Abstract
Background
Long-term cognitive impairment frequently occurs after critical illness; no treatments are known to improve long-term cognition.
Research Question
Does a single high-dose (540,000 International Units) enteral treatment of vitamin D3 given shortly after hospital admission in critically ill patients who are vitamin D deficient improve long-term global cognition or executive function?
Study Design and Methods
This study evaluated long-term cognitive outcomes among patients enrolled in a multicenter, blinded, randomized clinical trial comparing vitamin D3 treatment vs placebo in critically ill adults with vitamin D deficiency. Global cognition was measured by the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Executive function was measured with a composite score derived from three Delis-Kaplan Executive Function System subscales. Outcomes were assessed at a median of 443 days (interquartile range, 390-482 days) after randomization and were compared using multivariate proportional odds regression. Adjusted ORs of > 1.0 would indicate better outcomes in the vitamin D3 group compared with the placebo group.
Results
Ninety-five patients were enrolled, including 47 patients randomized to vitamin D3 treatment and 48 patients randomized to placebo. The adjusted median RBANS score at follow-up was 79.6 (95% CI, 73.0-84.0) in the vitamin D3 group and 82.1 (95% CI, 74.7-84.6) in the placebo group (adjusted OR, 0.83; 95% CI, 0.50-1.38). The adjusted median executive function composite scores were 8.1 (95% CI, 6.8-9.0) and 8.7 (95% CI, 7.4-9.3), respectively (adjusted OR, 0.72; 95% CI, 0.36-1.42).
Interpretation
In vitamin D-deficient, critically-ill adults, a large dose of enteral vitamin D3 did not improve long-term global cognition or executive function.
Trial Registry
ClinicalTrials.gov; No.: NCT03733418; URL: www.clinicaltrials.gov
Key Words: executive function, global cognition, ICU, vitamin D
Abbreviations: ADL, activities of daily living; D-KEFS, Delis-Kaplan Executive Function System; IADL, instrumental activities of daily living; IQR, interquartile range; LTCI, long-term cognitive impairment; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; SOFA, Sequential Organ Failure Assessment; VIOLET, Vitamin D to Improve Outcomes by Leveraging Early Treatment; VIOLET-BUD, Vitamin D to Improve Outcomes by Leveraging Early Treatment—Long-Term Brain Outcomes in Vitamin D Deficient Patients
Long-term cognitive impairment (LTCI) is a growing public health problem in survivors of critical illness. LTCI is a new deficit or a worsening of a pre-existing deficit in cognition that persists after an acute or critical illness. One in four patients discharged from the ICU experiences LTCI similar in severity to mild Alzheimer disease.1 Many patients discharged from the ICU demonstrate impairments in executive function,1 one type of cognitive dysfunction that leads to increased disability, lower quality of life, and reduced employment.2 No pharmacologic interventions have been shown to preserve long-term cognition or executive function after critical illness.
Systemic and CNS inflammation are increased in response to a critical illness and may be important drivers for LTCI and dementia development.3 Therefore, vitamin D administration early in the acute phase of critical illness potentially could preserve long-term cognition, especially in those who are vitamin D deficient. Vitamin D is a pleiotropic secosteroid hormone that attenuates systemic inflammatory responses,4 downregulates systemic inflammation by inhibiting the peripheral proinflammatory cytokine release (eg, tumor necrosis factor-α, IL-6, IL-12),5,6 crosses the blood-brain barrier,7 decreases microglial production of proinflammatory cytokines,8 and inhibits CNS inflammation.8
Observational data suggest that vitamin D deficiency is associated with worse long-term cognition9, 10, 11 and an increased risk of Alzheimer dementia.11 In community-dwelling older adults, Annweiler et al12 reported that vitamin D supplementation improved cognitive performance, including executive function. However, whether the administration of vitamin D3 to vitamin D-deficient patients with critical illness improves cognitive outcomes has not been tested rigorously. Therefore, we sought to determine if administration of a single high-dose (540,000 International Units) enteral vitamin D3 (cholecalciferol) treatment early during hospitalization to critically ill, vitamin D-deficient adults improves long-term global cognition or executive function.
Methods
Study Design
This was an ancillary study to the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET; Clinicaltrials.gov Identifier: NCT03096314) randomized control trial, conducted by the National Heart, Lung and Blood Institute Prevention and Early Treatment of Acute Lung Injury network. The parent trial showed that vitamin D3 treatment rapidly corrected vitamin D deficiency, but did not improve 90-day all-cause mortality.13
The current VIOLET Long-term Brain Outcomes in Vitamin D Deficient Patients (VIOLET-BUD; Clinicaltrials.gov Identifier: NCT03733418) ancillary study added long-term cognitive assessments to a subset of patients randomized in VIOLET. VIOLET-BUD assessed cognitive outcomes at 7 of the 42 Prevention and Early Treatment of Acute Lung Injury network sites participating in the VIOLET trial across the United States: Beth Israel Deaconess Medical Center, Brigham and Women’s Hospital, Intermountain Healthcare, Montefiore Medical Center, Oregon Health and Science University, University of Colorado, and Vanderbilt University Medical Center. Cognitive assessments occurred from November 2018 through October 2019. The governing single institutional review board at Vanderbilt University Medical Center approved this ancillary study. At long-term follow-up, all patients or their legally authorized representatives provided informed consent to participate.
