Abstract
Depression is associated with abnormalities in Hypothalamic-Pituitary-Adrenal (HPA) axis functioning and neural circuitry that underlie the stress response. Resting-state functional connectivity (RSFC) captures intrinsic connections between frontolimbic brain regions that may set the stage for the rallying and regulating of the HPA axis system. This study examined the association between cortisol stress response and frontolimbic (amygdala and ventral and dorsal medial prefrontal cortex [vmPFC and dmPFC respectively]) RSFC in 88 (Age: M = 15.95, SD = 2.04; 71.60% female) adolescents with (N=55) and without (N=33) major depressive disorder (MDD). We collected salivary cortisol in the context of a modified Trier Social Stress Test (TSST) paradigm. Key findings were that adolescents with depression and healthy controls showed different patterns of association between amygdala and vmPFC RSFC and HPA functioning: while healthy controls showed a positive relationship between frontolimbic connectivity and cortisol levels that may indicate coordination across neural and neuroendocrine systems, adolescents with depression showed a minimal or inverse relationship, suggesting poor coordination of these systems. Results were similar when examining non-suicidal self-injury subgroups within the MDD sample. These findings suggest that the intrinsic quality of this frontolimbic connection may be related to HPA axis functioning. In MDD, inverse associations may represent a compensatory response in one system in response to dysfunction in the other. Longitudinal multilevel research, however, is needed to disentangle how stress system coordination develops in normal and pathological contexts and how these systems recover with treatment.
Keywords: HPA-axis, RSFC, depression, adolescence, Trier social stress test
1. Introduction
To ensure survival, neural and neuroendocrine systems activate to detect and respond appropriately to threats. Ideally, these biological resources disengage once the threat has subsided. Research investigating individual indexes of neural or neuroendocrine functioning has established a relationship between depression and a lower ability to rally and shut down the stress response (Lu et al., 2012; Stetler & Miller, 2011). However, more work is needed to advance our understanding of the coordination between neural and neuroendocrine systems. Research incorporating multiple-level analysis approaches will enhance understanding of how stress responses across multiple systems are orchestrated within the context of adaptive and maladaptive functioning. This knowledge will be critical when planning potential interventions.
Patients with depression show abnormal patterns of hypothalamic pituitary adrenal (HPA) axis stress reactivity and recovery. Cortisol, a neurohormonal index of the HPA axis cascade, is secreted in response to stress. In healthy individuals, cortisol levels increase in response to stress followed by a rapid decrease in cortisol (i.e., recovery) when the threat is withdrawn. In individuals with depression, however, response to stress is associated with 1) a flat cortisol secretion pattern, and 2) higher cortisol levels during the recovery phase (Burke et al., 2005; Zorn et al., 2017). Non-suicidal self-injury (NSSI), a behavior common among adolescents with depression, is associated with a unique pattern of blunted HPA axis functioning (Klimes-Dougan et al., 2019). These patterns suggest an association between depression, difficulty engaging the HPA axis stress response, and dysfunction in the negative feedback loop that turns off the HPA axis stress response once the stressor ends.
Dysfunction in frontolimbic neurocircuitry, a circuit critical for threat detection and stress response modulation, has been shown in depression. The amygdala, a part of the limbic system, is implicated in bottom-up salience-driven emotion processing and the activation of the stress response, including the release of glucocorticoids (Arnsten, 2009; Radley et al., 2015; Roozendaal et al., 2002). Depression is associated with amygdala hyperactivity (Suslow et al., 2010; Victor et al., 2010). Amygdala volume, metabolism, and activation to emotional stimuli have all shown to positively correlate with cortisol levels in depression (Drevets et al., 2002; Klimes-Dougan et al., 2014), similarly to in healthy controls (Root et al., 2009; van Stegeren et al., 2007). Positive associations between cortisol levels and amygdala activation to emotional stimuli may represent joint activation of the fight-or-flight response, which may be excessive in depression and lead to a chronic state of stress. Despite evidence of an association between cortisol and amygdala activation, we have yet to explore how cortisol may relate to amygdala connectivity profiles, providing a more nuanced understanding of the potential role of regulatory circuits.
The prefrontal cortex (PFC) plays a role in regulating neuroendocrine, autonomic, and behavioral stress responses, including HPA axis functioning (Diorio et al., 1993; McKlveen et al., 2015). The medial PFC (mPFC) in particular has been implicated in regulating the HPA-axis (Diorio et al., 1993). The dorsal mPFC (dmPFC) contributes to the appraisal and expression of negative emotion and is related to inhibition of the HPA axis (Diorio et al., 1993; Etkin et al., 2011; Sullivan & Gratton, 2002). The ventral mPFC (vmPFC), through connections with limbic regions, contributes to the regulation of negative emotion and serves to activate the HPA axis. The mPFC is part of the default mode network (DMN), a set of brain regions that tend to be more active during rest and that are implicated in self-reflection (Buckner et al., 2008; Kaiser et al., 2015; Raichle, 2015). The PFC is often hypoactive in depression (Lee et al., 2008; Snyder, 2013). Cortisol non-suppression in unmedicated depression has been associated with medial PFC hypometabolism (Aihara et al., 2007), but healthy individuals have shown mixed patterns (Root et al., 2009; Wang et al., 2005). Positive associations between cortisol reactivity and PFC activation may indicate joint activation of the HPA axis and PFC to elicit regulatory mechanisms. Negative associations, however, may signal that one system may modulate the stress response in the other (e.g., PFC signaling the HPA axis to stop cortisol release). Those with depression may have difficulty coordinating PFC and HPA axis functioning to regulate the stress response.
Frontolimbic functional connectivity (FC) patterns have been less thoroughly examined in relation to HPA axis functioning, an approach which may explain the mixed findings on the association between cortisol and the frontolimbic circuit. Resting state functional connectivity (RSFC) permits examination of functional threat system disruption in the absence of specific threat cues. Connectivity between brain regions can enhance or impair how efficiently and effectively brain regions coordinate efforts to achieve an appropriate response, like turning off a stress reaction. The PFC tends to show negative connectivity with the amygdala (Kim, Loucks, et al., 2011), which may represent emotion regulation feedback mechanisms through which the PFC downregulates the amygdala (Frank et al., 2014; Urry et al., 2006). Depressed individuals, however, show reduced frontolimbic connectivity at rest and during emotional processing whereas healthy controls show strong PFC-amygdala FC (Anand et al., 2007; Dannlowski et al., 2009). Notably, stress can dampen PFC-mediated top-down processing and heighten limbic-driven bottom-up processing of emotionally salient information (Arnsten, 2009; Frank et al., 2014; Price & Drevets, 2010, 2012). Greater cortisol responses have been associated with greater frontolimbic resting state functional connectivity (Peters et al., 2019; Quaedflieg et al., 2015; Thomason et al., 2011). Cortisol release may interact with prefrontal recruitment to regulate and shut down limbic responses.
2. Purpose of study
The purpose of this study was to expand on our previous work that examined the interplay between amygdala activation and structure and HPA axis functioning (Klimes-Dougan et al., 2014) to examine how frontolimbic RSFC is associated with HPA axis functioning in adolescents with depression. The relationship between RSFC and cortisol response has not been previously examined among adolescents with MDD. We focus on RSFC as an index of the quality of the intrinsic connection between the vmPFC and dmPFC and the amygdala, which may reflect the capacity to rally and regulate the HPA system. Because the dmPFC and vmPFC play different roles in stress reactivity and regulation, we examined amygdala connectivity with the dmPFC and vmPFC separately to investigate their unique associations with HPA axis functioning. We hypothesized that healthy controls would show a positive association between frontolimbic RSFC and HPA axis reactivity, signifying synchronized up- and downregulation capacities, but patients with MDD would show a negative association with excessive cortisol being associated with poor connectivity, signifying dysregulation. Further, because the blunted pattern of HPA reactivity associated with non-suicidal self-injury (NSSI) among adolescents with MDD may influence the results (Klimes-Dougan et al., 2019), we also conducted exploratory follow-up analyses to assess the coordination between RSFC and HPA functioning in adolescents with and without NSSI.
