Abstract
Background
The abundance of publications of COVID‐19‐induced chilblains has resulted in a confusing situation.
Methods
This is a prospective single‐institution study from 15 March to 13 May 2020. Thirty‐two patients received PCR nasopharyngeal swabs. Of these, 28 patients had a thoracic CT‐scan, 31 patients had blood and urine examinations, 24 patients had skin biopsies including immunohistochemical and direct immunofluorescence studies, and four patients had electron microscopy.
Results
COVID‐19‐induced chilblains are clinically and histopathologically identical to chilblains from other causes. Although intravascular thrombi are sometimes observed, no patient had a systemic coagulopathy or severe clinical course. The exhaustive clinical, radiological, and laboratory work‐up in this study ruled‐out other primary and secondary causes. Electron microscopy revealed rare, probable viral particles whose core and spikes measured from 120 to 133 nm within endothelium and eccrine glands in two cases.
Conclusion
This study provides further clinicopathologic evidence of COVID‐19‐related chilblains. Negative PCR and antibody tests do not rule‐out infection. Chilblains represent a good prognosis, occurring later in the disease course. No systemic coagulopathy was identified in any patient. Patients presenting with acral lesions should be isolated, and chilblains should be distinguished from thrombotic lesions (livedo racemosa, retiform purpura, or ischemic acral necrosis).
Keywords: chilblains, coagulation, coagulopathy, COVID‐19, COVID‐toes, CT‐scan, direct immunofluorescence study, electron microscopy, histopathology, hypercoagulable, immunohistochemistry, interferon, livedo racemosa, lupus erythematosus, paraviral, retiform purpura, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), thrombi
Abbreviations
- ACE2
Angiotensin‐converting enzyme 2
- ANA
Antinuclear antibody
- DIF
Direct immunofluorescence
- EBV
Epstein‐Barr virus
- EM
Electron microscopy
- IFN
Interferon
- IHC
Immunohistochemistry
- LE
Lupus erythematosus
- PAS
Periodic acid‐Schiff
- PCR
Polymerase chain reaction
1. INTRODUCTION
In a short period, numerous publications have followed the initial report 1 of the histopathology of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) or COVID‐19‐induced chilblains, also called “COVID toes.” 1
With the abundance of publications on this subject, numerous misconceptions have arisen, most originating from case reports and small series containing only clinical pictures with no biopsy or laboratory work‐up. Thus, sometimes a diagnosis of COVID‐19‐induced chilblains has been made without excluding lupus erythematosus (LE), a systemic coagulopathy, or other recent viral infection using blood tests. Based upon reports of COVID‐19‐induced hypercoagulable states, some authors have assumed that COVID‐19‐induced chilblains are the same, 2 lumping chilblains with acral ischemic lesions 3 and correlating all acral eruptions with disease severity. 4 However, chilblains have never been reported as an isolated cutaneous finding in severely ill patients. 5 , 6 , 7 , 8 , 9
Further confusion has ensued after identification of microthrombi in skin biopsies, again assuming coagulation activation. 10 Numerous authors have even concluded that COVID‐19‐induced chilblains are unrelated to infection because of negative PCR swabs and serologies. Some have even attributed chilblains to a senescent lifestyle during the quarantine. 11 , 12 , 13 , 14 The abundance of publications and lack of clinicopathological correlation have resulted in an incomprehensible situation for dermatopathologists and clinicians.
The objectives of this study are to provide (1) a thorough assessment of a set of patients with clinical, histopathological, radiological, and laboratory analyses, and (2) an interpretation of our findings based on a critical synthesis of published literature. We will show that the most important component in the work‐up of a patient presenting with acral, vasculitic lesions, possibly from COVID‐19, is ruling‐out a hypercoaguable state, which has a poor prognosis. In contrast, young people presenting with COVID‐19‐induced chilblains are usually asymptomatic or pauci‐symptomatic, representing a late stage of the infection with a good prognosis.
2. METHODS
2.1. Study design
An Institutional Review Board (IRB) approved this prospective, single‐institution study. Data were collected during three clinic visits at baseline, 2 weeks, and 4 weeks, including (1) clinical pictures—all three visits; (2) COVID‐19 PCR nasopharyngeal swab—baseline; (3) thoracic CT‐scan—baseline; (4) skin biopsy for light, direct immunofluorescence (DIF), and electron microscopy (EM)—baseline; and (5) blood tests and two urine examinations—first and second visits.
2.2. Patients
The required inclusion criteria were as follows: (1) Developing the first episode of chilblains between 15 March and 13 May 2020; (2) absence of prior history of chilblains in the past winter/summer; (3) persistence of lesions >48 hours despite warming and drying affected areas; (4) dermatological evaluation confirming chilblains in the absence of features of LE, systemic sclerosis, a photosensitive eruption, or other dermatosis within the affected area. Exclusion criteria included identification of antinuclear antibody (ANA) >1:160 or any titer of ANA with identification of a specific ANA pattern, or serological evidence of recent infection.
2.3. Assessments
Among 32 patients (all newly reported except one case 1 ), we obtained the following: Clinical history with pictures in 32, 31, and 24 patients, respectively, at baseline, first visit, and second visit; nasopharyngeal swab in 32 patients; thoracic CT‐scan in 28 patients; skin biopsy with light microscopy, immunohistochemistry (IHC); DIF studies (IgG, IgA, IgM, C3, fibrinogen) in 24 patients; and EM in four patients; Two blood tests and two urine examinations in 31 and 24 patients, respectively, at the first and second visit. Blood tests included complete blood count, erythrocyte sedimentation rate, C‐reactive protein, ferritin levels, direct Coombs examination, haptoglobin, antiphospholipid antibodies (lupus anticoagulant, anti‐cardiolipin antibodies, anti beta2 glycoprotein I antibodies), coagulation studies (prothrombin time, activated partial thromboplastin time, thrombin time, d‐dimers, protein C and S, anti‐thrombin III, plasminogen, homocysteine, and factor II mutation), renal, hepatic, and thyroid function tests, vitamin D levels, titer and identification of ANA, complement levels, anti‐neutrophil cytoplasmic antibodies (ANCA), cryoglobulinemia, serum viscosity, immunoglobulin (Ig) G and IgM serologies for Epstein‐Barr virus (EBV), parvovirus B19, hepatitis B, hepatitis C, cytomegalovirus, coxsackie and Mycoplasma pneumoniae, anti‐streptolysin O antibody tests, and IgA and IgG serologies for COVID‐19. Proteinuria and hematuria were assessed in two urine specimens. Light microscopy included H&E, periodic acid‐Schiff (PAS), and Alcian‐blue stains and IHC for CD3, CD4, CD8, Granzyme B, CD20, CD123, CD68, CD163, myeloperoxidase, and CD56. Three patients were excluded because of ANA 1:160 with anti‐scl70 antibodies, ANA 1:320 with anti‐RNP antibodies, and ANA 1:320 with anti‐SSA (Ro) and anti‐SSA52 (Ro52) antibodies.
