Abstract
Background
This systematic and meta-analytic review aimed to investigate the effects of physical exercise on the working memory of older adults, and to identify the moderators of these effects.
Methods
We searched six electronic databases for randomized controlled trials on the effects of physical exercise on working memory that were published before or on May 15, 2020. The PEDro scale was used to evaluate the methodological quality of the included studies. Stata 14.0 software was used to perform the meta-analysis, subgroup analysis, and publication bias testing.
Results
A total of 28 studies and 2156 participants were included. The methodological quality of the included studies was fair to excellent, and there was no publication bias. Overall, we found that physical exercise had a significant effect on working memory in older adults (standardized mean difference = 0.30, p < 0.0001). The effects of physical exercise on working memory were moderated by exercise frequency, intensity, type, duration, cognitive status, and control subgroup (active/passive), but not by intervention period or age of participant.
Conclusion
Physical exercise can effectively improve the working memory of older adults. The recommended physical exercise is multi-component exercise or mind–body exercise of moderate intensity for 45–60 min 3 times a week, for more than 6 months.
Supplementary Information
The online version contains supplementary material available at 10.1186/s11556-021-00272-y.
Keywords: Physical exercise, Working memory, Older adults, Randomized controlled trial, meta-analysis
Background
Working memory (WM) refers to a system in which individuals temporarily store and manipulate information during complex cognitive tasks [1]. WM is considered to be a core cognitive function, because it underlies the brain’s ability to simultaneously store and manipulate information. WM is closely related to activity of the frontal and parietal networks, and the prefrontal cortex (PFC) in particular is considered to be an important brain area involved in WM [2]. Within the brain network, PFC is associated with the executive processing components, while the medial temporal cortex and hippocampus are associated to encoding and retrieval [3]. Parietal brain regions are associated with the temporary storage components [4], where the integration of visuospatial and associative information takes place [5].
WM is a core cognitive function. Age-related neural changes in brain networks results in a WM performance decline with increasing age. The best WM performance has been reported to be at about the age of 30 years, and to decrease significantly after the age of 60 years [6]. Both human and animal studies have found that PFC activity decreases with age [7, 8]. Mattay et al. found that while there was no difference in the performance of the 1-back task between younger and older people, the older group exhibited more activation in the bilateral frontal cortex; that study also found that older people performed worse on the 2-back task than younger people, and this was accompanied by less PFC activation [9].
An increasing amount of research has shown that physical exercise can improve cognitive functioning. This is especially true for executive functioning, which is closely related to frontal lobe activity [10]. Physical exercise is considered to be a safe treatment option for WM decline [11]. In a randomized controlled trial (RCT) with 120 older adults, Erikson et al. found that aerobic exercise (AE) training increased the size of the anterior hippocampus, and that this was associated with improvements in spatial memory [12]. Ikudome et al. found that even simple resistance exercise (RE), which uses only body mass for resistance, may be an effective method for preventing the age-related cognitive decline of inhibitory control and WM in older people [13]. In another study, Yang et al. allocated 52 older women into a Tai Chi Chuan group, square dancing group, and control group. After the 6-month intervention period, the reaction time and accuracy rate of the n-back task in the Tai Chi Chuan and square dancing groups improved alongside a P3 amplitude increase and latency decrease, which indicated that the Tai Chi Chuan and square dancing interventions improved the WM of older women [14]. Weuve et al. found that higher levels of activity were associated with a better backward memory span, and also observed less cognitive decline among more active women [15]. Hatta et al. examined the effects of physical activity on WM (which was measured using the Sternberg task) in older adults, and found both behavioral and neurophysiological evidence for the positive role of exercise [16]. Namely, the high exercise group had significantly faster reaction times and a larger P3 amplitude than the low exercise group, but there was no significant between-group difference in latency. Chang et al. used the same research design and found that the high exercise group had a significantly larger N1 amplitude than the low physical activity group.
However, some studies have revealed different results. Kramer et al. found no improvements in the accuracy of n-back task or in digit span test (DST) performance after an AE intervention [17]. Gothe et al. found that the reaction time and accuracy of the n-back task after 20 min of yoga were better than those observed after moderate-intensity AE [18]. One reason for the inconsistency of these research results may be the variability in the exercise features (e.g., frequency, intensity, duration, type, and intervention period), which could engage mechanisms underlying WM improvements in different ways. Another potential reason for these different research results is the use of different WM measurement tools, such as the DST, n-back task, and Sternberg task. WM is a complex advanced cognitive function. Baddeley has argued that WM consists of at least three parts – central execution, phonetic loop, and visual-spatial storage [19] – which involve multiple processes, such as encoding, maintenance, updating, attention, and inhibition. Each measurement tool assesses different sub-components of WM. Thus, it is difficult to comprehensively investigate the intervention effect of physical exercise on WM using one single paradigm. Finally, individual differences such as age, cognitive status, and education level will also affect the efficacy of an intervention.
Previous meta-analyses have paid little attention to the intervention effect of physical exercise on WM, especially in older adults. The populations included in these reviews were either patients with Parkinson’s disease and schizophrenia [20] or healthy older adults [21]. No meta-analysis has considered participants with normal cognition and patients with mild cognitive impairment (MCI) at the same time. Some reviews have indicated that age, the type of WM test, and exercise intensity moderate the relationship between physical exercise and WM. However, these prior reviews have offered relatively little information about the optimal prescription of physical exercise features for improving WM [22, 23]. To address these gaps in the literature and provide a theoretical basis for accurate exercise prescription, this study analyzed the effects of exercise interventions on WM and examined whether these effects are moderated by variations in the features of physical exercise.
Methods
This study was performed and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses [24]. We pre-registered our meta-analytic review at PROSPERO (CRD42021230431).
We searched six electronic databases (PubMed, Embase, The Cochrane Library, Web of Science, PsycINFO, China National Knowledge Infrastructure) from inception to April 13, 2020. According to the reviewer’s suggestion, we conducted a new literature search on April 12, 2021. Two researchers (CZD and YJL) independently used the following search terms (among others) for retrieval: “exercise”, “physical activity”, “fitness”, “aerobic exercise”, “cardiovascular exercise”, “resistance training”, “stretching”, “mind–body exercise”, “flexibility exercise”, “cognitive function”, “executive function”, “working memory”, “old people”, “old adults”, “randomized controlled trial”. The retrieval strategy adopted the combination of subject words and free words, and was determined after repeated prechecking. Language and publication types were not limited in the literature retrieval step.
|
Search strategy of Pubmed #1 physical exercise OR physical activity OR exercise OR fitness OR training OR aerobic exercise OR cardiovascular exercise OR resistance training OR stretching OR mind-body exercise OR flexibility exercise #2 working memory OR cognitive task OR executive function OR executive control OR updating OR short-term memory #3 old people OR elderly OR old age OR the aged OR senior citizen #4 randomized controlled trial OR controlled clinical trial OR RCT OR clinical intervention #5 #1 AND #2 AND #3 AND #4 |
Eligibility criteria
Two researchers (CZD and YJL) independently screened the literature according to the inclusion and exclusion criteria. After the screening, any discrepancy between the two researchers was resolved through discussions with the other two researchers (SDH and YJL) until consensus was reached.
The inclusion criteria were as follows: (1) the subjects were older adults; (2) the intervention was AE, RE, multi-component exercise (MCE), or mind–body exercise (MBE); (3) all or some of the outcome indicators were WM; (4) the study was an RCT.
We set the following exclusion criteria: (1) the subjects were older adults with dementia or mental disorders; (2) the intervention program contained confounding factors other than exercise, such as cognitive training, vitamin supplements, and drugs; (3) the study data could not be extracted, even after contacting the authors; (4) publications that were qualitative studies, case studies, reviews, non-intervention studies, or conference papers.
Data extraction
Two researchers (CZD and YJL) independently extracted the relevant information using a standardized form. Where data were missing or could not be extracted due to insufficient statistical reporting, we contacted the author(s) to request the missing data.
