Abstract
Aim
Coronavirus disease (COVID‐19) is characterized by pneumonia with secondary damage to multiple organs including the liver. Liver injury (elevated alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) often correlates with disease severity in COVID‐19 patients. The aim of this study is to identify pathological microthrombi in COVID‐19 patient livers by correlating their morphology with liver injury, and examine hyperfibrinogenemia and von Willebrand factor (vWF) as mechanisms of their formation.
Methods
Forty‐three post‐mortem liver biopsy samples from COVID‐19 patients were obtained from Papa Giovanni XXIII Hospital in Bergamo, Italy. Three morphological features of microthrombosis (sinusoidal erythrocyte aggregation [SEA], platelet microthrombi [PMT], and fibrous thrombi) were evaluated.
Results
We found liver sinusoidal microthrombosis in 23 COVID‐19 patients (53%) was associated with a higher serum ALT and AST level compared to those without (ALT: 10‐fold, p = 0.04; AST: 11‐fold, p = 0.08). Of 43 livers, PMT and SEA were observed in 14 (33%) and 19 (44%) cases, respectively. Fibrous thrombi were not observed. Platelet microthrombi were associated with increased ALT (p < 0.01), whereas SEA was not (p = 0.73). In COVID‐19 livers, strong vWF staining in liver sinusoidal endothelial cells was associated with significantly increased platelet adhesion (1.7‐fold, p = 0.0016), compared to those with weak sinusoidal vWF (2‐fold, p < 0.0001). Sinusoidal erythrocyte aggregation in 19 (83%) liver samples was mainly seen in zone 2. Livers with SEA had significantly higher fibrinogen (1.6‐fold, p = 0.031) compared to those without SEA in COVID‐19 patients.
Conclusions
Liver PMT is a pathologically important thrombosis associated with liver injury in COVID‐19, while SEA is a unique morphological feature of COVID‐19 patient livers. Sinusoidal vWF and hyperfibrinogenemia could contribute to PMT and SEA formation.
Keywords: endotheliopathy, fibrinogen, neutrophil, platelet, SARS‐CoV‐2 virus, vWF
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- COVID‐19
coronavirus disease 19
- DIC
disseminated intravascular coagulation
- IL‐6
interleukin‐6
- LSEC
liver sinusoidal endothelial cell
- PBS
phosphate‐buffered saline
- PMT
platelet microthrombi
- PTAH
phosphotungstic acid hematoxylin
- SARS‐CoV‐2
sever acute respiratory syndrome coronavirus 2
- SEA
sinusoidal erythrocyte aggregation
- SEM
standard error of the mean
- SOS
sinusoidal obstruction syndrome
- vWF
von Willebrand factor
INTRODUCTION
Severe acute respiratory syndrome CoV‐2 infection causes excessive levels of pro‐inflammatory cytokines and pneumonia with secondary impacts on multiple organs, including the liver. Although respiratory failure is the primary cause of morbidity and mortality in COVID‐19, liver injury (elevated serum ALT and AST) is common, and often correlates with severity of disease.1 Tsutsumi et al.2 reported that coagulopathy is associated with liver injury in patients with COVID‐19.
Several groups have reported liver sinusoidal microthrombosis with unique histological features in COVID‐19 patients.3, 4, 5, 6 Sonzogni et al. reported liver sinusoidal thrombosis in 13 of 48 patients with COVID‐19 (27%). In this study, sinusoids with enlarged lumens occupied by erythrocytes mixed with lymphocytes and granulocytes were considered to have thrombosis (i.e. SEA).3 Lagana et al. reported liver sinusoidal thrombosis with differing histology (fibrous thrombus) in 6 of 40 post‐mortem liver tissues from COVID‐19 patients (15%).4 Zhao et al. reported a third morphology of liver sinusoidal thrombosis in 12 of 17 autopsy cases (70%) in COVID‐19, CD61+ platelets aggregated in the sinusoids defined as PMT.5 Similarly, the presence of PMT was reported in a liver biopsy from a living COVID‐19 patient by Fiel et al.6 Collectively, these studies showed the presence of sinusoidal microthrombi in 15%–70% of patients, leaving uncertainty about their true frequency and significance. Here we compare different morphologies of microthrombi (SEA, PMT, and fibrous thrombi) within one set of patients and correlate with liver injury. We also explore hyperfibrinogenemia and elevated vWF as mechanisms of microthrombosis in the liver.