2.4. Statistics
Statistical analysis was conducted using IBM SPSS Statistics Version 22 (IBM Corp. IBM SPSS Statistics for Mac, Version 22.0. Armonk, New York) or Prism GraphPad Software Inc, with graphs made using Prism GraphPad Software Inc. (San Diego, California). Nonparametric tests were used to determine P value (as n < 30), Mann‐Whitney U test for continuous variables, and Fisher's exact test for discrete variables with a P value cutoff of P < .05.
3. RESULTS
3.1. Clinical findings
Summarized clinical and laboratory findings are presented in Table 1 and Figure 1, and correlation between clinical findings and clinical evolution is presented in Figure 2.
TABLE 1.
Clinical features and laboratory tests of patients with COVID‐19‐induced chilblains
| Clinical characteristics and complementary studies | Included patients | Patients after exclusion criteria | ||||
|---|---|---|---|---|---|---|
| Clinical history | Age |
Males 10 to 53 y (mean 29.62) Females 11 to 70 y (mean 36.88) |
||||
| Gender | Female 19/32 (59.37%) | Male 13/32 (40.63%) | Female 16/29 (55.17%) | Male 13/29 (44.83%) | ||
| Race/ethnicity | White 28/32 (87.5%), North African 4/32 (12.5%) | White 26/29 (89.65%), North African 3/29 (10.35%) | ||||
| Raynaud | 5/32 (15.62%) | 3/29 (10.71%) | ||||
| Smoking | 3/32 (9.37%) | 2/29 (7.14%) | ||||
| Photosensitivity | 0/32 | 0/29 | ||||
| Arthralgia/arthritis | 0/32 | 0/29 | ||||
| Lupus, systemic sclerosis or other autoimmune disease | 0/32 | 0/29 | ||||
| Thrombosis | 0/32 | 0/29 | ||||
| Exposure to cold | 1/32 (3.12%) | 1/29 (3.57%) | ||||
| Decrease of physical activities during lockdown | 3/32 (9.37%) | 2/29 (7.14%) | ||||
| Contact with hospital, nursing home, assisted living facility | 1/32 (3.12%) | 1/29 (3.57%) | ||||
| Contact with patient suspected of COVID‐19 infection | 13/32 (40.62%) | 10/29 (35.71%) | ||||
| Contact with confirmed COVID‐19‐infected patient | 4/32 (12.5%) | 3/29 (10.71%) | ||||
| Delay between general symptoms and chilblains | Symptoms preceded chilblains by 24 d (7 patients), were concurrent with chilblains (10 patients), or followed chilblains by 5 d (3 patients) | |||||
| Delay between chilblains and baseline visit | 19.69 d in 32 patients | 16.92 d in 29 patients | ||||
| Symptomatic chilblains (pain and/or pruritus) | 24/32 (75%) | 21/29 (72.41%) | ||||
| Clinical examination | Temperature | 37.47°C in 31 patients (35.6°C‐37.3°C) | 36.51°C in 28 patients (35.6°C‐37.3°C) | |||
| Oxygen saturation | 97.83% in 30 patients (95%‐100%) | 97.77 in 27 patients (95%‐100%) | ||||
| Heart rate | 84.64/min in 31 patients (60‐118/min) | 85.07/min in 28 patients (60‐118/min) | ||||
| Respiration rate | 17.72/min in 11 patients (15‐19/min) | 17.72 in 11 patients (15‐19/min) | ||||
| Clinical evolution | Complete resolution without recurrence and total duration |
After 2 wk: 14/31 (45.16%) Total duration: 32.42 d |
After 6 wk: 15/24 (62.5%) |
After 2 wk: 11/28 (39.28%) Total duration: 32.75 d |
After 6 wk: 12/21 (57.14%) | |
| Partial resolution without recurrence | After 2 wk: 12/31 (38.70%) | After 6 wk: 5/24 (20.84%) | After 2 wk: 12/28 (42.85%) | After 6 wk: 5/21 (23.8%) | ||
| No improvement | After 2 wk: 2/31 (6.46%) | After 6 wk: 2/24 (8.33%) | After 2 wk: 2/28 (7.15%) | After 6 wk: 2/21 (9.53%) | ||
| Recurrence | After 2 wk: 3/31 (9.68%) | After 6 wk: 2/24 (8.33%) | After 2 wk: 3/28 (10.72%) | After 6 wk: 2/21 (9.53%) | ||
| Other studies | Positive COVID‐19 PCR of nasopharyngeal swab | 2/32 (6.25%) | 1/29 (3.45%) | |||
| Abnormal findings on CT‐scan | 0/28 | 0/25 | ||||
| Abnormal laboratory tests | Positive COVID‐19 serology | 6/31 (19.35%) | 6/31 (19.35%) | |||
| Other abnormal findings | See legend | |||||
Notes: Clinical history and physical were recorded at baseline, 2‐week, and 4‐week visits. In addition to patients lost to follow‐up, three patients were excluded because of positive ANA. In the remaining 29 patients, age ranged in males 10 to 53 years (mean 29.62, SD 14.85, 95% CI from 20.64 to 38.59) and females 11 to 70 years (mean 36.88, SD 18.08, 95% CI from 27.24 to 46.51); U Mann‐Whitney P = 0.28 (nonsignificant difference). General symptoms were seen in 20 patients, preceding chilblains by 24 days (7 patients [pt]) (range from 11 to 40 days, mean 24, SD 9.96, 95% CI from 14.78 to 33.22); concurrent (10 pt); and following chilblains by 5 days (3 pt) (range from 2 to 7 days, mean 5, SD 2.65, 95% CI from 1.57 to 11.57). General symptoms were fever (1 pt >38°C), sore throat (3 pt), headache (7 pt), dry cough (8 pt), dyspnea (2 pt), nasal congestion (4 pt), loss of taste (1 pt), anosmia (1 pt), abdominal pain (2 pt), diarrhea (2 pt), myalgia (5 pt), night sweats (1 pt), fatigue (4 pt), and weight loss (1 pt). Nasopharyngeal swab was positive in two patients (1 pt excluded); All thoracic CT scans were normal; COVID‐19 serology positive in four patients after 2 weeks (three with IgA and one with IgG and IgA) and in two patients after 4 weeks (only IgA). Blood and urine tests were normal or negative after exclusion criteria except increased erythrocyte sedimentation rate (35 mm/h; 18‐102 mm/h) (6 pt), thrombocytopenia (1 pt) (128 000/mm3), increased ferritin (3 pt) (324.33 ng/mL; 297‐340 ng/mL), increased gamma glutamyl transferase (1 pt) (120 IU/lt), increased aspartate transaminase (2 pt) (43 IU/lt; 40‐46 IU/lt), increased alanine transaminase (2 pt) (83 IU/lt; 57‐109 IU/lt, vitamin D depletion in 15 patients (20.43 ng/mL; 9‐28,1 ng/mL), ANA 1:80 (10 pt) to 1:160 (2 pt) without SLE‐specific antibodies, ANA negative (12 pt), positive lupus anticoagulant (1 pt), Mycoplasma pneumoniae IgM with negative thoracic CT‐scan (3 pt asymptomatic), and increased anti‐streptolysin O antibodies (7 pt asymptomatic).
