuya Y, Ikuta S, Maruyama T, Nitta M, Saito T, Tsuzuki S, and others. 2019. Tumor recurrence patterns after surgical resection of intracranial low-grade gliomas. J Neurooncol 144(3):519–28. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H.2007. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54(4):559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geer CP, Grossman SA. 1997. Interstitial fluid flow along white matter tracts: a potentially important mechanism for the dissemination of primary brain tumors. J Neurooncol 32(3):193-201. [DOI] [PubMed] [Google Scholar]
- Giese A, Westphal M.1996. Glioma invasion in the central nervous system. Neurosurgery 39(2):235–50. [DOI] [PubMed] [Google Scholar]
- Gotz M, Stoykova A, Gruss P.1998. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron 21(5):1031–44. [DOI] [PubMed] [Google Scholar]
- Hartmann D, Sievers J, Pehlemann FW, Berry M.1992. Destruction of meningeal cells over the medial cerebral hemisphere of newborn hamsters prevents the formation of the infrapyramidal blade of the dentate gyrus. J Comp Neurol 320(1):33–61. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Morizane A, Koyanagi M, Ono Y, Sasai Y, Hashimoto N, and others. 2008. Meningeal cells induce dopaminergic neurons from embryonic stem cells. Eur J Neurosci 27(2):261–8. [DOI] [PubMed] [Google Scholar]
- Hu X, Deng Q, Ma L, Li Q, Chen Y, Liao Y, and others. 2020. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res 30(3):229–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T, Fraser PA, Cserr HF. 1991. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res 545(1-2):103–13. [DOI] [PubMed] [Google Scholar]
- Ide T, Uchida K, Suzuki K, Kagawa Y, Nakayama H.2011. Expression of cell adhesion molecules and doublecortin in canine anaplastic meningiomas. Vet Pathol 48(1):292–301. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, and others. 2012. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. 1970. On the mode of entry of blood vessels into the cerebral cortex. J Anat 106(Pt 3):507–20. [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. 1997. More hippocampal neurons in adult mice living in an enriched environment. Nature 386(6624):493–5. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Song H, Gage FH. 2015. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol 7(9):a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, and others. 2007. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells 25(9):2146–57. [DOI] [PubMed] [Google Scholar]
- Khan S. 2019. IGFBP-2 Signaling in the brain: from brain development to higher order brain functions. Front Endocrinol (Lausanne) 10:822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsmore KM, Logsdon DK, Floyd DH, Peirce SM, Purow BW, Munson JM. 2016. Interstitial flow differentially increases patient-derived glioblastoma stem cell invasion via CXCR4, CXCL12, and CD44-mediated mechanisms. Integr Biol (Camb) 8(12):1246–1260. [DOI] [PubMed] [Google Scholar]
- Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, and others. 2010. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells 28(3):545–54. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, and others. 2010. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell 7(2):163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokovay E, Wang Y, Kusek G, Wurster R, Lederman P, Lowry N, and others. 2012. VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell 11(2):220–30. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Muragaki Y, Iseki H, Mitsuhashi N, Okada Y.2012. Patterns of intracranial glioblastoma recurrence after aggressive surgical resection and adjuvant management: retrospective analysis of 43 cases. Neurol Med Chir (Tokyo) 52(8):577–86. [DOI] [PubMed] [Google Scholar]
- Kumar M, Csaba Z, Peineau S, Srivastava R, Rasika S, Mani S, and others. 2014. Endogenous cerebellar neurogenesis in adult mice with progressive ataxia. Ann Clin Transl Neurol 1(12):968–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, and others. 2007. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci 27(46):12623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts D, Storkebaum E, Morimoto M, Del-Favero J, Desmet F, Marklund SL, and others. 2003. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet 34(4):383–94. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, and others. 2011. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69(5):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. 1990. CNS stem cells express a new class of intermediate filament protein. Cell 60(4):585–95. [DOI] [PubMed] [Google Scholar]
- Lim M, Xia Y, Bettegowda C, Weller M.2018. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 15(7):422–442. [DOI] [PubMed] [Google Scholar]
- Lin R, Cai J, Nathan C, Wei X, Schleidt S, Rosenwasser R, and others. 2015. Neurogenesis is enhanced by stroke in multiple new stem cell niches along the ventricular system at sites of high BBB permeability. Neurobiol Dis 74:229–39. [DOI] [PubMed] [Google Scholar]
- Llorens-Bobadilla E, Zhao S, Baser A, Saiz-Castro G, Zwadlo K, Martin-Villalba A.2015. Single-cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell 17(3):329–40. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A.1996. Chain migration of neuronal precursors. Science 271(5251):978–81. [DOI] [PubMed] [Google Scholar]
- Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J.2017. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest 127(9):3210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M. 2012. The human brain intracerebral microvascular system: development and structure. Front Neuroanat 6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martano G, Borroni EM, Lopci E, Cattaneo MG, Mattioli M, Bachi A, and others. 2019. Metabolism of stem and progenitor cells: proper methods to answer specific questions. Front Mol Neurosci 12:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Arikawa-Hirasawa E.2012. Heparan sulfate niche for cell proliferation in the adult brain. Neurosci Lett 510(2):67–72. [DOI] [PubMed] [Google Scholar]
- Mercier F, Hatton GI. 2000. Immunocytochemical basis for a meningeo-glial network. J Comp Neurol 420(4):445–65. [DOI] [PubMed] [Google Scholar]
- Mercier F, Hatton GI. 2001. Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: roles in stem cell proliferation and morphological plasticity? J Comp Neurol 431(1):88–104. [DOI] [PubMed] [Google Scholar]
- Mongiat LA, Esposito MS, Lombardi G, Schinder AF. 2009. Reliable activation of immature neurons in the adult hippocampus. PLoS One 4(4):e5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Tanaka K, Buffo A, Wurst W, Kuhn R, Gotz M.2006. Inducible gene deletion in astroglia and radial glia—a valuable tool for functional and lineage analysis. Glia 54(1):21–34. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. 2008. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132(4):598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota B, Herculano-Houzel S.2015. Brain structure. Cortical folding scales universally with surface area and thickness, not number of neurons. Science 349(6243):74–7. [DOI] [PubMed] [Google Scholar]
- Nakagomi T, Molnar Z, Nakano-Doi A, Taguchi A, Saino O, Kubo S, and others. 2011. Ischemia-induced neural stem/progenitor cells in the pia mater following cortical infarction. Stem Cells Dev 20(12):2037–51. [DOI] [PubMed] [Google Scholar]
- Nakagomi T, Molnar Z, Taguchi A, Nakano-Doi A, Lu S, Kasahara Y, and others. 2012. Leptomeningeal-derived doublecortin-expressing cells in poststroke brain. Stem Cells Dev 21(13):2350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya S, Esumi S, Ohta K, Fukuda T, Ito T, Imayoshi I, and others. 2013. Amygdala kindling induces nestin expression in the leptomeninges of the neocortex. Neurosci Res 75(2):121–9. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Akima M, Hatori T, Nagayama T, Zhang Z, Ihara F.2003. Microvasculature of the human cerebral white matter: arteries of the deep white matter. Neuropathology 23(2):111–8. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, Muller F.1986. The meninges in human development. J Neuropathol Exp Neurol 45(5):588–608. [PubMed] [Google Scholar]
- Paredes MF, Li G, Berger O, Baraban SC, Pleasure SJ. 2006. Stromal-derived factor-1 (CXCL12) regulates laminar position of Cajal-Retzius cells in normal and dysplastic brains. J Neurosci 26(37):9404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr AM, Tator CH. 2007. Intrathecal epidermal growth factor and fibroblast growth factor-2 exacerbate meningeal proliferative lesions associated with intrathecal catheters. Neurosurgery 60(5):926–33. [DOI] [PubMed] [Google Scholar]
- Pedroni A, Minh do D, Mallamaci A, Cherubini E.2014. Electrophysiological characterization of granule cells in the dentate gyrus immediately after birth. Front Cell Neurosci 8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricevic J, Forempoher G, Ostojic L, Mardesic-Brakus S, Andjelinovic S, Vukojevic K, and others. 2011. Expression of nestin, mesothelin and epithelial membrane antigen (EMA) in developing and adult human meninges and meningiomas. Acta Histochem 113(7):703–11. [DOI] [PubMed] [Google Scholar]
- Pino A, Fumagalli G, Bifari F, Decimo I.2017. New neurons in adult brain: distribution, molecular mechanisms and therapies. Biochem Pharmacol 141: 4–22. [DOI] [PubMed] [Google Scholar]
- Popescu BO, Gherghiceanu M, Kostin S, Ceafalan L, Popescu LM. 2012. Telocytes in meninges and choroid plexus. Neurosci Lett 516(2):265–9. [DOI] [PubMed] [Google Scholar]
- Qazi H, Shi ZD, Tarbell JM. 2011. Fluid shear stress regulates the invasive potential of glioma cells via modulation of migratory activity and matrix metalloproteinase expression. PLoS One 6(5):e20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin D, Gan Y, Shao K, Wang H, Li W, Wang T, and others. 2008. Mouse meningiocytes express Sox2 and yield high efficiency of chimeras after nuclear reprogramming with exogenous factors. J Biol Chem 283(48):33730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM. 2000. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci 20(13):5012–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radakovits R, Barros CS, Belvindrah R, Patton B, Muller U.2009. Regulation of radial glial survival by signals from the meninges. J Neurosci 29(24):7694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. 2002. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci 3(1):65–71. [DOI] [PubMed] [Google Scholar]
- Reina-De La Torre F, Rodriguez-Baeza A, Sahuquillo-Barris J.1998. Morphological characteristics and distribution pattern of the arterial vessels in human cerebral cortex: a scanning electron microscope study. Anat Rec 251(1):87–96. [DOI] [PubMed] [Google Scholar]
