Abstract
Introduction:
Strategy training, a rehabilitation intervention, reduces disability and improves functional skills associated with goal-directed behavior. Stroke lesions impacting selected ventromedial regions of interest associated with initiation of goal-directed behavior may attenuate intervention response. If so, strategy training may not be optimal for people with stroke lesions in these regions.
Objective:
To examine whether ventromedial regions of interest attenuate changes in disability status attributed to strategy training.
Design:
Secondary analysis of data from two randomized controlled clinical trials.
Setting:
Inpatient stroke rehabilitation.
Participants:
People with acute stroke diagnosis and available diagnostic studies enrolled in inpatient rehabilitation randomized controlled studies between 2009 and 2017.
Intervention:
Participants were randomized to strategy training or a control condition in addition to usual care during inpatient rehabilitation.
Main Outcome Measures:
Diagnostic magnetic resonance imaging studies were retrieved from electronic medical records and stroke lesion location was characterized by a neuroradiologist. Intervention response was defined by Functional Independence Measure change scores of 22 points or greater.
Results:
Only 186 of 275 participants had diagnostic studies available;13 patients showed no apparent lesion on their diagnostic study. Among 173 cases, 156 had complete data at discharge (strategy training n=71, control n=85). Twenty-five cases had a lesion within a region of interest (strategy training n=14, control n=11). Intervention response was attenuated in the strategy training group for those with lesions in regions of interest [χ2(1, n=71)=4.60, p=.03], but not for those in the control group [Fisher’s exact test, n=85, p=.19).
Conclusions:
Lesions in ventromedial regions of interest may attenuate response to strategy training.
Keywords: Stroke, Diagnostic Imaging, Activities of Daily Living, Neurological Rehabilitation, Strategy Training
Introduction
Strategy training is a rehabilitation approach that trains persons with stroke-related disability to actively identify and analyze problems with everyday skilled activities and develop strategies to address these problems with the support of self-evaluation, self-monitoring, global strategy use, guided discovery, and iterative practice.1 Strategy training shows promise for promoting independence with daily self-care and mobility activities necessary to live in the community after stroke.2,3 Evidence also suggests that strategy training improves motor performance4 and executive functions,2,5 and stimulates goal-directed behavior after stroke.6
These observations may illuminate a greater understanding of the neurological circuitry underlying goal-directed behavior. Previous animal and clinical studies have demonstrated that post-stroke deficits in the initiation of goal-directed behavior can be attributed to lesions in the ventromedial frontal cortex (anterior cingulate), the ventral striatum (nucleus accumbens), and the medial dorsal thalamic nuclei.7,8 Of note, these same regions of interest are critical to improvements in skilled movements, executive functions, and mood.9,10 Given the association between strategy training and goal-directed behavior, stroke lesions impacting these ventromedial regions of interest may attenuate outcomes associated with strategy training. The purpose of this manuscript was to compare strategy training responsiveness, defined as change in the primary outcome independence in daily self-care and mobility activities, between participants who did and did not have a stroke lesion impairing the anterior cingulate, nucleus accumbens, or the medial dorsal thalamic nuclei. Our hypothesis was that participants who had lesions affecting one or more of these regions of interest would likely show attenuated response to the strategy training intervention than participants who did not have a lesion affecting any of these regions.
Methods
Study Design
We completed a secondary analysis of existing data retrieved from two randomized controlled clinical studies (NCT02755805, NCT01934621). These two studies examined strategy training compared to a control condition (reflective listening) using the same selection criteria, intervention methods, and outcome measures, as described in the following paragraphs. All procedures for the previous two studies and the current analyses were approved by the University Institutional Review Board.
Participants
The two studies recruited participants who were diagnosed with stroke and admitted to acute inpatient rehabilitation facilities within a single academic medical center between the years of 2009 and 2017. Eligible participants had: 1) acute stroke as primary diagnosis, and 2) cognitive impairments (Executive Interview, 14-item version11,12 score ≥ 3). Potential participants were excluded if they had: 1) a dementia diagnosis, 2) inability to follow two-step commands 80% of the time, 3) severe aphasia (Boston Diagnostic Aphasia Examination Severity Scale13 score <2), 4) major depressive, bipolar, or psychotic disorder (Primary Care Evaluation of Mental Disorders14) or 5) drug or alcohol abuse disorder within 3 months prior to screening (Mini-International Neuropsychiatric Interview).15
To describe the sample we collected participant demographic information (age, gender, race, education), medical history (characterized with the Charlson Comorbidity Index16), stroke characteristics (stroke type, hemisphere, and severity characterized by the National Institutes of Health Stroke Scale17). We also characterized motor function (Chedoke-McMaster Stroke Assessment Measure18), cognitive function (Repeatable Battery for the Assessment of Neuropsychological Status19–21), mood (Patient Health Questionnaire22), and quality of life (Stroke Impact Scale23,24).
