Abstract
Background and Purpose:
Strokes can be distinguished from benign peripheral causes of acute vestibular syndrome using bedside oculomotor tests (head impulse test, nystagmus, test-of-skew). Using head impulse test, nystagmus, test-of-skew is more sensitive and less costly than early magnetic resonance imaging for stroke diagnosis in acute vestibular syndrome but requires expertise not routinely available in emergency departments. We sought to begin standardizing the head impulse test, nystagmus, test-of-skew diagnostic approach for eventual emergency department use through the novel application of a portable video-oculography device measuring vestibular physiology in real time. This approach is conceptually similar to ECG to diagnose acute cardiac ischemia.
Methods:
Proof-of-concept study (August 2011 to June 2012). We recruited adult emergency department patients with acute vestibular syndrome defined as new, persistent vertigo/dizziness, nystagmus, and (1) nausea/vomiting, (2) head motion intolerance, or (3) new gait unsteadiness. We recorded eye movements, including quantitative horizontal head impulse testing of vestibulo-ocular-reflex function. Two masked vestibular experts rated vestibular findings, which were compared with final radiographic gold-standard diagnoses. Masked neuroimaging raters determined stroke or no stroke using magnetic resonance imaging of the brain with diffusion-weighted imaging obtained 48 hours to 7 days after symptom onset.
Results:
We enrolled 12 consecutive patients who underwent confirmatory magnetic resonance imaging. Mean age was 61 years (range 30–73), and 10 were men. Expert-rated video-oculography–based head impulse test, nystagmus, test-of-skew examination was 100% accurate (6 strokes, 6 peripheral vestibular).
Conclusions:
Device-based physiological diagnosis of vertebrobasilar stroke in acute vestibular syndrome should soon be possible. If confirmed in a larger sample, this bedside eye ECG approach could eventually help fulfill a critical need for timely, accurate, efficient diagnosis in emergency department patients with vertigo or dizziness who are at high risk for stroke.
Introduction:
Vertigo and dizziness account for 2.6 million US emergency department (ED) visits per year, ≈4% attributable to stroke.1 Roughly 250 to 500 000 involve a high-risk-for-stroke clinical presentation known as acute vestibular syndrome (AVS).2 AVS is a well-defined clinical syndrome of severe, continuous vertigo, or dizziness, nausea or vomiting, gait instability, head motion intolerance, and nystagmus lasting days to weeks.2–4 Most AVS patients have a benign peripheral vestibular cause (vestibular neuritis or nonbacterial labyrinthitis), but ≈25% have brain stem or cerebellar strokes.2 Other central causes, such as multiple sclerosis, are uncommon.2 Distinguishing dangerous central from benign peripheral vestibular causes can be challenging. Half of stroke patients presenting with AVS have no focal neurological signs.2 In the first 24 hours after onset of symptoms, computed tomography accurately detects cerebellar hemorrhages but not ischemic strokes (16% sensitivity).5 Even magnetic resonance imaging of the brain with diffusion-weighted imaging (MRI-DWI) identifies only ≈80% of posterior fossa infarctions in the first day.2
Three bedside eye movement findings (Head Impulse test, Nystagmus, Test-of-Skew [HINTS]) differentiate central from peripheral causes of AVS, even outperforming early MRI (Table I in the online-only Data Supplement).2 This is not surprising, given that structural anatomic changes from brain ischemia generally lag physiological dysfunction. These eye movement tests are rapid and noninvasive but unfamiliar to most nonspecialists. The horizontal head impulse test (h-HIT) is the single best predictor of stroke; a bilaterally normal result in AVS increases the odds of stroke 18-fold.2 Of the 3 tests, the h-HIT is the most technically demanding to perform, and interpretation varies with expertise.6 Even neuro-otology subspecialists can be deceived when interpreting the h-HIT nonquantitatively because it relies on perception of a fast, corrective eye movement during the test (Figure 1A, red chevrons) that may sometimes be hidden (covert saccades).7
Figure 1.
