ABSTRACT
The cell walls and capsules of Cryptococcus neoformans, a yeast-type fungal pathogen, are rich in polysaccharides. Dectin-2 is a C-type lectin receptor (CLR) that recognizes high-mannose polysaccharides. Previously, we demonstrated that Dectin-2 is involved in cytokine production by bone marrow-derived dendritic cells (BM-DCs) in response to stimulation with C. neoformans. In the present study, we analyzed the role of Dectin-2 in the phagocytosis of C. neoformans by BM-DCs. The engulfment of this fungus by BM-DCs was significantly decreased in mice lacking Dectin-2 (Dectin-2 knockout [Dectin-2KO]) or caspase recruitment domain-containing protein 9 (CARD9KO), a common adapter molecule that delivers signals triggered by CLRs, compared to wild-type (WT) mice. Phagocytosis was likewise inhibited, to a similar degree, by the inhibition of Syk, a signaling molecule involved in CLR-triggered activation. A PI3K inhibitor, in contrast, completely abrogated the phagocytosis of C. neoformans. Actin polymerization, i.e., conformational changes in cytoskeletons detected at sites of contact with C. neoformans, was also decreased in BM-DCs of Dectin-2KO and CARD9KO mice. Finally, the engulfment of C. neoformans by macrophages was significantly decreased in the lungs of Dectin-2KO mice compared to WT mice. These results suggest that Dectin-2 may play an important role in the actin polymerization and phagocytosis of C. neoformans by DCs, possibly through signaling via CARD9 and a signaling pathway mediated by Syk and PI3K.
KEYWORDS: Cryptococcus neoformans, immunology, innate immunity, phagocytosis
INTRODUCTION
Cryptococcus neoformans, a yeast-type fungus with a thick capsule, multiplies in the fecal sediments of birds such as pigeons. This fungal pathogen infects hosts via an airborne route and causes meningoencephalitis in patients with impaired cell-mediated immunity such as AIDS (1, 2). While in the environment between hosts, C. neoformans has a thinner capsule, which is convenient for entering the alveolar spaces; after entering a new host, the fungal yeast cells generate a thick capsule, which makes it easier for them to resist phagocytosis by neutrophils and macrophages (3). C. neoformans can even grow within macrophages due to a mechanism that permits them to escape from being killed by these cells (3). Th1-mediated cellular immunity plays a central role in protecting hosts against this fungal pathogen, whereas neutrophils and antibodies do not (4). Critical factors in the differentiation of Th1 cells for this purpose include both interleukin-12 (IL-12) and interferon gamma (IFN-γ) in addition to antigen presentation to naive T cells by dendritic cells (DCs) (5–7). Innate immune cells such as macrophages and DCs recognize pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs) (8, 9). It is important to clarify the mechanisms by which these cells detect C. neoformans via PRRs, as this will reveal how the Th1-mediated host protective response is developed during cryptococcal infection.
Previous studies (10, 11) have demonstrated that Toll-like receptor 9 (TLR9) plays critical roles in the development of the Th1-mediated host protective response via recognition of DNA from C. neoformans. In contrast, deficiency of either TLR2 or TLR4 does not affect host protection against this fungal pathogen (12). Because C. neoformans is rich in polysaccharides in its cell walls and capsules, we can predict that C-type lectin receptors (CLRs), molecules that sense polysaccharides (13), are involved in the recognition of this fungus by immune cells. Yamamoto and coworkers have demonstrated that caspase recruitment domain-containing protein 9 (CARD9), a common adaptor molecule that delivers signals triggered by CLRs, plays a critical role in the Th1-mediated host protective response to cryptococcal infection (14), which suggests the possible involvement of a particular CLR in this response. Among the CLRs, dendritic cell-associated C-type lectin 1 (Dectin-1) contributes to host defense against infection with Candida albicans, Pneumocystis carinii, and Aspergillus fumigatus through its recognition of β-1,3-glucan (15–17), whereas Dectin-2 plays a critical role in defense against infection with fungal pathogens, including C. albicans and A. fumigatus, through its recognition of high-mannose polysaccharides (18, 19). In our previous studies (20), however, deficiency of either Dectin-1 or Dectin-2 did not affect host defense against cryptococcal infection, although Th2 cytokines and mucin production in the lungs were increased in Dectin-2-deficient mice during infection (21). In addition, Dectin-2 deficiency reduces cytokine production by macrophages and dendritic cells (DCs) upon in vitro stimulation with C. neoformans (21). To activate these cells, Dectin-2 must recognize a particular polysaccharide; in the case of C. neoformans, it recognizes mannoprotein (21). Our previous study also demonstrated that macrophage inducible C-type lectin (Mincle) recognized its ligands within C. neoformans yeast cells (22). In spite of these intriguing findings, it is not yet understood precisely how CLRs are involved in the host defense and immune response against cryptococcal infection.
DCs are particularly necessary for initiating Th1 cell differentiation through antigen presentation and delivery of costimulatory and cytokine signals to naive T cells (23). To begin these processes, the DCs must first engulf and digest C. neoformans cells, which enables them to process the antigens (23). In previous studies, mannose receptor (MR) and dendritic cell-specific ICAM3 grabbing nonintegrin (DC-SIGN) have been reported to play a role in the phagocytosis of this fungus by macrophages and DCs through their binding to the mannoproteins on the yeast cell surfaces (24). In the present study, we explore the mechanism underlying the phagocytosis of C. neoformans by DCs, with attention given to the possible roles of CLRs, including Dectin-2, macrophage inducible C-type lectin (Mincle), and CARD9, in the engulfment of this fungal pathogen.
RESULTS
Effect of capsules on the phagocytosis of C. neoformans by BM-DCs.