Participants
Details of the VIOLET parent study’s eligibility criteria have been described previously and are provided in the online Supplemental Methods.13 In brief, patients were included in VIOLET if they were age 18 years of age or older, admitted to the ICU, or were going to be admitted to the ICU from the ED, had one or more risk factor for ARDS and mortality, and had vitamin D deficiency, defined as a serum 25-hydroxyvitamin D level of < 20 mg/dL measured at the local clinical laboratory. Patients were excluded if they were unable to be randomized within 12 h of ICU admission decision, they were unable to take study medication by mouth or enteral tube, they had serum calcium level of > 10.2 mg/dL or ionized calcium level of > 5.2 mg/dL, they had a kidney stone in past year or history of multiple prior kidney stone episodes, life-sustaining treatment was being withheld, they had an expected survival of < 48 h, or they were pregnant.
Patients who were known to be alive at the time of VIOLET-BUD screening were contacted by phone by local site study coordinators approximately 1 year after VIOLET enrollment. Patients were excluded from the VIOLET-BUD ancillary study if they did not speak English, were deaf, or were blind because the neuropsychological raters spoke only English and the tests had visual and aural components. For those who met VIOLET-BUD eligibility criteria and agreed to participate, the study coordinators obtained informed consent verbally over the phone. Study participants then signed the informed consent document before outcome ascertainment at the local site.
Intervention, Randomization, and Masking
As part of the VIOLET parent trial, a central electronic system randomized enrolled patients to receive vitamin D3 vs placebo at a 1:1 ratio using permutated blocks and stratified according to site. Participants randomized to the vitamin D3 group received a single enteral (oral or enteral tube) dose of 540,000 International Units vitamin D3 (cholecalciferol) vs placebo within 2 h after randomization. This high-dose regimen has been shown to replete vitamin D effectively for up to 1 month.14 Patients randomized to the control group received a placebo solution matched in appearance to the vitamin D3 solution. Patients, the clinical and research teams, and outcome assessors all were blinded to treatment assignment.
Outcomes
The prespecified coprimary outcomes for VIOLET-BUD were long-term global cognition and executive function. Originally, the objective was to conduct the follow-up assessments 12 ± 4 months after randomization. To maximize the number of included patients, VIOLET-BUD expanded the follow-up window during the study to include all VIOLET trial patients at sites participating in VIOLET-BUD trial, including those who were randomized more than 16 months before long-term outcome follow-up.
Global cognition was measured using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) by assessing immediate and delayed memory, attention, visuospatial construction, and language.15 It has been validated in patients with mild cognitive impairment, moderate to severe traumatic brain injuries, vascular dementias, and Alzheimer disease.15, 16, 17, 18, 19 The RBAN’s population age-adjusted mean ± SD for the global cognition score and for the individual cognitive domains is 100 ± 15; scores range from 40 to 160, with higher values indicating better cognition.15 In 821 ICU patients, RBANS scores ranged from 48 to 119, indicating that floor and ceiling effects are highly unlikely.1 To quantify executive function, we used tests from the Delis-Kaplan Executive Function System (D-KEFS), which is a comprehensive neuropsychological assessment of executive functions.20 Specifically, we conducted the D-KEFS proverb test subscale (conceptual flexibility), the D-KEFS trail making number-letter switching subscale (inhibition), and the D-KEFS verbal fluency category switching subscale (flexibility and set-shifting). The D-KEF subscales’ population age-adjusted mean ± SD is 10 ± 3. An executive function composite score was calculated from these three D-KEFS subscales, with scores ranging from 1 to 18 and higher, with higher values indicating better executive function. For this report, age-adjusted scores for the RBANS and its individual cognitive domains and the D-KEF subscales were reported.
For assessments in VIOLET-BUD, study participants returned to the local study site where they originally were enrolled in VIOLET, or they were assessed in their homes. Neuropsychologists from the Vanderbilt University Medical Center Critical Illness, Brain Dysfunction, and Survivorship Center conducted the neuropsychological tests via videophone with the assistance of local proctors, a validated method for such testing.21 Before VIOLET-BUD, we conducted a pilot study to confirm feasibility of using inexpensive high-definition web cameras while fine-tuning our methods.22
The local study team also collected additional outcome measures, including functional status as measured by the Katz activities of daily living (ADL) scale and Lawton instrumental activities of daily living (IADL) scale, current level of employment as measured by the Outcomes After Critical Illness and Surgery Return to Work survey,23 and nursing home placement.
