Abstract
Aims.
We aim to establish the feasibility and acceptability of the Tele-STELLA (Support via Telehealth: Living and Learning with Advancing Alzheimer’s Disease and Related Dementias) intervention. We will also assess the efficacy of the intervention in reducing the frequency of behavioral symptoms of dementia as well as family Care Partner reactivity to the symptoms.
Design.
This is a multi-component, quasi-experimental study that focuses on facilitating effective management of behavioral symptoms that occur in the later stages of dementia.
Methods.
Family Care Partners (n=124) for persons with Alzheimer’s disease will participate in two eight-week videoconferencing components which address behavioral symptoms--in both the persons with Alzheimer’s disease and their Care Partners. In the first component (“Nova”) Care Partners work with one nurse for an hour/week for four weeks, then they join a small group for another four weeks. In the second component (“Constellation”) Care Partners work in a larger group to hone skills and knit supportive relationships. Behavioral symptom frequency, and Care Partner reactivity to the behaviors, will be measured prior to, during, and after the intervention. The study is funded by the United States National Institute on Aging (R01AG067546); funding was initiated February, 2021.
Discussion.
Tele-STELLA fills a gap in current videoconference-based psychoeducational interventions in that it offers real-time interaction with nurses and peers. The intervention was designed with feedback by pilot participants. This study will assess Tele-STELLA in its current, novel format, thus preparing it for a larger, future randomized controlled trial.
Impact.
Tele-STELLA addresses symptoms that occur in the later stages of dementia, providing families with tools to facilitate effective behavioral management. Because Tele-STELLA is implemented via videoconferencing, it targets Care Partners who face barriers to support, such as cost and transportation.
Trial Registration.
This trial is registered with ClinicalTrials.gov (#NCT04627662).
Keywords: Dementia, Caregiving, Family Care, Technology
INTRODUCTION
Caring for a family member with dementia is a complex social, psychological and physical experience that can have both positive and negative consequences. Adults who care for a family member with Alzheimer’s disease and related dementias (ADRD) may find a sense of meaning, opportunity, and power in their evolving role, but they may also find caregiving burdensome. This burden is associated with the behavioral symptoms, such as agitation, pacing, and depression, that most people with ADRD experience along the disease trajectory. These symptoms are distressing for the person with ADRD, as well as their Care Partners, who also experience behavioral symptoms, such as depression, anxiety, and grief (Kales et al., 2015; Kolanowski et al., 2017; Lindauer & Harvath, 2014, 2015; Ornstein & Gaugler, 2012).
Interventions that reduce psychological burden are available to support many family members who care for those with ADRD (referred to here as “Care Partners”) in the United States and around the world (Alzheimer’s Association, 2020; Chien et al., 2011; Selwood et al., 2007), but access to them is limited by several factors, including geographic distance, financial resources, and stigma (Herrmann et al., 2018; Karlin et al., 1999). Technological strategies are needed reduce barriers to support the families of the 50 million people around the world with dementia (Alzheimer’s Disease International, 2019).
In the last decade, nurse scientists have met this challenge by incorporating internet-based videoconferencing technology into caregiver interventions, making them more accessible for families living with ADRD (Hepburn et al., 2021; Hopwood et al., 2018; Lindauer et al., 2018; Lindauer et al., 2019). These interventions combine nursing expertise with person-centered foci, resulting in interventions that improve caregiver well-being.
More broadly, interventions designed by allied health scientists have small to moderate effects on reducing Care Partner burden and depression and good user acceptance (Boots et al., 2014; Hopwood et al., 2018). However, despite evidence that Care Partners prefer individualized interventions with real-time counselors, most telehealth interventions are group-based, asynchronous, and not tailored to stages of disease (Hopwood et al., 2018).
To address the need for personalized, synchronous educational interventions for families caring for those in moderate to late-stage dementia, we designed Tele-STELLA (Support via Telehealth: Living and Learning with Advancing Alzheimer’s Disease and Related Dementias). Tele-STELLA uses videoconferencing to connect Care Partners with nurse interventionists who guide them in individualized strategies to reduce the impact of upsetting behavioral symptoms of ADRD on the family (Lindauer et al., 2018; Lindauer et al., 2019; Teri et al., 2005). While our work on telehealth-based support began in 2016, the COVID-19 pandemic has accelerated acceptance of this mode of intervention. Here we discuss our protocol which has been designed with input from Care Partners.
BACKGROUND
Tele-STELLA evolved from the established intervention, Staff Training in Assisted-Living Residences-Caregivers (STAR-C). The original intervention employs interventionists who meet with Care Partners, in person, one-to-one, in their own homes to provide instruction in an analysis-based approach to behavior modification. STAR-C has been tested in the community setting and found to be effective (Teri et al., 2005). However, sustainability of STAR-C can be challenging due to the cost and geographic distance (McCurry et al., 2017).
To address access and cost barriers, we designed and tested two telehealth versions of the STAR-C intervention. Both mirrored STAR-C in that Care Partners met with interventionists one-to-one for eight weeks to address behavioral symptoms; however, unlike the original STAR-C intervention (Teri et al., 2005), the pilot versions were conducted via videoconferencing. Pilot testing found that the telehealth interventions were acceptable to Care Partners and preferred over a potential in-home intervention. We found that frequency of behavioral symptoms, and Care Partner reactivity to the symptoms, improved significantly (Lindauer et al., 2018; Lindauer et al., 2019).
In post-intervention focus groups, Care Partners revealed that they liked the one-to-one format, but felt abandoned at the conclusion of the pilot interventions. The early versions of the Tele-STELLA intervention did not include meaningful opportunities for Care Partners to interact with each other post-intervention (Lindauer et al., 2018; Lindauer et al., 2019).
Early versions of the Tele-STELLA intervention were acceptable to Care Partners, but we did face technological barriers. Limited broadband access impeded use of the videoconferencing mode for some. And, like others, we found that telehealth use was a cultural shift for our participants, requiring much support and coaching (Foster & Sethares, 2014).
The pilot work informed the revision of the intervention to include one-to-one and multiple group sessions. In its current form, Tele-STELLA is a multi-component intervention that is designed for families living in the later stages of dementia, where behavioral symptoms can be more prominent (Kolanowski et al., 2017). Tele-STELLA begins with one-to-one sessions with each Care Partner and nurse interventionist (“Guide”), then links Care Partners to each other in a meaningful way to sustain support post intervention.
THE STUDY
Aims
The first aim of this study is to establish the feasibility and acceptability of Tele-STELLA. We will assess the feasibility of implementing Tele-STELLA across three sites, test the acceptability of the intervention, and evaluate the fidelity of intervention to the protocol.
The second aim is to test the efficacy of Tele-STELLA in reducing the frequency of behavioral and psychological symptoms of dementia and Care Partner reactivity to them. We hypothesize that Care Partners who complete the Tele-STELLA intervention will report a significant reduction in the frequency of behavioral symptoms and their reactivity to the symptoms.
