Abstract
This study systematically reviewed the published literature on the objective characterization of myofascial pain syndrome and myofascial trigger points using imaging methods. PubMed, Embase, Ovid and the Cochrane Library databases were utilized, while citation searching was conducted in Scopus. Citations were restricted to those published in English and in peer-reviewed journals between 2000–2021. Out of 1,762 abstracts screened, 69 articles underwent full-text review and 33 were included. Imaging data assessing myofascial trigger points (MTrPs) or myofascial pain syndrome (MPS) were extracted and important qualitative and quantitative information on general study methodologies, study populations, sample sizes, and MTrP/MPS evaluation were tabulated. Methodological quality of eligible studies was assessed based on the QUADAS (Quality Assessment of Diagnostic Accuracy Studies) criteria. Biomechanical properties and blood flow of active and latent MTrPs assessed via imaging were found to be quantifiably distinct from those of healthy tissue. While these studies show promise, more studies are needed. Future studies should focus on assessing diagnostic test accuracy and testing the reproducibility of results to establish the best performing methods. Increasing methodological consistency would further motivate implementing imaging methods in larger clinical studies. Considering the evidence on efficacy, cost, ease of use and time constraints, US-based methods are currently the imaging modalities of choice for MPS/MTrP assessment.
Keywords: Trigger Points, Myofascial Pain Syndromes, Diagnostic Imaging, Systematic Review
Introduction
Chronic musculoskeletal pain is estimated to affect 10–20% of the general population and as such constitutes a major public health problem.1 Among patients presenting with chronic musculoskeletal pain, the prevalence of myofascial pain syndrome (MPS) can be as high as 85% or even 95%.1,2 Central to this syndrome are myofascial trigger points (MTrPs), hypersensitive nodules within a taut band of skeletal muscle.3–11 MTrPs were described as far back as the 16th century12 however the most comprehensive work on which current practice and research is based was produced by Travell and Simons in their Trigger Point Manuals, first published in 1983.3 MTrPs were classified into active and latent trigger points. Active MTrPs by definition produce spontaneous pain, whereas latent MTrPs reproduce the patients’ familiar pain only on stimulation.3,4 Commonly, signs and symptoms associated with MTrPs are assessed with manual palpation through a physical examination. The presence of a local twitch response on stimulation of active MTrPs is a frequent diagnostic sign accepted by practitioners.5 MPS also may cause central and peripheral sensitization, with manifestations that include tenderness, referred pain, allodynia and hyperalgesia.13 Motor symptoms (e.g., muscle stiffness, weakness, decreased range of motion) and autonomic dysfunction (e.g., vasodilatation, vasoconstriction) have also been reported.6
Manual palpation through a physical examination constitutes the current standard for diagnosis and assessment of MTrPs and continues to be the standard against which new evaluation methods are assessed. Systematic reviews by Rathbone et al.14 and Lucas et al.15 however found that inter-rater reliability estimates for manual detection of MTrPs varied widely and concluded that physical examination is unreliable. To address these limitations, researchers have begun to develop objective measures and techniques based on in vivo imaging to improve the diagnosis and assessment of MTrPs and MPS. These imaging methods are mostly based on ultrasound (US), magnetic resonance imaging (MRI) and infrared thermography, and are the focus of this review. The broader goal of developing quantitative imaging biomarkers is to establish reliable and objective diagnostic measures and to objectively compare treatment effectiveness.
US with conventional grayscale (B-mode) scanning may show MTrPs as hypoechoic regions with a heterogeneous echotexture.16 More recently, vibration sonoelastography has been used to measure the stiffness of taut bands and MTrPs. In this method, first developed by Lerner et al.17, an external vibration source (typically 100 Hz from a hand-held massager) propagates shear-waves in the tissue of interest.17,18 The vibration pattern (amplitude and phase) is recorded using Doppler techniques and is then displayed as a color variance image.18 A stiff lesion is visualized as a local decrease in the peak vibration amplitude.18 Other US-based techniques include shear-wave elastography (where the vibrations are produced by the hand-held US probe) and compression sonoelastography (where tissue is compressed manually using the US probe). Beyond image intensity-based measures, the utilization of image texture analysis and other image enhancement techniques to distinguish between clinically relevant groups is growing.9,16,19–22
MRI has also been used to visualize MTrPs, which present as areas of focal signal alterations, including a higher T2 signal intensity, on conventional examinations.22,23 Magnetic resonance elastography (MRE), where an external vibration source is activated by the MRI scanner, has also been used.24 MRE includes the use of motion-encoding (magnetic field) gradients from the scanner, in addition to the normal phase-encoding and slice selection gradients, in order to capture the motion of the shear-waves as they propagate through tissue.24,25 The resultant MRE images or “elastograms” display tissue stiffness in a 2D map of color contrast.
Infrared thermography has also been studied as a tool to identify and characterize MTrPs. This technique involves using thermal cameras to detect the infrared radiation emitted by the body. The images displayed can be used to distinguish hotter or cooler areas and are called thermograms.26 The imaging of participants for MTrPs should involve careful control of the environmental temperature and any thermogenic substances participants may have recently ingested. Simple foam markers are typically used to mark the location of MTrPs found during the physical examination.
The goal of this article is to systematically review the literature for peer-reviewed studies that use imaging techniques in the assessment of MTrPs and MPS. The following sections describe our search strategy, inclusion and exclusion criteria, and the methods used for data extraction and synthesis. Results are discussed separately for each imaging modality, followed by the results of study quality assessment. Finally, the strengths and weaknesses of the current evidence in the diagnosis and assessment of MTrPs and MPS are discussed, as well as the performance and clinical viability of these methods.
Methods
Search Strategy
A series of exploratory searches were conducted in PubMed for records that objectively characterized MTrPs using imaging. These searches included terms such as “myofascial trigger points”, “myofascial taut bands”, “taut bands”, “MRI”, “ultrasound”, “sonography”, and others. These were subsequently combined into terms like “(myofascial trigger points) AND (MRI OR sonography OR sonoelastography)” to return more relevant records. For our final search, the authors were assisted by an expert health sciences librarian. Searches were conducted in PubMed, Embase, Ovid MEDLINE®, and the Cochrane Library on 01/11/2021. Records were limited to English language publications and to studies published in the 21st century (between the years 2000–2021). This study conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines27 and reports the required information accordingly (see Supplementary Checklist). In accordance with these, the full electronic search strategy for PubMed is provided in the Supplemental Document Section S.1.
Forwards and backwards citation searching subsequently conducted by the lead author and the librarian using a series of key seed articles identified through the electronic database searches. After removing duplicates, all the abstracts from these two sources were screened by two authors to produce a list of articles for full-text review. The final list of studies was then produced based on the inclusion and exclusion criteria, provided next.
Inclusion Criteria
Included studies fulfilled the following criteria:
Quantitatively characterized MTrPs or MPS using imaging techniques;
Used imaging techniques that produced at least a two-dimensional image; and
Were full-text, peer-reviewed journal publications of original research.
In order to increase the amount of relevant data on the characterization of MTrPs, randomized control trials and other treatment evaluation studies that used objective measures to assess MTrPs or MPS were also included if they met the inclusion and exclusion criteria. Studies that included participants with fibromyalgia alongside those with MTrPs or MPS were also included.
Exclusion Criteria
A significant number of studies returned in our search did not specifically characterize or quantify MTrPs using objective means. Some examined other aspects of myofascial tissue or used imaging solely to assist in treatment (e.g., US-guided injections); others focused on imaging the central nervous system to quantify central sensitization, on inter-rater reliability of palpation techniques, treatment evaluations (using only clinical outcomes), or used cadavers. These studies were excluded during the abstract screening phase and later during full-text review.
We also excluded studies that:
Were conducted in non-human subjects.
Had a sample size of five or fewer participants.
Data Extraction
After full-text screening, tables were constructed for each imaging modality to display important qualitative and quantitative information about general study methodologies, study populations, sample sizes, and MTrP/MPS properties measured. Specifically, details on the imaging techniques, use of control groups, populations from which participants were recruited, sampling methods, number of subjects, numbers of males and females, number of anatomic sites examined, images processed, treatments administered, and use of Travell and Simons’ criteria were extracted.3 For randomized controlled trials and studies that focused on treatments, the baseline quantitative values of MTrPs were prioritized. The sensitivity and specificity of diagnosis of MTrPs using imaging or other techniques were also extracted if tested in the studies.
