Abstract
Background:
Psychological stress and poor sleep are associated with a wide range of negative health outcomes, which are thought to be mediated in part by alterations in immune processes. However, the molecular bases of links among stress, sleep, and immune processes are not completely understood, particularly during adolescence when sensitivity to stress and problems with sleep tend to increase. In the current study, we investigated whether various stressors (daily stress, major life events, perceived stress), sleep indices (duration, efficiency), and their interactions (e.g., moderating effects) are associated with expression of genes bearing response elements for transcription factors that regulate inflammatory and anti-viral processes.
Method:
Eighty-seven late adolescents completed daily checklists of their social experiences across a 15-day period and reported on their major life events during the previous year. They also completed actigraphy-based assessments of sleep quality and duration during 8 consecutive nights. An average of 5.5 months later, participants reported on their global perceptions of stress during the previous month and provided blood samples for genome-wide expression profiling of mRNA from peripheral blood mononuclear cells (PBMCs).
Results:
Higher levels of daily interpersonal stress and shorter sleep duration were associated with upregulation of inflammation-related genes bearing response elements for proinflammatory transcription factor nuclear factor kappa B (NF-κB). Shorter sleep duration was also linked to downregulation of antiviral-related genes bearing response elements for interferon response factors (IRFs). Lastly, there was a significant interaction between daily stress and shorter sleep duration, such that the association between daily stress and inflammation-related gene expression was exacerbated in the context of shorter sleep duration. Results were independent of sex, ethnicity, parent education, body mass index, and smoking and alcohol history.
Conclusion:
Everyday interpersonal stress and shortened sleep can be consequential for upstream NF-κB signaling pathways relevant to inflammatory processes during late adolescence. Notably, the occurrence of both may lead to even greater activation of NF-κB signaling.
Keywords: Psychosocial stress, Actigraphy, Inflammation, Antiviral, Gene expression, Social genomics
1. Introduction
An extensive body of research has shown that higher levels of stress and difficulties with sleep undermine health (Cohen et al., 2007; Stenholm et al., 2019). For instance, individuals who grew up in adverse circumstances, care for chronically-ill family members, have few or difficult social relationships, or come from socioeconomically disadvantaged backgrounds are at increased risk for medical conditions such as cardiovascular disease, infectious diseases, and depression, and for premature mortality (Adler and Rehkopf, 2008; Cohen et al., 2007; Ehrlich et al., 2015; Godbout and Glaser, 2006; Holt-Lunstad et al., 2015). Similarly, persons with clinical or subclinical sleep problems such as insomnia, difficulties falling and staying asleep, and shorter sleep duration, are prone to developing the same health problems (Itani et al., 2017; Lovato and Gradisar, 2014; Prather et al., 2015).
Gene expression profiling studies suggest that these associations may arise in part through the activation of a conserved transcriptional response to adversity (CTRA). The CTRA is characterized by upregulation of pro-inflammatory gene expression, as mediated by increased signaling of the transcription factor nuclear factor-kappa B (NF-κB), and downregulation of antiviral gene expression, as mediated by decreased signaling of interferon response factors (IRFs) (Cole, 2014; Irwin and Cole, 2011; Irwin and Opp, 2017). Importantly, NF-κB regulates the production of pro-inflammatory cytokines, and greater NF-κB activity can increase inflammation. If sustained, heightened inflammation, in turn, can increase risk for a wide array of diseases (Nathan and Ding, 2010). IRFs regulate the transcription of interferons, which are critically involved in host resistance to viral pathogens (Fensterl and Sen, 2009). As such, decreased IRF activity may reflect compromised anti-viral responses (Sloan et al., 2007). A chronically activated CTRA, then, may contribute to increased risk for infectious diseases and inflammation-related chronic conditions. Previous work in humans and animals have linked chronic psychosocial stress (e.g., social isolation, socioeconomic disadvantage, bereavement, and early deprivation) and poor sleep (i.e., sleep deprivation) to up-regulated pro-inflammatory gene expression and other aspects of the CTRA profile (Cole, 2014; Irwin and Opp, 2017). The majority of these studies, however, focus on adult samples. Consequently, little is known about stress- and sleep-related inflammatory processes during the earlier decades of life.
Adolescence may be an especially important period to examine these dynamics given that adolescents in the US have similar or higher levels of stress as adults (American Psychological Association, 2014) and that adolescence is marked by socioemotional and biological changes relevant to stress and sleep. In particular, past research has shown that compared to adults and children, adolescents demonstrate heightened emotional and neurobiological responses to psychosocial stress (Hare et al., 2008; Somerville, 2013). Past research also suggests that affective processes change over the course of adolescence and pubertal development, with positive affect and sensitivity to positive emotional stimuli decreasing and negative affect and attentional bias towards negative emotional stimuli increasing in some adolescents (Bor et al., 2014; McLaughlin et al., 2015; Weinstein et al., 2007; Yang et al., 2018). Coinciding with these socioemotional changes are biological changes in the circadian rhythm (circadian phase delay) and environmental changes (e.g., greater access to technology, more social opportunities, earlier school start times) that contribute to adolescents’ tendency to have later bedtimes, shorter sleep duration, and more disrupted sleep (Carskadon, 2011; Maoloney et al., 2013; Owens and Group, 2014). Of note is that these changes–heightened reactions to psychosocial threats and negative affect, decreased positive affect, and increased poor sleep–can have negative downstream consequences for inflammatory processes (Carroll et al., 2011; Chiang et al., 2015a, b; Irwin and Opp, 2017; Sin et al., 2015).
To our knowledge, only two studies have investigated how stress and/or sleep may contribute to the activation of the CTRA profile. One of these studies showed that interpersonal stress was associated with upregulation of NF-κB signaling in female adolescents (Miller et al., 2009). The other study showed that children with obstructive sleep apnea had greater expression of inflammation-related genes relative to those without sleep apnea (Khalyfa et al., 2009). These studies begin to suggest that higher levels of stress and sleep difficulties may activate inflammatory aspects of the CTRA profile. However, whether these findings extend to activation of anti-viral aspects of the CTRA, other dimensions of stress (i.e., daily stress, life events, perceived stress), and more normative patterns of sleep continuity (i.e., duration of sleep, sleep maintenance or efficiency) in everyday life remains unknown.
Whether stress and sleep interact to influence inflammatory and anti-viral processes is also not entirely clear. This may be an important oversight given that both stress and sleep have been linked to heightened inflammation, as noted above, and that there is robust evidence that stress and sleep disturbance frequently co-occur (Åkerstedt et al., 2012; Kashani et al., 2012). Additionally, past theoretical and empirical work suggest that sleep might moderate the effects of stress on inflammatory activity. Biobehavioral and psychological responses to stress depend on appraisals of threat (Lazarus and Folkman, 1984), and poor sleep can increase threatening appraisals of stressors, thereby potentiating affective and biological responses to stress (Minkel et al., 2014, 2012) that can upregulate inflammatory processes (Carroll et al., 2011; Chiang et al., 2015b; Sin et al., 2015). Indeed, previous studies have shown that psychosocial stressors, such as peer victimization and family-related stress, are more strongly associated with depressed mood and HPA axis functioning in youth who have less sleep and have greater sleep difficulties (Chiang et al., 2017b, 2016).
