Abstract
Sessile plants encode a large number of small peptides and cell surface-resident receptor kinases, most of which have unknown functions. Here, we report that the Arabidopsis receptor kinase MALE DISCOVERER 1-INTERACTING RECEPTOR-LIKE KINASE 2 (MIK2) recognizes the conserved signature motif of SERINE-RICH ENDOGENOUS PEPTIDEs (SCOOPs) from Brassicaceae plants as well as proteins present in fungal Fusarium spp. and bacterial Comamonadaceae, and elicits various immune responses. SCOOP signature peptides trigger immune responses and altered root development in a MIK2-dependent manner with a sub-nanomolar sensitivity. SCOOP12 directly binds to the extracellular leucine-rich repeat domain of MIK2 in vivo and in vitro, indicating that MIK2 is the receptor of SCOOP peptides. Perception of SCOOP peptides induces the association of MIK2 and the coreceptors SOMATIC EMBRYOGENESIS RECEPTOR KINASE 3 (SERK3) and SERK4 and relays the signaling through the cytosolic receptor-like kinases BOTRYTIS-INDUCED KINASE 1 (BIK1) and AVRPPHB SUSCEPTIBLE1 (PBS1)-LIKE 1 (PBL1). Our study identifies a plant receptor that bears a dual role in sensing the conserved peptide motif from phytocytokines and microbial proteins via a convergent signaling relay to ensure a robust immune response.
Subject terms: Pattern recognition receptors in plants, Plant signalling, Biotic
Peptide signals generated during plant microbe interactions can trigger immune responses in plants. Here the authors show that SCOOP12, a member of a family of peptides present in Brassicaceae plants, and SCOOP12-like motifs in Fusarium fungi, can trigger immune responses following perception by the MIK2 receptor kinase.
Introduction
Sessile plants have evolved a large number of cell surface-resident receptor proteins to sense and respond to developmental and environmental cues. Many of these receptors are receptor kinases (RKs) with an extracellular domain perceiving the cognate ligands, a transmembrane domain, and a cytoplasmic kinase domain activating downstream signaling1–4. Plant RKs are classified into different subfamilies based on their extracellular domains. Leucine-rich repeat-RKs (LRR-RKs) with a few to more than 30 extracellular LRRs represent the largest subfamily RKs in plants2,5. LRR-RKs are found to perceive plant growth hormone brassinosteroids (BRs), various endogenous and exogenous peptide ligands involved in plant growth, reproduction, cell differentiation, immunity, and beyond1,5, hydrogen peroxide6 and quinone7. Upon the ligand perception, LRR-RKs often heterodimerize with SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE (SERK) subfamily LRR-RKs, e.g., BRASSINOSTEROID INSENSITIVE 1 (BRI1)-ASSOCIATED RECEPTOR KINASE 1 (BAK1)/SERK3 and SERK4, leading to the trans-phosphorylation between the cytoplasmic kinase domains of receptors and SERK coreceptors, and the subsequent activation of the cytoplasmic signaling events8,9.
Some plant LRR-RKs are pattern recognition receptors (PRRs) that recognize pathogen- or microbe-associated molecular patterns (PAMPs or MAMPs) or damage-associated molecular patterns (DAMPs) and initiate pattern-triggered immunity (PTI)10–13. For examples, FLAGELLIN-SENSITIVE 2 (FLS2) and ELONGATION FACTOR-TU (EF-Tu) RECEPTOR (EFR) recognize bacterial flagellin and EF-Tu, respectively14,15; PEP1 RECEPTOR 1 (PEPR1)/PEPR2 and RLK7 recognize plant endogenous peptides PLANT ELICITOR PEPTIDE 1 (Pep1), and PAMP-INDUCED SECRETED PEPTIDE 1 (PIP1)/PIP2, respectively16–19. Despite the different origins, MAMPs and DAMPs activate the conserved downstream signaling events, including cytosolic calcium influx, reactive oxygen species (ROS) burst, mitogen-activated protein kinase (MAPK) activation, ethylene production, transcriptional reprogramming, callose deposition, and stomatal closure to prevent pathogen entry11,13,20–22. Accumulating evidence indicates that the immune response triggered by DAMPs is to amplify MAMP-activated defense since peptidic DAMPs are usually induced upon MAMP perception10,20,21. Some plant secreted peptides, such as SERINE-RICH ENDOGENOUS PEPTIDE 12 (SCOOP12), have been shown to induce typical MAMP/DAMP-triggered immune response, the receptors of which have not been identified23.
The LRR-RK MALE DISCOVERER 1 (MDIS1)-INTERACTING RECEPTOR-LIKE KINASE 2 (MIK2) was initially identified as a component of a receptor heteromer involving in guided pollen tube growth by perceiving the female attractant peptide LURE124. However, the physical interaction between MIK2 and LURE1 was not detected25; instead, LURE1 bound directly to another LRR-RK PRK625, and the pollen-specific PRK6 was shown to be the receptor of LURE1 family peptides biochemically and genetically25–27. Thus, the ligand of MIK2 remains enigmatic. In addition, MIK2 plays a role in response to diverse environmental stresses, including cell wall integrity sensing, salt stress tolerance, and resistance to the soil-borne fungal pathogen Fusarium oxysporum28–31. A recent study suggested that MIK2 functions as a PRR perceiving an unknown peptide elicitor from F. oxysporum to activate plant immunity32. Here, we provide genetic and biochemical evidence to show that MIK2 is the bona fide receptor of SCOOP family peptides. SCOOP12 directly binds to the extracellular domain of MIK2 and induces MIK2 dimerization with BAK1/SERK4 coreceptors. Interestingly, SCOOP-LIKE signature motifs are also present in the genomes of a wide range of Fusarium spp. and some Comamonadaceae bacteria. Fusarium SCOOP-LIKE peptides activate the MIK2-BAK1/SERK4-dependent immune responses, pointing to a dual role of MIK2 in the perception of both MAMPs and DAMPs.
Results
The kinase domain of MIK2 elicits specific responses
We have previously identified RLK7 as the receptor of the secreted peptides PIP1 and PIP219. RLK7 belongs to the subfamily XI of LRR-RKs, including PEPRs and MIK2 (Supplementary Fig 1a). Similar to RLK7 and PEPR1, MIK2 is transcriptionally upregulated upon treatments of flg22, a synthetic 22-amino-acid peptide derived from bacterial flagellin, and elf18, a synthetic 18-amino-acid peptide derived from bacterial EF-Tu (Supplementary Fig. 1a). To explore the specific function of the extracellular and the cytosolic kinase domains of LRR-RKs, we generated a chimeric receptor consisting of the extracellular domain (ECD) of RLK7 and the transmembrane and cytoplasmic kinase domains (TK) of MIK2 (RLK7ECD-MIK2TK). The chimeric RLK7ECD-MIK2TK and the full-length RLK7 (RLK7ECD-RLK7TK) under the control of the cauliflower mosaic virus (CaMV) 35S promoter were transformed into the rlk7 mutant (Fig. 1a).
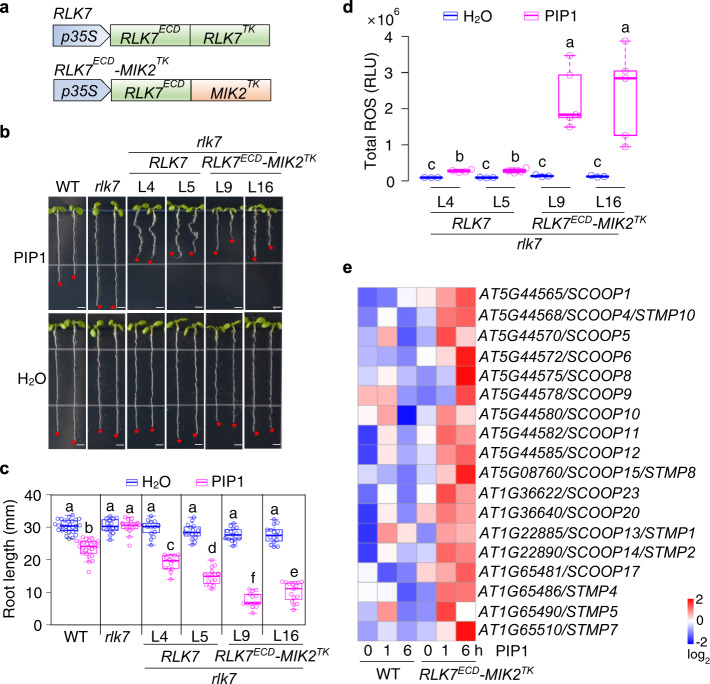
Fig. 1. Activation of the cytosolic kinase domain of MIK2 induces SCOOP expression.
a Diagram of the RLK7ECD-MIK2TK chimeric receptor gene. RLK7ECD-MIK2TK chimeric gene encoding RLK7 extracellular domain (RLK7ECD) and MIK2 transmembrane and cytoplasmic kinase domains (MIK2TK) and the full-length RLK7 (RLK7ECD-RLK7TK) driven by the CaMV35S promoter were transformed into rlk7. b, c RLK7ECD-MIK2TK/rlk7 transgenic seedlings show more severe root growth inhibition to PIP1 treatment than RLK7/rlk7 seedlings. Images of 10-day-old seedlings of Arabidopsis WT (Col-0), rlk7, two representative lines (L9 and L16) of RLK7ECD-MIK2TK/rlk7, and two lines (L4 and L5) of RLK7/rlk7 grown on ½MS plates with or without 1 µM PIP1: red stars indicate the root tips, scale bar, 4 mm (b; quantification of root length of seedlings (c)). d PIP1 treatment induces a more pronounced ROS production in RLK7ECD-MIK2TK/rlk7 than that in RLK7/rlk7 transgenic seedlings. One-week-old seedlings grown on ½MS plates were treated with H2O or 100 nM PIP1, and ROS production was calculated as the total relative luminescence units (RLU) (see “Methods” for details). e PIP1 treatment induces the expression of SCOOP genes in RLK7ECD-MIK2TK/rlk7 transgenic plants but not in WT plants. Ten-day-old seedlings grown on ½MS plates were treated with 1 µM PIP1 for 0, 1, and 6 h for RNA-Seq analysis. Heatmap represents adjusted log2-transformed transcript levels of SCOOPs and STMPs (see “Methods” for details). The box plots in (c) and (d) show the first and third quartiles as bounds of box, split by the medians (lines), with whiskers extending 1.5-fold interquartile range beyond the box, and minima and maxima as error bar. Different letters indicate a significant difference with others (P < 0.05, one-way ANOVA followed by Tukey’s test, n = 28, 26, 15, 15, 15, 13, 15, 16, 15, 14, 15, 15 for plots from left to right in (c), n = 3, 4, 3, 4, 3, 5, 3, 5 for plots from left to right in (d)). The experiments in (b–d) were repeated three times with similar results.
Compared to the rlk7 mutant, both RLK7/rlk7 and RLK7ECD-MIK2TK/rlk7 transgenic seedlings restored the sensitivity to PIP1 treatment exhibiting root growth retardation (Fig. 1b, c), indicating that the chimeric RLK7ECD-MIK2TK receptor is functional. Notably, RLK7ECD-MIK2TK/rlk7 seedlings showed more substantial root growth retardation to PIP1 treatment than RLK7/rlk7 (Fig. 1b, c). Besides, PIP1 treatment caused brown roots and darkened root-hypocotyl junctions in RLK7ECD-MIK2TK/rlk7 seedlings, but not in RLK7/rlk7 seedlings (Supplementary Fig. 1b). Furthermore, PIP1 treatment triggered a robust production of ROS in RLK7ECD-MIK2TK/rlk7 seedlings compared to that in RLK7/rlk7 seedlings, which weakly induced ROS production (Fig. 1d)19. We also generated a chimeric receptor carrying the ectodomain of PEPR1 and the transmembrane and cytoplasmic kinase domains of MIK2 (PEPR1ECD-MIK2TK) and transformed it into the pepr1/pepr2 (pepr1,2) mutant (Supplementary Fig. 1c). The PEPR1ECD-MIK2TK/pepr1,2 transgenic seedlings also showed severe root growth retardation, brown roots, and darkened root-hypocotyl junctions compared to WT seedlings upon Pep1 treatment (Supplementary Fig. 1d, e). Thus, the MIK2 kinase domain likely triggers specific responses differently from those mediated by the RLK7 and PEPR1 kinase domains. However, we cannot completely rule out the possibility that this was attributed to the increased expression of MIK2TK in RLK7ECD-MIK2TK/rlk7 and PEPR1ECD-MIK2TK/pepr1,2 transgenic plants.
MIK2TK-specific responses prompted us to investigate if MIK2, like RLK7 and PEPR1, acts as a receptor to perceive an endogenous peptide ligand(s). DAMP peptide signaling often induces the expression of themselves or precursors, thereby providing positive feedback for immune signal amplification10,13. We thus conducted an RNA-sequencing (RNA-Seq) analysis and focused on small peptide genes. Among 51 small peptide genes induced by PIP1 treatment in RLK7ECD-MIK2TK/rlk7, 18 genes encode SCOOPs23, SECRETED TRANSMEMBRANE PEPTIDEs (STMPs)33, and SCOOP/STMP-like proteins (Fig. 1e, Supplemental Fig. 2a, Supplementary Data 1). SCOOPs and STMPs have been recently identified as two groups of secreted peptides with roles in plant growth and defense23,33. SCOOPs and STMPs share certain degrees of similarities at the amino (N)-terminal signal peptide and/or the carboxyl (C)-terminal domain with overlapping family members, such as SCOOP4/STMP10, SCOOP13/STMP1, and SCOOP14/STMP2 (Supplementary Fig. 2b).
