Abstract
Objectives:
Most patients with Campylobacter infection do not require antibiotics; however, they are indicated in severe cases. Clinical breakpoints for many antibiotics are not yet established by the CLSI, making antibiotic selection for resistant infections challenging. During an outbreak of pet store puppy-associated XDR Campylobacter jejuni infections resistant to seven antibiotic classes, several patients required antibiotics. This study aimed to determine MICs of the outbreak strain for various antibiotics and describes the successful treatment of two patients using imipenem/cilastatin, a drug not traditionally used for Campylobacter infections.
Methods:
We used whole-genome multilocus sequence typing (wgMLST) to determine the genetic relatedness of Campylobacter isolates collected from two human patients’ stool samples with the outbreak strain. We performed extended antimicrobial susceptibility testing on 14 outbreak isolates and 6 control strains to determine MICs for 30 antibiotics (14 classes).
Results:
Isolates from both patients were highly related to the outbreak strain by wgMLST. MICs indicated resistance of the outbreak strain to most antibiotic classes, except phenicols, glycylcyclines and carbapenems. Due to potential side effects of phenicols and safety issues precluding use of glycylcyclines such as tigecycline when alternatives agents are available, we used carbapenems to treat patients who were severely ill from the outbreak strain infections.
Conclusion:
Stewardship and clinical vigilance are warranted when deciding whether and how to treat patients with suspected C. jejuni diarrhoea with antibiotics. Clinicians should maintain a high index of suspicion for XDR Campylobacter when patients fail to improve and consider the use of carbapenems in such settings.
Keywords: Extensively drug-resistant, XDR, Campylobacter jejuni, Zoonosis, Emerging infection
1. Introduction
Campylobacter is a leading cause of diarrhoeal illness, causing an estimated 1.5 million illnesses annually in the USA [1]. Campylobacter is a genus of Gram-negative, microaerophilic bacteria with more than 20 species, but only a few among those are pathogenic for humans. Approximately 90% of human Campylobacter infections are caused by Campylobacter jejuni, which causes infectious diarrhoea (often bloody) and rarely can lead to serious bloodstream infections, particularly in immunocompromised individuals [2].
Campylobacteriosis is a zoonotic infection that can be acquired from a variety of sources, including contact with animals such as puppies [3]. When antibiotics are indicated, macrolides and fluoroquinolones are the recommended agents [4]. However, the prevalence of antibiotic-resistant C. jejuni is increasing [5]. For example, resistance to ciprofloxacin increased from 18% in 1997 to 28% in 2017 [6].
In August 2017, the Florida Department of Health identified a C. jejuni outbreak linked to puppies from a national pet store chain based in Ohio. Subsequent investigation revealed more than 100 patients from 18 US states linked to the outbreak [7]. Antimicrobial susceptibility testing (AST) performed by officials at the National Antimicrobial Resistance Monitoring System (NARMS) at the US Centers for Disease Control and Prevention (CDC) indicated that the outbreak isolates were resistant to multiple classes of antibiotics including macrolides, fluoroquinolones, lincosamides, ketolides and tetracyclines; 10 of 12 isolates tested were also resistant to aminoglycosides [8–10].
This resistance profile raised urgent concerns about the optimal management of patients infected with this extensively drug-resistant (XDR) Campylobacter strain [11]. Multiple small studies have previously described in vitro susceptibility of C. jejuni to carbapenems [12–15]. However, clinical resistance breakpoints for many antimicrobials, including carbapenems, are yet to be established. For drugs that do have clinical breakpoints, interpretative criteria available from the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) differ [16, 17]. Also, clinical experience regarding successful use of carbapenems against XDR Campylobacter strains is limited.
During this multistate outbreak of XDR Campylobacter, several patients with severe infections required antibiotic treatment. Here we describe extended AST of the outbreak strain, which led to successful treatment of two severely ill patients associated with the outbreak using imipenem/cilastatin. A detailed account of the outbreak investigation is provided by Montgomery et al. [7].
2. Materials and methods
2.1. Antimicrobial susceptibility testing
2.1.1. Routine antimicrobial susceptibility testing
During the outbreak, state health department laboratories submitted a subset of 14 outbreak-associated isolates to NARMS for AST. Control strains of C. jejuni were selected for their known susceptibility to most antimicrobials on the NARMS panel. AST was performed for 14 outbreak-associated isolates on the standard panel used for testing Campylobacter spp. (Sensititre ™ Campylobacter CAMPY; Thermo Fisher Scientific, Cleveland, OH, USA) [18]. Nine antibiotics, including azithromycin, ciprofloxacin, clindamycin, erythromycin, florfenicol, gentamicin, nalidixic acid, telithromycin and tetracycline, were evaluated by the broth microdilution method in accordance with the manufacturer’s instructions. The minimum inhibitory concentration (MIC) for each drug was used to categorise isolates as susceptible or resistant based on epidemiologic cut-off values (ECOFFs) or NARMS consensus interpretative criteria [18]. Fourteen outbreak isolates were submitted to the US Food and Drug Administration (FDA) Center for Veterinary Medicine NARMS laboratory for additional AST.