The overall purpose of the current investigation was to examine whether stress and sleep separately, and together, shape inflammatory processes during adolescence. We examined these questions using data from a laboratory-based sub-study embedded in a larger three-wave longitudinal study of psychosocial contributions to adolescent health. In prior analyses of data from the sub-study, we showed that greater levels of early adversity were associated with greater circulating IL-6 responses to a standardized laboratory-based social stressor among adolescents with greater adiposity (Chiang et al., 2017a). We also showed that greater major life events, daily interpersonal stress, and early adversity were each associated with reduced cortisol (but not IL-6) responses to the same laboratory-based stressor (Chiang et al., 2018 in press). Results from Wave 1 of the larger parent study showed that daily interpersonal stress was not associated with systemic inflammation, as measured by C-reactive protein (CRP; Chiang et al., 2015a). They also showed that actigraphy based shorter sleep duration was linked with higher CRP in younger adolescents (Park et al., 2016) and that lower sleep efficiency (an index of sleep quality) potentiated links between family-related stress and HPA functioning and negative mood (Chiang et al., 2017b, 2016).
We build on these previous findings in the current study by first examining whether stress and sleep were each associated with the expression of inflammation- and antiviral-related genes and then asking whether sleep moderated the associations between stress and gene expression. We used bioinformatics to probe whether empirically observed differences in gene expression as a function of stress or sleep were potentially mediated by NF-κB (inflammatory) or interferon response factor (antiviral) signaling. We examined three types of stressors: daily interpersonal stress, perceived stress, and major life events. These stressor classes are distinct in that daily interpersonal stress represents the acute, more mundane, and specific social stressors in everyday life (e.g., having an argument), perceived stress represents global levels of feelings of stress, and major life events represent more severe events or situations that most people find distressing (e.g., death of a family member, loss of job). With respect to measures of sleep, we focused on actigraphy-based estimates of sleep duration and sleep efficiency. These sleep indices have been widely used in previous work and shown to be useful indices of sleep quantity and quality (Doane et al., 2015; Ohayon et al., 2017). Based on prior work reviewed above, we hypothesized that both stress and sleep would be associated with elevated CTRA expression (higher inflammatory / NF-κB activity and lower antiviral / IRF activity). Additionally, we hypothesized that the association between stress and the CTRA profile would be exacerbated in the context of poorer sleep (i.e., shorter sleep duration, lower sleep efficiency).
2. Methods
2.1. Participants
Participants were 91 late adolescents from a larger three-wave longitudinal study that originally recruited 316 adolescents from the 10th and 11th grades of four Los Angeles high schools during the first wave of data collection. At Wave 2, when participants were in 12th grade and one year post high school, 214 (67.7%) of the original 316 participants provided data. Thirty-four newly added participants from one of our previous studies (Tsai et al., 2013) or from one of the four high schools were recruited during Wave 2 to refresh the sample. Thus, 248 participants provided data for Wave 2. The current investigation is based on a subset of 91 participants recruited from the pool of Wave 2 participants and draws on daily interpersonal stress, major life events, and sleep data collected during Wave 2 of the parent study and perceived stress and gene expression data collected during the laboratory sub-study. The subset of participants of the current study did not differ from the larger Wave 2 sample on demographic variables, including age, gender, parent education, and ethnicity (ps > .210) or on primary variables of interest assessed during Wave 2 of the parent study, including major life events, daily interpersonal stress, sleep duration, and sleep efficiency (ps > .176). Two participants did not have blood samples, leaving a total of 89 samples available for gene expression assays. Among these 89 samples, eight had missing data on sleep parameters, three had missing data on daily interpersonal stress, and two had missing data on covariates. Thus, the final analytic samples across genomic analyses ranged from 79 to 87. Sample characteristics are displayed in Table 1.
Table 1.
Sample characteristics and Descriptive Statistics.
| Variable | Mean (SD) / n (%) |
|---|---|
| Age | 18.37 (.51) |
| Gender (n (%)) | |
| Female | 50 (56.18%) |
| Male | 39 (43.82%) |
| Ethnicity (n (%)) | |
| European-American | 32 (36.96%) |
| Latino | 57 (64.04%) |
| Parent education | 7.43 (2.02) |
| Less than high school diploma (n (%)) | 13 (14.77%) |
| High school diploma (n (%)) | 7 (5.68%) |
| Trade or vocational school or some college (n (%)) | 34 (38.64%) |
| College degree or higher (n (%)) | 34 (38.64%) |
| BMI | 25.15 (5.98) |
| Smoking history (n (%)) (ever smoked more than 2 puffs) | 25 (28.09%) |
| Alcohol history (n (%)) (ever had more than a few sips) | 51 (57.30%) |
| Stress | |
| Daily interpersonal stress (proportion of days, 0 = 0 days, 1 = 100% of days) | .24 (.21) |
| Major life events (count of events during past 12 months) | 3.16 (2.04) |
| Perceived stress (0 = never, 4 = very often) | 16.18 (6.62) |
| Sleep | |
| Duration (hours) | 7.48 (1.07) |
| Efficiency | 93.19 (4.75) |
Note. Categories for parent education were 1=some elementary school, 2=completed elementary school, 3=some junior high school, 4=completed junior high school, 5=some high school, 6=graduated high school, 7=trade or vocational school, 8=some college, 9=graduated from college, 10=some medical, law, or graduate school, and 11=graduated from medical, law, or graduate school. Education was averaged across parents.
2.2. Procedures
At each wave, participants and their parents completed a series of questionnaires and a daily diary protocol in which participants reported on their social and affective experiences each night before going to bed for 15 days and on their previous night’s sleep each morning for eight days. An average of 5.5 (SD = 2.7) months after completing Wave 2, participants of the current study completed a laboratory-based sub-study. Visits were scheduled between 12 pm and 2 pm, but several visits (n = 5) began at 3 or 4 pm. Participants were instructed to refrain from eating or drinking anything (except water) the hour prior to the visit. Upon arrival, participants provided written consent and a nurse assessed height, weight, and vital signs, and inserted an indwelling intravenous catheter in the antecubital vein of the non-dominant arm. In order to facilitate acclimation to the catheter and clinical environment, participants then viewed a neutral-content video for 20 min, after which baseline blood samples were collected into CPT tubes for the assessment of RNA. Participants then completed a standardized laboratory-based stress task (Trier Social Stress Test; TSST) and a set of psychosocial questionnaires, and provided several saliva and blood samples for the assessment of cortisol and IL-6 responses to the stress task. The additional blood samples were not included in the present investigation because they were unavailable for genomic assays. All study procedures were approved by the UCLA Institutional Review Board.
2.3. Measures
2.3.1. Stress
Measures of stress included daily interpersonal stress, major life events, and global levels of perceived stress.
2.3.1.1. Daily interpersonal stress.
Information on daily interpersonal stress was collected during Wave 2 of the parent study. Each night during the 15-day daily diary period, participants indicated on bedtime diary checklists whether they had experienced any of eight negative social interactions across the domains of family, peers, and school. Items included: argued with a parent, argued with another family member, argued with a friend, punished by a parent, parents argued, something bad happened to a family member, had an argument or was punished by an adult at school, and was insulted, threatened, or made fun of by someone at school. Following previous research (Hill et al., 2018; Surachman et al., 2018), we computed a summary score reflecting the proportion of days participants experienced some degree of stress in order to capture the recurrence or chronicity of daily stress. Endorsed items were summed and recoded as 0 or 1 for each day to indicate whether any one of the stressors occurred that day; recoded scores were then averaged across days to index the proportion of days that at least one stressor occurred.
