Abstract
Background
Several skin manifestations have been reported since the start of the COVID‐19 pandemic: chilblains‐like, livedoid lesions, urticaria‐like, pseudo‐Kawasaki disease, and others. Histopathologic images of these lesions most often show aspects of endothelitis, images similar to autoimmune vasculitis. Cutaneous lesions are often negative at RT‐PCR for SARS‐CoV‐2 virus.
Method and Results
We reviewed recent articles on the mechanisms of COVID‐19 and we synthesized main pathways of inflammatory cascade. After the penetration into the cells of the respiratory epithelium, SARS‐CoV‐2 virus initiates a “cytokine storm” well described in previous publications: the expression of interferon type I (IFN‐I) is one of the key elements of the antiviral response in COVID‐19 patients, IFN‐I expression seems to play an important role in the induction of interleukin 6 (IL‐6), chemotactic factors such as Granulocyte‐Macrophage Colony‐Stimulating Factor (GM‐CSF) and the consequent activation of monocyte‐macrophage system followed by the expression of TNF‐alpha, and finally by the induction of coagulation factors by both extrinsic and intrinsic pathways.
Conclusions
The simplified synthesis of the main pathophysiological mechanisms of COVID‐19 could help us to understand at least partially the importance of macrophage activation and its vascular involvement in many skin lesions that remain often negative at in situ tests for SARS‐CoV‐2.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the virus responsible for the epidemic of coronavirus disease‐19 (COVID‐19), which began at the end of 2019 in Wuhan, China, declared a pandemic by the World Health Organization on March 11, 2020. An important part of patients positive at the RT‐PCR nasal test for SARS‐CoV‐2 is being asymptomatic. 1 Symptomatic COVID‐19 patients most frequently present general symptoms (fever, fatigue, and anorexia), dysgeusia, anosmia, and respiratory signs (cough, dyspnea, pneumonia, and in the most severe cases, a severe acute respiratory syndrome). Dermatological manifestations of COVID‐19 described in case‐series published over the past 12 months were most often (according to the prevalence of these manifestations 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 ): chilblains‐like lesions (toes 88%, fingers 24% 11 ), diffuse erythematous rash, erythema multiforme‐like, punctiform purpura lesions, urticaria‐like, varicella‐like, and ischemic acrosyndromes. 11 In a large series of chilblains observed in France during the COVID‐19 pandemic, most cases of skin lesions were negative to RT‐PCR and serological tests. 11 Skin RT‐PCR tests were made in most reports some days after their onset, which was the reason for consulting a dermatologist, with or without other symptoms. 8 , 9 , 10 , 11 The histopathology, immunofluorescence, and immunohistochemistry examination of chilblains in symptomatic COVID‐19 patients showed an aspect of endothelitis similar to that of autoimmune vasculitis, with negative results for in situ test for SARS‐CoV‐2. 12 RT‐PCR results for SARS‐CoV‐2 were also negative in papulosquamous lesions in severe cases of COVID‐19. 13 In patients with more ischemic and/or more necrotic acrosyndromes, SARS‐CoV‐2 was detected by immunohistochemistry, and the presence of viral particles was seen in electron microscopy. 14
Simplified physiopathology of vascular lesions induced by SARS‐CoV‐2
SARSC‐CoV‐2 uses two receptors to enter host cells 15 : angiotensin‐converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) expressed by human epithelial cells (Fig. 1). SARS‐CoV‐2 then enters cells (endocytosis), its RNA is lysed into nonstructural proteins, and then RNA polymerase initiates RNA synthesis and assembly of new viruses 16 (Fig. 2a).
Figure 1.