2.1.2. Extended susceptibility testing at the FDA
Standard Campylobacter broth microdilution methods were applied to six dehydrated Sensititre ™ panels in accordance with current CLSI guidelines: three panels (CMV4AGNF, CMV3AGPF and CMV2DW; Sensititre TM) were used routinely for NARMS testing at that time; the other three are routinely used in clinical laboratories (GN4F, STP6F and ANO2B; Sensititre TM). For each Campylobacter isolate, a 0.5 McFarland standard (108 CFU/mL) bacterial suspension was prepared in 5 mL of Sensititre ™ cation-adjusted Mueller–Hinton broth (MHB). The suspension was then diluted to 105 CFU/mL by transferring 100 μL of the above suspension into 11 mL of MHB with lysed horse blood. Suspensions were inoculated into each well of the dehydrated Sensititre ™ panels at a volume of 100 μL, covered with a perforated seal, and incubated in a humid microaerophilic environment for 24 h at 42 °C. Quality control (QC) strains were included for each test simultaneously under recommended standard conditions for each QC strain (C. jejuni ATCC 33560, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, Klebsiella pneumoniae ATCC 700603 and Streptococcus pneumoniae ATCC 49619) in order to validate the method by ensuring the performance of each MIC panel used in the tests. MICs were determined using the unaided eye. Five NARMS surveillance C. jejuni strains known to be susceptible to the antibiotics on the standard NARMS Campylobacter (CAMPY) panel were also included as control strains for validation. If multiple MICs resulted when the same antibiotic class was tested on more than one panel, we reported the more specific dilution range (e.g. ≤0.12 μg/mL rather than ≤0.5 μg/mL) or the higher MIC value. No result for the same antibiotic differed by more than a single dilution.
Due to the treatment needs of patients infected in this outbreak, time was of the essence in finding a suitable treatment agent. Agar dilution, the gold standard of AST, would have required time-consuming testing and the availability of antimicrobial powders. Therefore, we designed testing methods based on supplies available both to clinical and NARMS laboratories, applied CLSI guidelines, and used QC organisms, including known susceptible Campylobacter isolates, for validation.
2.2. Resistance determination
The MIC for each drug was used to categorise isolates as susceptible or resistant. For azithromycin, chloramphenicol, ciprofloxacin, clindamycin, erythromycin, florfenicol, gentamicin, nalidixic acid, telithromycin and tetracycline, we used EUCAST ECOFFs [19, 20]. We considered isolates with MICs at or below the ECOFF (‘wild-type’) to be susceptible, and isolates with MICs above the ECOFF (‘non-wild-type’) to be resistant. We used CLSI criteria for Enterobacterales for ampicillin, ampicillin/sulbactam, amoxicillin/clavulanic acid, aztreonam, cefepime, cefotaxime, ceftazidime, cefuroxime, ertapenem, imipenem, levofloxacin, meropenem, minocycline, piperacillin/tazobactam, trimethoprim/sulfamethoxazole and tobramycin, and CLSI criteria for anaerobes for metronidazole and moxifloxacin to define isolates as susceptible or resistant to these agents [21].
We defined XDR Campylobacter as isolates resistant to macrolides, fluoroquinolones (the antimicrobial classes recommended for treatment [4]) and three or more additional CLSI-designated antimicrobial classes.
2.3. Identification of cases associated with the multistate outbreak
During the outbreak investigation, the Ohio Department of Health (ODH) co-ordinated with local health departments to identify additional cases and requested specimen submission to ODH’s state laboratory. The ODH laboratory performed whole-genome sequencing on all resulting isolates, and the CDC’s laboratory used whole-genome multilocus sequence typing (wgMLST) to compare genetic relatedness. Cases were considered outbreak-associated if the corresponding isolate was found to be highly related to other outbreak isolates by wgMLST [7, 10, 11]. The National Center for Biotechnology Information (NCBI) accession numbers for the sequenced isolates are as follows: Patient # 1, SAMN08014319; Patient # 2, SAMN09008083; and puppy of Patient # 1, SAMN08025804.
2.4. Ethics review
A Human Subjects Advisor from CDC’s National Center for Emerging and Zoonotic Infectious Diseases reviewed the proposal for this investigation and determined that it did not meet the definition of research under 45 CFR 46.102(d). The Institutional Review Board of Bon Secours Mercy Health made a similar determination and issued a waiver of informed consent.
