Highlights
-
•
Rice is a staple food for more than three billion people, and rice cultivars have evolved over thousands of years of adaptation to different environmental stresses in different regions. Domestication of rice cultivation led to the diversity of cultivars though phenotypic selection for desirable characters. India is blessed with great diversity of rice germplasm, and these are still conserved for many reasons. The aim of the study was to show the seedling-stage salt tolerance of a total of 50 indigenous rice genotypes from coastal Tamil Nadu, India. Using a hydroponic system, we studied the different agronomic characters from seedling to plant growth hight 14 days after exposure to six different concentrations of saline solution. Rice genotypes showed significant interaction and differential response towards salinity were assessed at the molecular level using simple sequence repeat (SSR) markers linked with salt-tolerance QTL. We found wide genetic distance among the genotypes studied. The combination of morphological findings and molecular assessment revealed better salt-tolerance in a few genotypes. This is, to the best of our knowledge, the first study on the indigenous rice landraces of coastal Tamil Nadu, India.
Keywords: Indigenous rice, Haplotype, Genetic diversity, Salt tolerance, SalTol, Simple sequence repeat (SSR), QTL
Abstract
We evaluated the seedling-stage salt tolerance of a total of 50 indigenous rice genotypes from coastal Tamil Nadu. Using a hydroponic system, we studied the different agronomic characters 14 days after exposure to six different concentrations of saline solution. Shoot and root length as well as plant biomass at seedling stage decreased with increasing salinity. Genotypes showing significant interaction and differential response towards salinity were assessed at the molecular level using 20 simple sequence repeat (SSR) markers linked with salt-tolerance QTL. These genotypes were grouped into eleven clusters based on molecular diversity analysis and eight clusters based on D2 statistical analysis. We found wide genetic distance among the genotypes studied. Simple correlation analysis revealed highly significant associations among the traits studied. The combination of morphological findings and molecular assessment revealed better salt-tolerance in a few genotypes viz. Kuzhi adichan, Poonkar, Kallundai, and Sornamugi.
Introduction
Rice, a staple food for more than three billion people worlds over [29, 41, 69] belongs to the genus Oryza of the family Poaceae. Consisting of two cultivated species, O. sativa and O. glaberrima, and 22 wild species, Oryza is a large genus of predominantly tropical aquatic or semi-aquatic grasses. O. sativa is grown worldwide, while O. glaberrima is mostly confined to West Africa [39, 79, 82]. Domesticated since early Holocene (∼10000 cal years before present), rice cultivation has led to cultivar diversity through phenotypic selection [8, 56] for desirable characters such as grain yield and grain quality [51, 62]. Being widely cultivated, adaptive evolution of rice cultivars over thousands of years has distributed them over wide range of environs, such as deep water to montane ecologies [27, 74].
Being one of the primary centers of origin, India is bestowed with wide diversity of rice landraces, wild congeners and modern cultivars [52, 71]. Estimated between 75000 to 100000, the number of indigenous landraces in India has shrunk over time [85], although a considerable portion is still conserved for a variety of reasons [46]. In areas of conservation, farmers cherish profound knowledge of landraces such as their uses, properties and peculiarities [10]. In Tamil Nadu, popularly called as the ‘rice granary of South India’, there were about 400 traditional landraces in vogue since olden times, which are still extant and used in households [67]. These landraces brandish several features such as pest and disease resistance (Sigappu Kuruvikar) and tolerance to flood (Samba Mosanam), drought (Vadan Samba) and salt (Kalarpalai). Some others are suitable for special uses such as fodder and thatching (Kullakar), stamina boosters (Mappillai Samba) and for medicinal uses (Pitchavarii, Navara and Neelan Samba). Understanding their importance, most of these landraces are now conserved in gene banks across the India, so that they are not destroyed due to climatic vagaries and catastrophes [54].
The practicality of conservation of specialty rice is particularly realized during disasters such as tsunamis, floods, cyclones, etc. when conventional cultivars fail to save the situation. For instance, when the coastal Tamil Nadu was devastated by the Indian Ocean tsunami on 26th December 2004, the salt water ingression from the bay has destroyed most of the standing crops in the affected region. The relief process could accommodate only certain traditional landraces which came to the rescue of farmers. However, soil salinization continues to happen even without disasters along the coastal as well as inlands of India, due to poor quality of irrigation water. Globally, rising salinization limits rice production significantly in those areas, which according to an estimate, accounts for about 33% of irrigated land [12, 64, 70].
Rice is sensitive to salt buildup in the soil, particularly during seedling and reproductive stages. Although seedling stage tolerance can support plants to establish under saline soils, the reproductive stage tolerance is essential for realizing yield under salt stress [88]. Comparatively, seedling phase is recognized as the most critical stage to salt stress [63, 65, 75], than the termianl phase. Although, several studies have examined the seedling stage salt tolerance [6, 14, 21, 22, 24, 26, 31, 38, 77], a recent review by Ganie et al. [18] concludes that only few studies are available towards identification of rice genotypes for seedling and reproductive stage salinity tolerance. As the first step towards this, it is essential to understand the variability and diversity of genotypes at the seedling stage [28, 78, 86, 87], and then to proceed for screening for reproductive stage salt tolerance.
Analysis of genetic diversity, once possible only by the use of phenotypic traits, was eased by the advent of molecular markers. However, use of morphological characters in the classification of rice accessions has particularly been felt cumbersome because of the inefficiency of the technique [5, 80]. This emphasizes the utility of molecular markers in such studies, especially when the trait in question has no significant morphological diversity associated with such as salt tolerance. Among the DNA based markers, PCR based systems such as simple sequence repeat (SSR), hold high promise in genetic mapping, diversity profiling as well as in marker-assisted selection [2, 16, 68]. Several marker systems including SSRs have extensively been used in mapping seedling stage salt tolerance in rice, which has resulted in the discovery of a prominent QTL, Saltol on chromososme 1. Babu et al. [7] elucidated the haplotype diversity using 20 QTL linked SSR markers distributed across the Saltol region, which resulted in identification of a highly conserved set of markers associated with Saltol, such as RM 8094, RM 3412 and RM 493. Several other studies also have emphasized the significance of these Saltol markers as candidates for marker-assisted selection [3, 17, 30, 35, 48]. In order to identify other genomic regions, particularly related to reproductive stage salt tolerance many studies have employed different approaches and across many genotypes [63, 66, 13, 1, 60].
In the present study, we have examined a set of rice landraces, hitherto unexplored, collected from coastal areas of Tamil Nadu for the seedling stage salt tolerance under salinity conditions to estimate the extent of their genetic diversity. The aim was to characterize their salt tolerance and to identify the allelic diversity as well as to assess the extent of association with the seedling characters.
Materials and methods
Plant materials and collection site
Forty-seven traditional rice landraces (Table 1) collected from farmers located around Thiruthuraipoondi were used in the study. Thiruthuraipoondi is a coastal town in the Coromandel coast of India, belonging to Tiruvarur district of Tamil Nadu. Located in the headland into the Bay of Bengal close to Point Calimere, the collection site is spread around the geocoordinates of 10.53°N and 79.65°E, 4 m above mean sea level. The site spans into two districts of Thiruvarur and Nagapattinam and is characterized by saline rich soils due to sea water ingression. Further, areas closer to the Bay suffered damages of 2004 Indian Ocean Tsunami, that had sent large volume of seawater inland. Soil types at the collection site were predominantly coastal alluvium and red loam. Three cultivars, CSR10, TRY1 (tolerant) and IR64 (sensitive) were also included in the study.