Extraction contents and coding were as follows. First, we captured the basic details of each study, including the names and nationalities of authors and the year of publication. Second, we collated and processed the basic details of the subjects, including cognitive status, sample size, age, and education level. Third, we captured data on the five following exercise prescription variables: frequency, intensity, duration, type, and intervention period [23]. Exercise frequency was classified according to the number of exercise sessions per week, as follows: low frequency: ≤ 2 times; moderate frequency: 3–4 times; high frequency: ≥ 5 times. Exercise intensity was classified as low, moderate, vigorous. Exercise type was classified as AE, RE, MCE, or MBE. Exercise duration (the minutes each session lasted) was classified as follows: short: ≤ 45 min; moderate: > 45 min to ≤60 min; long: > 60 min. Intervention period was classified according to the length of the intervention period, as follows: short: 4–12 weeks; mid-length: 13–24 weeks; long: > 24 weeks. Fourth, the control group was classified as follows: active control subgroup (who participated in stretching, health education, and/or social assembly) and passive control subgroup (who received no intervention). Finally, the main outcome index was DST result, and the secondary outcome indexes were the n-back, verbal span, Corsi block-tapping, executive control (EC), spatial span (SS), and letter-number sequence tasks. All behavioral measures of WM were extracted in the form of the mean and standard deviation.
Assessment of study quality
Methodological quality was independently evaluated by two researchers (CZD and YJL) using the Physiotherapy Evidence Database (PEDro) scale [25]. The PEDro scale comprises the 11 following items: eligibility criteria, randomization, concealed allocation, similar baseline, blinding of subjects, blinding of therapists, blinding of assessors, more than 85% retention, intent-to-treat analysis, between-group comparison, point measure, and measures of variability. The “eligibility criteria” item is not scored. One point is assigned to each item for which relevant information is explicitly presented, and the maximum score for any given study is 10 (9–10 = excellent quality, 6–8 = good quality, 4–5 = fair quality, < 4 = poor quality).
Statistical analysis
Stata 14.0 software (Stata, Texas, USA) was utilized for data analysis. Extracted data included the mean (M) and standard deviation (SD) of each group at postintervention, and the sample size. The standardized mean difference was selected as the magnitude of effect sizes (ESs). ESs were calculated by Cohen’s d, taking 0.2, 0.5, and 0.8 as the respective thresholds for small, medium, and large effects [26]. Heterogeneity was calculated using Higgins’s I2 statistics, taking 75, 50, and 25% as the respective thresholds for high, medium, and low ratios of inter-study heterogeneity [27]. Publication bias was tested using the Egger test in Stata 14.0.
After calculating the overall ES for WM, subgroup analyses were conducted for the measures of WM (e.g., DST-Backward (DSB), DST-Forward (DSF), n-back, and spatial span tasks), exercise prescription features (frequency, intensity, type, duration, length), and participant characteristics (age, control group, and cognitive status). We provided Forest plots of subgroups. Funnel plots of the ES against the standard error of the ES were visually inspected for small-sample bias, and Egger’s test values with 95% confidence intervals for funnel plot asymmetry were calculated.
Results
Literature search
Figure 1 summarizes the flow of the literature search and study selection. The initial search returned 5340 articles. After removing 1475 duplicate articles and 3690 articles according to the inclusion/exclusion criteria and abstract screening, 28 articles were finally included in this review.
Fig. 1.
Literature Selection Flow Diagram
Study characteristics
Table 1 presents the characteristics of all 28 studies included in this review. The sample size ranged from 19 to 210. The overall sample size was 2063, including 1016 participants in the experimental groups and 1047 in the control groups. Among the 28 studies included, participants of 11 articles were patients with MCI, and participants of 17 articles were normal older adults. Participants’ age ranged from 62 to 86 years. Participants were mainly female, except the Norouzi et al. study which only included men as the research subjects, Liu-ambrose and Damirchi only included women as the research subjects, and the remaining studies had no sex-based restrictions. The studies were performed in 16 countries, including Asian countries (16 papers, accounting for 57.1%), America (5 papers, accounting for 17.9%), European countries (5 papers, accounting for 17.9%), and Australia (2 papers, accounting for 7.1%).
Table 1.
Basic characteristics of the literature included in the study(M ± SD)
| Study | Country | Cognitive status | Sample(E/C) | Age(E/C) | Female proportion(E/C) | Education level(E/C) |
|---|---|---|---|---|---|---|
| Brown 2009a [49] | Astralia | N | 66/34 | 79.5 ± 5.9/78.1 ± 6.4 | 86.6/86.8 | 10.6 ± 2.4/10.3 ± 2.3 |
| Brown 2009b [49] | Astralia | N | 26/34 | 81.5 ± 6.9/78.1 ± 6.4 | 91.6/86.8 | 10.2 ± 1.9/10.3 ± 2.3 |
| Eggenberger 2016 [28] | Switzerland | N | 19/14 | 72.8 ± 5.9/77.8 ± 7.4 | 63.2/64.3 | 13.4 ± 1.8/13.6 ± 2.1 |
| Gothe 2016 [62] | America | N | 61/57 | C62.1 ± 5.8/62.0 ± 5.4 | 80.3/75.4 | >college degree 77/56.2 |
| Albinet 2016 [29] | America | N | 19/17 | 67 ± 5/66 ± 5 | 68.4/76.5 | 11.9 ± 3.9/11.6 ± 2.1 |
| Kalbe 2018 [50] | German | N | 18/17 | 68.2 ± 8.0/67.5 ± 5.9 | 61.1/58.8 | 14.4 ± 3.5/14.5 ± 2.9 |
| Liu-ambrose 2010a [37] | Canada | N | 46/47 | 69.4 ± 3.0/70.0 ± 3.3 | 100/100 | >high school 98.2/98 |
| Liu-ambrose 2010b [37] | Canada | N | 42/47 | 69.5 ± 2.7/70.0 ± 3.3 | 100/100 | >high school 98.2/98 |
| Hariprasad 2013 [43] | India | N | 60/60 | 75.74/74.78 | 58.1/62.1 | 13.1 ± 4.1/11.4 ± 4.4 |
| Norouzi 2019 [38] | Iran | N | 20/20 | 68.3 ± 4.1/68.1 ± 3.7 | 0/0 | NR |
| Nouchi 2013 [51] | Japan | N | 32/32 | 66.8 ± 4.6/67.1 ± 2.8 | NR | 13.4 ± 1.9/13.2 ± 2.0 |
| Lachman 2006 [39] | America | N | 102/108 | 75.3 ± 7.4/74.6 ± 6.5 | Total 77.6 | 14.3 ± 2.7/13.9 ± 3.1 |
| Ferreira 2015 [30] | Brazil | N | 22/22 | 66.2 ± 5.6/69.2 ± 4.8 | 68.2/86.3 | 12.9 ± 2.7/12.9 ± 2.5 |
| Vaughn 2014 [52] | Astralia | N | 25/23 | 69.0 ± 3.1/68.8 ± 3.5 | NR | 12.3 ± 2.9/12.7 ± 3.5 |
| Fabre 2002 [31] | France | N | 8/8 | 65.4 ± 2.2 /65.7 ± 1.5 | 87.5/75 | 11.2 ± 1.3/12.1 ± 1.4 |
| Shan 2016 [44] | China | N | 25/20 | 61.2 ± 5.3/59.1 ± 4.9 | 68/85 | 9.4 ± 3.1/8.8 ± 3.2 |
| Li 2016 [45] | China | N | 28/29 | 66.6 ± 4.0/65.9 ± 5.1 | NR | >high school 50/48.3 |
| Yang 2019 [46] | China | N | 13/13 | 66.3 ± 4.3/65.9 ± 3.5 | 76.9/76.9 | >high school 100/100 |
| Hong 2017b [40] | Korea | N | 13/12 | 75.9/73.2 | 83.3/46.1 | NR |
| Hong 2017a [40] | Korea | MCI | 10/12 | 77.9/75.9 | 70/75 | NR |
| Bae 2019 [53] | Japan | MCI | 41/42 | 75.5 ± 6.0/76.4 ± 5.0 | 43.9/52.4 | 11.1 ± 2.2/10.9 ± 2.3 |
| Damirchi 2018a [54] | Iran | MCI | 11/9 | 68.8 ± 3.7/69.1 ± 4.9 | 100/100 | 3.4 ± 1.0/3.2 ± 1.2 |
| Damirchi 2018b [54] | Iran | MCI | 13/9 | 67.8 ± 4.7/69.1 ± 4.9 | 100/100 | 2.8 ± 0.9/3.2 ± 1.2 |
| Donnezan 2018 [28] | France | MCI | 18/14 | 77.1 ± 1.44/79.2 ± 4 | NR | 6.1 ± 0.4/5.8 ± 0.4 |
| Yoon 2018 [41] | Korea | MCI | 20/23 | 73.8 ± 4.4/74.0 ± 4.3 | 70/69.6 | 8.1 ± 3.5/9.8 ± 4.4 |
| Eggermont 2009 [32] | Korea | MCI | 51/46 | 85.4 | NR | NR |
| Lam 2010 [47] | China | MCI | 135/194 | 77.2 ± 6.3/78.3 ± 6.6 | NR | 4.1 ± 4.3/2.6 ± 3.2 |
| Scherder 2005 [33] | Netherlands | MCI | 15/15 | 84 ± 6.4/86 ± 5.1 | 86.7/93.3 | 2.6 ± 1.1/2.7 ± 1.7 |
| Sungkarat 2016 [48] | Thailand | MCI | 33/33 | 68.3 ± 6.7/67.5 ± 7.3 | 93.9/78.8 | 11.4 ± 5.1/9.3 ± 5.5 |
| Zhu 2018 [34] | China | MCI | 29/31 | 70.3 ± 6.7/69 ± 7.3 | 51.7/67.7 | >high school 86.2/90.3 |
| Lü 2015 [42] | China | MCI | 22/23 | 69 ± 3.83/70.43 ± 5.53 | 72.7/69.6 | 9.8 ± 2.8/9.5 ± 2.6 |
| Nishiguchi 2015 [35] | Japan | N | 24 /24 | 73.0 ± 4.8/73.5 ± 5.6 | 73.0 ± 4.8/73.5 ± 5.6 | 12.2 ± 2.2/13.0 ± 2.5 |
N normal cognition, MCI Mild Cognitive Impairment, NR No Reported, E/C Experiment group/Control group
As shown in Table 2, all or some of the exercise variables were reported in the 28 included studies. There were four types of physical exercise in the 28 included studies, as follows: AE (n = 9) [28–36], RE (n = 6) [37–42], MBE (n = 7) [18, 43–48], and MCE (n = 6) [49–54]. Exercise frequency varied from one to five times/week, with three times/week being the most common; exercise duration varied from 30 min to 90 min, with 60 min being most common; and exercise program length varied from 4 weeks to 52 weeks, with 24 weeks being most common. The index of exercise intensity varied between studies, but most studies adopted moderate-intensity exercise. Among the 28 studies, 15 included a passive control group and 13 included an active control group (social activities, n = 5; health education, n = 3; stretching exercises, n = 4; and cognitive training, n = 1).