Multiple SCOOP peptides activate diverse plant immune and physiological responses
Fourteen SCOOPs (SCOOP1-14) have been identified in the Arabidopsis genome23. Some peptide-encoding genes induced in RLK7ECD-MIK2TK/rlk7 seedlings upon PIP1 treatment, such as AT1G36640, AT1G36622, STMP4, 5, and 7, bear sequence similarities with SCOOPs (Fig. 1e). We thus searched the Arabidopsis genome and identified additional nine SCOOP family members, namely SCOOP15-23 (Fig. 2a, Supplementary Fig. 2b). SCOOP15 was also named STMP833. Thus, the Arabidopsis genome encodes at least 23 SCOOP isoforms characterized by a conserved C-terminus with an SxS motif (where S is serine and x is any amino acid) (Fig. 2a, Supplementary Fig. 2b)23. Some SCOOPs, including SCOOP6, 7, 10, 11, and 15, contain two copies (an uppercase A or B was added for each copy) of the conserved domain with an SxS motif (Fig. 2a, Supplementary Fig. 2b). The expression pattern of SCOOP genes differs in various plant tissues (Fig. 2b). Of those, SCOOP10 has the highest expression in mature rosette leaves and shoots of seedlings. In contrast, SCOOP12 and 13 have the highest expression in roots and flowers, respectively (Fig. 2b), suggesting that different SCOOPs may bear tissue-specific functions.
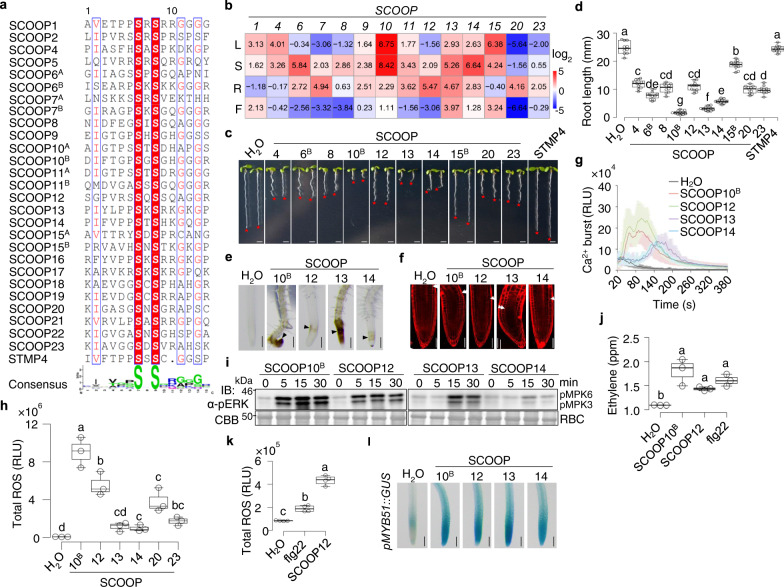
Fig. 2. SCOOP peptides activate plant immune responses.
a Alignment of SCOOP SxS motifs. Two SxS motifs in SCOOP6, 10, and 11 are respectively indicated with A and B. The conserved and similar residues are in red and yellow, respectively. b Expression of SCOOPs in different tissues. Heatmap represents adjusted log2 transformed transcript levels in 4-week-old leaves (L), 7-week-old flowers (F), and 10-day-old shoots (S) and roots (R). Data represent averages of relative expression levels of SCOOPs normalized to UBQ10 from three independent repeats. c, d SCOOPs inhibit root growth. Images of 10-day-old seedlings with 1 µM peptides: Red stars indicate the root tips, scale bar, 4 mm (c); quantification of root length (d). e SCOOPs cause root browning. Microscopic images of root tips with arrowheads indicating browning. Scale bar, 500 µm. f SCOOPs distort root meristems. Confocal images of root tips of 5-day-old seedlings with 1 µM peptides for 48 h and propidium iodide (PI) staining. Arrowheads indicate boundaries of root meristems and elongation zones. Scale bar, 100 µm. g SCOOPs trigger cytosolic Ca2+ increases. p35S::Aequorin seedlings were treated with 100 nM SCOOPs. Cytosolic Ca2+ concentration is indicated as means ± SE of RLU (n = 4, SCOOP10B and SCOOP12; n = 3, H2O, SCOOP13, and SCOOP14). h SCOOPs induce ROS production. One-week-old seedlings were treated with 100 nM peptides, and ROS production was calculated as total RLU. i SCOOPs induce MAPK activation. Ten-day-old seedlings were treated with 1 µM peptides, and MAPK activation was analyzed by α-pERK1/2 immunoblotting (IB). Coomassie brilliant blue (CBB) staining of Rubisco (RBC) for protein loading. Molecular weight (kDa) was labeled on the left of all immunoblots. j SCOOPs induce ethylene production. Ethylene concentration in leaf disks was indicated as parts per million (ppm). k SCOOP12 induces a robust ROS production in roots. Detached roots from 1-week-old seedlings were treated with 100 nM peptides. l SCOOPs induce expression of pMYB51::GUS in roots. Microscopic images of root tips of 1-week-old pMYB51::GUS seedlings treated with 1 µM SCOOPs for 3 h and subjected to GUS staining. Scale bar, 1 mm. Box plots in (d), (h), (j), and (k) show the first and third quartiles as bounds of box, split by the medians (lines), with whiskers extending 1.5-fold interquartile range beyond the box, and minima and maxima as error bar. Different letters indicate a significant difference with others (P < 0.05, one-way ANOVA followed by Tukey’s test, n = 10, 11, 11, 10, 11, 11, 11,10, 11, 10, 10, 9 for plots from left to right in (d); n = 3 for plots in (h) and (j); n = 4 for plots in (k). Experiments in (b–e), and (g–l) were repeated three times, and (f) twice with similar results.
SCOOPs possess an N-terminal signal peptide directing peptide secretion, variable regions, and a C-terminal conserved region (Supplementary Fig. 2b). Protein structure prediction indicates that SCOOP12 without the signal peptide forms an α-helix followed by a loop-like structure (Supplementary Fig. 3a). Secreted peptides are typically translated as precursor proteins and subsequently secreted into apoplasts for proteolytic processing34. To determine whether SCOOP12 is secreted and processed, we generated pSCOOP12::SCOOP12-GFP/scoop12 transgenic plants, in which SCOOP12 fused with GFP at the C-terminus under the control of its native promoter was transformed into the scoop12 mutant. SCOOP12-GFP signals were detected in the plasma membrane as well as apoplasts in leaf cells of transgenic plants (Supplementary Fig. 3b). Because the active SCOOP12 peptide is predicted to localize on SCOOP12 C-terminus and separates it from two flanking variable regions (Supplementary Fig. 3c), we speculate that SCOOP12 might be proteolytically processed at these sites34. In line with our prediction, multiple bands were detected for SCOOP12-GFP in transgenic plants by an immunoblotting analysis (Supplementary Fig. 3d). Together, our data hint that SCOOP12-GFP is likely secreted and proteolytically processed in Arabidopsis.
To determine the SCOOP12 processing sites, we followed an established protocol35,36 and incubated the recombinant HIS-SCOOP12 proteins with the protein extracts isolated from Arabidopsis seedlings. Apparently, the HIS-SCOOP12 proteins were proteolytically processed upon incubation with Arabidopsis protein extracts (Supplementary Fig. 3e). The processed products were subjected to a nano-flow liquid chromatography-tandem mass spectrometry (Nano-LC-MS/MS) analysis. A SCOOP12 fragment from leucine 44 (L44) to lysine 78 (Y78) was identified (Supplementary Fig. 3f), suggesting that SCOOP12 was cleaved after arginine 43 (R43) in a dibasic cleavage site (Supplementary Fig. 3g). Importantly, when the dibasic residues of R42R43 were substituted with alanine residues (A42A43), one of the processed bands of SCOOP12-GFP expressed in protoplasts was abolished (Supplementary Fig. 3h), further implicating the cleavage of SCOOP12 after R43. The data are consistent with the predicted cleavage site between the variable region I and the conserved domain (Supplementary Fig. 3c). We noted that the predicted cleavage site derived from the in vitro assay using plant extracts might not be fully representative of the situation in vivo.
SCOOP12 has been shown to regulate defense response and root elongation in Arabidopsis23. We tested whether other SCOOPs also have the activity to induce immune-related responses in Arabidopsis. Based on the sequence characteristics and expression patterns, we synthesized ten SCOOP peptides (SCOOP4, 6B, 8, 10B, 12, 13, 14, 15B, 20, and 23) corresponding to the SxS motif. We also synthesized the STMP4 peptide, which has an amino acid deletion in the conserved SxS motif. Treatment of all synthesized SCOOP peptides, but not STMP4, inhibited root growth to various degrees, with SCOOP10B and 13 being the most active (Fig. 2c, d). A close-up view indicated that prolonged treatments of SCOOP10B, 12, 13, and 14 peptides in WT seedlings led to brown roots, especially at the root tips of seedlings (Fig. 2e), and distorted root meristems (Fig. 2f), which were not reported for flg22 or Pep1 peptides16,37. The browning sites are different in 35S::RLK7ECD-MIK2TK transgenic plants treated with PIP1 (Supplementary Fig. 1b) and WT plants treated with SCOOPs (Fig. 2e). It is not clear the cause of root browning but could be associated with ROS production and cell wall modifications, such as lignification and suberification, upon SCOOP perception.
Similar to flg22, SCOOP peptides induced various hallmarks of PTI responses, including the cytosolic calcium increase (Fig. 2g), ROS production (Fig. 2h), MAPK activation (Fig. 2i), and ethylene production (Fig. 2j). SCOOP10B and 12 exhibited more potent activities than others in triggering PTI responses (Fig. 2g–i). SCOOP13 and 14 had relatively weak activities for Ca2+ and ROS bursts but were potent in inhibiting root growth (Fig. 2c, d, g, h). Besides, SCOOP10B, 12, 13, and 14 triggered the MAPK activation in both shoots and roots (Supplementary Fig. 3i). SCOOP12-induced ROS production in roots was significantly higher than that elicited by flg22 (Fig. 2k). Further, SCOOP10B, 12, 13, and 14 treatments induced the promoter activities of MYB51, a marker gene for root immunity38, in the roots of pMYB51::GUS transgenic plants (Fig. 2l). The data indicate that SCOOPs play a role in immune activation in both shoots and roots.
MIK2 mediates SCOOP-induced responses
Next, we tested if MIK2 is required for SCOOP-triggered responses. We isolated two alleles of T-DNA insertional mutants, mik2-1 (SALK_061769) and mik2-2 (SALK_046987). Both mik2-1 and mik2-2 were insensitive to the treatment of SCOOP10B (Fig. 3a, b). The root growth retardation became more pronounced with the increased concentrations of SCOOP10B from 1 to 100 nM, which was completely abolished in mik2-1 (Fig. 3c, d). In addition, the mik2-1 and mik2-2 mutants were insensitive to the growth inhibition triggered by the other nine SCOOPs (Fig. 3e, Supplementary Fig. 4a). However, the mik2-1 mutant responded to the Pep1 treatment similarly to WT plants (Supplementary Fig. 4b). Besides, MIK2-LIKE (MIK2L), the closest homolog of MIK2, and other members of subfamily XI LRR-RKs, including RLK719, PEPR117, PEPR218, HAESA (HAE), and HAESA-LIKE2 (HSL2)39, were not involved in responsiveness to SCOOP10B (Supplementary Fig. 4c). The fls2 mutant responded to SCOOP10B similarly to WT plants (Supplementary Fig. 4c), supporting that the activities in synthesized SCOOP peptides were unlikely due to the contamination of flg22. The data indicate that the root growth inhibition triggered by SCOOP peptides genetically depends on MIK2, but not other members of the subfamily XI LRR-RKs. Similar to SCOOPs, the apparent MIK2 orthologs with ≥60% of amino acid identity to Arabidopsis MIK2 were identified only in Brassicaceae plants (Supplementary Fig. 4d)23,33. In contrast to the tissue-specific expression of SCOOPs, MIK2 is highly expressed in shoots, roots, and leaves (Supplementary Fig. 4e), consistent with a previous report28.
