Abstract
Diffusion properties notably determine the behavior of biomembranes. Here we report the concurrent nanoscale fine-mapping of membrane topography, diffusivity, and packing order in live mammalian cells through a synergy of single-molecule and super-resolution methods. By identifying a bright, lipophilic fluorescence turn-on probe that enables sustained single-molecule imaging of cellular membranes under stroboscopic excitation, we accumulate the positions and transient displacements of >106 probe molecules to achieve super-resolution topography and diffusivity mapping. We thus determine a trend that the membrane diffusivity drops with increased lipid packing order when comparing the endoplasmic reticulum (ER) membrane, plasma membrane, and nanodomains induced by cholera toxin B. Utilizing our nanoscale mapping capability, we further unveil reduced diffusivity in the ER membrane at ER-plasma membrane contact sites. By next integrating spectrally resolved single-molecule imaging, we show this localized diffusion slowdown is not due to altered lipid packing order, but may instead be attributed to local protein crowding. Our integrated multidimensional single-molecule approach thus unveils and differentiates between nanoscale diffusional heterogeneities of different origins in live-cell membranes.
Graphical Abstract
INTRODUCTION
Diffusion properties both determine the behavior of biomembranes and provide a valuable window into their dynamic structures.1–5 However, it remains difficult to obtain a continuous fine map of local diffusivity for cellular membranes. Typical diffusivity measurements based on fluorescence recovery after photobleaching (FRAP) and fluorescence correlation spectroscopy (FCS) afford limited spatial resolutions and/or mapping capabilities.6,7 Although single-particle tracking (SPT) offers nanoscale resolutions,4,8,9 historically it relies on sparse sampling so that only a few particles are tracked for each cell. The recent rise of single-molecule localization microscopy (SMLM) super-resolution methods10–13 has inspired new ways to achieve SPT at high densities. Photoactivation-based approaches14–16 allow temporal separation of single molecules within the diffraction limit, thus enabling the tracking of thousands of molecules over time. Methods based on in situ fluorescent probe exchange17–19 further overcome the limited photoactivation cycles of each probe. However, emphasis has been on obtaining trajectories for single protein molecules, which diffuse slow and often do not uniformly sample the membrane.
We recently reported single-molecule displacement/diffusivity mapping (SMdM), for super-resolution mapping of the intracellular diffusion rates of unbound proteins.20 In contrast to SPT, in which each particle is tracked over many frames as it visits random locations, SMdM flips the question to evaluate, for each fixed location, how different (yet identical) single particles move locally by accumulating their transient displacements in short time windows. This location-centered strategy is powerful for spatial mapping. By eliminating the need to track long trajectories, SMdM is also ideal for systems in which fluorophores only emit transiently. PAINT (points accumulation for imaging in nanoscale topography)13 presents such a case for SMLM of the lipid membrane: As single probe molecules randomly enter the membrane phase from the aqueous medium, they turn on fluorescence emission and reside for ~10 ms before exiting,21 thus allowing unbiased, high-density membrane sampling.13
Here we exploit the transient intra-membrane diffusion of single probe molecules in PAINT to achieve SMdM diffusivity mapping for cellular membranes. By identifying a bright fluorescence turn-on probe that enables sustained PAINT-type single-molecule imaging for >105 frames under stroboscopic illumination, we accumulate the super-resolved positions and transient displacements of >106 molecules in live-cell membranes. We thus achieve continuous fine mapping of the cellular membrane diffusivity to unveil heterogeneities at the nanoscale, and further integrate spectrally resolved SMLM (SR-SMLM)22,23 to examine the possible roles of lipid packing order and protein crowding in local diffusivity differences.
RESULTS
For effective probing of both the topography and diffusion properties of cellular membranes, we started by identifying the commercial dye BDP-TMR-alkyne (Figure 1a) as an optimal probe. BDP-TMR has been reported as a water-soluble dye with a high affinity to lipid bilayers.24 We found that when excited at 560 nm, BDP-TMR-alkyne exhibited ~10-fold fluorescence turn-on in organic phases vs. in the aqueous medium (Figure 1b), owing to substantially reduced and blue-shifted absorption in the aqueous phase (Figure S1). When added into the cell medium at 3.3 nM and excited at 560 nm, it emitted strongly over low backgrounds as single molecules transiently entered cellular membranes (Figure 1c). In comparison to Nile Red, the typical PAINT probe for lipid membranes,13,25,26 BDP-TMR-alkyne was notably brighter and yielded superior single-molecule images (Figure 1c–e), consistent with its measured higher fluorescence quantum yield (~95%) and extinction coefficient (Figure S1). When excited by stroboscopic pulses of τ = 1 ms duration to minimize motion blur, a substantial fraction of the molecules emitted ~1,000 photons (Figure 1e), thus enabling reliable three-dimensional (3D) localization through single-molecule image shape.27 Similar to Nile Red, BDP-TMR-alkyne dynamically partitioned between the medium and membrane phases so that single molecules stayed in the membrane for ~10 ms,21 a timescale ideal for the <10 ms displacement time window of SMdM (below). The rapid membrane-medium exchange created a steady state, so that continuous imaging was readily achieved for >1.6×105 frames with no appreciable drop in the single-molecule count (Figure 1f), from which 106–107 molecules were accumulated across the view. It is further worth mentioning that these results were achieved in the regular cell media without the need for an oxygen scavenger, other additives, or photoactivation.
Figure 1.
