Significance
During vitellogenesis of oviparous animals, vitellogenin receptor (VgR)–mediated endocytosis is crucial for the incorporation of yolk proteins into oocytes. VgR also serves as a gatekeeper for vertical transmission of pathogenic microbes and symbionts. Juvenile hormone (JH) acts as a gonadotrophic hormone and stimulates this process in many insect species, but the regulatory mechanisms remain elusive. We find that JH activates the GPCR-PLC-PKC-ι cascade and phosphorylates VgR in an EGF-precursor homology domain. Endosomal acidification leads to VgR dephosphorylation. Phosphorylation is essential for VgR to translocate from oocyte cytoplasm to the membrane to bind with vitellogenin on the membrane and to internalize vitellogenin in oocytes. Moreover, JH-promoted VgR phosphorylation for intracellular recycling and vitellogenin deposition is evolutionarily conserved across divergent insect orders.
Keywords: vitellogenin receptor, vitellogenin, juvenile hormone, endocytosis, protein kinase C
Abstract
Vitellogenin receptor (VgR) plays a pivotal role in ovarian vitellogenin (Vg) uptake and vertical transmission of pathogenic microbes and Wolbachia symbionts. However, the regulatory mechanisms of VgR action as an endocytic receptor and translocation from oocyte cytoplasm to the membrane remain poorly understood. Here, by using the migratory locust Locusta migratoria as a model system, we report that juvenile hormone (JH) promotes VgR phosphorylation at Ser1361 in the second EGF-precursor homology domain. A signaling cascade including GPCR, PLC, extracellular calcium, and PKC-ι is involved in JH-stimulated VgR phosphorylation. This posttranslational regulation is a prerequisite for VgR binding to Vg on the external surface of the oocyte membrane and subsequent VgR/Vg endocytosis. Acidification, a condition in endosomes, induces VgR dephosphorylation along with the dissociation of Vg from VgR. Phosphorylation modification is also required for VgR recycling from oocyte cytoplasm to the membrane. Additionally, VgR phosphorylation and its requirement for Vg uptake and VgR recycling are evolutionarily conserved in other representative insects including the cockroach Periplaneta americana and the cotton bollworm Helicoverpa armigera. This study fills an important knowledge gap of low-density lipoprotein receptors in posttranslational regulation, endocytosis, and intracellular recycling.
In female reproduction of oviparous animals from worms to chickens, vitellogenesis is a prerequisite to egg production and embryonic growth after oviposition. During insect vitellogenesis, the yolk protein precursor, vitellogenin (Vg) is mainly synthesized in the fat body and secreted into the hemolymph. By the aid of hemolymph circulation, Vg is transported through intercellular spaces (termed patency) in the follicle epithelium and reaches the oocyte membrane, thereby being incorporated into oocytes by Vg receptor (VgR)-mediated endocytosis (1–4). In addition to carrying Vg for oocyte uptake, VgR is found to serve as a gatekeeper that regulates the vertical transmission of pathogenic microbes and Wolbachia symbionts (5–7). In the small brown planthopper Laodelphax striatellus, the rice stripe virus hitchhikes on Vg and enters developing oocytes through VgR-mediated endocytosis (8–10). Wolbachia symbionts in L. striatellus also achieve maternal transmission through the Vg/VgR machinery (7). Depletion of VgR in the tick Haemaphysalis longicornis leads to blocked transmission of the parasite Babesia gibsoni from the midgut into oocytes (11). In another tick, Rhipicephalus microplus, VgR knockdown causes inhibited vertical transmission of Babesia bovis into eggs (12). Insect VgRs are classified into the low-density lipoprotein receptor (LDLR) family (13–15). In VgR-mediated Vg internalization, the Vg/VgR complex on the oocyte membrane clusters in clathrin-coated pits and invaginates into the cytoplasm (16, 17). The complex then pinches off to form intracellular coated vesicles (4, 18). Vg and VgR are dissociated by ATP-dependent acidification after being transformed into the endosome or transitional yolk body. Thereafter, VgR travels back to the oocyte membrane, whereas Vg is crystallized and stored as vitellin (Vn) in yolk bodies for future embryo development (18–21). While extensive studies have been conducted to elucidate Vg synthesis and Vg-dependent microbe transmission, the molecular base of VgR-mediated Vg and microbe endocytosis, particularly the intracellular recycling of VgR, is poorly understood.
Juvenile hormone (JH), a sesquiterpenoid hormone produced in corpora allata, is known to stimulate vitellogenesis and egg development in a variety of insect species (2, 22, 23). The molecular action of JH relies on its intracellular receptor complex comprising two bHLH-PAS transcription factors, Methoprene-tolerant (Met) and Taiman (Tai) (24–26). In adult insects, JH requires Met/Tai in achieving its previtellogenic and vitellogenic effects on fat body competency and Vg synthesis (27–30). JH also induces the dimerization of Met with Cycle that up-regulates gene transcription for previtellogenic development of the mosquito Aedes aegypti (31). Moreover, JH stimulates Met phosphorylation via a signaling cascade, including receptor tyrosine kinase (RTK), phospholipase C (PLC), inositol trisphosphate (IP3), calcium/calmodulin-dependent protein kinase II (CaMKII), and protein kinase C (PKC), that enhances its transcriptional activity during the previtellogenic development of Ae. aegypti (32, 33). JH also triggers the phosphorylation of Serine/Arginine-rich (pre-mRNA [messenger RNA]) Splicing Factor (SRSF) via the RTK-PI3K-Akt cascade, which induces the generation of the A/B isoforms of Tai to potentiate the transcriptional activity of 20-hydroxyecdysone (20E)-ecdysone receptor (EcR)/ultraspiracle (USP) in Ae. aegypti vitellogenesis (34). In the cotton bollworm Helicoverpa armigera, JH induces Met phosphorylation, consequently increasing the interaction of Met with Tai and promoting the transcription of the JH early response gene, Kr-h1 (35). In the migratory locust Locusta migratoria, JH activates the GPCR/RTK-PLC-IP3R-PKC signaling pathway that stimulates the phosphorylation of the Na+/K+-ATPase α-subunit for patency induction and Vg transportation to the oocyte surface (36). Despite the characterization of JH action in insect previtellogenesis and vitellogenesis, little is known about the mechanisms of JH regulation in Vg uptake and VgR functioning.
L. migratoria, bearing panoistic ovaries and synchronously matured oocytes, has been a long-standing model for studying JH-dependent reproduction (1, 2, 37). In L. migratoria, JH acts independently of 20E in promoting Vg synthesis in the fat body, secretion into the hemolymph, and uptake by maturing oocytes (38–40). To elucidate the mechanisms of JH action in Vg uptake by maturing oocytes, we employed a quantitative phosphorproteomic approach to identify phosphoprotein profiles in the ovaries of L. migratoria. Phosphorylated VgR and its interaction with PKC were identified in vitellogenic ovaries. We found that JH promoted VgR phosphorylation at Ser1361 via the GPCR-PLC-PKC-ι signaling cascade. VgR phosphorylation was indispensable for its binding with Vg at the oocyte membrane and its recycling from oocyte cytoplasm to the membrane. Additionally, VgR phosphorylation and its requirement in Vg uptake have been found to be evolutionarily conserved in other insects including the American cockroach Periplaneta americana, which has panoistic ovaries, and H. armigera, which possesses meroistic ovaries.
