Abstract
Integrins are cell adhesion and signalling proteins crucial to a wide range of biological functions. Effective marketed treatments have successfully targeted integrins αIIbβ3, α4β7/α4β1 and αLβ2 for cardiovascular diseases, inflammatory bowel disease/multiple sclerosis and dry eye disease, respectively. Yet, clinical development of others, notably within the RGD-binding subfamily of αv integrins, including αvβ3, have faced significant challenges in the fields of cancer, ophthalmology and osteoporosis. New inhibitors of the related integrins αvβ6 and αvβ1 have recently come to the fore and are being investigated clinically for the treatment of fibrotic diseases, including idiopathic pulmonary fibrosis and nonalcoholic steatohepatitis. The design of integrin drugs may now be at a turning point, with opportunities to learn from previous clinical trials, to explore new modalities and to incorporate new findings in pharmacological and structural biology. This Review intertwines research from biological, clinical and medicinal chemistry disciplines to discuss historical and current RGD-binding integrin drug discovery, with an emphasis on small-molecule inhibitors of the αv integrins.
Subject terms: Drug discovery and development, Drug discovery and development, Receptor pharmacology
Integrins are key signalling molecules that are present on the surface of subsets of cells and are therefore good potential therapeutic targets. In this Review, Hatley and colleagues discuss the development of integrin inhibitors, particularly the challenges in developing inhibitors for integrins that contain an αv-subunit, and suggest how these challenges could be addressed.
Introduction
Integrin proteins are ubiquitous, heterodimeric, transmembrane glycoprotein receptors that primarily act as signalling proteins in mammals1. Each consists of an α-subunit and a β-subunit, of which there are 18 and 8 variants, respectively, creating the 24 known heterodimers (Fig. 1). The α- and β-subunits are bound in a noncovalent complex with the ligand-binding site at the interface. Integrins act as adhesion receptors, with the unusual ability to signal in both directions across the plasma membrane2. These events are called ‘inside-out’ signalling3 and ‘outside-in’ signalling4, resulting either from binding to extracellular ligands or from interacting with the cytoskeleton via the integrin intracellular domains. Integrins can therefore enable human cells to respond to changes in the extracellular environment (via outside-in signalling) and can influence the extracellular environment itself (via inside-out signalling). Information from outside the cell is communicated intracellularly when the ligand binds to the receptor, resulting in changes in cell polarity, cytoskeletal structure, gene expression, cell survival and proliferation5. In the opposite direction, intracellular activators such as talin-1 (ref.6) bind to the cytoplasmic tail of the β-subunit, evoking a conformational change that shifts the integrin into a high-affinity state, which more readily binds to extracellular ligands and thus promotes cell migration and extracellular matrix (ECM) assembly and remodelling7.
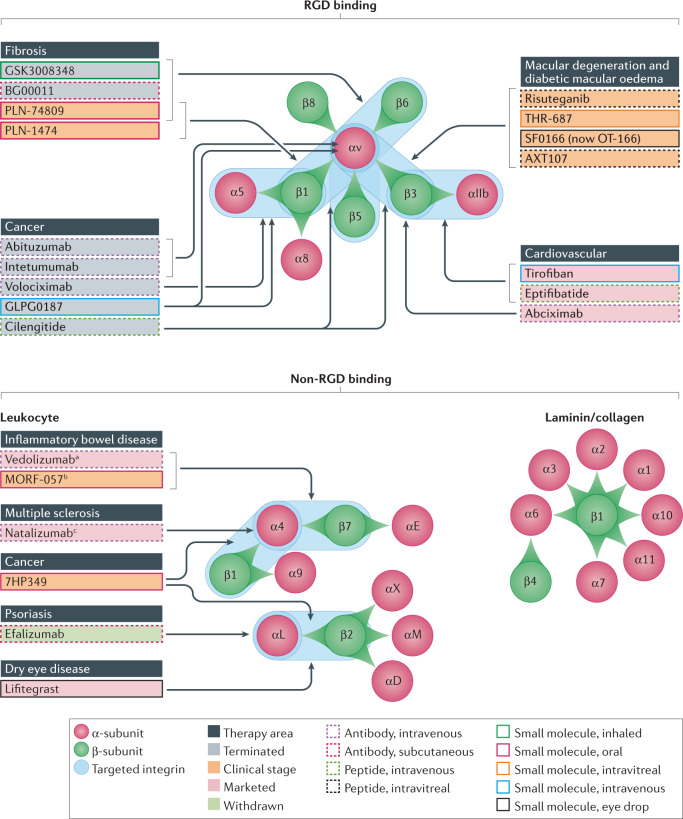
Fig. 1. The integrin family and targeted therapies.
All 24 distinct integrin heterodimers, formed from one α-subunit and one β-subunit, are represented and grouped according to their broad classification by cognate ligand or cellular expression. Therapeutically targeted integrins are highlighted in blue along with the therapeutic areas that are of current interest. Additionally, a select number of therapies in development, and marketed or terminated drugs and their modalities, are shown. Arrows are intended to demonstrate which integrin targets are thought to be key and their purpose is not to capture all known integrin activities. See Tables for additional information. aApproved for ulcerative colitis and Crohn’s disease. bBeing investigated clinically for ulcerative colitis. cAlso approved for Crohn’s disease in the USA.
The integrin proteins are classified into families that consist of receptors with related properties (Fig. 1). For example, all eight members of the RGD-binding family of integrins recognize the amino acid binding motif Arg–Gly–Asp (RGD) in their endogenous ligands. The related integrins α4β7 and α4β1 are therapeutic targets that are expressed on leukocytes and also recognize short peptide sequences, one of which is Leu–Asp–Val (LDV)8,9. In addition, families of integrins that bind to either collagen10 or laminin11 have wide-ranging roles in disease, but to date have not been extensively targeted12,13.
The inhibition of integrins has led to several marketed drugs, and many others are being investigated preclinically in both academic and industry settings. Since 2015, there have been at least 130 clinical trials of integrin-targeted therapies (https://www.clinicaltrials.gov/.clinicaltrials.gov and https://www.clinicaltrialsregister.eu/ctr-search/search using search term “integrin”). (This number is an estimation because there are clinical trials with integrin molecules that are not returned with this search term. Databases interrogated December 2020.) In total, six integrin inhibitor drugs, targeting four integrins (αIIbβ3 (also known as glycoprotein IIb/IIIa), α4β7, α4β1 and αLβ2), have been marketed (Table 1). Three of these drugs are antibodies and three are small molecules, but none is delivered by the oral route — contributing factors for the lack of orally available small molecules include the polar pharmacophore in those molecules coupled with complex pharmacology for the target pathway14. Intravenously administered inhibitors of the RGD-binding integrin αIIbβ3 were some of the first developed, exemplified by two small molecules, tirofiban (Aggrastat) and eptifibatide (Integrilin), alongside the antibody abciximab (ReoPro). All three therapies are prescribed for acute coronary syndrome and for the treatment of thrombotic cardiovascular events15 (Box 1; Table 1). Additionally, drug discovery programmes centred on the integrin αLβ2 (which is expressed on leukocytes) delivered a marketed small molecule, lifitegrast, for the topical treatment of dry eye disease16. Inhibitors of αLβ2 have also been investigated for autoimmune diseases and inflammatory disorders17.
Table 1.
Approved integrin-targeting drugs
| Generic name (brand name; manufacturer) | Chemotype; route of administration | Target; mechanism of action | Indication | Dose185 | Date of regulatory approval |
|---|---|---|---|---|---|
| Lifitegrast (Xiidra; Novartis) | Small molecule; topical | αLβ2 (LFA-1) antagonist; prevents lymphocyte adhesion, thereby reducing T cell-mediated inflammation | Dry eye disease | 1 drop in each eye every 12 h | July 2016 |
| Vedolizumab (Entyvio; Takeda) | Biologic (humanized mAb); i.v. infusion | α4β7 antagonist; inhibits binding to MADCAM1, thereby preventing T cells from homing to the gut | Ulcerative colitis and Crohn’s disease | 300 mg infused over 30 min at weeks 0, 2, 6 and every 8 weeks thereafter | May 2014 |
| Natalizumab (Tysabri; Biogen) | Biologic (humanized mAb); i.v. infusion | Pan-α4 antagonist; inhibits ligand binding to α4β7 and α4β1, thus reducing homing of T cells to the gut (in Crohn’s disease) and across the blood–brain barrier (in multiple sclerosis) | Multiple sclerosis and Crohn’s disease | 300 mg infused over 1 h every 4 weeks | November 2004 |
| Efalizumab (Raptiva; Genentech/Merck Serono) | Biologic (humanized mAb); s.c. injection | αL antagonist; targets lymphocyte-specific αLβ2, preventing lymphocyte activation and migration | Plaque psoriasis | 0.7 mg kg−1 followed by 1 mg kg−1 weekly | October 2003 (withdrawn 2009) |
| Tirofiban (Aggrastat; Medicure & Correvio) | Small molecule; i.v. infusion | αIIbβ3 antagonist, RGD mimetic; prevents platelet aggregation by inhibiting binding to fibrinogen | Acute coronary syndrome and thrombotic cardiovascular events | 25 mg kg−1 followed by 0.15 mg kg−1 min−1 for 18 h | August 1998 |
| Eptifibatide (Integrilin; Takeda, GSK, Merck) | Small molecule (heptapeptide); i.v. injection | αIIbβ3 antagonist, RGD mimetic; prevents platelet aggregation by inhibiting binding to fibrinogen | Acute coronary syndrome and thrombotic cardiovascular events | 180 mg kg−1 followed by 2 mg kg−1 min−1 for up to 72 h | May 1998 |
| Abciximab (ReoPro; Centocor, Inc./Eli Lilly/Janssen Biotech, Inc.) | Biologic (antigen-binding fragment); i.v. injection | Pan-β3 antagonist; inhibits binding of integrin αIIbβ3 to fibronectin, thus preventing platelet aggregation | Acute coronary syndrome and thrombotic cardiovascular events | 0.25 mg kg−1, followed by 10 mg kg−1 min−1 for 12 h | December 1994 |
Successful drugs gaining regulatory approval are tabulated in order of approval date, with most recent first. GSK, GlaxoSmithKline; i.v., intravenous; mAb, monoclonal antibody; MADCAM1, mucosal addressin cell adhesion molecule 1; RGD, Arg–Gly–Asp; s.c., subcutaneous.
The remaining marketed integrin drugs, vedolizumab (Entyvio) and natalizumab (Tysabri), are antibodies that act principally on the leukocyte integrins α4β7 and α4β1 and are used for treating ulcerative colitis, Crohn’s disease18 and multiple sclerosis19. Combined sales of these two molecules have reached more than $US4 billion per year20,21, underlining the impact of integrin inhibitors in treating disease. However, this class of molecules suffered a setback in 2009 when efalizumab, which targeted αL integrins (these are also expressed predominantly on leukocytes), was withdrawn from the market because of multiple cases of progressive multifocal leukoencephalopathy (PML), believed to be associated with inhibition of α4-containing integrins and αLβ2 (ref.22) (Box 2). Similarly, natalizumab was withdrawn from the market in early 2005 after its use was associated with PML in patients with multiple sclerosis; natalizumab returned the following year with a black box warning about the increased risk of PML, after a detailed review of all clinical trial data. These high-value biological targets continue to be investigated clinically with the risk of PML in mind. Indeed, two orally delivered antagonists of α4β7 are in clinical trials: a small molecule from Morphic Therapeutics (which is in phase I) and a peptide from Protagonist Therapeutics (which is in phase II) (Table 2).
Table 2.
Selected clinical studies with pending data
| Generic name (sponsor) | Modality | Delivery route | Primary integrin target | Indication | ClinicalTrials.gov identifiers | Study statusa |
|---|---|---|---|---|---|---|
| IDL-2965 (Indalo Therapeutics) | Small molecule | Oral | αvβ1, αvβ3, αvβ6 | IPF186, NASH187 | NCT03949530 | Terminated |
| PLN-74809 (Pliant Therapeutics) 15 | Small molecule | Oral | αvβ6, αvβ1 | IPF, primary sclerosing cholangitis | NCT04072315, NCT04396756, NCT04480840 | Recruiting |
| PLN-1474 (Pliant Therapeutics) | Small molecule | Oral | αvβ1 | End-stage liver fibrosis in NASH | Not available | Recruiting |
| PN-943 (Protagonist Therapeutics) | Peptide | Oral | α4β7 | Ulcerative colitis | NCT04504383 | Recruiting |
| CAR-T therapy (The Sixth Affiliated Hospital of Wenzhou Medical University) | Cell-based therapy | i.v. | β7 | Relapsed/refractory multiple myeloma | NCT03778346 | Recruiting |
| 7HP349 (7 Hills Pharma) | Small molecule | Oral | αLβ2, α4β1 | Solid tumours | NCT04508179 | Recruiting |
| MORF-057 (Morphic Therapeutics) | Small molecule | Oral | α4β7 | Healthy volunteers | NCT04580745 | Active, not recruiting |
| JSM-6427 (Jerini AG & Shire Pharmaceuticals, now Takeda Pharma) 3 | Small molecule | Parenteral | α5β1 (also binds αvβ6/8) | Age-related macular degeneration | NCT00536016 | Completed |
| OS2966 (OncoSynergy) | mAb | Intratumoural infusion | β1 | Glioma | NCT04608812 | Recruiting |
| AXT-107 (AsclepiX Therapeutics) | Peptide | Intravitreal injection | αvβ3, α5β1 | DME, nAMD | NCT04697758, NCT04746963 | Recruiting |
Emerging integrin-targeting therapies that are currently in clinical trials, or that have been in clinical trials but have not published any findings. This table details novel potential drugs intended as disease therapy, rather than those with potential diagnostic or prognostic value. Studies that have a clinical trials identifiers (NCT numbers) are indicated, together with the associated study status according to the latest data from www.clinicaltrials.gov. Numbers in bold refer to the molecule structures shown in Figs 2,3. DME, diabetic macular oedema; IPF, idiopathic pulmonary fibrosis; i.v., intravenous; mAb, monoclonal antibody; nAMD, neovascular age-related macular degeneration; NASH, nonalcoholic steatohepatitis. aStudy status information correct as of May 2021.
Excitingly, new molecules that target αv-containing integrins are now entering clinical trials for fibrotic diseases, including idiopathic pulmonary fibrosis (IPF) and nonalcoholic steatohepatitis (NASH), which have high and increasing23,24 unmet medical need. Because integrin proteins are pivotal to numerous biological pathways25 and they bind to a variety of endogenous ligands, inhibition of a single integrin or integrin family could treat a range of diseases26, such as multiple fibrotic diseases or multiple types of cancer.
It is therefore timely to discuss the progress made in drug discovery for the RGD-binding integrins, mainly the αv integrin subfamily, as detailed in this Review. Many of the lessons learned from this class of molecules are also pertinent to drug discovery and integrins in general. We discuss the different modalities adopted in integrin drug discovery, alongside their mechanisms, and diseases that might be treated by inhibition of these integrins. We also assess the factors to consider in integrin inhibitor drug design — the unusual physicochemical properties, how to achieve selectivity, the optimal modes of administration and the pharmacology. The latest developments will be discussed alongside the learnings from past programmes and clinical terminations to provide insight on the progress made to identify new, safe and effective treatments. We conclude with a summary of the key challenges and prospects for therapies that target integrins.
