Abstract
US is widely used in breast imaging for diagnostic purposes and is also used increasingly for supplemental screening in women with dense breasts. US frequently depicts masses that are occult on mammography, even after tomosynthesis, and the vast majority of such masses are benign. Many masses seen only on screening US are easily recognized as benign simple cysts. Probably benign, BI-RADS 3, or low suspicion, BI-RADS 4A masses are also common and often prompt short-interval follow-up or biopsy, respectively, yet the vast majority of these are benign. This review details appropriate characterization, classification, and new approaches to the management of probably benign masses seen on screening US that can reduce false positives and, thereby, reduce costs and patient anxiety.
Keywords: probably benign, breast cancer, BI-RADS 3, screening, breast ultrasound
Key Messages.
Many findings historically assessed as BI-RADS 3, probably benign, on screening breast US can now nearly always be assessed as BI-RADS 2, benign, including complicated cysts with debris, clustered microcysts, and multiple bilateral circumscribed masses.
Circumscribed, oval, hypoechoic, and/or isoechoic masses with minimal posterior enhancement or no posterior features, typical of a fibroadenoma, can be assessed as BI-RADS 3 on screening breast US and be safely reassessed at annual screening; malignancy rate across series at 6 months is comparable to a BI-RADS 1 or 2 assessment at only 8/3918 (0.2%) and, at 2 years of follow-up, only 17/4364 (0.39%).
Increase in diameter of a BI-RADS 3 mass of more than 20% in six months (44% in 12 months) should prompt biopsy as should stiffness on elastography, though most malignancies will show additional suspicious findings.
Greater caution is warranted for a BI-RADS 3 assessment in women over age 60, with a corresponding new finding on mammography, or in the setting of concurrent breast malignancy.
What Is a BI-RADS 3 Finding on Screening US?
A probably, benign Breast Imaging Reporting and Database System (BI-RADS) 3 (1), assessment was first codified for mammographic findings proven to have a risk of malignancy greater than typically benign findings but less than 2% (2–5). A mammographic BI-RADS 3 assessment is intended for baseline screening findings (or when no prior comparisons are available), after full diagnostic workup. Surveillance with initial short-interval, usually 6-month, follow-up, has been shown to be a safe alternative to biopsy, with stage distribution of the few cancers found at follow-up not worse than cancers undergoing immediate biopsy (2–5). For stable findings, continued diagnostic follow-up at 12 and 24 months is the usual protocol (6), with downgrade to BI-RADS 2, benign, for interval decrease in size, or BI-RADS 1, negative, if the finding resolves.
A common BI-RADS 3 finding on screening mammography is a solitary circumscribed oval mass that is hypo- or isoechoic on targeted US, compatible with a fibroadenoma or complicated cyst with debris, representing 589/3184 (18.5%) of probably benign findings in the initial series of Sickles (2). A malignancy rate less than 2% at 2 years of follow-up, as well as necessity of the 6-month follow-up, have been shown for BI-RADS 3 mammographic findings in the National Mammography Database (7). Not surprisingly, for findings recalled on screening mammography then assessed as BI-RADS 3, the malignancy rate increases linearly with increasing age with only 0.5% of such findings malignant in women in their 30s, 0.9% in women in their 40s, exceeding 2% above age 60, and as high as 4.6% for in women in their 80s (8). Analysis of malignancy rates for BI-RADS 3 findings on screening US as a function of patient age has not been reported, but similar results are expected.
Stavros et al (9) first proposed criteria for a benign versus malignant assessment of solid nodules on US. In their analysis of 750 solid masses, including 625 benign lesions, four features were associated with a ≤2% risk of malignancy: uniform hyperechogenicity; ellipsoid (oval) shape; two or three gentle lobulations; and a thin “pseudocapsule.” None of these suspicious features could be present: spiculation, taller-than-wide orientation (now known as “not parallel” to the skin surface or “vertical” orientation), angular margins, posterior shadowing, branch pattern, marked hypoechogenicity, calcifications, duct extension, or microlobulation. Skaane and Engedal (10) found that the combination of oval or round shape and echogenic pseudocapsule, as well as lack of any suspicious features, had a negative predictive value (NPV) of 96% in nonpalpable masses and 100% in palpable masses among 142 fibroadenomas and 196 invasive ductal carcinomas evaluated sonographically. Rahbar et al (11) and Baker et al (12) found, unfortunately, that some radiologist observers had difficulty consistently applying these criteria. In BI-RADS: US (13,14) mass margins are classified as either circumscribed or not, with spiculated, angular, microlobulated, or indistinct margins considered not circumscribed; pseudocapsule is an outdated term indicating a circumscribed margin.
Holzer-Fruehwald et al (15) were not able to identify a size threshold below which sonographically depicted masses were always benign though both increasing size and increasing age increased likelihood of malignancy. They did show a NPV of 99.1% (111/112) for a circumscribed oval or round mass lacking an echogenic rim and suggested BI-RADS 2 assessment for such findings; 0/28 masses assessed as BI-RADS 3 in that series were malignant. An echogenic rim is a suspicious finding (16,17) and typically reflects nests of tumor cells invading fat, though it can be due to edema such as with an abscess or fat necrosis. It is also important to recognize that round masses are considered to have a “not parallel” orientation and are therefore suspicious (unless due to a simple cyst) (14).
In the multicenter American College of Radiology Imaging Network (ACRIN) 6666 protocol of screening US in women with dense breasts and elevated risk, which began in 2004, prospective criteria were defined for a BI-RADS 3 assessment. These included: (1) a solitary circumscribed oval or gently lobulated (ie, 2–3 gentle lobulations) hypoechoic or isoechoic mass with no posterior features or minimal enhancement (Figure 1); (2) clustered microcysts (Figure 2); (3) probable fat necrosis (Figure 3); and (4) postoperative scar (if there was diagnostic uncertainty) (18). Probable fat necrosis is typically superficial, within the subcutaneous fat, and it may be seen without known trauma especially in a woman taking anticoagulation medication. Fat necrosis quickly evolves, with expected decrease or resolution observable by very short-interval follow-up US in 6 weeks to 3 months, and will not be further discussed. Postsurgical scars are nearly always recognizable by history and clinical examination and, with typical appearance on US, are benign findings and also will not be further discussed. Based on more recent studies, the vast majority of clustered microcysts can now be assessed as benign findings, BI-RADS 2. This review will show that both multiple and solitary circumscribed oval hypoechoic and/or isoechoic masses with no posterior features or minimal posterior enhancement seen on screening US can be safely followed at one year.
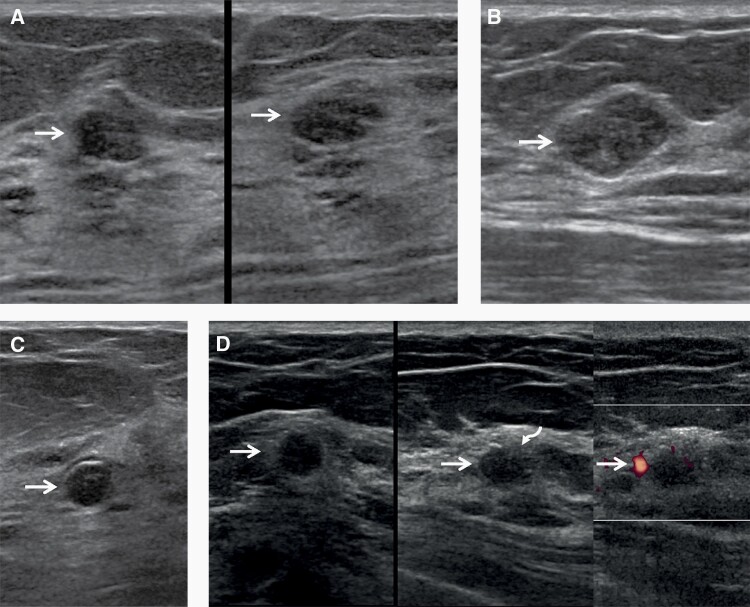
Figure 1.
