Abstract
Substitution of the thymidine moiety in DNA by C5-subsituted halogenated thymidine analogs causes significant augmentation of radiation damage in living cells. However, the molecular pathway involved in such radiosensitization process has not been clearly elucidated to date in solution at room temperature. So far, low-energy electron (LEEs; 0–20 eV) under vacuum condition and solvated electrons (esol−) in solution are shown to produce the σ-type C5-centered pyrimidine base radical through dissociative electron attachment involving carbon-halogen bond breakage. Formation of this σ-type radical and its subsequent reactions are proposed to cause cellular radiosensitization. Here, we report time-resolved measurements at room temperature showing that a radiation-produced quasi-free electron (eqf−) in solution promptly breaks the C5-halogen bond in halopyrimidines forming the σ-type C5 radical via an excited transient anion radical. These results demonstrate the importance of ultrafast reactions of eqf− which are extremely important in Chemistry, Physics, and Biology, including tumor radiochemotherapy.
Keywords: Radiosensitization, Pulse radiolysis, Halopyrimidines, Quasi-free electron (eqf−), Dissociative electron attachment
Graphical Abstract
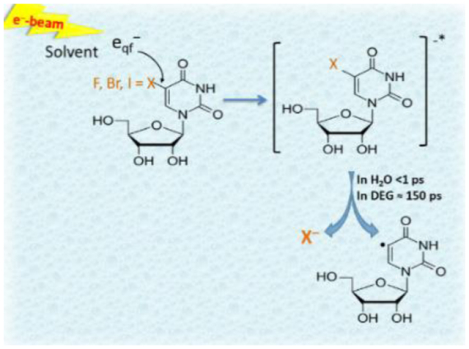
Introduction:
Minimizing the damage within the non-target volume of the surrounding normal tissues as well as maximizing the damage at and within the tumor are major challenges to tumor radiotherapy. Incorporation of halopyrimidine derivatives in which the halogen atom is at C5 of the pyrimidine base, e.g., 5-bromodeoxyuridine (Br5Urd), into the DNA of proliferating cells, lead to a significant increase in the ionizing radiation-induced strand breaks and cell death along with chromosomal aberration and micronuclei formation.1−23 As a result, these compounds could be used to treat rapidly growing tumors surrounded with normal tissues with limited proliferation. For example, radiotherapy with intravenous injection of these 5-halouridines into the poorly radioresponsive tumors, e.g., glioblastoma multiforme could have high therapeutic efficacy.1–3 Because of problems of delivery and severe normal tissue toxicity, clinical trials employing halopyrimidines were abandoned.4,5 However, basic research employing halogeneated bases including halopyrimidines is still continued to find strategies for mitigating the tissue toxicity.6,7 The principal action of sensitization of halopyrimidines have been elucidated by comparing the reaction of thymidine (m5dUrd) and radiation-produced electrons (in the gas phase, or at low temperature in solid state by low energy electron (LEE) or presolvated electron (epre−) or at room temperature by solvated electron (esol−) (vide infra)) with the corresponding reactions of substituted halopyrimidine derivatives (Scheme 1)8–9,10,11,12,13,14,15,16,17. Electron spin resonance spectroscopic (ESR) studies at low temperature homogeneous glassy or supercooled solutions have established that in halopyrimidine derivatives (e.g., 5-bromo-2′-deoxyuridine, Br5dUrd), the initial step at 77 K involves very fast epre− transfer to Br5dUrd, leading to the formation of the transient negative anions, ((Br5dUrd)•−, TNIs), which are π-anion radicals13,17–18,19. The TNI of Br5dUrd irreversibly decays to a bromide anion (Br−) along with the highly reactive σ-type uracil-5-yl radical (dUC5•) (Scheme 1). The σ-type dUC5• 8,10,16 being a vinyl-type radical, it can undergo addition to C=C double bond leading to interstrand cross-links17 as well as can lead to strand break formation via H-atom abstraction from sugar moiety.8–9,10,11,12,13,14,15,16,17,20 Note that low energy electron (LEE)-mediated radiosensitization was demonstrated in Br5dUrd-incorporated single or double-stranded DNA-oligomers of defined sequences in various studies and H-atom abstraction by dUC5• from the backbone sugar moiety and/or addition to the double bonds of the bases leading to form crosslinks have been proposed to cause the radiosensitization.8,10–11,12,13,14,15,16,17
Scheme 1.
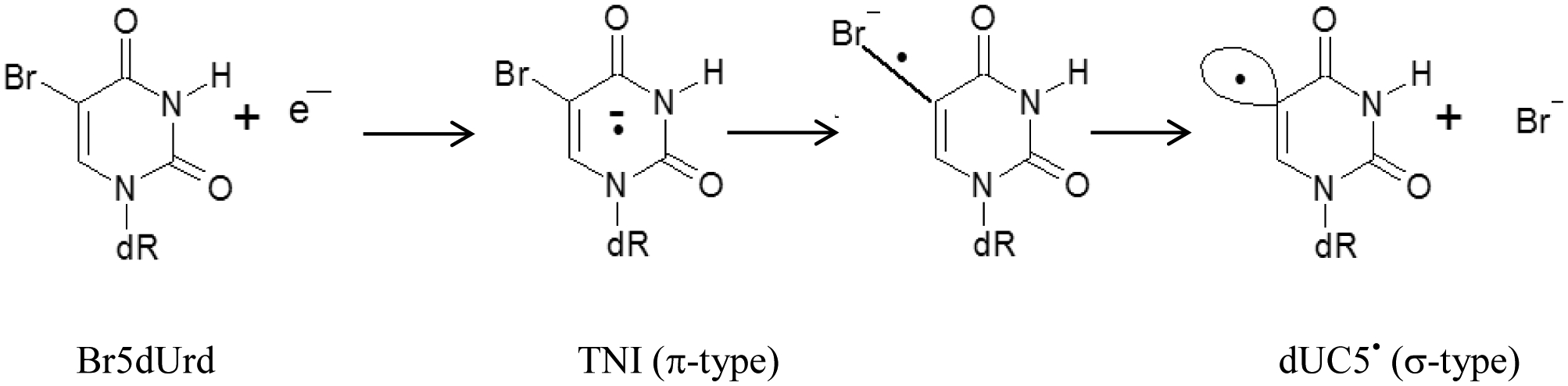
Proposed formation mechanism of the σ-type uracil-5-yl radical (UC5•) via DEA of Br5dUrd at low temperature.16,18 TNI = Transient negative ion at ground state. dR = 2′-deoxyribose.