FIGURE 1.
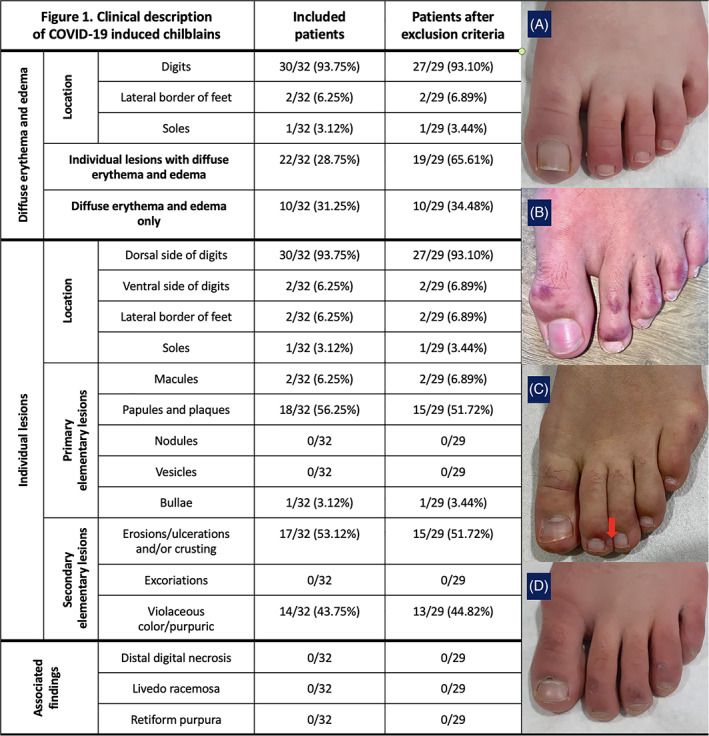
Clinical description of COVID‐19‐induced chilblains. The table portion of the figure summarizes the cutaneous clinical findings. Data are included for patients before and after three patients were excluded from the study. In this study, the cutaneous lesions were all located exclusively on the feet. One patient had both hand and feet involvement but was excluded because of identification of anti‐scl70 antibodies. All lesions were located on the dorsum of the digits and particularly on the distal phalanges except in two cases with eroded crusted lesions on the ventral side of the digits. A set of clinical photos are included (A–D). A, A diffuse edematous lesion on an erythematous background. B, Individual, erythematous, violaceous and purpuric papules, and plaques, with fewer macules. C, Necrotic bulla (arrow) on an erythemato‐violaceous background. D, Resolving lesions with residual erosions and desquamation
FIGURE 2.
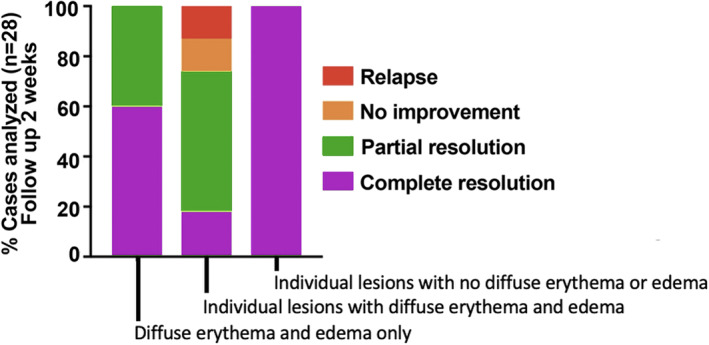
Correlation between clinical findings and clinical evolution of COVID‐19‐induced chilblains. Patients with diffuse background erythemato‐violaceous swelling (with or without individual lesions) had a more persistent or relapsing course compared with patients with only individual lesions. The worst course was seen in patients with both diffuse, erythematous background and individual lesions (P < 0.0001)
3.2. Histopathological findings
H&E, PAS, and Alcian‐blue stain photomicrographs are shown in Figure 3. A summary of histopathologic features is presented in Table 2; IHC findings are shown in Figure 4; and DIF findings are shown in Table 3. Finally, a clinico‐histopathological correlation of the most significant results is presented in Figure 5.
FIGURE 3.