Methodological quality and risk of bias were assessed with the QUADAS (Quality Assessment of Diagnostic Accuracy Studies) tool, as recommended by Cochrane Collaboration for diagnostic test accuracy studies.28 This formal assessment was performed only for studies that attempted to distinguish between clinically-relevant groups using objective means which could eventually lead to diagnostic test accuracy measurements, or those studies that did evaluate these measures (receiver operating characteristic (ROC)-based analysis, sensitivity, specificity, etc.). These studies were selected as their methods addressed the research question most directly, i.e., the objective assessment of MTrPs and MPS.
Results
Literature Search
A PRISMA flow diagram (Figure 1) summarizes the study selection process. Searching of electronic databases provided a total of 887 citations (188 PubMed, 287 Embase, 186 Ovid MEDLINE, 226 Cochrane Library). Citation searching in Scopus produced an additional 1,439 results. After removing duplicates, 69 articles underwent full-text screening and 36 were excluded (relevance (13), review articles (3), posters and study protocols (7), conference abstracts/proceedings (5), supplement abstracts (1), sample size (5), non-human subjects (2)). Thirty-three studies are included in this article. An overview of included studies is provided in Supplemental Document, Section S.2. There were no studies that explicitly used computed tomography (CT), positron emission tomography (PET) or single-photon emission computed tomography (SPECT) that met our study inclusion criteria. The specific measurement or imaging techniques used varied widely, as did other methodological aspects including study design, use of control groups and sample size. US was by far the most frequently used modality in the included studies, followed by thermography and MRI.
Figure 1 –
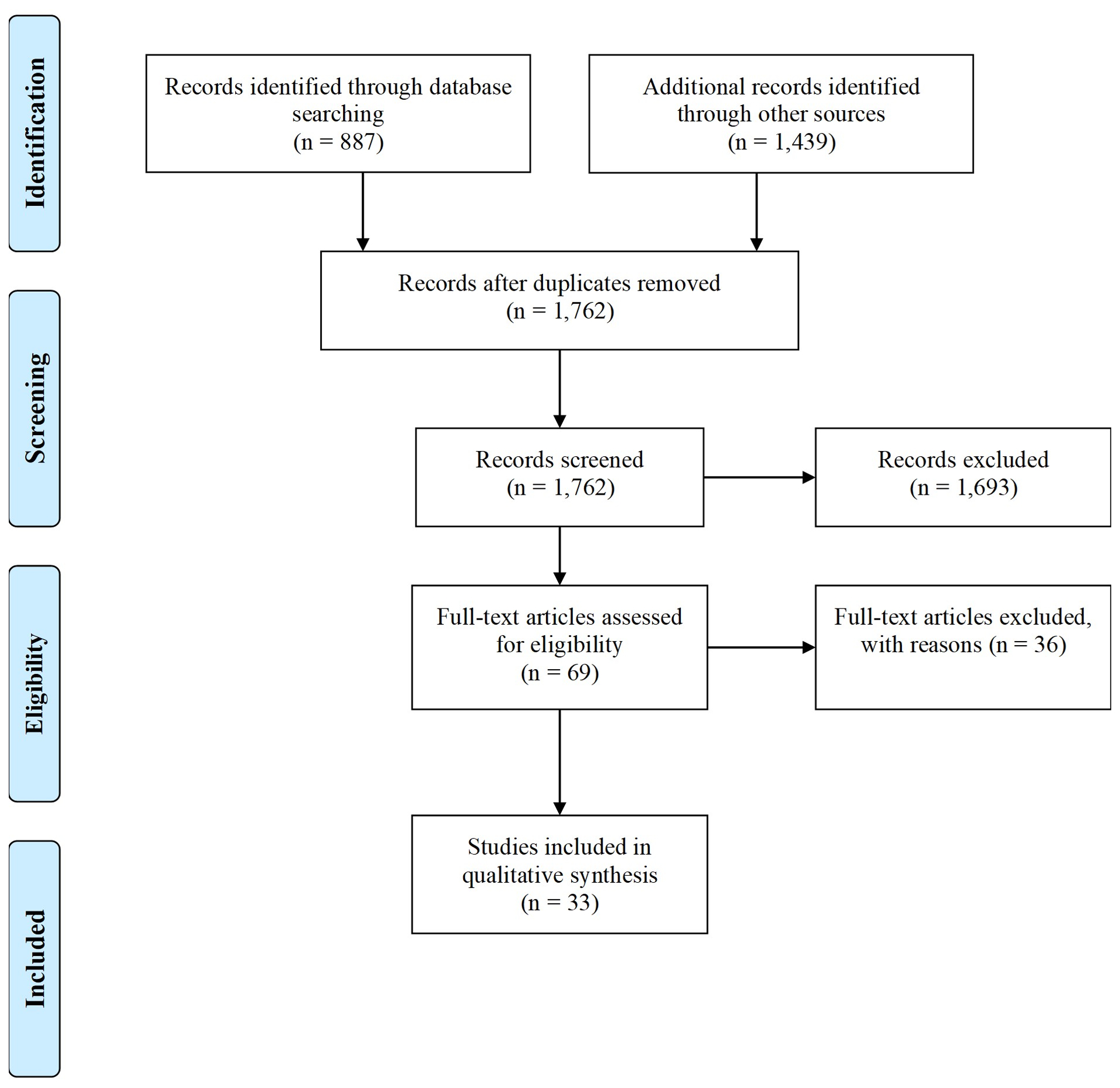
PRISMA flow diagram depicting the study selection process.27
Ultrasound Studies
Table 1 provides a summary of the studies that used US-based methods. The most common techniques used were conventional B-mode sonography and vibration sonoelastography. Doppler imaging was used in three studies21,29,30 to examine the blood flow in myofascial tissue. Three studies used other techniques (Table 1) or did not describe their procedures in detail.31–33
Table 1 –
Ultrasound studies by imaging technique
| B-mode only (N = 9) | |||||
|---|---|---|---|---|---|
| Author | Population | Total No. of participants No. of sites/images Males : Females |
Use of Control Groups | Use of Travell and Simons’ criteria | MTrP property measured |
| Cojocaru et al. 2015 | Patients with low back pain | N = 8 Not reported Not reported |
No control group of participants No mention of control sites | Not reported | Area |
| Da Silva et al. 2020 | Patients with shoulder pain and MTrPs in upper trapezius muscles | N = 40 Not reported 14:26 |
No control group of participants No comparison to normal tissue |
Yes | Area |
| Jafari et al. 2017 | Cervicogenic headache patients | N = 19 Not reported All female |
No control group of participants Adjacent muscle tissue used for comparison | Yes | Elastic modulus, area |
| Jafari et al. 2018 | Patients with a MTrP in the SCMa muscle | N =29 Not reported All female |
No control group of participants Adjacent muscle tissue used for comparison | Yes | Elastic modulus |
| Khumbare et al. 2020 | Patients with MPS and MTrPs aged 20 to 50 | N = 32 N = 282 images 8:7 (controls) |
Fifteen healthy controls simultaneously recruited, no mention of age or sex-matching | Not reported | Texture analysis features |
| Khumbare et al. 2017 | Neck pain patients aged between 20 and 65 years | N = 30 Not reported 5:10 (symptomatic) |
Fifteen healthy controls simultaneously recruited, no mention of age or sex-matching | Yes | Texture analysis features |
| Taheri et al. 2016 | Participants with symptoms of MPS aged 30–50, BMI 22–25 kg/m2 | N = 15 Not reported 4:11 |
Four healthy controls found among the fifteen participants | Yes | Area, Echogenicity |
| Togha et al. 2020 | Cervicogenic headache patients | N = 29 Not reported All female |
No specific control group of participants, comparison between treatment groups | Yes | Elastic modulus, area |
| Zale et al. 2015 | Participants aged 18–65 | N = 25 N = 500 sites 11:14 |
Eight healthy controls found among the twenty-five participants | Yes | Area |
| Vibration Sonoelastography (external, mechanically induced vibrations) (N = 6) | |||||
| Author | Population | Total No. of participants No. of sites/images Males : Females |
Use of Control Groups | Use of Travell and Simons’ criteria | MTrP property measured |
| Ballyns et al. 2011 | Cervical pain patients with an active or latent MTrP in the upper trapezii muscles | N = 44 N = 169 sites Not reported |
No control group of participants, sites without MTrPs served as controls | Yes | Areas of increased stiffness from elastograms (quantified with scoring system) |
| Ballyns et al. 2012 | Cervical pain patients with an active MTrP in the upper trapezii muscles | N = 22 Not reported 11:11 |
Nine healthy participants with no neck pain, no mention of matching | Yes | Shear wave velocity, shear modulus |
| Sikdar et al. 2009 | Participants with at least one active MTrP in the upper trapezius muscles | N = 9 N = 33 sites 2:7 |
No control group of participants, adjacent muscle tissue was used for comparison | Yes | Area and stiffness (quantified with a scoring system) |
| Takla et al. 2016 | Current physical therapy patients aged 25–45 | N = 50 N = 312 MTrPs Not reported |
No control group of participants, adjacent muscle tissue was used for comparison | Yes | Stiffness (as strain scores/ratios) |
| Turo et al. 2013 | Participants with neck pain for past three months and at least one MTrP in the upper trapezius muscles | N = 29 N = 232 images 13:16 |
Fifteen healthy controls simultaneously recruited, no mention of age or sex-matching | Yes | Entropy, stiffness |
| Turo et al. 2015 | Participants with at least one palpable nodule in the upper trapezius muscle | N = 48 N = 96 sites 18:30 |
No control group of participants | Yes | Stiffness via a mechanical heterogeneity index (fraction of elastogram area with decreased vibration) |
| Shear Wave Elastography (SWE) (N = 2) | |||||
