Abstract
Background:
Prostate cancer progression is navigated by the androgen receptor (AR) and transforming-growth factor-β (TGF-β) signaling.Wepreviously demonstrated that aberrant TGF-β signaling accelerates prostate tumor progression in a transgenic mouse model of prostate cancer via effects on epithelial-mesenchymal transition (EMT), driving castration-resistant prostate cancer (CRPC).
Methods:
This study examined the antitumor effect of the combination of TGF-β receptor I (TβRI) inhibitor, galunisertib, and FDA-approved antiandrogen enzalutamide, in our pre-clinical model. Age-matched genotypically characterized DNTGFβRII male mice were treated with either galunisertib and enzalutamide, in combination or as single agents in three “mini”-trials and the effects on tumor growth, phenotypic EMT, and actin cytoskeleton were evaluated.
Results:
Galunisertib in combination with enzalutamide significantly suppressed prostate tumor growth, by increasing apoptosis and decreasing cell proliferation of tumor cell populations compared to the inhibitor as a monotherapy (P < 0.05). The combination treatment dramatically reduced cofilin levels, actin cytoskeleton regulator, compared to single agents. Treatment with galunisertib targeted nuclear Smad4 protein (intracellular TGF-β effector), but had no effect on nuclear AR. Consequential to TGF-β inhibition there was an EMT reversion to mesenchymal-epithelial transition (MET) and re-differentiation of prostate tumors. Elevated intratumoral TGF-β1 ligand, in response to galunisertib, was blocked by enzalutamide.
Conclusion:
Our results provide novel insights into the therapeutic value of targeting TGF-β signaling to overcome resistance to enzalutamide in prostate cancer by phenotypic reprogramming of EMT towards tumor re-differentiation and cytoskeleton remodeling. This translational work is significant in sequencing TGF-β blockade and antiandrogens to optimize therapeutic response in CRPC.
Keywords: EMT, enzalutamide, TGF-β inhibition strong, therapeutic response
1 |. INTRODUCTION
Prostate cancer is the most frequently diagnosed cancer and the third leading cause of cancer deaths in males at 26 730 deaths in the United States in 2017, behind colorectal and lung and bronchus cancers.1 There is an estimated incidence of 161 360 cases of prostate cancers in the United States for 2018, accounting for approximately 19.3% of all estimated new cases of cancer in men in the United States.1,2 Our understanding of the use of systemic therapies to inhibit prostate tumor growth and progression stems from Huggins’ seminal discovery on the inhibitory effects of castration-induced androgen depletion (ADT).3 ADT as a strategy aimed at abrogating the androgen receptor (AR)-mediated tumor growth has dominated the therapeutic landscape in advanced prostate cancer for decades4; however, it invariably leads to emergence of castration-resistant prostate cancer (CRPC) and lethal disease.4,5 Indeed, 10–20% of prostate cancers progress to CRPC within 5 years of diagnosis (as indicated by increasing serum levels of prostate specific antigen (PSA), despite castrate levels of testosterone and progression to metastases), and 84% of newly diagnosed CRPC have metastatic disease.6,7 The median survival of patients following diagnosis of castration resistance and treatment with taxane-chemotherapy ranges between 15 and 36 months, a remarkably low survival that urgently needs improvement.4,8 The FDA-approved second generation antiandrogens abiraterone and enzalutamide have transformed the treatment landscape for mCRPC patients, but resistance arises with emergence to recurrent disease even in chemotherapy-naïve patients.9–13
At the molecular level the androgen receptor (AR) is a critical driver of therapeutic response in patients with mCRPC and persistent AR signaling and AR splice variants are validated therapeutic targets in CRPC.14,15 Phenotypically the process of epithelial-mesenchymal transition (EMT) is a significant contributor to prostate cancer progression to metastasis and therapeutic resistance in patients treated with AR-directed therapies.16–19 Targeting androgen signaling (antiandrogens) and microtubules (taxane chemotherapy) has survival benefits in patients with metastatic CRPC (mCRPC), but therapeutic resistance develops resulting in lethal disease.20,21 Growing evidence has established that docetaxel (1st line chemotherapy) inhibits AR nuclear localization in androgen-sensitive prostate tumors, while in CRPC AR splice variants remain capable of nuclear trafficking contributing to taxane therapeutic resistance.22,23 Furthermore, targeting the AR has been shown to confer cross-resistance between the antiandrogen enzalutamide and docetaxel, but not cabazitaxel (2nd line chemotherapy) in CRPC.24 Recent work from this laboratory revealed that Cabazitaxel led to reversion of EMT to mesenchymal-epithelial-transition (MET), kinesin-mediated multi-nucleation, and glandular re-differentiation while retaining nuclear AR in pre-clinical models of advanced prostate cancer.25
Transforming growth factor-β (TGF-β) has tumor-inhibitory activity in the early stages of prostate tumorigenesis, but it promotes migration and invasion in late stages toward metastasis.26,27 TGF-β1 signaling impairs growth by inhibiting proliferation and inducing apoptosis, and advances invasion and metastatic progression through two transmembrane serine/threonine kinase receptors, type I and type II receptors (TβRI and TβRII).26,28 The TGF-β intracellular signaling network centers around the receptor-activated Smads and Smad4 (primarily in cytoplasm) that upon nuclear import induce transcriptional regulation.28,29 The role of TGF-β as an inhibitor of epithelial cell proliferation in normal homeostasis and a potent inducer of EMT is well-established.26,30–32 Conditional abrogation of the essential signaling effector for TGF-β, TβRII receptor leads to tumorigenic growth and metastatic spread of epithelial tissues in several human cancers, including prostate tumors.33–35 Once this pathway is disrupted TβRI becomes a potent tumor promoter and the resistant tumor cells secrete high levels of TGF-β leading to EMT induction.35 Perturbation of epithelial homeostasis via EMT renders a critical venue for epithelial derived tumors to rapidly metastasize and acquire therapeutic resistance.29,36 We previously demonstrated that aberrant TGF-β signaling accelerates prostate tumorigenesis in a pre-clinical model via effects on EMT.35
Therapeutic targeting of TGF-β1 signaling during tumor progression has emerged as an attractive platform for cancer treatment including prostate tumors and hepatocellular carcinoma.37–39 The novel TGF-β receptor I (TRI) kinase inhibitor, LY2157299 monohydrate (galunisertib), has demonstrated safety and efficacy in the treatment of patients with hepatocellular carcinoma and glioblastoma,40,41 but has not yet been tested in men with mCRPC. Our recent studies using a disrupted TGFβ signaling-EMT driven mouse model37 demonstrated that under ADT, aberrant TGF-β signaling leads to AR activation and β-catenin nuclear localization, an adaptation mechanism contributing to the emergence of CRPC.42 Thus inhibition of TGF-β signaling is a promising strategy for overcoming resistance in mCRPC. The present study investigated the therapeutic efficacy of the TβRI inhibitor, galunisertib, as a monotherapy or in combination with the antiandrogen enzalutamide in our pre-clinical model. We found that TGF-β signaling blockade sensitizes prostate tumors to enzalutamide via impacting EMT to MET interconversion and causing glandular re-differentiation.