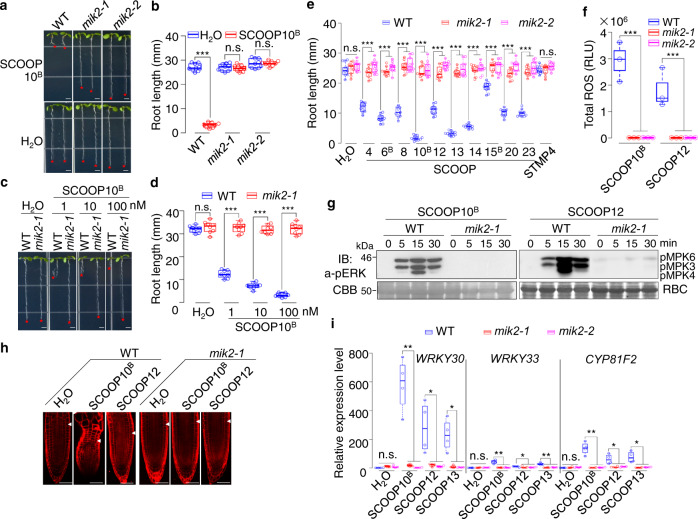
Fig. 3. SCOOP-triggered responses depend on MIK2.
a–d SCOOP10B-triggered root growth inhibition is blocked in mik2 mutants. Images of 10-day-old WT and mik2 mutant seedlings with or without 1 µM SCOOP10B (a) or different concentrations of SCOOP10B (c). Quantification of root length of seedlings is shown (b, d) (***P < 0.001, n.s., no significant differences, two-tailed Student’s t-test, n = 9 in (b), n = 8 in (d)). Scale bar, 4 mm. e The mik2 mutants block the root growth inhibition induced by different SCOOPs. Quantification of root length of 10-day-old WT and mik2 seedlings with or without 1 µM SCOOPs (***P < 0.001, n.s., no significant differences, two-tailed Student’s t-test, n = 9, plots 1–3, 5, 8–10, 12, 22–24, 27, 29, 31, 32, 36; n = 10, plots 4, 6, 7, 11, 15, 16, 18, 19, 21, 25, 30, 33; n = 8, plots 14, 17, 20, 26, 34, 35). f SCOOP12-induced ROS production is blocked in mik2 mutants. One-week-old seedlings were treated with 100 nM SCOOP10B or SCOOP12, and ROS production was calculated as total RLU (***P < 0.001, two-tailed Student’s t-test, n = 3). g SCOOP10B and SCOOP12-induced MAPK activation is blocked in mik2-1. Ten-day-old seedlings were treated with or without 1 µM SCOOP12. The MAPK activation was analyzed by immunoblotting (IB) with α-pERK1/2 antibodies. The protein loading is shown by CBB staining for RBC. h The mik2-1 mutant blocks SCOOP-induced root meristem distortion. Confocal images of root tips of 5-day-old seedlings with or without treatment of 1 µM peptides for 48 h followed by PI staining. Arrowheads indicate the boundary of the root meristem and the elongation zone. Scale bar, 50 µm. i SCOOP-induced defense gene expression is blocked in mik2 mutants. Ten-day-old seedlings were treated with or without 1 µM SCOOP10B, 12, or 13 for 1 h and subjected to RT-qPCR analysis. Expression of genes was normalized to that of UBQ10 (*P < 0.05, **P < 0.01, n.s., no significant differences, two-tailed Student’s t-test, n = 4). The box plots in (b), (d), (e), (f), and (i) show the first and third quartiles as bounds of box, split by the medians (lines), with whiskers extending 1.5-fold interquartile range beyond the box, and minima and maxima as error bar. The experiments in (a–d), (f), (h), and (i) were repeated three times, and (e) and (g) twice with similar results.
We also determined whether SCOOP-mediated other physiological and immune responses depended on MIK2. The SCOOP10B- and 12-induced ROS production (Fig. 3f) and MAPK activation (Fig. 3g) were abolished in mik2 mutants. The SCOOP10B- and 12-induced distortion of root meristems was not observed in mik2-1 (Fig. 3h). RNA-Seq analyses indicate that SCOOP12 treatment for 1 h regulates the expression of 2249 genes (1642 upregulated and 607 downregulated, fold change ≥2 or ≤0.5, p-value ≤0.01) in WT plants compared to 24 regulated genes in mik2, suggesting that SCOOP12-regulated gene transcription is almost completely abolished in mik2 (Supplementary Fig. 4f, Supplementary Data 2). Reverse transcription-quantitative polymerase chain reactions (RT-qPCR) confirmed that the upregulation of three immune-related marker genes, WRKY30, WRKY33, and CYP81F2, by SCOOP10B, 12, and 13 in WT seedlings was abolished in mik2 mutants (Fig. 3i). Taken together, these data indicate that SCOOP-triggered responses depend on MIK2.
We next compared genes upregulated by SCOOP12 with genes upregulated by the MIK2 kinase domain in RLK7ECD-MIK2TK/rlk7 transgenic plants upon PIP1 treatment (Supplementary Data 2). Interestingly, MIK2 kinase domain-upregulated genes in PIP1-treated RLK7ECD-MIK2TK/rlk7 transgenic plants largely overlapped (86.0%, 1036 out of 1205) with SCOOP12-MIK2-upregulated genes compared to its 61.9% (746 out of 1205) overlapping with PIP1-upregulated genes in WT plants (Supplementary Fig. 4g). A heatmap analysis indicates that SCOOP12-regulated genes were clustered together with MIK2 kinase domain-regulated genes in RLK7ECD-MIK2TK/rlk7 plants upon PIP1 treatment compared to PIP1-regulated genes in WT (Supplementary Fig. 4h). Similar to PIP1 treatment in RLK7ECD-MIK2TK/rlk7 transgenic plants (Fig. 1e), SCOOP12 treatment induced the expression of different SCOOP genes in WT plants (Supplementary Fig. 4i). The data further support largely overlapping responses between SCOOP12 perception and MIK2 activation.
MIK2 is the receptor of SCOOP12
Since SCOOPs trigger MIK2-dependent responses, we investigated whether SCOOP12 could bind to MIK2. We synthesized the red fluorescent tetramethylrhodamine (TAMRA)-labeled SCOOP12 peptides at its N-terminus (TAMRA-SCOOP12) and determined the ability of WT and mik2 plants for binding to TAMRA-SCOOP12 in vivo. TAMRA-SCOOP12 peptides were bioactive as they triggered MIK2-dependent root growth inhibition and MAPK activation, despite to a slightly lesser extent than SCOOP12 (Supplementary Fig. 5a–c). Red fluorescent signals were detected in root tips and leaf protoplasts in WT plants, but not in mik2-1 upon treatment of TAMRA-SCOOP12 (Fig. 4a). Importantly, pretreatment of unlabeled SCOOP12, but not flg22, markedly reduced red fluorescent signals of TAMRA-SCOOP12 in WT seedlings (Fig. 4b), indicating specific and MIK2-dependent binding of SCOOP12 in vivo.
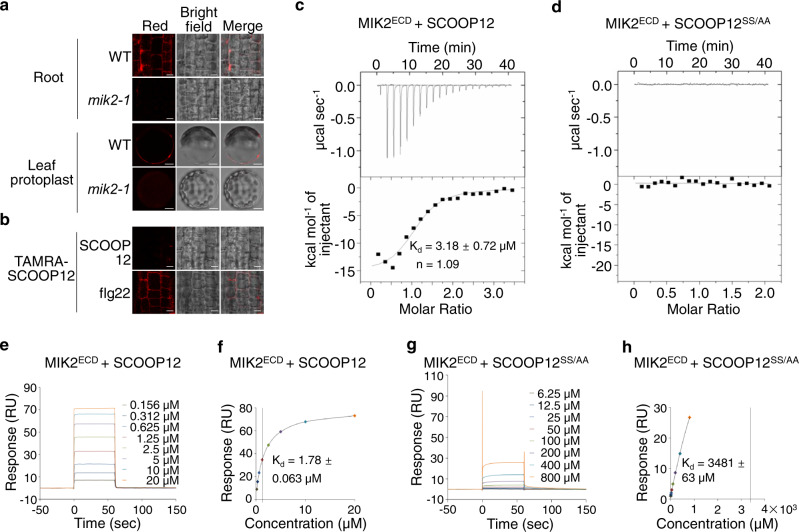
Fig. 4. SCOOP12 binds to MIK2.
a TAMRA-SCOOP12 peptides label roots and protoplasts from WT but not mik2-1. Confocal images of roots or protoplasts of WT and mik2-1 incubated with 100 nM TAMRA-SCOOP12 (see “Methods” for details). Scale bar, 4 mm (top), 10 µm (bottom). b SCOOP12 but not flg22 peptides compete for the binding of TAMRA-SCOOP12 to roots. Confocal images of roots of WT seedlings incubated with 100 nM TAMRA-SCOOP12 in the presence of 1 µM SCOOP12 or flg22 peptides. Scale bar, 4 mm. c, d SCOOP12 but not SCOOP12SS/AA binds to MIK2ECD with ITC assays. SCOOP12 (c) or SCOOP12SS/AA (d) was titrated into a solution containing MIK2ECD in ITC cells (see “Methods” for details). The top panels show raw data curves, and the bottom panels show the fitted integrated ITC data curve. The calculated binding kinetic dissociation constant (Kd values ± fitting errors) for SCOOP12 with MIK2ECD is 3.18 ± 0.72 µM, and the stoichiometry of binding (n) is approximately equal to one ligand molecule per receptor molecule (c). No binding was detected for SCOOP12SS/AA with MIK2ECD (d). e, f SCOOP12 binds to MIK2ECD with SPR assays. MIK2ECD proteins were immobilized on a sensor chip, and SCOOP12 peptides were used as flow-through analyte for SPR assays (see “Methods” for details). e The SPR sensorgram profile of SCOOP12 peptides at gradient concentrations flowing through the MIK2ECD immobilized chip. f The steady-state affinity (binding at equilibrium) indicated by a calculated Kd of 1.78 µM. g, h SCOOP12SS/AA does not bind to MIK2ECD with SPR assays. Similar assays using SCOOP12SS/AA peptides were performed as in (e) and (f). The SPR sensorgram profile (g) indicates nearly no binding between MIK2ECD and SCOOP12SS/AA with a Kd of 3481 µM (h). The above experiments were repeated twice with similar results.
To test whether SCOOP12 directly binds to MIK2 in vitro, we employed isothermal titration calorimetry (ITC) analysis using the extracellular LRR domain of MIK2 (MIK2ECD) expressed in insect cells. ITC quantitatively measures the binding equilibrium by determining the thermodynamic properties of protein-protein interaction in solution. As shown in Fig. 4c, MIK2ECD bound SCOOP12 potently with a dissociation constant (Kd) of 3.18 µM. Alanine (A) substitutions on two conserved serine (S) residues in the SxS motif of SCOOP12 (SCOOP12SS/AA) abolished its activities to inhibit root growth through MIK2 (Supplementary Fig. 5d, e)23. No binding was detected between MIK2ECD and SCOOP12SS/AA (Fig. 4d). The data indicate that MIK2 recognizes and binds to SCOOP12 directly, and the conserved SxS motif is essential for SCOOP12 binding to MIK2. In addition, we performed surface plasmon resonance (SPR) assays, in which affinity-purified MIK2ECD proteins isolated from insect cells were immobilized by an amine-coupling reaction on a sensor chip, and synthesized SCOOP12 peptides were used as the flow-through analytes. The SPR sensorgram showed the profile of SCOOP12 peptides at gradient concentrations flowing through MIK2ECD peptides immobilized on the chip (Fig. 4e). The analysis of the binding at equilibrium for SCOOP12 and MIK2ECD indicated a calculated Kd of 1.78 µM (Fig. 4f). A similar analysis of the SPR sensorgram using the MIK2ECD sensor chip flowing through with SCOOP12SS/AA peptides (Fig. 4g) indicated nearly no binding between MIK2ECD and SCOOP12SS/AA with a Kd of 3481 µM (Fig. 4h). Together, our data indicate that the extracellular domain of MIK2 directly binds to SCOOP12, and the conserved SxS motif of SCOOP12 is critical for its binding to MIK2. It is noted that the binding constants calculated from the in vitro binding assays are significantly different from the effective concentrations estimated from the physiological experiments. The difference might be due to conditions between in vivo and in vitro assays and the involvement of other cellular components in regulating SCOOP12-MIK2 binding in vivo40.
BAK1 and SERK4 are coreceptors of MIK2 in mediating SCOOP-triggered immunity
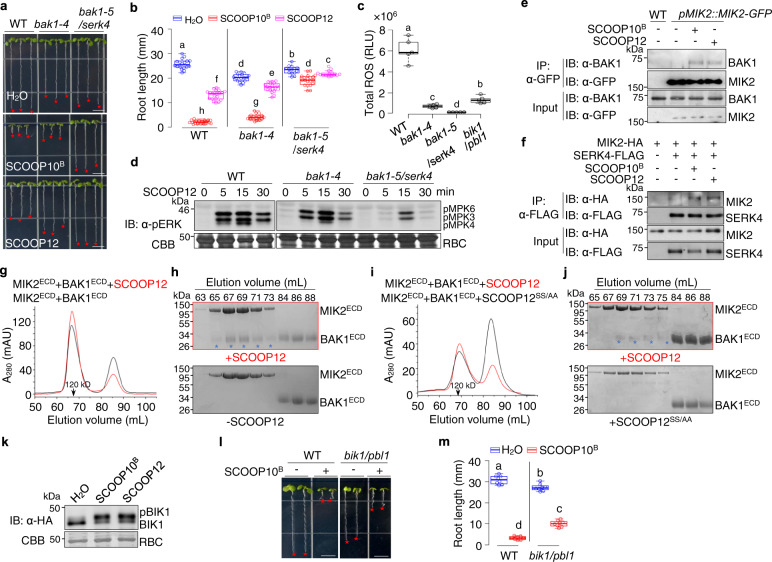
Ligand perception by LRR-RK receptors often recruits the BAK1/SERK4 family coreceptors to activate downstream signaling8,9. In line with a previous study23, SCOOP10B- and 12-induced root growth inhibition was partially compromised in bak1-4 compared to WT (Fig. 5a, b). Consistent with the redundant functions of BAK1 and SERK4, the SCOOP-mediated root growth inhibition was alleviated in the bak1-5/serk4 mutant (Fig. 5a, b). Similarly, SCOOP12-induced ROS production was reduced in bak1-4 and abolished in bak1-5/serk4 (Fig. 5c). In addition, SCOOP12- or 13-induced MAPK activation was reduced in bak1-4 and substantially blocked in bak1-5/serk4 (Fig. 5d, Supplementary Fig. 6a). To examine whether the SCOOP perception induces MIK2 and BAK1 association, we performed co-immunoprecipitation (co-IP) assays in the mik2-1 mutant transformed with GFP-tagged MIK2 under the control of its native promoter (pMIK2::MIK2-GFP/mik2) with α-BAK1 antibodies. The association between MIK2 and BAK1 was barely detectable in the absence of peptide treatment but was stimulated upon the treatment of SCOOP10B or 12 (Fig. 5e). SCOOP10B- and 12-induced MIK2-BAK1 association was also observed in protoplasts co-expressing the hemagglutinin (HA)-tagged MIK2 (MIK2-HA) and FLAG epitope-tagged BAK1 (BAK1-FLAG) (Supplementary Fig. 6b). SERK4 was also associated with MIK2 after SCOOP peptide treatments (Fig. 5f).
