Abstract
A simple and green colorimetric sensing assay strategy for highly efficient determination of melamine has been fabricated, which is based on the redox reaction of gallic acid with Ag+. Monodispersed Ag nanoparticles (AgNPs) were obtained using gallic acid as a reducing and stabilizing agent. However, the aggregate behavior of AgNPs was observed, while the melamine was present in the reaction medium. As a result, the color of the solution changed from vivid yellow to brown, and the density of the color was quantitatively correlated with the melamine concentration. The aggregation of AgNPs could be attributable to the formation of hydrogen bonds between melamine and gallic acid. The designed sensor exhibited a good detection limit of 0.099 μM (0.012 ppm), which was much lower than the safety limit in China (1.0 ppm) and EU (2.0 ppm). Additionally, the sensing assay displayed good selectivity toward melamine over other coexisting substances. Consequently, the proposed colorimetric sensor was successfully used for the determination of melamine detection in raw milk samples.
1. Introduction
Melamine (1,3,5-triazine-2,4,6-triamine), a desirable protein substitute, is predominately emerged in milk, infant formula, and pet food due to its high nitrogen content (66% by mass).1−3 Besides, the addition of melamine could increase the protein content in milk, which can mislead the consumers in the milk product market.4 However, while melamine concentration is higher than the safety limit [2.5 ppm of United States Food and Drug Administration (US FDA) and 1.0 ppm of infant formula in China],5 an insoluble compound with cyanuric acid that induces kidney stones could be formed, leading to renal failure and even death in humans.6,7 For example, serious renal failure and deaths of infants have been reported due to the addition of melamine into infant formula in 2008.8 The serious detrimental consequences of melamine contamination can pose to public health. Therefore, it is essential to develop reliable methods for the detection of melamine in milk industry.
Until now, a number of analytical methods such as mass spectroscopy (MS), liquid chromatography–MS (LC–MS), gas chromatography–MS, micellar LC, high-performance LC, and nuclear magnetic resonance spectroscopy have been well explored for the detection of melamine in food samples.9−13 These methods have the advantages of high specificity and sensitivity. However, the properties such as high instrumentation costs, complicated preconcentration, and time consumption hindered their utilization in melamine detection.14 Overall, it is of particular importance to establish a simple, low-cost, intrinsic, and sensitive method for the detection of melamine.
Recently, benefiting from the advantages of being simple and cost-effective and having good sensitivity and selectivity for real-time sample analysis,15−17 the metallic nanoparticle-based colorimetric sensing assays have been drawn considerable attention in biological science and analytical chemistry. Among the nanoparticle-based colorimetric assays, gold nanoparticles (AuNPs)18 and silver nanoparticles (AgNPs)19 have been widely used for the design of visual sensors for melamine detection owing to the simple preparation, stability, and biocompatibility. The colorimetric sensing assay technique involves three steps, including synthesis of Au/Ag nanoparticles, functionalization of nanoparticles, and detection of melamine.20 These two nanoparticles aggregate because of the interaction by hydrogen-bonding recognition, electrostatic, or donor–acceptor interaction. The shifting of resonant excitation is due to the introduction of melamine, resulting in the change of the color of the solution from bright yellow to pale red.21 For example, Inamuddin and Kanchi22 first synthesized the AgNPs, then modified the AgNPs with Colocasia esculenta, and finally detected the melamine in biological samples. Noticeably, the whole protocol was still time-consuming and required complex procedures and toxic reagents, which limited the applicability of the approaches.23,24 Therefore, it is necessary to establish a more facile and rapid sensing assay to achieve simple sensing of melamine in the raw milk sample.
Gallic acid possesses hydroxyl groups in the molecular structure and can reduce metal ions to metal nanoparticles. Herein, we employed a colorimetric sensor for highly selective detection of melamine. In this research, gallic acid acted as a reducing and functionalizing agent for AgNPs. More importantly, the synthesis and functionalization of AgNPs and the analysis of melamine could be completed in a simple process. In this study, the as-prepared colorimetric sensing assay provided a simple and convenient method for the detection of melamine.