2 |. MATERIALS AND METHODS
2.1 |. Mouse model of prostate tumor progression
Mice are maintained under environmentally controlled conditions and subject to a 12 h light/dark cycle with food and water ad libitum. The transgenic adenocarcinoma of mouse prostate (TRAMP) model (C57BL/6-Tg-TRAMP-8247Ng/J; Jackson Laboratories, Bar Harbor, ME; Stock #: 003135) is a widely studied and well-characterized model of prostate cancer progression to metastasis. The dominant-negative TGF-β receptor II (DNTGFβRII) mice were obtained from Dr. Lalage Wakefield (National Cancer Institute, Bethesda, MD). We have previously generated TRAMP DNTGFβRII mice in the C57BL/6 background.35,42 The TRAMP DNTGFRβII transgenic mice develop prostate adenocarcinoma earlier, with the malignant phenotype histopathologically evident at 12wks compared to age-matched TRAMP TGFβRII WT.34 At 16–18 weeks TRAMP DNTGFβRII developed neuroendocrine type adenocarcinoma with local invasion. All procedures and animal handling techniques were approved by the University of Kentucky Institutional Animal Care and Use Committee and in adherence to the NIH Guide for Care and Use of Laboratory Animals.
2.2 |. Drugs and dosing treatment
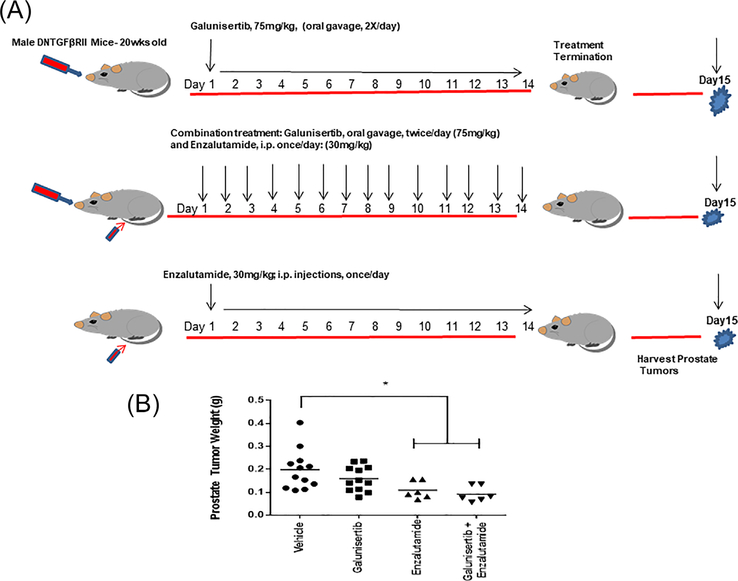
MDV3100 (enzalutamide) was obtained from Selleck Chemicals (Houston, TX). For in vivo use enzalutamide is prepared in dimethyl-sulphoxide (DMSO), diluted with PBS and injected intraperitoneally (for 2 weeks). The TβRI kinase inhibitor, galunisertib, (LY2157299 monohydrate), was provided by Lilly Pharmaceuticals, is given via oral gavage (twice/day for 2 weeks). The dosing regime is schematically illustrated on Figure 1 (panel A). Vehicle control consisted of 1% Carboxymethylcellulose, 0.5% Sodium Lauryl Sulfate, 0.085% Povidone (for mini-trials 1 and 2) and of 25% DMSO and 75% PBS (Trial 3).
FIGURE 1.
Treatment regimen and effect of TGF-β blockade on prostate tumor growth. Panel A, Dosing regimen of galunisertib administration (75 mg/kg body weight, twice/day via oral gavage for 2 weeks) as a monotherapy or in combination with the antiandrogen, enzalutamide. Vehicle control consisted of 1% Carboxymethylcellulose, 0.5% Sodium Lauryl Sulfate, 0.085% Povidone (for mini-trials 1 and 2) and of 25% DMSO and 75% PBS (Trial 3). Panel B, indicates the effect of the various treatments on prostate tumor weight. Galunisertib alone did not exert a significant effect on prostate tumor growth, but in response to the combination with enzalutamide there was a significant reduction in prostate tumor weight compared to vehicle control. (*), statistically significant difference at P < 0.05.
2.3 |. Immunohistochemical analysis
Tissue specimens from transgenic mouse prostate tumors were formalin fixed and paraffin-embedded; serial sections (5 μ) are subjected to immuno-histochemical analysis using the following antibodies: The rabbit monoclonal antibodies against E-cadherin and cytokeratin-18 were obtained from Cell Signaling Technology (Inc. Danvers, MA); rabbit polyclonal antibodies against nuclear antigen, Ki-67 (cell proliferation), and N-cadherin (EMT marker) were from Abcam (Inc. Cambridge, MA); the rabbit polyclonal antibody against the androgen receptor (AR) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA); the rabbit polyclonal antibody against cofilin (cytoskeleton regulator) was from Sigma Life Science (St. Louis, MO). The antibody against TGFβ 1 (ligand) obtained from Abcam Incorporated. Prostate tissue sections were exposed to specific primary antibody and immunostaining was detected by biotinylated goat anti-rabbit IgG and horseradish peroxidase-streptavidin conjugate (Millipore, Billerica, MA). Color development was performed using a FAST 3,3’-diaminobenzidine-based kit (Sigma-Aldrich) and counterstained with hematoxylin. The incidence of apoptosis was evaluated in situ using the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (Millipore). Prostate tissue sections were counterstained with methyl green and TUNEL-positive cells were counted per high power field as previously described.42 Numerical values represent the average number of positive cells counted from three different fields per section. Images are captured with an Olympus BX51 microscope (Olympus America) (Table 1).
TABLE 1.
List of antibodies used in the study
| Antibodies | Company |
|---|---|
| Rabbit polyclonal AR Antibody (N-20); sc-816 | Santa Cruz Biotechnology, Santa Cruz, CA |
| In Situ Apoptosis Detection Kit; S7100 | Millipore, Billerica, MA |
| Rabbit polyclonal N-Cadherin antibody; ab18203 | Abcam, Inc. Cambridge, MA |
| Rabbit polyclonal Ki67 antibody; ab15580 | Abcam, Inc. Cambridge, MA |
| Rabbit polyclonal Smad4 antibody; ab40759 | Abcam, Inc. Cambridge, MA |
| Rabbit polyclonal TGFβ1 antibody; ab25121 | Abcam, Inc. Cambridge, MA |
| Rabbit polyclonal Cytokeratin 18 antibody; ab189444 | Abcam, Inc. Cambridge, MA |
| Rabbit polyclonal cofiln antibody; C8736 | Sigma Life Science, St. Louis, MO |
| Rabbit monoclonal E-cadherin antibody; 3195S | Cell Signaling Technology, Inc. Danvers, MA |
| Rabbit monoclonal GAPDH antibody; 2118S | Cell Signaling Technology, Inc. Danvers, MA |
| Rabbit monoclonal H3 antibody; 4499S | Cell Signaling Technology, Inc. Danvers, MA |
2.4 |. Western blotting
Protein samples were prepared using the NE-PER nuclear-cytoplasmic fraction kit (Thermo Scientific, Rockford, IL). Protein content was quantified using the Pierce BCA Protein Assay Kits (Thermo Scientific) and protein samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (4–15% SDS-polyacrylamide gels; Bio-Rad, Hercules, CA), and transferred to Hybond-C membranes (Amersham Pharmacia Biotech, Piscataway, NJ). Membranes were blocked in 5% milk in Tris-buffered saline containing 0.05% Tween 20, and following incubation with the respective primary antibody (overnight at 4°C), membranes were exposed to species-specific horseradish peroxidase-labeled secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Signal detection was achieved with HyGLO Quick Spray Chemiluminescent HRP Antibody Detection Reagent (Denville scientific, Metuchen, NJ). Protein expression was determined by using the same antibodies as for immuno-histochemical analysis and specific rabbit polyclonal antibodies against Smad4 (Abcam, Inc.). Whole and cytoplasmic protein levels were normalized to glyceraldehyde 3-phosphate dehydrogenase expression (GAPDH), using the rabbit monoclonal antibodies against GAPDH (Cell Signaling Technology, Inc.). Nuclear protein expression was normalized to histone H3 antibody (Cell Signaling Technology, Inc.).