3. Method
3.1. Participants
The sample of participants in the current report consisted of 88 adolescents between 12 and 20 years old (Age: M = 15.95, SD = 2.04; 71.60% female; 69.32% White). Fifty-five met the criteria for a current diagnosis of MDD and 33 were healthy controls. Healthy controls had no current or lifetime history of any psychiatric diagnoses. See Table 1 for participant demographics.
Table 1.
Demographics
| Healthy control | MDD | Comparisons | |
|---|---|---|---|
|
| |||
| N | 33 | 55 | |
| Sex | |||
| % Female | 66.70% | 74.50% | X2(2) = .63, p = .42 |
| N | 22 F, 11 M | 41 F, 14 M | |
| Age | |||
| M (SD) | 16.04 (2.20) | 15.90 (1.96) | F(1, 86) = .09, p=.76 |
| Range | 12.30 – 19.80 | 12.30 – 20.00 | |
| IQ | |||
| M (SD) | 109.48 (12.06) | 108.16 (14.88) | F(1, 86) = .19, p = .67 |
| Range | 83 – 134 | 80 – 140 | |
| Race | |||
| White | 63.60% | 72.70% | X2(2) = .80, p = .37 |
| Minority Status | 36.40% | 27.30% | |
| Black/African American | 3.0% | 10.90% | |
| Asian American | 9.10% | 0.00% | |
| Native American | 3.00% | 1.80% | |
| Other | 21.20% | 14.50% | |
| Medication Status ☨ | |||
| % Medicated | 0% | 23.64% | |
| N | 0 medicated | 13 medicated | |
| NSSI | |||
| % NSSI | 0% | 58.18% | |
| N | 0 NSSI | 32 NSSI | |
| BDI | |||
| M (SD) | 2.60 (3.23) | 24.10 (12.27) | t(86) = 9.84, p < .001 |
| Range | 0.0 – 16.0 | .67 – 52.33 | |
Note.
Medication status is based on antidepressant usage
MDD = major depressive disorder; NSSI = nonsuicidal self-injury; BDI = Beck Depression Inventory.
3.2. Procedures
Adolescents were recruited using community postings and through clinics at the University of Minnesota, Twin Cities (UMN) and the neighboring communities. Inclusion criteria for the MDD group included a primary diagnosis of MDD. Inclusion criteria for the HC group included absence of any DSM-IV Axis I diagnosis. For exploratory analyses on NSSI groups, inclusion criteria for the MDD/NSSI group included a diagnosis of MDD and endorsement of any lifetime self-injurious behavior and inclusion criteria for the MDD/noNSSI group included a diagnosis of MDD and no lifetime self-injurious behavior. Exclusion criteria for both groups included current or past diagnoses of bipolar disorder, schizophrenia, a neurological disorder, pervasive developmental disorder, or a chronic or serious medical condition, or an IQ under 80 based on the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999).
All participants completed informed consent and/or assent (if under 18-years-old) and received compensation. Participants completed up to three study visits that involved 1. diagnostic interviews, neurocognitive assessments, and intelligence assessments, 2. an acute stressor paradigm (the Trier Social Stress Test; TSST), and 3. MRI scanning. We included participants who completed the TSST and had a complete set of usable structural and resting state functional MRI data (see Klimes-Dougan et al., 2014 for more detail). This study was approved by the Institutional Review Board at the University of Minnesota.
3.3. Measures
3.3.1. Psychiatric diagnoses and symptoms.
The Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (KSADS-PL; Kaufman et al., 1997), a semi-structured diagnostic interview, was used to diagnose Axis I disorders based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR; American Psychiatric Association, 2000). We used the KSADS-PL to determine MDD status as well as medication use and the presence of NSSI. Trained clinical psychologists, child psychiatrists, or advanced clinical psychology doctoral students supervised by a senior clinician conducted independent KSADS-PL interviews with participants and a legal guardian if the participant was under 18 years of age. If both a parent and child interview were conducted, consensus was established between child and parent reports to determine diagnoses. We established interrater reliability among KSADS-PL evaluators based on ratings for four training tapes. There was 100% agreement for depression diagnoses, 84.63% for depression symptoms, and 96% agreement for screening symptoms for all other psychiatric diagnoses.
At each study visit, participants completed the Beck Depression Inventory II (BDI), an 18-item self-report questionnaire measuring depression symptoms on a 4-point Likert scale, with higher scores reflecting more severe depressive symptoms (Beck et al., 1996). The BDI has well-established reliability and validity (Osman et al., 2004). We computed an overall BDI total score for each participant by averaging the total scores of all completed BDIs.
3.3.2. Estimated IQ.
We estimated IQ based on performance on the vocabulary and matrix reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). The WASI has been shown to be valid and reliable (Axelrod, 2002; Hays et al., 2002).
3.4. Neuroimaging
3.4.1. MRI Data Acquisition.
We acquired MRI data using a 12-channel receive only head coil supplied by Siemens on the 3T TIM Trio scanner at the Center for Magnetic Resonance Research at the UMN. A T1 weighted MP-RAGE scan and a six-minute, eyes-open resting state functional MRI (rsfMRI) scan along with a field map acquisition were acquired for each subject. The scan parameters for the T1 weighted MP-RAGE scan include TR=2530ms; TE=1100ms; TI=1100ms, voxel size=1mm isotropic. The rsfMRI scan used an echo planar imaging (EPI) whole brain acquisition with TR = 2000ms; TE = 30ms; voxel size 3.43×3.43×4mm; 34 slices; 180 volumes. The EPI sequence was modified to record the time courses from the Siemens supplied respiratory bellows and pulse oximetry monitors during fMRI scan. The field map scan had voxel parameters which matched the rsfMRI acquisition. See Cullen et al. (2014) for more details.
3.4.2. MRI Data Preprocessing.
FreeSurfer version 5.3.0 (https://surfer.nmr.mgh.harvard.edu/) was applied to the T1 weighted scan for each subject. Tools from the FMRIB software library (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) and custom MATLAB tools were used for fMRI image processing. Initial preprocessing steps included brain extraction, motion correction, denoising procedure using the RETROICOR (Glover et al., 2000) algorithm applied to the respiration and pulse oximetry traces, geometric distortion caused by magnetic field inhomogeneity using the field map, and regression of eight nuisance variables: white matter time series and cerebral spinal fluid time series (ROIs from the FreeSurfer parcellation), and the six time series generated in the motion correction (from FSL MCFLIRT) as parameters. Data scrubbing was then performed following Power and colleagues (Power et al., 2012). An average of 11.75 volumes were rejected (SD = 14.44; range; 0 to 56). Global signal regression was not applied given concerns about inducing erroneous negative correlations (Murphy et al., 2009; Murphy & Fox, 2017; Power et al., 2012; Satterthwaite et al., 2012). See Cullen et al. (2014) for more details.
3.4.2. RSFC Analysis.
Using a seed-based, whole-brain approach, FreeSurfer-based and subject-specific right and left amygdala ROIs were registered to the pre-processed rsfMRI data, and average time series of voxels in these regions were extracted after spatially smoothing (5mm kernel), prewhitening, and registration to standard space (MNI 152). See Cullen et al. (2014) for more details. Masks of the dmPFC (“superior frontal gyrus”) and vmPFC (“frontal medial cortex”) were created using FSL’s Harvard-Oxford Cortical Structural Atlas. Mean z-scores were extracted from individual amygdala RSFC maps using separate dmPFC and vmPFC masks.
3.5. Assessment and Analysis of HPA Axis Functioning
Trier Social Stress Test (TSST).