| Author | Population | Total No. of participants No. of sites/images Males : Females |
Use of Control Groups | Use of Travell and Simons’ criteria | MTrP property measured |
| Grabowski et al. 2018 | Participants with a single unilateral latent MTrP in the infraspinatus muscle | N = 18 N = 9 sites 6:12 |
Healthy controls, age and sex matched | Yes | Shear wave speed (SWS) |
| Maher et al. 2013 | Women with palpable MTrPs in the upper trapezius muscle | N = 7 N = 7 MTrPs All female |
No control group of participants | Not reported | Elastic modulus |
| Compression sonoelastography (manual technique) (N = 2) | |||||
| Author | Population | Total No. of participants No. of sites/images Males : Females |
Use of Control Groups | Use of Travell and Simons’ criteria | MTrP property measured |
| Calvo-Lobo et al. 2017 | Non-specific lumbopelvic pain patients | N = 10 N = 60 sites 9:1 |
No control group of participants, sites without MTrPs served as controls | Not reported | Stiffness (measured as manual strain index) |
| Müller et al. 2015 | Women aged 20–40 years with at least one active MTrP in both the right and left trapezius muscles | N = 24 N = 48 MTrPs All female |
No specific control group of participants, comparison between treatment groups | Yes | Stiffness (measured as strain ratio), area |
| Doppler (N = 3) | |||||
| Author | Population | Total No. of participants No. of sites/images Males : Females |
Use of Control Groups | Use of Travell and Simons’ criteria | MTrP property measured |
| Ballyns et al. 2011 | Cervical pain patients with an active or latent MTrP in the upper trapezii muscles | N = 44 N = 169 points Not reported |
No control group of participants. sites without TrPs served as controls. | Yes | Resistive Index, Pulsatility Index |
| Sikdar et al. 2009 | Participants with at least one active MTrP in the upper trapezius muscles | N = 9 N = 33 sites 2:7 |
No control group of participants, adjacent muscle tissue was used for comparison | Yes | Resistive Index |
| Sikdar et al. 2010 | Participants with at least one active MTrP in the upper trapezius muscles | N = 16 N = 74 sites Not reported |
No control group of participants, adjacent muscle tissue was used for comparison | Yes | Resistive Index, Pulsatility Index, Acceleration Time, and Time Averaged Peak Velocity |
| Other Studies (N = 3) | |||||
| Author | Population | Total No. of participants No. of sites/images Males : Females |
Use of Control Groups | Use of Travell and Simons’ criteria | MTrP property measured |
|
Aridici et al. 2016 B-mode, sonoelastography (details not specified) Treatment evaluation (HPPTb ultrasound vs. DNc) |
Participants with at least one A-MTrP in the upper trapezius muscle | N = 61 Not reported 8:53 |
No specific control group of participants, comparison between treatment groups | Yes | Stiffness (pixel intensities in MTrP ROIs) |
|
Kumbhare et al. 2018 B-mode sonography with 10s video at 30 fps |
Neck pain patients with palpable taut band or nodule in the upper trapezius muscle, aged between 20–65 | N = 61 N = 18,300 ROIs (300 images per subject * 61 subjects) Not reported |
Twenty-four aged-matched controls | Yes | Texture analysis features |
| Behr et al. 2015 B-mode sonography with video |
Musculoskeletal pain outpatient clinic patients with regional neck pain and a palpable TrP in the upper trapezius aged 20–65 | N = 69 N = 917 images 38:31 |
Twenty-nine healthy controls, aged matched | Yes | Texture analysis features used in binary SVMd classifiers of MTrPs and normal muscle |
Sternocleidomastoid.
High- power pain threshold.
Dry needling.
Support vector machine.
Twelve US studies quantified stiffness of myofascial tissue and MTrPs using several different measures such as elastic modulus, shear modulus, shear-wave speed, strain ratios, strain scores and several custom methods and scoring systems. For example, areas of low vibration in elastogram images (corresponding to increased stiffness) were used in two studies.29,34 Similarly, Turo et al.35 constructed a mechanical heterogeneity index where the fraction of an elastogram image area with reduced vibration was calculated. Sikdar et al.30 used a scoring system to classify elastograms into categories of increasing myofascial pathology, based on increasing areas of stiff tissue and increasing frequency of stiff nodules.
Studies that derived the elastic moduli of MTrPs had consistent results. Jafari et al.36 found mean values of 12.3 – 14.7 kPa in MTrPs compared to 5.0–8.0 kPa in adjacent muscle. In another study, the same group found similar values (13.4 kPa in MTrPs vs. 7.1 kPa in adjacent muscle).37 The pre-treatment stiffness measurements in the study by Togha et al.10 (average 12.3 – 14.8 kPa) corroborated these values. Other numerical values reported were the shear-wave speed and shear modulus. Both Ballyns et al.9 and Grabowski et al.38 reported significantly increased average shear-wave speeds in the muscle tissue of participants with MPS or MTrPs compared to controls. These values were in the range 2.0–5.0 m/s.9,38 The shear modulus was measured by Maher et al.39 and Ballyns et al.9, who report values of approximately 13.6 kPa and 3.5 kPa, respectively.
Several studies did not report the exact quantitative values for their measures of stiffness, but rather reported whether MTrPs were identifiable with their measures through several comparisons. When comparing MTrPs with adjacent muscle tissue, two studies found no significant difference in shear-wave speed,9,38 one found an increased elastic modulus (P<0.01),37 two found increased stiffness (P<0.002),29,30 and one found reduced strain scores (P<0.0001).40 Two studies found active MTrPs to be distinguishable from latent MTrPs,29,41 whereas others did not find statistically significant differences.30,40
Five studies used image enhancement techniques including texture analysis and entropy filtering.19,32–34,42 Predictor texture features in the study by Kumbhare et al.19 were able to classify healthy and MPS participants with a sensitivity and specificity of 95.6% and 97.3%. Behr et al.33 reported similar results to achieve a sensitivity and specificity of 88% and 86%. Turo et al.34 achieved a sensitivity and specificity of 69% and 81%, respectively, using entropy filtering after image acquisition.
