Abstract
Cardiac rehabilitation is a complex intervention that seeks to improve the functional capacity, wellbeing and health-related quality of life of patients with heart disease. A substantive evidence base supports cardiac rehabilitation as a clinically effective and cost-effective intervention for patients with acute coronary syndrome or heart failure with reduced ejection fraction and after coronary revascularization. In this Review, we discuss the major contemporary challenges that face cardiac rehabilitation. Despite the strong recommendation in current clinical guidelines for the referral of these patient groups, global access to cardiac rehabilitation remains poor. The COVID-19 pandemic has contributed to a further reduction in access to cardiac rehabilitation. An increasing body of evidence supports home-based and technology-based models of cardiac rehabilitation as alternatives or adjuncts to traditional centre-based programmes, especially in low-income and middle-income countries, in which cardiac rehabilitation services are scarce, and scalable and affordable models are much needed. Future approaches to the delivery of cardiac rehabilitation need to align with the growing multimorbidity of an ageing population and cater to the needs of the increasing numbers of patients with cardiac disease who present with two or more chronic diseases. Future research priorities include strengthening the evidence base for cardiac rehabilitation in other indications, including heart failure with preserved ejection fraction, atrial fibrillation and congenital heart disease and after valve surgery or heart transplantation, and evaluation of the implementation of sustainable and affordable models of delivery that can improve access to cardiac rehabilitation in all income settings.
Subject terms: Cardiovascular diseases, Rehabilitation
In this Review, Taylor and colleagues provide a summary of the current evidence base supporting the use of cardiac rehabilitation, an overview of international guidelines for cardiac rehabilitation and a discussion of major contemporary issues facing cardiac rehabilitation delivery around the world.
Key points
Cardiac rehabilitation is a complex, multicomponent intervention that includes exercise training and physical activity promotion, health education, cardiovascular risk management and psychological support, personalized to the individual needs of patients with heart disease.
Data from randomized, controlled trials support cardiac rehabilitation as a clinically effective and cost-effective intervention for patients with acute coronary syndrome or heart failure with reduced ejection fraction and after coronary revascularization.
Despite this robust evidence base and strong guideline recommendations, global access to cardiac rehabilitation is persistently poor, with scarce cardiac rehabilitation provision in low-income and middle-income settings.
Home-based and technology-based models of cardiac rehabilitation with appropriate quality assurance as an alternative or adjunct to traditional, centre-based programmes are needed to improve access to cardiac rehabilitation.
Cardiac rehabilitation programmes need to cater for and manage the needs of the increasing number of patients with heart disease who present with two or more chronic diseases.
Further research needs to strengthen the evidence base for cardiac rehabilitation in patients with heart failure with preserved ejection fraction, atrial fibrillation or congenital heart disease and after cardiac valve surgery or heart transplantation.
Introduction
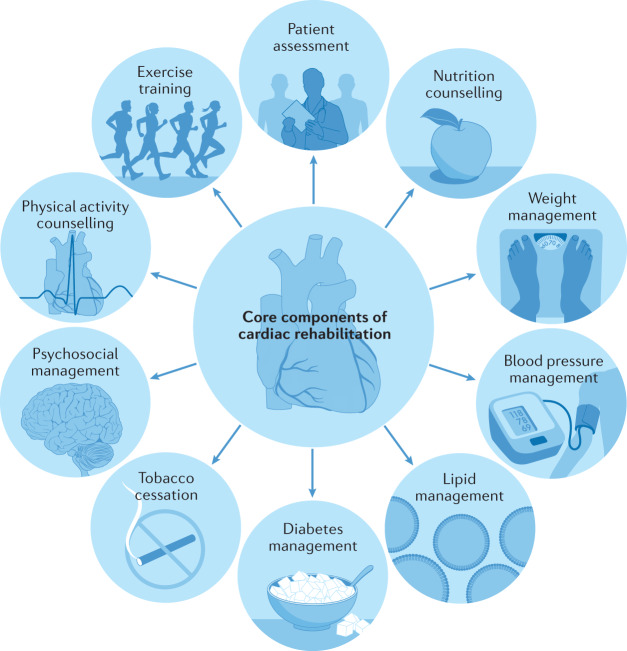
Cardiac rehabilitation is a complex intervention that includes exercise training, physical activity promotion, health education, cardiovascular risk management and psychological support, personalized to the individual needs of patients with diagnosed heart disease1 (Fig. 1). In addition to secondary prevention and improvement in cardiovascular prognosis, a focus of modern cardiac rehabilitation has been the drive to improve patient wellbeing and health-related quality of life2–4.
Fig. 1. Components of comprehensive cardiac rehabilitation.
A schematic summary of the major components of comprehensive cardiac rehabilitation. Adapted by permission from BMJ Publishing Group Limited. [Advances in rehabilitation for chronic diseases: improving health outcomes and function. Richardson C.R., Franklin B., Moy M.L., Jackson E.A., 365, l2191, 2019].
Introduced in the late 1960s, the recommendation for the provision of cardiac rehabilitation was, at that time, confined to low-risk patients who had survived an acute myocardial infarction (MI). With the development of an evidence base over the past two decades supporting the benefits of cardiac rehabilitation, contemporary clinical guidelines now routinely recommend the referral to comprehensive cardiac rehabilitation across a wider range of cardiac diagnoses, including acute coronary syndrome, heart failure with reduced ejection fraction (HFrEF) and coronary revascularization (percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery).
An important emphasis of contemporary guidelines, including the 2020 position statement from the European Association of Preventive Cardiology (EAPC)5, the 2017 guidance from the British Association for Cardiovascular Prevention and Rehabilitation6 and the 2020 position statement from the Secondary Prevention and Rehabilitation Section of EAPC, is the importance of quality assurance in cardiac rehabilitation delivery7 (Box 1). Key quality assurance elements include the involvement of a multidisciplinary team (including cardiologists, general practitioners and physicians with special interest, nurse specialists, physiotherapists, dietitians and psychologists) trained in the core competencies and effective delivery of the various core elements of a comprehensive cardiac rehabilitation programme (that is, exercise training and promotion, risk factor and self-management education, and psychological support)1,6, following a detailed initial assessment of the patient. Initially, cardiac rehabilitation was primarily practised as an exercise training intervention alone8. Although exercise training remains a central component of cardiac rehabilitation, the comprehensive model of modern cardiac rehabilitation is central to enabling patients to reduce their cardiovascular risk, foster and maintain their health-promotion behavioural patterns, increase their mental wellbeing, reduce their disability and promote an active lifestyle — with the overall aim of improving wellbeing and health-related quality of life. In response to the continuing evolution of cardiac rehabilitation practice and policy, this Review provides a state-of-the-art contemporary overview.
In this Review, we provide a detailed summary of the current evidence base supporting the use of cardiac rehabilitation, an overview of key international guidelines and position statements for cardiac rehabilitation and a synopsis of four key contemporary issues facing cardiac rehabilitation delivery across the globe: improving poor uptake, the effects of the coronavirus disease 2019 (COVID-19) pandemic, managing patient multimorbidity, and the provision of cardiac rehabilitation in low-income and middle-income countries (LMICs). We conclude with our recommendations for future research.
Box 1 Quality assurance standards according to BACPR6.
The British Association of Cardiopulmonary Rehabilitation (BACPR) has six standards for cardiovascular prevention and rehabilitation.
Standard One. The delivery of six core components by a qualified and competent multidisciplinary team led by a clinical coordinator.
Standard Two. Prompt identification, referral and recruitment of eligible patient populations.
Standard Three. Early initial assessment of individual patient needs, which informs the agreed personalized goals, which are reviewed regularly.
Standard Four. Early provision of a structured cardiovascular prevention and rehabilitation programme, with a defined pathway of care, which meets the individual’s goals and is aligned with patient preference and choice.
Standard Five. Upon programme completion, a final assessment of individual patient needs and demonstration of sustainable health outcomes.
Standard Six. Registration and submission of data to the National Audit for Cardiac Rehabilitation and participation in the National Certification Programme.
Box 1 adapted courtesy of British Association for Cardiovascular Prevention and Rehabilitation.
Overview of the evidence base
Our evidence overview is based on Cochrane systematic reviews and meta-analyses of cardiac rehabilitation. Cochrane reviews, with their rigorous methodological requirements and inclusion of only randomized controlled trials (RCTs), are internationally regarded as providing the highest quality of evidence for interventions. We focus on Cochrane reviews that compare the effects of exercise-based cardiac rehabilitation (exercise interventions alone or a comprehensive programme) with a control group (who did not receive cardiac rehabilitation). Key outcome findings (mortality, cardiovascular events, hospitalizations and health-related quality of life) for each indication are presented in Table 1 and summarized below. Researchers used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to summarize the certainty of the evidence for each outcome9 (Box 2).
Table 1.
