Abstract
Background and purpose
Neuropathological studies can elucidate the mechanisms of nervous system damage associated with SARS‐CoV‐2 infection. Despite literature on this topic is rapidly expanding, correlations between neurological symptoms and brain pathology findings in COVID‐19 patients remain largely unknown.
Methods
We performed a systematic literature review on neuropathological studies in COVID‐19, including 438 patients from 45 articles published by April 22, 2021. We retrieved quantitative data regarding demographic, clinical, and neuropathological findings. We carried out a Wilcoxon rank sum test or χ2 test to compare patients' subgroups based on different clinical and brain pathology features.
Results
Neuropathological findings in COVID‐19 patients were microgliosis (52.5%), astrogliosis (45.6%), inflammatory infiltrates (44.0%), hypoxic‐ischemic lesions (40.8%), edema (25.3%), and hemorrhagic lesions (20.5%). SARS‐CoV‐2 RNA and proteins were identified in brain specimens of 41.9% and 28.3% of subjects, respectively. Detailed clinical information was available from 245 patients (55.9%), and among them, 96 subjects (39.2%) had presented with neurological symptoms in association with typical COVID‐19 manifestations. We found that: (i) the detection rate of SARS‐CoV‐2 RNA and proteins in brain specimens did not differ between patients with versus those without neurological symptoms; (ii) brain edema, hypoxic‐ischemic lesions, and inflammatory infiltrates were more frequent in subjects with neurological impairment; (iii) neurological symptoms were more common among older individuals.
Conclusions
Our systematic revision of clinical correlates in COVID‐19 highlights the pathogenic relevance of brain inflammatory reaction and hypoxic‐ischemic damage rather than neuronal viral load. This analysis indicates that a more focused study design is needed, especially in the perspective of potential therapeutic trials.
Keywords: brain, COVID‐19, neurological symptoms, neuropathology, SARS‐CoV‐2
The evidence from brain pathology that edema, hypoxic‐ischemic lesions, inflammatory infiltrates, but not viral RNA or proteins, are associated with the presence of neurological symptoms in COVID‐19 patients corroborates the hypothesis that neurological impairment is likely due to brain inflammatory and hypoxic‐ischemic damage rather than to the direct cytopathic effects of SARS‐CoV‐2. Older individuals could show a higher risk of neurological manifestations in the event of SARS‐CoV‐2 infection.

INTRODUCTION
The coronavirus disease 2019 (COVID‐19) is an ongoing viral pandemic that emerged from Wuhan province in China and quickly spread to the rest of the world [1]. This infection is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), an enveloped, positive‐sense, single‐stranded RNA betacoronavirus responsible for over 194 million confirmed infections and more than 4 million deaths worldwide as of July 27, 2021 (https://covid19.who.int). In most cases, COVID‐19 presents with fever and upper respiratory symptoms, especially dry cough and often shortness of breath. However, 5% of patients may develop a critical illness with severe pneumonia leading to acute respiratory distress syndrome, disseminated coagulopathy, multiple organ dysfunction syndrome, and eventually death [2, 3]. Morbidity and mortality are more common in the elderly and in patients with comorbidities, including cardiovascular disease, arterial hypertension, obesity, and diabetes mellitus [4]. Nevertheless, young people with no comorbidities can also develop a critical illness [5].
Although involvement of the respiratory system is the most relevant clinical feature in COVID‐19 patients, neurological symptoms have been widely reported [6, 7, 8]. Neurological manifestations associated with SARS‐CoV‐2 infection can affect both the central and peripheral nervous system and range widely from mild taste and smell disturbances to more serious conditions, such as acute cerebrovascular disease, disseminated encephalomyelitis, or Guillain‐Barré syndrome [6, 7, 8, 9, 10].
The underlying pathogenic mechanisms of neurological involvement are still unclear. Both direct cytopathic effects mediated by SARS‐CoV‐2 replication and indirect effects due to respiratory failure with brain hypoxia, cytokine reaction, or parainfectious autoimmune response have been proposed [11]. Although direct and indirect mechanisms could coexist in COVID‐19 patients with neurological impairment, in most cases the prevailing pathophysiology remains to be addressed.
Only a few authors have identified SARS‐CoV‐2 in the cerebrospinal fluid of COVID‐19 patients [12, 13, 14]. However, in most clinical studies reporting neurological symptoms, the virus was neither searched nor detected in the cerebrospinal fluid.
In this critical review, we focused on neuropathological findings and their clinical correlations in COVID‐19. Although the number of these postmortem studies is not comparable to the number of reports on neurological manifestations, we believe that an in‐depth characterization of SARS‐CoV‐2–related brain pathology, including assessment of viral proteins and RNA in brain specimens, is crucial for understanding the etiology of neurological symptoms in COVID‐19 patients. Moreover, incorporation of neuropathological and molecular findings from brain tissue of subjects with COVID‐19 and neurological symptoms could also provide valuable clues for the best management practices and for guiding future research.