2. Results and Discussion
2.1. Mechanism of the Colorimetric Sensor
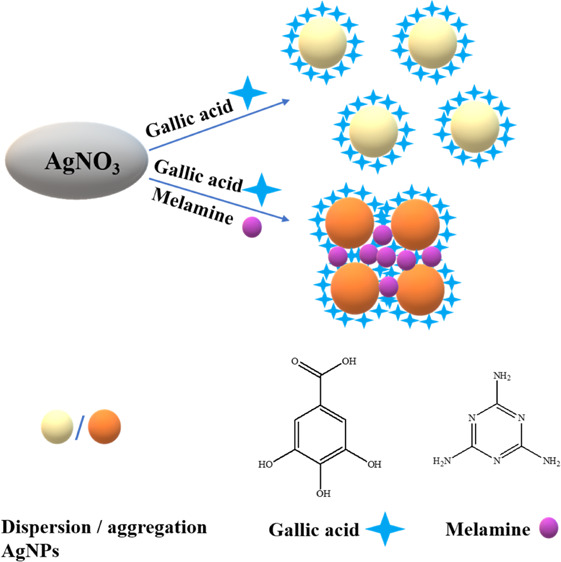
It is reported that gallic acid can reduce Ag+ to AgNPs without the addition of any other reducing materials. In this paper, the role of gallic acid during the formation of AgNPs was investigated by mixing gallic acid and AgNO3 in alkaline solution (Scheme 1).
Scheme 1. Mechanism Assay for the Colorimetric Detection of Melamine with the AgNPs@Gallic Acid System.
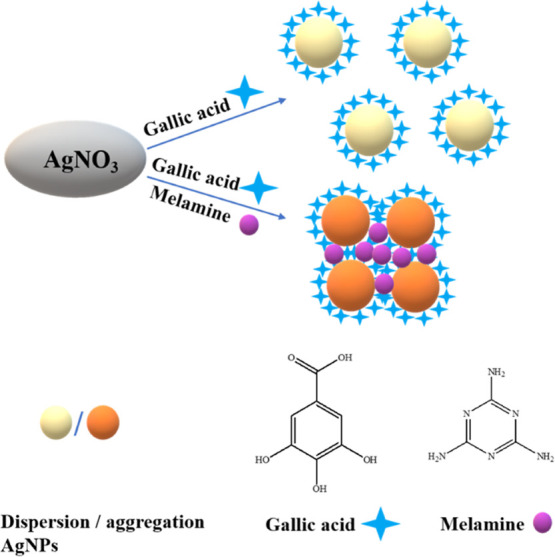
The prepared AgNPs reduced by gallic acid exhibited a vivid yellow color (left, inset of Figure 1) and showed a sharp absorption peak characteristic of AgNPs at 410 nm (Figure 1). The results indicated the formation of monodispersed AgNPs. The absorbance decreased dramatically (Figure 1), and the color of the mixture turned brown (right, inset of Figure 1) with the addition of melamine. We speculate that this phenomenon occurred because the AgNPs aggregated in the presence of melamine in the solution (Scheme 1).
Figure 1.
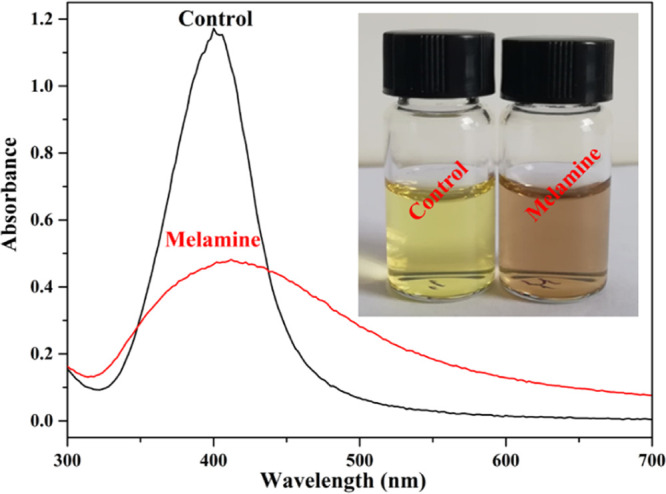
UV–visible absorption spectra of AgNPs@Gallic acid sensor assays in the absence and presence of melamine. Inset Photos: Colorimetric response of sensor assays.