Results
JH Induces VgR Phosphorylation in the Ovaries of Vitellogenic Females.
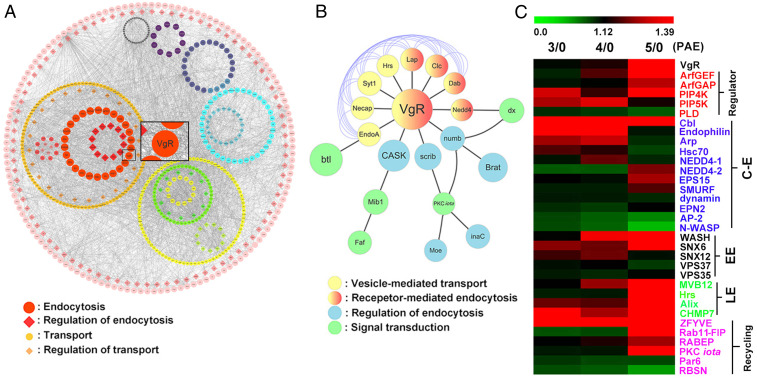
The first gonadotrophic cycle of adult female locusts under this study was about 8 d. Adult females underwent previtellogenic development within 3 d post adult eclosion (PAE), followed by Vg synthesis starting at ∼4 d PAE. To elucidate protein phosphorylation involved in JH-dependent female production, we initially performed quantitative phosphoproteomic analysis using proteins extracted from the ovaries of adult female locusts at 0, 3, 4, and 5 d PAE, respectively. A total of 6,973 phosphoric sites with 2,655 proteins were identified (SI Appendix, Table S1). Compared to the day of adult ecdysis, 590 more phosphoric sites with 466 proteins were detected in the ovaries of adult females at 3 d PAE (SI Appendix, Table S1). In the ovaries of 4-d-old adult females, 730 up-regulated phosphoric sites with 497 proteins were identified (SI Appendix, Table S1). With respect to the ovaries of adult females at 5 d PAE, 840 up-regulated phosphoric sites were detected with 557 proteins (SI Appendix, Table S1). Integrated protein–protein interaction network (PPI) analysis of up-regulated phosphoproteins by Cytoscape software revealed VgR phosphorylation in the ovaries of adult females on day 5 (Fig. 1A). Further analysis of phosphorylated VgR (p-VgR)–based network showed interaction of PKC with VgR (Fig. 1B), suggesting the possible involvement of PKC in VgR phosphorylation. Heatmap analysis by Multiple Experiment Viewer (MeV) software displayed that phosphoprotein abundance related to Vg uptake and VgR recycling increased at 5 d PAE (Fig. 1C). Multiple kinases were predicted for triggering the phosphorylation of these proteins (SI Appendix, Table S2). Interestingly, the dynamics of PKC iota (PKC-ι) phosphorylation appeared to have a similar pattern with VgR phosphorylation (Fig. 1C). The results suggest the involvement of PKC-ι in VgR phosphorylation during L. migratoria vitellogenesis.
Fig. 1.
Identification of p-VgR and its interacting proteins in the ovaries of vitellogenic females. (A) Detection of p-VgR by phosphoproteomic analysis with proteins extracted from the ovaries of 5-d-old adult females. Phosphorylated proteins involved in endocytosis were selected by PPI analysis using the cutoff criteria of fold change > 1.2 and P < 0.05 compared to that on the day of adult ecdysis. (B) VgR interaction network in ovarian Vg uptake. (C) Relative abundance of phosphorylated proteins during VgR-mediated Vg uptake at 3 to 5 d PAE compared to 0 d PAE. C-E, clathrin-dependent endocytosis; EE, early endosome; LE, late endosome.
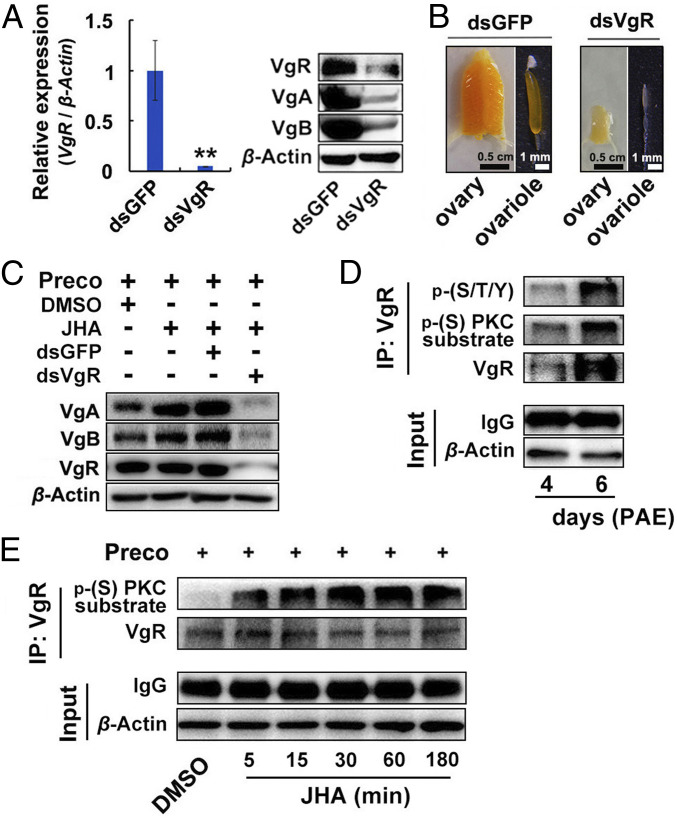
As VgR function in L. migratoria vitellogenesis had not been previously investigated by loss-of-function approach, we conducted VgR (GenBank: MH208453) RNA interference (RNAi) in vitellogenic adult females. L. migratoria has two Vg genes, VgA and VgB, that are coordinately induced by JH and expressed in similar patterns (36). Depletion of VgR caused markedly reduced accumulation of Vg protein in the ovaries (Fig. 2A). Consequently, the growth of primary oocytes and ovaries of VgR-depleted adult females was blocked (Fig. 2B). To further characterize the role of VgR in JH-induced Vg uptake in ovaries, a JH-deprived condition was achieved by precocene treatment, followed by application of the JH mimic, methoprene, to restore the JH activity. As shown in Fig. 2C, application of methoprene on precocene-treated adult females stimulated Vg deposition in the ovaries, whereas VgR knockdown inhibited the capacity of JH in induction of ovarian Vg uptake. These results reinforce our view that VgR plays a pivotal role in JH-dependent Vg incorporation in the ovaries of vitellogenic females.