Intervention Procedures
All eligible participants were randomly assigned to receive either strategy training or a control condition in addition to their usual clinical inpatient rehabilitation services. Intervention procedures are described in detail elsewhere.2,6 Briefly, strategy training is a meta-cognitive approach that trains people with stroke-related cognitive impairments in strategies for identifying and addressing challenges with daily living skills. Participant-selected activity-based goals, self-evaluation and self-monitoring, global strategy use (i.e., goal-plan-do-check), and guided discovery (i.e., structured facilitation of learning) are combined to promote problem solving and independence. Strategy training differs from task-specific training, which is frequently used in stroke rehabilitation.25 Strategy training guides the participant to develop self-assessment, strategy development, and problem-solving skills while learning to perform meaningful daily activities; task-specific training often directs the participant in task execution based on the therapist’s assessment, strategy development, and problem-solving skills. The control condition in the randomized controlled studies was a reflective listening program that focused on the stroke participants’ reflections on their stroke, rehabilitation, and recovery. No active feedback or problem-solving strategies were administered in the control condition.
Outcome Measures
The primary outcome measure for the present study was the Functional Independence Measure (FIM)26 total score, administered by trained raters at study baseline (proximal to the start of the study intervention, within the first 3 days of rehabilitation admission) and at rehabilitation discharge. The FIM assesses independence with basic self-care and mobility activities, with scores ranging from 18 (dependent) to 126 (independent).
Stroke Lesion Location Characterization
Diagnostic magnetic resonance imaging studies were collected from the medical record for both studies, if available. We selected diagnostic studies that were completed after initial medical intervention and within the first week of stroke. We collaborated with a neuroradiologist to characterize stroke lesion location after viewing axial diffusion weighted images, apparent diffusion coefficient images, axial flair images, and axial T2 images (Figure 1). For those cases with available diagnostic studies, stroke lesion location was coded as 1) affecting one or more regions of interest (anterior cingulate, nucleus accumbens, medial dorsal thalamic nuclei), 2) not affecting one or more regions of interest, or 3) not apparent on the available diagnostic study. We elected a priori to group these regions of interest together given the strong interconnectivity among these regions.27,28 The neuroradiologist was blinded to intervention allocation and outcome.
Figure 1.
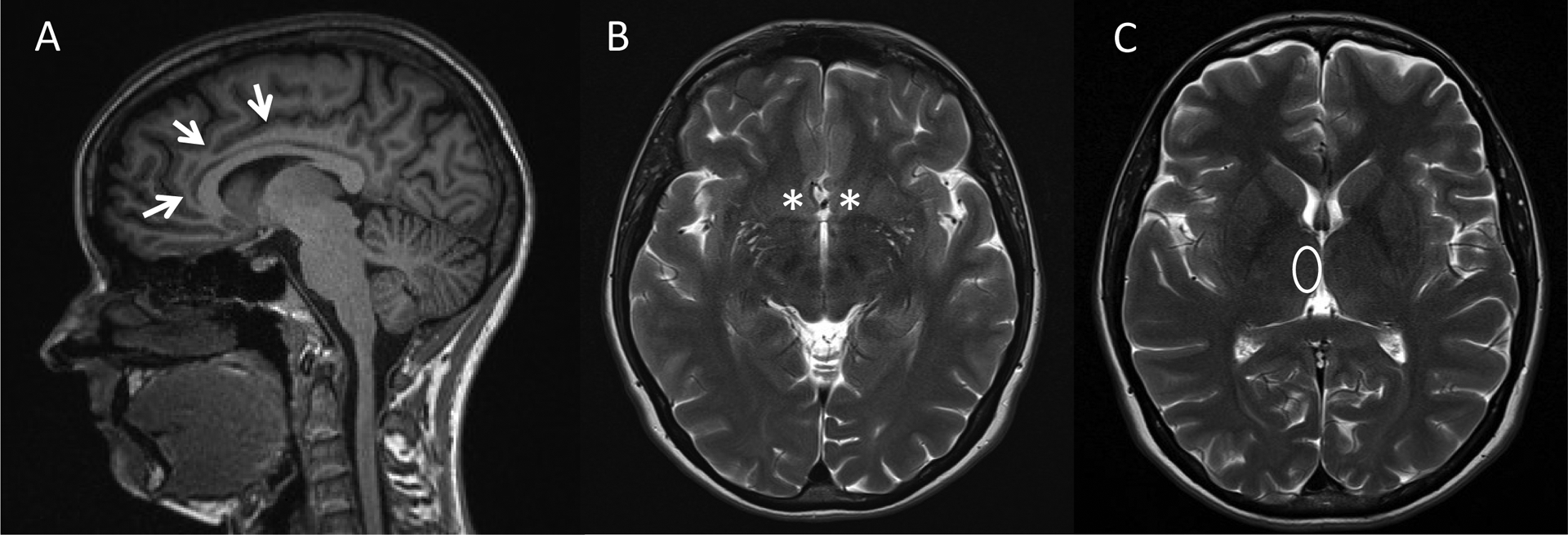
Regions of Interest
Note. Areas of interest assessed on MRI (a) T1 sagittal image with arrows on the anterior cingulate gyrus, (b) T2 axial image with asterisks on the bilateral nucleus accumbens, (c) T2 axial image with oval over the medial dorsal thalamic nucleus.