Regulatory
Aligning with the United States’ National Institutes of Health’s (NIH) multi-site, single Institutional Review Board (IRB) policy (National Institutes of Health, 2016, June 21), a single IRB was used to review Tele-STELLA (WCG-IRB Protocol # 20210754). A Data Safety Monitoring Plan is in place to oversee the safe conduct of the study. A physician with dementia expertise is the Safety Officer.
Design
Tele-STELLA is a prospective, quasi-experimental clinical trial (ClinicalTrials.gov #NCT04627662). Care Partners for people with dementia will receive the Tele-STELLA intervention to facilitate effective management of behavioral symptoms of ADRD. Detailed study characteristics are outlined in Table 1(Gaugler et al., 2020).
Table 1.
Tele-STELLA Intervention Characteristics (Gaugler et al., 2020)
| Dimension | Definition | Options | |
|---|---|---|---|
| Mode | Contact method between Care Partner (CP) and Guide | • Secure videoconferencing
with CP to deliver intervention • Telephone: Contact CPs, use for intervention if unable to connect via videoconferencing • Email: Delivery of surveys, study information, retention letters and general communication with CPs • Texting: Communication with CPs, Guides, other team members |
|
| Delivery Materials | Materials used in delivery of Tele-STELLA | • Tele-STELLA Handbook for
CPs • Guide Manual for interventionist |
|
| Fidelity Assessment Materials | Used by investigator to assess Guide fidelity to intervention | • Content Adherence Checklist | |
| Location | Where participants are recruited and where Tele-STELLA is delivered | • CPs recruited and
pre-screened from three ADRC’s located in Oregon, Georgia and
Kentucky • Full screen, consent, and implementation at OADRC • CPs from the three ADRCs access Tele-STELLA on a computer in their own homes |
|
| Schedule | Duration and intensity of Tele-STELLA | Nova Component | • Four weekly one-to-one sessions (one
Guide to one CP). Sessions to last up to 1 hour • Four one-to-several sessions (one Guide to up to four CPs). Sessions last up to 1 hour |
| • The Constellation • Component |
• A repeat of the same curriculum, in large group sessions, similar to the ECHO model | ||
| Scripting | Degree of scripted material for Guide | • Screening interview
scripted • Intervention informed by weekly topics and goals. Key points highlighted, but not scripted • Expectation that Guides will assist CPs in addressing at least 2 behavioral symptoms • Guides encouraged to tailor intervention to CP needs |
|
| Sensitivity to CP characteristics | Degree that Tele-STELLA is personalized to CP’s background, culture and experience | • Highly personalized to CP
within the intervention framework • CP identifies target behavioral symptoms (both CP’s and Care Recipient’s) • Guide assists CP in identifying strategies to address symptoms |
|
| Interventionist characteristics | Qualifications and training; concordance with CP characteristics | • Guides: Nurse
professionals • Experience working with families • Trained in STELLA intervention • Undergo ongoing fidelity assessments • Completion of unconscious bias training • Competent in videoconferencing • Sensitivity to cultural values of participants • Attend weekly meetings with team |
|
| Adaptability | Extent to which STELLA can be modified (what and when) | • Mode of delivery: Study
technicians set up and test internet connection at study start. If
internet fails, CPs can use phone • Duration of sessions: Up to 60 minutes • Location: CP can be at any private location • Content: Personalize to CP • Group size: - Nova: One-Guide-to-several CPs: Can be as few as 2 CPs or as many as 5 - Constellation: One to three Guides depending on Constellation Group size. - Constellation can have as many as 20 Care Partners • Guide: Guides may change over intervention. CPs will be advised of this |
|
| Treatment Delivery | Documentation of Guide adherence to treatment plan | • Number and duration of
sessions • Content covered • Guides use Tracking Logs to note implementation of intervention components and CP attendance |
|
| Treatment Receipt | Degree that CPs implemented ABC plan | • Guide verifies CP ABC
plan • CP reports use of ABC plan during sessions • Fidelity assessments of video recordings indicate CP receipt of information |
|
| Treatment Enactment | Degree that ABC plan is applied in real-world setting | • Guide appraisal of
knowledge and use of CP’s ABC plan • Surveys indicate CPs contact each other • Fidelity assessments of video recordings indicate CP receipt of information |
|
| Treatment content strategies | Strategies aimed at improving outcomes | • Information on skills and
support provided via instruction and Tele-STELLA
Handbook • Facilitation of development of ABC plan • Facilitation of communication and support between CPs |
|
| Mechanisms of Action-Objective | Objective assessment of processes that affect desired treatment outcomes | • Surveys delivered to CPs
via email prior to and after the intervention and weekly
sessions • CPs complete surveys in privacy -Behavioral symptoms frequency -CP reactivity to BPSD -Depression -Quality of Life -Grief -Use of psychoactive medications -Out-of-pocket Costs |
|
Tele-STELLA employs a person-centered perspective that is grounded in nursing’s tradition of holistic care. Kales’ et al. framework indicates behavioral symptoms arise from unmet needs, overburdened Care Partners and environmental factors, all within the context of cultural background and beliefs (Kales et al., 2015). Behavioral symptoms are bidirectional in that the behaviors of the person with ADRD affect the Care Partner’s behaviors and vice versa (Isik et al., 2018; Kolanowski et al., 2017).
Tele-STELLA will be implemented in three US states: Oregon, Kentucky and Georgia. The primary site is the Layton Aging and Alzheimer’s Disease Center (aka, Oregon Alzheimer’s Disease Research Center, OADRC) located at Oregon Health & Science University (OHSU). All study activities (e.g., consenting, intervention, data collection) will be managed by this site. Teams at the Alzheimer’s Disease Research Centers (ADRCs) at the University of Kentucky (UK) and Emory University (Emory) will recruit and pre-screen participants from their respective states. All study activities will occur via phone, videoconferencing, mail, email and/or text. There will be no in-person visits.
Participants.
A power analysis revealed we will need 124 Care Partners to find a significant difference on the reactivity portion of the Revised Memory and Behavior Problems Checklist (RMBPC) (Teri et al., 1992). With 124 participants, we’ll have 90% power to detect a 2-point reduction in Care Partner reactivity over the Tele-STELLA intervention period, using multiple comparison adjusted p-value of p=0.016 (0.05/3). To allow for 20% attrition, we aim to enroll 150 Care Partners. We will also enroll the 150 Care Recipients of the Care Partners, but will not collect data from them. We will collect data about them from the Care Partners.
We will recruit participants from the catchment areas of the three ADRCs. These three sites were chosen due to their expertise in recruiting underrepresented families in ADRD research: rural, African American and sexual and gender minority (SGM) populations (Bardach et al., 2020; Kumar et al., 2020; Patterson et al., 2017).
The Tele-STELLA sample will be made up of participants who self-identify as a Care Partner for a family member or close friend with moderate to severe ADRD. Care Partners will be asked to choose the dementia stage that best aligns with their Care Recipient’s current state (Box 1). Care Recipients will be enrolled with their Care Partners, but will not engage in any intervention activities.