Images of a 57-year-old woman with multiple bilateral masses on screening US, including cysts, presumed fibroadenomas, and invasive ductal carcinoma with ductal carcinoma in situ (IDC-DCIS). Oval, circumscribed hypoechoic masses (arrows) are seen in the left breast at 2 o’clock (A) (left, radial; right, antiradial) and the right breast at 2 o’clock (B) with no posterior features, typical of BI-RADS 3, probably benign masses on screening US. These can be followed at one year, and were both stable at subsequent 12- and 24-month screening US examinations. A benign rim-calcifying cyst (C, arrow) was seen in the right breast at 12 o’clock. Multiple simple cysts were also present (not shown). A hypoechoic oval mass (arrows) with a subtle echogenic rim on the radial image and focally indistinct margin (curved arrow) was seen in the left breast at 10 o’clock (D) (left, radial; center, antiradial; right, power Doppler). This is a BI-RADS 4A, low suspicion mass, which underwent targeted US then core-needle biopsy and excision showing a 1.2 cm grade 2 IDC-DCIS, estrogen receptor positive, progesterone receptor weakly positive, human epidermal growth factor 2 negative, with negative sentinel node biopsy.
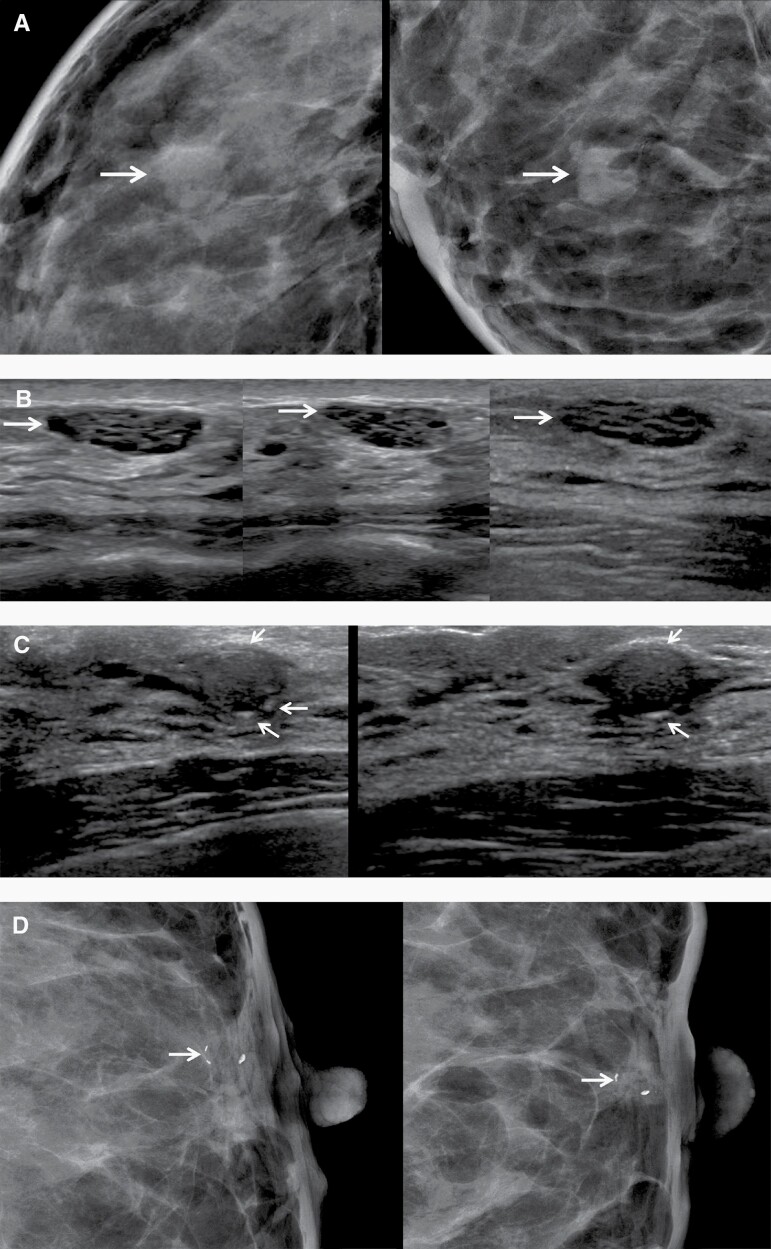
Figure 2.
Images of a 45-year-old woman with bilateral masses appropriately assessed as BI-RADS 2—clustered microcysts and a rim-calcifying cyst. A: Close-ups of screening right craniocaudal (CC, left) and mediolateral oblique (MLO, right) mammograms show heterogeneously dense parenchyma and a new oval mass (arrows). B: Screening radial (left) and antiradial (middle) US shows a corresponding mass due to clustered microcysts (arrows) with posterior enhancement. Artifactual internal echoes are reduced on harmonic imaging (right, radial, arrow). This should have been assessed as a benign finding, but instead underwent US-guided core-needle biopsy confirming fibrocystic change. Clustered microcysts are sometimes appropriately assessed as BI-RADS 3 with surveillance in 6 months if they are too small or deep for definitive characterization. C: Radial (left) and antiradial (right) screening US of the retroareolar left breast shows a circumscribed oval hypoechoic mass with peripheral echogenic foci compatible with rim calcifications (arrows), a benign finding. D: Close-ups of screening CC (left) and MLO (right) mammograms show corresponding rim calcifications (arrows). This also should have been assessed as a benign finding but underwent US-guided core-needle biopsy, yielding sclerotic cyst wall.
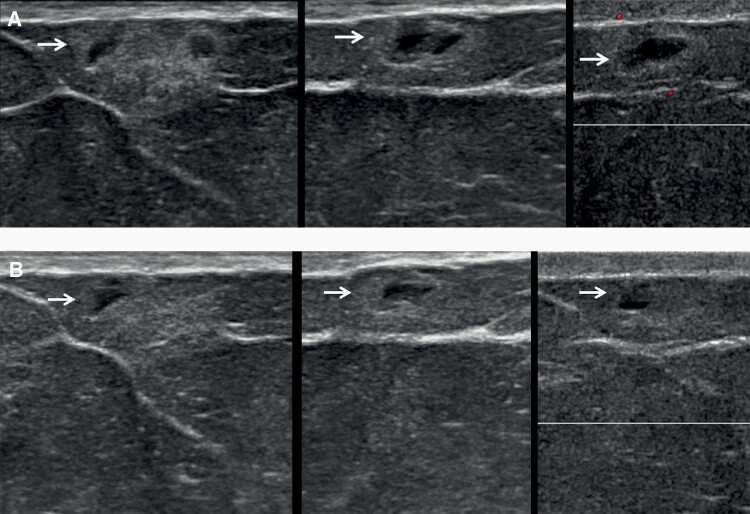
Figure 3.
Images of a 63-year-old woman with incidental presumed fat necrosis on screening breast US, decreasing on very short-interval follow-up. A: Initial antiradial (left), radial (center), and power Doppler US (right) images show focal anechoic masses with surrounding echogenic rim (arrows) within the subcutaneous fat, suggestive of fat necrosis despite lack of specific history of trauma, assessed as BI-RADS 3 with recommendation for follow-up US in 6–8 weeks. B: Targeted antiradial (left), radial (center), and Doppler US (right) images 6 weeks later shows interval decrease in the mass (arrows), now appropriately assessed as BI-RADS 2, benign finding.