Patients were considered to have pre-existing dementia if they had a history of dementia documented in the medical record or were taking an acetylcholinesterase inhibitor before randomization. Intelligence was estimated using the Barona index based on age, sex, region of residence, years of education, and highest occupation.24 Severity of illness at enrollment was characterized using the Sequential Organ Failure Assessment (SOFA). Infection was defined by the presence of sepsis or pneumonia at enrollment. Charlson Comorbidity Index was collected to characterize comorbidity burden.25
Statistical Analysis
The data analysis plan was prespecified and registered on March 3, 2020 (https://osf.io/zg2ny/). Measures of central tendency and dispersion were reported as medians and interquartile ranges (IQRs). To determine if single, high-dose enteral vitamin D3 treatment improved long-term global cognition and executive function compared with placebo, we used proportional odds regression models because the outcomes were continuous, ordinal, and not normally distributed. Although randomization accounts for group imbalances, adjusting for risk factors associated with the outcomes reduces measurement error and increases statistical power.26 The models were adjusted for age at enrollment (continuous), pre-existing cognitive impairment (dichotomous), baseline acute illness severity (SOFA score, ordinal), infection defined as sepsis or pneumonia at enrollment (dichotomous), and pre-enrollment intelligence (Barona Index score, ordinal). These covariates were chosen a priori based on literature review and expert opinion suggesting potential association with LTCI after critical illness. Postrandomization covariates (eg, interventions provided during hospitalization) were not adjusted for because they potentially may be affected by the randomized intervention and may be on the causal pathway. To account for correlation among patients within a given site, SEs were adjusted using the Huber-White sandwich estimate.27 No values were missing for the primary independent variable (vitamin D3 treatment vs placebo) or dependent variables. Missing values were rare for covariables (< 2%); model-based single imputation was used to impute values for these missing values. Adjusted medians and ORs with their 95% CIs are reported.28 For the proportional odds logistic regression models, the adjusted ORs indicated the odds of having higher scores of the outcome (better outcome) when treated with vitamin D3 compared with placebo. That is, adjusted ORs of more than 1.0 indicated higher odds of better outcomes in the vitamin D3 group.
VIOLET-BUD was designed to enroll 140 patients with completed follow-up data (70 patients per group) to detect a six-point difference in RBANS between treatment groups with a two-tailed α value of 0.05 and 80% power. The six-point difference is approximately half of the RBANS SD of 12.4 obtained from our previous studies.1 Half of an SD often is considered the minimum clinically important difference threshold,29 but notably, some consider an RBANS difference of eight points to be the threshold.30 VIOLET-BUD did not reach the original sample size goal because the VIOLET parent trial stopped enrollment early at an interim analysis because of futility of the main trial primary end point (90-day mortality). The final sample size in VIOLET BUD was 95 patients, which provided 80% power to detect at least a 7.2-point difference in RBANS scores between the vitamin D3 treatment and placebo groups.
We performed two prespecified sensitivity analyses by including additional covariates in the primary models: adjustment for time between randomization and neuropsychological assessment and baseline 25-hydroxyvitamin D level measured at the local laboratory. Because the VIOLET parent study did not observe a significant difference in 90-day mortality between the vitamin D3 and placebo groups,13 we did not complete analyses accounting for differential mortality.
As prespecified in the data analysis plan, we evaluated for potential heterogeneity of treatment effect by evaluating for interactions between treatment group and each of the following baseline characteristics: age, pre-existing cognitive impairment (eg, dementia), baseline severity of illness (SOFA score), infection (pneumonia or sepsis diagnosis), and estimated intelligence (Barona Index). Because detecting a statistical interaction may require significantly greater statistical power than detecting a main effect, we considered an interaction term P value of less than .20 to suggest the potential presence of effect modification.
Additional prespecified secondary analyses were performed to determine how vitamin D3 treatment affected individual RBANS cognitive domains (eg, immediate and delayed memory, attention, visuospatial construction, and language) and executive function subscales (D-KEFS proverbs, number-letter switching, and verbal fluency category switching subscales). We also determined if vitamin D3 treatment was associated with functional status (Katz ADL and Lawton IADL scales). Because of the low number of events, descriptive statistics were used to describe loss of employment and nursing home placement. Proportional odds assumptions were checked graphically and were not violated. All statistical analyses were conducted with R version 3.6.2 statistical software (R Foundation for Statistical Computing).
Results
Population
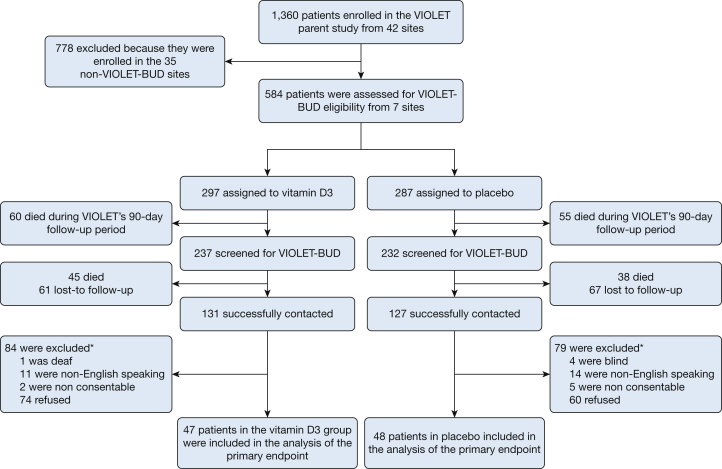
A total of 1,360 patients were enrolled in VIOLET at 42 centers, and 584 patients were enrolled in VIOLET at the seven sites participating in VIOLET-BUD (Fig 1). Of these, 105 of 297 patients (35.4%) in the vitamin D3 group and 93 of 287 patients (32.4%) in the placebo group died before follow-up could be completed. A total of 95 patients were enrolled successfully in VIOLET-BUD and completed the long-term assessments, including 47 patients in the vitamin D3 group and 48 patients in the placebo group. The median follow-up times were 443 days (IQR, 384-484 days) and 442 days (IQR, 401-477 days) in the vitamin D3 and placebo groups, respectively. Patients who were randomized to the vitamin D3 group were older and less likely to be Black (Table 1). Other patient characteristics, including dementia, years of education, estimated intelligence, comorbidity index, SOFA score, and proportion of infection, were similar between the two groups.