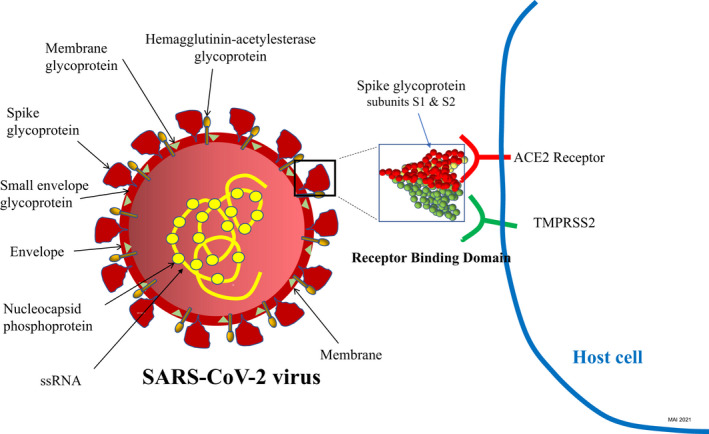
SARS‐CoV‐2 structure and its receptor binding domain (RBD) using human angiotensin‐converting enzyme 2 (ACE2) receptor and transmembrane protease serine 2 (TMPRSS2) for cellular entry; mRNA, messenger ribonucleic acid
Figure 2.
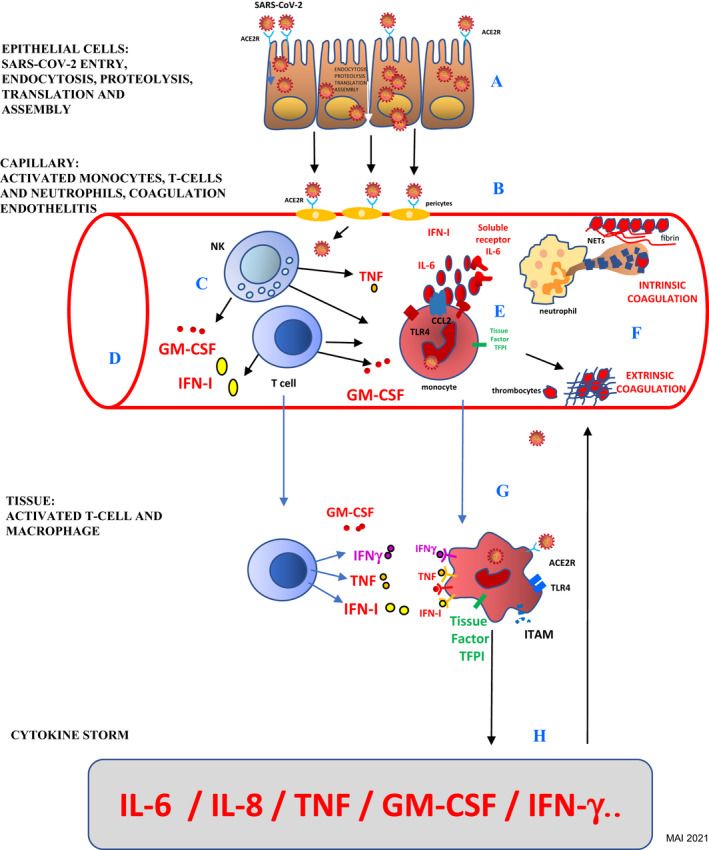
SARS‐CoV‐2 induced endothelitis and macrophage activation: (A) viral endocytosis, proteolysis, translation, assembly, and exocytosis; (B) capillaries' pericytes strongly express ACER2; (C) T‐Cells and NK cells activation; (D) induction of IFN‐I and GM‐CSF; (E) monocytes activate intravascular coagulation; (F) extrinsic and intrinsic coagulation; (G) tissue macrophage activation; (H) cytokine storm aggravate endothelitis. ACE2R, angiotensin‐converting enzyme 2 receptor; CCL2, chemokine ligand 2; GM‐CSF, granulocyte‐macrophage stimulating factor; IFNg, interferon gamma; IFN‐I, interferon type I; IL‐6, interleukin 6; ITAM, immunoreceptor tyrosine‐based activation motif; NETs, neutrophil extracellular traps; PAMPs, pathogen‐associated molecular patterns; P‐s, P‐selectin; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TFPI, tissue factor pathway inhibitor; TLR, toll‐like receptor; TLR4, toll‐like receptor 4; TNF, tumor necrosis factor; vWF, von Willebrand factor
Pericytes (Rouget's cells) 17 that envelop the endothelial cells of small blood vessels express high levels of ACE2 receptors and by consequence facilitate SARS‐CoV‐2 entrance 18 (Fig. 2b). The distribution and density of ACE2 receptor expression underline the important role of endothelitis and endothelial cells' damage and the consequent microvascular dysfunction in the pathophysiology of COVID‐19. 19 Significant expression of the ACE2 receptor has also been found in monocytes, macrophages, T‐cells, myocytes, and neuronal cells. 18
The activation of interferon type I (IFN‐I) expression in COVID‐19 can be compared to the “IFN‐I signature” in systemic lupus erythematosus (SLE). 20 , 21 The IFN signature in SLE is because of the activation of several types of IFN‐producing cells, endogenous inducers of IFN‐I, and autoimmune mechanisms. 22 , 23 The clinical and histopathological similarities between COVID‐19 chilblains‐like lesions with those seen in SLE could be related to the antiviral and immunostimulatory properties of IFN‐I and its role in SLE microangiopathy. 24 , 25
SARS‐CoV‐2 initiates the expression of IFN‐inducible genes as an antiviral response. The activation of these genes in COVID‐19 patients exacerbates the “cytokine storm,” especially linked to the induction of IFN‐I and GM‐CSF, followed by the T‐cell and NK responses 26 , 27 (Fig. 2c,d).