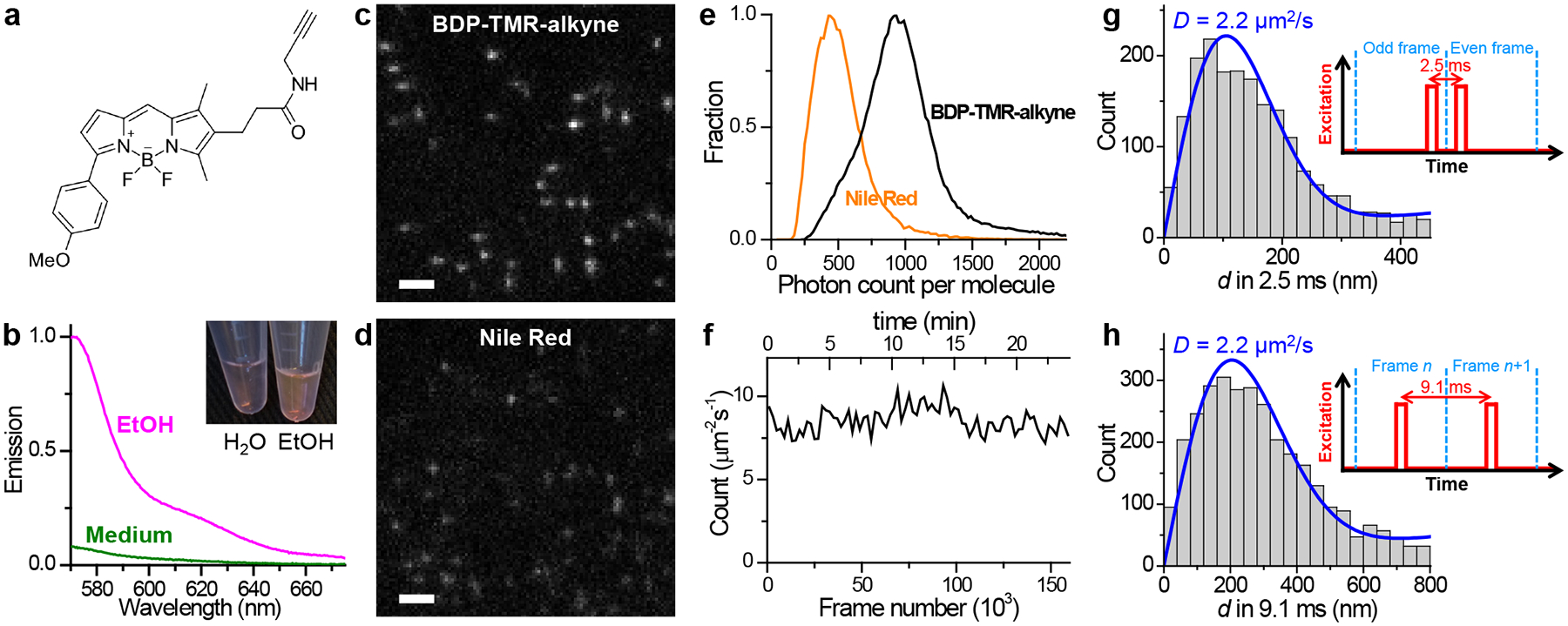
BDP-TMR-alkyne for stroboscopic PAINT and SMdM of cellular membranes. (a) Chemical structure of BDP-TMR-alkyne. (b) Fluorescence emission spectra of 3.3 nM BDP-TMR-alkyne in the aqueous cell medium (green curve) vs. in ethanol (magenta curve), when excited at 560 nm. Inset: photo of 10 μM solutions of BDP-TMR-alkyne in water vs. in ethanol. (c,d) Typical single-molecule images of BDP-TMR-alkyne (c) vs. Nile Red (d) in cell membranes recorded under identical conditions with stroboscopic excitation pulses of τ = 1 ms duration. A cylindrical lens was applied to introduce image elongations in vertical or horizontal directions for molecules of different depths. (e) Distribution of single-molecule photon counts for the two cases. (f) Time-dependent count of the detected BDP-TMR-alkyne single molecules per μm2 per second at the plasma membrane of a COS-7 cell, showing no noticeable drop over 1.6×105 frames (24 min at 110 fps). (g,h) Typical distributions of transient single-molecule displacement d of BDP-TMR-alkyne at the COS-7 cell plasma membrane under two stroboscopic excitation schemes (insets): repeated tandem excitation pulses at a Δt = 2.5 ms center-to-center separation (g), vs. excitation at the middle of every frame at 110 fps (Δt = 9.1 ms) (h). Blue curves: MLE (maximum likelihood estimation) fits to our model, with resultant diffusion coefficient D labeled. Scale bars: 2 μm (c,d).
For SMdM, we implemented two different excitation schemes while continuously recording in the wide-field at 110 frames per second (fps). Whereas the application of asymmetrically timed pulse pairs across tandem frames20 at a Δt = 2.5 ms center-to-center separation helped limit the transient single-molecule displacement d to <~200 nm (Figure 1g) for more localized statistics, exciting at the middle of each frame (Figure 1h) allowed d in Δt = 9.1 ms time windows to be extracted between consecutive frames. Fitting the d distributions obtained under the two excitation schemes to a modified two-dimensional (2D) random-walk model (Methods and Reference 20) gave similar typical diffusion coefficients of ~2.2 μm2/s for cell plasma membranes (Figure 1gh), comparable to that previously measured by SPT for other lipophilic dyes.15,28 We further obtained comparable results for excitation pulses of different duration τ (Figures S2 and S3), thus demonstrating the robustness of our measurements with varied parameters.
The possibility to accumulate a large amount of single-molecule data through stroboscopic PAINT of BODIPY-TMR-alkyne enabled continuous fine-mapping of membrane topography and diffusivity. Resultant 3D-SMLM images (Figure 2ab, Figure S2, and Figure S3) well resolved how the dorsal (top) plasma membrane raised out of the focal range when far away from the cell edge, as well as how the intracellular organelle membranes, e.g., that of the endoplasmic reticulum (ER), undulated in height and possibly contacted the plasma membrane (arrowheads in Figure 2b). The ventral (bottom) plasma membrane was lowly labeled owing to its inaccessibility to the probe-containing medium, similar to our observations for other PAINT probes.26,29 Meanwhile, spatially binning the accumulated transient single-molecule displacement d into 100×100 nm2 bins enabled their individual fitting (Methods and Reference 20) to obtain the local diffusivity D, hence color-coded SMdM super-resolution maps (Figure 2c, Figure S2, and Figure S3). Typical D values of 2.0–2.5 μm2/s were thus found for the plasma membrane. Treating the cell with cholera toxin B subunit (CTB), a cross-linker of the ganglioside GM1, gave rise to ~200 nm-sized nanodomains of substantially reduced local diffusivity down to ~1 μm2/s (Figure 2e), which partially co-localized with CTB (arrows in Figure 2de; see also Figure S4 for a comparison between CTB-treated and untreated cells under a stretched color scale to emphasize regions of low diffusivity). This result echoes our recent SR-SMLM results with Nile Red, which showed that CTB induces similarly-sized nanodomains of low chemical polarity in the plasma membrane, attributable to increased lipid packing order due to the local sequestration of GM1 and cholesterol.26 Cholesterol-induced packing order and reduced membrane fluidity are well documented at the bulk level.30–33
Figure 2.