Fig. 5. SCOOPs induce MIK2-BAK1/SERK4 complex formation and relay signaling through BIK1 and PBL1.
a, b SCOOP-triggered root growth inhibition is compromised in bak1-4 and bak1-5/serk4. Images of 10-day-old seedlings with or without 1 µM SCOOP10B or SCOOP12. Scale bar, 4 mm (a). Quantification of root length (b). c SCOOP12-induced ROS is compromised in bak1-4, bak1-5/serk4, and bik1/pbl1. One-week-old seedlings were treated with 100 nM SCOOP12, and ROS production was calculated as total RLUs. d SCOOP12-induced MAPK activation is compromised in bak1-4 and bak1-5/serk4. The assay was done as in Fig. 3g. e SCOOPs induce MIK2-BAK1 association. Leaves of pMIK2::MIK2-GFP/mik2-1 plants were treated with or without 1 μM SCOOPs for 30 min. Total proteins were subjected for immunoprecipitation (IP) with α-GFP agarose beads, and the immunoprecipitated proteins were detected with α-BAK1 or α-GFP antibodies (top two). The input controls before IP are shown on the bottom two panels. f SCOOPs induce MIK2-SERK4 association. Protoplasts expressing MIK2-HA and SERK4-FLAG, or a control vector (Ctrl) were treated with or without 1 μM SCOOPs for 15 min. IP and IB were performed as (e). g, h SCOOP12 induces MIK2ECD-BAK1ECD interaction. Gel filtration chromatography analysis using MIK2ECD and BAK1ECD shows the elution profiles in the presence (red) or absence (black) of 0.1 mg SCOOP12 (g). SDS-PAGE and CBB staining show the eluted fractions (top panel with SCOOP12; bottom panel without SCOOP12). Stars indicate the eluted BAK1ECD complexing with MIK2ECD (h). i, j SCOOP12SS/AA does not induce MIK2ECD-BAK1ECD interaction. Experiments were performed as in (g) and (h). k SCOOPs induce BIK1 phosphorylation. Protoplasts expressing BIK1-HA were treated with 1 μM SCOOP10B or SCOOP12 for 15 min. Proteins were subjected to IB using α-HA antibodies (top), and CBB staining for RBC as loading controls (bottom). Phosphorylated BIK1 (pBIK1) was indicated as a band mobility shift. l, m SCOOP10B-triggered root growth inhibition is compromised in bik1/pbl1. Images of 10-day-old seedlings with or without 1 µM SCOOP10B. Scale bar, 4 mm (l). Quantification of root length (m). The box plots in (b), (c), and (m) show the first and third quartiles as bounds of box, split by the medians (lines), with whiskers extending 1.5-fold interquartile range beyond the box, and minima and maxima as error bar. A significant difference is shown between different letters (P < 0.05, one-way ANOVA followed by Tukey’s test, n = 15, b, 5, c, and 8, m). Experiments in (a–d), (l), and (m) were repeated three times and (e–k) twice with similar results.
To test whether the extracellular domains of MIK2 and BAK1 are sufficient to form a SCOOP12-induced complex in vitro, we performed a gel filtration assay of BAK1ECD and MIK2ECD purified from insect cells in the presence of SCOOP12 or SCOOP12SS/AA peptides. The results show that MIK2ECD and BAK1ECD proteins co-migrated in the presence of SCOOP12 (Fig. 5g, h), indicating that SCOOP12 induces the dimerization of MIK2ECD and BAK1ECD. The protein complex was eluted mainly at the position corresponding to a size of a monomeric MIK2ECD-BAK1ECD (~120 kDa) (Fig. 5g, h), suggesting that SCOOP12 may not induce the homodimerization of the MIK2ECD-BAK1ECD complex. In contrast, SCOOP12SS/AA, which cannot bind to MIK2ECD, was unable to induce the dimerization between MIK2ECD and BAK1ECD (Fig. 5i, j), indicating that the SCOOP12-MIK2 binding is required for the MIK2-BAK1 interaction.
SCOOP-MIK2-BAK1 relays the signaling through the BIK1 family RLCKs
MAMP/DAMP perception activates receptor-like cytoplasmic kinases (RLCKs), including BOTRYTIS-INDUCED KINASE 1 (BIK1) and its close homolog AVRPPHB SUSCEPTIBLE1 (PBS1)-LIKE 1 (PBL1)41,42. SCOOP10B or 12 treatments led to the phosphorylation of BIK1, as indicated by a protein band mobility shift in the immunoblot (Fig. 5k). SCOOP12-induced ROS burst and SCOOP10B-induced growth inhibition were significantly compromised in the bik1/pbl1 mutant (Fig. 5c, l, m). Yet, the bik1/pbl1 mutant did not completely block SCOOP response, likely due to the functional redundancy of RLCKs41,42 or the existence of an RLCK-independent pathway downstream of SCOOP-MIK2. Nevertheless, the MIK2-BAK1 receptor complex activation by SCOOPs triggers phosphorylation of BIK1 and PBL1 in transducing signaling to downstream events. The activation of NADPH oxidase RBOHD by BIK1 is required for PRR-induced ROS production43,44. Likewise, SCOOP12-induced ROS production was abolished in the rbohD mutant (Supplementary Fig. 6c). BAK1 and BIK1/PBL1 are also required for the MIK2-dependent response triggered by a Fusarium elicitor32. Collectively, our results indicate that the SCOOP-MIK2-BAK1 receptorsome activates the conserved signaling pathways shared by other LRR-RK containing PRR complexes.
Microbial SCOOP-LIKE peptides trigger a MIK2-dependent immune response
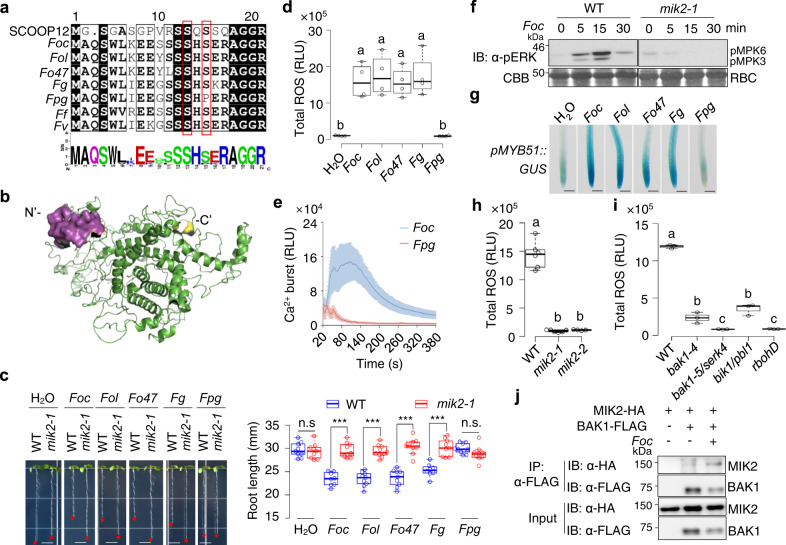
A recent report showed that the mik2 mutants were insensitive to an unidentified proteinous elicitor isolated from several Fusarium spp., suggesting that MIK2 may perceive a peptide elicitor from Fusarium32. This prompted us to examine whether SCOOP-LIKE (SCOOPL) peptides are encoded in the genomes of Fusarium spp. We blast-searched the genome of F. graminearum, a Fusarium strain with a well-annotated genome sequence45, using SCOOP10B and 12 as queries. A 21-amino-acid peptide sequence in the N-terminus of an uncharacterized protein (FGSG_07177) showed a high similarity to SCOOP12 (Fig. 6a, Supplementary Fig. 7a–c). FGSG_07177 is predicted as a transcription regulator with a GAL4-like DNA-binding domain (Supplementary Fig. 7b). Structural modeling indicates that the SCOOPL motif is located on the surface of FGSG_07177 (Fig. 6b). Blast-searching showed that the SCOOPL sequence is highly conserved among all Fusarium species surveyed, and all searched SCOOPLs localize in the N-terminus of FGSG_07177 orthologs, which appear to be a single copy gene in different Fusarium species (Fig. 6a, Supplementary Fig. 7c). Alignment of 22 SCOOPL sequences in FGSG_07177 orthologs from different Fusarium species revealed seven groups, most of which, except for F. pseudograminearum, contain an SxS motif as Arabidopsis SCOOPs (Fig. 6a, Supplementary Fig. 7c). The second conserved serine residue of F. pseudograminearum SCOOPL (FpgSCOOPL) is substituted by a proline (Fig. 6a, Supplementary Fig. 7c). RT-qPCR analyses indicated that the FGSG_07177 homologous gene (FOXB_11846) in Fo5176, a virulent strain on Arabidopsis and belonging to F. oxysporum f. sp. conglutinans46, is expressed, and its expression was upregulated at 48 h upon fungal inoculation in Arabidopsis roots (Supplementary Fig. 7d).
Fig. 6. Fusarium SCOOP-like peptides activate MIK2-dependent immune responses.
a Alignment of SCOOPL SxS motifs from different Fusarium strains with Arabidopsis SCOOP12. Foc, F. oxysporum f. sp. conglutinans Fo5176; Fol, F. oxysporum f. sp. lycopersici MN25; Fo47, F. oxysporum 47; Fg, F. graminearum PH-1; Fpg, F. pseudograminearum; Ff, F. fujikuroi; Fv, F. venenatum. Two conserved serine (S) residues are boxed in red. b The surface accessibility of FgSCOOPL. FgSCOOPL peptide is highlighted in magenta, the C-terminus in yellow, and the rest in green. c Fusarium SCOOPL peptides, except FpgSCOOPL, inhibit root growth in a MIK2-dependent manner. Images of 10-day-old WT and mik2-1 seedlings with or without 1 µM Fusarium SCOOPL peptides. Scale bar, 4 mm (left). Quantification of seedling root length (right) (***P < 0.001, n.s., no significant differences, two-tailed Student’s t-test, n = 9, 9, 8, 9, 9, 10, 9, 9, 8, 9, 10, 9 for plots from left to right). d Fusarium SCOOPLs, except FpgSCOOPL, induce ROS production. WT seedlings were treated with or without 1 µM peptides, and ROS was calculated as total RLUs. Different letters indicate a significant difference with others (P < 0.05, one-way ANOVA followed by Tukey’s test, n = 4). e FocSCOOPL triggers the cytosolic Ca2+ increase. p35S::Aequorin seedlings were treated with or without 1 µM FocSCOOPL or FpgSCOOPL. Cytosolic Ca2+ concentration was indicated as means of RLU. The data are shown as mean ± SE (n = 9 and 6 for FocSCOOPL and FpgSCOOPL). f FocSCOOPL-induced MAPK activation is blocked in mik2-1. Ten-day-old seedlings were treated with or without 1 µM FocSCOOPL. g Fusarium SCOOPLs induce pMYB51::GUS expression in roots. Images of root tips of 1-week-old pMYB51::GUS seedlings upon treatments with or without 1 µM SCOOPLs for 3 h and subjected to GUS staining. Scale bar, 1 mm. h, i FocSCOOPL-induced ROS production is blocked in mik2 (h), and in bak1-4, bak1-5/serk4, bik1/pbl1, and rbohD mutants (i). One-week-old seedlings were treated with 1 µM FocSCOOPL, and ROS production was calculated as total RLUs. Different letters indicate a significant difference with others (P < 0.05, one-way ANOVA followed by Tukey’s test, n = 6, 6, 4 for plots from left to right in (h), n = 3 in (i)). j FocSCOOPL induces MIK2-BAK1 association. The experiment was performed as in Fig. 5f. Box plots in c, d, h, and i show the first and third quartiles as bounds of box, split by the medians (lines), with whiskers extending 1.5-fold interquartile range beyond the box, and minima and maxima as error bar. Experiments in (c–j) were repeated three times with similar results.
To examine whether Fusarium SCOOPL peptides trigger immune responses as Arabidopsis SCOOPs, we synthesized five Fusarium SCOOPL peptides from F. oxysporum f. sp. conglutinans strain Fo5176 (Foc), F. oxysporum f. sp. Lycopersici (Fol), F. oxysporum strain Fo47 (Fo47), F. graminearum (Fg), and F. pseudograminearum (Fpg). Four out of five Fusarium SCOOPLs, except FpgSCOOPL, which has a polymorphism in the second conserved serine, were able to induce growth inhibition (Fig. 6c), ROS production (Fig. 6d), Ca2+ influx (Fig. 6e, Supplementary Fig. 7e, f), MAPK activation (Fig. 6f), and MYB51 promoter activation in roots (Fig. 6g). FgSCOOPL and FocSCOOPL induced the cytosolic Ca2+ increase at the concentration of 10 nM and 100 nM, respectively (Supplementary Fig. 7e, f). Fusarium SCOOPL appeared to trigger a weaker ROS production than Arabidopsis SCOOP10B and 12 (Supplementary Fig. 7g). The Fusarium SCOOPL-induced growth inhibition, MAPK activation, and ROS production were abolished in the mik2 mutants (Fig. 6c, f, h). Moreover, the FocSCOOPL-induced ROS production was compromised in the bak1-5/serk4, bik1/pbl1, and rbohD mutants (Fig. 6i). FocSCOOPL also induced MIK2-BAK1 association in Arabidopsis protoplasts (Fig. 6j). Thus, Fusarium SCOOPLs trigger similar responses with Arabidopsis SCOOPs in a MIK2-dependent manner. We knocked out the SCOOPL sequence in FOXB_11846 of Fo5176 to test if the SCOOPL plays a role in its pathogenicity (Supplementary Fig. 8a, b). Two independent Fo5176 SCOOPL knock-out lines showed elevated virulence in Arabidopsis compared to WT Fo5176 (Supplementary Fig. 8c, d). In addition, as reported previously28, the mik2-1 mutant was markedly more susceptible to F. oxysporum infections than WT plants (Supplementary Fig. 8e, f).