Figure 1.
Consolidated Standards of Reporting Trials diagram showing patient progression through the study. ∗Some patients had multiple exclusions, and therefore the sum of the individual exclusions exceeds the total excluded.
Table 1.
Patient Characteristics at Randomization
| Characteristic | Vitamin D3 (n = 47) | Placebo (n = 48) |
|---|---|---|
| Demographic | ||
| Age, y | 56.0 (39.5-65.0) | 46.0 (37.5-63.5) |
| Female sex | 21 (44.7) | 25 (52.1) |
| Race/ethnicity | ||
| Black | 4 (8.5) | 8 (16.7) |
| Hispanic | 5 (10.5) | 5 (10.4) |
| Facility residence before hospitalization | ||
| Home | ||
| Independent | 39 (83.0) | 39 (81.2) |
| With help) | 5 (10.6) | 7 (14.6) |
| With professional help | 2 (4.3) | 0 (0.0) |
| Skilled nursing facility | 1 (2.1) | 2 (4.2) |
| Dementia | 5 (10.6) | 4 (8.3) |
| Education, y | 14 (12-16) | 14 (12-16) |
| Barona Index | 108 (102-113) | 109 (102-113) |
| Clinical | ||
| Charlson Comorbidity Index | 3.00 (1.00-4.50) | 2.00 (0.00-3.75) |
| BMI, kg/m2 | 30.9 (25.3-34.0) | 30.7 (27.6-34.5) |
| Lung injury risk factors | ||
| Pneumonia | 19 (40.4) | 15 (31.2) |
| Sepsis | 21 (44.7) | 17 (35.4) |
| Shock | 18 (38.3) | 16 (33.3) |
| Mechanical ventilation for acute respiratory failure | 3 (6.4) | 8 (16.7) |
| Aspiration | 0 (0.0) | 2 (4.2) |
| Lung contusion | 4 (8.5) | 3 (6.2) |
| Pancreatitis | 0 (0.0) | 2 (4.2) |
| Smoke inhalation | 0 (0.0) | 0 (0.0) |
| Infection (sepsis or pneumonia diagnosis) | 30 (63.8) | 30 (62.5) |
| Illness severity | ||
| SOFA score | 4.00 (2.00-7.00) | 5.00 (2.00-7.00) |
| LIPS | 5.50 (3.00-7.50) | 5.00 (3.25-7.00) |
| Mechanical ventilation | 7 (14.9) | 9 (18.8) |
| Vasopressor use | 11 (23.4) | 14 (29.2) |
| Vital signs | ||
| Pulse rate, beats/min | 83 (75.5-98.0) | 95.5 (86-114) |
| BP, mm Hg | ||
| Systolic | 113 (102.5-129) | 112 (104-134) |
| Diastolic | 65 (52-75) | 66 (57-79) |
| Respiratory rate, per min | 20 (18-24.5) | 19 (16-25) |
| Oxygen saturation, % | 97 (94-98) | 97 (94-98) |
| Vitamin D related | ||
| 25-hydroxyvitamin D, ng/mL | 15.2 (12.9-17.4) | 13.1 (12.9-15.9) |
| eGFR, mL/min/1.73 m2 | 43.0 (32.9-85.8) | 53.6 (33.2-83.3) |
Data are presented as No. (%) or median (interquartile range). eGFR = estimated glomerular filtration rate; LIPS = Lung Injury Prediction Score; SOFA = Sequential Organ Failure Assessment.
Primary Analysis
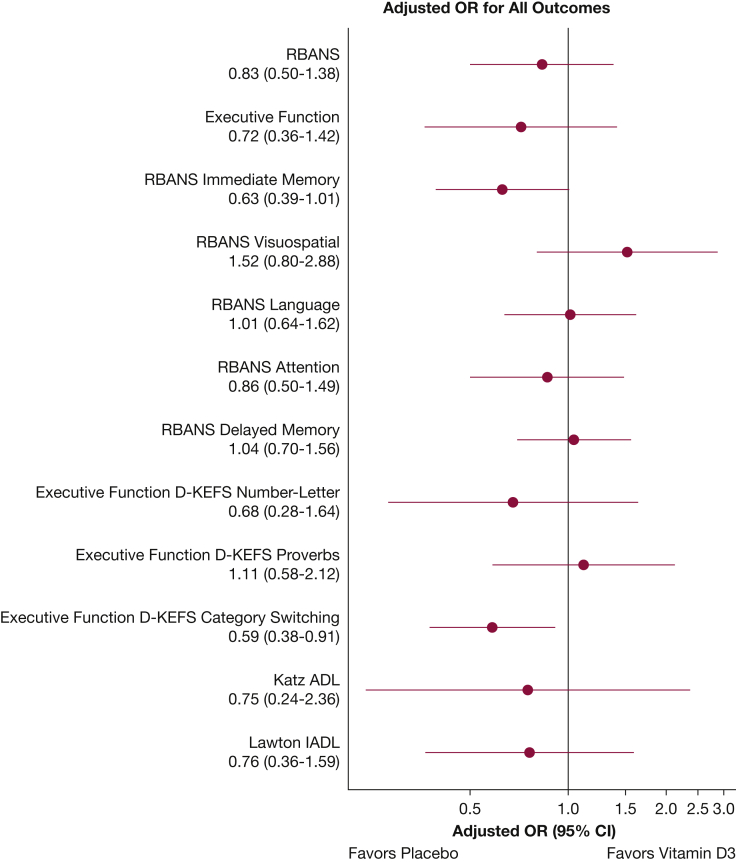
The adjusted median RBANS score was 79.6 (95% CI, 73.0-84.0) in the vitamin D3 group and 82.1 (95% CI, 74.7-84.6) in the placebo group (adjusted OR, 0.83; 95% CI, 0.50-1.38) (Table 2, Fig 2). The adjusted median executive function composite score was 8.1 (95% CI, 6.8-9.0) in the vitamin D3 group and 8.7 (95% CI, 7.4-9.3) in the placebo group (adjusted OR, 0.72; 95% CI, 0.36-1.42).