ESR studies of the halouracil base (Br5U) and the nucleoside (Br5dUrd) in homogeneous frozen aqueous solution along with DFT calculations have shown that the base radical (uracil-5-yl radical, UC5•, formed from Br5U) or nucleoside radical (uracil-5-yl radical, dUC5•, produced from Br5dUrd) can undergo a hydration reaction yielding 5-UHOH• (reducing) and 6-UHOH• (oxidizing) upon warming at ca. 150 K.16,18 Note that these reactions have not been reported by pulse radiolysis in aqueous solutions at ambient temperature. However, contrary to the halouracil base and nucleoside,16,18 ESR studies of Br5dUrd incorporated γ-irradiated (irradiated at 77 K) hydrated DNA showed almost no conversion of TNI to dUC5• and the TNI did not undergo any hydration reaction upon progressive warming to ca.150 K.21
Indeed, the radiation-produced secondary electrons (SEs) are among the most abundant reactive species (~ 4 × 104 electrons per 1 MeV).22–23,24 Over a timescale (~10−16−10−15 s), SEs can evolve to several distinct states (LEEs (eqf−) → epre− → esol−) that are likely responsible for radiation damage. eqf− stands for low kinetic energy electron, which is not trapped by solvent, and epre− is a trapped electron without being fully solvated.24 Via elastic scattering with the surrounding solvent molecules, SEs result in the formation of ballistic low-energy electrons (LEEs or quasi-free electrons (eqf−): 0–20 eV), which rapidly thermalize (eth−) and relax to presolvated (epre−) and eventually to the solvated electron, esol−, in a potential-energy well.24–25,26
So far, studies of LEEs-induced fragmentation are limited to the gas phase under vacuum conditions and to the solid state at low temperature.8,9,10,11,12,13,14,15,16,18,21,24 Therefore, these investigations cannot fully account for the processes in solutions at room temperature, a better model for understanding the reactions of radiation-produced electrons in cells. Note that a substantial role of esol− played in the radiation-induced decomposition of halopyrimidne monomers (bases, nucleosides, nucleotides) and halopyrimidine-incorporated oligomers of defined sequences have been established from steady-state 60Co γ-radiolysis experiments under •OH-scavenging conditions.9,11,20
esol−, an almost negligible factor to cause damage of native DNA,20,24,27 was shown to increase the yields of strand breaks, especially of single-strand breaks, once labeled with halopyrimidines.9,10,20 Furthermore, measured rates of esol− induced halogen anion elimination from the π-anion radical (TNI) have been reported by various groups.20,28,29 Nanosecond pulse radiolysis with optical detection showed that the lifetimes of TNIs at 330 nm formed via the reaction of esol− with various halouracils in water follow the following order 5FU•− (15μs)> 5ClU•− (4.9μs) > 5BrU•− (7.0±0.5 ns)> 5IU•− (1.7±0.3 ns).28 On the other hand, femtosecond laser spectroscopic experiments proposed that epre−, the short-lived precursors of esol−, result in a faster C-X bond breakage via dissociative electron attachment (DEA) generating dUC5• in competition to the solvation of radiation-produced SEs, and thereby accounting for radiosensitization by halopyrimidines.8–9,10,11,12,13,14,15,16,17,20,30–31,32 However, the concentration of the solute used in femtosecond experiments was very low; hence, the possibility of reaction of the solute with esol− can be questioned. In contrast, our picosecond pulse radiolysis results clearly established that such ultrafast reduction only occurred to a significant extent at higher nucleoside concentrations (≥ 50 mM).33 Moreover, in a simple system such as the inorganic solute [Ag(CN)2]− in water, it was observed that the reaction of the electron before solvation forms an excited TNI, which dissociates rapidly before relaxation.34
Spectroscopic findings in diethylene glycol (DEG) revealed that eqf− were exclusively able to cause a dissociative electron attachment (DEA).24,35 A combination of picosecond pulse radiolysis and DFT calculations showed that the excited TNI undergoes a concentration-independent N1-C1′ glycosidic bond scission with a time constant of ca. 350 ps.35 Unfortunately, there is no literature on eqf− mediated radiosensitization is available to date. Furthermore, it is still puzzling to determine the relative contributions of the effects of states of SEs (eqf−, epre−, esol−) towards halopyrimidine-mediated radiosensitization in irradiated biological systems owing to the involvement of both long and short ranges of electron transfer processes.8,36 For instance, in a cell that mostly consists of water, radiosensitization occurs essentially via esol− through its long-range diffusive interaction.37 Furthermore, the probability of hitting the target molecule directly with a non-thermal SE in diluted media is very low owing to its nanometer-scale path length.8 However, we should recognize that the DNA in the cell nucleus is a dense and highly packed structure, and as a result, the collision of eqf− with dsDNA in its immediate vicinity becomes effective.24, 33,35,38
From the viewpoint of physicochemical events in the interaction of radiation with a molecule, the critical factors determining a probable role in radiosensitization of halopyrimidines cannot omit the extent and rates of various states of SEs (eqf−, epre− and esol−) transfer along with the release of halide ions from the corresponding TNI (Scheme 1). To elucidate this, comprehensive time-resolved studies on the reactions of eqf−, epre− and esol− with halopyrimidine nucleosides vis-à-vis the corresponding reactions of those states of SEs with unsubstituted DNA-segments are essential as benchmarks. These benchmark studies will lead to the experimental characterization of respective metastable TNIs upon the attachment of eqf−, epre−, esol− with halopyrimidines. In addition, these studies should point to which of these particular state(s) of radiation-produced SEs are decisive for effective bond rupture in TNIs. These results will further point out the role of eqf− towards selecting a chemical agent as an effective radiosensitizer.
As mentioned above, the objective of this work is to identify the fundmental mechanism of the reactions of eqf−, epre− and esol− with halopyrimidines in solution at ambient temperature by pulse radiolysis combined with pulse-probe absorption spectroscopy. Also our previous works have established that pulse radiolysis displays unique advantages over the femtosecond laser spectroscopy for such purpose, i.e., electrons generated by ~MeV electron pulse are closer to those employed in radiobiological studies than the electrons produced by multi-photon ionization.24,33–34,35,38
Experimental:
Compounds used:
All the compounds used in our experiments are obtained from Sigma Aldrich. These compounds are used without any further purification.
Gamma-irradiation:
Methodology of gamma-irradiation of samples has been mentioned in the Results and Discussion section (discussion of the results of Figure 1 and Figure S1, along with that of Table 1).
Figure 1.
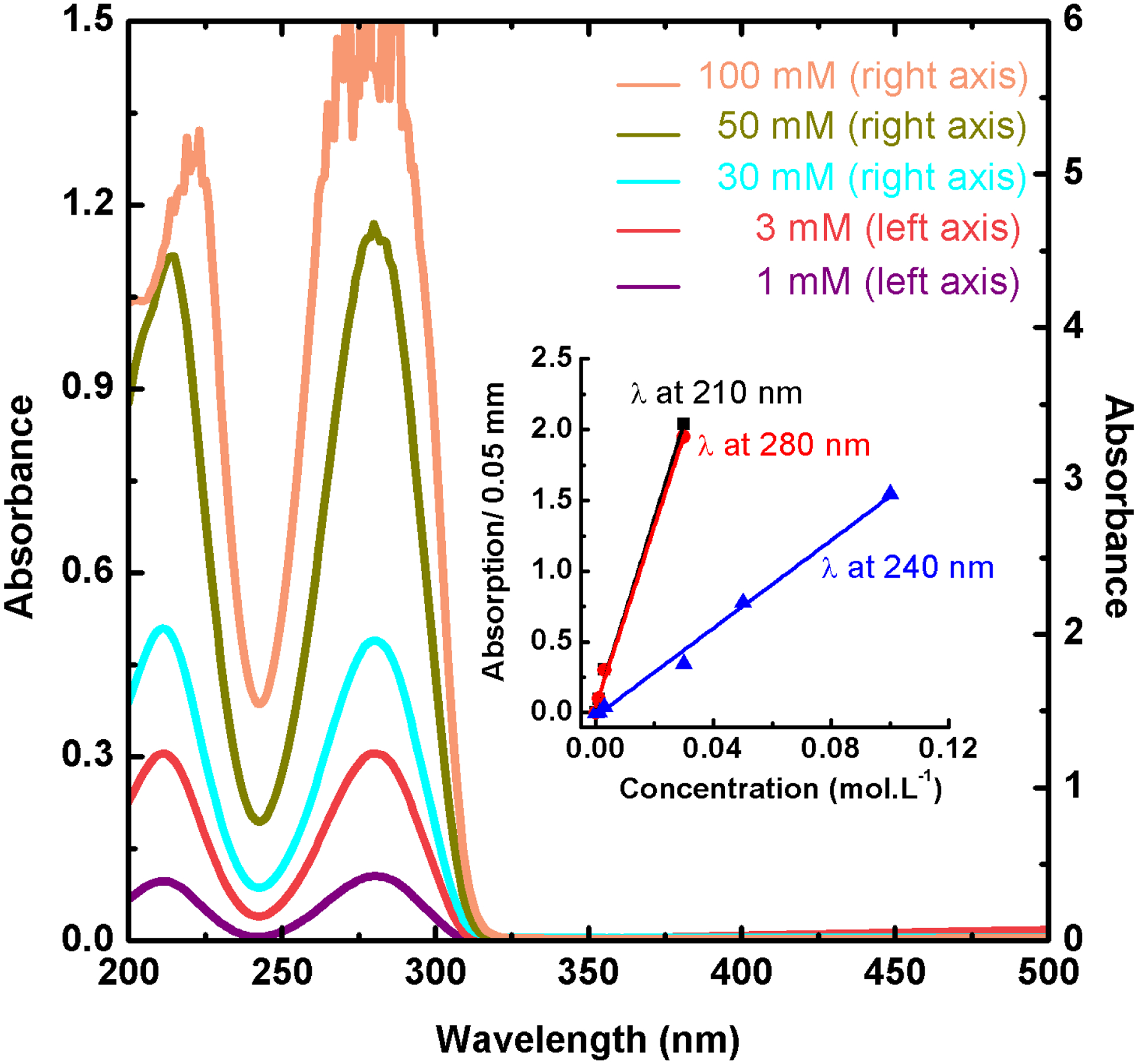
Steady state UV-Vis absorption spectra of 1, 3, 30, and 100 mM of Br5Urd in diethylene glycol (DEG) solution with optical path length of less than 100 μm. Insert: The absorption maximum at 280 and 210 nm and minimum at 240 nm of Br5Urd in DEG as a function of its concentrations. Note that the absorbance value higher than 3 are wrong due to the incorrect response of the spectrometer. The linearity of the absorption with concentrations of BrdU up to 100 mM shows that Br5Urd does not aggregate in DEG. Linear fit of abs at 210 nm: R2 = 0.9967, RMSE= 0.00297; Linear fit of abs at 280 nm: R2 = 0.9963, RMSE= 0.00311; Linear fit of abs at 240 nm: R2 = 0.9925, RMSE= 0.00281
Table 1.