Box 1 Therapeutic successes targeting blood cell-specific integrins.
The restricted expression of specific integrins on blood cells that mediate adhesion and migration has led to a longstanding interest in targeting integrins in cardiovascular and autoimmune diseases. Loss of functional integrin αIIbβ3 in patients with the clotting defect Glanzmann thrombasthenia highlighted this receptor as a potential target to treat cardiovascular conditions that involve abnormal clotting. Subsequent work identified the specific RGD motif in fibrinogen that binds to αIIbβ3 (ref.199), which initiated early target-led drug discovery programmes. These efforts led to the first approved integrin inhibitor, abciximab (ReoPro), a humanized antibody fragment with antagonist activity towards β3. Following the success of abciximab, two other αIIbβ3 inhibitors were approved, one peptide (eptifibatide) and one small molecule (tirofiban). All these drugs achieve their antithrombotic effects by preventing αIIbβ3 on activated platelets from binding to fibrinogen, thereby preventing platelet aggregation. Expectations for the utility of this class of therapies as antithrombotics was high because they inhibit coagulation irrespective of which pathway or pathways led to platelet activation. However, this enthusiasm waned when several orally available αIIbβ3 ligand mimetics were found to agonize αIIbβ3 when binding, thereby causing paradoxical platelet activation that potentially led to the increased cardiovascular mortality observed in clinical trials200. Lack of efficacy, together with low rates of receptor occupancy at trough concentrations, meant that platelet aggregation became a risk, halting the progression of oral compounds. Although these drugs are still useful in the acute treatment of patients receiving percutaneous angioplasty, this subclass of approved antiplatelet drugs has been superseded by other rapidly acting antithrombotics. However, recently, an αIIbβ3-binding small molecule, RUC-4 (ref.63) (the structure is shown as 12 in Fig. 2), that does not induce platelet-activating conformational changes in αIIbβ3 has shown some promise in early-stage clinical trials (ClinicalTrials.gov identifier: NCT03844191)65. This molecule is compatible with subcutaneous injection (so it can be administered before hospital admission), and competes with the magnesium ions required for conformational change, thus locking αIIbβ3 in its inactive state. This recent development seeks to overcome the major prevailing drawbacks associated with oral antithrombotics and currently approved αIIbβ3 inhibitors and reignites interest in αIIbβ3 inhibitors for the treatment of cardiovascular disease.
Box 2 Targeting the α4 integrins in multiple sclerosis and inflammatory bowel disease.
The leukocyte-specific integrins — αLβ2, αMβ2, αDβ2, αEβ7 and α4β7 — remain attractive targets to modulate immune cell-mediated diseases as each of them has a key role in immune cell function. Additionally, although it is not exclusively expressed in leukocytes, α4β1 is required for the adhesion of immune cells to vascular cell adhesion molecule 1 (VCAM1), which is expressed on inflamed endothelium; this interaction is important for the infiltration of immune cells into the central nervous system (CNS). Blockade of α4 prevented paralysis in a T cell-induced rat model of multiple sclerosis, and blockade of α4β1 specifically inhibited binding of immune cells to inflamed brain vessels201. This study provided the rationale for targeting α4β1 in immune-mediated CNS diseases. Similarly, blocking the binding of α4β7 (the predominant leukocyte gut-homing receptor) to mucosal addressin cell adhesion molecule 1 (MADCAM1) prevents inflammation known to cause irritable bowel syndrome (IBS).
The pan-α4 inhibitor natalizumab (Tysabri) reduces clinical relapses in patients with multiple sclerosis. Natalizumab also reduces the severity of Crohn’s disease, a subtype of inflammatory bowel disease (IBD), and increases the likelihood of clinical remission. However, the α4β7-specific biologic vedolizumab (Entyvio; approved in 2014) has largely replaced natalizumab in IBD indications because vedolizumab is less likely than natalizumab to cause progressive multifocal leukoencephalopathy (PML) and can be delivered subcutaneously (which is more convenient than the intravenous dosing required for natalizumab). Recent phase II results in treating ulcerative colitis with abrilumab (AMG181), another α4β7-specific antibody, in around 350 patients (ClinicalTrials.gov identifier: NCT01694485) suggest that this compound induces remission and mucosal healing in patients with moderate to severe ulcerative colitis without any incidence of PML202. Phase III trials of AMJ300, an oral α4-inhibiting prodrug, are also underway in patients with ulcerative colitis. The active metabolite has the same mechanism of action as natalizumab and may cause PML at similar frequency to natalizumab, but AMJ300 has a shorter half-life than natalizumab and could therefore be quickly removed from the patient’s body if PML occurs203. AMJ300 has also shown some efficacy in rodent models of multiple sclerosis, but development has so far focused on IBD indications.
Drugs that target β7, which affect both αEβ7 binding to E-cadherin and α4β7 binding to MADCAM1, are actively being developed for IBD indications. Unlike pan-α4 inhibitors, these drugs do not inhibit α4β1, so they should have a low risk of causing PML, and have the additional benefits (and risks) of reducing lymphocyte retention in the gut via αEβ7 inhibition. One such subcutaneously administered antibody, etrolizumab, showed promise in phase II studies in ulcerative colitis204,205, but failed to meet its primary end point versus placebo as maintenance therapy in a phase III study192. Despite the success of anti-integrin therapies in treating IBD, they remain a third-line treatment behind corticosteroid and antitumour necrosis factor therapies.
Targeting αv-containing integrins
Historically, much of RGD-binding integrin drug discovery has focused on αvβ3 for cancer27, ophthalmology28 and osteoporosis29,30; αvβ3 remains the most studied integrin in the past two decades (see Supplementary information for data comparing publication trends in integrin research). The rationale for targeting αvβ3 in cancer comes from its known role in tumour angiogenesis and its upregulation on endothelial cells; inhibitors of αvβ3 have had positive effects in preclinical models31. However, these studies have not translated effectively to the clinical setting22. For ophthalmic diseases, integrin inhibitors could reduce various pathologies associated with eye disease, such as inflammation, vascular leakage, angiogenesis and fibrosis. Integrin inhibitors have been effective in several preclinical models, and promising results have been reported thus far from clinical trials32. Indeed, most of the current αvβ3 clinical investigations centre on treating eye diseases (age-related macular degeneration (AMD) and diabetic macular oedema (DME))33 using topically dosed or intravitreally injected small molecules and peptides, although these molecules also inhibit other αv integrins and/or α5β1 to varying degrees (Table 3). The molecules that have progressed the furthest in the clinic are risuteganib (Luminate, Allegro Ophthalmics, structure 4 in Fig. 2), the fluorinated MK-0429 analogue SF0166 (Scifluor Life Sciences; now OT-166, OcuTerra Therapeutics, 11) for treating AMD and DME34, and the pan-αv/α5β1 inhibitor, THR-687 (Oxurion/Galapagos), which is currently in phase I trials for DME. JSM-6427 (Takeda, proposed structure shown in 3) is a peptidic small molecule that has been evaluated preclinically for treating ocular neovascular diseases35 and progressed to phase I clinical trials for AMD. However, no data have been reported since 2010. Most recently, a 20-mer synthetic peptide that targets both αvβ3 and α5β1 (AXT-107, developed by AsclepiX Therapeutics), derived from the non-collagenous domain of collagen IV, has entered clinical trials for treating retinal vascular diseases36,37.
Table 3.
Selected integrin inhibitors in clinical trials with reported clinical safety and efficacy data
| Name (sponsor) | Modality | Delivery route | Population or indication | Integrin targets | Highest human dose reported | ClinicalTrials.gov identifiers | Safety and efficacy |
|---|---|---|---|---|---|---|---|
| BG00011 (Biogen) | Humanized mAb | s.c. | IPF | αvβ6 | 56 mg weekly | NCT03573505 | Toxicity observed174 |
| Intetumumab (CNTO-95) (Centocor) | Humanized mAb | i.v. | Melanoma, prostate cancer | Pan-αv | 10 mg kg−1 every 3 weeks | NCT00246012, NCT00537381 | Tolerated but no efficacy188,189 |
| Abituzumab (DI17E6) (Merck KGaA) | Humanized mAb | i.v. | Colorectal cancer | Pan-αv | 1,500 mg every 4 weeks | NCT01008475 | Acceptable tolerability; did not meet primary end points106 |
| GLPG0187 (Galapagos NV) 7 | Small molecule | Continuous infusion | Solid tumours | Pan-αv, α5β1 | 400 mg daily | NCT01313598 | Well tolerated; no efficacy42 |
| GSK3008348 (GSK) 5 | Small molecule | Inhalation | Healthy volunteers | αvβ6 | 3 mg | NCT02612051 | Well tolerated190 |
| Cilengitide (EMD Serono) 1 | Cyclic peptide | i.v. | Glioblastoma | αvβ3, αvβ5 | 2,000 mg twice a week | NCT00689221 | Well tolerated; no efficacy39 |
| MK-0429 (Merck & Co.) 13 | Small molecule | Oral | Metastatic bone disease | Pan-αv | 1,600 mg twice daily for 4 weeks | NCT00302471 | Safe191 |
| Etrolizumab (Roche) | Humanized mAb | s.c. | Ulcerative colitis | α4β7 | 105 mg every 4 weeks | NCT02136069 | Well tolerated192 |
| VPI-2690B (Vascular Pharmaceuticals) | Humanized mAb | s.c. | Diabetic nephropathy | αvβ3 | 48 mg every 2 weeks | NCT02251067 | Safe; significant levels of drug exposure193 |
| THR-687 (Oxurion) | Small molecule | Intravitreal injection | DME | Pan-αv, α5β1 | 2.5 mg | NCT03666923 | Safe and well tolerated194 |
| SF0166/OT-166 (Scifluor Life Sciences/OcuTerra Therapeutics) 11 | Small molecule | Topical | Age-related macular degeneration, DME | αvβ3, αvβ6, αvβ8 | 5% solution twice a day for 28 days | NCT02914613, NCT02914639 | Well tolerated; one potential mild-to-moderate drug-related adverse event195 |
| Risuteganib (Luminate, ALG-1001; Allegro Ophthalmics) 4 | Small molecule | Intravitreal injection | DME, dry age-related macular degeneration | αvβ3, αvβ5, α5β1 | 1.0 mg | NCT03626636 | Well tolerated196 |
| PLN-74809 (Pliant Therapeutics) 15 | Small molecule | Oral | IPF | αvβ6, αvβ1 | 40 mg daily for 7 days197 | NCT04396756 | Good tolerability |
| SB-273005 (GSK) 14 | Small molecule | Oral | Osteoporosis | αvβ3/αvβ5 | 2,000 mg daily | Historic | No toxicity in humans; dose-dependent heart valve lesions in mice (species-specific)198 |
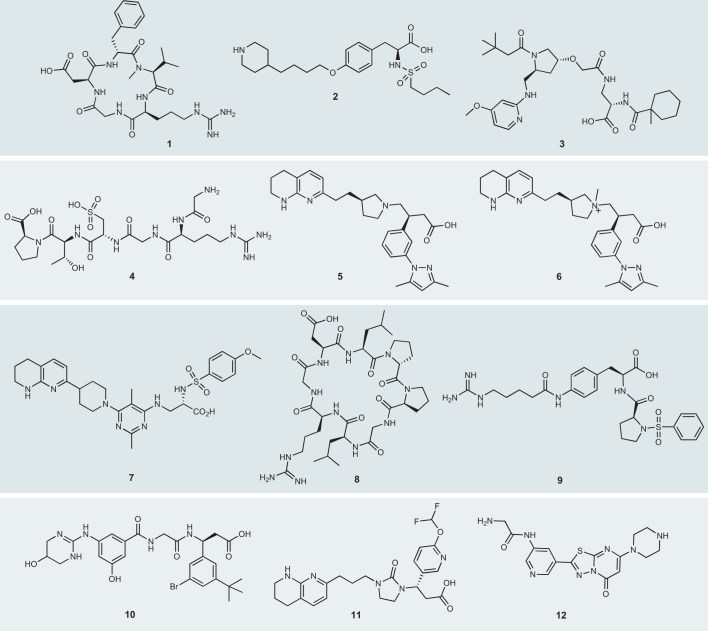
Fig. 2. Selected small molecules with parenteral properties that bind to RGD integrins.
The small molecules shown here have either been evaluated in the clinic or have been examined in preclinical models and are administered by a parenteral route with advantageous potency or selectivity profiles. The molecules are: 1 cilengitide, 2 tirofiban, 3 JSM-6427 (proposed structure); 4 risuteganib (Luminate), 5 GSK3008348, 6 αvβ6 integrin small-molecule inhibitor, 7 GLPG0187, 8 acyclic peptide selective for αvβ8, 9 αvβ1 integrin small-molecule inhibitor, 10 CWHM-12, 11 SF0166 (now OT-166), 12 RUC-4. RGD, Arg–Gly–Asp.
Although molecules that target αvβ3 integrins generally have an acceptable safety profile (Table 3), interest in using them to tackle cancer has waned, mainly owing to lack of efficacy. Broad reasons for failure may encompass several factors, such as redundancy, promiscuity and compensation mechanisms38. The most studied αvβ3 inhibitor molecule and furthest progressed is the cyclic peptide, cilengitide (Merck KGaA, 1), which also inhibits αvβ5 and α5β1 and has been assessed in approximately 30 different clinical trials for cancer. Ultimately, however, cilengitide fell short in phase III trials owing to a lack of efficacy against glioblastomas, with no improvement in overall survival39,40. Antibodies that target αvβ3, such as etaracizumab (MEDI-522; Abergrin) also advanced to clinical trials for several diseases, including cancer41, but progression has halted. GLPG0187 (7), a broad-spectrum αv inhibitor, also failed to show signs of efficacy in a phase Ib trial in patients with solid tumours, again, despite being well tolerated42.
Meanwhile, alternative approaches to αvβ3 inhibition are offering greater promise for treating the same or similar diseases: there are numerous promising therapies for treating glioblastomas43, cathepsin K inhibitors could be useful in osteoporosis44 and agents targeting vascular endothelial growth factor A (VEGFA), such as ranibizumab, are already available for treating AMD45. Any new drug discovery programme focused on the αvβ3 integrin will therefore require convincing target validation and demonstrable evidence that preclinical models are predictive of effects in the clinic. Academic research on the role of αvβ3 in cancer and other areas continues, aided by several high-quality tools that are available to test integrin-mediated mechanisms in new disease models to potentially deliver more effective therapies.
In recent years, αvβ6 and the well-established target α4β7 have attracted substantial interest as therapeutic targets. Several new inhibitors of the αvβ6 and/or αvβ1 integrins (most of which are small molecules) have progressed to clinical trials in the past 5 years, and α4β7-directed therapies (mostly antibodies) have advanced to late-stage clinical trials. The change in focus from αvβ3 to αvβ6 also switches the focus of downstream effector pathways from angiogenesis to modulation or inhibition of the transforming growth factor β (TGFβ) pathway (Box 3). The increased interest in α4β7 has presumably come from the clear benefit to patients from prescribed drugs, such as natalizumab, and the need for alternative therapies after several terminated clinical trials and withdrawals, specifically in ulcerative colitis and multiple sclerosis.