Table 1.
Details of 50 traditional rice genotypes collected from farmers of coastal Thiruthuraipoondi, Tamil Nadu, India used for the analysis of salt tolerance.
| Sl. No | Code | Genotypes | Sl. No | Code | Genotypes |
| 1 | G1 | Sivapu Kavuni | 26 | G26 | Marathondi |
| 2 | G2 | Selam Samba | 27 | G27 | Sornamugi |
| 3 | G3 | Valan | 28 | G28 | Kalundai |
| 4 | G4 | Arupatham Kuruvai | 29 | G29 | Boommi |
| 5 | G5 | Karudan Samba | 30 | G30 | Karuvachi |
| 6 | G6 | Navara | 31 | G31 | Poonkar |
| 7 | G7 | Karunkuruvai | 32 | G32 | Kattu Yanam |
| 8 | G8 | Kalan Namak | 33 | G33 | Karupu Kavuni |
| 9 | G9 | Seeraga Samba | 34 | G34 | Kuzhi Adichan |
| 10 | G10 | Milagu Samba | 35 | G35 | Mapillai Samba |
| 11 | G11 | Kaivarai Samba | 36 | G36 | Athur Kichadi |
| 12 | G12 | Kudaivazhai | 37 | G37 | Manjal Pooni |
| 13 | G13 | Rajamudi | 38 | G38 | Illapai Poo Samba |
| 14 | G14 | Pal Kudaivazhai | 39 | G39 | Sorna Masuri |
| 15 | G15 | Chinnar | 40 | G40 | Kichadi Samba |
| 16 | G16 | Ottadam | 41 | G41 | Mysore Malli |
| 17 | G17 | Vadan Samba | 42 | G42 | Kullakar |
| 18 | G18 | Sinkini Kar | 43 | G43 | Perunkar |
| 19 | G19 | Thulasi Vasam | 44 | G44 | Thooyamalli |
| 20 | G20 | Kanda Sali | 45 | G45 | Basumathi |
| 21 | G21 | Raja Mannar | 46 | G46 | Soor Kuruvai |
| 22 | G22 | Thanga Samba | 47 | G47 | Kattupooni |
| 23 | G23 | Neelanj Samba | 48 | G48 | Csr10 |
| 24 | G24 | Kothamali Samba | 49 | G49 | Try1 |
| 25 | G25 | Koondukar | 50 | G50 | Ir64 |
Phenotypic screening for salt tolerance
The salt stress experiments were conducted at Department of Genetics and Plant Breeding, Faculty of Agriculture, Annamalai University, Chidambaram, Tamil Nadu, India. The rice seeds were surface sterilized with 0.1% sodium hypochlorite or 70 % ethanol for 30 s andwashed repeatedly with sterile water and placed on germination paper and incubated at 27°C for 48 hours to germinate. To prevent damage to the pre-germinated seeds, they were kept loosely covered until transferred to polystyrene foam floats lines with a nylon net at the bottom. Prior to this, the floats were punched with 15mm diameter holes in a matrix of 8 × 12. The floats with seedlings were allowed to float over Yoshida nutrient solution [83] filled in a plastic crate. For preparing the Yoshida nutrient solution, the stocks containing macro and micronutrients were prepared initially. The culture solution was constituted by taking 1.25 ml of stock in one litter water and adjusting the pH to 5.0-5.1 daily. The culture solutions were replaced at weekly intervals. Salt stress was imposed on the 8th day after sowing by adding NaCl into Yoshida nutrient solution to make anelectrical conductivity (EC) of 4 dSm−1 in the beginning and is slowly raised to 7, 10, 13 and 16 dSm−1 by 14th days, which is maintained until final scoring. All 50 rice accessions were raised separately using such a hydroponic system, under normal (unstressed) and saline (stressed) conditions. Three cultivars, IR64 (sensitive), CSR10 and TRY1 (highly tolerant) were used as checks, and three replications were maintained.
Phenotyping for salt tolerance
To monitor various traits associated with salt-tolerance, observations were recorded on both salt-stressed and unstressed plants after exposing for 21 days of salt stress in the hydroponic system. The length of the root of each plant was measured and recorded in cm. The length of the shoot of each plant was measured and recorded in cm. Germination percentage was estimated as the number of seeds germinated out of the total number of seeds sown. Seedling vigor was expressed by the total length of the seedling multiplied with germination percentage. Seedlings were dried using a microwave oven and the dry weight was recorded in grams for each plant of the seedling collected, using a microwave oven to dry the seedlings, and was expressed in grams
Molecular analysis
Isolation of genomic DNA and quantification
Leaf tissue samples (2 g) from young, fresh, 10-15 days old leaves were collected and immediately stored at -20°C. A modified CTAB method (CTAB, (2% (w/v) CTAB; 20 mM EDTA, pH 8.0; 100 mM Tris–HCl, pH 8.0; 1.4 M NaCl); CTAB/NaCl solution (10% (w/v) CTAB; 0.7 M NaCl mixed at 65°C with stirring); TE buffer (10 mM Tris–HCl, pH 8.0; 1.0 mM EDTA, pH 8.0), chloroform:isoamyl alcohol (24:1, v/v), iso-propanol, 70% ethanol,2-mercaptoethanol (2ME), liquid nitrogen, sodium acetate and RNase) was used to isolate the genomic DNA from the leaf samples [15]. Then, purity of the DNA was tested by running the extracted genomic DNA samples on 0.8% agarose gel stained with 6ul/100ml ethidium bromide in 1 × TBE (Tris base, Boric acid, 0.5M EDTA) gel buffer. The gels were visualized and photographed under UV light (VilberLourmat, France). For spectrophotometric analysis, five μl of DNA was diluted to 3.0 ml of TE buffer. The spectrophotometer readings were recorded at 260 and 280 nm. DNA concentration was calculated using OD values at 260 nm.
Simple sequence repeats marker analysis
Twenty SSR markers spanning about 5.6 Mbp on chromosomes 1 and 6 were reported to be associated with salt tolerance in several previous investigations [3, 76]. These 20 QTL linked and unlinked SSR markers were used for the analysis of population structure were used in this study. Out of 20 SSR markers, five were located around the Saltol region of chromosome 1 [33]. The SSR primer sequences are given in Supplementary Table S1. Primers were synthesized using the services of M/S Eurofins Genomics India Pvt. Ltd. Chennai.
Polymerase chain reaction using simple sequence repeat markers
Polymerase chain reaction (PCR) amplifications were performed in a reaction volume of 10 μl containing 1 μl of genomic DNA (25 ng/μl) as template, 1.0 μl each of forward and reverse primers (10 ng/μl), 1 μl of dNTPs (10 mM), 0.5 units of Taq DNA polymerase, 1.0 μL of 10X PCR buffer and the rest was milliQ water. After initial denaturation at 94°C for 3 min, PCRs were run for 30 cycles with a denaturation step of 1 min at 94°C, annealing for 1 min at 55-60°C, and extension at 72°C for 2 min. A final extension was followed at 72°C for 10 min. The amplified PCR products (10 μl) were run on 1.5% (w/v) agarose gel at 120 V in 1 X TBE buffer. The size of the fragments was estimated using a 100 bp ladder (Genei, Bangalore) as size marker. The gels were photographed using the Gel Documentation System (VilberLourmat, France). All PCR reactions were done in triplicate to ensure the reproducibility and reliability of the results. Only highly reproducible and polymorphic primers were included in the study.