Screening US Technique
The suggested protocol for handheld screening US in ACRIN 6666 was transverse and sagittal survey scanning, in the supine oblique position for the outer breast and supine position for the inner breast (19). The axilla can be included electively and was documented in slightly more than 33% of examinations in ACRIN 6666 but did not improve cancer detection at the participant level (20). When Lee et al (21) reviewed screening US examinations that included the axilla, 14/4009 (3.5/1000) baseline examinations showed abnormal axillary findings as did 19/8835 (2.2/1000) incidence screens, and all 33 recalled axillary findings proved to be false positives. Among 13 573 US examinations of the axilla in women with negative mammography and screening breast US reported by Youn et al (22), 14 (1.0/1000) women underwent axillary biopsy with two found malignant: one metastatic endometrial carcinoma and one lymphoma. As such, scanning the axilla is often limited to ipsilateral axillary US in women with suspicious breast findings on mammography and/or US.
To appropriately assess a breast mass on US, the margins must be carefully evaluated in three dimensions, as on mammography/tomosynthesis. For handheld US (HHUS), this is best accomplished by requiring orthogonal views be obtained for all lesions other than simple cysts, as was performed in the ACRIN 6666 protocol. A set of images without and with calipers should be obtained so that margins are fully visible at the time of interpretation. One of these images should be along the longest horizontal diameter of the mass; this will often be in the radial plane along the duct system. To facilitate subsequent comparison and reporting, measurements should be reported with the largest horizontal diameter first, then the anteroposterior (vertical) diameter on that same image, then the orthogonal horizontal diameter (14); thus, for a vertically oriented mass, the largest diameter will be the second measurement reported.
Orthogonal Doppler images are also standard for masses other than simple cysts (19) and can be helpful in certain circumstances. Internal vascularity in an anechoic mass excludes a cyst and raises concern for malignancy. A hilar vessel can help confirm an intramammary node. Prominent internal vascularity in a solid circumscribed mass can occasionally be seen and also increases concern for malignancy (Figure 4). When performed, power Doppler is encouraged as it is more sensitive than color Doppler to the slow flow typically observed in breast masses. Acquisition of the full set of required diagnostic images in orthogonal views without and with calipers is standard practice for HHUS and does not constitute “additional imaging” (23). Spatial compounding facilitates evaluation of lateral mass margins (perpendicular to the beam) but, due to its use of multiple off-angle beams, reduces any posterior features. Harmonic imaging will accentuate posterior features and reduce artifactual internal echoes and can be helpful in fully documenting a given mass (Figure 2), though its use is discouraged for surveying due to pronounced posterior shadowing from ligaments and loss of signal beyond about 2 to 2.5 cm depth, depending on the insonating frequency used. Because a full set of diagnostic images is routinely obtained at HHUS, a BI-RADS 3 assessment can be given directly on screening US without the need for immediate recall for additional imaging. Across four series of technologist-performed screening US, only 50/16 676 (0.3%) were given a BI-RADS 0, incomplete, assessment requiring immediate additional imaging to render a final assessment (24). Any time the patient is asked to return for any additional imaging (e.g. at 6 months) prior to the next routine screening, this constitutes a “positive test” for screening audit purposes (25).
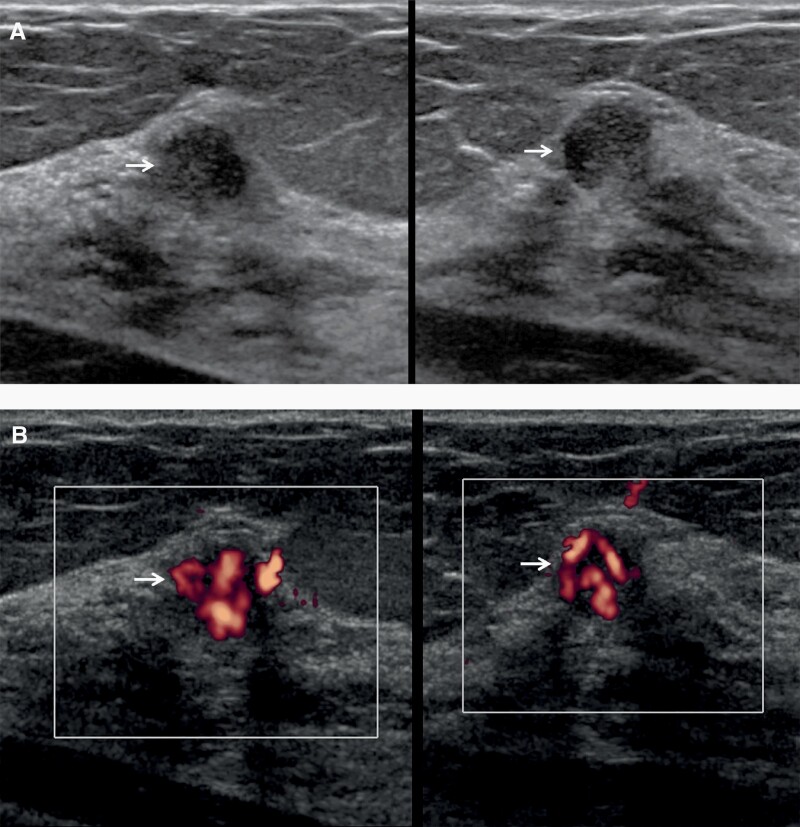
Figure 4.
Images of a 68-year-old woman with technologist-performed screening US-detected cancer. A: Radial (left) and antiradial (right) US images show a round hypoechoic mass (arrows) with partially circumscribed and partially indistinct margins. Strong internal vascularity was evident in the mass on power Doppler images (B, arrows), a suspicious finding. This was assessed as BI-RADS 3 (with recommendation for 6-month follow-up) by one radiologist and recommended for immediate additional imaging by the second radiologist as part of a research protocol. At targeted US, it was assessed as BI-RADS 4A, low suspicion. US-guided core-needle biopsy showed atypical ductal hyperplasia and papilloma, upgraded to nuclear grade 2 ductal carcinoma in situ at excision.
For automated US (AUS), transverse images are directly obtained and reviewed. Associated distortion can be well seen in the reconstructed coronal plane. The orthogonal sagittal plane is reconstructed and may lack sufficient detail for adequate margin characterization: for many BI-RADS 3 and 4A masses on AUS, immediate additional imaging with targeted HHUS may be needed. In one study, Jia et al (26) performed both HHUS and AUS on 937 women with dense breasts in a prospective multicenter experience in China. There were 20 patients with findings assessed as BI-RADS 3 on AUS but assessed as BI-RADS 4A, low suspicion, due to focally indistinct margins only perceptible on HHUS; all were benign on biopsy. Overall prevalence and outcomes of BI-RADS 3 use in that series were not clearly stated, though one malignancy was assessed as BI-RADS 3 on AUS. In a series of 394 women assessed as BI-RADS 3 directly on AUS, 2 (0.5%) were found to have malignancy in another quadrant on 2-year follow-up, prompting recommendation for annual follow-up (27). Further validation of use of BI-RADS 3 assessments directly on AUS is needed.
Prevalence, Biopsy Rate, and Malignancy Rate of BI-RADS 3 Masses on Screening US
BI-RADS 3 masses are very common on screening US, reported in from 0.6% (28,29) to over 20% of women (30–33) at the time of initial imaging (Table 1). Nam et al (34) reviewed and reclassified lesions seen on screening US and identified BI-RADS 3 lesions in 41.5% of women. The average age of women with BI-RADS 3 masses was younger, at 54.1 years, versus 55.9 years (P < 0.001) among all participants in the ACRIN 6666 study (18). BI-RADS 3 findings are more common in women with dense breasts than in those with nondense breasts in series where the latter were included and results detailed (30). Across technologist-performed screening US, the average rate of BI-RADS 3 was 6.2% (24) and did not change with incidence screens in the report by Weigert et al (35). Biopsy rate of BI-RADS 3 findings on screening US ranges up to 17% (18), similar to that of BI-RADS 3 findings on mammography (7).