To further verify the speculation, the morphology of AgNPs@gallic acid in the absence and presence of melamine was investigated by transmission electron microscopy (TEM) and high-resolution TEM (HRTEM). As shown in Figure 2a,c, the lattice spacing of 0.26 nm (Figure 2b,d) corresponded to the (004) plane of Ag nanocrystals.25 Also, the ringlike lattice structure in the selected area electron diffraction (SAED) pattern of the nanoparticles (inset, Figure 2a) suggested its crystal feature. What is more, the AgNPs were monodispersed with the absence of melamine (Figure 2a). The nanoparticles aggregated when melamine was added to the solution (Figure 2c). In addition, the field-emission scanning electron microscopy (FESEM) of the AgNPs is shown in Figure S1. Obviously, the monodispersed AgNPs aggregated in the presence of melamine. All the abovementioned results were in good agreement with our speculation that the color of the solution changed from vivid yellow to brown due to the aggregation of AgNPs because of melamine. Afterward, Fourier transform infrared (FTIR) spectra were used to analyze the characteristic peaks of the AgNPs. As shown in Figure S2, the peaks around 2924 and 2854 cm–1 were attributed to the stretch vibration of aliphatic C–H, whereas the peaks around 3280 and 1629 cm–1 were ascribed to the stretch vibration of catechol–OH and aromatic rings, respectively.26 These peaks were observed in both pure gallic acid and AgNPs@gallic acid, suggesting that the AgNPs were modified by gallic acid. These results proved that the AgNPs@gallic acid was successfully synthesized.
Figure 2.
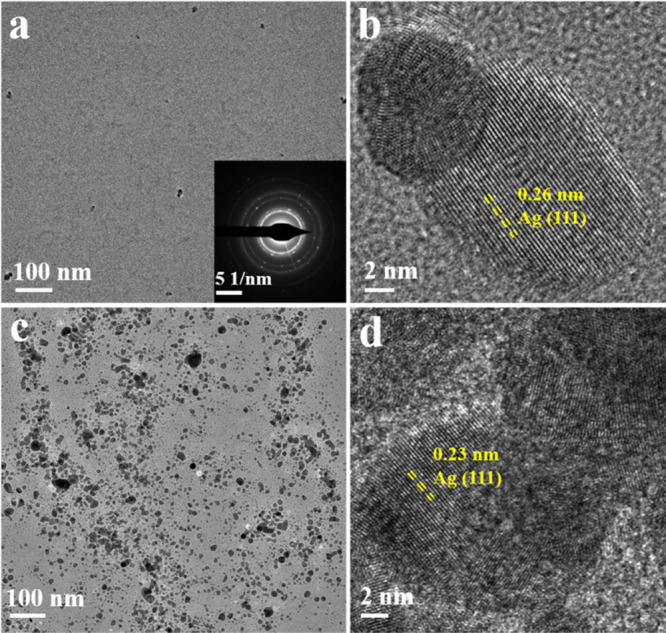
TEM (a,c) and HRTEM (b,d) of AgNPs@gallic acid in the absence (a,b) and presence (c,d) of melamine. The inset in (a) shows the SAED pattern of AgNPs@gallic acid.
Herein, we also investigated the reason for the aggregation of the AgNPs. In the melamine sensing assay of AgNPs@gallic acid, gallic acid exhibited reducing ability due to the presence of −OH groups, which promoted its antioxidant ability. Therefore, gallic acid functioned as reducing and stabilizing agents for the preparation of AgNPs (Figure S3), and the as-prepared AgNPs displayed an absorption peak at 410 nm in UV–vis absorption curve. However, two different kinds of interactions prevailed when melamine was present in the reaction medium. The first interaction was the hydrogen bonding interaction between melamine and gallic acid27 and the other interaction was the formation of the coordination bond between melamine and Ag+.28 The multiple −OH groups in gallic acid served as binding sites with melamine (Figure S4). Melamine could interact with gallic acid through hydrogen bonding, lowering the availability of reducing agents for reduction of Ag+ to Ag0. Simultaneously, nitrogen atoms of amines and triazine groups in melamine could interact with Ag+via acceptor–donor interaction.29 Therefore, melamine acted as a barrier according to the above-discussed interactions. Two conditions with melamine existed in solution. The first condition was that the barrier was not strong enough to prevent the close proximity of reducing agents and Ag+ ions when the concentration of melamine was low. In this condition, the aggregated AgNPs were formed. The second condition was that gallic acid and Ag+ ions were efficiently masked when the concentration of melamine was high,30 and the formation of AgNPs was interrupted.
2.2. Optimization of Experimental Conditions
Several factors, such as pH of the reaction medium, the concentration of AgNO3 and gallic acid and reaction time, might affect the sensitivity of the sensor assay. Therefore, these parameters were well optimized for the sensitive detection of melamine based on the characteristic peak at 410 nm.
2.2.1. Effect of pH
The pH could affect the interaction of gallic acid-functionalized AgNPs with the melamine,31 and therefore, the influence of the pH value on the oxidizing ability of Ag+ toward gallic acid and melamine was investigated. The UV–vis spectra were recorded, and the profile of the change in absorbance against the NaOH concentration is shown in Figure S5. Obviously, the profile absorbance change was achieved when NaOH concentration was 1300 nM, indicating the highest sensitivity for melamine detection. The binding reaction between gallic acid and melamine could be achieved at a fast reaction rate at higher pH, which was due to the formation of cyanuric acid, ammelide, or ammeline through the replacement of −NH2 in melamine with −OH groups. The results demonstrated that the as-prepared sensor assay could be utilized in this NaOH concentration, and 1300 nM was selected for the subsequent studies.