Care Partners must provide care for the person with ADRD (e.g., assistance with activities of daily living, medication management, care oversight) for at least 4 hours/week. Due to the COVID-19 pandemic and other unforeseen circumstances, this care does not need to be in-person (Table 2). Care Partners experience burden regardless of the location of the person with ADRD, thus they do not have to live with them to be included in the study (Schulz et al., 2004).
Table 2.
Inclusion/Exclusion Criteria
| Participant | Inclusion | Exclusion |
|---|---|---|
| Care Recipient with ADRD | • Diagnosis of ADRD, moderate to late
stages as defined by CP (Box 1) • Exhibits 2 or more behaviors, including those listed on RMBPC, that occur 3 or more times/week at study enrollment, that are bothersome to the CP • Family member of CP (relative, spouse or friend that is considered family) • Provides informed consent to participate in the research • Lives in defined ADRC catchment area |
• Dementia not related to
ADRD • Unable to leave CP during Tele-STELLA sessions • Early stage dementia, as define by CP (Box 1) |
| Care Partner (CP) | • Adult caring for family member with
ADRD • Provides care for at least 4 hours/week • Age of 18 years or older • Speaks and understands English • Owns a telephone (smartphone, cell-phone or landline) • Has email and mailing address to receive computer, study materials and surveys • Provides informed consent to participate in the research • Lives in ADRC catchment area |
• Unable to find activity for CR during
Tele-STELLA sessions • Completed similar telehealth intervention within the last year • Hearing and/or vision problems severe enough to prevent participation • Refuses to be video-recorded during Tele-STELLA sessions • Unwilling or unable to adequately follow study instructions and participate in study procedures |
All Care Partners must be able to identify two or more distressing behavioral symptoms in their Care Recipient as described on the RMBPC (Teri et al., 1992).
Recruitment Methods.
Care Partners will be recruited from clinic sites, ADRC research cohorts, electronic registries, community advisory committees, advocacy organizations, faith communities and social media advertisements.
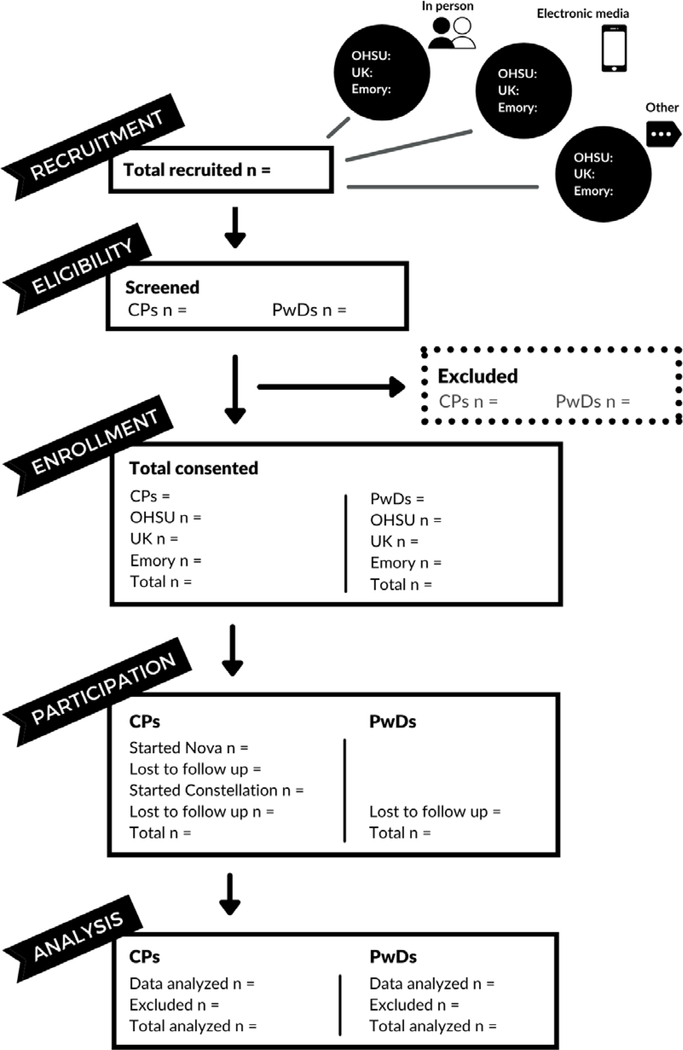
The RAs at the three ADRCs will deploy screening questions (Box 2), via electronic link or RA contact, to interested Care Partners. If individuals pass the pre-screen, the OADRC team will complete the full screen with potential participants, and if eligible, proceed with the consent procedures. We will track our recruitment and consent rates using the modified CONSORT diagram (Figure 1) (Guthrie & Bogue, 2015).
Figure 1.
Modified CONSORT Diagram for Tele-STELLA (Guthrie & Bogue, 2015)
Consent.
The OADRC team will consent participants (the Care Partners and the persons with dementia) by phone or videoconferencing, following ethical practices to ensure understanding and authentic consent. The person with ADRD will not partake in the Tele-STELLA intervention, but we will collect and analyze data collected about them. Our previous experience has taught us that the person with ADRD may enter the room of the Care Partner during Tele-STELLA sessions and inadvertently appear in the videorecordings. Thus, we will seek the consent of the person with ADRD or their legally authorized representative to participate in this study.
Procedures.
Care Partners will complete the two components of Tele-STELLA: Nova and Constellation (Figures 2 and 3). All sessions will be conducted via synchronous videoconferencing with “Guides,” nurses and allied health professionals with experience in caring for older adults and those with dementia. Each component (Nova and Constellation) is made up of weekly one-hour sessions over eight weeks. The Nova component is designed to introduce Care Partners to Tele-STELLA, first in a one-to-one format, then in small groups. The Constellation component is the same intervention, but provided at a group level, after Nova. The Tele-STELLA Handbook, provided to Care Partners, contains lessons and space for notes (A. Lindauer, Tran, L., Tarter, R., McAteer, P., 2021). The Guide Manual provides guidance for the nurse interventionists (A. Lindauer, Tarter, R., 2021).
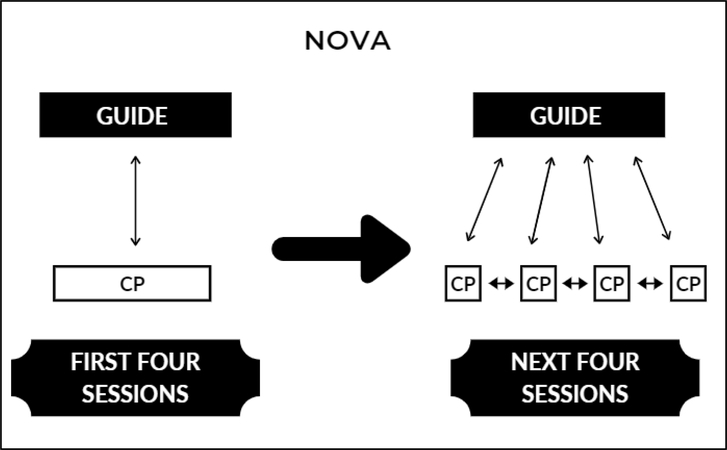
Figure 2.
