Summary
Haemato‐oncological patients are at risk in case of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection. Currently, vaccination is the best‐evaluated preventive strategy. In the present study, we aimed to assess serological response, predictive markers, and safety of BNT162b2 in haemato‐oncological patients. A total of 259 haemato‐oncological patients were vaccinated with two 30 µg doses of BNT162b2 administered 21 days apart. Serological response was assessed by ELECSYS® Anti‐SARS‐CoV‐2‐S immunoassay before vaccination, and at 3 and 7 weeks after the first dose (T1, T2). Safety assessment was performed. At T2 spike protein receptor binding domain (S/RBD) antibodies were detected in 71·4% of haematological and in 94·5% of oncological patients (P < 0·001). Haematological patients receiving systemic treatment had a 14·2‐fold increased risk of non‐responding (95% confidence interval 3·2–63·3, P = 0·001). Subgroups of patients with lymphoma or chronic lymphocytic leukaemia were at highest risk of serological non‐response. Low immunoglobulin G (IgG) level, lymphocyte‐ and natural killer (NK)‐cell counts were significantly associated with poor serological response (P < 0·05). Vaccination was well tolerated with only 2·7% of patients reporting severe side‐effects. Patients with side‐effects developed a higher S/RBD‐antibody titre compared to patients without side‐effects (P = 0·038). Haematological patients under treatment were at highest risk of serological non‐response. Low lymphocytes, NK cells and IgG levels were found to be associated with serological non‐response. Serological response in oncological patients was encouraging. The use of BNT162b2 is safe in haemato‐oncological patients.
Keywords: COVID‐19, BNT162b2 mRNA vaccine, serological response, immune cells, chronic lymphocytic leukaemia
Introduction
The coronavirus disease 2019 (COVID‐19) has been responsible for >163 million infections worldwide, >3·39 million of which were fatal (2·1%), thus it continues to pose a global threat. 1
In daily clinical practice various risk groups have emerged to be at high risk of severe and prolonged disease courses and increased mortality, especially the group of haemato‐oncological patients with their multifactorial immune dysfunction caused by treatment and underlying disease. Previous data has shown a significant heterogeneity of risk of a severe clinical course of COVID‐19 between different cancer entities. Previous studies have shown that patients with haematological disease, lung carcinoma, carcinoma with pulmonary metastases or generally patients with advanced disease have a high COVID‐19‐associated mortality. 2 Prediction of developing severe COVID‐19 disease is hardly possible. Recently, a correlation between low levels of natural killer (NK) cells and severe COVID‐19 disease has been postulated. 3
Intensive research was conducted into the rapid vaccine development against severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). Since its approval in December 2020, the BNT162b2 mRNA COVID‐19 vaccine (BNT162b2) from Pfizer/BioNTech has been administered widely in the general population. 4 Efficacy and safety data in haemato‐oncological patients are still limited as they have not been included in clinical trials. An initial efficacy study in patients with chronic lymphocytic leukaemia (CLL) showed a limited serological response of 40%. A correlation between poor serological response and systemic treatment, known from established vaccines such as the influenza vaccine, has previously been demonstrated 5 and a possible booster effect of immune checkpoint inhibitors on vaccine response was discussed. 6 Safety data in patients with cancer under immune checkpoint inhibitors showed satisfactory outcomes. 7 However, comprehensive data on safety and efficacy in haemato‐oncological patients under different treatments and with regard to immune cell counts are still pending. Overall, many unanswered questions remain about the risk–benefit ratio of BNT162b2‐vaccination in patients with cancer and haematological malignancy.
Therefore, the aim of the present study was to investigate the serological response and safety of BNT162b2 in a cohort of haemato‐oncological patients. The impact of the immune cell counts on antibody formation was of particular interest, as a potential predictor. Finally, the effect of different treatments on serological response and safety was evaluated.
Patients and methods
Objectives, participants and oversight
At our centre, the Academic Teaching Hospital Feldkirch, high‐risk immunocompromised haemato‐oncological patients had the opportunity to voluntarily participate in two vaccination campaigns. The first campaign started on 10 January and ended on 6 March. The second campaign started on 28 February and ended on 28 March. During the campaigns 259 patients were vaccinated with BNT162b2 (COMIRNATY®) with a dosing interval of 21 days and a respective dose of 30 μg, except one patient receiving the vaccination 55 days apart due to intermittent autologous stem cell transplantation.
Patients had to be without signs of infection to be amenable for vaccination. Oncological treatment was continued as planned. The vaccine was applied into the deltoid muscle.