METHODS
Materials
Post‐mortem liver biopsy samples from COVID‐19 patients were obtained from Drs. Aurelio Sonzogni and Lisa Licini from Papa Giovanni XXIII Hospital in Bergamo, Italy. As reported previously,3 autopsies were performed after a median time of 6 h from death. Tissues were fixed in 10% buffered formalin for more than 48 h and embedded in paraffin. The patients consisted of 30 men and 13 women. The mean age was 72 ± 12 years (range, 32–90 years). All patients did not have previous history of liver disease and did not develop clinical signs or symptoms of liver failure. Laboratory data was obtained from clinical charts: we analyzed the highest value of serum AST and ALT during the hospital stay. All patients were negative for hepatitis C virus antibodies; one patient was hepatitis B surface antigen positive/hepatitis B virus DNA negative. The liver tissues from 12 healthy adults were used as controls (five cases from Johns Hopkins University Hospital [immunohistochemistry experiments] and six cases from Yale University [immunofluorescence experiments]). Our studies were approved by the institutional review boards of Yale University and Johns Hopkins University, and received preliminary ethical approval followed by a resolution by the Hospital General Officer of Papa Giovanni XXIII Hospital, and were carried out in accordance with the Declaration of Helsinki.
Histological examination
Hematoxylin–eosin staining, PTAH staining, and immunohistochemical staining were carried out using paraffin‐embedded liver specimens. For immunohistochemical staining, we used an automated immunostainer (intelliPATH FLX; Biocare Medical) and the following antibodies: polyclonal rabbit anti‐human vWF antibody (A0082; dilution 1:4000; Dako), monoclonal mouse anti‐human CD61 antibody (M0753; dilution 1:20; Dako), and polyclonal rabbit anti‐human fibrinogen (ab34269; dilution 1:50; Abcam) (Table S1). Pathologists with experience in COVID‐19 liver histopathology examined histological findings blindly (A.S. and R.K.). CD61 is a specific marker of platelets and megakaryocytes. In normal liver, some LSECs express vWF, and its intensity is weaker or comparable to those of vascular endothelial cells.7 von Willebrand factor‐weak‐positive LSECs are defined as its expression being similar or weaker than those in vascular endothelial cells; vWF‐strong‐positive LSECs are defined as its expression being stronger than those in vascular endothelial cells. The vWF positive area and CD61 positive area in each specimen were measured in four randomly selected regions with ×200 power field in an unbiased fashion using Fiji (ImageJ). The average number of neutrophils in the sinusoid at four randomly selected sinusoidal regions with ×200 power field was evaluated to assess neutrophil accumulation in the sinusoids.
Immunofluorescence
We detected vWF, platelets, and fibrinogen by immunofluorescent labeling of paraffin‐embedded human liver specimens. Paraffin sections were deparaffinized with xylene and dehydrated with graded ethanol. For immunofluorescence with vWF and CD41 (integrin αIIb), we undertook heat‐induced epitope retrieval with citrate buffer (0.1 M citric acid and 0.1 M sodium citrate). CD41 is a specific marker of platelets and megakaryocytes. For immunofluorescence for fibrinogen, we used proteinase K‐induced epitope retrieval. The sections were incubated with blocking buffer (5% normal donkey serum and 0.3% Triton X‐100 in PBS) for 1 h and incubated with primary antibodies overnight at 4°C. The following antibodies were used: polyclonal rabbit anti‐human vWF antibody (A0082, dilution 1:200; Dako), monoclonal mouse anti‐human CD41 (integrin αIIb) antibody (sc‐365938, dilution 1:100; Santa Cruz Biotechnology), and polyclonal rabbit anti‐human fibrinogen antibody (A0080, dilution 1:100; Dako) (Table S1). After washing three times with PBS, each for 5 min, primary antibodies were detected using Alexa Fluor 488‐conjugated donkey anti‐rabbit IgG, Alexa Fluor 647‐conjugated or Fluor 555‐conjugated donkey anti‐mouse IgG, or Alexa Fluor 647‐conjugated donkey anti‐rabbit IgG secondary antibody (dilution 1:300; Invitrogen), respectively. All samples were mounted with Fluoroshield containing DAPI (Sigma‐Aldrich) and observed with a fluorescence microscope (Zeiss Observer Z1; Zeiss). The fibrinogen positive area in each specimen was measured in six randomly selected regions with ×200 power field in an unbiased fashion using Fiji (ImageJ).