Diagram of Nova Component (CP=Care Partner)
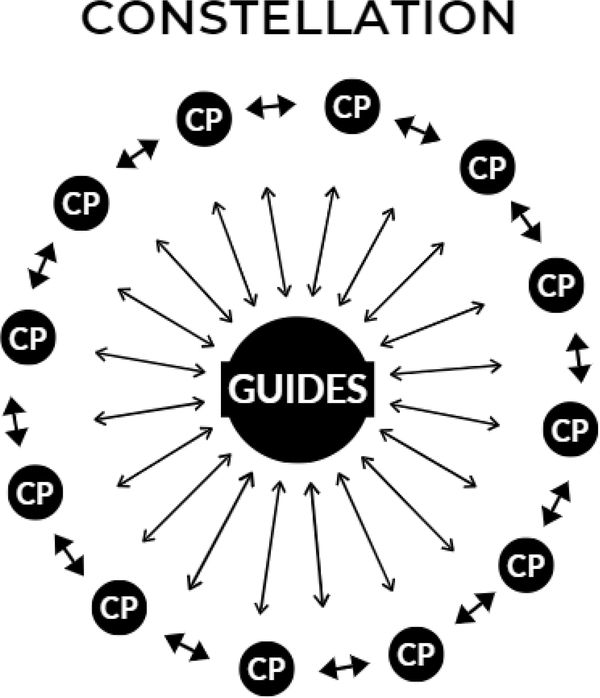
Figure 3.
Hub and Spokes Diagram of Constellation Component (CP=Care Partner)
The Nova component.
In the first four weeks of Nova, one Care Partner will meet with one Guide via synchronous videoconferencing (Figure 2). The one-to-one sessions orient the Care Partners to the learning environment, allow for mutual trust to develop between the Care Partners and Guide, and give Care Partners the authority to select the behavioral symptoms they want to address (Knowles, 2005).
During the first Nova session the Care Partners and Guides will document the behaviors the Care Partners want to work on while in Nova. Then the Care Partners will learn the Tele-STELLA ABC approach, which focuses on describing the behavior in detail, then identifying the activators and consequences of the behavior. With a deeper understanding of the behavior, Care Partners can develop strategies to prevent or modify it (Beck, 2011). After the Care Partners work on the behavioral symptoms of their Care Recipients, they will be asked to consider and address their own behavioral symptoms (e.g., depression, pre-death grief (Lindauer & Harvath, 2014)).
At Week 5, after the 4-week induction period, up to five Care Partners will join one Guide, via videoconferencing, for the remaining four weeks in Nova (Figure 2). In these small group sessions the Guide presents didactic information on pleasant events (Teri & Logsdon, 1991), affective symptoms associated with providing care, and Care Partner coping. Care Partners will be encouraged to share their stories about their experiences, their ABC plans, and their successes and challenges.
The Constellation component.
After completing the Nova component, Care Partners will join the Tele-STELLA Constellation via synchronous videoconferencing (Figure 3). Up to 20 Care Partners will meet as a group with up to three Tele-STELLA Guides for one-hour, weekly sessions for eight weeks to review and discuss the key lessons of Tele-STELLA. Any Care Partner who completes the Nova component can join a Constellation. Up to four Constellations will be provided per year. Care Partners can join any Constellation, but they need to start it within one year of starting Nova.
Similar to the ECHO model (Extension of Community Healthcare Outcomes) (Arora et al., 2010; Lindauer et al., 2020), each Constellation session starts with a brief didactic lesson presented by the Guides (the “hub”) for the Care Partners in the “spokes” (Figure 3). Following the lessons, Care Partners will share their stories about their family’s experience with ADRD and the Tele-STELLA ABC approach.
Accommodations.
We anticipate barriers to successful scheduling and will make the following accommodations:
Allow up to two weeks break between Nova session #4 (one-to-one) and #5 (small group).
Care Partners can delay joining a Constellation for up to a year after starting Nova.
Care Partners can skip up to 2 Nova sessions and 2 Constellation sessions and still remain in the study.
If there are more Care Partners than available Guides, the Care Partners may have to wait to join the Nova component.
These accommodations are designed to mirror the “real life” experience of busy Care Partners, whose time demands may not perfectly align with study goals. The scheduling flexibility allows pragmatic implementation, but could impact our ability to standardize study conditions. To address inconsistencies in timing, the analyses will incorporate information about the length of time between Nova and Constellation as well as the relationship between the number of sessions attended and the outcome scores on the RMBPC (Teri et al., 1992).
Focus groups.
Any Care Partner who signed a consent, whether or not they participated in the intervention, can participate in a focus group. Focus group leaders--nurses with focus group experience--will facilitate discussion about the Care Partners’ experiences with, or barriers to, Tele-STELLA participation. The focus groups will be videorecorded and transcribed. Transcripts will be analyzed for themes of acceptability (Lindauer et al., 2018; Lindauer et al., 2019).
Fidelity Assessment
We will assess treatment fidelity for adherence to the study protocol, as well as Care Partner receipt of the Tele-STELLA intervention, using procedures tested in our second pilot (Lindauer et al., 2019; Onken et al., 2014). We will record Tele-STELLA sessions, and content experts will view approximately four videos per Guide per year. Feedback will be provided to the Guides, as needed, to improve treatment fidelity.
All of the above procedures will be implemented with the first cohort (up to 36 Care Partners). We will assess enrollment processes, completion rates, acceptability and preliminary efficacy. Should findings indicate a need for substantial revisions, the study team will discuss the revisions and implement changes that align with the theoretical basis of the intervention (Kales et al., 2015). We will implement the revised Tele-STELLA until up 124 Care Partners have completed the full intervention.
Data Collection
Demographic information will be collected upon enrollment. Care Recipient ADRD diagnosis, stage and diagnosis year will be documented. The main outcomes, behavioral symptom frequency and Care Partner reactivity, will be assessed with the 24-item RMBPC (Teri et al., 1992). Tele-STELLA requires Care Partners to work on their specific family behavioral symptoms. The Care Partners will rate the frequency of the target behaviors specific to their family, and their reactivity to them, before and after the Nova and Constellation components.
Surveys with the study measures will be emailed to the Care Partners. The six “Planet” surveys and a weekly “Orbit” surveys (Table 3) were developed with the Oregon Roybal Center for CAre Support Translational Research Advantaged by Integrating Technology (ORCASTRAIT) (NIA P30 AG024978). The Planet surveys measure variables such as Care Partner depression, grief and care recipient behavioral frequency, and will be distributed six times during each Care Partner’s study engagement timeframe (Table 4). These surveys will take approximately 45 minutes to complete. The Orbit survey collects weekly data on caregiver physical and emotional strain, and out-of-pocket caregiving costs. It will take approximately 10–15 minutes to fill out. All surveys can be completed on smart phones, computers or tablets.
Table 3.