We assessed salivary cortisol in the context of a modified version of the TSST, which participants completed in the afternoon (Mean = 14:51:40; range = 12:51:00 to 16:28:00) on a separate visit from the MRI scan. The TSST was administered at the second study visit so that participants could become familiar with the testing site at the first visit. Participants began the TSST upon arrival. Participants were given 5 minutes to prepare a 5-minute speech to describe themselves as though they were applying for a job. Participants were then taken to a room to deliver the speech to two confederates dressed in white lab coats. The participant was provided with a microphone and speeches were video recorded. The confederates maintained a neutral expression and provided minimal feedback during the speech. Following the speech, the participants completed a 5-minute serial subtraction task with corrective feedback which was also performed in front of the confederates. Participants were debriefed after the task.
Five salivary samples were collected across the task: before speech preparation (time point A; 0 min), after the TSST was completed (time point B; 20 min), at 35 min (time point C), at 50 min (time point D), and at 65 min (time point E). Labeled samples were stored in a −25 degree Celsius freezer and were shipped to Universität Trier in Trier, Germany, for analysis using assay methods consistent with Dressendörfer et al (1992). Cortisol values were winsorized to be within three standard deviations of the mean. Variables of interest included: area under the curve with respect to increase (AUCi) and ground (AUCg; Pruessner et al., 2003) as well as the peak cortisol value (“Highest”; Klimes-Dougan et al., 2018; Vajravelu et al., 2015). While they were correlated (AUCg and Highest: r = .87, p < .001; AUCg and AUCi: r = .30, p = .004; and AUCi and Highest: r = .25, p = .02), these three values provide slightly different indexes of cortisol; these three variables characterize the overall pattern of cortisol response to a stressor over time (AUCg), the increase in cortisol to a stressor over time relative to pre-task cortisol levels (AUCi), and the peak amount of cortisol secretion across the TSST paradigm (Highest).
3.6. Statistical Analyses
R Statistical Software was used to conduct analyses (R Core Team, 2013). Multiple linear regression models were run predicting HPA axis functioning from group (HC or MDD), amygdala and mPFC RSFC (vmPFC and dmPFC separately), and their interaction. RSFC values were mean-centered. For the main analyses, we applied the Bonferroni correction to correct for multiple comparisons (right and left amygdala, dmPFC and vmPFC, AUCg, AUCi, and highest cortisol level) for an adjusted p-value of .004. A series of follow-up analyses were conducted given different patterns of HPA axis functioning in adolescents with MDD with versus without a history of self-injury (Klimes-Dougan et al., 2019). These analyses were conducted using subsamples of the MDD groups with (MDD/NSSI) and without non-suicidal self-injury (MDD/noNSSI). We report findings with uncorrected p-values that were significant or marginally significant in the Results section and tables. Because time of day has shown to affect reactivity to the TSST (Goodman et al., 2017) and there are also significant sex effects in HPA axis responses to the TSST (Zorn et al., 2017) as well as sex differences in mPFC involvement with the HPA axis (Buchanan et al., 2010), we ran supplementary models controlling for time of cortisol collection and sex in our analyses. The patterns of results remained the same in models including time or gender (see supplementary materials). Follow-up analyses were conducted with medicated and unmedicated subsamples of the MDD group to determine whether results were related to antidepressant medication usage. Both MDD groups with and without medication showed the same pattern of results (see supplementary materials).
4. Results
4.1. Demographics and Descriptive Results
There were no significant differences between the MDD and HC group in age, sex, or IQ (all p’s > .14; See Table 1). As expected, self-reported depressive symptoms on the BDI were higher in the MDD than HC, t(86) = 9.84, p < .001. No group differences were found with HPA axis functioning or with frontolimbic connectivity when analyzed separately. There were also no significant differences in TSST start time or in wake time. See Table 2 for more details.
Table 2.
HPA Axis Functioning and Frontolimbic Connectivity
| Healthy control | MDD | Comparisons | |
|---|---|---|---|
|
| |||
| TSST Start Time | |||
| M (SD) | 15:01(1:11) | 14:45 (1:13) | t(86) = .99, p = .32 |
| Range | 12:51 – 16:14 | 13:00 – 16:28 | |
| TSST AUCg | |||
| M (SD) | 18.09 (11.65) | 20.29 (12.68) | t(86) = .82, p = .42 |
| Range | 1.74 – 42.38 | 2.30 – 53.48 | |
| TSST AUCi | |||
| M (SD) | 3.16 (9.70) | .92 (13.45) | t(86) = .83, p = .41 |
| Range | −26.83 – 20.19 | −32.23 – 40.36 | |
| TSST Highest | |||
| M (SD) | .42 (.25) | .44 (.25) | t(86) = .40, p = .69 |
| Range | .03 – 1.00 | .04 – .94 | |
| L Amygdala-vmPFC RSFC | |||
| M (SD) | .30 (.80) | .39 (.78) | t(86) = .51, p = .61 |
| Range | −1.23 – 3.04 | −1.34 – 2.23 | |
| R Amygdala-vmPFC RSFC | |||
| M (SD) | .42 (.74) | .33 (.90) | t(86) = .45, p = .65 |
| Range | −1.23 – 2.26 | −1.66 – 2.95 | |
| L Amygdala-dmPFC RSFC | |||
| M (SD) | .09 (.50) | .07 (.50) | t(86) = .24, p = .81 |
| Range | −1.07 – .92 | −.94 – 1.03 | |
| R Amygdala-dmPFC RSFC | |||
| M (SD) | .06 (.59) | .04 (.50) | t(86) = .13, p = .90 |
| Range | −1.32 – 1.23 | −1.18 – 1.10 | |
Note. MDD = major depressive disorder, TSST = Trier Social Stress Test; AUCg = area under the curve with respect to ground; AUCi = area under the curve with respect to increase; vmPFC = ventral medial prefrontal cortex; dmPFC = dorsal medial prefrontal cortex; RSFC = resting state functional connectivity (values indicate z values)
4.2. MDD and Amygdala and Dorsal and Ventral Medial Prefrontal Cortex Resting State Connectivity and Cortisol Levels to the TSST
vmPFC.
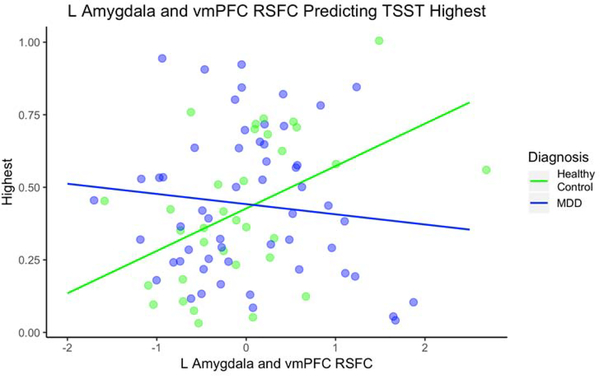
The models for the R amygdala - vmPFC RSFC and group did not significantly predict AUGg, AUCi or Highest cortisol. The interaction between L amygdala - vmPFC RSFC and group predicted Highest cortisol, F(3, 84) = 2.79, p < .05 (See Figure 3 and Table 3). For HC, every 1 unit increase in L amygdala and mPFC RSFC was associated with a .15 increase in Highest. For MDD, every 1 unit increase in L amygdala and mPFC RSFC was associated with a .03 decrease in Highest. The group by RSFC interaction improved the model compared to the model without the interaction, F(2, 85) = 7.12, p = .01. The interaction between L amygdala - vmPFC RSFC and group predicted AUCg in the same direction as Highest cortisol but the overall model was only marginally significant, R2adj = .04, F(3, 84) = 2.25, p = .09 (see Table 3). The model for AUCi was not significant.
Fig. 3.