2.3.1.2. Major life events.
The number of major life events across the domains of family, friends, and school/work that occurred during the previous 12 months was assessed using a 21-item events checklist that participants completed during Wave 2 of the parent study. Items were adapted from previous measures of stressful events that have been associated with negative outcomes (Conger et al., 2002; Hammen, 1991). Example items included parents divorced or separated, a parent lost his/her job, a family member became seriously ill, a close friend moved quite far away, you had a serious falling out or ended a friendship with a close friend, you were suspended or expelled in school, your grades in school went down a lot, you lost your job. Affirmative responses were summed across items, such that higher scores reflected greater exposure to stressful events.
2.3.1.3. Perceived stress.
The 10-item Perceived Stress Scale (PSS; Cohen et al., 1983) administered during the laboratory visit was used to assess how often participants experienced feelings of stress during the past month on a 5-point scale (0 = never, 4 = very often). Example items include how often participants felt “nervous or stressed” and “unable to control the important things in your life.” Responses were summed across items, and there was good internal consistency in the present sample (α = .87).
2.3.2. Sleep
Sleep was assessed during Wave 2 of the parent study using actigraphy (Micro Motionlogger Sleep Watch, Ambulatory Monitoring, Inc.). For eight consecutive nights, adolescents were instructed to wear actigraph watches on their non-dominant wrists and to press a button on the actigraph watch to indicate turning off the lights to sleep, getting out of bed in the middle of the night, and getting out of bed in the morning. Action4 (Ambulatory Monitoring, Inc.) software was used to code and score actigraphy data. The in-bed period was computed as the duration between the first event marker indicating when lights were turned off to sleep and the last event marker indicating when participants got out of bed in the morning. For event markers that were absent, daily self-reports were used to determine times that adolescents turned off the lights and got out of bed in the morning.
Sleep duration and efficiency were calculated by first using the Sadeh actigraph scoring algorithm to score one-minute epochs (Acebo et al., 2005; Sadeh et al., 1994). Sleep onset time was based on the first of at least three consecutive minutes of sleep, and sleep offset time was based on the last five or more consecutive minutes of sleep. Total sleep duration for each night was computed as the total number of minutes scored as sleep during the in-bed period and sleep efficiency was calculated as the percentage of actual sleep during the in-bed period. The intraclass correlations for sleep duration and efficiency were 0.25 and .28, respectively. Sleep indices were averaged across the eight nights to compute adolescents’ average sleep duration and sleep efficiency.
2.3.3. Gene expression profiling
Blood samples for RNA were collected during the laboratory visit. Within two hours of sample collection, PBMCs were separated from red blood cells, lysed in RNA-stabilization buffer (RLT, Qiagen Inc.), and stored at −80 °C. When all samples were available, total RNA was extracted (Qiagen RNeasy) and checked for suitable mass (> 200 ng by NanoDrop ND1000 spectrophotometry) and integrity (RNA integrity number > 3 by Agilent TapeStation capillary electrophoresis). Samples were assayed by RNA sequencing (RNAseq) in the UCLA Neuroscience Genomics Core Laboratory using Lexogen QuantSeq 3′ FWD cDNA library synthesis and multiplex DNA sequencing on an Illumina HiSeq 4000 instrument with single-strand 65 nt sequence reads (all following the manufacturer’s standard protocol). Samples yielded > 10 million sequence reads, each of which was mapped to the RefSeq human genome sequence using the STAR aligner (Dobin et al., 2013) to generate transcript counts per million total transcripts (TPM).
2.3.4. Covariates
Potential confounding variables included sociodemographic characteristics (gender, age, ethnicity, parental education), body mass index (BMI), smoking behavior, and alcohol consumption, as these factors have been previously related to inflammatory, stress, and/or sleep processes (Bøe et al., 2012; Dietch et al., 2017; O’Connor et al., 2009; Umberson et al., 2014). Participants self-reported their gender and their parents reported on participants’ date of birth (from which age was computed) during Wave 1 of the larger study. Determination of ethnicity was based on participant self-reports and parent-reports of the birth countries of participants’ parents and grandparents. Parents also reported the highest level of education they and their spouses completed on an 11-point scale (1 = some elementary school, 11 = graduated from medical, law, or graduate school) and responses were averaged across parents. BMI was computed from measures of height and weight collected during the laboratory visit, and information on smoking and drinking history were collected from self-reports of whether participants had ever taken more than two puffs of cigarettes and more than a few sips of alcohol during the Wave 2 visit of the parent study.
2.4. Statistical approach
Analysis of differential gene expression was conducted separately for each measure of stress and sleep. In order to manage concerns about multiple hypothesis testing using a limited sample size, primary moderation analyses focused on interaction effects between sleep and stress dimensions that showed main effects on inflammatory and/or interferon signaling. Post-hoc ancillary analyses explored potential interactions between other stress and sleep measures.
TPM values were floored at 1 and log2-transformed for analysis by standard linear statistical models relating transcript abundance to measures of stress and/or sleep while excluding genes with minimal variation in expression (SD < .5 log2 expression units). Among the 60,679 gene transcripts assayed, 17,218 showed above-minimal variability and entered into these quantitative analyses. In each analysis, all genes showing a point estimate of > 1.5-fold difference in average transcript abundance over a 4-SD range of predictor value (i.e., sleep or stress measure) served as input into higher-order bioinformatics analyses using TELiS promoter sequence analysis (Cole et al., 2005) to test 2 a priori-specified hypotheses regarding activity of transcription control pathways relevant to CTRA biology: 1) the positive CTRA component of inflammation (NF-κB, as indicated by differential expression of genes bearing transcription factor-binding motifs matching the TRANSFAC position-specific weight matrix V$NFKAPPAB65_01) and, 2) the inverse CTRA component of innate antiviral responses (interferon response factors, IRFs, as indicated by V$IRF2_01).
TELiS analyses were conducted using 9 different parametric combinations of promoter DNA sequence length (−300, −600, and −1000 to +200 nucleotides surrounding the RefSeq-designated transcription start site) and transcription factor-binding motif (TFBM) detection stringency (TRANSFAC mat_sim values of 0.80, .90, and .95) (Cole et al., 2005). Log2-transformed TFBM ratios (comparing prevalence in promoters of up- vs. down-regulated genes) were averaged across the 9 parametric combinations and tested for statistical significance using standard errors derived from bootstrap resampling of linear model residual vectors (controlling for potential correlation across genes). Individual genes were not tested for statistically significant differences in expression because the goal of this study was solely to assess differences in average expression of sets of genes that reflect common transcription factor influences, and for this application point estimate-based screening has been shown to provide more reliable results than gene screening based on gene-specific p-values (Cole et al., 2005).