Allele scoring
Qualitative multistate traits that depict an array of characters were converted into binary characters [73] based on the variations present. Only clear and unambiguous bands were scored. Markers were scored based on the size of the marker of the corresponding band among the accessions.
Genetic diversity
The genetic diversity of the fifty genotypes was determined from the polymorphic molecular marker pattern by estimating the genetic distance using the DICE dissimilarity coefficient. Cluster analysis was performed on a dissimilarity matrix of simple matching coefficients using the unweighted neighbor-joining algorithm, DARwin version 5.0.158 [55] with 7000 permutations. The variances and the corresponding standard errors of the mean were computed from the deviations of the individual values [53].
Marker statistics
For a set of accessions, genetic diversity parameters such as the number of alleles per locus, allele frequency, heterozygosity and PIC values were estimated using the POWERMARKER Version 3.25 [40, 81]. SSR allelic composition for each genotype at every marker locus was determined by counting the number of alleles per locus and the allele frequencies, and the PIC values were determined using the formula
where k is the number of alleles, Pi and Pj are frequency of the i-th and j-th alleles in the population, respectively [09]. Allele frequency represents the frequency of a particular allele for each marker. Heterozygosity is the proportion of heterozygous individuals in the population, and PIC value that represents the amount of polymorphism within a population was estimated based on the Botstein et al. [9].
Haplotyping Saltol marker
The haplotype diversity analysis of SalTol QTL-linked markers was done according to McCartney et al. [45], taking CSR10 and IR 64 as reference alleles for tolerance and susceptibility, respectively.
Results
Ranking of genotypes
The ranking of genotypes was done separately for saline induced and normal situations. Genotypes were ranked based on their highest contribution of characters towards genetic diversity, as suggested by Zeng et al. [84].
Ranking for six seedling characters viz., shoot length, root length, total seedling length, dry matter production and seedling vigor was calculated. In our experiments, characters studied along with their contribution to total divergence indicated that the contribution of dry matter production was highest, followed by root length, seedling vigour and shoot length under stress conditions. Hence, these characters - dry matter production, shoot length or seedling vigor - could be taken as selection index based on per se performance in both stress and normal conditions. This method of ranking could be effective in the isolation of genotypes with more appropriation rather than the commonly employed method of using per se performance and critical difference value without considering the magnitude of contribution of individual traits towards total genetic divergence.
Genetic divergence based on Tocher’s method
Genetic divergence is considered the basis of any crop improvement programme to select parents with more diversity. The genotypes selected under different ecological and agro-climatic conditions are likely to represent a different set of gene complexes even for the same level of mean performance. Among several methods of multivariate analysis for the study of saline related seedling characters, genotypes are measured for genetic divergence between the populations. Mahalanobis D2 statistics [43] have been very effective and useful in selecting genotypes for stress tolerance. The method permits precise comparison among all possible pairs of a population in any given group of genotypes.
The genetic divergence of the 50 genotypes grown under salt stress ranged from 0.00 to 143.37 and under normal conditions it ranged from 0.00 to 20.85. This indicates that a sufficient amount of genetic divergence had occurred among the genotypes studied.
Using Tocher's method [59], the 50 genotypes were grouped into eight clusters under saline conditions and only two clusters under normal condition. The larger number of clusters in saline condition, indicate the influence of genetic divergence on stress response.
The number of genotypes in different clusters in both situations also varied. The Clusters IV, V, VI, VII, and VIII under saline conditions, consisted of only 2 genotypes each. The low number of genotypes in these clusters is due to some barriers such as selection for diverse adaptive gene complexes. Under normal conditions, cluster II consists of 49 genotypes and cluster I was monogenotypic. The clustering pattern of rice genotypes indigenous to different geographical locations suggests that geographical distribution does not necessarily determine genetic divergence. Other researchers have also emphasized this [61, 72].
Molecular diversity of simple sequence repeats markers linked with salt tolerance
Twenty SSR markers tightly linked with salt-tolerant QTLs present on chromosome one and six were used for screening 50 genotypes of rice landraces. Two SSR markers, viz.RM336 and RM8007, did not give any amplification, and RM513 marker amplification was monomorphic. The rest of the 17 SSR markers produced proper polymorphic amplicons in 50 genotypes. Alleles generated by 17 SSR markers revealed consistently well-resolved and reproducible alleles with clear allele patterns among all the 50 rice genotypes (Supplementary Fig. S1).
A total of 43 alleles was recorded in 17 polymorphic SSR markers in the 50 accessions studied (Table 2). The number of microsatellite alleles of the markers ranged between two (RM5365, RM6711, RM10825, RM10843) and five (RM8053) alleles with an average of 2.70 alleles per locus. Marker RM10825 had the lowest amplicon size (80-90 bp) and RM493 the highest amplicon size (300 bp). PIC values varied from 0.25 to 0.53 with an average of 0.3665. RM1287 had the highest value, while RM10843 had the lowest PIC value. The allelic frequencies of major alleles of these 17 marker loci ranged from 0.82 (RM10843) to 0.47 (RM483). Heterozygosity ranged from 0.800 (RM7075, RM493, RM483) to 0.00 (RM10843, RM10864) with an average of 0.0341. A 100 percent homozygosity was noticed in five marker loci. Expected genetic diversity varied from 0.59 (RM1287) to 0.28 (RM10843) with the mean value of 0.0346.
Table 2.
List of 17 simple sequence repeat (SSR) markers (tightly linked with salt-tolerant quantitative trait loci (QTLs) that present on Saltol regions of rice chromosome) with major allele frequency, allele no, gene diversity, heterozygosity and polymorphism information content (PIC) value, used for the analysis 50 traditional rice genotypes at the seedling stage for salt tolerance under salinity conditions.