Tele-STELLA Survey Descriptions
| Mercury | |
| Revised Memory & Behavioral Problems Checklist (Teri et al., 1992) | Measure of frequency and reactivity to behavioral and psychological symptoms of dementia |
| Center for Epidemiologic Studies Depression Scale (Andresen et al., 2013) | 10-item depression measure |
| Marwit Meuser Caregiver Grief Index-Short Form (Marwit & Meuser, 2005) | Care Partner pre-death grief |
| Placement plan scale (Morycz, 1985) | One-item assessment of plans for placement of family member with dementia |
| QoL AD(Logsdon et al., 2002) (Care Partner) | 13-item assessment of quality of life for the Care Partner |
| QoL AD(Logsdon et al., 2002) (Care Recipient) | 13-item assessment of quality of life completed by the Care Partner on behalf of the person with dementia. |
| Venus Survey | |
| Mercury Survey plus: | |
| Tele-STELLA contact | Contact with other Care Partners |
| Tele-STELLA Nova Experience Survey | 16-item + comments on satisfaction, privacy, ease of use |
| Earth Survey | |
| Mercury surveys plus: | |
| Tele-STELLA contact | Contact with other Care Partners |
| Mars Survey | |
| Mercury Survey plus: | |
| Tele-STELLA contact | Contact with other Care Partners |
| Tele-STELLA Constellation Experience Survey | 16-item + comments on satisfaction, privacy, ease of use |
| Weekly Orbit Survey: Distributed weekly during full intervention | |
| Measures Care Partner emotional and physical strain; frequency of contact with clinical providers; frequency of as-needed medication use for behavioral symptoms; out-of-pocket costs. | |
Table 4.
Tele-STELLA Care Partner Activities
| Activity | Due |
|---|---|
|
MecurySurvey (Survey 1) |
Prior to Nova Session 1 |
|
Nova Component One Guide to one Care Partner |
After Mercury Survey completed |
|
Nova Component One Guide to 5 Care Parnters |
After Nova Session 4 |
|
Venus Survey (Survey 2) |
Within one week of completion of Nova |
|
Earth Survey (Survey 3) |
1 month after last Nova Session 8 |
|
Earth Survey (Survey 4) |
1 week prior to Constellation |
|
Constellation Component 3 Guides to 20 Care Partners |
Within 1 year of Nova |
|
Mars Survey (Survey 5) |
Within one week of completion of
Constellation |
|
Earth Survey (Survey 6) |
2 months after last Constellation
Session |
| Orbit Survey: Distributed
weekly
Orbit surveys start after enrollment and continue until all surveys are completed | |
Technology
Tele-STELLA will be implemented using real-time videoconferencing via OHSU’s Webex system. This platform is secure as independently validated by OHSU’s information technology and legal departments.
Our previous pilot experience emphasized the need to retain a strong technical team to support the Guides and the Care Partners. Along with technical staff, the study employs one analyst to meet with each Care Partner prior to and during the intervention to test their videoconferencing capability and address connection problems.
Care Partners may use their own computers, tablets, or smartphones for this study. They will have a Webex link to access the study’s videoconferencing site. Care Partners who do not have a device to access the study, or adequate internet capability, may borrow a Chromebook (Google, 2021). The Chromebooks have a simplified user interface that allows access to the Tele-STELLA study only. Chromebooks will be managed by the technology team who has the capability to track the location of the Chromebooks and decommission them if they are lost (Dodge, 2019; Lindsey J, 2019; Nguyen, 2020). If all efforts to establish a videoconferencing link fail, Care Partners may access the Webex link via phone.
The Webex recording system will be used to record and store the Tele-STELLA sessions. OHSU Security Engineering department has agreed to ensure that this study’s policies and procedures meet strict Information Security Directives.
The Guides will connect with the Care Partners via the Webex system. Guides may complete the visit from their homes, offices, or other private locations. They will use headphones so that others in the Guides’ households will not overhear the sessions.
Study data will be collected and managed using Qualtrics (Qualtrics, 2021) and REDCap electronic data capture tools hosted at OHSU. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies (Harris et al., 2019). Copies of data will be transferred to a secure OHSU server via Application Programming Interface call. Other electronic data will be stored on a secure, password protected OHSU server. Access to data is restricted to study personnel with username/password authentication.
Data Analysis
For Aim 1 (feasibility and acceptability of Tele-STELLA), we will assess data from the surveys to characterize the sample, assess program acceptability, and measure treatment fidelity. For acceptability, we will analyze the Tele-STELLA Experience Survey (Lindauer et al., 2018; Lindauer et al., 2019) results using descriptive statistics.
A narrative analysis approach will be used to analyze the qualitative focus group data. The transcripts from the videorecordings will be examined to identify contextual features, themes of acceptance, or lack thereof (Kelly & Howie, 2007). These qualitative findings will be considered in relation to the quantitative data using a parallel mixed analysis approach (Teddlie & Tashakkori, 2009). This will be followed by an analysis that looks at areas of data convergence and divergence. We have used this approach in our pilots, which guided development of Tele-STELLA (Lindauer et al., 2018; Lindauer et al., 2019).
For the fidelity assessments, Cohen’s kappa coefficient will be used to calculate content expert inter-rater agreement, with the following coefficients: Almost perfect 0.81–1.00; Substantial 0.61–0.80; Moderate, 0.41–0.60, Fair 0.21–0.40; Slight/poor, <0.20 (Landis & Koch, 1977).
For Aim 2, to assess the efficacy of Tele-STELLA in reducing the frequency of behavioral symptoms and Care Partner reactivity to the symptoms, we will analyze pre/post changes on the RMBPC (Teri et al., 1992) before and after the Nova and Constellation components. Our hypothesis is that Care Partner reactivity to behavioral symptoms (RMBPC reactivity subscale score (Teri et al., 1992)) will be reduced (statistically significantly different from null) after Nova (Teri et al., 2005). We expect that the scores will be further reduced after Constellation sessions. Changes in RMBPC (Teri et al., 1992) reactivity subscale score at post Nova session (time 1) and post Constellation session (time 2) in relation to the reactivity subscale baseline score (time 0) will be examined using pre-post paired t-tests (Munro, 2005), using type-I error rate <=0.05 as a statistical significance.
We will then assess whether the above changes in the Care Partner reactivity subscale on the RMBPC (Teri et al., 1992) are associated with the following variables, controlling for age, sex, number of hours caregiving/week, rural status and education: a. number of Tele-STELLA sessions each Care Partner attended, b. the number of days prior to starting Nova sessions, and c. the number of days prior to starting Constellation sessions.
We will assess changes in the RMBPC reactivity subscale (Teri et al., 1992) over time between groups (rural White, African American; urban African American, White, SGM), with the hypothesis that there are no significant differences across groups. We will test whether the efficacy of our intervention differs across groups using mixed models for repeated measures (MMRM) with the outcome being RMBPC reactivity subscale and including variables which indicate each group and their interactions with assessment time points (baseline, post-Nova, post-Constellation).
We will also assess efficacy in the domains of Care Partner depression, QOL and pre-death grief before and after each component (Nova and Constellation), with pre-post paired t tests.46
If some participants participate by phone only, we will compare their changes in reactivity scores with those completing the study via videoconferencing by including a variable for indicating phone use only in the model.