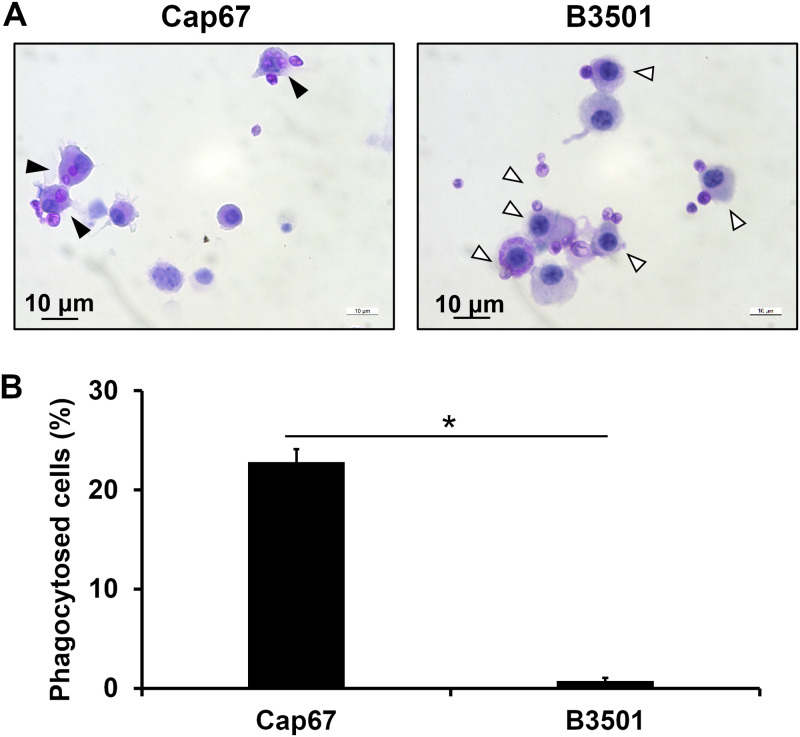
To elucidate the effect of cryptococcal capsules on the phagocytosis of this fungus by BM-DCs, the numbers of BM-DCs engulfing yeast cells were compared after BM-DCs were incubated with B3501 and Cap67, an acapsular mutant of B3501. This experiment was performed using the periodic acid Schiff (PAS) staining method. As shown in Fig. 1A and B, approximately 20% of BM-DCs had engulfed Cap67 cells during incubation, whereas no B3501 cells were engulfed. These results indicate that the capsule of C. neoformans makes it strongly resistant to phagocytosis by DCs. Therefore, Cap67, but not B3501, was used in the subsequent experiments, which were designed to mimic the situation just after C. neoformans invades the alveolar spaces, when these yeast cells have no capsules or thinly expressed capsules (3).
FIG 1.
Effect of capsules on the phagocytosis of C. neoformans by BM-DCs. BM-DCs from WT mice were cultured with C. neoformans Cap67 or B3501 for 2 h. (A) These cells were spun down onto a glass slide, stained with PAS, and observed under a light microscope. Closed arrows, phagocytosed cells; open arrows, cells attached to fungi on their surfaces. (B) The proportions of phagocytosed cells are shown. Each column represents the mean ± SD of triplicate cultures. *, P < 0.05.
Role of cations in the phagocytosis of C. neoformans by BM-DCs.
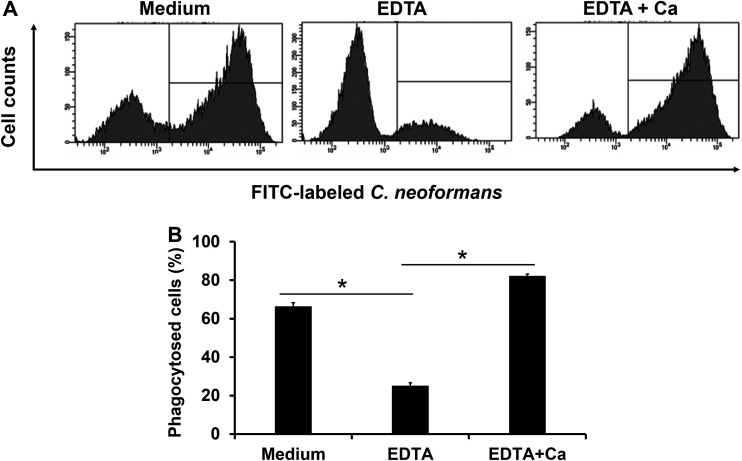
Since the Ca2+ ion is required for the binding of polysaccharides to CLRs, we examined the effect of EDTA, a chelator of cations, on the phagocytosis of C. neoformans by BM-DCs. This experiment was performed using flow cytometry. As shown in Fig. 2A and B, phagocytosis of the yeast cells by BM-DCs was strongly inhibited by the addition of EDTA, while the replacement of the Ca2+ ion led to complete recovery of this response up to the levels seen in the absence of EDTA. These results suggest that certain CLRs may be involved in the phagocytosis of this fungus by BM-DCs.
FIG 2.
Role of cations in the phagocytosis of C. neoformans by BM-DCs. BM-DCs from WT mice were pretreated with 10 mM EDTA. These BM-DCs were cultured with FITC-labeled C. neoformans Cap67 in the culture medium supplemented with 10 mM CaCl2. (A) Phagocytosed cells were analyzed using flow cytometry. Representative histograms are shown. (B) The proportions of phagocytosed cells are shown. Each column represents the mean ± SD of triplicate cultures. *, P < 0.05.
Contribution of CLRs and CARD9 to the phagocytosis of C. neoformans by BM-DCs.
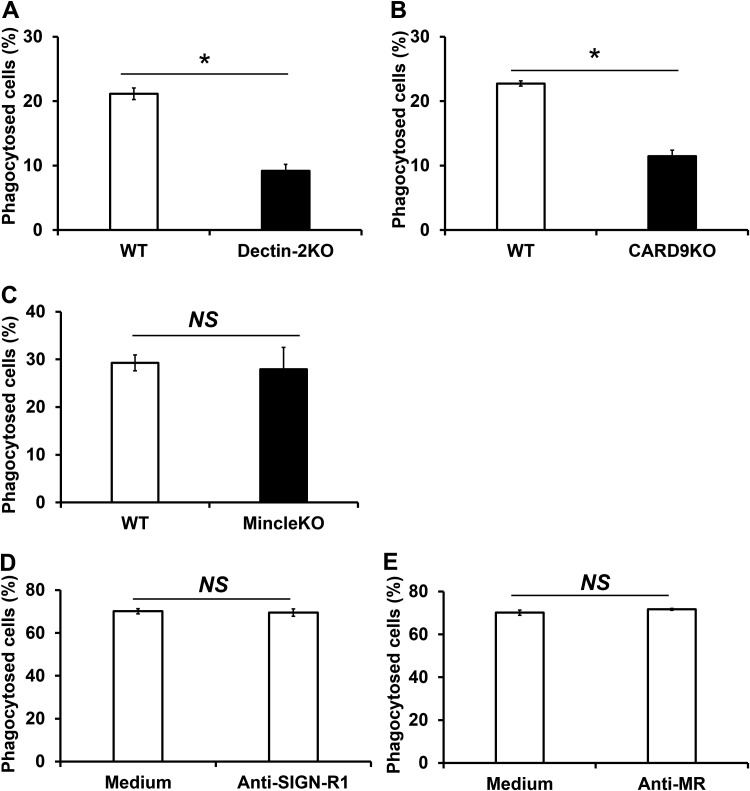
To address the possible involvement of CLRs in the phagocytosis of C. neoformans by BM-DCs, we evaluated the effect of Dectin-2, Mincle, and CARD9 deficiency on this response. These experiments were analyzed using the PAS staining method and flow cytometry. The PAS staining data are presented in Fig. 3. As shown in Fig. 3A and B, phagocytosis of this fungus was significantly decreased in Dectin-2KO mice compared with WT mice, as well as in CARD9KO mice compared with WT mice. Deficiency of Mincle, on the other hand, did not suppress the phagocytosis of C. neoformans by BM-DCs (Fig. 3C). Additionally, Dectin-2, Mincle, and CARD9 deficiency did not affect the phagocytosis of capsular strain B3501 (see Fig. S1 in the supplemental material).