All analyses controlled for gender, ethnicity, body mass index (kg/m2), smoking history, heavy alcohol consumption history, and parental educational attainment. We also considered including medical history, physical activity, and current sleep duration and efficiency (as measured via self-report using the Pittsburgh Sleep Quality Index) as additional covariates by examining their correlations with primary variables of interest. Medical history, physical activity, and self-reported current sleep efficiency were not correlated with any of the primary variables of interest (ps > .10). Self-reported current sleep duration was marginally correlated only with actigraphy-based sleep duration assessed via actigraphy (p = .054). Consequently, we examined whether primary results involving actigraphy-based measures of sleep duration changed when controlling for current self-reported sleep duration in ancillary analyses. Results were not altered, and therefore, we report results without current self-reported sleep as a covariate.
3. Results
Characteristics of the sample are presented in Table 1. Adolescents reported experiencing at least one interpersonal stress on 24% of days (equal to 3.6 of 14 days) and approximately three major life events in the previous year. They also perceived moderate levels of global stress. There was considerable variability for all three measures of stress. Average levels of sleep duration fell within the recommended range of 7–10 h for 18- to 25-year-olds (Hirshkowitz et al., 2015), though closer to the lower bound, and overall, adolescents in the current sample had high sleep efficiency.
Bivariate correlations among stress and sleep parameters are displayed in Table 2. As expected, the three stress measures (i.e., daily interpersonal stress, major life events, perceived stress) were only moderately correlated, suggesting that they do indeed represent partially distinct domains of stressful experience. Similarly, sleep duration and sleep efficiency were only moderately correlated. Stress measures and sleep parameters, however, were not associated with one another.
Table 2.
Bivariate correlations among stress and sleep indices.
| Daily stress | Major life events | Perceived stress | Sleep duration | |
|---|---|---|---|---|
| Daily stress | ||||
| Major life events | .45*** | |||
| Perceived stress | .39*** | .27** | ||
| Sleep duration | .15 | .09 | .14 | |
| Sleep efficiency | −.06 | −.12 | −.04 | .27* |
Note.
p < .05;
p < .01;
p < .001.
3.1. Stress and gene expression
Daily interpersonal stress was associated with > 1.5-fold differential activity of 1096 gene transcripts (629 up-regulated and 467 down-regulated; Supporting Information Table 1a). Bioinformatic analysis of transcription factor-binding motifs (TFBMs) in the promoters of these genes suggested that increased levels of daily stress was tied to increased activity of NF-κB (mean log2-trasnformed TFBM ratio in up- vs down-regulated genes = .565 ± standard error .245, p = .022) but no significant difference in activity of IRF family factors (.278 ± .364, p = .447: Fig. 1).
Fig. 1.
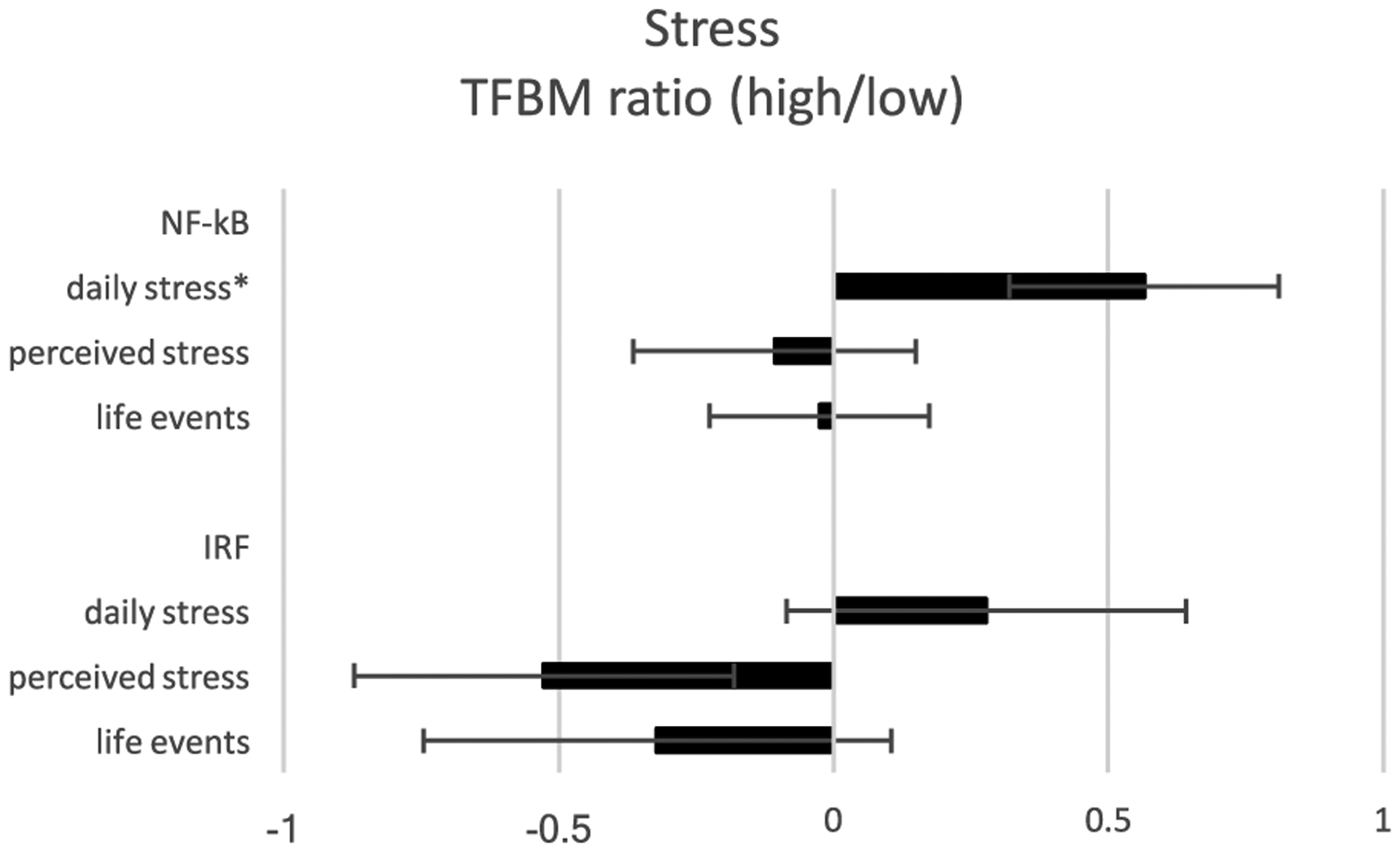
Bioinformatic analysis of proinflammatory and antiviral transcription control pathways in association with various measures of stress. Data represent log2-transformed ratios of transcription factor-binding motifs (TFBMs) for proinflammatory (NF-κB) and antiviral (IRF) transcription factors in the promoters of differentially expressed genes (determined as > 1.5-fold greater difference in average transcript abundance in relation to various measures of stress).
Reports of major life events were associated with > 1.5-fold differential activity of 1242 gene transcripts (443 up-regulated and 779 down-regulated; Supporting Information Table 1b), and bioinformatics analyses failed to indicate any significant contribution from NF-κB (−0.025 ± .200, p = .676) or IRF factors (−.321 ± .426, p = .465; Fig. 1).
Perceived stress scale scores were associated with > 1.5-fold differential activity of 1171 gene transcripts (323 up-regulated and 848 down-regulated; Supporting Information Table 1c), but bioinformatics analyses again failed to indicate any significant contribution to this pattern from differential activity of NF-κB (−0.107 ± .257, p = .676) or IRF factors (−.527 ± .346, p = .130: Fig. 1).