2.5 |. Statistical analysis
The numerical data are analyzed for statistical significance using the unpaired t-test by GraphPadprism 6. Values are expressed as the mean ± standard error of the mean (SEM). Statistical differences between the various groups were considered significant at a P value of <0.05.
3 |. RESULTS
Persistent and/or aberrant AR signaling drives resistance to enzalutamide and prostate tumor progression to recurrent disease.13,38 In order to exploit the therapeutic response of advanced hormone-naïve prostate tumors to the TβRI inhibitor galunisertib, we conducted three “mini”-trials in age-matched (20-wk-old) littermates of DNTGFβTRII mice: (i) galunisertib alone (monotherapy) with a vehicle control; (ii) galunisertib alone (monotherapy) or on combination with the antiandrogen enzalutamide; and (iii) enzalutamide monotherapy with a vehicle control (Supplementary Figure S1, panel A). Statistical evaluation of the treatment effects on prostate tumor growth revealed a significant suppression of tumor growth in response to galunisertib in combination with enzalutamide, compared to TGF-β inhibitor alone (Figure 1, panel B). There was no apparent toxicity in terms of a significant effect on total body weight of mice after at the administered drug doses/treatments in the three “mini”-trials (Supplementary Figure S1, panel B).
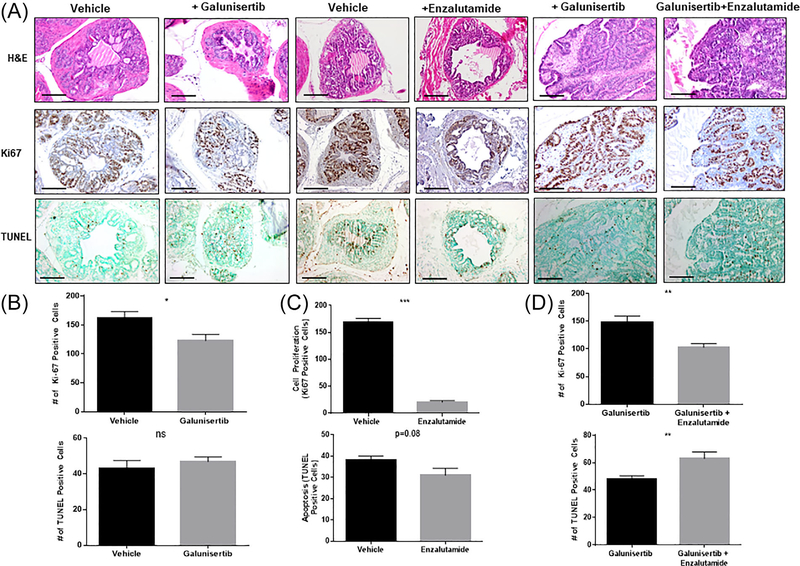
Representative images of the comparative assessment of histolopathology, cell proliferation, and incidence of apoptosis in serial sections of prostate tumors derived from 20-wk-old DNTGFβRII mice after treatment with galunisertib alone or in combination with enzalutamide are shown on Figure 2 (panels A and B, magnification ×100). Panel C reveals the effect of the various treatments on prostate tumor weight; as shown, treatment with the antiandrogen and the combination of TGF-β blockade and enzalutamide resulted in a significant decrease in prostate tumor weight compared to vehicle control or galunisertib monotherapy (P < 0.05). Quantitative analysis of the Ki-67 immunoreactivity among the prostate tumor epithelial cells revealed a significant decrease in the proliferative index after treatment with galunisertib (alone), compared to the vehicle control mice and combination therapy treated mice (Figure 2, panels B and D, respectively; *P < 0.05). Treatment of age matched DNTGFβTRII mice with enzalutamide alone led to a significant reduction in the proliferative capacity of prostate tumor cells compared to vehicle control tumors (P <0.05), while there was no significant effect on the incidence of apoptosis between the two groups (Figure 2, panel C). There was a significant increase in the number of apoptotic (TUNEL-positive) cells among the prostate tumor cell populations in response to galunisertib and enzalutamide combination compared to galunisertib alone (Figure 2, panel D). Interestingly, there was no significant difference in the incidence of apoptosis after TGF-β blockade (monotherapy) compared to vehicle control mice (Figure 2, panel B).
FIGURE 2.
Effect of TGFβRI inhibitor (galunisertib) on prostate tumor cell proliferation and apoptosis. Panel A shows representative images of comparative assessment of H&E histological staining, cell proliferation, and apoptosis in serial sections of prostate tumors derived from the (20-wk-old) DNTGFβRII mice after treatment with enzalutamide, galunisertib as single agents or combination treatment. On day 15 posttreatment mice were euthanized and prostate tumors were surgically excised and subjected to immunohistochemical analysis (per Figure 1A) Quantitative analysis of the Ki-67 immunoreactivity among the prostate tumor epithelial cells indicated a significantly lower proliferative index in prostate tumors from galunisertib—and enzalutamide alone—treated mice compared to vehicle control mice (panels B and C respectively; *P < 0.05). While the number of apoptotic (TUNEL-positive) cells among the prostate tumor cell populations after treatment with either the TβRI inhibitor or enazultamide as single agents was not affected, comparative analysis of the apoptotic response to the combination of galunisertib and enzalutamide treatment revealed a significant increase in apoptosis of prostate tumors (Figure 2, panels A and D). Numerical data represent the average scoring of three fields per individual section, assessed by two independent observers. Values represent the average ± SEM (standard error of the mean). Statistically significant difference between groups is set at P < 0.05.
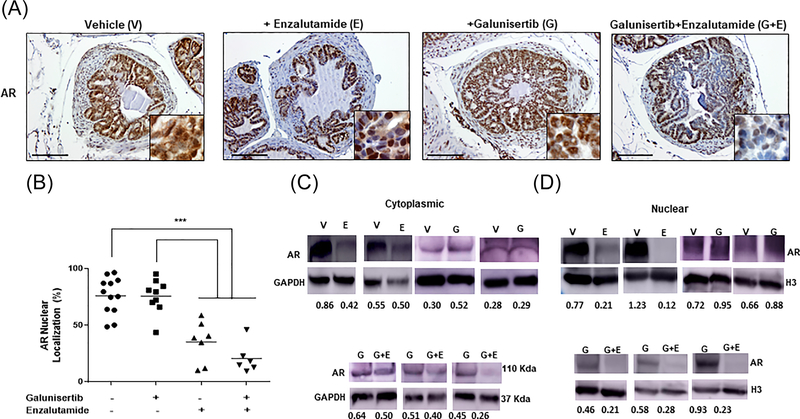
We subsequently examined the impact of TGFβ signaling blockade on AR expression and nuclear localization in prostate tumors derived from aged-matched mice from the three mini-trials. As shown on Figure 3, treatment with galunisertib monotherapy had no significant effect on AR immunoreactivity and nuclear localization compared to vehicle controls (Panel A, magnification ×100, insert × 400). The nuclear staining intensity and topological distribution of AR after TGF-β signaling blockade were remarkably similar to the patterns obtained for the tumors derived from the control mice (Figure 3, panel A). Treatment with enzalutamide (antiandrogen monotherapy) significantly reduced nuclear AR levels compared to controls, as expected (Figure 3, panel B). In response to the combination of galunisertib and enzalutamide there was a marked decrease of nuclear AR levels compared to galunisertib monotherapy (Figure 3, panel B). The combination treatment also caused a further decrease in nuclear AR localization compared to the antiandrogen alone (P < 0.5). Western blot analysis of subcellular fractions (cytoplasmic and nuclear) from prostate tumor lysates from the individual age-matched groups revealed that neither the cytoplasmic nor nuclear AR expression was affected by the TβRI inhibitor alone compared to vehicle controls (Figure 3, panel D). Enzalutamide alone significantly reduced nuclear AR levels as revealed by Western blot analysis (Figure 3, panels A–C). Treatment with the combination of galunisertib and enzalutamide led to a significant downregulation of AR levels in nuclear fractions of prostate tumors that was significantly different from enzalutamide monotherapy (Figure 3, panels B and D).