Box 3 Activation of TGFβ via the αv integrins.
All five of the Arg–Gly–Asp (RGD)-binding αv-containing integrins have been shown to activate the pro-fibrotic mediator transforming growth factor β (TGFβ)206, releasing this growth factor from its inactive state, in which it is bound to latency-associated peptide (LAP) and tethered to the extracellular matrix (ECM). Only the TGFβ1 and TGFβ3 isoforms are activated by integrins, as the TGFβ2–LAP complex lacks the RGD sequence207. The activation of latent TGFβ by the myofibroblast integrins αvβ1, αvβ3 and αvβ5 requires a contractile cytoskeleton, and when fibroblasts differentiate into myofibroblasts, contractility increases, especially after stimulation with growth factors. In similar fashion, αvβ6 on epithelial cells requires tractive force and cytoskeletal integrity to release TGFβ from pro-TGFβ153.
Integrins can activate TGFβ via a protease-dependent or protease-independent pathway. In the case of αvβ8, proteolytic activity is required and matrix metalloproteinase 14 (MMP14) is simultaneously recruited to the LAP RGD site208. Protease-independent TGFβ activation by integrins requires these two molecules to be in close proximity, coupled with tractive force, resulting in the presentation of TGFβ to its cognate receptor209. This can perpetuate a feedforward loop whereby TGFβ upregulates integrin expression and a repertoire of ECM proteins, leading to continual and self-sustaining growth factor activation. The differentiation of myofibroblasts is induced by active TGFβ, and this process is mediated in part through SMAD and the canonical TGFβ pathway. However, the process may also be stimulated by non-canonical activation of focal adhesion kinase (FAK) through interactions with integrins210. Therefore, the pro-fibrotic effects of TGFβ on fibroblasts are likely caused by integrins on epithelial cells and fibroblasts, which release the active growth factor, as well as autonomous, non-canonical, pro-fibrotic pathways through TGFβ-induced αvβ1 and FAK.
Modalities and mechanisms
Integrins can be targeted with a range of mechanisms designed to activate (as agonists) or inactivate (as silent antagonists or inhibitors) the integrin complex, inhibit a secondary biological process initiated by the integrin (as functional antagonists), deliver a cytotoxic drug (as a drug conjugate) in a cell-specific manner or direct chimeric antigen receptor T (CAR T) cells for immunotherapy46–48. The majority of integrin drug discovery initiatives have set out to target the orthosteric binding sites (endogenous ligand-binding sites) on integrins, which are formed when the α- and β-subunits bind to each other in a noncovalent complex. However, several drug discovery programmes that targeted the orthosteric site have not been successful, which highlights the potential risks of this approach. Some of the approved therapies exert their effects via allosteric interactions with the α-subunits of the integrins they target; for example, natalizumab exclusively targets the α4-subunit of α4β1 at an allosteric site (ref.49) (Table 1).
Integrins can exist in an activated or inactivated state, in which they demonstrate high or low affinity for ligands, respectively50. Extracellular ligand binding to the orthosteric site induces intracellular signalling, but also shifts the integrin from a low-affinity to a high-affinity state7. Under normal physiological conditions, this enables cells to respond to changes in the extracellular environment, but this response can become exaggerated and unwanted under pathophysiological conditions51. Therefore, an antagonist molecule designed to bind to an integrin and prevent an endogenous ligand from binding may also have a direct agonist effect on the integrin if it causes this shift in affinity. These effects have been observed for marketed αIIbβ3 RGD mimetics such as eptifibatide52, which induced severe thrombocytopenia in a small group of patients53, a phenomenon that was particularly evident during clinical trials with oral inhibitors54,55. In a related observation, low concentrations of αvβ3 integrin RGD mimetics stimulate, rather than inhibit, tumour growth and angiogenesis in preclinical models56,57. Agonist effects can therefore also occur at low receptor occupancy for multiple members of the integrin family, perhaps in a manner analogous to the two-state model of activation of another family of membrane-bound receptors, the G protein-coupled receptors58. Importantly, these agonist effects may have caused or contibuted to the clinical failure of cilengitide (1)56. Paradoxically, however, low-dose activation of an integrin receptor may indeed have therapeutic potential: in preliminary studies with cilengitide at low doses, its pro-angiogenic effects enhanced the delivery and potency of the chemotherapy agent gemcitabine to tumours59.
In light of this potential issue, academic groups have used structure-guided design to identify silent small-molecule integrin antagonists. Initial research hypothesized that the limitations manifested by thrombocytopenia and/or increased bleeding times with αIIbβ3 inhibitors were partially because the inhibitors induced high-affinity conformations of the integrin60. Inhibitors that minimize the agonist effects by stabilizing the low-affinity conformation have been designed and identified. One of the first breakthroughs in this area came with the amine ligands RUC-1 (ref.61) and RUC-2 (ref.62), which are non-RGD mimetics that bind to the orthosteric site in αIIbβ3 and induce smaller conformational changes in the β3-subunit than marketed agents do. This observation has been backed up preclinically with the next molecule in the series, RUC-4 (12), which has reduced bleeding and the appropriate pharmacokinetic properties required for an antithrombotic drug, and may therefore be effective in the clinical setting63. RUC-4 is currently in a phase II clinical trial (NCT04284995) by CeleCor Therapeutics64 to assess the pharmacokinetic and pharmacodynamic properties of a single subcutaneous injection in patients with a myocardial infarction65. This is a promising approach, and applying this binding principle to other αv-containing integrins could provide opportunities to develop new non-zwitterionic chemotypes that do not induce potentially undesired conformational states. To our knowledge, such molecules are still in preclinical development, but the results are eagerly anticipated66.
Another group, meanwhile, reported the first crystal structure of a mutant of fibronectin bound to αvβ3 that acts as a ‘pure’ antagonist67. Other pure αvβ3 small-molecule antagonists have been designed, using cryogenic electron microscopy imaging of integrin conformations, that had limited access to the high-affinity conformation of αvβ3 and did not enhance angiogenesis at low concentrations60. Using similar methodology, small-molecule pure antagonists for αIIbβ3 were designed that have reduced bleeding in preclinical models68. These molecules are traditional RGD ligand mimetics, unlike the RUC series of compounds. Additionally, a high molecular weight polypeptide disintegrin, TMV-7, which recognizes the αIIb β-propeller domain, does not induce a conformational change in the β3-subunit, and maintains the antithrombotic effects with little tendency for bleeding69. Several exciting avenues are currently being investigated in the pursuit of safer and more effective integrin inhibitors.
Inhibitors such as TMV-7 that bind allosterically may have fewer unwanted side effects. With this approach in general, the affinity state of the receptor is also probably less relevant, because the binding site is distinct from the orthosteric site. Allosteric molecules could block integrin activation either by occluding the orthosteric site or by inducing a conformational change that shifts the integrin to a low-affinity state. However, and especially for antibodies, reduced selectivity may result if the integrin being targeted contains either an α- or β-subunit that pairs with multiple other β- or α-subunits. For example, abituzumab, an antibody that binds to an allosteric site on the αv-subunit and blocks the RGD site, likely inhibits all αv integrins, and this may broaden the risk of on-target toxic effects and thereby potentially reduce the therapeutic window.
The levels of several integrins are increased on tumours, so targeting these integrins with a conjugated cytotoxic molecule could be an effective strategy to specifically target tumour cells70. Instead of directly inhibiting an integrin, this novel approach, using drug conjugates that target αvβ6, takes advantage of high local expression in diseased tissue compared with relatively low levels elsewhere. Interestingly, the β7 subunit, in combination with a number of tumour cell antigens, has also been targeted with a CAR-T cell approach in multiple myeloma46 in an effort to improve the immunosuppressive microenvironment of tumours (Table 2).
Modifying molecules to reduce their half-lives in the systemic circulation can also ameliorate some potential toxicity. For example, the high-affinity αvβ6-binding small-molecule RGD mimetic, GSK3008348 (5), is internalized and degraded in cells, thereby reducing both lung and systemic drug load following inhaled administration71. This molecule is both an agonist of αvβ6 and a functional antagonist of TGFβ, as it indirectly inhibits TGFβ signalling by reducing TGFβ activation. Ligand-induced internalization could also be exploited to improve selectivity. For example, if αvβ6 was the only αv-containing integrin to be internalized quickly and return to the cell surface slowly following RGD binding, inhibitors targeting this integrin via an RGD-mimetic interaction would have an additional selectivity bias. The functional consequences of this approach would depend on the type and duration of signalling initiated as a result of internalization, which are not known, but to date there has been no evidence of negative effects.
The general advantages and disadvantages of small versus large integrin inhibitor molecules have been described elsewhere72. However, given the seemingly delicate balance between beneficial and detrimental effects derived from full and partial engagement of integrins, from a safety perspective, small molecules may well be better than antibodies because they are cleared from the body in hours, whereas antibody clearance takes days or weeks. It is also easier to have periods of time when no drug is present (for example, if adverse effects arise) and manage receptor occupancy levels with small molecules than with antibodies. Small molecules can also be dosed at home via the oral or inhaled route of administration, whereas antibodies are often dosed at a clinical site by subcutaneous or intraperitoneal injection.
With the breadth and complexity of biology and mechanisms at play within the integrin family, it is likely that only a small component of integrin biology is understood, meaning further research is required to fill the gaps. Do the phenomena observed with certain integrins after drug binding — agonism, internalization and downregulation — also occur with other integrins? More research and an improved set of tool molecules73 are required to further dissect these complexities. Indeed, current RGD-binding integrin drug discovery efforts, irrespective of their clinical successes, are likely to catalyse future research because they will deliver a new set of well-characterized tools. These investigations could lead to the successful generation of integrin-targeting drugs.
Diseases involving integrins
For the subfamily of integrins that contain the αv-subunit, a plethora of target validation studies have been completed in fibrotic diseases and oncology. In this section, we summarize the target validation studies that support the hypotheses that integrin inhibition will have a therapeutic benefit in fibrotic diseases, cancer and viral infections.
Pulmonary fibrosis
Currently, clinical investigations for the antifibrotic potential of integrin inhibitors centre on IPF, a debilitating chronic condition of unknown aetiology. Several αv-containing integrins (αvβ1 (refs74,75), αvβ5 (ref.76) and αvβ6 (ref.77)) are upregulated in IPF. αvβ1 and αvβ6 are the most thoroughly validated targets; levels of αvβ6 also have potential prognostic value78. Multiple integrins may be involved in IPF: αvβ6 drives TGFβ activation in alveolar epithelial cells while αvβ1 mirrors this in myofibroblasts, which characterize and contribute to the development of fibrotic diseases. The contribution of the integrins and cell types to IPF depends on the disease stage; αvβ6 is implicated in the early phase, during epithelial damage, after which αvβ1 in myofibroblasts drives the fibrotic foci75.
αvβ6 was the first αv integrin to be identified as crucial in IPF. Gene knockout and pharmacological intervention studies in the bleomycin-induced mouse model of lung fibrosis with a selective αvβ6 monoclonal antibody, BG00011 (ref.79) (known as STX-100 and 3G9 preclinically), demonstrated that αvβ6 deletion or inhibition could prevent the development of fibrosis or reverse established fibrosis, respectively. Later work showed that blocking αvβ1 with a small-molecule inhibitor (9) in the bleomycin model could reverse established fibrosis75, but the lack of selectivity of this molecule, which also binds to non-αv-containing integrins, suggests that further target validation work is required74. It is worth noting that genetic deletion of αvβ1 is not possible because homozygous β1-null mice do not survive through to birth80 and therefore a key preclinical tool in the target validation armoury for αvβ1 is missing. The pan-αv inhibitor MK-0429 (13 in Fig. 3) also reduces fibrosis progression in the bleomycin-induced mouse model81, supporting the notion that αvβ1 and αvβ6 have important roles in fibrosis, but the data from this inhibitor does not differentiate their contributions. However, as the drug was dosed 5 days after bleomycin treatment in this study, during the inflammatory phase of the model rather than in the subsequent fibrotic stage, the results should be treated with caution.
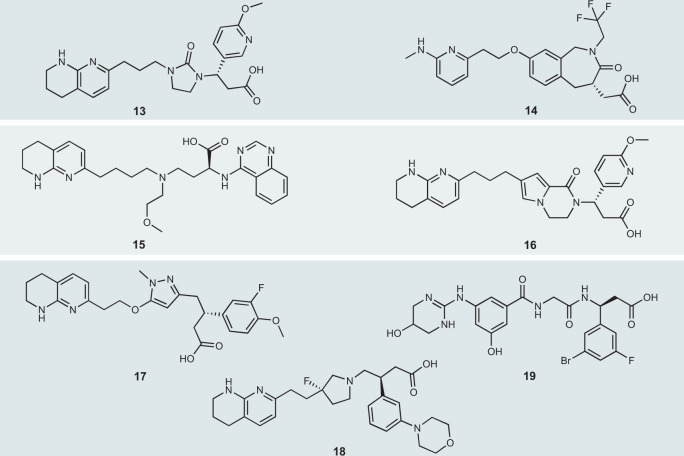
Fig. 3. Selected small molecules with oral properties that bind to RGD integrins.
The small molecules shown here have either been evaluated in the clinic or have been examined in preclinical models and are administered by a parenteral route with advantageous potency or selectivity profiles or are predicted to have these attributes based on their physicochemical properties. The molecules are: 13 MK-0429, 14 SB-273005, 15 PLN-74809 (proposed structure), 16 an example of the series from Bristol-Myers Squibb patent WO2019/094319, 17 an example of the series from St Louis University and Indalo Therapeutics patent WO2018/132268, 18 an example from GlaxoSmithKline’s patent WO2016/046226, 19 an example of the series from St Louis University patent WO2017/117538. RGD, Arg–Gly–Asp.
There is some preclinical evidence that α3β1 (ref.82), α4β1 (ref.83) and α8β1 (ref.84) integrins play a role in pulmonary fibrosis, and that αvβ8 is important in small airway fibrosis associated with chronic obstructive pulmonary fibrosis and asthma85. However, these are challenging integrins to target because few selective small molecules and antibody tools exist, and perhaps, as a consequence, to the best of our knowledge, there are no advanced IPF drug discovery campaigns focused on these integrins.
Hepatic fibrosis
Hepatic fibrosis is associated with the end stage of chronic liver diseases such as chronic virus-induced hepatitis B and hepatitis C, and nonalcoholic fatty liver disease (NAFLD). NAFLD encompasses a range of chronic liver diseases related to obesity, steatosis (accumulation of fat) and NASH that can lead to fibrosis, cirrhosis and hepatocellular carcinoma. In end-stage liver disease, αvβ6 protein levels are increased in bile duct epithelia and transitional hepatocytes, and the αvβ6 mRNA levels increase with disease progression in patients with hepatitis C86. In vivo target validation studies for individual integrins have been confined to less-relevant disease models such as the chemically induced carbon tetrachloride (CCl4) and the surgery-based bile-duct ligation (BDL) models, which are not particularly representative of end-stage human liver fibrosis. As with IPF, both αvβ1 and αvβ6 have been implicated in the development of liver fibrosis, with evidence from genetic deletion or pharmacological inhibition with small molecules or monoclonal antibodies in animal models75,87,88. In addition, pan-αv blockade attenuated fibrosis in a more relevant model of NASH89 — the choline-deficient, amino‐acid-defined, high‐fat diet model — and further interrogation of this model with selective tools may aid selection of the optimum integrin or integrins to target. The most advanced integrin-directed therapy for NASH is the selective αvβ1 inhibitor PLN-1474, which was recently acquired by Novartis AG from Pliant Therapeutics (Table 2).