Six studies also examined the effect of treatment on stiffness measures of myofascial tissue. Three studies found statistically significant decreases in stiffness (various measures) after treatment with dry needling or high power US treatment.31,35,39 Three other studies found no difference in stiffness after treatment with ischemic compression, dry needling or acupuncture.10,36,43
The lowest active MTrP areas reported (means, 3.40 – 5.35 mm2)36 and the highest (means, 0.48, 0.49 cm2)44 varied by around one order of magnitude. Only one study compared the areas of active MTrPs and latent MTrPs and found no significant difference.30
Four studies examined the change in MTrP area with treatment.10,36,43,45 The treatments used were ischemic compression, acupuncture, injections, and dry needling. All studies reported a decrease in area post-treatment and three studies reported a statistically significant decrease. Only two of these studies utilized control groups with sham treatment10,43 and only one found the decrease in area to be significant when compared to the control group.10
Fourteen out of 23 studies reported on the echogenicity of MTrPs. Of these, 11 studies simply commented that MTrPs could be visualized as focal hypoechoic regions on B-mode US. Three studies explored the echogenicity further and found differences in mean-echo intensity and grey-scale values of cases compared to controls.40,42,44
Studies that used Doppler US found significant alterations in the blood flow in and around MTrPs. The specific results of these studies can be viewed in Table 2. The prevalence of retrograde blood flow was found to be higher at active compared to normal sites.9,21,29,30 Two studies also reported an increased pulsatility index (PI) in active MTrPs compared to normal sites and no difference in the resistive index (RI).21,29 Peak systolic velocity and minimum diastolic velocity were able to differentiate between active and latent MTrPs.21
Table 2 –
Doppler measurements of blood flow in and around MTrPs.
| Author | Measures | Comparison / Site | Results |
|---|---|---|---|
| Ballyns et al. 2011 | Pulsatility Index (PI) Resistive Index (RI) |
A-MTrPsa and L-MTrPsb vs. normal A-MTrPsa vs. normal All three |
Occurrence of retrograde flow higher (P<0.01) PI higher at A-MTrPsa (P<0.05) No difference in RI between sites (P>0.05) |
| Sikdar et al. 2009 | Resistive Index (RI) Frequency of retrograde diastolic flow |
A-MTrPsa L-MTrPsb Normal sites |
69% retrograde diastolic flow 16.7% retrograde diastolic flow 7% retrograde diastolic flow |
| Sikdar et al. 2010 | Pulsatility Index (PI) Resistive Index (RI) Peak systolic velocity (PSV) Minimum diastolic velocity (MDV) Acceleration time (AT) Time averaged peak velocity (TAPV) |
A-MTrPsa vs. normal sites A-MTrPsa vs. L-MTrPsb L-MTrPsb vs. normal sites |
PI and PSV increased; MDV decreased (P<0.05) PSV increased; MDV decreased (P<0.05); no difference in PI. No difference in PSV, MDV or PI No differences in RI, AT or TAPV between sites |
Active myofascial trigger points.
Latent myofascial trigger points.
Infrared Thermography Studies
Table 3 provides a summary of the studies that used infrared thermography. Four out of seven studies reported using Travell and Simons’ criteria for identifying MTrPs.
Table 3 –
Infrared Thermography Studies
| Infrared thermography (N = 7) | |||||
|---|---|---|---|---|---|
| Author | Population | Total No. of participants No. of sites/images Males : Females |
Use of Control Groups | Use of Travell and Simons’ criteria | MTrP property measured |
| Cojocaru et al. 2015 | Patients with low back pain | N = 8 Not reported Not reported |
No control group of participants. No mention of control sites | Not reported | Temperature for the whole lumbar region and for MTrPs |
| Gabrhel et al. 2013 | Patients of both sexes with pelvic-femoral pain admitted to a rehabilitation medicine clinic | N = 83 Not reported 42:41 |
No control group of participants. Contralateral unaffected regions used for comparison | Yes | Temperature differences in gluteal region Sensitivity and specificity |
| Girasol et al. 2018 | Participants of both sexes aged 18–45 with chronic neck pain of more than 3m duration with an A-MTrP in the upper trapezius muscle | N = 40 Not reported 2:38 |
No control group of participants. No mention of control sites | Yes | Temperature, correlation with other variables |
| Haddad et al. 2012 | Female participants with masticatory pain with not history of systemic problems and no history of TMDs | N = 26 N = 780 ROIs All female |
No control group | Not reported | Temperature of regions of masseter muscle correlated with PPT Sensitivity and specificity |
| Magalhães et al. 2015 | Participants from a university community aged 18–45 with BMI 20–25 | N = 15 Not reported 2:13 |
No specific control group of participants, comparison between treatment groups | Yes | Temperature differences after application of compressive forces |
| Skorupska et al. 2015 | Chronic sciatica patients aged 30–60 with unilateral leg pain for > 3 months | N = 50 Not reported 21:29 |
No specific control group of participants, comparison between TrP-positive and TrP-negative individuals | Yes | Temperature pre- and post-treatment with DNa |
| Zhang et al. 2009 | Healthy participants without any signs or symptoms of musculoskeletal pain | N = 15 Not reported 11:4 |
No control group of participants, contralateral unaffected regions used for comparison | Not reported | Temperature differences Temperature pre- and post-treatment with glutamate injections |
Dry needling.
The studies all utilized very similar methods for their data acquisition. Most cameras used had very similar precision and emissivity where reported (approximately 0.05 °C and 0.98, respectively), and 4 out of 7 studies reported using acclimatization periods before imaging the participants. These periods varied and lasted 15–30 minutes at temperatures of 21–24 °C. In terms of the studied populations and comparisons between groups, muscles, or tissues however, the studies varied greatly. Some studies placed polystyrene markers over the MTrPs and recorded temperature directly over these, whereas others compared average temperatures of whole muscles or body regions with and without MTrPs. Some measured temperatures pre- and post-treatment and others temperature differences between affected and unaffected sides, or between MTrPs and contralateral areas of the same size. One study tested the correlations of temperature with typical measures known to be associated with the presence of MTrPs such as pain, pressure pain threshold, range of motion and muscle electrical activity.46 They found a significant negative correlation between base muscle electrical activity and temperature.
Studies that tested the ability of infrared thermography to identify trigger points found contradictory results. Zhang et al.47 found no difference in temperature over the forearms with latent MTrPs compared to those without them. Gabrhel et al.48 and Haddad et al.49, on the other hand, found increased temperatures over MTrPs. The former found that MTrPs had increased temperatures (range 0.8–1.5 °C) compared to equivalent contralateral unaffected areas. The latter two found that a normalized temperature gradient between equivalent contralateral sites could predict/detect the presence of a MTrP in that site with a sensitivity and specificity of 62.5% and 71.3%, respectively.
Two studies used infrared thermography to examine the effects of various treatments on temperature over or near MTrPs.45,50 A significant increase in areas of higher temperature (compared to pre-treatment values) was observed in MTrP-positive and not in MTrP-negative patients that underwent dry needling treatment in the study by Magalhães et al.50 Cojocaru et al.45 also reported temperature changes after injections into MTrPs in the lumbar region.
MRI Studies
MTrP studies using MRI involved two techniques: conventional, anatomical MRI and MRE. Table 4 provides a summary of the studies that used these methods. Three of 4 studies that used MRI did not report the use of Travell and Simons’ criteria in the identification of the MTrPs or taut bands. All studies used similar scanner equipment with a field strength of 1.5 T, except for one study which used a 3.0 T scanner. Scan parameters and pulse sequences also varied.