Statistical analysis
All data are shown as the mean ± SEM. Statistical significance was determined by one‐way ANOVA with Tukey's test or Welch's t‐test as appropriate based on normality and variance. The χ2 test was used for categorical variables. Statistical analysis was carried out using GraphPad Prism 9 (GraphPad) or the JMP software package (release 15.0; SAS Institute). Significance was defined as *p < 0.05, **p < 0.01, ***p < 0.001.
RESULTS AND DISCUSSION
For comparison to previous studies and utilizing hematoxylin−eosin and PTAH staining, we defined microthrombi containing predominantly erythrocytes as SEA (Figures 1a and S1) and microthrombi composed predominantly of fibrin as fibrous thrombi. Platelet microthrombi is defined as a predominance of aggregated platelets determined by immunolabeling with CD61 or CD41 (platelet markers) (Figures 1b, S2 and 3).
FIGURE 1.
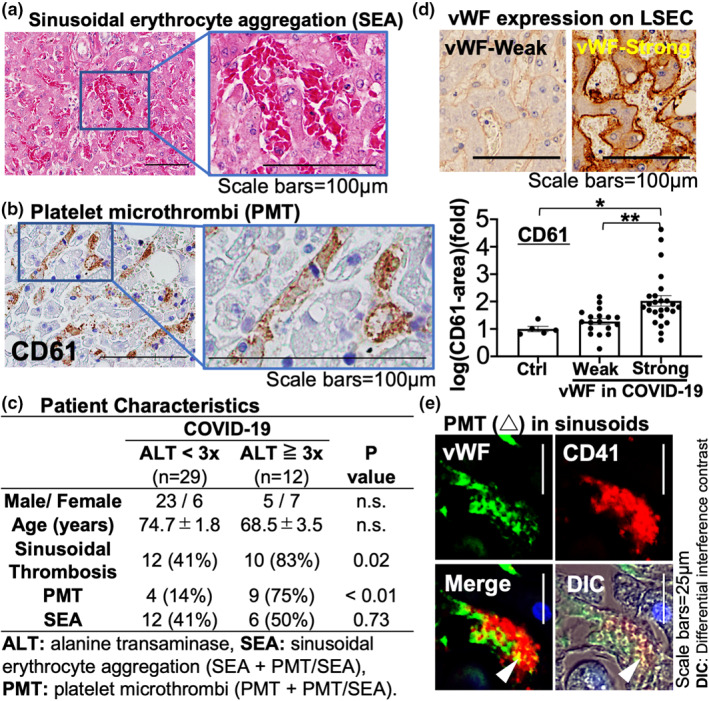
Characteristics of liver sinusoidal microthrombosis in coronavirus disease (COVID‐19) patients. (a) Hematoxylin–eosin staining showing sinusoidal erythrocyte aggregation (SEA). (b) Immunolabeling of CD61 showing platelet microthrombi (PMT). (c) Summary of sinusoidal microthrombosis in livers from 43 COVID‐19 patients with or without liver injury, defined as alanine aminotransferase (ALT) greater than three times the upper limit of normal. (d) von Willebrand factor (vWF) immunolabeling in livers from COVID‐19 patients and its quantification: control (Ctrl; n = 5) versus vWF‐strong liver sinusoidal endothelial cells (LSECs) (n = 25) versus vWF‐weak LSECs (n = 18). *p < 0.05, **p < 0.01. (e) PMT (arrowhead), CD41+ platelets (red) are attached on the vWF‐expressing LSECs (green). DIC, differential interference contrast; n.s., not significant
We found liver sinusoidal microthrombosis in 23 COVID‐19 patients (53%) which was associated with a higher serum ALT and AST level compared to those without (ALT: 10‐fold, p = 0.04, AST: 11‐fold, p = 0.08) (Table 1). In the 35 of COVID‐19 patients that could be evaluated, six patients (17%) met the DIC criteria of the International Society on Thrombosis and Haemostasis. Three of these six patients had sinusoidal microthrombus. However, the serum ALT value and frequency of sinusoidal microthrombi in the COVID‐19 patients with DIC did not increase compared to those without. Of 43 livers from COVID‐19 patients, PMT and SEA were observed in 14 (33%) and 19 (44%) of cases, respectively (Table 2). Fibrous thrombi were not observed in our cases (Figure S1). There was a significant increase in the presence of steatosis (p = 0.02) in livers with PMT and a trend toward increased presence of intrasinusoidal neutrophils in livers with PMT (p = 0.06) or SEA (p = 0.08), compared to control livers (Table 2).