2.2.2. Effect of Concentration of Gallic Acid and AgNO3
In this proposed sensor, gallic acid not only acted as reducing and stabilizing agents but also acted as the binding site for melamine. Therefore, the concentration of gallic acid played a crucial role in the sensitivity and linear range of the sensor. As shown in Figure S6, the value of ΔA410 increased with the concentration of gallic acid from 4 to 8 μM and reached the maximum value at 8 μM, indicating the best sensitivity toward melamine. Therefore, the concentration of gallic acid at 8 μM was selected as the optimum concentration in the subsequent experiments.
Due to the interactions between melamine and Ag+, the effect of AgNO3 concentration on the sensing ability was also optimized. AgNO3 with different concentrations (60, 70, 80, 90, 100, 110, 120, 130, 140, and 150 μM) was tested, while the concentration of gallic acid was fixed at 8 μM. According to Figure S7, a maximum value of ΔA410 was observed at an AgNO3 concentration of 90 μM. As a result, 90 μM was chosen as the optimum concentration of AgNO3.
2.2.3. Effect of the Reaction Time
The reaction time for the oxidation of gallic acid was also studied to investigate the response of the designed sensing assay. Absorbance data for A410 of AgNPs in the presence and absence of melamine were collected every 2 min within 27 min. As shown in Figure S8, the number of nanoparticles was low, which was due to the fact that melamine acts as a barrier when they are present in the solution. In addition, the absorbance data of the system reached a stable value at 10 min, demonstrating that the oxidization reaction was completed or reached equilibrium. Therefore, 10 min was chosen as the incubation time for the sensing assay in the following experiment. Overall, the visual detection process could be achieved quickly in the typical colorimetric sensor.
2.3. Sensitive Detection of Melamine under the Optimized Conditions
To evaluate the sensitivity of the melamine sensing assay, the change of the absorbance intensity at 410 nm under optimized conditions in response to the melamine concentration in the range from 0 to 10 μM was utilized to measure the quantitative determination using UV–vis absorption spectrophotometry. The absorbance intensity decreased (Figure 3) with the increase of melamine concentrations, while the color of the solution changed from yellow to brown (Figure S9). The spectral curves and corresponding calibration (inset of Figure 3) demonstrated that the good linearity was obtained between the melamine concentrations and ΔA410 in the two concentration ranges. The linear equation was y = 0.0754 + 0.09552x – 0.00525x2 with a correlation coefficient R2 = 0.992. The limit of detection (LOD) was calculated to be 0.099 μM (0.012 ppm) according to the following equation32
| 1 |
where Sd represents the standard deviation of the blank solution of the sensor and s is the slope of the calibration curve.
Figure 3.
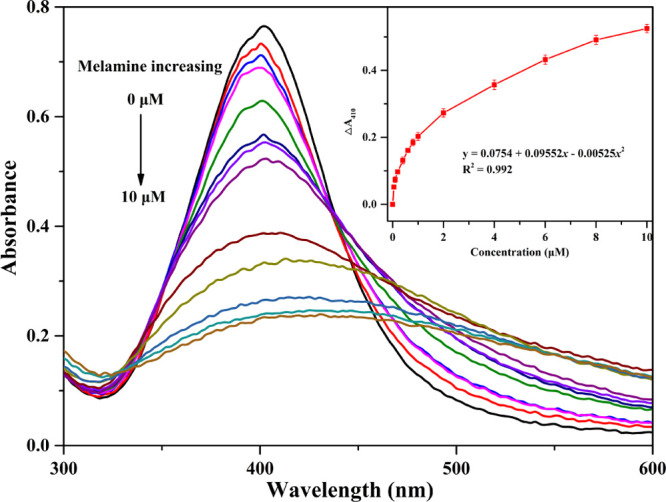
Absorption spectra of AgNPs@gallic acid assays in the presence of various concentrations of melamine. Inset: linear relationship of ΔA410 vs melamine concentrations.
The LOD of the sensing assay was much lower than the safety limit of melamine in EU (2.0 ppm) and China (1.0 ppm) and also compared to those of the colorimetric melamine sensors in the previous reported methods (Table S1). Overall, the proposed colorimetric method was not only fast but also simple when taking all procedures into consideration.