METHODS
This systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines [15] (Figure 1). Studies eligible for inclusion were those dealing with neuropathological characteristics of COVID‐19 patients. Studies published by April 22, 2021 were identified from the National Library of Medicine's MEDLINE database. We utilized the following comprehensive Medical Subject Headings terms: (COVID‐19 OR SARS‐CoV‐2 OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease 2019" OR "2019 novel coronavirus" OR "2019 nCoV") AND (CNS OR PNS OR brain OR cerebrum OR cerebral OR cerebellum OR cerebellar OR brainstem OR thalamus OR thalamic OR hippocampus OR hippocampal OR pons OR pontine OR medulla oblongata OR neurological OR nervous system OR neuron OR nerve OR neural OR encephalitis). Reference lists of identified articles were further reviewed to search for additional studies. Randomized controlled trials, nonrandomized controlled trials, case‐control studies, cohort studies, cross‐sectional studies, case series, case reports, brief reports, and letters to the editor were all included in the study. Exclusion criteria were: (i) articles not in English, (ii) animal studies, (iii) reviews and other types of articles (e.g., editorials, conference abstracts, commentaries) not reporting original findings. We did not apply any restrictions on age, sex, or ethnicity of patients in the studies.
FIGURE 1.
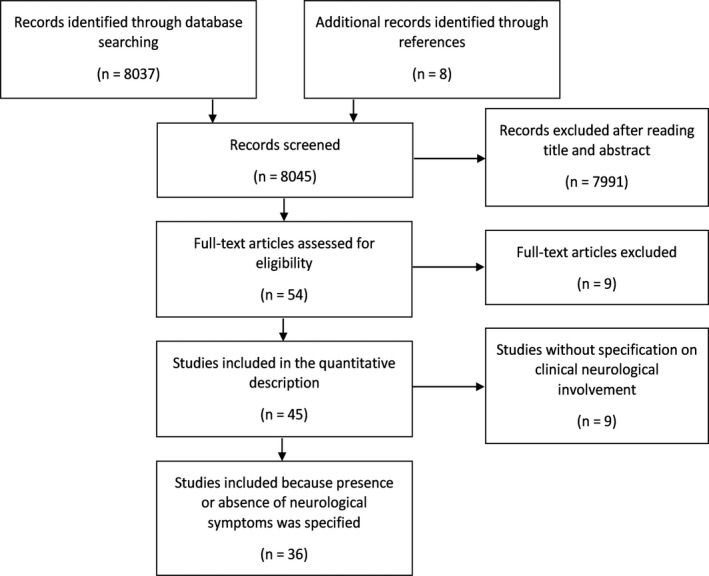
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram.
All abstracts or full articles without electronic abstracts were reviewed independently by two different authors, who both went through each phase of the review independently (screening, eligibility, inclusion, and quality assessment) to identify potentially relevant studies and evaluate their reliability and risk of bias. The Murad tool for noncomparative cohorts and case reports or case series, and the Newcastle‐Ottawa Scale for comparative cohorts and cross‐sectional studies were used for quality assessment of the included studies [16, 17]. We considered “poor,” “moderate,” or “good” quality when three or fewer, four, or five of the criteria were fulfilled in the Murad tool, respectively, and three points or fewer, four to five, or six and above in the Newcastle‐Ottawa Scale, respectively. Disagreement was resolved by discussion with the other investigator.
The following main features from individual patients were considered when reported: (i) demographic characteristics (i.e., age and gender), (ii) presence or absence of cardiovascular risk factors from medical history (at least one among arterial hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, or previous stroke), (iii) presence or absence of neurological symptoms concurrent to or following the onset of typical COVID‐19 manifestations, (iv) presence or absence of SARS‐CoV‐2 RNA or proteins in brain specimens, and (v) presence or absence of main alterations at gross and microscopic examination of brain samples (i.e., edema, hypoxic‐ischemic lesions, hemorrhagic lesions, microgliosis, astrogliosis, and inflammatory infiltrates). Patients' subgroups featured by different brain pathology findings were compared by age, gender, and presence or absence of cardiovascular risk factors or neurological symptoms. Comparisons of brain pathology findings based on detection rate of SARS‐CoV‐2 RNA or proteins in brain specimens were also carried out. Differences between subgroups were analyzed by means of Wilcoxon rank sum test or Pearson χ2 test, as appropriate. Level of statistical significance was set at a 0.05. Because of the exploratory nature of these analyses, we did not use correction for multiple comparisons to avoid type II error. Statistical analyses were performed using JMP Pro 14.0 (SAS Institute, Cary, NC).
RESULTS
We selected a final number of 45 articles, which provided a cumulative sample of 438 COVID‐19 patients with available brain pathology (see Appendix S1 for the list of articles, and Appendix S2 for a summary of the main characteristics of these studies). The quality was rated “good” in 18 articles (40.0%), “moderate” in 20 articles (44.4%), and “poor” in seven articles (15.6%) (see Appendix S2 for details). In one article, the histopathological examination was performed on in vivo brain samples obtained from surgical intervention, whereas in all other articles the neuropathology was investigated on postmortem brain specimens. SARS‐CoV‐2 RNA was searched on brain samples in 24 studies, using real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) in 22 articles and through in situ hybridization in nine articles. Of note, both techniques of SARS‐CoV‐2 RNA detection were used in seven studies. SARS‐CoV‐2 proteins were searched in 14 articles by means of immunohistochemistry. Gross and microscopic brain pathology was analyzed in 39 articles. Only four studies investigated the presence of viral particles using electron microscopy. Information on clinical neurological involvement could be retrieved in 36 of the 45 selected articles, thus the correlation between presence/absence of neurological symptoms and neuropathological features was evaluated in these 36 articles. Findings from the different studies are shown in Tables 1, 2, 3 in the form of proportions, namely as the number of cases with a particular neuropathological feature divided by the total sample of interest.