Fig. 2.
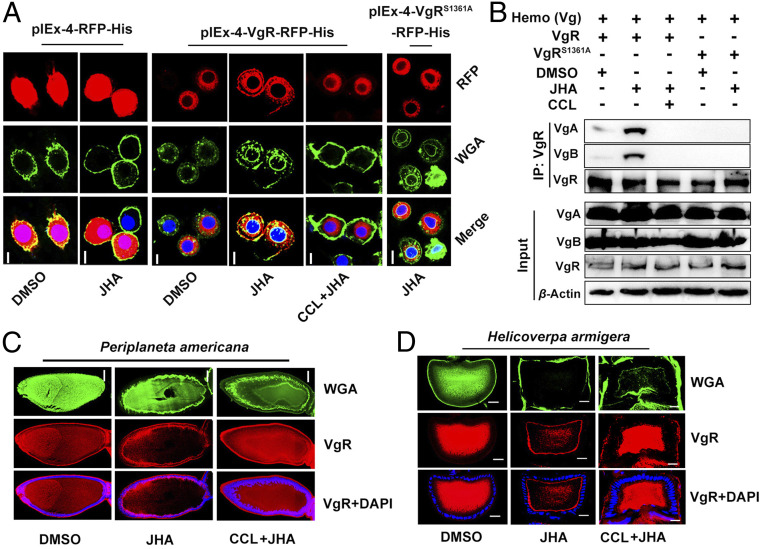
Effect of VgR knockdown on ovarian Vg uptake and responsiveness of VgR phosphorylation to JH. (A, Left) Knockdown efficiency of VgR in the ovaries of 6-d-old adult females measured by qRT-PCR. (Right) Relative levels of VgR, VgA, and VgB in the ovaries subjected to VgR knockdown. (B) Representative phenotypes of ovaries and ovarioles of VgR-depleted adult females on day 6. (Scale bars: ovary, 0.5 cm; ovariole, 1 mm.) (C) Inhibition of VgR knockdown on JH-induced ovarian Vg uptake in 6-d-old adult females. Preco, precocene; JHA, methoprene. (D) Immunoprecipitation and Western blot showing PKC-mediated VgR phosphorylation in the ovaries of adult females. (E) JH induction on PKC-mediated VgR phosphorylation. **P < 0.01 compared to the dsGFP control. n = 5.
Since PKC, presumably PKC-ι, was predicted to interact with p-VgR (Fig. 1 B and C), we next carried out immunoprecipitation assays using antibodies against phospho-(S/T/Y) and phospho-(Ser) PKC substrate. p-VgR was detected in protein extracts from the ovaries of adult females at 4 d PAE, and the abundance of p-VgR apparently increased on day 6 (Fig. 2D). As hemolymph JH titers progressively elevated during the previtellogenic stage and rose to a peak in the vitellogenic phase (41), the enhanced abundance of p-VgR in the ovaries of 6-d-old adult females appeared to correlate with the increased JH titers in vitellogenic females. To confirm the responsiveness of VgR phosphorylation to JH, we performed immunoprecipitation using antibodies against phospho-(Ser) PKC substrate and VgR. The protein extracts were isolated from the ovaries of JH-deprived adult females at 6 d PAE as well as those further treated with methoprene for 5 to 180 min. As shown in Fig. 2E, p-VgR abundance was extremely low in precocene-treated adult females. Additional application of methoprene on JH-deprived adult females stimulated VgR phosphorylation, starting from 5 min exposure (Fig. 2E). Collectively, the above data in this section document that JH induces VgR phosphorylation via PKC in the ovaries of vitellogenic females of L. migratoria.
JH Stimulates VgR Phosphorylation at Amino Acid Residue Ser1361.
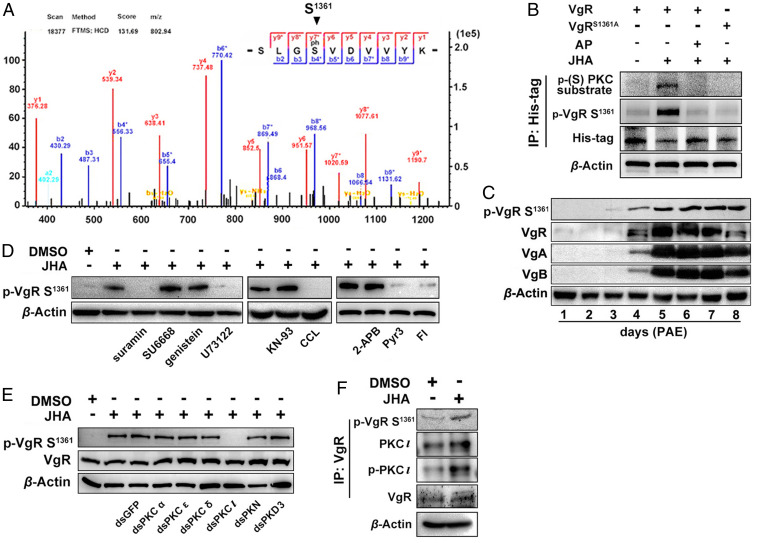
To identify the VgR phosphorylation site, we performed a liquid chromatography/tandem mass spectrometry (LC-MS/MS) analysis using proteins isolated from the ovaries of vitellogenic females at 5 d PAE. As shown in Fig. 3A, VgR was phosphorylated at Ser1361 that localizes in the second epidermal growth factor (EGF)-precursor homology domain (SI Appendix, Fig. S1). We next performed immunoprecipitation using protein extracts from Sf9 cells overexpressed with wild-type His-VgR or mutated His-VgRS1361A to verify VgR phosphorylation at Ser1361. Immunoprecipitations with His-tag antibody, followed by Western blots with phospho-VgR (Ser1361) antibody or phospho-(Ser) PKC substrate antibody, demonstrated that VgR phosphorylation was removed after alkaline phosphatase (AP) treatment. Mutational VgRS1361A could not be recognized by either phospho-PKC substrate or phospho-VgR (Ser1361) antibody (Fig. 3B). Taken together, these observations indicate that VgR phosphorylation occurs at Ser1361. To reveal the dynamics of p-VgR during the first gonadotrophic cycle of adult females, Western blots were conducted with the phospho-VgR (Ser1361) antibody and protein extracts from the ovaries of adult females at 0 to 8 d PAE. As illustrated in Fig. 3C, p-VgR was very weak on day 3, increased on day 4, and reached a peak at 5 to 8 d PAE. The elevated abundance of p-VgR appeared to correlate with the increased titer of JH from the previtellogenic to vitellogenic stages (41). Also, the fluctuation of p-VgR abundance tended to synchronize with that of Vg proteins (Fig. 3C). These observations together suggest a link of VgR phosphorylation at Ser1361 with JH and vitellogenesis.
Fig. 3.