| S. No | Marker | Major allele frequency |
Allele No |
Gene diversity |
Heterozygosity | PIC |
|---|---|---|---|---|---|---|
| 1 | RM3412 | 0.7100 | 3.0000 | 0.4143 | 0.0200 | 0.3486 |
| 2 | RM483 | 0.4700 | 4.0000 | 0.5855 | 0.0800 | 0.5138 |
| 3 | RM493 | 0.5400 | 3.0000 | 0.5049 | 0.0800 | 0.4011 |
| 4 | RM562 | 0.6100 | 4.0000 | 0.5527 | 0.0200 | 0.5145 |
| 5 | RM1287 | 0.5400 | 3.0000 | 0.5907 | 0.0400 | 0.5350 |
| 6 | RM5365 | 0.8100 | 2.0000 | 0.3022 | 0.0600 | 0.2604 |
| 7 | RM6711 | 0.7600 | 2.0000 | 0.3579 | 0.0400 | 0.2983 |
| 8 | RM7075 | 0.6600 | 3.0000 | 0.4971 | 0.0800 | 0.4542 |
| 9 | RM8046 | 0.8100 | 2.0000 | 0.3018 | 0.0200 | 0.2604 |
| 10 | RM8115 | 0.7800 | 3.0000 | 0.3442 | 0.0000 | 0.3020 |
| 11 | RM10720 | 0.5600 | 2.0000 | 0.4829 | 0.0000 | 0.3714 |
| 12 | RM10825 | 0.5400 | 2.0000 | 0.4869 | 0.0000 | 0.3734 |
| 13 | RM10843 | 0.8200 | 2.0000 | 0.2897 | 0.0400 | 0.2516 |
| 14 | RM10864 | 0.7000 | 2.0000 | 0.4116 | 0.0000 | 0.3318 |
| 15 | RM10871 | 0.8000 | 2.0000 | 0.3136 | 0.0000 | 0.2688 |
| 16 | RM8053 | 0.7100 | 5.0000 | 0.4369 | 0.0400 | 0.3943 |
| 17 | RM8094 | 0.6500 | 2.0000 | 0.4465 | 0.0600 | 0.3515 |
| Mean | 0.6747 | 2.7059 | 0.4306 | 0.0341 | 0.3665 |
The marker index (MI) was lowest for RM562 (0.77) and highest for RM8094 (1.92). The SSR marker RM483 was the best in this analysis based on PIC coupled with MI value, followed by RM1287 and RM8046. Higher PIC with higher MI value indicates that all these primers are capable of distinguishing salt tolerance among genotypes.
Clustering analysis for simple sequence repeat markers
Clustering analysis based on the unweighted pair group with arithmetic mean (UPGMA) using DARwin with Euclidean distance matrix, the 50 rice genotypes were grouped into 11clusters (Fig. 1 and Table 3). The dendrogram based on UPGMA grouped the 50 genotypes that were demarcated at a similarity coefficient of 0.50. Cluster I was the largest with thirteen genotypes followed by cluster II with eight genotypes and cluster V with six genotypes. The dendrogram revealed that the genotypes originated from different groups, indicating non-parallelism of genetic diversity between genotypes from different geographical origins. Maximum inter-cluster distance was observed between the clusters II and VIII followed by clusters II and IV and cluster IV and VIII under saline situation.
Fig. 1.
Dendrogram based on simple sequence repeat (SSR) markers tightly linked with salt-tolerant quantitative trait loci (QTLs) that present on Saltol regions of rice chromosome one and six, alleles profile among 50 indigenous genotypes collected from farmers of coastal Thiruthuraipoondi, Tamil Nadu, India.
Table 3.
Details of 50 traditional rice genotypes clusters produced by 17 simple sequence repeat (SSR) markers tightly linked with salt-tolerant quantitative trait loci (QTLs) that present on Saltol regions of rice chromosome using the unweighted pair group with arithmetic mean (UPGMA) method.
| Cluster Number | Number of Genotypes | Name of the Genotype |
|---|---|---|
| I (a) | 5 | CSR10, Sornamugi, ottadam, poonkar and kalundai |
| I(b) | 7 | Seeraga samba, Kalan namak, Thulasi vasam, Kuzhi Adichan, Navara, Kichadi Samba and Karunkuruvai |
| II | 8 | Chinnar, Pal kudaivazhai, Mysore malli, Karuvachi, Perunkar, Mappilai Samba, Karudan samba and Arupatham kuruvai |
| III | 1 | Kandasali |
| IV | 3 | Basumathi, sinkinikar and karupukavuni |
| V | 6 | Athur kichadi, Thanga samba, Thooyamalli, Sorna masuri, Kothamali Samba and Boomi |
| VI | 3 | Kudaivazhai, Valan and Milagu samba |
| VII | 4 | Marathondi, Koondukar, Kattu ponni and Kattuyanam |
| VIII | 4 | Selam samba, Sivapu kavuni, IR64 and Neelaj samba |
| IX | 1 | Manjal pooni |
| X | 2 | TRY1, Rajamudi |
| XI | 1 | Soor kuruvai |
The genotypes Sornamugi, Ottadam, Poongar, Kalundai and Rajamudi are always grouped with CSR10 and TRY1 (tolerant check) in the same cluster. This suggests that these four genotypes should be considered as salt-tolerant genotypes.
Cluster distance
Cluster analysis grouped the fifty accessions into eight clusters with considerable variation in the morphological properties. Maximum intra-cluster distance was observed in cluster VII which comprises of two genotypes under stress condition. This indicated high divergence among the genotypes within the cluster. Minimum inter-cluster distance was noticed between cluster I and VI and I and VIII under saline condition, indicating that the genotypes in these clusters might have evolved by similar evolutionary process.
The genotypes found in different clusters under saline situation fall into the same clusters in normal condition. The genotypes which recorded high per se performance with maximum inter-cluster distance under saline condition was selected for further improvement. Based on the present study, five genotypes, Kalundai, Poongar, Kuzhiadichan, Rajamudi and Vadan Samba, were selected from different clusters considering the intra and inter cluster distance along with high per se performance. Improvement of these genotypes would lead to greater opportunity for maximum utilization and exploitation of saline tolerance. These could also be used as donors for of favorable genes in ruling rice genotypes either through recombination or through heterosis breeding.
Molecular diversity and haplotype analysis of SalTol
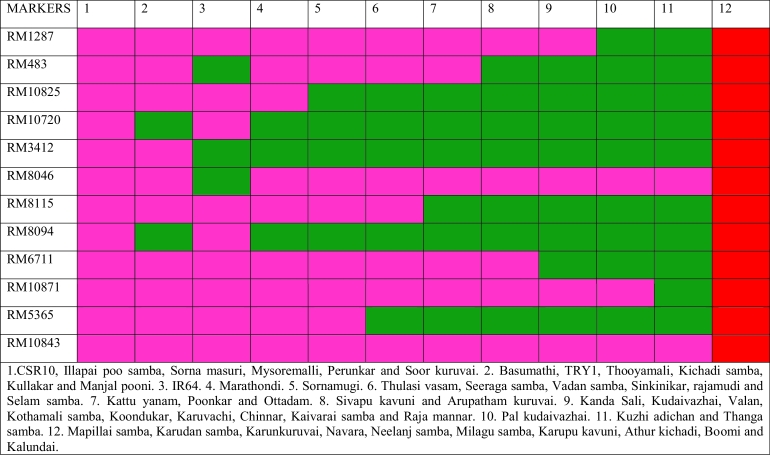
In the present study of 50 genotypes, out of 20 SSR markers, 17 markers showed polymorphism, which spanned around 3Mbp in the different salt-tolerant regions on chromosome 1. Because of the large size of saltol region based on CSR10, it is challenging to conserve haplotypes among the rice gene pool. The markers used were, as expected, found to be highly polymorphic among 50 rice genotypes screened, Fig. 2, Fig. 3, indicate that saltol haplotype is not well conserved across rice gene pool.
Fig. 2.
Haplotypes of simple sequence repeat (SSR) markers tightly linked with salt-tolerant quantitative trait loci (QTLs) that present on Saltol regions of rice chromosome one and six in 50 rice genotypes collected from farmers of coastal Thiruthuraipoondi, Tamil Nadu, India. Numbers G1–G50 represent different rice genotypes as defined in Table 1.
Fig. 3.
Twelve rice haplotypes produced by key simple sequence repeat (SSR) markers tightly linked with salt-tolerant quantitative trait loci (QTLs) that present on Saltol regions of rice chromosome one with reference CSR10.