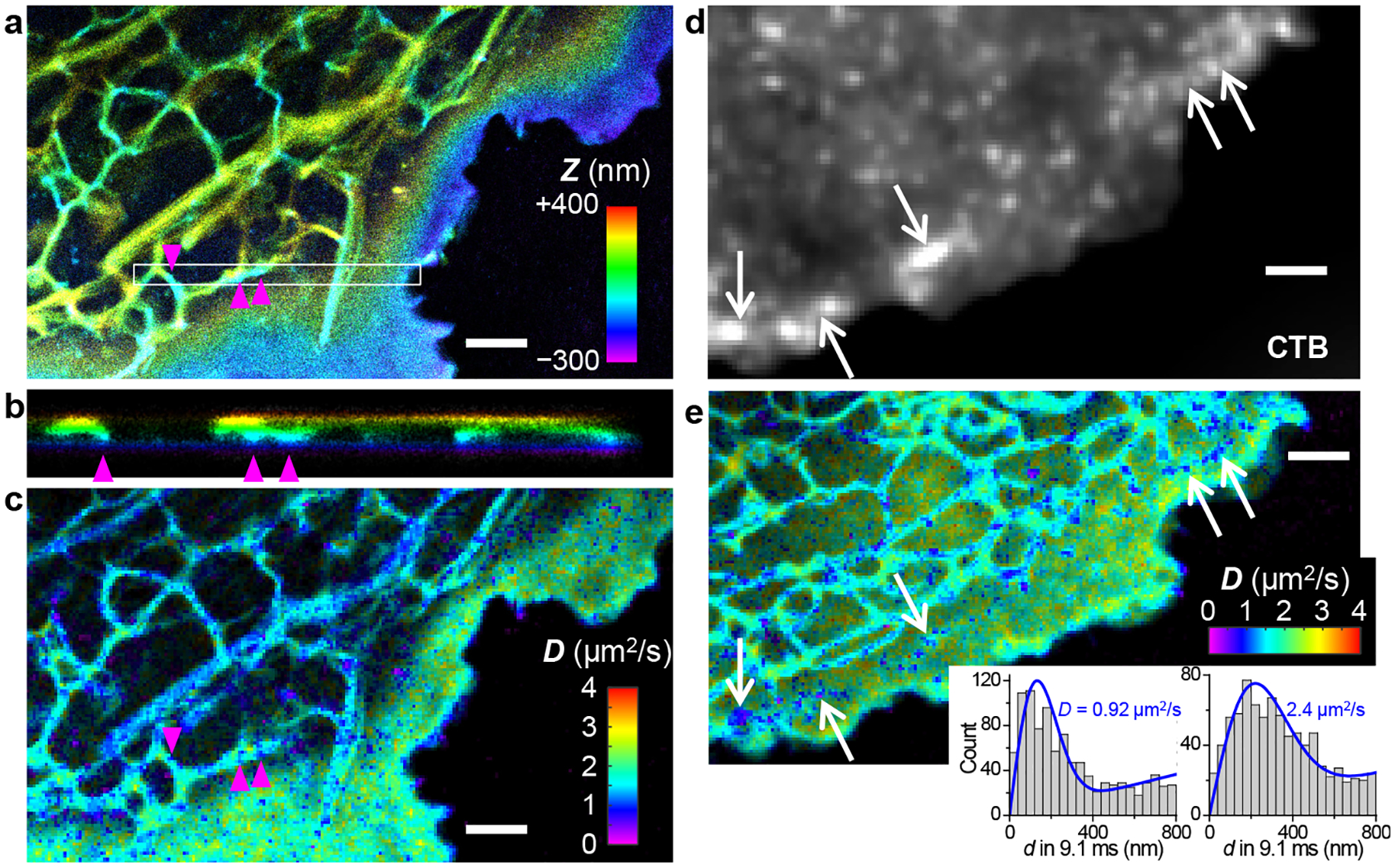
Concurrent 3D-SMLM and SMdM diffusion mapping of cellular membranes through stroboscopic PAINT of BDP-TMR-alkyne. (a) 3D-SMLM image obtained in a live COS-7 cell. Color presents depth (z); purple/blue: closest to the coverslip; red/yellow: farthest away. (b) Cross-sectional view along the box in (a) in the xz direction. Arrowheads point to possible contacts between ER and the ventral plasma membrane. (c) SMdM diffusivity map constructed from the same single-molecule data. Color presents local diffusivity D. (d) Epifluorescence image of dye-tagged CTB applied to another COS-7 cell. (e) BDP-TMR-alkyne SMdM diffusivity map of the same cell. Insets: typical distributions of single-molecule displacements for the low-diffusivity nanodomains (left) versus other parts of the plasma membrane (right), and their MLE fits for D (blue curves). For both cells, stroboscopic excitation of τ = 1 ms duration was applied at the middle of every frame at 110 fps (Δt = 9.1 ms). Scale bars: 2 μm.
For the ER-tubule membrane, typical D values appeared slightly lower than the plasma membrane (Figure 2ce), apparently against trend with their lower cholesterol level and packing order.26,34,35 2D plotting of single-molecule displacements showed a preferred angular distribution along the tubule (Figure 3a), indicating the isotropic 2D fitting model we originally used unsuitable. This observation underscores a geometrical effect that underestimates motion perpendicular to the tubule length: in the extreme case, a molecule that traveled a round trip along the tubule circumference in Δt would give a zero displacement.
Figure 3.