Identification of VgR phosphorylation site and its kinase. (A) VgR phosphorylation at Ser1361 identified by LC-MS/MS analysis of ovarian protein extracts from vitellogenic females. (B) Verification of VgR phosphorylation at Ser1361. Proteins extracted from Sf9 cells transfected with wild-type pIEx-4-VgR-RFP-His (VgR) versus mutated pIEx-4-VgRS1361A-RFP-His (VgRS1361A). AP for 30 min. JHA, methoprene for 30 min; p-VgR S361, phospho-VgR (Ser1361) antibody. (C) Relative abundance of p-VgR, VgR, VgA, and VgB in the ovaries of adult females during the first gonadotrophic cycle. (D) Effects of GPCR (suramin), RTK (SU6668 and genistein), PLC (U73122), CaMKII (KN-93), PKC (CCL), IP3R (2-APB), TRPC3 channel inhibitor (Pyr3), and T-type voltage-gated calcium channel (Fl) inhibitors on JH-stimulated VgR phosphorylation. (E) Effect of PKC isoform knockdown on VgR protein abundance and JH-stimulated VgR phosphorylation. (F) Immunoprecipitation and Western blot showing JH-induced interaction of PKC-ι and VgR in the ovaries of vitellogenic females.
JH Promotes VgR Phosphorylation via the GPCR-PLC-PKC-ι Signaling Cascade.
To unveil the molecules potentially involved in the regulation of JH-dependent VgR phosphorylation at Ser1361, we selected a series of inhibitors to treat JH-deprived 4-d-old adult females with additional methoprene administration. As shown in Fig. 3D, methoprene treatment of JH-deprived adult females induced VgR phosphorylation at Ser1361. Further application of GPCR inhibitor (suramin), PLC inhibitor (U73122), and PKC inhibitor (chelerythrine chloride [CCL]) abolished JH-induced VgR phosphorylation (Fig. 3D). However, injection of RTK inhibitors (SU6668 and genistein) or CaMKII inhibitor (KN-93) had no obvious effect on JH-induced VgR phosphorylation (Fig. 3D). The data indicate that JH activates a signaling cascade including GPCR, PLC, and PKC that mediates VgR phosphorylation at Ser1361. Moreover, application of transient receptor potential canonical (TRPC) channels inhibitor (Pyr3) and T-type voltage-gated calcium channel inhibitor (flunarizine dihydrochloride [FL]) attenuated methoprene-induced VgR phosphorylation (Fig. 3D). In contrast, treatment of inositol trisphosphate receptor (IP3R) inhibitor (2-aminoethoxy-diphenyl borate [2-APB]) had no distinct effect (Fig. 3D). The results suggest that JH-stimulated VgR phosphorylation relies on extracellular calcium but not intracellular calcium stores.
To determine the involvement of PKC isoforms in JH-stimulated VgR phosphorylation, we performed RNAi screening of genes coding for PKC-α (GenBank: MN793141), PKC-δ (GenBank: MN793140), PKC-ε (GenBank: MN793143), PKC-ι (GenBank: MN793142), PKN (GenBank: MN793144), and PKD3 (GenBank: MN793145) in adult females on day 4 (SI Appendix, Fig. S2). A Western blot using the ovarian protein extracts and phospho-VgR (Ser1361) antibody exhibited that depletion of PKC-ι completely blocked JH-stimulated VgR phosphorylation, whereas knockdown of PKC-α, PKC-ε, PKC-δ, PKN, or PKD3 had no effect on VgR phosphorylation (Fig. 3E). Notably, the abundance of total VgR proteins had no apparent change after knockdown of PKC-ι or other PKC isoforms (Fig. 3E). We next characterized JH-stimulated interaction of PKC-ι and VgR during locust vitellogenesis using protein extracts from the ovaries of 4-d-old adult females treated with methoprene for 24 h. Immunoprecipitation with anti-VgR antibody followed by Western blots with antibodies against p-VgR (Ser1361), PKC-ι, or phospho-PKC-ι (p-PKC-ι) revealed that methoprene stimulated the interaction of p-PKC-ι with VgR for VgR phosphorylation (Fig. 3F). These observations indicate that JH-stimulated VgR phosphorylation at Ser1361 is triggered by PKC-ι.
VgR Phosphorylation Is Required for Its Binding to Vg and Intracellular Recycling.
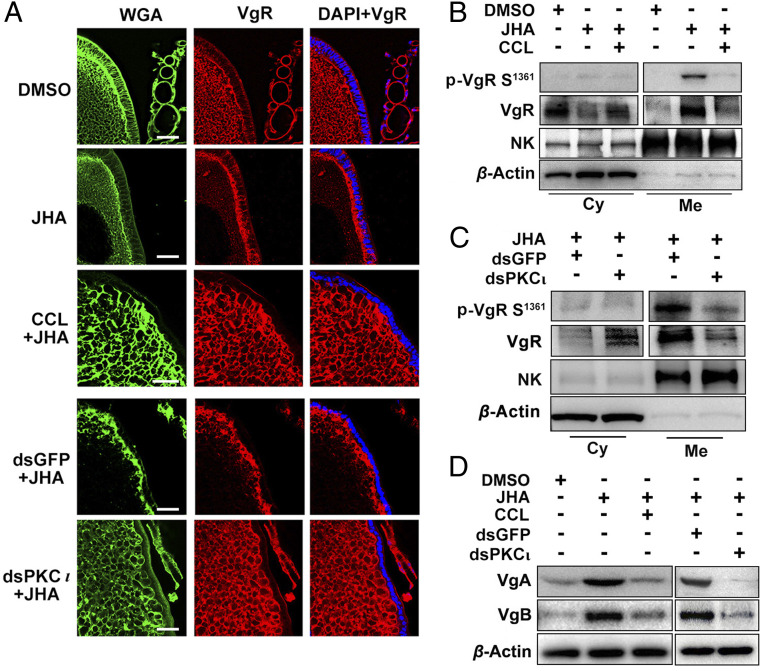
As VgR phosphorylation by PKC-ι was concomitant with vitellogenesis, we next asked how p-VgR participated in Vg deposition in oocytes. To visualize the subcellular distribution of VgR in oocytes, we performed immunostaining of primary oocytes of adult females at 4 to 6 d PAE. VgR principally distributed in oocyte cytoplasm on day 4 but dominantly localized in oocyte membrane on days 5 to 6 (SI Appendix, Fig. S3). These observations indicate the intracellular translocation of VgR during JH-stimulated vitellogenesis in locusts. We next conducted immunostaining of primary oocytes of 4-d-old adult females injected with CCL or double-stranded RNA of PKC-ι (dsPKC-ι) and further treated with methoprene. In the dimethylsulfoxide (DMSO)–treated controls, VgR primarily localized in oocyte cytoplasm (Fig. 4A). Application of methoprene promoted VgR migration toward the oocyte periphery (Fig. 4A). However, pretreatment of CCL or knockdown of PKC-ι inhibited the capacity of methoprene to induce VgR migration from oocyte cytoplasm to the membrane (Fig. 4A), suggesting an essential role of PKC-ι in VgR translocation. Subsequent Western blots using the phospho-VgR (Ser1361) antibody confirmed the accumulation of p-VgR on the oocyte membrane after JH induction (Fig. 4B). The abundance of p-VgR was remarkably reduced in oocyte membrane extracts from adult females subjected to CCL treatment prior to methoprene administration (Fig. 4B). As well, compared to the double-stranded RNA of green fluorescent protein (dsGFP) controls, the levels of p-VgR were markedly reduced in oocyte membrane extracts from PKC-ι–depleted adult females (Fig. 4C). Notably, while methoprene application facilitated Vg deposition in oocytes, CCL treatment or PKC-ι knockdown suppressed JH-stimulated Vg sequestration in oocytes (Fig. 4D). These observations suggest that VgR phosphorylation is essential for ovarian Vg uptake and VgR migration from oocyte cytoplasm to the membrane.