2.4. Selectivity Study of the Optimized Sensor
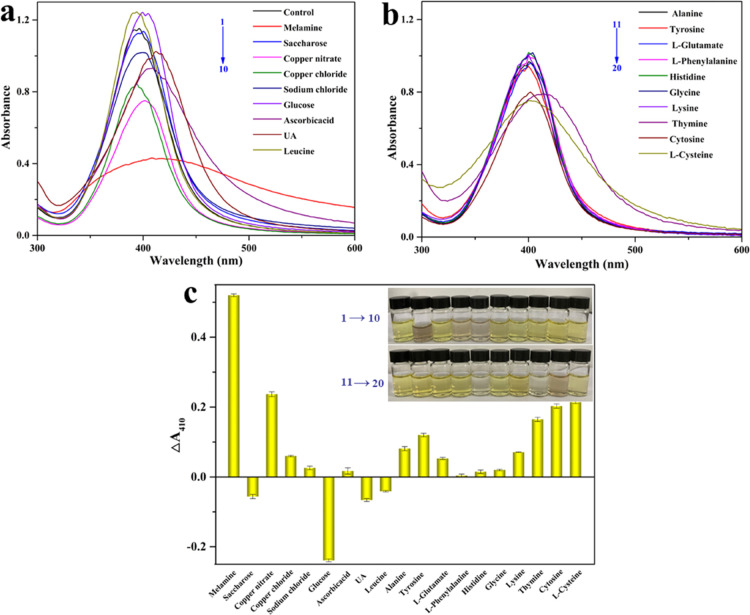
The interferences of the common analytes presented in the milk were needed to be investigated to validate good applicability of the optimized calorimetric sensing assay. Hence, the effect of thymine, cytosine, inorganic biomolecules on the melamine detection was investigated. ΔA410 of the sensor assays was used to evaluate the selectivity of the proposed sensor assays. As shown in Figure 4, melamine leaded to significant enhancement and color darkening, which could be observed by the naked eye (inset in Figure 4c). Also, copper nitrate, copper chloride, and cytosine have a slight effect on the absorbance ratio. The results indicated that a remarkably high selectivity of the proposed sensor strategy toward melamine over other analytes in the pretreated milk. The excellent antiinterference of the sensor might be because of the fact that gallic acid could bind to Ag+.33 Thus, the proposed sensor was appropriate for the selective colorimetric sensing assay of melamine in the pretreated milk.
Figure 4.
(a,b) Selectivity evaluation and (c) ΔA410 of the sensor system AgNPs@gallic acid in the presence of various analytes. The inset in (c): photographs depicting the corresponding color changes.
2.5. Detection of Melamine in Real Samples
We measured the melamine concentration in raw milk with the proposed sensor to test the practical applications of the calorimetric sensor. The milk samples were spiked with three known concentrations of melamine (0.8, 1.0, and 2.0 ppm) to validate the developed strategy and analyzed by the method given in Section 2.3, and all the measurements were repeated three times. The results of the determination were shown in Table 1. The satisfactory recoveries and relative standard deviation (RSD) values were about 93.6–101.1 and 2.65–4.07% on average, respectively. The good synchroneity indicated that the sensing assay could be satisfied with the required percentage recoveries applied for the detection of melamine in raw samples.
Table 1. Recovery Test of Melamine in Raw Milk at Different Spiking Concentrations of Melamine.
| sample number | added (μM) | found (μM) | recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 0.8 | 0.809 | 101.1 | 2.65 |
| 2 | 1.0 | 0.936 | 93.6 | 4.07 |
| 3 | 2.0 | 1.900 | 95.0 | 3.02 |
3. Conclusions
In summary, a new convenient and economical colorimetric melamine sensor based on gallic acid-stabilized AgNPs was successfully fabricated. Herein, the gallic acid acted as reducing and stabilizing agents for AgNPs. The well-dispersed AgNPs could be obtained after modification by gallic acid. The coordinate bonding between melamine and Ag+ caused the aggregation behavior of AgNPs. As a consequence, the color of the solution changed from vivid yellow to brown, which made the melamine determination convenient. More importantly, the detection of melamine could be achieved in a simple process with a detection limit of 0.099 μM (0.012 ppm). Simultaneously, the sensor displayed excellent selectivity in the proposed sensing strategy. The proposed colorimetric sensing assay was convenient and time-saving for the determination of melamine in raw milk.