Calculated radiolytic yields of Br− formed upon gamma irradiation of Br5Urd solutions in diethylene glycol (DEG) and water (see Figure S1).
| Concentration of 5-Bromouridine (Br5Urd) Under N2 | G value (×10−6 mol J−1) ± 0.04 | |
|---|---|---|
| In DEG with 0.5 M | 0.3 mM | 0.22 |
| t-Butanol | 0.3 M | 0.36 |
| In water with 2 M | 0.3 mM | 0.29 |
| t-Butanol | 0.1 M | 0.41 |
Pulse radiolysis:
The picosecond (ps) pulse radiolysis transient absorbance measurements were performed at the electron facility ELYSE (Université Paris-Saclay).24,33,35,38 The transient absorption pulse-probe setup is based on the laser-electron intrinsic synchronization resulting from the laser triggered photocathode of the accelerator. For the present study, a broadband probe detection scheme was used, the principle of which has already been described.33 A supercontinuum, generated by focusing ~1 μJ of the laser source into a 6 mm thick CaF2 disk, was used as the optical probe covering a broad spectral range (380–1500 nm). A reference signal is split off from the broadband probe before the fused silica optical flow cell. Each of the probe and reference beams were coupled into the optical fibers, transmitted to a spectrometer, and dispersed onto a CCD.33 The combination of the broadband probe and the multichannel detector allows the entire transient spectrum to be recorded in one shot; as a result, the transient spectrum is independent of the shot-to-shot fluctuations and possible long-term drifts of the electron source. For data acquisition, we cautiously maintained the same radiation dose (55.3 ± 0.5 Gy) per electron pulse that was deposited on the samples to minimize the absorbance fluctuation.33 All measurements were performed employing a fused silica flow cell with a 5 mm optical path collinear to the electron pulse propagation. The electron pulses were of ~ 4 nC, with an electron energy of 6–8 MeV, delivered at a repetition frequency of 10 Hz. The measurements were performed at 22.5°C.33
Following our previous studies, the experimental data matrices were analyzed by a multivariate curve resolution alternating least squares (MCR-ALS) approach.24,33,35,38 The number of the absorbing species in a data matrix is assessed by a Singular Value Decomposition of the matrix, and an MCR-ALS analysis with the corresponding number of species is performed. Positivity constraints are imposed for both spectra and kinetics, and the shape of the spectra can also be imposed.24,33,35,38
Results & Discussion:
Since halouracil bases (X5U), halouracil ribonucleosides (X5Urd) and halouracil-2′-deoxynucleosides (X5dUrd) produced the same radical site on the base, uracil-5-yl radical, UC5• (Scheme 1),20,28 we have employed X5Urd in our studies (Figure 2, vide infra). Also, employment of nucleosides ensures high solubility of the compounds that is needed for our pulse radiolysis experiments to study the reactions of eqf− and of epre− with halouracil base moieties.
Figure 2.
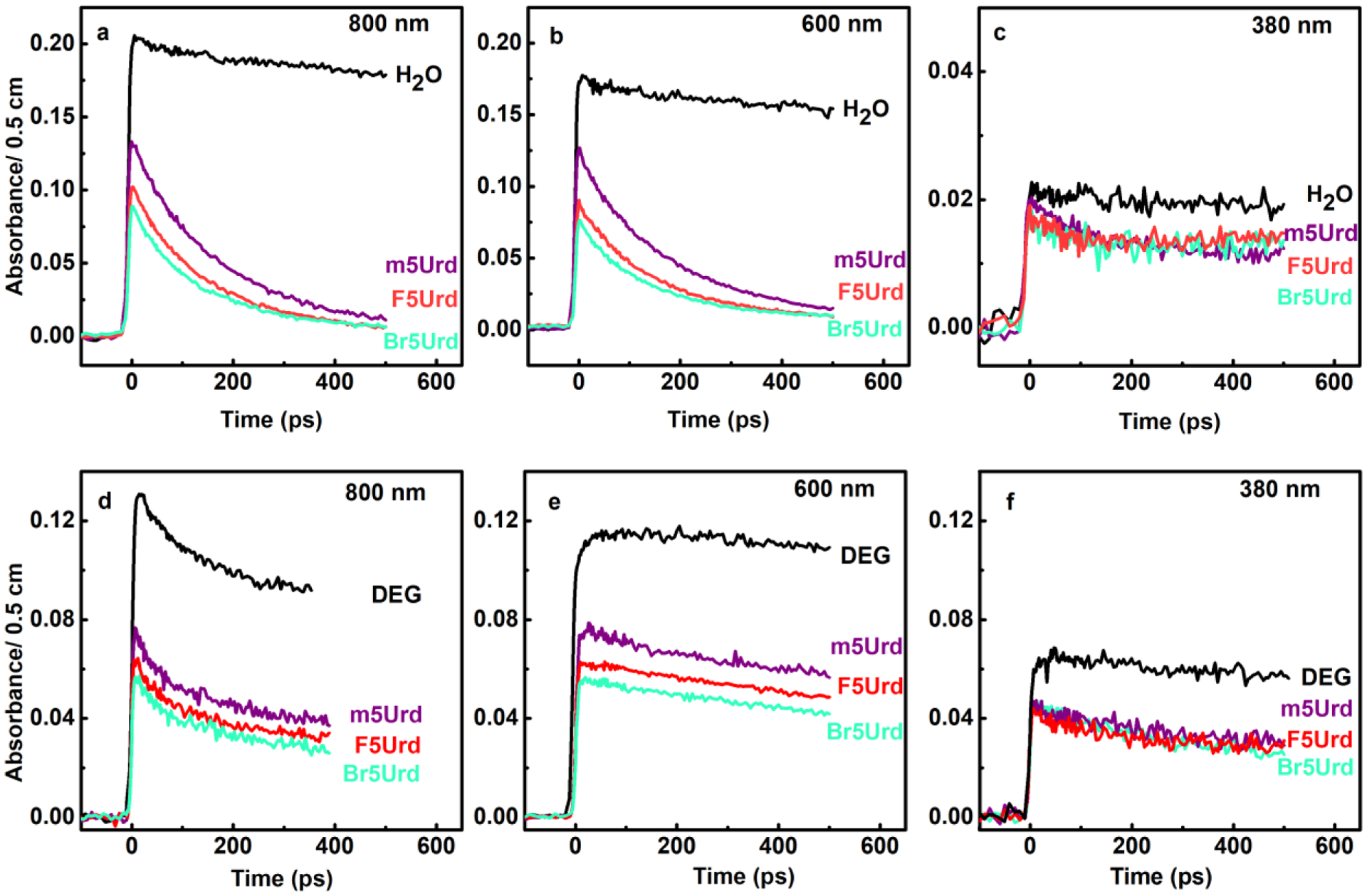
Initial absorption and the decay observed in neat water (top) and in neat DEG (bottom) in the presence of 200 mM m5Urd, Br5Urd and F5Urd at different wavelengths.