Statistical Analyses
We performed all statistical analyses using SPSS software version 26 (IBM Corp., Armonk, NY) with a significance level of α=.05. We computed descriptive statistics to summarize particpants’ characteristics. We also compared participants included and excluded from the analyses based on availability of diagnostic studies in the medical record. To characterize intervention response, we calculated FIM change scores from baseline to inpatient rehabilitation discharge. Responders were those who achieved the minimally clinical important difference of 22 points or greater change on FIM total score.29,30 We examined associations between lesion location and intervention response at rehabilitation discharge using Pearson chi-square or Fisher’s exact tests, as appropriate.
Results
Diagnostic magnetic resonance imaging studies were available for 186 out of 275 participants in the two clinical studies (Figure 2). Diagnostic studies were not available if clinical imaging was performed at a community hospital outside the health system, or if clinical imaging other than magnetic resonance imaging was performed per clinical protocols based on patient need. Of the 186 participants, 13 had no apparent lesion on the study. These were excluded from further analysis. When comparing characteristics of participants who were included and excluded from the current analysis (n=173 and n=102 respectively), we noted that excluded participants had significantly greater chronicity prior to receiving intervention (days, excluded participants M=18.74, SD=20.40, included participants M=13.05, SD=10.32; t262=3.01, p=.01) and had higher education (years, excluded participants M=14.38, SD=2.40; included participants M=13.63, SD=2.66; t267=2.33, p=.02).
Figure 2.
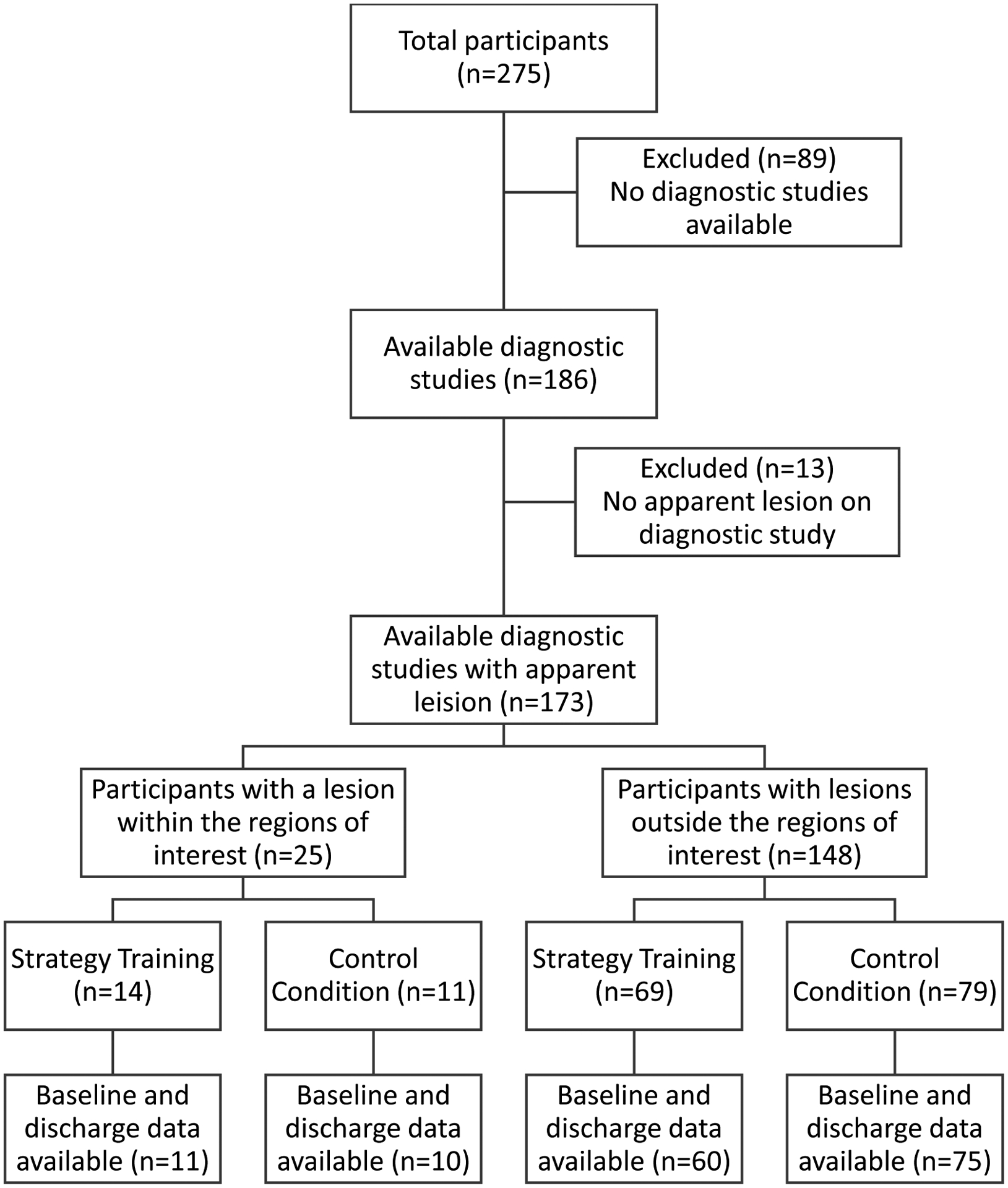
Flow Chart of Reporting Analysis Cases
The table provides characteristics of the participants with available diagnostic studies and apparent lesions (n=173), by intervention group. Of the 173 participants, 25 had a lesion within one or more of the regions of interest (nucleus accumbens, anterior cingular cortex, medial dorsal thalamic nuclei), and 148 had lesions outside these regions. At baseline, participants with lesions within the regions of interest had greater stroke severity (NIHSS score M=7.96, SD=4.37, t163=2.31, p=.02), motor impairment (CMA score M=14.55, SD=6.12, t154=2.20, p=.03), cognitive impairment (RBANS score, M=65.89, SD=11.97, t128= 2.88, p<.01), and disability (FIM score