Table 2.
Intervention Characteristics Included in the Study
| Study | Exercise type | Exercise prescription variables | Outcomes | ||||
|---|---|---|---|---|---|---|---|
| Experiment group | Control group | Length (week) | Frequency (times/week) | Session time (minites/times) | Intensity | ||
| Brown 2009a [49] | MCE | Wait list | 24 | 2 | 60 | NR | (1) |
| Brown 2009b [49] | MCE | Wait list | 24 | 2 | 60 | NR | (1) |
| Eggenberger 2016 [28] | AE | Balance | 8 | 3 | 30 | NR | (6) |
| Gothe 2016 [62] | MBE | Stretching | 8 | 3 | NR | NR | (3), (5) |
| Albinet 2016 [29] | AE | Stretching | 20 | 2 | 60 | 40–65%HRR | (3), (5), (7) |
| Kalbe 2018 [50] | MCE | Cognitive training | 7 | 2 | 90 | NR | (1) |
| Liu-ambrose 2010a [37] | RE | Balance | 52 | 1 | 60 | 80–100%1RM | (5) |
| Liu-ambrose 2010b [37] | RE | Balance | 52 | 2 | 60 | 80–100%1RM | (5) |
| Hariprasad 2013 [43] | MBE | Wait list | 24 | 1 | 60 | NR | (1), (7) |
| Norouzi 2019 [38] | RE | Social visit | 4 | 3 | 60–80 | NR | (3) |
| Nouchi 2013 [51] | MCE | Wait list | 4 | 3 | 30 | 60–80%HRmax | (1) |
| Lachman 2006 [39] | RE | Wait list | 24 | 3 | 30 | 10RM | (1) |
| Ferreira 2015 [30] | AE | Social visit | 24 | 3 | 40–50 | 60–80%HRR | (1) |
| Vaughn 2014 [52] | MCE | Wait list | 16 | 2 | 60 | NR | (8) |
| Fabre 2002 [31] | AE | Draw and sing | 8 | 2 | 60 | NR | (1) |
| Shan 2016 [44] | MBE | Wait list | 12 | 5 | 60 | NR | (1) |
| Li 2016 [45] | MBE | Health education | 24 | 3 | 60 | 55–75%HRmax | (1) |
| Yang 2019 [46] | MBE | Daily activities | 8 | 3 | 45 | NR | (3) |
| Hong 2017b [40] | RE | Wait list | 12 | 2 | 60 | 15RM | (1) |
| Hong 2017a [40] | RE | Wait list | 12 | 2 | 60 | 15RM | (1) |
| Bae 2019 [53] | MCE | Health education | 24 | 2 | 90 | NR | (4) |
| Damirchi 2018a [54] | MCE | Wait list | 24 | 3 | 45 | 55–75%HRR, RPE13–15 | (1) |
| Damirchi 2018b [54] | MCE | Wait list | 24 | 3 | 45 | 55–75%HRR, RPE13–15 | (1) |
| Donnezan 2018 [28] | AE | Wait list | 12 | 2 | 60 | 60% HRmax | (1) |
| Yoon 2018 [41] | RE | Stretching | 16 | 3 | 60 | RPE12–13 | (1) |
| Eggermont 2009 [32] | AE | Social visit | 6 | 5 | 30 | NR | (1) |
| Lam 2010 [47] | MBE | Stretching | 8 | 3 | 30 | NR | (1) |
| Scherder 2005 [33] | AE | Wait list | 6 | 3 | 30 | NR | (1) |
| Sungkarat 2016 [48] | MBE | Health education | 15 | 3 | 50 | NR | (1) |
| Zhu 2018 [34] | AE | General care | 12 | 3 | 35 | 60–80% HRmax | (1) |
| Lü 2015 [42] | RE | Wait list | 12 | 3 | 60 | NR | (1) |
| Nishiguchi 2015 [35] | AE | Wait list | 12 | 1 | 90 | NR | (3) |
Digit Span;(2) Sternberg task;(3)N-back task;(4) Corsi block-tapping task;(5) Verbal Span;(6) Executive Control task;(7) Spatial Span; (8)Letter-Number Sequencing test; HRR heart rate reserve, HRmax Maximum heart rate, RPE rating of perceived exertion, VO2max Maximum oxygen uptake, “RM” Repetition Maximum
Methodological quality
The methodological quality of the included studies is reported in Table 3. The PEDro scores of the included studies ranged from 5 to 10 points, with an average of 7 points. The overall methodological quality was fair to excellent, with PEDro scores ≥6 for 15 studies (good), PEDro scores of 4–5 for 7 studies (fair), and PEDro scores of 9–10 for 6 studies (excellent). All the included studies carried out randomization, between-group comparisons, point measure, and measures of variability. A total of 16 studies used concealed allocation, 6 studies used blinding of assessors, blinding of subjects, and blindness of therapists, and 12 studies used an intent-to-treat analysis.
Table 3.