Discussion
Rice is a crop that is most sensitive to salinity stress at the seedling and reproductive stages [25, 28, 50, 57]. A quick way to screen for and select stress-tolerant genotypes is to survey diversity in the gene pool [4, 11, 49]. Wild rice varieties denote enormous genetic diversity and different molecular functions for many agronomic traits [37]. For example, only one QTL was major, and 22 QTLs were minor contributing significantly in the phenotypic variation of salt tolerance traits among the rice recombinant inbred lines (RILs) [44]. However, traditional rice varieties of coastal area in TamilNadu, India are distinct regarding their phenotypic response to salinity. Using these accessions, we can identify more suitable QTLs for salinity tolerance to introgress into high yield rice varieties. Therefore, we screened a set of 50 rice accessions comprising salt-tolerant breeding lines, high yielding varieties, and salt-tolerant landraces using a hydroponic system in laboratory conditions at different salinity levels of 0, 4, 7, 13, 16 dsm−1.
Previous studies, which considered only the salt tolerance index, had suggested that none of the genotypes could be selected as tolerant for most of the characters studied. However, considering the per se and highest contribution of the genotypes under stress and normal conditions, we see that Kuzhiadican, Poongar and Sornamugi are the most tolerant genotypes at the seedling stage.
Wide variations in standard evaluation system (SES) scores from 1 to 9 were observed from the 21 days onwards till the 7th day after of salinization. Based on salinity scores recorded, the 50 rice accessions were divided into five groups, including 7 highly tolerant, 8 tolerant, 15 moderately tolerant, 14 susceptible and 6 highly susceptible accessions. The normal distribution of salinity score, observed in the 50 rice accessions used in this experiment, is suggestive of the polygenic nature of the trait, thus confirming earlier findings [14, 20, 34, 42, 47].
The present investigation revealed 17 SSR markers, and 43 alleles in the 50 genotypes. Among these markers, the number of alleles per locus varied between two and five, with an average of 2.7059 per locus. This low number of alleles per locus, indicates low diversity among genotypes, as has been reported earlier [58].
The PIC values of the 50 rice accessions studied ranged from 0.2516 to 0.5350 with an average of 0.3665. Our study revealed that the primers RM483, RM562, and RM1287 show a number of alleles with PIC value of more than 0.5. This indicates the efficiency of these primers to detect heterogeneous accession, agreeing with the findings of Giarrocco [19]. Though it can be argued that the use of a higher number of markers to characterize accession would be more effective in describing genotypes, our results show that a fewer number can be as efficient for identifying salt-tolerant genotypes. In the present study, out of 20 SSR markers, only RM8094 and RM3412 could discriminate the salt-tolerant genotypes from the susceptible genotypes.
Analysis of genetic divergence in the 50 genotypes revealed the superiority of SSR markers over morphological traits, in elucidating genetic relatedness more precisely. Eleven clusters were obtained using SSR markers as compared to only eight clusters obtained using morphological traits.
Similar comparisons of haplotypes for seedling stage salinity tolerance were done in previous studies [7, 17, 23, 30, 35, 48]. Babu et al. [7] could delineate 14 haplotypes for six informative Saltol associated markers analyzed across 23 rice genotypes. Kordrostami et al. [30] identified 14 haplotypes involving 12 Saltol associated markers across 44 rice genotypes, Krishnamurthy et al. [[32]; 2016b; [36]] analyzed 21 SSR markers across 94 rice genotypes and could identify 11 seedling stage salinity tolerant genotypes containing regions other than Saltol controlling their salinity tolerance. As in these previous studies, we have found genotypes with probable novel allele regions which can be considered candidates for improving seedling stage salinity tolerance.
To compare the presence of 12 key markers RM1287, RM483, RM10825, RM10720, RM3412, RM8046, RM8115, RM8094, RM6711, RM10871, RM5365 and RM10843 for salt tolerance, 12 haplotypes were identified among 50 genotypes, based on marker banding patterns (Supplementary Fig. S1). Forty genotypes had different combinations of CSR10 alleles at different loci, while ten genotypes did not share any allele (Haplotype 12) with CSR10. From the comparison of haplotypes with high frequency of CSR10 alleles it can be deduced that marker RM10843 showed association with high salt tolerance response. The marker RM10843 is present in salt-tolerant genotypes Kuzhiadichan, Poongar, and Sornamugi also.
Some of the highly sensitive lines such as IR64, Soorkuruvai and Karunkuruvai also had alleles similar to CSR10 at this locus. Genotypes which carried alleles similar to CSR10 at marker loci RM6711, RM10871 and RM10843 showed differential reactions to salinity stress, which indicated that no single marker had a strong positive association with salt tolerance. The marker RM8046 helped distinguish salt-tolerant genotypes from a sensitive genotype IR64, which had alleles similar to CSR10. It is essential to validate gene-linked markers between donor and recurrent parent since it is used in marker-assisted backcross breeding. The other highly tolerant genotypes, namely Boomi and Garudan Samba did not possess any allele similar to CSR10 that could explain the tolerance, implying that they may possess novel QTLs alleles for salt tolerance.
To conclude, the genotypes, Kuzhiadichan, Sornamugi and Poongar, possessed a high degree of salinity tolerance and, hence, can be used as new donors for the trait. In the other salt-tolerant genotypes, Boomi and Garudan Samba, the trait does not seem to be linked to saltol locus, and, therefore, they can become new sources for mapping QTL for seedling stage salinity tolerance. In future, these genotypes will be tested for their reproductive stage salinity tolerance.
Author contribution
VM performed the experiments and wrote the manuscript in collaboration with RM. AY, AS, MS, RM, and AR designed the experiments. RM, AS, AR, PMAS, MS and AY edited and revised the manuscript.
Declaration of Competing Interest
All the authors have declared no conflict of interest.
Acknowledgments
We sincerely thank Dr K. K. Vinod, Division of Genetics, ICAR-Indian Agricultural Research Institute, New Delhi, 110012, India for technical support.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.btre.2021.e00666.