The formation of dimer and multimers are possible at high concentration of Br5Urd. To be sure that there is no such aggregation of Br5Urd in concentrated solution, we presented at first the absorption spectra of Br5Urd at a different concentration by using an optical path lower than 100 μm (Figure 1). We did not observe any change in the absorption band. Therefore, for the present work, we have the same single and unaggregated Br5Urd molecule at different concentrations (Figure 1).
We performed subsequent steady-state gamma irradiation studies at two different concentrations in DEG and water solutions by measuring the amount of formed Br− in solutions of Br5Urd using the potentiometric titration method previously reported.39 Determination of the amount of Br− in solutions of Br5Urd in DEG and in water upon gamma irradiation leads to an approximate first estimate of dissociative electron attachment contribution.
Table 1 exhibits the radiolytic yields of Br− in solutions of Br5Urd at different concentrations in DEG and water obtained at irradiation doses ranging from 50 Gy to 2000 Gy (see Figure S1). The experiments were carried out in DEG under N2 in the presence of 0.5 M tertiary-Butanol (t-butanol). The experiments of Br5Urd in water solutions were carried out under N2 in the presence of 2 M t-butanol or 1 mM concentrations of Br5Urd. We found that the yield of Br− formation is equivalent to that of the hydrated or solvated electrons (eaq− or esol−) and that is 2.7 ×10−7 mol J−1.20 This result is in agreement with HPLC measurement previously reported by Ye et al.40 At higher Br5Urd concentration, the yield of Br− was measured as around 4 ×10−7 mol J−1; this result shows that all electrons (esol−or its precursors, eqf− and epre−) contribute only to the formation of Br− in an aqueous solution.
We performed similar measurements in DEG, and we found similar results. We note that the yield of the electron in this solvent having lower polarity is lower than in water.35,41 In agreement, our results showed that in DEG, the scavenging of the electron (solvated or not solvated) resulted in the formation of Br− with slightly lower yield. From our pulse radiolysis measurements, it is evident that at high Br5Urd concentration, the radiation-produced SE is scavenged prior to its solvation, (see below and Figure S2). This means electron scavenging only induces Br− formation with no efficient attack on other bonds. Mass spectrometry analysis was performed in an earlier study on radiolysis of Br5dUrd in water solution.40 The experiment did not show any unaltered base release from the gamma radiolysis of Br5dUrd and confirmed the formation of Br−. After being sure about the Br− formation as a single product via reaction of precursors of the solvated electron with Br5dUrd in water and DEG, we have employed pulse radiolysis to explain the mechanism of dissociative electron detachment (Scheme 1).
To carry out pulse radiolysis of halopyrimidines, we selected target molecules of bromo- and fluorouridine (Br5Urd and F5Urd and the electron affinity of the base - BrU » FU)14,42,43 along with ribothymidine or 5-methyluridine (m5Urd) (Scheme 2) due to their high solubility (> 0.3 mol L−1). This high solubility enables us to model the cellular milieu (where the local concentration is high) better and to perform sets of measurements of both short and long-range addition of radiation-produced SE to the compounds. Following previous studies including ours,29,33,38 pulse radiolysis were carried out in solutions of m5Urd, F5Urd and Br5Urd in water and in diethylene glycol (DEG) at different concentrations under ambient temperature.
Scheme 2.
Structure of X5Urd derivatives that are used in this work. X = Br, 5-Bromouridine (Br5Urd); X = F, 5-Flurouridine (F5Urd), and X = CH3, 5-methyluridine (ribothymidine, m5Urd).
With our time resolution (5 ps), eqf− and epre− reactivity in aqueous solution are indistinguishable because the hydration of electron is complete within 1 ps, beyond instrument resolution.8,24–25,26,27,29,33,35 Owning to a much slower e− solvation in DEG, the lifetime of epre− absorbing around 800 nm is long enough for spectral identifications, and becomes possible to discriminate the activity between eqf− and epre−.29,35 For example, the pulse–probe data obtained for 0.3 M DEG solutions of m5Urd, F5Urd and Br5Urd are reported in Figure S3. As a result, direct characterizations of distinct states of TNI become achievable.
The extents of precursors of esol− (eqf− and epre−) transfer to F5Urd and Br5Urd were studied by observing the changes at the beginning of the infrared region, i.e., the 800 nm absorption (Figure 2a and Figure 2e). In aqueous solution, initial absorbance correlates directly with the radiolytic yield of esol− at the end of the pulse (5 ps). As a result, the substantial signal quenching demonstrates the proportion of total eqf− and epre− captured by halopyrimidines prior to their conversion to eaq−. At identical concentration (200 mM) shown in Figure 2, effective electron interaction that is completed in less than 1 ps, shows that the efficiency of electron capture by X5Urd follows the order as Br5Urd > F5Urd > m5Urd. This observation establishes that halogen substitution at C5 of the pyrimidine base greatly enhances the ultrafast electron scavenging. Moreover, our results establish that Br5Urd is a better eqf− and epre− acceptor than F5Urd thereby supporting the DFT calculated electron affinity and experiments involving LEE.33,35,37,38,42,43 On the other hand, rates of the diffusion-controlled reactions between eaq− and X5Urd could be directly acquired from the decay profile in hundreds of ps as shown in Figure 2f. These results establish that eaq− reactivity to halopyrimidines followed the order as F5Urd> Br5Urd> m5Urd, which is different from those with epre− and eqf− (Br5Urd > F5Urd > m5Urd).
The solvation dynamics of the radiation-produced electron is observed to be slower in DEG (~150 ps) than that in water (~1 ps).24,35,41,44 Therefore, the instrument resolution of electron pulse (5 ps) enables us to resolve the transitions from epre− to esol−. The electron solvation process is manifested by the decay at 800 nm where only epre− absorbs (Figure 2a) and by the simultaneous build-up kinetics of esol− at 600 nm (Figure 2b and Figure 2c). These kinetics are the outcome of a time-dependent blue spectral shift (Figure S3), a characteristic feature of electron solvation that has been generally observed in polar solvents such as water and alcohols.24,35,41,44 Furthermore, normalized kinetics above the wavelength of 700 nm, regardless of concentration (lower than 0.3 M) and nature of the compound (m5Urd, F5Urd and Br5Urd) dissolved in DEG, are observed to be very similar to those in neat DEG. Therefore, this observation shows that even at concentrations up to 0.3 mol L−1, the halogen substitution in X5Urd (X5Urd, X=Br, F) strikingly causes a little effect on the relaxation of epre− to esol− thereby confirming our previous work.24,33,35,38
The previously reported laser flash photolysis results30–32 on the reaction of epre− with X5Urd suggested a fraction of epre− could induce carbon-halogen bond scission via DEA forming the σ-type uracil-5-yl radical (UC5•), the radical species responsible to initiate the radiation sensitization process. In contrast, our time-resolved radiolysis results, in addition to our previous results established that epre− is more likely to undergo the solvation and does not cause the C5-X bond scission in X5Urd. However, these authors neither reported the absorption spectra in the visible and near-infrared regions nor the kinetics of esol− and epre−. We believe that these parameters are crucial to verify the presence of esol− and epre− in the UV region. In addition, the pump and probe wavelengths employed in these experiments are very close in the UV region; consequently, this inconsistency is possibly attributed to the optical fluctuations caused by the intense femtosecond laser. Hence, our results reported in this work along with our previous results24,33,35,38 establish that the earlier reported results on the reaction of epre− with X5Urd30–32 are incorrect.
The reactions of eqf− (the precursor of epre−) with various uridine analogs studied in this work (X5Urd, X= CH3 (m5Urd), Br (Br5Urd), F (F5Urd)) can be inferred from the quenching of initial infrared absorbance (i.e., at 800 nm). From the decrease of the initial intensity of the absorbance at 800 nm, the order of eqf− activity in DEG was observed to be consistent with those of eqf− in water. Furthermore, due to the higher viscosity of DEG than that of water,24,35 the decay of esol− in DEG is not noticeable in the presence of solutes (X5Urd, X= CH3 (m5Urd), Br (Br5Urd), F (F5Urd)) during the first 500 ps (Figure 2b and Figure 2f).