FIGURE 3.
Effect of TGF-β signaling blockade on nuclear AR expression. Panel A shows representative images for AR immunoreactivity in prostate tumors after the various treatments (Figure 1A). Treatment with enzalutamide as a single agent and in combination with galunisertib reulted in a marked reduction of nuclear AR levels (magnification ×100, insert ×400). Panel B reveals the numerical analysis of the immuoreactivity data indicating that in response to enzalutamide alone and the combination of galunisertib and enzalutamide there was a significant decrease of nuclear AR levels in prostate tumors. Galunisertib monotherapy had no significant effect on AR protein levels and nuclear localization compared to vehicle control mice (panels A and B). Panels C and D indicate representative Western blots profiling AR expression in cytoplasmic and nuclear fractions from prostate tumor lysates; treatment with the TβRI inhibitor alone had no marked effect on AR levels compared to controls, while the antiandrogen, enzalutamide alone reduced nuclear AR. The combination treatment of enzalutamide and galunisertib led to a marked decrease in nuclear AR compared to galunisertib monotherapy (panels C and D). Western blotting was performed as described in section 2.
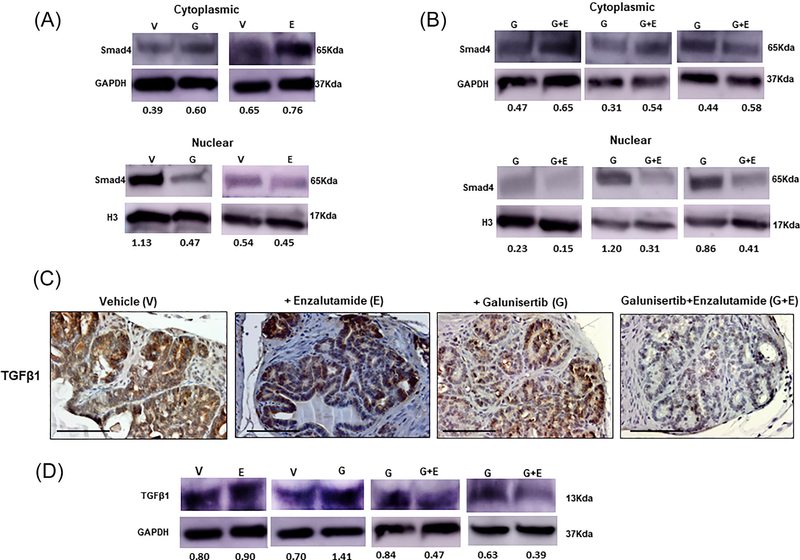
The action of the TβRI inhibitor on the intracellular TGF-β effector Smad4, was then interrogated in prostate tumors. In response to galunisertib there was a marked decrease in Smad4 protein levels in both the cytoplasmic and nuclear fractions of prostate tumor lysates compared to tumors from untreated control mice (Figure 4, panel A). Comparative Western analysis of tumors from the second mini-preclinical trial showed that the combination of galunisertib and enzalutamide resulted in a significant downregulation of nuclear Smad4 compared to galunisertib given as monotherapy (Figure 4, panel B). Enzalutamide alone did not have an effect on Smad4 levels (Figure 4, panel B).
FIGURE 4.
Impact of galunisertib and antiandrogen treatment on Smad4. Panel A, Western blot analysis that blocking the TßRI receptor, galunisertib (G) given alone markedly reduces Smad4 protein levels in both the cytoplasmic and nuclear fractions of prostate tumor lysates compared to tumors from control mice, while enazultamide monotherapy (E) has no effect on Smad 4 expression or localization. Panel B shows the results from Western blot analysis of prostate tumor lysates (second “mini”-trial) revealing that the combination treatment of galunisertib with enzaluamide (G+E) led to a further decrease in nuclear Smad 4 compared to galunisertib alone (higher than twofold). The results of protein profiling by Western blotting are representative of three independent experiments for each of the three clinical mini-trials. Protein expression was assessed by densitometric analysis of band intensity (numerical values shown at the bottom of blot). Panels C and D indicate the results of the immunohistochemical staining and Western blot analysis, respectively for TGF-β1 ligand expression in prostate tumors after the various treatments. In response to galunisertib alone there was enhanced intensity in the immunoreactivity of TGF-β1, compared to controls. Treatment with combination of galunisertib and enzalutamide led to a dramatic decrease in tissue protein TGF-β1 levels compared to controls or either drug given alone (Panel C; magnification ×400). The numerical results of TGF-β1 protein expression from Western analysis of total cell lysates from treated and untreated prostate tumors are shown at the bottom of the blots (panel D). Enzalutamide alone (E) did not have a detectable effect on TGF-β1 expression, while in response to galunisertib there was a twofold increase in ligand levels compared to controls; in contrast the combination treatment (G+E) led to a marked reduction of TGF-β1, compared to drug alone (E).
Interestingly enough, we detected a considerable elevation in the intratumoral tissue levels of the ligand TGF-β1 in response to galunisertib treatment of prostate tumors, by both immunohistochemical and Western blot analysis (Figure 4, panels C and D, respectively). This increase in TGFβ1 after treatment with the TβRI inhibitor was suppressed to low levels after the combination treatment with enzalutamide (Figure 4, panels C and D), indicating that the feedback regulated increase in ligand levels by the TβRI inhibitor, is antagonized by the antiandrogen (Figure 4, panels C and D). Evaluation of the circulating plasma levels of TGF-β1 in treated and untreated transgenic mice did not show significant differences (Supplementary Table S1) possibly due to the small number of samples analyzed.