Appendix. Supplementary materials
References
- 1.Agrama HA, Eizenga GC. Molecular diversity and genome-wide linkage disequilibrium patterns in a worldwide collection of Oryza sativa and its wild relatives. Euphytica. 2008;160:339–355. doi: 10.1007/s10681-007-9535-y. [DOI] [Google Scholar]
- 2.Agre AP. Classification of elite cassava varieties (Manihot esculenta Crantz) cultivated in Benin Republic using farmers’ knowledge, morphological traits and simple sequence repeat (SSR) markers. Genet. Resour. Crop Evol. 2018;65:513–525. doi: 10.1007/s10722-017-0550-0. [DOI] [Google Scholar]
- 3.Alam R, Sazzadur Rahman M, Seraj ZI, Thomson MJ, Ismail AM, Tumimbang-Raiz E, Gregorio GB. Investigation of seedling-stage salinity tolerance QTLs using backcross lines derived from Oryza sativa L. Pokkali. Plant Breed. 2011;130:430–437. doi: 10.1111/j.1439-0523.2010.01837.x. [DOI] [Google Scholar]
- 4.Ali J. Harnessing the hidden genetic diversity for improving multiple abiotic stress tolerance in rice (Oryza sativa L.) PLoS One. 2017;12 doi: 10.1371/journal.pone.0172515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali MN, Yeasmin L, Gantait S, Goswami R, Chakraborty S. Screening of rice landraces for salinity tolerance at seedling stage through morphological and molecular markers. Physiol. Mol. Biol. Plants. 2014;20:411–423. doi: 10.1007/s12298-014-0250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ammar MHM. Mapping of QTLs Controlling Na+, K+ and CI− Ion Concentrations in Salt Tolerant Indica Rice Variety CSR27. J. Plant Biochem. Biotechnol. 2009;18:139–150. doi: 10.1007/BF03263312. [DOI] [Google Scholar]
- 7.Babu N. Marker based haplotype diversity of Salto/QTL in relation to seedling stage salinity tolerance in selected genotypes of rice. Indian J. Genet. Plant Breed. 2014;74:16–25. doi: 10.5958/j.0975-6906.74.1.003. [DOI] [Google Scholar]
- 8.Bajracharya J, Rana RB, Gauchan D, Sthapit BR, Jarvis DI, Witcombe JR. Rice landrace diversity in Nepal. Socio-economic and ecological factors determining rice landrace diversity in three agro-ecozones of Nepal based on farm surveys. Genet. Resour. Crop Evol. 2010;57:1013–1022. doi: 10.1007/s10722-010-9544-x. [DOI] [Google Scholar]
- 9.Botstein D, White RL, Skolnick M, Davis RW. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980;32:314–331. [PMC free article] [PubMed] [Google Scholar]
- 10.Burman D. Participatory evaluation guides the development and selection of farmers' preferred rice varieties for salt- and flood-affected coastal deltas of South and Southeast Asia. Field Crops Res. 2018;220:67–77. doi: 10.1016/j.fcr.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen G. Variation in the Abundance of OsHAK1 Transcript Underlies the Differential Salinity Tolerance of an indica and a japonica Rice Cultivar. Front. Plant Sci. 2018;8:2216. doi: 10.3389/fpls.2017.02216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui D. Association mapping of salinity and alkalinity tolerance in improved japonica rice (Oryza sativa L. subsp. japonica Kato) germplasm. Genet. Resour. Crop Evol. 2015;62:539–550. doi: 10.1007/s10722-014-0179-1. [DOI] [Google Scholar]
- 13.De Leon TB, Linscombe S, Gregorio G, Subudhi PK. Genetic variation in Southern USA rice genotypes for seedling salinity tolerance. Front. Plant Sci. 2015;6:374. doi: 10.3389/fpls.2015.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Leon TB, Linscombe S, Subudhi PK. Molecular Dissection of Seedling Salinity Tolerance in Rice (Oryza sativa L.) Using a High-Density GBS-Based SNP Linkage Map. Rice. 2016;9:52. doi: 10.1186/s12284-016-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990:13–15. [Google Scholar]
- 16.Ganie SA, Karmakar J, Roychowdhury R, Mondal TK, Dey N. Assessment of genetic diversity in salt-tolerant rice and its wild relatives for ten SSR loci and one allele mining primer of salT gene located on 1st chromosome. Plant Syst. Evol. 2014;300:1741–1747. doi: 10.1007/s00606-014-0999-7. [DOI] [Google Scholar]
- 17.Ganie SA, Borgohain MJ, Kritika K, Talukdar A, Pani DR, Mondal TK. Assessment of genetic diversity of Saltol QTL among the rice (Oryza sativa L.) genotypes. Physiol. Mol. Biol. Plants. 2016;22:107–114. doi: 10.1007/s12298-016-0342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganie SA, Molla KA, Henry RJ, Bhat KV, Mondal TK. Advances in understanding salt tolerance in rice. Theor. Appl. Genet. 2019;132:851–870. doi: 10.1007/s00122-019-03301-8. [DOI] [PubMed] [Google Scholar]
- 19.Giarrocco LE. Assessment of the Genetic Diversity in Argentine Rice Cultivars with SSR Markers. Crop Sci. 2007;47:853–858. doi: 10.2135/cropsci2005.07.0198. [DOI] [Google Scholar]
- 20.Gregorio GB, Senadhira D. Genetic analysis of salinity tolerance in rice (Oryza sativa L.) Theor. Appl. Genet. 1993;86:333–338. doi: 10.1007/bf00222098. [DOI] [PubMed] [Google Scholar]
- 21.Gregorio GB, Senadhira D, Mendoza RD, Manigbas NL, Roxas JP, Guerta CQ. Progress in breeding for salinity tolerance and associated abiotic stresses in rice. Field Crops Res. 2002;76:91–101. doi: 10.1016/S0378-4290(02)00031-X. [DOI] [Google Scholar]
- 22.Hossain H, Rahman MA, Alam MS, Singh RK. Mapping of Quantitative Trait Loci Associated with Reproductive-Stage Salt Tolerance in Rice. J Agron Crop Sci. 2015;201:17–31. doi: 10.1111/jac.12086. [DOI] [Google Scholar]
- 23.Islam ASMF, Ali MR, Gregorio GB, Islam MR. Genetic diversity analysis of stress tolerant rice (Oryza sativa L.) Afr. J. Biotechnol. 2012;11:15123–15129. [Google Scholar]
- 24.Jahan N. QTL analysis for rice salinity tolerance and fine mapping of a candidate locus qSL7 for shoot length under salt stress. Plant Growth Regul. 2019 doi: 10.1007/s10725-019-00566-3. [DOI] [Google Scholar]
- 25.Jamil M, Rha ES. Response of transgenic rice at germination and early seedling growth under salt stress. Pakistan J. Biol. Sci. 2007;10:4303–4306. doi: 10.3923/pjbs.2007.4303.4306. [DOI] [PubMed] [Google Scholar]
- 26.Jing W, Deng P, Cao C, Zhang W. Fine mapping of qSKC-1, a major quantitative trait locus for shoot K(+) concentration, in rice seedlings grown under salt stress. Breed Sci. 2017;67:286–295. doi: 10.1270/jsbbs.16190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Julia CC, Waters DLE, Wood RH, Rose TJ. Morphological characterisation of Australian ex situ wild rice accessions and potential for identifying novel sources of tolerance to phosphorus deficiency. Genet. Resour. Crop Evol. 2016;63:327–337. doi: 10.1007/s10722-015-0252-4. [DOI] [Google Scholar]