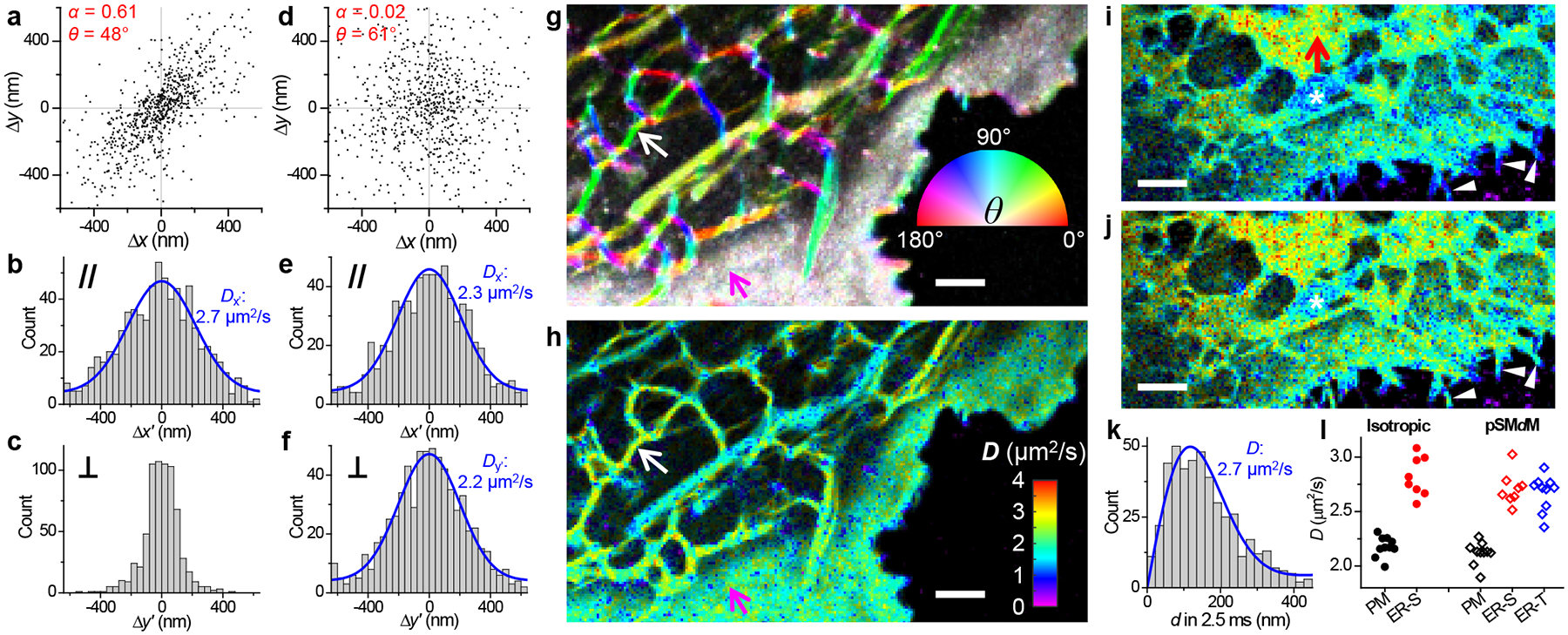
Principal-direction SMdM (pSMdM) analysis. (a) 2D plot of single-molecule displacements in Δt = 9.1 ms for BDP-TMR-alkyne at an ER tubule [white arrows in (g,h)]. A principal direction θ of 48° is calculated with an anisotropy α of 0.61. (b,c) 1D distributions of the displacements in (a) projected along (b) and perpendicular (c) to θ. Blue curve in (b): MLE fit to an 1D diffusion model with resultant D = 2.7 μm2/s. (d) 2D plot of single-molecule displacements in Δt = 9.1 ms for BDP-TMR-alkyne at the plasma membrane [magenta arrows in (g,h)]. α = 0.02 and θ = 61°. (e,f) 1D distributions of the displacements in (d) projected along (e) and perpendicular (f) to θ. Blue curves: MLE fits to a 1D diffusion model, with resultant D = 2.3 and 2.2 μm2/s. (g) Color map presenting the calculated local θ (hue; inset color wheel) and α (color saturation; range 0–0.5) for the same region as Figure 2c. (h) pSMdM diffusivity map based on 1D diffusion fits along local principal directions. (i,j) SMdM (i) and pSMdM (j) diffusivity maps of another cell, acquired with tandem excitation pulses at Δt = 2.5 ms center-to-center separation, and presented on the same D color scale as (h). Asterisks mark a mitochondrion, for which D values are underestimated. (k) Distribution of single-molecule displacements in Δt for a region in the ER sheet [red arrow in (i)]. Blue curve: MLE fit, yielding D = 2.7 μm2/s. (l) Statistics for D values in the two samples obtained with isotropic SMdM (filled circles) and pSMdM (open diamonds). Each data point corresponds to an ~0.15 μm2 area containing signals solely from the plasma membrane (black), an ER sheet (red), or an ER tubule (blue). Scale bars: 2 μm (g,h,i,j).
We reason that the true diffusivity may still be obtained by examining displacements along the tubule. To this end, we started by determining the local displacement anisotropy α and principal direction θ (Figure 3a; Methods). Projecting single-molecule displacements along and perpendicular to θ gave contrasting distributions: the former (Figure 3b) represents nonrestraint diffusion along the tubule, which could be fitted to a modified one-dimensional (1D) random-walk model (Methods and Reference 20) to extract a D value (Figure 3b), whereas the latter (Figure 3c) is convolved with tubule width. In comparison, displacements at the plasma membrane were isotropic (Figure 3d), and comparable D values were obtained when the 1D diffusion model was applied to projections along and perpendicular to the near-arbitrarily defined θ (Figure 3ef). Applying the above analysis to every spatial bin showed well-defined directional preferences along ER tubules in the resultant θ-α color maps (Figure 3g and Figure S2). Projecting the single-molecule displacements in each bin along its θ direction next enabled their individual fitting to the 1D diffusion model.
Resultant principal-direction SMdM (pSMdM) diffusivity maps (Figure 3h, Figure S2, and Figure S3) showed D values higher in the ER-tubule membrane (~2.7 μm2/s; Figure 3b for typical 1D distribution of projected displacements) than in the plasma membrane (~2.2 μm2/s; Figure 3ef for typical 1D distributions of projected displacements), in line with their expected lower lipid packing order and hence possibly higher fluidity. To further substantiate this finding, we next compared results on 2D ER sheets, which we observed in a small fraction of the cells. Isotropic SMdM (Figure 3i; see Figure 3k for typical distribution of single-molecule displacements) and pSMdM (Figure 3j) both showed typical D values of ~2.7 μm2/s (Figure 3l), comparable to the pSMdM results above on the 1D ER tubules, yet substantially higher than the isotropic SMdM and pSMdM results on the plasma membrane (both at ~2.2 μm2/s; Figure 3l). Together, these results indicate significantly higher diffusivity in the ER membrane when compared to the plasma membrane. Separately, for the occasionally observed thin cell protrusions, pSMdM helped recover D values comparable to the typical plasma membrane, when isotropic SMdM yielded artificially reduced D due to the 1D geometry (white arrowheads in Figure 3ij). pSMdM, however, did not overcome the complex, cristae geometry of mitochondrial membranes, for which D values were still underestimated (e.g., marked by asterisks in Figure 3ij and Figure 4), awaiting future investigations.
Figure 4.