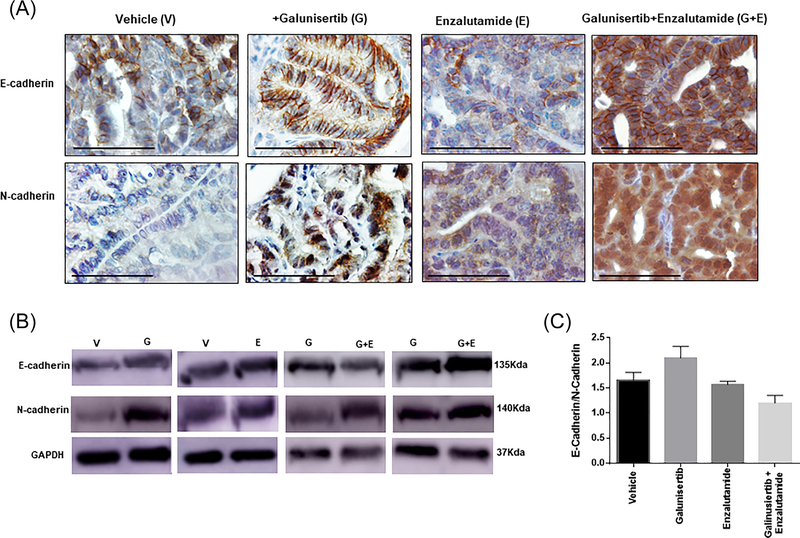
The clinical heterogeneity in progression to metastatic CRPC reflects the diversity of molecular and phenotypic adaptations in the tumor landscape.4 Previously we demonstrated that prostate cancer cells surviving taxane chemotherapy and antiandrogen therapy undergo perturbations at the phenotypic level that promote their re-differentiated phenotype via EMT conversion to MET, impacting therapeutic resistance in pre-clinical models of advanced prostate cancer.25 Driven by this evidence we subsequently interrogated the EMT landscape in prostate tumors to determine the consequences of TGFβ1 blockade on the EMT phenotypic landscape in our preclinical model of advanced prostate cancer. The results shown on Figure 5 (panel A) indicate characteristic representative images of the immunostaining profile of E-cadherin and N-cadherin expression (protein effectors of EMT) in response to treatment (2-wks) of DNTGFβRII mice (20 weeks old) with the TβRI inhibitor alone (monotherapy), the antiandrogen alone (enzalutamide) as monotherapy, or the combination of galunisertib and enzalutamide. Galunisertib given as a monotherapy leads to a remarkable increase in E-Cadherin expression compared to vehicle control tumors (Figure 5, panel A). In response to the combination treatment of galunisertib and enzalutamide there was a further dramatic increase in E-cadherin paralleled by an increase in N-cadherin immunoreactivity in serial sections of prostate tumors. These changes in EMT effectors in response to treatment were confirmed by Western blot analysis of prostate tumor cell lysates from the various mini-trials. Representative profiles (by Western blotting) shown on Figure 5 (panel B), indicate that galunisertib alone (G) resulted in an increase in both E-cadherin and N-cadherin compared to vehicle control mice (V). In response to the combination treatment of galunisertib and enzalutamide (G+E), there was an additional increase in expression of both EMT proteins, E-cadherin and N-cadherin (Figure 5, panel B). Determination of the E-cadherin/N-cadherin expression ratio (based on the densitometric analysis of the protein bands) indicated a ratio increase in response to galunisertib treatment, compared to vehicle controls and the galunisertib and enzalutamide combination treatment (Figure 5C). These findings indicate induction of MET (conversion of EMT to MET) after TGF-β signaling blockade of prostate tumors.
FIGURE 5.
Galunisertib treatment promotes EMT to MET phenotypic conversion in prostate tumors in vivo. Panel A shows representative images of E-cadherin and N-cadherin immunostaining in serial sections of prostate tumors from vehicle control and galunisertib-treated mice, given as a single agent (magnification, ×400). Panel B shows the results of Western blot analysis of expression profile of EMT protein effectors E-cadherin and N-cadherin in total cell lysates of prostate tumors from vehicle control, and inhibitor treated as monotherapy and combination of galunisertib and enzalutamide in DNTGFβRII mice. GAPDH was used as a loading control. Panel C indicates the ratio of relative expression of E-Cadherin over N-cadherin in prostate tumor cell lysates from the Western blot analysis and subsequent densitometric analysis of protein bands for each respective protein. TGFβRI inhibitor led to an increase in E-cadherin paralleled by increased N-cadherin protein levels in prostate tumors (favoring MET reversal of TGF-β-mediated EMT in response to galunisertib). Values represent the mean intensity of bands from samples from the three treatment mini-trials ± SEM.
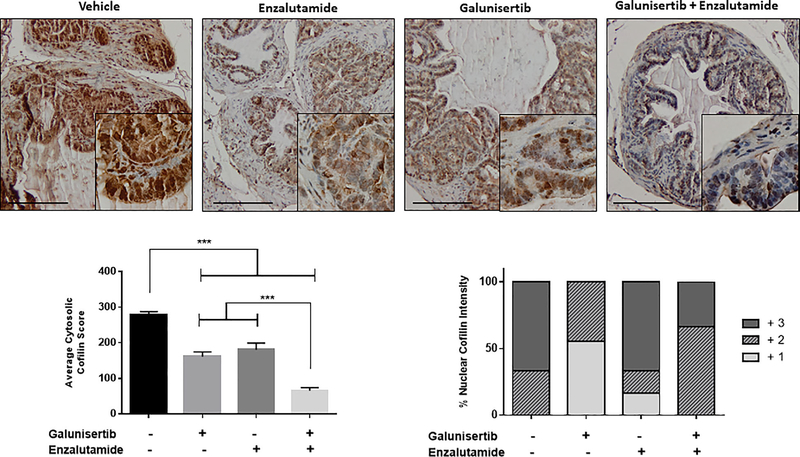
Cofilin a small apoptosis protein and the main regulator of the actin cytoskeleton dynamics was previously shown by our group to be a non-Smad effector of TGF-β signaling in prostate cancer progression and invasion.27,43 Considering that EMT engages the actin cytoskeleton to confer the invasive and migratory properties of prostate tumor cells,35,36 we subsequently investigated the impact of TGF-β signaling inhibition and antiandrogen on cofilin expression and intracellular localization. The results shown on Figure 6, indicate that TGF-β blockade with galunisertib leads to a significant depletion of the cytosolic levels of cofilin (P < 0.05), and a marked decrease in the nuclear intensity of the protein (P < 0.05). Enzalutamide given as a monotherapy also significantly downregulated cytosolic cofilin levels compared to controls, but had no marked effect on nuclear cofilin immunoreactivity (Figure 6, panels B and C, respectively). In response to the combination treatment of galunisertib and enzalutamide, there was a significantly higher reduction in cytoplasmic cofilin expression in prostate tumors compared to the effect by either drug given as single drug treatment (galunisertib or enzalutamide) indicating a synergistic impact on the actin cytoskeleton remodeling (Figure 6, panel B; P < 0.05). Significantly, the combination treatment did not affect the nuclear cofilin expression/localization compared to the controls (Figure 6, panel C).
FIGURE 6.
Combination of TGF-β signaling blockade and antiandogen impacts actin cytoskeleton organization in prostate tumors. Panel A reveals representative images of cofilin (TGF-β effector and actin cytoskeleton regulator) immunoreatctivity in prostate tumors after various treatments. Combination treatment of galunisertib (Tβ1RI inhibitor) and enzalutamide resulted in dramatic depletion of cofilin protein levels compared to each drug alone or vehicle control (Magnification ×200, insert ×1000). Panels B and C reveal the numerical analysis of the immunoreactivity scoring (from panel A) of the cytosolic cofilin levels and nuclear intensity of the protein, respectively. In response to the combination treatment of galunisertib and enzalutamide, there was a significant decrease in cytoplasmic cofilin expression in prostate tumors compared to vehicle controls and single drug treatment (galunisertib or enzalutamide) (P < 0.05). Galunisertib as a monotherapy resulted in decreased nuclear cofilin intensity compared to controls and the other treatments (Panel C). Numerical values represent the average scoring of cofilin immunoreactivity from three fields per tumor section, assessed by two independent observers (panel B) ±SEM. *** indicates statistically significant differences between treatments at P < 0.05. The nuclear cofilin intensity was determined based on the distribution of increasing staining intensity +1 (low), +2 (moderate),+3 (highest) among nuclei of prostate tumor sections, as shown.