Table 4 –
MRI Studies by Imaging Technique
| Conventional MRI (N = 2) | |||||
|---|---|---|---|---|---|
| Author | Population | Total No. of participants No. of sites/images Males : Females |
Use of Control Groups | Use of Travell and Simons’ criteria | MTrP property measured |
| Luiza da Silva Queiroz et al. 2020 | Female migraine patients (ICHD-3), aged 18–30 | N = 14 Not reported All female |
Six controls without migraine | Yes | T1signal intensity at and around MTrPs with and without gadolinium contrast |
| Sollmann et al. 2019 | Migraine patients (ICHD-3) with clinically confirmed MTrPs in the upper trapezius muscles | N = 10 N = 38 images 1:9 |
No control group of participants, adjacent muscle tissue used for comparison | Not reported | T2 mapping with prepared TSE sequence |
| Magnetic Resonance Elastography (N = 2) | |||||
| Author | Population | Total No. of participants No. of sites/images Males : Females |
Use of Control Groups | Use of Travell and Simons’ criteria | MTrP property measured |
| Chen et al. 2008 | Women with and without myofascial pain | N = 8 Not reported All female |
Four healthy controls recruited, no mention of age matching | Not reported | Shear stiffness in taut bands, in adjacent muscle and in controls |
| Chen et al. 2016 | Participants with myofascial pain in trapezius muscles aged 21–70 years | N = 65 N = 140 MRE scans 20:45 |
No control group of participants, adjacent or contralateral muscle tissue used for comparison | Not reported | Stiffness and stiffness ratio of taut bands and adjacent muscle, intra- and inter-rater reliability, comparison of MRE with clinical detection of taut bands |
Two studies used conventional, anatomical MRI to characterize MTrPs.22,51 One study found that MTrPs could not be identified with the use of T1-weighted imaging with or without gadolinium contrast.51 Sollmann et al.22 utilized a T2-prepared, turbo-spin-echo pulse sequence to produce T2 maps for MTrPs. Compared to adjacent tissue, MTrP regions were found to have significantly higher mean T2 signal intensity.22 Fifteen of 16 clinically identified MTrPs were identified successfully using the T2 maps.22
Two studies by Chen et al.7,52 used MRE and calculated the stiffness of taut bands. They found values of 11.5 and 10.9 kPa, respectively. Moreover, they found that these regions were consistently stiffer than surrounding muscle tissue and stiffer than similar regions in control participants. Chen et al.7 however found that there was poor agreement between MRE and clinical evaluation in identifying taut bands, with just 63% of taut bands clinically identified being confirmed on MRE. They also measured intra- and inter-rater reliability of taut band identification via MRE and found near perfect kappa scores.7
Methodological Quality and Risk of Bias
Studies whose methods focused on objectively distinguishing between clinically relevant groups were assessed for methodological quality and risk of bias. Thirteen studies fit these criteria. Figures 2 and 3 show the methodological quality of each individual study and the global quality across studies based on each QUADAS item, respectively.
Figure 2 –
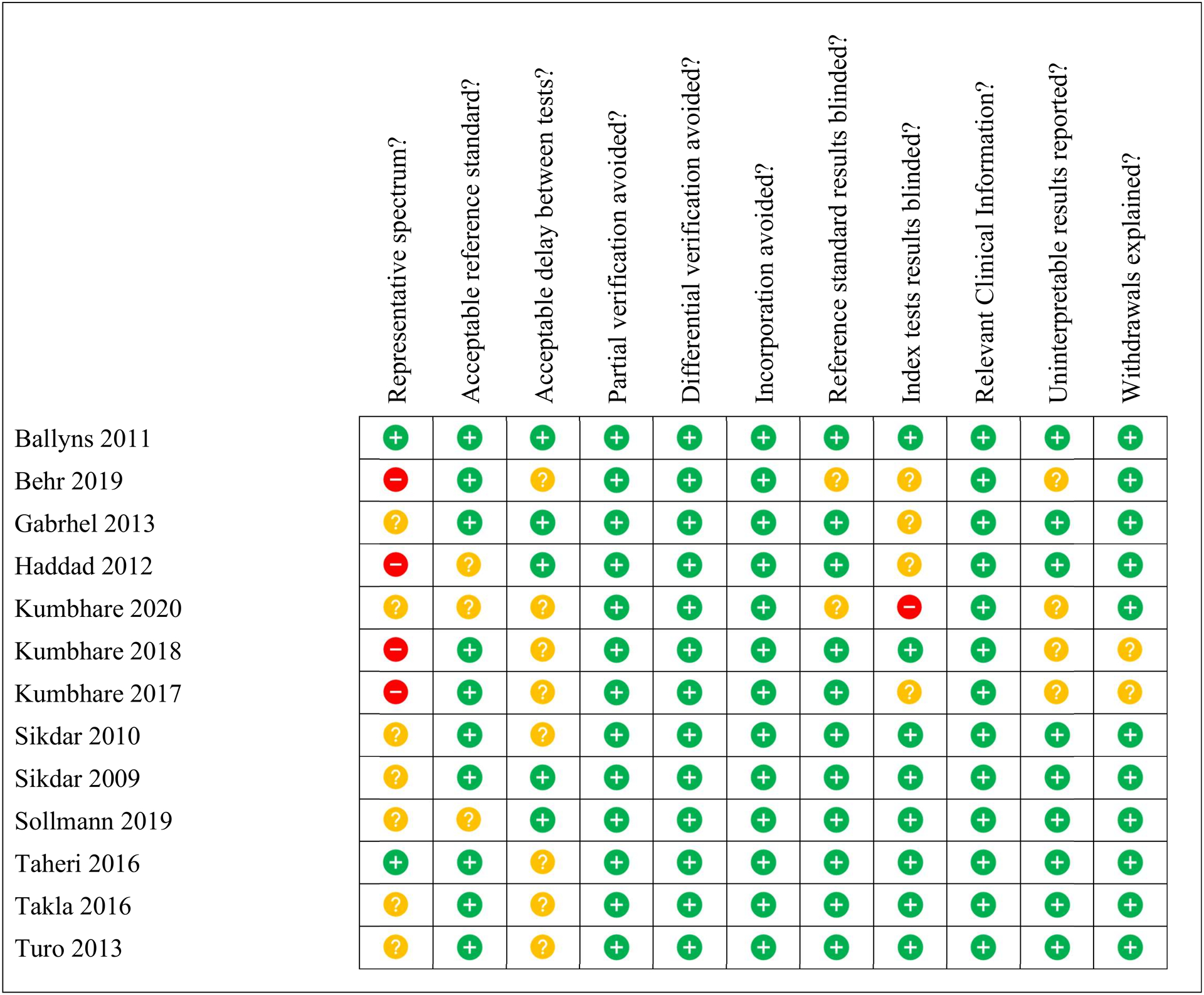
QUADAS (Quality Assessment of Diagnostic Accuracy Studies)28 outcomes for each study. The quality of the study and the likelihood of bias in the results is indicated by the colour: green (low chance, high quality), yellow (uncertain chance and quality), red (high chance, low quality).