Interestingly, a SCOOPL motif also exists in the C-terminus of an unknown protein conserved in different genus or species of the bacterial Comamonadaceae family, including Acidovorax sp., Curvibacter sp., Comamonas sp., Ramlibacter sp., Limnohabitans sp., Verminephrobacter eiseniae, Hydrogenophaga sp., and Polaromonas sp. (Supplementary Fig. 9a). SCOOPLs from a strain of Curvibacter sp. (Cu) and Verminephrobacter eiseniae (Ve), but not from Acidovorax temperans (At) or Acidovorax avenae (Aa) induced growth inhibition and MYB51 promoter activation in roots (Supplementary Fig. 9b–d). The inactivity of AtSCOOPL and AaSCOOPL may be due to amino acid variations in the fourth residue in AtSCOOPL and in the first conserved serine residue in AaSCOOPL (Supplementary Fig. 9a). The CuSCOOPL- or VeSCOOPL-induced growth inhibition was blocked in mik2-1 (Supplementary Fig. 9b, c). Thus, SCOOPL signature motifs are highly conserved in different microbes, and they may serve as MAMPs perceived by the plant MIK2 receptor.
Discussion
The Arabidopsis genome encodes more than 1000 putative secreted peptide genes, most of which have unknown functions34. Similarly, plants have also evolved a large number of RKs, with only a few having defined ligands and functions3. In this study, we report that LRR-RK MIK2 recognizes multiple plant endogenous peptides of SCOOP family members, leading to a series of PTI responses, including cytosolic Ca2+ influx, ROS burst, MAPK activation, ethylene production, and defense-related gene expression. SCOOP12 directly binds to the extracellular LRR domain of MIK2 in vivo and in vitro, indicating that MIK2 is a bona fide receptor of SCOOPs. The SxS signature motif is essential for SCOOP functions and MIK2 binding. Perception of SCOOPs by MIK2 induces the heterodimerization of MIK2 with BAK1 and SERK4, and BAK1/SERK4 are required for SCOOP-triggered responses, indicating that BAK1/SERK4 are coreceptors of MIK2 in perceiving SCOOPs. SCOOPs have been identified as secreted peptides specific to the Brassicaceae family23. Interestingly, the SCOOP active motif was also found in a conserved yet uncharacterized protein ubiquitously present in fungal Fusarium spp. and bacterial Comamonadaceae. Both Fusarium and Comamonadaceae SCOOP-LIKE peptides with the SxS signature activate the MIK2-dependent immune responses. Thus, our data reveal a dual role of MIK2 in perceiving the conserved SCOOP signature motif from plants and microbes in triggering plant immunity (Supplementary Fig. 10a). Our study is consistent with an accompanying report in which MIK2 was identified as a receptor of plant SCOOPs and mediated response to Fusarium SCOOP-like peptides47.
Plant plasma membrane-resident RKs perceive diverse exogenous and endogenous signals via the extracellular domain and activate intracellular responses through the cytoplasmic kinase domain1–3. The chimeric receptors with the swapped extracellular and intracellular domains between different RKs have been used to study the specificity of signal perception and signaling activation48–52. The extracellular domains of RKs determine the ligand-binding specificity50,51,53. The intracellular kinase domains of different RKs also trigger specific responses48. For example, the chimeric receptor of the EFR extracellular domain and WALL-ASSOCIATED KINASE 1 (WAK1) cytoplasmic kinase domain triggers defense responses that are activated by oligogalacturonides (OGs), the proposed ligand of WAK146. We show that the chimeric receptor of RLK7ECD-MIK2TK or PEPR1ECD-MIK2TK activates some immune responses that are not observed upon RLK7 or PEPR1 activation by the corresponding ligands. More importantly, the immune responses triggered by RLK7ECD-MIK2TK or PEPR1ECD-MIK2TK largely overlapped with those activated by SCOOPs, the ligands of MIK2. For example, RLK7 activation by ligand PIP1 moderately inhibits root growth and weakly induces ROS production19. In contrast, the RLK7ECD-MIK2TK activation by PIP1 causes severe growth inhibition and robust ROS production, similar to SCOOP treatments. PIP1-activated responses are BIK1-independent19, whereas SCOOPs induce BIK1 phosphorylation and BIK1-dependent responses. Thus, upon ligand perception by the extracellular domain, the cytosolic kinase domain of RKs activates some convergent and unique signaling events, likely through differential phosphorylation events and interaction with various partners.
Fourteen SCOOPs have been previously identified in Arabidopsis, and SCOOP12 can activate defense response and regulate root elongation23. Our study extends the SCOOP family to 23 members, and all tested ones with the conserved SxS motif activate plant immune response. It remains unknown why plants have evolved so many SCOOPs and the functional specificity for different SCOOPs. Notably, SCOOP genes show different expression patterns, with some highly expressed in leaves and some in roots. SCOOP12, the most studied SCOOP, is highly expressed in roots (Fig. 2b), and was indicated to regulate root development and resistance to necrogenic bacterium Erwinia amylovora and necrotrophic fungus Alternaria brassicola23. However, MIK2 is ubiquitously expressed in different plant tissues (Supplementary Fig. 4e, and http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi)28, and regulates many plant processes, including cell wall damage response, salt tolerance, and pollen tube development24,28. It is likely that specific SCOOPs might be recognized by MIK2 in different plant tissues to regulate distinct physiological responses. SCOOP12 triggers a robust immune response in roots, and physiological changes, such as darkened hypocotyl-root junctions and distorted meristems in roots. In addition, the mik2 mutant showed increased susceptibility to the root-invading pathogen F. oxysporum (Supplementary Fig. 8e, f)28. However, the enhanced susceptibility to F. oxysporum has not been observed in the scoop12 mutant, likely due to the functional redundancy of multiple SCOOPs in Arabidopsis. Alternatively, it could be attributed to SCOOPL genes in F. oxysporum.
SCOOPL sequences are also present in a wide range of fungal Fusarium spp. and bacterial Comamonadaceae. In Fusarium spp., the SCOOPL motif we identified is located in the N-terminus of a highly conserved GAL4 DNA-binding domain-containing protein with uncharacterized functions. It is possible that SCOOPL motifs are also present in some other proteins in Fusarium spp47. Some of these SCOOPLs are active in triggering MIK2- and BAK1/SERK4-dependent immune responses (Fig. 6c, h, i). Knock-out of the SCOOPL in Fo5176 enhanced the fungal pathogenicity in Arabidopsis (Supplementary Fig. 8c, d). Therefore, Fusarium SCOOPLs may be recognized as MAMPs by plants. It will also be interesting to determine whether Fusarium SCOOPL is the proteinous elicitor isolated from several Fusarium spp., which triggers a MIK2-dependent immunity32. It remains unknown whether this protein is secreted and processed during Fusarium infection and how MIK2 perceives it. Notably, elf18, an 18-amino acid synthetic peptide, is derived from the conserved bacterial translation elongation factor EF-Tu, which can be detected on the extracellular surface and secretome of bacteria54–56. Some SCOOPL peptides from Fusarium spp. and Comamonadaceae have amino acid variations in the conserved SxS motif and cannot activate the immune response. This is consistent with the observation that the SxS motif is required for plant SCOOP peptide functions and binding to MIK2. The variations may be due to the pathogens’ evolutionary pressure to escape from hosts’ perception of immune elicitation.
It has been reported that some pathogens and nematodes deploy mimics of plant endogenous peptides, such as CLAVATA3/ESR (CLE), PLANT PEPTIDE CONTAINING SULFATED TYROSINE (PSY), and RAPID ALKALINIZATION FACTOR (RALF), to promote the pathogenicity by hijacking plant peptide-receptor signaling57–60. For example, RALF secreted from F. oxysporum induces plant receptor FERONIA-dependent extracellular alkalization to favor fungal multiplication57. In contrast, similar to plant SCOOPs, microbial SCOOPLs activate the MIK2-dependent immune responses, suggesting that SCOOPLs act as MAMPs rather than virulence factors. Compared to the wide distribution of SCOOPLs in fungal Fusarium spp. and bacterial Comamonadaceae, plant SCOOPs are only present in Brassicaceae plants (Supplementary Fig. 4d)23,33. This indicates that plant SCOOPs may have evolved later than microbial SCOOPLs. In addition, gene duplications of SCOOPs are common in Brassicaceae species23, an indicative for highly evolved genes. For example, twelve SCOOPs exist as tandem repeats on Arabidopsis chromosome 523. Thus, plant SCOOPs may have convergently evolved to mimic microbial SCOOPLs and amplify SCOOPL-triggered immunity. Phylogenetic analysis indicates that peptide motifs of Arabidopsis SCOOPs, Fusarium and Comamonadaceae SCOOPLs might have evolved independently (Supplementary Fig. 10b). Moreover, plant SCOOPs are derived from small peptide precursor proteins, whereas Fusarium and Comamonadaceae SCOOPLs reside in proteins belonging to distinct families. The divergence of protein families harboring SCOOP/SCOOPL suggests that SCOOPs and SCOOPLs might have evolved convergently but unlikely by horizontal gene transfers61.
Methods
Plant materials and growth conditions
The Arabidopsis thaliana accession Columbia-0 (Col-0) was used as wild type (WT). T-DNA insertion mutants of mik2-1 (SALK_061769), mik2-2 (SALK_046987), mik2-like (SALK_112341), and rlk7-3 (SALK_120595) were obtained from the Nottingham Arabidopsis Stock Centre (NASC). The fls2 (SALK_141277), bak1-4, bik1-1/pbl1-1, bak1-5/serk4-1, rbohd mutants, and the transgenic line expressing p35S::GFP have been reported previously19,62,63. The hae/hsl2 mutant was kindly provided by Dr. Reidunn B. Aalen (University of Oslo, Norway), the pepr1-2/pepr2-2 (pepr1,2) mutant by Dr. Zhi Qi (Inner Mongolia University, China), the scoop12 mutant by Dr. Sébastien Aubourg (INRA, France), a transgenic line expressing p35S::Aequorin by Dr. Marc Knight (Durham University, UK), transgenic line expressing pMYB51::GUS by Dr. Frederick M. Ausubel (Harvard Medical School, USA). Plants were grown in soil (Metro Mix 366, Sunshine LP5 or Sunshine LC1, Jolly Gardener C/20 or C/GP) in a growth room at 20–23 °C, 50% humidity, and 75–100 μE m−2 s−1 light with a 12-h light/12-h dark photoperiod for 4–5 weeks before protoplast isolation, or ethylene measurement. Seedlings used for analyses of root growth inhibition, MAPK activation, ROS production, cytosolic Ca2+ concentration increase, gene transcription, and GUS staining were grown on half-strength Murashige and Skoog (1/2MS) plates containing 0.5% (w/v) sucrose, 0.75% (w/v) agar, and 2.5 mM MES, pH 5.8, under the same conditions as plants grown in soil.
Plasmid construction and generation of transgenic plants
The BAK1 (AT4G33430), SERK4 (AT2G13790), BIK1 (AT2G39660), and MIK2 (AT4G08850) coding sequences (CDSs) were amplified by PCR from Col-0 cDNA using the primers containing BamHI at the 5′ end and StuI at the 3′ end. The PCR products were digested by BamHI and StuI, followed by ligation into the pHBT-FLAG or pHBT-HA vector to generate pHBT-35S::BAK1-FLAG, pHBT-35S::SERK4-FLAG, pHBT-35S::BIK1-HA, pHBT-35S::MIK2-HA, and pHBT-35S::MIK2-GFP constructs24,41,62. MIK2ECD (residues 1–707) and BAK1ECD (residues 1–220) were amplified by PCR from Col-0 cDNA and cloned into pFastBac-1 vector with a C-terminal 6× HIS tag25,62. The fusion protein vectors carrying MIK2ECD or BAK1ECD were used for insect cell expression. To obtain the binary vector pCAMBIA1300-35S::RLK7, a 2907-bp RLK7 CDS was PCR-amplified from WT cDNA using gene-specific primers with KpnI and SalI at the 5′ and 3′ ends, respectively, followed by KpnI and SalI digestion and ligation into the pCAMBIA1300 vector. To generate the binary vector pCAMBIA1300 carrying the RLK7ECD-MIK2TK chimeric receptor gene, an 1824-bp fragment encoding RLK7ECD was PCR-amplified from WT cDNA using a gene-specific forward primer with KpnI at the 5′ end and a gene-specific reverse primer with 8-bp overlapping sequence from the 5′ end of MIK2TK, and a 1011-bp fragment encoding MIK2TK using a gene-specific forward primer with 8-bp overlapping sequence from the 3′ end of RLK7 ECD and a gene-specific reverse primer with SalI at the 5′ end. RLK7ECD was fused with MIK2TK and inserted into the pUC19 vector using in-fusion recombinant enzymes (Clontech). After digestion with KpnI and SalI, the RLK7ECD-MIK2TK chimeric receptor gene was inserted into a pCAMBIA1300 vector with the CaMV 35 S promoter and the HA tag at the 3′ end to generate pCAMBIA1300-p35S::RLK7ECD-MIK2TK. A similar strategy was used to generate pCAMBIA1300-35S::PEPR1ECD-MIK2TK. A 2307-bp fragment encoding PEPR1ECD was amplified for fusion with MIK2TK. To obtain pHBT-35S::MIK2-HA constructs, the MIK2 CDS was PCR-amplified from WT cDNA using gene-specific primers with BamHI and StuI at 5′ and 3′ ends, respectively, followed by BamHI and StuI digestion and ligation into the pHBT vector with HA sequence at the 3′ end. To generate the binary vector pCAMBIA1300-pMIK2::MIK2-GFP, a 2000-bp promoter sequence upstream of the start codon of MIK2 was PCR-amplified and subcloned into pHBT-35S::MIK2-GFP between XhoI and BamHI sites. The pMIK2::MIK2-GFP-NOS fragment was further PCR amplified and inserted into pCAMBIA1300 between EcoRI and SalI using in-fusion recombinant enzymes. To generate the binary vector pCAMBIA-pSCOOP12::SCOOP12-GFP, a 2481-bp fragment containing the SCOOP12 promoter and coding sequence was PCR-amplified using Col-0 genomic DNA as a template. The fragment was further inserted into NcoI-digested pCAMBIA1300-GFP using in-fusion recombinant enzymes. The primers used for cloning and sequencing were described in Supplementary Data 3, and the Sanger-sequencing verified all insertions in different vectors.