Methodological Quality Assessment for Inclusion in the study
| Study | Item1 | Item2 | Item3 | Item4 | Item5 | Item6 | Item7 | Item8 | Item9 | Item10 | Sum score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brown 2009 [49] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Eggenberger 2016 [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| Gothe 2016 [62] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| Albinet 2016 [29] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Kalbe 2018 [50] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 7 |
| Liu-ambrose 2010 [37] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 8 |
| Hariprasad 2013 [43] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Norouzi 201 9[38] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Nouchi 2013 [51] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Lachman 2006 [39] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Ferreira 2015 [30] | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Vaughn 2014 [52] | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Fabre 2002 [31] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Shan 2016 [44] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| Li 2016 [45] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Yang 2019 [46] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Hong 2017 [40] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Bae 2019 [53] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 8 |
| Damirchi 2018 [54] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Donnezan 2018 [28] | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
| Yoon 2018 [41] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Eggermont 2009 [32] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Lam 2010 [47] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| Scherder 2005 [33] | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Sungkarat 2016 [48] | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Zhu 2018 [34] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| Lü 2015 [42] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Nishiguchi 2015 [35] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
Item 1, randomization; Item 2, concealed allocation; Item 3, similar baseline; Item4, blinding of subjects; Item 5, blinding of therapists; Item 6, blinding of assessors; Item 7, more than 85% retention; Item 8, intent-to-treat analysis; Item 9, between-group comparison; Item 10, point measure and measures of variability; 1, explicitly described and present in details; 0, absent, inadequately described, or unclear
Meta-analysis
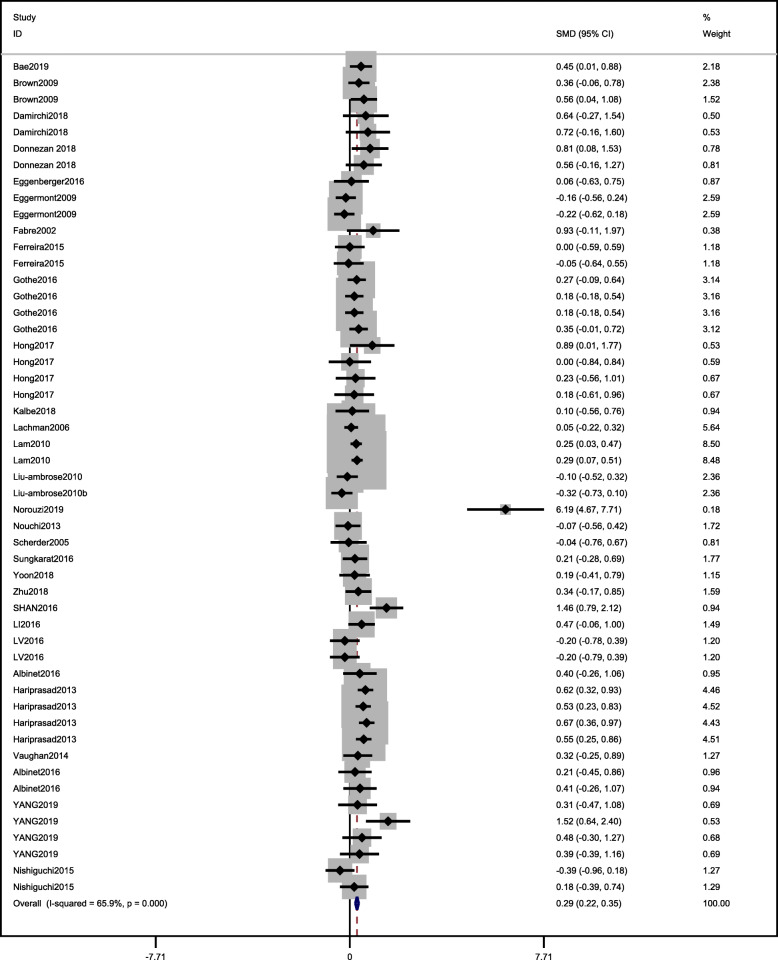
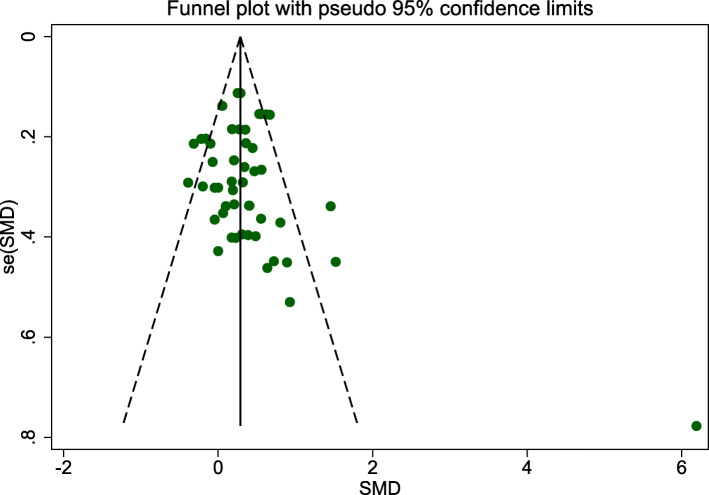
A total of 51 effects were included in the meta-analysis, and the overall ES was 0.29, p < 0.001, with a significant difference between the experimental and control groups. This indicates that exercise significantly improved WM in older adults. The heterogeneity test revealed a moderate degree of heterogeneity in the included studies (Table 4 and Fig. 2), so a random effect model was used to synthesize the data. The funnel plot in Fig. 3 was symmetrical, which indicates that there was no publication bias. Egger’s test showed that there was no publication bias in this study, which indicates that the small sample size in one of the included study did not affect the results (t = 1.46, p > | t | = 0.149 > 0.05; Table 5).
Table 4.
Summary of Subgroup Analysis Results
| Moderator | Grouping standard | n (ES) | Heterogeneity test results | The results of meta-analysis | ||
|---|---|---|---|---|---|---|
| I2 | P | ES,95%CI | P | |||
| Measurement of WM testing | Digital Span Forward | 13 | 86.1% | < 0.001 | SMD = 0.38(0.24,0.51) | < 0.001 |
| Digital Span Backward | 21 | 34.6% | 0.061 | SMD = 0.19(0.09,0.29) | < 0.001 | |
| N-back (Accuracy) | 6 | 7.4% | 0.369 | SMD = 0.21(0.002,0.41) | 0.048 | |
| N-back (Reaction Time) | 3 | 68.6% | 0.041 | SMD = 0.55(0.14,0.95) | 0.008 | |
| Spatial Span | 3 | 0% | 0.735 | SMD = 0.59(0.39,0.80) | < 0.001 | |
| Miscellaneous | 5 | 0% | 0.978 | SMD = 0.22(0.01,0.43) | 0.038 | |
| Type | MCE | 9 | 0% | 0.639 | SMD = 0.38(0.2,0.56) | < 0.001 |
| AE | 13 | 0% | 0.464 | SMD = 0.04(−0.12,0.19) | 0.66 | |
| MBE | 17 | 45.6% | 0.21 | SMD = 0.41(0.33,0.5) | < 0.001 | |
| RE | 12 | 86.0% | < 0.001 | SMD = 0.06(−0.10,0.21) | 0.50 | |
| Duration (minutes) | Short (≤45 min) | 8 | 24.6% | 0.233 | SMD = 0.12(0.008,0.24) | 0.037 |
| Moderate (45–60 min) | 38 | 40.1% | 0.006 | SMD = 0.37(0.28,0.45) | < 0.001 | |
| Long (> 60 min) | 5 | 93.7% | < 0.001 | SMD = 0.33(0.06,0.59) | < 0.017 | |
| Frequency (week/times) | Low (1–2times) | 21 | 44.9% | 0.014 | SMD = 0.37(0.27,0.48) | < 0.001 |
| Moderate (3–4times) | 27 | 69.0% | < 0.001 | SMD = 0.25(0.16,0.34) | < 0.001 | |
| High (≥5times) | 3 | 90.0% | < 0.001 | SMD = 0.06(−0.20,0.32) | 0.628 | |
| Intervention period (weeks) | Short (≤12 weeks) | 19 | 75.2% | < 0.001 | SMD = 0.24(0.15,0.33) | < 0.001 |
| Moderate(12–24 weeks) | 2 | 15.2% | 0.261 | SMD = 0.33(0.23,0.44) | < 0.001 | |
| Long (> 24 weeks) | 4 | 85.0% | < 0.001 | SMD = 0.33(0.15,0.50) | < 0.001 | |
| Intensity | Low | 29 | 54.8% | < 0.001 | SMD = 0.32(0.25,0.40) | < 0.001 |
| Moderate | 14 | 81.3% | < 0.001 | SMD = 0.31(0.16,0.47) | < 0.001 | |
| Vigorous | 8 | 0% | 0.577 | SMD = -0.002(−0.20,0.19) | 0.987 | |
| Control group | Active | 28 | 70.6% | < 0.001 | SMD = 0.22(0.14,0.30) | < 0.001 |
| Passive | 23 | 54.9% | 0.001 | SMD = 0.38(0.28,0.48) | < 0.001 | |
| Cognitive status | MCI | 17 | 27.5% | 0.141 | SMD = 0.22(0.14,0.30) | < 0.001 |
| Normal | 32 | 73.5% | < 0.001 | SMD = 0.30(0.23,0.36) | < 0.001 | |
| Age | > 76 yrs | 18 | 51.9% | 0.006 | SMD = 0.33(0.24,0.42) | < 0.001 |
| ≤75 yrs | 33 | 70.7% | < 0.001 | SMD = 0.24(0.14,0.33) | < 0.001 | |
Fig. 2.
forest plot
Fig. 3.
funnel plot
Table 5.