Chronic kidney disease
In comparison with the target validation data that implicate αv-containing integrins in lung and liver fibrosis, the kidney has attracted less attention, possibly because there are no fibrosis-specific surrogate end points for clinical trials, so demonstrating clinical efficacy in short-term studies is difficult90. Several chronic renal diseases such as glomerulonephritis, diabetes, IgA nephropathy and Alport syndrome are associated with fibrotic changes and increased αvβ6 expression in epithelial cells91. Although some evidence supports a role for αvβ1 in kidney fibrosis92, most target validation studies suggest that αvβ6 is more relevant91,93. A breakthrough in understanding how to test this hypothesis clinically is needed. Interestingly, Merck recently patented an historic pan-αv molecule, MK-0429 (13), for chronic kidney disease94. This patent was supported by preclinical evidence; efficacy was demonstrated in a rat in vivo model of diabetic nephropathy95.
Skin fibrosis
Skin fibrosis may be present in systemic sclerosis (SSc, a connective tissue disease), hypertrophic scarring and keloid lesions. There is some evidence that integrins have a role in skin fibrosis, specifically SSc, as fibroblasts isolated from disease samples showed elevated levels of activated β3-subunits96. In addition, the dual αvβ3/αvβ5 inhibitor cilengitide blocked cutaneous fibrosis when therapeutically administered in a murine model of SSc97. Interestingly, these effects were thought to be due to inhibition of integrin signalling pathways rather than blockade of TGFβ activation. It is possible that a pan-αv integrin inhibitor could be of value in SSc-associated interstitial lung disease (SSc-ILD); in this disease, αvβ6 is hypothesized to contribute to disease in the lung98, and αvβ3 and/or αvβ5 may drive the skin manifestations. However, an investigational study using the pan-αv monoclonal antibody abituzumab was terminated for lack of eligible patients (NCT02745145).
Cancer
Numerous integrins, namely αvβ3, αvβ5 and α5β1, have been investigated as potential therapeutic targets for various cancers for more than 25 years. The role of integrins in cancer22,99,100 has been reviewed extensively elsewhere but we highlight some of the key findings for inhibitors of αv-containing integrins.
Although the rationale for targeting αvβ3 and αvβ5 in cancer was plausible based on the preclinical data and the role of these molecules in tumour angiogenesis, this approach has not led to clinical success. Additional cell types may need to be targeted in the tumour microenvironment for these inhibitors to have a benefit38. αvβ6 is also upregulated in several tumours101 and could also have prognostic potential102–105. Clinical trials have investigated the pan-αv antibodies abituzumab and intetumumab in colorectal carcinoma106 and melanoma107, respectively, as monotherapies, in combination with chemotherapy or in combination with chemotherapy and an epidermal growth factor receptor inhibitor. Although neither set of studies achieved statistically significant efficacy in its primary end points, there was some evidence of improvement in patients with colorectal carcinoma that expressed high levels of αvβ6 (ref.106). This suggests that selecting patients who have high αvβ6 expression in the target organ could maximize the chances of clinical efficacy. The selective imaging tools now available108 (Table 4) could form part of a triage strategy in future trials and for other indications in which αvβ6 is a potential key driver of disease. With this rationale, selectively targeting αvβ6 with antibodies or peptides may have utility in treating pancreatic cancer109 or breast cancer110 or as conjugates to antitumour agents111.
Table 4.
Recent clinical imaging studies targeting integrins
| Tracer (sponsor) | Imaging modality | Primary integrin target | Study aim | Indication | ClinicalTrials.gov identifier | Study statusa |
|---|---|---|---|---|---|---|
| 68Ga-NOTA-3P-TATE-RGD (Peking Union Medical College Hospital) | PET/CT | αvβ3 | Target expression | Lung cancer | NCT02817945 | Unknown |
| [18F]Fluciclatide (GE Healthcare) | PET | αvβ3/αvβ5 | Target expression and reproducibility | Solid tumours | NCT00918281 | Completed |
| [18F]FBA-A20FMDV2 (GSK) | PET | αvβ6 | Target expression and engagement | IPF | NCT02052297, NCT03069989 | Terminated |
| [18F]FBA-A20FMDV2 (Queen Mary University of London) | PET | αvβ6 | Target expression | Cancer | NCT04285996 | Active, not recruiting |
| [18F]αvβ6-BP (University of California, Davis) | PET/CT | αvβ6 | Target expression | Multiple cancers | NCT03164486 | Recruiting |
| [18F]FP-R01-MG-F2 (Pliant Therapeutics and Stanford University) | PET/CT/MRI | αvβ6 | Target expression and engagement | IPF, primary sclerosing cholangitis, pancreatic cancer | NCT03183570, NCT02683824, NCT04072315 | Recruiting |
| 99mTc-3PRGD2 (RDO Pharm) | SPECT/CT | Pan-αv | Target expression | Lung cancer | NCT03974685, NCT04233476 | Completed/recruiting |
| 99mTc-RWY (Peking University) | SPECT/CT | α6 | Target expression | Breast cancer | NCT04289532 | Completed |
The table lists selected tracer compounds that are included in studies registered on www.clinicaltrials.gov. These compounds are under investigation as tools to measure target expression and/or target engagement primarily in cancer and fibrotic indications. CT, computed tomography; GSK, GlaxoSmithKline; IPF, idiopathic pulmonary fibrosis; PET, positron emission tomography; SPECT, single-photon emission computerized tomography. aStudy status information correct as of May 2021.
Specific subsets of cancers are targeted by immunotherapies that disrupt the programmed cell death protein 1 (PD1)–PD1 ligand 1 (PDL1) axis. These surface proteins constitute a receptor–ligand pair and are members of a family of checkpoint inhibitors that moderate immune function and halt the development of a T cell response112. This checkpoint protects the host from autoimmunity and inappropriate immune responses but is inappropriately activated in some tumour microenvironments; overexpression of PDL1 in tumour cells induces tolerance by binding to PD1 on T cells, thereby inhibiting cytotoxic T cell activation and proliferation, and cytokine secretion. Tumour cells thus evade immune detection113. A new class of anticancer immunotherapies that target susceptible PDL1-expressing cancers has emerged over the past decade, and several biologic agents that target this pathway have been approved in cancer114.
Recent work highlights three promising independent mechanisms by which integrins may be targeted to treat cancer: reducing the expression of PDL1 in cancer cells, reducing the levels of TGFβ in the tumour microenvironment and targeting leukocyte integrins to prevent T cells from homing to tumours. Integrins participate in regulation of PDL1 expression and are thus an important constituent of the immune evasion apparatus. αvβ3 positively regulates PDL1 expression in the tumour microenvironment115, and in murine studies αvβ3 depletion restricts the growth of primary tumours116. This is particularly relevant because most patients with cancer who receive anti-PD1 or anti-PDL1 therapies do not respond to treatment117; anti-αvβ3 therapy may sensitize tumours to disruption of this axis and therefore be useful in combination with PD1- or PDL1-targeted agents. Because overexpression of αvβ3 is a common feature in some cancer types and is often associated with poor prognosis, targeting αvβ3 in these cancers could unmask tumours protected by PDL1 overexpression, rendering them susceptible to treatment with an anti-PD1 therapy. Selecting patients on the basis of αvβ3 expression could increase the likelihood of a positive outcome. Inhibiting integrins other than αvβ3 may also sensitize tumours to checkpoint inhibitors. For example, inhibition of αvβ6 induced T cell-mediated immunity in immunotherapy-resistant tumour models118.
Similar to αvβ3, blockade of αvβ8 has been shown to potentiate a cytotoxic T cell response in tumours, although these effects seem to be independent of the PD1–PDL1 axis. In contrast to αvβ3, αvβ8 expression in tumours does not usually correlate with PDL1 expression, although αvβ8 expression on cancer cells drives tumour growth in vivo. αvβ8 promotes tumorigenesis through a mechanism different from that of αvβ3 that may involve TGFβ. In this alternative mechanism of immune evasion, active TGFβ is released from its latent form, which is present on immune cells, by binding to αvβ8 on tumour cells119 or potentially on immune cells120,121. Active TGFβ in the tumour stroma can prevent the penetration of T cells into the tumour and thus protect tumours from T cell attack122,123.
The success of strategies that directly target αvβ3 and αvβ8 will likely be tied to the expression profiles of these integrins on individual tumours, whereas improving T cell adhesion and activation by targeting leukocyte adhesion integrins should be less dependent on the precise mechanisms by which individual tumours evade host immunity. Allosteric activation of the leukocyte-specific integrins αLβ2 and α4β1 in T cells with the small molecule 7HP349 enhanced T cell activation and adhesion, and thereby improved the penetration of T cells into tumours in mouse models of melanoma and colon carcinoma124. This compound is now in phase I trials (Table 2).
Efforts are also being made to conjugate integrin-binding small molecules, peptides and antibodies to bioactive moieties to target specific tissues. Integrins are cell surface receptors and are overexpressed in specific diseased tissues, and are therefore perfect candidates for the application of this technology. So far, efforts have centred on RGD-binding approaches to target delivery of drug conjugates to tumours, and αvβ3 has been the primary focus owing to its role in the development of tumour vasculature. Proof-of-concept experiments with nanoparticles as the drug moieties and various cyclic RGD peptides as the αvβ3-targeting component demonstrated that these molecules rapidly localized to tissues and induced targeted cytotoxicity in vivo125–127. The potential to deliver other tools, such as diagnostic imaging ligands, to tissues is also of interest99,128. Studies have successfully delivered therapeutic and imaging compounds to specific tumours and improved the uptake and activity of the bioactive cargo in tumours. Altering the targeting moiety and conjugate design can give this system remarkable flexibility and precision, and this conjugate approach may well lead to successful treatments. Indeed, a recent clinical trial (NCT04389632) has been initiated to investigate an antibody–drug conjugate that recognizes β6 to selectively target solid tumours129.
Viral infections
In light of the SARS-CoV-2 pandemic of 2020 and beyond it is appropriate to highlight the less explored role that integrins can have in virus transmission. In this regard viruses use various mechanisms, including binding to integrins, to gain cell entry or attachment. By expressing relevant peptide sequences on their surfaces, viruses can bind to integrins to invade host cells, activate intracellular signalling events and mediate disease pathogenesis130. Viruses commonly co-opt the RGD recognition sequence, which, in principle, can therefore be targeted to intervene in a range of viral infections131. Notably, RGD-binding integrins are used, amongst others, by Zika virus (αvβ5)132, rotavirus (αvβ3)133, Ebola virus (α5β1)134 and foot-and-mouth-disease virus (αvβ6)135 to gain entry to host cells. However, a potential drawback for the design of integrin inhibitors for this use is the risk of redundancy, whereby alternative cell entry mechanisms are available to viruses to enable rapid evasion from drugs.
Recently, it was postulated that RGD-binding integrins are also co-receptors for angiotensin-converting enzyme 2 (ACE2)136, which is used by SARS-CoV-2 for host cell entry; thus, integrin inhibitors could have utility in multiple types of viral infection137. In in vitro experiments, the noncompetitive α5β1 inhibitor, ATN-161, reduced infection138. Interestingly, Pliant Therapeutics is investigating its dual αvβ6/αvβ1 small-molecule inhibitor in a phase II clinical trial (NCT04565249) in patients with COVID-19 and acute respiratory distress syndrome (ARDS).
Drug design for RGD-binding integrins
Small-molecule inhibitor properties
Small molecules are important for two key reasons: first, as tools and probes139 (including imaging molecules; Table 4) and secondly as safe and efficacious marketed drugs. Tool molecules, ideally with suitable drug-like characteristics, can answer key mechanistic and biological questions in preclinical settings. For example, integrin inhibitors from UCSF, c8 (9), and from St Louis University, CWHM-12 (10), have been used extensively in target validation studies for fibrotic diseases. However, converting a tool or an early lead into a drug molecule is a challenging and time-consuming business, and all the knowledge and design is ultimately combined into a single entity, from perhaps many hundreds or even thousands of molecules profiled. Many of the recent integrin clinical candidates are small molecules that have undergone extensive optimization processes and can offer significant benefits, such as in activity and pharmacokinetic profiles, compared with other modalities, including antibodies or larger conjugate molecules. In this section, we therefore discuss the properties of these molecules in detail, the selectivity challenges and how emerging new research could alter future drug design.
It is evident from the low number of molecules that have been developed from research activities and are clinically successful that small-molecule integrin inhibitor drug design is not straightforward. However, for integrins, emerging insights from structural biology and pharmacology research may be game changing. Clearly, the validation of the target in clinically predictive disease models is crucial, but additionally, a key factor to now consider is how molecules affect the integrin conformational states and the relationship — if any — between these states and safety and efficacy. This relationship has not been routinely investigated and research is ongoing, but ideally such efforts would be part of any integrin lead optimization programme, although such endeavours are likely to be resource intensive. The conformational states induced by αvβ3 and αIIbβ3 inhibitors and subsequent signalling is potentially paralleled by other integrins, including other RGD-binding integrins and α4β7.
There are a substantial number of inhibitors described for integrins, a small subset of which are suitable for clinical evaluation. The vast majority of RGD-binding integrin inhibitors are RGD mimetics with physicochemical properties that are not compatible with oral bioavailability. Much of modern drug design is facilitated and informed by heuristics: Lipinski’s ‘Rule of Five’140,141 is perhaps the most widely used set of guidelines for drug design. Although these tools are blunt and simplified142, they are useful because they are easily applied and allow the rapid design of drug-like molecules. There is also a growing recognition that molecules outside Lipinski drug space, so called ‘beyond Rule of Five’, are of value for unusual or less tractable targets143,144. Compared with marketed oral drugs, most αv integrin inhibitors, including those that can be orally dosed, display molecular descriptors that conform less to the Rule of Five and Veber’s rules145 owing to the intrinsic polar nature of the RGD pharmacophore, with increased molecular weight, an increased number of hydrogen bond donors or acceptors, a higher number of rotatable bonds and larger polar surface area (PSA) (see Supplementary information for a plot of the physicochemical properties of integrin molecules compared with FDA marketed oral drugs). There are also large differences in the properties of integrin inhibitors according to the route of administration — those delivered parenterally (Fig. 2) and those delivered orally (Fig. 3). Only molecules from the parenteral class of RGD-binding integrin inhibitors have become marketed drugs (namely tirofiban and eptifibatide), which may reflect the challenges of obtaining high-quality orally bioavailable inhibitors in this physicochemical space (as discussed earlier). Compromises often have to be made to the preferred pharmacological profile to obtain molecules with sufficient permeability and/or bioavailability for clinical use. Approaches to mask the polarity as ester prodrugs have also enabled moderate oral bioavailability for several αIIbβ3-inhibitor small molecules146,147, which demonstrates that ionization, polarity and lipophilicity are important for oral absorption of integrin inhibitors. Although prodrugs are more complex to progress to the market than single drug entities, they can maintain the key potency and selectivity requirements that may otherwise be compromised in favour of oral absorption.