Table 2.
Primary and Secondary Outcomes
| Outcome | Vitamin D3 | Placebo | Adjusted OR (95% CI) |
|---|---|---|---|
| Primary | |||
| RBANS, adjusted | 79.6 (73.0-84.0) | 82.1 (74.7-84.6) | 0.83 (0.50-1.38) |
| Executive function score, adjusted | 8.1 (6.8-9.0) | 8.7 (7.4-9.3) | 0.72 (0.36-1.42) |
| Secondary | |||
| RBANS cognitive domain | |||
| Immediate memory, adjusted | 84.1 (80.2-89.0) | 87.6 (83.0-93.9) | 0.63 (0.39-1.01) |
| Visuospatial, adjusted | 70.7 (66.0-75.9) | 68.7 (64.6-73.9) | 1.52 (0.80-2.88) |
| Language, adjusted | 95.7 (90.1-97.4) | 95.6 (90.0-97.5) | 1.01 (0.64-1.62) |
| Attention, adjusted | 87.9 (80.7-95.0) | 90.1 (82.4-96.1) | 0.86 (0.50-1.49) |
| Delayed memory, adjusted | 85.1 (81.4-91.3) | 84.6 (81.4-91.0) | 1.04 (0.70-1.56) |
| Executive function subdomains | |||
| D-KEFS number-letter switching, adjusted | 7.8 (6.0-9.0) | 8.5 (7.3-9.8) | 0.68 (0.28-1.64) |
| D-KEFS proverbs, adjusted | 9.1 (7.8-10.1) | 8.9 (7.6-9.9) | 1.11 (0.58-2.12) |
| D-KEFS category switching, adjusteda | 8.6 (7.3-9.7) | 9.2 (7.9-10.8) | 0.59 (0.38-0.91) |
| Katz ADL | 6 (6.0-6.0) | 6.0 (6.0-6.0) | 0.75 (0.24-2.36) |
| Lawton IADL | 8.0 (7.9-8.0) | 8.0 (8.0-8.0) | 0.76 (0.36-1.59) |
| Loss of employmentb | 7 (35.0) | 10 (34.5) | — |
| Skilled nursing facility residence | 1 (2.1) | 1 (2.1) | — |
Data are presented as No. (%) or median (interquartile range), unless otherwise indicated. Adjusted medians and adjusted ORs with 95% CIs were calculated using proportional odds logistic regression28 adjusted for age, dementia, severity of illness, infection (sepsis or pneumonia), and estimated intelligence. Adjusted ORs of more than 1.0 indicate better outcomes in the vitamin D3 group compared with the placebo group. Proportional logistic regression was not performed for the loss of employment and skilled nursing facility residence because of the small number of outcomes. Global cognition was measured using the RBANS, which has a score range from 40 to 160; 100 is considered normal, and higher values are better. The executive function score ranges from 1 to 18; 10 is considered normal, and higher values indicate better executive function. This score uses the D-KEFS proverb test subscale, trail making number-letter switching subscale, and verbal fluency subscale. ADL = activities of daily living; D-KEFS = Delis-Kaplan Executive Function System; IADL = instrumental activities of daily living; — = adjusted odds ratio not calculated due to the small number of events; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status.
P < .05.
Of the 49 patients who were fully or partially employed.
Figure 2.
Graph showing adjusted ORs with their 95% CIs for the cognitive and secondary outcomes. Adjusted ORs of more than 1.0 indicate better outcomes in the vitamin D3 group compared with the placebo group. ADL = activities of daily living; D-KEFS = Delis-Kaplan Executive Function System; IADL = instrumental activities of daily living; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status.
Secondary and Sensitivity Analyses
Patients who received vitamin D3 showed significantly lower D-KEFS verbal fluency category switching subscale scores compared with those in the placebo group (adjusted OR, 0.59; 95% CI, 0.38-0.91). Other secondary outcomes were similar between the vitamin D3 and placebo groups, including immediate memory, visuospatial, language, attention, delayed memory, D-KEFS number-letter switching, D-KEFS proverbs, and functional status as measured by the Katz ADL and Lawton IADL scales (Table 2).
In sensitivity analyses, adding adjustment for time from randomization to neuropsychological testing and baseline 25-hydroxyvitamin D levels did not change the results of the primary analysis substantially (e-Table 1). The heterogeneity of treatment effect analysis can be seen in e-Figures 1-5.