TABLE 1.
Cumulative brain pathology findings and comparisons based on neurological symptoms in COVID‐19 patients
| Brain pathology findingsa | Total sample | Neurological symptoms | p value | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| SARS‐CoV−2 RNA | Yes | 104/248 (41.9%) | 41/78 (52.6%) | 37/78 (47.4%) | 0.097 |
| No | 144/248 (58.1%) | 23/60 (38.3%) | 37/60 (61.7%) | ||
| SARS‐CoV−2 proteins | Yes | 51/179 (28.3%) | 17/29 (58.6%) | 12/29 (41.4%) | 0.929 |
| No | 128/179 (71.7%) | 60/104 (57.7%) | 44/104 (42.3%) | ||
| Edema | Yes | 73/289 (25.3%) | 25/45 (55.6%) | 20/45 (44.4%) | 0.024 |
| No | 216/289 (74.7%) | 46/127 (36.2%) | 81/127 (63.8%) | ||
| Hypoxic‐ischemic lesionsb | Yes | 138/338 (40.8%) | 53/109 (48.6%) | 56/109 (51.4%) | <0.001 |
| No | 200/338 (59.2%) | 27/112 (24.1%) | 85/112 (75.9%) | ||
| Hemorrhagic lesionsc | Yes | 57/278 (20.5%) | 16/39 (41.0%) | 23/39 (59.0%) | 0.261 |
| No | 221/278 (79.5%) | 43/137 (31.4%) | 94/137 (68.6%) | ||
| Microgliosis | Yes | 147/280 (52.5%) | 25/67 (37.3%) | 42/67 (62.7%) | 0.318 |
| No | 133/280 (47.5%) | 34/113 (30.1%) | 79/113 (69.9%) | ||
| Astrogliosis | Yes | 130/285 (45.6%) | 23/58 (39.7%) | 35/58 (60.3%) | 0.292 |
| No | 155/285 (54.4%) | 36/114 (31.6%) | 78/114 (68.4%) | ||
| Inflammatory infiltratesd | Yes | 144/332 (44.0%) | 30/64 (46.9%) | 34/64 (53.1%) | 0.004 |
| No | 188/332 (56.0%) | 30/116 (25.9%) | 86/116 (74.1%) | ||
Data are reported as number of patients (percent). p values were obtained by comparing patients with versus those without neurological symptoms using the Pearson χ2 test. Significant p values are bolded.
aViral particles were found by means of electron microscopy in three out of four subjects.
bIntraparenchymal intravascular microthrombi with focal microscopic cortical or deep recent infarcts were detected in 47 patients (13.9%).
cPerivascular microhemorrhages were reported in 20 subjects (7.2%).
dPerivascular inflammatory cell infiltration or endotheliitis suggesting vasculitis were observed in 122 patients (36.7%).
TABLE 2.
Comparisons of brain pathology findings based on SARS‐CoV‐2 RNA in COVID‐19 patients
| Brain pathology findings | SARS‐CoV−2 RNA | p value | ||
|---|---|---|---|---|
| Yes | No | |||
| Edema | Yes | 26/64 (40.6%) | 38/64 (59.4%) | 0.663 |
| No | 38/86 (44.2%) | 48/86 (55.8%) | ||
| Hypoxic‐ischemic lesions | Yes | 39/86 (45.3%) | 47/86 (54.7%) | 0.951 |
| No | 35/78 (44.9%) | 43/78 (55.1%) | ||
| Hemorrhagic lesions | Yes | 14/35 (40.0%) | 21/35 (60.0%) | 0.465 |
| No | 61/130 (46.9%) | 69/130 (53.1%) | ||
| Microgliosis | Yes | 36/90 (40.0%) | 54/90 (60.0%) | 0.146 |
| No | 38/74 (51.4%) | 36/74 (48.6%) | ||
| Astrogliosis | Yes | 35/88 (39.8%) | 53/88 (60.2%) | 0.161 |
| No | 39/77 (50.6%) | 38/77 (49.4%) | ||
| Inflammatory infiltrates | Yes | 34/86 (39.5%) | 52/86 (60.5%) | 0.131 |
| No | 40/78 (51.3%) | 38/78 (48.7%) | ||
Data are reported as number of patients (percent). p values were obtained using the Pearson χ2 test.
TABLE 3.
Comparisons of brain pathology findings based on SARS‐CoV‐2 proteins in COVID‐19 patients
| Brain pathology findings | SARS‐CoV−2 proteins | p value | ||
|---|---|---|---|---|
| Yes | No | |||
| Edema | Yes | 21/38 (55.3%) | 17/38 (44.7%) | <0.001 |
| No | 21/88 (23.9%) | 67/88 (76.1%) | ||
| Hypoxic‐ischemic lesions | Yes | 20/83 (24.1%) | 63/83 (75.9%) | 0.705 |
| No | 19/71 (26.8%) | 52/71 (73.2%) | ||
| Hemorrhagic lesions | Yes | 1/14 (7.1%) | 13/14 (92.9%) | 0.241 |
| No | 28/140 (20.0%) | 112/140 (80.0%) | ||
| Microgliosis | Yes | 17/100 (17.0%) | 83/100 (83.0%) | 0.429 |
| No | 12/54 (22.2%) | 42/54 (77.8%) | ||
| Astrogliosis | Yes | 17/107 (15.9%) | 90/107 (84.1%) | 0.159 |
| No | 12/47 (25.5%) | 35/47 (74.5%) | ||
| Inflammatory infiltrates | Yes | 17/108 (15.7%) | 91/108 (84.3%) | 0.133 |
| No | 12/46 (26.1%) | 34/46 (73.9%) | ||
Data are reported as number of patients (percent). p values were obtained using the Pearson χ2 test. Significant p values are bolded.