3.2. Sleep and gene expression
Sleep duration was associated with > 1.5-fold differential activity of 1431 gene transcripts (682 up-regulated and 749 down-regulated; listed in Supporting Information Table 2a), and promoter-based bioinformatic analyses linked shorter sleep duration to increased activity of CTRA-related transcriptional dynamics, including greater activity of the pro-inflammatory transcription factor, NF-κB (.700 ± .266, p = .009), and lower activity of the IRF family of antiviral transcription factors (−1.054 ± .361, p = .004; Fig. 2).
Fig. 2.

Bioinformatic analysis of proinflammatory and antiviral transcription control pathways in association with sleep indices. Data represent log2-transformed ratios of transcription factor-binding motifs (TFBMs) for pro-inflammatory (NF-κB) and antiviral (IRF) transcription factors in the promoters of differentially expressed genes (determined as > 1.5-fold greater difference in average transcript abundance in relation to sleep indices).
Sleep efficiency was associated with > 1.5-fold differential activity of 2060 gene transcripts (1672 up-regulated and 388 down-regulated; Supporting Information Table 2b), but bioinformatics analyses of these genes failed to indicate differential activity of either NF-κB (.231 ± .210, p = .273) or IRF family factors (−.695 ± .474, p = .144; Fig. 2).
Similar results emerged from analyses that included both sleep duration and sleep efficiency in the same analytic model (sleep duration: NF-κB: .596 ± .187, p = .002; IRF: −1.249 ± .368, p = .001; sleep efficiency: NF-κB: .129 ± .202, p = .523; IRF: −.724 ± .435, p = .097). Similar results from separate and mutually adjusted analyses were not surprising because sleep duration was only moderately correlated with sleep efficiency (r = .27, p = .02; Table 2).
3.3. Sleep as a moderator of stress-related differences in gene expression
To determine whether the pro-inflammatory effects associated with sleep duration might exaggerate the pro-inflammatory effects associated with daily interpersonal stress, we tested for moderation of the stress effect by sleep duration using a product-term interaction in the context of a model including main effects of both variables (after adjusting for the same covariates used in previous models). Among the 2405 genes showing > 1.5-fold differential expression in association with the Sleep duration x Daily interpersonal stress interaction term (1894 up-regulated and 511 down-regulated; Supporting Information Table 3), promoter-based bioinformatics analyses indicated increased activity of NF-κB (.503 ± .173, p = .004) but no differential activity of IRF factors (.107 ± .348, p = .760).
Although not part of our pre-specified analysis plan, we also conducted post-hoc ancillary analyses testing for potential interactions involving other combinations of sleep measures (either duration or efficiency) and stress measures. Results showed no significant interaction between sleep duration and life events, or between sleep efficiency and daily stress, life events, or perceived stress. These analyses did indicate an effect of shorter sleep duration in accentuating perceived stress-related decreases in IRF activity (.873 ± .394, p = .027) but not NF-κB activity (.068 ± .180, p = .708).
4. Discussion
To our knowledge, the present study is one of the first to examine whether experiences of everyday stress and sleep are each, and together, linked to inflammation- and antiviral-related gene expression in late adolescents. We found that greater daily interpersonal stress and shorter sleep duration were each associated with greater pro-inflammatory gene expression and with increased signaling of the proinflammatory transcription factor, NF-κB. Shorter sleep duration was also associated with downregulation of antiviral gene expression and decreased signaling of IRFs. Notably, increased daily stress and shorter sleep duration interacted, such that daily stress was more strongly associated with NF-κB target gene activity among adolescents with shorter sleep duration. These patterns of findings were not observed for stressful life events, perceived stress, and sleep efficiency.
The association between daily interpersonal stress and differential expression of genes with response elements for NF-κB is consistent with previous gene expression profiling studies of adults showing a similar pattern in relation to other, more major adversities (e.g., caring for a family member with chronic illness, loss of a spouse, low social status) (Cole, 2014). The current study extends these past findings by demonstrating that exposure to more normative, mundane stressors in everyday life, such as arguments with and punishment by parents, can accumulate across days to have similar consequences for inflammatory signaling during adolescence. It also mirrors previous findings linking daily stress to elevated protein markers of inflammation in adults and adolescents (Chiang et al., 2012; Fuligni et al., 2009), and extends these basic links to the expression of inflammation-related genes and elucidates the gene regulatory pathways involved, namely increased signaling of NF-κB. Contrasting previous gene expression profiling studies (Cole, 2014), we did not observe any associations between stress and downregulation of antiviral gene expression mediated by IRF activity. Why we observed this discrepancy with prior work is not entirely clear. It may be that only more major or chronic adversities like those examined in prior studies (e.g., poverty, social isolation, early deprivation) impact IRF-related gene expression and the less severe stressors examined in the current study have less impact.
Interestingly, the effects of stress on NF-κB target gene expression were specific to daily stress—parallel associations were not observed for perceived stress and life events. A critical distinction between the measure of daily stress and the measures of perceived stress and life events is that the former focused on interpersonal stressors that more directly involved the participants. By contrast, the measure of perceived stress focused on global feelings of distress not necessarily tied to any particular stressors, and the measure of life events included both interpersonal and non-interpersonal episodic stressors. It may be that interpersonal stressors are particularly consequential for certain health-related outcomes, including the expression of inflammatory genes. Indeed, stressors involving interpersonal loss, social status, and social rejection are more strongly related to depression and HPA axis functioning (Dickerson and Kemeny, 2004; Slavich and Irwin, 2014). Furthermore, the CTRA profile has been linked particularly to adversities that are interpersonal in nature—for instance, social isolation or poor social relationships, low social status, social rejection, bereavement, and care-giving for a chronically ill family member (Cole, 2014). Of course, follow-up studies comparing transcriptional profiles associated with interpersonal vs. non-interpersonal stressors are needed to verify this hypothesis.
How might daily interpersonal stress come to affect inflammatory gene expression? Putative pathways involve the sympathetic nervous system (SNS) and the HPA axis. Stress activates the SNS, and when the SNS neurotransmitter norepinephrine binds to beta-adrenergic receptors on immune cells, it can act to increase transcription of proinflammatory genes (Irwin and Cole, 2011; Rohleder, 2014). In contrast with the promotive effects of the SNS’s norepinephrine, cortisol–the hormonal output of the HPA axis–suppresses the production of pro-inflammatory cytokines when bound to its corresponding glucocorticoid receptors in immune cells (Irwin and Cole, 2011; Rohleder, 2014). Repeated or chronic exposure to stress may foster a state of glucocorticoid resistance, such that immune cells become less sensitive to cortisol’s inhibitory signals (Miller et al., 2002). Thus, it may be that stressors occurring day after day affect inflammatory gene expression by repeatedly engaging the SNS and/or by decreasing glucocorticoid receptor sensitivity. It will be important for future work to replicate the present findings and advance them by investigating these potential pathways.