M=60.38, SD=17.79, t161=2.58, p=.01) than participants with lesions outside these regions (NIHSS score M=6.10, SD=3.59; CMA score M=17.37, SD=5.48; RBANS score M=74.58, SD=11.89; FIM score M=70.86, SD=18.45). There was also a marginal difference in stroke type [χ2(1, n=173)=3.71, p=.05], with hemorrhagic stroke being two times more likely to generate a lesion in a region of interest.
Table.
Characteristics of participants with available diagnostic studies and apparent lesions
| Strategy Training (n=83) | Control Condition (n=90) | ||||
|---|---|---|---|---|---|
| M(SD) or % (n) | Missing % (n) | M(SD) or % (n) | Missing % (n) | ||
| Age, years | 68.19 (13.01) | 0 (0) | 68.94 (12.50) | 0 (0) | t171= 0.39 |
| Sex, male | 46 (38) | 0 (0) | 56 (50) | 0 (0) | χ21= 1.65 |
| Education, years | 13.91 (2.71) | 5 (4) | 13.37 (2.61) | 1 (1) | t166= 1.32 |
| Race | χ25= 4.04 | ||||
| African American or Black | 19 (16) | 0 (0) | 16 (14) | 0 (0) | |
| Caucasian/White | 80 (66) | 0 (0) | 79 (71) | 0 (0) | |
| Comorbidity, CCI | 2.51 (1.82) | 8 (7) | 2.64 (1.91) | 6 (5) | t159= 0.41 |
| Stroke type, ischemic | 83 (69) | 0 (0) | 74 (67) | 0 (0) | χ21= 1.94 |
| Stroke hemisphere, right | 55 (46) | 0 (0) | 58 (52) | 0 (0) | χ 2 2 = 0.10 |
| Stroke severity, NIHSS‡ | 6.56 (3.62) | 7 (6) | 6.23 (3.91) | 2 (2) | t163= 0.56 |
| Stroke chronicity, days | 14.12 (13.21) | 6 (5) | 12.12 (6.82) | 1 (1) | t165= 1.25 |
| Stroke impact, SIS8 | 23.68 (8.63) | 24 (20) | 21.73 (8.12) | 17 (15) | t136= 1.37 |
| Motor function, CMA | 17.01 (5.89) | 15 (12) | 16.93 (5.45) | 6 (5) | t154= 0.09 |
| Cognitive function, RBANS | 73.79 (11.22) | 33 (27) | 73.07 (13.00) | 18 (16) | t128= 0.33 |
| Depressive symptoms, PHQ‡ | 6.45 (4.05) | 4 (3) | 6.14 (4.22) | 2 (2) | t166= 0.49 |
| Lesion location | |||||
| Nucleus accumbens | 4 (3) | 0 (0) | 1 (1) | 0 (0) | χ21= 120 |
| Anterior cingulate | 10 (8) | 0 (0) | 3 (3) | 0 (0) | χ21= 2.88 |
| Mid-dorsal thalamic nuclei | 4 (3) | 0 (0) | 9 (8) | 0 (0) | χ21= 2.02 |
| Outside regions of interest | 83 (69) | 0 (0) | 88 (79) | 0 (0) | χ21= 0.75 |
| Disability, FIM, baseline | 68.11 (19.40) | 10 (8) | 70.34 (18.08) | 2 (2) | t161= 0.76 |
| Disability, FIM, change | 20.82 (12.12) | 15 (12) | 19.80 (11.33) | 6 (5) | t154= 0.54 |
| Responder, FIM change≥22 | 41 (34) | 15 (12) | 40 (34) | 6 (5) | χ21= 0.98 |
| IRF length of stay, days | 19.20 (10.58) | 0 (0) | 19.44 (7.38) | 0 (0) | t171= 0.17 |
| Intervention sessions | 5.67 (3.63) | 8 (7) | 7.40 (3.25) | 8 (7) | t157= 3.17 |
p≤.05.
p≤.01.