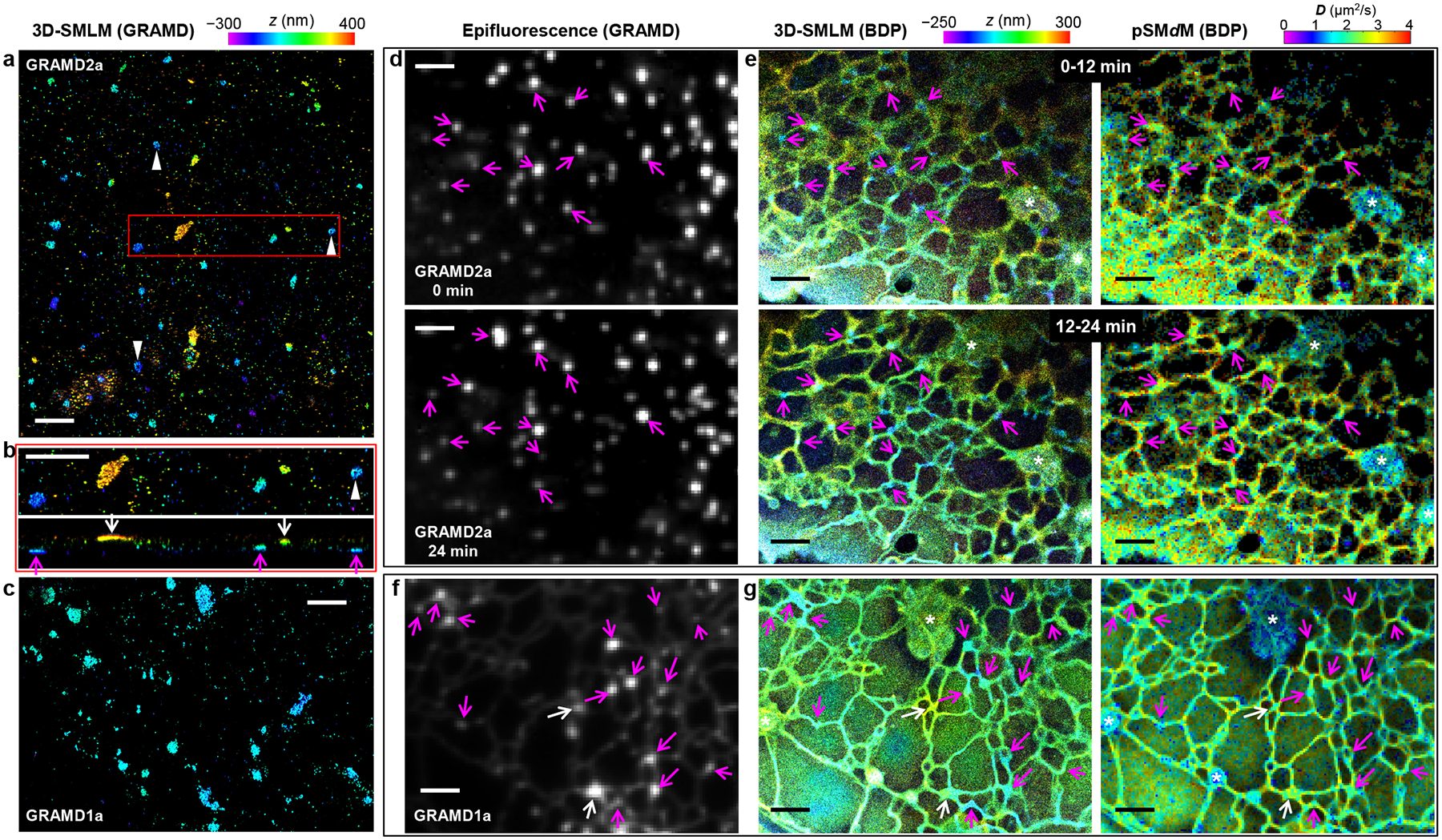
Concurrent SMdM and 3D-SMLM, together with fluorescent protein markers, indicate reduced membrane diffusivity at ER-PM contact sites. (a) 3D-SMLM image of immunolabeled GRAMD1a-GFP expressed in a COS-7 cell. Color presents depth (color scale on the top). (b) Zoom-in of the red box in (a) (top) and its vertical view in the xz direction (bottom). Magenta and white arrows point to apparent contact sites at the ventral and dorsal plasma membranes, respectively. (c) 3D-SMLM image of immunolabeled GRAMD1a-GFP at the ventral plasma membrane of another COS-7 cell. (d) Live-cell epifluorescence images of GRAMD2a-GFP expressed in a COS-7 cell, taken before (top) and after (bottom) stroboscopic PAINT of BDP-TMR-alkyne. (e) 3D-SMLM (left) and pSMdM (right) images (separate color scales on the top) of the same region via stroboscopic PAINT of BDP-TMR-alkyne, for two consecutive 12-min periods. Data were acquired with tandem excitation pulses of τ = 1 ms duration at Δt = 2.5 ms. Arrows in (d,e) point to example GRAMD2a-positive ER-PM contacts. Asterisks mark mitochondria. (f) Epifluorescence image of GRAMD1a-GFP expressed in another live COS-7 cell. (g) 3D-SMLM (left) and pSMdM (right) images of BDP-TMR-alkyne of the same region. Data were acquired with excitation pulses of τ = 1 ms duration at the middle of each frame at Δt = 9.1 ms. Magenta and white arrows in (f,g) point to example GRAMD1a-positive contact sites at the ventral and dorsal membranes, respectively. Scale bars: 2 μm.
Closer examination of both the isotropic and principal-direction SMdM data showed the ER diffusivity sporadically exhibit local reductions in D, often at tubule junctions and termini close to the plasma membrane (Figures 2, 3, S2, and S3). To examine the possibility that these low-diffusivity foci corresponded to ER-plasma membrane (ER-PM) contact sites,36–38 we expressed in the cells green fluorescent protein (GFP)-tagged GRAMD1a or GRAMD2a, which localizes to a subset of the EP-PM contacts.39 3D-SMLM through immunolabeling GFP in fixed cells showed GRAMD2a as ~100–500 nm patches at both the dorsal and ventral plasma membranes (Figure 4ab), often of slightly elongated shapes and occasionally with hollow centers (arrowheads). GRAMD1a similarly appeared as nanoscale patches, but with somewhat higher structural heterogeneities (Figure 4c).
To co-visualize these EP-PM contact site markers with membrane topography and diffusivity in live cells, we recorded epifluorescence images of GRAMD2a-GFP both before and after a long stroboscopic PAINT recording of BDP-TMR-alkyne over 24 min (Figure 4d). The single-molecule data were divided into two image stacks for the 0–12 min and 12–24 min periods, and each processed into matching 3D-SMLM and pSMdM images (Figure 4e). We thus found the GRAMD2a-GFP foci before and after the single-molecule recording separately matched the first and second 3D-SMLM images for ER tubule junctions/termini of low z values (arrows), indicative of contacts to the ventral plasma membrane. Notably, the concurrently obtained SMdM images showed substantially (~30%) reduced D at the same sites (Figure 4e), thus indicating that the ER-PM contacts maintained locally reduced membrane diffusivity as they reorganized in the live cell. In a different experiment on a thinner cell, GRAMD1a-GFP labeling (Figure 4f) and 3D-SMLM (Figure 4g) helped identify ER-PM contact sites at both the ventral (magenta arrows) and dorsal (white arrows) plasma membranes, and SMdM indicated similar diffusion slowdowns for both cases (Figure 4g).