Fig. 4.
Effect of VgR phosphorylation on its intracellular migration and Vg deposition in oocytes. (A) Effect of PKC inhibitor (CCL) treatment and PKC-ι knockdown (dsPKC-ι) on JH-induced VgR translocation in primary oocytes. Green, Alexa Fluor 488-WGA for membrane staining. Red, VgR staining by anti-VgR antibody. Blue, DAPI staining for nuclei. (Scale bars, 25 μm.) (B and C) Western blots showing the effect of PKC inhibitor treatment (B) and PKC-ι knockdown (C) on JH-induced VgR translocation. Cy, cytoplasm fraction. Me, membrane fraction. NK, Na+/K+-ATPase as a marker of membrane protein extracts. (D) Effect of PKC inhibitor treatment and PKC-ι knockdown on JH-induced Vg uptake in the ovaries of 6-d-old adult females pretreated with precocene that was further injected with dialyzed hemolymph Vg proteins obtained from 6-d-old adult females under normal development.
To further define the requirement of phosphorylation for VgR translocation, we performed immunostaining of Sf9 cells transfected with pIEx-4-RFP-His, pIEx-4-VgR -RFP-His, or pIEx-4-VgRS1361A-RFP-His. When the pIEx-4-RFP-His empty vector was transfected, no translocation was observed with RFP under methoprene treatment (Fig. 5A). Interestingly, methoprene induced the migration of recombinant RFP-VgR fusion proteins toward the membrane (Fig. 5A). Pretreatment of PKC inhibitor CCL hindered methoprene-induced RFP-VgR translocation to the cell membrane (Fig. 5A). Additionally, recombinant RFP-VgRS1361A failed to migrate from the cytoplasm to the membrane after methoprene treatment (Fig. 5A). We next performed immunoprecipitation and Western blots to further determine the requirement of VgR phosphorylation for Vg binding. Protein extracts from Sf9 cells overexpressed with VgR or VgRS1361A were incubated with dialytic hemolymph Vg proteins from adult females at 6 d PAE. In the protein mixture containing overexpressed VgR, application of methoprene induced the interaction of Vg and VgR (Fig. 5B). However, methoprene-induced Vg–VgR interaction was blocked by CCL pretreatment (Fig. 5B). Nevertheless, Vg failed to bind to mutational VgRS1361A in the presence of methoprene (Fig. 5B). The three-dimensional structural prediction showed that VgRS1361D (Ser1361 is mutated to aspartic acid to mimic phosphorylation of serine residue) but not unphosphorylated VgR or VgRS1361A binds to Vg (SI Appendix, Fig. S4). Taken together, these data provide further evidence that PKC-ι–mediated VgR phosphorylation at Ser1361 is a prerequisite for its binding to Vg and translocation to the cell membrane. As Vg is dissociated from VgR in endosomes (4, 6, 15), we next explored the effect of acidification on VgR dephosphorylation using Sf9 cells transfected with pIEx-4-VgR-RFP-His vector and treated with methoprene. As shown in SI Appendix, Fig. S5, application of HCl at 10−4 M resulted in VgR dephosphorylation.
Fig. 5.
Subcellular translocation and Vg binding of phosphorylated VgR. (A) Translocation of recombinant VgR in Sf9 cells transfected with wild-type pIEx-4-VgR-RFP-His versus mutational pIEx-4-VgRS1361A-RFP-His. Red, RFP fusion proteins; green, Alexa Fluor 488-WGA; blue, DAPI staining; JHA, methoprene. (Scale bars, 10 μm.) (B) Requirement of VgR phosphorylation for its binding to Vg. Proteins extracted from Sf9 cells transfected with pIEx-4-VgR-RFP-His (VgR) or pIEx-4 -VgRS1361A-RFP-His (VgRS1361A). Hemo (Vg), dialyze hemolymph Vg proteins from 6-d-old adult females. (C and D). Conservation of JH-induced VgR phosphorylation and p-VgR translocation in the oocytes of 4-d-old adult females of the cockroach P. americana (C) and 2-d-old adult females of the cotton bollworm H. armigera (D). Red, VgR. (Scale bars, 100 μm.)
JH-Stimulated VgR Phosphorylation and Intracellular Recycling Are Evolutionarily Conserved.
JH-stimulated ovarian Vg uptake has been reported in insects across divergent orders (42, 43). We chose the blattarian P. americana and lepidopteran H. armigera to examine the conservation of VgR phosphorylation and p-VgR recycling. Immunostaining was conducted using the VgR antibody and primary oocytes of vitellogenic females injected with DMSO, methoprene, or CCL plus methoprene. For the cockroach P. americana, the DMSO control had VgR dominantly localizing in the oocyte cytoplasm, whereas methoprene treatment stimulated VgR moving to the oocyte membrane (Fig. 5C). However, pretreatment of CCL blocked methoprene-induced VgR translocation from oocyte cytoplasm to the membrane (Fig. 5C). Similar results were observed with the cotton bollworm H. armigera (Fig. 5D). These observations suggest that JH-stimulated VgR phosphorylation and translocation are conserved in other insect species, at least including P. americana and H. armigera.
Discussion
Despite the fact that VgR plays a pivotal role in endocytosis of Vg as well as vertical transmission of pathogenic microbes and Wolbachia symbionts into maturing oocytes (6, 9, 13, 15), its regulatory mechanism remains poorly understood. Studies on transcriptional regulation of VgR have been mainly reported in the fruit fly Drosophila melanogaster, the mosquito Ae. Aegypti, and the red fire ant Solenopsis invicta (44–46). At the posttranscriptional and/or translational level, miR-2739 and miR-167 coordinately regulate VgR expression by binding directly to its 3′-untranslated region in the silkworm Bombyx mori (47). The work presented here showed that VgR was posttranslationally regulated by phosphorylation at Ser1361. Exogenous application of methoprene induced VgR phosphorylation. Pharmacological and loss-of-function approaches further demonstrated that JH activated a signaling cascade including GPCR, PLC, extracellular Ca2+, and PKC-ι and thus triggered VgR phosphorylation. These observations provide evidence that JH promotes PKC-ι–triggered VgR phosphorylation for insect vitellogenesis. During the previtellogenic development of Ae. aegypti, JH can activate the RTK-PLC-IP3-CaMKII-PKC cascade and stimulates Met phosphorylation (32, 33). JH also activates a pathway including GPCR, PLC, IP3R, and PKC, which promotes Na+/K+-ATPase phosphorylation for Vg transportation through patency in the follicular epithelium of L. migratoria (36). Our present study, therefore, extends the view of intrinsic regulation of VgR as well as nongenomic action of JH during insect reproduction.