Molecular factors, such as pathogen‐associated molecular patterns (PAMPs), damage‐associated molecular patterns (DAMPs), interluekin‐6 (IL‐6), and chemotactic factors such as granulocyte‐macrophage stimulating factor (GM‐CSF), activate the monocyte‐macrophage system. 1 Activated pericytes and endothelial cells express chemotactic factors and adhesion molecules leading to the recruitment of monocytes and neutrophils. Monocytes activate the extrinsic coagulation pathway inducing (via IL‐6‐stimulated tissue factor coagulation factor III) deposits of fibrinogen and the appearance of thrombi (Fig. 2e). Extracellular neutrophil traps (NETs) activate the intrinsic coagulation pathway activating platelets that adhere and aggregate forming thrombi (Fig. 2f). 28
The activation of macrophages is mainly linked to the expression of IL‐6 and GM‐CSF. The IFN‐I expression has already been shown to be one of the most important mechanisms in the induction of skin vascular lesions in COVID‐19. 1 , 5 , 26 , 29
IFN type I expression and tissue macrophage's activation (Fig. 2g) aggravate the “cytokine storm” 26 which worsens vascular impact (Fig. 2h).
On the contrary, in SARS‐CoV‐2‐induced pulmonary distress, the suppression of first‐line interferon responses and abrogation of NK and T‐cells' responses suggest a role for type 2 pneumocyte gp130 receptor expression and important IL‐6 release. 27
In most COVID‐19 cases, the skin signs as chilblains appear several weeks after SARS‐CoV‐2 infection, explaining partially the negative nasal and skin RT‐PCR tests, underlining again that these skin lesions are rather induced by the SARS‐CoV‐2 “cytokine storm” and less by a direct virus‐induced cytopathic effect.
Conclusion
The physiopathology of COVID‐19 presented synthetically in this article supports the hypothesis concerning the mechanisms of the induction of skin lesions in which interferon type I response and immune response inducing a vascular involvement play a central role in the induction of chilblains‐like lesions, livedoid lesions, and urticaria‐like lesions. 3 , 5 , 12 , 13 In recent reports, the presence of SARS‐CoV‐2 was seen by electron microscopy in lesions of endothelitis, 14 but in the majority of published articles, the skin lesions as chilblains are mostly negative being induced "at a distance" by the virus that is triggering an immune reaction responsible for the initiation of the “cytokine storm” and for the release of activators of the monocyte‐macrophage system, explaining at least partially the endothelitis and chilblains‐like lesions that are often negative at the screening by RT‐PCR in situ test for SARS‐CoV‐2.
Conflict of interest: The author declares no conflict of interest for this manuscript. Dr. Ionescu is an investigator for Psoriasis National Register France Psobioteq (no honoraria), investigator and speaker for Uriage cosmetics (honoraria), and was previously a speaker for Novartis, Celgene (honoraria).
Funding source: None.