Lower scores=better health.
CCI=Charlson Comorbidity Index. NIHSS= National Institutes of Health Stroke Scale. CMA=Chedoke-McMaster Stroke Assessment Measure (total of last 5-item sum score). RBANS=Repeatable Battery for the Assessment of Neuropsychological Status Index Score. PHQ9=Patient Health Questionnaire-9 (9-item version). SIS8=Stroke Impact Scale, Section 8 Daily Activity Participation (8-item version). ADL = Activities of Daily Living. FIM = Functional Independence Measure. IRF = Inpatient Rehabilitation Facility.
Figure 3 provides the frequencies and proportions of participants who met and did not meet criteria for intervention response (i.e., responders demonstrated FIM score change ≥ 22) by lesion location. Analyses revealed that for participants who received strategy training, the proportions of response and non-response differed based on whether the stroke lesion was located within and outside the regions of interest [χ2(1, n=71)=4.60, p=.03; ⱷ=.26]. A closer look suggests that lesions within regions of interest attenuated strategy training intervention response. In contrast, among participants who received the control condition, the proportions of response and non-response did not differ based on lesion location [Fisher’s exact test, n=85, p=.19; ⱷ=.15].
Figure 3.
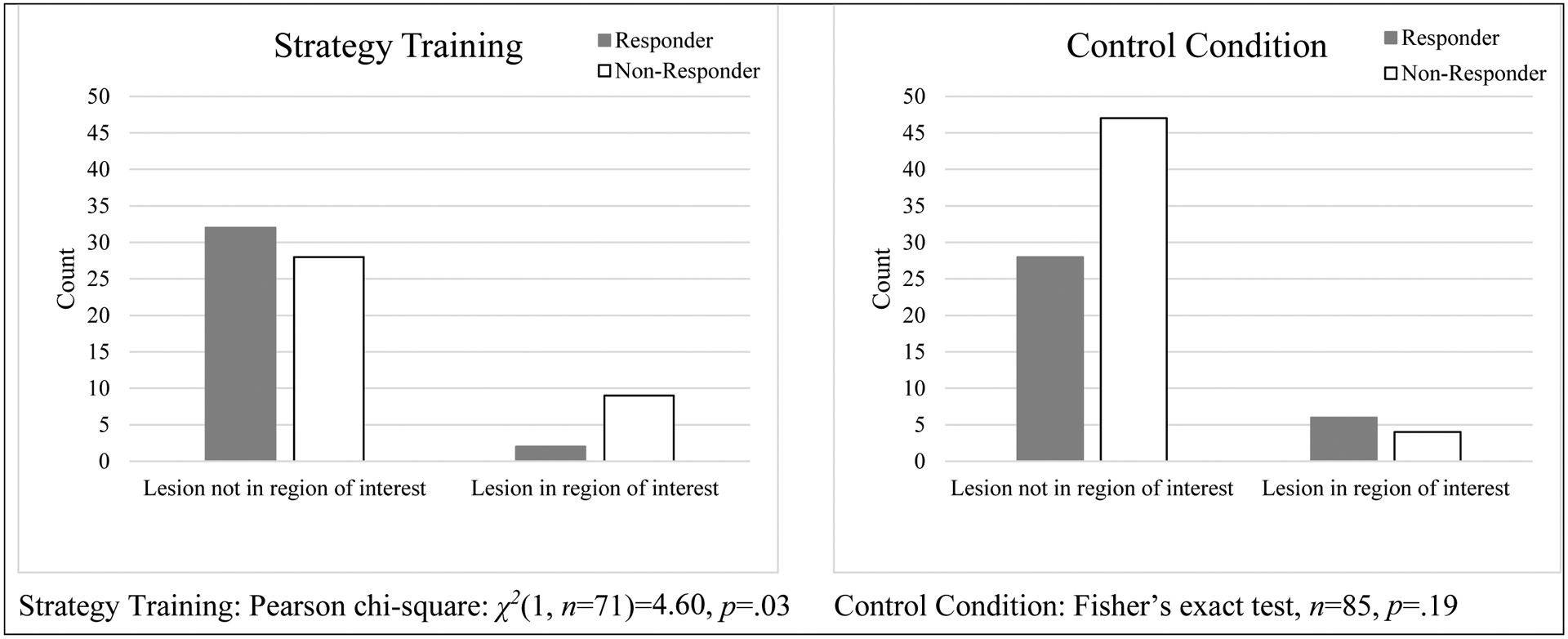
Frequencies of responders by group, and by lesion location
Note. Valid sample at discharge, n=156 of 173 (χ21= 0.30, p=.59). Chi-square and Fisher’s exact tests examined [Response (yes, no) vs Lesion (yes, no)] within each intervention group.