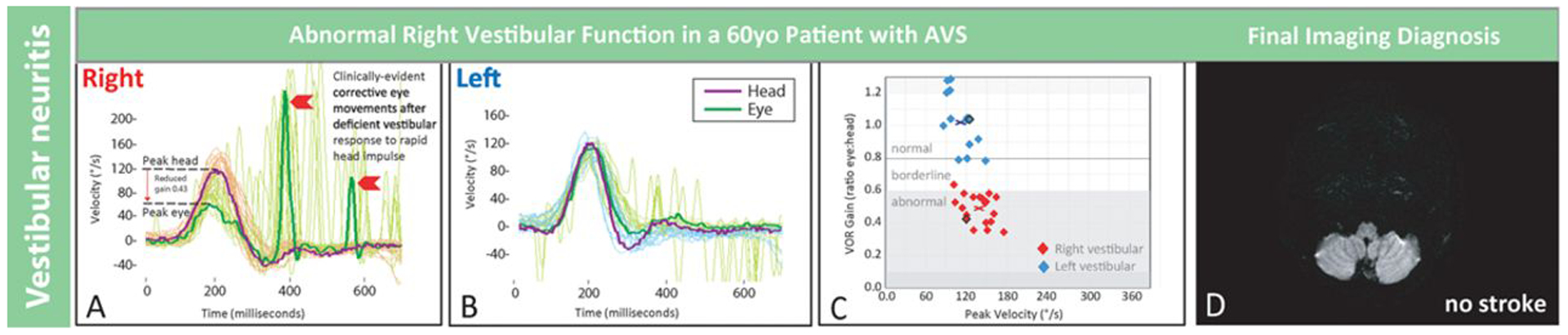
Quantitative head impulse and magnetic resonance imaging (MRI) results in an older patient with vestibular neuritis. Shown are results from patient No. 8 in Figure 3, a 60-year-old man with vestibular neuritis. Clinical presenting features were vertigo, nausea, and gait disturbance without general neurological or auditory symptoms or signs. There was unidirectional nystagmus and no skew deviation. Shown are physiological tracings from multiple rightward (A) and leftward (B) horizontal head impulse test (h-HIT) maneuvers temporally superimposed with a single maneuver highlighted darker. The rightward h-HIT response is abnormal (A). It has both a quantitative abnormality (reduced vestibulo-ocular reflex [VOR] gain during the head movement, downward red arrow) and a qualitative, clinically evident abnormality (fast, corrective eye movements to realign the eye on the target after the head stops moving, red chevrons). The leftward h-HIT response is normal (B). Each h-HIT result is mapped in the corresponding VOR gain plot (C), where the central x denotes the mean right- (red) or left-sided (blue) VOR gain across h-HIT trials. A single representative axial MRI-diffusion-weighted images (DWI) through the inferior cerebellum shows no stroke (D). Note that only the abnormal h-HIT result differed from clinical findings in patient No. 2, the young patient with a large, inferior cerebellar stroke in Figure 2. AVS indicates acute vestibular syndrome and °/s, degrees per second.
An easy-to-use, lightweight, portable, noninvasive video-oculography device has been developed that accurately measures the h-HIT vestibulo-ocular reflex (VOR) under controlled, laboratory conditions.8 If this device works similarly in clinical practice, it could lead to earlier stroke diagnosis and more efficient ED testing and triage decisions for AVS patients. If fully automated, the device might eventually be used even without onsite expertise. This approach is conceptually analogous to diagnosis of myocardial infarction by electrocardiography in patients with high-risk chest pain. As a crucial first step toward automation for use in frontline care settings, we sought to determine whether the device could be used to help discriminate central from peripheral causes in ED patients with AVS.