Results with respect to sleep revealed that shorter sleep duration was associated with increased expression of NF-κB target genes and decreased expression of IRF target genes. These findings converge with past observational studies linking shorter sleep duration to heightened circulating levels of inflammatory markers from peripheral blood in healthy youth (Burdayron and McGrath, 2017; Hall et al., 2015; Martinez-Gomez et al., 2011; Park et al., 2016) and with past studies demonstrating that partial sleep deprivation activates NF-κB signaling in adults (Irwin and Opp, 2017). It also converges with prior experimental work on sleep and viral processes. Those studies linked shorter sleep duration to greater susceptibility to developing a clinical cold after viral exposure (Prather et al., 2015) and to decreased antibody responses to influenza and hepatitis A vaccinations (Lange et al., 2003; Spiegel et al., 2002). The current study extends these prior findings to upstream inflammatory and antiviral processes at the genomic level. To our knowledge, this is one of the first studies to identify an association between sleep duration and patterns of gene expression in adolescents. It is also worth noting that in contrast to previous laboratory studies tying experimentally-induced partial sleep loss to NF-κB signaling (Irwin and Opp, 2017) and to immune responses to vaccines, the current findings were observed for participants’ everyday patterns of sleep in their naturalistic context. As such, our findings suggest that even sleep loss occurring in adolescents’ everyday lives can be consequential for molecular inflammatory and antiviral processes.
The effect of sleep on inflammatory and antiviral gene expression was observed only for sleep duration and not for sleep efficiency. It may be that sleep efficiency has little impact on these immune processes in young individuals. Alternatively, sleep efficiency may modulate inflammatory and antiviral activity, but its potential links with them may be more difficult to detect during adolescence given that adolescents tend to be efficient sleepers (de Zambotti et al., 2015; Javaheri et al., 2008). Links between sleep efficiency and immune dynamics, then, may emerge in the context of more severe loss of sleep efficiency or after prolonged periods of compromised sleep efficiency. Future experimental studies manipulating sleep efficiency and fragmentation are needed to clarify whether sleep efficiency does indeed have consequences for inflammatory and antiviral processes.
A primary and novel contribution of the present investigation is the examination of the interactive effect between stress and sleep on inflammatory gene expression. We found that shorter sleep duration potentiated the association between daily interpersonal stress and inflammatory gene expression (or conversely, that longer sleep duration mitigated this association). A number of studies have similarly found sleep to moderate other effects of stress. In particular, sleep efficiency has been shown to moderate the effects of daily family stress on daily negative affect and HPA axis functioning (Chiang et al., 2017b, 2016). The current study suggests that for upstream molecular inflammatory dynamics, sleep duration, rather than sleep efficiency may be the more important moderator. Shorter sleep duration may accentuate transduction of the everyday social environment through upregulation of SNS activity and/or alterations in HPA axis functioning (Irwin and Opp, 2017). SNS activation decreases during sleep, and thus shorter sleep duration may prolong SNS activation. As noted above, this in turn can upregulate inflammatory processes (Irwin and Cole, 2011). Shorter sleep duration has also been associated with altered HPA axis functioning (Irwin and Opp, 2017), which may have implications for glucocorticoid resistance and effective termination of inflammation (though this remains to be tested). These sleep-associated alterations of the SNS and HPA axis may foster an underlying biological vulnerability that exacerbates the link between daily stress and expression of genes targeted by NF-κB. Shorter sleep duration may also exacerbate the observed effects of daily stress on gene expression through psychological and behavioral pathways–enhancing threat appraisals, prolonging negative affective reactivity due to compromises in emotion regulation, increasing consumption of unhealthy foods, and decreasing physical activity. Indeed, prior work has linked shorter sleep duration to greater perceptions of threat (Minkel et al., 2012), more difficulty in regulating negative emotions (Baum et al., 2014; Palmer and Alfano, 2017; Tempesta et al., 2018), and higher levels of adiposity (Itani et al., 2017); these in turn are thought to and/or have been linked to inflammation (e.g., Appleton et al., 2013; Chiang et al., 2015; O’Connor et al., 2009).
In understanding the implications of stress and sleep for inflammation-related gene expression, it is important to note that expression of inflammation-related genes was assessed an average of 5.5 months after the assessment of daily interpersonal stress and sleep. Although this prospective design may temper concerns about the directionality of findings, it raises other questions about the study’s findings. One concern is that the expression of genes and transcriptional changes are dynamic and can be transient (Bar-Joseph et al., 2012), which raises the question of why daily stress and sleep would be related to gene expression several months later. We may have observed these links because daily assessments may be a sampling of adolescents’ typical experiences. If so, we would expect some level of temporal stability. Indeed, past research has shown that daily stress is relatively stable across a 4-year period from late adolescence into young adulthood (Howland et al., 2017). Similarly, in the current study, we observed good week-to-week stability in daily stress (r = .67). Short-term stability in sleep during late adolescence is less clear and an important avenue for future research, but if sleep patterns are also relatively stable across several months, our daily assessments of stress and sleep may closely represent or serve as proxies for those experiences occurring closer in time to when gene expression was measured. Another possibility is that our daily assessments of sleep may reflect repeated stress and poor sleep; and to the extent that these experiences become chronic, they may lead to sustained overexpression of certain inflammation- and/or antiviral-related genes. These hypotheses remain speculative and will need to be tested in future work.
Several other study limitations should also be considered when interpreting the present findings. First, the sample size was limited. Although sufficient to test this study’s a priori hypotheses regarding gene set-based bioinformatic assessments of CTRA-related transcription factor activity, the current sample size was not powered for hypothesis-free discovery of statistically significant associations between individual gene transcripts and stress or sleep parameters. This report tests several distinct substantive hypotheses (i.e., regarding effects of sleep and of stress in relationship to inflammatory and to antiviral gene expression) and it will be important to replicate each of these substantive findings in future research. Second, this study recruited a localized population of community-dwelling adolescents. Future research in larger samples from other sociodemographic settings will be required to identify additional transcriptional correlates of stress and sleep beyond the CTRA-related transcriptional dynamics examined here and to evaluate the generalizability of the present findings. Third, the current study included no measures of adolescents’ circadian preference or rhythm, which may play an important role in the dynamics among stress, sleep, and inflammatory processes in light of prior work demonstrating a shift in circadian rhythms during adolescence and that a preference for a delayed sleep-wake schedule is linked to worse mental and physical health outcomes (Arora and Taheri, 2015; Baron and Reid, 2014). Fourth, with the exception of IL-6, we did not assess any downstream signaling molecules or biological markers in the NF-κB and IRF pathways. We previously reported no association between the same measure of daily interpersonal stress and circulating levels of IL-6 (Chiang et al., 2018), which may seem to contrast the study’s current findings with respect to daily stress and inflammation-related gene expression. However, it is critical to note that we only assessed IL-6 whereas the expression of inflammation-related genes and upregulated NF-κB activity may represent a multitude of proinflammatory signals. Furthermore, multiple tissues, including immune and adipose tissue, release IL-6 and as such, the cellular origin of circulating IL-6 cannot be ascertained. By contrast, the methods used in the current study allowed us to specifically probe immune cells. Given these issues, future studies should extend our findings and identify the downstream consequences of the observed transcriptomic differences. Lastly, the current sample was healthy with very few participants displaying clinical illness. Consequently, the clinical health significance of the current findings also remains unknown.