TABLE 1.
Relationship between liver sinusoidal microthrombosis and liver injury in patients with coronavirus disease (COVID‐19)
| COVID‐19 | p‐value | ||
|---|---|---|---|
| Sinusoidal microthrombosis | |||
| None | Present | ||
| (n = 20) | (n = 23) | ||
| Male/female | 16/4 | 14/9 | n.s. |
| Age (years) | 74.1 ± 1.4 | 69.7 ± 3.2 | n.s. |
| Laboratory | |||
| AST (U/L) | 85.6 ± 4.3 | 945.6 ± 465.6 | 0.08 |
| ALT (U/L) | 63.8 ± 5.5 | 655.7 ± 275.8 | 0.04 |
| Histology | |||
| Steatosis (>5%) | 7 (35%) | 13 (57%) | n.s. |
| Advanced fibrosis | 0 | 1 (4%) | n.s. |
| Neutrophil accumulation | 9.1 ± 0.9 | 11.4 ± 1 | 0.09 |
| Portal inflammation | 6 (30%) | 4 (17%) | n.s. |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; n.s., not significant.
TABLE 2.
Summary for type of liver sinusoidal microthrombosis in patients with coronavirus disease (COVID‐19)
| COVID‐19 | |||||
|---|---|---|---|---|---|
| Sinusoidal microthrombosis | |||||
| Nonea | PMTb | SEAc | p‐value | ||
| (n = 20) | (n = 14) | (n = 19) | a versus b | a versus c | |
| Male/female | 16/4 | 10/4 | 11/8 | n.s. | n.s. |
| Age (years) | 74.1 ± 1.4 | 69.7 ± 3.2 | 68.9 ± 3.9 | n.s. | n.s. |
| Laboratory | |||||
| AST (U/L) | 85.6 ± 4.3 | 1597.3 ± 774.3 | 856.3 ± 521.3 | 0.08 | n.s. |
| ALT (U/L) | 63.8 ± 5.5 | 655.7 ± 275.8 | 615.9 ± 1318.1 | 0.04 | 0.09 |
| Histology | |||||
| Steatosis (>5%) | 7 (35%) | 11 (79%) | 10 (53%) | 0.02 | n.s. |
| Advanced fibrosis | 0 (0%) | 0 (0%) | 1 (5%) | n.s. | n.s. |
| Neutrophil accumulation | 9.1 ± 0.9 | 12.6 ± 1.5 | 11.7 ± 1.1 | 0.06 | 0.08 |
| Portal inflammation | 6 (30%) | 2 (14%) | 4 (21%) | n.s. | n.s. |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; COVID‐19, coronavirus disease 19; PMT, platelet microthrombi; SEA, sinusoidal erythrocyte aggregation.
(None): Without sinusoidal microthrombosis
(PMT): PMT + PMT/SEA.
(SEA): SEA + PMT/SEA.
We have assessed whether demographic parameters could influence the occurrence of PMT or SEA (Table S2). The demographic parameters, including age, sex, diabetes, obesity, dyslipidemia, hypertension, intensive care unit admission, and DIC, did not significantly influence the presence of sinusoidal thrombosis, PMT, or SEA. However, these results could potentially be due to the small sample size available for the analysis of each parameter. Further analyses with a larger samples size could clarify these results.