References
- 1. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol 2020; 20: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bouaziz JD, Duong T, Jachiet M, et al. Vascular skin symptoms in COVID‐19. J Eur Acad Dermatol Venereol 2020; 34: e451–e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection induced chilblains: a case report with histopathologic findings. JAAD Case Rep 2020; 6: 489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masson A, Bouaziz JD, Sulimovic L, et al. Chilblains is a common cutaneous finding during the COVID‐19 pandemic. J Am Acad Dermatol 2020; 83: 667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rybojad M. COVID‐19: des signes cutanés témoins d'une atteinte vasculaire au premier. Réalités Thérapeutiques DV 2020; 292: 7–15. [Google Scholar]
- 6. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol 2020; 34: e212–e213. [DOI] [PubMed] [Google Scholar]
- 7. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy 2020; 75: 1730–1741. [DOI] [PubMed] [Google Scholar]
- 8. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol 2020; 82: e177. 10.1016/j.jaad.2020.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marzano AV, Genovese G, Fabbrocini G, et al. Varicella‐like exanthem as a specific COVID‐19‐associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol 2020; 83: 280–285. 10.1016/j.jaad.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia. Zhonghua Xue Ye Xue Za Zhi 2020; 41: E006. 10.3760/cma.j.issn.0253-2727.2020.0006 [DOI] [PubMed] [Google Scholar]
- 11. Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID‐19 outbreak occur in patients who are negative for COVID‐19 on polymerase chain reaction and serology testing. Br J Dermatol 2020; 183: 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanitakis J, Lesort C, Danset M, et al. Chilblain‐like acral lesions during the COVID‐19 pandemic ("COVID toes"): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol 2020; 83: 870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol 2020; 156: 819–820. [DOI] [PubMed] [Google Scholar]
- 14. Colmenero I, Santonja C, Alonso‐Riaño M, et al. SARS‐CoV‐2 endothelial infection causes COVID‐19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol 2020; 183: 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cascella M, Rajnik M, Cuomo A, et al. Features, evaluation and treatment coronavirus (COVID‐19). StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 16. Sanders JM, Monogue ML, Jodlowski TZ, et al. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19). A review. JAMA 2020; 323: 1824–1836. [DOI] [PubMed] [Google Scholar]
- 17. Dore‐Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des 2008; 14: 1581–1593. [DOI] [PubMed] [Google Scholar]
- 18. Chen W, Lan Y, Yuan X, et al. Detectable 2019‐nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microb Infect 2020; 9: 469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Becker RC. COVID‐19 update: Covid‐19‐associated coagulopathy. J Thromb Thrombolysis 2020; 50: 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 2003; 197: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baechler EC, Batliwalla FM, Karypis G, et al. Interferon‐inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Nat Acad Sci 2003; 100: 2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon‐regulated genes in SLE. Autoimmunity 2003; 36: 481–489. [DOI] [PubMed] [Google Scholar]
- 23. Rönnblom L, Leonard D. Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci Med 2019; 6: e000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Munoz J, Marque M, Dandurand M, et al. Interféronopathies de type I. Ann Dermatol Venereol 2015; 142: 653–663. [DOI] [PubMed] [Google Scholar]
- 25. Kolivras A, Aeby A, Crow Y. Cutaneous histopathological findings of Aicardi‐Goutières syndrome, overlap with chilblain lupus. J Cutan Pathol 2008; 35: 774–777. [DOI] [PubMed] [Google Scholar]
- 26. Blanco‐Melo D, Nilsson‐Payant BE, Liu WC, et al. Imbalanced host response to SARS‐CoV‐2 drives development of COVID‐19. Cell 2020; 181: 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McGonagle D, Sharifa K, O'Regand A, et al. The role of cytokines including interleukin‐6 in COVID‐19 induced pneumonia and macrophage activation syndrome‐like disease. Autoimmun Rev 2020; 19: 102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iba T, Levy JH, Raj A, et al. Advance in the management of sepsis‐induced coagulopathy and disseminated intravascular coagulation. J Clin Med 2019; 8: 728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Velazquez‐Salinas L, Verdugo‐Rodriguez A, Rodriguez LL, et al. The role of interleukin 6 during viral infections. Front Microbiol 2019; 10: 1057. [DOI] [PMC free article] [PubMed] [Google Scholar]