Demographic data were documented by chart review. In addition, the following laboratory data were collected: total immunoglobulin G (IgG), anti‐SARS‐CoV‐2‐antibodies against viral spike protein (S/RBD‐antibodies), neutrophils count, lymphocytes count, cluster of differentiation 4 (CD4+) count, cytotoxic T cell (CD8+) count and NK cell count. NK cells and lymphocytes were distinguished into two groups respectively, one below and one above the lowest reference value of our medical central laboratory (74 cell/µl for NK cells and 0·7 g/l for lymphocytes). IgG was divided into two groups (> or ≤ 500 mg/dl) in accordance with Herishanu et al. 5
A standardised safety questionnaire, in accordance with the registration study by Polack et al., 4 was used to evaluate local or systemic reactions within 7 days after vaccination during outpatient visits. The severity of the side‐effects was graded in accordance with the Common Terminology Criteria of Adverse Events (CTCAE) ranging from 1 to 5. 8
The ELECSYS® Anti‐SARS‐CoV‐2‐S immunoassay from Roche, for the quantitative in vitro determination of S/RBD‐antibodies to the SARS‐CoV‐2 spike (S) protein receptor binding domain (RBD) in human serum was utilised. Using a recombinant protein, which represents in a double antigen sandwich format, the RBD of the SARS‐CoV‐2‐S antigen. The assay allows the detection of high‐affinity antibodies to SARS‐CoV‐2. To identify those patients who had had prior contact with the virus and had undergone a silent infection, the presence of antibodies against SARS‐CoV‐2 nucleocapsid antigen (NC‐antibodies) was tested at baseline if patients showed positive S/RBD‐antibodies. Per definition, at values >0·82 binding activity units per millilitre (BAU/ml), S/RBD‐antibodies are detectable. The clinical sensitivity of the assay is 98·8% with a 95% confidence interval (95% CI) of 98·1–99·3% and a clinical specificity of 99·96% (95% CI 99·91–100%), the analytical specificity is 99·96% (95% CI 99·7–100%). The assay correlates particularly well with the vesicular stomatitis virus (VSV)‐based pseudo‐neutralisation assay with a positive predictive agreement of 92·3% (95% CI 63·97–99·81%). 9 Baseline laboratory assessment (T0) was documented if available. Serological response was assessed just before the second dose (T1) and 4–5 weeks after the second dose (T2) by determination of S/RBD‐antibodies.
During the observation range infections with SARS‐CoV‐2 or incidence of COVID‐19 disease, assessed via a positive SARS‐CoV‐2 polymerase chain reaction (PCR) test result, were documented. A PCR test was performed in cases of suspected SARS‐CoV‐2 infection (e.g. present respiratory or gastrointestinal infection symptoms). Furthermore, occurrence and cause of death was noted.
The present study was conducted in accordance with the Declaration of Helsinki of 1975 (revised 2013) and Good Clinical Practice. The study protocol was approved by the Institutional Review Board and the Ethics Committee of the Medical University of Innsbruck (EC No: 1088/2021).
Statistical evaluation
The main objective of this study was to assess safety and serological response of BNT162b2 vaccination in haemato‐oncological patients. Sample size was not pre‐specified. Baseline characteristics of included patients were described using percentages, means and standard deviations (SD). The S/RBD‐antibody titres are given as means (SDs) and medians [interquartile ranges (IQRs)] for the three time‐points assessed and compared between patient groups with analysis of variance (ANOVA) testing. Serological non‐responding was defined as no detectable S/RBD‐antibodies at T2. This outcome was described using two logistic regression analyses: first for all patients and second for patients with haematological disease only. Age, sex, tumour entities and therapy served as covariates for these analyses. Odds ratios (ORs) and their 95% CIs were estimated to predict the risk of serological non‐responding. In addition, IgG at baseline was evaluated in the same model as a potential predictor for serological responding. The role of baseline immune status expressed as low levels of neutrophils, lymphocytes, CD4+, CD8+ and NK cells was analysed with ANOVA and for categorical variables with chi‐square testing. Safety assessment was performed using cross‐tabulation with chi‐square testing and ANOVA testing for age differences. A two‐sided P < 0·05 was considered to indicate statistical significance. Statistical analyses were performed with the IBM Statistical Package for the Social Sciences (SPSS®), version 26 (IBM, Armonk, NY, USA).
Results
Demographic characteristics are shown in Table I. All patients were observed from the day of first vaccination with a median follow‐up period of 49 days. Five patients died during the study period, one died from COVID‐19 disease (time of infection was within 7 days after the first vaccination), the remaining four patients died from complications of the underlying disease. Patient flow is shown in Figure 1.