- 28.Kakar N, Jumaa SH, Redoña ED, Warburton ML, Reddy KR. Evaluating rice for salinity using pot-culture provides a systematic tolerance assessment at the seedling stage. Rice. 2019;12:57. doi: 10.1186/s12284-019-0317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khumto S, Sreethong T, Pusadee T, Rerkasem B, Jamjod S. Variation of floral traits in Thai rice germplasm (Oryza sativa) Genet. Resour. Crop Evol. 2018;65:1123–1132. doi: 10.1007/s10722-017-0600-7. [DOI] [Google Scholar]
- 30.Kordrostami M, Rabiei B, Hassani Kumleh H. Association analysis, genetic diversity and haplotyping of rice plants under salt stress using SSR markers linked to SalTol and morpho-physiological characteristics. Plant Syst. Evol. 2016;302:871–890. doi: 10.1007/s00606-016-1304-8. [DOI] [Google Scholar]
- 31.Koyama ML, Levesley A, Koebner RMD, Flowers TJ, Yeo AR. Quantitative Trait Loci for Component Physiological Traits Determining Salt Tolerance in Rice. Plant Physiol. 2001;125:406. doi: 10.1104/pp.125.1.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnamurthy S, Pundir P, Singh Y, Sharma S, Sharma P, Sharma D. Yield Stability of Rice Lines for Salt Tolerance Using Additive Main Effects and Multiplicative Interaction Analysis -AMMI. J. Soil Salinity and Water Quality. 2015;7:98–106. [Google Scholar]
- 33.Krishnamurthy S, Sharma S, Mishra V, Tiwari S, Batra V, Singh N. Assessment of genetic diversity in rice genotypes for salinity tolerance using Saltol markers of Chromosome 1. Indian J. Genet. Plant Breed. 2014;74:243–247. doi: 10.5958/0975-6906.2014.00167.9. [DOI] [Google Scholar]
- 34.Krishnamurthy SL, Sharma PC, Batra V, Kumar V, Rao LVS. Effect of salinity and use of stress indices of morphological and physiological traits at the seedling stage in rice. Indian J. Exp. Biol. 2016;54:843–850. [PubMed] [Google Scholar]
- 35.Krishnamurthy SL, Sharma SK, Kumar V, Tiwari S, Singh NK. Analysis of genomic region spanning Saltol using SSR markers in rice genotypes showing differential seedlings stage salt tolerance. J. Plant Biochem. Biotechnol. 2016;25:331–336. doi: 10.1007/s13562-015-0335-5. [DOI] [Google Scholar]
- 36.Krishnamurthy SL. Analysis of Stability and G × E Interaction of Rice Genotypes across Saline and Alkaline Environments in India. Cereal Res. Commun. 2016;44:349–360. doi: 10.1556/0806.43.2015.055. [DOI] [Google Scholar]
- 37.Kumar K. High resolution genetic mapping and identification of a candidate gene(s) for the purple sheath color and plant height in an interspecific F2 population derived from Oryza nivara Sharma & Shastry × Oryza sativa L. cross. Genet Resour Crop Evol. 2019 doi: 10.1007/s10722-019-00869-4. [DOI] [Google Scholar]
- 38.Lin HX. QTLs for Na+ and K+ uptake of the shoots and roots controlling rice salt tolerance. Theor. Appl. Genet. 2004;108:253–260. doi: 10.1007/s00122-003-1421-y. [DOI] [PubMed] [Google Scholar]
- 39.Linares OF. African rice (Oryza glaberrima): History and future potential. Proc. Natl Acad. Sci. USA. 2002;99:16360–16365. doi: 10.1073/pnas.252604599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu K, Muse SV. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21:2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- 41.Ma JF. An efflux transporter of silicon in rice. Nature. 2007;448:209–212. doi: 10.1038/nature05964. [DOI] [PubMed] [Google Scholar]
- 42.Ma NL. Susceptibility and tolerance of rice crop to salt threat: Physiological and metabolic inspections. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahalanobis P. Proceedings of the Indian Science Congress (Calcutta) 1928. Statistical study of the Chinese head; pp. 107–122. [Google Scholar]
- 44.Mazumder A, Rohilla M, Bisht DS, Krishnamurthy SL, Barman M, Sarma RN, Sharma TR, Mondal TK. Identification and mapping of quantitative trait loci (QTL) and epistatic QTL for salinity tolerance at seedling stage in traditional aromatic short grain rice landrace Kolajoha (Oryza sativa L.) of Assam, India. Euphytica. 2020;216:75. [Google Scholar]
- 45.McCartney CA, Somers DJ, Fedak G, Cao W. Haplotype diversity at fusarium head blight resistance QTLs in wheat. Theor. Appl. Genet. 2004;109:261–271. doi: 10.1007/s00122-004-1640-x. [DOI] [PubMed] [Google Scholar]
- 46.Mehta PS, Ojha SN, Negi KS, Verma SK, Rayal A, Tyagi RK. On-farm status of rice (Oryza sativa L.) genetic resources in Garhwal Himalaya of Uttarakhand, India. Genet. Resour. Crop Evol. 2014;61:1279–1294. doi: 10.1007/s10722-014-0110-9. [DOI] [Google Scholar]
- 47.Moeljopawiro S, Ikehashi H. Inheritance of salt tolerance in rice. Euphytica. 1981;30:291–300. doi: 10.1007/BF00033990. [DOI] [Google Scholar]
- 48.Mohammadi-Nejad G, Singh R, Arzani A, REZAEI A, SABOURI H, Gregorio G. Evaluation of salinity tolerance in rice genotypes. Int. J. Plant Prod. 2010;4:199–208. [Google Scholar]
- 49.Mohammadi R, Mendioro MS, Diaz GQ, Gregorio GB, Singh RK. Mapping quantitative trait loci associated with yield and yield components under reproductive stage salinity stress in rice (Oryza sativa L.) J. Genet. 2013;92:433–443. doi: 10.1007/s12041-013-0285-4. [DOI] [PubMed] [Google Scholar]
- 50.Munns R, Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 51.Pachauri V, Taneja N, Vikram P, Singh NK, Singh S. Molecular and morphological characterization of Indian farmers rice varieties (Oryza sativa L.) Australian J. Crop Sci. 2013;7:923. [Google Scholar]
- 52.Pandey A, Bisht IS, Bhat KV, Mehta PS. Role of informal seed system in promoting landrace diversity and their on-farm conservation: a case study of rice in Indian Himalayas. Genet. Resour. Crop Evol. 2011;58:1213–1224. doi: 10.1007/s10722-010-9654-5. [DOI] [Google Scholar]
- 53.Panse V, Sukhatme PV. Indian Council of Agricultural Research Publications; New Delhi: 1978. Statistical methods for agricultural workers. [Google Scholar]
- 54.Peres S. Saving the gene pool for the future: Seed banks as archives Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of. Biol. Biomed. Sci. 2016;55:96–104. doi: 10.1016/j.shpsc.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Perrier X, Jacquemoud-Collet JP. DARwin Software. 2006 http://darwinciradfr/darwin [Google Scholar]
- 56.Pinto TT, Ogliari JB, Maghelly OR. Phenotypic characterization of dryland rice (Oryza sativa L.) germplasm conserved in situ (on farm) in a crop-diversity microcenter in southern Brazil. Genet. Resour. Crop Evol. 2019;66:415–427. doi: 10.1007/s10722-018-0720-8. [DOI] [Google Scholar]
- 57.Radanielson AM, Angeles O, Li T, Ismail AM, Gaydon DS. Describing the physiological responses of different rice genotypes to salt stress using sigmoid and piecewise linear functions. Field Crops Res. 2018;220:46–56. doi: 10.1016/j.fcr.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ram SG, Thiruvengadam V, Vinod KK. Genetic diversity among cultivars, landraces and wild relatives of rice as revealed by microsatellite markers. J. Appl. Genet. 2007;48:337–345. doi: 10.1007/bf03195230. [DOI] [PubMed] [Google Scholar]