Age was indicated for 302 subjects (median: 69 years; range: 5−98 years). Gender was reported for 318 patients (220 males, 69.2%). Medical history was available from 334 individuals (76.3%), and in particular, cardiovascular risk factors were present in 244 subjects (73.1%). Information on presence or absence of neurological symptoms or disorders associated with COVID‐19 could be retrieved only for 245 patients (55.9%), and among them, 96 subjects (39.2%) presented with neurological symptoms (see Table 4 for details). Patients with neurological symptoms were older than those without neurological impairment (median age: 69 vs. 66 years, respectively; p = 0.038), whereas no significant differences were observed in regard to gender (56 males/26 females vs. 106 males/43 females, respectively; p = 0.596), and presence (Y) or absence (N) of cardiovascular risk factors (54 Y/18 N vs. 106 Y/42 N, respectively; p = 0.598).
TABLE 4.
Characterization of neurological symptoms or disorders in COVID‐19 patients
| Neurological symptoms/disorders | No. of patients, n = 96 | % |
|---|---|---|
| Altered mental status | 62 | 64.6 |
| Headache | 13 | 13.5 |
| Pupillary abnormalities | 7 | 7.3 |
| Speech alterations | 5 | 5.2 |
| Seizure | 5 | 5.2 |
| Hypogeusia/ageusia | 5 | 5.2 |
| Postural instability/gait disorders | 4 | 4.2 |
| Hyposmia/anosmia | 3 | 3.1 |
| Stroke | 2 | 2.1 |
| Hypoxic encephalopathy | 2 | 2.1 |
| Necrotizing encephalopathy | 1 | 1.0 |
| Sensory symptoms | 1 | 1.0 |
| Lower limb weakness | 1 | 1.0 |
Detection of SARS‐CoV‐2 RNA was not associated with the presence of neurological symptoms, gender, or history of cardiovascular risk factors (Tables 1 and 5). Instead, a significant difference emerged when considering age, because patients with SARS‐CoV‐2 RNA‐positive brain specimens were older than subjects with negative brain samples (median age: 71.5 vs. 66 years) (Table 5).
TABLE 5.
Comparisons of brain pathology findings with age, gender, and presence of cardiovascular risk factors in COVID‐19 patients
| Brain pathology findings | Age | Gender | Cardiovascular risk factors |
|---|---|---|---|
| SARS‐CoV‐2 RNA | <0.001 | 0.163 | 0.689 |
| SARS‐CoV‐2 proteins | 0.244 | 0.006 | 0.500 |
| Edema | 0.728 | 0.527 | 0.402 |
| Hypoxic‐ischemic lesions | 0.227 | 0.345 | <0.001 |
| Hemorrhagic lesions | 0.165 | 0.664 | 0.114 |
| Microgliosis | 0.003 | 0.611 | 0.906 |
| Astrogliosis | 0.027 | 0.659 | 0.751 |
| Inflammatory infiltrates | 0.009 | 0.780 | 0.659 |
Data are reported as p values, which were obtained using the Pearson χ2 test. Significant p values are bolded.
Identification of SARS‐CoV‐2 proteins was not related to the presence of neurological symptoms, age, or cardiovascular risk factors, whereas we found an association with gender (Tables 1 and 5). Brain samples positive for SARS‐CoV‐2 proteins were more frequently identified in females (23 out of 54, 42.6%) than in males (23 out of 106, 21.7%).
Presence of edema was not related to age, gender, or history of cardiovascular risk factors, but was associated with the presence of neurological symptoms (Tables 1 and 5). Subjects with edema more frequently had neurological manifestations.
Detection of hypoxic‐ischemic lesions was not associated with age or gender, but differed based on the presence of neurological symptoms and cardiovascular risk factors (Tables 1 and 5). In particular, neurological symptoms were more often reported in subjects with hypoxic‐ischemic lesions. Similarly, hypoxic‐ischemic lesions were more common in the patients’ group with cardiovascular risk factors (101 out of 201, 50.2%), with respect to subjects without cardiovascular risk factors (18 of 76, 23.7%).
Identification of hemorrhagic lesions did not differ with regard to the presence of neurological symptoms, age, gender, or history of cardiovascular risk factors (Tables 1 and 5).
Evidence of microgliosis or astrogliosis was not related to the occurrence of neurological symptoms, gender, or cardiovascular risk factors (Tables 1 and 5). Instead, these brain pathology findings were associated with patients' age, because subjects with microgliosis or astrogliosis were older than patients without these neuropathological alterations (median age: 71 vs. 64 years for microgliosis, 70 vs. 63 years for astrogliosis; Table 5).
Identification of inflammatory infiltrates was not related to gender or history of cardiovascular risk factors, but was associated with the presence of neurological symptoms and patients' age (Tables 1 and 5). Neurological symptoms were more often observed in individuals with inflammatory infiltrates. Moreover, subjects with inflammatory infiltrates were older than those without this neuropathological feature (median age: 71 vs. 64 years).