Upon extending the timescale to μs range, additional experiments were performed to shed light on the reactions of esol− in DEG and water at lower concentrations of solutes (X5Urd) (1–5 ×10−3 mol L−1). This is illustrated by taking Br5Urd as an example. The absorption spectra and kinetics of the esol− and of the anion radical (Br5Urd•−, TNI) are reported in Figure S4. The diffusive esol− mediated transfer to each compound in DEG was observed to follow a decreasing order of efficiency m5Urd > F5Urd >Br5Urd - which is also observed to be the same for the corresponding diffusive esol− mediated transfer to each compound in water. These results, therefore, clearly establish that contrary to the previously published results,30,31 no reaction of either eqf− or epre− with X5Urd cannot be detected at low concentrations of X5Urd. Consequently, our spectroscopic observations in two different solvents (H2O and DEG) establish that eqf− scavenging occurs on a femtosecond timescale and only at high concentration, nearly with an estimated time reaction (~10−15 s) that is around several orders of magnitude shorter than those involving esol−.
Our results for halopyrimidines in Figure 2 clearly show that a larger extent of eqf− attachment for Br5Urd than that F5Urd (See also Figure S5). In contrast, the rate constant of the esol− reaction with F5Urd was found to be slightly larger than that with Br5Urd. We emphasize that such difference in rate is associated with various variables, including the energy of the delocalized electron and its state of solvation, electron mobility, the nature of the acceptor molecule as well the degree of solvation etc. The energy level of eqf− above the conduction band is a few eV higher than the ground states of the electron, esol−.8,22,33,34–35,36,41 Delocalized radius and mobility of electron are significantly reduced during electron solvation.10,41 Furthermore, it was also reported that the electron affinity of the pyrimidine ring increased due to halogen-replacement of the methyl group.20,21,38,42,43 Consequently, the direct observation of electron attachment enables us to conclude that the radiation-produced eqf− transfer process is an important part of the mechanism of radiosensitization by halopyrimidines.
The unusual kinetic phenomena in the reactions of electrons in liquids with varying dielectric constant could have important implications for anticancer treatment. For radiosensitization to occur, electron transfer principally acts as the initial event leading to the release of halide ions and subsequent molecular damages induced by the σ-UC5• (Scheme 1).16,20,21 Our findings suggest that the attachment of esol− with halopyrimidines takes place at a much slower rate in solution; consequently, the advantage ofemploying a halogen-incorporated nucleoside as the esol− acceptor is not obvious.20,46
| (1) |
| (2) |
Prior to its diffusion of esol− to the sites for an effective sensitization to occur, it is very likely that esol− is quenched at diffusion-controlled rates (reactions 1 and 2) by certain rich electron-affinic sites such as oxygen and proteins that are abundant in living cells.20 Therefore, due to these competitive reactions, the extent of esol− mediated production of uracil-5-yl radical would be less and consequently, the augmentation of DNA radiation damage, according to our results, can be limited.
Our results shown in Figure 2 establish that in contrast to esol−, ultrafast eqf− attachment in the liquid phase at room temperature that are consistent with LEEs under vacuum, occurs effectively when immediately formed. Thus, eqf− can play a significant role in damaging the target molecules in the vicinity. In addition, the reported larger value in electron affinity of the Br atom than that of the F atom was confirmed in eqf− sensitization and agreed with the other experiments and theory.8–9,10,11,12,13,14,15,16,18,20,21,42,43 Our results point out that such efficient radiation-produced electron-mediated sensitization should become more plausible in highly concentrated solutions and predicts that a considerable degree of halopyrimidine incorporation in DNA of hypoxic tumor cells would be for most effective radiosensitization.1–3,12,20, 47
For clarity, let us note three different reduced species (i.e., three different TNI): X5Urd•−, X5Urd•−*, and X5Urd•−**. The first one (X5Urd•−) is formed by esol−, the second one (X5Urd•−*) is by epre− and eqf− with low kinetic energy, and the last one (X5Urd•−**) is formed only by eqf− with enough kinetic energy to overcome the C5-X bond dissociation barrier. Now, the key issue here is to characterize the nature of the product of the reaction between eqf− and X5Urd. Spectral characters of X5Urd•−** and X5Urd•− were sorted by using global analysis24,33,35,38,41,48 of spectro-kinetic data matrix obtained during pulse-probe measurements (Figure S3 and Figure 3).
Figure 3.
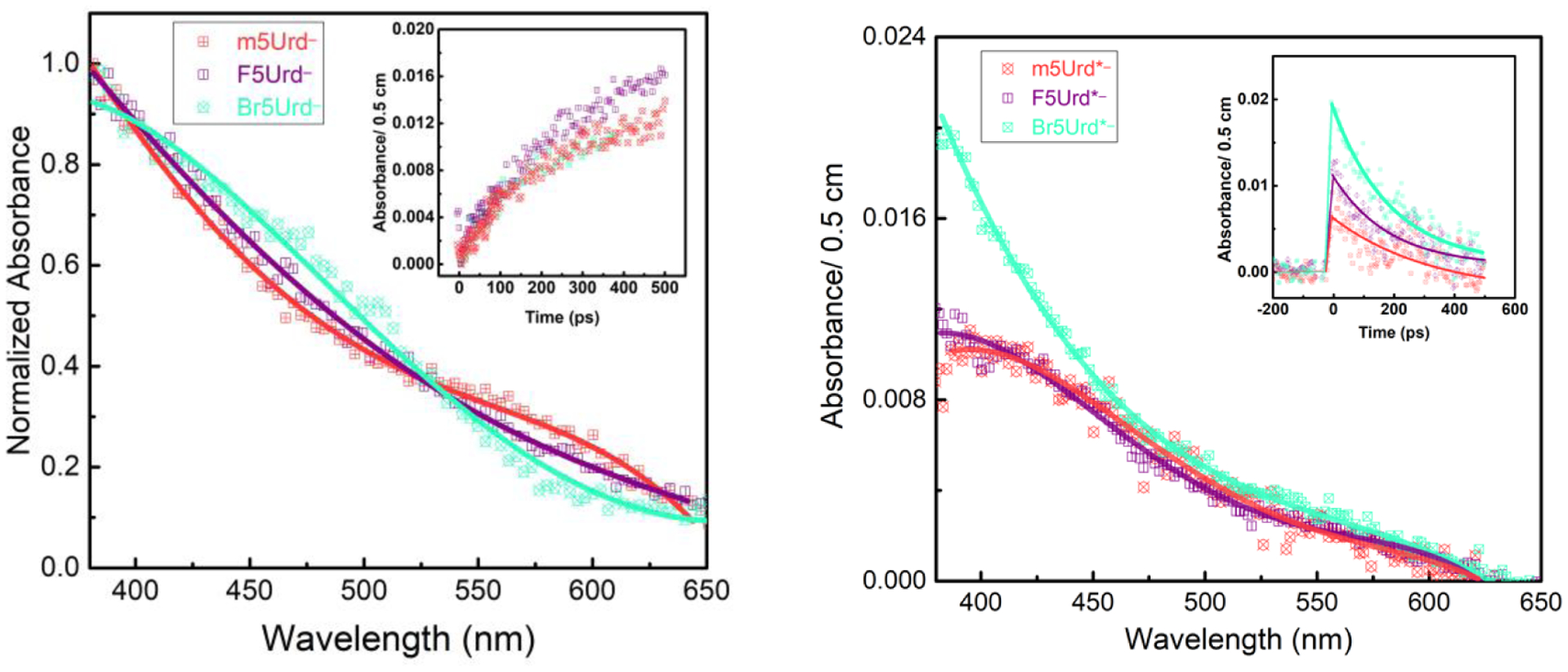
Left: Transient absorption spectra and bond-breaking rate of F5Urd•−** and Br5Urd•−** in comparison with m5Urd•−*. The decreasing order is as follow: Br5Urd•−* > F5Urd•−* > m5Urd•−*.Right: Transient absorption spectra and the formation rate of F5Urd•− and Br5Urd•− in comparison with m5Urd•−.