RGD versus non-RGD
Almost all RGD-binding integrin inhibitors are RGD mimetics featuring a mimetic for the guanidine of arginine and a β-arylpropionic acid or α-amino-carboxylic acid to replace the aspartic acid72 (Figs 2,3). Such compounds are zwitterionic or amphoteric and thus usually charged at physiological pH; this constrains molecular design, and obtaining good oral pharmacokinetics can be challenging. We are unaware of any small-molecule modulators of αv integrins that bind outside the orthosteric ligand-binding site, although several large molecules — including an αvβ6 antibody (BG00011, 3G9; Biogen)81 and ProAgio (an αvβ3-binding protein)148 — have been described. Also, as previously mentioned, ATN-161 is a small-molecule peptide that binds outside the RGD-binding site of α5β1. Consequently, new chemotypes that are non-zwitterionic — either basic or acidic but not both — would be useful. Such chemotypes would have different physicochemical characteristics from traditional RGD mimetics, and would almost certainly change the nature of the pharmacokinetic design challenge. However, few non-RGD-mimetic compounds have progressed. Many feature functionality that is incompatible with oral bioavailability or are unlikely to demonstrate high passive permeability, and some include structural motifs that are found in pan-assay interference compounds (PAINS)149.
RUC-4 (12) is a notable exception. This αIIbβ3 inhibitor does not have a carboxylic acid (which otherwise forms a strong interaction with the magnesium ion in the binding site), and thus is basic and has non-zwitterionic properties. In the ligand binding site, the primary amine displaces the magnesium ion, locking the receptor in an inactive conformation62,63. In addition, GlaxoSmithKline (GSK) designed potent and selective αvβ1 inhibitors150 that, whilst conforming to the general RGD-mimetic motif, were non-zwitterionic, featuring a phenyl urea as a non-basic arginine mimetic. Non-zwitterionic molecules may offer good permeability and absorption while still acting as ligands in the RGD-binding site.
Numerous approaches to identify new small-molecule chemotypes can be considered. Structure-based design could be useful because αvβ3 and αvβ6 crystal structures, as well as homology models for other RGD-binding integrins, are available151. Crystal structures of integrins in the inactive unbound forms through to the activated ligand-bound forms have been published and these structures have elucidated fundamental principles of integrin activation4,49,50,61,62,67,68,152,153. The conformation of the tertiary structure of integrins can change considerably, although the actual binding site regions change less dramatically. Recent publications suggest that ligands for different conformations of αvβ3 and αIIbβ3 can be designed, including peptidic pure antagonists67 or small molecules60–62. It remains to be seen what effect these compounds have clinically, but pure antagonism of these integrins could overcome previously encountered problems and thus trigger significant renewed interest. In our own experience, however, even if the potency and selectivity of RGD-integrin inhibitors can be rationalized from modelling studies in hindsight, the design of inhibitors de novo from modelling and docking studies is challenging154. Similarly, despite NMR studies that identify ligand-binding interactions with αvβ6 (ref.155), αIIbβ3 (ref.156) and αvβ3 (ref.157) in a cellular environment — which more closely reproduces physiological binding — these studies have predominantly provided insights retrospectively.
Combined computational and NMR studies enabled the design of a potent and selective αvβ6 small peptide from a nonapeptide158. In this novel approach, a computationally driven algorithm, using a docking score as proxy for binding activity, successfully predicted active analogues. But by far the most common and successful approach (based on new patent filings) has used existing, published knowledge159. As a result, many of these chemotypes look similar to each other, and the structural space is becoming crowded.
Selectivity
The desired integrin selectivity can greatly affect the design of integrin inhibitors. No αv-targeting molecules are clinically proven, so pharmacological effects cannot be assigned to specific selectivity profiles. In treating fibrotic diseases, for example, it is unclear which αv-containing integrins are pivotal in any particular disease in humans and this is complicated by differing integrin expression levels across the tissues and organs, and in animal models. Validation studies conducted with non-selective tools may therefore shed little light on the precise selectivity requirements to treat a specific disease. However, because non-selective molecules appear to be safe in the clinic (Table 3), investing the time and resources to identify the specific selectivity profile in validation studies may not always be necessary. For some applications, though, selectivity is probably important.
Many inhibitors of αv-containing integrins share key binding characteristics72. The homologous RGD pharmacophore recognition sequence in these integrin binding sites, especially in integrins in which the α-subunit binding domains or the β-subunits are similar, makes the design of highly selective small molecules difficult, even with the help of ligand–protein X-ray crystal structures and the construction of homology models. As a result, a range of selectivity profiles is attainable, but designing molecules that target one specific integrin and not others is challenging.
Inhibitors of αv integrins can often be assigned to one of four broad ‘selectivity buckets’, which indicate likely selectivity profiles: selective dual αvβ3 and αvβ5 inhibitors; pan-αv inhibitors; selective dual αvβ6 and αvβ8 inhibitors; and selective αvβ1 inhibitors (selective for αvβ1 over the other αv-containing integrins). Molecules in this final group may inhibit other β1-containing integrins, and it is too early to assign additional αv selectivity patterns to these inhibitors, but will also constitute a bucket. Although most compounds fall into one of these buckets, the selectivity profile of a compound may have been either intentional or merely tolerated. Obtaining selectivity for αvβ3 and αvβ5 over αvβ6 and αvβ8 or vice versa is relatively straightforward, but obtaining selectivity within these buckets or pairs is challenging, owing to the subtle differences in binding site architecture, and is less well understood. However, for examples where selectivity has indeed been achieved within a bucket, changes are often made to the parts of the molecule that bind to the ‘specificity-determining loop’72.
Few small molecules with selectivity for αvβ5 (particularly over αvβ3) or αvβ8 (particularly over αvβ6) exist, although an acyclic peptide (8)160 that is selective for αvβ8, and αvβ5-selective molecules161, have been identified. Interestingly, the current clinical molecule, SF0166 (11), is reported to be selective for αvβ3 over αvβ5 by virtue of only small structural changes34. Additionally, a few αvβ6 inhibitors have good selectivity over αvβ8, such as compounds from GSK (5, 6)154,162. Antibodies that bind to the various RGD integrins have already been described and have a range of selectivity profiles72.
Interestingly, some of the small-molecule αvβ3 inhibitor lead molecules came from αIIbβ3 compounds and some of the αvβ6 leads originated from in-house αvβ3 leads163, so molecules with different selectivity profiles from the desired profile can be useful starting points.
Given the current clinical potential of αvβ6 inhibitors, can selective αvβ6 small-molecule inhibitors be developed using the wealth of information already available for published αvβ3 molecules? Some specific design tricks could be used to switch selectivity. Many αvβ6 inhibitors (for example, molecules 5, 15 and 18) have basic linker regions, whereas αvβ3/αvβ5 inhibitors (for example, 13 and 14) often have neutral, non-ionizable linkers. The basic group interacts with a threonine residue that is present in αvβ6 but absent in αvβ3 and αvβ5. The molecule (6), which is analogous to the inhaled candidate (5), contains a methyl quaternized nitrogen162 in the linker region, and the presence of this ionized nitrogen increases the potency and selectivity for αvβ6. Unfortunately, these advantages come at the expense of low permeability and reduced oral bioavailability, so this molecule (6) and similar molecules are more suitable for parenteral administration. As with all small-molecule drug design, the properties of molecules are intertwined, and structural changes to optimize selectivity may make optimization of other parameters, such as oral bioavailability, more challenging.
Similarly, certain structural motifs have affinity for specific subunits. The bulky sulfonamide contained in (9), or similar analogues, binds strongly to the β1-subunit and consequently this molecule interacts with several other β1-containing integrins, such as α4β1 (ref.164), α2β1 (ref.165), and αvβ1 (ref.166).
In the absence of an αvβ1-selective antibody, small-molecule tools (such as 9) have proved invaluable for initial target validation studies in fibrotic diseases. However, because it also binds to other β1-containing integrins, this molecule may be less useful to pinpoint the importance of specific integrins as relevant targets74. This integrin cross-reactivity highlights the need for comprehensive cross-screening. However, such screening is less available: unlike kinase screening panels, which are commercially available (example firms are MRC PPU and Eurofins; see Related links); integrin screening panels are not. Furthermore, the observed potency and selectivity of ligands also depend on the type of assay, conditions and set-up, which can make inter-compound comparisons less robust. We recently tested a comprehensive set of tools that bind αv-containing integrins in the same assays72, and the profiles of peptidic tools have also been compared167.
Several molecules have activity at multiple αv integrins, including αvβ6, and are orally bioavailable. Such molecules have been developed by Pliant (15)168, GSK (18)169 and St Louis University (19)170, and show good permeability. In each of these examples, the linker is either neutral, or the pKa of the basic nitrogen in the linker is modulated by virtue of a proximal fluorine or ether functional group. Molecular conformational flexibility can also allow intramolecular folding to ‘hide’ ionized functionality171,172, but this can complicate the molecular design even further.
Clinical data
The key integrin inhibitors in ongoing clinical studies are summarized in Table 2 and those that have completed clinical trials are listed in Table 3 with highest clinical dose, safety and efficacy information. The majority of studies target or have targeted the αv-containing integrins, particularly αvβ6 and αvβ3. The most advanced clinical molecule currently in clinical trials is the oral dual αvβ1/αvβ6 inhibitor, PLN-74809 (15; Pliant Therapeutics), which is currently in phase II for IPF (NCT04072315). Targeting the lung specifically has also been investigated with the inhaled and selective αvβ6 small molecule GSK3008348 (5; GSK)71 but this programme has been strategically placed on hold, despite observed target engagement in a phase Ib study of IPF using an imaging readout173 (Table 4). The development of the αvβ6-selective antibody from Biogen (BG00011) was recently terminated because of undisclosed safety concerns174, and the development of the pan-αv integrin inhibitor IDL-2965 (NCT03949530) for NASH was also terminated. It remains to be seen whether these setbacks will deter companies such as GSK, Pliant Therapeutics, Bristol-Myers Squibb and Morphic Therapeutics, which hold patents describing molecules with varying αv activities (molecules 15–18)159.
Both pan-αv antibodies and small molecules appear to be well tolerated in humans (Table 3), although the lack of efficacy for several molecules suggests that target engagement may have been insufficient to test for potential mechanism-based toxicity. Although the clinical data from BG00011, which was terminated for undisclosed safety reasons, raises potential safety concerns about selective αvβ6 inhibition, small doses of the inhaled selective inhibitor 5 were well tolerated. It is possible that sustained αvβ6 inhibition detrimentally affects the anti-inflammatory and protective roles of this integrin, and in an already compromised fibrotic lung, this could become a risk. The corollary, therefore, is that there is only a small therapeutic window for targeting αvβ6 in the lung, so the dose and therapeutic modality are particularly important. A study in chronic allograft dysfunction was also withdrawn for BG00011 (then named STX-100, NCT00878761) before recruitment of patients, potentially as a result of preclinical work indicating a protective role for αvβ6 (ref.175).
Although the marketed drug abciximab therapeutically targets αIIbβ3, it also appreciably inhibits αvβ3 (ref.176), adding further evidence that targeting αvβ3 is safe in humans. Cilengitide, too, has been widely explored clinically and seems to have failed for efficacy rather than safety reasons. Although the dosing route is not correlated with toxicity, inhaled dosing may be advantageous for treating diseases of the lung, as it could result in lower systemic drug levels.
As for other therapeutic areas, the generation of translational biomarkers, the demonstration of target engagement and the development of pharmacokinetic and pharmacodynamic relationships are key to understanding the chances of success. In the integrin field, there are few historical studies in which these characteristics have been evaluated, but studies have recently begun to take this translational approach. For example, the target engagement of GSK3008348 (5) was demonstrated using an αvβ6-specific radiolabelled peptide ligand in bleomycin-treated mice via single-photon emission computerized tomography (SPECT)71 and confirmed in patients with IPF via positron emission tomography (PET)173. In addition, the functional consequences of αvβ6 integrin inhibition (using levels of phosphorylated SMAD, a marker for TGFβ activation) have been measured in bronchoalveolar lavage fluid from healthy subjects or patients with IPF, and used as a pharmacodynamic measurement to optimize the dose177.
Reagents to image αv-containing integrins (Table 4) have been used to define integrin expression levels and to determine whether a drug engages its target. As integrins themselves could have prognostic value — for example, levels of αvβ6 in IPF and cancer — imaging an integrin in patients could be used to track disease progression. This approach could also be used to select for patients with either high levels of target expression or rapidly progressing forms of disease. For example, a retrospective analysis of αvβ6 expression in patients with colorectal carcinoma who received the pan-αv antibody abituzumab suggested that individuals with high expression levels were more likely to benefit from the therapy106.
Challenges and prospects
Considering how many drug discovery projects and clinical studies have focused on integrins over the past 30 years, the number of approved therapies has been disappointing5,26. Translating the potential, and indeed the validity, of the preclinical integrin data into clinically efficacious drugs is clearly not without complexity. These clinical failures likely reflect a combination of challenges that are generic to drug development and those that are target-class specific. Many of the integrin inhibitors that were tested historically had suboptimal pharmacokinetics, with routes of administration and dosing regimens that led to poor exposure and target coverage, which is a generic challenge for small-molecule drug development14,72. This, coupled with a lack of clinical pharmacodynamic biomarkers to measure target engagement, left the relevance of integrins in the disease of interest unknown, as molecules often failed to test the hypothesized mechanism. Conversely, target coverage as a result of poor pharmacokinetics was less of an issue for the large molecules (such as antibodies) that were tested in the clinic, and indeed these have arguably shown more potential. A retrospective analysis of the abituzumab clinical trial data, for example, suggested that the drug had some clinical benefit in patients with higher expression of αvβ6 (ref.106). Therefore, identifying patients with higher target expression levels might improve success, and although this seems be a logical approach for any target class, it has rarely been employed for integrins. This is probably because, in practice, it is difficult to measure expression levels in the clinic without negatively affecting patient recruitment and the time needed to complete studies, and because the ultimate market for the drug will be smaller if the label granted is narrowed to only those patients who express high levels of integrins.
As with any drug discovery area, some potentially interesting compounds have not progressed because of strategic decisions. Even if a compound has demonstrated some potential and there is a clear next step, the programme may no longer be attractive. Projects can also be placed on hold or terminated if a company refocuses their goals, as when AstraZeneca shelved their dual αvβ6/αvβ8 monoclonal antibody (RAD264)178, or loses confidence in a target because of negative clinical results or safety concerns from competitor trials.
A further generic challenge is the translation of preclinical in vivo pharmacodynamic studies to the clinic. These studies are still of value for investigating pharmacokinetic and pharmacodynamic relationships in a whole-body system, but their capacity to accurately represent human biology, especially human diseases, is often questionable and the data generated can therefore become supportive instead of essential179. Even safety studies in animal models of the disease are not ideal because the models themselves are not necessarily translatable to the human disease and the animals can be highly compromised. These limitations to preclinical pharmacodynamic and safety studies can result in on-target safety issues in the clinic, such as those observed with the α4 inhibitors and the αvβ6 monoclonal antibody BG00011, which can be difficult to predict or discover early in the process. Studies in diseased human tissue, especially in fibrosis, may therefore further aid validation and translation as more relevant disease systems and surrogates are developed71,180,181.