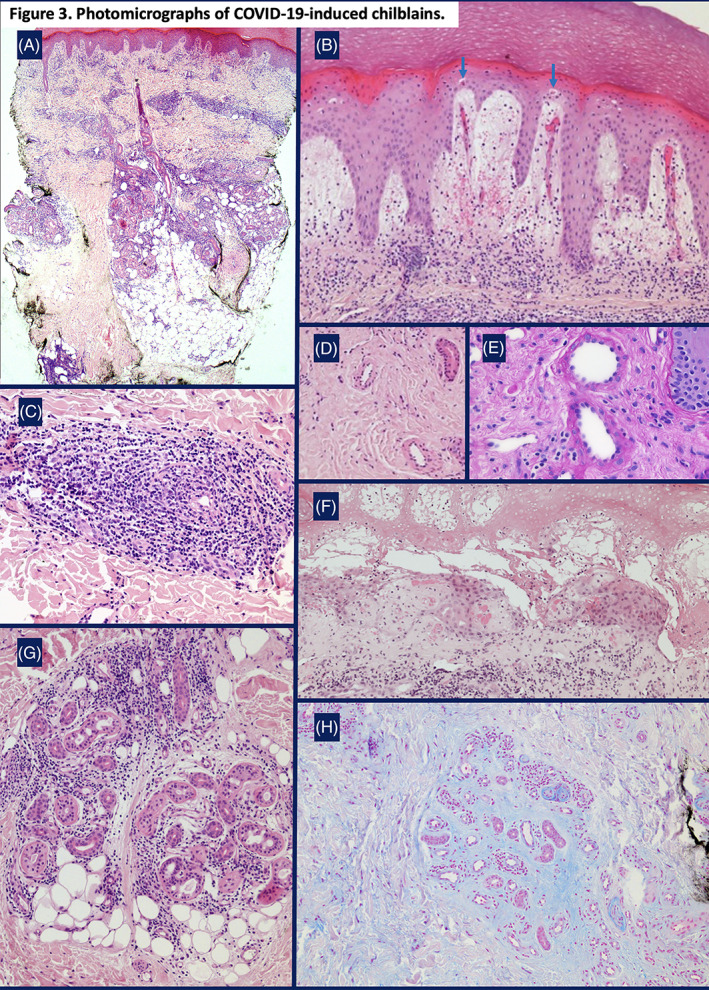
Photomicrographs of COVID‐19‐induced chilblains. A, A superficial and deep dermal perivascular lymphocytic infiltrate. There is a peri‐eccrine component with focal extension into the subcutis (H&E, ×400); B, Marked papillary dermal edema, dilated vessels, red cell extravasation, and apoptotic keratinocytes within the lower portion of the epidermis (arrows) (H&E, ×200); C, A lymphocytic infiltrate tightly‐cuffing small‐sized vessels whose walls are devoid of fibrin (H&E, ×200); D, Post‐capillary venules with thickened walls (H&E, ×100); E, Post‐capillary venules with thickened walls devoid of fibrin. There are prominent, bulging endothelial nuclei (PAS, ×400); F, Fibrin in the papillary dermis with overlying epidermal necrosis (seen clinically as a bulla) (H&E, ×200); G, A peri‐eccrine lymphocytic infiltrate (H&E, ×200); H, Abundant mucin deposition in the dermis, accentuated around eccrine glands (Alcian‐blue stain, ×100)
TABLE 2.
Histopathologic findings of COVID‐19‐induced chilblains
| % of patients (n = 21) with this finding present or absent | |||
|---|---|---|---|
| Present | Absent | ||
| Epidermal changes | Acanthosis | 86 | 14 |
| Hyperkeratosis | 100 | 0 | |
| Parakeratosis |
14 (upper) 43 (lower) |
43 | |
| Parakeratosis with exudate |
38 (moist) 19 (dry) |
43 | |
| Spongiosis | 33 | 67 | |
| Exocytosis | 48 | 52 | |
| Interface dermatitis | Vacuolar interface |
62 (focal) 19 (diffuse) 19 (continuous) |
0 |
| Number of apoptotic keratinocytes (×20) |
48 (1 keratinocyte) 38 (2–3 keratinocytes) 14 (≥4 keratinocytes) |
0 | |
| Basal membrane thickening |
48 (focal) 10 (diffuse) |
42 | |
| Lichenoid infiltrate | 0 | 100 | |
| Pigmentary incontinence | 5 (focal) | 95 | |
| Papillary dermal changes | Papillary dermal edema | 28 | 72 |
| Red cell extravasation | 76 | 24 | |
| Fibrin deposition | 14 | 86 | |
| Lymphocytic vasculitis | Perivascular lymphocytic infiltrate |
14 (discrete) 43 (moderate) 43 (intense) |
0 |
| Post‐capillary venule wall infiltration | 86 | 14 | |
| Swollen endothelial cells | 57 | 43 | |
| Vessel wall thickening | 86 | 14 | |
| Fibrin deposition | 5 (focal) | 95 | |
| Red cell extravasation | 43 | 57 | |
| Intraluminal thrombi formation | 5 (focal) | 95 | |
| Lymphocytic infiltrate | Superficial infiltrate | 95 | 5 |
| Deep infiltrate | 95 | 5 | |
| Distribution |
21 (top heavy) 5 (bottom heavy) |
74 (no difference) | |
| Perivascular |
14 (discrete) 43 (moderate) 43 (intense) |
0 | |
| Interstitial |
48 (discrete) 24 (moderate) 10 (intense) |
18 | |
| Peri‐eccrine |
42 (discrete) 26 (moderate) 26 (intense) |
6 | |
| Other | Interstitial mucin deposition |
71 (focal) 29 (diffuse) |
0 |
| Peri‐eccrine mucin deposition |
25 (focal) 71 (diffuse) 25 (intense) |
5 | |
| Collagen necrobiosis | 0 | 100 | |
| Subcutaneous infiltration |
31 (discrete) 31 (moderate) 7 (intense) |
31 | |
Notes: A skin biopsy was performed in 24 patients, but three patients were excluded because of a positive ANA. Thus, 21 patients are reported. Of note, the exclusion of these three patients did not statistically affect our results. Each histopathologic feature is recorded as “Present” or “Absent” in a percentage of the patients. Subcharacteristics are noted in parentheses.
FIGURE 4.

Immunohistochemical examination of COVID‐19‐induced chilblains. A summary of immunohistochemical (IHC) findings in 21 patients who received a biopsy. The lymphocytic infiltrate was mostly composed by CD3+ T lymphocytes with subpopulations of CD4+, CD8+, and granzyme B+ T lymphocytes. Few CD68+ and CD163+ histiocytes were present, and they were negative for myeloperoxidase. CD123+ plasmacytoid cells assessed for their distribution, whether they were isolated or present as aggregates
TABLE 3.
Direct immunofluorescence study findings in COVID‐19‐induced chilblains
| % of patients (n = 21) with positive DIF | ||||||
|---|---|---|---|---|---|---|
| IgG | IgA | IgM | C3 | Fibrinogen | ||
| Blood vessel walls | Superficial dermis | 0 | 0 | 23 (focal) | 23 (focal) |
35 (focal) 35 (moderate) |
| Deep dermis | 0 | 0 | 23 (focal) | 0 |
35 (focal) 8 (moderate) |
|
| Dermoepidermal junction | 0 | 0 | 0 | 8 (granular) |
4 (linear continuous) 4 (linear discontinuous) |
|
Note: Results are summarized for 21 patients in the study. Three patients who had a DIF study were excluded because of a positive ANA.
FIGURE 5.