The transient absorption spectra of excited anion radicals (X5Urd•−**) are close to each other with an additional band at around 550 nm to that of m5Urd•−**. Clearly, all the anion radicals rapidly undergo a single exponential decay with a lifetime of around 100 ps, and in comparison, the decay rates are observed to be in decreasing order of Br5Urd•−**> F5Urd•−**> m5Urd•−**. The excited states can alternatively release the energy to the surrounding solvent environment, yielding the ground states (X5Urd•−) with an energy lower than the bottom of the conduction band (epre− + X5Urd→ X5Urd•−*→X5Urd•−). The latter resulting radical is of the same nature as the one formed by esol− (esol− + X5Urd → X5Urd•−). However, we did not observe this relaxation channel in our experiment contrary to that reported previously by Zimbrick et al.46 which has been well-accepted in the literature.1–2,3,4,5,6,7,20 The decomposition of halopyrimidine labelled-DNA not only depends on the extent of electron transfer but also strongly on the stability of X5Urd•−** or X5Urd•−. Our recent investigations have established that reaction of eqf− with rT forms the excited ribothymidine anion radical, rT•−** (i.e., X5Urd•−** X=Me), and rT•−** decays rapidly via C-N bond breakage with a time constant of about 350 ps.35 In contrast, the ground state of rT•− is found to be stable up to tens of μs.35 Numerous theoretical and experimental studies have established that the C-X bond is more vulnerable to electron attack than to the C-C and C-N bonds8–19,20,21,40,42–46 and in agreement with γ-radiolysis measurements showing that the yield of Br− is equal to that of electron.20,40 It is, therefore, rationalized that two main fragmentation channels can occur for X5Urd•−**, but the debromination to form the σ-type allylic dUC5• radical is the predominant pathway (Scheme 1).
Thus, our results show that eqf− is able to induce the elimination of halogen rather than causing N1–C1′ glycosidic bond cleavage in halopyrimidines, and the DEA process favors the C-Br bond breakage more than the C-F bond scission.
On the other hand, the halogen-substituted nucleoside π-anion radicals upon epre− and esol− attachment obtained from water and DEG are found to be relatively stable. As shown in Figure 3 (right), the initial fast rise in absorbance corresponds to the formation of anion radicals in their ground states, X5Urd•−. The decay kinetics of X5Urd•− at a longer timescale suggests that X5Urd•− does not undergo bond cleavage up to μs (Figure S4). One may argue that these results can be attributed to the formation of dUC5• while the decay of the X5Urd•− can proceed with similar absorption, i.e., with a similar molar extinction coefficient. This pathway can be easily ruled out because dUC5• only absorbs below 275 nm.28 In addition, the spectra of X5Urd•− that is different from that of X5Urd•−** remain unchanged as a function of time. In order to check the lifetime of X5Urd•−, complementary experiments were carried out in very dilute solutions (~10−3 M), and results show that it takes a few μs with nearly several order of magnitudes higher for X5Urd•− to dissociate than for the same dissociation happened in case of X5Urd•−**. In both water and DEG, no evidence has been found for epre− mediated C5-X bond scission in X5Urd•−*; X5Urd•−* most probably relaxes to the ground state, i.e., to X5Urd•−. Therefore, our observations establish that X5Urd•−** formed by the reaction of radiation-produced eqf− with X5Urd, promptly undergoes C-X bond breakage. On the other hand, epre− and esol− are not able to overcome the energy barrier to facilitate the bond breakage in X5Urd•−* and in X5Urd•−. The findings also clarify for the first time that the degree of solvation is the key issue to determine the energy states of TNIs of halopyrimidines and, therefore to the occurrence of bond cleavage. Our results are in good agreement with studies of slow electrons (near 0 eV) mediated sensitization that obtained under vacuum conditions as well as recent micro-hydration investigation of electron-induced DNA damage.49,50 In addition, our observation clearly point out that epre− only plays a minor role in radiation sensitization by electron-affinic compounds like halopyrimidines. Thus, our results establish that the hypothesis that came out from the femtosecond laser-photolysis studies proposing epre− could play a major role in radiation sensitization30–31,32 is incorrect. Most importantly, the present experiments were performed in bulk solutions that might better represent the situations in cells and can be used as benchmarks for evaluation of any potential radiosensitizers.
Moreover, the exponentially-growing V79–171 cells whose DNA is substituted by Br5dUrd and I5dUrd in place of thymidine (Thd), when irradiated in the presence and in absence of 1 M acetone, it was found that acetone removed the majority of the double strand breaks and approximately half of the increase in cell killing.9,51 Therefore, employing our results, we interpret that these reductions in radiosensitizations are due to the efficient scavenging of eqf− by 1 M acetone present in these cells.
It is important to note that during incorporation of halopyridines in the cellular DNA, thymidylate synthetase causes efficient hepatic dehalogenation. This process hinders the accumulation of therapeutically suuficient amount of halopyrimidines and consequently, limits the use of halopyrimidines as effective radiosensitizers for tumor radiotherapy. Thus, some groups are synthesizing halopyridine-like molecules with similar or even better DEA efficiency, but without the drawbacks of dehalogenation by thymidylate synthetase. In other words, new base and nucleoside derivatives are synthesized that have similar or better DEA yields in the 0–3 eV region than the haloprimidines; in addition, these derivatives would not loose the C5-modification of the thymine base by thymidylate synthetase.52–58 Based on our previous works and on the current work, Our work predicts that DEA process of these halopyridine-like molecules in solution should predominantly be governed by eqf−.
Conclusions:
In conclusion, our results show that the mechanism of radiosensitization by halopyrimidines in the liquid phase could involves an ultrafast quasi-free electron (eqf−) transfer pathway. This ultrafast pathway, at first, leads to the formation of a metastable TNI (X5Urd•−**), subsequently, X5Urd•−** undergoes the C5-X (X= Br) bond breakage with a time constant of about 100 ps in DEG. It is important to note that in aqueous solution, we were not able to observe the formation of X5Urd•−** and its fast dissociation. A faster thermal relaxation of X5Urd•−** or dissociation can explain this fact. Moreover, eqf− induced C5-Br bond cleavage in Br5Urd is favored over N1–C1′ glycosidic bond scission in rT due to higher electron affinity of Br5Urd than rT and substitution of the electron-donating CH3 group by electronegative Br atom makes the C-Br bond scission in Br5Urd•−** very efficient. In contrast, both epre− and esol− transfer to X5Urd (X = Br, F, -CH3) take place at one to three orders of magnitude slower rates than that to Br5Urd, leading to the formation of ground states of TNI (X5Urd•−) with a lifetime of tens of μs. Thus, our findings from pulse radiolysis comprehensively elucidate the molecular basis for assessment of any sensitization effect operating via radiation-induced quasi-free electron transfer.
Supplementary Material
Acknowledgements
This work is supported by National Natural Science Foundation of China (Grant No. 11975122 and Grant No. 21906083) and Jiangsu Province Science Foundation for Youths (Grant No. BK20190384). AA thanks the National Cancer Institute of the National Institutes of Health (Grant RO1CA045424), the National Science Foundation under Grant No. CHE- 1920110, and to REF, CBR at OU for support. In addition, the authors warmly thank Pr Pedro D’Oliveira and Dr Israël Mbomekalle for supporting in Br− measurement protocole and Ms. Isabelle De Waele and Ms. Mireille Benoit for their help in short-path optical cell measurements.
Footnotes
Supporting information:
(a) Curves showing the concentrations (in μM) of gamma-radiation produced Br− in solutions of Br5Urd in DEG and in water as a function of absorbed dose (in Gy) with corresponding linear regression analyses for determination of G-values. (b) Decay of solvated electron in DEG. (c) Two-dimensional absorption matrix data obtained by picosecond pulse radiolysis. (d) Kinetics and the absorption spectra of the solvated electron and the Br5U•− in water and in DEG. (e) The initial yields and kinetics of solvated electrons in solutions of Urd, Br5Urd, and F5Urd in DEG at different concentrations. The Supporting Information is available free of charge at http://pubs.acs.org/.
The authors declare no competing financial interest.
