Dear Editor,
Blumenthal et al. 1 reported cases of delayed large local reaction to the mRNA‐1273 Moderna COVID‐19 vaccine, which was called the ‘Moderna arm’ for the first time. Intriguingly, this phenomenon was observed primarily in Moderna vaccine but rarely reported in BNT162b2 COVID‐19 vaccine by Pfizer‐BioNTech. 2 , 3 , 4 Because the reports were limited to mRNA vaccines, a delayed‐type hypersensitivity reaction to the excipient polyethylene glycol (PEG) in both mRNA vaccines was suggested as one potential aetiology. 4 , 5
Unlikely these mRNA vaccines, ChAdOx1 nCoV‐19 (AZD1222) is a replication‐defective chimpanzee adenovirus‐vectored vaccine, and it includes polysorbate 80 as an excipient. 6 Recently, we have observed delayed cutaneous reactions around the injection site of the ChAdOx1 nCoV‐19 vaccine. We report four cases with such reactions that developed at least three days (range, Day 4–17) after the first dose of vaccination (Table 1). All the patients were female healthcare workers (1 physician, 2 nurses and 1 laboratory technician) at our university hospitals and were ethnically Korean. They did not have a previous history of hypersensitivity reactions to drugs or any vaccine. Only one patient had allergic rhinitis but did not require regular medication. Routine blood tests including complete blood cell counts, C‐reactive protein and serum IgE were unremarkable. All patients had a large delayed skin reaction that started with erythematous swelling (Fig. 1a–d), and three developed systemic symptoms including fever, chill and myalgia from the day of vaccination and that resolved before the delayed skin reactions. Thus, at the development of delayed local skin reactions, none of the patients experienced concurrent systemic symptoms. The delayed skin lesions resolved after short‐term treatment, such as oral antihistamines or topical or oral corticosteroids, while the duration of skin reaction varied from 4 to 18 days. Skin biopsy from three patients showed superficial perivascular and perifollicular lymphocytic infiltration with sparse eosinophils (Fig. 1e–k). These findings suggest that the delayed local cutaneous reactions are mediated by hypersensitivity mechanisms. However, the exact pathophysiological mechanism remains unclear if they are true hypersensitivity reactions. One reason for this controversy is that the skin tests (patch, prick, and intradermal test) of the patient who was injected BNT162b2 vaccine were all negative. 2 In the aspect of hypersensitivity reaction, unlike PEG, polysorbate 80 in ChAdOx1 nCoV‐19 vaccine has been used in other vaccines before, it is still unclear which antigen in the vaccine caused these reactions.
Table 1.
Demographic and clinical data of subjects with delayed cutaneous reaction to ChAdOx1 nCoV‐19 vaccine*
| Patient | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Age, years | 44 | 30 | 58 | 53 |
| Sex | Female | Female | Female | Female |
| Past medical history | Allergic rhinitis | No | Dyslipidemia | Vitiligo |
| Current medication | No | No | Atorvastatin | No |
| Past history of cutaneous reaction to vaccination | No | No | No | No |
| Day of skin reaction onset after COVID‐19 vaccination | 4 | 5 | 17 | 14 |
| Symptoms and signs of cutaneous reaction | Erythema, swelling, pain, tenderness | Erythema, swelling, pain, tenderness, pruritus | Erythema, swelling, pain, tenderness | Erythema, swelling, pruritus |
| Lesion size, cm | 10 × 10 | 15 × 8 | 9 × 7.5 | 18 × 10 |
| Immediate injection site reaction after vaccination | No | No | No | No |
| Immediate systemic symptoms after vaccination | Fever (38.5°C), chill, myalgia | Fever (38.4°C), chill, fatigue, headache | Fever (38.1°C), chill, fatigue, headache, myalgia | Fatigue, myalgia |
| Concurrent systemic symptoms with delayed skin reaction | No | No | No | No |
| Treatment | Oral predinisolone 30 mg for 4 days | Oral antihistamine, topical corticosteroid for 3 days | Oral antihistamine, topical corticosteroid for 4 days | Oral antihistamine, topical corticosteroid for 4 days |
| Duration of skin reaction, days | 4 | 17 | 7 | 6 |
| Treatment response | Complete resolution | Complete resolution | Complete resolution | Complete resolution |
None of the patients had known previous SARS‐CoV‐2 infection. The clinical data were reported by patients, and symptoms were evaluated by a dermatologist or allergist.
Figure 1.