4. Experimental Section
4.1. Chemicals and Reagents
Silver nitrate (AgNO3), gallic acid (C19H19N7O6), trichloroacetic acid (C2HCl3O2), sodium hydroxide (NaOH), and melamine (C3H6N6) pure were purchased from Aladdin Corporation. All of these reagents were of analytical grade and directly used as received. Raw milk was purchased from the local supermarket. Ultrapure water from a Millipore system (18.2 MΩ cm) was employed throughout the whole experiment.
4.2. Instruments
UV–vis absorption spectra were recorded using a Cary 50 UV–vis spectrophotometer (Varian, Inc.) with a quartz cuvette (path length, 1 cm). The FTIR spectrum was measured on the GX FTIR system (Japan) in the range of 400–4000 cm–1. TEM was collected by the Tecnai G2 F20 (FEI) instrument operated at an accelerating voltage of 200 kV.
4.3. Colorimetric Detection of Melamine
First, 30 μL of gallic acid (8 μM) and 30 μL of NaOH (1.3 mM) were added in 3 mL of water in a 5 mL centrifuge tube. Then, 30 μL of melamine solutions with different concentrations from 0 to 10.0 μM and 60 μL of AgNO3 (0.09 mM) were added into the tube in sequence and kept for 15 min. Finally, the solution was transferred into a quartz cuvette, and UV–vis absorption spectra were recorded to monitor the colorimetric changes. All the experiments were repeated three times.
4.4. Pretreatment of the Raw Milk Sample
Milk samples were pretreated to remove the interfering proteins in raw milk, which might interfere with the colorimetric sensor. Specifically, 10% of 2 mL of trichloroacetic acid was added into 5 mL of raw milk. Then, the solution was sonicated for 20 min and stirred for 10 min to ensure the complete precipitation of proteins in the samples. Subsequently, the mixture was centrifuged at 10 000 rpm for 15 min to separate the precipitate. The obtained supernatant portion was adjusted to pH 7.0 with 1 M NaOH. For recovery experiments, the corresponding amount of melamine was dissolved in the raw milk samples and the spiked-milk samples were performed with the same described procedure as the blank raw milk sample. Finally, the concentration of melamine in the samples was calculated using the linear regression equations, and the recoveries were calculated according to eq 2
| 2 |
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21764007, 21765011, and 21802057), the Natural Science Foundation of the JiangXi Province of China (20161BAB203082), and Jiangxi Provincial Department of Education’s Item of Science and Technology (GJJ201019).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03927.
FESEM of AgNPs; FTIR spectra; schematic illustration; effect of NaOH, gallic acid, AgNO3, and reaction time; photographs of AgNPs@gallic acid solution; and comparison of various colorimetry sensing assays for melamine (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ren H.; Li M.; Fu Y.; Jin L. Silver nanoclusters functionalized by chromotropic acid and layered double hydroxides for the turn-on detection of melamine. J. Mater. Chem. C 2016, 4, 6104–6109. 10.1039/c6tc01264g. [DOI] [Google Scholar]
- Cao H.; Hu X.; Hu C.; Zhang Y.; Jia N. A novel solid-state electrochemiluminescence sensor for melamine with Ru(bpy)32+/mesoporous silica nanospheres/Nafion composite modified electrode. Biosens. Bioelectron. 2013, 41, 911–915. 10.1016/j.bios.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Xin H.; Stone R. Chinese probe unmasks high-tech adulteration with melamine. Science 2008, 322, 1310–1311. 10.1126/science.322.5906.1310. [DOI] [PubMed] [Google Scholar]
- Chien C.-Y.; Wu C.-F.; Liu C.-C.; Chen B.-H.; Huang S.-P.; Chou Y.-H.; Chang A.-W.; Lee H.-H.; Pan C.-H.; Wu W.-J.; Shen J.-T.; Chang M.-Y.; Huang C.-H.; Shiea J.; Hsieh T.-J.; Wu M.-T. High melamine migration in daily-use melamine-made tableware. J. Hazard. Mater. 2011, 188, 350–356. 10.1016/j.jhazmat.2011.01.128. [DOI] [PubMed] [Google Scholar]