FIG 3.
Contribution of CLRs and CARD9 to the phagocytosis of C. neoformans by BM-DCs. (A to C) BM-DCs prepared from WT and Dectin-2KO (A), CARD9KO (B), and MincleKO (C) mice were cultured with C. neoformans Cap67 for 2 h. These cells were spun down onto a glass slide, stained with PAS, and observed under a light microscope. (D and E) BM-DCs from WT mice were preincubated with anti-MR Ab or anti-SIGN-R1 Ab for 30 min before phagocytosis assay. Phagocytosed cells were analyzed using flow cytometry. The proportions of phagocytosed cells are shown. Each column represents the mean ± SD of triplicate cultures. *, P < 0.05. NS, not significant.
We also explored the possible involvement of the CLRs MR and SIGN-R1 by examining the effects of antibody (Ab) against each receptor. These experiments were analyzed using the PAS staining method and flow cytometry. The flow cytometric data are presented in Fig. 3. As shown in Fig. 3D and E, the addition of Ab against either MR or SIGN-R1 had no suppressive effect on the engulfment of C. neoformans by BM-DCs, whereas these antibodies did efficiently block the phagocytosis of C. albicans (Fig. S2).
These results indicate that Dectin-2 and its adaptor molecule CARD9, but not Mincle, MR, or SIGN-R1, are involved in the engulfment of C. neoformans by BM-DCs.
Effect of inhibitors of Syk and PI3K on the phagocytosis of C. neoformans by BM-DCs.
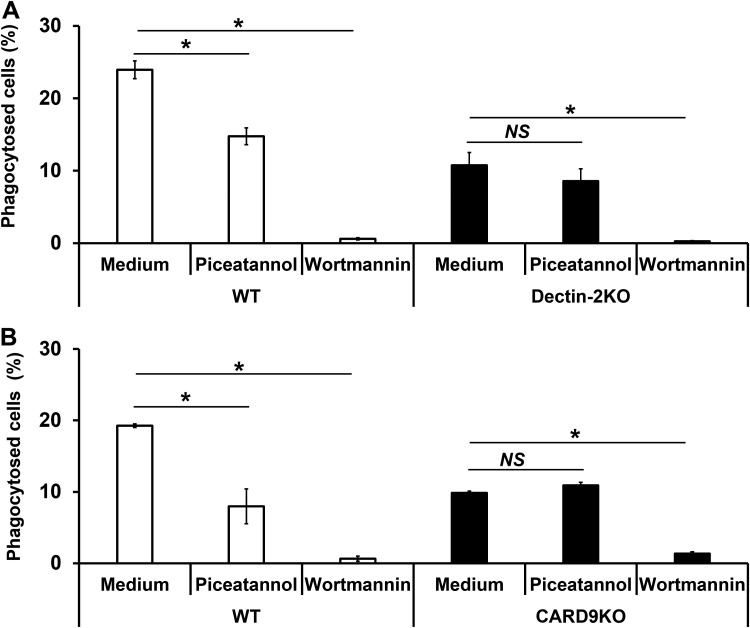
Dectin-2 is known to induce the activation of DCs through signaling delivered by Syk and PI3K (25, 26). Therefore, we next evaluated the effect of inhibiting these signaling molecules on the phagocytosis of C. neoformans by BM-DCs. This experiment was performed using the PAS staining method. As shown in Fig. 4A and B, BM-DCs in WT mice treated with piceatannol, a Syk inhibitor, exhibited significantly reduced levels of phagocytosis of the fungus, comparable to the levels seen in Dectin-2KO mice and CARD9KO mice. In contrast, wortmannin, a PI3K inhibitor, completely abrogated phagocytosis by BM-DCs in both KO genotypes as well as in WT mice (Fig. 4A and B). These results suggest that Dectin-2 may trigger signaling for the phagocytosis of this fungus through the Syk-CARD9 pathway and that PI3K may be involved in this Dectin-2-mediated phagocytosis pathway. Furthermore, PI3K may be essential for phagocytosis pathways mediated by other receptors as well as that mediated by Dectin-2.
FIG 4.
Effect of inhibitors of Syk and PI3K on the phagocytosis of C. neoformans by BM-DCs. BM-DCs prepared from WT and Dectin-2KO (A) and CARD9KO (B) mice were preincubated with piceatannol or wortmannin for 30 min. The treated BM-DCs were cultured with C. neoformans Cap67 for 2 h. These cells were spun down onto a glass slide, stained with PAS, and observed under a light microscope. The proportions of phagocytosed cells are shown. Each column represents the mean ± SD of triplicate cultures. *, P < 0.05. NS, not significant.
Involvement of Dectin-2 and CARD9 in actin polymerization.