There are also class-specific challenges to therapeutically targeting integrins. The lack of selective drug-like tools for this target class, especially because the same subunit is part of multiple different integrins and there are many subunits within integrin subfamilies, has made interpretation and therefore translation of preclinical data difficult. Similarly, measuring expression levels of integrins within these subfamilies is difficult if heterodimer-specific antibodies do not exist. This makes correlating expression levels of the key integrins in the organ of interest in in vivo models with levels in diseased human tissue a challenge, which likely contributes to the failures in this field. Developing small molecules with high affinity and selectivity for an integrin, while maintaining the required physicochemical properties and optimal pharmacokinetic profile for oral dosing, has also been difficult. Even circumventing this with large molecules, such as biologics, is not always successful.
New target validation tools for drug discovery using technologies based on genomics, transcriptomics, proteomics and metabolomics will allow more detailed and global analyses of disease cohorts. For example, genome-wide association studies (GWAS) can identify genetic variations that occur more frequently in people with a disease than in those without. Targets with genetic associations are estimated to be twice as likely to succeed in clinical development182. Although there are few associations between diseases and changes in integrin-related genes, there is an indirect association between αvβ6 and IPF. A variation of AKAP13 was identified as a potential genetic risk factor in IPF183, and AKAP13 mediates activation of TGFβ downstream of αvβ6 (ref.184). Although these tools have yet to be fully applied to integrin target validation, they will undoubtedly be an important part of this process in the future.
Although integrins fell out of favour as targets for drug development, there has been renewed interest and investment in the integrins as drug targets, particularly for fibrotic diseases. To be successful, current integrin programmes should note the key lessons from the many integrin-targeted trials that have already been completed. Future integrin-targeted programmes should focus on the development of molecules that enable hypothesized mechanisms to be fully tested; robust target validation; clinical studies that measure target engagement; and translational biomarkers to measure clinical efficacy. With these changes, indications including fibrosis and cancer, which were previously targeted with little success, may be re-evaluated with improved therapeutic agents.
Supplementary information
Acknowledgements
The authors thank Megan Cully for editorial support.
Author contributions
R.J.S., J.A.R and S.J.F.M. and R.J.D.H. researched data, discussed the content, wrote the article and edited/reviewed the manuscript before submission. R.G.J. edited/reviewed the manuscript before submission.
Competing interests
R.J.D.H. and S.J.F.M. hold GSK shares and are applicants on GSK integrin patents. R.J.S. and J.A.R. hold GSK shares and are currently Galecto, Inc. employees and shareholders. R.G.J. reports grants from GlaxoSmithKline, grants and personal fees from Pliant Therapeutics, grants from Biogen, during the conduct of the study; personal fees from Galapagos, other from Galecto, personal fees and other from GlaxoSmithKline, personal fees and other from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Pliant, personal fees from Bristol-Myers Squibb, personal fees from Chiesi, personal fees from Roche/Promedior, personal fees and other from RedX, other from NuMedii, other from Nordic Biosciences, personal fees from Veracyte, personal fees from PatientMPower, personal fees from Resolution Therapeutics, personal fees from Vicore, outside the submitted work; he is supported by a National Institute of Health Research Professorship (NIHR ref: RP-2017-08-ST2-014). He is a trustee for Action for Pulmonary Fibrosis.
Footnotes
Peer review information
Nature Reviews Drug Discovery thanks Scott Turner, Zhiyuan Zhong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Eurofins kinase screening: https://www.eurofinsdiscoveryservices.com/services/in-vitro-assays/kinases/screening-profiling-services/
MRC PPU kinase screening: http://www.kinase-screen.mrc.ac.uk/
Supplementary information
The online version contains supplementary material available at 10.1038/s41573-021-00284-4.
References
- 1.Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 3.Faull RJ, Ginsberg MH. Inside-out signaling through integrins. J. Am. Soc. Nephrol. 1996;7:1091–1097. doi: 10.1681/ASN.V781091. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, et al. Requirement of alpha and beta subunit transmembrane helix separation for integrin outside-in signaling. Blood. 2007;110:2475–2483. doi: 10.1182/blood-2007-03-080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell. Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klapholz B, Brown NH. Talin — the master of integrin adhesions. J. Cell Sci. 2017;130:2435–2446. doi: 10.1242/jcs.190991. [DOI] [PubMed] [Google Scholar]
- 7.Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 2013;14:503–517. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J. Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruoslahti E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 10.Gullberg DE, Lundgren-Akerlund E. Collagen-binding I domain integrins — what do they do? Prog. Histochem. Cytochem. 2002;37:3–54. doi: 10.1016/s0079-6336(02)80008-0. [DOI] [PubMed] [Google Scholar]
- 11.Aumailley M. The laminin family. Cell Adh. Migr. 2013;7:48–55. doi: 10.4161/cam.22826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeltz C, Gullberg D. The integrin-collagen connection — a glue for tissue repair? J. Cell Sci. 2016;129:653–664. doi: 10.1242/jcs.180992. [DOI] [PubMed] [Google Scholar]
- 13.Stipp CS. Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets. Expert Rev. Mol. Med. 2010;12:e3. doi: 10.1017/S1462399409001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox D. How not to discover a drug — integrins. Expert Opin. Drug Discov. 2020;16:197–211. doi: 10.1080/17460441.2020.1819234. [DOI] [PubMed] [Google Scholar]
- 15.Fullard JF. The role of the platelet glycoprotein IIb/IIIa in thrombosis and haemostasis. Curr. Pharm. Des. 2004;10:1567–1576. doi: 10.2174/1381612043384682. [DOI] [PubMed] [Google Scholar]
- 16.Perez VL, Pflugfelder SC, Zhang S, Shojaei A, Haque R. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul. Surf. 2016;14:207–215. doi: 10.1016/j.jtos.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Mitroulis I, et al. Leukocyte integrins: role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol. Ther. 2015;147:123–135. doi: 10.1016/j.pharmthera.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon FH, et al. A randomized placebo-controlled trial of a humanized monoclonal antibody to alpha4 integrin in active Crohn’s disease. Gastroenterology. 2001;121:268–274. doi: 10.1053/gast.2001.26260. [DOI] [PubMed] [Google Scholar]
- 19.Léger OJ, et al. Humanization of a mouse antibody against human alpha-4 integrin: a potential therapeutic for the treatment of multiple sclerosis. Hum. Antibodies. 1997;8:3–16. [PubMed] [Google Scholar]
- 20.Biogen reports record revenues for both the full year and fourth quarter of 2017, $12.3 billion and $3.3 billion, respectively. Businesswirehttps://www.businesswire.com/news/home/20180125005353/en/Biogen-Reports-Record-Revenues-Full-Year-Fourth (2018).
- 21.Tong, A. Takeda reports second PhIII win for subcutaneous Entyvio as regulators review expanded use. Endpointshttps://endpts.com/takeda-reports-second-phiii-win-for-subcutaneous-entyvio-as-regulators-review-expanded-use/ (2019).
- 22.Raab-Westphal S, Marshall JF, Goodman SL. Integrins as therapeutic targets: successes and cancers. Cancers. 2017;9:110. doi: 10.3390/cancers9090110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie B, et al. Idiopathic pulmonary fibrosis registry china study (PORTRAY): protocol for a prospective, multicentre registry study. BMJ Open. 2020;10:e036809. doi: 10.1136/bmjopen-2020-036809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abeysekera K, et al. Prevalence of steatosis and fibrosis in young adults in the UK: a population-based study. Lancet Gastroenterol. Hepatol. 2020;5:295–305. doi: 10.1016/S2468-1253(19)30419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar CC. Signaling by integrin receptors. Oncogene. 1998;17:1365–1373. doi: 10.1038/sj.onc.1202172. [DOI] [PubMed] [Google Scholar]
- 26.Goodman SL, Picard M. Integrins as therapeutic targets. Trends Pharmacol. Sci. 2012;33:405–412. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedlander M, et al. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc. Natl Acad. Sci. USA. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilder RL. Integrin alpha V beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Ann. Rheum. Dis. 2002;61:ii96–ii99. doi: 10.1136/ard.61.suppl_2.ii96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura I, Duong LT, Rodan SB, Rodan GA. Involvement of alpha(v)beta3 integrins in osteoclast function. J. Bone Miner. Metab. 2007;25:337–344. doi: 10.1007/s00774-007-0773-9. [DOI] [PubMed] [Google Scholar]
- 31.Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br. J. Cancer. 2004;90:561–565. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Hove, I. et al. Targeting RGD-binding integrins as an integrative therapy for diabetic retinopathy and neovascular age-related macular degeneration. Prog. Retin. Eye Res.10.1016/j.preteyeres.2021.100966 (2021). [DOI] [PubMed]
- 33.Bhatwadekar AD, Kansara V, Luo Q, Ciulla T. Anti-integrin therapy for retinovascular diseases. Expert Opin. Investig. Drugs. 2020;29:935–945. doi: 10.1080/13543784.2020.1795639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Askew BC, Furuya T, Edwards DS. Ocular distribution and pharmacodynamics of SF0166, a topically administered αvβ3 integrin antagonist, for the treatment of retinal diseases. J. Pharmacol. Exp. Ther. 2018;366:244–250. doi: 10.1124/jpet.118.248427. [DOI] [PubMed] [Google Scholar]
- 35.Zahn G, et al. Preclinical evaluation of the novel small-molecule integrin alpha5beta1 inhibitor JSM6427 in monkey and rabbit models of choroidal neovascularization. Arch. Ophthalmol. 2009;127:1329–1335. doi: 10.1001/archophthalmol.2009.265. [DOI] [PubMed] [Google Scholar]
- 36.Silva R, et al. Tyrosine kinase blocking collagen IV-derived peptide suppresses ocular neovascularization and vascular leakage. Sci. Transl. Med. 2017;9:eaai8030. doi: 10.1126/scitranslmed.aai8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirando AC, et al. A collagen IV-derived peptide disrupts α5β1 integrin and potentiates Ang2/Tie2 signaling. JCI Insight. 2019;4:e122043. doi: 10.1172/jci.insight.122043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alday-Parejo B, Stupp R, Rüegg C. Are integrins still practicable targets for anti-cancer therapy? Cancers. 2019;11:978. doi: 10.3390/cancers11070978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stupp R, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100–1108. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 40.Mason WP. End of the road: confounding results of the CORE trial terminate the arduous journey of cilengitide for glioblastoma. Neuro. Oncol. 2015;17:634–635. doi: 10.1093/neuonc/nov018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hersey P, et al. A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin alpha(v)beta(3), + or − dacarbazine in patients with stage IV metastatic melanoma. Cancer. 2010;116:1526–1534. doi: 10.1002/cncr.24821. [DOI] [PubMed] [Google Scholar]
- 42.Cirkel GA, et al. A dose escalating phase I study of GLPG0187, a broad spectrum integrin receptor antagonist, in adult patients with progressive high-grade glioma and other advanced solid malignancies. Invest. New Drugs. 2016;34:184–192. doi: 10.1007/s10637-015-0320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shergalis A, Bankhead A, III, Luesakul U, Muangsin N, Neamati N. Current challenges and opportunities in treating glioblastoma. Pharmacol. Rev. 2018;70:412–445. doi: 10.1124/pr.117.014944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa AG, Cusano NE, Silva BC, Cremers S, Bilezikian JP. Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nat. Rev. Rheumatol. 2011;7:447–456. doi: 10.1038/nrrheum.2011.77. [DOI] [PubMed] [Google Scholar]
- 45.Ammar MJ, Hsu J, Chiang A, Ho AC, Regillo CD. Age-related macular degeneration therapy: a review. Curr. Opin. Ophthalmol. 2020;31:215–221. doi: 10.1097/ICU.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 46.Hosen N, et al. The activated conformation of integrin β7 is a novel multiple myeloma-specific target for CAR T cell therapy. Nat. Med. 2017;23:1436–1443. doi: 10.1038/nm.4431. [DOI] [PubMed] [Google Scholar]
- 47.Wallstabe L, et al. CAR T cells targeting αvβ3 integrin are effective against advanced cancer in preclinical models. Adv. Cell Gene Ther. 2018;1:e11. doi: 10.1002/acg2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whilding LM, et al. Targeting of aberrant αvβ6 integrin expression in solid tumors using chimeric antigen receptor-engineered T cells. Mol. Ther. 2017;25:259–273. doi: 10.1016/j.ymthe.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Y, Schürpf T, Springer TA. How natalizumab binds and antagonizes α4 integrins. J. Biol. Chem. 2013;288:32314–32325. doi: 10.1074/jbc.M113.501668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramsay AG, et al. HS1-associated protein X-1 regulates carcinoma cell migration and invasion via clathrin-mediated endocytosis of integrin alphavbeta6. Cancer Res. 2007;67:5275–5284. doi: 10.1158/0008-5472.CAN-07-0318. [DOI] [PubMed] [Google Scholar]
- 52.Bassler N, et al. A mechanistic model for paradoxical platelet activation by ligand-mimetic alphaIIb beta3 (GPIIb/IIIa) antagonists. Arterioscler. Thromb. Vasc. Biol. 2007;27:e9–e15. doi: 10.1161/01.ATV.0000255307.65939.59. [DOI] [PubMed] [Google Scholar]
- 53.Aster RH, Curtis BR, McFarland JG, Bougie DW. Drug-induced immune thrombocytopenia: pathogenesis, diagnosis, and management. J. Thromb. Haemost. 2009;7:911–918. doi: 10.1111/j.1538-7836.2009.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat. Rev. Drug Discov. 2016;15:173–183. doi: 10.1038/nrd.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cannon CP, et al. Oral glycoprotein IIb/IIIa inhibition with orbofiban in patients with unstable coronary syndromes (OPUS-TIMI 16) trial. Circulation. 2000;102:149–156. doi: 10.1161/01.cir.102.2.149. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds AR, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat. Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 57.Weis SM, Stupack DG, Cheresh DA. Agonizing integrin antagonists? Cancer Cell. 2009;15:359–361. doi: 10.1016/j.ccr.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Ehlert FJ, Suga H, Griffin MT. Analysis of agonism and inverse agonism in functional assays with constitutive activity: estimation of orthosteric ligand affinity constants for active and inactive receptor states. J. Pharmacol. Exp. Ther. 2011;338:671–686. doi: 10.1124/jpet.111.179309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong PP, et al. Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer Cell. 2015;27:123–137. doi: 10.1016/j.ccell.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 60.Li J, et al. Novel pure αVβ3 integrin antagonists that do not induce receptor extension, prime the receptor, or enhance angiogenesis at low concentrations. ACS Pharmacol. Transl. Sci. 2019;2:387–401. doi: 10.1021/acsptsci.9b00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu J, et al. Closed headpiece of integrin αIIbβ3 and its complex with an αIIbβ3-specific antagonist that does not induce opening. Blood. 2010;116:5050–5059. doi: 10.1182/blood-2010-04-281154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu J, et al. Structure-guided design of a high-affinity platelet integrin αIIbβ3 receptor antagonist that disrupts Mg²+ binding to the MIDAS. Sci. Transl. Med. 2012;4:125ra32. doi: 10.1126/scitranslmed.3003576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J, et al. RUC-4: a novel αIIbβ3 antagonist for prehospital therapy of myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2014;34:2321–2329. doi: 10.1161/ATVBAHA.114.303724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.CeleCor initiates CELEBRATE, a pivotal clinical trial of subcutaneously administered Zalunfiban (RUC-4) in STEMI patients treated in ambulances. https://www.celecor.com/blog/https/wwwcelecorcom (2021).