References:
- (1).Dewey WC; Humphrey RM Increase in Radiosensitivity to Ionizing Radiation Related to Replacement of Thymidine in Mammalian Cells with 5-Bromodeoxyuridine. Radiat. Res 1965, 26, 538–553. [PubMed] [Google Scholar]
- (2).Dewey WC; Sedita BA; Humphrey RM Radiosensitization of X Chromosome of Chinese Hamster Cells Related to Incorporation of 5-Bromodeoxyuridine. Science 1966, 152, 519. [DOI] [PubMed] [Google Scholar]
- (3).Sano K; Hoshino T; Nagai M Radiosensitization of Brain Tumor Cells with a Thymidine Analogue (Bromouridine). J. Neurosurg 1968, 28, 530–538. [DOI] [PubMed] [Google Scholar]
- (4).Dabaja BS; McLaughlin P; Ha CS; Pro B; Meyers CA; Seabrooke LF; Wilder RB; Kyritsis AP; Preti HA; Yung WKA et al. Primary central nervous system lymphoma: Phase I evaluation of infusional bromodeoxyuridine with whole brain accelerated fractionation radiation therapy after chemotherapy. Cancer 2003, 98, 1021–1028. [DOI] [PubMed] [Google Scholar]
- (5).Prados MD; Seiferheld W; Sandler HM; Buckner JC; Phillips T; Schultz C; Urtasun R; Davis R; Gutin P; Cascino TL et al. Phase III randomized study of radiotherapy plus procarbazine, lomustine, and vincristine with or without BUdR for treatment of anaplastic astrocytoma: final report of RTOG 9404. Int. J. Radiat. Oncol. Biol. Phys 2004, 58, 1147–1152. [DOI] [PubMed] [Google Scholar]
- (6).Vogel S; Ebel K; Heck C; Schürmann RM; Milosavljević AR; Giuliani A; Bald I Vacuum-UV induced DNA strand breaks – influence of the radiosensitizers 5-bromouracil and 8-bromoadenine. Phys. Chem. Chem. Phys 2019, 21, 1972–1979. [DOI] [PubMed] [Google Scholar]
- (7).Schürmann R; Bald I Effect of adsorption kinetics on dissociation of DNA-nucleobases on gold nanoparticles under pulsed laser illumination. Phys. Chem. Chem. Phys 2017, 19, 10796–10803. [DOI] [PubMed] [Google Scholar]
- (8).Alizadeh E; Sanche L Precursors of Solvated Electrons in Radiobiological Physics and Chemistry. Chem. Rev 2012, 112, 5578–5602. [DOI] [PubMed] [Google Scholar]
- (9).Webb CF; Jones GDD; Ward JF; Moyer DJ; Aguilera JA; Ling LL Mechanisms of Radiosensitization in Bromodeoxyuridine-substituted Cells. Int. J. Radiat Biol 1993, 64, 695–705. [DOI] [PubMed] [Google Scholar]
- (10).Beach C; Fuciarelli AF; Zimbrick JD Electron Migration along 5-Bromouracil-Substituted DNA Irradiated in Solution and in Cells. Radiat. Res 1994, 137, 385–393. [PubMed] [Google Scholar]
- (11).Cecchini S; Girouard S; Huels MA; Sanche L; Hunting DJ Single-Strand-Specific Radiosensitization of DNA by Bromodeoxyuridine. Radiat. Res 2004, 162, 604–615. [DOI] [PubMed] [Google Scholar]
- (12).Park Y; Polska K; Rak J; Wagner JR; Sanche L Fundamental Mechanisms of DNA Radiosensitization: Damage Induced by Low-Energy Electrons in Brominated Oligonucleotide Trimers. J. Phys. Chem. B 2012, 116, 9676–9682. [DOI] [PubMed] [Google Scholar]
- (13).Schürmann R; Tsering T; Tanzer K; Denifl S; Kumar SVK; Bald I Resonant Formation of Strand Breaks in Sensitized Oligonucleotides Induced by Low-Energy Electrons (0.5–9 eV). Angew. Chem. Int. Ed 2017, 56, 10952–10955. [DOI] [PubMed] [Google Scholar]
- (14).Abdoul-Carime H; Huels MA; Illenberger E; Sanche L Sensitizing DNA to Secondary Electron Damage: Resonant Formation of Oxidative Radicals from 5-Halouracils. J. Am. Chem. Soc 2001, 123, 5354–5355. [DOI] [PubMed] [Google Scholar]
- (15).Chomicz L; Rak J; Storoniak P Electron-Induced Elimination of the Bromide Anion from Brominated Nucleobases. A Computational Study. J. Phys. Chem. B 2012, 116, 5612–5619. [DOI] [PubMed] [Google Scholar]
- (16).Chomicz L; Petrovici A; Archbold I; Adhikary A; Kumar A; Sevilla MD; Rak J An ESR and DFT study of hydration of the 2′-deoxyuridine-5-yl radical: a possible hydroxyl radical intermediate. Chem. Commun 2014, 50, 14605–14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Cecchini S; Girouard S; Huels MA; Sanche L; Hunting DJ Interstrand Cross-Links: A New Type of γ-Ray Damage in Bromodeoxyuridine-Substituted DNA. Biochemistry 2005, 44, 1932–1940. [DOI] [PubMed] [Google Scholar]
- (18).Sevilla MD; Failor R; Zorman G Radicals formed after electron attachment to 5-halouracils in aqueous glasses. J. Phys. Chem 1974, 78, 696–699. [Google Scholar]
- (19).Bernhard WA in Radical and Radical Ion Reactivity in Nucleic Acid Chemistry, (Greenberg MM (Ed.)), 2009, pp. 41–68. [Google Scholar]
- (20).von Sonntag C Free-Radical-Induced DNA Damage and Its Repair, 1 ed., Springer, Berlin, Heidelberg, 2006. [Google Scholar]
- (21).Razskazovskiy Y; Swarts SG; Falcone JM; Taylor C; Sevilla MD Competitive Electron Scavenging by Chemically Modified Pyrimidine Bases in Bromine-Doped DNA: Relative Efficiencies and Relevance to Intrastrand Electron Migration Distances. J. Phys. Chem. B 1997, 101, 1460–1467. [Google Scholar]
- (22).Cobut V; Frongillo Y; Patau JP; Goulet T; Fraser MJ; Jay-Gerin JP Radiat. Phys. Chem 1998, 51, 229–243; [Google Scholar]
- (23).Pimblott SM; LaVerne JA Production of low-energy electrons by ionizing radiation. Radiat. Phys. Chem 2007, 76, 1244–1247. [Google Scholar]
- (24).Ma J; Denisov SA; Adhikary A; Mostafavi M, Ultrafast Processes Occurring in Radiolysis of Highly Concentrated Solutions of Nucleosides/Tides. Int. J. Mol. Sci 2019, 20, 4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Elkins MH; Williams HL; Shreve AT; Neumark DM Relaxation Mechanism of the Hydrated Electron. Science 2013, 342, 1496. [DOI] [PubMed] [Google Scholar]
- (26).Stähler J; Deinert J-C; Wegkamp D; Hagen S; Wolf M Real-Time Measurement of the Vertical Binding Energy during the Birth of a Solvated Electron. J. Am. Chem. Soc 2015, 137 (10), 3520–3524. [DOI] [PubMed] [Google Scholar]
- (27).Nabben FJ; Karman JP; Loman H Inactivation of Biologically Active DNA by Hydrated Electrons. Int. J. Radiat. Biol 1982, 42, 23–30. [DOI] [PubMed] [Google Scholar]
- (28).Rivera E; Schuler RH Intermediates in the reduction of 5-halouracils by eaq−. J. Phys. Chem 1983, 87, 3966–3971. [Google Scholar]
- (29).Takeda N; Poliakov PV; Cook AR; Miller JR Faster Dissociation: Measured Rates and Computed Effects on Barriers in Aryl Halide Radical Anions. J. Am. Chem. Soc 2004, 126, 4301–4309. [DOI] [PubMed] [Google Scholar]
- (30).Wang C-R; Nguyen J; Lu Q-B Bond Breaks of Nucleotides by Dissociative Electron Transfer of Nonequilibrium Prehydrated Electrons: A New Molecular Mechanism for Reductive DNA Damage. J. Am. Chem. Soc 2009, 131, 11320–11322. [DOI] [PubMed] [Google Scholar]
- (31).Wang CR; Hu A; Lu QB Direct observation of the transition state of ultrafast electron transfer reaction of a radiosensitizing drug bromodeoxyuridine. J. Chem. Phys 2006, 124, 241102. [DOI] [PubMed] [Google Scholar]