The binary vectors were introduced into Agrobacterium tumefaciens strain GV3101 for the floral dipping method-based Arabidopsis transformation. Transgenic plants were selected with 20 µg/mL hygromycin B. Multiple transgenic lines in the T1 generation were analyzed by immunoblotting for protein expression. Two lines with a 3:1 segregation ratio for hygromycin resistance in the T2 generation were selected to obtain homozygous seeds.
To generate a vector for recombinant protein expression of SCOOP12 in Escherichia coli, a 147-bp fragment encoding SCOOP12 lacking the N-terminal signal peptide was PCR-amplified from pCAMBIA-pSCOOP12::SCOOP12-GFP using gene-specific primers with BamHI and HindIII at 5′ and 3′ ends, respectively, followed by BamHI and HindIII digestion and ligation into pET28a-Avi-HIS6-SUMO64 to create pET28a-Avi-HIS6-SUMO-SCOOP12.
To build pHBT-SCOOP12-GFP vector, 237-bp SCOOP12 CDS was PCR-amplified from pCAMBIA-pSCOOP12::SCOOP12-GFP using gene-specific primers with BamHI and StuI at the 5′ and 3′ ends, followed by BamHI and StuI digestion and ligation into the pHBT vector before GFP sequence. The pHBT-SCOOP12R42R43/AA-GFP was generated by Platinum Pfx DNA polymerase-mediated site-directed mutagenesis with pHBT-SCOOP12-GFP as a template.
Generation of FocSCOOPL deletion mutants
The FocSCOOPL deletion mutants were generated with a homologous recombination method. A gene replacement cassette containing a geneticin resistance gene (NEO) and a 1098-bp fragment upstream and a 920-bp fragment downstream of SCOOPL in F. oxysporum 5176 (Fo5176) was generated with PCR amplification. Fo5176 protoplasts were isolated via treating fresh mycelia with 2 mg/mL driselase (D9515, Sigma, MO, USA), 20 mg/mL lysozyme (L-040-25, GOLDBIO, USA), and 15 mg/mL cellulose (200605-02, Yakult, Japan). The gene replacement cassette was introduced into protoplasts using a polyethylene glycol (PEG)-mediated protoplast transformation method65. Briefly, the gene replacement cassette was gently mixed with protoplasts in STC solution (1.2 M sorbitol, 10 mM Tris-HCl, pH 7.5, 10 mM CaCl2) and 20 min later, SPTC solution (1.2 M sorbitol, 10 mM Tris-HCl, pH 7.5, 10 mM CaCl2, 40% PEG4000) was added to the mixture with STC/SPTC (3:1). The mixture was incubated at room temperature for 30 min and then transferred into 20 mL regeneration medium (1.0 M sucrose, 0.1% yeast extract, and 0.1% tryptone) overnight at 25 °C. The regenerated protoplasts were screened with 100 µg/mL geneticin and verified by PCR assays. Primers used for PCR amplification and identification of deletion mutants were listed in Supplementary Data 3.
Peptide synthesis
PIP1, Pep1, flg22, and all non-labeled SCOOPs and SCOOP-LIKE peptides were synthesized at ChinaPeptides (Shanghai, China). TAMRA-labeled SCOOP12 peptides were synthesized at Biomatik (Delaware, USA). The sequences of synthesized peptides were listed in Supplementary Data 4.
Root growth assay
Cold stratified seeds were surface-sterilized with 70% (v/v) ethanol for 5 min and were sown on ½MS plates with or without peptides at indicated concentrations. Ten-day-old seedlings grown on plates vertically in a growth chamber were photographed, and the root lengths of seedlings were measured using Image J (http://rsb.info.nih.gov/ij/).
RNA sequencing (RNA-Seq)
Ten-day-old WT Col-0, mik2-1, and RLKECD-MIKTK (L16) seedlings grown on ½MS plates were incubated in 1 mL of liquid ½MS medium overnight. Seedlings were then treated with or without 1 μM PIP1 or SCOOP12 for 1 or 6 h and harvested for RNA isolation. Total RNAs (5 μg) from two biological replicates were pooled for cDNA library construction. cDNA library preparation and sequencing were carried out on an Illumina HiSeq 4000 platform with 150-nucleotide pair-end reads in LC-BIO (Hangzhou, China). Total reads were mapped to the Arabidopsis genome (TAIR10; www.arabidopsis.org) with Hisat, and the read counts for every gene were generated with StringTie. The expression levels of genes were calculated by quantifying the cDNA fragments per kilobase of transcript per million fragments mapped (FPKM). Differentially expressed genes (DEGs) between different treatments were defined by fold change of read counts at 1 and 6 h compared to that at 0 h (differential expression analysis using edgeR, fold change ≥ 2 with p-value ≤ 0.01). DEGs were listed in Supplementary Data 2.
RNA isolation and reverse transcription-quantitative polymerase chain reactions (RT-qPCR) analysis
For detection of gene expression in Arabidopsis, the total RNA was extracted from 10-day-old seedlings grown on ½MS plates, or from rosette leaves or inflorescences of 4- or 7-week-old soil-grown plants using TRIzol reagent (Invitrogen). For detecting gene expression in F. oxysporum during infections, 3-week-old Arabidopsis seedlings were gently taken out of soil, and roots were inoculated with 1 × 107/mL F. oxysporum spores after washing with sterile water to clear off soil chunks. After incubating on sterile wet papers for 0–48 h, F. oxysporum-infected Arabidopsis roots were collected for total RNA extraction using TRIzol reagent (Invitrogen). One microgram of total RNA was reverse-transcribed to synthesize the first-strand cDNA with M-MuLV Reverse Transcriptases (Thermo Fisher Scientific) and oligo(dT) primers following by RNase-free DNase I (Thermo Fisher Scientific) treatment. RT-qPCR analyses were performed on a QuantStudio™ 3 Real-Time PCR Detection System (Thermo Fisher Scientific) using Faster Universal SYBR® Green Master (Roche) and gene-specific primers. The expression of each gene in Arabidopsis and F. oxysporum was normalized to the expression of Arabidopsis UBQ10 and F. oxysporum GAPDH, respectively. The primers used for RT-qPCR were listed in Supplementary Data 3.
ROS assay
ROS burst was determined by a luminol-based assay. Five one-week-old seedlings grown on ½MS plates were incubated in 200 μL ddH2O overnight in a 1.5 mL centrifuge tube. Then, ddH2O was replaced by 200 µL of reaction solution containing 50 µM of luminol, and 10 µg/mL of horseradish peroxidase (Sigma-Aldrich) supplemented with or without 100 nM or 1 μM peptide. Luminescence was measured immediately after adding the solution with a luminometer (Glomax 20/20n, Promega) with a one-second interval for 15 min. The total values of ROS production were indicated as means of the relative light units (RLUs).
Measurement of cytosolic Ca2+ concentration
One-week-old seedlings expressing p35S::Aequorin grown vertically on ½MS plates were put into a 1.5 mL centrifuge tube containing 200 μL solution with 1 mM KCl and 1 mM CaCl2. Aequorin was reconstituted by treating the seedlings with coelenterazine-h (Promega, Beijing, China) in the dark overnight at a final concentration of 10 µM. Luminescence was measured with a luminometer (Glomax 20/20n, Promega) with a one-second interval for 10 min. The values for cytosolic Ca2+ concentrations were indicated as means of RLUs.
Measurement of ethylene production
Twelve leaves of 4-week-old plants grown on soil were excised into leaf disks of 0.25 cm2, followed by overnight incubation in a 25 mL glass vial with 2 mL of ddH2O for recovery. Then, ddH2O was replaced by 1 mL of peptides at 1 µM, and the vials were capped immediately with a rubber stopper and incubated at 23 °C with gentle agitation for 4 h. One mL of the vial headspace was injected into a TRACETM 1310 Gas Chromatograph (Thermo Fisher Scientific) with FID supported by Chromeleon 7 for quantitation.
Histochemical detection of GUS activity
Two-week-old seedlings grown on ½MS plates were immersed and vacuumed in the GUS staining solution (10 mM EDTA, 0.01% [v/v] Silwet L-77, 2 mM potassium ferricyanide, 2 mM potassium ferrocyanide, and 2 mM 5-Bromo-4-chloro-3-indolyl-β-D-glucuronic acid in 50 mM sodium phosphate buffer, pH 7.0) for 5 min, followed by incubation at 37 °C for 12–24 h. Stained samples were fixed with a 3:1 ethanol:acetic acid solution overnight and cleared by lactic acid and photographed with the Olympus SZX10 stereoscopic microscope equipped with DP27 camera.
Microscopy assays
For the observation of propidium iodide (PI) stained roots, 5-day-old seedlings grown on ½MS plates were mounted in ddH2O containing 10 μM PI for 20 min before imaged with the Leica SP8 confocal microscope. Images were captured at 543 nm laser excitation and 578–700 nm emission. For the observation of TAMRA-SCOOP12 labeled roots and protoplasts, 5-day-old seedlings grown on ½MS plates or protoplasts isolated from leaves of 4-week-old soil-grown plants were treated with 100 nM TAMRA-SCOOP12 with or without 1 μM SCOOP12 or flg22 for 5 min in liquid ½MS or W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, 2 mM MES, pH 5.7), followed by washing with ½MS or W5 solution for three times before imaged with the Leica SP8 confocal microscope. Images were captured at 552 nm laser excitation and 570–620 nm emission. To observe SCOOP12-GFP in Arabidopsis pSCOOP12::SCOOP12-GFP/scoop12 transgenic plants, detached leaves were imaged under the Leica SP8 confocal microscope directly or 3 min after 5% sodium chloride (NaCl) treatment. Images were captured at 472 nm laser excitation and 493–547 nm emission. For all the observations, the pinhole was set at one Airy unit, and the imaging processing was carried out by using the Leica Application Suite X (LAS X) software.
MAPK assay
Ten-day-old seedlings grown vertically on ½MS plates were transferred to ddH2O overnight for recovery. Then, ddH2O was replaced by 100 nM peptides for the indicated time. Each sample containing three seedlings was grounded in 40 μL of extraction buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 1% [v/v] Triton X-100, 1 mM Na3VO4, 1 mM NaF, 1 mM DTT, 1:200 complete protease inhibitor cocktail from Sigma). The supernatant was collected after 13,000 × g centrifugation for 5 min at 4 °C and protein samples with 1× SDS buffer were loaded on 10% (v/v) SDS-PAGE gel before transfer to a PVDF membrane, which was then blotted using α-pERK1/2 antibodies (1:1500; Cell Signaling, Cat # 9101) for the detection of phosphorylated MPK3, MPK4, and MPK6.
BIK1 phosphorylation assay
WT protoplasts were transfected with pHBT-35S::BIK1-HA for 12 h followed by treatment with 1 μM SCOOP peptides for 15 min. Total proteins were isolated with extraction buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 1% [v/v] Triton X-100, 1 mM Na3VO4, 1 mM NaF, 1 mM DTT, 1:200 complete protease inhibitor cocktail from Sigma). The supernatant collected after 13,000 × g centrifugation for 5 min at 4 °C was loaded on 10% SDS-PAGE gel before transfer to a PVDF membrane, which was then blotted with α-HA antibodies (1:3000; Roche, Cat # 12013819001).
Co-immunoprecipitation (co-IP) assay and detection of SCOOP12-GFP proteins
For co-IP assays in protoplasts, protoplasts were co-transfected with pHBT-35S::MIK2-HA and pHBT-35S::BAK1-FALG or pHBT-35S::SERK4-FLAG at 50 μg DNA for 500 μL protoplasts at the density of 2 × 105/mL for each sample and were incubated for 12 h. After treatment with 1 µM SCOOP peptides for 15 min, samples were collected by centrifugation at 200 × g for 2 min and lysed in 300 μL IP buffer (20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1 mM EDTA, 2 mM DTT, 10% [v/v] Glycerol, 0.5% [v/v] Triton X-100 and protease inhibitor cocktail from Sigma) by vortexing. After centrifugation at 10,000 × g for 10 min at 4 °C, 30 μL of supernatant was collected for input controls. The remaining supernatant was pre-incubated with protein-G-agarose beads at 4 °C for 1 h with gentle shaking at 100 × g on a rocker. IP was carried out with α-FLAG agarose for 3 h at 4 °C. Beads were collected by 500 × g centrifugation for 2 min and washed three times with washing buffer (20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1 mM EDTA, 0.1% [v/v] Triton X-100) and once with 50 mM Tris-HCl, pH 7.5. Immunoprecipitated proteins and input proteins were analyzed by immunoblotting with α-HA (1:3000; Roche, Cat # 12013819001) or α-FLAG antibodies (1:3000; Sigma-Aldrich, Cat # A8592).