Evidence for cardiac rehabilitation: summary of Cochrane review findings
| Condition reviewed (year) | Details | Mortality | CVD morbidity | Hospitalization | Health-related quality of life | Ref. |
|---|---|---|---|---|---|---|
| Coronary heart disease (2021) | 84 trials; median follow-up 6 months; 23,172 participants, primarily after MI or revascularization |
All-cause: RR 0.87, 95% CI 0.73–1.04 (25 trials; 9,946 participants; good certainty) CVD: RR 0.88, 95% CI 0.68–1.15 (five trials; 5,360 participants; moderate certainty) |
CABG surgery: RR 0.99, 95% CI 0.78–1.27 (20 trials; 4,473 participants; moderate certainty) PCI: RR 0.86, 95% CI 0.63–1.19 (13 trials; 3,465 participants; moderate certainty) Fatal or non-fatal MI: RR 0.72, 95% CI 0.55–0.93 (22 trials; 7,432 participants; moderate certainty) |
All-cause: RR 0.58, 95% CI 0.43–0.77 (14 trials; 2,030 participants; low certainty) CVD-related: RR 0.80, 95% CI 0.41–1.59 (six trials; 1,087 participants; low certainty) |
SF-12/36, PCS: MD 1.23, 95% CI 1.04–3.50 (four trials; 1,104 participants; no GRADE assessment) SF-12/36, MCS: MD 2.33, 95% CI 1.02–3.63 (four trials; 1,104 participants; no GRADE assessment) |
10 |
| Heart failure (2019) | 44 trials; median follow-up 6 months; 5,783 participants, primarily with HFrEF | All-cause: RR 0.89, 95% CI 0.66–1.21 (17 trials; 2,596 participants; low certainty) | NR |
All cause: RR 0.70, 95% CI 0.60–0.83 (20 trials; 2,142 participants; moderate certainty) HF-related: RR 0.59, 95% CI 0.42–0.84 (14 trials; 1,114 participants; low certainty) |
MLwHF: MD –7.1, 95% CI –10.5 to –3.7 (17 trials; 1,995 participants; low certainty) | 15 |
| Atrial fibrillation (2017) | Six trials; follow-up from 8 weeks to 6 months; 421 participants | All-cause: RR 1.00, 95% CI 0.06–15.78 (six trials; 421 participants; very low certainty) | Serious adverse eventsa: RR 1.01, 95% CI 0.98–1.05 (five trials; 381 participants; very low certainty) | NR |
SF-36 physical: MD 1.96, 95% CI –2.50 to 6.42 SF-36 mental: MD 1.99, 95% CI –0.48 to 4.46 (two trials; 224 participants; very low certainty) |
17 |
| Congenital heart disease (2020) | 15 trials, median follow-up not reported; 924 participants | NR | NR | NR | SF-36 total score, MLwHF, EQ5D VAS: SMD 0.76, 95% CI –0.13 to 1.65 (three trials; 163 participants; very low certainty) | 18 |
| Implantable cardioverter–defibrillator (2019) | Eight trials; median follow-up 3 months; 1,730 participants | All cause: RR 1.96, 95% CI 0.18–21.26 (one trial; 196 participants; low certainty) | Serious adverse eventsa: RR 1.05, 95% CI 0.77–1.44 (two trials; 356 participants; low certainty) | NR | NR | 19 |
| Heart transplantation (2017) | 10 trials; median follow-up 3 months; 300 participants | NR | NR | NR | NR | 20 |
| Valve surgery (2020) | Six trials; follow-up 3–14 months; 364 participants | All-cause: RR 0.83, 95% CI 0.26–2.68 (two trials; 131 participants; very low certainty) | NR | All-cause: RR 2.72, 95% CI 0.11–65.56 (one trial; 122 participants; very low certainty) |
SF-36 physical: MD –0.87, 95% CI –3.57 to 1.83 SF-36 mental: MD –1.45, 95% CI –4.70 to 1.80 (two trials, 150 participants; very low certainty) |
21 |
All outcomes are pooled outcomes at 6–12 months of follow-up, and quality assessment is based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system, unless otherwise stated. CABG, coronary artery bypass graft; CVD, cardiovascular disease; EQ5D VAS, EuroQoL Visual Analogue Scale; HFrEF, heart failure with reduced ejection fraction; MCS, mental component score; MD, mean difference; MI, myocardial infarction; MLwHF, Minnesota Living with Heart Failure questionnaire; NR, not reported; PCI, percutaneous coronary intervention; PCS, physical component score; RR, relative risk; SF, Short-Form; SMD, standardized mean difference. aSerious adverse events defined as any untoward medical occurrence that was life-threatening, resulting in death or that was persistent or leading to substantial disability; any medical event that had jeopardized the patient or required intervention to prevent it; any hospital admission or prolongation of existing hospital admission.
Box 2 The GRADE system.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system9 is a framework for rating the quality of evidence, applied to each outcome in a Cochrane systematic review, because the quality of evidence often varies between outcomes. GRADE has four levels of evidence (also known as certainty in evidence or quality of evidence).
Very low: the true effect is probably markedly different from the estimated effect.
Low: the true effect might be markedly different from the estimated effect.
Moderate: the authors believe that the true effect is probably close to the estimated effect.
High: the authors have high confidence that the true effect is similar to the estimated effect.
Evidence from randomized controlled trials starts at high quality. The certainty in the evidence is increased or decreased for several reasons. Reasons why certainty can be rated down:
Risk of bias: when the results of a study do not represent the truth because of inherent limitations in the design or conduct of a study
Imprecision: the rating focuses on the 95% confidence interval around the best estimate of the intervention effect
Inconsistency: assessed by similarity of point estimates and the overlap of their confidence intervals (statistical heterogeneity)
Indirectness: if the patients or intervention studied are different from those for whom the recommendation applies or the reported outcome is a surrogate for a different outcome
Publication bias: when the trial outcome influences the decision whether to publish or otherwise distribute the finding
Reasons why certainty can be rated up:
Large magnitude of effect
Dose–response gradient
Coronary heart disease
The 2021 update10 of the 2016 version11 of the Cochrane review of cardiac rehabilitation for coronary heart disease included 23,172 patients with MI (40 RCTs) or stable angina pectoris (five RCTs), after revascularization (14 RCTs) or in mixed populations. Meta-analysis of trials with outcomes up to 12 months of follow-up showed no effect of cardiac rehabilitation compared with control on all-cause mortality or the risk of revascularization. Participation in cardiac rehabilitation resulted in reductions in the risk of fatal or non-fatal MI and all-cause hospitalization. Although 29 trials collected health-related quality-of-life data, pooling of data was limited owing to variation in the outcome measures. Pooled analysis across three trials showed that cardiac rehabilitation improved generic health-related quality of life, assessed with the Short-Form 36 or 12 (mental component score), but had weak evidence of an improvement in the physical component score. Twenty of the 29 trials reported higher levels of health-related quality of life in one or more subscales with exercise-based cardiac rehabilitation than with control at follow-up. Outcome evidence assessed by GRADE was judged to be of ‘moderate’ certainty, downgraded owing to poor reporting on the randomization process (selection bias), lack of blinding (detection bias) and wide 95% confidence intervals (imprecision). Meta-regression (trial-level) analyses indicated that the benefits of cardiac rehabilitation seemed to be consistent across types and settings of cardiac rehabilitation (home versus centre, exercise-only versus comprehensive cardiac rehabilitation programmes, aerobic versus aerobic plus resistance training, dose of aerobic exercise) and study characteristics (single-centre versus multicentre).
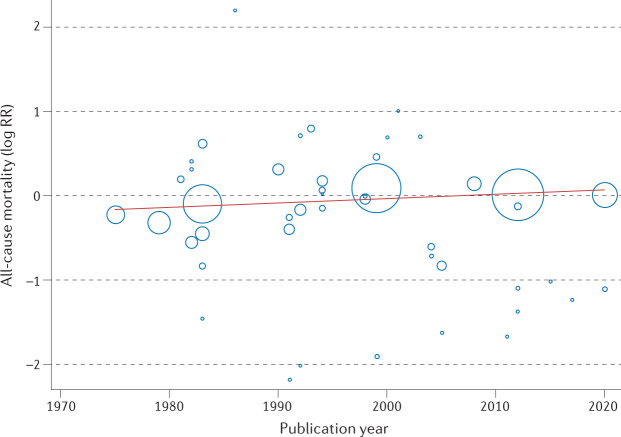
This Cochrane review has been criticized for the inclusion of older RCTs that might not reflect contemporary practice and studies that might not have used robust quality assurance in terms of the delivery of the cardiac rehabilitation intervention — for example, the UK-based, multicentre RAMIT trial12,13. Given that trials included in the Cochrane review span the period 1974–2020, the authors sought to address this issue by undertaking an assessment of the change in cardiac rehabilitation outcome over time. Interestingly, weak evidence exists of a reduction (slope 1.005, 95% CI 0.0098–1.0118, P = 0.13) in the all-cause mortality effect (log relative risk) of cardiac rehabilitation over time (Fig. 2). The authors interpreted this absence of an improvement in the effect of cardiac rehabilitation on all-cause mortality over the past 2–3 decades as reflecting the evolution of usual care and the introduction of life-saving therapies, including thrombolysis and secondary prevention drugs, such as β-blockers and statins. Interestingly, the 2020 meta-analysis of the CROSII study14, which included RCTs and prospective and retrospective cohort studies, reported a reduction in mortality with cardiac rehabilitation in patients with acute coronary syndrome or after revascularization, with an index event in 1995 or later. However, with the inclusion of observational evidence, the prognostic benefit reported by the CROSII study is subject to selection bias and confounding.
Fig. 2. Cardiac rehabilitation and all-cause mortality in patients with coronary heart disease: 1970–2020.
Meta-regression analysis of the treatment effect of cardiac rehabilitation on all-cause mortality over time in patients with coronary heart disease. The area of each data point is inversely related to the standard error of log relative risk (RR). The absence of an improvement in the effect of cardiac rehabilitation on all-cause mortality over the past 2–3 decades might reflect the evolution of usual care and the introduction of life-saving therapies, including thrombolysis and secondary prevention drugs.
Heart failure
A 2019 Cochrane review of cardiac rehabilitation in heart failure included 44 RCTs in 5,783 participants, predominantly with HFrEF15. This meta-analysis showed that participation in cardiac rehabilitation was associated with reduced rates of all-cause and heart-failure-specific hospitalization and improved health-related quality of life compared with control, whereas no significant effect of cardiac rehabilitation on all-cause mortality was detected. Pooled data across the 17 trials reporting the Minnesota Living with Heart Failure questionnaire (a disease-specific, health-related quality-of-life measure) showed not only a significant improvement with cardiac rehabilitation (mean difference –7.1, 95% CI −10.5 to –3.7), but also a magnitude of effect that is deemed ‘clinically important’ (an increase in the score by ≥5 points, compared with control)16. Certainty of outcomes was judged to be low to moderate, downgraded primarily owing to selection bias, imprecision (wide 95% confidence intervals or lack of events) and detection bias or placebo effects (health-related quality of life). Meta-regression analyses indicated that the benefits of cardiac rehabilitation for heart failure were consistent, irrespective of the nature of the cardiac rehabilitation or the setting.
Atrial fibrillation
The 2017 Cochrane review of cardiac rehabilitation in atrial fibrillation included six RCTs in 421 patients with various types of atrial fibrillation17. Given the small number of trials and reported clinical events, the effect of cardiac rehabilitation in this patient population in terms of the key outcomes of mortality, cardiovascular events, hospitalizations and health-related quality of life are all uncertain, with moderate to very low certainty (downgraded primarily owing to imprecision as a result of the small evidence base). Peak oxygen uptake (aerobic exercise capacity) was, on average, 3.76 ml/kg/min (95% CI 1.37–6.15 ml/kg/min) higher with cardiac rehabilitation than with the control (moderate quality of evidence).