Results of Egger’s Test
| Std_EFF | Coef. | Std.Err. | t | P > |t| | 95%CI |
|---|---|---|---|---|---|
| Slope | 0.0991962 | 0.1395748 | 0.71 | 0.481 | −0.1816588, 0.3800512 |
| Bias | 0.8738566 | 0.5967201 | 1.46 | 0.149 | −0.3252973, 2.073011 |
Subgroup analysis
WM measurements
The subgroup analysis revealed that the six WM measurements [55] significantly moderated the effect of exercise on WM (Q(5) = 16.63, p = 0.005). The ES of the SS results (Cohen’s d = 0.59) was greater than that of the n-back (Cohen’s d = 0.55), DSF (Cohen’s d = 0.38) and DSB results (Cohen’s d = 0.19).
Exercise prescription variables
The type of exercise intervention significantly moderated the effect of exercise on WM (Q (3) = 27.10, p < 0.001). The subgroup analysis revealed that the ES for older adults engaged in MBE (Cohen’s d = 0.41) was larger than for those engaged in MCE (Cohen’s d = 0.38). The ESs of AE (Cohen’s d = 0.04) and RE (Cohen’s d = 0.06) were not significantly different.
Exercise duration significantly moderated the effect of exercise on WM (Q (2) = 11.74, p < 0.003). The results of the subgroup analysis indicated that the ES for older adults engaged in a moderate-duration exercise (45–60 min) (Cohen’s d = 0.37) was larger than that for long-duration exercise (≥ 60 min) (Cohen’s d = 0.33) and short-duration exercise (≤ 45 min) (Cohen’s d = 0.12).
Exercise frequency significantly moderated the effect of exercise on WM (Q (2) = 8.29, p = 0.016). The subgroup analysis indicated that the ES for older adults engaged in a low-frequency exercise (1 or 2 times/week) (Cohen’s d = 0.37) was larger than that for those engaged in moderate-frequency exercise (3 or 4 times/week) (Cohen’s d = 0.25) or high-frequency exercise (≥ 5 times/week) (Cohen’s d = 0.06).
Exercise intensity significantly moderated the effect of exercise on WM (Q (2) = 9.39, p = 0.009). The subgroup analysis indicated that the ES for older adults engaged in a low-intensity exercise (Cohen’s d = 0.32) was larger than that for those engaged in moderate-intensity exercise (Cohen’s d = 0.31) or high-intensity exercise (Cohen’s d = − 0.002).
There were no significant differences in the ESs according to intervention period (Q (2) = 1.93, p = 0.381).
The active/passive control group significantly moderated the effect of exercise on WM (Q (1) = 5.85, p = 0.016).
Subject characteristics
There were no significant differences in the ESs according to cognitive status (Q (2) = 3.20, p = 0.074).
There were no significant differences in the ESs according to age (Q (1) = 2.07, p = 0.15].
Discussion
Overall analysis of exercise intervention effects
To the best of our knowledge, this is the first meta-analysis of RCTs investigating the effects of exercise prescription on WM. It is important to further our understanding on how exercise prescription could moderate the intervention effect. A previous meta-analysis revealed that regular physical exercise can improve WM in older adults [56], but included participants of all ages, from adolescents to older adults, and only 5 of the included studies were with older adults. The number of included studies in that meta-analysis was small, which limits the generalizability of those results. Additionally, no previous meta-analysis has investigated whether cognitive status influences the effect of exercise on WM in older adults with cognitive impairment.
The present meta-analysis included 28 studies and synthesized 51 ESs. The results further confirmed that exercise significantly improves WM in older adults, with a positive, significant small ES. Based on the results of this review, we believe that exercise is an effective way to improve WM in older adults, which is generally consistent with the results of previous meta-analyses [10, 23]. However, the current research found a moderate heterogeneity between the included studies, which may be caused by factors such as different WM measurement tools, the cognitive status of older adults, and the specific features of physical exercise.
Subgroup analysis of exercise intervention effects
WM measurements
This study found that the intervention effect of physical exercise on the WM of older adults was moderated by the WM measurement tools. WM comprises many sub-components, such as encoding, maintaining, and manipulating information [57]. Different measurement tools differ in their investigation of these different WM subcomponents. For example, the DSF and verbal span tasks mainly assess retention in WM. The n-back and DSB tasks not only assess memory retention, but also the manipulation of WM. The tools used to assess WM are diverse, and can be divided into two categories – task span and n-back tasks [58]. In the included studies, WM was mostly tested using the DST, because the DSF task does not involve additional manipulations of the memory content. The DSB task not only assesses retention, but also manipulation. By comparing the differences between the two tasks, the intervention effect of a single component can be determined.
The current study found that the intervention effect of physical exercise on the DSF task is better than that on the DSB task, which is similar to previous results [59]. This shows that the intervention effect of physical exercise on relatively simple WM is better, but the intervention effect on task manipulation is poor, which may because the scoring method of the DSF is not very sensitive and cannot reflect the changes in WM. The use of a more accurate digit-letter sequence task could be explored in this context [60].
The n-back task is arguably the most commonly used continuous updating test and shows acceptable convergence with conceptually distinct measures of WM, including complex span and serial reordering tasks [61]. This task can be classified as 1-back, 2-back, and 3-back. The subjects respond according to whether the current information is the same as the previous information. This study found significant ESs of the n-back reaction time and accuracy. However, few studies on this were included, so this explanation should be treated with some caution. Furthermore, the difficulty of n-back task could also affect the intervention effect, and the intervention effect on the accuracy of the relatively simple 1-back task is not as good as that on 2-back accuracy [46, 62].
Exercise prescription variables
The current meta-analysis also evaluated the effects of exercise prescription on the exercise effects on WM. The present results revealed that the type of physical exercise is a potential regulatory variable in this relationship.
The moderating effect of exercise type
Our findings indicate that exercise type moderates the influence of exercise on WM. Exercise type is an important feature of physical exercise, and most of the earlier studies adopted an AE intervention [63]. The intervention effects of RE [64], MCE [65], and MBE [66] have also been confirmed. However, it is worth noting that the present results revealed no significant intervention effects of AE and RE on WM, but did find significant effects of MCE and MBE. Previous meta-analyses also reported that AE and RE do not improve WM in older adults [55, 67, 68], but, when combined, they become effective in improving WM [69]. As the most commonly used method of physical exercise intervention, many studies have shown that AE and RE cause changes in brain function [70, 71] and can improve cognitive functioning [72, 73]. The inconsistency in the results of previous studies may due to the multi-component nature of WM, the various WM measurement tools, and large individual differences in cognitive functioning.
Compared with a single form of exercise, MCE and MBE are relatively complex, in that they involve multi-point memory and adopt characteristics of aerobic, resistance, balance, and stretching movements. The effects of various forms of exercise may produce complementary neurobiological and physiological effects on WM, especially when the form of exercise engages similar systems to those engaged in WM tasks. Tai Chi Chuan perfectly integrates traditional philosophy, the theory of traditional Chinese medicine, and the five-element theory; it also combines physical movement with respiration, mind with consciousness, consciousness with the body, and qi with the body. It strives to achieve a unity of mind, consciousness, strength, qi(a Chinese concept of energy), and shape, while constantly adjusting the direction, range, power, and speed of movement. This practice requires not only memory, but also a variety of higher-level cognitive functions to maintain postural stability. It has also been reported to improve brain structure and cognitive function by improving cardiovascular function and coordination ability [74]. The amplitude and latency of event-related potentials in older adults who have been practicing Tai Chi Chuan for a long time have been reported to significantly change [75]. Several previous experimental studies [10, 74] and meta-analyses [23, 67, 76, 77] have shown that MCE and MBE may have a greater positive impact on the cognitive function of older adults than other types of exercise.
The moderating effect of exercise frequency
The subgroup analysis indicated that exercise frequency moderates the influence of exercise on WM. Low- and moderate-frequency exercise had a positive exercise effect on WM in older adults, while high-frequency exercise had no such positive effect. The results of a previous meta-analysis indicated that both high-frequency and low-frequency physical exercise can improve cognitive functioning in older adults [78]. The difference between these previous results and those of the current study may be related to the lack of literature on an exercise frequency of 5 times or more per week. Our result may not represent a true effect, and the results should be interpreted with caution, and more research is needed.
The moderating effect of exercise intensity
The subgroup analysis indicated that exercise intensity moderates the influence of exercise on WM. There was heterogeneity in the intervention effect between different exercise intensities. Moderate- and low-intensity exercise was found to effectively improve WM in older adults, while high-intensity physical exercise had no intervention effect. Several meta-analyses have made the same conclusions [79, 80]. It has been agreed that moderate-intensity physical exercise can effectively improve WM in older adults, which is also in line with the exercise intensity advocated by the American Sports Medical Association and the World Health Organization.