Table I.
Demographic characteristics of the patients.
| Characteristic | Value* |
|---|---|
| Number of patients | 259 |
| Gender, n (%) | |
| Female | 110 (42·5) |
| Male | 149 (57·5) |
| Age, years, mean (SD) | 65·1 (12·2) |
| Tumour entity, n (%) | |
| Solid | 136 (52·5) |
| Gastrointestinal cancer | 50 (36·8) |
| Breast cancer | 39 (28·7) |
| Lung cancer | 19 (14) |
| Others† | 28 (20·9) |
| Metastatic tumour status | 117 (86) |
| Haematological | 123 (47·5) |
| Multiple myeloma | 42 (34·1) |
| CLL, lymphoma and Waldenström macroglobulinaemia‡ | 47 (38·2) |
| AML/MDS/MPN§ | 34 (26·2) |
| SCT, n (%)¶ | 20 (16·3) |
| Time form SCT to first vaccination, months, median (IQR)‖ | 42·5 (11–109) |
| Therapy, n (%) | |
| Chemotherapy | 72 (27·8) |
| Immunotherapy | 27 (10·4) |
| Targeted therapy | 92 (35·5) |
| Close surveillance | 68 (26·3) |
AML, acute myeloid leukaemia; CLL, chronic lymphocytic leukaemia; IQR, interquartile range; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasia; SCT, stem cell transplantation; SD, standard deviation.
Percentages may not total 100 due to rounding.
This group comprises (in descending order): melanoma, sarcoma, neuroendocrine tumour, cancer of unknown primary, thymic carcinoma, adrenal carcinoma, and germ cell tumour.
This group comprises (in descending order): low‐grade non‐Hodgkin lymphoma [CLL, follicular lymphoma, hairy cell leukaemia, marginal zone lymphoma, mantle cell lymphoma, mucosa‐associated lymphoid tissue (MALT) lymphoma]; high‐grade non‐Hodgkin lymphoma (diffuse large B‐cell lymphoma), Hodgkin lymphoma, Waldenström macroglobulinaemia, Castleman disease, T‐cell lymphoma.
This group comprises (in descending order): MPN (chronic myeloid leukaemia, polycythaemia vera, essential thrombocythemia, primary myelofibrosis), AML, MDS.
18 patients with autologous SCT, two patients with allogeneic SCT.
Median time is given in months with IQR. In total, six patients received vaccination within 1 year after SCT and one patient received SCT between the two vaccinations.
Fig 1.
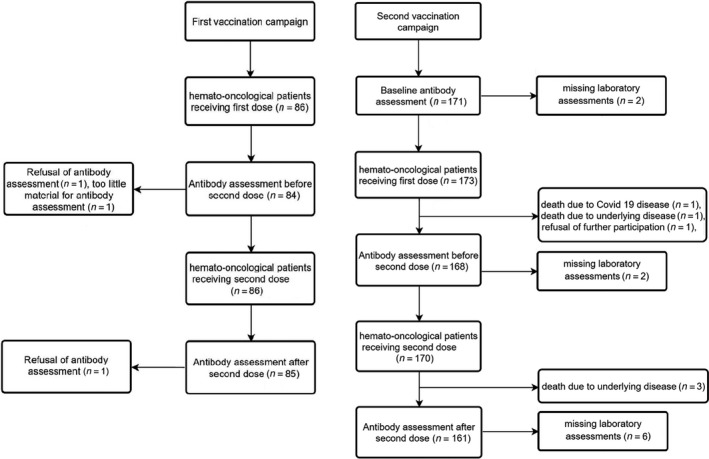
Patient flow in first and second vaccination campaign. The patient flowchart shows the two vaccination campaigns in our study. The number of patients at key target points such as date of vaccination and antibody determination are indicated. Time points and cause of dropouts are shown in the timeline.
Serological response
Baseline laboratory assessments were performed in 171 patients, 6·4% (eight oncological and three haematological patients) of which showed prior presence of S/RBD‐antibodies. Only one of these patients was known to have had COVID‐19 disease, the remaining had undergone a silent infection. All 11 patients had NC‐antibodies and showed a boosting effect after vaccination with a median (IQR) titre increase at 2572 (2572–25720) BAU/ml [mean (SD) 2426·12 (461·32) BAU/ml].