- (32).Wang C-R; Lu Q-B Real-Time Observation of a Molecular Reaction Mechanism of Aqueous 5-Halo-2′-deoxyuridines under UV/Ionizing Radiation. Angew. Chem. Int. Ed 2007, 46 (33), 6316–6320. [DOI] [PubMed] [Google Scholar]
- (33).Ma J; Wang F; Denisov SA; Adhikary A; Mostafavi M Reactivity of prehydrated electrons toward nucleobases and nucleotides in aqueous solution. Sci. Adv 2017, 3, e1701669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Wang F; Archirel P; Muroya Y; Yamashita S; Pernot P; Yin C; El Omar AK; Schmidhammer U; Teuler J-M; Mostafavi M Effect of the solvation state of electron in dissociative electron attachment reaction in aqueous solutions. Phys. Chem. Chem. Phys 2017, 19, 23068–23077. [DOI] [PubMed] [Google Scholar]
- (35).Ma J; Kumar A; Muroya Y; Yamashita S; Sakurai T; Denisov SA; Sevilla MD; Adhikary A; Seki S; Mostafavi M Observation of dissociative quasi-free electron attachment to nucleoside via excited anion radical in solution. Nat. Commun 2019, 10, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Alizadeh E; Orlando TM; Sanche L Biomolecular Damage Induced by Ionizing Radiation: The Direct and Indirect Effects of Low-Energy Electrons on DNA. Annu. Rev. Phys. Chem 2015, 66, 379–398. [DOI] [PubMed] [Google Scholar]
- (37).von Sonntag C Radiation-Induced DNA Damage: Indirect Effects: Recent Trends in Radiation Chemistry, (Wishart JR., Rao BSM (Eds.)), World Scientific Publishing Co., Singapore, New Jersey, London, 2010. [Google Scholar]
- (38).Ma J; Denisov SA; Marignier J-L; Pernot P; Adhikary A; Seki S; Mostafavi M Ultrafast Electron Attachment and Hole Transfer Following Ionizing Radiation of Aqueous Uridine Monophosphate. J. Phys. Chem. Lett 2018, 9, 5105–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Hanna JG; Jura J Determination of Trace Amounts of Inorganic Chloride in Ethylene Glycol. Anal. Chem 1959, 31, 1820–1821. [Google Scholar]
- (40).Ye MY; Shen Y Electrospray Ionization Mass Spectrometry and HPLC Determination of the Products in the Radiolysis of 5-Bromouracil, its Nucleoside and Nucleotide Derivatives. J. Liq. Chromatogr 1994, 17, 773–791. [Google Scholar]
- (41).Kenney-Wallace GA; Jonah CD Picosecond spectroscopy and solvation clusters. The dynamics of localizing electrons in polar fluids. J. Phys. Chem 1982, 86, 2572–2586. [Google Scholar]
- (42).Wetmore SD; Boyd RJ; Eriksson LA A theoretical study of 5-halouracils: electron affinities, ionization potentials and dissociation of the related anions. Chem. Phys. Lett 2001, 343, 151–158. [Google Scholar]
- (43).Modelli A; Bolognesi P; Avaldi L Temporary Anion States of Pyrimidine and Halopyrimidines. J. Phys. Chem. A 2011, 115, 10775–10782. [DOI] [PubMed] [Google Scholar]
- (44).Gauduel Y; Migus A; Chambaret JP; Antonetti A Femtosecond reactivity of electron in aqueous solutions. Rev. Phys. Appl. (Paris) 1987, 22, 1755–1759. [Google Scholar]
- (45).Gu J; Leszczynski J; Schaefer HF Interactions of Electrons with Bare and Hydrated Biomolecules: From Nucleic Acid Bases to DNA Segments. Chem. Rev 2012, 112, 5603–5640. [DOI] [PubMed] [Google Scholar]
- (46).Zimbrick JD; Ward JF; Myers LS Studies on the Chemical Basis of Cellular Radiosensitization by 5-bromouracil Substitution in DNA. Int. J. Radiat. Biol 1969, 16, 505–523. [DOI] [PubMed] [Google Scholar]
- (47).Kummar S; Anderson L; Hill K; Majerova E; Allen D; Horneffer Y; Ivy SP; Rubinstein L; Harris P; Doroshow JH et al. First-in-Human Phase 0 Trial of Oral 5-Iodo-2-Pyrimidinone-2′-Deoxyribose in Patients with Advanced Malignancies. Clin. Cancer Res 2013, 19, 1852–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Ruckebusch C; Sliwa M; Pernot P; de Juan A; Tauler R Comprehensive data analysis of femtosecond transient absorption spectra: A review. J. Photochem. Photobiol. C: Photochem. Rev 2012, 13, 1–27. [Google Scholar]
- (49).Abdoul-Carime H; Gohlke S; Fischbach E; Scheike J; Illenberger E Thymine excision from DNA by subexcitation electrons. Chem. Phys. Lett 2004, 387, 267–270. [Google Scholar]
- (50).Kočišek J; Pysanenko A; Fárník M; Fedor J Microhydration Prevents Fragmentation of Uracil and Thymine by Low-Energy Electrons. J. Phys. Chem. Lett 2016, 7, 3401–3405. [DOI] [PubMed] [Google Scholar]
- (51).Jones GDD; Ward JF; Limoli CL; Moyer DJ; Aguilera JA Mechanisms of Radiosensitization in Iododeoxyuridine-Substituted Cells. Int. J. Radiat Biol 1995, 67, 647–653. [DOI] [PubMed] [Google Scholar]
- (52).Sosnowska M; Makurat S; Zdrowowicz M; Rak J 5-selenocyanatouracil: a potential hypoxic radiosensitizer. electron attachment induced formation of selenium centered radical. J. Phys. Chem. B 2017, 121, 6139–6147. [DOI] [PubMed] [Google Scholar]
- (53).Makurat S; Zdrowowicz M; Chomicz-Manka L; Kozak W; Serdiuk IE; Wityk P; Kawecka A; Sosnowska M; Rak J 5-Selenocyanato and 5-trifluoromethanesulfonyl derivatives of 2’-deoxyuridine: synthesis, radiation and computational chemistry as well as cytotoxicity. RSC Adv 2018, 8, 21378–21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Meißner R; Makurat S; Kozak W; Limão-Vieira P; Rak J; Denifl S Electron-induced dissociation of the potential radiosensitizer 5-selenocyanato-2’-deoxyuridine. J. Phys. Chem. B 2019, 123, 1274–1282. [DOI] [PubMed] [Google Scholar]
- (55).Zdrowowicz M; Chomicz L; Zyndul M; Wityk P; Rak J; Wiegand TJ; Hanson CG; Adhikary A; Sevilla MD 5-Thiocyanato-2’-deoxyuridine as a possible radiosensitizer: electron-induced formation of uracil-C5-thiyl radical and its dimerization. Phys. Chem. Chem. Phys 2015, 17, 16907–16916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ameixa J; Arthur-Baidoo E; Meißner R; Makurat S; Kozak W; Butowska K; Ferreira da Silva F; Rak J; Denifl S Low–energy electron–induced decomposition of 5–trifluoromethanesulfonyl–uracil: A potential radiosensitizer. J. Chem. Phys 2018, 149, 164307. [DOI] [PubMed] [Google Scholar]
- (57).Mudgal M; Dang TP; Sobczak AJ; Lumpuy DA; Dutta P; Ward S; Ward K; Alahmadi M; Kumar A; Sevilla MD et al. Site of Azido Substitution in the Sugar Moiety of Azidopyrimidine nucleosides Influences the Reactivity of Aminyl Radicals Formed by Dissociative Electron Attachment. J. Phys. Chem. B 2020, 124, 11357–11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Wen Z; Peng J; Tuttle P; Ren Y; Garcia C; Debnath D; Rishi S; Hanson C; Ward S; Kumar A et al. Electron-mediated Aminyl and Iminyl Radicals from C5-Azido-Modified Pyrimidine Nucleosides Augment Radiation Damage to Cancer Cells. Org. Lett 2018, 20, 7400–7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