Congenital heart disease
The 2020 Cochrane review focused on physical activity interventions across 15 RCTs in 924 adults and children with various forms of congenital heart disease18. Owing to the absence of trials reporting events, the authors concluded that there was no basis to determine the effect of cardiac rehabilitation in terms of either mortality or hospitalizations. In addition, evidence supporting the effect of cardiac rehabilitation on health-related quality of life was uncertain (very low quality of evidence owing to a small evidence base). Small improvements in both peak oxygen uptake (mean difference 1.89 ml/kg/min, 95% CI 0.22–3.99 ml/kg/min; 14 trials, 732 patients) and muscle strength (mean difference 17.1 N/m, 95% CI 3.4–30.8 N/m) were reported with cardiac rehabilitation (both moderate quality of evidence).
After cardioverter–defibrillator implantation
The 2019 Cochrane review included eight RCTs in 1,730 individuals with an implanted cardioverter–defibrillator, primarily for an indication of heart failure19. Owing to the small number of trials and reported events, the effect of cardiac rehabilitation on mortality, adverse events and health-related quality of life were all uncertain (low to very low quality of evidence). Low-quality evidence indicated that participating in cardiac rehabilitation resulted in a small increase in exercise capacity (determined by peak oxygen uptake) compared with control (mean difference 0.91 ml/kg/min, 95% CI 0.60–1.21 ml/kg/min; seven trials, 1,485 patients).
After heart transplantation
The 2017 Cochrane review included ten RCTs in 300 individuals after heart transplantation20. Cardiac rehabilitation increased peak oxygen uptake compared with the no-exercise control group (mean difference 2.5 ml/kg/min, 95% CI 1.63–3.36 ml/kg/min; nine trials, 284 patients, moderate quality of evidence). Although a meta-analysis was not possible owing to the lack of consistency of outcome reporting, the three individual trials that reported health-related quality of life showed no consistent advantage of cardiac rehabilitation over control. Owing to the small number of trials and reported events, a meta-analysis was not undertaken, and the effect of cardiac rehabilitation on all-cause mortality and hospitalizations was uncertain.
After valve surgery
The 2021 Cochrane review included six RCTs in 364 patients who had received either open or percutaneous heart valve surgery21. Owing to the lack of trials and outcome data, the authors were unable to conclude definitively the effect of cardiac rehabilitation in this population in terms of mortality, hospitalization or health-related quality of life (all very low quality of evidence). Cardiac rehabilitation increased peak oxygen uptake for all but the submaximal measures (mean difference 2.38 ml/kg/min, 95% CI 0.36–4.40 ml/kg/min; five trials, 294 patients, moderate quality of evidence) compared with no exercise.
General quality of evidence
Although systematic reviews and meta-analyses of RCTs are the gold standard for establishing the effects of intervention, a consistent limitation identified across the Cochrane reviews was the potential risk of bias and lack of consistency of outcomes reported by RCTs on cardiac rehabilitation to date. Therefore, improvement in the certainty of the evidence base for cardiac rehabilitation in the future depends on the conduct and reporting of high-quality RCTs, including the consistent collection and reporting of outcome measures, such as health-related quality of life (Box 3). It is important to recognize the limitations of meta-regression analyses and that this analysis can be subject to ecological fallacy, that is, study-level assessment of the relationships between study characteristics and patient outcomes does not necessarily reflect the true (patient-level) association22. For example, both meta-regression analyses reported in the Cochrane reviews on coronary heart disease and heart failure indicate that the benefit of cardiac rehabilitation is not affected by the study-level dose of exercise prescription. However, other (patient-level) data show that the dose of exercise is very important and that cardiac rehabilitation might result in no benefits when the prescription of exercise is too low in intensity or is of insufficient duration23,24. A more detailed review on this topic was published previously5.
Although developing areas for the application of cardiac rehabilitation, such as cardio-oncology and patients with left ventricular assist devices or spontaneous coronary artery dissection, have not been the subject of a Cochrane review, reviews of the evidence base for cardiac rehabilitation in these indications have been reviewed previously25–27.
Box 3 Future research recommendations.
The following key priorities for future cardiac rehabilitation research are drawn from current Cochrane reviews, clinical guidelines and other sources cited in this Review. These priorities apply to the following indications: heart failure with preserved ejection fraction, stable angina pectoris, atrial fibrillation, congenital heart disease and heart transplantation.
Future evidence collection should take the form of well-reported, large, multicentre, randomized, controlled trials, adequately powered and deemed high in quality and low in risk of bias, and should collect data on key outcomes, including mortality, hospitalization, health-related quality of life, health-care and societal costs and cost-effectiveness.
Given the current suboptimal uptake of cardiac rehabilitation, future trials of alternative models of cardiac rehabilitation delivery that can improve patient access and adherence are needed, including home-based and mobile, computer and digital technology-assisted programmes, as an alternative to or alongside traditional, centre-based models of delivery, especially for marginalized groups, for example, elderly individuals, women, and those from ethnic minorities and socioeconomically deprived groups. These trials need to consider assessing the patient-level and system-level outcome, including safety, costs and the quality assurance of programme delivery.
Development and evaluation of rehabilitation programmes that serve the needs of patients with cardiac disease who present with multimorbidity (the presence of two or more long-term conditions).
Development and evaluation of affordable and sustainable cardiac rehabilitation for patients with cardiac disease in low-income and middle-income countries.
Cost-effectiveness
In addition to clinical efficacy (‘effectiveness’) and safety, with the growing cost pressures on health-care systems across the world, the costs and cost-effectiveness of cardiac rehabilitation need to be considered. A 2018 systematic review of the cost-effectiveness of cardiac rehabilitation identified 19 economic studies28. Seven of these studies compared cardiac rehabilitation with no cardiac rehabilitation and the remaining studies compared intervention types within cardiac rehabilitation, for example, home-delivered or digitally delivered versus centre-based programmes. To facilitate comparison across studies, the authors converted all costs into 2016 US$, with the use of the consumer price index and purchasing power parity conversion. Most of the studies concluded that cardiac rehabilitation was cost-effective compared with no cardiac rehabilitation (incremental cost-effectiveness ratios (ICERs) ranged from US$1,065 to US$71,755 per quality-adjusted life-year (QALY)). In the UK, an acceptable level of cost-effectiveness is judged to be intervention with an ICER between £20,000 and £30,000 per QALY or lower, that is, ~US$25,000 to ~US$45,000 per QALY or lower29. Although generally cost-effective, the authors of the review concluded that further research was required to determine the most cost-effective design of cardiac rehabilitation, for example, a comparison of the cost-effectiveness of different modes of delivery (centre-based, home-based or using mobile technology) and combinations of interventions.
Clinical guideline recommendations
Reflecting the RCT evidence presented above, current clinical guidelines consistently provide a strong recommendation for cardiac rehabilitation referral for patients with MI or heart failure and after revascularization (CABG surgery or PCI). Table 2 summarizes the guideline statements from the ESC30,31, AHA/ACC32,33, National Institute for Health and Care Excellence (NICE)34,35 in the UK, and National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand36,37.
Table 2.
Cardiac rehabilitation recommendations in international guidelines for CHD and HF
| Region (society) | Recommendations for rehabilitation | Class of recommendation | Level of evidence | Comments | Ref. |
|---|---|---|---|---|---|
| CHDa | |||||
| USA (AHA/ACC) | All eligible patients with ACS or whose status is immediately post-coronary artery bypass surgery or post-PCI should be referred to a comprehensive outpatient cardiovascular rehabilitation programme either before hospital discharge or during the first follow-up office visit | I | A | A home-based cardiac rehabilitation programme can be substituted for a supervised, centre-based programme for low-risk patients | 32 |
| All eligible outpatients with the diagnosis of ACS, coronary artery bypass surgery or PCI and/or peripheral artery disease within the past year should be referred to a comprehensive outpatient cardiovascular rehabilitation programme | I | A | |||
| All eligible outpatients with the diagnosis of chronic angina within the past year should be referred to a comprehensive outpatient cardiovascular rehabilitation programme | I | B | |||
| UK (NICEb) | All individuals after a myocardial infarction should be given advice and offered a cardiac rehabilitation programme with an exercise component | NA | NA | NA | 34 |
| Programmes should include physical activity (adapted to clinical condition and ability), lifestyle advice (including advice on driving, flying and sexual activity), stress management and health education | NA | NA | |||
| Australia and New Zealand (NHFA and CSANZ) | Attendance at cardiac rehabilitation or a structured secondary prevention service for all patients hospitalized with ACS | I | A | Individualization of cardiac rehabilitation or secondary prevention service referral. A wide variety of prevention programmes improve health outcomes in patients with coronary disease. After discharge from hospital, patients with ACS and, where appropriate, their companions should be referred to an individualized preventive intervention according to their personal preference and values and the available resources. Services can be based in the hospital, primary care, the local community or the home | 36 |
| Europe (ESC) | Exercise-based cardiac rehabilitation is recommended in patients with chronic coronary syndrome. For full details, see 10.1093/eurheartj/ehz425 | I | A | Benefits of cardiac rehabilitation occur both after an acute myocardial infarction and after revascularization | 30 |
| HFc | |||||
| USA (AHA/ACC) | Exercise training (or regular physical activity) is safe and effective for patients with HF who are able to participate to improve functional status | I | A | NA | 33 |
| Cardiac rehabilitation can be useful in clinically stable patients with HF to improve functional capacity, exercise duration, HRQOL and mortality | IIa | B | |||
| UK (NICEb) | Recommends offering individuals with HF a personalized, exercise-based cardiac rehabilitation programme, unless their condition is unstable | NA | NA | Emphasis on specificity of, and improving access to, rehabilitation for the patients, including offering choice of venue for rehabilitation, delivering a comprehensive programme and being sensitive to the needs of the individual | 35 |
| The programme should be preceded by an assessment to ensure that it is suitable for the person, provided in a format and setting (at home, in the community or in the hospital) that is easily accessible for the person, include a psychological and educational component, may be incorporated within an existing cardiac rehabilitation programme and should be accompanied by information about support available from health-care professionals when the individual is participating in the programme | NA | NA | |||
| Australia and New Zealand (NHFA and CSANZ) | Regular performance of moderate intensity (that is, breathing more quickly but able to hold a conversation) continuous exercise is undertaken by patients with stable chronic HF, particularly in those with reduced LVEF, to improve physical functioning and quality of life, and to reduce hospitalizations | Strong | High | Exercise studies in HF have been largely conducted in patients with HFrEF aged <70 years. However, evidence has emerged for the benefits of exercise training in patients with HFpEF, which is more prevalent in older patients with HF and in women | 37 |
| Europe (ESC) | Regular aerobic exercise is encouraged in patients with HF. For full details, see: 10.1093/eurheartj/ehw128 | I | A | Most of the evidence available in the Cochrane review is from patients with HFrEF | 31 |
ACS, acute coronary syndrome; CCS, chronic coronary syndrome; CHD, coronary heart disease; CSANZ, Cardiac Society of Australia and New Zealand; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HRQOL, health-related quality of life; LVEF, left ventricular ejection fraction; NHFA, National Heart Foundation of Australia; NICE, National Institute for Health and Care Excellence; PCI, percutaneous coronary intervention. aIncludes ACS, acute myocardial infraction, post-revascularization, stable angina and PCI. bUnlike other guidelines, evidence informing the UK NICE guidance is assessed based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria and the class/level approach is not used. cIncludes HFrEF and HFpEF. AHA/ACC guidelines adapted with permission from refs32,33, Elsevier. NICE guidelines adapted with permission from refs34,35, NICE. NHFA/CSANZ guidelines adapted with permission from refs36,37, Wiley.