At T1 S/RBD‐antibodies were present in 43·4% of haematological patients and in 60% of oncological patients (P = 0·006). At T2, the rate of serological response was 71·4% in haematological patients and 94·5% in oncological patients (P < 0·001). Figure 2 shows S/RBD‐antibody titres at T2 regarding underlying disease and treatment. Figure 3 shows the S/RBD‐antibody titres at T0, T1 and T2. At T2, 41 patients did not respond to vaccination, 34 (82·9%) of them being haematological patients [nine patients had myeloma, 23 had lymphoma or CLL, one had acute myeloid leukaemia (AML) and one had myeloproliferative neoplasia]. Of the remaining seven oncological patients one had gastrointestinal cancer, four had breast cancer, one had melanoma and one had adrenal carcinoma.
Fig 2.
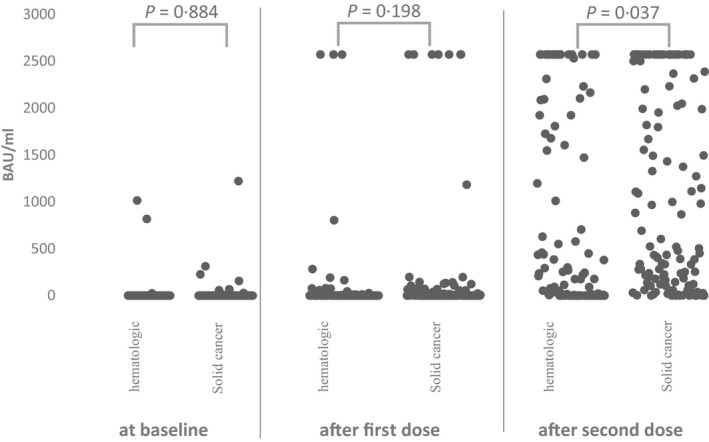
Scatter plot of spike protein receptor binding domain (S/RBD)‐antibody titres at baseline (T0), after fist dose (T1) and after second dose (T2) divided by tumour entity. The absolute antibody titre in binding activity units per millilitre (BAU/ml) at the time of baseline‐, T1‐ and T2‐analysis is shown descriptively by the scatter plot. Each time‐point was divided into the tumour entities (haematological malignancy or solid tumour). The differences between antibody titres at each time‐point were tested and indicated with P values using analysis of variance (ANOVA).
Fig 3.
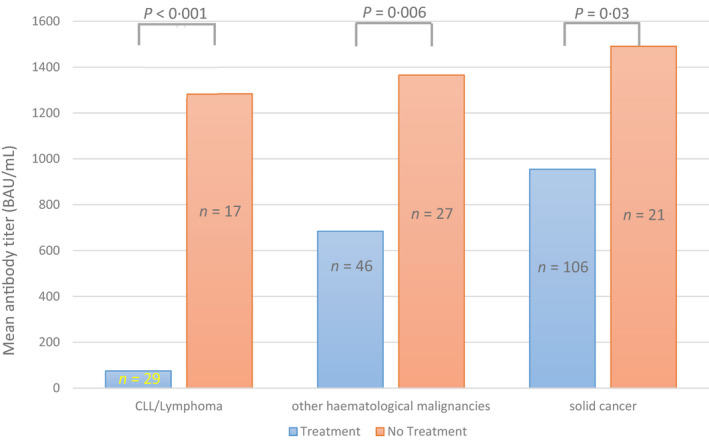
Serological response after second vaccine dose (T2) regarding treatment and tumour entity. Mean antibody titre in binding activity units per millilitre (BAU/ml) for T2 is shown in relation to tumour entities and treatment. Tumour entity was differentiated into patients with lymphoma or chronic lymphoid leukaemia (CLL), patients with other haematological malignancies, and patients with underlying solid cancer. These groups were divided into patients under treatment and patients under close surveillance (no treatment). The number of patients (n) in each group is shown. The indicated P value between treatment groups is reported using analysis of variance (ANOVA).
The haematological patients had a 6·4‐fold increased risk of serological non‐response (95% CI 2·7–15·4, P < 0·001). In the case of systemic treatment risk for non‐response in these patients it elevated up to a 14·2‐fold increase (95% CI 3·2–63·3, P = 0·001). Performing subgroup analyses, we found patients with lymphoma or CLL to have a 12‐fold increased risk of serological non‐response (95% CI 5·4–26·5, P < 0·001) compared to other haematological patients and risk increased 20‐fold in cases of systemic treatment (95% CI 3·5–114·4, P = 0·001). In a binary logistic model these results were adjusted for age and gender. Furthermore, we found patients with side‐effects to have significantly higher S/RBD‐antibody titres compared to patients without side‐effects (median S/RBD‐antibody titre of 440·02 BAU/ml compared to 209·57 BAU/ml, P = 0·038). These results are shown in Table II. Regarding therapy, 32 of the 34 haematological non‐responders (94·1%) received either chemotherapy, immunotherapy, or targeted therapy during the study period. All seven oncological non‐responders received systemic treatment during the study period. Looking more closely at seronegative patients receiving systemic treatment, we noted significantly decreased lymphocytes count and NK cell count (P for interaction treatment × serological non‐response = 0·037 and 0·018 respectively) in comparison to patients under close surveillance. There was no significant difference regarding CD4+ and CD8+ cell counts.