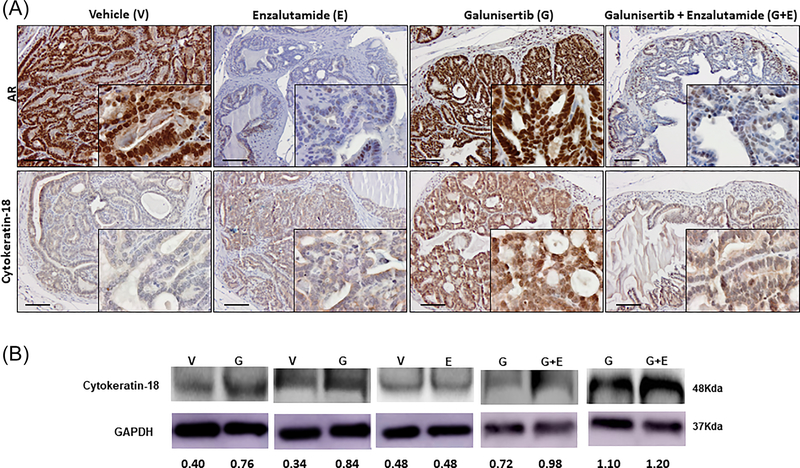
In order to examine whether reversal of EMT to MET may lead to re-differentiated epithelial cell phenotype that can be targeted by sequential therapy (TGF-β signaling blockade followed by antiandrogen), we used a marker for cell differentiation, cytokeratin-18, to examine its distribution of expression in response to treatment. Figure 7 shows representative images revealing the immunoreactivity and expression pattern of AR and cytokeratin-18 proteins (upper and lower panel, respectively as indicated), in serial sections of prostate tumors after treatment with the TβR1 inhibitor alone or in combination with the antiandrogen, enzalutamide. Blockade of TGFβ signaling resulted in a selective expression of cytokeratin-18 among the prostate tumor cell populations retaining nuclear AR levels (after 2 weeks of galunisertib treatment). Treatment with the antiandrogen markedly decreased nuclear AR levels, with no apparent effect on cytokeratin-18 immunoreactivity (Figure 7, Panel A). Evaluation of the impact of treatment on cytokeratin-18 via Western blot analysis (Figure 7, Panel B) revealed that TGFβ signaling blockade upregulated cytokeratin-18 protein in prostate tumor cell lysates compared to vehicle control, while enzalutamide had no effect on cytokeratin-18.
FIGURE 7.
TGF-β blockade induces prostate tumor cell differentiation independent of AR. Panel A shows representative characteristic images of cytokeratin-18 immunoreactivity and AR expression and nuclear localization in prostate tumor sections. Upper panel, reveals AR immunoreactivity and lower panel cytokeratin-18 immunoreactivity in serial sections of prostate tumors after various treatments, vehicle control (V), enzalutamide monotherapy (E), galunisertib monotherapy (G), and the combination treatment of galunisertib and enzalutamide (G+E) (dosing as described in section 2 for 2 weeks). Galunisertib monotherapy led to a marked increase in cytokeratin-18 immunoreactivity indicating tumor re-differentiation, compared to the enzalutamide alone and vehicle control (Magnification ×200, insert ×1000). Panel B indicates the cytokeratin-18 expression profile in response to various treatments as detected by Western blot analysis. The results shown are representative of three independent experiments for each of the three “mini”-trials. Protein expression was determined by densitometric analysis of band intensity (numerical values shown at the bottom of blot). Galunisertib alone (G) resulted in approximately twofold increase in cytokeratin-18 expression, compared to vehicle controls (V), while there was no apparent difference with enzalutamide alone (E). The combination of galunisertib and enzalutamide (G+E) had no major effect on cytokeratin-18 levels.
4 |. DISCUSSION
Therapeutic resistance to ADT is virtually inevitable but occurs after a unique timeline of therapy for each prostate cancer patient after biochemical recurrence or diagnosis of advanced disease.20,21 In view of our recent work demonstrating that TGF-β signaling is involved in the emergence of a castration-resistant state in a pre-clinical model of EMT-driven prostate tumor progression,42 defining the molecular events through which TGF-β signaling contributes to mCRPC will provide novel targetable interactions. This study provides evidence on the therapeutic impact of the combination of Tβ1RI inhibitor galunisertib, with the FDA-approved antiandrogen (enzalutamide) via an action bypassing the AR. Combination of galunisertib with enzalutamide led to a marked reduction in prostate tumor growth associated with EMT conversion to MET, without a significant change in nuclear AR. Our results support the potential therapeutic value of targeting TGF-β signaling under castrate androgen levels for patients with CRPC. Translation of these pre-clinical findings in the clinic proceeds through a multisite randomized trial of galunisertib and enzalutamide in men with metastatic CRPC (NCT02452008).
Therapeutic inhibition of oncogenic drivers induces dramatic secretome changes in drug-sensitive cancer cells, enabling a tumor microenvironment that nurtures expansion of resistant clones that are susceptible to combination therapy.44 The massive heterogeneity and plasticity that characterize prostate tumors fuels the contribution of regulatory changes imposed by the microenvironment, such as the EMT phenotype and the actin cytoskeleton remodeling, to therapeutic resistance.27,35,36 The present study defines for the first time the consequences of inhibition of TGF-β signaling on regulators of two dynamic processes, EMT interconversion to MET and the actin cytoskeleton organization, leading to re-differentiation of prostate tumors in an in vivo model. Heterogeneity in apoptotic thresholds may underlie therapeutic resistance45 and TGF-β causes tumor suppression via a lethal EMT programing.46 The events of dramatic cofilin depletion (compromising the integrity of cytoskeleton) and phenotypic changes (promoting MET) may drive an enhanced response to the combination strategy of galunisertib and the antiandrogen in advanced tumors. Thus we submit that the phenotypic “identity shift” toward MET in prostate tumors in response to TGF-β signaling blockade may sensitize prostate tumor cell populations to other TGF-β-navigated events, “conditioning” the microenvironment to overcome therapeutic resistance29 (Figure 8). Diverse TGF-β directed mechanisms leading to nuclear AR accumulation in the absence of androgens (castrate-resistant disease), including ZEB transcription factors engaged by nuclear import and export signals facilitating EMT,47 steroid receptor co-activators48,49 and integrin αvβ6-mediated JNK1 activation,50 indirectly support our findings. One must also recognize the impact of EMT “thresholds” and cytoskeleton dynamics on apoptosis outcomes and tumor therapeutic response.25,35,46
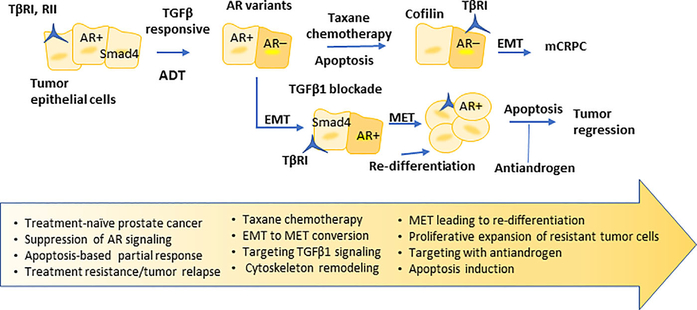
FIGURE 8.
Schematic illustration of overcoming prostate tumor therapeutic resistance after ADT, and taxane chemotherapy with TGFβ1 signaling blockade. In response to TGFβ1 signaling inhibition, prostate tumor cells undergo EMT to MET conversion, reverting to an epithelial phenotype and glandular re-differentiation while retaining nuclear AR activity. Certain tumor cells may exhibit selective actin cytoskeleton remodeling that would sensitize them to sequential drug action. These tumor cells are now “primed” to be targeted by antiandrogen (sequential to galunisertib treatment) to undergo apoptosis thus driving tumor regression.