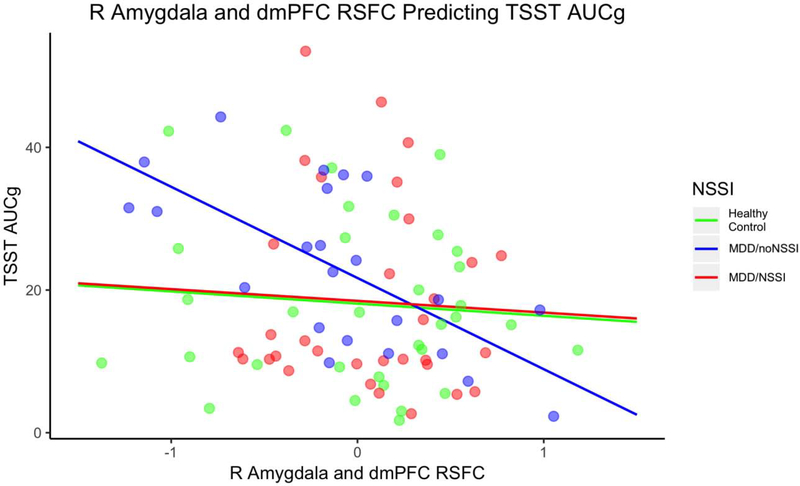
R Amygdala and dmPFC RSFC Predicting TSST AUCg for NSSI Subgroups. Note. MDD = major depressive disorder; NSSI = nonsuicidal self-injury; TSST = Trier Social Stress Test; AUCg = area under the curve with respect to ground; dmPFC = dorsal medial prefrontal cortex; RSFC = resting state functional connectivity; RSFC are z values centered at the mean. The interaction between R amygdala - dmPFC RSFC and group predicted AUCg with the healthy control group showing a positive relationship and the MDD/noNSSI and MDD/NSSI groups showing a negative relationship.
Table 3.
Summary of Multiple Regression Models
| AUCg | Highest | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | β | SE | p value | β | SE | p value |
|
| ||||||
| L Amygdala-vmPFC RSFC predicting TSST for MDD | ||||||
| L Amygdala-vmPFC RSFC | 6.20 | 2.65 | .02* | .15 | .05 | .01* |
| Diagnosis | 1.92 | 2.65 | .47 | .02 | .05 | .78 |
| L Amygdala-vmPFC × MDD | −7.80 | 3.37 | .02* | −.18 | .07 | .01* |
| Adjusted R2 | .04 | .06 | ||||
|
| ||||||
| R amygdala-dmPFC RSFC predicting TSST for MDD | ||||||
| R amygdala-dmPFC RSFC | −1.71 | 3.55 | .63 | .01 | .07 | .85 |
| Diagnosis | 2.14 | 2.63 | .42 | .02 | .05 | .70 |
| R amygdala-dmPFC × MDD | −7.21 | 4.83 | .14 | −.18 | .10 | .07 |
| Adjusted R2 | .06 | .04 | ||||
|
| ||||||
| L amygdala-vmPFC RSFC predicting TSST for NSSI | ||||||
| L amygdala-vmPFC RSFC | .15 | .05 | .01* | |||
| MDD/noNSSI | .05 | .07 | .43 | |||
| MDD/NSSI | −.02 | .06 | .78 | |||
| L amygdala-vmPFC × MDD/noNSSI | −.16 | .08 | .05 | |||
| L amygdala-vmPFC × MDD/NSSI | −.22 | .08 | .01* | |||
| Adjusted R2 | .05 | |||||
|
| ||||||
| R amygdala-dmPFC RSFC predicting TSST for NSSI | ||||||
| R amygdala-dmPFC RSFC | −1.71 | 3.52 | .63 | |||
| MDD/noNSSI | 3.59 | 3.23 | .27 | |||
| MDD/NSSI | .38 | 2.95 | .90 | |||
| R amygdala-dmPFC × MDD/noNSSI | −11.07 | 5.50 | .05* | |||
| R amygdala-dmPFC × MDD/NSSI | 0.06 | 6.31 | .99 | |||
| Adjusted R2 | .08 | |||||
Note. MDD = major depressive disorder; NSSI = nonsuicidal self-injury; TSST = Trier Social Stress Test; AUCg = area under the curve with respect to ground; vmPFC = ventral medial prefrontal cortex; dmPFC = dorsal medial prefrontal cortex; RSFC = resting state functional connectivity; RSFC are z values centered at the mean. The reference group is the healthy control group.
indicates p < .05.
dmPFC.
For the R amygdala - dmPFC RSFC, there was a trend toward an interaction between RSFC and group predicting AUCg (F(3, 84) = 2.78, p < .05) and Highest cortisol (F(3, 84) = 2.12, p = .10) for the TSST (see Table 3). The model for AUCi was not significant. For the L amygdala - dmPFC RSFC analyses, none of the models were significant.
4.3. Followup Analyses for NSSI subgroups and Amygdala and Medial Prefrontal Cortex Resting State Connectivity and Cortisol Levels to the TSST
NSSI.
There were no significant differences between the subgroups of MDD, MDD/NSSI, MDD/noNSSI, and HC groups in age, sex, or IQ, (all p’s > .24). There were no significant group differences in HPA axis functioning or in RSFC, p’s > 17. The MDD/noNSSI and MDD/NSSI groups did not differ in depression scores, p > .05.
vmPFC.
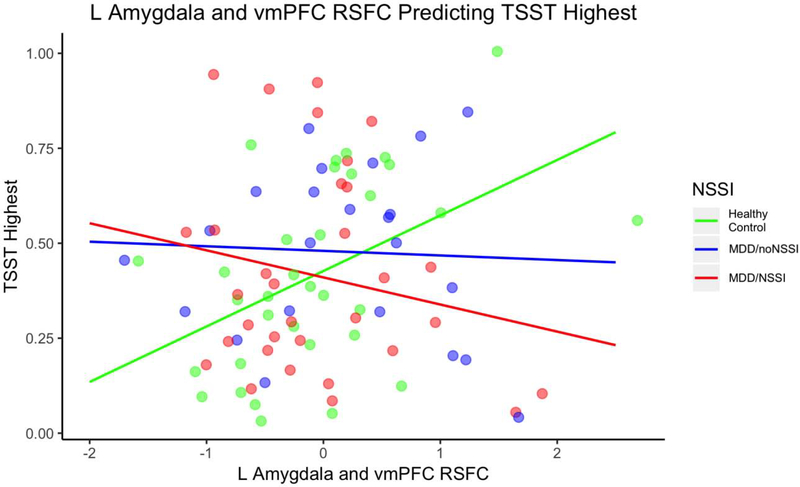
The models for the R amygdala - vmPFC RSFC and subgroup (HC, MDD/NSSI and MDD/noNSSI) did not significantly predict AUGg, AUCi or Highest cortisol. The interaction between L amygdala - vmPFC RSFC and subgroup marginally predicted Highest cortisol, F(5, 82) = 2.00, p = .09 (See Figure 2 and Table 3). For HC, every 1 unit increase in L amygdala and vmPFC RSFC was associated with a .15 increase in Highest. For MDD/noNSSI, every 1 unit increase in L amygdala and vmPFC RSFC was associated with a .01 decrease in Highest. For MDD/NSSI, every 1 unit increase in L amygdala and vmPFC RSFC was associated with a .07 decrease in Highest. The group by RSFC interaction significantly improved the model compared to the model without the interaction, F(2, 82) = 4.02, p = .02. These patterns were in the same direction as the previously reported L amygdala finding for the two-group comparison, but the negative association between RSFC and HPA axis functioning were more pronounced for the MDD/NSSI group. There was no significant interaction for AUCg or AUCi.
Fig. 2.
L Amygdala and vmPFC RSFC Predicting TSST Highest for NSSI Subgroups. Note. NSSI = nonsuicidal self-injury; MDD = major depressive disorder without NSSI; TSST = Trier Social Stress Test; vmPFC = ventral medial prefrontal cortex; RSFC = resting state functional connectivity; RSFC are z values centered at the mean. The interaction between R amygdala - mPFC RSFC and group predicted AUCg with the healthy control group showing a positive relationship and the MDD/noNSSI and MDD/NSSI groups showing a negative relationship.
dmPFC.