- 65.Kereiakes DJ, et al. First human use of RUC-4: a nonactivating second-generation small-molecule platelet glycoprotein IIb/IIIa (integrin αIIbβ3) inhibitor designed for subcutaneous point-of-care treatment of ST-segment-elevation myocardial infarction. J. Am. Heart Assoc. 2020;9:e016552. doi: 10.1161/JAHA.120.016552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morphic Therapeutic. MINT platform and strategy. https://morphictx.com/our-technology/mint-platform-and-strategy (2018).
- 67.Van Agthoven JF, et al. Structural basis for pure antagonism of integrin αVβ3 by a high-affinity form of fibronectin. Nat. Struct. Mol. Biol. 2014;21:383–388. doi: 10.1038/nsmb.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adair BD, et al. Structure-guided design of pure orthosteric inhibitors of αIIbβ3 that prevent thrombosis but preserve hemostasis. Nat. Commun. 2020;11:398. doi: 10.1038/s41467-019-13928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuo YJ, Chen YR, Hsu CC, Peng HC, Huang TF. An αIIbβ3 antagonist prevents thrombosis without causing Fc receptor γ-chain IIa-mediated thrombocytopenia. J. Thromb. Haemost. 2017;15:2230–2244. doi: 10.1111/jth.13803. [DOI] [PubMed] [Google Scholar]
- 70.Marelli UK, Rechenmacher F, Sobahi TR, Mas-Moruno C, Kessler H. Tumor targeting via integrin ligands. Front. Oncol. 2013;3:222. doi: 10.3389/fonc.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.John AE, et al. Translational pharmacology of an inhaled small molecule αvβ6 integrin inhibitor for idiopathic pulmonary fibrosis. Nat. Commun. 2020;11:4659. doi: 10.1038/s41467-020-18397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hatley R, et al. An αv-RGD integrin inhibitor toolbox: drug discovery insight, challenges and opportunities. Angew. Chem. Int. Ed. Engl. 2018;57:3298–3321. doi: 10.1002/anie.201707948. [DOI] [PubMed] [Google Scholar]
- 73.Arrowsmith CH, et al. The promise and peril of chemical probes. Nat. Chem. Biol. 2015;11:536–541. doi: 10.1038/nchembio.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilkinson AL, Barrett JW, Slack RJ. Pharmacological characterisation of a tool αvβ1 integrin small molecule RGD-mimetic inhibitor. Eur. J. Pharmacol. 2019;842:239–247. doi: 10.1016/j.ejphar.2018.10.045. [DOI] [PubMed] [Google Scholar]
- 75.Reed NI, et al. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci. Transl. Med. 2015;7:288ra79. doi: 10.1126/scitranslmed.aaa5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scotton CJ, Chambers RC. Bleomycin revisited: towards a more representative model of IPF? Am. J. Physiol. Lung Cell Mol. Physiol. 2010;299:L439–L441. doi: 10.1152/ajplung.00258.2010. [DOI] [PubMed] [Google Scholar]
- 77.Horan GS, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am. J. Respir. Crit. Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 78.Saini G, et al. αvβ6 integrin may be a potential prognostic biomarker in interstitial lung disease. Eur. Respir. J. 2015;46:486–494. doi: 10.1183/09031936.00210414. [DOI] [PubMed] [Google Scholar]
- 79.Weinreb PH, et al. Function-blocking integrin alphavbeta6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J. Biol. Chem. 2004;279:17875–17887. doi: 10.1074/jbc.M312103200. [DOI] [PubMed] [Google Scholar]
- 80.Stephens LE, et al. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 81.Zhang J, et al. Discovery of a new class of integrin antibodies for fibrosis. Sci. Rep. 2021;11:2118. doi: 10.1038/s41598-021-81253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim KK, et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J. Clin. Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Q, et al. Effect of antibody against integrin alpha4 on bleomycin-induced pulmonary fibrosis in mice. Biochem. Pharmacol. 2000;60:1949–1958. doi: 10.1016/s0006-2952(00)00491-3. [DOI] [PubMed] [Google Scholar]
- 84.Volkert G, et al. Contribution of the α8 integrin chain to the expression of extracellular matrix components. Cell Commun. Adhes. 2014;21:89–98. doi: 10.3109/15419061.2013.876012. [DOI] [PubMed] [Google Scholar]
- 85.Kitamura H, et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J. Clin. Invest. 2011;121:2863–2875. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Popov Y, et al. Integrin alphavbeta6 is a marker of the progression of biliary and portal liver fibrosis and a novel target for antifibrotic therapies. J. Hepatol. 2008;48:453–464. doi: 10.1016/j.jhep.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 87.Henderson NC, et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 2013;19:1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang B, et al. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46:1404–1412. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ulmasov B, et al. An inhibitor of arginine-glycine-aspartate-binding integrins reverses fibrosis in a mouse model of nonalcoholic steatohepatitis. Hepatol. Commun. 2018;3:246–261. doi: 10.1002/hep4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klinkhammer BM, Goldschmeding R, Floege J, Boor P. Treatment of renal fibrosis-turning challenges into opportunities. Adv. Chronic Kidney Dis. 2017;24:117–129. doi: 10.1053/j.ackd.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 91.Hahm K, et al. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am. J. Pathol. 2007;170:110–125. doi: 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang Y, et al. Pharmacologic blockade of αvβ1 integrin ameliorates renal failure and fibrosis in vivo. J. Am. Soc. Nephrol. 2017;28:1998–2005. doi: 10.1681/ASN.2015050585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma LJ, et al. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(−/−) mice. Am. J. Pathol. 2003;163:1261–1273. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cox, J. M. et al. Composition and methods for treating chronic kidney disease. US 20190307735 (2019).
- 95.Zhou X, et al. An integrin antagonist (MK-0429) decreases proteinuria and renal fibrosis in the ZSF1 rat diabetic nephropathy model. Pharmacol. Res. Perspect. 2017;5:e00354. doi: 10.1002/prp2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gerber EE, et al. Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature. 2013;503:126–130. doi: 10.1038/nature12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bagnato GL, et al. Dual αvβ3 and αvβ5 blockade attenuates fibrotic and vascular alterations in a murine model of systemic sclerosis. Clin. Sci. 2018;132:231–242. doi: 10.1042/CS20171426. [DOI] [PubMed] [Google Scholar]
- 98.Katsumoto TR, Violette SM, Sheppard D. Blocking TGFβ via inhibition of the αvβ6 integrin: a possible therapy for systemic sclerosis interstitial lung disease. Int. J. Rheumatol. 2011;2011:208219. doi: 10.1155/2011/208219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat. Rev. Cancer. 2018;18:533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015;25:234–240. doi: 10.1016/j.tcb.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nieberler M, et al. Exploring the role of RGD-recognizing integrins in cancer. Cancers. 2017;9:116. doi: 10.3390/cancers9090116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bates RC, et al. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J. Clin. Invest. 2005;115:339–347. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elayadi AN, et al. A peptide selected by biopanning identifies the integrin alphavbeta6 as a prognostic biomarker for nonsmall cell lung cancer. Cancer Res. 2007;67:5889–5895. doi: 10.1158/0008-5472.CAN-07-0245. [DOI] [PubMed] [Google Scholar]
- 104.Hazelbag S, et al. Overexpression of the alpha v beta 6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J. Pathol. 2007;212:316–324. doi: 10.1002/path.2168. [DOI] [PubMed] [Google Scholar]
- 105.Zhang ZY, et al. Integrin alphavbeta6 acts as a prognostic indicator in gastric carcinoma. Clin. Oncol. 2008;20:61–66. doi: 10.1016/j.clon.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 106.Élez E, et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial. Ann. Oncol. 2015;26:132–140. doi: 10.1093/annonc/mdu474. [DOI] [PubMed] [Google Scholar]
- 107.O’Day S, et al. CNTO 95 Investigators. A randomised, phase II study of intetumumab, an anti-αv-integrin mAb, alone and with dacarbazine in stage IV melanoma. Br. J. Cancer. 2011;105:346–352. doi: 10.1038/bjc.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Keat N, et al. A microdose PET study of the safety, immunogenicity, biodistribution, and radiation dosimetry of 18F-FB-A20FMDV2 for imaging the integrin αvβ6. J. Nucl. Med. Technol. 2018;46:136–143. doi: 10.2967/jnmt.117.203547. [DOI] [PubMed] [Google Scholar]
- 109.Reader CS, et al. The integrin αvβ6 drives pancreatic cancer through diverse mechanisms and represents an effective target for therapy. J. Pathol. 2019;249:332–342. doi: 10.1002/path.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moore KM, et al. Therapeutic targeting of integrin αvβ6 in breast cancer. J. Natl Cancer Inst. 2014;106:dju169. doi: 10.1093/jnci/dju169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moore KM, et al. Integrin αvβ6-specific therapy for pancreatic cancer developed from foot-and-mouth-disease virus. Theranostics. 2020;10:2930–2942. doi: 10.7150/thno.38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 113.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 2019;12:92. doi: 10.1186/s13045-019-0779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brown NF, Marshall JF. Integrin-mediated TGFβ activation modulates the tumour microenvironment. Cancers. 2019;11:1221. doi: 10.3390/cancers11091221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vannini A, et al. αvβ3-integrin regulates PD-L1 expression and is involved in cancer immune evasion. Proc. Natl Acad. Sci. USA. 2019;116:20141–20150. doi: 10.1073/pnas.1901931116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bagati A, et al. Integrin αvβ6-TGFβ-SOX4 pathway drives immune evasion in triple-negative breast cancer. Cancer Cell. 2021;39:54–67.e9. doi: 10.1016/j.ccell.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takasaka N, et al. Integrin αvβ8-expressing tumor cells evade host immunity by regulating TGF-β activation in immune cells. JCI Insight. 2018;3:e122591. doi: 10.1172/jci.insight.122591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dodagatta-Marri E, et al. Integrin αvβ8 on T cells suppresses anti-tumor immunity in multiple models and is a promising target for tumor immunotherapy. Cell Rep. 2021;36:109309. doi: 10.1016/j.celrep.2021.109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reszka-Blanco, N. et al. Inhibition of integrin αvβ8 enhances immune checkpoint induced anti-tumor immunity by acting across immunologic synapse in syngeneic models of breast cancer. Poster 1559. https://investor.morphictx.com/static-files/cc336336-fe88-4947-a8d9-5afbb5b4691c (2021).
- 122.Tauriello D, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 123.Mariathasan S, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hailemichael Y, et al. Potentiating immune checkpoint blockade therapeutic efficacy using a small molecule activator of integrin cell adhesion receptors [abstr.] Cancer Res. 2019 doi: 10.1158/1538-7445.AM2019-5010. [DOI] [Google Scholar]
- 125.Hood JD, et al. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- 126.Shan D, et al. RGD-conjugated solid lipid nanoparticles inhibit adhesion and invasion of αvβ3 integrin-overexpressing breast cancer cells. Drug Deliv. Transl. Res. 2015;5:15–26. doi: 10.1007/s13346-014-0210-2. [DOI] [PubMed] [Google Scholar]
- 127.Zhang T, et al. Dual-targeted hybrid nanoparticles of synergistic drugs for treating lung metastases of triple negative breast cancer in mice. Acta Pharmacol. Sin. 2017;38:835–847. doi: 10.1038/aps.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gajbhiye KR, Gajbhiye V, Siddiqui IA, Gajbhiye JM. cRGD functionalised nanocarriers for targeted delivery of bioactives. J. Drug Target. 2019;27:111–124. doi: 10.1080/1061186X.2018.1473409. [DOI] [PubMed] [Google Scholar]
- 129.Patnaik A, et al. A phase 1 study of SGN-B6A, an antibody-drug conjugate targeting integrin beta-6, in patients with advanced solid tumors (SGNB6A-001, Trial in Progress) J. Clin. Oncol. 2021;39(15_Suppl):TPS3144. [Google Scholar]
- 130.Maginnis MS. Virus-receptor interactions: the key to cellular invasion. J. Mol. Biol. 2018;430:2590–2611. doi: 10.1016/j.jmb.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hussein HA, et al. Beyond RGD: virus interactions with integrins. Arch. Virol. 2015;160:2669–2681. doi: 10.1007/s00705-015-2579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang S, et al. Integrin αvβ5 internalizes Zika virus during neural stem cells infection and provides a promising target for antiviral therapy. Cell Rep. 2020;30:969–983.e4. doi: 10.1016/j.celrep.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guerrero CA, et al. Integrin alpha(v)beta(3) mediates rotavirus cell entry. Proc. Natl Acad. Sci. USA. 2000;97:14644–14649. doi: 10.1073/pnas.250299897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schornberg KL, et al. Alpha5beta1-integrin controls ebolavirus entry by regulating endosomal cathepsins. Proc. Natl Acad. Sci. USA. 2009;106:8003–8008. doi: 10.1073/pnas.0807578106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kotecha A, et al. Rules of engagement between αvβ6 integrin and foot-and-mouth disease virus. Nat. Commun. 2017;8:15408. doi: 10.1038/ncomms15408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sigrist CJ, Bridge A, Le Mercier P. A potential role for integrins in host cell entry by SARS-CoV-2. Antivir. Res. 2020;177:104759. doi: 10.1016/j.antiviral.2020.104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Makowski L, Olson-Sidford W, W-Weisel J. Biological and clinical consequences of integrin binding via a rogue RGD motif in the SARS CoV-2 spike protein. Viruses. 2021;13:e146. doi: 10.3390/v13020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Beddingfield BJ, et al. The integrin binding peptide, ATN-161, as a novel therapy for SARS-CoV-2 infection. JACC Basic Transl. Sci. 2021;6:1–8. doi: 10.1016/j.jacbts.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Garbaccio RM, Parmee ER. The impact of chemical probes in drug discovery: a pharmaceutical industry perspective. Cell Chem. Biol. 2016;23:10–17. doi: 10.1016/j.chembiol.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 140.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 141.Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov. Today Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 142.Cumming JG, Davis AM, Muresan S, Haeberlein M, Chen H. Chemical predictive modelling to improve compound quality. Nat. Rev. Drug Discov. 2013;12:948–962. doi: 10.1038/nrd4128. [DOI] [PubMed] [Google Scholar]
- 143.Egbert M, Whitty A, Keserű GM, Vajda S. Why some targets benefit from beyond rule of five drugs. J. Med. Chem. 2019;62:10005–10025. doi: 10.1021/acs.jmedchem.8b01732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.DeGoey DA, Chen HJ, Cox PB, Wendt MD. Beyond the Rule of 5: lessons learned from AbbVie’s drugs and compound collection. J. Med. Chem. 2018;61:2636–2651. doi: 10.1021/acs.jmedchem.7b00717. [DOI] [PubMed] [Google Scholar]
- 145.Veber DF, et al. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 146.Nicholson NS, et al. SC-54684A: an orally active inhibitor of platelet aggregation. Circulation. 1995;91:403–410. doi: 10.1161/01.cir.91.2.403. [DOI] [PubMed] [Google Scholar]
- 147.Nicholson NS, et al. Orbofiban: an orally active GPIIb/IIIa platelet receptor antagonist. Med. Res. Rev. 2001;21:211–226. doi: 10.1002/med.1007. [DOI] [PubMed] [Google Scholar]
- 148.Turaga RC, et al. Rational design of a protein that binds integrin αvβ3 outside the ligand binding site. Nat. Commun. 2016;7:11675. doi: 10.1038/ncomms11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miller LM, Pritchard JM, Macdonald S, Jamieson C, Watson A. Emergence of small-molecule non-RGD-mimetic inhibitors for RGD integrins. J. Med. Chem. 2017;60:3241–3251. doi: 10.1021/acs.jmedchem.6b01711. [DOI] [PubMed] [Google Scholar]
- 150.Hatley R, et al. The design of potent, selective and drug-like RGD αvβ1 small-molecule inhibitors derived from non-RGD α4β1 antagonists. ChemMedChem. 2019;14:1315–1320. doi: 10.1002/cmdc.201900359. [DOI] [PubMed] [Google Scholar]
- 151.Zheng Y, Leftheris K. Insights into protein-ligand interactions in integrin complexes: advances in structure determinations. J. Med. Chem. 2020;63:5675–5696. doi: 10.1021/acs.jmedchem.9b01869. [DOI] [PubMed] [Google Scholar]
- 152.Xiong JP, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dong X, et al. Force interacts with macromolecular structure in activation of TGF-β. Nature. 2017;542:55–59. doi: 10.1038/nature21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Procopiou PA, et al. Discovery of (S)-3-(3-(3,5-dimethyl-1 H-pyrazol-1-yl)phenyl)-4-((R)-3-(2-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)ethyl)pyrrolidin-1-yl)butanoic acid, a nonpeptidic αvβ6 integrin inhibitor for the inhaled treatment of idiopathic pulmonary fibrosis. J. Med. Chem. 2018;61:8417–8443. doi: 10.1021/acs.jmedchem.8b00959. [DOI] [PubMed] [Google Scholar]
- 155.Sorge JL, Wagstaff JL, Rowe ML, Williamson RA, Howard MJ. Q2DSTD NMR deciphers epitope-mapping variability for peptide recognition of integrin αvβ6. Org. Biomol. Chem. 2015;13:8001–8007. doi: 10.1039/c5ob01237f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Claasen B, Axmann M, Meinecke R, Meyer B. Direct observation of ligand binding to membrane proteins in living cells by a saturation transfer double difference (STDD) NMR spectroscopy method shows a significantly higher affinity of integrin alpha(IIb)beta3 in native platelets than in liposomes. J. Am. Chem. Soc. 2005;127:916–919. doi: 10.1021/ja044434w. [DOI] [PubMed] [Google Scholar]
- 157.Vasile F, et al. Insight to the binding mode of triazole RGD-peptidomimetics to integrin-rich cancer cells by NMR and molecular modeling. Bioorg. Med. Chem. 2016;24:989–994. doi: 10.1016/j.bmc.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 158.Di Leva FS, et al. From a helix to a small cycle: metadynamics-inspired αvβ6 integrin selective ligands. Angew. Chem. Int. Ed. Engl. 2018;57:14645–14649. doi: 10.1002/anie.201803250. [DOI] [PubMed] [Google Scholar]
- 159.Nanthakumar, C. B., Hatley, R. J. D. & Slack, R. J. in Anti-fibrotic Drug Discovery (eds Brenneman, J. & Iyer, M. R.) ch. 2, 37–75 (Royal Society of Chemistry, 2020).