We found that PMT was associated with increased liver injury (p < 0.01), whereas SEA was not (p = 0.73) (Figure 1c). Platelet microthrombi is also known to exist in other pathological conditions, including thrombotic microangiopathy,8 chronic hepatitis/cirrhosis,9 and SOS.10 Platelet microthrombi formation in SOS depends on unusually large vWF multimer release from injured LSECs.10 von Willebrand factor mediates platelet adhesion to endothelial cells. In COVID‐19 livers, some patients showed strong vWF staining in LSECs (Figure 1d) which was associated with significantly increased platelet adhesion (1.7‐fold, p = 0.0069) (Figure 1d,e), compared to those with weak sinusoidal vWF expression and control livers 2‐fold, p = 0.023) (Figure 1d).
Hepatic steatosis with mild inflammatory infiltration has been also reported previously.3, 11 Although the underlying mechanism remains unknown, we speculate that elevated IL‐6 and the subsequent inflammatory signaling could mediate the induction of steatosis. The potential involvement of IL‐6 signaling in the generation of steatosis can be suggested by the positive correlation between steatosis and PMT. Our very recent paper reported that IL‐6 is capable of inducing endotheliopathy (pro‐inflammatory and pro‐coagulant states) in LSECs.12 Given that PMT (platelet attachment, neutrophil recruitment, and fibrin deposition) is likely the result of endotheliopathy, PMT could be the result of IL‐6 signaling. Further investigations will be needed to determine whether IL‐6 directly or indirectly induces steatosis in COVID‐19 patients.
Sinusoidal erythrocyte aggregation is an aggregation of erythrocytes mixed with fibrin(ogen), leukocytes, and/or platelets, which attach to the surface of LSECs. We observed SEA in 19 (83%) of liver samples with sinusoidal microthrombosis, mainly located in zone 2 (Figures 1a, left panel, S4). This is distinct from congestive hepatopathy, which is typically observed in zone 3. Generally, erythrocyte aggregation does not occur even in vascular congestion, because a net negative surface charge on erythrocytes by sialic acid prevents it. However, in the presence of macromolecules such as fibrinogen, which is known to interact with sialic acid on the surface of erythrocytes, erythrocytes aggregate (rouleaux formation).13 Our study showed that livers with SEA had significantly higher levels of fibrinogen (1.6‐fold, p = 0.031) compared to those without SEA in COVID‐19 patients (Figures 2a , Figures S5). Fibrinogen is produced predominantly in hepatocytes and secreted into the blood. It is cleaved by thrombin to form fibrin, leading to the formation of thrombosis with erythrocytes and platelets. The plasma concentration of fibrinogen increases during systemic inflammatory conditions and coagulopathy, a typical feature in COVID‐19.14 Of note, fibrin(ogen) colocalizing with platelets (CD41) was significantly higher in the area where PMT and SEA coexist (1.6‐fold, p = 0.04), compared to that with SEA alone (Figure 2b).
FIGURE 2.

Hepatic fibrinogen levels are associated with sinusoidal erythrocyte aggregation (SEA) formation in coronavirus disease (COVID‐19). (a) Fibrinogen deposit in the sinusoids. In COVID‐19 patients, fibrinogen area is larger in liver tissues with SEA (n = 19) than those without (w/o) SEA (n = 24) in COVID‐19 or control livers (n = 6). (b) Coimmunolabeling of fibrinogen (red) and platelets (green, CD41) in COVID‐19 liver. Fibrin(ogen) deposition in liver sinusoids (inset) and on the platelet microthrombi (PMT) in liver sinusoid (arrowhead). SEA alone (n = 9) versus SEA/PMT (n = 10). (c) Potential mechanism of the formation of liver sinusoidal microthrombosis (PMT and SEA) in COVID‐19. Ctrl, control; DIC, differential interference contrast; Fbg, fibrinogen; LSEC, liver sinusoidal endothelial cell; Plt, platelet; RBC, red blood cell; vWF, von Willebrand factor. *p < 0.05, **p < 0.01
In conclusion, liver PMT is the most pathologically important morphology of microvascular thrombosis in COVID‐19 because it is associated with liver injury. Platelet microthrombi potentially occurs due to strong expression of sinusoidal vWF. We propose that SEA is a unique morphological feature of COVID‐19 livers due to hyperfibrinogenemia (Figure 2c). This stands in contrast to patients with thrombotic microangiopathy or chronic hepatitis/cirrhosis, in which unchanged or diminished fibrinogen production, respectively, is common.8 The limitation of this study is its retrospective nature, meaning that variable factors including medical treatment, demographics among patients, timing of blood sample collection, and liver biopsy were not controlled. The serum AST and ALT values were obtained 6.3 days (average) before death. Thus, we cannot rule out the possibility that systemic circulatory disturbance before death may have affected the serum AST/ALT values. Although the thrombotic phenomena in the liver vasculature associated with liver injury in COVID‐19 are generally not of significant concern in the acute setting, evidence from lung injury suggests the possibility of fibrosis or long‐term consequences.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Supporting information
Supplementary Material 1
ACKNOWLEDGMENTS
This study was supported by NIH grants (R56DK121511) to Yasuko Iwakiri, AASLD Clinical, Translational, and Outcomes Research Award to Matthew J. McConnell, a grant of The International Research Fund for Subsidy of Kyushu University School of Medicine Alumni to Reiichiro Kondo. The authors thank the Clinical and Molecular Core of the Silvio O. Conte Digestive Diseases Research Core Center (NIH 30 DK034989).