For co-IP assays in transgenic seedlings, leaves of 4-week-old pMIK2::MIK2-GFP transgenic plants were hand-inoculated with 1 μM SCOOP10B or SCOOP12 and were treated for 30 min, followed by total protein isolation with extraction buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% [v/v] NP-40 and 1:200 protease inhibitor cocktail from Sigma). Co-IP was carried out the same as for co-IP with protoplasts except that GFP-Trap agarose beads (Chromotek) were used instead. Immunoprecipitated proteins and input proteins were analyzed by immunoblotting with α-GFP (1:3500; Roche, Cat # 11814460001) or α-BAK1 antibodies (1:2000)64.
To detect SCOOP12-GFP proteins, five of one-week-old pSCOOP12::SCOOP12-GFP/scoop12, p35S::GFP, and WT seedlings grown on ½MS plates were harvested and grounded in 40 μL of extraction buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 1% [v/v] Triton X-100, 1 mM Na3VO4, 1 mM NaF, 1 mM DTT, 1:200 complete protease inhibitor cocktail from Sigma). The supernatant was collected after 13,000 × g centrifugation for 5 min at 4 °C, and protein samples were separated on 15% (v/v) SDS-PAGE and subjected to immunoblotting (IB) with α-GFP antibodies (1:3500; Roche, Cat #11814460001).
In vitro protein cleavage assay
Recombinant HIS-SCOOP12 proteins were expressed in E. coli BL21 strain with an isopropyl β-D-1-thiogalactopyranoside (IPTG)-inducible system and purified with Ni-NTA agarose (QIAGEN, #166048536) in buffer containing 50 mM Tris-HCl pH 7.5 and 300 mM NaCl. For in vitro protein cleavage assay, 5 μg of purified HIS-SCOOP12 proteins were incubated with 10 μL protein extracts isolated from 10-day-old Arabidopsis WT seedlings or protein extraction buffer (50 mM HEPES, pH 7.4, 10 mM EDTA, and 1× protease inhibitor cocktail, Sigma) following a published protocol36 for 5 h at room temperature with rotation. The reaction was quenched by adding SDS loading buffer followed by separation on 15% (v/v) SDS-PAGE and subjected to immunoblotting (IB) with α-HIS antibodies (1: 2000; Roche, Cat #11965085001) or Coomassie Brilliant Blue (CBB) staining. The processed SCOOP12 bands were cut from SDS-PAGE for LC-MS/MS analysis.
LC-MS/MS analysis
The processed SCOOP12 bands were in-gel digested following a published protocol66, except that digestion was conducted with Lys-C (Promega, Madison, WI, USA). The digested peptides were desalted using micro ZipTip mini-reverse phase (Millipore). In brief, after wetting (with 50% acetonitrile) and equilibrating a ZipTip (with 0.1% formic acid), the peptides were bound to C-18 material. A subsequent washing step with 0.1% formic acid was followed by a final elution with 60% acetonitrile and 0.1% formic acid. The samples were lyophilized to dryness using a labconco speedvac (Centrivap, Labconco Inc., USA). The resulting peptides were resuspended in 0.1% formic acid for mass spectrometric analysis. The mass spectrometry data acquisition was performed on an EASY-nLC 1200 ultraperformance liquid chromatography system (Thermo Scientific Inc., San Jose, CA) connected to an Orbitrap Q-Exactive HF instrument equipped with a nano-electrospray source (Thermo Scientific Inc., San Jose, CA)67. The peptide samples were loaded to a C18 trapping column (75 μm i.d. × 2 cm, Acclaim PepMap® 100 particles with 3 μm size and 100 Å pores) and then eluted using a C18 analytical column (75 μm i.d. × 25 cm, 2 μm particles with 100 Å pore size). The flow rate was set at 250 nL/min with solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid and 99.9% acetonitrile) as the mobile phases. MS/MS analysis was carried out in positive mode applying data-dependent acquisition. Survey full-scan MS spectra scan range was 375–1575 m/z, with a resolution of 60,000 at 200 m/z. The precursor isolation window was set at 4 m/z, the automatic gain control (AGC) was 3e6, and the maximum injection time (IT) was 80 ms. The MS/MS resolution was 15,000, and the mass range was 200–2000 m/z. The minimum AGC target was 8.0e3, and the IT was 55 ms. The normalized collision energy was set to be 28 with apex trigger of top 7 precursor ions. The tandem mass spectra were extracted from the Xcalibur .raw files and converted into mgf files using Proteome Discoverer 2.4 (Thermo Fisher Scientific Inc., San Jose, CA). All MS/MS samples were analyzed using Mascot Daemon (Matrix Science, London, UK; version 2.4.0). Mascot was set up to search the Arabidopsis TAIR10 database assuming the digestion enzyme semi-Lys C. Decoy database searching was enabled. Carbamidomethyl of cysteine was specified in Mascot as a fixed modification. The raw MS/MS spectra were extracted and exported from the raw data file using Xcalibur Qualbrower and annotated manually with the assistance of Mascot and MS Product from ProteinProspector.
Protein expression and purification
The ECD domains of MIK2 (MIK2ECD) and BAK1 (BAK1ECD) fused with a 6 × HIS tag at the C-terminus were expressed using the Bac-to-Bac baculovirus expression system (Invitrogen) in High Five cells at 22 °C25. One liter of cells (1.8 × 106 cells per mL cultured in the medium from Expression Systems) was infected with 20 mL baculovirus and the media were harvested after 48 h of infection. The secreted MIK2ECD-6 × HIS and BAK1ECD-6 × HIS proteins were purified using Ni-NTA (Novagen) and size-exclusion chromatography (Hiload 200, GE Healthcare) in buffer containing 10 mM Bis-Tris pH 6.0 and 100 mM NaCl.
Isothermal titration calorimetry (ITC) assays
The binding affinities of MIK2ECD with SCOOP12 or SCOOP12SS/AA peptides were measured on a MicroCalorimeter ITC200 (Microcal LLC) at 25 °C25. All protein samples used in the ITC assay were dialyzed in the buffer containing 200 mM HEPES, pH 7.0, and 100 mM NaCl. Protein concentration was determined by absorbance spectroscopy at 280 nm. SCOOP12 or SCOOP12SS/AA peptides (600 μM) were injected (1.5 μL per injection) into the stirred calorimeter cells containing 30 μM MIK2ECD with 20 × 2 μL at 2.5-min intervals. ITC data were analyzed using MicroCal Origin 7.0.
Surface plasmon resonance (SPR) assays
The binding kinetics and affinities of MIK2ECD with SCOOP12 or SCOOP12SS/AA peptides were assessed on a Biacore T200 instrument (GE Healthcare, Pittsburgh, PA) with CM5 sensor chips. The MIK2ECD proteins were exchanged to 10 mM sodium acetate (pH 5.5), and the peptides used for SPR were dissolved in HBS-EP+ (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.05% [v/v] surfactant P20 (GE Healthcare)). About 4700 response units of MIK2ECD proteins were immobilized on a CM5 chip, and a blank channel was used as a negative control. SCOOP12 or SCOOP12SS/AA peptides were diluted into indicated concentrations and injected at a flow rate of 30 μL min−1 for 2 min, followed by dissociation for 5 min. After dissociation, 5 mM NaOH was injected for 30 s to remove any non-covalently bound proteins from the chip surface. The binding kinetics was analyzed with the software Biaevaluation® Version 4.1 using the 1:1 Langmuir binding model.
Gel filtration assays
The purified MIK2ECD proteins (0.5 mg for each) and BAK1ECD proteins (0.2 mg for each) were incubated together with SCOOP12 or SCOOP12SS/AA (0.1 mg for each) in a buffer containing 10 mM Bis-Tris, pH 6.0, 100 mM NaCl on ice for 1 h. Then, each of the mixtures was separated by gel filtration (Hiload 200, GE Healthcare) with a flow rate of 0.8 mL/min and an injection volume of 40 mL at 4 °C, and the peak fractions were separated on SDS-PAGE followed by Coomassie blue (CBB) staining.
Fusarium oxysporum disease assay
F. oxysporum strain Fo5176 was grown on potato dextrose agar plates (Difco) for 4 d at 28 °C. The hyphae of Fo5176 were inoculated in potato dextrose broth (Difco) and incubated in a shaker (120 rpm) at 28 °C for 5 d. The spores were collected by filtering through Miracloth (Millipore) followed by centrifugation at 3000 × g for 15 min, resuspended with sterile water, and adjusted to a final concentration of 107 spores/mL. Three-week-old Arabidopsis seedlings were gently taken out of the soil, and roots were washed with sterile water to clear off soil chunks, blotted using paper towels, immersed in Fo5176 spore suspension or water (mock) for 5 min, and then planted back in pots with pre-wet fresh soil. The inoculated seedlings were covered with a dome for two days and incubated at 28 °C, 65% humidity, and 100 μE m−2 s−1 light with a 12-h light/12-h dark photoperiod. The disease severity was evaluated using the percentage of chlorotic leaves in each plant68.
Phylogenetic analysis and graphical illustration
Protein sequences were retrieved from the NCBI database. Multiple sequence alignments were generated with ClustalW69 and displayed in ESPript3 (espript.ibcp.fr). The consensus of sequences was analyzed by WebLogo (http://weblogo.berkeley.edu/logo.cgi). Phylogenetic analysis was carried out using MEGAX with the neighbor-joining method with 1000 bootstrap replicates70. The phylogenetic tree was visualized in an interactive tree of life (iTOL, https://itol.embl.de/). Homology modeling and visualization of F. graminearum protein FGSG_07177 were performed with Pymol (The PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC.) with the centromere DNA-binding protein complex CBF3 subunit B (PDB-ID, c6f07B) as the template. Three-dimensional structure of SCOOP12 was predicted by I-TASSER (https://zhanglab.ccmb.med.umich.edu/I-TASSER/).
Statistical analysis
Statistical analyses were all done in Excel with built-in formulas. The p values were calculated with two-tailed unpaired Student’s t-test analysis for binary comparison or one-way ANOVA and Tukey’s post hoc honest significance test to compare more than two genotypes or treatments. The measurements shown in box plots display the first and third quartiles and split by medians (center lines), with whiskers extending to 1.5-fold the interquartile range from the 25th and 75th percentiles. Dots represent outliers. Asterisks illustrate the p values: ***P < 0.001, **P < 0.01, and *P < 0.05.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank the Arabidopsis Biological Resource Center (ABRC) for providing the Arabidopsis T-DNA insertion lines, Dr. Reidunn B. Aalen (University of Oslo, Norway), Dr. Zhi Qi (Inner Mongolia University, China), Dr. Frederick M. Ausubel (Harvard Medical School, USA), Dr. Marc Knight (Durham University, UK), and Dr. Sébastien Aubourg (INRA, France) for providing Arabidopsis mutants or transgenic lines, Dr. Lijun Ma (University of Massachusetts Amherst, USA) for providing F. oxysporum strain Fo5176, Dr. Weicai Yang (IGDB, CAS) for providing MIK2-HA construct, and Dr. Pingwei Li (Texas A&M University, USA) for providing pET28a-Avi-His6-SUMO vector. We also thank Dr. Cyril Zipfel (University of Zurich, Switzerland), and Dr. Ralph Hückelhoven (Technical University of Munich, Germany) for discussions, Dr. Jinrong Xu (Purdue University, USA), and Dr. Huiquan Liu (Northwest A&F University, China) for assisting on the Fusarium genome sequence analysis, Dr. Wei Zhang (Shandong University, China) for technique supports, and members of the laboratories of L.S. and P.H. for discussions and comments of the experiments. The work was supported by National Science Foundation (NSF) (IOS-1951094) and NIH (R01GM092893) to P.H., NIH (R01GM097247), the Robert A. Welch Foundation (A-1795) to L.S., Natural Science Foundation of Shandong Province (ZR2020MC022) to S.H., National Natural Science Foundation of China (NSFC) to S.H. (31500971) and Z.H. (31971119), and Youth Innovation Technology Project of Higher School in Shandong Province to S.H. (2020KJF013) and R.M. (2019KJD003).
Source data
Author contributions
S.Hou, D.Liu., P.H., and L.S. conceived, designed experiments, analyzed data, and wrote the manuscript; S.Hou identified SCOOP and SCOOP-LIKE sequences, generated Arabidopsis transgenic and mik2 mutant lines, performed physiological analysis, RNA-Seq and RT-qPCR analyses, SPR assay, ROS production assay, and cytosolic calcium assay; D.Liu performed immunoblotting analysis, protein-protein interaction analysis, ethylene production measurement, microscopy assays, homology modeling, and statistical analysis; S.Huang under the supervision of Z.H. and J.C. purified MIK2ECD and BAK1ECD proteins and performed ITC and gel filtration analyses; D.Luo contributed to the root growth and ROS production assays, generated pMIK2::MIK2-GFP/mik2-1 transgenic lines; Z.L. performed phylogenetic analysis, sequence alignment analysis, the Fusarium genome sequence analysis, partial SPR assay, generation of FocSCOOPL deletion mutants, and RT-qPCR analysis of FOXG_11846 expression in F. oxysporum during infection; P.W. performed F. oxysporum disease assays; Q.X. and S.C. performed LC-MS/MS analysis; R.M., Z.H., and J.C. analyzed the data.
Data availability
The raw RNA-seq data is available in the NCBI database with accession numbers GSE159580 and GSE166301. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review informationNature Communications thanks Yusuke Saijo, Kiwamu Tanaka and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shuguo Hou, Derui Liu, Shijia Huang.