ETHICAL CONSIDERATIONS
Tele-STELLA is multi-component intervention for Care Partners for persons with moderate to advanced ADRD. While the persons with ADRD are not involved in the intervention, we will collect data about them and they may be exposed to the intervention while their Care Partner is engaged in it. They may overhear their Care Partners discussing their behaviors with other study participants, which may be distressing for them. Care Recipients may appear on the videorecordings, jeopardizing their privacy. To minimize these risks, we will ask Care Partners to identify an activity (e.g., a TV show, visiting with another family member) that will keep the person with ADRD occupied and out of computer range during the Tele-STELLA visits. Because of these risks, we will seek Care Recipient consent for this study. If the Care Recipients are decisionally impaired, we will obtain the informed consent from Care Recipients’ legally authorized representatives.
All participants will be assigned a unique identifier that will be used instead of their name or other identifying information. However, video-recordings will show their faces and their names and any information discussed during the sessions will be heard in the audio. In this case, the information will be identifiable as coming from them and will not be private.
Care Partners will be asked to share their phone numbers and email addresses with other participants. We will ask all participants to refrain from sharing this information with others outside Tele-STELLA. The phone numbers and email addresses will not be confidential. Information about confidentiality is described in detail in the consent forms.
VALIDITY AND RELIABILITY
Measures with sound reliability were chosen to assess the primary outcomes (frequency of behaviors and Care Partner reactivity to the behaviors). The RMBPC (Teri et al., 1992) is among the most commonly-used measures of burden in caregiver research and has strong internal consistency (α=.86) (Jones et al., 2012). All surveys will a be administered in electronic form to limit social desirability and recall biases (Althubaiti, 2016).
Tele-STELLA is not a randomized controlled trial (RCT), which may limit its external validity. However, a strength of Tele-STELLA is its ecological validity, meaning it is pragmatic and adaptable to every-day life. Care Partners do not need to live with their Care Recipients, which liberalizes participation for Care Partners whose family members live in separate homes or facilities. Once in the study, allowances are made for Care Partners to stay in the study if they miss sessions or skip surveys. Accommodations are made for those who may not have computers or easy access to the internet. Survey administration permits Care Partners to complete the assessments in the privacy of their homes, on their own schedules. Finally, recognizing that the study may add additional burden to Care Partners, we provide up to $100 US to encourage participation throughout the study period.
DISCUSSION
The Tele-STELLA study is a prospective, multi-component, psychoeducational intervention designed by nurses to facilitate effective management of behavioral symptoms common in the later stages of dementia. The intervention is delivered entirely via videoconferencing with no in-person activities. This addresses a current gap in which interventions are not often tailored to dementia stage (Hopwood et al., 2018). And because the Tele-STELLA intervention is driven by the needs of the Care Partners, it is uniquely personalized to their specific concerns.
Tele-STELLA broadens nursing’s long history of individualized care to include family Care Partners, and is a natural extension of the profession’s philosophy of holistic care. Nursing has pioneered, implemented, and researched many important strategies to relieve ADRD-related caregiver strain (Buckwalter et al., 1999; Fortinsky et al., 2014; Hepburn et al., 2021). The Tele-STELLA intervention aligns this history and with the literature that indicates multicomponent, personalized internet-based interventions are most helpful in improving Care Partner well-being.
In concordance with person-centered nursing care in dementia (Fazio et al., 2018), the design of the study was informed by Care Partners who had participated in the pilot versions of Tele-STELLA (Lindauer et al., 2018; Lindauer et al., 2019). In designing Tele-STELLA, we paid careful attention to incorporating the Care Partner feedback, while at the same time considering rigor and pragmatism. We paid particular attention to the need for randomization and intervention group size.
We recognize designing Tele-STELLA as an RCT would contribute to external validity. However, an RCT at the early stage of the intervention may yield limited fidelity and questionable internal validity resulting in limited prospects for large-scale testing for effectiveness and scalability. Further, randomizing participants to an intervention with limited testing is ethically unsound. Thus, Tele-STELLA is a NIH Stage 1 non-randomized study which focuses on refining and modifying the intervention, materials and methods, as well as assessing preliminary efficacy and fidelity (Onken et al., 2014).
Next, determining group size was challenging. Care Partners in the focus groups asked for one-to-one sessions, but they also wanted multiple group sessions. The literature indicates that support groups, both in-person and via telehealth, can be helpful for Care Partners, but further suggests that stressed Care Partners may be reluctant to join groups or may have unrealistic expectations about the benefits of group work (Steffen, 2012). Coupling our experience with the literature on group size, we decided to include a 4-week, one-to-one induction period, which may familiarize Care Partners with the protocol and, ideally, allay concerns about group participation.
We also grappled with the need for the larger group sessions (Constellation). An important step in intervention development is to design interventions for communities beyond the research milieu (Onken et al., 2014). Of concern is that the benefit of the Tele-SELLA would drop off the “implementation cliff” (Weisz, 2014) when implemented at the community level and lose some of its efficacy. The Constellation component will provide valuable lessons in preparing Tele-STELLA for community use and, ideally, limit the loss of efficacy. Constellation could increase capacity for Care Partner support, which is critical to address family needs with the expanding prevalence of dementia around the world.
Worldwide, it is expected the number of people with dementia will increase to 82 million in 2030 (Alzheimer’s Disease International, 2019). Nurses are, and will continue to be, critical team members in dementia care. Nurses need interventions that are adaptable to different cultures, Care Partner needs, and geographic variabilities to support the millions of Care Partners across the globe. The detailed Tele-STELLA protocol and manuals provide instruction to future teams, and the personalized approach allows adaptation across communities, languages and values.
LIMITATIONS
We anticipate that we well face technological and scheduling challenges as we test the Tele-STELLA. The accommodations discussed above address scheduling issues. We engaged a strong technology team to anticipate and address technological concerns. Of note, while many classic, in-person Care Partner study reports document efforts to ameliorate transportation costs, few address the “engine” that permits this access. Comments about the safety, privacy and function of automobiles and public transport are rarely addressed in the extant literature. We hope future advances in internet connectivity will make documentation of technology access strategies similarly unnecessary.
In addition to the above challenges, we will likely need to modify the Tele-STELLA based on our initial findings. For example, this is the first time the Constellation component will be tested, and it is not known if it will contribute to the efficacy of the intervention. However, we know that group programing, such as Constellation, facilitates adult learning (Knowles, 2005), and peer group programming reduces isolation and reinforces positive change (Lukens & McFarlane, 2004). Considering the worldwide need for dementia education and support, Constellation could facilitate rapid dispersion of Tele-STELLA, if proven effective.
CONCLUSION
While many Care Partner support programs are available, both on- and offline, ours is one of the few to address the needs of families in the moderate to late stages, via telehealth. Our foundational work, initiated in 2016, has prepared this study for large scale testing. And while we anticipate the COVID-19 pandemic to resolve, other pandemics, natural disasters, and ADRD itself, will continue to limit in-person family support. Tele-STELLA has the potential to provide education and support to any family Care Partner with an internet connection, anywhere in the world.