To understand whether the observed diffusion slowdown at ER-PM contact sites could be related to changes in the lipid packing order as we showed above for the CTB treatment, we next integrated SMdM with SR-SMLM22,23 using the solvatochromic membrane probe Nile Red, which detects variations in lipid packing order through shifts in emission spectrum due to changes in the membrane chemical polarity.23,26,35 Although the lower single-molecule brightness of Nile Red (Figure 1de) was not optimal for 3D localization, it worked well for SMdM with stroboscopic excitation pulses of τ = 2 ms duration (Figure 5a). Reduced local diffusivity was thus similarly observed at ER-PM contact sites marked by GRAMD2a-GFP (Figure 5c). Meanwhile, the collected single-molecule emission spectra at these sites were identical to adjacent ER regions (Figure 5bd), indicating no significant changes in the local lipid order.23,26 In comparison, markedly bluer emission was observed for the plasma membrane (Figure 5bd) accompanying its lower diffusion rate, as expected from cholesterol-associated packing order.26,35 The ER-PM contact sites are characterized by a wealth of trans-ER-membrane proteins that interact with plasma-membrane components to achieve key functions as lipid exchange and Ca2+ regulation,36–38 and electron microscopy indicates high protein densities.40,41 It is thus possible that macromolecular crowding in the ER membrane could create a maze that impedes diffusion1,42–44 without altering the local lipid packing order.
Figure 5.
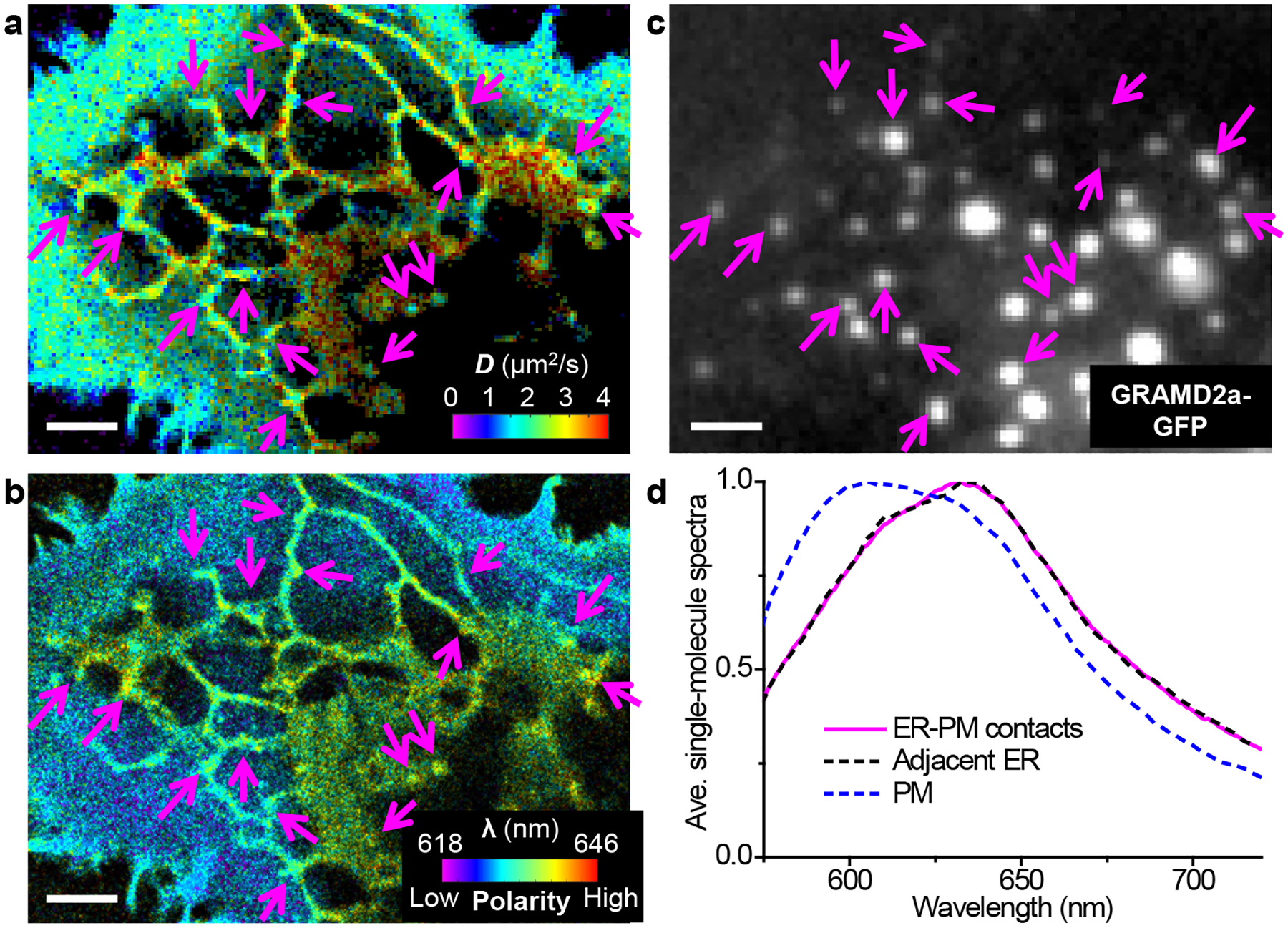
Concurrent SMdM and SR-SMLM of Nile Red indicate that the reduced diffusivity at ER-PM contacts is not associated with significant changes in lipid order. (a,b) Concurrently acquired pSMdM (a; color presents local diffusivity) and SR-SMLM (b; color presents the spectral mean of locally accumulated single molecules) images of Nile Red in a live COS-7 cell. Data were acquired with tandem excitation pulses of τ = 2 ms duration at Δt = 2.5 ms. (c) Epifluorescence image of GRAMD2a-GFP expressed in this cell. Arrows point to example GRAMD2a-positive ER-PM contact sites where substantial reductions in D are noticed in the pSMdM image. (d) Averaged local single-molecule spectra at the ER-PM contacts (magenta line) vs. adjacent ER regions (black dashed line), as well as at the plasma membrane (blue dashed line). Scale bars: 2 μm (a–c).
DISCUSSION
Although SPT has been valuable in probing diffusion at the nanoscale, its starting point is often to obtain long single-particle trajectories to enable their individual fitting to diffusion models. In contrast, SMdM focuses on examining, for every fixed location, how different (yet identical) molecules move within short time windows, with the explicit goal for a continuous, 2D spatial map of local diffusivity. The need to only detect single molecules in short time windows permits the use of probes that only emit transiently, yet at the same time, to obtain good local statistics demands high counts of single molecules. In this work, we thus started by identifying a new probe, BDP-TMR-alkyne, that exhibited excellent fluorescence turn-on and bright emission in the membrane phase while also enabling sustained stroboscopic PAINT experiments over long periods. High densities of single-molecule positions and displacements were thus obtained to achieve nanoscale fine-mapping of membrane topography and diffusivity. While not yet explored in this study, the alkyne functional group of this probe may be further utilized, e.g., click-reactions to specific lipids.