Heterogeneity in TGF-β responsiveness might exist among prostate tumor cell populations, with the ligand TGF-β1 (or TGF-β3) simultaneously inducing apoptosis and limiting proliferation, while in other subsets promoting invasive properties and driving metastatic spread. A recent study (by an independent group), provides new mechanistic insights into to the ability of TGF-β signaling to regulate FOXA binding to TGF-β gene enhancer and resulting in significant changes in Slug, an EMT transcriptional repressor.51 This evidence supports the functional relevance of the molecular activation of TGF-β3 signaling by FOXA toward EMT induction that can be targeted by galunisertib to impair invasion and metastasis. TGF-β mediated EMT has been linked to tumor chemoresistance, but targeting EMT alone might be functionally counterproductive by enabling proliferation of surviving disseminated cells.52 Indeed a switch from E- to N-cadherin predicts clinical prostate tumor progression, recurrence, and mortality,16,17 and therapeutic targeting of N-cadherin in CRPC emerges as an effective strategy blocking metastasis.18 Moreover, EMT-associated markers are detected in circulating tumor cells in prostate cancer patients.41 Based on our data, we propose a novel action by the TGF-β signaling inhibitor in changing the phenotypic landscape of these hormone-naïve tumors not by abrogating EMT induction but causing its reversal to MET. Changes in the interconversion rates of EMT to MET may generate metabolic heterogeneities in prostate cancer cells that transient as they might be, could impact tumor cell responsiveness and therapeutic resistance. Although TGF-β-induced EMT in prostate tumorigenesis can be compromised via effects of the AR axis on TGF-β effectors SMADs 3, 4, that negatively regulate AR-transcription,43 in our study we found that inhibition of TGF-β signaling has no effect on nuclear AR. Since Smad4 translocates to the nucleus in response to TGF-β by binding to Importin 7/8,45 the effect of TGF-β blockade on the association of Importin7 and Smad4 in prostate tumor cells is being examined.53
A limitation in our study is that we did not interrogate a correlation between the acquisition of the neuroendocrine phenotype and the dynamics of EMT-MET conversion in the context of the tumor microenvironment during prostate cancer progression. This begs the question as to whether a phenotypic reversion of the neuroendocrine phenotype back to the original epithelial phenotype is possible in transgenic mice of advanced age. To this end we are currently investigating the effects of the TGF-β signaling inhibition in younger mice (14–16 weeks of age) and will follow the impact on survival and neuroendocrine differentiation up to 35 weeks of age. Studies in squamous cell carcinoma revealed a non-genetic paradigm for TGF-β signaling in causing diversity of response to anti-cancer therapeutics among tumor-initiating stem cells.54 It was demonstrated that tumor microenvironment generates heterogeneity in TGF-β signaling at the tumor-stroma interface, with non-responding progenitor cells proliferating faster, promoting tumor growth, while TGF-β responding cells aberrantly differentiating, but both contributing to therapeutic resistance. Ongoing studies in our laboratory aim to characterize the coordinated molecular events engendering the phenotypic alterations dictated by TGF-β signaling in prostate tumors with acquisition of response by mesenchymal cells upon conversion to epithelial cells.
In summary, this study supports the therapeutic efficacy of galusinertib in combination with enzalutamide, through phenotypic reprograming of TGF-β-mediated EMT to MET, compromising the cytoskeleton organization, and causing re-differentiation of prostate tumors. Our findings are significant in addressing the clinical challenge of lethal prostate cancer occurring after ADT and taxane chemotherapy with biochemical recurrence and progression to metastases (mCRPC).20,55 Thus upon treatment with the TβRI inhibitor, EMT is converted to MET among some prostate cell populations that revert, reverting to an epithelial phenotype for glandular re-differentiation and retain intact nuclear AR activity; another cell subset may undergo apoptosis (as schematically illustrated on Figure 8). A population of cells with MET phenotype adapt to TGF-β signaling inhibition escaping apoptosis, undergoing re-differentiation and resuming proliferation with progression. These tumor cells can then be effectively targeted by the antiandrogen enzalutamide, thus impairing tumor relapse and progression to CRPC (Figure 8). Perturbation of potential interactions between Smad4 with AR or AR-independent effectors,56 or with cofilin (cytoskeleton remodeling) by antiandrogens may confer a new targetable partnership to overcome resistance. The ongoing clinical trial in patients with advanced CRPC will enable a new therapeutic platform for sequencing TGF-β signaling blockade with antiandrogens, with optimized anti-tumor action and survival benefit in patients with lethal disease. The current clinical knowledge of sequencing treatment strategies in patients with mCRPC21 directs our efforts towards exploiting the temporal interconversions of EMT to MET, in sequencing TGF-β signaling blockade with antiandrogens to impair lethal prostate cancer and develop personalized signatures to predict therapeutic resistance in mCRPC.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge support of this work through funding from a Schwab Foundation Grant (NK, CP), National Institutes of Health grant K23CA197526 (CP), the James F. Hardymon Endowment in Urologic Research at the University of Kentucky (NK), and the National Center for Advancing Translational Sciences, UL1TR000117 (CAW). The authors thank Lorie Howard for assisting in the manuscript submission.
Funding information
National Institutes of Health, Grant number: K23CA197526; National Center for Advancing Translational Sciences, Grant number: UL1TR000117; Schwab Foundation
Abbreviations:
- AR
androgen receptor
- CRPC
castration-resistant prostate cancer
- DMSO
dimethylsulfoxide
- EMT
epithelial-mesenchymal transition
- FDA
food and drug adminstration
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase expression
- MET
mesenchymal epithelial transition
- PBS
phosphate buffer saline
- PSA
prostate specific antigen
- TGF-β
transforming-growth factor-β
- TRAMP
transgenic adenocarcinoma of mouse prostate
- TβRI
TGF-β receptor I
- TβRII
TGF-β receptor II
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling
Footnotes
CONFLICTS OF INTEREST
None of the authors declares conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Solo K, Valant J, Todd MB, Mehra M. Prevalence of prostate cancer clinical states and mortality in the United States: Estimates using a dynamic progression model. PLoS ONE. 2015;10:e0139440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huggins C, Stevens RE Jr., Hodges CV. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–223. [Google Scholar]
- 4.Crawford ED, Petrylak D, Sartor O. Navigating the evolving therapeutic landscape in advanced prostate cancer. Urol Oncol. 2017;35S:S1–S13. [DOI] [PubMed] [Google Scholar]
- 5.Kirby M, Hirst C, Crawford ED. Characterizing the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180–1192. [DOI] [PubMed] [Google Scholar]
- 6.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level <or =4.0ng per milliliter. N Engl J Med. 2014;350:2239–2246. [DOI] [PubMed] [Google Scholar]
- 7.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–1764. [DOI] [PubMed] [Google Scholar]
- 8.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376: 1147–1154. [DOI] [PubMed] [Google Scholar]
- 9.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. [DOI] [PubMed] [Google Scholar]
- 10.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. [DOI] [PubMed] [Google Scholar]
- 11.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 12.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azad AA, Eigl BJ, Murray RN, Kollmannsberger C, Chi KN. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur Urol. 2015;67:23–29. [DOI] [PubMed] [Google Scholar]
- 14.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. [DOI] [PubMed] [Google Scholar]