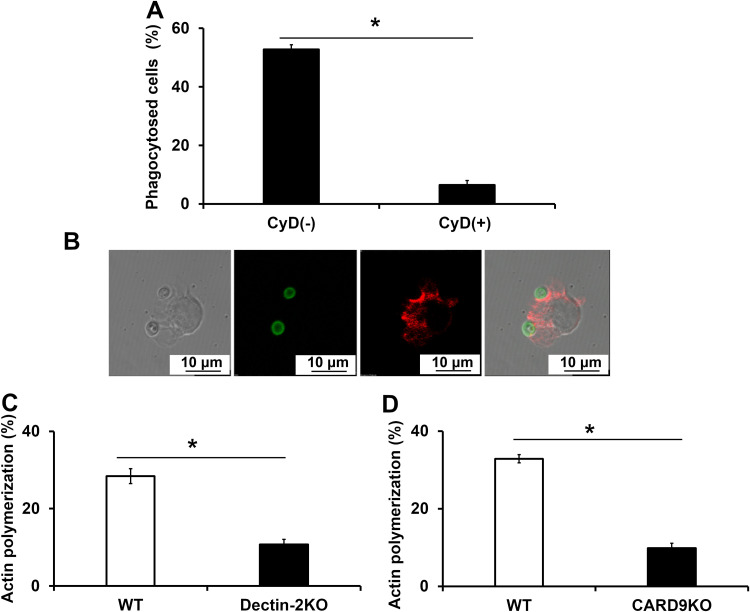
Actin polymerization at the contact site usually triggers phagocytosis through cytoskeleton conformational change. To confirm the role of actin polymerization in the phagocytosis of C. neoformans, we examined the effect of cytochalasin D, an inhibitor of actin polymerization, on this response. This experiment was performed using flow cytometry. As shown in Fig. 5A, the addition of cytochalasin D to the cultures strongly inhibited the engulfment of C. neoformans by BM-DCs, demonstrating that actin polymerization is a necessary part of the phagocytosis of this fungus. We also used confocal microscopy to visualize actin polymerization in BM-DCs after incubation with C. neoformans. As shown in Fig. 5B, actin polymerization was detected only at the submembranous areas where BM-DCs were in direct contact with fungal yeast cells. Actin polymerization was significantly attenuated in Dectin-2KO and CARD9KO mice compared with WT mice (Fig. 5C and D). These results indicate that the phagocytosis of C. neoformans by BM-DCs is mediated by Dectin-2 and CARD9 and that it occurs through actin polymerization.
FIG 5.
Involvement of Dectin-2 and CARD9 in actin polymerization. (A) BM-DCs from WT mice were pretreated with cytochalasin D (CyD) for 30 min. These BM-DCs were cultured with FITC-labeled C. neoformans Cap67 for 2 h. Phagocytosed cells were analyzed using flow cytometry. (B, C, D) BM-DCs prepared from WT and Dectin-2KO and CARD9KO mice were cultured with FITC-labeled C. neoformans Cap67 for 30 min. BM-DCs were spun down onto a glass slide, stained with Acti-stain 555 phalloidin, and observed under a confocal laser scanning microscope. BM-DCs with strong actin polymerization (red) at sites where they were bound to C. neoformans (green) were counted, and the percentages of all cells with this characteristic were calculated. Each column represents the mean ± SD of triplicate cultures. *, P < 0.05.
Effect of complement molecules on the Dectin-2-mediated phagocytosis of C. neoformans by BM-DCs.
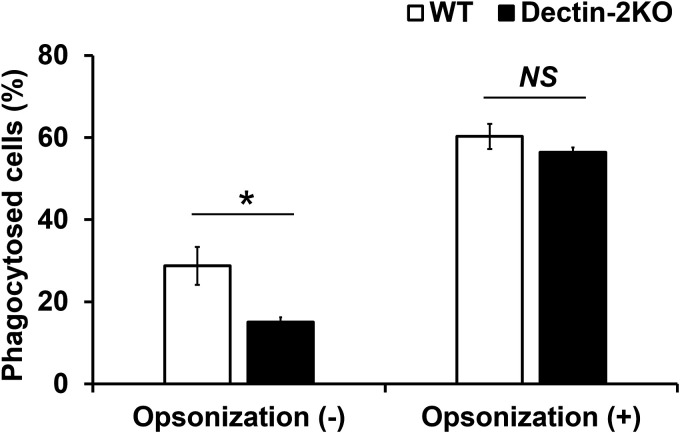
Phagocytosis is promoted not only by PRRs such as CLRs but also by the complement system. We examined how Dectin-2-mediated phagocytosis was affected under conditions of opsonization by complement molecules. To induce opsonization, C. neoformans was preincubated with murine serum. This experiment was analyzed using the PAS staining method and flow cytometry. The PAS staining data are presented in Fig. 6. Phagocytosis of C. neoformans by BM-DCs occurred at equivalent levels in WT and Dectin-2KO mice after opsonization by serum opsonins (Fig. 6). These results indicate that Dectin-2-dependent phagocytosis of C. neoformans is functional only in the absence of serum opsonin-dependent opsonization.
FIG 6.
Effect of complement on the Dectin-2-mediated phagocytosis of C. neoformans by BM-DCs. C. neoformans Cap67 was preincubated with serum derived from WT mice for 1 h. These cells were cultured with BM-DCs prepared from WT and Dectin-2KO mice for 2 h. These cells were spun down onto a glass slide, stained with PAS, and observed under a light microscope. The proportions of phagocytosed cells are shown. Each column represents the mean ± SD of triplicate cultures. *, P < 0.05. NS, not significant.
Involvement of Dectin-2 in the phagocytosis of C. neoformans in the lungs.
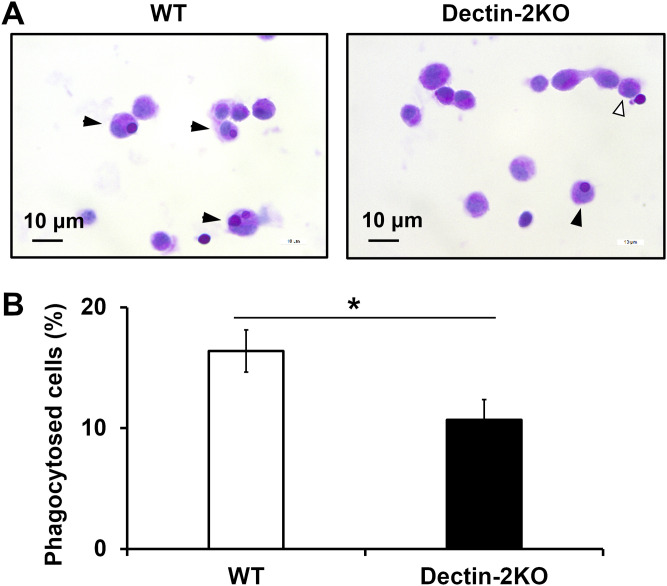
The results shown in Fig. 6 suggest that phagocytosis triggered by Dectin-2 may not work during in vivo infection, when complement-mediated opsonization is usually activated. To address this possibility, we next examined whether Dectin-2 was involved in the phagocytosis of C. neoformans during in vivo infection in the lungs. The number of macrophages engulfing yeast cells was evaluated in bronchoalveolar lavage fluid (BALF) collected from mice 3 h after infection with C. neoformans. This experiment was performed using the PAS staining method. As shown in Fig. 7A and B, fewer C. neoformans cells were engulfed in Dectin-2KO mice than were engulfed in WT mice. These results suggest that Dectin-2 may actually be involved in the phagocytosis of C. neoformans in infected lungs.