The resultant high-resolution diffusivity maps first resolved locally reduced D in CTB-induced nanodomains in the plasma membrane, a result echoing our recent SR-SMLM observation of locally reduced chemical polarities due to CTB-sequestrated GM1 and cholesterol.26 Whereas by globally altering the cholesterol level, previous work has examined how cholesterol-induced packing order reduces the fluidity of both cell and model membranes at the bulk level,30–33 SMdM helped unveil this effect locally for the nanoscale, presumably more orderly packed phases induced by CTB.
The apparent dependency of diffusivity on the local cholesterol level next prompted us to scrutinize diffusivity in the ER membrane, where a lower cholesterol level and packing order are expected when compared to the plasma membrane.26,34,35 We thus identified a geometrical effect that led to the underestimation of D in 1D structures like ER tubules, and devised a new data analysis framework, pSMdM, to overcome this issue by projecting single-molecule displacements along local principal directions. By fitting the projected displacements to a modified 1D random-walk model, we thus obtained comparable D values for the 1D ER tubules and the occasionally observed 2D ER sheets, while also showing them to be substantially higher than the plasma membrane of the same cells. Together with the CTB results above, we thus determined a trend that the membrane diffusivity drops with increased lipid packing order.
Combining the D mapping capability of SMdM with 3D localization while further incorporating specific fluorescent protein markers, we next unveiled reduced diffusivity in the ER membrane at ER-PM contact sites. By next integrating SR-SMLM, we showed that this effect was not due to altered local lipid order, hence implicating protein crowding as a possible source of the diffusion slowdown. Experimental work on cells and model lipid bilayers, together with theory, has well addressed how protein crowding impedes membrane diffusion at the bulk level.1,4–44 However, as previous diffusion measurements offer limited mapping capabilities, how such effects might locally modulate membrane diffusivity remains elusive. By offering fine maps of local diffusivity, SMdM unveiled local diffusion slowdowns at the ER-PM contact sites, consistent with their high protein densities observed in electron microscopy.40,41 Such effects may help stabilize the contact sites and assist material exchanges36–38 between the ER and the plasma membrane, the potential consequences of which call for future experimental and theoretical investigations. Meanwhile, future SMdM and SR-SMLM studies would benefit from solvatochromic membrane probes that specifically label either the plasma membrane or the ER membrane alone.
Together, as a timely integration of emerging modes of functional single-molecule and super-resolution approaches,45 our work thus unveiled and differentiated between nanoscale diffusional heterogeneities of different origins in live-cell membranes.
Supplementary Material
ACKNOWLEDGMENTS
We thank W. Li and S. Moon for discussion. This work was supported by the National Science Foundation (CHE-1554717), the National Institute of General Medical Sciences of the National Institutes of Health (DP2GM132681), the Beckman Young Investigator Program, and the Packard Fellowships for Science and Engineering, to K.X. K.X. is a Chan Zuckerberg Biohub investigator.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Materials and methods; Spectral properties of BDP-TMR-alkyne in different solvents; Additional stroboscopic PAINT and SMdM results of BDP-TMR-alkyne on COS-7 cells, acquired with varied excitation pulse durations and separations; Comparison of SMdM results for CTB-treated and untreated cells on a stretched D scale (PDF)
REFERENCES
- (1).Ramadurai S; Holt A; Krasnikov V; van den Bogaart G; Killian JA; Poolman B Lateral diffusion of membrane proteins. J. Am. Chem. Soc 2009, 131, 12650–12656. [DOI] [PubMed] [Google Scholar]
- (2).Honigmann A; Mueller V; Ta H; Schoenle A; Sezgin E; Hell SW; Eggeling C Scanning STED-FCS reveals spatiotemporal heterogeneity of lipid interaction in the plasma membrane of living cells. Nat. Commun 2014, 5, 5412. [DOI] [PubMed] [Google Scholar]
- (3).Trimble WS; Grinstein S Barriers to the free diffusion of proteins and lipids in the plasma membrane. J. Cell Biol 2015, 208, 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Albrecht D; Winterflood CM; Sadeghi M; Tschager T; Noe F; Ewers H Nanoscopic compartmentalization of membrane protein motion at the axon initial segment. J. Cell Biol 2016, 215, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Jacobson K; Liu P; Lagerholm BC The lateral organization and mobility of plasma membrane components. Cell 2019, 177, 806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lippincott-Schwartz J; Snapp E; Kenworthy A Studying protein dynamics in living cells. Nat. Rev. Mol. Cell Biol 2001, 2, 444–456. [DOI] [PubMed] [Google Scholar]
- (7).Machan R; Wohland T Recent applications of fluorescence correlation spectroscopy in live systems. FEBS Lett. 2014, 588, 3571–3584. [DOI] [PubMed] [Google Scholar]
- (8).Kusumi A; Tsunoyama TA; Hirosawa KM; Kasai RS; Fujiwara TK Tracking single molecules at work in living cells. Nat. Chem. Biol 2014, 10, 524–532. [DOI] [PubMed] [Google Scholar]
- (9).Cognet L; Leduc C; Lounis B Advances in live-cell single-particle tracking and dynamic super-resolution imaging. Curr. Opin. Chem. Biol 2014, 20, 78–85. [DOI] [PubMed] [Google Scholar]
- (10).Rust MJ; Bates M; Zhuang XW Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Betzig E; Patterson GH; Sougrat R; Lindwasser OW; Olenych S; Bonifacino JS; Davidson MW; Lippincott-Schwartz J; Hess HF Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [DOI] [PubMed] [Google Scholar]
- (12).Hess ST; Girirajan TPK; Mason MD Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J 2006, 91, 4258–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Sharonov A; Hochstrasser RM Wide-field subdiffraction imaging by accumulated binding of diffusing probes. Proc. Natl. Acad. Sci. U. S. A 2006, 103, 18911–18916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Manley S; Gillette JM; Patterson GH; Shroff H; Hess HF; Betzig E; Lippincott-Schwartz J High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 2008, 5, 155–157. [DOI] [PubMed] [Google Scholar]