Table II.
Binary logistic regression model: risk factors for serological non‐response.
| Characteristic | Predictor | B | SE | OR (95% CI) | P * |
|---|---|---|---|---|---|
| Age | Per year | 0·027 | 0·015 | 1·0 (1·0–1·1) | 0·73 |
| Gender | Male (vs. female) | 0·115 | 0·385 | 1·1 (0·5–2·4) | 0·764 |
| Underlying disease | Haematological (vs. solid cancer) | 1·862 | 0·444 | 6·4 (2·7–15·4) | <0·001 |
| Therapy | ST (vs. CS) | 2·096 | 0·743 | 8·1 (1·9–34·9) | 0·005 |
| Subgroup analysis of haematological patients, adjusted for age and gender (CLL or lymphoma vs. others) | |||||
| Underlying disease | CLL, lymphoma (vs. others) | 2·483 | 0·405 | 12 (5·4–26·5) | <0·001 |
| Therapy | ST (vs. CS) | 2·994 | 0·891 | 20 (3·5–114·4) | 0·001 |
B, regression coefficient B; CLL, chronic lymphocytic leukaemia; CI, confidence interval; CS, close surveillance; OR, Odds ratio; SE, standard error; ST, systemic treatment.
Significance: P < 0·05.
In addition, our data showed a higher rate of serological response in patients with high IgG levels before vaccination. Divided into groups [IgG ≤550 mg/dl (n = 36), 14·2%; IgG >550 mg/dl (n = 218), 85·8%], patients with IgG >550 mg/dl had a 4·9‐fold improved chance of serological response (95% CI 2·1–11·6, P < 0·001, adjusted for age, gender, and tumour entity in the binary logistic model). An IgG ≤550 mg/dl correlated significantly with tumour entity. In detail, 80·6% of 36 patients with IgG ≤550 mg/dl had underlying haematological disease (P < 0·001). Furthermore, there was a correlation with treatment, as 88·8% of patients with an IgG of ≤550 mg/dl were under treatment (P = 0·025), of which 22 were under targeted therapy (P = 0·007). Results are presented in Figure 4.
Fig 4.
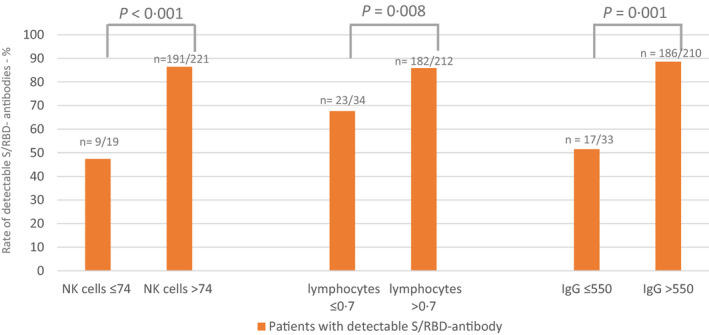
The spike protein receptor binding domain (S/RBD)‐antibody response with respect to natural killer (NK)‐cell count, lymphocytes count and immunoglobulin G (IgG) level. Patients with baseline NK‐cell count and lymphocytes count below and above the lower reference value (74 cell/µl for NK cells and 0·7 g/l for lymphocytes) are presented according to the percentage of patients with serological response (detectable S/RBD‐ antibodies >0·82 binding activity units per millilitre [BAU/ml]) using cross tabulation. Patients with IgG > and ≤550 mg/dl are also differentiated by percentage of serological response. The number of cases in the different groups are given in numbers (n).
Only one patient in our cohort (the patient was not seropositive at T2), had a positive SARS‐CoV‐2 PCR test result 42 days after the second vaccination and needed hospitalisation due to COVID‐19.
Safety results
Overall, 61·6% of patients reported side‐effects, whereby no significant difference according to prior seropositivity, tumour entity or therapy was found (all P > 0·05). In general, nine of 11 patients who were seropositive at baseline reported side‐effects, none of which were graded as severe.