Table 1.
Rate of BI-RADS 3 (BR3) Assessments on Screening US and Malignancy Rates
| Author, year | N Screens | N Women |
N BR3 Women (%) |
N Biopsied BR3 Women (%) | N Women Followed | Duration of Follow-up | N Women With Cancer by 6 Months | N Women With Cancer by 2 Years | N Invasive Cancers/Total Cancers (%) | N Node Negative (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Technologist Performed | ||||||||||
| Kaplan (69) | 1862 | 1862 | 72 (3.9) | 0 | 43 | 6 mo | 0 | 0 | NA | NA |
| 19 | 12 mo | |||||||||
| Hooley 2012 (31) | 935 | 935 | 187 (20.0) | 22 lesions, 17 (9.1) women | 145 | 6 mo | 0a | NR | 1 melanomaa | NA |
| 178 | 15 mo | |||||||||
| Parris 2013 (70) | 5519 | 5519 | 452 (8.2) | NR | 0 | NA | NR | NR | NA | NA |
| Destounis 2017 (29) | 5434 | 4898 | 94b (1.9) | 4 (4.3) | 89 | NR | 0 | NR | 0 | NA |
| Weigert 2017 (35) | 13 516 | 2706 | 868 (6.4) | NR | 0 | NA | NR | NR | NA | NA |
| Physician Performed | ||||||||||
| Kolb 1998 (71) | 3626 | 3626 | 92 (2.5) | NR | 86 | 4–6 mo | 0 | NR | 0 | NA |
| deFelice 2007 (72) | 1754 | 1754 | 149 (8.5) | 149 (100)c | 0 | NA | 3 (2.0) | NA | 3/3 (100) | NR |
| Youk 2011 (73) | 1368 | NS | 120 (8.8) | NR | 119 | 24 mo | 1 (0.8) | 1 total (dx at 6 mo) | 1/1 (100) | 1/1 (100) |
| Barr 2013 (18) | 7473 | 2662 | 519d (19.5) | 124 (16.6)d lesions | 512 lesions | 22 mo | 1 (0.1) | 6/636 (0.9%) lesions | 5/6 (83) lesions 2–18 mm, median 10 mm | 4/5 (80) |
| Chang 2015 (30) | 990e | 990e | 282 (28.5) | 14 (5.0) | 0 | 12 mo | 0 | NA | NA | NA |
| Moon (32) | 2005 | 2005 | 533 (26.6) | NR | 533 | 12 mo | 1/533 | NA | 1/1 (100) | 1/1 (100) |
| 12 mm ILC | ||||||||||
| Nam 2016 (34) | NR | 1661 | 689 (41.5) | 31 | 653f | 24 mo | 1/653 (0.2) | 1 (0.2)f | 1/1 (100) | 1/1 (100) |
| Chae (64) | 12 187 | 12 187 | 1783 (14.6) | 63 (3.5) | 1164g | 24 mo | NR | 8/1164 (0.7)g | 6/8 (75) | 6/6 (100) |
| Moon (33) | 3523 | 3523 | 820 (23.3) | 15h | 445 | 3–60 mo, mean 24.5 mo | 0/345 | 0/445h | NA | NA |
| Buchberger (28) | 66 680 | 66 680 | 397 (0.6)i | NR | 1255 | 12 mo | 1i | 1i | 1/1 (100) | NR |
| Overall | 125 882 | 108 156 | 7057 (6.5) | 8/3918 (0.2) | 17/4364 (0.39) | 18/21 (86) | 13/14 (93) |
Abbreviations: NA, not applicable; NR, not reported.
aOne new lesion was seen at time of 6-month follow-up that proved to be metastatic melanoma 2 months later.
bA total of 101 women were assessed as BI-RADS 3 after mammography and US; 94 were due only to US.
c97 “complex” cysts (now termed complicated cysts) underwent direct fine-needle aspiration biopsy, all benign; 52 hypoechoic cysts versus solid masses underwent fine-needle aspiration biopsy, 38 proved solid; 8 with cellular atypia had core biopsy yielding 3 malignancies (2 invasive ductal carcinoma (IDC) and 1 invasive lobular carcinoma).
dIn year 1 of ACRIN 6666, 2659 women had US screening and 358 (13.5%) women were assessed as BI-RADS 3 with 506 lesions; in year 2, 2493 women had US screening and 121 (4.9%) were assessed BI-RADS 3 with 142 lesions; in year 3, 2321 women had screening US, of whom 86 (3.7%) were assessed BI-RADS 3 with 97 lesions; overall, 2662 unique women had a total of 7473 screening US examinations and 519 unique women were given a BI-RADS 3 assessment for 745 lesions.
eIn total, 1526 women had prevalence screening US, of whom only 990 had dense breasts; 340/1526 (22.3%) of all women were given BI-RADS 3 assessments.
fIn total, 653 had follow-up at 24 months or biopsy.
gIn total, 1017 women had one BR3 lesion and 147 had multiple; malignancy rate 4/184 (2.2%) if mammographic abnormality also; 4/980 (0.4%) if US abnormality only.
hIn total, 345 had initial follow-up at 3–9 months and 100 more had initial follow-up at 9–15 months; three new lesions developing at >15 month follow-up were malignant: 1 ductal carcinoma in situ and 2 node-negative invasive ductal carcinomas. A total of 15 lesions were biopsied after the initial follow-up, based on growth (n = 6) or new findings (n = 9); not clear how many total lesions in denominator.
iIn total,1255 women were given BI-RADS 3 assessments after combined mammography and US; 397 were prompted by US alone. One 6 mm IDC was diagnosed at 6-month follow-up. Another 13 mm IDC was assessed as probably benign on mammography and downgraded to BI-RADS 2, benign, based on US, then diagnosed at the next screen at 14 months but is not included here as it was not BR3 on US.
In a review of cancers seen only on screening US, Berg and Vourtsis (36) reported an average incremental cancer detection rate of 2.0 to 2.7 per 1000 for physician performed and technologist-performed HHUS respectively and 2.5 per 1000 for AUS; 88% (631/719) of cancers found only on US were invasive and, where detailed, 90% (497/554) were node negative. While not all series include results of surveillance of BI-RADS 3 findings, across those that do, malignancy rate at 6 months was only 8/3918 (0.2%) (Table 1). At 2 years of follow-up, 17/4364 (0.39%) proved malignant. The cancers observed after BI-RADS 3 assessment were nearly all invasive and 13/14 (93%), where reported, were node negative.
Across three annual rounds of screening US in ACRIN 6666, 519/2662 (19.5%) women received a BI-RADS 3 assessment on a total of 745 lesions (18). On the first screen, 358/2659 (13.5%) women had BI-RADS 3 findings on US, as did 121/2493 (4.9%) newly on the second screen, and 86/2321 (3.7%) newly on the third annual screen. Thus, as expected, the overall frequency of new BI-RADS 3 lesions decreased with incidence screening, but a BI-RADS 3 assessment did continue to be used even for new findings with no increase in malignancy rate. BI-RADS 3 findings represented about a quarter of new lesions at each screen. Of the 519 women with a BI-RADS 3 finding, 43 (8.3%) had new BI-RADS 3 lesions at multiple time points. Of the 745 lesions, 124 (16.6%) underwent US-guided biopsy, showing a variety of entities: 37 benign cystic lesions including ruptured cysts, 28 fibrosis or other fibrocystic change, 25 fibroadenomas, 14 benign breast tissue, four each sclerosing adenosis and benign papilloma, three fat necrosis, four “other,” and five malignancies. Patient preference and/or risk factors prompted the majority of biopsies, with only four prompted by growth or suspicious change. Pseudoangiomatous stromal hyperplasia can also manifest as a circumscribed oval hypoechoic mass.