Figure 3 –
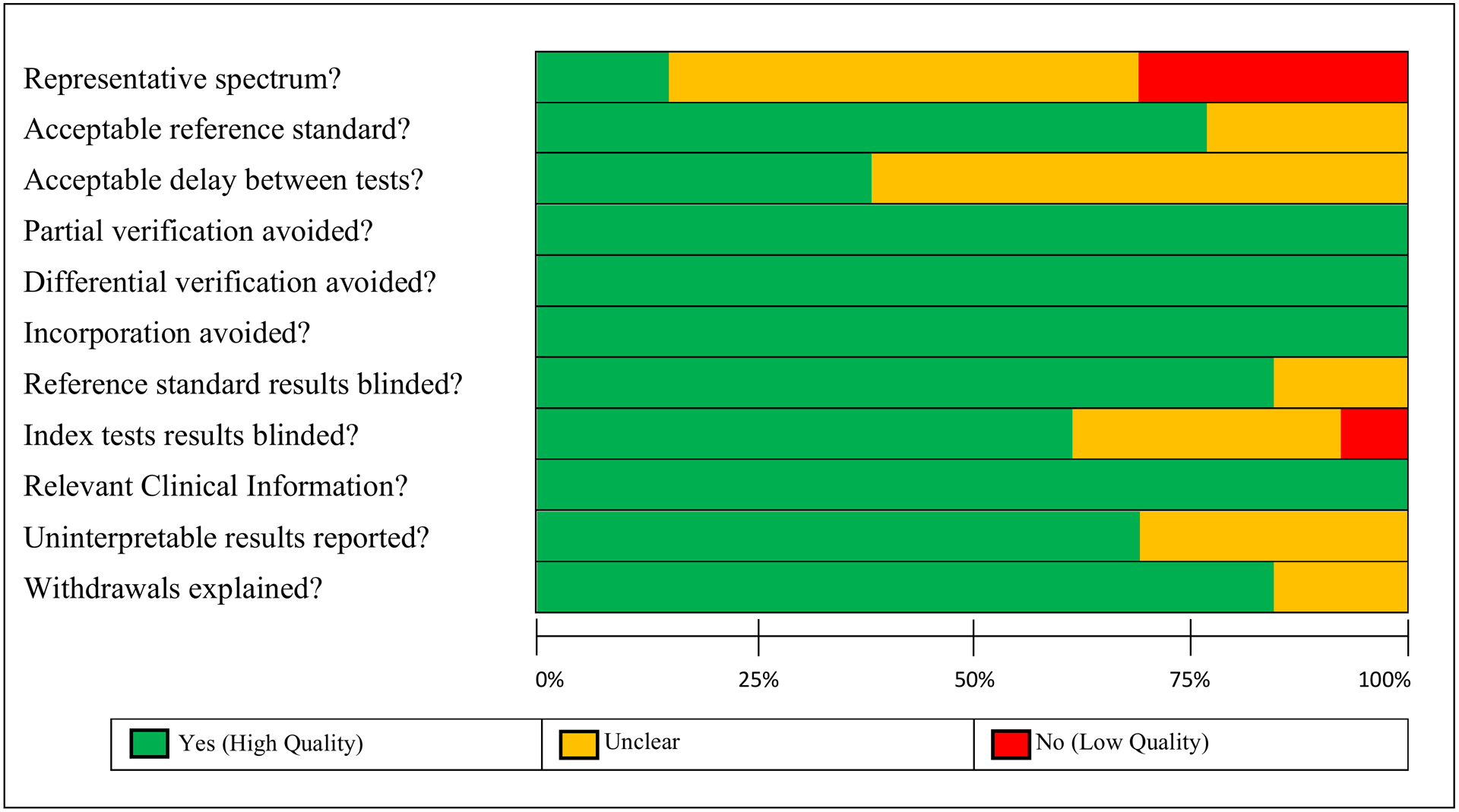
QUADAS quality assessment across the 15 studies. The figure shows the proportion of high (green), uncertain (yellow) and low (red) quality study methodologies for each QUADAS item.
Overall, methodological quality was sound amongst these 13 studies. The most problematic item was the spectrum of participants. Four studies did not use sequential sampling of participants and instead recruited their controls separately, which can introduce bias. Nine studies did not report the time interval between physical examination and their assessment techniques. Regarding the reference standard, adherence to Travell and Simons’ criteria (the only reference standard available) was very consistent. Where adherence to these criteria was not reported, most studies did take care to mention the involvement of experienced physiotherapists or physicians. Other studies however, merely mentioned that “clinical criteria” for diagnosis were followed. Partial verification, differential verification and incorporation bias were successfully addressed in the methodology, minimizing the possibility of these sources of bias in all 13 studies. Reporting of uninterpretable results and participant dropouts was good, though 4 studies did not comment on this (Figure 2). Finally, blinding of both reference and index tests was conducted in most studies, with a few not reporting it clearly and only one study where the index test was not carried out blind to the reference test results.19
Discussion
Findings and Etiology
Findings of studies included in this review indicate conclusively that MTrPs and taut bands do exist and are indeed local areas of increased muscle stiffness. In particular, this is evidenced by the close agreement in the magnitude of the stiffness values of MTrPs and taut bands compared to adjacent muscle tissue across different studies using different imaging modalities. Jafari et al.37 and Togha et al.10 both measured an elastic modulus for MTrPs of 13–14 kPa using US and Chen et. al.7 found a value of 10.9 kPa using MRE. Values for adjacent muscle tissue also showed close agreement across these studies (ranging between 5–7 kPa).7,10,37 In addition, most studies measuring stiffness (using various measures) reported statistically significant differences between MTrPs/taut bands and adjacent muscle, and between cases and controls. This close agreement and cross-study consistency also lends credibility to these methods in accurately and reproducibly measuring the structural properties of soft tissue in vivo, which is essential to improving the objective assessment of MTrPs.
Other important findings are those from studies using Doppler US. MTrPs have altered blood flow characterized by higher systolic velocities and frequent retrograde flow, which may be caused by local constriction due to the taut band or altered due to inflammation or autonomic changes.21,29,30 This is consistent with Simons’ integrated hypothesis on the etiology of MTrPs, where excess acetylcholine release initiates the abnormal contraction in a taut band.3,4 The resulting ischemia and hypoxia are theorized to increase the release of substances that sensitize and activate peripheral nociceptors and cause pain. Evidence from other sources examining molecular biomarkers20,53,54 support this hypothesis by showing elevated levels of cytokines, bradykinin, substance P, and others in the vicinity of MTrPs along with calcitonin gene-related peptide which is released on depolarization of nociceptive neurons.8 These substances are also thought to cause autonomic modulation, which increases the release of acetylcholine and perpetuates the abnormal muscle contraction.8 An acidic pH has been measured in vivo in MTrPs20,55, and this has been shown to cause muscle pain.8,56 In addition, an acidic pH may inhibit the function of acetylcholine esterase, increasing the levels of acetylcholine and further perpetuating contraction.8
More research is needed on the specific mechanisms that initiate and perpetuate the MTrP in MPS,8 which in the future could lead to the development of targeted therapies. Furthermore, should any of these markers prove to have high specificity for the disease, it could be possible to create targeted radioligands and use molecular imaging techniques such as PET.57,58 Recently, systems capable of systemic PET imaging at significantly lower dose have become available59,60 and could provide a unique molecular-level in vivo picture of MTrPs and MPS pathophysiology.
Viability and Performance of Methods
Imaging modalities and techniques discussed in this review showed the potential to characterize tissue with MTrPs. However, they differ in their effectiveness and their likelihood to be adopted in a clinical setting.
The most successful US-based technique appears to be sonoelastography, as most studies utilizing this technique were able to detect differences between clinically relevant groups and identify MTrPs within normal tissue. Among these studies, it is likely that those using vibration sonoelastography and shear-wave elastography have more reliable results due to their use of mechanically induced stimuli. However, only one study that utilized US elastography assessed the diagnostic test accuracy of their methods.40
Other promising techniques involve post-imaging enhancement of simple B-mode US images through texture analysis and entropy filtering. Although the results of these studies achieved desirable diagnostic test statistics, their image processing techniques require specialized software and know-how that may not be readily accessible in a clinical setting. In the future it is likely that region-of-interest delineation and texture analysis will become more viable thanks to the implementation of machine learning. Elastography-based methods, on the other hand, could be implemented using frequently used US equipment, where available. More research is needed to establish the diagnostic accuracy of these elastography methods. The ability of thermography to detect MTrPs may need to be evaluated further, as current studies report contradictory results. Only two studies reported the ability to detect MTrPs.48,49 This is likely due to its focus on surface skin temperature. It should be noted that these methods also require image processing after acquisition. Studies using MRI showed the ability to image MTrPs when using MRE and T2 mapping.7,22,52 A routine T1-weighted MRI acquisition is not effective for detecting or assessing MTrPs.51
The likelihood of the discussed imaging methods to be adopted in a clinical setting is dependent on cost, ease of use and time constraints, in addition to performance factors. MRI-based methods generally suffer from relatively high costs and accessibility constraints. Thermography-based methods appear to have limited efficacy based on this review of the literature. Thus, US’s ease of use, low cost and clinical availability make it generally the current imaging modality of choice for MPS/MTrP assessment. Some US machines may be upgraded to perform sonoelastography, enabling current practitioners to expand their assessment tools. The upgrades are specific to each manufacturer and costs can vary significantly.61 For a review of sonoelastography techniques with corresponding technologies, practitioners are encouraged to consult the review by Sigrist et al.62 as well as the manufacturers of their current equipment.
Limitations and Risk of Bias
Among the studies assessed formally using the QUADAS tool, quality was generally high, and the risk of bias was accordingly low, as discussed. Notably, however, care must be taken in the interpretation of two of the quality items.