- Vasimalai N.; Abraham John S. Picomolar melamine enhanced the fluorescence of gold nanoparticles: Spectrofluorimetric determination of melamine in milk and infant formulas using functionalized triazole capped gold nanoparticles. Biosens. Bioelectron. 2013, 42, 267–272. 10.1016/j.bios.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Kamran A.; Rizvi S. M. A.. Reason and trends for using packaged milk in Pakistan: study of urban Pakistani consumers. In Proceedings of the Sixth International Conference on Management Science and Engineering Management; Xu J., Yasinzai M., Lev B., Eds.: New York, 2013; pp 909–924. [Google Scholar]
- Feng D.; Wu Y.; Tan X.; Chen Q.; Yan J.; Liu M.; Ai C.; Luo Y.; Du F.; Liu S.; Han H. Sensitive detection of melamine by an electrochemiluminescence sensor based on tris(bipyridine) ruthenium (II)-functionalize metal-organic frameworks. Sens. Actuators, B 2018, 265, 378–386. 10.1016/j.snb.2018.03.046. [DOI] [Google Scholar]
- Li N.; Liu T.; Liu S. G.; Lin S. M.; Fan Y. Z.; Luo H. Q.; Li N. B. Visible and fluorescent detection of melamine in raw milk with one-step synthesized silver nanoparticles using carbon dots as the reductant and stabilizer. Sens. Actuators, B 2017, 248, 597–604. 10.1016/j.snb.2017.03.068. [DOI] [Google Scholar]
- Zhao X.; Chen L.; Li B. Magnetic molecular imprinting polymers based on three-dimensional (3D) graphene-carbon nanotube hybrid composites for analysis of melamine in milk powder. Food Chem. 2018, 255, 226–234. 10.1016/j.foodchem.2018.02.078. [DOI] [PubMed] [Google Scholar]
- Regasa M. B.; Refera Soreta T.; Femi O. E.; C. Ramamurthy P. Development of molecularly imprinted conducting polymer composite film-based electrochemical sensor for melamine detection in infant formula. ACS Omega 2020, 5, 4090–4099. 10.1021/acsomega.9b03747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C.; Liu C.; Li Y.; Guo Y.; Luo F.; Wang P.; Guo L.; Qiu B.; Lin Z. Homogeneous electrochemical biosensor for melamine based on DNA triplex structure and exonuclease III-assisted recycling amplification. Anal. Chem. 2016, 88, 10176–10182. 10.1021/acs.analchem.6b02753. [DOI] [PubMed] [Google Scholar]
- Miao H.; Fan S.; Wu Y.-N.; Zhang L.; Zhou P.-P.; Chen H.-J.; Zhao Y.-F.; Li J.-G. Simultaneous determination of melamine, ammelide, ammeline, and cyanuric acid in milk and milk products by gas chromatography-tandem mass spectrometry. Biomed. Environ. Sci. 2009, 22, 87–94. 10.1016/s0895-3988(09)60027-1. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Yan X. Simultaneous determination of melamine and 5-hydroxymethylfurfural in milk by capillary electrophoresis with diode array detection. J. Agric. Food Chem. 2009, 57, 8742–8747. 10.1021/jf9021916. [DOI] [PubMed] [Google Scholar]
- Su H.; Fan H.; Ai S.; Wu N.; Fan H.; Bian P.; Liu J. Selective determination of melamine in milk samples using 3-mercapto-1-propanesulfonate-modified gold nanoparticles as colorimetric probe. Talanta 2011, 85, 1338–1343. 10.1016/j.talanta.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Zorainy M.; Boffito D. C.; Gobara M.; Baraka A.; Naeem I.; Tantawy H. Synthesis of a novel Ce(iii)/melamine coordination polymer and its application for corrosion protection of AA2024 in NaCl solution. RSC Adv. 2021, 11, 6330–6345. 10.1039/d0ra08587a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q.; Wei H. P.; Du S.; Li H. B.; Ji Z. P.; Hu X. Y. Detection of subnanomolar melamine based on electrochemical accumulation coupled with enzyme colorimetric assay. J. Agric. Food Chem. 2013, 61, 1810–1817. 10.1021/jf304034e. [DOI] [PubMed] [Google Scholar]
- Shaban S. M.; Lee J. Y.; Kim D.-H. Dual-surfactant-capped Ag nanoparticles as a highly selective and sensitive colorimetric sensor for citrate detection. ACS Omega 2020, 5, 10696–10703. 10.1021/acsomega.9b04199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul I. E.; Rajeshwari A.; Prathna T. C.; Raichur A. M.; Chandrasekaran N.; Mukherjee A. Colorimetric detection of melamine based on the size effect of AuNPs. Anal. Methods 2015, 7, 1453–1462. 10.1039/c4ay02622e. [DOI] [Google Scholar]