Discussion
In this ancillary study of a randomized trial, we found no evidence that early administration of high-dose (540,000 International Units) enteral vitamin D3 (cholecalciferol) improved long-term cognition or executive function in critically ill patients with vitamin D deficiency. We also observed that the vitamin D3 group showed significantly lower D-KEFS verbal fluency category switching subscale scores, indicating potential harm. Because this was part of our secondary analyses, this finding should be interpreted with caution. We did not observe any effect of early vitamin D3 administration on the individual cognitive domains such as immediate and delayed memory, attention, visuospatial construction, language, or most of the executive function subscales. The point estimates generally favored placebo over vitamin D3 treatment for most outcomes that we assessed. Taken together with the original randomized control trial that reported an absence of effect of vitamin D3 on 90-day mortality,13 these findings suggest that high-dose vitamin D3 administration is unlikely to have a role in the management of critically ill patients who are vitamin D deficient to improve mortality or morbidity.
Previous observational studies regarding vitamin D3 administration and cognitive function were conducted in community-dwelling older adults. These studies reported that patients with higher vitamin D intake were less likely to be cognitively impaired,31 less likely to demonstrate Alzheimer dementia,32 and had better cognitive performance, including executive functioning.12 In the current study, we observed no effect of vitamin D3 administration on long-term cognition in a cohort of critically ill patients. The lack of effect is unlikely to be secondary to insufficient plasma 25-hydroxyvitamin D levels; in the intervention arm of VIOLET, 87% had plasma 25-hydroxyvitamin D concentrations of ≥ 20 mg/dL on day 3,13 which is considered the threshold for vitamin D deficiency. Only 6% of patients in the placebo arm showed plasma 25-hydroxyvitamin D concentrations of ≥ 20 mg/dL on day 3.13 Previous studies have shown that plasma 25-hydroxyvitamin D concentrations plateau at 3 days and persist for up to 1 month after a single 540,000-International Unit dose of vitamin D3.14,33 Although vitamin D3 was given within 12 h of the clinician’s decision to admit the patient to the ICU, it may have been given too late in the disease course, when systemic and CNS inflammation is already well established. However, it would be extremely difficult to administer vitamin D3 earlier in the course of critical illness than was achieved in VIOLET.
The adjusted median RBANS score was 79.6 and 82.1 in the vitamin D3 and placebo groups, respectively, in a geographically diverse cohort. These scores are approximately 1.5 SD less than the age-adjusted population mean of 100 ± 15 and are similar to scores observed in patients with mild cognitive impairment.15 These findings are remarkably similar to those of the Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU) study, which evaluated global cognition in 821 patients in the ICU; they also observed a median RBANS score of 80 at 12 months.1 Our study confirmed that critically ill patients are at particularly high risk for developing LTCI and underscores the need for interventions designed to mitigate this adverse outcome.
One novel aspect of our study is the implementation of videophone-assisted neuropsychological testing using inexpensive high-definition web cameras. This allowed for centrally located raters to administer cognitive testing in seven geographically diverse sites across the United States. Several studies have reported the results for neurocognitive testing conducted by videoconferencing to be highly correlated with face-to-face evaluations.21,34,35 Galusha-Glasscock et al21 observed that the videoconference-assisted RBANS score, our primary cognitive measure, was highly correlated with the in-person RBANS score with a correlation coefficient of 0.88 (P < .0001) in patients with and without dementia. This approach could facilitate future ICU clinical trials that assess cognition as an outcome.
No differences were observed in the Katz ADL and Lawton IADL scores between the vitamin D3 and placebo arms, but the medians and IQRs were close to their maximum scores, indicating ceiling effects. These functional status measures were designed for geriatric patients, and the median ages were 56 and 46 years for the vitamin D3 and placebo groups, respectively. It is possible that an effect may have been found if we had used a more robust basic ADL assessment such as the Barthel Index (score ranges from 0 to 100)36 or instrumental ADL assessment such as the Functional Activities Questionnaire (scores range from 0 to 30).37 Alternatively, the 6-min walk test could be used to objectively assess.38
Our study has several limitations. We required enrollment within 12 h of the decision to admit a patient to an ICU; this ensured early treatment among patients who were enrolled, but also resulted in some patients not being enrolled because of an inability to obtain consent for trial participation in this narrow window. The narrow window for consent may have led to the preferential enrollment of less severely ill patients who could consent for themselves over more severely ill patients who required surrogate decision-makers for consent. We did not provide long-term vitamin D3 supplementation because the intervention was a single dose. However, the loss of cognition predominantly occurs within the first month of the acute illness,39 and our high-dose regimen has been shown to replete vitamin D effectively during this period.14 It is unlikely that that long-term vitamin D3 supplementation would have markedly improved long-term cognition. We also enrolled and collected data for VIOLET-BUD in those who survived; this potentially introduced additional selection bias. However, mortality was similar between the vitamin D3 and placebo groups, minimizing this bias. With 95 patients enrolled, some baseline characteristics may have been imbalanced despite randomization. Our primary analysis included multivariate adjustment for important baseline characteristics, but residual effects from baseline imbalances could have affected the point estimates. Because the VIOLET parent trial stopped enrollment early due to futility, VIOLET-BUD did not meet its original enrollment goal. However, we enrolled enough patients to detect an eight-point difference in RBANS, which some consider to be the minimum clinically important difference.30 Most point estimates also favored placebo, making it unlikely that increasing the number of patients enrolled would have demonstrated beneficial effects for vitamin D3 treatment.