First, Item 1: the representative spectrum. This item has two components: whether the patients recruited are representative of those who would receive the test/assessment in practice and the sampling method used to recruit participants. All 13 studies assessed had a representative sample of participants by our estimation, however some used sampling methods where cases and controls were recruited separately. This can introduce bias when measuring diagnostic test accuracy. However, most of the studies that received a low-quality score on this item were not designed for this purpose; they were designed as initial assessments of their methods in detecting MTrPs. Therefore, the overall quality of this item is, in fact, higher than represented. This item was incorporated as it provides a useful metric on where the evidence is during the current translational phase to more widespread clinical use. Moreover, it provides a standard for future research to work towards.
Second is Item 2: the reference standard. Although our assessment indicates a low risk of bias for this item, care must be taken when interpreting diagnostic test accuracy results in these studies. This is because Travell and Simons’ criteria are the only available reference standard, are subjective in nature and suffer from inadequate inter-rater reliability.14,15 Therefore, results reporting the sensitivity and specificity are unlikely to be biased but will have a significant amount of uncertainty.
Regarding the other items, there is a very low risk of bias from Items 4, 5 and 6, as well as from uninterpretable results or withdrawals. The likelihood of bias from including information not usually available in the clinic is also low among these 13 studies.
Blinding seems to have been conducted in all studies, except for one (Figure 2). Several studies failed to report this explicitly however, and future studies can improve upon this. Overall, the authors estimate a low chance of bias from blinding (Items 7 and 8, Figure 2).
Lastly, 8 studies failed to report the delay between physical examination and the imaging assessments. As MPS and MTrPs are associated with chronic pain, it seems unlikely that symptoms might fluctuate significantly, however, there is the possibility of some bias being introduced here. Future studies should take care to report this time interval as well as minimizing it.
Among the remaining studies, overall methodologies were adequate for each specific study’s design and purpose. Some weaknesses were observed, however. Multiple studies did not make use of controls and, among those that did, many recruited them separately instead of consecutively. Further, few studies utilized matching for the controls recruited. A number of studies could have improved on other key methodological aspects, such as providing more details on the populations used for recruitment and on the physical examination (including use of Travell and Simons’ criteria), reporting on the experience of examiners, and using a more balanced number of male and female participants.
The most important limitation in interpreting the results presented in this article is the heterogeneity of study methodology. Methods for assessing MTrPs and MPS varied in terms of imaging modalities and measurement techniques, however particular methodologies of quantification of images did as well. A prime example is how authors chose to quantify elastography images (several custom scoring systems were used29,30,35) and the particular measures of stiffness they chose (strain ratios, shear-wave speed, shear stiffness, elastic modulus, etc. (Table 1)). The comparisons made against MTrPs also varied widely. Studies compared them against adjacent muscle tissue, to corresponding healthy muscle tissue in controls, to contralateral sites, or a comparison was made between active and normal standardized locations in both healthy controls and participants with MTrPs/MPS. This complicates comparison of results across studies even when their measurement techniques are very similar. Sex-specific differences may also complicate interpretation. The numbers of male and female participants in the studies varied significantly, and although it is unclear whether there are sex differences in MTrPs and MPS,1 there are known sex differences in pain sensitivity and risk of developing clinical pain.63 The anatomical locations of MTrPs also varied, further complicating interpretation. For these reasons, it remains challenging to establish quantitative thresholds or cut-off points that could be used quickly and efficiently to assess or diagnose MTrPs, to discriminate between clinically relevant groups in terms of severity, or to assess likely response to treatment. Some methods do show the ability to distinguish between active MTrPs and latent MTrPs and between cases and controls, for example those studies that used Doppler and texture analysis.19,21,30,32,42 However, these results are yet to be replicated or to be tested in different or broader populations.
Lastly, there appears to be an unmet need for additional epidemiological and public health research on MPS and MTrPs. Little appears to be known on such key matters as remission rates, recurrence, rates of misdiagnosis, adherence to treatment, and quality of life impact. Even estimates of prevalence are uncertain. There are therefore many opportunities for future research in establishing what approach is needed to combat this disease and its large contribution to the burden of chronic pain.
Conclusion
This systematic review provides guidance for future imaging research in MPS and MTrPs, where there is a need for biomarkers to improve diagnosis and treatment assessment through objective means. Future research should focus on establishing the best performing methods and measures, and on testing the reproducibility of results. This, in turn, will lead to more established measures of MTrPs that could be tested in larger clinical studies. The development of an objective diagnostic reference standards appears to be possible, and several methods in this review present promising results on the road to this goal. At this time, considering efficacy, cost, ease of use and time constraints US-based methods including elastography and Doppler are likely the techniques of choice for MTrP/MPS assessment. More research on individual and population level outcomes of MPS and MTrP patients is however warranted to determine how these and other reviewed methods can be best deployed to ease the burden of these chronic pain disorders.
Supplementary Material
Author Disclosures:
The authors would like to acknowledge the support from the National Institutes of Health (NIH) grant R01AR076088. We declare that we have no competing interests and derive no financial benefits from this work. We certify that this work is original and has not been published or presented elsewhere.
References
- 1.Gerwin RD Classification, epidemiology, and natural history of myofascial pain syndrome. Curr Pain Headache Rep 5, 412–420 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Fishbain DA, Goldberg M, Meagher BR, Steele R & Rosomoff H Male and female chronic pain patients categorized by DSM-III psychiatric diagnostic criteria. Pain 26, 181–197 (1986). [DOI] [PubMed] [Google Scholar]
- 3.Travell JG & Simons DG Myofascial pain and dysfunction: the trigger point manual. (Williams & Wilkins, 1983). [Google Scholar]
- 4.Gerwin RD, Dommerholt J & Shah JP An expansion of Simons’ integrated hypothesis of trigger point formation. Current Science Inc 8, 468–475 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Yap EC Myofascial pain - An overview. Annals of the Academy of Medicine Singapore 36, 43–48 (2007). [PubMed] [Google Scholar]
- 6.Dommerholt J, Bron C & Franssen J Myofascial trigger points: An evidence-informed review. Journal of Manual and Manipulative Therapy 14, 203–221 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Q et al. Quantification of Myofascial Taut Bands. Arch Phys Med Rehabil 97, 67–73 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jafri MS Mechanisms of Myofascial Pain. Int Sch Res Notices 2014, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballyns JJ et al. Office-Based Elastographic Technique for Quantifying Mechanical Properties of Skeletal Muscle. J Ultrasound Med 31, 1209–1219 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Togha M, Bahrpeyma F, Jafari M & Nasiri A A sonographic comparison of the effect of dry needling and ischemic compression on the active trigger point of the sternocleidomastoid muscle associated with cervicogenic headache: A randomized trial. BMR 1–11 (2020) doi: 10.3233/BMR-171077. [DOI] [PubMed] [Google Scholar]
- 11.Simons DG New views of myofascial trigger points: etiology and diagnosis. Arch Phys Med Rehabil 89, 157–159 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Ruhmann W The Earliest Book on Rheumatism. Br J Rheumatism 11, 140–162 (1940). [Google Scholar]
- 13.Srbely JZ, Dickey JP, Lee D & Lowerison M Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. Journal of Rehabilitation Medicine 42, 463–468 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Rathbone ATL, Grosman-Rimon L & Kumbhare DA Interrater Agreement of Manual Palpation for Identification of Myofascial Trigger Points: A Systematic Review and Meta-Analysis. Clin J Pain 33, 715–729 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Lucas N, Macaskill P, Irwig L, Moran R & Bogduk N Reliability of physical examination for diagnosis of myofascial trigger points: a systematic review of the literature. Clin J Pain 25, 80–89 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Srbely JZ, Kumbhare D & Grosman-Rimon L A narrative review of new trends in the diagnosis of myofascial trigger points: diagnostic ultrasound imaging and biomarkers. J Can Chiropr Assoc 60, 220–225 (2016). [PMC free article] [PubMed] [Google Scholar]