3. Results
3.1. Clinical association
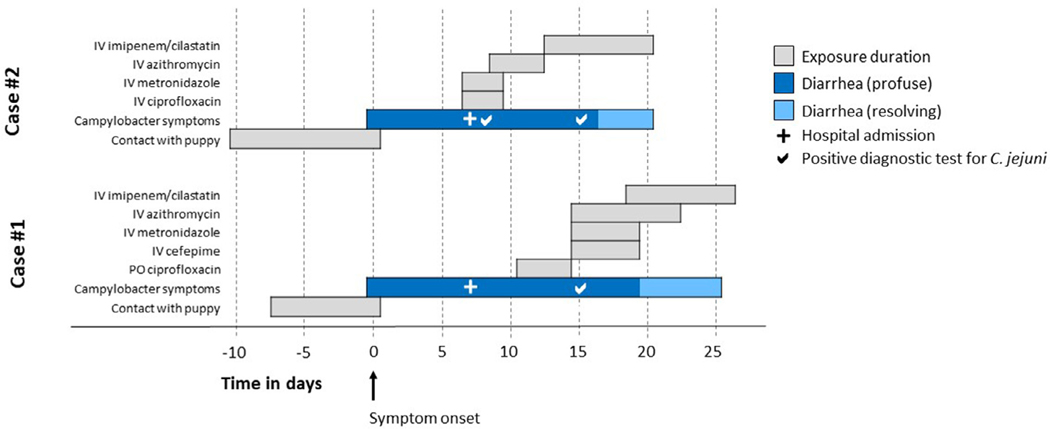
We demonstrate the clinical use of this information in the successful treatment of two patients who were severely ill from infection with the outbreak-related XDR Campylobacter strain and required aggressive antimicrobial treatment. The clinical course of these patients, including key exposures, symptom onset and antibiotic courses, are summarised in Fig. 1.
Fig 1.
Clinical course of two patients showing the duration of diarrhoea and key exposures in days. PO, oral; IV, intravenous.
3.1.1. Patient #1
Patient #1 was a 67-year-old man with a history of coronary artery disease, type 2 diabetes mellitus, essential hypertension, obstructive sleep apnoea and previous bariatric surgery (gastric sleeve), who developed worsening watery diarrhoea 7 days after purchasing a puppy from a pet store. The patient presented to the emergency department after 4 days of worsening diarrhoea. He was initially treated with oral ciprofloxacin (500 mg twice daily) without improvement and was transferred to the intensive care unit when he developed diabetic ketoacidosis. Antibiotics were switched to intravenous (IV) cefepime (20 0 0 mg every 12 h), IV azithromycin (500 mg daily) and IV metronidazole (500 mg every 8 h) without much improvement in his condition. Stool PCR test was positive for Campylobacter spp., and stool culture yielded C. jejuni. The patient’s family alerted the infectious diseases (ID) physician to a news story based on CDC’s web posting about the antibiotic-resistant Campylobacter outbreak linked to pet store puppies; the ID physician contacted the ODH and CDC. Given high suspicion that this case was linked to the outbreak, a 7-day course of IV imipenem/cilastatin (1000 mg every 8 h) was initiated. The patient’s diarrhoea improved after 48 h and resolved 7 days after starting imipenem/cilastatin (Fig. 1). Before discharge, the patient was advised about hygiene measures to avoid re-infection. His puppy’s stool was also cultured and tested positive for C. jejuni. Isolates from the patient and puppy were later tested at the CDC and FDA. The patient was seen in the outpatient clinic 1 week after discharge and reported no recurrence of diarrhoea. He was followed up until 1 year after hospitalisation and remained asymptomatic.
3.1.2. Patient #2
Patient #2 was a 60-year-old woman with a history of pancreatic cancer, pancreaticoduodenectomy and cholecystectomy, who developed severe non-bloody diarrhoea and low back pain 10 days after purchasing a puppy from a local pet store. The patient was hospitalised with dizziness, high fever and worsening diarrhoea. She was treated with IV ciprofloxacin (400 mg every 12 h) and IV metronidazole (500 mg every 8 h); IV azithromycin (500 mg daily) was added after 2 days of non-improvement. Stool culture yielded C. jejuni. As she had failed to improve on standard antibiotic therapy, concerns were raised for antibiotic resistance; hence, a 7-day course of IV imipenem/cilastatin (10 0 0 mg every 8 h) was initiated. Her diarrhoea improved within 48 h and resolved completely within 7 days of starting imipenem/cilastatin (Fig. 1). She was discharged after a 2-week hospital stay and was given similar instructions as Patient #1. Her puppy was reported to have diarrhoea soon after purchase but no testing was done for Campylobacter. The patient refused further testing for her puppy and was lost to follow-up after discharge. Her stool isolate was later tested at the CDC and FDA.
3.2. Outbreak association and antimicrobial susceptibility testing
Isolates from both patients and from the puppy of Patient #1 were found to be highly related to each other and the outbreak strain by wgMLST [11]. All three isolates (from two patients and one puppy) underwent AST with 30 different antibiotics from 14 antibiotic classes and had susceptibility patterns that were similar to each other and consistent with the outbreak strain (Table 1).
Table 1.