Physical exercise can result in structural brain changes, such as increased hippocampal volume [12] and gray matter volume [66]. Many studies have found that exercise intensity plays an important role in improving cognitive performance [81, 82]. A recent meta-analysis found that both high-intensity and low-intensity physical exercise improved executive functioning, with no significant differences between the two intensities [83]. However, only 2 of the included studies adopted high-intensity physical exercise. Thus, this result should be interpreted with caution, and more studies are needed.
The moderating effect of exercise duration
The subgroup analysis indicated that exercise duration moderates the influence of exercise on WM, whereby the effect of exercise tends to increase with a longer duration. Most researchers have implemented sessions that last 30–60 min; however, some research has failed to clearly state the exercise duration or to distinguish between the warm-up, main exercise, and cool-down of each session. Many studies have suggested that 20 min of physical exercise can significantly improve cognitive functioning in older adults [72, 84]. Exercise durations that are too short are insufficient to induce changes in body arousal level, brain structure, and function. However, exercise sessions that are too long may cause excessive fatigue in older adults, and does not induce brain plasticity. Therefore, it is important to define the duration that will most effectively induce such changes [68]. Future studies should thus clarify the intervention effect according to exercise duration.
The moderating effect of intervention period
The subgroup analysis indicated that the intervention period does not moderate the effect of exercise on WM. The short, medium, and long intervention periods could all improve WM in older adults. The current findings replicate several earlier studies. For example, one study reported no relationship between exercise effect and intervention period [79], but some studies have proposed that the effect of a long intervention period [85] or short intervention period [86] is better.
The most commonly used intervention periods in the included studies were 8 weeks, 12 weeks, and 24 weeks; those more than 24 weeks were rare, so these results should be interpretated with caution. While a long intervention period may improve cognitive performance, the cognitive performance of older adults may decline over time, thus offsetting the effect of the intervention. Future studies need to prolong the length of exercise and increase the number of follow-ups to evaluate whether cognitive status differences between intervention and control groups increase with age.
Subject characteristics
The subgroup analysis indicated that the cognitive status of subjects did not moderate the influence of exercise on WM. However, this study found that a larger effect of the intervention in older adults with normal cognition than in older adults with MCI. As a possible moderating variable, many researchers have examined the role of cognitive status in the effect of interventions on cognition. While research has revealed significant intervention-related improvements in healthy older adults, older patients with MCI, and even patients with dementia, these results have not been consistent in the cognitive domain or in the magnitudes of improvement [12, 78, 87]. The discrepancy of these results may be caused by the small number of included studies.
The subgroup analysis indicated that age does not moderate the effect of exercise on WM. These results are not consistent with those of Colcombe and Kramer, who found that physical exercise has the greatest impact on cognitive function in adults aged 66–70 years, followed by those in the 71–80 years bracket, and has the least impact on the cognitive function of adults aged 55–65 years [10]. The reason for this inconsistency may be different in the measurement tools of cognitive sub-domain.
Strengths and limitations
This study’s primary strength was the exclusive inclusion of RCTs. In previous studies, the inclusion of cross-sectional studies have introduced confounding variables that affect the authenticity of the research results. Another strength of this study is that it analyzed the moderating effect of exercise prescription features. These results thus provide a theoretical basis for identifying optimal exercise prescription parameters.
This meta-analysis also has several limitations that should be overcome in future research. First, while there is a variety of measurement tools to assess WM, this study mainly used DST results as the primary outcome. Second, the included studies have some methodological flaws, such as the absence of blinding. Third, there are no standards for exercise intensity and exercise duration in the literature, which makes it difficult to determine the most effective intervention parameters.
Conclusion
This systematic meta-analytic review indicates that exercise is a promising way to improve WM in older adults. We found that the best physical exercise prescription for improving WM in older adults is moderate intensity MBE or MCE sessions of 45–60 min performed 3–4 times a week, for at least 12 weeks. The effect of the intervention was not affected by age or cognitive status. However, due to the limited inclusion of studies, the optimal exercise prescription needs to be confirmed in future work.
Supplementary Information
Acknowledgments
Not applicable.
Abbreviations
- AE
Aerobic exercise
- DST
Digit Span Test
- DSF
Digit Span Test-Forward
- DSB
Digit Span Test-Backward
- ES
Effect size
- EC
Executive control
- M
Mean
- MBE
Mind–body exercise
- MCI
Mild cognitive impairment
- MCE
Multi-component exercise
- PEDro
Physiotherapy Evidence Database
- PFC
Prefrontal cortex
- RE
Resistance exercise
- RCT
Randomized controlled trial
- SS
Spatial span
- SD
Standard deviation
- WM
Working memory
Authors’ contributions
All authors reviewed and approved the final manuscript. Two researchers (CZD and YJL) independently screened the literature according to the inclusion and exclusion criteria. Two researchers (CZD and SDH) independently extracted the relevant information using a standardized form. CZD conceived and wrote the manuscript; YJL and SDH collected the data; CZT and WX analyzed the data and wrote the manuscript.
Funding
This work was supported by the Key Laboratory Project of Shanghai Science and Technology Commission (11DZ2261100), and the National Social Science Foundation(20BTY116).
Availability of data and materials
All the data is in the supplementary data file.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Each of the authors confirms that this manuscript has not been previously published and is not currently under consideration by any other journal. Additionally, all of the authors have approved the contents of this paper and have agreed to submission policies of the European Review of Aging and Physical Activity.
Competing interests
All authors have no conflicts of interest relevant to the content of this review.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cai Zhidong, Email: 1911111023@sus.edu.cn.
Xing Wang, Email: 18930132117@163.com.
Jilin Yin, Email: yinjilin88@163.com.
Dehai Song, Email: shitinghuijun@163.com.
Zhitong Chen, Email: chenzhitong@lixin.edu.cn.
References
- 1.Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1016/j.cub.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Ku YX. Cognitive and neural mechanisms underlying working memory. Sheng Li Xue Bao. 2019;71(1):173–185. doi: 10.13294/j.aps.2019.0004. [DOI] [PubMed] [Google Scholar]
- 3.Rosen VM, Engle RW. The role of working memory capacity in retrieval. J Experiment Psychol Gen. 1997;126(3):211–227. doi: 10.1037//0096-3445.126.3.211. [DOI] [PubMed] [Google Scholar]
- 4.Smith EE, Jonides J. Working memory: a view from neuroimaging. Cogn Psychol. 1997;33(1). 10.1006/cogp.1997.0658. [DOI] [PubMed]
- 5.Eriksson J, Vogel EK, Lansner A, Bergström F, Nyberg L. Neurocognitive architecture of working memory. Neuron. 2015;88(1):33–46. doi: 10.1016/j.neuron.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott EM, Cherry KE, Brown JS, Smitherman EA, Jazwinski SM, Yu Q, Volaufova J. Working memory in the oldest-old: evidence from output serial position curves. Mem Cogn. 2011;39(8):1423–1434. doi: 10.3758/s13421-011-0119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'esposito M, Postle BR. The cognitive neuroscience of working memory. Annu Rev Psychol. 2015;66(1):115–142. doi: 10.1146/annurev-psych-010814-015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy KM, Rodrigue KM, Bischof GN, Hebrank AC, Reuter-Lorenz PA, Park DC. Age trajectories of functional activation under conditions of low and high processing demands: an adult lifespan fmri study of the aging brain. Neuroimage. 2015;104:21–34. doi: 10.1016/j.neuroimage.2014.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392(1–2):32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 11.Mcsween MP, Coombes JS, Mackay CP, et al. The immediate effects of acute aerobic exercise on cognition in healthy older adults: a systematic review. Sports Med. 2019;49(1):67–82. doi: 10.1007/s40279-018-01039-9. [DOI] [PubMed] [Google Scholar]