- 17.Lerner RM, Parker KJ, Holen J, Gramiak R & Waag RC Sono-Elasticity: Medical Elasticity Images Derived from Ultrasound Signals in Mechanically Vibrated Targets. in Acoustical Imaging: Proceedings of the Sixteenth International Symposium, June 10–12, 1987 (ed. Kessler LW) 317–327 (Springer US, 1988). doi: 10.1007/978-1-4613-0725-9_31. [DOI] [Google Scholar]
- 18.Taylor LS, Porter BC, Rubens DJ & Parker KJ Three-dimensional sonoelastography: principles and practices. Phys Med Biol 45, 1477–1494 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Kumbhare D, Shaw S, Ahmed S & Noseworthy MD Quantitative ultrasound of trapezius muscle involvement in myofascial pain: comparison of clinical and healthy population using texture analysis. J Ultrasound 23, 23–30 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah JP et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil 89, 16–23 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Sikdar S, Ortiz R, Gebreab T, Gerber LH & Shah JP Understanding the vascular environment of myofascial trigger points using ultrasonic imaging and computational modeling. Conf Proc IEEE Eng Med Biol Soc 2010, 5302–5305 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sollmann N et al. Quantitative magnetic resonance imaging of the upper trapezius muscles – assessment of myofascial trigger points in patients with migraine. The Journal of Headache and Pain 20, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landgraf MN et al. Alterations in the trapezius muscle in young patients with migraine--a pilot case series with MRI. Eur. J. Paediatr. Neurol 19, 372–376 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Di Ieva A et al. Magnetic resonance elastography: A general overview of its current and future applications in brain imaging. Neurosurg. Rev 33, 137–145 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Muthupillai R et al. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 269, 1854–1857 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Lahiri BB, Bagavathiappan S, Jayakumar T & Philip J Medical applications of infrared thermography: A review. Infrared Physics & Technology 55, 221–235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG & Group TP Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine 6, e1000097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reitsma JB et al. Chapter 9: Assessing methodological quality. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (2009). [Google Scholar]
- 29.Ballyns JJ et al. Objective sonographic measures for characterizing myofascial trigger points associated with cervical pain. J Ultrasound Med 30, 1331–1340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sikdar S et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil 90, 1829–1838 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aridici R et al. Comparison of the Efficacy of Dry Needling and High-Power Pain Threshold Ultrasound Therapy with Clinical Status and Sonoelastography in Myofascial Pain Syndrome. Am J Phys Med Rehabil 95, e149–158 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Kumbhare DA, Ahmed S, Behr MG & Noseworthy MD Quantitative Ultrasound Using Texture Analysis of Myofascial Pain Syndrome in the Trapezius. Crit Rev Biomed Eng 46, 1–31 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Behr M, Noseworthy M & Kumbhare D Feasibility of a Support Vector Machine Classifier for Myofascial Pain Syndrome: Diagnostic Case-Control Study. J Ultrasound Med 38, 2119–2132 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Turo D et al. Ultrasonic characterization of the upper trapezius muscle in patients with chronic neck pain. Ultrason Imaging 35, 173–187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turo D et al. Novel Use of Ultrasound Elastography to Quantify Muscle Tissue Changes After Dry Needling of Myofascial Trigger Points in Patients With Chronic Myofascial Pain. J Ultrasound Med 34, 2149–2161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jafari M, Bahrpeyma F & Togha M Effect of ischemic compression for cervicogenic headache and elastic behavior of active trigger point in the sternocleidomastoid muscle using ultrasound imaging. J Bodyw Mov Ther 21, 933–939 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Jafari M, Bahrpeyma F, Mokhtari-Dizaji M & Nasiri A Novel method to measure active myofascial trigger point stiffness using ultrasound imaging. J Bodyw Mov Ther 22, 374–378 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Grabowski PJ, Slane LC, Thelen DG, Obermire T & Lee KS Evidence of Generalized Muscle Stiffness in the Presence of Latent Trigger Points Within Infraspinatus. Arch Phys Med Rehabil 99, 2257–2262 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Maher RM, Hayes DM & Shinohara M Quantification of dry needling and posture effects on myofascial trigger points using ultrasound shear-wave elastography. Arch Phys Med Rehabil 94, 2146–2150 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Takla MKN, Razek NMA, Kattabei O & El-Lythy MAF A comparison between different modes of real-time sonoelastography in visualizing myofascial trigger points in low back muscles. J Man Manip Ther 24, 253–263 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calvo-Lobo C et al. Tensiomyography, sonoelastography, and mechanosensitivity differences between active, latent, and control low back myofascial trigger points: A cross-sectional study. Medicine (Baltimore) 96, e6287 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumbhare D, Shaw S, Grosman-Rimon L & Noseworthy MD Quantitative Ultrasound Assessment of Myofascial Pain Syndrome Affecting the Trapezius: A Reliability Study. J Ultrasound Med 36, 2559–2568 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Müller CEE, Aranha MFM & Gavião MBD Two-dimensional ultrasound and ultrasound elastography imaging of trigger points in women with myofascial pain syndrome treated by acupuncture and electroacupuncture: a double-blinded randomized controlled pilot study. Ultrason Imaging 37, 152–167 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Taheri N et al. Ultrasonography in Diagnosis of Myofascial Pain Syndrome and Reliability of Novel Ultrasonic Indexes of Upper Trapezius Muscle. Ortop Traumatol Rehabil 18, 149–154 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Cojocaru MC et al. Trigger points--ultrasound and thermal findings. J Med Life 8, 315–318 (2015). [PMC free article] [PubMed] [Google Scholar]
- 46.Girasol CE, Dibai-Filho AV, de Oliveira AK & de Jesus Guirro RR Correlation Between Skin Temperature Over Myofascial Trigger Points in the Upper Trapezius Muscle and Range of Motion, Electromyographic Activity, and Pain in Chronic Neck Pain Patients. J. Manip. Physiol. Ther 41, 350–357 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Ge H-Y, Yue S-W, Kimura Y & Arendt-Nielsen L Attenuated skin blood flow response to nociceptive stimulation of latent myofascial trigger points. Arch Phys Med Rehabil 90, 325–332 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Gabrhel J, Popracová Z, Tauchmannová H & Chvojka Z Thermal findings in pain syndromes of the pelvic-femoral region. Thermology International 23, 157–163 (2013). [Google Scholar]
- 49.Haddad DS, Brioschi ML & Arita ES Thermographic and clinical correlation of myofascial trigger points in the masticatory muscles. Dentomaxillofac Radiol 41, 621–629 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magalhães MF et al. Evolution of Skin Temperature after the Application of Compressive Forces on Tendon, Muscle and Myofascial Trigger Point. PLoS One 10, e0129034 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luiza da Silva Queiroz M et al. MRI in migraineurs: are there abnormalities in the area where the myofascial trigger points are palpable and in volume measurements? J Bodyw Mov Ther 24, 260–266 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Chen Q, Basford J & An K-N Ability of magnetic resonance elastography to assess taut bands. Clin Biomech (Bristol, Avon) 23, 623–629 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Stefano R et al. Image analysis quantification of substance P immunoreactivity in the trapezius muscle of patients with fibromyalgia and myofascial pain syndrome. J. Rheumatol 27, 2906–2910 (2000). [PubMed] [Google Scholar]
- 54.Grosman-Rimon L et al. Circulating biomarkers in acute myofascial pain A case-control study. Medicine (United States) 95, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah JP, Phillips TM, Danoff JV & Gerber LH An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J. Appl. Physiol 99, 1977–1984 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Sluka KA et al. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106, 229–239 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Shen B et al. Visualizing Nerve Injury in a Neuropathic Pain Model with [18F]FTC-146 PET/MRI. Theranostics 7, 2794–2805 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoon D, Kogan F, Gold GE & Biswal S Identifying Musculoskeletal Pain Generators Using Clinical PET. Semin Musculoskelet Radiol 24, 441–450 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Badawi RD et al. First Human Imaging Studies with the EXPLORER Total-Body PET Scanner*. J Nucl Med 60, 299–303 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaudhari AJ et al. Total-Body PET Imaging of Musculoskeletal Disorders. PET Clin 16, 99–117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.RECOMMENDATIONS: NON-INVASIVE ASSESSMENT OF LIVER DISEASE STAGE AT BASELINE AND DURING FOLLOW UP. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection (World Health Organization, 2015). [PubMed] [Google Scholar]
- 62.Sigrist RMS, Liau J, Kaffas AE, Chammas MC & Willmann JK Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 7, 1303–1329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bartley EJ & Fillingim RB Sex differences in pain: a brief review of clinical and experimental findings. BJA: British Journal of Anaesthesia 111, 52 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