Minimum inhibitory concentrations (MICs) and interpretations for Campylobacter jejuni isolates from Patient #1, Patient #2 and the puppy belonging to Patient #1
| Antibiotic class | Antibiotic | MIC (Mμ/mL) Patient #1 |
Patient #2 | Puppy | Intermediate range (μg/mL) | Resistant range (μg/mL) |
|---|---|---|---|---|---|---|
|
|
||||||
| Aminoglycosides | Gentamicina | >32 | >32 | >32 | N/A | ≥4 |
| Tobramycin b, c | >4 | >4 | > 4 | 8 | ≥16 | |
| β- | Amoxicillin/clavulanic acid b | 4/2 | 4/2 | 4/2 | 16/8 | ≥32/16 |
| Lactam | Ampicillin/sulbactam b, c | >8/4 | >8/4 | > 8/4 | 16/8 | ≥32/16 |
| combinations | Piperacillin/tazobactamb | 64/2 | 64/2 | 64/2 | 32/4–64/4 | ≥ 128/4 |
| Carbapenems | Ertapenemb | 0.25 | <0.12 | <0.12 | 1 | ≥ 2 |
| Imipenemb | 0.12 | 0.12 | 0.12 | 2 | ≥4 | |
| Meropenemb | 0.06 | 0.06 | 0.06 | 2 | ≥4 | |
| Cephems | Cefuroximeb, c, d | >4 | >4 | > 4 | 8–16 | ≥32 |
| Cefotaximeb | 4 | 4 | 4 | 2 | ≥4 | |
| Ceftazidimeb | 16 | 8 | 8 | 8 | ≥16 | |
| Ceftriaxoneb | 16 | 16 | 16 | 2 | ≥4 | |
| Cefepimeb | 1 | 1 | 1 | 4–8 | ≥16 | |
| Folate pathway antagonists | Trimethoprim/sulfamethoxazoleb | >4/76 | >4/76 | >4/76 | N/A | ≥4/76 |
| Glycylcyclines | Tigecycline | ≤0.008 | ≤0.008 | ≤0.008 | N/A | N/A |
| Ketolides | Telithromycin a | >8 | >8 | > 8 | N/A | ≥8 |
| Lincosamides | Clindamycin a | 4 | 8 | 8 | N/A | ≥1 |
| Macrolides | Azithromycin a | >64 | >64 | > 64 | N/A | ≥0.5 |
| Erythromycin a | >64 | >64 | > 64 | N/A | ≥8 | |
| Tylosin | >16 | >16 | > 16 | N/A | N/A | |
| Monobactams | Aztreonamb | >16 | >16 | > 16 | 8 | ≥16 |
| Nitroimidazoles | Metronidazole e | >16 | >16 | > 16 | 16 | ≥32 |
| Phenicols | Chloramphenicol a | ≤2 | ≤2 | ≤2 | N/A | ≥32 |
| Florfenicol a | 1 | 1 | 1 | N/A | ≥8 | |
| Quinolones | Ciprofloxacin a | 16 | 16 | 16 | N/A | ≥1 |
| Levofloxacinb | >4 | >4 | > 4 | 1 | ≥2 | |
| Moxifloxacin e | 2 | 2 | 2 | 4 | ≥8 | |
| Nalidixic acid a | >64 | >64 | > 64 | N/A | ≥32 | |
| Tetracyclines | Minocyclineb | 2 | 2 | 4 | 8 | ≥16 |
| Tetracycline a | >64 | >64 | > 64 | N/A | ≥2 | |
N/A, not applicable; CLSI; Clinical and Laboratory Standards Institute.
NOTE: MICs at or above the intermediate or resistant MIC ranges are shown in boldface.
Resistant range for these agents was defined by MICs above the epidemiological cut-off values (ECOFFs) established by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [19]. EUCAST uses the terms ‘wild-type’ and ‘non-wild-type’ instead of susceptible and resistant; ECOFFs should not be used to predict clinical efficacy.
Intermediate and resistant ranges for these agents were defined based on CLSI breakpoints for Enterobacterales [21] and should not be used to predict clinical efficacy.
It is not possible to distinguish whether the isolate MICs fall into the intermediate or resistant range based on the dilutions tested.
Intermediate and resistant ranges differ based on the route of transmission; these values are for oral administration.
Intermediate and resistant ranges for these agents were defined based on CLSI breakpoints for anaerobes [21] from the CLSI M100Ed30 and should not be used to predict clinical efficacy.