The interaction between R amygdala – dmPFC RSFC and subgroups (HC, MDD/NSSI, and MDD/noNSSI) predicted AUCg, F(5, 82) = 2.45, p = 0.04 (see Figure 3 and Table 3). For HC, every 1 unit increase in R amygdala and dmPFC RSFC was associated with a 1.71 decrease in AUCg. For MDD/noNSSI, every 1 unit increase in R amygdala and dmPFC RSFC was associated with a 12.78 decrease in AUCg. For the MDD/NSSI group, every 1 unit increase in R amygdala and dmPFC RSFC was associated with a 1.65 decrease in AUCg. The group by RSFC interaction marginally improved the model, F(2, 82) = 2.33, p = .10. The models for AUCi and highest were not significant. These patterns were in the same direction as the R amygdala finding for the two-group comparison for the MDD/noNSSI group, but the MDD/NSSI group did not differ from HC. For the L amygdala - dmPFC RSFC analyses, none of the models were significant.
5. Discussion
There is mounting evidence of disruptions in neural and neuroendocrine functioning in adolescents diagnosed with depression. Multilevel analytic approaches are needed to advance our understanding of the coordination between the brain and the body within the context of stress activation and regulation. This study examined the relationship between key neural and neuroendocrine patterns in adolescents with and without MDD. Despite the evidence that there are no significant differences in frontolimbic connectivity or neuroendocrine functioning between the groups, the pattern of coordination across systems differs with healthy controls displaying positive and adolescents with MDD displaying minimal or a negative correspondence between resting frontolimbic connectivity and cortisol levels in the context of the TSST. In terms of reactivity, the vmPFC RSFC generally showed stronger group differences in associations with AUCg and Highest cortisol levels compared to the dmPFC, although findings were not significant for the dmPFC. Results suggest that patterns were stronger when accounting for NSSI.
This is the first study that has examined the coordination between neural connectivity and neuroendocrine systems within the context of adolescent depression. Although both groups show some coordination between neural and neuroendocrine stress systems, they show opposing relationships. Efficient systems for processing threats in our environment would involve good regulatory control over limbic regions, so that appropriate appraisal and resource allocation takes place, at times implicating rallying metabolic resources, including the HPA axis system. Our results provide support for this prediction. Greater intrinsic ventral frontolimbic connections being related to greater cortisol reactivity in healthy controls suggests intact regulatory processes and coordinated upregulation and downregulation of these two biological systems in response to stress. The HPA axis may release high levels of cortisol in response to a stressor to rally resources, trusting that feedforward amygdala to vmPFC connectivity will cue the vmPFC to appropriately downregulate the amygdala and HPA axis as needed once the stressor has passed. Frontolimbic connectivity in the context of this recovery period rather than at rest likely would be more aptly suited to investigate this hypothesis.
In contrast, patients with MDD show a minimal or negative association between neural and HPA stress systems, possibly reflecting an attenuated association between intrinsic connections in the amygdala to vmPFC emotion regulation brain circuit and cortisol reactivity. Weak coordination between these two stress systems may make it harder to engage regulation processes in response to stressors, resulting in perseverative negative emotions or rumination that are strong contributors to depression (Fried & Nesse, 2015). Notably, the DMN, which encompasses the mPFC, is implicated in rumination (Hamilton et al., 2015). This may contribute to the high cortisol levels during the recovery period found in depression because the vmPFC is not operating to downregulate this stress response. The extent to which this pattern extends to other regulatory contexts is not clear. Peters et al. (2019) found the opposite relationship with the dmPFC, finding that greater baseline cortisol is associated with greater positive amygdala connectivity with the dmPFC, in an older sample (21 to 30 years of age) with remitted depression. Not only do these studies differ with regard to task demands (baseline versus TSST cortisol), the results may be related to developmental changes in connectivity with adolescents showing inverted relationships when compared with young adults (Burkhouse et al., 2019). Dysregulated HPA functioning has shown to impact the development of brain circuits. In females with early life stress, greater childhood afternoon basal cortisol levels predicted heightened negative amygdala-vmPFC RSFC in adolescence (Burghy et al., 2012). Similarly, allostatic overload of the HPA axis may prompt early maturation of frontolimbic connectivity (Gee et al., 2013) and a compensatory overreliance on frontolimbic pathways to regulate feelings of stress in MDD.
Findings were specific to AUCg and highest cortisol levels and not with AUCi. AUCg and highest are influenced by both basal cortisol levels and reactivity to a stressor whereas AUCi is specific to cortisol level increase to a stressor. Significant associations may have only been found with AUCg and highest in this study because RSFC and cortisol were not assessed simultaneously. Real-time engagement of the frontolimbic circuit during a stressor may be more isolated to cortisol reactivity. RSFC, in contrast, as a correlation between the activity patterns of different brain regions at rest, can be thought to reflect inherent networks or the quality of a communication pathway. Greater frontolimbic RSFC may enable quicker regulatory feedback processes from the mPFC in response to feedforward amygdala activity. These pathways likely affect basal cortisol levels and cortisol levels in response to stress. As such, RSFC should be related to basal cortisol and cortisol reactivity (reflected in AUCg) whereas other neural metrics, like brain activation or task-based connectivity, may be more specific to cortisol reactivity as indexed by AUCi.
Exploratory analyses on NSSI and RSFC predicting cortisol levels within the MDD group largely showed a similar negative association between frontolimbic RSFC and TSST cortisol levels (AUCg and highest) in the MDD and MDD/NSSI groups for the vmPFC. The MDD/NSSI group showed a numerically stronger negative association between RSFC and cortisol. For the dmPFC, however, the MDD/NSSI group did not differ from healthy controls in the relationship between RSFC and cortisol reactivity whereas the MDD group showed a negative correspondence between RSFC and cortisol reactivity. NSSI may uniquely involve abnormal coordination between the HPA axis and amygdala connectivity with the vmPFC but not the dmPFC. Given the vmPFC’s role in emotion regulation, this inverse coordination between vmPFC connectivity and the HPA axis may interfere with the success of coping strategies to stress, resulting in reliance on less adaptive coping strategies, like self-injury. On one hand, negative connectivity being associated with greater cortisol reactivity may be driven by excessive amygdala feedforward activity, indicating an excessive joint interoceptive signal of stress that may maintain negative mood (Cicchetti & Dawson, 2002). On the other hand, positive connectivity being associated with lower cortisol reactivity may suggest that excessive recruitment of the vmPFC by the amygdala interferes with HPA system recruitment efficiency and successful rallying of the HPA axis in response to social stress. Patients with MDD with and without NSSI may expend a lot of time and effort regulating negative emotions to compensate for emotion regulation difficulties. This “overuse” of the amygdala-vmPFC pathway may disrupt connections with the HPA axis, leaving the HPA axis less attuned to potential signaling from the frontolimbic circuit. Inverse associations in depression may also reflect an adaptive response in one system but dysfunction in the other and may contribute to emotional numbing as neural and neuroendocrine systems are not jointly signaling affect. This numbing is a commonly cited reason for engaging in self-harm (Edmondson et al., 2016). See Klimes-Dougan (2019) for further discussion.
These findings should be considered in the context of the study limitations. While this sample was considerably larger than most studies considering coordination across neural and neuroendocrine systems, it still had a relatively limited sample size, particularly for evaluating interaction effects and secondary analyses with the NSSI subgroup. Given that our results did not survive our conservative correction for multiple comparisons, these results should be replicated in larger samples to better understand the relationships between HPA axis and brain functioning in samples with and without depression. Given a polythetic approach to the diagnosis of depression, there is considerable heterogeneity across these youth. Meaningful subgroups may exist. Here we have considered depressed adolescents who do or do not engage in NSSI as one subgroup, but there are likely additional considerations, such as comorbid anxiety, which also affects frontolimbic connectivity and HPA axis functioning (Kim, Gee, et al., 2011; Vreeburg et al., 2010). Although we conducted analyses to look at the interaction between RSFC and NSSI status to predict HPA axis functioning, our NSSI group were all adolescents diagnosed with MDD. Additional characteristics should be attended to in future research given that NSSI is not a behavior limited to MDD. Also, NSSI may include vastly different levels of frequency and severity across individuals. Although we covered a broad span of adolescence (12 to 20), a wider age range should be examined in the future using longitudinal designs to fully understand the relationship between these systems as the prefrontal cortex continues to develop up to age 25 (Arain et al., 2013). The associations between fully developed stress systems may differ from those still undergoing development. Likewise, longitudinal designs covering the course of depression and recovery will also inform how poor coordination across these systems can either increase risk for depression or follow the development of depression and whether treatment can normalize coordination across these systems.