Contributor Information
Shuguo Hou, Email: hou_shuguo@126.com.
Libo Shan, Email: lshan@tamu.edu.
Ping He, Email: pinghe@tamu.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-25580-w.
References
- 1.Tang D, Wang G, Zhou JM. Receptor kinases in plant-pathogen interactions: more than pattern recognition. Plant Cell. 2017;29:618–637. doi: 10.1105/tpc.16.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hohmann U, Lau K, Hothorn M. The structural basis of ligand perception and signal activation by receptor kinases. Annu Rev. Plant Biol. 2017;68:109–137. doi: 10.1146/annurev-arplant-042916-040957. [DOI] [PubMed] [Google Scholar]
- 3.Dievart A, Gottin C, Perin C, Ranwez V, Chantret N. Origin and diversity of plant receptor-like kinases. Annu Rev. Plant Biol. 2020;71:131–156. doi: 10.1146/annurev-arplant-073019-025927. [DOI] [PubMed] [Google Scholar]
- 4.Escocard de Azevedo Manhaes AM, Ortiz-Morea FA, He P, Shan L. Plant plasma membrane-resident receptors: surveillance for infections and coordination for growth and development. J. Integr. Plant Biol. 2021;63:79–101. doi: 10.1111/jipb.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkhadir Y, Yang L, Hetzel J, Dangl JL, Chory J. The growth-defense pivot: crisis management in plants mediated by LRR-RK surface receptors. Trends Biochem Sci. 2014;39:447–456. doi: 10.1016/j.tibs.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu F, et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature. 2020;578:577–581. doi: 10.1038/s41586-020-2032-3. [DOI] [PubMed] [Google Scholar]
- 7.Laohavisit A, et al. Quinone perception in plants via leucine-rich-repeat receptor-like kinases. Nature. 2020;587:92–97. doi: 10.1038/s41586-020-2655-4. [DOI] [PubMed] [Google Scholar]
- 8.Ma X, Xu G, He P, Shan L. SERKing coreceptors for receptors. Trends Plant Sci. 2016;21:1017–1033. doi: 10.1016/j.tplants.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Liebrand TW, van den Burg HA, Joosten MH. Two for all: receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci. 2014;19:123–132. doi: 10.1016/j.tplants.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Gust AA, Pruitt R, Nurnberger T. Sensing danger: key to activating plant immunity. Trends Plant Sci. 2017;22:779–791. doi: 10.1016/j.tplants.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Couto D, Zipfel C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016;16:537–552. doi: 10.1038/nri.2016.77. [DOI] [PubMed] [Google Scholar]
- 12.Zhou JM, Zhang Y. Plant immunity: danger perception and signaling. Cell. 2020;181:978–989. doi: 10.1016/j.cell.2020.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka, K. & Heil, M. Damage-associated molecular patterns (DAMPs) in plant innate immunity: applying the danger model and evolutionary perspectives. Annu. Rev. Phytopathol. 10.3389/fpls.2019.00646 (2021). [DOI] [PubMed]
- 14.Gomez-Gomez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell. 2000;5:1003–1011. doi: 10.1016/S1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 15.Zipfel C, et al. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 16.Huffaker A, Pearce G, Ryan CA. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl Acad. Sci. USA. 2006;103:10098–10103. doi: 10.1073/pnas.0603727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi Y, Pearce G, Ryan CA. The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc. Natl Acad. Sci. USA. 2006;103:10104–10109. doi: 10.1073/pnas.0603729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA. PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell. 2010;22:508–522. doi: 10.1105/tpc.109.068874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou S, et al. The secreted peptide PIP1 amplifies immunity through receptor-like kinase 7. PLoS Pathog. 2014;10:e1004331. doi: 10.1371/journal.ppat.1004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu X, Feng B, He P, Shan L. From chaos to harmony: responses and signaling upon microbial pattern recognition. Annu. Rev. Phytopathol. 2017;55:109–137. doi: 10.1146/annurev-phyto-080516-035649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou S, Liu Z, Shen H, Wu D. Damage-associated molecular pattern-triggered immunity in plants. Front Plant Sci. 2019;10:646. doi: 10.3389/fpls.2019.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saijo Y, Loo EP, Yasuda S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018;93:592–613. doi: 10.1111/tpj.13808. [DOI] [PubMed] [Google Scholar]
- 23.Gully K, et al. The SCOOP12 peptide regulates defense response and root elongation in Arabidopsis thaliana. J. Exp. Bot. 2019;70:1349–1365. doi: 10.1093/jxb/ery454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T, et al. A receptor heteromer mediates the male perception of female attractants in plants. Nature. 2016;531:241–244. doi: 10.1038/nature16975. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, et al. Structural basis for receptor recognition of pollen tube attraction peptides. Nat. Commun. 2017;8:1331. doi: 10.1038/s41467-017-01323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeuchi H, Higashiyama T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature. 2016;531:245–248. doi: 10.1038/nature17413. [DOI] [PubMed] [Google Scholar]
- 27.Zhong, S. et al. Cysteine-rich peptides promote interspecific genetic isolation in Arabidopsis. Science364, 10.1126/science.aau9564 (2019). [DOI] [PMC free article] [PubMed]
- 28.Van der Does D, et al. The Arabidopsis leucine-rich repeat receptor kinase MIK2/LRR-KISS connects cell wall integrity sensing, root growth and response to abiotic and biotic stresses. PLoS Genet. 2017;13:e1006832. doi: 10.1371/journal.pgen.1006832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engelsdorf, T. et al. The plant cell wall integrity maintenance and immune signaling systems cooperate to control stress responses in Arabidopsis thaliana. Sci. Signal11, eaao3070 (2018). [DOI] [PubMed]
- 30.Chaudhary A, et al. The Arabidopsis receptor kinase STRUBBELIG regulates the response to cellulose deficiency. PLoS Genet. 2020;16:e1008433. doi: 10.1371/journal.pgen.1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Julkowska MM, et al. Natural variation in rosette size under salt stress conditions corresponds to developmental differences between Arabidopsis accessions and allelic variation in the LRR-KISS gene. J. Exp. Bot. 2016;67:2127–2138. doi: 10.1093/jxb/erw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coleman AD, et al. The Arabidopsis leucine-rich repeat receptor-like kinase MIK2 is a crucial component of early immune responses to a fungal-derived elicitor. N. Phytol. 2021;229:3453–3466. doi: 10.1111/nph.17122. [DOI] [PubMed] [Google Scholar]
- 33.Yu Z, et al. The Brassicaceae-specific secreted peptides, STMPs, function in plant growth and pathogen defense. J. Integr. Plant Biol. 2020;62:403–420. doi: 10.1111/jipb.12817. [DOI] [PubMed] [Google Scholar]
- 34.Olsson V, et al. Look closely, the beautiful may be small: precursor-derived peptides in plants. Annu. Rev. Plant Biol. 2019;70:153–186. doi: 10.1146/annurev-arplant-042817-040413. [DOI] [PubMed] [Google Scholar]
- 35.Ni J, Guo Y, Jin H, Hartsell J, Clark SE. Characterization of a CLE processing activity. Plant Mol. Biol. 2011;75:67–75. doi: 10.1007/s11103-010-9708-2. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Ni J, Denver R, Wang X, Clark SE. Mechanisms of molecular mimicry of plant CLE peptide ligands by the parasitic nematode Globodera rostochiensis. Plant Physiol. 2011;157:476–484. doi: 10.1104/pp.111.180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18:265–276. doi: 10.1046/j.1365-313X.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 38.Millet YA, et al. Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell. 2010;22:973–990. doi: 10.1105/tpc.109.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenvik GE, et al. The EPIP peptide of INFLORESCENCE DEFICIENT IN ABSCISSION is sufficient to induce abscission in arabidopsis through the receptor-like kinases HAESA and HAESA-LIKE2. Plant Cell. 2008;20:1805–1817. doi: 10.1105/tpc.108.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, et al. Allosteric receptor activation by the plant peptide hormone phytosulfokine. Nature. 2015;525:265–268. doi: 10.1038/nature14858. [DOI] [PubMed] [Google Scholar]
- 41.Lu D, et al. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc. Natl Acad. Sci. USA. 2010;107:496–501. doi: 10.1073/pnas.0909705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, et al. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe. 2010;7:290–301. doi: 10.1016/j.chom.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Kadota Y, et al. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell. 2014;54:43–55. doi: 10.1016/j.molcel.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Li L, et al. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe. 2014;15:329–338. doi: 10.1016/j.chom.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Cuomo CA, et al. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science. 2007;317:1400–1402. doi: 10.1126/science.1143708. [DOI] [PubMed] [Google Scholar]
- 46.Fokkens L, et al. A chromosome-scale genome assembly for the Fusarium oxysporum strain Fo5176 to establish a model Arabidopsis-fungal pathosystem. G3. 2020;10:3549–3555. doi: 10.1534/g3.120.401375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhodes J, et al. Perception of a divergent family of phytocytokines by the Arabidopsis receptor kinase MIK2. Nat. Commun. 2021;12:705. doi: 10.1038/s41467-021-20932-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brutus A, et al. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl Acad. Sci. USA. 2010;107:9452–9457. doi: 10.1073/pnas.1000675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwessinger B, et al. Transgenic expression of the dicotyledonous pattern recognition receptor EFR in rice leads to ligand-dependent activation of defense responses. PLoS Pathog. 2015;11:e1004809. doi: 10.1371/journal.ppat.1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas NC, et al. The rice XA21 ectodomain fused to the Arabidopsis EFR cytoplasmic domain confers resistance to Xanthomonas oryzae pv. oryzae. PeerJ. 2018;6:e4456. doi: 10.7717/peerj.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albert M, et al. A two-hybrid-receptor assay demonstrates heteromer formation as switch-on for plant immune receptors. Plant Physiol. 2013;163:1504–1509. doi: 10.1104/pp.113.227736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng B, et al. EMS1 and BRI1 control separate biological processes via extracellular domain diversity and intracellular domain conservation. Nat. Commun. 2019;10:4165. doi: 10.1038/s41467-019-12112-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albert M, et al. Arabidopsis thaliana pattern recognition receptors for bacterial elongation factor Tu and flagellin can be combined to form functional chimeric receptors. J. Biol. Chem. 2010;285:19035–19042. doi: 10.1074/jbc.M110.124800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunze G, et al. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell. 2004;16:3496–3507. doi: 10.1105/tpc.104.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He Y, Wang H, Chen L. Comparative secretomics reveals novel virulence-associated factors of Vibrio parahaemolyticus. Front. Microbiol. 2015;6:707. doi: 10.3389/fmicb.2015.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harvey KL, Jarocki VM, Charles IG, Djordjevic SP. The diverse functional roles of elongation factor Tu (EF-Tu) in microbial pathogenesis. Front. Microbiol. 2019;10:2351. doi: 10.3389/fmicb.2019.02351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masachis S, et al. A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat. Microbiol. 2016;1:16043. doi: 10.1038/nmicrobiol.2016.43. [DOI] [PubMed] [Google Scholar]
- 58.Pruitt RN, et al. A microbially derived tyrosine-sulfated peptide mimics a plant peptide hormone. N. Phytol. 2017;215:725–736. doi: 10.1111/nph.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang X, et al. Nematode-encoded RALF peptide mimics facilitate parasitism of plants through the FERONIA receptor kinase. Mol. Plant. 2020;13:1434–1454. doi: 10.1016/j.molp.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 60.Chen S, et al. In planta processing and glycosylation of a nematode CLAVATA3/ENDOSPERM SURROUNDING REGION-like effector and its interaction with a host CLAVATA2-like receptor to promote parasitism. Plant Physiol. 2015;167:262–272. doi: 10.1104/pp.114.251637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Vries S, Stukenbrock EH, Rose LE. Rapid evolution in plant-microbe interactions—an evolutionary genomics perspective. N. Phytol. 2020;226:1256–1262. doi: 10.1111/nph.16458. [DOI] [PubMed] [Google Scholar]
- 62.Meng X, et al. Ligand-induced receptor-like kinase complex regulates floral organ abscission in Arabidopsis. Cell Rep. 2016;14:1330–1338. doi: 10.1016/j.celrep.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma X, et al. Ligand-induced monoubiquitination of BIK1 regulates plant immunity. Nature. 2020;581:199–203. doi: 10.1038/s41586-020-2210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu J, et al. The malectin-like receptor-like kinase LETUM1 modulates NLR protein SUMM2 activation via MEKK2 scaffolding. Nat. Plants. 2020;6:1106–1115. doi: 10.1038/s41477-020-0748-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Z, et al. A phosphorylated transcription factor regulates sterol biosynthesis in Fusarium graminearum. Nat. Commun. 2019;10:1228. doi: 10.1038/s41467-019-09145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gabelica V, et al. Advantages and drawbacks of nanospray for studying noncovalent protein-DNA complexes by mass spectrometry. Rapid Commun. Mass Spectrom. 2002;16:1723–1728. doi: 10.1002/rcm.776. [DOI] [PubMed] [Google Scholar]
- 67.Guan Q, et al. Comparative proteomics of Mesembryanthemum crystallinum guard cells and mesophyll cells in transition from C3 to CAM. J. Proteom. 2021;231:104019. doi: 10.1016/j.jprot.2020.104019. [DOI] [PubMed] [Google Scholar]
- 68.Wang P, et al. The cotton wall-associated kinase GhWAK7A mediates responses to fungal wilt pathogens by complexing with the chitin sensory receptors. Plant Cell. 2020;32:3978–4001. doi: 10.1105/tpc.19.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 70.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The raw RNA-seq data is available in the NCBI database with accession numbers GSE159580 and GSE166301. Source data are provided with this paper.