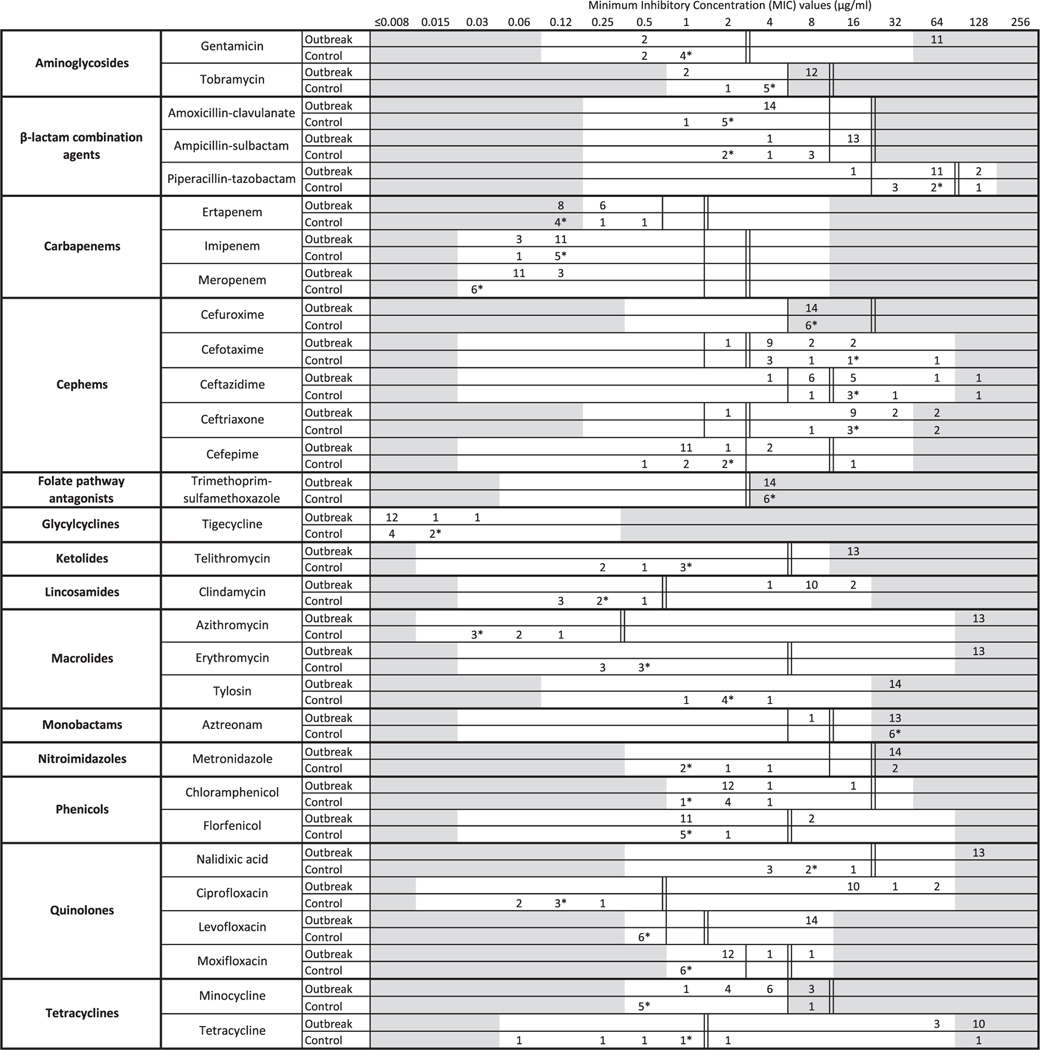
Outbreak isolates had MICs indicating resistance to most antibiotic classes, except phenicols, glycylcyclines and carbapenems (Fig. 2). Also, average MICs of control strains and the CLSI standard QC strain C. jejuni ATCC 33560 were lower than the MICs of outbreak isolates for aminoglycosides, ketolides, lincosamides, macrolides, quinolones, tetracyclines, β-lactam combination agents, cephems (except cefepime), monobactams and nitroimidazoles.
Fig. 2.
Squashtogram showing minimum inhibitory concentrations (MICs) for 14 antibiotic classes in two populations of Campylobacter jejuni isolates: outbreak isolates (n = 14a) and control isolates (n = 6). * Indicates the MIC of the commercially available strain C. jejuni ATCC 33560. a One isolate was not tested for susceptibility to azithromycin, ciprofloxacin, clindamycin, erythromycin, florfenicol, gentamicin, nalidixic acid, telithromycin and tetracycline. NOTE: Interpretation of the squashtogram: a squashtogram is a visual aid for the interpretation of MIC values. This squashtogram shows the distribution of MICs for antimicrobial agents tested and allows an immediate comparative summary of resistance for specific categories of isolates. Results for 14 outbreak-associated isolates, 5 control isolates and the commercially available strain ATCC 33560 are shown here. The number of isolates falling into each MIC category is shown in a horizontal bar chart. For most antimicrobial agents tested, three categories (susceptible, intermediate and resistant) are used to interpret MICs. For each antibiotic, ∥(double lines) are used to mark the breakpoint for resistance interpretation and | (single line) is used to mark the breakpoint for intermediate interpretation (where applicable). MICs for tigecycline and tylosin are reported without breakpoints. Dilutions that were not tested are shaded in grey; when isolates were resistant to the highest dilution tested, results were reported in the next highest dilution (shaded grey).