Discussion
Our results provide support for the hypothesis that lesions in ventromedial regions may be associated with attenuated strategy training intervention response. Participants who had lesions within regions of interests were less likely to meet criteria for intervention response in strategy training compared to those in the control condition. The attenuated strategy training response may be due to the clinical impairments and disability associated with ventromedial lesions. In this sample, participants with ventromedial lesions tended to have more severe neurological impairments and activities of daily living disabilities at the onset of inpatient rehabilitation than participants with lesions affecting other regions. This is consistent with previous studies on stroke-related apathy and depression that frequent emerge from ventromedial lesions after stroke.31–33 Of note, previous studies of strategy training identified stroke severity as the only moderator of strategy training response.3,34
The disability severity at inpatient rehabilitation admission for people with ventromedial lesions is problematic because high disability severity is associated with poor rehabilitation outcomes.35 The hypoactivation of the ventromedial regions may impair motor skill learning and contribute to attenuated rehabilitation outcomes.10 Certainly, animal studies have demonstrated the importance of these regions in the use of contextual cues and mnemonic memory retrieval during learning.36 Collectively these data suggest that participants with ventromedial lesions are at risk for severe and persistent disability and require early identification and tailored rehabilitation if we are to ameliorate these tendencies.
Our findings would suggest that alternative intervention strategies may be required to enhance rehabilitation outcomes for people with ventromedial lesions after stroke. Previous studies suggest that behavioral interventions, particularly those that use directed or rhythmic cues or positive reinforcement,37–40 show promise. These behavioral interventions may be provided either alone or in combination with neuromodulation approaches (e.g., high frequency repeated transcranial magnetic stimulation, cranial electrotherapy, and deep brain stimulation41–44). The common elements among these behavioral interventions (i.e., directed task-practice, directed cues, and focused feedback with rewards) are inherently different from the elements in strategy training (i.e., guided task-practice, guided cues, and elicited self-assessment and self-monitoring). These differences may inform the personalization of rehabilitation intervention protocols to optimize outcomes for people with ventromedial lesions after stroke.
Our study had 25 of 173 participants with ventromedial lesions, 14 in strategy training, and 11 in control condition. Thus, the incidence of cases was 14.5% (16.8% in strategy training, 12.2% in control condition). It is difficult to compare this incidence with previous research, but a scan of published studies suggests 1 to 2% of acute stroke cases may have ventromedial lesions.45–47 The higher incidence in our sample may be due to the fact that we recruited in inpatient rehabilitation, where there is likely to be a higher proportion of people with elevated impairment and disability severity, thus justifying the need for these services. It is also possible that lesions affecting these regions are under-documented given the focus on prominent cortical regions of interest in the frontal, temporal and parietal lobes given the frequency of middle cerebral artery stroke cases.25
We excluded cases with unavailable diagnostic studies or cases with available diagnostic studies with no apparent lesions. For the most part, missing diagnostic studies were performed in hospitals outside the health system, many of them in community-based hospitals participating in the acute stroke tele-health intervention program. Nonetheless, this resulted in the exclusion of cases that were significantly more chronic (i.e., on average 5 additional days between stroke onset and inpatient rehabilitation admission). Chronicity may be a proxy indicator for medical complexity. These participants may have been less likely to respond to intervention because they were more chronic, or because they had greater medical complexity. Another difference between included and excluded participants was observed in years of education. However, although these differences were statistically significant, there were not clinically meaningful. Additional investigation is warranted.
The study findings should be interpreted with caution. While we detected a reliable association between lesion location and strategy training intervention response, this study does not support causality. We conducted a secondary analysis of existing data from a single institution using available diagnostic studies. While a useful starting point, these methods contributed to several limitations. First, our analyses identified meaningful differences between participants who did and did not have available diagnostic studies. Second, we involved only a single neuroradiologist in the review of diagnostic images. Third, there were relatively few cases with lesions in the selected regions of interest. This low number prohibiting multivariate analyses that might have controlled for baseline differences. Previous studies suggest that logistic regression analyses with relatively few “events” are highly vulnerable to underestimation.48 Collectively, these design elements may limit the generalizability of study findings. Future studies should use prospective, randomized designs with multiple sites and larger samples, as well as research quality imaging studies to assess causal relationships between lesion location and intervention response.
Conclusion
A secondary analysis of participants from two clinical studies suggests that strategy training outcomes may be attenuated among people with lesions in ventromedial regions associated with initiation of goal-directed behavior.