After the first dose, adverse events occurred in 42% of patients, with pain at the injection site the most reported, followed by fatigue. Severe headache and severe general muscle pain were reported once after the first dose. After the second vaccination, adverse events were documented in 41·7% of patients. The most frequent one was fatigue, followed by pain at the injection site. Fever occurred more frequently after the second dose compared to after the first dose (6·7% compared to 0·4%, P < 0·001). Side‐effect rates are shown in detail in Figure 5.
Fig 5.
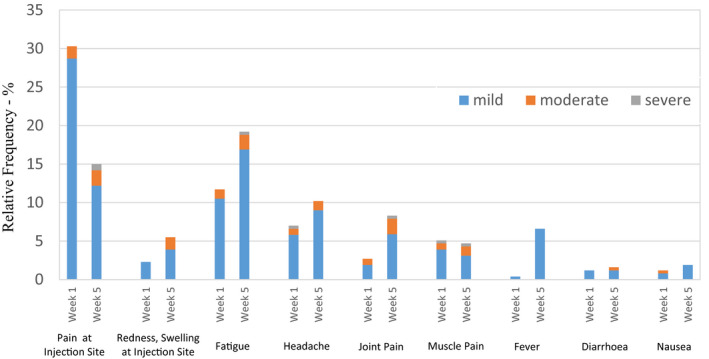
Occurrence of local and systemic side‐effects (within 7 days of vaccination). Relative incidence of local and systemic adverse events are expressed as percentages, at 1 week and 5 weeks after the first dose. Thus, side‐effects for the 7 days following each dose are displayed and differentiated by severity using the Common Terminology Criteria of Adverse Events (CTCAE). No Grade IV or V adverse reactions were reported.
Women reported significantly more side‐effects after the second partial vaccination than men (55·1% compared to 33·6%, P < 0·001). Apart from this, no other severe side‐effect was recorded, no thrombotic event or anaphylactic reaction occurred and no inpatient treatment was required due to an adverse event.
Overall, younger age showed to be significantly associated with the occurrence of adverse events. Patients with side‐effects were significantly younger than patients without (62·77 vs. 68·54 years, P < 0·001).
Discussion
In the present study, we show serological SARS‐CoV‐2 antibody response, potential predictive markers of response and safety in haemato‐oncological patients. In our present patient cohort, vaccination with BNT162b2 was safe. In detail, our present study indicates a substantially poor serological response‐rate of 71·4% in patients with underlying haematological disease. These patients have a 6·4‐fold increased risk of not developing S/RBD antibodies, which increases under treatment up to a 14·2‐fold risk. Among haematological patients, patients with CLL and lymphoma emerged as those at highest risk of non‐response independent of treatment. However, in the case of systemic treatment, the risk of serological non‐response further increases up to 20‐fold. Our present findings are consistent with the recently published results of a meta‐analysis by Vijenthira et al. 10 where patients with underlying B‐cell lymphoma receiving anti‐CD20 therapy were poor vaccination responders considering their seroconversion rates after administration of the pandemic influenza vaccine. This is also supported by the data of Ghione et al. 11 who reported a poor response rate after COVID‐19 vaccination in patients treated with anti‐CD20 therapy. In their study, the vaccination response depended significantly on the time interval between vaccination and last anti‐CD20 therapy. The data of Herishanu et al. 5 support our present findings as well, although their work compared haematological patients with healthy participants. Bird et al. 12 found patients with myeloma receiving treatment at the time of vaccination to have lower response rates, which is consistent with our present results of decreased serological responses in haematological patients under systemic treatment. In the past, data of established virus vaccines like pneumococcal polysaccharide vaccine (PPSV23), hepatitis B vaccine and seasonal influenza vaccine already showed a reduced immunogenicity in patients with haematological malignancies. 13 , 14 , 15 , 16
Immunological dysfunction is known in patients with CLL and myeloma. This is either caused by innate immunodeficiency or induced by treatment leading to a lower rate of immunogenicity after vaccination. 12 , 17 , 18 , 19 , 20 A correlation between serological response and high NK‐cell count has already been described in pneumococcal polysaccharide vaccine (PPSV23). 21 The correlation between reduced antibody response in patients under treatment as well as low NK‐cell and lymphocytes counts found in our present study could be interpreted as the immunosuppressive effect of systemic treatment used, especially in haematological diseases. This is in line with previous data indicating an impact of NK cells on severe course of COVID‐19 disease. 3 Thus, NK cells and lymphocytes played an essential role in the immune response of COVID‐19 vaccination in our present cohort. Furthermore, the impact of a high IgG level (>550 mg/dl), which improves response 4·9‐fold, could be interpreted as a surrogate for the immune status of the patient. Therefore, measurement of IgG may be pivotal for the further management of COVID‐19 vaccination in haemato‐oncological patients.