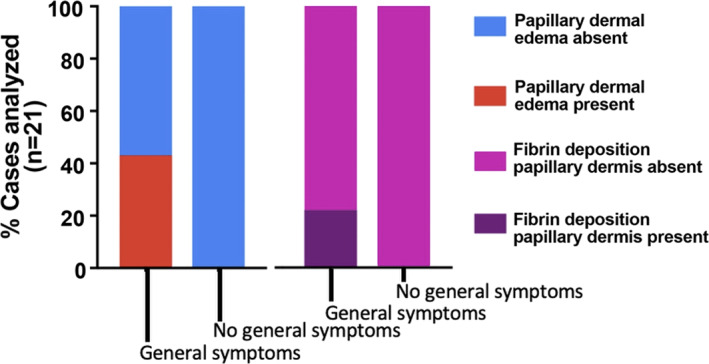
A clinicopathologic correlation of COVID‐19‐induced chilblains. Association of clinical symptoms with the most predominant histopathologic findings of papillary dermal edema and fibrin deposition. Patients who developed systemic symptoms showed more intense papillary dermal edema (P < 0.0001) and papillary dermal fibrin deposition (P < 0.0001). There was no association with the presence of lymphocytic vasculitis, interface changes, or peri‐eccrine involvement
3.3. Electron microscopy
Ultrastructural examination in two patients revealed rare, probable viral structures within the cytoplasm of endothelial cells and the secretory portion of eccrine glands with an electro‐lucent core surrounded by a capsule and numerous spikes as shown in Figure 6. Size ranged from 120 to 133 nm. No probable viral structures were identified in fibroblasts, lymphocytes, histiocytes, or keratinocytes.
FIGURE 6.
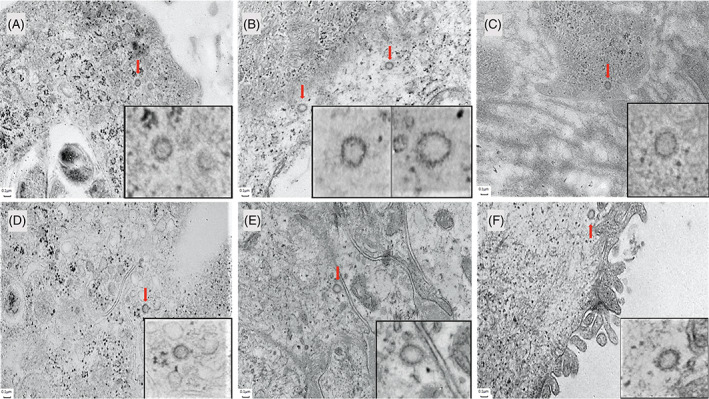
Electron microscopy showing probable viral particles in COVID‐19‐induced chilblains. A, Endothelial cell. Probable viral particle (arrow) with a core and spikes measuring 0.099 μm and 0.031 μm, respectively (×8000); B, Endothelial cell. Probable viral particles (arrow) with a core and spikes measuring 0.101 and 0.032 μm, respectively (left particle), and 0.089 and 0.032 μm, respectively (right particle) (×8000); C, Endothelial cell. Probable viral particle (arrow) with a core and spikes measuring 0.95 and 0.027 μm, respectively (×8000); D, Eccrine gland cell. Probable viral particle (arrow) with a core and spikes measuring 0.098 and 0.022 μm, respectively (×8000); E, Eccrine gland cell with magnification. Probable viral particle (arrow) with a core and spikes measuring 0.98 and 0.030 μm, respectively (×8000). F, Eccrine gland cell. Probable viral particle (arrow) with a core and spikes measuring 0.101 and 0.030 μm, respectively (×8000)
4. DISCUSSION
Our findings relate to the expansive existing, published literature to date as follows:
With this set of patients and the complete clinical and laboratory work‐up, there is even stronger evidence that COVID‐19 may cause chilblains, especially in young people. The published experience shows that COVID‐19‐induced chilblains, unlike primary, cold‐induced chilblains, have been mostly reported in warmer climates such as Spain, Italy, and France and sometimes in family clusters, with no history of cold exposure or Raynaud phenomenon. 6 , 15 Most reports have been in white people, with rare reports in Hispanic and Black people. 16 No cases have been reported in a large series from China. 17 Secondary chilblain LE was ruled‐out in our study because of negative ANA and DIF studies and the absence of other features of chronic cutaneous LE. 18 Secondary chilblains from a coagulopathy, including cryoglobulinemia, and monogenic interferonopathies (familial chilblain lupus, Aicardi‐Goutières syndrome, stimulator of IFN genes‐associated [STING] vasculopathy with onset in infancy [SAVI]) 19 were not evident. There are reports of COVID‐19 coinfection with other viruses and bacteria, such as EBV, parvovirus B19, Mycoplasma, and Streptococcus, 15 , 16 , 17 , 20 which were excluded in our study.
In two of our cases, EM identified rare structures highly suspicious for COVID‐19 in endothelium and eccrine glands (Figure 6). Similar structures have been published by Colmenero et al. 21 However, other cellular structures, such as coated vesicles, can mimic viral particles, and definitive identification requires the use of immunogold studies. In contrast to COVID‐toes, EM on autopsy lung tissue from fatal disease has shown abundant virus. 22 , 23
We did not employ IHC diagnostics, but IHC has been reported by others. COVID‐19 spike protein has been detected with IHC in endothelial cells and the secretory portion of sweat glands, proposing sweat as a possible contagion. 21 , 24 , 25 Spike protein alone was detected with IHC in endothelial and eccrine cells, however, without detection of spike RNA by PCR. 26 IHC directed against COVID‐19 nucleocapsid protein has been reported as negative. 22
Numerous reports of reactivation of chilblains during the second COVID‐19 wave in patients who had comparable lesions in the same anatomic areas in the previous pandemic wave, together with the occurrence of new cases during the second wave of COVID‐19, strongly suggests that chilblains are closely related to SARS‐CoV‐2 infection. 23 , 27 , 28 , 29 Three recent cases of BNT162b2 mRNA COVID‐19 vaccine (Pfizer)‐induced chilblains further reinforce the hypothesis of their relationship with SARS‐CoV‐2. 30 , 31 , 32
-
2.