- Reiss K, Mentlein R, Sievers J, Hartmann D.2002. Stromal cell-derived factor 1 is secreted by meningeal cells and acts as chemotactic factor on neuronal stem cells of the cerebellar external granular layer. Neuroscience 115(1):295–305. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Baeza A, Reina-De La, Torre F, Ortega-Sanchez M, Sahuquillo-Barris J.1998. Perivascular structures in corrosion casts of the human central nervous system: a confocal laser and scanning electron microscope study. Anat Rec 252(2):176–84. [DOI] [PubMed] [Google Scholar]
- Rosenblatt S, Irikura K, Caday CG, Finklestein SP, Moskowitz MA. 1994. Basic fibroblast growth factor dilates rat pial arterioles. J Cereb Blood Flow Metab 14(1):70–4. [DOI] [PubMed] [Google Scholar]
- Russi AE, Brown MA. 2015. The meninges: new therapeutic targets for multiple sclerosis. Transl Res 165(2):255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S, Lamanna WC, Esko JD. 2011. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3(7):a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler N, Mayer J, Saenger S, Gimsa U, Walz C, Brenmoehl J, and others. 2017. Phenotype analysis of male transgenic mice overexpressing mutant IGFBP-2 lacking the Cardin-Weintraub sequence motif: reduced expression of synaptic markers and myelin basic protein in the brain and a lower degree of anxiety-like behaviour. Growth Horm IGF Res 33:1–8. [DOI] [PubMed] [Google Scholar]
- Schlager C, Korner H, Krueger M, Vidoli S, Haberl M, Mielke D, and others. 2016. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530(7590):349–53. [DOI] [PubMed] [Google Scholar]
- Shibata-Germanos S, Goodman JR, Grieg A, Trivedi CA, Benson BC, Foti SC, and others. 2020. Structural and functional conservation of non-lumenized lymphatic endothelial cells in the mammalian leptomeninges. Acta Neuropathol 139(2):383–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, and others. 2009. Retinoic acid from the meninges regulates cortical neuron generation. Cell 139(3):597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers J, von Knebel Doeberitz C, Pehlemann FW, Berry M.1986. Meningeal cells influence cerebellar development over a critical period. Anat Embryol (Berl) 175(1):91–100. [DOI] [PubMed] [Google Scholar]
- Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, and others. 2020. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 577(7792):689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine CA, Munson JM. 2019. Convection-enhanced delivery: connection to and impact of interstitial fluid flow. Front Oncol 9:966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm R, Hollt V.2007. CXC chemokine receptor 4 regulates neuronal migration and axonal pathfinding in the developing nervous system: implications for neuronal regeneration in the adult brain. J Mol Endocrinol 38(3):377–82. [DOI] [PubMed] [Google Scholar]
- Suter T, DeLoughery ZJ, Jaworski A.2017. Meninges-derived cues control axon guidance. Dev Biol 430(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G, Devenport D, Godt D, Brown NH. 2007. Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol 9(12):1413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi K, Tanaka Y, Nakano-Doi A, Sakuma R, Kamachi S, Shirakawa M, and others. 2017. Identification of multipotent stem cells in human brain tissue following stroke. Stem Cells Dev 26(11):787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritos N, Kitraki E, Phillipidis H, Stylianopoulou F.1998. Beta-adrenergic receptors mediate a stress-induced decrease in IGF-II mRNA in the rat cerebellum. Cell Mol Neurobiol 18(5):525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, De Prijck S, and others. 2019. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci 22(6):1021–35. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. 2002. Functional neurogenesis in the adult hippocampus. Nature 415(6875):1030–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Caulfield ME, Hatfield JK, Brown MA. 2015. Dynamic changes in meningeal inflammation correspond to clinical exacerbations in a murine model of relapsing-remitting multiple sclerosis. J Neuroimmunol 278:112–22. [DOI] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. 2000. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol 42(2):248–57. [PubMed] [Google Scholar]
- Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, and others. 2004. Nestin expression–a property of multi-lineage progenitor cells? Cell Mol Life Sci 61(19-20):2510–22. [DOI] [PubMed] [Google Scholar]
- Zappone MV, Galli R, Catena R, Meani N, De Biasi S, Mattei E, and others. 2000. Sox2 regulatory sequences direct expression of a β-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development 127(11):2367–2382. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, and others. 2015. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347(6226):1138–42. [DOI] [PubMed] [Google Scholar]
- Zhang ET, Inman CB, Weller RO. 1990. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat 170:111–23. [PMC free article] [PubMed] [Google Scholar]
- Zhou QG, Lee D, Ro EJ, Suh H.2016. Regional-specific effect of fluoxetine on rapidly dividing progenitors along the dorsoventral axis of the hippocampus. Sci Rep 6:35572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, and others. 1994. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron 12(1):11–24. [DOI] [PubMed] [Google Scholar]
- Zujovic V, Thibaud J, Bachelin C, Vidal M, Deboux C, Coulpier F, and others. 2011. Boundary cap cells are peripheral nervous system stem cells that can be redirected into central nervous system lineages. Proc Natl Acad Sci U S A 108(26):10714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]