ACKNOWLEDGEMENTS:
Funding provided by the University of Pittsburgh School of Health Sciences Competitive Medical Research Fund, the University of Pittsburgh Medical Center Rehabilitation Institute, and the Eunice K. Shriver National Center for Medical Rehabilitation Research (K12 HD055931; R01 HD074693). In addition, the authors would like to thank Katherine Grunewald, Lauren Kenney, Emily Krut, Julia Kychun, and Nicole Rosenbaum for their assistance with study analyses.
Footnotes
CONFLICTS OF INTEREST: The authors have no commercial interests to disclose.
References
- 1.Meichenbaum DH. Cognitive behavior modification: An integrative approach. In. New York: Plenum; 1977. [Google Scholar]
- 2.Skidmore ER, Dawson DR, Butters MA, et al. Strategy Training Shows Promise for Addressing Disability in the First 6 Months After Stroke. 2015;29(7):668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skidmore ER, Butters M, Whyte E, Grattan E, Shen J, Terhorst L. Guided Training Relative to Direct Skill Training for Individuals With Cognitive Impairments After Stroke: A Pilot Randomized Trial. Arch Phys Med Rehabil. 2017;98(4):673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEwen S, Polatajko H, Baum C, et al. Combined Cognitive-Strategy and Task-Specific Training Improve Transfer to Untrained Activities in Subacute Stroke:An Exploratory Randomized Controlled Trial. 2015;29(6):526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf TJ, Polatajko H, Baum C, et al. Combined Cognitive-Strategy and Task-Specific Training Affects Cognition and Upper-Extremity Function in Subacute Stroke: An Exploratory Randomized Controlled Trial. Am J Occup Ther. 2016;70(2):7002290010p7002290011–7002290010p7002290010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skidmore ER, Whyte EM, Butters MA, Terhorst L, Reynolds CF 3rd. Strategy Training During Inpatient Rehabilitation May Prevent Apathy Symptoms After Acute Stroke. Pm r. 2015;7(6):562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76(3):470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rochat L, Van der Linden M, Renaud O, et al. Poor reward sensitivity and apathy after stroke: implication of basal ganglia. Neurology. 2013;81(19):1674–1680. [DOI] [PubMed] [Google Scholar]
- 9.Le Heron C, Apps MAJ, Husain M. The anatomy of apathy: A neurocognitive framework for amotivated behaviour. Neuropsychologia. 2018;118(Pt B):54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widmer M, Lutz K, Luft AR. Reduced striatal activation in response to rewarding motor performance feedback after stroke. Neuroimage Clin. 2019;24:102036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc. 1992;40(12):1221–1226. [DOI] [PubMed] [Google Scholar]
- 12.Larson EB, Heinemann AW. Rasch analysis of the Executive Interview (The EXIT-25) and introduction of an abridged version (The Quick EXIT). Arch Phys Med Rehabil. 2010;91(3):389–394. [DOI] [PubMed] [Google Scholar]
- 13.Roth C. Boston Diagnostic Aphasia Examination. New York, NY: Springer New York; 2011. [Google Scholar]
- 14.Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. Jama. 1994;272(22):1749–1756. [PubMed] [Google Scholar]
- 15.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–33;quiz 34–57. [PubMed] [Google Scholar]
- 16.Tessier A, Finch L, Daskalopoulou SS, Mayo NE. Validation of the Charlson Comorbidity Index for predicting functional outcome of stroke. Arch Phys Med Rehabil. 2008;89(7):1276–1283. [DOI] [PubMed] [Google Scholar]
- 17.Brott T, Adams HP Jr., Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. [DOI] [PubMed] [Google Scholar]
- 18.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24(1):58–63. [DOI] [PubMed] [Google Scholar]
- 19.Larson E, Kirschner K, Bode R, Heinemann A, Goodman R. Construct and predictive validity of the repeatable battery for the assessment of neuropsychological status in the evaluation of stroke patients. J Clin Exp Neuropsychol. 2005;27(1):16–32. [DOI] [PubMed] [Google Scholar]
- 20.Larson EB, Kirschner K, Bode RK, Heinemann AW, Clorfene J, Rebecca Goodman RG. Brief Cognitive Assessment and Prediction of Functional Outcome in Stroke. Topics in Stroke Rehabilitation. 2003;9(4):10–21. [DOI] [PubMed] [Google Scholar]
- 21.Wilde MC. The validity of the repeatable battery of neuropsychological status in acute stroke. Clin Neuropsychol. 2006;20(4):702–715. [DOI] [PubMed] [Google Scholar]
- 22.Williams LS, Brizendine EJ, Plue L, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke. 2005;36(3):635–638. [DOI] [PubMed] [Google Scholar]
- 23.Duncan PW, Bode RK, Min Lai S, Perera S. Rasch analysis of a new stroke-specific outcome scale: the Stroke Impact Scale. Arch Phys Med Rehabil. 2003;84(7):950–963. [DOI] [PubMed] [Google Scholar]