European, US and Australian/New Zealand guidelines all give cardiac rehabilitation their highest recommendation (class I: evidence and/or general agreement that a given treatment or procedure is beneficial, useful and effective and should be recommended) on the basis of an evidence rating of level A (data derived from multiple RCTs or meta-analyses) or level B (data derived from a single RCT or large non-randomized studies). The NICE recommendations are based on both clinical effectiveness and cost-effectiveness. Although these latter recommendations do not use the class and level approach, a strong recommendation for cardiac rehabilitation is made.
Given the small number of RCTs in patients with stable angina or heart failure with preserved ejection fraction, the European and Australian/New Zealand recommendations focus on HFrEF, and a level B rating is given for angina by the AHA/ACC. These guidelines recommend the need to conduct further research in these indications. The importance of a comprehensive nature of modern cardiac rehabilitation delivery is emphasized by the UK NICE guidance, recommending that programmes comprise physical activity, lifestyle advice, stress management and health education components.
Given the current underuse (referral and uptake) of cardiac rehabilitation services, with only a minority of eligible patients participating in cardiac rehabilitation over the past decade, the clinical guidelines emphasize the importance of alternative models of cardiac rehabilitation delivery to the traditional, centre-based programmes. The Australian/New Zealand, UK and US guidance all include a formal recommendation for consideration of home-based delivery to improve access to cardiac rehabilitation. The 2019 American Association of Cardiovascular and Pulmonary Rehabilitation, AHA and ACC joint scientific statement notes that although home-based cardiac rehabilitation is a common model in Canada and Europe, it is less common in the USA and emphasizes the need for quality assurance for the delivery of home-based cardiac rehabilitation programmes in their country38.
Given the more limited RCT evidence, current guidelines for the management of other cardiac indications, such as atrial fibrillation and congenital heart disease, provide no strong recommendation for or against the use of cardiac rehabilitation. Future high-quality RCTs of cardiac rehabilitation in these indications are needed to inform future guideline updates and clinical policy and practice. Although not the focus of this Review, a previous comparison of cardiac rehabilitation guidelines provides details of the differences and consensus in recommendations for exercise testing, prescription and monitoring39.
Major contemporary issues
Improving poor uptake
Despite the evidence for benefits of cardiac rehabilitation and strong guideline recommendations, the uptake of cardiac rehabilitation remains poor. Although the availability of cardiac rehabilitation is virtually absent in some global localities, in many areas, including Europe, North America and Australasia, a fairly small proportion of patients with acute coronary syndrome or HFrEF or who have undergone revascularization are currently referred for cardiac rehabilitation.
The latest data from the 2019 UK National Audit of Cardiac Rehabilitation (NACR) reported that 68,074 out of 135,861 (50%) individuals with a main diagnosis of coronary heart disease received cardiac rehabilitation (MI 29%, PCI 51% and CABG surgery 75%)40. For heart failure, the national level of cardiac rehabilitation attendance was <10%40. Cardiac rehabilitation participation rates in the USA are very low, ranging from 19% to 34% in national analyses, with large state-by-state geographical variations and differences according to cardiac diagnosis41. Consistent with the findings of many national and single-centre studies of cardiac rehabilitation, the 2019 UK NACR data show that certain groups are much less likely to attend cardiac rehabilitation than others, that is, older individuals, women, non-white and ethnic minority groups and patients with multimorbidity (defined as the presence of two or more long-term conditions)40.
The basis of suboptimal uptake of cardiac rehabilitation is complex and multilayered and reflects potential barriers at the level of the clinician, the patient and the health service (Table 3). At the clinician level, the absence of education on cardiac rehabilitation in their general medical and cardiology training might result in the low rate of referral by physicians41–43. For patients, a range of factors might influence their individual decision to act on this referral and attend a cardiac rehabilitation programme, such as inconvenience (and costs) of transport to attend a centre-based programme held during the ‘9–5’ working day, especially if these individuals are in employment. At the health service level, barriers can include the capacity and funding of cardiac rehabilitation programmes. For example, the 2019 UK NACR showed that group-based, supervised cardiac rehabilitation was the most common mode of delivery of cardiac rehabilitation, with 75.4% of patients receiving this method of cardiac rehabilitation compared with only 8.8% taking up home-based cardiac rehabilitation40. Barriers at these three levels are probably interactive. For example, travelling to centres and a dislike of group-based cardiac rehabilitation sessions are known to be particularly relevant for certain groups of patients, including women, ethnic minorities and people from areas of high deprivation who are elderly, living with complex health conditions or living in rural areas42,43. Cardiac rehabilitation is a crucial environment to contribute to the optimization of a patient’s cardiovascular risk, with opportunities for screening, education and medical treatment (exercise, nutrition, smoking cessation and medications). Despite these potential benefits of risk-factor reduction, the results from the EUROASPIRE III study44 indicated the underuse of cardiac rehabilitation, with poor referral and low participation rates and wide variations between European countries. Some of the key proposed solutions to these patient, clinician and health service barriers to accessing cardiac rehabilitation are summarized in Box 4.
Table 3.
Barriers to accessing CR and potential solutions
| Barriers | Proposed solution | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incorporate CR into educational curriculum | Automated referral system | Public education to create informed public | Counselling by clinicians | Weekend/evening sessions | Home-based CR | Evidence-based tele-rehabilitation and mobile health technologies | Multi-tiered programmes | Alternative reimbursement and insurance policies | Task distribution | Use of existing physical infrastructure | Involve caregivers (family and friends) | Align incentives with service delivery | Improve revenue collection | ||
| Physician level | Lack of CR in cardiology training | ✓ | |||||||||||||
| Lack of endorsement or referral | ✓ | ✓ | ✓ | ||||||||||||
| Patient level | Lack of awareness | ✓ | ✓ | ||||||||||||
| Motivation | ✓ | ✓ | |||||||||||||
| Time | ✓ | ✓ | ✓ | ||||||||||||
| Transport | ✓ | ✓ | |||||||||||||
| Affordability | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| System and service level | Lack of resources | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Lack of reimbursement | ✓ | ✓ | ✓ | ✓ | |||||||||||
| COVID-19 pandemic | Temporary closure of CR centres | ✓ | ✓ | ||||||||||||
| Reduced capacities after partial re-opening of CR centres | ✓ | ✓ | |||||||||||||
| Anxiety of patients about commuting regularly to hospital | ✓ | ✓ | ✓ | ||||||||||||
CR, cardiac rehabilitation. Adapted from ref.43, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
Given that the potential loss to patients of important gains in health-related quality of life and the rise in pressures and costs on health-care systems as a result of increased unplanned hospitalization, poor participation (uptake) of cardiac rehabilitation is an increasingly important policy priority. For example, in the UK, the NHS England Long Term Plan45 was published in 2019, with the aim to increase the overall national uptake of cardiac rehabilitation to 85% (from the current 50%) of all eligible patients by 2028. In the USA, a road map was proposed to achieve >70% participation in cardiac rehabilitation by 2022, with the aim of saving 25,000 lives and preventing 180,000 hospitalizations per year46.
An example of innovative service development is the Rehabilitation Enablement in Chronic Heart Failure (REACH-HF) programme of facilitated cardiac rehabilitation. This comprehensive programme of cardiac rehabilitation for use at home comprises a heart failure manual, a relaxation CD, a choice of exercise (walking programme or a chair-based DVD), a progress tracker for patients, and a family and friends resource for caregivers. A UK-based, multicentre RCT in 216 individuals with HFrEF confirmed that the addition of REACH-HF to usual care compared with usual care alone was effective in improving the primary outcome of health-related quality of life, which was assessed using the Minnesota Living with Heart Failure questionnaire (–5.7 points, 95% CI −10.6 to −0.7 points, P = 0.025)47. Subsequent economic modelling on the basis of the results from the trial confirmed the acceptable cost-effectiveness of the REACH-HF programme, with an ICER of £1,720 per QALY48. Given its clinical effectiveness and cost-effectiveness, the REACH-HF programme is now being rolled out into routine care across the UK and the Republic of Ireland to improve access to and uptake of cardiac rehabilitation49,50.
A growing body of research now shows that home-based models of cardiac rehabilitation delivery achieve similar gains in patient efficacy and safety to traditional, centre-based programmes at similar cost-effectiveness and, indeed, might lead to higher levels of patient adherence51,52. As with many previous trials of cardiac rehabilitation, studies of home-based cardiac rehabilitation have focused on low–moderate risk populations. Several cardiac rehabilitation programmes are now using a hybrid approach to deliver cardiac rehabilitation. For example, this approach initially offers patients centre-based cardiac rehabilitation and then evolves to longer-term maintenance through technology-supported, home-based sessions53. The effectiveness of these innovative models is likely to depend on active, ongoing contact between patients and health-care professionals through more traditional methods, such as home visits and telephone consultations, or the use of technology-based solutions, which include web-based video calls and social networking platforms54.