4. Discussion
For most patients, C. jejuni infection is self-limiting and antibiotic treatment typically offers only a modest benefit [22]. Antibiotic treatment is generally reserved for patients with severe or prolonged disease or for those with risk factors for complicated infection [23]. The two patients described here had severe C. jejuni infections in the setting of their relatively older ages and preexisting co-morbid conditions. We faced several challenges in the successful treatment of these two patients. The first was realising that their illnesses were associated with a large pet store puppy-related outbreak of a rare XDR Campylobacter strain. The CDC’s public outbreak notification was critical in alerting the first patient’s family, who in turn notified infectious disease physician, resulting in a discussion with the CDC response team. The second challenge was that this XDR Campylobacter strain showed resistance to all first- and second-line agents. AST for alternative agents was not available at the hospital’s reference clinical laboratories owing to lack of breakpoints. Federal health officials, however, were already conducting additional AST on the outbreak isolates to determine the most appropriate antibiotic therapy. Third, there have been only a few reports of the successful use of carbapenems to treat Campylobacter, making treatment decisions challenging.
Treatment options for C. jejuni infections are limited at baseline by resistance mechanisms present in most isolates. Campylobacter jejuni isolates show intrinsic resistance to glycopeptides (vancomycin) and polymyxins (colistin), and nearly all isolates carry resistance mechanisms for folate pathway inhibitors (trimethoprim) and express a β-lactamase that limits susceptibility to penicillins (amoxicillin, ampicillin, ticarcillin) [24, 25]. In addition, C. jejuni isolates encode efflux pumps with differential expression that can work synergistically with other resistance mechanisms to limit the effectiveness of antimicrobials based on molecule size and charge, potentially resulting in high-level resistance to multiple antimicrobial classes [25, 26]. Resistance to recommended treatment drugs is common; during 2004–2015, the years leading up to the outbreak, 23.5% of isolates were resistant to fluoroquinolones, macrolides or both based on NARMS surveillance data [27]. We used the term XDR rather than multidrug-resistant for this particular outbreak strain because isolates from this strain had such an extensive resistance that they could not be treated with fluoroquinolones, macrolides and at least three other antimicrobial classes. Using our definition of XDR, only 1.0% of surveillance isolates met the XDR criteria during the same time period and only 0.3% of surveillance isolates shared the same resistance profile to the antimicrobials on the CAMPY panel as the isolates from the patients described in this report. In the future, as clinical breakpoints for additional antimicrobial classes are adopted by the CLSI, a formal definition for XDR C. jejuni following the guidelines developed for other pathogens may become feasible [28].
Based on the extended AST performed on outbreak isolates, this XDR C. jejuni strain showed apparent invitro resistance to antimicrobials from 11 of 14 classes tested. It has been suggested that multidrug-resistant strains could be treated with glycylcyclines (tigecycline) or gentamicin in conjunction with carbapenem antibiotics [29]; however, this XDR strain appears resistant to gentamicin, and the FDA recommends reserving tigecycline for use in situations when alternative treatments are not suitable [30]. Glycylcyclines (tigecycline) and phenicols (chloramphenicol), classes to which the patients’ isolates appeared susceptible, are only available intravenously in the USA and are typically reserved for situations when safer drugs are ineffective [31, 32]. Although AST indicated potential in vitro susceptibility of the outbreak strain to cefepime, MICs of other third- and fourth-generation cephalosporins were relatively high. Also, Patient #1 clinically failed to improve with IV cefepime; therefore, cefepime was not used to treat Patient #2. Carbapenems, including imipenem/cilastatin, have a better safety profile than other alternatives and are widely available. Carbapenem treatment has also been suggested based on agar dilution studies of XDR Campylobacter [33]. Hence, imipenem/cilastatin was used to treat the two severely ill patients described in this report; both had successful outcomes. These findings echo previous case reports of successful use of carbapenems under similar circumstances [34–37].
5. Conclusion
These cases illustrate that a careful history of pet exposure can help establish a diagnosis and guide treatment. Application of antimicrobial stewardship principles and careful clinical vigilance are warranted when deciding whether or not to empirically treat patients with antibiotics. Patients who are severely ill or immuno-compromised and are diagnosed with Campylobacter infection by PCR-based assays should have stool cultures and AST done whenever feasible to determine the best treatment course. Clinicians should maintain a high index of suspicion for XDR Campylobacter when patients fail to improve as expected and should consider the use of carbapenems in such settings.
Acknowledgments
The authors gratefully acknowledge the contributions of multiple individuals, including: Christopher Juergens, Kim Jurell, Sean Kirby, Prasad Kudalkar, Paras Patel, Jeffrey Stotz and Saiyid-Naufal Zaidi from Mercy Health for their roles in diagnosis and treatment of the patients; Sherif Zaki from the US Centers for Disease Control and Prevention (CDC) for pathology evaluation of clinical specimens; Rachael D. Aubert, Colin Basler, Christy T. Bennett, Jessica C. Chen, Samuel J. Crowe, Staci Dixon, Natasha Dowell, Jason P. Folster, Aimee Geissler, Michael Jhung, Lavin A. Joseph, Lia Koski, Mark Laughlin, Megin Nichols, Scott Robertson, Rachel Silver, Lauren M. Stevenson, Preethi Sundararaman, Laura Whitlock and Ian Williams from the CDC and the Ohio Department of Health, Ohio Department of Agriculture, City of Hamilton Health Department, Butler County General Health District and Hamilton County Public Health for their contributions to the outbreak investigation and response; and Sherry Ayers and Shenia Young from the US Food and Drug Administration (FDA) for their help with the extended antimicrobial susceptibility testing of isolates related to the outbreak.