Reclassifying BI-RADS 3 Findings as BI-RADS 2
There are many findings that were historically assessed as BI-RADS 3, probably benign, for which there are now sufficient data validating a BI-RADS 2, benign assessment. Among such lesions that can be considered benign are complicated cysts with debris, clustered microcysts, and multiple bilateral circumscribed masses.
Complicated cysts with debris must be distinguished from complex cystic and solid masses, including intracystic masses, as complex cystic and solid masses should be assessed as suspicious findings, BI-RADS 4, 4B moderate suspicion, with likelihood of malignancy averaging 36% (37). Complicated cysts with debris are easily recognized as such when there are mobile internal echoes or a fluid-debris level, and these are assessed as benign findings. Rim calcification is sometimes evident and also indicates benignity (Figures 1, 2). Complicated cysts can mimic solid, oval, circumscribed masses when hypoechoic. Even so, across the literature, only 4/1343 (0.3%) of complicated cysts with debris proved malignant on follow-up. This rate parallels the rate of malignancy after a BI-RADS 2 assessment. Complicated cysts with debris were observed in 376/2662 (14%) of women in the ACRIN 6666 protocol; of these 376 women, 301 (80%) also had at least one simple cyst and 84 (22%) of women had multiple bilateral simple cysts (37). Among the 745 BI-RADS 3 lesions in ACRIN 6666, 183 (24.6%) were described as complicated cysts with debris and 1/183 (0.5%) proved malignant (18). Hooley et al (31) detailed that 131/187 (70.0%) BI-RADS 3 assessments on screening US were for complicated cysts with debris and included 79 complicated cysts in the setting of multiple simple cysts. Another 19 of the 131 complicated cysts were ≤5 mm. If these 98 complicated cysts were reclassified as benign findings, overall BI-RADS 3 rate would have dropped from 187/935 (20.0%) to 89/935 (9.5%). Complicated cysts with debris can be assessed as BI-RADS 2, benign findings.
Clustered microcysts represent distended acini within the terminal duct lobular unit. The resulting oval or microlobulated mass can be seen on mammography and US and should have circumscribed margins. Occasionally, fluid-debris levels or milk of calcium can be seen within individual microcysts. Microcysts can be lined with bland or tall, columnar, apocrine metaplastic epithelium. Clustered microcysts were seen in 104/2662 (3.9%) of screening US examinations in ACRIN 6666 (37) and 110/1900 (5.8%) of all breast US examinations (38) and they are especially common in perimenopausal women, with mean age of 48 years (range 32 to 71). Across seven series (37–43), only 1/507 (0.2%) clustered microcystic mass was found to be malignant, again consistent with BI-RADS 2 assessment. Indeed, that one lesion was from ACRIN 6666 and may not have even represented the finding that ultimately was diagnosed as invasive lobular carcinoma. As discussed in (44), there is one additional report from Japan (45) of 8 malignancies among 52 women with clustered microcysts, including six ductal carcinomas in situ (DCIS) and two invasive carcinomas; however, the three cases illustrated in that publication all had associated solid components and did not meet strict criteria for clustered microcysts. Harmonic imaging can help clear artifactual internal echoes and allow recognition of the typical appearance (Figure 2). Internal vascularity can be seen on Doppler along the thin septations between adjacent acini. Uncommonly, it can be difficult to recognize clustered microcysts as such when they are deep or very small (or both), and a BI-RADS 3 assessment is reasonable in these situations. Caution should be exercised in postmenopausal women with a new mass on mammography or coarse heterogeneous or pleomorphic calcifications that appears to represent clustered microcysts on US as there is increased risk of DCIS or other malignancy (45). In general, clustered microcysts should be assessed as BI-RADS 2, benign findings.
As for mammography (46), multiple bilateral circumscribed solid-appearing masses on US can be assessed as BI-RADS 2, benign findings (47), without need for recall. There should be at least three masses overall and at least one in each breast. Documentation of multiple bilateral circumscribed masses may be easier with AUS than HHUS, though measurements still should be compared, and each mass must be carefully scrutinized with either approach (Figure 1). In the ACRIN 6666 trial, no malignancies were observed among 153 such findings in 135 women: the malignancy rate was 0% (95%CI 0 to 2.4%), indicating a BI-RADS 2, benign, assessment is appropriate. Of the 135 women with multiple bilateral circumscribed masses, 82 (61%) also had solitary suspicious findings, and in 2/82 (2.4%) such women, the solitary finding was malignant. Caution is needed in this setting: Song et al (48) confirmed that multiple masses can be distracting and 12/72 (17%) cancers missed on screening US were in that setting.
Reducing Surveillance of Probably Benign Findings Detected on Screening US
Based on the very low rate of malignancy of 0.9% (6/636 with follow-up) among BI-RADS 3 findings in ACRIN 6666, with only one malignancy diagnosed because of suspicious change at six-month follow-up, it was suggested that 12-month follow-up, at the time of annual screening US, may be sufficient. Similarly, Moon et al (33) found no malignancies at 3–15 months of follow-up, with three lesions developing after 15 months that were malignant among 445 women followed, and also suggested that initial 12-month follow-up may be appropriate. Overall, as stated, across 12 series, at the 6-month follow-up, 8/3918 (0.2%) BI-RADS 3 findings on screening US proved malignant as did 17/4364 (0.39%) at two years of follow-up (Table 1). This is very similar to the 0.08 to 0.35% observed interval malignancy rates at one year among women with dense breasts assessed as BI-RADS 1 or 2 on mammography in the Breast Cancer Surveillance Consortium (49).
Upgrading to Biopsy
Stiffness on elastography (when available) should be considered prior to surveillance. At follow-up, growth and other suspicious changes should also be evaluated. In the BE1 multinational study of shear-wave elastography (SWE) (50), there were 181 oval, circumscribed masses with no suspicious features, of which 144 had been assessed as BI-RADS 3 (including four malignancies) and 37 as BI-RADS 4A, low suspicion. Twelve of the 144 BI-RADS 3 masses, including all four malignancies (two grade 3 and two grade 2 invasive ductal carcinomas), were stiff both visually and quantitatively (> 80 kPa, velocity 5.2 m/sec) on SWE and 28/37 (76%) masses assessed as 4A were soft and could have been downgraded to surveillance, for an overall improvement in specificity from 140/177 (79.1%) to 160/177 (90.4%, P < 0.001). Prospectively, in a study from South Korea, masses otherwise assessed as BI-RADS 4A that were softer than a cutoff of 30 kPa (3.2 m/sec) on SWE could be downgraded to surveillance, with dramatic improvement in specificity of screening US and no loss in sensitivity (51). Strain elastography can also improve assessment of probably benign and low suspicion masses, with ratio of horizontal diameter on elastography to diameter on B-mode (E:B ratio) the most accurate of the various measures that can be obtained (52). In a prospective multicenter trial, 219/221 (99.1%) malignant lesions had E:B ratio of 1.0 or greater and 361/413 (87.4%) benign lesions had ratios <1.0 (53). Zheng et al showed that among 494 BI-RADS 4A, low suspicion, masses on US (including 49 malignant), that 290 (58.7%) could be downgraded to BI-RADS 3 (of which 289, 99.7%, were benign) by use of strain elastographic approaches (54). Elastographic methods do not penetrate well beyond 3 cm depth: its use is discouraged for evaluating deeper lesions (50).