Box 4 Strategies to facilitate increased referral to and enrolment and long-term participation in CR programmes.
Achieve strong endorsement of cardiac rehabilitation (CR) by referring clinicians (cardiologists, physicians and health-care professionals) by incorporating it into the hospital discharge plan.
Automatically refer all eligible patients for CR at the time of hospital discharge — giving patients a choice to attend a centre-based or home-based hybrid programme.
Provide CR information (printed and web links or videos) and education to inpatients before discharge from hospital.
Ensure good communication with the patient’s primary care physician or general practitioner so they are sent the discharge details with information on CR programmes — giving the option of centre-based CR or home-based CR (for low-to-moderate-risk patients) or a hybrid programme.
Schedule CR enrolment appointments via the patient’s preferred communication mode (telephone call, text message, e-mail or post).
Advise the patient’s primary care physician or general practitioner to refer patients for CR if the patient has not been referred — encouraging enrolment and participation.
Consider system-level, provider-level and patient-level financial incentives for referral to, enrolment in and completion of CR sessions.
Target and identify racial and ethnic minorities, women, older adults, and rural and socioeconomically deprived groups who are least likely to enrol in and complete CR.
Encourage long-term support through trained health-care professionals using face-to-face or web-based applications to track ongoing efforts for cardiovascular risk reduction, including physical activity and fitness, for example, by the primary-care team.
Box 4 adapted with permission from ref.38, Elsevier.
Effects of the COVID-19 pandemic
The COVID-19 pandemic has had a major global effect on the use and delivery of health and health-care systems. As we write this Review, the UK has become the first European country to officially record >125,000 deaths associated with COVID-19. Although vaccine rollout has begun, many countries across the world are having to take various public-health measures to suppress virus transmission rates, including lockdown measures, provision of social distancing guidance, track and trace of individuals with COVID-19 and quarantining of travellers between one country and another55.
Worse COVID-19 outcomes and increased risk of death are linked to pre-existing cardiovascular disease56–58, and these individuals are advised to shield or self-isolate at home to minimize the risk of infection59. The pandemic has led to the disruption of many hospital services, including non-urgent outpatient appointments and routine ambulatory care, which have been curtailed or minimized. For cardiac rehabilitation, the pandemic has accentuated existing barriers to access discussed above (Table 3). In Canada, the USA and Europe, many cardiac rehabilitation centres have been closed, with some countries observing an overall reduction in cardiac rehabilitation participation60,61. In addition, cardiac rehabilitation capacity has been reduced because rehabilitation staff are being deployed to the ‘front line’ of intensive medical care for COVID-19. The increased risk of infection can lead to patients with diagnosed heart disease being anxious about travelling to centres to undertake rehabilitation. The dramatic effect of the pandemic on access to cardiac rehabilitation is illustrated by the British Heart Foundation NACR61, which has observed more than a two-thirds decrease in cardiac rehabilitation attendance in patients with heart failure from the pre-COVID period (4,969 patients in May 2019 to January 2020) to the COVID period (1,474 patients in February 2020 to August 2020). However, this drop in uptake was associated with a substantial increase in the proportion of patients enrolling in home-based cardiac rehabilitation programmes, which increased from 22.2% to 72.4% in the same time frame.
Conventional cardiac rehabilitation services that have relied on patients attending group-based sessions in hospitals or community centres have been difficult to sustain, and renewed calls have been made for alternatives to centre-based cardiac rehabilitation59,60. Even before the outbreak of COVID-19, the uptake of cardiac rehabilitation in many countries was suboptimal.
As described above, increasing evidence supports the effectiveness and safety of mobile-technology-supported models of delivery62–64, which are recommended in international guidelines34–54 and receive reimbursement from external agencies65. Endorsement of remote delivery of cardiac rehabilitation in the COVID-19 era has come from various international sources47,48,54,55,60,61,66,67. EAPC has made an emphatic call for cardiac tele-rehabilitation to maintain the delivery of the core components of cardiac rehabilitation and has provided a practical guide for the set-up of a comprehensive cardiac tele-rehabilitation intervention during the COVID-19 pandemic60. However, concerns have been raised about equity in the use of technology to maintain access to outpatient care. Lower rates of technology and Internet use and access to these facilities have been documented in elderly individuals, those of lower socioeconomic status and ethnic minorities, mirroring the groups associated with limited enrolment and low levels of participation in cardiac rehabilitation68,69. In a cohort study of 2,940 patients scheduled to attend cardiology clinics at one centre in the USA in 2020, those individuals with lower income and who were non-English-speaking, female and/or older had more difficulty in engaging in care via telemedicine, suggesting that its rapid adoption exacerbates existing inequities70,71.
The pandemic has prompted providers of cardiac rehabilitation to seriously consider remote models of delivery, so that patients with heart disease can follow a self-care rehabilitation programme from their own home, which could also include support from their family and friends. A beacon model of innovative, evidence-based service delivery in the UK during the pandemic has been the REACH-HF programme72.
Managing multimorbidity
Cardiac rehabilitation has traditionally been commissioned and delivered as a ‘single disease’ service and focuses on the needs of patients with MI or heart failure or who have undergone revascularization. Although referred for cardiac rehabilitation for a specific indication, patients do not typically present with their single index disease alone, but instead have several long-term comorbidities. For example, the large, US-based, multicentre, randomized, controlled HF-ACTION trial73 of cardiac rehabilitation reported that in addition to an index diagnosis of heart failure, at entry to the study, a substantial proportion of the 2,331 patients with heart failure had several comorbidities, including 59% with hypertension, 21% with atrial fibrillation or flutter and 32% with diabetes mellitus. The 2019 UK NACR reported that approximately 50% of all 6,502 patients referred for cardiac rehabilitation had two or more comorbidities40.
The management of multimorbidity is an important challenge facing health-care systems74. Levels of multimorbidity are predicted to grow with population demographic changes and improved survival rates resulting in increased numbers of older individuals75,76. Importantly, patients with multimorbidity are at higher risk of dying prematurely, being admitted to hospital, having longer stays in hospital and having a reduced health-related quality of life75,76 than patients with only one chronic medical condition. The presence of multimorbidity seems to affect the provision of cardiac rehabilitation services. The 2019 UK NACR data set showed that multimorbidity was a strong risk factor for both non-use of cardiac rehabilitation and programme non-completion40. For example, a higher proportion of non-completers have symptoms of anxiety (5% higher) and depression (8% higher) than completers77.
The increasing burden and complexity of multimorbidity challenge our traditional model of cardiac rehabilitation. Although a core component of cardiac rehabilitation is a detailed patient assessment that includes the assessment of comorbidity, with its focus on single-disease management instead of individualized or personalized care, the delivery of existing programmes of cardiac rehabilitation might be failing to meet the health needs of patients with cardiac disease and multimorbidity. These patients have a high risk of non-referral to a cardiac rehabilitation programme. Furthermore, even if they are referred to rehabilitation, a high risk exists that the programmes will not fully address the needs of patients with multimorbidity. Instead, we need to adapt to the change in population demographics and look to provide a model of personalized multimorbidity rehabilitation that meets the needs of patients, irrespective of their index diagnosis, cardiovascular or otherwise. Arguments for this multimorbidity rehabilitative model approach are summarized in Box 5.
Although a move to a model of cardiac rehabilitation that more comprehensively addresses the needs of patients with heart disease and their multimorbidity is appealing, the evidence base for innovation remains limited. At present, only two small, developmental studies have specifically focused on multimorbidity rehabilitation. A pilot RCT evaluated the feasibility of 8 weeks of a ‘generic rehabilitation’ programme of supervised exercise and education (based on the principles of cardiac rehabilitation and pulmonary rehabilitation) or no rehabilitation control in 16 patients with multimorbidity at a single centre in Australia78. The researchers reported that 71% of patients completed the rehabilitation intervention and had a higher mean improvement in 6-min walking distance than the control population (44 m versus 23 m)78.
The Healthy and Active Rehabilitation Programme (HARP) was established in Ayrshire, Scotland, in 2015 (ref.79). The HARP model was developed to focus specifically on deprived and rural communities and those with high unscheduled care demand (that is, cardiac or pulmonary disease, cancer, stroke, diabetes and/or a high risk of falls). Developed from existing models of cardiac rehabilitation and pulmonary rehabilitation, HARP is based on a comprehensive patient assessment followed by a 10-week exercise and education programme. Interviews with patients with multimorbidity indicated that the HARP programme was well received and was perceived to improve confidence and motivation for physical activity and other healthy behaviours.
In the absence of an established evidence base, an urgent need exists for research into the acceptability, efficacy and cost-effectiveness of personalized models of rehabilitation for multimorbidity. Although we should not abandon our existing cardiac rehabilitation practice, there remains the challenge of more comprehensively meeting the needs of patients with cardiac disease and multimorbidity and developing a robust evidence base around these developments. A 2020 editorial identified key research questions around the future evolution of cardiac rehabilitation services for multimorbidity80.
Box 5 Adapting the traditional model of cardiac rehabilitation.
Advantages of adapting the traditional (single-index) cardiac rehabilitation model for patients with multimorbidity80.
Sustainability
In the current financially challenged health service, health-care commissioners and purchasers are likely to consider the expansion of disease-specific rehabilitation services as inefficient and unsustainable. Instead, they would be more attracted to a programme that caters for patients with multimorbidity as a more appropriate and cost-effective model of care.
Holistic
The failure to consider the effect of multimorbidity on the wellbeing and functionality of the patient and, for example, ‘just rehabilitate their heart failure’ is likely to diminish greatly the potential benefits of rehabilitation. Given that candidate patients for pulmonary and cardiac rehabilitation commonly have multiple chronic conditions, many of the important clinical problems that these patients face are probably not directly related to their cardiac or respiratory disease. We know from qualitative research that treating one condition at a time is inconvenient and unsatisfactory for patients with chronic conditions.
Inclusivity
Personalized multimorbidity rehabilitation presents an opportunity to develop a model by which to extend services to other important long-term conditions that would be amenable to rehabilitation, such as atrial fibrillation. Furthermore, this model could be extended to include other patient groups with, for example, transient ischaemic attack, mild stroke or peripheral vascular disease.