Despite these limitations, the present study provides preliminary evidence that seemingly mundane interpersonal stressors and shortened sleep in everyday life can have ramifications for inflammatory dynamics at the molecular level even in the early decades of life. Specifically, daily interpersonal stress and shorter sleep duration are both associated with increased expression of genes regulated by the pro-inflammatory transcription factor, NF-κB. Moreover, these two stimuli may have synergistic effects, such that expression of inflammatory-related genes may be greatest in the immune cells of late adolescents confronting more daily interpersonal stress and getting less sleep. Adolescence is marked by sensitivity to stress and an increased prevalence of shortened sleep. To the extent that these patterns continue for a protracted amount of time, their effects on pro-inflammatory signaling may have far-reaching implications for later health and disease.
Supplementary Material
Acknowledgements
Preparation of this manuscript was supported by the National Heart, Lung, and Blood Institute (F32-HL134276 to J.J.C.), National Institute on Aging (R01-AG051944, R01-AG056424, R01-AG052655 to M.R.I), National Cancer Institute (R01-CA160245, R01-CA207130, R01-CA203930 to M.R.I), and the National Institute on Drug Abuse (R01-DA032922 to M.R.I.). Research reported in this publication was supported jointly by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD062547; R24-HD041022), National Institute on Aging (U24AG047867, P30-AG028748, P30-AG017265), UCLA Cousins Center for Psychoneuroimmunology, University of California Institute for Mexico and the US, American Psychological Association, Division 38 of the American Psychological Association, the UK Economic and Social Research Council, and the Biotechnology and Biological Sciences Research Council (ES/M00919X/1).
Footnotes
Conflict of interest
The authors declare no conflict of interests.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.psyneuen.2018.11.026.
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA, 2005. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1-to 5-year-old children. Sleep 28, 1568–1577. [DOI] [PubMed] [Google Scholar]
- Adler NE, Rehkopf DH, 2008. US disparities in health: descriptions, causes, and mechanisms. Annu. Rev. Public Health 29, 235–252. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T, Orsini N, Petersen H, Axelsson J, Lekander M, Kecklund G, 2012. Predicting sleep quality from stress and prior sleep–a study of day-to-day covariation across six weeks. Sleep Med 13, 674–679. [DOI] [PubMed] [Google Scholar]
- American Psychological Association, 2014. Stress in America: Are Teens Adopting Adults’ Stress Habits. Stress in America Surveys http://www.apa.org/news/press/releases/stress/2013/stress-report.pdf.
- Appleton AA, Buka SL, Loucks EB, Gilman SE, Kubzansky LD, 2013. Divergent associations of adaptive and maladaptive emotion regulation strategies with inflammation. Health Psychol 32, 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora T, Taheri S, 2015. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int. J. Obes 39, 39–44. [DOI] [PubMed] [Google Scholar]
- Bar-Joseph Z, Gitter A, Simon I, 2012. Studying and modelling dynamic biological processes using time-series gene expression data. Nat. Rev. Genet 13, 552–564. [DOI] [PubMed] [Google Scholar]
- Baron KG, Reid KJ, 2014. Circadian misalignment and health. Int. Rev. Psychiatry 26,139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW, 2014. Sleep restriction worsens mood and emotion regulation in adolescents. J. Child Psychol. Psychiatry 55, 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøe T, Hysing M, Stormark KM, Lundervold AJ, Sivertsen B, 2012. Sleep problems as a mediator of the association between parental education levels, perceived family economy and poor mental health in children. J. Psychosom. Res 73, 430–436. [DOI] [PubMed] [Google Scholar]
- Bor W, Dean AJ, Najman J, Hayatbakhsh R, 2014. Are child and adolescent mental health problems increasing in the 21st century? A systematic review. Aust. N. Z. J. Psychiatry 48, 606–616. [DOI] [PubMed] [Google Scholar]
- Burdayron R, McGrath JJ, 2017. Our house is a zoo: household chaos, school start time, poor sleep, and their role in inflammation among children and adolescents. Sleep Med 40, e217–e218. [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, Marsland AL, 2011. Negative affective responses to a speech task predict changes in interleukin(IL)-6. Brain Behav. Immun 25, 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, 2011. Sleep in adolescents: the perfect storm. Pediatric Clin 58, 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Eisenberger NI, Seeman TE, Taylor SE, 2012. Negative and competitive social interactions are related to heightened proinflammatory cytokine activity. Proc. Natl. Acad. Sci 109, 1878–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Bower JE, Almeida DM, Irwin MR, Seeman T, Fuligni AJ, 2015a. Socioeconomic status, daily affective and social experiences, and inflammation during adolescence. Psychosom. Med 77, 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Bower JE, Taylor SE, 2015b. Early adversity, neural development, and inflammation. Dev. Psychobiol 57, 887–907. [DOI] [PubMed] [Google Scholar]
- Chiang JJ, Tsai KM, Park H, Bower JE, Almeida DM, Dahl RE, Irwin MR, Seeman TE, Fuligni AJ, 2016. Daily family stress and HPA axis functioning during adolescence: the moderating role of sleep. Psychoneuroendocrinology 71, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Bower JE, Irwin MR, Taylor SE, Fuligni AJ, 2017a. Adiposity moderates links from early adversity and depressive symptoms to inflammatory reactivity to acute stress during late adolescence. Brain Behav. Immun 66, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Kim JJ, Almeida DM, Bower JE, Dahl RE, Irwin MR, McCreath H, Fuligni J, 2017b. Sleep efficiency modulates associations between family stress and adolescent depressive smptoms and negative affect. J. Adolesc. Health 61, 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Ko A, Bower JE, Irwin ME, Taylor SE, Fuligni AJ, 2018. Stress, psychological resources, and HPA and inflammatory reactivity during late adolescence. Dev. Psychopathol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J. Health Soc. Behav 24, 385–396. [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE, 2007. Psychological stress and disease. J. Am.Med. Assoc 298, 1685–1687. [DOI] [PubMed] [Google Scholar]
- Cole SW, 2014. Human social genomics. PLoS Genet 10, e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Yan W, Galic Z, Arevalo J, Zack JA, 2005. Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics 21, 803–810. [DOI] [PubMed] [Google Scholar]
- Conger RD, Wallace LE, Sun Y, Simons RL, McLoyd VC, Brody GH, 2002. Economic pressure in African American families: a replication and extension of the family stress model. Dev. Psychol 38, 179–193. [PubMed] [Google Scholar]
- de Zambotti M, Baker FC, Colrain IM, 2015. Validation of sleep-tracking technology compared with polysomnography in adolescents. Sleep 38, 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, 2004. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull 130, 355–391. [DOI] [PubMed] [Google Scholar]
- Dietch JR, Taylor DJ, Smyth JM, Ahn C, Smith TW, Uchino BN, Allison M, Ruiz JM, 2017. Gender and racial/ethnic differences in sleep duration in the North Texas heart study. Sleep ealth 3, 324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane LD, Gress-Smith JL, Breitenstein RS, 2015. Multi-method assessments of sleep over the transition to college and the associations with depression and anxiety symptoms. J. Youth Adolesc 44, 389–404. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR, 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich KB, Hoyt LT, Sumner JA, McDade TW, Adam EK, 2015. Quality of relationships with parents and friends in adolescence predicts metabolic risk in young adulthood. Health Psychol 34, 896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensterl V, Sen GC, 2009. Interferons and viral infections. Biofactors 35, 14–20. [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Telzer EH, Bower J, Cole SW, Kiang L, Irwin MR, 2009. A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin American and European backgrounds. Psychosom. Med 71, 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Glaser R, 2006. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J. Neuroimmune Pharmacol 1, 421–427. [DOI] [PubMed] [Google Scholar]