Supplementary Material
Acknowledgment
The authors thank Dr. Linda Teri for her generous contributions to this work.
Funding Statement
Research reported in this publication was supported by the National Institute On Aging of the National Institutes of Health under Award Numbers R01AG067546, P30AG008017, P30AG066518. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest.
References
- Althubaiti A (2016). Information bias in health research: definition, pitfalls, and adjustment methods. Journal of Multidisciplinary Healthcare, 9, 211–217. 10.2147/JMDH.S104807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2020). Alzheimer’s disease facts and figures. Alzheimers Dement(Online). 10.1002/alz.12068 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Disease International. (2019). World Alzheimer Report 2019: Attitudes to dementia https://www.alzint.org/u/WorldAlzheimerReport2019.pdf
- Arora S, Kalishman S, Thornton K, Dion D, Murata G, Deming P, Parish B, Brown J, Komaromy M, Colleran K, Bankhurst A, Katzman J, Harkins M, Curet L, Cosgrove E, & Pak W (2010). Expanding access to hepatitis C virus treatment--Extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology, 52(3), 1124–1133. 10.1002/hep.23802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardach SH, Gibson A, Parsons K, Stauffer A, & Jicha GA (2020). Rural caregivers: Identification of informational needs through telemedicine questions. The Journal of Rural Health. 10.1111/jrh.12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JS (2011). Cognitive behavior therapy: Basics and beyond (2nd ed.). The Guilford Press. [Google Scholar]
- Boots LM, de Vugt ME, van Knippenberg RJ, Kempen GI, & Verhey FR (2014). A systematic review of Internet-based supportive interventions for caregivers of patients with dementia. International Journal of Geriatric Psychiatry, 29(4), 331–344. 10.1002/gps.4016 [DOI] [PubMed] [Google Scholar]
- Buckwalter KC, Gerdner L, Kohout F, Hall GR, Kelly A, Richards B, & Sime M (1999). A nursing intervention to decrease depression in family caregivers of persons with dementia. Archives of Psychiatric Nursing, 13(2), 80–88. 10.1016/s0883-9417(99)80024-7 [DOI] [PubMed] [Google Scholar]
- Chien LY, Chu H, Guo JL, Liao YM, Chang LI, Chen CH, & Chou KR (2011). Caregiver support groups in patients with dementia: A meta-analysis. International Journal of Geriatric Psychiatry, 26(10), 1089–1098. 10.1002/gps.2660 [DOI] [PubMed] [Google Scholar]
- Dodge HH, Hooker K, Antonucci TC. (2019). I-CONECT Project: Can social interaction improve cognitive functions among socially isolated older adults? Innovation in Aging, 3, S224. 10.1093/geroni/igz038.824 [DOI] [Google Scholar]
- Fazio S, Pace D, Flinner J, & Kallmyer B (2018, January 18). The fundamentals of person-centered care for individuals with dementia. Gerontologist, 58(suppl_1), S10–S19. 10.1093/geront/gnx122 [DOI] [PubMed] [Google Scholar]
- Fortinsky RH, Delaney C, Harel O, Pasquale K, Schjavland E, Lynch J, Kleppinger A, & Crumb S (2014). Results and lessons learned from a nurse practitioner-guided dementia care intervention for primary care patients and their family caregivers. Research in Gerontological Nursing, 7(3), 126–137. 10.3928/19404921-20140113-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MV, & Sethares KA (2014, November). Facilitators and barriers to the adoption of telehealth in older adults: an integrative review. Computers, Informatics, Nursing, 32(11), 523–533; quiz 534–525. 10.1097/CIN.0000000000000105 [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Statz TL, Birkeland RW, Louwagie KW, Peterson CM, Zmora R, Emery A, McCarron HR, Hepburn K, Whitlatch CJ, Mittelman MS, & Roth DL (2020). The ResidentialCare Transition Module: a single-blinded randomized controlled evaluation of a telehealth support intervention for family caregivers of persons with dementia living in residential long-term care. BMC geriatrics, 20(1), 133. 10.1186/s12877-020-01542-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Google. (2021). Chromebook. Retrieved April 3 from https://www.google.com/chromebook/
- Guthrie GE, & Bogue RJ (2015). Impact of a shared medical appointment lifestyle intervention on weight and lipid parameters in individuals with type 2 diabetes: A clinical pilot. Journal of the American College of Nutrition, 34(4), 300–309. 10.1080/07315724.2014.933454 [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, & Duda SN (2019). The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, 95, 103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn K, Nocera J, Higgins M, Epps F, Brewster GS, Lindauer A, Morhardt D, Shah R, Nash R, & Griffiths PC (2021). Results of a randomized trial testing the efficacy of Tele-Savvy, an online synchronous/asynchronous psychoeducation program for family caregivers of persons living with dementia. Gerontologist. 10.1093/geront/gnab029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann LK, Welter E, Leverenz J, Lerner AJ, Udelson N, Kanetsky C, & Sajatovic M (2018). A systematic review of dementia-related stigma research: Can we move the stigma dial? The American Journal of Geriatric Psychiatry, 26(3), 316–331. 10.1016/j.jagp.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Hopwood J, Walker N, McDonagh L, Rait G, Walters K, Iliffe S, Ross J, & Davies N (2018). Internet-based interventions aimed at supporting family caregivers of people with dementia: Systematic review. Journal of medical Internet research, 20(6), e216. 10.2196/jmir.9548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isik AT, Soysal P, Solmi M, & Veronese N (2018). Bidirectional relationship between caregiver burden and neuropsychiatric symptoms in patients with Alzheimer’s disease: A narrative review. International Journal of Geriatric Psychiatry. 10.1002/gps.4965 [DOI] [PubMed] [Google Scholar]
- Jones C, Edwards RT, & Hounsome B (2012). Health economics research into supporting carers of people with dementia: A systematic review of outcome measures. Health and Quality of Life Outcomes, 10, 142–7525-7510–7142. 10.1186/1477-7525-10-142 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales HC, Gitlin LN, & Lyketsos CG (2015). Assessment and management of behavioral and psychological symptoms of dementia. BMJ, 350, h369. 10.1136/bmj.h369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin NJ, Bell PA, Noah JL, Martichuski DK, & Knight BL (1999). Assessing Alzheimer’s support group participation: A retrospective follow-up. American Journal of Alzheimer’s Disease, 14(6), 326–333. https://www.scopus.com/inward/record.uri?eid=2-s2.0-0033404588&partnerID=40&md5=e2448001e8a7a93b96dd1900adc12734 [Google Scholar]
- Kelly T, & Howie L (2007). Working with stories in nursing research: procedures used in narrative analysis. International Journal of Mental Health Nursing, 16(2), 136–144. 10.1111/j.1447-0349.2007.00457.x [DOI] [PubMed] [Google Scholar]