Funding
None.
Footnotes
Ethical approval
Not required.
Declaration of Competing Interest
None declared.
References
- [1].Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 2011;17:7–15. doi: 10.3201/eid1701.p11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].US Centers for Disease Control and Prevention (CDC) Campylobacter (campy-lobacteriosis): information for health professionals, Atlanta, GA: CDC; 2020. https://www.cdc.gov/campylobacter/technical.html [accessed 25 October]. [Google Scholar]
- [3].Friedman CR, Hoekstra RM, Samuel M, Marcus R, Bender J, Shiferaw B, et al. Emerging Infections Program FoodNet Working Group. Risk factors for sporadic Campylobacter infection in the United States: a case–control study in FoodNet sites. Clin Infect Dis 2004;38(Suppl 3):S285–96. doi: 10.1086/381598. [DOI] [PubMed] [Google Scholar]
- [4].Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis 2017;65:e45–80. doi: 10.1093/cid/cix669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marotta F, Garofolo G, di Marcantonio L, Di Serafino G, Neri D, Romantini R, et al. Antimicrobial resistance genotypes and phenotypes of Campylobacter jejuni isolated in Italy from humans, birds from wild and urban habitats, and poultry. PLoS One 2019;14:e0223804. doi: 10.1371/journal.pone.0223804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].US Centers for Disease Control and Prevention (CDC) Antibiotic resistance threats in the United States, 2019, Atlanta, GA: US Department of Health and Human Services, CDC; 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf [accessed 25 October 2020]. [Google Scholar]
- [7].Montgomery MP, Robertson S, Koski L, Salehi E, Stevenson LM, Silver R, et al. Multidrug-resistant Campylobacter jejuni outbreak linked to puppy exposure—United States, 2016–2018. MMWR Morb Mortal Wkly Rep 2018;67:1032–5. doi: 10.15585/mmwr.mm6737a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].US Centers for Disease Control and Prevention (CDC) Campylobacter (campylobacteriosis), Atlanta, GA: CDC; 2020. https://www.cdc.gov/campylobacter/index.html [accessed 25 October]. [Google Scholar]
- [9].Payot S, Bolla JM, Corcoran D, Fanning S, Mégraud F, Zhang Q. Mechanisms of fluoroquinolone and macrolide resistance in Campylobacter spp. Microbes Infect 2006;8:1967–71. doi: 10.1016/j.micinf.2005.12.032. [DOI] [PubMed] [Google Scholar]
- [10].US Centers for Disease Control and Prevention (CDC) Multistate outbreak of multidrug-resistant Campylobacter infections linked to contact with pet store puppies, Atlanta, GA: CDC; 2017. 3 October- https://www.cdc.gov/campylobacter/outbreaks/puppies-9-17/index.html [accessed 25 October 2020]. [Google Scholar]
- [11].Joseph LA, Francois Watkins LK, Chen J, Tagg KA, Bennett C, Caidi H, et al. Comparison of molecular subtyping and antimicrobial resistance detection methods used in a large multistate outbreak of extensively drug-resistant Campylobacter jejuni infections linked to pet store puppies. J Clin Microbiol 2020;58e00771–20. doi: 10.1128/jcm.00771-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Post A, Martiny D, van Waterschoot N, Hallin M, Maniewski U, Bottieau E, et al. Antibiotic susceptibility profiles among Campylobacter isolates obtained from international travelers between 2007 and 2014. Eur J Clin Microbiol Infect Dis 2017;36:2101–7. doi: 10.1007/s10096-017-3032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Karikari AB, Obiri-Danso K, Frimpong EH, Krogfelt KA. Antibiotic resistance of Campylobacter recovered from faeces and carcasses of healthy livestock. Biomed Res Int 2017:40918562017. doi: 10.1155/2017/4091856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McDermott PF, Bodeis SM, Aarestrup FM, Brown S, Traczewski M, Fedorka-Cray P, et al. Development of a standardized susceptibility test for Campylobacter with quality-control ranges for ciprofloxacin, doxycycline, erythromycin, gentamicin, and meropenem. Microb Drug Resist 2004;10:124–31. doi: 10.1089/1076629041310064. [DOI] [PubMed] [Google Scholar]
- [15].Zhao S, Young SR, Tong E, Abbott JW, Womack N, Friedman SL, et al. Antimicrobial resistance of Campylobacter isolates from retail meat in the United States between 2002 and 2007. Appl Environ Microbiol 2010;76:7949–56. doi: 10.1128/aem.01297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0, 2020. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf [accessed 25 October 2020]. [Google Scholar]
- [17].Clinical and Laboratory Standards Institute (CLSI). Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteriaCLSI guideline M45. 3rd ed. Wayne, PA: CLSI; 2016. https://clsi.org/media/1450/m45ed3_sample.pdf [accessed 25 October 2020]. [Google Scholar]