Comparisons of brain pathology findings based on presence or absence of SARS‐CoV‐2 RNA or proteins in COVID‐19 patients are shown in Tables 2 and 3, respectively. The only significant association was found between edema and presence of SARS‐CoV‐2 proteins (Table 3). In particular, edema was more common among brain specimens positive for SARS‐CoV‐2 proteins.
Several studies analyzed samples from different brain areas, whereas in other cases the sampling site was not specified. A quantitative summary of these available neuropathological data is depicted in Tables 6 and 7.
TABLE 6.
Detection rate of SARS‐CoV‐2 RNA and proteins in COVID‐19 patients in different brain areas
| Brain areas | SARS‐CoV‐2 RNA, n = 104 | SARS‐CoV‐2 proteins, n = 51 |
|---|---|---|
| Cerebrum | 29 (27.9%) | 17 (33.3%) |
| Cerebellum | 28 (26.9%) | 2 (3.9%) |
| Brainstem | 38 (36.5%) | 17 (33.3%) |
| Olfactory bulb/nerve | 27 (26.0%) | 4 (7.8%) |
| Not specified region | 26 (25.0%) | 17 (33.3%) |
Data are reported as number of patients (percent).
TABLE 7.
Detection rate of brain pathology findings in COVID‐19 patients in different brain areas
| Brain areas | Edema, n = 73 | Hypoxic‐ischemic lesions, n = 138 | Hemorrhagic lesions, n = 57 | Microgliosis, n = 147 | Astrogliosis, n = 130 | Inflammatory infiltrates, n = 144 |
|---|---|---|---|---|---|---|
| Cerebrum | 30 (41.1%) | 119 (86.2%) | 21 (36.8%) | 58 (39.5%) | 26 (20.0%) | 29 (20.1%) |
| Cerebellum | 8 (11.0%) | 68 (49.3%) | 10 (17.5%) | 45 (30.6%) | 10 (7.7%) | 11 (7.6%) |
| Brainstem | 22 (30.1%) | 51 (37.0%) | 16 (28.1%) | 70 (47.6%) | 33 (25.4%) | 24 (16.7%) |
| Olfactory bulb/nerve | 2 (2.7%) | 0 | 0 | 19 (12.9%) | 9 (6.9%) | 13 (9.0%) |
| Not specified region | 44 (60.3%) | 16 (11.6%) | 25 (43.9%) | 64 (43.5%) | 81 (62.3%) | 113 (78.5%) |
Data are reported as number of patients (percent).
DISCUSSION
An increasing number of studies have shown that SARS‐CoV‐2 infection can be associated with both peripheral and central nervous system impairment, which may contribute to worsen the prognosis in COVID‐19 patients [6, 7]. Evidence on the spectrum of neuropathological findings in subjects who died from COVID‐19 appeared late because of initial uncertainties about the neurotropism of SARS‐CoV‐2 and a major focus on lung pathology. Nevertheless, the understanding of the neuropathology related to SARS‐CoV‐2 infection is rapidly evolving and it is more and more crucial to gather insights into the pathogenic mechanisms responsible for neurological manifestations in COVID‐19 patients. To our knowledge, this is the first systematic review that quantitatively reports available data on brain pathology and neurological symptoms, aiming at exploring clinical correlates of neuropathological findings in a large cumulative sample of COVID‐19 patients. The results of statistical analyses provide interesting clues on the possible pathophysiology underlying the neurological involvement in subjects with COVID‐19.
Detection rates of SARS‐CoV‐2 RNA and proteins did not differ between COVID‐19 patients with versus those without neurological symptoms. This first finding is in line with the prevailing hypothesis that, in most subjects, neurological symptoms associated with COVID‐19 do not arise from direct cytopathic effects mediated by SARS‐CoV‐2. Such a view is also consistent with the results of studies showing that the severity of neurological symptoms or neuropathological alterations does not correlate with SARS‐CoV‐2 detection in brain specimens [19, 20]. Of relevance, the relatively low identification rates (about 42%) and the low levels of viral RNA cast doubt on the real presence of SARS‐CoV‐2 in the brain. It has been argued that detection of SARS‐CoV‐2 RNA in brain samples could derive from hematogenous viral RNA or viral contamination during different stages of the autopsy [20]. This suggestion is further corroborated by the even lower detection rates of SARS‐CoV‐2 proteins (about 28%) as well as of viral RNA when using in situ hybridization, which is a more reliable detection technique with respect to the most widely used RT‐PCR [20].
On the other hand, edema, hypoxic‐ischemic lesions, and inflammatory infiltrates were more frequently observed in brain specimens from COVID‐19 patients with neurological impairment as compared to subjects without neurological symptoms. This evidence would support a role of brain inflammatory reaction and hypoxic‐ischemic damage rather than neuronal viral invasion in determining the neurological involvement associated with SARS‐CoV‐2 infection.
More than 25% of COVID‐19 patients have shown varying degrees of brain edema, leading to flattened brain surface, widened gyri, narrowed sulci, and meningeal congestion with diffuse discoloration of the gray–white matter junction [6, 21]. Although the pathogenesis of brain edema remains to be elucidated, the presence of inflammatory cell infiltration surrounding the edematous tissues suggests that edema might result from a host‐specific inflammatory response [6, 21]. SARS‐CoV‐2 can induce an exaggerated immune‐mediated response to viral infection capable of damaging damage blood vessel walls and increasing vascular permeability in the brain [22].