FIG 7.
Involvement of Dectin-2 in the phagocytosis of C. neoformans in the lungs during infection. WT and Dectin-2KO mice were infected intratracheally with C. neoformans Cap67. Then, 3 h after infection, BALF was collected. (A) The phagocytosed cells in BALF were spun down onto a glass slide, stained with PAS, and observed under a microscope. Closed arrows, phagocytosed cells; open arrows, cells attached to fungi on their surfaces. (B) The proportions of phagocytosed cells are shown. Each column represents the mean ± SD of three to four mice. *, P < 0.05.
DISCUSSION
The present study focused on the function of CLRs, especially Dectin-2, in the phagocytosis of C. neoformans by DCs and demonstrated that Dectin-2 contributes to the engulfment of this fungus. Previous studies have reported that Dectin-2 expressed on macrophages and neutrophils is involved in these cells’ uptake of cancer cells and Candida glabrata, which is consistent with our results (27, 28). In BM-DCs, the phagocytosis of C. neoformans was mediated by Dectin-2, but the presence or absence of Dectin-2 only changed the phagocytosis rate by approximately 50%, suggesting that some other receptor may be involved in the uptake of this fungus. Although β-glucosylceramide (β-GlcCer) and mannoprotein, both of which are components of this fungus, are recognized by Mincle and MR/SING-R1, respectively (24, 29), these CLRs were not found to contribute to the phagocytosis of C. neoformans by BM-DCs. A possible reason for this is that other fungal cell wall components, such as glucan and chitin, may mask these ligands from their receptors, preventing the receptors from interacting with the ligands on C. neoformans. Because macrophage receptors with collagenous structure (MARCO) and CD36, classified as scavenger receptors, have previously been reported to play roles in the phagocytosis of C. neoformans (30, 31), a similar involvement in the phagocytosis of this fungus by BM-DCs is also possible.
CARD9, a common adaptor molecule that delivers signals triggered by CLRs, also contributes to the phagocytosis of C. neoformans by BM-DCs. The engulfment of this fungus by BM-DCs from Dectin-2KO mice was reduced to a degree similar to that seen in BM-DCs from CARD9KO mice, suggesting that Dectin-2 may be a major player acting as the upstream receptor of CARD9 for this phagocytosis response. In line with this possibility, BM-DCs from MincleKO mice engulfed the fungus at the same rate as that seen in WT-derived BM-DCs. Furthermore, we addressed the possible involvement of signaling molecules such as Syk and PI3K in the phagocytosis of this fungus by examining the effects of their respective inhibitors. These molecules have been reported to play important roles in the production of cytokines by dendritic cells via a Dectin-2-dependent pathway (25, 26). In the present study, a Syk inhibitor significantly but partially ameliorated the phagocytosis of C. neoformans by BM-DCs, resulting in a reduction similar to that observed in BM-DCs from Dectin-2KO or CARD9KO mice. These results support the hypothesis that Dectin-2 is the principal player acting upstream of CARD-9-mediated signaling. In contrast, a PI3K inhibitor completely abrogated the phagocytosis of C. neoformans by BM-DCs, indicating that this molecule is essential for the phagocytosis response, although it remains unclear whether PI3K activation is triggered by Dectin-2 or by some other receptor (25, 26).
Interestingly, Dectin-2 and CARD9 might be among the factors that induce actin polymerization, which in turn triggers conformational change in cytoskeletons. Dectin-1, a CLR involved in the recognition of β-1,3-glucan, is known as a phagocytic receptor against Candida albicans, which activates the Rho family members Rac1 and Cdc42 through a PI3K-mediated signaling pathway (32, 33). These molecules contribute to actin polymerization, which eventually leads to phagocytosis by macrophages (34). Similarly, Dectin-2-mediated signaling may modulate some regulators of actin polymerization such as the Rho family. Further investigations are required to improve our understanding of the precise mechanisms involved in Dectin-2-mediated phagocytosis.
In in vivo infection, microorganisms are usually opsonized by activated complements (35). Therefore, we examined whether the Dectin-2-mediated phagocytosis of C. neoformans was still functional under conditions of opsonization by serum opsonins and found that the phagocytosis of yeast cells opsonized by serum opsonins was almost equivalent between WT and Dectin-2KO mice. These results raise the possibility that phagocytosis triggered by Dectin-2 may not work during in vivo infection. This possibility is unlikely, however, given that the phagocytosis of C. neoformans by macrophages is significantly attenuated in the lungs of Dectin-2KO mice compared with WT mice after infection with this fungal pathogen. There are several possible explanations for the difference between the results obtained in in vivo and in vitro infection. First, the yeast cells were exposed to excessive amounts of opsonins in serum and may have been opsonized at the optimal level to promote phagocytosis. In short-term in vivo infection, in contrast, yeast cells may not be fully opsonized to the optimal level for phagocytosis; under such conditions, Dectin-2-mediated signaling may support a lesser degree of phagocytosis. Second, BM-DCs were used in an in vitro phagocytosis assay; in vivo, however, macrophages are major participants in the phagocytosis of C. neoformans during infection. The different cell types targeted for analysis may have caused these different results, because different cells may have different levels of dependency on Dectin-2 for phagocytosis. In the early phase of an infection, Dectin-2 may work as an efficient phagocytic receptor against C. neoformans in the lung, although the presence of large quantities of opsonins may mask this contribution.