Additional methods are in development to better characterize low suspicion and probably benign masses seen sonographically. Optoacoustic (OA) imaging assesses deoxyhemoglobin (increased in cancers) relative to oxyhemoglobin and also patterns of vascularity—with increased boundary zone vessels favoring malignancy. There are preliminary results using OA to selectively upgrade BI-RADS 3 masses to biopsy and downgrade suspicious masses to surveillance (55,56); further study is needed. Contrast-enhanced US is also being explored to reduce benign biopsies. In the series of Lee et al (57), 36/109 benign masses recommended for biopsy did not enhance, but also 2/16 malignancies did not. Research is also ongoing to define the role of contrast-enhanced mammography to reduce biopsy of low-suspicion masses if they do not enhance (58), and it may be that otherwise probably benign lesions that do enhance should undergo biopsy, but further study is needed. For all such approaches, it is important to restrict potential down-classification to BI-RADS 3 to those masses that are assessed as BI-RADS 4A, low suspicion, or possibly BI-RADS 4B, moderate suspicion, based on B-mode imaging. Masses assessed on B-mode imaging as BI-RADS 4C, high-suspicion, or BI-RADS 5, highly suggestive of malignancy, should undergo biopsy.
Clinical circumstances are also important to consider. Surveillance is not a good option for women who plan pregnancy or who may be relocating out of the country, nor especially for women with synchronous breast cancer. In the series of Kim et al (59) evaluating incidental synchronous lesions found on whole breast US in women with recently diagnosed breast cancer, 56/482 (11.6%) of concurrent BI-RADS 3 lesions were malignant, including: 36/170 (21.2%) in the same quadrant; 12/122 (9.8%) in a different quadrant; and 8/190 (4.2%) in the contralateral breast.
Both benign and malignant lesions can grow. Gordon et al (60) followed 1070 masses after fine-needle aspiration biopsy results suggested fibroadenoma; interval enlargement was observed in 194 (18.1%) masses, in 187 women, for which detailed results were published. The 95th percentile for increase in diameter in 6 months was a 20% increase at all ages. Change in volume can also be assessed, using the formula 4/3 π (diameter1 × diameter2 × diameter3) divided by 2. Gordon et al (60) found that presumed fibroadenomas with growth in volume less than 16% per month in women under age 50 and less than 13% per month in women over age 50 could be safely followed, but increase in largest diameter of 20% in six months at any age had equivalent diagnostic accuracy.
Enlargement was much more common in women < age 50 than in older women. Two of 67 enlarging masses excised proved to be phyllodes tumors. It should be noted that at a 20% rate of growth per 6 months, a mass measuring 1 cm in largest diameter initially could measure up to 1.2 cm at 6 months, 1.4 cm at 12 months, 2.1 cm at 24 months, and 3.0 cm at 36 months. BI-RADS 3 masses larger than 2 cm are not more likely to be primary breast malignancies per se, but Jung et al (61) did find 2 malignant phyllodes tumors among 126 excised BI-RADS 3 masses larger than 2 cm after benign result on core-needle biopsy, as well as 10 benign phyllodes tumors. Absolute size is not a criterion for biopsy or surgical excision per se.
Based on the work of Gordon et al, a 20% increase in diameter in 6 months has also been used as a criterion to prompt biopsy in a circumscribed oval mass that is otherwise probably benign (and likely a fibroadenoma). Moon et al (62) reported on 214 probably benign sonographically depicted masses in 199 women, and found mean increase in diameter for 22 malignancies of 2.2 mm/month vs. 0.5 mm/month for 192 benign masses (P < 0.0001). They reported that 7/145 (4.8%) masses with growth alone were malignant versus 15/39 (38%) with other suspicious change, P = 0.0011. Ha et al (63) reported on 12 514 BI-RADS 3 lesions on US, of which 738 (5.9%) grew more than 20% in diameter in 6 months; 527 of the enlarging masses had biopsy or two-year follow-up. Of these 527 masses, 26 (4.9%) proved malignant: 8/420 (1.9%) of masses only showing growth were malignant, as were 18/107 (17%) of those with other suspicious change(s) including margins, shape, echotexture, or orientation (P = 0.009).
Correlation with mammography is mandatory when performing screening US. Screening US, of course, can characterize a new mammographically depicted mass as a cyst and avoid recall, and, similarly, a mass seen on screening US may correspond to a mass stable on mammography/tomosynthesis (sometimes only seen in retrospect) and therefore benign. A new “probably benign” mass seen on mammography does appear to have a higher risk of malignancy than a baseline finding (8). In the series of Chae et al (64) of 1164 women with a BI-RADS 3 assessment on US who had at least 2-year follow-up, the malignancy rate was 4/184 (2.2%) among those who also had an abnormality on mammography versus 4/980 (0.4%) when a finding was seen only on US (P = 0.025).
Artificial Intelligence
Finally, artificial intelligence and/or computer-assisted diagnosis (CADx) may help reduce false positives on screening US. BI-RADS 3 lesions can be selectively downgraded to BI-RADS 2, benign and selectively upgraded to BI-RADS 4, though in two studies to date, the net effect was an increase in recommended benign biopsies (65,66). It appears that inexperienced radiologists or nonradiologist physicians may benefit most from CADx (67,68), though further study focused on lesions near the threshold for biopsy is needed and is in process.
Conclusions
BI-RADS 3 circumscribed oval masses due to complicated cysts with debris and fibroadenomas are common on screening US. Most complicated cysts with debris can be recognized as benign findings, as can clustered microcysts and multiple bilateral circumscribed masses. Rarely, malignancies can appear circumscribed and hypoechoic, including triple receptor negative invasive ductal carcinoma, encapsulated papillary carcinoma, and DCIS. A BI-RADS 3 assessment with 12-month follow-up is reasonable for apparently solid, circumscribed masses on baseline screening HHUS and that follow-up can be performed as a screening examination provided the mass is redocumented. Orthogonal views, images without and with calipers and Doppler are necessary for complete characterization of masses other than simple cysts and should be included in HHUS documentation, to allow immediate final assessment for the vast majority of findings. While several studies report that new BI-RADS 3 masses detected on incidence screening US can also be followed at 12 months, greater caution is needed in that setting, and particularly if there is a corresponding new mass on mammography in a postmenopausal woman. Growth in diameter >20% in 6 months (annualized rate of 44%) should prompt biopsy, though the malignancy rate remains <2% in the absence of other suspicious change. The use of BI-RADS 3 directly on automated screening US is not yet well validated but may also be appropriate. For audit purposes, only additional testing prior to the next screen is considered “test positive”: BI-RADS 3 with 12-month follow-up would be audited as a negative screen and such use would improve the specificity of screening breast US.
Funding
This work was supported by grants from the National Cancer Institute, R01CA187593, and the Breast Cancer Research Foundation, BCRF 020-015.
Conflict of Interest Statement
The department receives a research grant from Koios Medical, Inc, for which W.A.B. is the principal Investigator. W.A.B. holds the position of associate editor for the Journal of Breast Imaging and was recused from reviewing or making decisions for this article.