COVID‐19‐induced chilblains are histopathologically identical to chilblains resulting from the many other primary and secondary causes. Our study demonstrates that it is not yet possible to determine the etiology of chilblains in any single patient. Chilblains are the phenotypic correlate of microangiopathic changes seen histopathologically (ie, lymphocytic vasculitis). Numerous publications have confirmed our histopathological findings of the resemblance to primary and secondary chilblains from other etiologies. There is commonly vacuolar interface change with necrotic keratinocytes, papillary dermal edema with extravasated erythrocytes, a superficial and deep perivascular lymphocytic infiltrate involving post‐capillary venule walls, and a deep, peri‐eccrine lymphocytic infiltrate. 10 , 33 , 34 , 35 , 36 , 37 , 38 Histopathologic distinction between primary (cold‐induced) and secondary (chilblain lupus) chilblains remains challenging and controversial, the former being favored by marked papillary dermal edema, limited vacuolar interface dermatitis, and the presence of a peri‐eccrine lymphocytic infiltrate. 39 , 40 , 41 , 42 , 43 In our cases, cold‐induced chilblains seemed unlikely as papillary dermal edema was absent in 72% of our cases, vacuolar interface changes were present in all cases, and DIF was negative in all cases (Tables 2 and 3). However, we do not believe that the distinction between primary and secondary chilblains can be made based only upon histopathological examination without taking into consideration the clinical context. Notable among our findings was thickening of post‐capillary venule walls (Figure 3D,E), which has been observed by others, and generous peri‐eccrine mucin deposition (Figure 3H). These findings could be explained by the known presence of the angiotensin‐converting enzyme‐2 (ACE2) receptor on endothelial cells and eccrine glands, because the ACE2 receptor has been identified as the binding target for COVID‐19. 44
Of note, the chilblains are histopathologically similar to the reported “erythema‐multiforme‐like” eruption with confluent, targetoid lesions, usually on hands and elbows. 45 , 46 These cases showed cytoplasmic granular positivity for spike protein in endothelial cells of the upper dermal vessels and epithelial cells of acrosyringia using IHC. These patients also have negative PCR swabs. 46
-
3.
No patients showed evidence for a systemic coagulopathy or a genetic susceptibility for a hypercoagulable state. Importantly, identification of microthrombi histopathologically is not definitive evidence of a systemic coagulopathy. Several reports of COVID‐19‐induced chilblains have demonstrated microthrombi, 21 , 33 , 34 leading some to conclude that there was a systemic coagulopathy, despite there being no confirmatory laboratory test (ie, D‐dimers). 10 The use of the term “acro‐ischemia” has been further confusing, because it suggests that all COVID‐19‐induced acral lesions result from a coagulopathy. 47 Microthrombi may, therefore, be found in primary and secondary chilblains, without there being systemic involvement. While primary and COVID‐19‐induced chilblains are not related to a coagulopathy, critically ill patients with a livedo‐like pattern (ie, acral livedo racemosa, retiform purpura, and ischemic acral necrosis) do have a coagulopathy and a severe clinical course. 17 These patients lack typical histopathological findings of chilblains. 48 Thus, it is important to distinguish the chilblains‐like pattern from the livedo‐like pattern, 49 because the latter indicates severe disease with a coagulopathy, often leading to death. 50
-
4.
Negative blood serologic tests and PCR swabs do not rule‐out COVID‐19 causality. In our study, COVID‐19 PCR on nasopharyngeal swab was positive in only one case and serologic studies were positive in six cases (Table 1). Of note, both patients with positive EM had negative PCR swab and serologies. Negative serologic tests cannot rule‐out COVID‐19 because effective protective immunity appears to be dependent on specific memory T‐cells and not a humoral antibody response. 51 , 52 , 53 Negative PCR results can be explained by the appearance of chilblains in the convalescent phase of the disease. Clinicians should understand that a negative swab or antibody test does not necessarily rule‐out COVID‐19 as a causative agent and cannot be used as an argument linking chilblains to lifestyle changes brought on by containment. 54
Negative serologies in patients with mild disease can be explained through type‐I interferon (IFN) pathway activation, thereby suppressing the humoral antibody response and explaining the reported rapid loss of antibodies, 55 especially in pauci‐symptomatic persons 56 In our initial report, 1 based on clinical and histopathological similarities to secondary chilblain LE and monogenic interferonopathies, we suspected viral‐induced type‐I IFN activation, an assertion that has since been supported by others. 35 , 44 , 57 , 58 , 59 Type‐I IFN levels fall with age and heightened production in young, healthy people leads to successful control of COVID‐19 but also results in chilblains, not as a direct viral response but as a paraviral immune response. 48 As the type‐I IFN pathway wanes in adulthood, COVID‐19 infection is not as successfully inhibited, resulting in more severe disease. 44 , 60
-
5.
COVID‐toes usually signal a good prognosis in asymptomatic patients. Clinically, the disease has different findings along its course, each with different prognostic significance. 5 , 48 COVID‐toes are a late, reassuring sign, which should be distinguished from systemic coagulopathy. Our findings of the chilblains appearing later after mild symptoms have been supported by others. 5 , 48 Despite the absence of disease severity in a subset of patients, COVID‐toes may persist more than 60 days (instead of 15 days), representing persistent inflammation. 61 Given the histopathologic similarity of the reported erythema multiforme‐like eruption with also negative PCR swabs, the erythema multiforme‐like eruption is also a good prognostic clue. All of these patients, however, are important in reaching “herd immunity” and ending the pandemic. 48 , 62 , 63 , 64
5. CONCLUSION
In summary, we have presented the largest series of presumptive COVID‐19‐induced chilblains to date with histopathological analysis and an exhaustive clinical, radiological, and laboratory work‐up. Our study provides strong evidence that the chilblains are not the result of other primary or secondary causes. All cases showed a close phenotypic and histopathologic correlation, and electron microscopy in a subset of the cases demonstrated probable viral particles. None of our patients had any evidence in favor of systemic coagulopathy, and all showed an indolent disease course. A presentation of any acral lesion during this period should signal the need to identify and isolate an asymptomatic patient, but dermatologists should distinguish simple chilblains from thrombotic lesions, such as distal livedo racemosa, retiform purpura, and ischemic acral necrosis, because the latter may portend a more severe clinical course.