Methods:
We conducted a proof-of-concept study at 2 tertiary-care medical centers. Results of quantitative head impulse video-oculography were compared with neuroimaging for acute stroke diagnosis. We enrolled adult ED patients with AVS seen <7 days since symptom onset and still symptomatic. We included patients with continuous vertigo or dizziness having lasted ≥1 hour plus pathological nystagmus and ≥1 of the following: (1) nausea or vomiting, (2) head motion intolerance, or (3) new gait or balance disturbance. We excluded patients with relevant previous vestibular or oculomotor disorder, acute drug/alcohol intoxication, or new head trauma. The study protocol was approved by the institutional review board at both sites.
We used a noninvasive, quantitative video-oculography device (ICS Impulse, GN Otometrics, Taastrup, Denmark, http://www.icsimpulse.com/) to measure and record VOR responses. The h-HIT was applied as a rapid horizontal head rotation from lateral to center, with the patient visually fixating on a target roughly 5 to 10 feet away. The device records head and eye velocity traces (Figures 1A, 1B, 2A, and 2B). It plots the VOR gain ratio for each h-HIT trial (Figures 1C and 2C), and then calculates a side-specific mean across all trials for that patient (x in Figures 1C and 2C). We separately calculated the relative right-left asymmetry in mean VOR gain ratio (Figure 3A). Examiners also looked for nystagmus in different gaze positions and ocular alignment (Figure 3B).3,4
Figure 2.
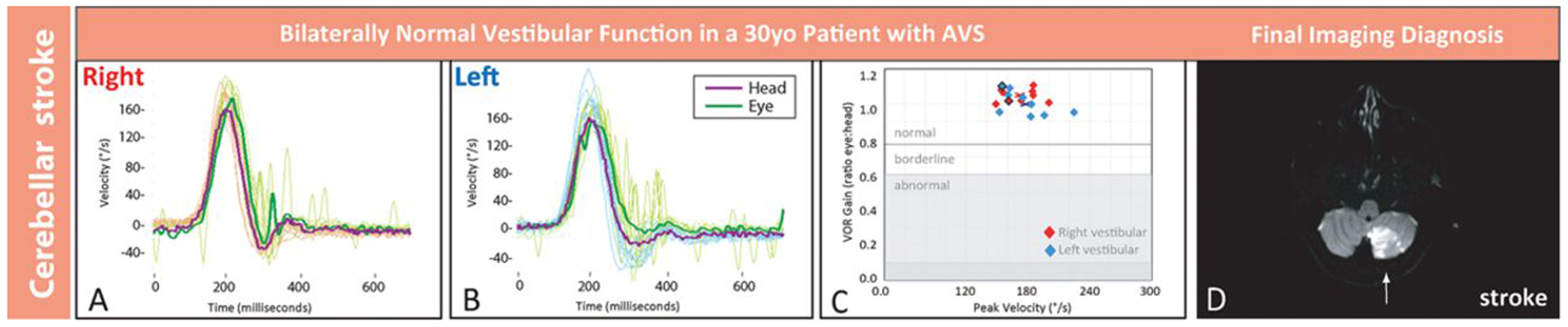
Quantitative head impulse and magnetic resonance imaging (MRI) results in a young patient with stroke. Shown are results from patient No. 2 in Figure 3, a 30-year-old man with inferior cerebellar stroke. Clinical presenting features were vertigo, nausea, and gait disturbance without general neurological or auditory symptoms or signs. There was unidirectional nystagmus and no skew deviation. Shown are physiological tracings from multiple rightward (A) and leftward (B) horizontal head impulse test (h-HIT) maneuvers temporally superimposed with a single maneuver highlighted darker. Tracings reveal normal h-HIT results bilaterally, with a vestibulo-ocular reflex (VOR) eye movement response that closely matches the head movement (A and B). Each h-HIT result is mapped in the corresponding VOR gain plot (C), where the central x denotes the mean right- (red) or left-sided (blue) VOR gain across h-HIT trials. A single representative axial MRI–diffusion-weighted images (DWI) through the inferior cerebellum shows a large acute, left posterior inferior cerebellar artery territory infarction (D, arrow). The stroke was extensive (maximal dimensions 3.0×5.0×4.4 centimeters), spanning 8 axial sections. Note that only the bilaterally normal h-HIT result differed from clinical findings in patient No. 8, the older patient with vestibular neuritis in Figure 1. AVS indicates acute vestibular syndrome; and °/s, degrees per second.