This study focused on amygdala to medial prefrontal cortex connectivity. Although the frontolimbic circuit is critical for the experience of negative emotions, like stress, and emotion regulation, future studies should also examine connectivity of other limbic regions, like the hypothalamus, the bed nucleus of the stria terminalis, hippocampus, insula and other regions implicated in HPA functioning. Furthermore, we chose to include multiple nuanced measures of HPA axis response to fully contextualize the TSST and to provide a basis set for future studies; we do note however, the high correlation between Highest and AUCg (r = .87, R2 = .76) suggests only a small amount of variance unique to each measure. Even within these statistical limitations, there are indeed, subtle differences in relations of these TSST measures with rsfMRI that can be pursued in future work. Paired t-tests revealed a significant difference between the pre-task TSST cortisol sample (M = .20, SD = .18) and an at-home control cortisol sample taken at approximately the same time as the TSST (M = .29, SD = .23), t(52) = 2.52, p = .02 (data that was available for a subset of the sample), thus suggesting that participants may have already been showing less activation of the HPA axis at the onset of the TSST. It remains unclear if indexes of HPA axis activation might generalize to contexts outside of the laboratory (particularly for AUCi). Moreover, our MRI and TSST visits occurred on separate days and thus reflected general interplay across system functioning that was not time-linked. Future research could advance these findings by examining cortisol reactivity to a social stressor in the context of an MRI scan to specifically understand how changes in frontolimbic connectivity before and after a stressor correspond with changes in HPA axis functioning. Psychosocial stress MRI paradigms exist (e.g., MIST; Dedovic et al., 2005), but challenges exist (e.g., relatively lower stress activation). Ideally, this approach would allow for more targeted investigations into the interplay between neural and neuroendocrine systems during stress activation and recovery. It is possible that a better estimate of RSFC could have clarified our findings. Our RSFC MRI sequence is relatively short (6 minutes) compared to current recommendations. Although RSFC MRI sequences as short as 5 minutes have been found to be reliable (Van Dijk et al., 2010), longer RSFC MRI sequences should be used in the future for maximally reliable RSFC measures (Birn et al., 2013; Noble et al., 2019; Zuo & Xing, 2014). We note that on average only about 7% of data was scrubbed because of movement, which is a very modest amount, while acknowledging that a few participants had a higher percentage of images scrubbed (e.g., up to 31%). There were no significant correlations between number of censored images and any TSST measures, precluding that our results were unduly influenced by movement or for corrections thereof. Further, although we focused on RSFC interplay with cortisol reactivity, RSFC interplay with cortisol recovery is also important to examine for a more complete understanding of stress system functioning. See supplementary materials for exploratory analyses on cortisol recovery. RSFC interplay with cortisol recovery across a longer interval of time with more frequent assessments of the recovery response should be explored more in depth in future work.
Findings of this study have important implications for the treatment and prevention of depression. Stress system abnormalities do not always resolve with remission from depression and may even precede the onset of a major depressive episode (Grynderup et al., 2013; Morris et al., 2014). Therefore, stress system dysfunction may be a risk factor for the development of depression and may contribute to the high relapse rates in depression. As such, stress system functioning may need to be a specific treatment target in order to facilitate remission and to prevent relapse. For example, those with depression may benefit from bio- or neurofeedback so they can become more attuned with their stress response and learn how to downregulate amygdala hyperactivity or hypercortisolemia when a stressor has concluded as well as coordinate the functioning of these two systems (Barreiros et al., 2019; Hamilton et al., 2016). Additionally, stress regulation techniques, such as mindfulness-based therapies (Kilpatrick et al., 2011), may provide individuals with depression tools they lack to jointly inhibit these stress systems as a whole. Both neural connectivity and cortisol reactivity patterns have also shown to predict treatment response to antidepressant medications (Klimes-Dougan et al., 2018), so these different patterns of interplay may also be relevant in determining personalized algorithms of treatment assignment (Gunlicks-Stoessel et al., 2020).
6. Conclusions
In conclusion, this study underscores the importance of using multiple levels of analysis to understand stress system functioning. Although the groups largely did not differ in frontolimbic RSFC or HPA functioning, patients with depression and healthy controls showed different patterns of coordination between RSFC and HPA functioning. The results add to the literature showing different patterns of interplay between neuroendocrine stress response and connectivity of the frontolimbic circuitry for youth with and without MDD. The minimal or inverse associations in patients with MDD may reflect poor coordination or a compensatory response that may have implications for treatment.
Supplementary Material
Fig. 1.
L Amygdala and vmPFC RSFC Predicting TSST Highest Cortisol. Note. MDD = major depressive disorder; TSST = Trier Social Stress Test; vmPFC = ventral medial prefrontal cortex; RSFC = resting state functional connectivity; RSFC are z values centered at the mean. The interaction between L amygdala - vmPFC RSFC and group predicted highest cortisol with the healthy control group showing a positive relationship and the MDD group showing a negative relationship.
Highlights.
Frontolimbic connectivity under psychosocial stress associates with cortisol levels.
Patterns of associations differ in adolescents with and without depression.
Positive correspondence in healthy controls suggests coordination across systems.
Negative correspondence in depressed youth suggests poor coordination across systems.
Multilevel approaches are critical for understanding stress system functioning.