Kolivras A, Thompson C, Pastushenko I, et al. A clinicopathological description of COVID‐19‐induced chilblains (COVID‐toes) correlated with a published literature review. J Cutan Pathol. 2022;49(1):17-28. 10.1111/cup.14099
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection‐induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;6(6):489‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fernandez‐Nieto D, Jimenez‐Cauhe J, Suarez‐Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID‐19 outbreak. J Am Acad Dermatol. 2020;83(1):e61‐e63. 10.1016/j.jaad.2020.04.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ortega‐Quijano D, Jimenez‐Cauhe J, Selda‐Enriquez G, Fernandez‐Guarino M, Fernandez‐Nieto D. Algorithm for the classification of COVID‐19 rashes. J Am Acad Dermatol. 2020;83(2):e103‐e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Visconti A, Bataille V, Rossi N, et al. Diagnostic value of skin manifestation of SARS‐CoV‐2 infection. Br J Dermatol. 2021;184(5):880‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71‐77. 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. López‐Robles J, de la Hera I, Pardo‐Sánchez J, Ruiz‐Martínez J, Cutillas‐Marco E. Chilblain‐like lesions: a case series of 41 patients during the COVID‐19 pandemic. Clin Exp Dermatol. 2020;45(7):891‐892. 10.1111/ced.14275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID‐19. J Eur Acad Dermatol Venereol. 2020;34(8):e346‐e347. 10.1111/jdv.16533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Landa N, Mendieta‐Eckert M, Fonda‐Pascual P, Aguirre T. Chilblain‐like lesions on feet and hands during the COVID‐19 pandemic. Int J Dermatol. 2020;59(6):739‐743. 10.1111/ijd.14937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saenz Aguirre A, De la Torre Gomar FJ, Rosés‐Gibert P, et al. Novel outbreak of acral lesions in times of COVID‐19: a description of 74 cases from a tertiary university hospital in Spain. Clin Exp Dermatol. 2020;45(8):1065‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanitakis J, Lesort C, Danset M, Jullien D. Chilblain‐like acral lesions during the COVID‐19 pandemic ("COVID toes"): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol. 2020;83(3):870‐875. 10.1016/j.jaad.2020.05.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herman A, Peeters C, Verroken A, Tromme I, Tennstedt D. Evaluation of chilblains as a manifestation of the COVID‐19 pandemic. JAMA Dermatol. 2020;156(9):998‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roca‐Ginés J, Torres‐Navarro I, Sánchez‐Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID‐19 pandemic. JAMA Dermatol. 2020;156(9):992‐997. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neri I, Virdi A, Corsini I, et al. Major cluster of paediatric 'true' primary chilblains during the COVID‐19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34(11):2630‐2635. 10.1111/jdv.16751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rouanet J, Lang E, Beltzung F, et al. Recent outbreak of chilblain‐like lesions is not directly related to SARS‐CoV‐2 infection. J Eur Acad Dermatol Venereol. 2020;34(11):689‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen T, Song J, Liu H, Zheng H, Chec C. Positive Epstein‐Barr virus detection in corona virus disease 2019 (COVID‐19) patients. Sci Rep. 2020;11(1):10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nirenberg MS, Herrera MDMR. Foot manifestations in a patient with COVID‐19 and Epstein‐Barr virus: a case study. Foot (Edinb). 2021;46(1):101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cordoro KM, Reynolds SD, Wattier R, McCalmont TH. Clustered cases of acral perniosis: clinical features, histopathology, and relationship to COVID‐19. Pediatr Dermatol. 2020;37(3):419‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hedrich CM, Fiebig B, Hauck FH, et al. Chilblain lupus erythematosus – a review of literature. Clin Rheumatol. 2008;27(8):949‐954. [DOI] [PubMed] [Google Scholar]
- 19. Munoz J, Marque M, Dandurand M, Meunier L, Crow YJ, Bessis D. Interféronopathies de type I [Type I interferonopathies]. Ann Dermatol Venereol. 2015;142(11):653‐663. [DOI] [PubMed] [Google Scholar]
- 20. Larkins N, Murray KJ. Major cluster of chilblain cases in a cold dry Western Australian winter. J Paediatr Child Health. 2013. Feb;49(2):144‐147. [DOI] [PubMed] [Google Scholar]
- 21. Colmenero I, Santonja C, Alonso‐Riaño M, et al. SARS‐CoV‐2 endothelial infection causes COVID‐19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183(4):729‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ko CJ, Harigopal M, Damsky W, et al. Perniosis during the COVID‐19 pandemic: negative anti‐SARS‐CoV‐2 immunohistochemistry in six patients and comparison to perniosis before the emergence of SARS‐CoV‐2. J Cutan Pathol. 2020;47(11):997‐1002. 10.1111/cup.13830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Signa S, Sementa AR, Coccia MC, et al. Recurrence of previous chilblain lesions during the second wave of COVID‐19: can we still doubt the correlation with SARS‐CoV‐2? J Eur Acad Dermatol Venereol. 2021;35:e475‐e477. 10.1111/jdv.17283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santonja C, Heras F, Núñez L, Requena L. COVID‐19 chilblain‐like lesion: immunohistochemical demonstration of SARS‐CoV‐2 spike protein in blood vessel endothelium and sweat gland epithelium in a polymerase chain reaction‐negative patient. Br J Dermatol. 2020;183(4):778‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gambichler T, Reuther J, Stücker M, et al. SARS‐CoV‐2 spike protein is present in both endothelial and eccrine cells of a chilblain‐like skin lesion. J Eur Acad Dermatol Venereol. 2020;35(3):e187‐e189. 10.1111/jdv.16970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ko CJ, Harigopal M, Gehlhausen JR, Bosenberg M, McNiff JM, Damsky W. Discordant anti‐SARS‐CoV‐2 spike protein and RNA staining in cutaneous perniotic lesions suggests endothelial deposition of cleaved spike protein. J Cutan Pathol. 2021;48(1):47‐52. 10.1111/cup.13866 [DOI] [PubMed] [Google Scholar]
- 27. Maanaoui S, Salez F, Carpentier O. Recurrence of chilblains during a second contact with SARCoV‐2: a case report. Br J Dermatol. 2021;185:227‐228. 10.1111/bjd.20070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Recalcati S, Barbagallo T, Tonolo S, Milani M, Fantini F. Relapse of chilblain‐like lesions during the second wave of coronavirus 19. J Eur Acad Dermatol Venereol. 2021;35(5):e315‐e316. 10.1111/jdv.17168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Piccolo V, Bassi A, Russo T, et al. Chilblain‐like lesions and COVID‐19: second wave, second outbreak. J Eur Acad Dermatol Venereol. 2021;35(5):e316‐e318. 10.1111/jdv.17145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pileri A, Guglielmo A, Raone B, Patrizi A. Chilblain lesions after COVID‐19 mRNA vaccine. Br J Dermatol. 2021;185(1):e3. 10.1111/bjd20060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davido B, Mascitti H, Fortier‐Beaulieu M, Jaffal K, de Truchis P. ‘Blue toes’ following vaccination with Pfizer BNT162b2 mRNA COVID‐19 vaccine. J Travel Med. 2021;28(4):taab024. 