- Knowles M, Holton EF III, Swanson RA. (2005). The adult learner (6th ed.). Elsevier. [Google Scholar]
- Kolanowski A, Boltz M, Galik E, Gitlin LN, Kales HC, Resnick B, Van Haitsma KS, Knehans A, Sutterlin JE, Sefcik JS, Liu W, Petrovsky DV, Massimo L, Gilmore-Bykovskyi A, MacAndrew M, Brewster G, Nalls V, Jao YL, Duffort N, & Scerpella D (2017). Determinants of behavioral and psychological symptoms of dementia: A scoping review of the evidence. Nursing Outlook, 65(5), 515–529. 10.1016/j.outlook.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar VV, Huang H, Zhao L, Verble DD, Nutaitis A, Tharwani SD, Brown AL, Zetterberg H, Hu W, Shin R, Kehoe PG, Quyyumi A, Nocera J, Kippels A, & Wharton W (2020). Baseline results: The association between cardiovascular risk and preclinical Alzheimer’s disease pathology (ASCEND) study. Journal of Alzheimer’s Disease, 75, 109–117. 10.3233/JAD-191103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis JR, & Koch GG (1977). The measurement of observer agreement for categorical data. Biometrics, 33(1), 159–174. https://www.ncbi.nlm.nih.gov/pubmed/843571 [PubMed] [Google Scholar]
- Lindauer A, Croff R, Mincks K, Mattek N, Shofner SJ, Bouranis N, & Teri L (2018). “It took the stress out of getting help”: The STAR-C-Telemedicine mixed methods pilot. Care Weekly, 2, 7–14. 10.14283/cw.2018.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer A, & Harvath TA (2014). Pre-death grief in the context of dementia caregiving: A concept analysis. Journal of Advanced Nursing, 70(10), 2196–2207. 10.1111/jan.12411 [DOI] [PubMed] [Google Scholar]
- Lindauer A, & Harvath TA (2015). The meanings caregivers ascribe to dementia-related changes in care recipients: A meta-ethnography. Research in Gerontological Nursing, 8(1), 39–48. 10.3928/19404921-20141121-01 [DOI] [PubMed] [Google Scholar]
- Lindauer A, McKenzie G, LaFazia D, McNeill L, Mincks K, Spoden N, Myers M, Mattek N, & Teri LL (2019). Using technology to facilitate fidelity assessments: The Tele-STAR caregiver intervention. Journal of Medical Internet Research,, 21(5), e13599. 10.2196/13599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer A, Tarter R (2021). Guide Manual. Available upon request: TeleSTELLA@ohsu.edu. [Google Scholar]
- Lindauer A, Tran L, Tarter R, McAteer P (2021). Tele-STELLA Handbook. Available upon request: TeleSTELLA@ohsu.edu [Google Scholar]
- Lindauer A, Wild K, Natonson A, Mattek N, Wolf M, Steeves-Reece A, & Messecar D (2020). Dementia 360 ECHO: Using technology to facilitate diagnosis and treatment. Gerontology & Geriatrics Education, 1–7. 10.1080/02701960.2020.1835658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey J GE, Nguyen K, Guerrero CL, Scavone C, Pandya S, Dorsey T, Dodge H. (2019). Overcoming the challenges of technology in gerontological research. Innovation in Aging, 3, S225. 10.1093/geroni/igz038.827 [DOI] [Google Scholar]
- McCurry SM, Logsdon RG, Mead J, Pike KC, La Fazia DM, Stevens L, & Teri L (2017). Adopting evidence-based caregiver training programs in the real world: Outcomes and lessons learned from the STAR-C Oregon translation study. Journal of Applied Gerontology, 36(5), 519–536. 10.1177/0733464815581483 [DOI] [PubMed] [Google Scholar]
- Munro BH (2005). Statisical methods for health care research (5th ed.). Lippincott, Williams and Wilkins. [Google Scholar]
- National Institutes of Health. (2016, June21). Final NIH Policy on the Use of a Single Institutional Review Board for Multi-Site Research. Retrieved NOT-OD-16–094 from https://grants.nih.gov/policy/humansubjects/single-irb-policy-multi-site-research.htm
- Nguyen K, Scavone C, Kaye JA, MacDonald M, & Dodge HH (2020). Successful transitions to remote assessments and technology installations due to COVID-19: the I-CONECT Project. Innovation in Aging, 4, S958. 10.1093/geroni/igaa057.3502 [DOI] [Google Scholar]
- Onken LS, Carroll KM, Shoham V, Cuthbert BN, & Riddle M (2014). Reenvisioning clinical science: Unifying the discipline to improve the public health. Clinical Psychological Science: A Journal of the Association for Psychological Science, 2(1), 22–34. 10.1177/2167702613497932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein K, & Gaugler JE (2012). The problem with “problem behaviors”: a systematic review of the association between individual patient behavioral and psychological symptoms and caregiver depression and burden within the dementia patient–caregiver dyad. International Psychogeriatrics, 24(10), 1536–1552. 10.1017/S1041610212000737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JG, Jabson JM, & Bowen DJ (2017). Measuring sexual and gender minority populations in health surveillance. LGBT Health, 4(2), 82–105. 10.1089/lgbt.2016.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualtrics. (2021). Survey Software. Retrieved April 7, 2021 from https://www.qualtrics.com
- Schulz R, Belle SH, Czaja SJ, McGinnis KA, Stevens A, & Zhang S (2004). Long-term care placement of dementia patients and caregiver health and well-being. JAMA : The Journal of the American Medical Association, 292(8), 961–967. 10.1001/jama.292.8.961 [doi] [DOI] [PubMed] [Google Scholar]
- Selwood A, Johnston K, Katona C, Lyketsos C, & Livingston G (2007). Systematic review of the effect of psychological interventions on family caregivers of people with dementia. Journal of Affective Disorders,, 101(1–3), 75–89. 10.1016/j.jad.2006.10.025 [DOI] [PubMed] [Google Scholar]
- Steffen AMM, K. R. (2012). Predicting attendance at dementia family support groups. American Journal of ALzheimer’s Disease & Other Dementias, 27(8), 633–639. 10.1177/1533317512465677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teddlie CT, & Tashakkori A (2009). Foundations of mixed methods research. Sage. [Google Scholar]
- Teri L, & Logsdon RG (1991). Identifying pleasant activities for Alzheimer’s disease patients: the pleasant events schedule-AD. The Gerontologist, 31(1), 124–127. [DOI] [PubMed] [Google Scholar]
- Teri L, McCurry SM, Logsdon R, & Gibbons LE (2005). Training community consultants to help family members improve dementia care: A randomized controlled trial. The Gerontologist, 45(6), 802–811. 10.1093/geront/45.6.802 [DOI] [PubMed] [Google Scholar]
- Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, & Vitaliano PP (1992). Assessment of behavioral problems in dementia: The Revised Memory and Behavior Problems Checklist. Psychology & Aging, 7(4), 622–631. 10.1037//0882-7974.7.4.622 [DOI] [PubMed] [Google Scholar]
- Weisz JR, Ng MY, Bearman SK (2014). Odd couple? Reenvisioning the relation between science and practice in the dissemination-implementation Era. Clinical Psychological Science : A Journal of the Association for Psychological Science, 2(1), 58–74. 10.1177/2167702613501307 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.