Acknowledgments
The authors thank the adolescents and families who participated in this research study and contributed to scientific efforts to increase understanding depression. The study was funded by a grant to Dr. Klimes-Dougan from the Deborah E. Powell Center for Women’s Health at the University of Minnesota, and by grants to Dr. Cullen including the National Institute of Mental Health (K23MH090421), the National Alliance for Research on Schizophrenia and Depression, the University of Minnesota Graduate School, and the Minnesota Medical Foundation.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aihara M, Ida I, Yuuki N, Oshima A, Kumano H, Takahashi K, Fukuda M, Oriuchi N, Endo K, Matsuda H, & Mikuni M (2007). HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Research: Neuroimaging, 155(3), 245–256. 10.1016/j.pscychresns.2006.11.002 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders, text revision. American Psychiatric Association. [Google Scholar]
- Anand A, Li Y, Wang Y, Gardner K, & Lowe MJ (2007). Reciprocal Effects of Antidepressant Treatment on Activity and Connectivity of the Mood Regulating Circuit: An fMRI Study. The Journal of Neuropsychiatry and Clinical Neurosciences, 19(3), 274–282. 10.1176/jnp.2007.19.3.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sandhu R, & Sharma S (2013). Maturation of the adolescent brain. Neuropsychiatric Disease and Treatment, 9, 449–461. 10.2147/NDT.S39776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10(6), 410–422. 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod BN (2002). Validity of the Wechsler Abbreviated Scale of Intelligence and Other Very Short Forms of Estimating Intellectual Functioning, Validity of the Wechsler Abbreviated Scale of Intelligence and Other Very Short Forms of Estimating Intellectual Functioning. Assessment, 9(1), 17–23. 10.1177/1073191102009001003 [DOI] [PubMed] [Google Scholar]
- Barreiros AR, Almeida I, Baía BC, & Castelo-Branco M (2019). Amygdala Modulation During Emotion Regulation Training With fMRI-Based Neurofeedback. Frontiers in Human Neuroscience, 13. 10.3389/fnhum.2019.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck depression inventory-II. San Antonio, 78(2), 490–498. [Google Scholar]
- Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, Nair VA, Meyerand ME, & Prabhakaran V (2013). The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage, 83, 550–558. 10.1016/j.neuroimage.2013.05.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Driscoll D, Mowrer SM, Sollers JJ, Thayer JF, Kirschbaum C, & Tranel D (2010). Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology, 35(1), 56–66. 10.1016/j.psyneuen.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The Brain’s Default Network: Anatomy, Function, and Relevance to Disease. Annals of the New York Academy of Sciences, 1124(1), 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, Davidson RJ, & Birn RM (2012). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience, 15(12), 1736–1741. 10.1038/nn.3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, & Mohr DC (2005). Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology, 30(9), 846–856. 10.1016/j.psyneuen.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Burkhouse KL, Stange JP, Jacobs RH, Bhaumik R, Bessette KL, Peters AT, Crane NA, Kreutzer KA, Fitzgerald K, Monk CS, Welsh RC, Phan KL, & Langenecker SA (2019). Developmental changes in resting-state functional networks among individuals with and without internalizing psychopathologies. Depression and Anxiety, 36(2), 141–152. 10.1002/da.22864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, & Dawson G (2002). Editorial: Multiple levels of analysis. Development and Psychopathology, 14(3), 417–420. 10.1017/S0954579402003012 [DOI] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, & Lim KO (2014). Abnormal Amygdala Resting-State Functional Connectivity in Adolescent Depression. JAMA Psychiatry, 71(10), 1138–1147. 10.1001/jamapsychiatry.2014.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, Hohoff C, Schöning S, Kersting A, Baune BT, Mortensen LS, Arolt V, Zwitserlood P, Deckert J, Heindel W, & Suslow T (2009). Reduced amygdala–prefrontal coupling in major depression: Association with MAOA genotype and illness severity. The International Journal of Neuropsychopharmacology, 12(01), 11. 10.1017/S1461145708008973 [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, & Pruessner JC (2005). The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry and Neuroscience, 30(5), 319–325. [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, & Meaney MJ (1993). The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience, 13(9), 3839–3847. 10.1523/JNEUROSCI.13-09-03839.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, & Strasburger CJ (1992). Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. The Journal of Steroid Biochemistry and Molecular Biology, 43(7), 683–692. 10.1016/0960-0760(92)90294-S [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, & Raichle ME (2002). Glucose metabolism in the amygdala in depression: Relationship to diagnostic subtype and plasma cortisol levels. Pharmacology Biochemistry and Behavior, 71(3), 431–447. 10.1016/S0091-3057(01)00687-6 [DOI] [PubMed] [Google Scholar]
- Edmondson AJ, Brennan CA, & House AO (2016). Non-suicidal reasons for self-harm: A systematic review of self-reported accounts. Journal of Affective Disorders, 191, 109–117. 10.1016/j.jad.2015.11.043 [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Hussein AA, Smart LM, & Sabatinelli D (2014). Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neuroscience & Biobehavioral Reviews, 45, 202–211. 10.1016/j.neubiorev.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Fried EI, & Nesse RM (2015). Depression sum-scores don’t add up: Why analyzing specific depression symptoms is essential. BMC Medicine, 13(1), 72. 10.1186/s12916-015-0325-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea M, Espert R, Salvador A, & Martí-Bonmatí L (2011). The sad, the angry, and the asymmetrical brain: Dichotic Listening studies of negative affect and depression. Brain and Cognition, 76(2), 294–299. 10.1016/j.bandc.2011.03.003 [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, & Tottenham N (2013). A Developmental Shift from Positive to Negative Connectivity in Human Amygdala-Prefrontal Circuitry. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 33(10), 4584–4593. 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Li T-Q, & Ress D (2000). Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine, 44(1), 162–167. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Janson J, & Wolf JM (2017). Meta-analytical assessment of the effects of protocol variations on cortisol responses to the Trier Social Stress Test. Psychoneuroendocrinology, 80, 26–35. 10.1016/j.psyneuen.2017.02.030 [DOI] [PubMed] [Google Scholar]
- Grynderup MB, Kolstad HA, Mikkelsen S, Andersen JH, Bonde JP, Buttenschøn HN, Kærgaard A, Kærlev L, Rugulies R, Thomsen JF, Vammen MA, Mors O, & Hansen ÅM (2013). A two-year follow-up study of salivary cortisol concentration and the risk of depression. Psychoneuroendocrinology, 38(10), 2042–2050. 10.1016/j.psyneuen.2013.03.013 [DOI] [PubMed] [Google Scholar]
- Gunlicks-Stoessel M, Klimes-Dougan B, VanZomeren A, & Ma S (2020). Developing a data-driven algorithm for guiding selection between cognitive behavioral therapy, fluoxetine, and combination treatment for adolescent depression. Translational Psychiatry, 10(1), 1–11. 10.1038/s41398-020-01005-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Glover GH, Bagarinao E, Chang C, Mackey S, Sacchet MD, & Gotlib IH (2016). Effects of salience-network-node neurofeedback training on affective biases in major depressive disorder. Psychiatry Research: Neuroimaging, 249, 91–96. 10.1016/j.pscychresns.2016.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays JR, Reas DL, & Shaw JB (2002). Concurrent validity of the Wechsler abbreviated scale of intelligence and the Kaufman brief intelligence test among psychiatric inpatients. Psychological Reports, 90(2), 355–359. 10.2466/pr0.2002.90.2.355 [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, & Pizzagalli DA (2015). Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry, 72(6), 603–611. 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, & Ryan N (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, & Davidson RJ (2008). Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology, 33(4), 517–529. 10.1016/j.psyneuen.2008.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Suyenobu BY, Smith SR, Bueller JA, Goodman T, Creswell JD, Tillisch K, Mayer EA, & Naliboff BD (2011). Impact of mindfulness-based stress reduction training on intrinsic brain connectivity. NeuroImage, 56(1), 290–298. 10.1016/j.neuroimage.2011.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, & Whalen PJ (2011). Anxiety Dissociates Dorsal and Ventral Medial Prefrontal Cortex Functional Connectivity with the Amygdala at Rest. Cerebral Cortex, 21(7), 1667–1673. 10.1093/cercor/bhq237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, & Whalen PJ (2011). The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behavioural Brain Research, 223(2), 403–410. 10.1016/j.bbr.2011.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimes-Dougan B, Begnel E, Almy B, Thai M, Schreiner MW, & Cullen KR (2019). Hypothalamic-pituitary-adrenal axis dysregulation in depressed adolescents with non-suicidal self-injury. Psychoneuroendocrinology, 102, 216–224. 10.1016/j.psyneuen.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Eberly LE, Westlund Schreiner M, Kurkiewicz P, Houri A, Schlesinger A, Thomas KM, Mueller BA, Lim KO, & Cullen KR (2014). Multilevel assessment of the neurobiological threat system in depressed adolescents: Interplay between the limbic system and hypothalamic–pituitary–adrenal axis. Development and Psychopathology, 26(4pt2), 1321–1335. 10.1017/S0954579414001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimes-Dougan B, Westlund Schreiner M, Thai M, Gunlicks-Stoessel M, Reigstad K, & Cullen KR (2018). Neural and neuroendocrine predictors of pharmacological treatment response in adolescents with depression: A preliminary study. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 81, 194–202. 10.1016/j.pnpbp.2017.10.015 [DOI] [PubMed] [Google Scholar]