- 15.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennbacken J, Tesan T, Wang W, Gustavsson H, Damber JE, Welen K. N-cadherin increases after androgen deprivation and is associated with metastasis in prostate cancer. Endocr Relat Cancer. 2010;17: 469–479. [DOI] [PubMed] [Google Scholar]
- 17.Gravdal K, Halvosten OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progression of prostate cancer. Clin Cancer Res. 2007;13:7003–7011. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka H, Kono E, Tran CP, et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med. 2010;16:1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sethi S, Macoska J, Chen W, Sarkar FH. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Trans Res. 2010;3:90–39. [PMC free article] [PubMed] [Google Scholar]
- 20.Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol. 2013;11:14–23. [PMC free article] [PubMed] [Google Scholar]
- 21.Sartor O, Gillessen S. Treatment sequencing in metastatic castrate-resistant prostate cancer. Asian J Androl. 2014;16:426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu M, Horbinski CM, Garzotto M, Qian D, Beer T, Kyprianou N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70:7992–8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darshan MS, Loftus MS, Thadani-Mulero M, et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res 2011;71: 6019–6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Soest RJ, de Morrée ES, Kweldam CF, et al. Targeting the androgen receptor confers in vivo cross-resistance between enzalutamide and docetaxel, but not cabazitaxel, in castration-resistant prostate cancer. Eur Urol. 2015;67:981–985. [DOI] [PubMed] [Google Scholar]
- 25.Martin SK, Pu H, Penticuff J, Cao Z, Horbinski C, Kyprianou N. Multinucleation and mesenchymal-to-epithelial transition alleviate resistance to combined cabazitaxel and antiandrogen therapy in advanced prostate cancer. Cancer Res. 2016;76:92–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu B, Kyprianou N. Transforming growth factor-β and cancer. In: Alison MR, ed. Molecular and Cellular Basis of Cancer. Louisville, KY: The Cancer Textbook; 2007;22:257–271. [Google Scholar]
- 27.Collazo J, Zhu B, Larkin S, et al. Cofilin regulates cellular invasion responses to TGF-β towards prostate cancer metastasis. Cancer Res. 2014;74:2362–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullen AC, Orlando DA, Newman JJ, et al. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell. 2011;147:565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massague J. TGF-β signaling in context. Nat Rev Mol Cell Biol. 2012;13: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li P, Yang R, Gao WQ. Contributions of epithelial-mesenchymal transition and cancer stem cells to the development of castration resistance of prostate cancer. Mol Cancer. 2014;13: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu GL, Yang HJ, Liu T, Lin YZ. Expression and significance of E-cadherin, N-cadherin, transforming growth factor-beta1 and Twist in prostate cancer. Asian Pac J Trop Medicine. 2014;7:76–82. [DOI] [PubMed] [Google Scholar]
- 32.Thuault U, Valcourt M, Kowanetz A, Moustakas A. TGF-β and smad signaling in transcriptome reprogramming during EMT transforming growth factor-β in cancer therapy. Humana Press. 2008;I:259–273. [Google Scholar]
- 33.Lu SL, Herrington H, Reh D, et al. Loss of transforming growth factor-β type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 2006;20:1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ijichi H, Chytil A, Gorska AE, et al. Aggressive pancreatic ductal ademocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-β signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pu H, Collazo J, Jones E, et al. Dysfunctional transforming growth factor-beta receptor II accelerates prostate tumorigenesis in the TRAMP mouse model. Cancer Res. 2009;69:7366–7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matuszak E, Kyprianou N. Androgen signaling in epithelial-mesenchymal transition in prostate cancer progression to metastasis. Expert Rev J Clinic Endocrinol Metabol. 2011;6:469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-beta signaling inhibitors for cancer therapy. Nat Rev Drug Discov. 2004;3: 1011–1022. [DOI] [PubMed] [Google Scholar]
- 38.Jones E, Pu H, Kyprianou N. Targeting TGF-β in prostate cancer: therapeutic possibilities during tumor progression. Expert Opin Ther Targets. 2009;13:227–234. [DOI] [PubMed] [Google Scholar]
- 39.Giannelli G, Villa E, Lahn M. Transforming growth factor-beta as a therapeutic target in hepatocellular carcinoma. Cancer Res. 2014;74: 1890–1894. [DOI] [PubMed] [Google Scholar]
- 40.Faivre SJ, Santoro A, Kelley RK, et al. Growth Factor-beta (TGF-β) Receptor I Kinase Inhibitor, LY2157299 Monohydrate, in Patients With Advanced Hepatocellular Carcinoma (HCC). 2014 Gastrointestinal Cancers Symposium, 2014; San Francisco. [Google Scholar]
- 41.Carpentier AF, Brandes AA, Kesari S, et al. Safety interim data from a three-arm phase II study evaluating safety and pharmacokinetics of the oral transforming growth factor-beta (TGF-β) receptor I kinase inhibitor LY2157299 monohydrate in patients with glioblastoma at first progression. J Clin Oncol. 2013;31:(suppl; abstr 2061). [Google Scholar]
- 42.Pu H, Begemann DE, Kyprianou N. Aberrant TGF-β signaling drives castration-resistant prostate cancer in a male mouse model of prostate tumorigenesis. Endocrinology. 2017;158:1612–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu B, Fukada K, Zhu H, Kyprianou N. Prohibitin and Cofilin are intracellular effectors of TGF-β signaling in prostate cancer cells. Cancer Res. 2006;66:8640–8647. [DOI] [PubMed] [Google Scholar]
- 44.Obenauf AC, Zou Y, Ji AL, et al. Therapy-induced tumor secretomes promote resistance and tumor progression. Nature. 2015;520:369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogden A, Rida PC, Reid MD, Kucuk O, Aneja R. Die-hard survivors: heterogeneity in apoptotic thresholds may underlie chemoresistance. Expert Rev Anticancer Ther. 2015;15:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.David CJ, Huang YH, Chen M, et al. TGF-β tumor suppression through a lethal EMT. Cell. 2016;164:1015–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gheldof A, Hulpiau P, van Roy F, de Craene B, Berx G. Evolutionary functional analysis and molecular regulation of the ZEB transcription factors. Cell Mol Life Sci. 2012;69:2527–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tien JC, Liu Z, Liao L, et al. The steroid receptor coactivator-3 is required for the development of castration-resistant prostate cancer. Cancer Res. 2013;73:3997–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peacock SO, Fahrenholtz CD, Burnstein KL. Vav3 enhances androgen receptor splice variant activity and is critical in castration-resistant prostate cancer growth and survival. Mol Endocrinol. 2012;26: 1967–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dutta A, Li L, Fedele C, et al. αvβ6 integrin is required for TGFβ1-mediated matrix metalloproteinase2 expression. Biochem J. 2015;466: 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin HJ, Zhao JC, Ogden I, Bergan R, Yu J. Androgen-receptor independent function of FoxA1 in prostate cancer metastasis. Cancer Res. 2013;73:3725–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armstrong AJ, Marengo MS, Oltean S, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao X, Chen C, Cottonham C, Xu L. Preferential utilization of Imp7/8 in nuclear import of Smads. J Biol Chem. 2008;283: 22867–22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oshomori N, Oristian D, Fuchs E. TGF-β promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell. 2015;160: 963–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pezaro CJ, Omlin AG, Altavilla A, et al. Activity of cabazitaxel in castration-resistant prostate cancer progressing after docetaxel and next-generation endocrine agents. Eur Urol. 2014;66: 459–465. [DOI] [PubMed] [Google Scholar]
- 56.Vidal SJ, Rodriguez-Bravo V, Aidan Quinn S, et al. A targetable GATA2-IGF2 axis confers aggressiveness in lethal prostate cancer. Cancer Cell. 2015;27:223–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.