Box 5 adapted with permission from ref.80, Oxford University Press.
Improving access in LMICs
It is estimated that by 2030, more than 80% of cardiovascular-related disability and death will occur in the 139 LMICs owing to increasing prevalence of risk factors, such as hypertension, smoking, diabetes and obesity81,82. Although secondary prevention strategies are vitally important to stemming this growing epidemic, cardiac rehabilitation programmes remain largely non-existent in the LMIC setting compared with high-income economies.
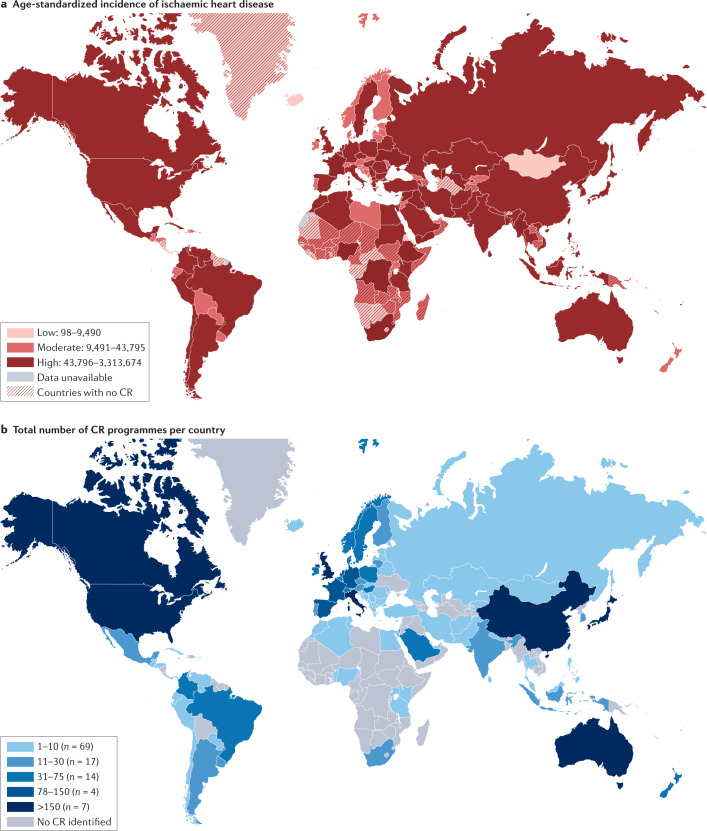
The global inequality in cardiac rehabilitation provision was quantified by the International Council of Cardiovascular Prevention and Rehabilitation (ICCPR) audit83. Published in 2019, this ICCPR study revealed that cardiac rehabilitation is available in only half of the countries of the world, and this geographical distribution of cardiac rehabilitation is negatively correlated with the incidence of ischaemic heart disease, according to the Global Burden of Disease study84 (Fig. 3).
Fig. 3. Global incidence of ischaemic heart disease and availability of cardiac rehabilitation.
a | Age-standardized incidence of ischaemic heart disease. b | Total number of cardiac rehabilitation (CR) programmes per country. CR is available in only approximately half of the countries of the world and, in broad terms, the geographical distribution of CR is negatively correlated with the incidence of ischaemic heart disease. Data from ref.83.
This inequality is put into sharp focus by the contrasting densities in cardiac rehabilitation provision of only one cardiac rehabilitation place available for every 66 patients with ischaemic heart disease in LMICs, compared with one place for every 3.4 patients in high-income counties85. For example, Bangladesh has only one cardiac rehabilitation programme across the whole country, whereas England has more than 200 cardiac rehabilitation programmes, despite a similar annual incidence of ischaemic heart disease (409,000 versus 318,284, respectively). Although the barriers to the availability of cardiac rehabilitation are complex (Table 3), crucial additional economic constraints include limited health-care system budgets plus the consequent need for patient out-of-pocket payment, for which public funding is not available or is limited43.
Although the evidence demonstrating the beneficial effects of cardiac rehabilitation to date has mainly been collected in RCTs conducted in high-income settings, there is now a growing body of literature from developing countries. An ongoing systematic review has identified 26 RCTs of cardiac rehabilitation in 6,380 patients (primarily with ischaemic heart disease or heart failure) conducted across eight LMICs (Bangladesh, Brazil, China, Egypt, India, Iran, Nigeria and Pakistan)86. Meta-analysis of these trials conducted in LMICs shows that the increase in exercise capacity with cardiac rehabilitation (mean increase in peak oxygen uptake of 3.1 ml/kg/min, 95% CI 2.6–3.6 ml/kg/min) compared with the control population who received no cardiac rehabilitation was similar to figures reported in trials of cardiac rehabilitation conducted in high-income countries (3.3 ml/kg/min, 95% CI 2.6–4.0 ml/kg/min)87.
A systematic review of economic evaluations of cardiac rehabilitation in LMICs found no studies from low-income countries88. However, five studies in middle-income settings in Latin America indicated that cardiac rehabilitation could be a cost-effective intervention. In Brazil, the mean cost per patient was US$503 for a 3-month cardiac rehabilitation programme, with a mean monthly saving in health-care costs of US$190 for cardiac rehabilitation, compared with an increase of US$48 in the control group receiving no cardiac rehabilitation. Given the limited health-care budgets in many LMICs, the researchers of this study emphasized the need for affordable cardiac rehabilitation models in this setting89.
Box 6 provides a case example of the development of cardiac rehabilitation in the LMIC setting of Bangladesh90. Expansion of cardiac rehabilitation services is urgently needed to mitigate the epidemic of cardiovascular diseases in LMICs. Unlike high-resource settings, in which cardiac rehabilitation has traditionally been delivered in the hospital setting, often with a team of highly specialist staff, considerations of affordability, scalability and the needs of the local populations and health-care systems demand alternative approaches for the provision of cardiac rehabilitation services in LMICs. This alternative approach includes home-based and community-based programmes supported by accessible digital technology (such as Internet and mobile phone accessibility) and cost-effective training programmes for health-care staff to ensure the quality of delivery of cardiac rehabilitation practice91,92. An imperative on the global health community is to incorporate novel cardiac rehabilitation delivery models into efforts directed at the secondary prevention of cardiovascular disease, in line with the WHO’s dual strategic targets of reducing mortality from non-communicable diseases by 25% by 2030 and overcoming the ever-increasing unmet need for rehabilitation worldwide, which is particularly profound in LMICs93,94.
Box 6 Case example: cardiac rehabilitation in Bangladesh.
In the past 10 years, Bangladesh has expanded the number of cardiovascular care facilities and improved service quality throughout the country. These facilities are run by public, private and autonomous sectors and include dedicated cardiac institutions and multi-speciality institutions with cardiac care facilities, with most located in the capital, Dhaka. Although the number of acute cardiac care facilities has increased, currently only one hospital-based cardiac rehabilitation programme is available in Bangladesh, based at the Ibrahim Cardiac Hospital & Research Institute (ICHRI).
From 2010, ICHRI introduced an exercise-based and education-based multidisciplinary cardiac rehabilitation programme for patients after cardiac surgery, consisting of a 30-min group exercise programme supported with a leaflet on sternum protection containing advice that can be followed at home. A single-centre, quasi-randomized controlled trial indicated that this cardiac rehabilitation programme was feasible and had potential benefits in terms of coronary heart disease risk factors, health-related quality of life, mental wellbeing and exercise capacity90. Following a 12-month clinical fellowship in Denmark and the UK in 2015, Jamal Uddin (a senior physiotherapist) started a comprehensive cardiac rehabilitation programme at the ICHRI. This programme consists of a group exercise programme from the seventh postoperative day, a risk-factor management educational class, dietary advice from dietitians and a manual to allow participants to maintain home-based cardiac rehabilitation. The manual includes upper-limb and lower-limb exercises, breathing exercises and aerobic exercise (a walking programme). The ICHRI also offers a 1-year cardiac rehabilitation follow-up (three follow-up visits within 1 year). During this follow-up, the patient first visits the cardiology unit and then the physiotherapy and cardiac rehabilitation unit for a cardiac fitness test and receives complete instructions for following an exercise programme.
A stakeholder round-table meeting was held in Dhaka on 30 November 2019: researchers, clinicians, health-care professionals and health-care policy-makers met to discuss affordable, flexible and feasible ways to scale up cardiac rehabilitation provision in Bangladesh and South Asia. This round-table meeting called for three key actions: expand and increase the reach of cardiac rehabilitation services through centre-based and home-based cardiac rehabilitation programmes; emphasize the importance of involving more cardiologists and cardiac surgeons to refer patients to cardiac rehabilitation; and offer inclusive, professional development training for health-care providers to promote the establishment of more cardiac rehabilitation programmes in Bangladesh.
Conclusions
Cardiac rehabilitation is a complex, multicomponent intervention that includes exercise training and physical activity promotion, health education, cardiovascular risk management and psychological support, personalized to the individual needs of patients diagnosed with heart disease. First introduced in the 1960s for low-risk patients who survived an acute MI, a growing body of RCT evidence over the past 3–4 decades now supports contemporary clinical guidelines, which recommend routine referral for cardiac rehabilitation across a range of cardiac diagnoses, including acute coronary syndrome, heart failure and after coronary revascularization (PCI or CABG surgery). As discussed in this Review, despite consistent and strong recommendations for cardiac rehabilitation referral in international clinical guidelines, contemporary cardiac rehabilitation practice faces a number of challenges. Global access to cardiac rehabilitation is persistently poor, with only 5–50% of eligible patients with cardiac disease receiving rehabilitation. Sadly, the ongoing COVID-19 pandemic has substantially added to this challenge: existing centre-based programmes have paused their services, with rehabilitation staff being relocated to critical care settings and patients being anxious about travelling to a centre for their rehabilitation. However, out of this ‘access challenge’ has come the opportunity to expedite the switch to (or combine) accessible home-based and technology-based models of cardiac rehabilitation, with appropriate quality assurance for their delivery. The development and provision of innovative models of delivery are likely to be especially important in LMICs, in which cardiac rehabilitation services are scarce, and scalable and affordable models are much needed. Key areas of research to support the future practice of cardiac rehabilitation are summarized in Box 3.