- Lee B-T, Seok J-H, Lee B-C, Cho SW, Yoon B-J, Lee K-U, Chae J-H, Choi I-G, & Ham B-J (2008). Neural correlates of affective processing in response to sad and angry facial stimuli in patients with major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 32(3), 778–785. 10.1016/j.pnpbp.2007.12.009 [DOI] [PubMed] [Google Scholar]
- Lu Q, Li H, Luo G, Wang Y, Tang H, Han L, & Yao Z (2012). Impaired prefrontal–amygdala effective connectivity is responsible for the dysfunction of emotion process in major depressive disorder: A dynamic causal modeling study on MEG. Neuroscience Letters, 523(2), 125–130. 10.1016/j.neulet.2012.06.058 [DOI] [PubMed] [Google Scholar]
- McKlveen JM, Myers B, & Herman JP (2015). The Medial Prefrontal Cortex: Coordinator of Autonomic, Neuroendocrine, and Behavioral Responses to Stress. Journal of Neuroendocrinology, 27(6), 446–456. 10.1111/jne.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Rao U, Wang L, & Garber J (2014). Cortisol reactivity to experimentally-manipulated psychosocial stress in young adults at varied risk for depression. Depression and Anxiety, 31(1). 10.1002/da.22125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, & Bandettini PA (2009). The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage, 44(3), 893–905. 10.1016/j.neuroimage.2008.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S, Scheinost D, & Constable RT (2019). A decade of test-retest reliability of functional connectivity: A systematic review and meta-analysis. NeuroImage, 203, 116157. 10.1016/j.neuroimage.2019.116157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Wiebking C, Feinberg T, & Panksepp J (2011). The “resting-state hypothesis” of major depressive disorder-a translational subcortical-cortical framework for a system disorder. Neuroscience and Biobehavioral Reviews, 35(9), 1929–1945. 10.1016/j.neubiorev.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Osman A, Kopper BA, Barrios F, Gutierrez PM, & Bagge CL (2004). Reliability and Validity of the Beck Depression Inventory—II With Adolescent Psychiatric Inpatients. Psychological Assessment, 16(2), 120–132. 10.1037/1040-3590.16.2.120 [DOI] [PubMed] [Google Scholar]
- Peters AT, Jenkins LM, Stange JP, Bessette KL, Skerrett KA, Kling LR, Welsh RC, Milad MR, Phan KL, & Langenecker SA (2019). Pre-scan cortisol is differentially associated with enhanced connectivity to the cognitive control network in young adults with a history of depression. Psychoneuroendocrinology, 104, 219–227. 10.1016/j.psyneuen.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, & Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, & Drevets WC (2010). Neurocircuitry of Mood Disorders. Neuropsychopharmacology, 35(1), 192–216. 10.1038/npp.2009.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, & Drevets WC (2012). Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences, 16(1), 61–71. 10.1016/j.tics.2011.12.011 [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, & Hellhammer DH (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. 10.1016/S0306-4530(02)00108-7 [DOI] [PubMed] [Google Scholar]
- Quaedflieg CWEM, Ven V. van de, Meyer T, Siep N, Merckelbach H, & Smeets T (2015). Temporal Dynamics of Stress-Induced Alternations of Intrinsic Amygdala Connectivity and Neuroendocrine Levels. PLOS ONE, 10(5), e0124141. 10.1371/journal.pone.0124141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ [Google Scholar]
- Radley J, Morilak D, Viau V, & Campeau S (2015). Chronic stress and brain plasticity: Mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neuroscience & Biobehavioral Reviews, 58, 79–91. 10.1016/j.neubiorev.2015.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, (2015). The Brain’s Default Mode Network. Annual Review of Neuroscience, 38(1), 433–447. 10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- Root JC, Tuescher O, Cunningham-Bussel A, Pan H, Epstein J, Altemus M, Cloitre M, Goldstein M, Silverman M, Furman D, LeDoux J, McEwen B, Stern E, & Silbersweig D (2009). Frontolimbic function and cortisol reactivity in response to emotional stimuli: NeuroReport, 20(4), 429–434. 10.1097/WNR.0b013e328326a031 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, & McGaugh JL (2002). Glucocorticoids interact with the basolateral amygdala β-adrenoceptor–cAMP/cAMP/PKA system in influencing memory consolidation. European Journal of Neuroscience, 15(3), 553–560. 10.1046/j.0953-816x.2001.01876.x [DOI] [PubMed] [Google Scholar]
- Rutherford HJV, & Lindell AK (2011). Thriving and Surviving: Approach and Avoidance Motivation and Lateralization. Emotion Review, 3(3), 333–343. 10.1177/1754073911402392 [DOI] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, & Gur RE (2012). Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. NeuroImage, 60(1), 623–632. 10.1016/j.neuroimage.2011.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139(1), 81–132. 10.1037/a0028727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler C, & Miller GE (2011). Depression and Hypothalamic-Pituitary-Adrenal Activation: A Quantitative Summary of Four Decades of Research: Psychosomatic Medicine, 73(2), 114–126. 10.1097/PSY.0b013e31820ad12b [DOI] [PubMed] [Google Scholar]
- Sullivan RM, & Gratton A (2002). Prefrontal cortical regulation of hypothalamic–pituitary–adrenal function in the rat and implications for psychopathology: Side matters. Psychoneuroendocrinology, 27(1–2), 99–114. 10.1016/S0306-4530(01)00038-5 [DOI] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schöning S, Ohrmann P, Bauer J, Pyka M, Kersting A, Arolt V, Heindel W, & Dannlowski U (2010). Automatic Mood-Congruent Amygdala Responses to Masked Facial Expressions in Major Depression. Biological Psychiatry, 67(2), 155–160. 10.1016/j.biopsych.2009.07.023 [DOI] [PubMed] [Google Scholar]
- Thomason ME, Hamilton JP, & Gotlib IH (2011). Stress-induced activation of the HPA axis predicts connectivity between subgenual cingulate and salience network during rest in adolescents: Intrinsic brain connectivity and HPA stress response in adolescents. Journal of Child Psychology and Psychiatry, 52(10), 1026–1034. 10.1111/j.1469-7610.2011.02422.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Reekum C. M. van, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, & Davidson RJ (2006). Amygdala and Ventromedial Prefrontal Cortex Are Inversely Coupled during Regulation of Negative Affect and Predict the Diurnal Pattern of Cortisol Secretion among Older Adults. Journal of Neuroscience, 26(16), 4415–4425. 10.1523/JNEUROSCI.3215-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajravelu ME, Tobolski J, Burrows E, Chilutti M, Xiao R, Bamba V, Willi S, Palladino A, Burnham JM, & McCormack SE (2015). Peak cortisol response to corticotropin-releasing hormone is associated with age and body size in children referred for clinical testing: A retrospective review. International Journal of Pediatric Endocrinology, 2015. 10.1186/s13633-015-0018-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, & Rombouts SARB (2007). Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiology of Learning and Memory, 87(1), 57–66. 10.1016/j.nlm.2006.05.008 [DOI] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Öhman A, & Drevets WC (2010). Relationship of Emotional Processing to Masked Faces in the Amygdala to Mood State and Treatment in Major Depressive Disorder. Archives of General Psychiatry, 67(11), 1128–1138. 10.1001/archgenpsychiatry.2010.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeburg SA, Zitman FG, van Pelt J, DeRijk RH, Verhagen JCM, van Dyck R, Hoogendijk WJG, Smit JH, & Penninx BWJH (2010). Salivary Cortisol Levels in Persons With and Without Different Anxiety Disorders: Psychosomatic Medicine, 72(4), 340–347. 10.1097/PSY.0b013e3181d2f0c8 [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, & Detre JA (2005). Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences, 102(49), 17804–17809. 10.1073/pnas.0503082102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1999). Manual for the Wechsler abbreviated intelligence scale (WASI). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, Gordon E, & Bryant RA (2006). Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. NeuroImage, 29(2), 347–357. 10.1016/j.neuroimage.2005.03.047 [DOI] [PubMed] [Google Scholar]
- Zorn JV, Schür RR, Boks MP, Kahn RS, Joëls M, & Vinkers CH (2017). Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology, 77, 25–36. 10.1016/j.psyneuen.2016.11.036 [DOI] [PubMed] [Google Scholar]
- Zuo X-N, & Xing X-X (2014). Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: A systems neuroscience perspective. Neuroscience & Biobehavioral Reviews, 45, 100–118. 10.1016/j.neubiorev.2014.05.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.