Hypoxic‐ischemic lesions were found in brain specimens of about 41% of cases. This finding is not unexpected, considering that COVID‐19 patients may develop severe hypoxia due to respiratory failure or as a complication of protracted hypotension during cardiac arrest [23]. Hypoxia per se might cause a hypercoagulable condition leading to microthrombotic brain vessel occlusion and ischemic damage [24]. Direct activation of the coagulation cascade by a cytokine storm and endothelial dysfunction may also contribute to a procoagulant state in COVID‐19 [25]. Of interest, the evidence of megakaryocytes in cortical capillaries from COVID‐19 patients could play a major role in causing brain ischemic alterations by obstructing microvascular blood flow [26]. It is noteworthy that hypoxic‐ischemic lesions were more commonly reported in patients with cardiovascular risk factors. On the one hand, hypoxic ischemic lesions could be at least partly preexisting in some cases. On the other hand, microvascular changes due to cardiovascular risk factors, such as arterial hypertension or diabetes mellitus, could foster the occurrence of hypoxic‐ischemic lesions in COVID‐19 patients. This latter hypothesis is in keeping with the evidence of an increased risk of vascular events in subjects with COVID‐19 [27], and with findings of worse outcome of COVID‐19 when premorbid vascular risk factors and diseases are present [28].
T‐cell lymphocytic infiltrates were found in 44% of the brain samples from COVID‐19 patients. Again, both direct viral infection and host‐specific inflammatory response could be involved [19, 29, 30]. The second hypothesis may be corroborated by the finding of no association between detection rate of SARS‐CoV‐2 RNA or proteins and inflammatory infiltration in our analysis. This suggestion is further supported by the evidence that inflammatory cell infiltration similar to that of COVID‐19 can be encountered in patients with sepsis or systemic inflammation [29].
The cumulative examination of brain specimens revealed hemorrhagic lesions with different extension, from perivascular microhemorrhages to larger intracerebral and subarachnoid hemorrhages, in more than 20% of subjects with COVID‐19, in the absence of any association with neurological manifestations. The mechanisms responsible for COVID‐19 patients' susceptibility to develop intracranial hemorrhages remain unclear [31]. The tropism of SARS‐CoV‐2 toward the endothelial cells via their angiotensin‐converting enzyme II receptors could play a role [32, 33], also according to the evidence of viral particles within endothelial cells and accumulation of inflammatory cells leading to the death of endothelial cells [25]. Additional pathogenic mechanisms underlying cerebral hemorrhagic lesions associated with SARS‐CoV‐2 infection could include disseminated intravascular coagulation and concomitant anticoagulation therapy [34].
A variable degree of microgliosis and astrogliosis was seen in about half of the cases, often in association with T‐cell lymphocytic infiltrates, but without any association with the presence of neurological symptoms. It has been supposed that in COVID‐19 patients, microglial activation could be induced by increased levels of systemic cytokines, including interleukin‐6 and interferon‐γ [35, 36]. Microglial activation would allow the phagocytosis of dying neurons as described in hypoxic and systemic inflammatory conditions [37, 38]. As for microgliosis, activation of astrocytes represents an essential part of the response of the central nervous system to injury, being involved in mechanisms of neural protection and repair after damage of different etiologies [39]. As both microgliosis and astrogliosis occur in a variety of medical conditions, and critical illness can contribute to their induction, the causal connection to SARS‐CoV‐2 infection remains uncertain [19, 37]. Our findings showed no association between detection rate of SARS‐CoV‐2 RNA or proteins and microgliosis or astrogliosis, thus supporting the hypothesis that these neuropathological alterations may represent nonspecific responses of the brain. In line with this view, Deigendesch et al. [29] failed to detect differences when comparing the extent of microglial activation between subjects with COVID‐19 and patients who died from septic conditions.
Our analysis for different brain areas has highlighted that microgliosis, and to a lesser extent astrogliosis and inflammatory cell infiltration, were more frequent in the brainstem. Whether this is a consequence of an increased vulnerability to inflammatory stress of brainstem structures, or of a greater susceptibility to a direct cytopathic viral damage, remains to be ascertained [19]. Furthermore, we observed a greater prevalence of brain specimens featured by microgliosis, astrogliosis, and inflammatory infiltrates among older patients, whereas no association between these neuropathological findings and gender or cardiovascular risk factors was found. This finding may be not surprising considering that age is a well‐recognized negative prognostic factor for morbidity and mortality in COVID‐19 [40]. Also, the detection rate of SARS‐CoV‐2 RNA, but not of SARS‐CoV‐2 proteins, was higher in the older population, though RNA levels were always low and more likely compatible with contamination from blood viremia [37, 41]. It may be supposed that an increased presence of viral RNA in the blood within brain samples could be detected in older patients, who more frequently present a severe disease and higher viral load [42]. Moreover, age might correlate with the evidence of neurological manifestations, as supported by our finding that patients with COVID‐19–related neurological symptoms were older than subjects who did not shown any evidence of neurological impairment. Thus, age could represent a risk factor not only for COVID‐19 severity [40], but also for the onset of neurological symptoms associated with SARS‐CoV‐2 infection.