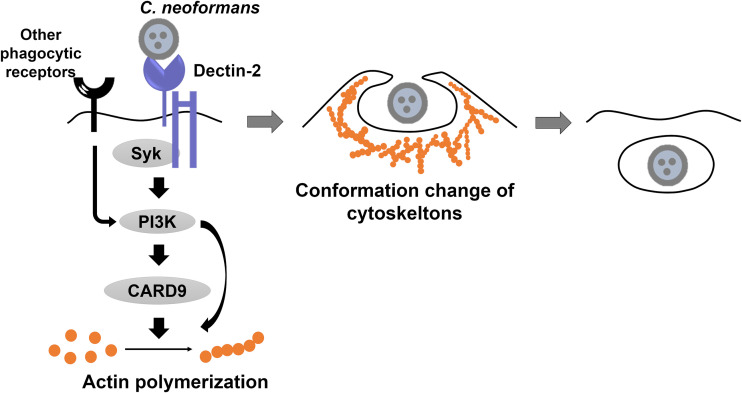
In conclusion, the present study demonstrates that Dectin-2 plays a critical role in the actin polymerization and phagocytosis of C. neoformans by BM-DCs, possibly through signal delivery by CARD9 and a signaling pathway mediated by Syk and PI3K, as summarized in Fig. 8. This promotion of phagocytosis may also operate in macrophages during infection with this fungal pathogen. Although Dectin-1 is reported to contribute to phagocytosis by macrophages (34), this is, to the best of our knowledge, the first report identifying the role of Dectin-2 in promoting actin polymerization and phagocytosis by DCs, in addition to its ability to induce the production of cytokines by macrophages and DCs (18, 21). Thus, this study provides clues to a better understanding of the mechanisms controlling the innate immune response during infection with C. neoformans.
FIG 8.
Summary of Dectin-2-induced phagocytosis of C. neoformans by DCs. Dectin-2 expressed on the cell surfaces of DCs recognizes C. neoformans. Dectin-2-mediated signaling for actin polymerization and phagocytosis is delivered by Syk and PI3K and leads in turn to the activation of CARD9, an adaptor molecule of this receptor, at sites of contact with C. neoformans. Following actin polymerization, cytoskeleton structures are changed, leading to the phagocytosis of the yeast cells by DCs. A phagocytic receptor other than Dectin-2 might be involved in the phagocytosis of C. neoformans triggered via PI3K-dependent signaling, possibly through a CARD9-independent pathway.
MATERIALS AND METHODS
Mice.
Dectin-2 knockout (Dectin-2KO), CARD9KO, and MincleKO mice were generated and backcrossed more than eight generations to C57BL/6 mice, as previously described (36–38). C57BL/6 mice (CLEA, Tokyo, Japan) were used as wild-type (WT) mice in this study, and male or female mice were used at 6 to 10 weeks of age. All mice were kept under specific-pathogen-free conditions at the Institute for Animal Experimentation, Tohoku University Graduate School of Medicine. All experimental procedures involving animals followed the Regulations for Animal Experiments and Related Activities at Tohoku University, Sendai, Japan, and were approved by the Institutional Animal Care and Use Committee at Tohoku University. All experiments were performed under anesthesia, and all efforts were made to minimize the suffering of the animals.
Microorganisms.
A serotype D strain of Cryptococcus neoformans known as B3501 (provided by K. J. Kwong-Chung, National Institutes of Health, Bethesda, MD, USA) and the capsule deletion strain Cap67 (a kind gift from S. M. Levitz, University of Massachusetts Medical School, Worcester, MA, USA) were cultured on potato dextrose agar (PDA; Eiken, Tokyo, Japan) plates for 2 to 3 days. As a control, Candida albicans was cultured separately on PDA plates for 1 day. Before use, fungi were washed three times with normal saline.
Preparation of BM-DCs.
WT, Dectin-2KO, CARD9KO, and MincleKO mice were sacrificed by cervical dislocation, and bone marrow cells were harvested from their femurs. After erythrocyte lysis with lysis buffer (0.83% NH4Cl, pH 7.2, and Tris-HCl; 9: 1), these bone marrow cells were cultured in 10 ml of RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO, USA) containing 10% fetal calf serum (FCS) (Biowest, Nuaillé, France), 100 U/ml penicillin G, 100 μg/ml streptomycin, 50 μM 2-mercaptoethanol (Sigma-Aldrich), and 20 ng/ml murine granulocyte-macrophage colony-stimulating factor (GM-CSF; Wako, Osaka, Japan). On day 3, 10 ml of the same medium was added; on day 6, half of the medium was changed. On day 8, floating cells were collected and used as bone marrow-derived dendritic cells (BM-DCs).
Phagocytosis assay.
BM-DCs (2 × 104 cells) from WT, Dectin-2KO, CARD9KO, and MincleKO mice were cocultured with C. neoformans (2 × 105 cells) for 2 h at 37°C in a 5% CO2 incubator. These cells were attached onto a slide glass by centrifugation at 1,000 × g for 5 min using a StatSpin CytoFuge 2 (Iris Sample Processing, Westwood, MA, USA) and stained with a PAS staining kit (Muto, Tokyo, Japan). Finally, 500 cells were counted under microscopic observation, and the proportion of phagocytosed cells was calculated as the phagocytosis rate. In a flow cytometric analysis, C. neoformans was labeled with fluorescein isothiocyanate (FITC; Dojin, Tokyo, Japan) for 10 min at room temperature and cocultured with BM-DCs. To deplete Ca2+, BM-DCs were pretreated with 10 mM EDTA; to supplement Ca2+, BM-DCs were incubated with C. neoformans in the culture medium described above, with 10 mM CaCl2 added (Wako). For opsonization, cells of the Cap67 strain (5 × 107 cells) were preincubated with serum derived from WT mice for 1 h at 37°C. After being washed three times, opsonized Cap67 cells were assayed for phagocytosis by BM-DCs.
Inhibitors and antibodies.
As inhibitors, BM-DCs were preincubated with either 50 μM piceatannol (Sigma-Aldrich), 1 μg/ml wortmannin (Adipogen Life Sciences, San Diego, CA, USA), or 10 μg/ml cytochalasin D (Wako) for 30 min on ice. As neutralizing antibodies, BM-DCs were incubated with either 200 ng/ml anti-MR antibody (Abcam, Cambridge, MA, USA) or anti-SIGN-R1 (DC-SIGN murine homologue) antibody (Hycult Biotech, Wayne, PA, USA).
Phagocytosis analysis by flow cytometry.
After BM-DCs were cocultured with fluorescein isothiocyanate (FITC)-labeled C. neoformans for 2 h at 37°C in a 5% CO2 incubator, the cultures were treated with 0.02% trypan blue at room temperature for 15 min to quench the fluorescein of extracellular C. neoformans. After this treatment, the cells were washed three times with phosphate-buffered saline (PBS) containing 1% FCS and 0.1% sodium azide (Sigma-Aldrich) and then stained with allophycocyanin (APC)-anti-mouse CD11c MAb (clone 145-2C11; BioLegend, San Diego, CA, USA). These cells were analyzed using a BD FACS Canto II flow cytometer (BD Bioscience, Franklin Lakes, NJ, USA). Data were collected from 20,000 to 30,000 cells using forward-scatter and side-scatter to set a gate on the dendritic cell population as CD11c+ cells. The internalized C. neoformans cells in BM-DCs were detected as FITC-positive cells.