- 12.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikudome S, Mori S, Unenaka S, Kawanishi M, Kitamura T, Nakamoto H. Effect of long-term body-mass-based resistance exercise on cognitive function in elderly people. J Appl Gerontol. 2017;36(12):1519–1533. doi: 10.1177/0733464815625834. [DOI] [PubMed] [Google Scholar]
- 14.Yang ZY, Mei J, Chen S, et al. Effect of tai Chi and square dance exercise on working memory of female elderly:an ERP study. J Tianjin Univ Sport. 2019;34(01):86–92. doi: 10.13297/j.cnki.issn1005-0000.2019.01.013. [DOI] [Google Scholar]
- 15.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 16.Hatta A, Nishihira Y, Kim SR, Kaneda T, Kida T, Kamijo K, Sasahara M, Haga S. Effects of habitual moderate exercise on response processing and cognitive processing in older adults. Jpn J Physiol. 2005;55(1):29–36. doi: 10.2170/jjphysiol.R2068. [DOI] [PubMed] [Google Scholar]
- 17.Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 18.Gothe N, Pontifex MB, Hillman C, McAuley E. The acute effects of yoga on executive function. J Phys Act Health. 2013;10(4):488–495. doi: 10.1123/jpah.10.4.488. [DOI] [PubMed] [Google Scholar]
- 19.Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 20.Firth J, Stubbs B, Rosenbaum S, Vancampfort D, Malchow B, Schuch F, Elliott R, Nuechterlein KH, Yung AR. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546–556. doi: 10.1093/schbul/sbw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teixeira-Santos AC, Moreira CS, Magalhães R, et al. Reviewing working memory training gains in healthy older adults: a meta-analytic review of transfer for cognitive outcomes. Neurosci Biobehav Rev. 2019;103:163–177. doi: 10.1016/j.neubiorev.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Ye M, Wang L, Xiong J, Zheng G. The effect of mind-body exercise on memory in older adults: a systematic review and meta-analysis. Aging Clin Exp Res. 2021;33(5):1163–1173. doi: 10.1007/s40520-020-01557-5. [DOI] [PubMed] [Google Scholar]
- 23.Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2017;52(3):154–160. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verhagen A. The delphi list : a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by delphi consensus. J Clin Epidemiol. 1998;51(12):1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J. Statistical power analysis for the behavioral sciences [M] 2. Mahwah: Lawrence Erlbaum Associates; 1988. pp. 490–500. [Google Scholar]
- 27.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res ed) 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Combourieu Donnezan L, Perrot A, Belleville S, Bloch F, Kemoun G. Effects of simultaneous aerobic and cognitive training on executive functions, cardiovascular fitness and functional abilities in older adults with mild cognitive impairment. Ment Health Phys Act. 2018;15:78–87. doi: 10.1016/j.mhpa.2018.06.001. [DOI] [Google Scholar]
- 29.Albinet CT, Abou-Dest A, Andre N, et al. Executive functions improvement following a 5-month aquaerobics program in older adults: role of cardiac vagal control in in hibition performance. Biol Psychol. 2016;115(3):69–77. doi: 10.1016/j.biopsycho.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira L, Tanaka K, Santos-Galduroz RF, et al. Respiratory training as strategy to prevent cognitive decline in aging: a randomized controlled trial. Clin Interv Aging. 2015;10:593–603. doi: 10.2147/cia.s79560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabre C, Chamari K, Mucci P, Massé-Biron J, Préfaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int J Sports Med. 2002;23(06):415–421. doi: 10.1055/s-2002-33735. [DOI] [PubMed] [Google Scholar]
- 32.Eggermont LHP, Swaab DF, Hol EM, Scherder EJA. Walking the line: a randomised trial on the effects of a short term walking programme on cognition in dementia. J Neurol Neurosurg Psychiatry. 2009;80(7):802–804. doi: 10.1136/jnnp.2008.158444. [DOI] [PubMed] [Google Scholar]
- 33.Scherder EJA, Paasschen JV, Deijen JB, et al. Physical activity and executive functions in the eldery with mild cognitive impairment. Aging Ment Health. 2005;9(3):272–280. doi: 10.1080/13607860500089930. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Y, Wu H, Qi M, Wang S, Zhang Q, Zhou L, Wang S, Wang W, Wu T, Xiao M, Yang S, Chen H, Zhang L, Zhang K, Ma J, Wang T. Effects of a specially designed aerobic dance routine on mild cognitive impairment. Clin Interv Aging. 2018;13:1691–1700. doi: 10.2147/cia.s163067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishiguchi S, Yamada M, Tanigawa T, Sekiyama K, Kawagoe T, Suzuki M, Yoshikawa S, Abe N, Otsuka Y, Nakai R, Aoyama T, Tsuboyama T. A 12-week physical and cognitive exercise program can improve cognitive function and neural efficiency in community-dwelling older adults: a randomized controlled trial. J Am Geriatr Soc. 2015;63(7):1355–1363. doi: 10.1111/jgs.13481. [DOI] [PubMed] [Google Scholar]
- 36.Eggenberger P, Wolf M, Schumann M, de Bruin ED. Exergame and balance training modulate prefrontal brain activity during walking and enhance executive function in older adults. Front Aging Neurosci. 2016;8. 10.3389/fnagi.2016.00066. [DOI] [PMC free article] [PubMed]
- 37.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions a 12-month randomized controlled trial. Arch Intern Med. 2010;170(2):170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norouzi E, Vaezmosavi M, Gerber M, et al. Dual-task training on cognition and resistance training improved both balance and working memory in older people. Phys Sportsmed. 2019;47(4):471–478. doi: 10.1111/jgs.13481. [DOI] [PubMed] [Google Scholar]
- 39.Lachman ME, Neupert SD, Bertrand R, Jette AM. The effects of strength training on memory in older adults. J Aging Phys Act. 2006;14(1):59–73. doi: 10.1123/japa.14.1.59. [DOI] [PubMed] [Google Scholar]
- 40.Hong SG, Kim JH, Jun T-W. Effects of 12-week resistance exercise on electroencephalogram patterns and cognitive function in the elderly with mild cognitive impairment: a randomized controlled trial. Clin J Sport Med. 2018;28(6):500–508. doi: 10.1097/jsm.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 41.Yoon DH, Lee JY, Song W. Effects of resistance exercise training on cognitive function and physical performance in cognitive frailty : a randomized controlled trail. J Nutr Health Aging. 2018;22(8):944–951. doi: 10.1007/s12603-018-1090-9. [DOI] [PubMed] [Google Scholar]
- 42.Lü J, Sun M, Liang l, et al. Effects of momentum-based dumbbell training on cognitive function in older adults with mild cognitive impairment: a pilot randomized controlled trial. Clin Interv Aging. 2015;11:9–16. doi: 10.2147/CIA.S96042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hariprasad VR, Koparde V, Sivakumar PT, et al. Randomized clinical trial of yoga-based intervention in residents from elderly homes: effects on cognitive function. Indian J Psychiatry. 2013;55(Suppl 3):S357–S363. doi: 10.4103/0019-5545.116308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SHAN YT. The effects of “24-style” simplified Taijiquan on memory, attention and executive function of the middle-aged and elderly in community——a randomized controlled study [D] Fuzhou: Fujian University of Tranditional Chinese Medicine; 2016. [Google Scholar]
- 45.LI SZ. Effects of Baduanjin on global cognitive function and memory in the elderly with mild cognitive impairment [D] Fuzhou: Fujian University of Tranditional Chinese Medicine; 2016. [Google Scholar]
- 46.Yang Y. Effects of 8-week Taijiquan on cognitive control and working memory of the elderly in community [D] Beijing: Capital University of Pysical Education and Sports; 2019. [Google Scholar]
- 47.Lam LC, Chau RC, Wong BM, et al. Interim follow-up of a randomized controlled trial comparing chinese style mind body (tai chi) and stretching exercises on cognitive function in subjects at risk of progressive cognitive decline. Int J Geriatr Psychiatry. 2011;26(7):733–740. doi: 10.1002/gps.2602. [DOI] [PubMed] [Google Scholar]
- 48.Sungkarat S, Boripuntakul S, Chattipakorn N, Watcharasaksilp K, Lord SR. Effects of tai chi on cognition and fall risk in older adults with mild cognitive impairment: a randomized controlled trial. J Am Geriatr Soc. 2016;65(4):721–727. doi: 10.1111/jgs.14594. [DOI] [PubMed] [Google Scholar]
- 49.Brown AK, Liu-Ambrose T, Tate R, Lord SR. The effect of group-based exercise on cognitive performance and mood in seniors residing in intermediate care and self-care retirement facilities: a randomised controlled trial. Br J Sports Med. 2009;43(8):608–614. doi: 10.1136/bjsm.2008.049882. [DOI] [PubMed] [Google Scholar]