Interestingly, p-VgR was abundant in the vitellogenic stage and accumulated on the oocyte membrane for Vg binding. Mutational VgRS1361A was unable to bind to Vg. Inhibition of VgR phosphorylation resulted in blocked Vg deposition in oocytes. These findings together indicate that VgR phosphorylation is essential for its binding to Vg and ovarian Vg uptake during the vitellogenic phase. Insect VgRs possess two EGF-precursor homology domains, and their phosphorylation site (Ser1361) resides in the second one. The EGF-precursor homology domains harbor six YWTD motifs that form a β-propeller involved in acid-dependent ligand dissociation in endosomes (6, 15, 48). Mutations of EGF-precursor homology domain restrict the acid-dependent ligand release in endosomes (19, 49). The EGF-precursor homology domains also help the ligand-binding domains to recognize and bind to appropriate ligands (6, 48). It is conceivable that VgR phosphorylation at Ser1361 in the EGF-precursor homology domain induces a conformational change that is optimal for ligand binding. We also observed that VgR was dephosphorylated at low pH. It has been previously reported that acidification-induced conformational change of LDLRs results in the dissociation of ligands from the ligand-binding domains (50, 51). It seems likely that endosomal pH leads to VgR dephosphorylation, followed by the release of Vg from VgR. Nevertheless, we cannot rule out the possibility of simultaneous occurrence of VgR dephosphorylation and Vg/VgR dissociation. Our phosphoproteomic and LC-MS/MS analyses revealed that the abundance of phosphorylated proteins in the ovaries of adult female locusts considerably increased in the vitellogenic stage. The data suggest that JH simultaneously regulates multiple proteins in modulating Vg uptake and VgR recycling. Phosphorylation likely induces the conformational change and functional activity of these proteins, which orchestrates the formation of clathrin-coated pits, vesicles, and endosomes for Vg uptake and VgR recycling.
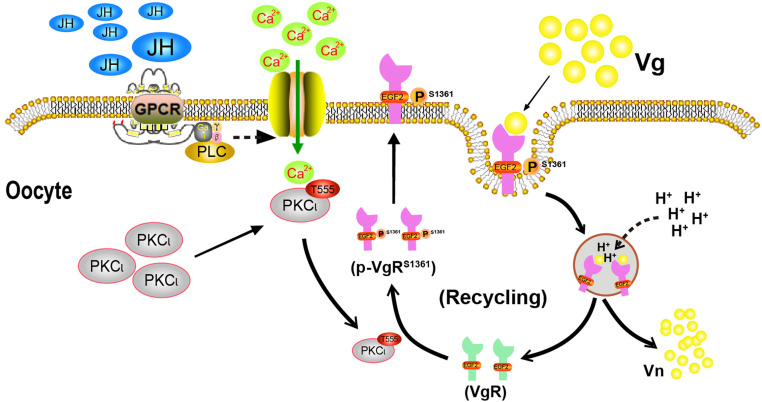
VgRs are highly conserved in their modular structure and Vg uptake across diverse insect orders (15, 20). In this study, we additionally demonstrated that JH-stimulated VgR phosphorylation and intracellular translocation were evolutionarily conserved in other representative insects including P. americana and H. armigera. The results suggest a conservated mechanism in VgR regulation. This evidence demonstrates the phosphorylation-mediated recycling of LDLRs during oocyte maturation and egg development. On the basis of our findings, we propose a model for the regulation of VgR in JH-stimulated ovarian Vg uptake (Fig. 6). JH activates a signaling cascade including GPCR, PLC, extracellular Ca2+, and PKC-ι and thus triggers VgR phosphorylation at Ser1361 in the second EGF-precursor homology domain. Phosphorylated VgR migrates from the oocyte cytoplasm to the membrane, where it binds to Vg for endocytosis. In intracellular endosomes, VgR is dephosphorylated and Vg is dissociated from dephosphorylated VgR upon acidification. Released Vg is crystallized and stored as Vn in yolk bodies, whereas dissociated VgR is phosphorylated by PKC-ι and then returns to the oocyte membrane surface for ligand binding and potential microbe transmission.
Fig. 6.
A proposed model for JH-stimulated VgR phosphorylation, Vg internalization, and VgR recycling. JH promotes VgR phosphorylation at Ser1361 via a signaling cascade including GPCR, PLC, extracellular Ca2+, and PKC-ι. Phosphorylated VgR migrates from oocyte cytoplasm to the membrane for Vg binding and subsequent VgR/Vg endocytosis. Endosomal acidification induces VgR dephosphorylation and Vg/VgR dissociation. Released Vg is stored as Vn in yolk bodies. Cytoplasmic VgR is phosphorylated and returns to oocyte surface.
Materials and Methods
Insects.
The gregarious phase of L. migratoria was maintained as previously reported (36). JH-deprived adult females were obtained by intra-abdominal injection of precocene III (Sigma-Aldrich) at 500 μg/locust within 6 h PAE. s-(+)-methoprene (Abcam) was injected at 150 μg per locust. In pharmacological experiments with inhibitors, adult females on day 4 were injected with suramin (150 μg/locust), SU6668 (3 μg/locust), genistein (6 μg/locust), U73122 (10 μg/locust), KN-93 (10 μg/locust), CCL (5 μg/locust), 2-APB (18 μg/locust), Pyr3 (10 μg/locust), or FL (50 μg/locust), followed by methoprene or DMSO treatment for 30 min.
Phosphoproteomic and LC-MS/MS Analyses.
Phosphoproteomic and LC-MS/MS analyses were performed by PTM Biolab. Briefly, ovarian proteins were extracted from adult females at 0, 3, 4, and 5 d PAE, followed by trypsin digestion and tandem mass tag labeling. Phosphopeptides were enriched by the biomaterial-based posttranslational modification enrichment approach and lyophilized for LC-MS/MS analysis. The PPI network was analyzed via the String website (https://string-db.org/) and visualized with Cytoscape (version 3.7.1). The heatmap was generated by MeV software (version 4.9.0). Kinase–substrate interaction was predicted by NetPhos 3.1 software.
Antiserum Preparation.