Actin polymerization analysis.
BM-DCs and FITC-labeled C. neoformans were cocultured for 30 min at 37°C in a 5% CO2 incubator and then fixed with 4% paraformaldehyde (Wako). After the cells were attached on glass slides, the cells were permeated by 0.5% Triton X-100 (Sigma-Aldrich) at room temperature for 5 min and stained with 100 μM Acti-stain 555 phalloidin (Cytoskeleton, Inc., Denver, CO, USA). ProLong Gold (Invitrogen, Carlsbad, CA, USA) was used as a mounting agent. To analyze the actin polymerization induced by C. neoformans, BM-DCs with strong actin polymerization at the sites bound to C. neoformans were counted, and the proportion of these cells out of the total cell number was calculated. A confocal microscope system C2si (Nikon, Tokyo, Japan) was used for observation.
In vivo phagocytosis assay.
Mice were inoculated intratracheally with Cap67 (1 × 106 cells/mouse) in a volume of 50 μl. Three hours after infection, mice were anesthetized with an intramuscular injection of 0.3 mg midazolam (Teva-Takeda, Aichi, Japan) and 0.02 mg medetomidine (Orion, Espoo, Finland) and an intraperitoneal injection of 25 mg pentobarbital/kg of body weight (Abbott Laboratory, North Chicago, IL, USA), and their chests were opened. PBS was instilled into the trachea through a cannula to collect bronchoalveolar lavage fluid (BALF). Phagocytosed cells in the BALF were analyzed by phagocytosis assay with PAS staining under microscopic observation.
Statistical analysis.
Data were analyzed using JMP Pro v11.2.0 software (SAS Institute Japan, Tokyo, Japan). Data are expressed as the mean ± the standard deviation (SD). Differences between groups were examined for statistical significance using Welch’s t test. A P value of less than 0.05 was considered significant. In Fig. 4, Bonferroni adjustment was used for multiple comparisons.
ACKNOWLEDGMENTS
This work was supported in part by a Grant-in-Aid for Scientific Research (B) (18H02851 and 21H02965) and Early-Career Scientists (19K17920 and 21K16314) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, by the Research Program on Emerging and Reemerging Infectious Diseases from the Japan Agency for Medical Research and Development, AMED (JP20fk0108094 and JP21fk0108094), by the Strategic International Collaborative Research Program (SICORP), AMED (JP20jm0210073 and JP21jm0210073), by the MSD Life Science Foundation, Public Interest Incorporated Foundation (ID-014), and by the Joint Usage/Research Program of the Medical Mycology Research Center, Chiba University (20-02 and 21-04).
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Yuki Kitai, Email: yuki.m4058@gmail.com.
Liise-anne Pirofski, Albert Einstein College of Medicine.
REFERENCES
- 1.Cunha BA. 2001. Central nervous system infections in the compromised host: a diagnostic approach. Infect Dis Clin North Am 15:567–590. 10.1016/S0891-5520(05)70160-4. [DOI] [PubMed] [Google Scholar]
- 2.Jarvis JN, Harrison TS. 2007. HIV-associated cryptococcal meningitis. AIDS 21:2119–2129. 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- 3.Feldmesser M, Tucker S, Casadevall A. 2001. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol 9:273–278. 10.1016/s0966-842x(01)02035-2. [DOI] [PubMed] [Google Scholar]
- 4.Koguchi Y, Kawakami K. 2002. Cryptococcal infection and Th1-Th2 cytokine balance. Int Rev Immunol 21:423–438. 10.1080/08830180213274. [DOI] [PubMed] [Google Scholar]
- 5.Kawakami K, Qureshi MH, Koguchi Y, Zhang T, Okamura H, Kurimoto M, Saito A. 1999. Role of TNF-α in the induction of fungicidal activity of mouse peritoneal exudate cells against Cryptococcus neoformans by IL-12 and IL-18. Cell Immunol 193:9–16. 10.1006/cimm.1999.1460. [DOI] [PubMed] [Google Scholar]
- 6.Decken K, Kohler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately MK, Alber G. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun 66:4994–5000. 10.1128/IAI.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawakami K, Koguchi Y, Qureshi MH, Miyazato A, Yara S, Kinjo Y, Iwakura Y, Takeda K, Akira S, Kurimoto M, Saito A. 2000. IL-18 contributes to host resistance against infection with Cryptococcus neoformans in mice with defective IL-12 synthesis through induction of IFN gamma production by NK cells. J Immunol 165:941–947. 10.4049/jimmunol.165.2.941. [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov R. 2009. Approaching the asymptote: 20 years later. Immunity 30:766–775. 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820. 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, Miyazato A, Xiao G, Hatta M, Inden K, Aoyagi T, Shiratori K, Takeda K, Akira S, Saijo S, Iwakura Y, Adachi Y, Ohno N, Suzuki K, Fujita J, Kaku M, Kawakami K. 2008. Deoxynucleic acids from Cryptococcus neoformans activate myeloid dendritic cells via a TLR9-dependent pathway. J Immunol 180:4067–4074. 10.4049/jimmunol.180.6.4067. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Ishii K, Nakamura Y, Miyazato A, Maki A, Abe Y, Miyasaka T, Yamamoto H, Akahori Y, Fue M, Takahashi Y, Kanno E, Maruyama R, Kawakami K. 2012. Toll-like receptor 9-dependent activation of bone marrow-derived dendritic cells by URA5 DNA from Cryptococcus neoformans. Infect Immun 80:778–786. 10.1128/IAI.05570-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura K, Miyagi K, Koguchi Y, Kinjo Y, Uezu K, Kinjo T, Akamine M, Fujita J, Kawamura I, Mitsuyama M, Adachi Y, Ohno N, Takeda K, Akira S, Miyazato A, Kaku M, Kawakami K. 2006. Limited contribution of Toll-like receptor 2 and 4 to the host response to a fungal infectious pathogen, Cryptococcus neoformans. FEMS Immunol Med Microbiol 47:148–154. 10.1111/j.1574-695X.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- 13.Willment JA, Brown GD. 2008. C-type lectin receptors in antifungal immunity. Trends Microbiol 16:27–32. 10.1016/j.tim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto H, Nakamura Y, Sato K, Takahashi Y, Nomura T, Miyasaka T, Ishii K, Hara H, Yamamoto N, Kanno E, Iwakura Y, Kawakami K. 2014. Defect of CARD9 leads to impaired accumulation of gamma interferon-producing memory phenotype T cells in lungs and increased susceptibility to pulmonary infection with Cryptococcus neoformans. Infect Immun 82:1606–1615. 10.1128/IAI.01089-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. 2007. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 8:31–38. 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki H, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. 2007. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol 8:39–46. 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 17.Werner JL, Metz AF, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Trindade IF, Brown GD, Steele C. 2009. Requisite role for the Dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol 182:4938–4946. 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, Kimberly RP, Underhill D, CruzPD, Jr, Ariizumi K. 2006. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem 281:38854–38866. 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 19.McGreal EP, Rosas M, Brown GD, Zamze S, Wong SY, Gordon S, Martinez-Pomares L, Taylor PR. 2006. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 16:422–430. 10.1093/glycob/cwj077. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura K, Kinjo T, Saijo S, Miyazato A, Adachi Y, Ohno N, Fujita J, Kaku M, Iwakura Y, Kawakami K. 2007. Dectin-1 is not required for the host defense to Cryptococcus neoformans. Microbiol Immunol 51:1115–1119. 10.1111/j.1348-0421.2007.tb04007.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura Y, Sato K, Yamamoto H, Matsumura K, Matsumoto I, Nomura T, Miyasaka T, Ishii K, Kanno E, Tachi M, Yamasaki S, Saijo S, Iwakura Y, Kawakami K. 2015. Dectin-2 deficiency promotes Th2 response and mucin production in the lungs after pulmonary infection with Cryptococcus neoformans. Infect Immun 83:671–681. 10.1128/IAI.02835-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato Y, Sato K, Yamamoto H, Kasamatsu J, Miyasaka T, Tanno D, Miyahara A, Kagesawa T, Oniyama A, Kawamura K, Yokoyama R, Kitai Y, Umeki A, Ishizuka S, Takano K, Shiroma R, Nakahata N, Kawakami K, Kanno E, Tanno H, Yamasaki S, Hara H, Ishii K, Kawakami K. 2020. Limited role of Mincle in the host defense against infection with Cryptococcus deneoformans. Infect Immun 88:400–420. 10.1128/IAI.00400-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252. 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 24.Mansour MK, Latz E, Levitz SM. 2006. Cryptococcus neoformans glycoantigens are captured by multiple lectin receptors and presented by dendritic cells. J Immunol 176:3053–3061. 10.4049/jimmunol.176.5.3053. [DOI] [PubMed] [Google Scholar]
- 25.Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, Moita LF, Taylor PR, Reis e Sousa C. 2009. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med 206:2037–2051. 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MJ, Yoshimoto E, Saijo S, Iwakura Y, Lin X, Katz HR, Kanaoka Y, Barrett NA. 2016. Phosphoinositide 3-kinase δ regulates Dectin-2 signaling and the generation of Th2 and Th17 immunity. J Immunol 197:278–287. 10.4049/jimmunol.1502485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ifrim D, Bain J, Reid D, Oosting M, Verschueren I, Gow NA, Krieken JH, Brown GD, Kullberg BJ, Joosten L, Meer JW, Koentgen F, Erwig L, Quintin J, Netea M. 2014. Role of Dectin-2 for host defense against systemic infection with Candida glabrata. Infect Immun 82:1064–1073. 10.1128/IAI.01189-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura Y, Inoue A, Hangai S, Saijo S, Negishi H, Nishio J, Yamasaki S, Iwakura Y, Yanai H, Taniguchi T. 2016. The innate immune receptor Dectin-2 mediates the phagocytosis of cancer cells by Kupffer cells for the suppression of liver metastasis. Proc Natl Acad Sci U S A 113:14097–14102. 10.1073/pnas.1617903113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagata M, Izumi Y, Ishikawa E, Kiyotake R, Doi R, Iwai S, Omahdi Z, Yamaji T, Miyamoto T, Bamba T, Yamasaki S. 2017. Intracellular metabolite β-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity. Proc Natl Acad Sci U S A 114:E3285–3294. 10.1073/pnas.1618133114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J, Flaczyk A, Neal LM, Fa Z, Eastman AJ, Malachowski AN, Cheng D, Moore BB, Curtis JL, Osterholzer JJ, Olszewski MA. 2017. Scavenger receptor MARCO orchestrates early defenses and contributes to fungal containment during cryptococcal infection. J Immunol 198:3548–3557. 10.4049/jimmunol.1700057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, Moore KJ, Calderwood SB, Hacohen N, Luster AD, El Khoury J. 2009. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med 206:637–653. 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor PR, Brown GD, Herre J, Williams DL, Willment JA, Gordon S. 2004. The role of SIGNR1 and the β-glucan receptor (Dectin-1) in the nonopsonic recognition of yeast by specific macrophages. J Immunol 172:1157–1162. 10.4049/jimmunol.172.2.1157. [DOI] [PubMed] [Google Scholar]
- 33.Herre J, Marshall AS, Caron E, Edwards AD, Williams DL, Schweighoffer E, Tybulewicz V, Sousa CR, Gordon S, Brown GD. 2004. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood 104:4038–4045. 10.1182/blood-2004-03-1140. [DOI] [PubMed] [Google Scholar]
- 34.Hoppe AD, Swanson JA. 2004. Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol Biol Cell 8:3509–3519. 10.1091/mbc.e03-11-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nauta AJ, Roos A, Daha MR. 2004. A regulatory role for complement in innate immunity and autoimmunity. Int Arch Allergy Immunol 134:310–323. 10.1159/000079261. [DOI] [PubMed] [Google Scholar]
- 36.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, Iwakura Y. 2010. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32:681–691. 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Hara H, Ishihara C, Takeuchi A, Imanishi T, Xue L, Morris SW, Inui M, Takai T, Shibuya A, Saijo S, Iwakura Y, Ohno N, Koseki H, Yoshida H, Penninger JM, Saito T. 2007. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat Immunol 8:619–629. 10.1038/ni1466. [DOI] [PubMed] [Google Scholar]
- 38.Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E, Sakuma M, Tateno H, Uno J, Hirabayashi J, Mikami Y, Takeda K, Akira S, Saito T. 2009. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci U S A 106:1897–1902. 10.1073/pnas.0805177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download IAI.00330-21-s0001.pdf, PDF file, 0.02 MB (24KB, pdf)
Supplemental material. Download IAI.00330-21-s0002.pdf, PDF file, 0.02 MB (23.2KB, pdf)