References
- 1.D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 2.Sickles EA. Periodic mammographic follow-up of probably benign lesions: results in 3184 consecutive cases. Radiology 1991;179(2):463–468. [DOI] [PubMed] [Google Scholar]
- 3.Sickles EA. Nonpalpable, circumscribed, noncalcified solid breast masses: likelihood of malignancy based on lesion size and age of patient. Radiology 1994;192(2):439–442. [DOI] [PubMed] [Google Scholar]
- 4.Varas X, Leborgne JH, Leborgne F, Mezzera J, Jaumandreu S, Leborgne F. Revisiting the mammographic follow-up of BI-RADS category 3 lesions. AJR Am J Roentgenol 2002;179(3):691–695. [DOI] [PubMed] [Google Scholar]
- 5.Vizcaíno I, Gadea L, Andreo L, et al. ; Sceening Program Working Group . Short-term follow-up results in 795 nonpalpable probably benign lesions detected at screening mammography. Radiology 2001;219(2):475–483. [DOI] [PubMed] [Google Scholar]
- 6.Sickles EA. Probably benign breast lesions: when should follow-up be recommended and what is the optimal follow-up protocol? Radiology 1999;213(1):11–14. [DOI] [PubMed] [Google Scholar]
- 7.Berg WA, Berg JM, Sickles EA, et al. Cancer yield and patterns of follow-up for BI-RADS category 3 after screening mammography recall in the national mammography database. Radiology 2020;296(1):32–41. [DOI] [PubMed] [Google Scholar]
- 8.Lee CS, Berg JM, Berg WA. Cancer yield exceeds 2% for BI-RADS 3 probably benign findings in women older than 60 years in the national mammography database. Radiology 2021;299(3):550–558. [DOI] [PubMed] [Google Scholar]
- 9.Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology 1995;196(1):123–134. [DOI] [PubMed] [Google Scholar]
- 10.Skaane P, Engedal K. Analysis of sonographic features in the differentiation of fibroadenoma and invasive ductal carcinoma. AJR Am J Roentgenol 1998;170(1):109–114. [DOI] [PubMed] [Google Scholar]
- 11.Rahbar G, Sie AC, Hansen GC, et al. Benign versus malignant solid breast masses: US differentiation. Radiology 1999;213(3):889–894. [DOI] [PubMed] [Google Scholar]
- 12.Baker JA, Kornguth PJ, Soo MS, Walsh R, Mengoni P. Sonography of solid breast lesions: observer variability of lesion description and assessment. AJR Am J Roentgenol 1999;172(6):1621–1625. [DOI] [PubMed] [Google Scholar]
- 13.Mendelson EB, Baum JK, Berg WA, Merritt CRB, Rubin E.. Breast Imaging Reporting and Data System, BI-RADS: Ultrasound. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 14.Mendelson EB, Böhm-Vélez M, Berg WA, et al. ACR BI-RADS® Ultrasound. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 15.Holzer-Fruehwald L, Meissnitzer M, Weber M, Holzer S, Hergan K, Weismann C. Can cut-off-values for tumor size or patient age in breast ultrasound reduce unnecessary biopsies or is it all about bi-rads? A retrospective analysis of 763 biopsied T1-sized lesions. Ultrasound Int Open 2017;3(3):E94–E98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durmus T, Stöckel J, Slowinski T, Thomas A, Fischer T. The hyperechoic zone around breast lesions - an indirect parameter of malignancy. Ultraschall Med 2014;35(6):547–553. [DOI] [PubMed] [Google Scholar]
- 17.Elverici E, Barça AN, Aktaş H, et al. Nonpalpable BI-RADS 4 breast lesions: sonographic findings and pathology correlation. Diagn Interv Radiol 2015;21(3):189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barr RG, Zhang Z, Cormack JB, Mendelson EB, Berg WA. Probably benign lesions at screening breast US in a population with elevated risk: prevalence and rate of malignancy in the ACRIN 6666 trial. Radiology 2013;269(3):701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berg WA, Blume JD, Cormack JB, et al. ; ACRIN 6666 Investigators . Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008;299(18):2151–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg WA, Mendelson EB. Should the axilla be included in screening US? response. Radiology 2015;274(2):624. [PubMed] [Google Scholar]
- 21.Lee SH, Yi A, Jang MJ, Chang JM, Cho N, Moon WK. Supplemental screening breast US in women with negative mammographic findings: effect of routine axillary scanning. Radiology 2018;286(3):830–837. [DOI] [PubMed] [Google Scholar]
- 22.Youn I, Yoon JH, Youk JH, et al. Necessity of axillary scanning after negative finding on both mammography and subsequent breast ultrasound. Ultrasound Med Biol 2018;44(1):71–77. [DOI] [PubMed] [Google Scholar]
- 23.Berg WA, Mendelson EB. How should screening breast US be audited? The patient perspective. Radiology 2014;272(2):309–315. [DOI] [PubMed] [Google Scholar]
- 24.Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology 2014;272(1):12–27. [DOI] [PubMed] [Google Scholar]
- 25.Sickles EA, D’Orsi CJ.. Follow-up and Outcome Monitoring. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 26.Jia M, Lin X, Zhou X, et al. Diagnostic performance of automated breast ultrasound and handheld ultrasound in women with dense breasts. Breast Cancer Res Treat 2020;181(3):589–597. [DOI] [PubMed] [Google Scholar]
- 27.Barr RG, DeSivestri A, Golatta M.. Outcome of return to routine screening for BI-RADS 3 lesions detected at supplemental automated whole-breast ultrasound in women with dense breasts: a prospective study [published online ahead of print July 14, 2021]. Amer J Roentgenol 2021. doi: 10.2214/AJR.21.26180 [DOI] [PubMed] [Google Scholar]
- 28.Buchberger W, Geiger-Gritsch S, Knapp R, Gautsch K, Oberaigner W. Combined screening with mammography and ultrasound in a population-based screening program. Eur J Radiol 2018;101:24–29. [DOI] [PubMed] [Google Scholar]
- 29.Destounis S, Arieno A, Morgan R. New York state breast density mandate: follow-up data with screening sonography. J Ultrasound Med 2017;36(12):2511–2517. [DOI] [PubMed] [Google Scholar]
- 30.Chang JM, Koo HR, Moon WK. Radiologist-performed hand-held ultrasound screening at average risk of breast cancer: results from a single health screening center. Acta Radiol 2015;56(6):652–658. [DOI] [PubMed] [Google Scholar]
- 31.Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology 2012;265(1):59–69. [DOI] [PubMed] [Google Scholar]
- 32.Moon HJ, Jung I, Park SJ, Kim MJ, Youk JH, Kim EK. Comparison of cancer yields and diagnostic performance of screening mammography vs. supplemental screening ultrasound in 4394 women with average risk for breast cancer. Ultraschall Med 2015;36(3):255–263. [DOI] [PubMed] [Google Scholar]
- 33.Moon HJ, Kim MJ, Yoon JH, Kim EK. Follow-up interval for probably benign breast lesions on screening ultrasound in women at average risk for breast cancer with dense breasts. Acta Radiol 2018;59(9):1045–1050. [DOI] [PubMed] [Google Scholar]
- 34.Nam SY, Ko EY, Han BK, Shin JH, Ko ES, Hahn SY. Breast imaging reporting and data system category 3 lesions detected on whole-breast screening ultrasound. J Breast Cancer 2016;19(3):301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weigert JM. The connecticut experiment; the third installment: 4 years of screening women with dense breasts with bilateral ultrasound. Breast J 2017;23(1):34–39. [DOI] [PubMed] [Google Scholar]
- 36.Berg WA, Vourtsis A. Screening breast ultrasound using hand-held or automated technique in women with dense breasts. J Breast Imaging 2019;1(4):283–296. [DOI] [PubMed] [Google Scholar]
- 37.Berg WA, Sechtin AG, Marques H, Zhang Z. Cystic breast masses and the ACRIN 6666 experience. Radiol Clin North Am 2010;48(5):931–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berg WA. Sonographically depicted breast clustered microcysts: is follow-up appropriate? AJR Am J Roentgenol 2005;185(4):952–959. [DOI] [PubMed] [Google Scholar]