- (15).Shim SH; Xia C; Zhong G; Babcock HP; Vaughan JC; Huang B; Wang X; Xu C; Bi GQ; Zhuang X Super-resolution fluorescence imaging of organelles in live cells with photoswitchable membrane probes. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 13978–13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hoze N; Nair D; Hosy E; Sieben C; Manley S; Herrmann A; Sibarita JB; Choquet D; Holcman D Heterogeneity of AMPA receptor trafficking and molecular interactions revealed by superresolution analysis of live cell imaging. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 17052–17057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Giannone G; Hosy E; Levet F; Constals A; Schulze K; Sobolevsky AI; Rosconi MP; Gouaux E; Tampe R; Choquet D; Cognet L Dynamic superresolution imaging of endogenous proteins on living cells at ultra-high density. Biophys. J 2010, 99, 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Winckler P; Lartigue L; Giannone G; De Giorgi F; Ichas F; Sibarita JB; Lounis B; Cognet L Identification and super-resolution imaging of ligand-activated receptor dimers in live cells. Sci. Rep 2013, 3, 2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Masson JB; Dionne P; Salvatico C; Renner M; Specht CG; Triller A; Dahan M Mapping the energy and diffusion landscapes of membrane proteins at the cell surface using high-density single-molecule imaging and Bayesian inference: application to the multiscale dynamics of glycine receptors in the neuronal membrane. Biophys. J 2014, 106, 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Xiang L; Chen K; Yan R; Li W; Xu K Single-molecule displacement mapping unveils nanoscale heterogeneities in intracellular diffusivity. Nat. Methods 2020, 17, 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Gao F; Mei E; Lim M; Hochstrasser RM Probing lipid vesicles by bimolecular association and dissociation trajectories of single molecules. J. Am. Chem. Soc 2006, 128, 4814–4822. [DOI] [PubMed] [Google Scholar]
- (22).Zhang Z; Kenny SJ; Hauser M; Li W; Xu K Ultrahigh-throughput single-molecule spectroscopy and spectrally resolved super-resolution microscopy. Nat. Methods 2015, 12, 935–938. [DOI] [PubMed] [Google Scholar]
- (23).Yan R; Moon S; Kenny SJ; Xu K Spectrally resolved and functional super-resolution microscopy via ultrahigh-throughput single-molecule spectroscopy. Acc. Chem. Res 2018, 51, 697–705. [DOI] [PubMed] [Google Scholar]
- (24).Hughes LD; Rawle RJ; Boxer SG Choose your label wisely: water-soluble fluorophores often interact with lipid bilayers. PLoS One 2014, 9, e87649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Lew MD; Lee SF; Ptacin JL; Lee MK; Twieg RJ; Shapiro L; Moerner WE Three-dimensional superresolution colocalization of intracellular protein superstructures and the cell surface in live Caulobacter crescentus. Proc. Natl. Acad. Sci. U. S. A 2011, 108, E1102–E1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Moon S; Yan R; Kenny SJ; Shyu Y; Xiang L; Li W; Xu K Spectrally resolved, functional super-resolution microscopy reveals nanoscale compositional heterogeneity in live-cell membranes. J. Am. Chem. Soc 2017, 139, 10944–10947. [DOI] [PubMed] [Google Scholar]
- (27).Huang B; Wang W; Bates M; Zhuang X Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 2008, 319, 810–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Nishimura SY; Lord SJ; Klein LO; Willets KA; He M; Lu Z; Twieg RJ; Moerner WE Diffusion of lipid-like single-molecule fluorophores in the cell membrane. J. Phys. Chem. B 2006, 110, 8151–8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Danylchuk DI; Moon S; Xu K; Klymchenko AS Tailor-made switchable solvatochromic probes for live-cell super-resolution imaging of plasma membrane organization. Angew. Chem. Int. Ed 2019, 58, 14920–14924. [DOI] [PubMed] [Google Scholar]
- (30).Cooper RA Influence of increased membrane cholesterol on membrane fluidity and cell function in human red blood cells. J. Supramol. Struct 1978, 8, 413–430. [DOI] [PubMed] [Google Scholar]
- (31).Johnson ME; Berk DA; Blankschtein D; Golan DE; Jain RK; Langer RS Lateral diffusion of small compounds in human stratum corneum and model lipid bilayer systems. Biophys. J 1996, 71, 2656–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Korlach J; Schwille P; Webb WW; Feigenson GW Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc. Natl. Acad. Sci. U. S. A 1999, 96, 8461–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Goodwin JS; Drake KR; Remmert CL; Kenworthy AK Ras diffusion is sensitive to plasma membrane viscosity. Biophys. J 2005, 89, 1398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).van Meer G; Voelker DR; Feigenson GW Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol 2008, 9, 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Klymchenko AS Solvatochromic and fluorogenic dyes as environment-sensitive probes: design and biological applications. Acc. Chem. Res 2017, 50, 366–375. [DOI] [PubMed] [Google Scholar]
- (36).Saheki Y; De Camilli P Endoplasmic reticulum-plasma membrane contact sites. Annu. Rev. Biochem 2017, 86, 659–684. [DOI] [PubMed] [Google Scholar]
- (37).Wu H; Carvalho P; Voeltz GK Here, there, and everywhere: The importance of ER membrane contact sites. Science 2018, 361, eaan5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Chen YJ; Quintanilla CG; Liou J Recent insights into mammalian ER-PM junctions. Curr. Opin. Cell Biol 2019, 57, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Besprozvannaya M; Dickson E; Li H; Ginburg KS; Bers DM; Auwerx J; Nunnari J GRAM domain proteins specialize functionally distinct ER-PM contact sites in human cells. eLife 2018, 7, e31019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Fernández-Busnadiego R; Saheki Y; De Camilli P Three-dimensional architecture of extended synaptotagmin-mediated endoplasmic reticulum–plasma membrane contact sites. Proc. Natl. Acad. Sci. U. S. A 2015, 112, E2004–E2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Wu YM; Whiteus C; Xu CS; Hayworth KJ; Weinberg RJ; Hess HF; De Camilli P Contacts between the endoplasmic reticulum and other membranes in neurons. Proc. Natl. Acad. Sci. U. S. A 2017, 114, E4859–E4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Frick M; Schmidt K; Nichols BJ Modulation of lateral diffusion in the plasma membrane by protein density. Curr. Biol 2007, 17, 462–467. [DOI] [PubMed] [Google Scholar]
- (43).Goose JE; Sansom MSP Reduced lateral mobility of lipids and proteins in crowded membranes. PLoS Comput. Biol 2013, 9, e1003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Guigas G; Weiss M Effects of protein crowding on membrane systems. Biochim. Biophys. Acta 2016, 1858, 2441–2450. [DOI] [PubMed] [Google Scholar]
- (45).Yan R; Wang B; Xu K Functional super-resolution microscopy of the cell. Curr. Opin. Chem. Biol 2019, 51, 92–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