Acknowledgements
The authors thank J. Uddin (Physiotherapy Unit, Department of Cardiac Surgery Ibrahim Cardiac Hospital & Research Institute, Dhaka, Bangladesh) for drafting the content of Box 6, G. Dibben (MRC/CSO Social and Public Health Sciences Unit, University of Glasgow, UK) for preparing Fig. 2 for initial submission and U. Ahmed (MRC/CSO Social and Public Health Sciences Unit, University of Glasgow, UK) for editorial review of the text.
Author contributions
All the authors researched data for the article, contributed substantially to discussions of its content, wrote the article, and reviewed and/or edited the manuscript before submission.
Competing interests
R.S.T. is a member of the ESC Association of Cardiovascular Nursing and Allied Professions (ACNAP) Science Committee 2020–2022 and lead investigator for the following ongoing funded projects: ‘Implementation of an evidence-based cardiac rehabilitation home programme for heart failure patients and their caregivers in Scotland: SCOT:REACH-HF project’, funded by Heart Research UK; ‘A randomized controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: the REACH-HFpEF Study’, funded by NIHR HTA Programme (NIHR130487). H.M.D. is a co-opted member of the British Association of Cardiovascular Prevention and Rehabilitation (BACPR) and a co-lead for the ongoing funded research projects: ‘D REACH-HF: Digital Rehabilitation Enablement in Chronic Heart Failure’, funded by the British Heart Foundation, Hope for Hearts fund’; ‘Extending the reach and implementation of the successful REACH-HF programme with a digitally delivered training programme’, funded by NIHR Programme Development Grant (NIHR202040). S.J.D.M. is a researcher on the following ongoing funded research projects: ‘D REACH-HF: Digital Rehabilitation Enablement in Chronic Heart Failure’, funded by the British Heart Foundation, Hope for Hearts fund’; ‘Extending the reach and implementation of the successful REACH-HF programme with a digitally delivered training programme’, funded by NIHR Programme Development Grant (NIHR202040).
Footnotes
Peer review information
Nature Reviews Cardiology thanks D. Forman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Richardson CR, Franklin B, Moy ML, Jackson EA. Advances in rehabilitation for chronic diseases: improving health outcomes and function. BMJ. 2019;365:l2191. doi: 10.1136/bmj.l2191. [DOI] [PubMed] [Google Scholar]
- 2.Dempster M, Donnelly M. Measuring the health related quality of life of people with ischaemic heart disease. Heart. 2000;83:641–644. doi: 10.1136/heart.83.6.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor R, Dibben G, Faulkner J, Dalal H. More evidence of cardiac rehabilitation: need to consider patient quality of life. Can. J. Cardiol. 2021 doi: 10.1016/j.cjca.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Cook R, Davidson P, Martin R, et al. Cardiac rehabilitation for heart failure can improve quality of life and fitness. BMJ. 2019;367:l5456. doi: 10.1136/bmj.l5456. [DOI] [PubMed] [Google Scholar]
- 5.Ambrosetti M, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2021;28:460–495. doi: 10.1177/2047487320913379. [DOI] [PubMed] [Google Scholar]
- 6.BACPR. The BACPR Standards and Core Components for Cardiovascular Disease Prevention and Rehabilitation 2017 (3rd Edition) https://www.bacpr.com/resources/BACPR_Standards_and_Core_Components_2017.pdf (2017).
- 7.Abreu A, et al. Standardization and quality improvement of secondary prevention through cardiovascular rehabilitation programmes in Europe: the avenue towards EAPC accreditation programme: a position statement of the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology (EAPC) Eur. J. Prev. Cardiol. 2021;28:496–509. doi: 10.1177/2047487320924912. [DOI] [PubMed] [Google Scholar]
- 8.Kavanagh, T. in Cardiac Rehabilitation (eds Jones, D. & West, R.) 4–30 (BMJ Publishing Group, 1995).
- 9.Guyatt GH, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faulkner, J. et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. (in press). [DOI] [PMC free article] [PubMed]
- 11.Anderson L, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2016;3:CD001800. doi: 10.1002/14651858.CD001800.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West RR, Jones DA, Henderson AH. Rehabilitation after myocardial infarction trial (RAMIT): multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart. 2012;98:637–644. doi: 10.1136/heartjnl-2011-300302. [DOI] [PubMed] [Google Scholar]
- 13.Rauch B, et al. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: a systematic review and meta-analysis of randomized and non-randomized studies - The Cardiac Rehabilitation Outcome Study (CROS) Eur. J. Prev. Cardiol. 2016;23:1914–1939. doi: 10.1177/2047487316671181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salzwedel A, et al. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: update of the Cardiac Rehabilitation Outcome Study (CROS-II) Eur. J. Prev. Cardiol. 2020;27:1756–1774. doi: 10.1177/2047487320905719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long L, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst. Rev. 2019;1:CD003331. doi: 10.1002/14651858.CD003331.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Thoracic Society. Minnesota Living with Heart Failure questionnaire http://qol.thoracic.org/sections/instruments/ko/pages/mlwhfq.html (2004).
- 17.Risom SS, et al. Exercise-based cardiac rehabilitation for adults with atrial fibrillation. Cochrane Database Syst. Rev. 2017;2:CD011197. doi: 10.1002/14651858.CD011197.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams CA, et al. Physical activity interventions for people with congenital heart disease. Cochrane Database Syst. Rev. 2020;10:CD013400. doi: 10.1002/14651858.CD013400.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen KM, et al. Exercise-based cardiac rehabilitation for adult patients with an implantable cardioverter defibrillator. Cochrane Database Syst. Rev. 2019;2:CD011828. doi: 10.1002/14651858.CD011828.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson L, et al. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst. Rev. 2017;4:CD012264. doi: 10.1002/14651858.CD012264.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham LN, et al. Exercise-based cardiac rehabilitation for adults after heart valve surgery. Cochrane Database Syst. Rev. 2021;5:CD010876. doi: 10.1002/14651858.CD010876.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins, J. P. T. Cochrane handbook for systematic reviews of interventions version 6.2. Cochrane Training https://training.cochrane.org/handbook (2021).
- 23.Scherrenberg M, et al. Is there an optimal dose of cardiac rehabilitation in coronary artery disease patients? Int. J. Cardiol. 2021;330:7–11. doi: 10.1016/j.ijcard.2021.01.065. [DOI] [PubMed] [Google Scholar]
- 24.Nichols S, et al. Routine exercise-based cardiac rehabilitation does not increase aerobic fitness: a CARE CR study. Int. J. Cardiol. 2020;305:25–34. doi: 10.1016/j.ijcard.2020.01.044. [DOI] [PubMed] [Google Scholar]
- 25.Ganga HV, et al. Supervised exercise training versus usual care in ambulatory patients with left ventricular assist devices: a systematic review. PLoS ONE. 2017;12:e0174323. doi: 10.1371/journal.pone.0174323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tajrishi FZ, et al. Spontaneous coronary artery dissection and associated myocardial bridging: current evidence from cohort study and case reports. Med. Hypotheses. 2019;128:50–53. doi: 10.1016/j.mehy.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Wang HL, et al. Exercise interventions in cardio-oncology populations: a scoping review of the literature. J. Cardiovasc. Nurs. 2021;36:385–404. doi: 10.1097/JCN.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 28.Shields GE, et al. Cost-effectiveness of cardiac rehabilitation: a systematic review. Heart. 2018;104:1403–1410. doi: 10.1136/heartjnl-2017-312809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ. 2004;329:224–227. doi: 10.1136/bmj.329.7459.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knuuti J, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 31.Ponikowski P, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 32.Smith SC, Jr, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a Guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. J. Am. Coll. Cardiol. 2011;58:2432–2446. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 33.Yancy CW, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 34.NICE. Acute coronary syndromes: NICE guideline [NG185]. https://www.nice.org.uk/guidance/NG185 (2020).
- 35.NICE. Chronic heart failure in adults: diagnosis and management: NICE guideline [NG106]. https://www.nice.org.uk/guidance/ng106 (2018). [PubMed]
- 36.Chew DP, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of acute coronary syndromes. Med. J. Aust. 2016;205:128–133. doi: 10.5694/mja16.00368. [DOI] [PubMed] [Google Scholar]
- 37.Atherton JJ, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ. 2018;27:1123–1208. doi: 10.1016/j.hlc.2018.06.1042. [DOI] [PubMed] [Google Scholar]
- 38.Thomas RJ, et al. Home-based cardiac rehabilitation: a scientific statement from the American association of cardiovascular and pulmonary rehabilitation, the American Heart Association, and the American College of Cardiology. J. Am. Coll. Cardiol. 2019;74:133–153. doi: 10.1016/j.jacc.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price KJ, et al. A review of guidelines for cardiac rehabilitation exercise programmes: Is there an international consensus? Eur. J. Prev. Cardiol. 2016;23:1715–1733. doi: 10.1177/2047487316657669. [DOI] [PubMed] [Google Scholar]
- 40.BHF. National Audit of Cardiac Rehabilitation (NACR) Quality and Outcomes Report 2019. https://www.bhf.org.uk/informationsupport/publications/statistics/national-audit-of-cardiac-rehabilitation-quality-and-outcomes-report-2019 (2019).
- 41.Peters AE, Keeley EC. Trends and predictors of participation in cardiac rehabilitation following acute myocardial infarction: data from the behavioral risk factor surveillance system. J. Am. Heart Assoc. 2017;7:e007664. doi: 10.1161/JAHA.117.007664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruano-Ravina A, et al. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int. J. Cardiol. 2016;223:436–443. doi: 10.1016/j.ijcard.2016.08.120. [DOI] [PubMed] [Google Scholar]
- 43.Ragupathi L, et al. Availability, use, and barriers to cardiac rehabilitation in LMIC. Glob. Heart. 2017;12:323–334.e10. doi: 10.1016/j.gheart.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Kotseva K, et al. Use and effects of cardiac rehabilitation in patients with coronary heart disease: results from the EUROASPIRE III survey. Eur. J. Prev. Cardiol. 2013;20:817–826. doi: 10.1177/2047487312449591. [DOI] [PubMed] [Google Scholar]
- 45.NHS. Long-term Plan. Cardiovascular disease. https://www.longtermplan.nhs.uk/online-version/chapter-3-further-progress-on-care-quality-and-outcomes/better-care-for-major-health-conditions/cardiovascular-disease/ (2019).