Gender differences in hospitalization and mortality rate of COVID‐19 patients have emerged since the beginning of the pandemic, with evidence that SARS‐CoV‐2 infection causes more severe symptoms and higher mortality among men [43, 44]. Differences in immune phenotypes between men and women are supposed to be involved [45]. An unexpected finding of our analysis was that detection rate of SARS‐CoV2 proteins was greater in brain specimens from women. In agreement, Mahallawi et al. [46] have shown that females have a higher SARS‐CoV‐2 viral load in the blood. Taking into account the observations in animal models that SARS‐CoV‐2 proteins can cross the blood–brain barrier [47], it may be speculated that a greater number of viral proteins could cross the blood–brain barrier in women given their higher SARS‐CoV‐2 viral load.
A strength of this review is that we followed a systematic approach, providing unprecedented quantitative neuropathological and clinical findings in COVID‐19 patients from extensive analysis of literature. However, several limitations need to be considered, many of which are related to the preliminary and descriptive nature of most of the included studies. Several articles analyzed single case reports or small case series, and appropriate age‐ and sex‐matched controls were lacking, thus limiting interpretation of the results. Additionally, discrepancies among studies may partly be explained by great variability in the sampling procedures and histological processing of brain specimens, differences in the site and extension of brain sampling, and heterogeneity of the enrolled subjects, making the comparison of findings less reliable. Furthermore, it is noteworthy that autopsy studies have the limit to refer to the most severe cases. Therefore, the neuropathological findings cannot be generalized to the entire population of COVID‐19 patients, who more often present less severe forms of disease. It is worth noting that the time elapsed from the onset of COVID‐19 and autopsy as well as between the onset of neurological symptoms and death may have influenced the detection rates of viral RNA or proteins and other neuropathological findings. Unfortunately, these pieces of information were not provided in the selected articles, thus preventing further elaboration on these issues. Hopefully, the cumulative findings from this review will stimulate the accurate collection of more detailed data on these specific aspects in future studies. Moreover, some of the articles included in this review failed to report patients' neurological symptoms, whereas the retrospective nature of the studies precluded the systematic use of validated and standardized tools to collect reliable clinical data, also including information on neurological manifestations. A separate analysis of neuropathological findings based on type of neurological involvement was hampered by the lack of detailed clinical information in a large number of the selected articles. In addition, almost all neurological symptoms or disorders in patients with available brain pathology refer to central nervous system impairment (Table 4), thus preventing any considerations about those subjects who reported peripheral neurological syndromes. Lastly, the very high number of articles retrieved with our search strategy may have caused mistakes in the exclusion process. To minimize such possibility, two authors independently analyzed the original list of articles to ensure data quality and accuracy.
In conclusion, although prevalence and meaning of neuropathological findings in patients who died from COVID‐9 should be interpreted with caution given the abovementioned limitations, the results of this systematic review shed light on the pathogenic underpinning of COVID‐19–related neurological complications. The evidence from brain pathology that edema, hypoxic‐ischemic lesions, inflammatory infiltrates, but not viral RNA or proteins, were associated with the presence of neurological symptoms in COVID‐19 patients corroborates the hypothesis that neurological impairment is likely due to brain inflammatory and hypoxic‐ischemic damage rather than to the controversial neurotropism of SARS‐CoV‐2. Moreover, older individuals could show a higher risk of neurological manifestations in the event of SARS‐CoV‐2 infection. Future studies are warranted to define the contribution of comorbidities, critical illness, treatments, genetic background, and immune status to the neuropathological findings in COVID‐19. Moreover, shared protocols for evaluation of brain specimens from COVID‐19 patients and accurate collection of clinical data will be necessary to confirm the present findings in larger patient cohorts and to provide more reliable information on the mechanisms of neurological impairment associated with SARS‐CoV‐2 infection. A global effort to collect clinical, instrumental, and neuropathological findings by means of multicenter studies is advisable to obtain an in‐depth characterization of pathophysiological correlates of both central and peripheral neurological disorders observed in COVID‐19.
CONFLICT OF INTERESTS
All authors declare no conflicts of interest regarding the content of this article.
AUTHOR CONTRIBUTIONS
Giuseppe Cosentino: conceptualization, methodology, supervision, writing–original draft preparation. Massimiliano Todisco: methodology, data curation, formal analysis, writing–original draft preparation. Noy Hota: data curation, formal analysis. Giovanni Della Porta: data curation, formal analysis. Patrizia Morbini: writing–review and editing. Cristina Tassorelli: writing–review and editing. Antonio Pisani: conceptualization, methodology, supervision, writing–review and editing.
ETHICAL STATEMENT
No ethical approval or informed consent was required given the nature of this study.
Supporting information
Appendix S1
Appendix S2
ACKNOWLEDGEMENT
Open Access Funding provided by Universita degli Studi di Pavia within the CRUI–CARE Agreement. [Correction added on 18 May 2022, after first online publication: CRUI funding statement has been added.]
Cosentino G, Todisco M, Hota N, et al. Neuropathological findings from COVID‐19 patients with neurological symptoms argue against a direct brain invasion of SARS‐CoV‐2: A critical systematic review. Eur J Neurol. 2021;28:3856–3865. 10.1111/ene.15045
G. Cosentino and M. Todisco contributed equally to this work.
Funding information
Open Access Funding provided by Universita degli Studi di Pavia within the CRUI–CARE Agreement.
WOA Institution: Universita degli Studi di Pavia.
Blended DEAL: CARE
DATA AVAILABILITY STATEMENT
All data supporting the findings of this study are available in this article.