Complementary DNA (cDNA) fragments of locust VgR and PKC-ι were amplified with the specific primers (SI Appendix, Table S3), cloned into pET-32a(+)-His, and confirmed by sequencing. The recombinant proteins were expressed in Rosetta host cells under isopropyl-β-d-thiogalactoside induction and purified by Ni2+-NTA affinity column (CWbiotech). Polyclonal antiserums were raised in New Zealand white rabbits using the purified proteins as previously described (52, 53). The antiserums of locust Vg, Na+/K+-ATPase, and β-actin were raised as previously described (36). The antibody against phospho-VgR S1361 was generated by PTM Biolab. Antibodies against phospho-(S/T/Y), phospho-(S) PKC substrate, phospho-PKC-ι, and His-tag were purchased from Abcam and Cell Signaling Technology.
Western Blot and Immunoprecipitation.
Protein extracts from insects and Sf9 cells were isolated in ice-cold lysis buffer containing 150 mM NaCl, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, 50 mM Tris⋅HCl (pH 7.4), and 1 mM phenylmethanesulfonyl fluoride plus protease and phosphatase inhibitor mixtures (Roche). Proteins were fractionated on 7.5% sodium dodecylsulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membrane (Millipore). Western blots were conducted using primary antibodies and corresponding horseradish peroxidase–conjugated secondary antibodies (ProteinTech) and visualized with the Electrochemiluminescence Detection Kit (Boster). For immunoprecipitation, precleared lysates were incubated with VgR or His-tag antibody at 4 °C overnight. The immunocomplexes were captured with protein-A agarose (Sigma-Aldrich) for 4 h at 4 °C and eluted in Laemmli sample buffer, followed by Western blots with antibodies.
RNAi and qRT-PCR.
cDNA templates were amplified by PCR, cloned into pGEM-T vector (Tiangen), and confirmed by sequencing. double-stranded RNAs (dsRNAs) were synthesized by in vitro transcription with T7 RiboMAX Express RNAi System (Promega). Newly emerged adult females were intra-abdominally injected with 15 µg dsRNA and boosted on day 3. Phenotypes were photographed by a Canon EOS550D camera and Leica M205C stereomicroscope. Total RNA was extracted from ovaries using TRIzol reagent (Invitrogen), and cDNA was reverse transcribed using the FastQuant RT Kit (with gDNase) (Tiangen). qRT-PCR was performed using a SuperReal PreMix Plus (with SYBR Green I) kit (Tiangen) with a Light Cycler 96 System (Roche), initiated at 95 °C for 2 min, followed by 40 cycles of 95 °C for 20 s, 58 °C for 20 s, and 68 °C for 20 s. Relative expression levels were calculated using the 2−∆∆Ct method and normalized with β-actin. Primers used for RNAi and qRT-PCR are listed in SI Appendix, Table S3.
Immunostaining.
Primary oocytes were dissected from adult female insects subjected to CCL administration, PKC-ι knockdown, and/or methoprene treatment. DMSO and dsGFP treatment were used as the controls. The sections were fixed in 4% paraformaldehyde and permeabilized in 0.3% Triton X-100. Immunostaining was conducted using VgR antibody and Alexa Fluor 555–conjugated secondary antibodies (Thermo Fisher Scientific). For staining of Sf9 cells transfected with recombinant vectors, VgR was visualized with RFP fusion. Membrane and nuclei were stained with 5 μg/mL Alexa Fluor 488 wheat germ agglutinin (WGA) and DAPI (Thermo Fisher Scientific), respectively. Images were captured with a ZEISS LSM 710 laser confocal microscope and processed with ZEN2012 software (Carl Zeiss).
Data Analysis.
Statistical analyses were performed by Student’s t test using the SPSS22.0 software. Significant difference was considered at P < 0.05. Values were reported as mean ± SE.
Supplementary Material
Acknowledgments
We thank Dongran Yang for technical help. This work was supported by National Natural Science Foundation of China Grants 31630070, U1804232, and 31601896.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2106908118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Wyatt G. R., Davey K. G., “Cellular and molecular actions of juvenile hormone. II. Roles of juvenile hormone in adult insects” in Advances in Insect Physiology, Evans P. D., Ed. (Academic Press, 1996), vol. 26, pp. 1–155. [Google Scholar]
- 2.Raikhel A. S., Brown M. R., Belles X., “Hormonal control of reproductive processes” in Comprehensive Molecular Insect Science, Gilbert L. I., Iatrou K., Gill S. S., Eds. (Elsevier, Boston, 2005), vol. 3, pp. 433–491. [Google Scholar]
- 3.Pszczolkowski M. A., Peterson A., Srinivasan A., Ramaswamy S. B., Pharmacological analysis of ovarial patency in Heliothis virescens. J. Insect Physiol. 51, 445–453 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Snigirevskaia E., Raikhel A. S., “Receptor-mediated endocytosis of yolk proteins in insect oocytes” in Progress in Vitellogenesis. Reproductive Biology of Invertebrates, Raikhel A. S., Adiyodi K. G., Adiyodi R. G., Eds. (Science Publishers Inc., 2005), vol. 12, pp. 199–228. [Google Scholar]
- 5.Huo Y., et al., Transovarial transmission of a plant virus is mediated by vitellogenin of its insect vector. PLoS Pathog. 10, e1003949 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell R. D. III, Sonenshine D. E., Pérez de León A. A., Vitellogenin receptor as a target for tick control: A mini-review. Front. Physiol. 10, 618 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Y., et al., Vertical transmission of Wolbachia is associated with host vitellogenin in Laodelphax striatellus. Front. Microbiol. 9, 2016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huo Y., et al., Insect tissue-specific vitellogenin facilitates transmission of plant virus. PLoS Pathog. 14, e1006909 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan H., et al., Rice stripe virus hitchhikes the vector insect vitellogenin ligand-receptor pathway for ovary entry. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180312 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He K., Lin K., Ding S., Wang G., Li F., The vitellogenin receptor has an essential role in vertical transmission of rice stripe virus during oogenesis in the small brown plant hopper. Pest Manag. Sci. 75, 1370–1382 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Boldbaatar D., et al., Tick vitellogenin receptor reveals critical role in oocyte development and transovarial transmission of Babesia parasite. Biochem. Cell Biol. 86, 331–344 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Hussein H. E., et al., Silencing expression of the Rhipicephalus microplus vitellogenin receptor gene blocks Babesia bovis transmission and interferes with oocyte maturation. Parasit. Vectors 12, 7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raikhel A. S., Dhadialla T. S., Accumulation of yolk proteins in insect oocytes. Annu. Rev. Entomol. 37, 217–251 (1992). [DOI] [PubMed] [Google Scholar]
- 14.Schonbaum C. P., Lee S., Mahowald A. P., The Drosophila yolkless gene encodes a vitellogenin receptor belonging to the low density lipoprotein receptor superfamily. Proc. Natl. Acad. Sci. U.S.A. 92, 1485–1489 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tufail M., Takeda M., Insect vitellogenin/lipophorin receptors: Molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect Physiol. 55, 87–103 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Raikhel A. S., Lea A. O., Hormone-mediated formation of the endocytic complex in mosquito oocytes. Gen. Comp. Endocrinol. 57, 422–433 (1985). [DOI] [PubMed] [Google Scholar]
- 17.Raikhel A. S., Accumulations of membrane-free clathrin-like lattices in the mosquito oocyte. Eur. J. Cell Biol. 35, 279–283 (1984). [PubMed] [Google Scholar]