Kondo R, Kawaguchi N, McConnell MJ, Sonzogni A, Licini L, Valle C, et al. Pathological characteristics of liver sinusoidal thrombosis in COVID‐19 patients: A series of 43 cases. Hepatol Res. 2021;51(9):1000–1006. 10.1111/hepr.13696
REFERENCES
- 1.Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal liver tests in COVID‐19: a retrospective observational cohort study of 1827 patients in a major U.S. hospital network. Hepatology. 2020;72:1169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsutsumi T, Saito M, Nagai H, Yamamoto S, Ikeuchi K, Lim LA, et al. Association of coagulopathy with liver dysfunction in patients with COVID‐19. Hepatol Res. 2021;51:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, et al. Hepatic pathology in patients dying of COVID‐19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao CL, Rapkiewicz A, Maghsoodi‐Deerwester M, Gupta M, Cao W, Palaia T, et al. Pathological findings in the postmortem liver of patients with coronavirus disease 2019 (COVID‐19). Hum Pathol. 2020;109:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiel MI, El Jamal SM, Paniz‐Mondolfi A, Gordon RE, Reidy J, Bandovic J, et al. Findings of severe hepatic severe acute respiratory syndrome coronavirus‐2 infection. Cell Mol Gastroenterol Hepatol. 2021;11:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli‐1 in normal human tissues. J Histochem Cytochem. 2006;54:385–95. [DOI] [PubMed] [Google Scholar]
- 8.Merrill JT, Erkan D, Winakur J, James JA. Emerging evidence of a COVID‐19 thrombotic syndrome has treatment implications. Nat Rev Rheumatol. 2020;16:581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo R, Yano H, Nakashima O, Tanikawa K, Nomura Y, Kage M. Accumulation of platelets in the liver may be an important contributory factor to thrombocytopenia and liver fibrosis in chronic hepatitis C. J Gastroenterol. 2013;48:526–34. [DOI] [PubMed] [Google Scholar]
- 10.Nishigori N, Matsumoto M, Koyama F, Hayakawa M, Hatakeyayama K, Ko S, et al. von Willebrand factor‐rich platelet thrombi in the liver cause sinusoidal obstruction syndrome following oxaliplatin‐based chemotherapy. PloS One. 2015;10:e0143136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baiocchini A, Del Nonno F, Taibi C, Visco‐Comandini U, D'Offizi G, Piacentini M, et al. Liver sinusoidal endothelial cells (LSECs) modifications in patients with chronic hepatitis C. Sci Rep. 2019;9:8760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConnell MJ, Kawaguchi N, Kondo R, Sonzogni A, Licini L, Valle C, et al. Liver injury in COVID‐19 and IL‐6 trans‐signaling‐induced endotheliopathy. J Hepatol. 2021. 10.1016/j.jhep.2021.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamee AP, Tansley GD, Simmonds MJ. Sublethal mechanical trauma alters the electrochemical properties and increases aggregation of erythrocytes. Microvasc Res. 2018;120:1–7. [DOI] [PubMed] [Google Scholar]
- 14.Helms J, Tacquard C, Severac F, Leonard‐Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1