REFERENCES
- 1. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med. 2020;382:2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolff D, Nee S, Hickey NS, Marschollek M. Risk factors for Covid‐19 severity and fatality: a structured literature review. Infection. 2021;49:15‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected With SARS‐CoV‐2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med. 2020;382:2268‐2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: a UK‐wide surveillance study. Lancet Psychiatry. 2020;7:875‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oliveira V, Seabra M, Rodrigues R, et al. Neuro‐COVID frequency and short‐term outcome in the Northern Portuguese population. Eur J Neurol. 2021;28:3360‐3368. 10.1111/ene.14874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Filosto M, Cotti Piccinelli S, Gazzina S, et al. Guillain‐Barré syndrome and COVID‐19: an observational multicentre study from two Italian hotspot regions. J Neurol Neurosurg Psychiatry. 2021;927:751‐756. [DOI] [PubMed] [Google Scholar]
- 10. Avenali M, Martinelli D, Todisco M, et al. Clinical and electrophysiological outcome measures of patients with post‐infectious neurological syndromes related to COVID‐19 treated with intensive neurorehabilitation. Front Neurol. 2021;12:643713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Natoli S, Oliveira V, Calabresi P, et al. Does SARS‐Cov‐2 invade the brain? Translational lessons from animal models. Eur J Neurol. 2020;27:1764‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Domingues RB, Mendes‐Correa MC, de Moura Leite FBV, et al. First case of SARS‐COV‐2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J Neurol. 2020;267:3154‐3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang YH, Jiang D, Huang JT. SARS‐CoV‐2 detected in cerebrospinal fluid by PCR in a case of COVID‐19 encephalitis. Brain Behav Immun. 2020;87:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. Int J Infect Dis. 2020;94:55‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wells GA, Shea B, O’Connell D, et al. The Newcastle‐Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Eur J Epidemiol. 2011;25:603‐605. [DOI] [PubMed] [Google Scholar]
- 18. Todisco M, Alfonsi E, Arceri S, et al. Isolated bulbar palsy after SARS‐CoV‐2 infection. Lancet Neurol. 2021;20:169‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID‐19 in Germany: a post‐mortem case series. Lancet Neurol. 2020;19:919‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thakur KT, Miller EH, Glendinning MD, et al. COVID‐19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain. 2021:awab148. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van den Enden AJM, van Gils L, Labout JAM, et al. Fulminant cerebral edema as a lethal manifestation of COVID‐19. Radiol Case Rep. 2020;15:1705‐1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li H, Liu L, Zhang D, et al. SARS‐CoV‐2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517‐1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kluge S, Janssens U, Welte T, et al. German recommendations for critically ill patients with COVID‐19. Med Klin Intensivmed Notfmed. 2020;115:111‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77‐83. [DOI] [PubMed] [Google Scholar]
- 25. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nauen DW, Hooper JE, Stewart CM, Solomon IH. Assessing brain capillaries in coronavirus disease 2019. JAMA Neurol. 2021;78:760‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scutelnic A, Heldner MR. Vascular events, vascular disease and vascular risk factors‐strongly intertwined with COVID‐19. Curr Treat Options Neurol. 2020;22:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Du H, Pan X, Liu N, et al. The effect of vascular risk factor burden on the severity of COVID‐19 illness, a retrospective cohort study. Respir Res. 2020;21:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deigendesch N, Sironi L, Kutza M, et al. Correlates of critical illness‐related encephalopathy predominate postmortem COVID‐19 neuropathology. Acta Neuropathol. 2020;140:583‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schurink B, Roos E, Radonic T, et al. Viral presence and immunopathology in patients with lethal COVID‐19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290‐e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fayed I, Pivazyan G, Conte AG, et al. Intracranial hemorrhage in critically ill patients hospitalized for COVID‐19. J Clin Neurosci. 2020;81:192‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID‐19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666‐1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharifi‐Razavi A, Karimi N, Rouhani N. COVID‐19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. 2020;35:100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Asakura H, Ogawa H. COVID‐19‐associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. 2021;113:45‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen X, Zhao B, Qu Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with Coronavirus Disease 2019. Clin Infect Dis. 2020;71:1937‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. EBioMedicine. 2020;55:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Al‐Dalahmah O, Thakur KT, Nordvig AS, et al. Neuronophagia and microglial nodules in a SARS‐CoV‐2 patient with cerebellar hemorrhage. Acta Neuropathol Commun. 2020;8:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15:209‐216. [DOI] [PubMed] [Google Scholar]
- 39. Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16:249‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Izcovich A, Ragusa MA, Tortosa F, et al. Prognostic factors for severity and mortality in patients infected with COVID‐19: a systematic review. PLoS One. 2020;15:e0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid‐19. N Engl J Med. 2020;383:989‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fajnzylber J, Regan J, Coxen K, et al. SARS‐CoV‐2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gebhard C, Regitz‐Zagrosek V, Neuhauser HK, et al. Impact of sex and gender on COVID‐19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meng Y, Wu P, Lu W, et al. Sex‐specific clinical characteristics and prognosis of coronavirus disease‐19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16:e1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID‐19 disease outcomes. Nature. 2020;588:315‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mahallawi WH, Alsamiri AD, Dabbour AF, et al. Association of viral load in SARS‐CoV‐2 patients with age and gender. Front Med. 2021;8:608215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rhea EM, Logsdon AF, Hansen KM, et al. The S1 protein of SARS‐CoV‐2 crosses the blood‐brain barrier in mice. Nat Neurosci. 2021;24:368‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Data Availability Statement
All data supporting the findings of this study are available in this article.