- 39.Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology 2003;227(1):183–191. [DOI] [PubMed] [Google Scholar]
- 40.Chang YW, Kwon KH, Goo DE, Choi DL, Lee HK, Yang SB. Sonographic differentiation of benign and malignant cystic lesions of the breast. J Ultrasound Med 2007;26(1):47–53. [DOI] [PubMed] [Google Scholar]
- 41.Daly CP, Bailey JE, Klein KA, Helvie MA. Complicated breast cysts on sonography: is aspiration necessary to exclude malignancy? Acad Radiol 2008;15(5):610–617. [DOI] [PubMed] [Google Scholar]
- 42.Goldbach AR, Tuite CM, Ross E. Clustered microcysts at breast US: outcomes and updates for appropriate management recommendations. Radiology 2020;295(1):44–51. [DOI] [PubMed] [Google Scholar]
- 43.Greenwood HI, Lee AY, Lobach IV, Carpentier BM, Freimanis RI, Strachowski LM. Clustered microcysts on breast ultrasound: what is an appropriate management recommendation? AJR Am J Roentgenol 2017;209(6):W395–W399. [DOI] [PubMed] [Google Scholar]
- 44.Berg WA. Reducing unnecessary biopsy and follow-up of benign cystic breast lesions. Radiology 2020;295(1):52–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka A, Imai A, Goto M, Konishi E, Shinkura N. Which patients require or can skip biopsy for breast clustered microcysts? Predictive findings of breast cancer and mucocele-like tumor. Breast Cancer 2016;23(4):590–596. [DOI] [PubMed] [Google Scholar]
- 46.Leung JW, Sickles EA. Multiple bilateral masses detected on screening mammography: assessment of need for recall imaging. AJR Am J Roentgenol 2000;175(1):23–29. [DOI] [PubMed] [Google Scholar]
- 47.Berg WA, Zhang Z, Cormack JB, Mendelson EB. Multiple bilateral circumscribed masses at screening breast US: consider annual follow-up. Radiology 2013;268(3):673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song SE, Cho N, Chu A, et al. Undiagnosed breast cancer: features at supplemental screening US. Radiology 2015;277(2):372–380. [DOI] [PubMed] [Google Scholar]
- 49.Kerlikowske K, Zhu W, Tosteson AN, et al. ; Breast Cancer Surveillance Consortium . Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med 2015;162(10):673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berg WA, Cosgrove DO, Doré CJ, et al. ; BE1 Investigators . Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 2012;262(2):435–449. [DOI] [PubMed] [Google Scholar]
- 51.Lee SH, Chang JM, Kim WH, et al. Added value of shear-wave elastography for evaluation of breast masses detected with screening US imaging. Radiology 2014;273(1):61–69. [DOI] [PubMed] [Google Scholar]
- 52.Barr RG, De Silvestri A, Scotti V, et al. Diagnostic performance and accuracy of the 3 interpreting methods of breast strain elastography: a systematic review and meta-analysis. J Ultrasound Med 2019;38(6):1397–1404. [DOI] [PubMed] [Google Scholar]
- 53.Barr RG, Destounis S, Lackey LB II, Svensson WE, Balleyguier C, Smith C. Evaluation of breast lesions using sonographic elasticity imaging: a multicenter trial. J Ultrasound Med 2012;31(2):281–287. [DOI] [PubMed] [Google Scholar]
- 54.Zheng X, Huang Y, Wang Y, et al. Combination of different types of elastography in downgrading ultrasound breast imaging-reporting and data system category 4a breast lesions. Breast Cancer Res Treat 2019;174(2):423–432. [DOI] [PubMed] [Google Scholar]
- 55.Neuschler EI, Butler R, Young CA, et al. A pivotal study of optoacoustic imaging to diagnose benign and malignant breast masses: a new evaluation tool for radiologists. Radiology 2018;287(2):398–412. [DOI] [PubMed] [Google Scholar]
- 56.Neuschler EI, Lavin PT, Tucker FL, et al. Downgrading and upgrading gray-scale ultrasound BI-RADS categories of benign and malignant masses with optoacoustics: a pilot study. AJR Am J Roentgenol 2018;211(3):689–700. [DOI] [PubMed] [Google Scholar]
- 57.Lee SC, Tchelepi H, Grant E, et al. Contrast-enhanced ultrasound imaging of breast masses: adjunct tool to decrease the number of false-positive biopsy results. J Ultrasound Med 2019;38(9):2259–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zuley ML, Bandos AI, Abrams GS, et al. Contrast enhanced digital mammography (CEDM) helps to safely reduce benign breast biopsies for low to moderately suspicious soft tissue lesions. Acad Radiol 2020;27(7):969–976. [DOI] [PubMed] [Google Scholar]
- 59.Kim SJ, Ko EY, Shin JH, et al. Application of sonographic BI-RADS to synchronous breast nodules detected in patients with breast cancer. AJR Am J Roentgenol 2008;191(3): 653–658. [DOI] [PubMed] [Google Scholar]
- 60.Gordon PB, Gagnon FA, Lanzkowsky L. Solid breast masses diagnosed as fibroadenoma at fine-needle aspiration biopsy: acceptable rates of growth at long-term follow-up. Radiology 2003;229(1):233–238. [DOI] [PubMed] [Google Scholar]
- 61.Jung HK, Moon HJ, Kim MJ, Kim EK. Benign core biopsy of probably benign breast lesions 2 cm or larger: correlation with excisional biopsy and long-term follow-up. Ultrasonography 2014;33(3):200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moon HJ, Kim EK, Kwak JY, Yoon JH, Kim MJ. Interval growth of probably benign breast lesions on follow-up ultrasound: how can these be managed? Eur Radiol 2011;21(5):908–918. [DOI] [PubMed] [Google Scholar]
- 63.Ha SM, Chae EY, Cha JH, Shin HJ, Choi WJ, Kim HH. Growing BI-RADS category 3 lesions on follow-up breast ultrasound: malignancy rates and worrisome features. Br J Radiol 2018;91(1087):20170787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chae EY, Cha JH, Shin HJ, Choi WJ, Kim HH. Reassessment and follow-up results of BI-RADS category 3 lesions detected on screening breast ultrasound. AJR Am J Roentgenol 2016;206(3):666–672. [DOI] [PubMed] [Google Scholar]
- 65.Berg WA, Gur D, Bandos AI, et al. Impact of original and artificially improved AI-based CADx on Breast US interpretation. J Breast Imag 2021;3(3):301–311. [DOI] [PubMed] [Google Scholar]
- 66.Philpotts LE, Cavallo JJ, Durand MA, Andrejeva-Wright L, Cotton CJ, Barinov L. Use of an artificial intelligence decision support platform for determining management of masses detected on screening breast ultrasound (abstr). Am Roentgen Ray Soc 2021. [Google Scholar]
- 67.Park HJ, Kim SM, La Yun B, et al. A computer-aided diagnosis system using artificial intelligence for the diagnosis and characterization of breast masses on ultrasound: Added value for the inexperienced breast radiologist. Medicine (Baltimore) 2019;98(3):e14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mango VL, Sun M, Wynn RT, Ha R. Should we ignore, follow, or biopsy? impact of artificial intelligence decision support on breast ultrasound lesion assessment. AJR Am J Roentgenol 2020;214(6):1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaplan SS. Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology 2001;221(3):641–649. [DOI] [PubMed] [Google Scholar]
- 70.Parris T, Wakefield D, Frimmer H. Real world performance of screening breast ultrasound following enactment of Connecticut Bill 458. Breast J 2013;19(1):64–70. [DOI] [PubMed] [Google Scholar]
- 71.Kolb TM, Lichy J, Newhouse JH. Occult cancer in women with dense breasts: detection with screening US–diagnostic yield and tumor characteristics. Radiology 1998;207(1):191–199. [DOI] [PubMed] [Google Scholar]
- 72.De Felice C, Savelli S, Angeletti M, et al. Diagnostic utility of combined ultrasonography and mammography in the evaluation of women with mammographically dense breasts. J Ultrasound 2007;10(3):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Youk JH, Kim EK, Kim MJ, Kwak JY, Son EJ. Performance of hand-held whole-breast ultrasound based on BI-RADS in women with mammographically negative dense breast. Eur Radiol 2011;21(4):667–675. [DOI] [PubMed] [Google Scholar]