- Song J.; Wu F.; Wan Y.; Ma L. Colorimetric detection of melamine in pretreated milk using silver nanoparticles functionalized with sulfanillic acid. Food Contr. 2015, 50, 356–361. 10.1016/j.foodcont.2014.08.049. [DOI] [Google Scholar]
- Xiao C.; Zhang X.; Liu J.; Yang A.; Zhao H.; Li X.; He Y.; Yuan Z. Sensitive colorimetric detection of melamine with 1,4-dithiothreitol modified gold nanoparticles. Anal. Methods 2015, 7, 924–929. 10.1039/c4ay02491e. [DOI] [Google Scholar]
- Liang L.; Zhen S.; Huang C. Visual and light scattering spectrometric method for the detection of melamine using uracil 5’-triphosphate sodium modified gold nanoparticles. Spectrochim. Acta, Part A 2017, 173, 99–104. 10.1016/j.saa.2016.08.049. [DOI] [PubMed] [Google Scholar]
- Inamuddin; Kanchi S. One-pot biosynthesis of silver nanoparticle using Colocasia esculenta extract: colorimetric detection of melamine in biological samples. J. Photochem. Photobiol., A 2020, 391, 112310. 10.1016/j.jphotochem.2019.112310. [DOI] [Google Scholar]
- Kumar S. V.; Huang N. M.; Lim H. N.; Zainy M.; Harrison I.; Chia C. H. Preparation of highly water dispersible functional graphene/silver nanocomposite for the detection of melamine. Sens. Actuators, B 2013, 181, 885–893. 10.1016/j.snb.2013.02.045. [DOI] [Google Scholar]
- Borase H. P.; Patil C. D.; Salunkhe R. B.; Suryawanshi R. K.; Salunke B. K.; Patil S. V. Biofunctionalized silver nanoparticles as a novel colorimetric probe for melamine detection in raw milk. Biotechnol. Appl. Biochem. 2015, 62, 652–662. 10.1002/bab.1306. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Fu Q.; Huang H.; Wei L.; Guo X. Silver-quantum-dot-modified MoO3 and MnO2 paper-like freestanding films for flexible solid-state asymmetric supercapacitors. Small 2019, 15, 1805235. 10.1002/smll.201805235. [DOI] [PubMed] [Google Scholar]
- Alam M. F.; Laskar A. A.; Ahmed S.; Shaida M. A.; Younus H. Colorimetric method for the detection of melamine using in-situ formed silver nanoparticles vis tannic acid. Spectrochim. Acta, Part A 2017, 183, 17–22. 10.1016/j.saa.2017.04.021. [DOI] [PubMed] [Google Scholar]
- Silly F.; Shaw A. Q.; Castell M. R.; Briggs G. A. D.; Mura M.; Martsinovich N.; Kantorovich L. Melamine structures on the Au (111) surface. J. Phys. Chem. C 2008, 112, 11476–11480. 10.1021/jp8033769. [DOI] [Google Scholar]
- Roy B.; Bairi P.; Nandi A. K. Supramolecular assembly of melamine and its derivatives: nanostructures to functional materials. RSC Adv. 2014, 4, 1708–1734. 10.1039/c3ra44524k. [DOI] [Google Scholar]
- Wang H.; Chen D.; Yu L.; Chang M.; Ci L. One-step, room temperature, colorimetric melamine sensing using an in-situ formation of silver nanoparticles through modified Tollens process. Spectrochim. Acta, Part A 2015, 137, 281–285. 10.1016/j.saa.2014.08.041. [DOI] [PubMed] [Google Scholar]
- El-Nagar G. A.; Sarhan R. M.; Abouserie A.; Maticiuc N.; Bargheer M.; Lauermann I.; Roth C. Efficient 3D-silver flower-like microstructures for non-enzymatic hydrogen peroxide (H2O2) amperometric detection. Sci. Rep. 2017, 7, 12181. 10.1038/s41598-017-11965-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.; Xiao Y.; Jiang X.; Li J.; Qin H.; Huang H.; Zhang Y.; He X.; Wang K. A ratiometric nanosensor based on conjugated polyelectrolyte-stabilized AgNPs for ultrasensitive fluorescent and colorimetric sensing of melamine. Talanta 2016, 151, 68–74. 10.1016/j.talanta.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Ayaz Ahmed K. B.; Mariappan M.; Veerappan A. Nanosilver cotton swabs for highly sensitive and selective colorimetric detection of sulfide ions at nanomolar level. Sens. Actuators, B 2017, 244, 831–836. 10.1016/j.snb.2017.01.077. [DOI] [Google Scholar]
- Ma Y.; Niu H.; Zhang X.; Cai Y. One-step synthesis of silver/dopamine nanoparticles and visual detection of melamine in raw milk. Analyst 2011, 136, 4192–4196. 10.1039/c1an15327g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.