Figure 3.
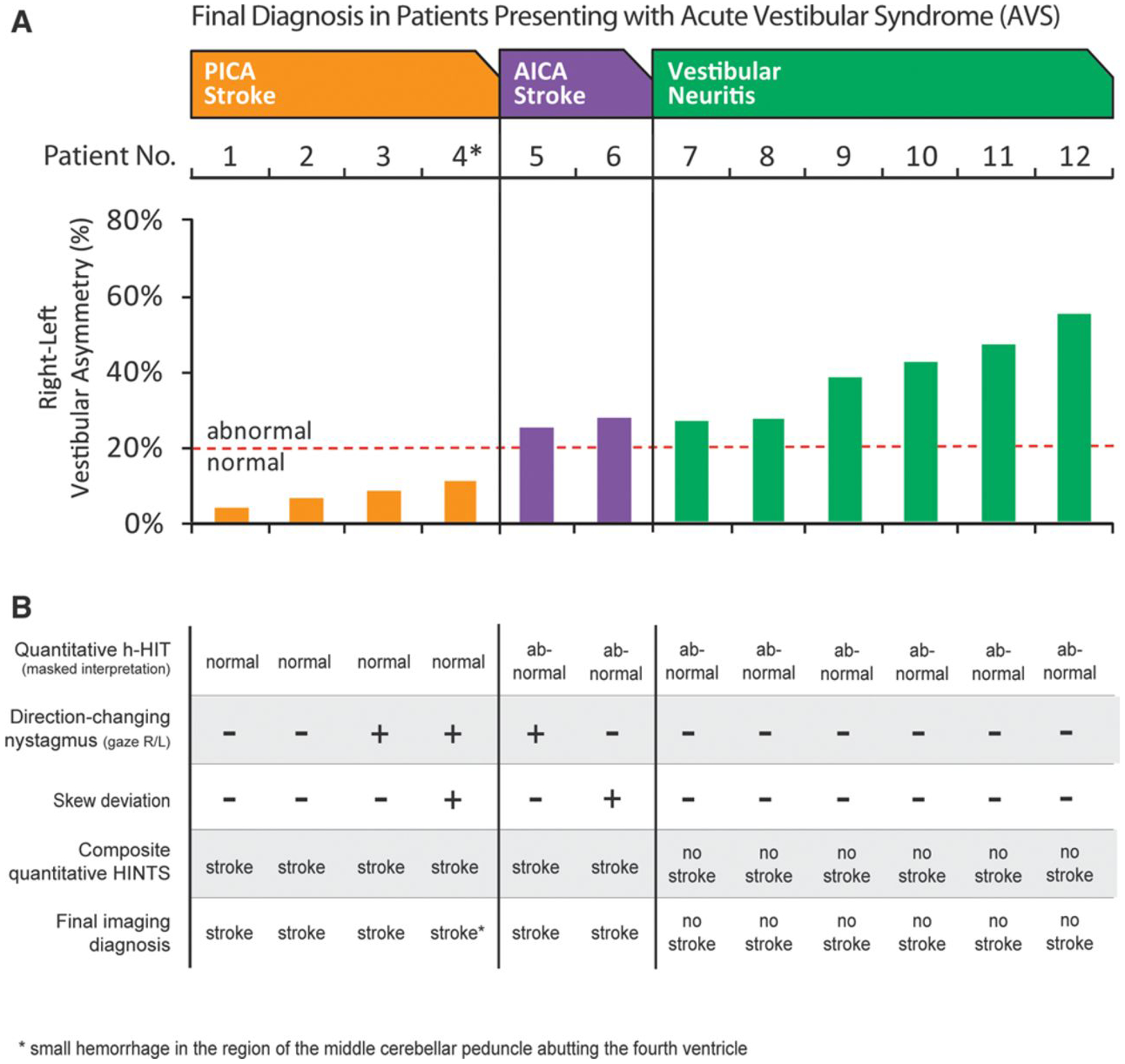
Vestibulo-ocular reflex (VOR) abnormalities and oculographic head impulse, nystagmus, test-of-skew (HINTS) results for 12 acute vestibular syndrome (AVS) patients. A, Relative right-left VOR asymmetry for each patient (absolute VOR gains shown in Figure II in the online-only Data Supplement). Patients are grouped by diagnosis and arrayed from least to greatest asymmetry within groups. B, Masked interpretations of all quantitative and clinical results as well as composite HINTS-based diagnosis. Bilaterally normal horizontal head impulse test (h-HIT) results or those with direction-changing nystagmus or skew-predicted radiographic stroke. Unilaterally abnormal h-HIT results indicated vestibular neuritis, absent direction-changing nystagmus, and skew. AICA indicates anterior inferior cerebellar artery; PICA, posterior inferior cerebellar artery; and R/L, right/left.
Two independent, expert masked neuro-otologists categorized each patient as a suspected central pattern (ie, bilaterally normal VOR), a suspected peripheral pattern (ie, unilaterally abnormal VOR), or equivocal, using only h-HIT results. They were then given the other expert clinical HINTS findings (nystagmus and skew) and asked to recode. Neuroimaging diagnoses (stroke versus no stroke) were rendered by 2 independent, masked neuroimaging experts. Stroke diagnoses were based on computed tomography or MRI showing acute hemorrhage, MRI-DWI showing acute ischemic stroke (any time after symptom onset), or delayed MRI-DWI showing no acute stroke (>48 hours but <7 days after symptom onset). For agreement between raters, Cohen κ was calculated using SPSS v17 (SPSS Inc, Chicago, IL).
Results:
We identified 14 consecutive AVS patients who underwent confirmatory neuroimaging between August 2011 and June 2012. One was excluded for failed device calibration and one for previous vestibular neuritis. Among 12 enrollees, median age was 62 years (range 30–73; interquartile range 59–69), and 10 were men. Eight were white, non-Hispanic, 2 were Hispanic, 1 was black, and 1 American Indian/Alaskan native. All patients tolerated and completed oculomotor testing without difficulty or complications. Even those with head motion intolerance had no difficulty with multiple h-HIT maneuvers (presumably because of the very small amplitude of head motion); they experienced more nausea when initially asked to sit up than during testing.
All subjects underwent MRI-DWI obtained ≈10 hours to 5 days after symptoms began. There was excellent agreement between masked neuroimaging raters (11/12; 92%; κ 0.83); after adjudication, all final results matched original nonstudy radiology reports (6 stroke and 6 nonstroke). Of the patients with a vascular pathogenesis, 5 were ischemic strokes and 1 was a small hemorrhage. All nonstroke patients had negative neuroimaging and were diagnosed clinically with vestibular neuritis.
Accuracy of central versus peripheral vestibular diagnosis by quantitative HINTS examination was 12 of 12 for both masked raters (κ 1.0). All expert h-HIT interpretations matched simple quantitative rules based directly on VOR gain measures (ie, patients with absolute gain <0.6 or right-left asymmetry >20% were all coded by experts as having an abnormal h-HIT). Exemplar h-HIT results are shown in Figures 1 and 2, raw quantitative data (absolute mean VOR gains) in Figure II in the online-only Data Supplement and aggregate results in Figure 3.