- 18.Raikhel A. S., et al., Molecular biology of mosquito vitellogenesis: From basic studies to genetic engineering of antipathogen immunity. Insect Biochem. Mol. Biol. 32, 1275–1286 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Mellman I., Fuchs R., Helenius A., Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 55, 663–700 (1986). [DOI] [PubMed] [Google Scholar]
- 20.Sappington T. W., Raikhel A. S., Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol. 28, 277–300 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Sappington T. W. R., Alexander S., Insect vitellogenin yolk protein receptors. Entomol. Pub. 12, 34 (2005). [Google Scholar]
- 22.Bellés X., “Vitellogenesis directed by juvenile hormone” in Reproductive Biology of Invertebrates, Raikhel A. S., Ed. (Science Publishers Inc., 2005), vol. 12, pp. 157–198. [Google Scholar]
- 23.Roy A., Palli S. R., Epigenetic modifications acetylation and deacetylation play important roles in juvenile hormone action. BMC Genomics 19, 934 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charles J. P., et al., Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl. Acad. Sci. U.S.A. 108, 21128–21133 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M., Mead E. A., Zhu J., Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl. Acad. Sci. U.S.A. 108, 638–643 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jindra M., Bellés X., Shinoda T., Molecular basis of juvenile hormone signaling. Curr. Opin. Insect Sci. 11, 39–46 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Gujar H., Palli S. R., Juvenile hormone regulation of female reproduction in the common bed bug, Cimex lectularius. Sci. Rep. 6, 35546 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo W., et al., Juvenile hormone-receptor complex acts on mcm4 and mcm7 to promote polyploidy and vitellogenesis in the migratory locust. PLoS Genet. 10, e1004702 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z., Yang L., Song J., Kang L., Zhou S., An isoform of Taiman that contains a PRD-repeat motif is indispensable for transducing the vitellogenic juvenile hormone signal in Locusta migratoria. Insect Biochem. Mol. Biol. 82, 31–40 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Zou Z., et al., Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc. Natl. Acad. Sci. U.S.A. 110, E2173–E2181 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin S. W., Zou Z., Saha T. T., Raikhel A. S., bHLH-PAS heterodimer of methoprene-tolerant and Cycle mediates circadian expression of juvenile hormone-induced mosquito genes. Proc. Natl. Acad. Sci. U.S.A. 109, 16576–16581 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu P., Peng H. J., Zhu J., Juvenile hormone-activated phospholipase C pathway enhances transcriptional activation by the methoprene-tolerant protein. Proc. Natl. Acad. Sci. U.S.A. 112, E1871–E1879 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ojani R., Liu P., Fu X., Zhu J., Protein kinase C modulates transcriptional activation by the juvenile hormone receptor methoprene-tolerant. Insect Biochem. Mol. Biol. 70, 44–52 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu P., Fu X., Zhu J., Juvenile hormone-regulated alternative splicing of the taiman gene primes the ecdysteroid response in adult mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 115, E7738–E7747 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y.-X., et al., Juvenile hormone induces methoprene-tolerant 1 phosphorylation to increase interaction with Taiman in Helicoverpa armigera. Insect Biochem. Mol. Biol. 130, 103519 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Jing Y.-P., An H., Zhang S., Wang N., Zhou S., Protein kinase C mediates juvenile hormone-dependent phosphorylation of Na+/K+-ATPase to induce ovarian follicular patency for yolk protein uptake. J. Biol. Chem. 293, 20112–20122 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy S., et al., “Chapter five—Regulation of reproductive processes in female mosquitoes” in Advances in Insect Physiology, Raikhel A. S., Ed. (Academic Press, 2016), vol. 51, pp. 115–144. [Google Scholar]
- 38.Song J., Wu Z., Wang Z., Deng S., Zhou S., Krüppel-homolog 1 mediates juvenile hormone action to promote vitellogenesis and oocyte maturation in the migratory locust. Insect Biochem. Mol. Biol. 52, 94–101 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Wu Z., Guo W., Xie Y., Zhou S., Juvenile hormone activates the transcription of cell-division-cycle 6 (Cdc6) for polyploidy-dependent insect vitellogenesis and oogenesis. J. Biol. Chem. 291, 5418–5427 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song J., Zhou S., Post-transcriptional regulation of insect metamorphosis and oogenesis. Cell. Mol. Life Sci. 77, 1893–1909 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo W., et al., Juvenile hormone-dependent Kazal-type serine protease inhibitor Greglin safeguards insect vitellogenesis and egg production. FASEB J. 33, 917–927 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Roy S., Saha T. T., Zou Z., Raikhel A. S., Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 63, 489–511 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Wu Z., Yang L., He Q., Zhou S., Regulatory mechanisms of vitellogenesis in insects. Front. Cell Dev. Biol. 8, 593613 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho K.-H., Cheon H.-M., Kokoza V., Raikhel A. S., Regulatory region of the vitellogenin receptor gene sufficient for high-level, germ line cell-specific ovarian expression in transgenic Aedes aegypti mosquitoes. Insect Biochem. Mol. Biol. 36, 273–281 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Schonbaum C. P., Perrino J. J., Mahowald A. P., Regulation of the vitellogenin receptor during Drosophila melanogaster oogenesis. Mol. Biol. Cell 11, 511–521 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen M. E., Lewis D. K., Keeley L. L., Pietrantonio P. V., cDNA cloning and transcriptional regulation of the vitellogenin receptor from the imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae). Insect Mol. Biol. 13, 195–204 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Chen E., et al., bmo-miR-2739 and the novel microRNA miR-167 coordinately regulate the expression of the vitellogenin receptor in Bombyx mori oogenesis. Development 147, dev183723 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Andersen O. M., Dagil R., Kragelund B. B., New horizons for lipoprotein receptors: Communication by β-propellers. J. Lipid Res. 54, 2763–2774 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis C. G., et al., Acid-dependent ligand dissociation and recycling of LDL receptor mediated by growth factor homology region. Nature 326, 760–765 (1987). [DOI] [PubMed] [Google Scholar]
- 50.Innerarity T. L., Structural biology. LDL receptor’s beta-propeller displaces LDL. Science 298, 2337–2339 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Jeon H., Blacklow S. C., An intramolecular spin of the LDL receptor beta propeller. Structure 11, 133–136 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Wu Z., He Q., Zeng B., Zhou H., Zhou S., Juvenile hormone acts through FoxO to promote Cdc2 and Orc5 transcription for polyploidy-dependent vitellogenesis. Development 147, dev188813 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Wu Z., Guo W., Yang L., He Q., Zhou S., Juvenile hormone promotes locust fat body cell polyploidization and vitellogenesis by activating the transcription of Cdk6 and E2f1. Insect Biochem. Mol. Biol. 102, 1–10 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.