- 24.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30(10):2131–2140. [DOI] [PubMed] [Google Scholar]
- 25.Winstein CJ, Stein J, Arena R, et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98–e169. [DOI] [PubMed] [Google Scholar]
- 26.Stineman MG, Shea JA, Jette A, et al. The Functional Independence Measure: tests of scaling assumptions, structure, and reliability across 20 diverse impairment categories. Arch Phys Med Rehabil. 1996;77(11):1101–1108. [DOI] [PubMed] [Google Scholar]
- 27.Felten DL. Autonomic-Hypothalamic-Limbic Systems. In: Felten DL, ed. Netter’s Atlas of Neuroscience. 3rd ed.: Elsevier; 2016:421–461. [Google Scholar]
- 28.Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annual review of psychology. 2015;66:25–52. [DOI] [PubMed] [Google Scholar]
- 29.Dromerick AW, Edwards DF, Diringer MN. Sensitivity to changes in disability after stroke: a comparison of four scales useful in clinical trials. J Rehabil Res Dev. 2003;40(1):1–8. [DOI] [PubMed] [Google Scholar]
- 30.Beninato M, Gill-Body KM, Salles S, Stark PC, Black-Schaffer RM, Stein J. Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Arch Phys Med Rehabil. 2006;87(1):32–39. [DOI] [PubMed] [Google Scholar]
- 31.Caeiro L, Ferro JM, Costa J. Apathy secondary to stroke: a systematic review and meta-analysis. Cerebrovasc Dis. 2013;35(1):23–39. [DOI] [PubMed] [Google Scholar]
- 32.Hama S, Yamashita H, Yamawaki S, Kurisu K. Post-stroke depression and apathy: Interactions between functional recovery, lesion location, and emotional response. Psychogeriatrics. 2011;11(1):68–76. [DOI] [PubMed] [Google Scholar]
- 33.Terroni L, Amaro E, Iosifescu DV, et al. Stroke lesion in cortical neural circuits and post-stroke incidence of major depressive episode: a 4-month prospective study. World J Biol Psychiatry. 2011;12(7):539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skidmore ER, Dawson DR, Butters MA, et al. Strategy Training Shows Promise for Addressing Disability in the First 6 Months After Stroke. Neurorehabil Neural Repair. 2015;29(7):668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil. 1996;77(12):1226–1232. [DOI] [PubMed] [Google Scholar]
- 36.Freeman JH Jr., Cuppernell C, Flannery K, Gabriel M. Context-specific multi-site cingulate cortical, limbic thalamic, and hippocampal neuronal activity during concurrent discriminative approach and avoidance training in rabbits. J Neurosci. 1996;16(4):1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubbard IJ, Carey LM, Budd TW, et al. A Randomized Controlled Trial of the Effect of Early Upper-Limb Training on Stroke Recovery and Brain Activation. Neurorehabil Neural Repair. 2015;29(8):703–713. [DOI] [PubMed] [Google Scholar]
- 38.Marcotte K, Laird L, Bitan T, et al. Therapy-Induced Neuroplasticity in Chronic Aphasia After Phonological Component Analysis: A Matter of Intensity. Front Neurol. 2018;9:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nardo D, Holland R, Leff AP, Price CJ, Crinion JT. Less is more: neural mechanisms underlying anomia treatment in chronic aphasic patients. Brain. 2017;140(11):3039–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitall J, Waller SM, Sorkin JD, et al. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: a single-blinded randomized controlled trial. Neurorehabil Neural Repair. 2011;25(2):118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hara T, Abo M, Sasaki N, et al. Improvement of higher brain dysfunction after brain injury by repetitive transcranial magnetic stimulation and intensive rehabilitation therapy: case report. Neuroreport. 2017;28(13):800–807. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki N, Hara T, Yamada N, Niimi M, Kakuda W, Abo M. The Efficacy of High-Frequency Repetitive Transcranial Magnetic Stimulation for Improving Apathy in Chronic Stroke Patients. Eur Neurol. 2017;78(1–2):28–32. [DOI] [PubMed] [Google Scholar]
- 43.Lempka SF, Malone DA Jr., Hu B, et al. Randomized clinical trial of deep brain stimulation for poststroke pain. Ann Neurol. 2017;81(5):653–663. [DOI] [PubMed] [Google Scholar]
- 44.Mallory GW, Abulseoud O, Hwang SC, et al. The nucleus accumbens as a potential target for central poststroke pain. Mayo Clin Proc. 2012;87(10):1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matos Casano HA, Tadi P, Ciofoaia GA. Anterior Cerebral Artery Stroke. In: StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2020. [PubMed] [Google Scholar]
- 46.Kumral E, Erdoğan CE, Bayam FE, Arslan H. Cingulate infarction: A neuropsychological and neuroimaging study. J Neurol Sci. 2019;402:1–6. [DOI] [PubMed] [Google Scholar]
- 47.Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100–108. [DOI] [PubMed] [Google Scholar]
- 48.King G, Zeng L. Logistic regression in rare events data. Political Analysis 2001;9(2):137–163. [Google Scholar]