Discussion:
Our proof-of-concept findings suggest that a portable video-oculography device could someday be used in the acute setting to help nonspecialist physicians diagnose stroke in patients with acute vertigo or dizziness. Testing using the device was well tolerated by patients. Training of our research associates to perform the h-HIT maneuver and to operate the device was not difficult. A simple interpretation algorithm would have accurately classified all patients as central (stroke) or peripheral (vestibular neuritis): step 1–quantitative h-HIT first, diagnosing stroke if bilaterally normal; step 2–in those with unilateral vestibular deficit, search for direction-changing nystagmus and skew, diagnosing neuritis if both are absent.
In the future, quantitative HINTS testing might be used in the ED to discharge AVS patients unlikely to have a stroke without any imaging, speed access to acute therapy without awaiting MRI, or select patients for enrollment in treatment trials for acute posterior circulation stroke. In settings without ready access to MRI, it could be used to determine need for transport to regional centers with higher levels of stroke care. Because of the ability to capture raw eye movement videos as well as quantitative measures, such a device might also be applied in prehospital (eg, ambulance) or community-based care settings (eg, urgent care centers) as part of remote telestroke consultation.
This proof-of-concept study is limited by our small sample. Investigators and technicians were experienced in performing h-HITs and assessing oculomotor function. Despite restricting our patient population to those with MRI-DWI during the optimal diagnostic window (48 hours to 7 days after symptom onset), false-negative neuroimaging remains a theoretical possibility. Quantitative results were not interpreted in real time. Our device software did not quantify nystagmus or skew deviation, although future versions will, and similar devices already do (http://eyeseecam.com/). These results only apply directly to a specific subset of patients with vertigo or dizziness (ie, AVS). Not all clinicians are familiar with selecting appropriate patients. Use of HINTS in all comers with dizziness, including transient or purely positional symptoms, would markedly increase MRI overuse.
Conclusions:
Device-based identification of vertebrobasilar stroke in AVS could help fulfill a critical need for timely, accurate, and efficient diagnosis in patients presenting acute vertigo or dizziness with high-risk clinical features. Widespread use in EDs or urgent care clinics might involve a fully automated eye ECG read by local providers with backup telediagnosis by offsite experts. Educational efforts to teach providers to recognize AVS might also be needed; patient selection is critical because the HINTS approach relies on a normal physiological finding, rather than an abnormal one, to identify patients in need of neuroimaging. Future research should include larger comparative observational studies and randomized trials to establish the accuracy, added value, and cost-effectiveness of this novel diagnostic method.
Supplementary Material
Sources of Funding:
ICS impulse devices were loaned to investigators by GN Otometrics. This work was supported by grants from the Swiss National Science Foundation (PBBEP2 136573), Agency for Healthcare Research and Quality (R18 HS017690), and National Institutes of Health (K23 RR024009, R01EY019347).
Footnotes
Disclosures:
None.
Supplemental Material
Online Supplement for “Quantitative Video-Oculography to Help Diagnose Stroke in Acute Vertigo and Dizziness: Toward an ECG for the Eyes” by Newman-Toker, et al.
References:
- 1.Newman-Toker DE, Hsieh YH, Camargo CA, Pelletier AJ, Butchy GT, Edlow JA. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. 2008;83:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarnutzer AA, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ. 2011;183:E571–E592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman-Toker DE, Kattah JC, Alvernia JE, Wang DZ. Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology. 2008;70(24 pt 2):2378–2385. [DOI] [PubMed] [Google Scholar]
- 4.Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorns-Häderli M, Straumann D, Palla A. Accuracy of the bedside head impulse test in detecting vestibular hypofunction. J Neurol Neurosurg Psychiatr. 2007;78:1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM. Head impulse test in unilateral vestibular loss: vestibulo-ocular reflex and catch-up saccades. Neurology. 2008;70:454–463. [DOI] [PubMed] [Google Scholar]
- 8.MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73:1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