In contrast to haematological patients, a favourable serological response rate of 94·5% can be recorded in patients with solid‐organ tumours, which is in line with the general population. 4 These data are supported by the findings of Massarweh et al. 22 who presented a serological response of 90% after BNT162b2 vaccination in an oncological cohort. Both haematological and oncological patients experience an immunosuppressive effect in the case of systemic treatment. Differences in serological response after vaccination for individual agents have been described, e.g. poor vaccination‐response in patients treated with CD38 monoclonal antibodies. 20 In our present cohort, systemic treatment was differentiated into three groups (immunotherapy, chemotherapy and targeted therapy) to obtain a sufficient number of patients in each group. No significant difference in serological response could be detected between these groups. This is in line with Bird et al. 12 However, haematological malignancies themselves lead to immunological dysfunction and thus an enhanced immunosuppression in those patients results in poorer serological response‐rates. 12 , 20
In general, seropositivity after the first dose was low in both the haematological and oncological patients. Nevertheless, a significant difference in favour of oncological patients was noted after one dose of BNT162b2 (serological response rate of 60% vs. 43·4%). These results are supported by the findings of Monin et al., 23 which showed a poor seroconversion rate after single‐dose vaccination in patients with cancer and particularly in those with haematological disease. Therefore, it is key to receive the recommended two doses of of BNT162b2 vaccine.
In our present patients with pre‐existing S/RBD‐antibodies a booster effect was shown already after the first vaccination dose. The side‐effect rate was higher in these seropositive patients, whereas no severe events occurred. This supports our approach of vaccinating patients regardless of detectable S/RBD‐antibodies before vaccination, aiming for a higher antibody response.
Our present study found lower side‐effect rates in haemato‐oncological patients compared to the general population represented by the reactogenicity subset in the registration study. However, the higher mean age in our present cohort has to be noted, as younger age is known to significantly correlate with higher side‐effect rates. 4 In addition, habituation to treatment induced side‐effects and disease‐related symptoms must be considered in haemato‐oncological patients, which can lead to a worse ability for side‐effect assignment. Overall, we found primarily mild local and systemic side‐effects after vaccination with BNT162b2. Only 2·3% of patients reported severe side‐effects. The data of short‐term safety reported by Waissengrin et al. 7 support our present results. In contrast to Polack et al., 4 no hospitalisation due to adverse events was necessary in our present cohort.
Furthermore, no differences in side‐effect or serological response rates were found regarding different systemic treatments. This is supported by Waissengrin et al. 7 who reported a good safety profile of BNT162b2 in patients treated with immune checkpoint inhibitors. Therefore, we can assume that the use of BNT162b2 is safe in haemato‐oncological patients regardless of oncological treatment.
Moreover, our present data show an association between side‐effect rate and S/RBD‐antibody titre level. This seems plausible, knowing that the inflammatory and immunological response following vaccination manifests in reactogenicity and thus is a normal sign of the developing immune response. 19
Certain limitations of our present study must be acknowledged such as the retrospective setting, the relatively small number of patients and baseline laboratory determination of S/RBD‐antibodies that was not available for all patients. Furthermore, no regular PCR‐based screening was performed in asymptomatic patients to potentially recognise silent SARS‐CoV‐2‐infections. Thus, we are not able to rule out a hidden booster effect of silent infections. The evaluation of safety and vaccination response was carried out over a limited period. We will closely follow‐up our patients to evaluate long‐term side‐effects of BNT162b2 and will determine S/RBD‐antibodies on a regular basis.
Conclusion
The serological response rate is low in haematological patients and decreases even more in the case of systemic treatment. Patients with CLL and lymphoma receiving systemic treatment are at the highest risk of not developing S/RBD‐antibodies. In these high‐risk patients’ routine evaluation of S/RBD‐antibody titres after vaccination is urgently needed to recommend, if necessary, rigorously maintaining the safety measures for patients with no antibody response. Our preliminary data suggest IgG level, lymphocyte and NK‐cell count as potential predictors for serological antibody response. In contrast, the response rate in oncological patients is comparable to the general population. Finally, the use of BNT162b2 is safe in patients with underlying haematological or oncological disease regardless of treatment regimen.
Conflict of interest
There are no conflict of interests with the present study.
Data availability statement
The primary original data can be requested from the corresponding author.
References
- 1. Johns Hopkins University Coronavirus Resource Center . COVID‐19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University; 2020. Available from: https://coronavirus.jhu.edu/map.html.