10.1093/jtm/taab024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Piccolo V, Bassi A, Argenziano G, et al. BNT162b2 mRNA COVID‐19 vaccine‐induced chilblain‐like lesions reinforces the hypothesis of their relationship with SARS‐CoV‐2. J Eur Acad Dermatol Venereol. 2021. 10.1111/jdv.17320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Masson A, Bouaziz JD, Sulimovic L, et al. Chilblains is a common cutaneous finding during the COVID‐19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83(2):667‐670. 10.1016/j.jaad.2020.04.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andina D, Noguera‐Morel L, Bascuas‐Arribas M, et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatr Dermatol. 2020;37(3):406‐411. 10.1111/pde.14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID‐19‐associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol. 2021;184(1):141‐150. 10.1111/bjd.19415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain‐like lesions: lights and shadows on the relationship with COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sohier P, Matar S, Meritet JF, Laurent‐Rousel S, Dupin N, Aractingi S. Histopathological features of chilblain‐like lesions developing in the setting of the COVID‐19 pandemic. Arch Pathol Lab Med. 2020. 10.5858/arpa.2020-0613-SA [DOI] [PubMed] [Google Scholar]
- 38. Freeman EE, McMahon DE, Lipoff JB, et al. Pernio‐like skin lesions associated with COVID‐19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83(2):486‐492. 10.1016/j.jaad.2020.05.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crowson AN, Magro CM. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28(4):478‐484. 10.1016/s0046-8177(97)90038-1 [DOI] [PubMed] [Google Scholar]
- 40. Boada A, Bielsa I, Fernández‐Figueras MT, Ferrándiz C. Perniosis: clinical and histopathological analysis. Am J Dermatopathol. 2010;32(1):19‐23. 10.1097/DAD.0b013e3181af1d24 [DOI] [PubMed] [Google Scholar]
- 41. Cribier B, Djeridi N, Peltre B, Grosshans E. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45(6):924‐929. 10.1067/mjd.2001.117861 [DOI] [PubMed] [Google Scholar]
- 42. Wang ML, Chan MP. Comparative analysis of chilblain lupus erythematosus and idiopathic perniosis: histopathologic features and immunohistochemistry for CD123 and CD30. Am J Dermatopathol. 2018;40(4):265‐271. [DOI] [PubMed] [Google Scholar]
- 43. Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89(2):207‐215. 10.1016/j.mayocp.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 44. Hubiche T, Cardot‐Leccia N, Duff F, et al. Chilblains appear as a manifestation of SARS‐CoV‐2 infection and reveal features of type I interferonopathy and micro‐vasculopathy. Lancet. 2020. 10.2139/ssrn.3586683 [DOI] [Google Scholar]
- 45. Gianotti R, Recalcati S, Fantini F, et al. Histopathological study of a broad spectrum of skin dermatoses in patients affected or highly suspected of infection by COVID‐19 in the northern part of Italy: analysis of the many faces of the viral‐induced skin diseases in previous and new reported cases. Am J Dermatopathol. 2020;42(8):564‐570. 10.1097/DAD.0000000000001707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Torrelo A, Andina D, Santonja C, et al. Erythema multiforme‐like lesions in children and COVID‐19. Pediatr Dermatol. 2020;37(3):442‐446. 10.1111/pde.14246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Piccolo V, Bassi A. Acral findings during the COVID‐19 outbreak: chilblain‐like lesions should be preferred to acroischemic lesions. J Am Acad Dermatol. 2020;83(3):e231. 10.1016/j.jaad.2020.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID‐19‐associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83(4):1118‐1129. 10.1016/j.jaad.2020.06.1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marzano AV, Cassano N, Genovese G, Moltrasio C, Vena GA. Cutaneous manifestations in patients with COVID‐19: a preliminary review of an emerging issue. Br J Dermatol. 2020;183(3):431‐442. 10.1111/bjd.19264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hay RJ. A viral rash: the impact of COVID‐19 infection on the skin. Br J Dermatol. 2020;183(1):1‐2. 10.1111/bjd.19188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Melgaço JG, Azamor T, Ano Bom APD. Protective immunity after COVID‐19 has been questioned: what can we do without SARS‐CoV‐2‐IgG detection? Cell Immunol. 2020;353:104114. 10.1016/j.cellimm.2020.104114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weinstein MC, Freedberg KA, Hyle EP, Paltiel AD. Waiting for certainty on Covid‐19 antibody tests – at what cost? N Engl J Med. 2020;383(6):e37. 10.1056/NEJMp2017739 [DOI] [PubMed] [Google Scholar]
- 53. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell. 2020;183(1):158‐168.e14. 10.1016/j.cell.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Freeman E, McMahon D, Fox L. Emerging evidence of the direct association between COVID‐19 and chilblains. JAMA Dermatol. 2021;157(2):238‐239. [DOI] [PubMed] [Google Scholar]
- 55. Fallet B, Narr K, Ertuna YI, et al. Interferon‐driven deletion of antiviral B cells at the onset of chronic infection. Sci Immunol. 2016;1(4):eaah6817. 1‐23. 10.1126/sciimmunol.aah6817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ibarrondo FJ, Fulcher JA, Goodman‐Meza D, et al. Rapid decay of anti‐SARS‐CoV‐2 antibodies in persons with mild Covid‐19. N Engl J Med. 2020;383(11):1085‐1087. 10.1056/NEJMc2025179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Quintana‐Castanedo L, Feito‐Rodríguez M, Fernández‐Alcalde C, et al. Concurrent chilblains and retinal vasculitis in a child with COVID‐19. J Eur Acad Dermatol Venereol. 2020;34(12):764‐766. 10.1111/jdv.16801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Battesti G, El Khalifa J, Abdelhedi N, et al. New insights in COVID‐19‐associated chilblains: a comparative study with chilblain lupus erythematosus. J Am Acad Dermatol. 2020;83(4):1219‐1222. 10.1016/j.jaad.2020.06.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aschoff R, Zimmermann N, Beissert S, Günter C. Type I interferon signature in chilblain‐like lesions associated with the COVID‐19 pandemic. Dermatopathology. 2020;7(3):57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rodríguez‐Villa Lario A, Vega‐Díez D, González‐Cañete M, et al. Histological findings in chilblain lupus‐like COVID lesions: in search of an answer to understand their aetiology. J Eur Acad Dermatol Venereol. 2020;34(10):572‐574. 10.1111/jdv.16733.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McMahon D, Gallman A, Hruza G, et al. Long COVID in the skin: a registry analysis of COVID‐19 dermatological duration. Lancet Infect Dis. 2021;21(3):313‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Colonna C, Genovese G, Monzani NA, et al. Outbreak of chilblain‐like acral lesions in children in the metropolitan area of Milan, Italy, during the COVID‐19 pandemic. J Am Acad Dermatol. 2020;83(3):965‐969. 10.1016/j.jaad.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles' heel of current strategies to control Covid‐19. N Engl J Med. 2020;382(22):2158‐2160. 10.1056/NEJMe2009758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gilbert M, Dewatripont M, Muraille E, Platteau JP, Goldman M. Preparing for a responsible lockdown exit strategy. Nat Med. 2020;26(5):643‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