- 160.Reichart F, et al. Selective targeting of integrin αvβ8 by a highly active cyclic peptide. J. Med. Chem. 2019;62:2024–2037. doi: 10.1021/acs.jmedchem.8b01588. [DOI] [PubMed] [Google Scholar]
- 161.Lippa RA, et al. Discovery of the first potent and selective αvβ5 integrin inhibitor based on an amide-containing core. Eur. J. Med. Chem. 2020;208:112719. doi: 10.1016/j.ejmech.2020.112719. [DOI] [PubMed] [Google Scholar]
- 162.Barrett TN, et al. Profile of a highly selective quaternized pyrrolidine betaine αvβ6 integrin inhibitor-(3S)-3-(3-(3,5-dimethyl-1H-pyrazol-1-yl)phenyl)-4-((1S and 1R,3R)-1-methyl-3-(2-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)ethyl)pyrrolidin-1-ium-1-yl)butanoate synthesized by stereoselective methylation. J. Med. Chem. 2019;62:7543–7556. doi: 10.1021/acs.jmedchem.9b00819. [DOI] [PubMed] [Google Scholar]
- 163.Anderson NA, et al. Discovery of an orally bioavailable pan αv integrin inhibitor for idiopathic pulmonary fibrosis. J. Med. Chem. 2019;62:8796–8808. doi: 10.1021/acs.jmedchem.9b00962. [DOI] [PubMed] [Google Scholar]
- 164.Hagmann WK, et al. The discovery of sulfonylated dipeptides as potent VLA-4 antagonists. Bioorg. Med. Chem. Lett. 2001;11:2709–2713. doi: 10.1016/s0960-894x(01)00544-3. [DOI] [PubMed] [Google Scholar]
- 165.Choi S, et al. Small molecule inhibitors of integrin alpha2beta1. J. Med. Chem. 2007;50:5457–5462. doi: 10.1021/jm070252b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Reed NI, et al. Exploring N-arylsulfonyl-l-proline scaffold as a platform for potent and selective αvβ1 integrin inhibitors. ACS Med. Chem. Lett. 2016;7:902–907. doi: 10.1021/acsmedchemlett.6b00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kapp TG, et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci. Rep. 2017;7:39805. doi: 10.1038/srep39805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cha, J. et al. Amino acid compounds and methods of use. Patent WO2019173653 (2019).
- 169.Anderson, N. A. et al. Novel compounds. Patent WO2016046226 (2016).
- 170.Ruminski, P. G. & Griggs, D. W. Meta-azacyclic amino benzoic acid derivatives as pan integrin antagonists. Patent WO2017117538 (2017).
- 171.Thompson, J. Joint RSC CICAG-BMCS Meeting, Sygnature Discovery, BioCity, Nottingham, UK. Investigating the Chameleonic Properties of αVβ6 Integrin Antagonists for the Treatment of IPF. https://www.rsc.org/images/CICAG_Newsletter_Winter_2019-20_tcm18-252021.pdf (2019)
- 172.Kuhn B, Mohr P, Stahl M. Intramolecular hydrogen bonding in medicinal chemistry. J. Med. Chem. 2010;53:2601–2611. doi: 10.1021/jm100087s. [DOI] [PubMed] [Google Scholar]
- 173.Maher TM, et al. A positron emission tomography imaging study to confirm target engagement in the lungs of patients with idiopathic pulmonary fibrosis following a single dose of a novel inhaled αvβ6 integrin inhibitor. Respir. Res. 2020;21:75. doi: 10.1186/s12931-020-01339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fierce Biotech. Biogen axes phase 2 study lung-scarring med due to safety concerns. https://www.fiercebiotech.com/biotech/biogen-axes-phase-2-study-lung-scarring-med-due-to-safety-concerns (2017).
- 175.Lo DJ, et al. Inhibition of αvβ6 promotes acute renal allograft rejection in nonhuman primates. Am. J. Transplant. 2013;13:3085–3093. doi: 10.1111/ajt.12467. [DOI] [PubMed] [Google Scholar]
- 176.Tam SH, Sassoli PM, Jordan RE, Nakada MT. Abciximab (ReoPro, chimeric 7E3 Fab) demonstrates equivalent affinity and functional blockade of glycoprotein IIb/IIIa and alpha(v)beta3 integrins. Circulation. 1998;98:1085–1091. doi: 10.1161/01.cir.98.11.1085. [DOI] [PubMed] [Google Scholar]
- 177.Raghu, G. et al. Randomized, Double-Blind, Placebo-Controlled, Multiple Dose, Dose-Escalation Study of BG00011 (Formerly STX-100) in Patients with Idiopathic Pulmonary Fibrosis (IPF). D14. ILD: Clinical Research. 10.1164/ajrccm-conference.2018.197.1_MeetingAbstracts.A7785 (2018).
- 178.Eberlein C, et al. A human monoclonal antibody 264RAD targeting αvβ6 integrin reduces tumour growth and metastasis, and modulates key biomarkers in vivo. Oncogene. 2013;32:4406–4416. doi: 10.1038/onc.2012.460. [DOI] [PubMed] [Google Scholar]
- 179.Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat. Rev. Drug Discov. 2011;10:712. doi: 10.1038/nrd3439-c1. [DOI] [PubMed] [Google Scholar]
- 180.Mercer PF, et al. Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax. 2016;71:701–711. doi: 10.1136/thoraxjnl-2015-207429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Paish HL, et al. A bioreactor technology for modeling fibrosis in human and rodent precision-cut liver slices. Hepatology. 2019;70:1377–1391. doi: 10.1002/hep.30651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nelson MR, et al. The support of human genetic evidence for approved drug indications. Nat. Genet. 2015;47:856–860. doi: 10.1038/ng.3314. [DOI] [PubMed] [Google Scholar]
- 183.Allen RJ, et al. Genetic variants associated with susceptibility to idiopathic pulmonary fibrosis in people of European ancestry: a genome-wide association study. Lancet Respir. Med. 2017;5:869–880. doi: 10.1016/S2213-2600(17)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Organ L, Porte J, John A, Jenkins RG. Investigating the role of AKAP13 in epithelial cells on TGF-β activation. Thorax. 2021;74:S91. [Google Scholar]
- 185.Hamilton R. J. (Ed. in Chief) Tarascon Pocket Pharmacopoeia (Jones & Bartlett Learning, 2020)
- 186.Kossen K, et al. IDL-2965: a selective, highly-potent, oral integrin antagonist for IPF. Eur. Respir. J. 2019;54:PA5374. [Google Scholar]
- 187. Kossen, K. et al. IDL-2965: a selective, highly potent, clinical-stage integrin antagonist for the treatment of NASH. Poster. https://www.postersessiononline.eu/173580348_eu/congresos/NAFLD2019/aula/-P05_17_NAFLD2019.pdf (2019).
- 188.Chu FM, et al. A phase 1, multicenter, open-label study of the safety of two dose levels of a human monoclonal antibody to human α(v) integrins, intetumumab, in combination with docetaxel and prednisone in patients with castrate-resistant metastatic prostate cancer. Invest. New Drugs. 2011;29:674–679. doi: 10.1007/s10637-010-9388-4. [DOI] [PubMed] [Google Scholar]
- 189.Heidenreich A, et al. A randomized, double-blind, multicenter, phase 2 study of a human monoclonal antibody to human αν integrins (intetumumab) in combination with docetaxel and prednisone for the first-line treatment of patients with metastatic castration-resistant prostate cancer. Ann. Oncol. 2013;24:329–336. doi: 10.1093/annonc/mds505. [DOI] [PubMed] [Google Scholar]
- 190.Maden CH, et al. Safety, tolerability and pharmacokinetics of GSK3008348, a novel integrin αvβ6 inhibitor, in healthy participants. Eur. J. Clin. Pharmacol. 2018;74:701–709. doi: 10.1007/s00228-018-2435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rosenthal MA, et al. Evaluation of the safety, pharmacokinetics and treatment effects of an alpha(v)beta(3) integrin inhibitor on bone turnover and disease activity in men with hormone-refractory prostate cancer and bone metastases. Asia Pac. J. Clin. Oncol. 2010;6:42–48. doi: 10.1111/j.1743-7563.2009.01266.x. [DOI] [PubMed] [Google Scholar]
- 192.Roche provides update on phase III studies of etrolizumab in people with moderately to severely active ulcerative colitis. https://www.roche.com/investors/updates/inv-update-2020-08-10.htm (2021).
- 193.Vascular Pharma. VPI-2690B. http://www.vascularpharma.com/science/vpi-2690b (2021).
- 194.Oxurion NV – Expert Presentation of Positive Topline Data from a Phase 1 Study evaluating THR-687 for the treatment of DME, at Angiogenesis, Exudation, and Degeneration 2020 Conference. https://www.oxurion.com/content/oxurion-nv-expert-presentation-positive-topline-data-phase-1-study-evaluating-thr-687 (2020).
- 195.SciFluor Announces Positive Top-Line Results of Phase 1/2 Study of SF0166 Eye Drops to Treat Wet Age-Related Macular Degeneration. https://www.businesswire.com/news/home/20171218005625/en/SciFluor-Announces-Positive-Top-Line-Results-Phase-12 (2019).
- 196.Maturi R, et al. Safety and efficacy of risuteganib in intermediate non-exudative age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2020;61:1944. [Google Scholar]
- 197.Turner S, et al. Late Breaking Abstract - PK/PD assessment of an oral, selective αVβ6/αVβ1 integrin dual antagonist, PLN-74809, for the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 2019;54:PA1298. [Google Scholar]
- 198.Dalmas Wilk DA, Scicchitano MS, Morel D. In vitro investigation of integrin-receptor antagonist-induced vascular toxicity in the mouse. Toxicol. Vitr. 2013;27:272–281. doi: 10.1016/j.tiv.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 199.Hawiger J, Kloczewiak M, Bednarek MA, Timmons S. Platelet receptor recognition domains on the alpha chain of human fibrinogen: structure-function analysis. Biochemistry. 1989;28:2909–2914. doi: 10.1021/bi00433a024. [DOI] [PubMed] [Google Scholar]
- 200.Chew DP, Bhatt DL, Sapp S, Topol EJ. Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists: a meta-analysis of phase III multicenter randomized trials. Circulation. 2001;103:201–206. doi: 10.1161/01.cir.103.2.201. [DOI] [PubMed] [Google Scholar]
- 201.Yednock TA, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 202.Sandborn WJ, et al. Efficacy and safety of abrilumab in a randomized, placebo-controlled trial for moderate-to-severe ulcerative colitis. Gastroenterology. 2019;156:946–957.e18. doi: 10.1053/j.gastro.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 203.Fukase H, Kajioka T, Oikawa I, Ikeda N, Furuie H. AJM300, a novel oral antagonist of α4-integrin, sustains an increase in circulating lymphocytes: a randomised controlled trial in healthy male subjects. Br. J. Clin. Pharmacol. 2020;86:591–600. doi: 10.1111/bcp.14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tang MT, et al. Nonclinical and clinical pharmacology, pharmacokinestics and pharmacodynamics of etrolizumab, an anti-β7 integrin therapy for inflammatory bowel disease. Aliment. Pharmacol. Ther. 2018;47:1440–1452. doi: 10.1111/apt.14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vermeire S, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384:309–318. doi: 10.1016/S0140-6736(14)60661-9. [DOI] [PubMed] [Google Scholar]
- 206.Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sheppard D. The role of integrins in pulmonary fibrosis. Eur. Respir. Rev. 2008;17:157–162. [Google Scholar]
- 208.Mu D, et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J. Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur. J. Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 210.Thannickal VJ, et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.