Interpretation
In conclusion, vitamin D3 repletion did not improve long-term global cognition or executive function in critically ill patients who were vitamin D deficient and at high risk of mortality. High-dose vitamin D3 administration is unlikely to have a role in the management of critically ill patients who are vitamin D deficient to improve mortality or morbidity.
Take-home Points.
Study Question: Does a single high-dose (540,000 International Units) enteral treatment of vitamin D3 given shortly after hospital admission in critically ill patients who are vitamin D deficient improve long-term global cognition or executive function?
Results: Using multivariate proportional odds logistic regression, the adjusted ORs for vitamin D3 treatment were 0.83 (95% CI, 0.50-1.38) and 0.72 (95% CI, 0.36-1.42) for global cognition and executive function, respectively; an OR of < 1.0 indicates better outcomes in the placebo group.
Interpretation: In vitamin D-deficient, critically-ill adults, a large dose of enteral vitamin D3 did not improve long-term global cognition or executive function.
Acknowledgments
Author contributions: J. H. H. takes responsibility for the content of the manuscript, including the data and analysis. J. H. H. was primarily responsible for obtaining funding. J. H. H., A. A. G., and W. H. S. conceived the study. J. H. H., A. A. G., E. M. C., J. C. J., and W. H. S. designed the study. J. H. H., A. A. G., S. M. B., A. B., E. M. C., A. A. H., P. C. H., J. C. J., A. K., and N. R. collected data and assisted in obtaining funding. O. M. O. and R. R. analyzed the data. All authors contributed to data interpretation, drafting, critical review, and final approval of the manuscript and are accountable for the accuracy and integrity of the results.
Financial/nonfinancial disclosures: None declared.
∗Vitamin D to Improve Outcomes by Leveraging Early Treatment Network Investigators: A full list of the VIOLET Investigators and members of the National Heart, Lung, and Blood Institute PETAL Clinical Trials Network is provided in e-Appendix 1.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors acknowledge the study personnel listed in the Supplemental Materials who assisted in data collection for this manuscript. The authors also thank Lora A. Reinick, MD, of the National Heart, Lung and Blood Institute, for her valuable assistance with this project especially with study design.
Additional information: The e-Appendix, e-Figures, and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by the National Heart, Lung, and Blood Institute [Grant R56HL141567]. Additional support was provided by the National Center for Advancing Translational Sciences [Grant 2 UL1 TR000445-06] and the Veterans Affairs Geriatric Research, Education, and Clinical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of Vanderbilt University Medical Center, the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Veterans Affairs.
Supplementary Data
References
- 1.Pandharipande P.P., Girard T.D., Jackson J.C. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norman B.C., Jackson J.C., Graves J.A. Employment outcomes after critical illness: an analysis of the Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors cohort. Crit Care Med. 2016;44(11):2003–2009. doi: 10.1097/CCM.0000000000001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simone M.J., Tan Z.S. The role of inflammation in the pathogenesis of delirium and dementia in older adults: a review. CNS Neurosci Ther. 2011;17(5):506–513. doi: 10.1111/j.1755-5949.2010.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Ambrosio D., Cippitelli M., Cocciolo M.G. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101(1):252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller K., Haahr P.M., Diamant M., Rieneck K., Kharazmi A., Bendtzen K. 1,25-Dihydroxyvitamin D3 inhibits cytokine production by human blood monocytes at the post-transcriptional level. Cytokine. 1992;4(6):506–512. doi: 10.1016/1043-4666(92)90012-g. [DOI] [PubMed] [Google Scholar]
- 6.Schleithoff S.S., Zittermann A., Tenderich G., Berthold H.K., Stehle P., Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 7.Balabanova S., Richter H.P., Antoniadis G. 25-Hydroxyvitamin D, 24, 25-dihydroxyvitamin D and 1,25-dihydroxyvitamin D in human cerebrospinal fluid. Klin Wochenschr. 1984;62(22):1086–1090. doi: 10.1007/BF01711378. [DOI] [PubMed] [Google Scholar]
- 8.Lefebvre d’Hellencourt C., Montero-Menei C.N., Bernard R., Couez D. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J Neurosci Res. 2003;71(4):575–582. doi: 10.1002/jnr.10491. [DOI] [PubMed] [Google Scholar]
- 9.Evans C.S., Self W., Ginde A.A., Chandrasekhar R., Ely E.W., Han J.H. Vitamin D deficiency and long-term cognitive impairment among older adult emergency department patients. West J Emerg Med. 2019;20(6):926–930. doi: 10.5811/westjem.2019.8.43312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annweiler C., Allali G., Allain P. Vitamin D and cognitive performance in adults: a systematic review. Eur J Neurol. 2009;16(10):1083–1089. doi: 10.1111/j.1468-1331.2009.02755.x. [DOI] [PubMed] [Google Scholar]
- 11.Balion C., Griffith L.E., Strifler L. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012;79(13):1397–1405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annweiler C., Fantino B., Gautier J., Beaudenon M., Thiery S., Beauchet O. Cognitive effects of vitamin D supplementation in older outpatients visiting a memory clinic: a pre-post study. J Am Geriatr Soc. 2012;60(4):793–795. doi: 10.1111/j.1532-5415.2011.03877.x. [DOI] [PubMed] [Google Scholar]