- 50.Kalbe E, Roheger M, Paluszak K, Meyer J, Becker J, Fink GR, Kukolja J, Rahn A, Szabados F, Wirth B, Kessler J. Effects of a cognitive training with and without additional physical activity in healthy older adults: a follow-up 1 year after a randomized controlled trial. Front Aging Neurosci. 2018;10(407):1–14. doi: 10.3389/fnagi.2018.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nouchi R, Taki Y, Takeuchi H, Sekiguchi A, Hashizume H, Nozawa T, Nouchi H, Kawashima R. Four weeks of combination exercise training improved executive functions, episodic memory, and processing speed in healthy elderly people: evidence from a randomized controlled trial. Age. 2014;36(2):787–799. doi: 10.1007/s11357-013-9588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaughan S, Wallis M, Polit D, Steele M, Shum D, Morris N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: a randomised controlled trial. Age Ageing. 2014;43(5):623–629. doi: 10.1093/ageing/afu010. [DOI] [PubMed] [Google Scholar]
- 53.Bae S, Lee S, Lee S, Jung S, Makino K, Harada K, Harada K, Shinkai Y, Chiba I, Shimada H. The effect of a multicomponent intervention to promote community activity on cognitive function in older adults with mild cognitive impairment: a randomized controlled trial. Complement Ther Med. 2019;42:164–169. doi: 10.1016/j.ctim.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 54.Damirchi A, Hosseini F, Babaei P. Mental training enhances cognitive function and bdnf more than either physical or combined training in elderly women with mci: a small-scale study. Am J Alzheimers Dis Other Demen. 2018;33(1):20–29. doi: 10.1177/1533317517727068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rathore A, Lom B. The effects of chronic and acute physical activity on working memory performance in healthy participants: a systematic review with meta-analysis of randomized controlled trials. Syst Rev. 2017;6(1):124. doi: 10.1186/s13643-13017-10514-13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 58.Redick TS, Lindsey DRB. Complex span and n-back measures of working memory: a meta-analysis. Psychon Bull Rev. 2013;20(6):1102–1113. doi: 10.3758/s13423-013-0453-9. [DOI] [PubMed] [Google Scholar]
- 59.Li WR, Ku YX. The influence of acute stress on working memory: physiological and psychological mechanisms. Adv Psychol Sci. 2020;28(9):1508–1524. doi: 10.3724/SP.J.1042.2020.01508. [DOI] [Google Scholar]
- 60.Wells EL, Kofler MJ, Soto EF, Schaefer HS, Sarver DE. Assessing working memory in children with adhd: minor administration and scoring changes may improve digit span backward's construct validity. Res Dev Disabil. 2018;72:166–178. doi: 10.1016/j.ridd.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmiedek F, Lövdén M, Lindenberger U. A task is a task is a task: putting complex span, n-back, and other working memory indicators in psychometric context. Front Psychol. 2014;5:1475. doi: 10.3389/fpsyg.2014.01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gothe NP, Keswani RK, Mcauley E. Yoga practice improves executive function by attenuating stress levels. Biol Psychol. 2016;121(Pt A):109–116. doi: 10.1016/j.biopsycho.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 63.Netz Y. Is there a preferred mode of exercise for cognition enhancement in older age?-a narrative review. Front Med. 2019;6(57):1–10. doi: 10.3389/fmed.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu-Ambrose T, Nagamatsu LS, Voss MW, Khan KM, Handy TC. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging. 2012;33(8):1690–1698. doi: 10.1016/j.neurobiolaging.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 65.Vedovelli K, Giacobbo BL, Corrêa MS, Wieck A, Argimon IIL, Bromberg E. Multimodal physical activity increases brain-derived neurotrophic factor levels and improves cognition in institutionalized older women. Geroscience. 2017;39(4):407–417. doi: 10.1007/s11357-017-9987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tao J, Liu J, Liu W, Huang J, Xue X, Chen X, Wu J, Zheng G, Chen B, Li M, Sun S, Jorgenson K, Lang C, Hu K, Chen S, Chen L, Kong J. Tai chi chuan and baduanjin increase grey matter volume in older adults: a brain imaging study. J Alzheimers Dis. 2017;60(2):389–400. doi: 10.3233/jad-170477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12–31. doi: 10.1016/j.arr.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Li Z, Peng X, Xiang W, et al. The effect of resistance training on cognitive function in the older adults: a systematic review of randomized clinical trials. Aging Clin Exp Res. 2018;30(11):1259–1273. doi: 10.1007/s40520-018-0998-6. [DOI] [PubMed] [Google Scholar]
- 69.Levin O, Netz Y, Ziv G. The beneficial effects of different types of exercise interventions on motor and cognitive functions in older age: a systematic review. Eur Rev Aging Phys Act. 2017;14(1):20. doi: 10.1186/s11556-017-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herold F, Törpel A, Schega L, Müller NG. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements - a systematic review. Eur Rev Aging Phys Act. 2019;16:10. doi: 10.1186/s11556-019-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voss WM, Prakash RS, Erickson KI, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2:32. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang Y-K, Etnier JL. Chronic exercise and cognitive function: an update of current findings. Int J Sport Exerc Psychol. 2019;17(2):85–88. doi: 10.1080/1612197X.2016.1223068. [DOI] [Google Scholar]
- 73.Zheng G, Xia R, Zhou W, Tao J, Chen L. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2016;50(23):14430–11450. doi: 10.1136/bjsports-2015-095699. [DOI] [PubMed] [Google Scholar]
- 74.Chang YK, Nien YH, Tsai CL, Etnier JL. Physical activity and cognition in older adults: the potential of tai chi chuan. J Aging Phys Act. 2010;18(4):451–472. doi: 10.1123/japa.18.4.451. [DOI] [PubMed] [Google Scholar]
- 75.Fong DY, Chi LK, Li F, Chang YK. The benefits of endurance exercise and tai chi chuan for the task-switching aspect of executive function in older adults: an erp study. Front Aging Neurosci. 2014;6(295):1–11. doi: 10.3389/fnagi.2014.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saez De Asteasu ML, Martinez-Velilla N, Zambom-Ferraresi F, et al. Role of physical exercise on cognitive function in healthy older adults: a systematic review of randomized clinical trials. Ageing Res Rev. 2017;37(5):117–134. doi: 10.1016/j.arr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 77.Wayne Effect of tai chi on cognitive performance in older adults: systematic review and meta-analysis. J Am Geriatr Soc. 2014;62(1):25–39. doi: 10.1111/jgs.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Groot C, Hooghiemstra AM, Raijmakers PGHM, van Berckel BNM, Scheltens P, Scherder EJA, van der Flier WM, Ossenkoppele R. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res Rev. 2016;25:13–23. doi: 10.1016/j.arr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Sanders LMJ, Hortobagyi T, La Bastide-Van Gemert S. Dose-response relationship between exercise and cognitive function in older adults with and without cognitive impairment: a systematic review and meta-analysis. PLoS One. 2019;14(1):e0210036. doi: 10.1371/journal.pone.0210036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naderi A, Shaabani F, Esmaeili A, Salman Z, Borella E, Degens H. Effects of low and moderate acute resistance exercise on executive function in community-living older adults. Sport Exerc Perform Psychol. 2019;8(1):106–122. doi: 10.1037/spy0000135. [DOI] [Google Scholar]
- 81.Borde R, Hortobágyi T, Granacher U. Dose–response relationships of resistance training in healthy old adults: a systematic review and meta-analysis. Sports Med. 2015;45(12):1693–1720. doi: 10.1007/s40279-015-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kramer AF, Erickson KI. Effects of physical activity on cognition, well-being, and brain: human interventions. Alzheimers Dement. 2007;3(2 Suppl):S45–S51. doi: 10.1016/j.jalz.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 83.Chou DME. The acute effect of high-intensity exercise on executive function: a meta-analysis. Perspect Psychol Sci. 2019;14(5):734–764. doi: 10.1177/1745691619850568. [DOI] [PubMed] [Google Scholar]
- 84.Chen FT, Etnier JL, Wu CH, Cho YM, Hung TM, Chang YK. Dose-response relationship between exercise duration and executive function in older adults. J Clin Med. 2018;7(9):9. doi: 10.3390/jcm7090279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ludyga S, Gerber M, Pühse U, Looser VN, Kamijo K. Systematic review and meta-analysis investigating moderators of long-term effects of exercise on cognition in healthy individuals. Nat Hum Behav. 2020;4(6):603–612. doi: 10.1038/s41562-020-0851-8. [DOI] [PubMed] [Google Scholar]
- 86.Xue Y, Yang Y, Huang T. Effects of chronic exercise interventions on executive function among children and adolescents: a systematic review with meta-analysis. Br J Sports Med. 2019;53(22):1397–1404. doi: 10.1136/bjsports-2018-099825. [DOI] [PubMed] [Google Scholar]
- 87.Forbes D, Thiessen EJ, Blake CM, et al. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2015. 10.1002/14651858.CD006489.pub34. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data is in the supplementary data file.