- 46.Ades PA, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin. Proc. 2017;92:234–242. doi: 10.1016/j.mayocp.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalal HM, et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: the REACH-HF multicentre randomized controlled trial. Eur. J. Prev. Cardiol. 2019;26:262–272. doi: 10.1177/2047487318806358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor RS, et al. The cost effectiveness of REACH-HF and home-based cardiac rehabilitation compared with the usual medical care for heart failure with reduced ejection fraction: a decision model-based analysis. Eur. J. Prev. Cardiol. 2019;26:1252–1261. doi: 10.1177/2047487319833507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purcell C, et al. Protocol for an implementation study of an evidence-based home cardiac rehabilitation programme for people with heart failure and their caregivers in Scotland (SCOT:REACH-HF) BMJ Open. 2020;10:e040771. doi: 10.1136/bmjopen-2020-040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daw P, et al. Getting evidence into clinical practice: protocol for evaluation of the implementation of a home-based cardiac rehabilitation programme for patients with heart failure. BMJ Open. 2020;10:e036137. doi: 10.1136/bmjopen-2019-036137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison AS, Doherty P. Does the mode of delivery in routine cardiac rehabilitation have an association with cardiovascular risk factor outcomes? Eur. J. Prev. Cardiol. 2018;25:1925–1933. doi: 10.1177/2047487318798923. [DOI] [PubMed] [Google Scholar]
- 52.Buckingham SA, et al. Home-based versus centre-based cardiac rehabilitation: abridged Cochrane systematic review and meta-analysis. Open Heart. 2016;3:e000463. doi: 10.1136/openhrt-2016-000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imran HM, et al. Home-Based cardiac rehabilitation alone and hybrid with center-based cardiac rehabilitation in heart failure: a systematic review and meta-analysis. J. Am. Heart Assoc. 2019;8:e012779. doi: 10.1161/JAHA.119.012779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas RJ, et al. Home-based cardiac rehabilitation: a scientific statement from the american association of cardiovascular and pulmonary rehabilitation, the American Heart Association, and the American College of Cardiology. J. Am. Coll. Cardiol. 2019;74:133–153. doi: 10.1016/j.jacc.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hartley DM, Reisinger HS, Perencevich EN. When infection prevention enters the temple: intergenerational social distancing and COVID-19. Infect. Control Hosp. Epidemiol. 2020;41:868–869. doi: 10.1017/ice.2020.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishiga M, et al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schimelpfenig, N. What does a stay-at-home order mean? 11 questions answered. Healthline. https://www.healthline.com/health-news/what-a-stay-at-home-order-means (2020).
- 59.Babu AS, Arena R, Ozemek C, Lavie CJ. COVID-19: a time for alternate models in cardiac rehabilitation to take centre stage. Can. J. Cardiol. 2020;36:792–794. doi: 10.1016/j.cjca.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scherrenberg M, et al. The future is now: a call for action for cardiac telerehabilitation in the COVID-19 pandemic from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2021;28:524–540. doi: 10.1177/2047487320939671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doherty, P. & Harrison, A. Investigation of the impact of COVID-19 on UK CR: what can we learn from this experience? [abstract]. Presented at the 2020 BACPR Annual Conference (2020).
- 62.Buckingham SA, et al. Home-based versus centre-based cardiac rehabilitation: abridged Cochrane systematic review and meta-analysis. Open Heart. 2016;3:e000463. doi: 10.1136/openhrt-2016-000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rawstorn JC, et al. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102:1183–1192. doi: 10.1136/heartjnl-2015-308966. [DOI] [PubMed] [Google Scholar]
- 64.Thomas RJ, et al. 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J. Am. Coll. Cardiol. 2018;71:1814–1837. doi: 10.1016/j.jacc.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 65.Vishwanath V, Beckman AL, Kazi DS. Reimagining cardiac rehabilitation in the era of coronavirus disease 2019. JAMA Health Forum. 2020;1:e201346. doi: 10.1001/jamahealthforum.2020.1346. [DOI] [PubMed] [Google Scholar]
- 66.Thomas E, Gallagher R, Grace SL. Future-proofing cardiac rehabilitation: transitioning services to telehealth during COVID-19. Eur. J. Prev. Cardiol. 2021;28:e35–e36. doi: 10.1177/2047487320922926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dalal H, et al. Correspondence to the EJPC in response to position paper by Ambrosetti M et al. 2020 Cardiovascular rehabilitation and COVID-19: the need to maintain access to evidence-based services from the safety of home. Eur. J. Prev. Cardiol. 2020 doi: 10.1177/2047487320923053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson, M. & Kumar, M. Digital divide persists even as lower-income Americans make gains in tech adoption. Pew Research Center. https://www.pewresearch.org/fact-tank/2019/05/07/digital-divide-persists-even-as-lower-income-americans-make-gains-in-tech-adoption/ (2021).
- 69.Balady GJ, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124:2951–2960. doi: 10.1161/CIR.0b013e31823b21e2. [DOI] [PubMed] [Google Scholar]
- 70.Eberly LA, et al. Telemedicine outpatient cardiovascular care during the COVID-19 pandemic: bridging or opening the digital divide? Circulation. 2020;142:510–512. doi: 10.1161/CIRCULATIONAHA.120.048185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nouri S, Khoong EC, Lyles CR, Karliner L. Addressing equity in telemedicine for chronic disease management during the Covid-19 pandemic. NEJM Catalyst. 2020 doi: 10.1056/CAT.20.0123. [DOI] [Google Scholar]
- 72.NICE. COVID-19 ready rehabilitation for heart failure: REACH-HF can deliver. https://www.nice.org.uk/sharedlearning/covid-19-ready-rehabilitation-for-heart-failure-reach-hf-can-deliver (2020).
- 73.O’Connor CM, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barnett K, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 75.Jani BD, et al. Relationship between multimorbidity, demographic factors and mortality: findings from the UK Biobank cohort. BMC Med. 2019;17:74. doi: 10.1186/s12916-019-1305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Makovski TT, et al. Multimorbidity and quality of life: systematic literature review and meta-analysis. Ageing Res. Rev. 2019;53:100903. doi: 10.1016/j.arr.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 77.Sumner J, Böhnke JR, Doherty P. Does service timing matter for psychological outcomes in cardiac rehabilitation? Insights from the National Audit of Cardiac Rehabilitation. Eur. J. Prev. Cardiol. 2018;25:19–28. doi: 10.1177/2047487317740951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barker K, et al. A rehabilitation programme for people with multimorbidity versus usual care: a pilot randomized controlled trial. J. Comorb. 2018;8:2235042X18783918. doi: 10.1177/2235042X18783918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cowie A, McKay J, Keenan A. Combined generic-specialist multimorbidity rehabilitation post acute cardiac event. Brit. J. Card. Nurs. 2018;13:340–347. [Google Scholar]
- 80.Taylor RS, Singh S. Personalised rehabilitation for cardiac and pulmonary patients with multimorbidity: time for implementation? Eur. J. Prev. Cardiol. 2020 doi: 10.1177/2047487320926058. [DOI] [PubMed] [Google Scholar]
- 81.GBD 2017 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.WHO. Global status report on noncommunicable diseases 2010. United Nations Digital Library https://digitallibrary.un.org/record/706319?ln=en (2011).
- 83.Turk-Adawi K, et al. Cardiac rehabilitation availability and density around the globe. EClinicalMedicine. 2019;13:31–45. doi: 10.1016/j.eclinm.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Global Health Data Exchange. Institute for Health Metrics and Evaluation. GBD results tool. http://ghdx.healthdata.org/gbd-results-tool (2021).
- 85.Pesah E, et al. Cardiac rehabilitation delivery in low/middle-income countries. Heart. 2019;105:1806–1812. doi: 10.1136/heartjnl-2018-314486. [DOI] [PubMed] [Google Scholar]
- 86.Taylor, R. S., Grace, S., Mamataz, T., Uddin, J. & Ibn Alam, S. Effects of cardiac rehabilitation in low- and middle-income countries: a systematic review and meta-analyses of randomised controlled trials. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=185296 (2020). [DOI] [PMC free article] [PubMed]
- 87.Uddin J, et al. Predictors of exercise capacity following exercise-based rehabilitation in patients with coronary heart disease and heart failure: a meta-regression analysis. Eur. J. Prev. Cardiol. 2016;23:683–693. doi: 10.1177/2047487315604311. [DOI] [PubMed] [Google Scholar]
- 88.Oldridge NB, Pakosh MT, Thomas RJ. Cardiac rehabilitation in low- and middle-income countries: a review on cost and cost-effectiveness. Int. Health. 2016;8:77–82. doi: 10.1093/inthealth/ihv047. [DOI] [PubMed] [Google Scholar]
- 89.Salvetti XM, Oliveira JA, Servantes DM, Vincenzo de Paola AA. How much do the benefits cost? Effects of a home-based training programme on cardiovascular fitness, quality of life, programme cost and adherence for patients with coronary disease. Clin. Rehabil. 2008;22:987–996. doi: 10.1177/0269215508093331. [DOI] [PubMed] [Google Scholar]
- 90.Uddin J, et al. Effect of home-based cardiac rehabilitation in a lower-middle income country: results from a controlled trial. J. Cardiopulm. Rehabil. Prev. 2020;40:29–34. doi: 10.1097/HCR.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 91.Grace SL, et al. Cardiac rehabilitation delivery model for low-resource settings: an International Council of Cardiovascular Prevention and Rehabilitation consensus statement. Prog. Cardiovasc. Dis. 2016;59:303–322. doi: 10.1016/j.pcad.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 92.Taylor R, Zwisler AD, Uddin J. Global health-care systems must prioritise rehabilitation. Lancet. 2021;396:1946–1947. doi: 10.1016/S0140-6736(20)32533-2. [DOI] [PubMed] [Google Scholar]
- 93.WHO. Global status report on noncommunicable diseases 2014. https://apps.who.int/iris/handle/10665/148114 (2014).
- 94.WHO. Rehabilitation 2030: a call for action. https://www.who.int/news-room/events/detail/2017/02/06/default-calendar/rehabilitation-2030-a-call-for-action (2017).