- 13.Ginde A.A., Brower R.G., Caterino J.M. Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. N Engl J Med. 2019;381(26):2529–2540. doi: 10.1056/NEJMoa1911124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amrein K., Schnedl C., Holl A. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312(15):1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 15.Randolph C., Tierney M.C., Mohr E., Chase T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 16.Duff K., Hobson V.L., Beglinger L.J., O’Bryant S.E. Diagnostic accuracy of the RBANS in mild cognitive impairment: limitations on assessing milder impairments. Arch Clin Neuropsychol. 2010;25(5):429–441. doi: 10.1093/arclin/acq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duff K., Humphreys Clark J.D., O’Bryant S.E., Mold J.W., Schiffer R.B., Sutker P.B. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol. 2008;23(5):603–612. doi: 10.1016/j.acn.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobson V.L., Hall J.R., Humphreys-Clark J.D., Schrimsher G.W., O’Bryant S.E. Identifying functional impairment with scores from the repeatable battery for the assessment of neuropsychological status (RBANS) Int J Geriatr Psychiatry. 2010;25(5):525–530. doi: 10.1002/gps.2382. [DOI] [PubMed] [Google Scholar]
- 19.McKay C., Casey J.E., Wertheimer J., Fichtenberg N.L. Reliability and validity of the RBANS in a traumatic brain injured sample. Arch Clin Neuropsychol. 2007;22(1):91–98. doi: 10.1016/j.acn.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Latzman R.D., Markon K.E. The factor structure and age-related factorial invariance of the Delis-Kaplan Executive Function System (D-KEFS) Assessment. 2010;17(2):172–184. doi: 10.1177/1073191109356254. [DOI] [PubMed] [Google Scholar]
- 21.Galusha-Glasscock J.M., Horton D.K., Weiner M.F., Cullum C.M. Video Teleconference administration of the Repeatable Battery for the Assessment of Neuropsychological Status. Arch Clin Neuropsychol. 2016;31(1):8–11. doi: 10.1093/arclin/acv058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han J.H., Collar E.M., Lassen-Greene C., Self W.H., Langford R.W., Jackson J.C. Feasibility of videophone-assisted neuropsychological testing for intensive care unit survivors. Am J Crit Care. 2020;29(5):398–402. doi: 10.4037/ajcc2020492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Needham D.M., Colantuoni E., Mendez-Tellez P.A. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124. doi: 10.1136/bmj.e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barona A., Reynolds C.R., Chastain R. A demographically based index of premorbid intelligence for the WAIS-R. J Consult Clin Psychol. 1984;35:341–345. [Google Scholar]
- 25.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez A.V., Steyerberg E.W., Habbema J.D. Covariate adjustment in randomized controlled trials with dichotomous outcomes increases statistical power and reduces sample size requirements. J Clin Epidemiol. 2004;57(5):454–460. doi: 10.1016/j.jclinepi.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 27.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48(4):817–838. [Google Scholar]
- 28.Liu Q., Shepherd B.E., Li C., Harrell F.E., Jr. Modeling continuous response variables using ordinal regression. Stat Med. 2017;36(27):4316–4335. doi: 10.1002/sim.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Copay A.G., Subach B.R., Glassman S.D., Polly D.W., Jr., Schuler T.C. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Phillips R., Qi G., Collinson S.L. The minimum clinically important difference in the repeatable battery for the assessment of neuropsychological status. Clin Neuropsychol. 2015;29(7):905–923. doi: 10.1080/13854046.2015.1107137. [DOI] [PubMed] [Google Scholar]
- 31.Annweiler C., Schott A.M., Rolland Y., Blain H., Herrmann F.R., Beauchet O. Dietary intake of vitamin D and cognition in older women: a large population-based study. Neurology. 2010;75(20):1810–1816. doi: 10.1212/WNL.0b013e3181fd6352. [DOI] [PubMed] [Google Scholar]
- 32.Annweiler C., Rolland Y., Schott A.M. Higher vitamin D dietary intake is associated with lower risk of Alzheimer’s disease: a 7-year follow-up. J Gerontol. 2012;67(11):1205–1211. doi: 10.1093/gerona/gls107. [DOI] [PubMed] [Google Scholar]
- 33.Amrein K., Sourij H., Wagner G. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care. 2011;15(2):R104. doi: 10.1186/cc10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munro Cullum C., Hynan L.S., Grosch M., Parikh M., Weiner M.F. Teleneuropsychology: evidence for video teleconference-based neuropsychological assessment. J Int Neuropsychol Soc. 2014;20(10):1028–1033. doi: 10.1017/S1355617714000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadsworth H.E., Galusha-Glasscock J.M., Womack K.B. Remote neuropsychological assessment in rural American Indians with and without cognitive impairment. Arch Clin Neuropsychol. 2016;31(5):420–425. doi: 10.1093/arclin/acw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahoney F.I., Barthel D.W. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 37.Pfeffer R.I., Kurosaki T.T., Harrah C.H., Jr., Chance J.M., Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 38.Needham D.M., Sepulveda K.A., Dinglas V.D. Core outcome measures for clinical research in acute respiratory failure survivors. An International Modified Delphi Consensus Study. Am J Respir Crit Care Med. 2017;196(9):1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inouye S.K., Marcantonio E.R., Kosar C.M. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12(7):766–775. doi: 10.1016/j.jalz.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.