- 2. Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS‐CoV‐2: A multicenter study during the COVID‐19 outbreak. Cancer Discov. 2020;10:783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kreutmair S, Unger S, Núñez NG, Ingelfinger F, Alberti C, De Feo D. Distinct immunological signatures discriminate severe COVID‐19 from non‐SARS‐CoV‐2‐driven critical pneumonia. Immunity. 2021;54:1578–93.e5. 10.1016/j.immuni.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Korompoki E, Gavriatopoulou M, Kontoyiannis DP. COVID‐19 vaccines in patients with cancer—A welcome addition, but there is need for optimization. JAMA Oncol. 2021. Online ahead of print. 10.1001/jamaoncol.2021.1218 [DOI] [PubMed] [Google Scholar]
- 7. Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short‐term safety of the BNT162b2 mRNA COVID‐19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. United States Department of Health and Human Services . Common terminology criteria for adverse events (CTCAE); 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
- 9. Roche Deutschland Holding GmbH . Elecsys anti‐SARS‐CoV‐2‐S; 2021. Available from: https://www.roche.de/diagnostik‐produkte/produktkatalog/tests‐parameter/elecsys‐anti‐sars‐cov‐2‐s/.
- 10. Vijenthira A, Gong I, Betschel SD, Cheung M, Hicks LK. Vaccine response following anti‐CD20 therapy: A systematic review and meta‐analysis of 905 patients. Blood Adv. 2021;5:2624–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghione P, Gu JJ, Attwood K, Torka P, Goel S, Sundaram S, et al. Impaired humoral responses to COVID‐19 vaccination in patients with lymphoma receiving B‐cell directed therapies. Blood. 2021. Online ahead of print. 10.1182/blood.2021012443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bird S, Panopoulou A, Shea RL, Tsui M, Saso R, Sud A, et al. Response to first vaccination against SARS‐CoV‐2 in patients with multiple myeloma. Lancet Haematol. 2021;8:e389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartkamp A, Mulder AH, Rijkers GT, van Velzen‐Blad H, Biesma DH. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B‐cell chronic lymphocytic leukaemia. Vaccine. 2001;19:1671–7. [DOI] [PubMed] [Google Scholar]
- 14. Svensson T, Kättström M, Hammarlund Y, Roth D, Andersson PO, Svensson M, et al. Pneumococcal conjugate vaccine triggers a better immune response than pneumococcal polysaccharide vaccine in patients with chronic lymphocytic leukemia A randomized study by the Swedish CLL group. Vaccine. 2018;36:3701–7. [DOI] [PubMed] [Google Scholar]
- 15. Pleyer C, Ali MA, Cohen JI, Tian X, Soto S, Ahn IE, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bosaeed M, Kumar D. Seasonal influenza vaccine in immunocompromised persons. Hum Vaccin Immunother. 2018;14:1311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griggio V, Perutelli F, Salvetti C, Boccellato E, Boccadoro M, Vitale C, et al. Immune dysfunctions and immune‐based therapeutic interventions in chronic lymphocytic leukemia. Front Immunol. 2020;11:594556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beck CR, McKenzie BC, Hashim AB, Harris RC, Zanuzdana A, Agboado G, et al. Influenza vaccination for immunocompromised patients: summary of a systematic review and meta‐analysis. Influenza Other Respir Viruses. 2013;7(Suppl 2):72–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hervé C, Laupèze B, Del Giudice G , Didierlaurent AM, Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pimpinelli F, Marchesi F, Piaggio G, Giannarelli D, Papa E, Falcucci P, et al. Fifth‐week immunogenicity and safety of anti‐SARS‐CoV‐2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol. 2021;14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miyasaka T, Aoyagi T, Uchiyama B, Oishi K, Nakayama T, Kinjo Y, et al. A possible relationship of natural killer T cells with humoral immune response to 23‐valent pneumococcal polysaccharide vaccine in clinical settings. Vaccine. 2012;30:3304–10. [DOI] [PubMed] [Google Scholar]
- 22. Massarweh A, Eliakim‐Raz N, Stemmer A, Levy‐Barda A, Yust‐Katz S, Zer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS‐CoV‐2 in patients undergoing treatment for cancer. JAMA Oncol. 2021. e212155. Online ahead of print. 10.1001/jamaoncol.2021.2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Monin L, Laing AG, Muñoz‐Ruiz M, McKenzie DR, del Molino del Barrio I, Alaguthurai T, et al. Safety and immunogenicity of one versus two doses of the COVID‐19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary original data can be requested from the corresponding author.