- 59.Rao C. John Wiley & Sons; New York (NY): 1952. Advanced statistical methods in biometric research. [Google Scholar]
- 60.Rashid M, Shahin I, Islam M, Lutful H. Genetic diversity analysis of rice landraces (Oryza sativa L.) for salt tolerance using SSR markers in Bangladesh. Fundamental Appl. Agric. 2018;3:460–466. [Google Scholar]
- 61.Rather AG, Zargar MA, Sheikh FA. Genetic divergence in rice (Oryza sativa L.) under temperate conditions. Indian J. Agric. Sci. 2001;71:344–345. [Google Scholar]
- 62.Ray A, Deb D, Ray R, Chattopadhayay B. Phenotypic characters of rice landraces reveal independent lineages of short-grain aromatic indica rice. AoB Plants. 2013;5 doi: 10.1093/aobpla/plt032. plt032-plt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reddy INBL, Kim B-K, Yoon I-S, Kim K-H, Kwon T-R. Salt Tolerance in Rice: Focus on Mechanisms and Approaches. Rice Sci. 2017;24:123–144. doi: 10.1016/j.rsci.2016.09.004. [DOI] [Google Scholar]
- 64.Reiahisamani N, Esmaeili M, Khoshkholgh Sima NA, Zaefarian F, Zeinalabedini M. Assessment of the oil content of the seed produced by Salicornia L., along with its ability to produce forage in saline soils. Genet. Resour. Crop Evol. 2018;65:1879–1891. doi: 10.1007/s10722-018-0661-2. [DOI] [Google Scholar]
- 65.Roy SJ, Negrão S, Tester M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014;26:115–124. doi: 10.1016/j.copbio.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Sakina A, Ahmed I, Shahzad A, Iqbal M, Asif M. Genetic variation for salinity tolerance in Pakistani rice (Oryza sativa L.) germplasm. J. Agron. Crop Sci. 2016;202:25–36. [Google Scholar]
- 67.Sathya A. The art of naming traditional rice varieties and landraces by ancient Tamils. Asian Agri-History. 2014;18:15–21. [Google Scholar]
- 68.Schmidt R, Caldana C, Mueller-Roeber B, Schippers JHM. The contribution of SERF1 to root-to-shoot signaling during salinity stress in rice. Plant Signal Behav. 2014;9:e27540. doi: 10.4161/psb.27540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shang F, Chen L, Meng X, Yang K, Wang J. Fine mapping and grain yield analysis of a major QTL controlling primary branch number in rice (Oryza sativa L.) Genet. Resour. Crop Evol. 2019 doi: 10.1007/s10722-019-00857-8. [DOI] [Google Scholar]
- 70.Shrivastava P, Kumar R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015;22:123–131. doi: 10.1016/j.sjbs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh N. Genetic diversity trend in Indian rice varieties: an analysis using SSR markers. BMC Genet. 2016;17:127. doi: 10.1186/s12863-016-0437-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh S, Sanghera GS, Ga P, Gn B. Genetic variability and heritability in rice (Oryza sativa L.) Environ. Ecol. 2005;23(3):549–551. [Google Scholar]
- 73.Sokal RR, Sneath PHA. WH Freeman & Co; New York: 1963. Principles of Numerical Taxonomy; p. 359. [Google Scholar]
- 74.Sweeney M, McCouch S. The complex history of the domestication of rice. Ann. Bot. 2007;100:951–957. doi: 10.1093/aob/mcm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tahjib-Ul-Arif M, Sayed MA, Islam MM, Siddiqui MN, Begum SN, Hossain MA. Screening of rice landraces (Oryza sativa L.) for seedling stage salinity tolerance using morpho-physiological and molecular markers. Acta Physiol. Plant. 2018;40:70. doi: 10.1007/s11738-018-2645-4. [DOI] [Google Scholar]
- 76.Thomson MJ, Ocampo D, Egdane J, Katimbang M, Singh R, Gregorio G, Ismail M. Proceedings of BioAsia, 6th Asian Crop Science Association. 2007. QTL mapping and marker-assisted backcrossing for improved salinity tolerance in rice; pp. 6–12. [Google Scholar]
- 77.Tiwari S. Mapping QTLs for Salt Tolerance in Rice (Oryza sativa L.) by Bulked Segregant Analysis of Recombinant Inbred Lines Using 50K SNP Chip. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ul Haq T, Gorham J, Akhtar J, Akhtar N, Steele KA. Dynamic quantitative trait loci for salt stress components on chromosome 1 of rice. Funct. Plant Biol. 2010;37:634–645. doi: 10.1071/FP09247. [DOI] [Google Scholar]
- 79.Veltman MA, Flowers JM, van Andel TR, Schranz ME. Origins and geographic diversification of African rice (Oryza glaberrima) PLoS One. 2019;14 doi: 10.1371/journal.pone.0203508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Virk PS, Zhu J, Newbury HJ, Bryan GJ, Jackson MT, BV Ford-Lloyd. Effectiveness of different classes of molecular marker for classifying and revealing variation in rice (Oryza sativa) germplasm. Euphytica. 2000;112:275–284. doi: 10.1023/A:1003952720758. [DOI] [Google Scholar]
- 81.Weir SB. 1996. Genetic data analysis II. Sinauer publishers. Sunderland, MA. [Google Scholar]
- 82.Yawen Z, Shiquan S, Zichao L, Zhongyi Y, Xiangkun W, Hongliang Z, Guosong W. Ecogeographic and genetic diversity based on morphological characters of indigenous rice (Oryza sativa L.) in Yunnan, China. Genet. Resour. Crop Evol. 2003;50:567–577. doi: 10.1023/A:1024436501289. [DOI] [Google Scholar]
- 83.Yoshida S, Forno DA, Cock JH, Gomez K. International Rice Research Institute; Ban4os (Philippines): 1976. Laboratory manual for physiological studies of rice Los. [Google Scholar]
- 84.Zeng L, Shannon MC, Grieve CM. Evaluation of salt tolerance in rice genotypes by multiple agronomic parameters. Euphytica. 2002;127:235–245. doi: 10.1023/A:1020262932277. [DOI] [Google Scholar]
- 85.Randhawa G.J., Bhalla S., Chalam V .C., Tyagi V, Verma D.D., Hota M. Document on biology of rice (Oryza sativa L.) in India. National Bureau of Plant Genetic Resources, New Delhi and Project coordinating and monitoring unit. Ministry of Environment and Forests,New Delhi. 2006:79. [Google Scholar]
- 86.Ahmed M.S.U., Khalequzzaman M., Bashar M.K., Shamsuddin A.K.M. Agro-morphological, physico-chemical and molecular characterization of rice germplasm with similar names of Bangladesh. Rice science. 2016;23(4):211–218. [Google Scholar]
- 87.Zhang J., Liu Y.X., Zhang N., Hu B., Jin T., Xu H., Qin Y., Yan P., Zhang X., Guo X., Hui J. NRT1. 1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nature biotechnology. 2019;37(6):676–684. doi: 10.1038/s41587-019-0104-4. [DOI] [PubMed] [Google Scholar]
- 88.Singh, V.K., Singh, B.D., Kumar, A., Maurya, S., Krishnan, S.G., Vinod, K.K., Singh, M.P., Ellur, R.K., Bhowmick, P.K. and Singh, A.K., 2018. Marker-assisted introgression of Saltol QTL enhances seedling stage salt tolerance in the rice variety “Pusa Basmati 1”. International journal of genomics, 2018. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.