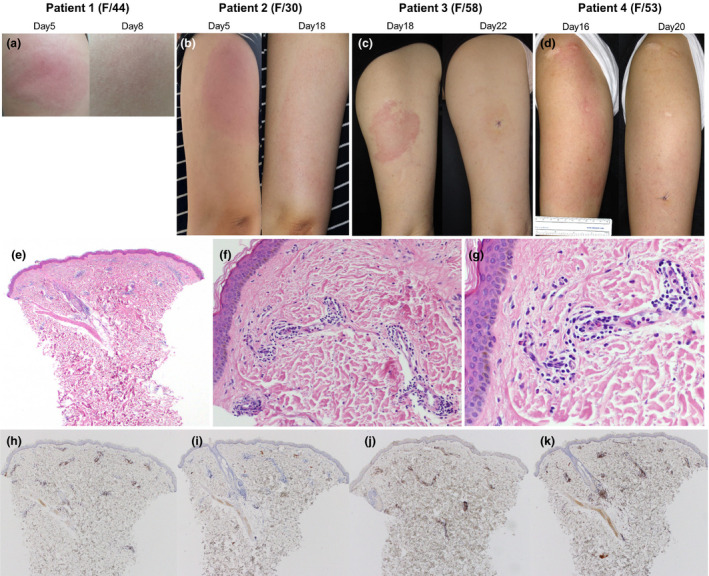
Clinical photographs and the representative histopathologic findings of delayed cutaneous reactions to the ChAdOx1 nCoV‐19 vaccine. (a) Patient 1 presented considerable induration with pain and tenderness on Day 5. After 3 days of oral prednisolone 30 mg, the skin lesion resolved. (b) Patient 2 started a painful erythematous swelling on Day 5. Without treatment, the skin lesion became larger (15 × 8 cm), but pain decreased, and pruritus occurred on Day 18. The skin lesion gradually improved after 3 days of oral antihistamine and topical corticosteroid. (c) Patient 3 presented with erythematous tender plaque on day 17. There was no immediate skin reaction after vaccination. On Day 18, the patient underwent skin biopsy and was prescribed oral antihistamine and topical corticosteroid. Four days later (on Day 22), the skin lesion was much improved. (d) Patient 4 suffered from 18 × 10 cm sized itchy erythematous swollen plaque on Day 16. There was no immediate cutaneous symptom after vaccination and no concurrent systemic symptom. (e–g) Skin biopsy specimen from patient 4 shows superficial perivascular and perifollicular lymphocytic infiltration and some eosinophils. Neutrophils are present inside dilated small vessels. Immunohistochemistry reveals mainly CD3+ T cells with sparse exocytosis (h) and few CD20+ B cells (i). There is a mixed population of CD4+ (j) and CD8+ (k) cells, but CD4+ is predominant. These findings are consistent with a delayed hypersensitivity reaction (e: H&E ×40, f: H&E ×200, g: H&E ×400, h: CD3 ×40, i: CD20 ×40, j: CD4 ×40, k: CD8 ×40).
Our report suggests that the delayed local cutaneous reactions to the COVID‐19 vaccines are not vaccine‐specific, since both mRNA vaccine and viral‐vector vaccine showed such reactions. Before implementing a mass vaccination campaign with various COVID‐19 vaccines, clinicians should be aware of the possible ‘COVID‐arm’ to avoid unnecessary tests or treatment. While the Centers for Disease Control and Prevention reported that patients who experienced delayed cutaneous reactions to the first dose of COVID‐19 vaccine could be safely vaccinated with the second dose, 7 more data are required to understand and manage the ‘COVID‐arm’ to various COVID‐19 vaccines.
Conflicts of interest
Jeong Eun Kim, Hyun Lee, Seung Sam Paik, Ji‐Yong Moon, Ho Joo Yoon and Sang‐Heon Kim declare to have no conflict of interest.
Funding source
None.
IRB approval
Yes (Number HYUH 2021‐03‐052).
Acknowledgement
The patients in this manuscript have given written informed consent to the publication of their case details. This research was supported by a grant from the Korean Academy of Asthma, Allergy and Clinical Immunology.
References
- 1. Blumenthal KG, Freeman EE, Saff RR et al. Delayed large local reactions to mRNA‐1273 vaccine against SARS‐CoV‐2. N Engl J Med 2021; 384: 1273–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baeck M, Marot L, Belkhir L. Delayed large local reactions to mRNA vaccines. N Eng J Med. 2021;384(24):e98. [DOI] [PubMed] [Google Scholar]
- 3. Fernandez‐Nieto D, Hammerle J, Fernandez‐Escribano M et al. Skin manifestations of the BNT162b2 mRNA COVID‐19 vaccine in healthcare workers. ‘COVID‐arm’: a clinical and histological characterization. J Eur Acad Dermatol Venereol. 2021;35(7):e425‐e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: A registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85(1): 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farinazzo E, Ponis G, Zelin E et al. Cutaneous adverse reactions after m‐RNA COVID‐19 vaccine: early reports from North‐East Italy. J Eur Acad Dermatol Venereol 2021; 35: e548–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Voysey M, Clemens SAC, Madhi SA et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention . https://www.cdc.gov/coronavirus/2019‐ncov/vaccines/safety/allergic‐reaction.html. Accessed 29th March 2021.