- [18].National Antimicrobial Resistance Monitoring System (NARMS). The National Antimicrobial Resistance Monitoring System manual of laboratory methods. 3rd ed. Washington, DC: US Food and Drug Administration; 2016. https://www.fda.gov/media/101423/download [accessed 25 October 2020]. [Google Scholar]
- [19].European Committee on Antimicrobial Susceptibility Testing (EU-CAST). Antimicrobial wild type distributions of microorganisms. https://mic.eucast.org/Eucast2/SearchController/search.jsp?action = init [accessed 25 October 2020]. [Google Scholar]
- [20].US Centers for Disease Control and Prevention (CDC). Antibiotics tested by NARMS. Atlanta, GA: CDC. https://www.cdc.gov/narms/antibiotics-tested.html [accessed 25 October 2020]. [Google Scholar]
- [21].Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testingCLSI supplement M100. 30th ed. Wayne, PA: CLSI; 2020. [Google Scholar]
- [22].Ternhag A, Asikainen T, Giesecke J, Ekdahl K. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin Infect Dis 2007;44:696–700. doi: 10.1086/509924. [DOI] [PubMed] [Google Scholar]
- [23].Wei L, Ratnayake L, Phillips G, McGuigan CC, Morant SV, Flynn RW, et al. Acid-suppression medications and bacterial gastroenteritis: a population-based cohort study. Br J Clin Pharmacol 2017;83:1298–308. doi: 10.1111/bcp.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lachance N, Gaudreau C, Lamothe F, Lariviere LA. Role of the β-lactamase of Campylobacter jejuni in resistance to β-lactam agents. Antimicrob Agents Chemother 1991;35:813–18. doi: 10.1128/aac.35.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Iovine NM. Resistance mechanisms in Campylobacter jejuni. Virulence 2013;4:230–40. doi: 10.4161/viru.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lin J, Michel LO, Zhang Q. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob Agents Chemother 2002;46:2124–31. doi: 10.1128/aac.46.7.2124-2131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].US Centers for Disease Control and Prevention (CDC) National Antimicrobial Resistance Monitoring System (NARMS) now: human data, Atlanta, GA: US Department of Health and Human Services, CDC; 2019. https://wwwn.cdc.gov/narmsnow/ [accessed 25 October 2020]. [Google Scholar]
- [28].Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- [29].Lehtopolku M, Nakari UM, Kotilainen P, Huovinen P, Siitonen A, Hakanen AJ. Antimicrobial susceptibilities of multidrug-resistant Campylobacter jejuni and C. coli strains: in vitro activities of 20 antimicrobial agents. Antimicrob Agents Chemother 2010;54:1232–6. doi: 10.1128/aac.00898-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].US Food and Drug Administration (FDA) FDA drug safety communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new Boxed Warning. FDA; 2013. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-increased-risk-death-iv-antibacterial-tygacil-tigecycline [accessed 25 October 2020]. [Google Scholar]
- [31].Cai Y, Wang R. Tigecycline: benefits and risks. Lancet Infect Dis 2011;11:804–5. doi: 10.1016/S1473-3099(11)70183-9. [DOI] [PubMed] [Google Scholar]
- [32].Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011;11:834–44. doi: 10.1016/S1473-3099(11)70177-3. [DOI] [PubMed] [Google Scholar]
- [33].Shin E, Hong H, Oh Y, Lee Y. First report and molecular characterization of a Campylobacter jejuni isolate with extensive drug resistance from a travel-associated human case. Antimicrob agents Chemother 2015;59:6670–2. doi: 10.1128/aac.01395-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pereira L, Sampaio S, Tavares I, Bustorff M, Pestana M. Bacteremia due to Campylobacter in renal transplantation: a case report and review of literature. Transpl Infect Dis 2014;16:1007–11. doi: 10.1111/tid.12302. [DOI] [PubMed] [Google Scholar]
- [35].Burch KL, Saeed K, Sails AD, Wright PA. Successful treatment by meropenem of Campylobacter jejuni meningitis in a chronic alcoholic following neurosurgery. J Infect 1999;39:241–3. doi: 10.1016/S0163-4453(99)90059-2. [DOI] [PubMed] [Google Scholar]
- [36].Kerstens PJ, Endtz HP, Meis JF, Oyen WJ, Koopman RJ, van den Broek PJ, et al. Erysipelas-like skin lesions associated with Campylobacter jejuni septicemia in patients with hypogammaglobulinemia. Eur J Clin Microbiol Infect Dis 1992;11:842–7. doi: 10.1007/bf01960888. [DOI] [PubMed] [Google Scholar]
- [37].Van den Eden EJ, Cabuy A, Daelemans R, Verhaegen J, Lins RL. Campylobacter jejuni peritonitis during chronic ambulatory peritoneal dialysis (CAPD). Perit Dial Int 1990;10:177–8. [PubMed] [Google Scholar]