- Hall MH, Lee L, Matthews KA, 2015. Sleep duration during the school week is associated with C-reactive protein risk groups in healthy adolescents. Sleep Med 16, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, 1991. Generation of stress in the course of unipolar depression. J. Abnorm.Psychol 100, 555–561. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ, 2008. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry 63, 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill PL, Sin NL, Turiano NA, Burrow AL, Almeida DM, 2018. Sense of purpose moderates the associations between daily stressors and daily well-being. Ann. Behav. Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L, 2015. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 1, 40–43. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D, 2015. Loneliness and social isolation as risk factors for mortality a meta-analytic review. Perspect. Psychol. Sci 10, 227–237. [DOI] [PubMed] [Google Scholar]
- Howland M, Armeli S, Feinn R, Tennen H, 2017. Daily emotional stress reactivity in emerging adulthood: temporal stability and its predictors. Anxiety Stress Coping 30, 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Cole SW, 2011. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol 11, 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Opp MR, 2017. Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology 42, 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani O, Jike M, Watanabe N, Kaneita Y, 2017. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med 32, 246–256. [DOI] [PubMed] [Google Scholar]
- Javaheri S, Storfer-Isser A, Rosen CL, Redline S, 2008. Sleep quality and elevated blood pressure in adolescents. Circulation 118, 1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani M, Eliasson A, Vernalis M, 2012. Perceived stress correlates with disturbed sleep: a link connecting stress and cardiovascular disease. Stress 15, 45–51. [DOI] [PubMed] [Google Scholar]
- Khalyfa A, Capdevila OS, Buazza MO, Serpero LD, Kheirandish-Gozal L, Gozal D, 2009. Genome-wide gene expression profiling in children with non-obese obstructive sleep apnea. Sleep Med 10, 75–86. [DOI] [PubMed] [Google Scholar]
- Lange T, Perras B, Fehm HL, Born J, 2003. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom. Med 65, 831–835. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S, 1984. Stress, Appraisal, and Coping Springer, New York. [Google Scholar]
- Lovato N, Gradisar M, 2014. A meta-analysis and model of the relationship between sleep and depression in adolescents: recommendations for future research and clinical practice. Sleep Med. Rev 18, 521–529. [DOI] [PubMed] [Google Scholar]
- Maoloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF, 2013. The micro-biome: stress, health and disease. Mamm. Genome 25, 49–74. [DOI] [PubMed] [Google Scholar]
- Martinez-Gomez D, Eisenmann JC, Gomez-Martinez S, Hill EE, Zapatera B, Veiga OL, Marcos A, 2011. Sleep duration and emerging cardiometabolic risk markers in adolescents. The AFINOS study. Sleep Med 12, 997–1002. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Garrad MC, Somerville LH, 2015. What develops during emotional development? A component process approach to identifying sources of psycho-pathology risk in adolescence. Dialogues Clin. Neurosci 17, 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK, 2002. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol 21, 531–541. [DOI] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Cole SW, 2009. Chronic interpersonal stress predicts activation of pro-and anti-inflammatory signaling pathways six months later. Psychosom. Med 71, 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel JD, Banks S, Htaik O, Moreta MC, Jones CW, McGlinchey EL, Simpson NS, Dinges DF, 2012. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion 12, 1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel J, Moreta MC, Muto J, Htaik O, Jones C, Basner M, Dinges D, 2014. Sleep deprivation potentiates HPA axis stress reactivity in healthy adults. Health Psychol 33, 1430–1434. [DOI] [PubMed] [Google Scholar]
- Nathan C, Ding A, 2010. Nonresolving inflammation. Cell 140, 871–882. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, 2009. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun 23, 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon M, Wickwire EM, Hirshkowitz M, Albert SM, Avidan A, Daly FJ,Dauvilliers Y, Ferri R, Fung C, Gozal D, 2017. National Sleep Foundation’s sleep quality recommendations: first report. Sleep Health 3, 6–19. [DOI] [PubMed] [Google Scholar]
- Owens JF, Group A.S.W., 2014. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics 134, e921–e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CA, Alfano CA, 2017. Sleep and emotion regulation: an organizing, integrative review. Sleep Med. Rev 31, 6–16. [DOI] [PubMed] [Google Scholar]
- Park H, Tsai KM, Dahl RE, Irwin MR, McCreath H, Seeman TE, Fuligni AJ, 2016. Sleep and inflammation during adolescence. Psychosom. Med 78, 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Janicki-Deverts D, Hall MH, Cohen S, 2015. Behaviorally assessed sleep and susceptibility to the common cold. Sleep 38, 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, 2014. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom. Med 76, 181–189. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, Carskadon MA, 1994. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 17, 201–207. [DOI] [PubMed] [Google Scholar]
- Sin NL, Graham-Engeland JE, Ong AD, Almeida DM, 2015. Affective reactivity to daily stressors is associated with elevated inflammation. Health Psychol 34, 1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR, 2014. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull 140, 774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW, 2007. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J. Neurosci 27, 8857–8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, 2013. The teenage brain: sensitivity to social evaluation. Curr. Drections Psychol. Sci 22, 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Sheridan JF, Van Cauter E, 2002. Effect of sleep deprivation on response to immunizaton. J. Am. Med. Assoc 288, 1471–1472. [DOI] [PubMed] [Google Scholar]
- Stenholm S, Head J, Kivimäki M, Magnusson Hanson LL, Pentti J, Rod NH, Clark AJ, Oksanen T, Westerlund H, Vahtera J, 2019. Sleep duration and sleep disturbances as predictors of healthy and chronic disease-free life expectancy between ages 50 and 75: a pooled analysis of three cohorts. J. Gerontol 74, 204–210. [DOI] [PubMed] [Google Scholar]
- Surachman A, Wardecker B, Chow SM, Almeida DM, 2018. Life course socioeconomic status, daily stressors, and daily well-being: examining chain of risk models. J. Gerontol.: Series B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempesta D, Socci V, De Gennaro L, Ferrara M, 2018. Sleep and emotional processing. Sleep Med. Rev 40, 183–195. [DOI] [PubMed] [Google Scholar]
- Tsai KM, Telzer EH, Gonzales NA, Fuligni AJ, 2013. Adolescents’ daily assistance to the family in response to maternal need. J. Marriage Fam 75, 964–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson D, Williams K, Thomas PA, Liu H, Thomeer MB, 2014. Race, gender, and chains of disadvantage: childhood adversity, social relationships, and health. J. Health Soc. Behav 55, 20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SM, Mermelstein RJ, Hankin BL, Hedeker D, Flay BR, 2007. Longitudinal patterns of daily affect and global mood during adolescence. J. Res. Adolesc 17, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang S, Lou Y, Long Q, Liang Y, Xie S, Yuan J, 2018. The increased sex differences in susceptibility to emotional stimuli during adolescence: an event-related potential study. Front. Hum. Neurosci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


